
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Endocrinol., 26 January 2023
Sec. Clinical Diabetes
Volume 14 - 2023 | https://doi.org/10.3389/fendo.2023.1094353
This article is part of the Research TopicThe Role of Diabetes in the Pathophysiology and Prognosis of Ischemic StrokeView all 13 articles
 Zheng Dai1,2†
Zheng Dai1,2† Haiming Cao3†
Haiming Cao3† Feng Wang2
Feng Wang2 Lei Li2
Lei Li2 Hongquan Guo4
Hongquan Guo4 Xiaohao Zhang3
Xiaohao Zhang3 Haichang Jiang2
Haichang Jiang2 Juehua Zhu5
Juehua Zhu5 Yongjun Jiang6*
Yongjun Jiang6* Dezhi Liu7*
Dezhi Liu7* Gelin Xu1,8,9*
Gelin Xu1,8,9*Background and Purpose: Hyperglycemia has been associated with unfavorable outcome of acute ischemic stroke, but this association has not been verified in patients with endovascular thrombectomy treatment. This study aimed to assess the impact of stress hyperglycemia ratio on early neurological deterioration and favorable outcome after thrombectomy in patients with acute ischemic stroke.
Methods: Stroke patients with endovascular thrombectomy in two comprehensive centers were enrolled. Early neurological deterioration was defined as ≥4 points increase of National Institutes of Health Stroke Scale (NIHSS) at 24 hours after endovascular procedure. Favorable outcome was defined as modified Rankin Scale (mRS) score of 0-2 at 90 days of stroke onset. Multivariate regression analysis was used to identify the predictors for early neurological deterioration and favorable outcome.
Results: Among the 559 enrolled, 74 (13.2%) patients developed early neurological deterioration. The predictors for early neurological deterioration were high stress hyperglycemia ratio at baseline (OR =5.77; 95% CI, 1.878-17.742; P =0.002), symptomatic intracranial hemorrhage (OR =4.90; 95% CI, 2.439-9.835; P <0.001) and high NIHSS score after 24 hours (OR =1.11; 95% CI, 1.071-1.151; P <0.001). The predictors for favorable outcome were stress hyperglycemia ratio (OR =0.196, 95% CI, 0.077-0.502; P =0.001), age (OR =0.942, 95% CI, 0.909-0.977; P =0.001), NIHSS score 24 hours after onset (OR =0.757, 95% CI =0.693-0.827; P <0.001), groin puncture to recanalization time (OR =0.987, 95% CI, 0.975-0.998; P =0.025), poor collateral status before treatment (ASITN/SIR grade 0-3, OR =62.017, 95% CI, 25.920-148.382; P <0.001), successful recanalization (mTICI 2b or 3, OR =7.415, 95% CI, 1.942-28.313; P =0.001).
Conclusion: High stress hyperglycemia ratio may be related to early neurological deterioration and decreased likelihood of favourable outcomes after endovascular thrombectomy in patients with acute ischemic stroke.
Endovascular thrombectomy has been involving as the first-line treatment for acute ischemic stroke caused by large artery occlusion (1–3). However, mechanical recanalization not always necessarily resulted in favorable outcome even when patients were treated within 6 hours of stroke onset (4, 5). Exploring the possible factors associated with early neurological deterioration (END), a strong predictor for functional outcomes, is of vital importance for continuously improving the efficacy of endovascular thrombectomy in stroke patients (6, 7).
Hyperglycemia was associated with END in patients with acute ischemic stroke (8–10). Hyperglycemia could destruct blood-brain barrier, aggravate ischemic lesion, increase risk of hemorrhage transformation after cerebral infarction, and reduce duration of ischemic penumbra existence (11–14). Glycated hemoglobin (HbA1c) is more stable than blood glucose level in patients with acute ischemic stroke, and stress hyperglycemia ratio, defined as the stress fasting glycemia/HbA1c ratio (SHR), may be more feasible for evaluating the functional outcome. Some studies observed that stroke patients with high SHR had decreased likelihood of favorable functional outcome and increased likelihood of recurrence and intracranial hemorrhage after recanalization treatment (15–17); others missed these phenomena (18). Therefore, the relationship between SHR and END or functional outcome after endovascular recanalization treatment in patients with acute ischemic stroke is far from determined. This study aimed to investigate the effects of SHR on END and functional outcome in patients with acute ischemic stroke and treated with endovascular thrombectomy.
