- 1Department of Endocrinology, Hebei General Hospital, Shijiazhuang, China
- 2Department of School of Post Graduate Studies, Hebei Medical University, Shijiazhuang, Hebei, China
- 3Department of Clinical Laboratory, Hebei General Hospital, Shijiazhuang, China
- 4Hebei Key Laboratory of Metabolic Diseases, Hebei General Hospital Shijiazhuang, Hebei, China
- 5Department of Internal Medicine, Hebei Medical University, Shijiazhuang, Hebei, China
Objective: The hemoglobin glycation index (HGI) reflects biological variability in hemoglobin A1c. Even so, studies on the relationship between HGI and non-alcoholic fatty liver disease (NAFLD) are limited. Therefore, this study aimed to explore the relationship between HGI and NAFLD. In addition, the study also aimed to provide new methods to identify patients with a high risk for the development of NAFLD.
Methods: This was a retrospective study based on physical examination data from Japan. Patients were divided into quartiles (Q1–Q4) according to their HGI level; the lowest quartile (Q1) was used as the reference group. Patents were also classified into two subgroups based on the presence or absence of NAFLD. Baseline characteristics between the groups were compared. Multivariate logistic regression analysis was used to investigate the association between the HGI and NAFLD. A mediation analysis examined the mediation relationship between HGI and NAFLD. Subgroup analyses were performed to the reliability of the results.
Results: A total of 14280 patients were eligible for inclusion in this study; 2515 had NAFLD. Patients in the NAFLD group had higher levels of HGI than patients in the non-NAFLD group. Increases in HGI correlated with an increased risk of NAFLD. After adjusting for confounding factors, the multivariate logistic regression analysis revealed that HGI was positively related to the prevalence of NAFLD. In addition, mediation analysis showed that body mass index (BMI) partly mediated the indirect impact of HGI on NAFLD preference. Subgroup analyses were performed according to age, sex, smoking status, and waist circumference. Our results indicated that HGI significantly correlated with NAFLD in patients with one of the following factors: age ≤60 years, BMI >28 kg/m2, female sex, a history of smoking, and abdominal obesity.
Conclusions: HGI was an independent risk factor for NAFLD, and BMI partly mediated the association between HGI and NAFLD.
1 Introduction
Non-alcoholic fatty liver disease (NAFLD) is a pathological syndrome characterized by excessive fat accumulation in hepatocytes in the absence of excessive alcohol consumption or other definite factors, including non-alcoholic fatty liver, non-alcoholic steatohepatitis (NASH), cirrhosis, and hepatocellular carcinoma (1–3). Previous studies have shown that NAFLD correlates with metabolic disorders related to insulin resistance (IR), such as type 2 diabetes mellitus, obesity, and metabolic syndromes. Additionally, NAFLD predisposes patients to atherosclerosis (4–7). Previous studies have shown that NAFLD correlates with increased cardiovascular disease (CVD) risks,chronic kidney disease and cancer (8–10). Globally, NAFLD is a common cause of chronic liver disease. The incidence of NAFLD increases annually, and the global prevalence of NAFLD is ~ 30% (11). The prevalence of NAFLD, in Asians is expected to increase by 20-35% during the next decade (12). This rapid increase in the incidences of NAFLD will significantly impact healthcare and economic systems worldwide.
Hemoglobin A1c (HbA1c) is a diagnostic tool for diabetes and pre-diabetes, and it reflects the mean plasma glucose levels over the last 2–3 months. Recent studies suggest that blood glucose levels and interindividual variations contribute to HbA1c levels (13, 14). Consequently, patients with similar mean plasma glucose levels may have differing HbA1c levels. mean plasma glucose levels account for approximately 60%–80% of the variance in HbA1c levels (15). Based on these limitations, Hempe et al (16) developed a mathematical method in 2002, known as the hemoglobin glycation index (HGI), to quantify the difference between measured and predicted HbA1c levels. Subsequent studies demonstrated that HGI correlates with the observed variabilities in HbA1c levels (17, 18). An assessment of HGI in the Actions to Control Cardiovascular Risk in Diabetes trial showed that patients with diabetes and a high HGI had increased risks for diabetic retinopathies and nephropathies. Similar findings were reported in the Diabetes Control and Complication Trial (19, 20). Previous studies have also demonstrated an association between HGI and the risk of CVD in type 2 diabetes mellitus patients (21–24). High HGI levels, even in non-diabetic patients, correlate with an increased risk for atherosclerosis (25, 26). Mi et al. (27) observed that HGI is an independent risk factor for the development of hypertension despite HbA1c levels. In another study, HGI positively correlated with an increased risk for CVD, independent of other glycemic control factors (26). In addition, a prospective cohort study in Korea showed that HGI correlates with significant morbidity in CVD in the presence of controlled HbA1c levels (23). These findings strongly suggest that HGI directly impacts CVD compared to other glucose‐controlling indexes.
Nevertheless, previous studies on HGI primarily focused on vascular complications associated with diabetes. As such, studies demonstrating a relationship between HGI and NAFLD are limited. Therefore, this study aimed to elucidate the association between HGI and NAFLD.
2 Materials and methods
2.1 Study design and the subjects
2.1.1 Data sources
Data for this study were derived from the “Ectopic fat obesity presents the greatest risk for incident type 2 diabetes: a population-based longitudinal study” research (28). Patient data for this study were collected between 2004 and 2015. The ethics committee of Murakami Memorial Hospital approved the study. In addition, written informed consent was obtained from each patient for the inclusion of their data in this study. The authors of the original study waived copyright and related ownership for these data. Therefore, we used these data for secondary analysis without infringing on the rights of the authors.
2.1.2 Inclusion and exclusion criteria
All patients who visited the Murakami Memorial Hospital for routine physical examinations between 2004 and 2015 were enrolled in this study. Patient data, inclusive of personal data and test indicators, were recorded. Patients with alcoholic liver disease, viral hepatitis, diabetes mellitus (including type 1 and type 2 diabetes, gestational diabetes, and special types of diabetes), fasting plasma glucose (FPG) ≥6.1 mmol/l HbA1c ≥6.5%, taking any medications at baseline, incomplete data, or excessive alcohol consumption (> 210 g per week in men and > 140 g per week in women during the past 12 months) were excluded from this study.
2.1.3 Data collection and measures
The following subject variables were included in the dataset: sex, age, smoking history, body mass index (BMI), waist circumference (WC), total cholesterol (TC), triglyceride (TG), high-density lipoprotein cholesterol (HDL-C), FPG, HbA1c, Aspartate aminotransferase (AST), alanine aminotransferase (ALT), γ-glutamyl transferase (GGT), systolic blood pressure (SBP), and diastolic blood pressure (DBP). In addition, a fatty liver was diagnosed through abdominal ultrasonography, performed by trained technicians.
2.2 Research methods
2.2.1 Calculation of HGI
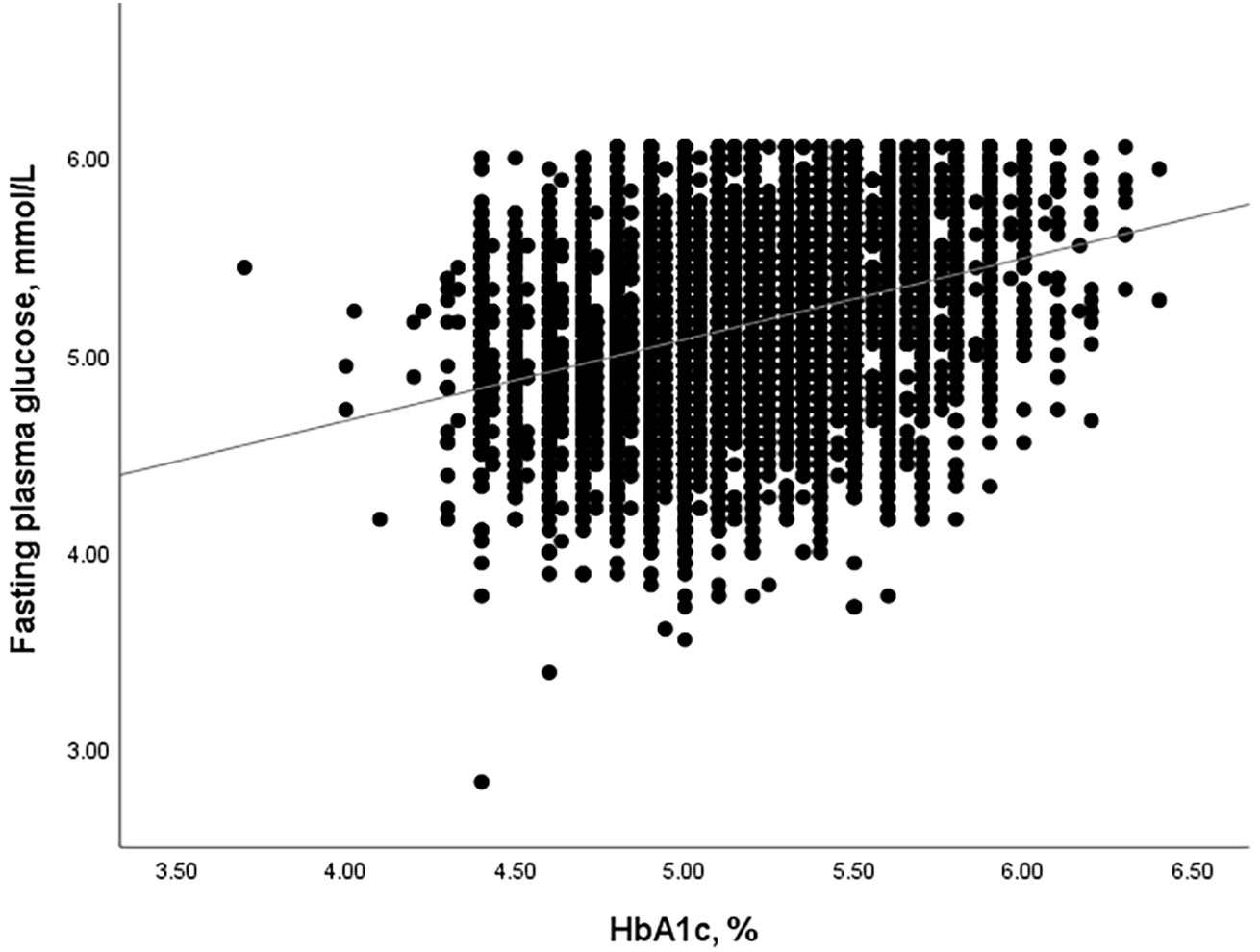
HGI was defined as the difference between the measured HbA1c levels and the predicted HbA1c levels (HGI = measured HbA1c - predicted HbA1c). Linear regression analysis was used to calculate the predicted HGI values (predicted HbA1c = 0.250 × FPG (mmol/L) + 3.889, r=0.321, and P<0.001, Figure 1).
