- 1Department of Gastrointestinal Surgery, The First People’s Hospital of Zunyi and Third Affiliated Hospital of Zunyi Medical University, Zunyi, Guizhou, China
- 2Department of Metabolic and Bariatric Surgery, The First Affiliated Hospital of Jinan University, Guangzhou, China
Background: Fibrosis stages affect clinical prognoses related to nonalcoholic fatty liver disease (NAFLD). However, data on the prevalence and clinical features of significant fibrosis are scarce in Chinese bariatric surgery patients. We aimed to investigate the prevalence of significant fibrosis in bariatric surgery patients and to identify its predictors.
Methods: We prospectively enrolled the patients performing intra-operative liver biopsies during bariatric surgery from a bariatric surgery center in a university hospital between May 2020 and January 2022. Anthropometric characteristics, co-morbidities, laboratory data and pathology reports were collected and analyzed. The performance of non‐invasive models was evaluated.
Results: Of 373 patients, 68.9%% had non-alcoholic steatohepatitis (NASH) and 60.9% exhibited fibrosis. Significant fibrosis was present in 9.1% of patients, advanced fibrosis in 4.0%, and cirrhosis in 1.6%. Multivariate logistic regression showed that increasing age (odds ratio [OR], 1.06; p=0.003), presence of diabetes (OR, 2.62; p=0.019), elevated c- peptide (OR, 1.26; p=0.025) and elevated aspartate aminotransferase (AST) (OR, 1.02; p=0.004) were independent predictors of significant fibrosis. The non-invasive models, AST to Platelet ratio (APRI), Fibrosis‐4 (FIB-4), and Hepamet fibrosis scores (HFS) provided greater accuracy for predicting significant fibrosis, compared to the NAFLD Fibrosis Score (NFS) and BARD score.
Conclusion: More than two-thirds of bariatric surgery patients had NASH and the prevalence of significant fibrosis was high. Elevated levels of AST and c- peptide, advanced age and diabetes indicated a higher risk of significant fibrosis. Non-invasive models, APRI, FIB-4 and HFS can be used to identify significant liver fibrosis in bariatric surgery patients.
Introduction
Nonalcoholic fatty liver disease (NAFLD), now known as metabolic-associated fatty liver disease (MAFLD), has become the most common cause of chronic liver disease worldwide (1, 2). Epidemiological research estimates a 25% prevalence in the general population, rising to 90% in patients undergoing bariatric surgery (3, 4). Some risk factors, including obesity, diabetes, hypertension, hyperlipidemia and metabolic syndrome are established indicators of NAFLD development (5). Thus, it is anticipated that as the prevalence of obesity and diabetes increases, so will that of NAFLD. Non-alcoholic steatohepatitis (NASH) is the active form of NAFLD. It is characterized by hepatocyte ballooning and lobular necroinflammation (which can occur with or without fibrosis) and may silently progress towards cirrhosis, end-stage liver disease, and even hepatocellular carcinoma (6, 7).
A strong correlation has been demonstrated between the degree of fibrosis and liver-specific morbidity and overall mortality in NAFLD patients (8). Furthermore, patients with significant fibrosis are most likely to experience complications and further progression of the hepatic disease (9). Unfortunately, most patients with fibrosis are asymptomatic and have normal transaminases. Thus, we need to detect risk factors for liver fibrosis, especially significant fibrosis, because distinguishing between NAFLD with or without significant fibrosis has important clinical significance for determining the prognosis (10, 11). Abdominal ultrasound is effective in detecting fatty liver but not liver fibrosis. To date, histologic evaluation of the liver biopsy remains the gold standard for diagnosing NASH and assessing the stage of fibrosis (6, 12). Nevertheless, liver biopsy is not a routine procedure due to its invasiveness, high costs, sampling variability and various potential complications. There are several non-invasive scoring systems specifically designed to identify the presence of advanced fibrosis which include: the aspartate aminotransferase (AST)-to-platelet ratio index (APRI) (13), BARD scoring system (14), NAFLD Fibrosis Score (NFS) (15) and Fibrosis-4(FIB-4) score (16). Some studies have shown that these non-invasive scoring systems were assessed to detect advanced fibrosis in morbid obesity or diabetes, but the application of these scores was from white and non-Asian populations (17, 18). Little is known about the reliability of non-invasive scoring systems to detect significant liver fibrosis in Chinese bariatric patients. (Reviewer #1). In addition, these scoring systems were developed using data from viral hepatitis patients and have yet to be validated for Chinese bariatric surgery patients. Thus, we determined to test the hypothesis that these algorithms were able to identify significant liver fibrosis among bariatric surgery patients.