Stroke patients with endovascular thrombectomy in two comprehensive centers were screened for eligibility during November 1, 2018 and May 31, 2022. Local ethic review board approved the study protocol. Due to its retrospective nature, patient consent was waived.
Patients were treated with endovascular thrombectomy if they: 1) aged 18 years or old; 2) had ischemic stroke caused by large artery occlusion in anterior or posterior circulation; 3) pre-stroke mRS score ≤2; and 4) had arterial sheath being placed in 6 hours of stroke onset or met the DAWN or DEFUSE criteria (19, 20). Patients were not treated with endovascular thrombectomy if they: 1) had a life expectancy <12 months; 2) had severe cardiopulmonary failure; 3) had a platelet count of <55×1000/mm3; or 4) had anemia (hemoglobin <100 g/l) or other conditions which may affect HbA1c measurement.
All thrombectomy procedures were performed with Solitaire (Covidien, Irvine, CA) and Catalyst6 devices (Stryker, Kalamazoo, MI) alone or in combination. Successful recanalization was defined as grade 2b-3 in modified thrombolysis in cerebral infarction (mTICI). Collateral circulation was evaluated using the American Society of Interventional and Therapeutic Neuroradiology/Society of Interventional Radiology collateral vessel grading system (ASITN/SIR), and categorized into grade 0 or 1, 2, and 3 or 4. The door to groin puncture time (DPT), groin puncture to final recanalization time (PRT), number of retriever passes, intravenous thrombolysis, and rescue treatment (including angioplasty, stenting, intra-artery thrombolysis) were recorded. Possible pre-procedure infarction were quantified using the Alberta Stroke Program Early CT Score (ASPECTS) or ASPECTS for posterior circulation (pc-ASPECTS) on non-contrast CT. CT was performed immediately and 24 hours after the endovascular procedures to detect possible intracranial hemorrhage. An extra CT scan was arranged whenever as the novel symptoms indicated.
Stroke severity was assessed using the National Institutes of Health Stroke Scale (NIHSS) score. Stress hyperglycemia ratio was defined as the stress fasting glycemia/HbA1c ratio (SHR). END was defined as an increase of 24-hour NIHSS score of ≥4 points after endovascular procedure (21). Favorable outcome was defined as a mRS score of 0-2 at 90 days of stroke onset. Symptomatic intracranial hemorrhage (sICH) was defined and classified according to the European Cooperative Acute Stroke Study (ECASS-III) criteria (22). Malignant brain edema was defined and classified according to the SITS-MOST (Safe Implementation of Thrombolysis in Stroke-Monitoring Study) protocol, and grade 3 was defined as malignant edema (23, 24).
Categorical variables were expressed as frequencies and percentages, and analyzed with χ2 or Fisher’s exact test. Quantitative variables were expressed as medians and interquartile ranges (IQRs), and were analyzed with Mann-Whitney U test. Receiver Operating Characteristic Curve (ROC) was constructed to explore the cutoff value of SHR for predicting favorable outcome. Multivariable logistical regression model was used to assess the potential factors associated with favorable outcome. Parameters with P <0.05 in univariate analysis entered in multivariate analysis. The covariates included in the multivariable logistical regression were mTICI score (2b or 3), MCE, sICH, NIHSS 24 hours after procedure, Pre-procedure ASPCET score, PTR, Pre-procedure ASITN/SIR score, homocysteine, retriever passes >3 times, lymphocyte, HbA1c, fasting blood glucose, glycosylated hemoglobin, SHR. Model 1 and Model 2 were diabetic group and non-diabetic group, respectively. Model 3 included fasting blood glucose and glycosylated hemoglobin as confounders, and model 4 excluded fasting blood glucose and glycosylated hemoglobin as confounders. P value of <0.05 was considered as statistically significant. Statistical analyses were performed using SPSS 25.0 (IBM, Armonk, NY).
A total of 559 stroke patients were enrolled. The median (IQR) age was 70 (63–77) years, and NIHSS score after 24 hours of thrombectomy was 12 (6-19). There were 357 (63.9%) male patients. Among the enrolled, 74 (13.2%) patients developed END. There were 69 (12.3%) patients occurred sICH in 24 hours, and 81 patients (14.5%) died in 90 days. Favorable outcome was obtained in 284 (50.8%) patients. There were 190 (34.0%) patients had high SHR. The ROC curve showed that the optimal cutoff value of SHR for predicting favorable outcome was 1.07, the sensitivity was 84.7%, the specificity was 52.5%, and the Youden index was 0.372 (Figure 1).