2.2.2 Statistical methods
Statistical analyses were performed with SPSS 25.0 statistical software. The results were expressed as the mean ± SD if the quantitative data were normally distributed. An independent sample t-test was used for intergroup comparisons, and multiple group comparisons were made with a one-way analysis of variance. Quantitative data that did not conform to the normal distribution are presented as the median (P25 and P75). The non-parametric Kruskal-Wallis test was used for comparisons between the different groups. Counting data were expressed as n (%), and a chi-square test was adopted. Pearson correlations were used for normally distributed data, while Spearman correlations were used for abnormally distributed data. Multivariate logistic regressions were performed to assess the associations between HGI and NAFLD. Subgroup analyses were performed to assess the robustness of the results in relation to specific variables.A likelihood ratio test was used to examine the interaction between TyG index and variables used for stratification. Finally, mediation analysis investigated factors that mediated the relationship between HGI and NAFLD. Differences were considered statistically significant at p < 0.05.
3 Results
3.1 Comparison of baseline characteristics between NAFLD and non-NAFLD groups
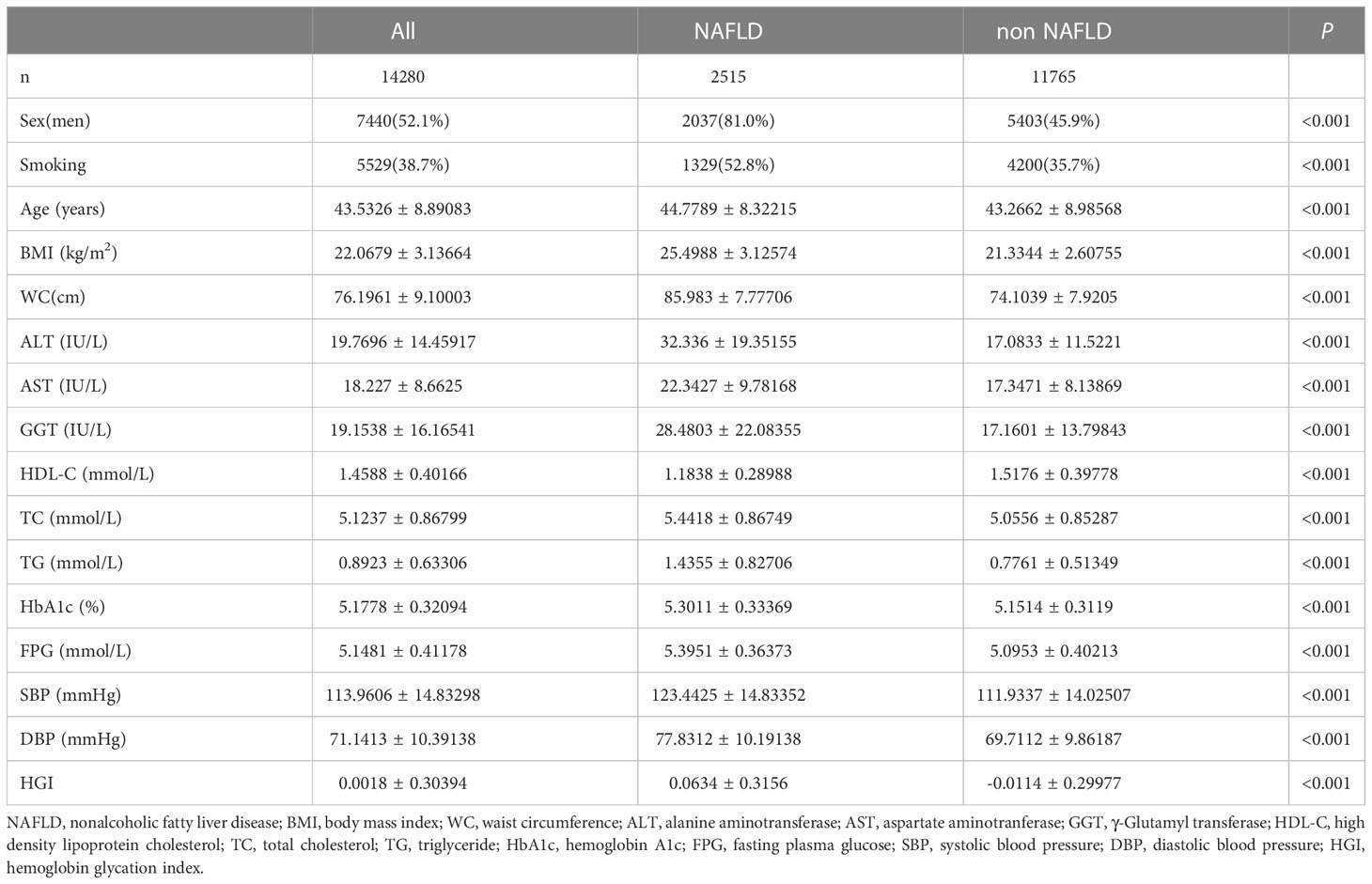
A total of 14280 patients were included in this study, including 7440 males and 6840 women, with an average age of 43.53 ( ± 8.89) years old; 11765 patients did not have NAFLD (non-NAFLD group), while 2515 patients had NAFLD (NAFLD group). At presentation, patients in the NAFLD group had significantly higher HGI, age, BMI, WC, TG, TC, AST, ALT, GGT, SBP, DBP, and lower HDL-C levels than patients in the non-NAFLD group. In addition, the number of smokers in the NAFLD group was higher than those in the non-NAFLD group (Table 1).