Currently, research data reporting on the prevalence and clinical characteristics of fibrosis mainly originate from Western countries (3, 19, 20). However, there has yet to be a study that specifically evaluates the prevalence of significant fibrosis (and its associated predictors) in the Chinese population. In fact, China is one of the countries with the largest population of obesity, and the obesity phenotype is mainly moderate obesity (21). In addition, given that Chinese eating habits and lifestyles are particularly distinctive compared with those of other nationalities, the prevalence of significant fibrosis may vary considerably compared with data published to date. Determining potential risk factors for significant fibrosis may help clinicians perform risk stratification of bariatric surgery patients with NAFLD, facilitating early identification of high-risk populations. Thus, this study aimed to evaluate prevalence and clinical predictors of hepatic fibrosis (confirmed by biopsy) experienced by Chinese bariatric surgery patients. In addition, we look to validate the reliability of the aforementioned, non-invasive fibrosis scoring algorithms.
Materials and methods
Study population
This is a prospective, observational study of a cohort of Chinese bariatric surgery patients. In this study, patients were recruited from a bariatric surgery center in a tertiary university hospital during the period May 2020-January 2022. Then inclusion criteria for this study were as follows: (1) patients who met metabolic surgery standard: body mass index (BMI) ≥ 32.5 kg/m2 or BMI ≥ 27.5 kg/m2 with poor weight loss by medications or lifestyle modification and with at least two components of metabolic syndrome or with comorbidities (22); (2) patients who had consented to a trans-operative liver biopsy. The exclusion criteria were:(1) patient had any history of alcoholism (average daily consumption of alcohol of 30 g/day for men and 20 g/day for women); (2) patients tested positive for viral hepatitis (B or C); (3) patients had incomplete pathology reports. (4) patients with diabetes take insulin treatment; (5) patients underwent preoperative weight loss or very low-calorie diets. (Reviewer #3) The study was approved by our hospital ethics committee (2019-024). Written informed consent was obtained from each participant or legal representatives before bariatric surgery.
Clinical and laboratory data
Clinical and laboratory data was sourced from a prospectively collected database (KY-2020-021). Demographic data (gender, age), anthropometric data (weight, BMI, waist circumference, hip circumference, waist to hip ratio) and the presence of co-morbidity (Metabolic syndrome, hypertension, type-2 diabetes mellitus(T2DM)) were analyzed. BMI was calculated by dividing body weight by the square of body height. Metabolic syndrome was defined as the presence of at least 3 of the 5 following criteria (23): (1) abdominal obesity (waist circumference ≥ 90 cm in man and ≥ 80 cm in women); (2) blood pressure ≥130/85 mmHg or taking antihypertensive drug; (2) serum triglycerides ≥1.7 mmol/L, or taking lipid-lowering drugs; (4) serum high-density lipoprotein cholesterol (HDL-c) <1.0 mmol/L for man and <1.3 mmol/L for women, or drug treatment for reduced HDL-c; (5) fasting plasma glucose (FPG) ≥5.6 mmol/L), or drug treatment for elevated glucose. Hypertension was diagnosed as patients with systolic/diastolic pressures ≥ 140/90 mmHg, or taking antihypertensive drugs. T2DM was defined in accordance with the clinical classification and diagnosis of diabetes (24).