Compared with patients without END, those with END had higher median NIHSS scores after 24 hours (29 vs. 11, P <0.001), higher homocysteine (16.4 vs. 14.6, P =0.029), higher fasting glucose levels (8.7 vs. 6.9, P <0.001), higher glycated hemoglobin (6.4 vs. 5.9, P =0036), higher SHR (1.4 vs. 1.1, P <0.001), lower pre-procedure ASPECTS score (8 vs. 9, P =0.001), lower pre-procedure ASITN grade (1 vs. 3, P <0.001), longer PRT (80 vs. 67, P =0.014), higher proportion of multiple retriever passes (16.2% vs. 8.2%, P =0.028), lower proportion of successful recanalization (83.8% vs. 91.3%, P =0.040), and higher proportion of malignant brain edema (28.4% vs. 15.5%, P =0.006). The proportion of sICH was higher (48.6% vs. 6.8%, P <0.001) in patient with END than that in patient without. Proportion of favorable outcome was lower (10.8% vs. 56.9%, P <0.001), and mortality (33.8% vs. 11.5%, P <0.001) was higher in patients with END. Moreover, subgroup analysis showed that proportion of favorable outcome was lower in patients with diabetes mellitus. (Table 1, Figure 2).
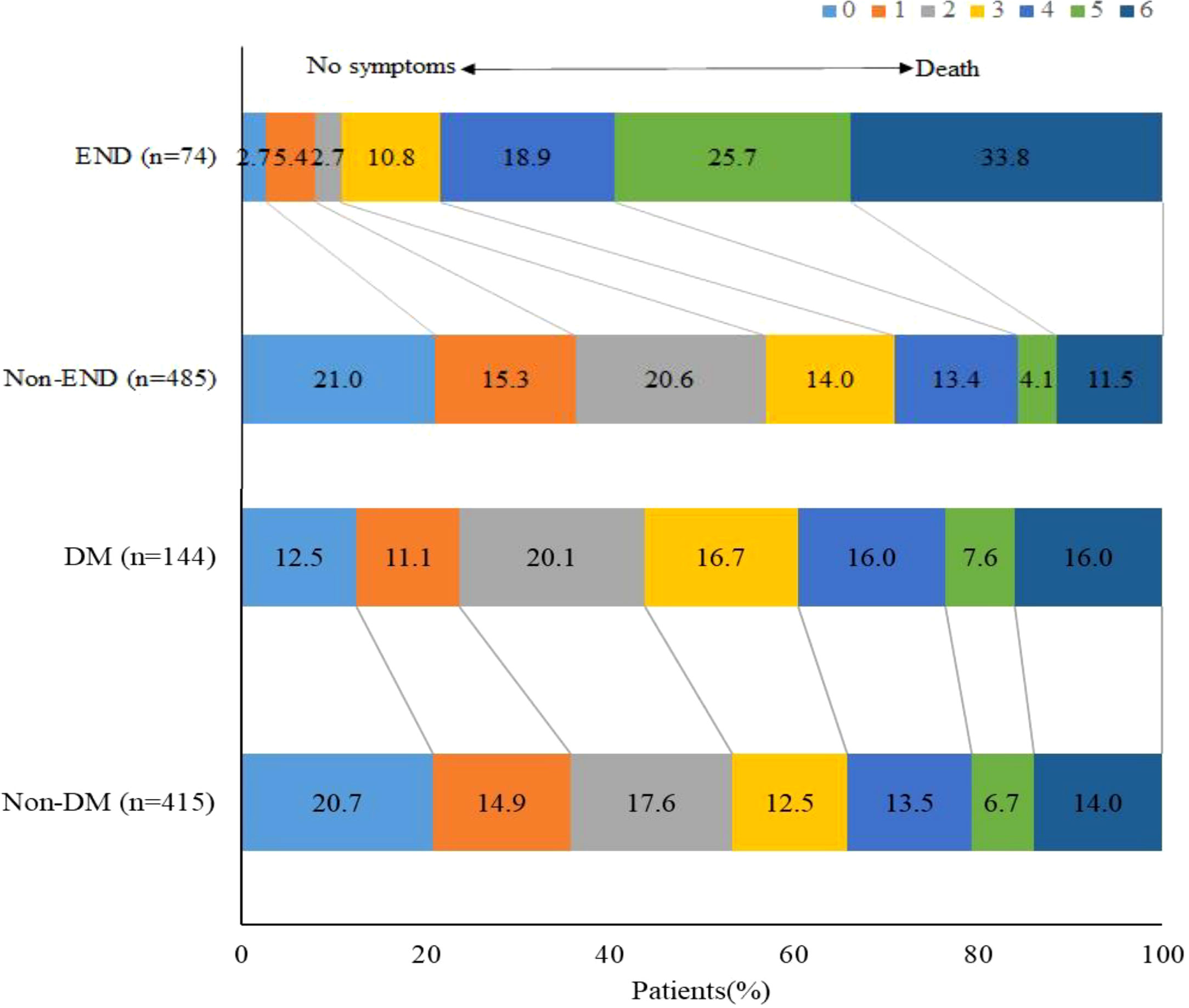
Figure 2 Functional outcomes according to END and DM. Distribution of modified Rankin Scale scores at 90 days.