3.2 Baseline characteristics of study subjects according to the levels of HGI
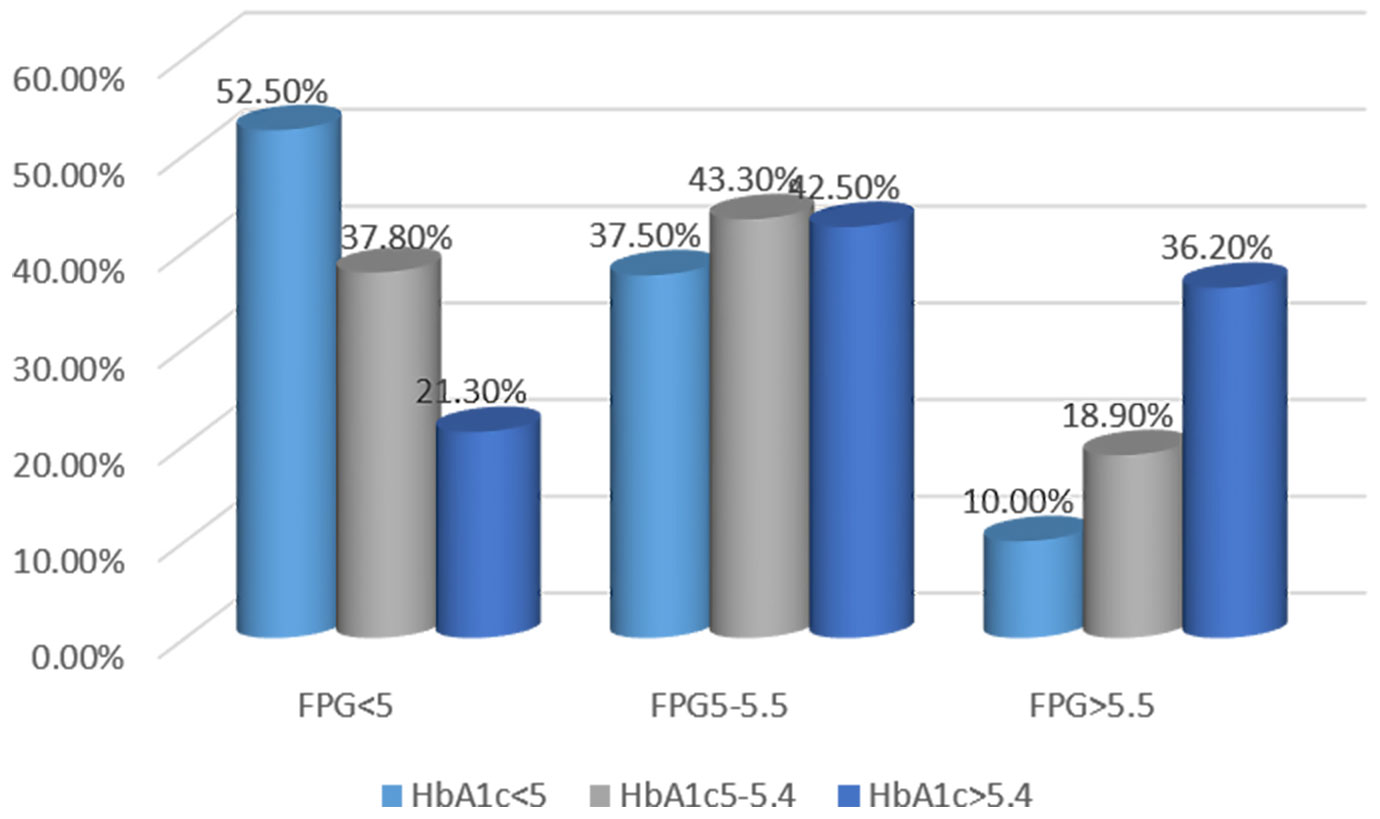
This study showed that 21.3% of patients had HbA1c levels > 5.4%, even in patients with FPG <5 mmol/L. HbA1c levels < 5% were identified in 10% of patients subjects with FPG >5.5 mmol/L. These results indirectly illustrate the need for further study on HGI (Figure 2).
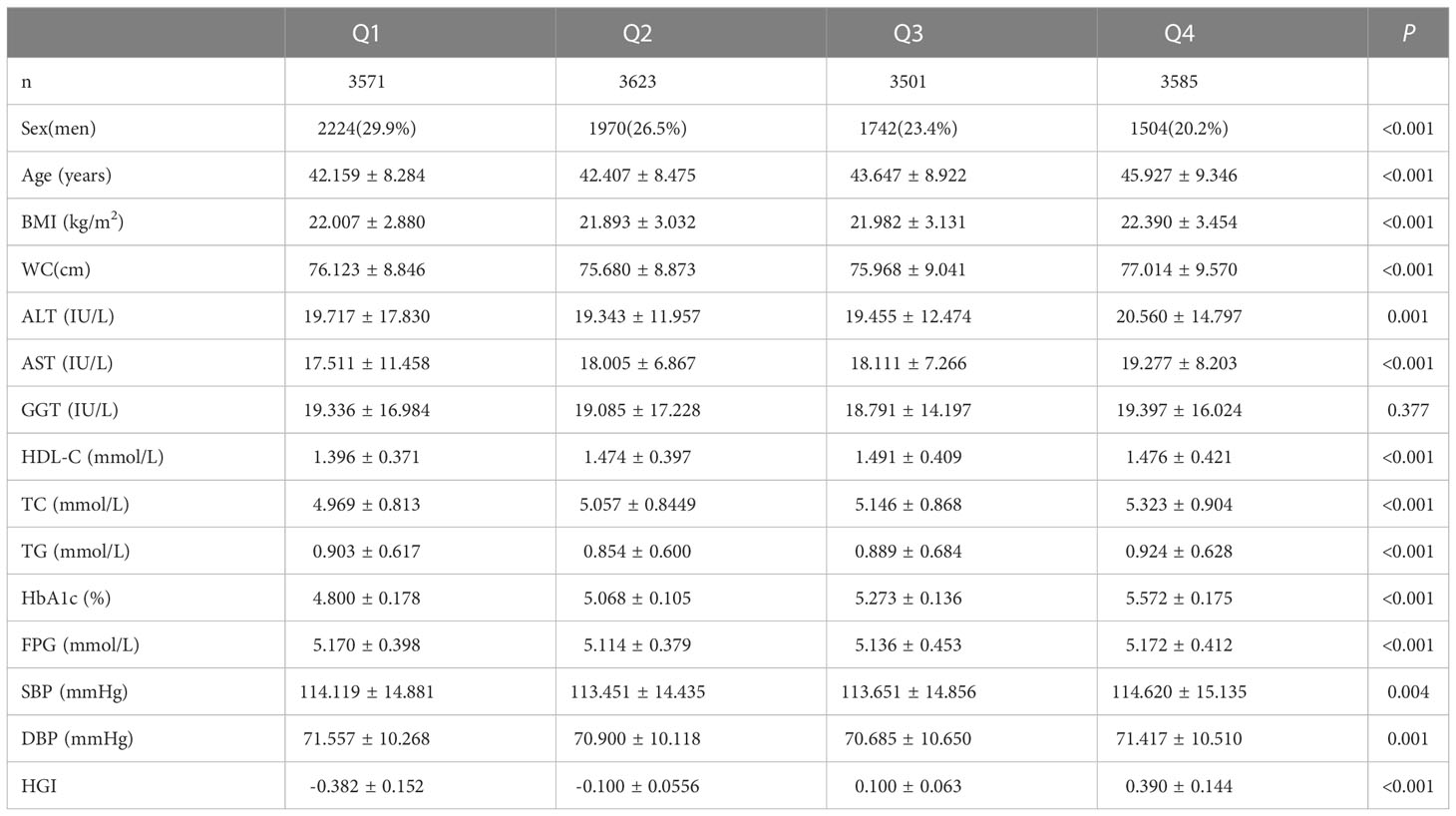
Subjects were grouped into four groups (Q1-Q4) in ascending order according to the interquartile range of the HGI. There were proportional increases between HGI and TC levels,HGI and the female proportion, while the number of smokers proportionally decreased. Statistical differences were noted between the groups. The age of patients in the Q4 group was significantly higher than that of patients in the Q1, Q2, and Q3 groups. Similarly, the age of patients in the Q3 group was higher than those in the Q2 and Q1 groups. WC and BMI were higher in the Q4 group than in the other groups (Q1–3). The Q4 group had higher ALT and SBP levels than the Q2 and Q3 groups. AST levels in the Q4 group were higher than in the Q1, Q2, and Q3 groups. However, AST levels in the Q3 group were higher than in the Q1 group. HDL-C levels in the Q2, Q3, and Q4 groups were higher than in the Q1 group. TG levels in the Q4 group were significantly higher than in the Q2 group. Similarly, TG levels in the Q2 group were higher than in the Q1 group. Finally, DBP levels in the Q4 group were higher than in the Q3 group (Table 2).