We also collected the following biochemical parameters: FPG; fasting plasma C-peptide; fasting plasma insulin; glycated hemoglobin (HbA1c); serum uric acid (SUA); creatinine; blood urea nitrogen (BUN); aminotransferase (ALT); aspartate aminotransferase (AST); γ-glutamyl transpeptidase (GGT); alkaline phosphatase (ALP); total bilirubin; direct bilirubin; indirect bilirubin; albumin; total cholesterol; triglycerides; HDL-C; low-density lipoprotein cholesterol (LDL-C)], and routine blood data pertaining to red blood cell (RBC), white blood cells (WBC) and platelet. Standard laboratory methods were used to carry out each of these biochemical tests. In addition, we also calculated homeostatic model assessment of insulin resistance (HOMA-IR) (insulin (mU/L) x FPG (mmol/L)/22.5) to indirectly assessed insulin resistance.
In addition, certain non-invasive fibrosis scores were computed using the relevant published formulas: APRI (AST to platelet ratio index) (13); FIB-4 (age, ALT, AST, platelet) (16); NFS (age, BMI, diabetes status, platelet, albumin) (15); BARD (BMI, AST/ALT ratio, T2DM) (14); Hepamet Fibrosis Score (HFS) was computed using a free web page: https://www.hepamet-fibrosis-score.eu/ (25)..
Histopathological evaluation
Liver specimens were obtained, in the form of a wedge biopsy from the left lobe of the liver, by the surgeon performing the bariatric surgery. Liver tissue specimens were routinely formalin-fixed, paraffin-embedded and then stained with hematoxylin-eosin. The biopsy specimen was at least 10 mm long or not less than 10 portal tracts. All histological examinations were performed by the same experienced pathologist, blinded for clinical and laboratory data. Histopathological analysis was performed according to the steatosis, activity, and fibrosis (SAF) score (26). Fibrosis was graded as 0–4 stages (27): F0 = no fibrosis, F1 = perisinusoidal or periportal fibrosis, F2 = perisinusoidal and portal/periportal fibrosis, F3 = bridged fibrosis, and F4 = cirrhosis. NASH was defined as steatosis (5% of hepatocytes), hepatocellular ballooning and lobular inflammation. Significant liver fibrosis was defined as stage 2 fibrosis or above.
Statistical analysis
Statistical analysis was performed using SPSS version 26.0 (SPSS Inc. Chicago, IL, USA), and MedCalc version 19.4.0 (Ostend, Belgium). Continuous data were presented as mean ± standard deviation (SD), whilst categorical data was given as a number (frequency or percentage). Pairwise comparisons of continuous data were performed using the t-test or Mann–Whitney test, whereas categorical data were compared using a chi-square test or Fisher’s exact test. Normality was assessed using the Kolmogorov-Smirnov test. To identify the predictive factors related to significant fibrosis, univariate logistic regression models were performed to identify each possible predictor. Then, multicollinearity was assessed using the variance inflation factor (VIF) method, with a VIF≥5 indicating the presence of multicollinearity, and no significant collinear variables were found. Finally, independent variables with statistically significant (P <0.05) were introduced into a multivariable logistic regression (backward selection method). An odds ratio (OR) with a 95% confidence interval (CI) was calculated.
In order to evaluate the performance of non-invasive scoring systems for detecting significant fibrosis, we calculated the area under the curve (AUC) of receiver operating characteristic curves (ROC) (AUROC), sensitivity, specificity, positive predictive value (PPV) and negative predictive value (NPV) along with their 95% CI. ROC curves were compared using the methods of Hanley & McNeil (28). Statistical significance was defined as a p<0.05.