In Model 3 with unadjusted fasting blood glucose and glycosylated hemoglobin being adjusted, SHR (OR =4.78; 95% CI, 1.38-16.60; P =0.014), sICH (OR =5.04; 95% CI, 2.49-10.21; P <0.001), and NIHSS score at 24 hours (OR =1.10; 95% CI, 1.06-1.15; P <0.001) were related to END. In Model 4 with fasting blood glucose and glycosylated hemoglobin being adjusted, SHR (OR =5.77; 95% CI, 1.88-17.74; P =0.002), sICH (OR =4.90; 95% CI, 2.44-9.84; P <0.001) and NIHSS score at 24 hours (OR =1.11; 95% CI, 1.07-1.15; P <0.001) were related to END (Figure 3).
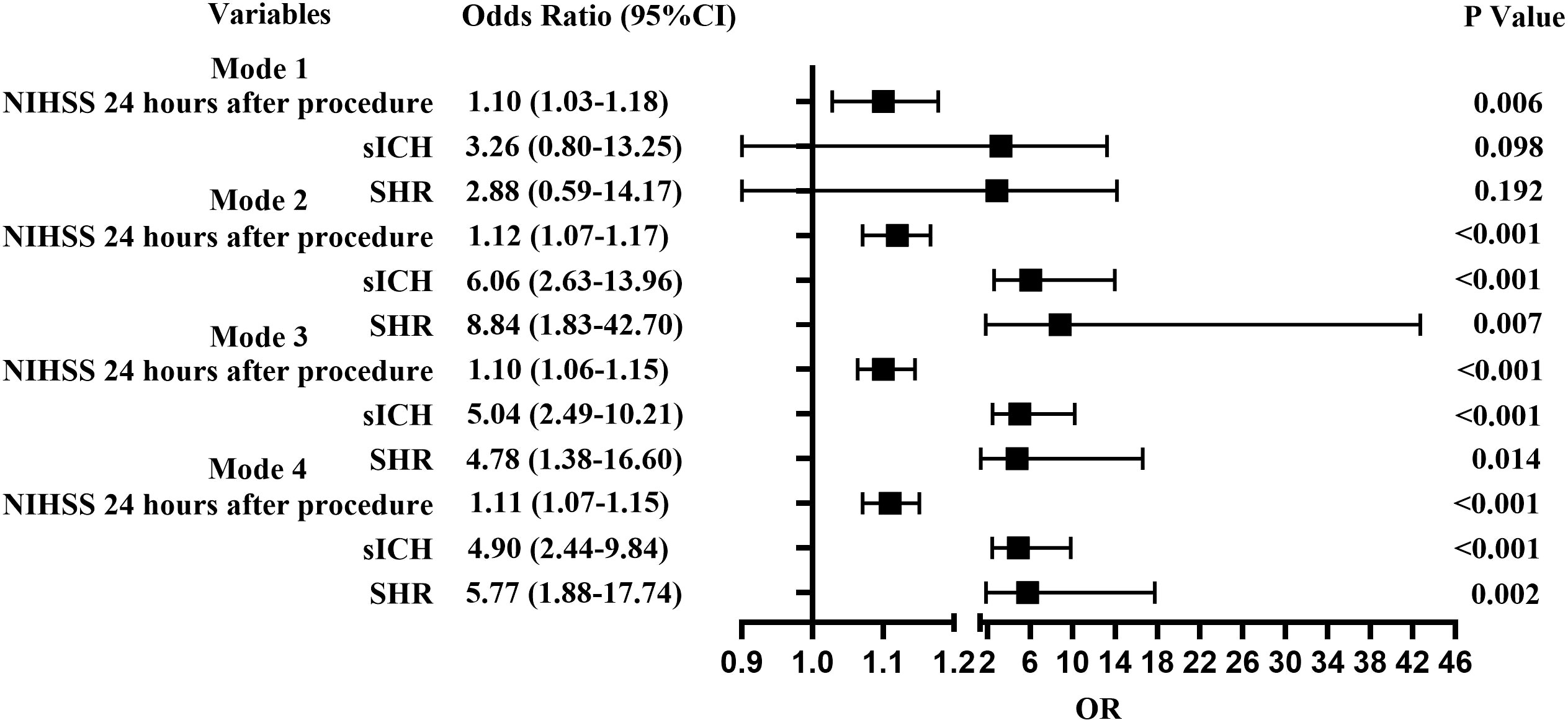
Figure 3 Forest plots for predictors of END. Model 1, Diabetic group; Model 2, Non-diabetic group; Model 3, Including fasting blood glucose and glycosylated hemoglobin as confounders; Model 4, Excluding fasting blood glucose and glycosylated hemoglobin as confounders.
When patients were stratified as with and without diabetes mellitus (DM) and adjusted for major confounding factors, multivariant analysis detected that sICH (OR =6.06; 95% CI, 2.63-13.96; P <0.001), 24-hour NIHSS score (OR =1.12; 95% CI, 1.07-1.17; P <0.001), and high SHR (OR =8.84; 95% CI, 1.83-42.70; P =0.007) could influence the development of END in patients without diabetes mellitus. High NIHSS score after 24 hours (OR =1.10; 95% CI, 1.03-1.18; P =0.006) could influence the development of END in patients with diabetes mellitus (Figure 3).
Multivariant analysis detected that SHR (OR =0.20, 95% CI, 0.08-0.50; P =0.001), age (OR =0.94, 95% CI, 0.91-0.98; P =0.001), baseline NIHSS score (OR =1.15, 95% CI, 1.05-1.25; P =0.002), NIHSS score after 24 hours (OR =0.76, 95% CI, 0.69-0.83; P <0.001), PRT (OR =0.99, 95% CI, 0.98-1.00; P =0.025), pre-procedure ASITN grade (OR =62.02, 95% CI, 25.92-148.38; P <0.001) and successful recanalization (OR =7.42, 95% CI, 1.94-28.31; P =0.001) were associated with favorable outcome (Table 2).
This study observed that stroke patients with high SHR had increased incidence of END and decreased likelihood of favorable outcome after endovascular treatment.