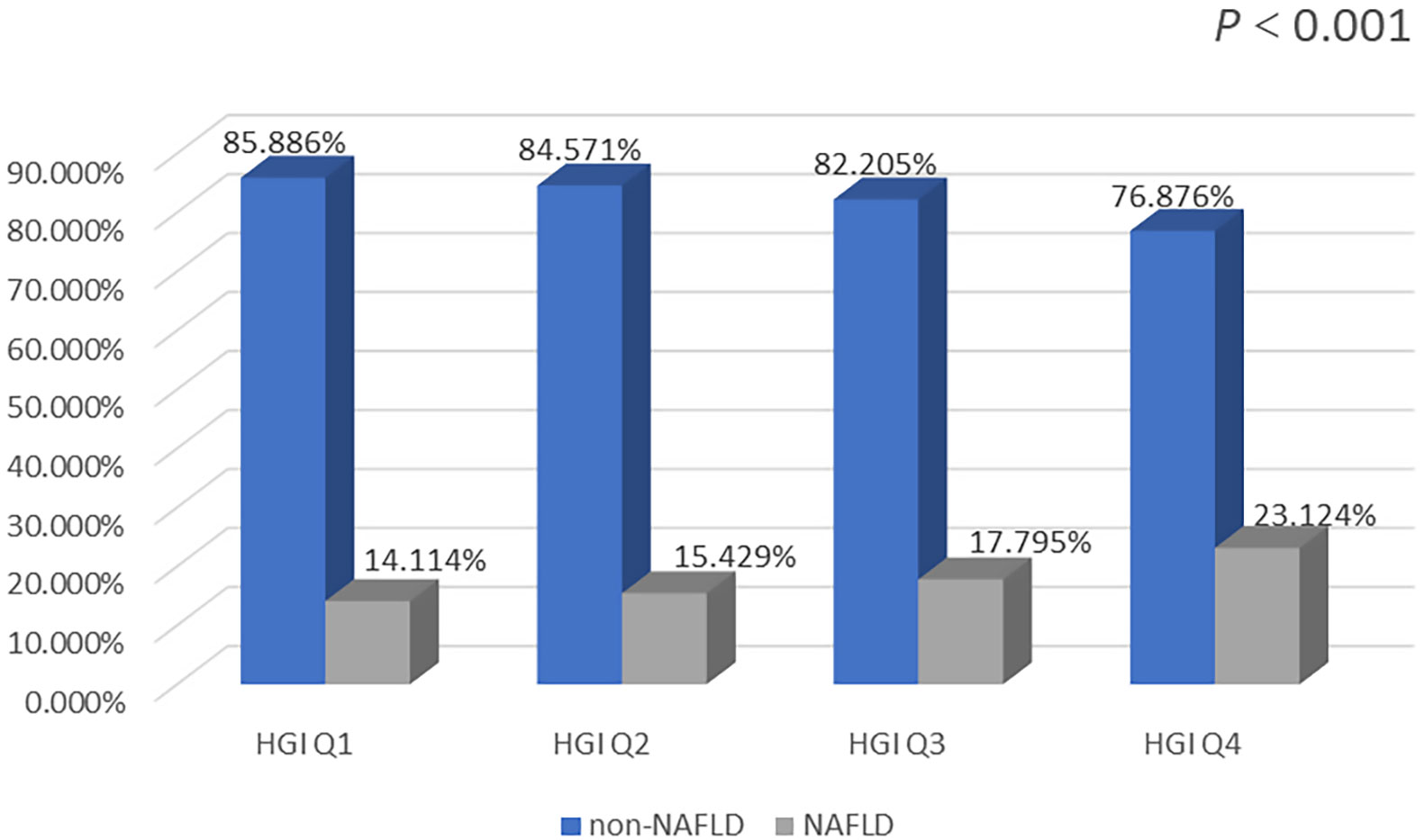
The prevalence of NAFLD was markedly higher in the Q2, Q3, and Q4 groups than in the Q1 group. In addition, the Q4 group had the highest prevalence of NAFLD. The prevalence of NAFLD increased significantly with increasing HGI (Figure 3).
3.3 The correlations between HGI and potential risk factors of NAFLD
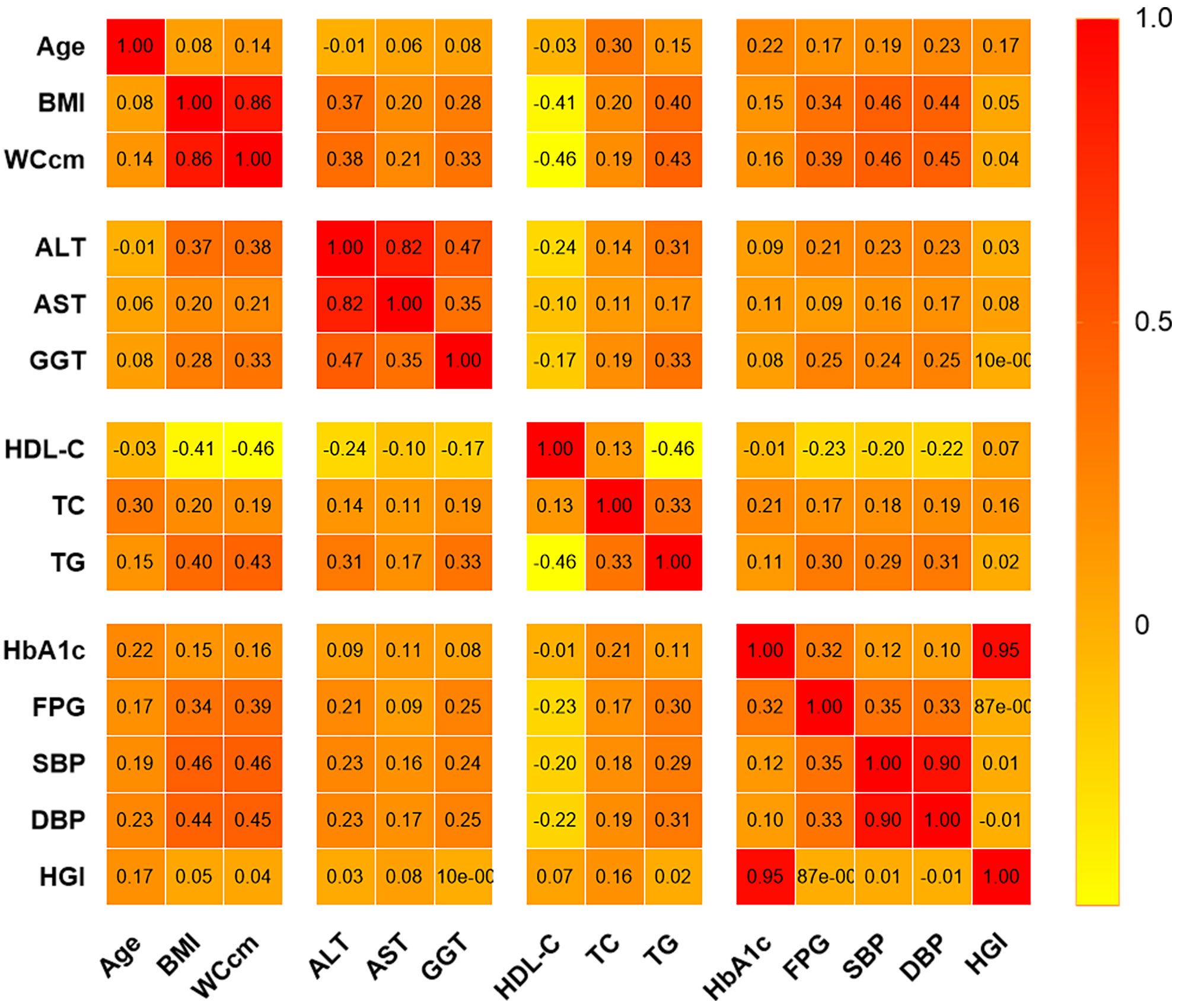
There were no significant correlations between HGI and GGT, DBP, or SBP. However, correlation analysis revealed a positive correlation between HGI and age (r=0.172, P<0.001), BMI (r=0.046, P<0.001), WC (r=0.038, P<0.001), ALT (r=0.025, P=0.003), AST (r=0.083, P<0.001), TC (r=0.161, P<0.001), HDL-C (r=0.071, P<0.001), and TG (r=0.017, P=0.045) (Figure 4).
3.4 The association between HGI and NAFLD in multivariable logistic regression analyses
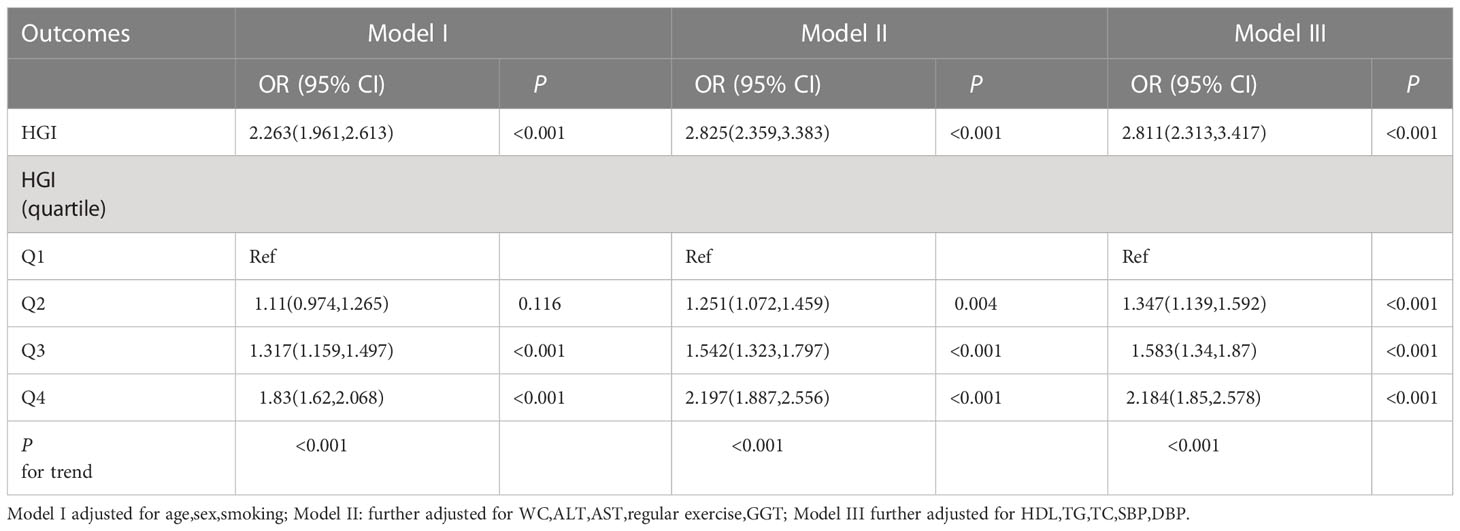
Multivariate logistic regression analysis showed that HGI was an independent risk factor for NAFLD (OR 2.811; 95% CI, 2.313-3.417; P < 0.001) after adjusting for sex, age, smoking, BMI, WC, ALT, AST, GGT, HDL-C, TC, TG, SBP, and DBP. In addition, HGI was analyzed as a categorical variable to ensure the robustness of the results. The results showed a significant positive correlation between HGI and the risk of NAFLD (Table 3).