SHR was determined as a better quantitative indicator for stress hyperglycemia than blood glucose level when evaluating the outcomes of critical illness (25). High SHR has been associated with increased risk of END and poor outcome in patients with intravenous thrombolysis. But no study on relationship between SHR and END has been reported in patients with endovascular thrombectomy (26). The underlying mechanism for SHR influencing END may be multifactorial. First, increased lactate productions may deteriorate ischemic condition, and disrupt neuron metabolism in penumbra areas (27). Second, stress hyperglycemia could aggravate hemorrhagic transformation after ischemic stroke by inducing mitochondrial dysfunction and endothelial cell apoptosis (28). Third, stress hyperglycemia may have adverse effects on collateral circulation (29). Fourth, the prothrombotic effect of stress hyperglycemia could result in thrombus extension and blood-brain barrier destruction (30).
Previous study confirmed that patients with high SHR had an increased risk of symptomatic intracranial hemorrhage and mortality after endovascular thrombectomy (15). This study associated SHR and unfavorable outcome in patients treated with endovascular thrombectomy. Several explanations may account for the association between SHR and unfavorable outcome after endovascular thrombectomy. First, acute stress response may lead to enhance inflammation reaction, which in turn leads to increased hepatic glycogenolysis, insulin resistance, cell endothelial injury, platelet aggregation, and mitochondrial dysfunction (11, 31). Second, stress hyperglycemia may directly damage ischemic brain tissue through lactic acid accumulation and intra-cellular acidosis, and aggravate ischemic injury (32). Third, stress hyperglycemia could generate reperfusion injury via oxidative stress and inflammatory process with increased expression of endothelial adhesion molecules and monomeric C-reactive protein (33). Fourth, stress hyperglycemia may disrupt blood-brain barrier and promote hemorrhagic transformation (34).