3.5 Mediated effect of BMI on the association between HGI and NAFLD
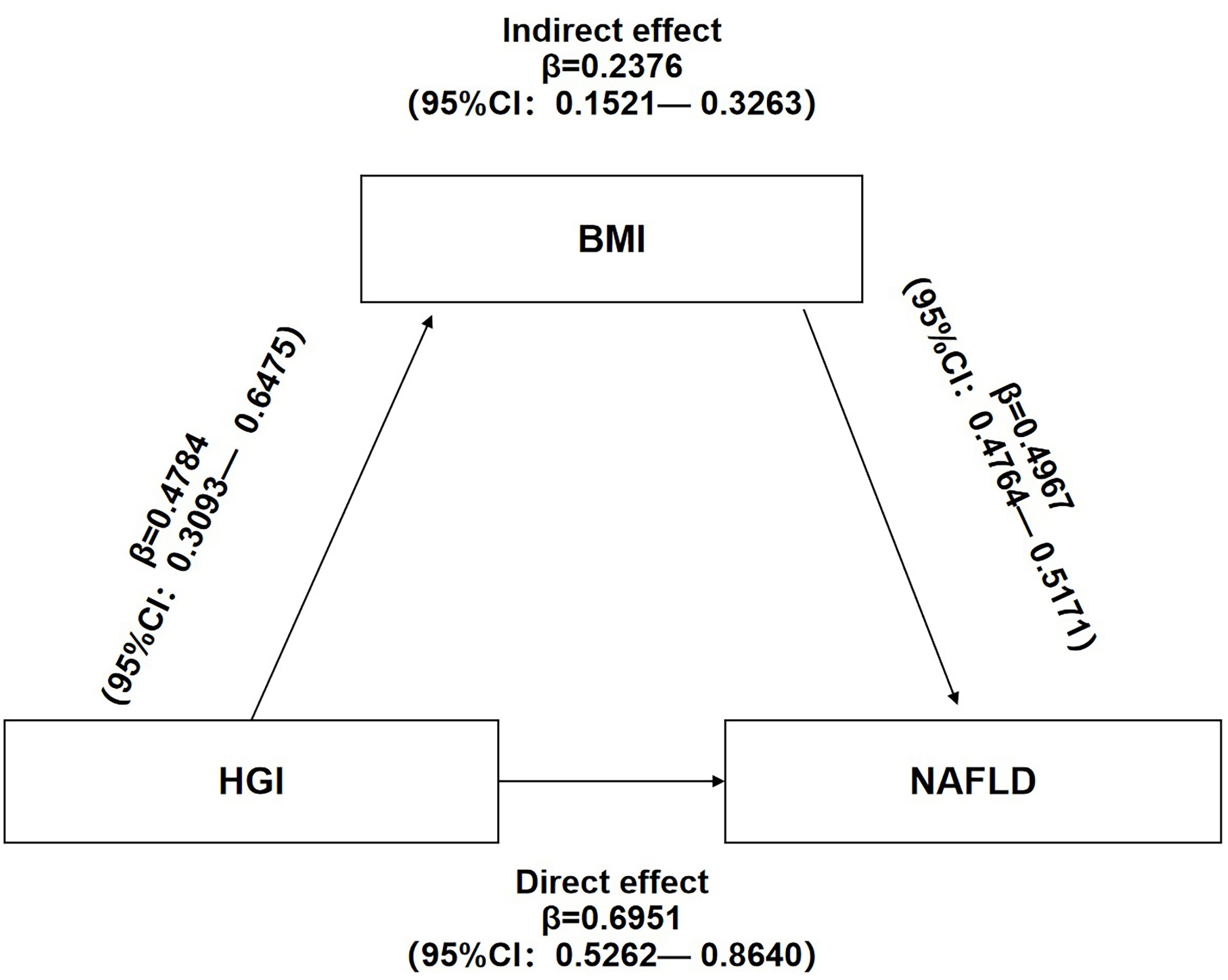
Mediation analysis of the relationship between HGI, BMI, and NAFLD showed that HGI and BMI were risk factors for NAFLD. Additionally, HGI positively correlated with BMI. These results suggest potential mechanisms in which BMI links HGI with NAFLD. Mediation analysis also showed that HGI significantly impacted the prevalence of NAFLD (β=0.6951,95%CI:0.5262-0.8640) and that BMI partly mediated the indirect impact of HGI on the incidence of NAFLD (β=0.2376,95%CI:0.1521-0.3263). A mediated percentage of 25.47% was observed in the model (Figure 5).
3.6 Subgroup analyses
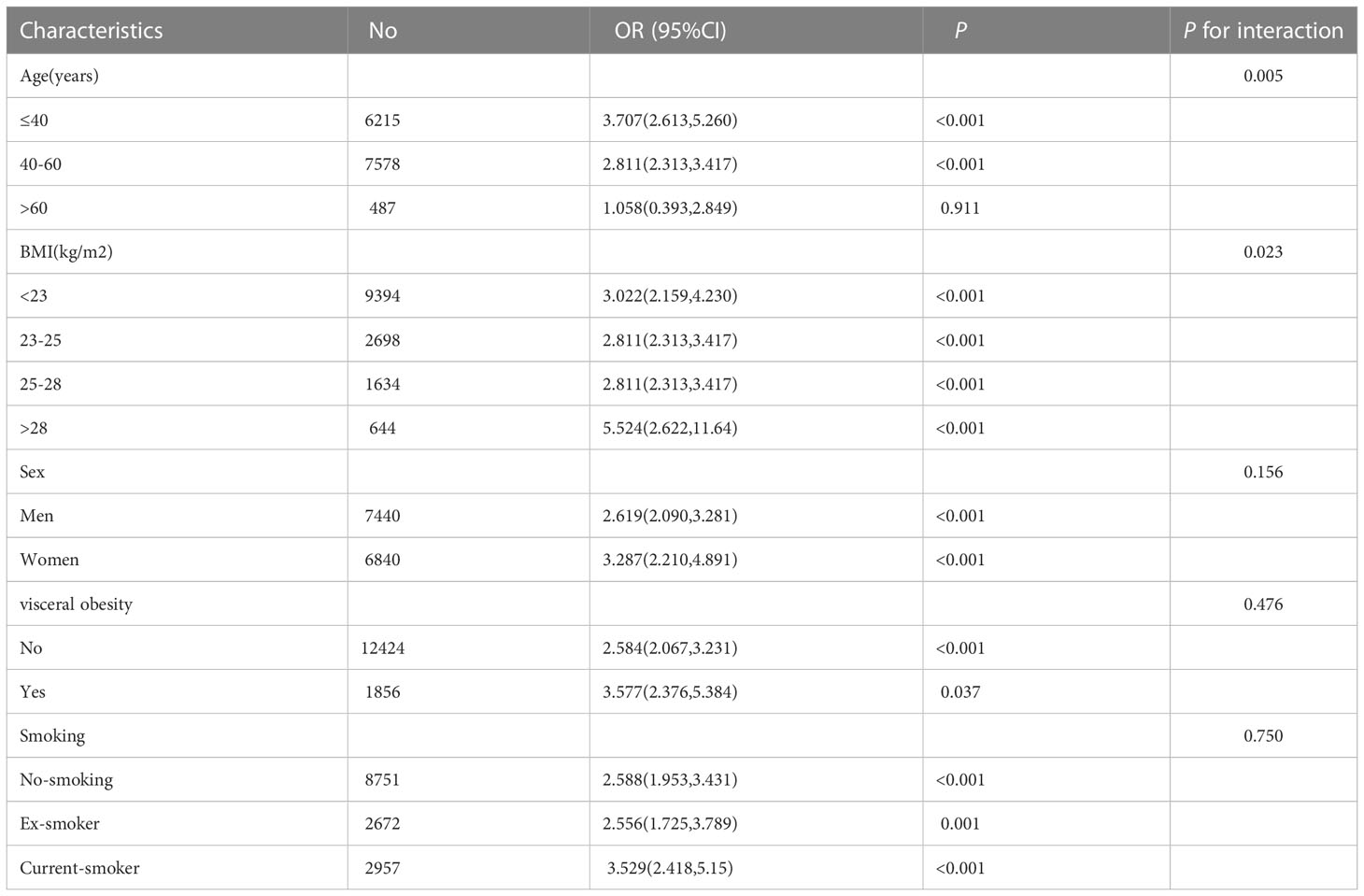
A subgroup analysis on age, sex, smoking, WC, and BMI in the study population was performed to determine variations in the association between HGI and NAFLD in different populations. The association of HGI and risk of NAFLD was stronger among those with a age less than 40 years and BMI>28kg/m2.No other significant interaction was observed in subgroup analyses. (Table 4).
4 Discussion
In this study, the physical examination population was evaluated. Higher HGI levels were observed in the NAFLD group than in the non-NAFLD group. Furthermore, HGI increased simultaneously with the prevalence of NAFLD. Multivariate logistic regression analysis showed that HGI was an independent risk factor for NAFLD. Further analysis showed that patients in the highest quartile of HGI had significantly higher risks for NAFLD than those in the three lower quartile groups. A study conducted by Fiorentino et al. (29) demonstrated that HGI correlated with hepatic steatosis; this finding was congruent with the findings of this study. Our results are similar to these two studies (30, 31).,elevated HGI levels are independently associated with NAFLD.We also found that BMI partly mediates the association between HGI and NAFLD.Our research demonstrates several new populations that may be at risk for NAFLD including people younger than 40 with high HGI and obese people with high HGI,which were not previously described.
Several mechanisms may explain the association between HGI and NAFLD. First, intracellular glucose levels were higher in patients with higher HGIs than those with lower HGIs. Increased intracellular glucose release a significant number of noxious metabolites. This release ultimately leads to liver injury (18). Chronic inflammation is considered a core pathogenesis of the development of NAFLD (32, 33). Fiorentino et al. (29) also observed that there is a significant increase in the levels of inflammation-related biomarkers in patients with higher HGIs.
Similarly, Hu et al. (30) reported a positive correlation between HGI and inflammatory biomarkers, including WBC and platelet counts. The US National Health and Nutrition Examination Survey results suggest that HGI independently associates with inflammatory biomarkers (such as C-reactive protein, polymorphonuclear leukocytes, and monocytes.) (34) Inflammation might impair insulin signaling and exacerbate fatty liver infiltration. Alternatively, inflammation induces oxidative stress, damages mitochondria, and causes ER stress. Defective mitochondria are associated with incomplete fat oxidation and the generation of toxic lipid intermediates. These toxic lipid intermediates generate many reactive oxygen species, which eventually promote the progression of liver steatosis into more severe forms of the disease (35, 36) and fuel the transition from NAFLD to non-alcoholic steatohepatitis, liver cirrhosis, and even hepatocellular carcinoma (35, 37, 38). Previous studies reported that HGI represents the degree of non-enzymatic hemoglobin glycation (26, 29, 39). Non-enzymatic hemoglobin glycation might play a key role in NAFLD pathogenesis (40, 41). Studies have also shown that HGI reflects the level of advanced glycation end products (AGEs) (42). AGEs may alter the structure of proteins and their functions, resulting in alterations that contribute to histopathological changes in the liver (42). Studies have also demonstrated that AGEs, in combination with the activation of the RAGE downstream pathways, trigger further inflammation and oxidative stress and impair insulin signaling. Therefore, AGEs increase the development and progression of NAFLD (43). IR is recognized as a potential pathogenesis of NAFLD. In addition, HGI may associate with IR. Furthermore, the degree of IR in patients with lower HGIs was less than not those in patients with higher HGIs (26).
As a result of obesity, an imbalance in pro- and anti-inflammatory adipokines is secreted from adipose tissue. This secretion contributes to the development of NAFLD (44). Similarly, a previous investigation indicated that several adipokines, including adiponectin and leptin, play a role in the pathogenesis of NAFLD, including hepatic fat accumulation, chronic inflammation, and IR (45, 46). BMI is an easy-to-implement body composition classification screening indicator. BMI classifies patients as underweight, normal weight, overweight, or obesity. BMI is widely employed as a surrogate indicator for measuring weight status (adjusted for height) and the percentage of fat mass (47–49). Among anthropometric measurements, BMI has the best discriminatory power (50). Therefore, BMI is the most commonly used anthropometric measure of obesity (51, 52). Numerous studies have shown a strong correlation between BMI and NAFLD; the higher the BMI, the higher the risk of developing NAFLD (53–55). Tang Z et al. (56) noted that increases in BMI correlated with NAFLD and that these increases were independent of the patient’s age. The study also revealed that BMI modulates the association between physical examinations and blood biochemistry parameters and NAFLD (56).Obesity-related inflammatory input has a well-established correlation with NAFLD (22, 57). BMI is a poor predictor of NAFLD severity (58). Even so, BMI was confirmed as the most useful predictive factor for NAFLD onset in both sexes (59). BMI had the highest area under the curve in the prediction of NAFLD (60). BMI is superior to WC in predicting the risk for NAFLD (61). A recent study showed a significant correlation between HGI and obesity (26), which was consistent with the previous findings of Yoo et al. (31) Correspondingly, Hu et al. (30) found that HGI levels had positive associations with BMI. Therefore, a mediator analysis was conducted to determine if BMI mediates the correlation between HGI and NAFLD. Our study showed that HGI and BMI were positively associated with NAFLD, and that HGI positively correlated with BMI. The mediation analysis results indicated that the relationship between the HGI and NAFLD was partly mediated by obesity. In addition, the correlation between the HGI and NAFLD was significantly higher in obese patients.
This study identified that increases in HGI correlated with increased incidences in females. Previous studies also showed gender differences in NAFLD related to gender-specific gene expression, sex hormones, and gender-related economic, behavioral, and environmental factors (62, 63)..
This study had limitations. First, this was a cross-sectional study, and thus could not establish a cause-effect relationship. Further prospective studies are required to validate these findings. Second, HGI levels may vary by population (64, 65). Therefore, the results obtained from this study conducted in Japan might not be applicable to other ethnicities. Third, fatty liver diagnosed by ultrasonography may provide an incorrect diagnosis compared to a liver biopsy. Finally, since the dataset was obtained from a public database, it could not be updated. Therefore, we could not investigate the effect of HGI on liver fibrosis. Some known risk factors of demographic data for NAFLD, such as dietary preferences, exercise habits, work types, and so on, were not included, which limits a comprehensive assessment of the correlation between risk factors and NAFLD.
5 Conclusion
In conclusion, HGI positively correlates with the prevalence of NAFLD, and BMI partly mediates the association between HGI and NAFLD.Our research demonstrates several new populations that may be at risk for NAFLD including people younger than 40 with high HGI and obese people with high HGI.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found below: https://doi.org/10.1038/s41366-018-0076-3.
Ethics statement
The studies involving human participants were reviewed and approved by Murakami Memorial Hospital. The patients/participants provided their written informed consent to participate in this study.
Author contributions
YX and YZ performed the data analysis and YX prepared the initial draft of the manuscript. LY and LH gave statistical advice throughout the data analyses and contributed to the manuscript. HM was responsible for the study concept, data analysis, and interpretation of the findings, and critically reviewed and revised the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This study was supported by Central Committee Guides Local Science and Technology Development Project (No. 226Z7721G).
Acknowledgments
The original data can be available by email request at any time (YX:eGluZ3lsOTVAMTYzLmNvbQ==).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
AGE, Advanced Glycation End Products; AST, Aspartate aminotransferase; ALT, Alanine Aminotransferase; CVD, Cardiovascular Disease; DBP, Diastolic Blood Pressure; FPG, Fasting Plasma Glucose; HGI, Hemoglobin Glycation Index; HbA1c, Hemoglobin A1c; GGT, γ-glutamyl transferase; HDL-C, High-Density Lipoprotein Cholesterol; IR, Insulin Resistance; NAFLD, Non-Alcoholic Fatty Liver Disease; SBP, Systolic Blood Pressure; TC, Total Cholesterol; TG, Triglyceride; WC, Waist Circumference.