Several limitations of this study should be address when interpreting the results. END was defined as NIHSS score increase within 24 hours after endovascular procedures, but this condition could occur a few days later. We did not monitor dynamics changes of SHR. The effects of antidiabetic agents and anesthesia were not assessed.
High stress hyperglycemia ratio may be related to early neurological deterioration and decreased likelihood of favorable outcomes after endovascular thrombectomy in patients with acute ischemic stroke.
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/supplementary material.
The studies involving human participants were reviewed and approved by the ethics committees of the Affiliated Wuxi People’s Hospital of Nanjing Medical University and the Affiliated Nanjing Hospital of Nanjing Medical University. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
ZD and HC contributed equally to the conception of the research and drafted the manuscript. LL, HJ, and HG acquired the data. XZ, JZ, and FW analyzed the data. YJ revised the manuscript and made contribution to the revision, GX and DL revised the manuscript and approved the final version of the manuscript. All authors contributed to the article and approved the submitted version.
This work was supported by Foundation of Wuxi Municipal Health Commission (#M202142); the National Science Foundation of China (81471181 and 81870933), Guangdong Basic and Applied Basic Research Foundation (2021A1515011351), Guangzhou Science and Technology Project (202102010127), and the Opening Lab Program of Guangzhou Medical University (0506308) to YJ.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Powers WJ, Rabinstein AA, Ackerson T, Adeoye OM, Bambakidis NC, Becker K, et al. Guidelines for the early management of patients with acute ischemic stroke: 2019 update to the 2018 guidelines for the early management of acute ischemic stroke: A guideline for healthcare professionals from the American heart Association/American stroke association. Stroke (2019) 50:e344–418. doi: 10.1161/STR.0000000000000211
2. Pierot L, Jayaraman MV, Szikora I, Hirsch JA, Baxter B, Miyachi S, et al. Standards of practice in acute ischemic stroke intervention: International recommendations. J neurointerv Surg (2018) 10:1121–6. doi: 10.1136/neurintsurg-2018-014287
3. Turc G, Bhogal P, Fischer U, Khatri P, Lobotesis K, Mazighi M, et al. European Stroke organisation (ESO) - European society for minimally invasive neurological therapy (ESMINT) guidelines on mechanical thrombectomy in acute ischaemic stroke endorsed by stroke alliance for Europe (SAFE). Eur Stroke J (2019) 4:6–12. doi: 10.1177/2396987319832140
4. Olivot JM, Heit JJ, Mazighi M, Raposo N, Albucher JF, Rousseau V, et al. What predicts poor outcome after successful thrombectomy in early time window? J neurointerv Surg (2022) 14:1051–5. doi: 10.1136/neurintsurg-2021-017946
5. Goyal M, Menon BK, van Zwam WH, Dippel DWJ, Mitchell PJ, Demchuk AM, et al. Endovascular thrombectomy after large-vessel ischaemic stroke: A meta-analysis of individual patient data from five randomised trials. Lancet (2016) 387:1723–31. doi: 10.1016/s0140-6736(16)00163-x
6. van de Graaf RA, Samuels N, Chalos V, Lycklama ANGJ, van Beusekom H, Yoo AJ, et al. Predictors of poor outcome despite successful endovascular treatment for ischemic stroke: Results from the MR CLEAN registry. J neurointerv Surg (2022) 14:660–5. doi: 10.1136/neurintsurg-2021-017726
7. Zhou T, Yi T, Li T, Zhu L, Li Y, Li Z, et al. Predictors of futile recanalization in patients undergoing endovascular treatment in the DIRECT-MT trial. J neurointerv Surg (2022) 14:752–5. doi: 10.1136/neurintsurg-2021-017765
8. Bhole R, Nouer SS, Tolley EA, Turk A, Siddiqui AH, Alexandrov AV, et al. Predictors of early neurologic deterioration (END) following stroke thrombectomy. J neurointerv Surg (2022) 18:neurintsurg–2022-018844. doi: 10.1136/neurintsurg-2022-018844
9. Zhang M, Xing P, Tang J, Shi L, Yang P, Zhang Y, et al. Predictors and outcome of early neurological deterioration after endovascular thrombectomy: A secondary analysis of the DIRECT-MT trial. J neurointerv Surg (2022) 10:neurintsurg–2022-018976. doi: 10.1136/neurintsurg-2022-018976
10. Huang ZX, Huang Y, Zeng J, Hao H, Petroski GF, Lu H, et al. Admission glucose levels may increase the risk for early neurological deterioration in females with acute ischemic stroke. Front Neurol (2020) 11:548892. doi: 10.3389/fneur.2020.548892
11. Kruyt ND, Biessels GJ, Devries JH, Roos YB. Hyperglycemia in acute ischemic stroke: Pathophysiology and clinical management. Nat Rev Neurol (2010) 6:145–55. doi: 10.1038/nrneurol.2009.231
12. Virani SS, Alonso A, Benjamin EJ, Bittencourt MS, Callaway CW, Carson AP, et al. Heartdisease and stroke statistics-2020 update: A report from the American heart association. Circulation (2020) 141:e139–596. doi: 10.1161/CIR.0000000000000757
13. Huo X, Liu R, Gao F, Ma N, Mo D, Liao X, et al. Effect of hyperglycemia at presentation on outcomes in acute large artery occlusion patients treated with solitaire stent thrombectomy. Front Neurol (2019) 10:71. doi: 10.3389/fneur.2019.00071
14. Arnold M, Mattle S, Galimanis A, Kappeler L, Fischer U, Jung S, et al. Impact of admission glucose and diabetes on recanalization and outcome after intra-arterial thrombolysis for ischaemic stroke. Int J Stroke (2014) 9:985–91. doi: 10.1111/j.1747-4949.2012.00879.x
15. Merlino G, Pez S, Gigli GL, Sponza M, Lorenzut S, Surcinelli A, et al. Stress hyperglycemia in patients with acute ischemic stroke due to large vessel occlusion undergoing mechanical thrombectomy. Front Neurol (2021) 12:725002. doi: 10.3389/fneur.2021.725002
16. Merlino G, Smeralda C, Gigli GL, Lorenzut S, Pez S, Surcinelli A, et al. Stress hyperglycemia is predictive of worse outcome in patients with acute ischemic stroke undergoing intravenous thrombolysis. J Thromb Thrombolysis (2021) 51:789–97. doi: 10.1007/s11239-020-02252-y
17. Shen CL, Xia NG, Wang H, Zhang WL. Association of stress hyperglycemia ratio with acute ischemic stroke outcomes post-thrombolysis. Front Neurol (2021) 12:785428. doi: 10.3389/fneur.2021.785428
18. Osei E, den Hertog HM, Berkhemer OA, Fransen PSS, Roos Y, Beumer D, et al. Admission glucose and effect of intra-arterial treatment in patients with acute ischemic stroke. Stroke (2017) 48:1299–305. doi: 10.1161/STROKEAHA.116.016071
19. Nogueira RG, Jadhav AP, Haussen DC, Bonafe A, Budzik RF, Bhuva P, et al. Thrombectomy 6 to 24 hours after stroke with a mismatch between deficit and infarct. N Engl J Med (2018) 378:11–21. doi: 10.1056/NEJMoa1706442
20. Albers GW, Marks MP, Kemp S, Christensen S, Tsai JP, Ortega-Gutierrez S, et al. Thrombectomy for stroke at 6 to 16 hours with selection by perfusion imaging. N Engl J Med (2018) 378:708–18. doi: 10.1056/NEJMoa1713973
21. Seners P, Ben Hassen W, Lapergue B, Arquizan C, Heldner MR, Henon H, et al. Prediction of early neurological deterioration in individuals with minor stroke and large vessel occlusion intended for intravenous thrombolysis alone. JAMA Neurol (2021) 78:321–8. doi: 10.1001/jamaneurol.2020.4557
22. Hacke W, Kaste M, Bluhmki E, Brozman M, Dávalos A, Guidetti D, et al. Thrombolysis with alteplase 3 to 4.5 hours after acute ischemic strokee. N Engl J Med (2008) 359:1317–29. doi: 10.1056/NEJMoa0804656