References
1. Kim KH, Lee MS. Pathogenesis of nonalcoholic steatohepatitis and hormone-based therapeutic approaches. Front endocrinology (2018) 9:485. doi: 10.3389/fendo.2018.00485
2. Haas JT, Francque S, Staels B. Pathophysiology and mechanisms of nonalcoholic fatty liver disease. Annu Rev Physiol (2016) 78:181–205. doi: 10.1146/annurev-physiol-021115-105331
3. Benedict M, Zhang X. Non-alcoholic fatty liver disease: An expanded review. World J Hepatol (2017) 9(16):715–32. doi: 10.4254/wjh.v9.i16.715
4. Birkenfeld AL, Shulman GI. Nonalcoholic fatty liver disease, hepatic insulin resistance, and type 2 diabetes. Hepatol (Baltimore Md) (2014) 59(2):713–23. doi: 10.1002/hep.26672
5. Younossi ZM. Non-alcoholic fatty liver disease - a global public health perspective. J hepatology (2019) 70(3):531–44. doi: 10.1016/j.jhep.2018.10.033
6. Younossi Z, Anstee QM, Marietti M, Hardy T, Henry L, Eslam M, et al. Global burden of NAFLD and NASH: trends, predictions, risk factors and prevention. Nat Rev Gastroenterol Hepatol (2018) 15(1):11–20. doi: 10.1038/nrgastro.2017.109
7. Mansour A, Mohajeri-Tehrani MR, Samadi M, Gerami H, Qorbani M, Bellissimo N, et al. Risk factors for non-alcoholic fatty liver disease-associated hepatic fibrosis in type 2 diabetes patients. Acta Diabetol (2019) 56(11):1199–207. doi: 10.1007/s00592-019-01374-x
8. Targher G, Byrne CD, Lonardo A, Zoppini G, Barbui C. Non-alcoholic fatty liver disease and risk of incident cardiovascular disease: A meta-analysis. J Hepatol (2016) 65(3):589–600. doi: 10.1016/j.jhep.2016.05.013
9. Mantovani A, Zaza G, Byrne CD, Lonardo A, Zoppini G, Bonora E, et al. Nonalcoholic fatty liver disease increases risk of incident chronic kidney disease: A systematic review and meta-analysis. Metabolism (2018) 79:64–76. doi: 10.1016/j.metabol.2017.11.003
10. Mantovani A, Dauriz M, Byrne CD, Lonardo A, Zoppini G, Bonora E, et al. Association between nonalcoholic fatty liver disease and colorectal tumours in asymptomatic adults undergoing screening colonoscopy: a systematic review and meta-analysis. Metabolism (2018) 87:1–12. doi: 10.1016/j.metabol.2018.06.004
11. Powell EE, Wong VW, Rinella M. Non-alcoholic fatty liver disease. Lancet (2021) 397(10290):2212–24. doi: 10.1016/S0140-6736(20)32511-3
12. Estes C, Chan HLY, Chien RN, Chuang WL, Fung J, Goh GB, et al. Modelling NAFLD disease burden in four Asian regions-2019-2030. Aliment Pharmacol Ther (2020) 51(8):801–11. doi: 10.1111/apt.15673
13. Chalew SA, McCarter RJ, Hempe JM. Biological variation and hemoglobin A1c: relevance to diabetes management and complications. Pediatr Diabetes (2013) 14(6):391–8. doi: 10.1111/pedi.12055
14. Li K, Song WJ, Wu X, Gu DY, Zang P, Gu P, et al. Associations of serum glucagon levels with glycemic variability in type 1 diabetes with different disease durations. Endocrine (2018) 61(3):473–81. doi: 10.1007/s12020-018-1641-1
15. Herman WH, Ma Y, Uwaifo G, Haffner S, Kahn SE, Horton ES, et al. Differences in A1C by race and ethnicity among patients with impaired glucose tolerance in the diabetes prevention program. Diabetes Care (2007) 30(10):2453–7. doi: 10.2337/dc06-2003
16. Hempe JM, Gomez R, McCarter RJ Jr., Chalew SA. High and low hemoglobin glycation phenotypes in type 1 diabetes: a challenge for interpretation of glycemic control. J Diabetes Complications (2002) 16(5):313–20. doi: 10.1016/S1056-8727(01)00227-6
17. Chalew SA, McCarter RJ, Thomas J, Thomson JL, Hempe JM. A comparison of the glycosylation gap and hemoglobin glycation index in patients with diabetes. J Diabetes Complications (2005) 19(4):218–22. doi: 10.1016/j.jdiacomp.2005.01.004
18. Chalew SA, McCarter RJ, Ory-Ascani J, Hempe JM. Labile A1C is inversely correlated with the hemoglobin glycation index in children with type 1 diabetes. Diabetes Care (2010) 33(2):273–4. doi: 10.2337/dc08-2220
19. Hempe JM, Liu S, Myers L, McCarter RJ, Buse JB, Fonseca V. The hemoglobin glycation index identifies subpopulations with harms or benefits from intensive treatment in the ACCORD trial. Diabetes Care (2015) 38(6):1067–74. doi: 10.2337/dc14-1844
20. McCarter RJ, Hempe JM, Gomez R, Chalew SA. Biological variation in HbA1c predicts risk of retinopathy and nephropathy in type 1 diabetes. Diabetes Care (2004) 27(6):1259–64. doi: 10.2337/diacare.27.6.1259
21. Cheng PC, Hsu SR, Cheng YC, Liu YH. Relationship between hemoglobin glycation index and extent of coronary heart disease in individuals with type 2 diabetes mellitus: a cross-sectional study. PeerJ (2017) 5:e3875. doi: 10.7717/peerj.3875
22. Ahn JS, Sinn DH, Min YW, Hong SN, Kim HS, Jung SH, et al. Non-alcoholic fatty liver diseases and risk of colorectal neoplasia. Alimentary Pharmacol Ther (2017) 45(2):345–53. doi: 10.1111/apt.13866
23. Kim MK, Jeong JS, Yun JS, Kwon HS, Baek KH, Song KH, et al. Hemoglobin glycation index predicts cardiovascular disease in people with type 2 diabetes mellitus: A 10-year longitudinal cohort study. J Diabetes Complications (2018) 32(10):906–10. doi: 10.1016/j.jdiacomp.2018.08.007
24. Jin JL, Sun D, Cao YX, Guo YL, Wu NQ, Zhu CG, et al. Triglyceride glucose and haemoglobin glycation index for predicting outcomes in diabetes patients with new-onset, stable coronary artery disease: a nested case-control study. Ann Med (2018) 50(7):576–86. doi: 10.1080/07853890.2018.1523549
25. Rhee EJ, Cho JH, Kwon H, Park SE, Park CY, Oh KW, et al. Association between coronary artery calcification and the hemoglobin glycation index: The kangbuk Samsung health study. J Clin Endocrinol Metab (2017) 102(12):4634–41. doi: 10.1210/jc.2017-01723
26. Marini MA, Fiorentino TV, Succurro E, Pedace E, Andreozzi F, Sciacqua A, et al. Association between hemoglobin glycation index with insulin resistance and carotid atherosclerosis in non-diabetic individuals. PloS One (2017) 12(4):e0175547. doi: 10.1371/journal.pone.0175547
27. Mi J, Song J, Zhao Y, Wu X. Association of hemoglobin glycation index and its interaction with obesity/family history of hypertension on hypertension risk: A community-based cross-sectional survey. BMC Cardiovasc Disord (2020) 20(1):477. doi: 10.1186/s12872-020-01762-0
28. Okamura T, Hashimoto Y, Hamaguchi M, Obora A, Kojima T, Fukui M. Ectopic fat obesity presents the greatest risk for incident type 2 diabetes: a population-based longitudinal study. Int J Obes (Lond) (2019) 43(1):139–48. doi: 10.1038/s41366-018-0076-3
29. Fiorentino TV, Marini MA, Succurro E, Andreozzi F, Sciacqua A, Hribal ML, et al. Association between hemoglobin glycation index and hepatic steatosis in non-diabetic individuals. Diabetes Res Clin Pract (2017) 134:53–61. doi: 10.1016/j.diabres.2017.09.017
30. Hu DS, Zhu SH, Li X, Chen QF, Lin CJ, Fang DH, et al. Association between hemoglobin glycation index and NAFLD in Chinese nondiabetic individuals. Can J Gastroenterol hepatology (2019) 2019:8748459. doi: 10.1155/2019/8748459
31. Yoo JH, Kang YM, Cho YK, Lee J, Jung CH, Park JY, et al. The haemoglobin glycation index is associated with nonalcoholic fatty liver disease in healthy subjects. Clin endocrinology (2019) 91(2):271–7. doi: 10.1111/cen.14001
32. Dowman JK, Tomlinson JW, Newsome PN. Pathogenesis of non-alcoholic fatty liver disease. Qjm (2010) 103(2):71–83. doi: 10.1093/qjmed/hcp158
33. Zhao L, Chen Y, Tang R, Chen Y, Li Q, Gong J, et al. Inflammatory stress exacerbates hepatic cholesterol accumulation via increasing cholesterol uptake and de novo synthesis. J Gastroenterol Hepatol (2011) 26(5):875–83. doi: 10.1111/j.1440-1746.2010.06560.x
34. Liu S, Hempe JM, McCarter RJ, Li S, Fonseca VA. Association between inflammation and biological variation in hemoglobin A1c in U.S. nondiabetic adults. J Clin Endocrinol Metab (2015) 100(6):2364–71. doi: 10.1210/jc.2014-4454
35. Zhang XQ, Xu CF, Yu CH, Chen WX, Li YM. Role of endoplasmic reticulum stress in the pathogenesis of nonalcoholic fatty liver disease. World J Gastroenterol (2014) 20(7):1768–76. doi: 10.3748/wjg.v20.i7.1768
36. Hribal ML, Procopio T, Petta S, Sciacqua A, Grimaudo S, Pipitone RM, et al. Insulin-like growth factor-I, inflammatory proteins, and fibrosis in subjects with nonalcoholic fatty liver disease. J Clin Endocrinol Metab (2013) 98(2):E304–308. doi: 10.1210/jc.2012-3290
37. Korf H, Boesch M, Meelberghs L, van der Merwe S. Macrophages as key players during adipose tissue-liver crosstalk in nonalcoholic fatty liver disease. Semin Liver Dis (2019) 39(3):291–300. doi: 10.1055/s-0039-1687851
38. Szabo G, Petrasek J. Inflammasome activation and function in liver disease. Nat Rev Gastroenterol Hepatol (2015) 12(7):387–400. doi: 10.1038/nrgastro.2015.94
39. Fiorentino TV, Marini MA, Succurro E, Sciacqua A, Andreozzi F, Perticone F, et al. Elevated hemoglobin glycation index identify non-diabetic individuals at increased risk of kidney dysfunction. Oncotarget (2017) 8(45):79576–86. doi: 10.18632/oncotarget.18572
40. Pereira E, Silvares RR, Flores EEI, Rodrigues KL, Ramos IP, da Silva IJ, et al. Hepatic microvascular dysfunction and increased advanced glycation end products are components of non-alcoholic fatty liver disease. PloS One (2017) 12(6):e0179654. doi: 10.1371/journal.pone.0179654
41. Mehta R, Shaw G, Masschelin P, Felix S, Otgonsuren M, Baranova A, et al. Polymorphisms in the receptor for advanced glycation end-products (RAGE) gene and circulating RAGE levels as a susceptibility factor for non-alcoholic steatohepatitis (NASH). PloS One (2018) 13(6):e0199294. doi: 10.1371/journal.pone.0199294
42. Felipe DL, Hempe JM, Liu S, Matter N, Maynard J, Linares C, et al. Skin intrinsic fluorescence is associated with hemoglobin A(1c )and hemoglobin glycation index but not mean blood glucose in children with type 1 diabetes. Diabetes Care (2011) 34(8):1816–20. doi: 10.2337/dc11-0049
43. Palma-Duran SA, Kontogianni MD, Vlassopoulos A, Zhao S, Margariti A, Georgoulis M, et al. Serum levels of advanced glycation end-products (AGEs) and the decoy soluble receptor for AGEs (sRAGE) can identify non-alcoholic fatty liver disease in age-, sex- and BMI-matched normo-glycemic adults. Metabolism (2018) 83:120–7. doi: 10.1016/j.metabol.2018.01.023
44. Cantero I, Abete I, Del Bas JM, Caimari A, Arola L, Zulet MA, et al. Changes in lysophospholipids and liver status after weight loss: the RESMENA study. Nutr Metab (Lond) (2018) 15:51. doi: 10.1186/s12986-018-0288-5
45. Tilg H. Adipocytokines in nonalcoholic fatty liver disease: key players regulating steatosis, inflammation and fibrosis. Curr Pharm Des (2010) 16(17):1893–5. doi: 10.2174/138161210791208929
46. Handa P, Maliken BD, Nelson JE, Morgan-Stevenson V, Messner DJ, Dhillon BK, et al. Reduced adiponectin signaling due to weight gain results in nonalcoholic steatohepatitis through impaired mitochondrial biogenesis. Hepatology (2014) 60(1):133–45. doi: 10.1002/hep.26946
47. Hall DM, Cole TJ. What use is the BMI? Arch Dis Child (2006) 91(4):283–6. doi: 10.1136/adc.2005.077339
48. Duren DL, Sherwood RJ, Czerwinski SA, Lee M, Choh AC, Siervogel RM, et al. Body composition methods: comparisons and interpretation. J Diabetes Sci Technol (2008) 2(6):1139–46. doi: 10.1177/193229680800200623
49. Porto LG, Nogueira RM, Nogueira EC, Molina GE, Farioli A, Junqueira LF, et al. Agreement between BMI and body fat obesity definitions in a physically active population. Arch Endocrinol Metab (2016) 60(6):515–25. doi: 10.1590/2359-3997000000220
50. Schwenger KJP, Kiu A, AlAli M, Alhanaee A, Fischer SE, Allard JP. Comparison of bioelectrical impedance analysis, mass index, and waist circumference in assessing risk for non-alcoholic steatohepatitis. Nutrition (2022) 93:111491. doi: 10.1016/j.nut.2021.111491
51. Chandalia M, Lin P, Seenivasan T, Livingston EH, Snell PG, Grundy SM, et al. Insulin resistance and body fat distribution in south Asian men compared to Caucasian men. PloS One (2007) 2(8):e812. doi: 10.1371/journal.pone.0000812
52. Petersen KF, Dufour S, Feng J, Befroy D, Dziura J, Dalla Man C, et al. Increased prevalence of insulin resistance and nonalcoholic fatty liver disease in Asian-Indian men. Proc Natl Acad Sci USA (2006) 103(48):18273–7. doi: 10.1073/pnas.0608537103
53. Almobarak AO, Barakat S, Khalifa MH, Elhoweris MH, Elhassan TM, Ahmed MH. Non alcoholic fatty liver disease (NAFLD) in a Sudanese population: What is the prevalence and risk factors? Arab J Gastroenterol (2014) 15(1):12–5. doi: 10.1016/j.ajg.2014.01.008
54. Abangah G, Yousefi A, Asadollahi R, Veisani Y, Rahimifar P, Alizadeh S. Correlation of body mass index and serum parameters with ultrasonographic grade of fatty change in non-alcoholic fatty liver disease. Iran Red Crescent Med J (2014) 16(1):e12669. doi: 10.5812/ircmj.12669
55. Loomis AK, Kabadi S, Preiss D, Hyde C, Bonato V, St Louis M, et al. Body mass index and risk of nonalcoholic fatty liver disease: Two electronic health record prospective studies. J Clin Endocrinol Metab (2016) 101(3):945–52. doi: 10.1210/jc.2015-3444
56. Tang Z, Pham M, Hao Y, Wang F, Patel D, Jean-Baptiste L, et al. Sex, age, and BMI modulate the association of physical examinations and blood biochemistry parameters and NAFLD: A retrospective study on 1994 cases observed at shuguang hospital, China. BioMed Res Int (2019) 2019:1246518. doi: 10.1155/2019/1246518
57. Woo Baidal JA, Lavine JE. The intersection of nonalcoholic fatty liver disease and obesity. Sci Trans Med (2016) 8(323):323rv321. doi: 10.1126/scitranslmed.aad8390
58. Klepper CM, Khurana T, Sun Q, Fei L, Crimmins NA, Siegel R, et al. BMI metrics are poor predictors of pediatric nonalcoholic fatty liver disease severity. Childhood Obes (Print) (2022). doi: 10.1089/chi.2021.0316
59. Miyake T, Kumagi T, Hirooka M, Furukawa S, Koizumi M, Tokumoto Y, et al. Body mass index is the most useful predictive factor for the onset of nonalcoholic fatty liver disease: A community-based retrospective longitudinal cohort study. J gastroenterology (2013) 48(3):413–22. doi: 10.1007/s00535-012-0650-8
60. Shengir M, Krishnamurthy S, Ghali P, Deschenes M, Wong P, Chen T, et al. Prevalence and predictors of nonalcoholic fatty liver disease in south Asian women with polycystic ovary syndrome. World J Gastroenterol (2020) 26(44):7046–60. doi: 10.3748/wjg.v26.i44.7046
61. Hu X, Huang Y, Bao Z, Wang Y, Shi D, Liu F, et al. Prevalence and factors associated with nonalcoholic fatty liver disease in shanghai work-units. BMC gastroenterology (2012) 12:123. doi: 10.1186/1471-230X-12-123
62. Kautzky-Willer A, Harreiter J, Pacini G. Sex and gender differences in risk, pathophysiology and complications of type 2 diabetes mellitus. Endocr Rev (2016) 37(3):278–316. doi: 10.1210/er.2015-1137
63. Ballestri S, Nascimbeni F, Baldelli E, Marrazzo A, Romagnoli D, Lonardo A. NAFLD as a sexual dimorphic disease: Role of gender and reproductive status in the development and progression of nonalcoholic fatty liver disease and inherent cardiovascular risk. Adv Ther (2017) 34(6):1291–326. doi: 10.1007/s12325-017-0556-1
64. Chalew S, Hamdan M. Racial disparity in HbA1c persists when fructosamine is used as a surrogate for mean blood glucose in youth with type 1 diabetes. Pediatr diabetes (2018) 19(7):1243–8. doi: 10.1111/pedi.12696
Keywords: hemoglobin glycation index, nonalcoholic fatty liver disease, body mass index, obesity, glycated hemoglobin A 1c
Citation: Xing Y, Zhen Y, Yang L, Huo L and Ma H (2023) Association between hemoglobin glycation index and non-alcoholic fatty liver disease. Front. Endocrinol. 14:1094101. doi: 10.3389/fendo.2023.1094101
Received: 09 November 2022; Accepted: 26 January 2023;
Published: 07 February 2023.
Edited by:
Andrei I. Tarasov, Ulster University, United KingdomReviewed by:
Wanzhu Jin, Chinese Academy of Sciences (CAS), ChinaBo Ban, Affiliated Hospital of Jining Medical University, China
Huabing Zhang, Peking Union Medical College Hospital (CAMS), China
Copyright © 2023 Xing, Zhen, Yang, Huo and Ma. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Huijuan Ma, aHVpanVhbm1hMTlAMTYzLmNvbQ==
 Yuling Xing
Yuling Xing Yunfeng Zhen
Yunfeng Zhen Liqun Yang1
Liqun Yang1 Huijuan Ma
Huijuan Ma