23. Wahlgren N, Ahmed N, Dávalos A, Ford GA, Grond M, Hacke W, et al. Thrombolysis with alteplase for acute ischaemic stroke in the safe implementation of thrombolysis in stroke-monitoring study (SITS-MOST): An observational study. Lancet (2007) 369:275–82. doi: 10.1016/s0140-6736(07)60149-4
24. Zhang X, Yan S, Zhong W, Yu Y, Lou M. Early nt-probnp (n-terminal probrain natriuretic peptide) elevation predicts malignant edema and death after reperfusion therapy in acute ischemic stroke patients. Stroke (2021) 52:537–42. doi: 10.1161/STROKEAHA.120.029593
25. Roberts GW, Quinn SJ, Valentine N, Alhawassi T, O'Dea H, Stranks SN, et al. Relative hyperglycemia, a marker of critical illness: Introducing the stress hyperglycemia ratio. J Clin Endocrinol Metab (2015) 100:4490–7. doi: 10.1210/jc.2015-2660
26. Wang L, Cheng Q, Hu T, Wang N, Wei X, Wu T, et al. Impact of stress hyperglycemia on early neurological deterioration in acute ischemic stroke patients treated with intravenous thrombolysis. Front Neurol (2022) 13:870872. doi: 10.3389/fneur.2022.870872
27. Bourcier R, Goyal M, Muir KW, Desal H, Dippel DWJ, Majoie C, et al. Risk factors of unexplained early neurological deterioration after treatment for ischemic stroke due to large vessel occlusion: a post hoc analysis of the HERMES study. J neurointerv Surg (2022), neurintsurg–2021-018214. doi: 10.1136/neurintsurg-2021-018214
28. Parsons MW, Barber PA, Desmond PM, Baird TA, Darby DG, Byrnes G, et al. Acute hyperglycemia adversely affects stroke outcome: a magnetic resonance imaging and spectroscopy study. Ann Neurol (2002) 52:20–8. doi: 10.1002/ana.10241
29. Wiegers EJA, Mulder M, Jansen IGH, Venema E, Compagne KCJ, Berkhemer OA, et al. Clinical and imaging determinants of collateral status in patients with acute ischemic stroke in Mr clean trial and registry. Stroke (2020) 51:1493–502. doi: 10.1161/STROKEAHA.119.027483
30. Lemkes BA, Hermanides J, Devries Jh, Holleman F, Meijers Jc, Hoekstra JB. Hyperglycemia: a prothrombotic factor? J Thromb Haemost (2010) 8:1663–9. doi: 10.1111/j.1538-7836.2010.03910.x
31. Dungan KM, Braithwaite SS, Preiser JC. Stress hyperglycaemia. Lancet (2009) 373:1798–807. doi: 10.1016/S0140-6736(09)60553-5
32. Capes SE, Hunt D, Malmberg K, Pathak P, Gerstein HC. Stress hyperglycemia and prognosis of stroke in nondiabetic and diabetic patients: a systematic overview. Stroke (2001) 32:2426–32. doi: 10.1161/hs1001.096194
33. Gasche Y, Copin JC, Sugawara T, Fujimura M, Chan PH. Matrix metalloproteinase inhibition prevents oxidative stress-associated blood-brain barrier disruption after transient focal cerebral ischemia. J Cereb Blood Flow Metab (2001) 21:1393–400. doi: 10.1097/00004647-200112000-00003
Keywords: acute ischemic stroke, early neurological deterioration, endovascular thrombectomy, large artery occlusion, stress hyperglycemia ratio
Citation: Dai Z, Cao H, Wang F, Li L, Guo H, Zhang X, Jiang H, Zhu J, Jiang Y, Liu D and Xu G (2023) Impacts of stress hyperglycemia ratio on early neurological deterioration and functional outcome after endovascular treatment in patients with acute ischemic stroke. Front. Endocrinol. 14:1094353. doi: 10.3389/fendo.2023.1094353
Received: 10 November 2022; Accepted: 13 January 2023;
Published: 26 January 2023.
Edited by:
Sun Wen, The First Affiliated Hospital of University of Science and Technology of China Anhui Provincial Hospital, ChinaReviewed by:
Pan Zhang, University of Science and Technology of China, ChinaCopyright © 2023 Dai, Cao, Wang, Li, Guo, Zhang, Jiang, Zhu, Jiang, Liu and Xu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Gelin Xu, Z2VsaW54dUBuanUuZWR1LmNu; Dezhi Liu, eXpsZHpAMTI2LmNvbQ==; Yongjun Jiang, amlhbmd5am5qdUBnbWFpbC5jb20=
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.