Results
Clinical baseline characteristics
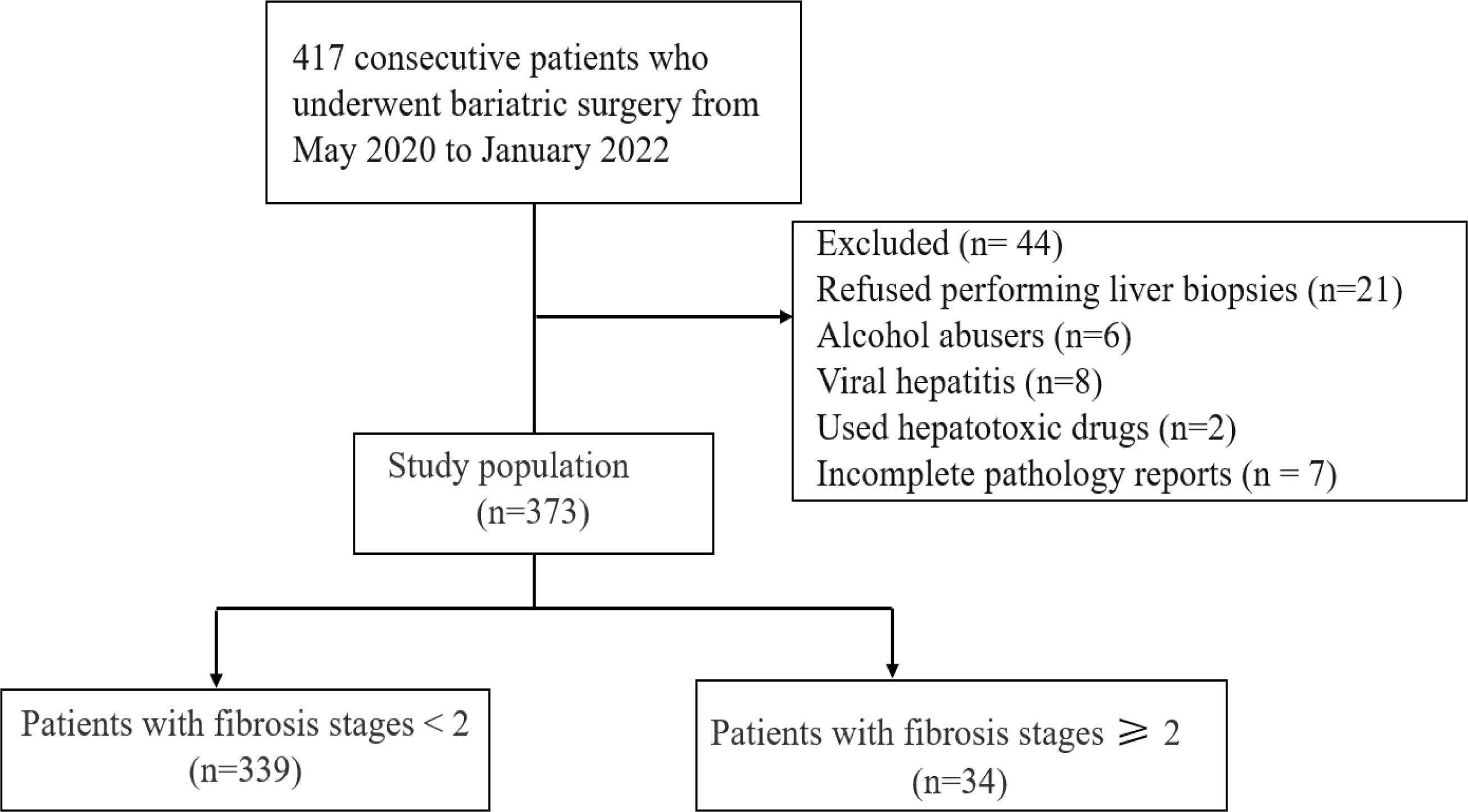
Of the 417 consecutive patients who underwent bariatric surgery between May 2020 and January 2022, 44 patients exhibited criteria (detailed in methods) that meant they were excluded from our study. In total, 373 patients were recruited into this study, including 126 (33.7%) male patients and 247 (66.3%) female patients. Flow diagram of the study is shown in Figure 1.
The mean age and BMI of the study population were 30.9 ± 9.0 years and 39.4 ± 7.6 kg/m2, respectively. Patients with significant fibrosis tended to be older. They also exhibited: a higher prevalence of T2DM; higher levels of fasting plasma glucose, c-peptide, HbA1c, ALT, AST, GGT, and lower platelet counts compared to patients without significant fibrosis (p<0.05). When the non-invasive scoring systems were applied to our data, the results revealed that the significant fibrosis group had significantly higher scores than the patients assigned to the non-significant fibrosis group. A more detailed description of the study population is displayed in Table 1.

Table 1 Baseline characteristics of all patients and those with significant fibrosis and without significant fibrosis.
Prevalence of steatosis and significant fibrosis
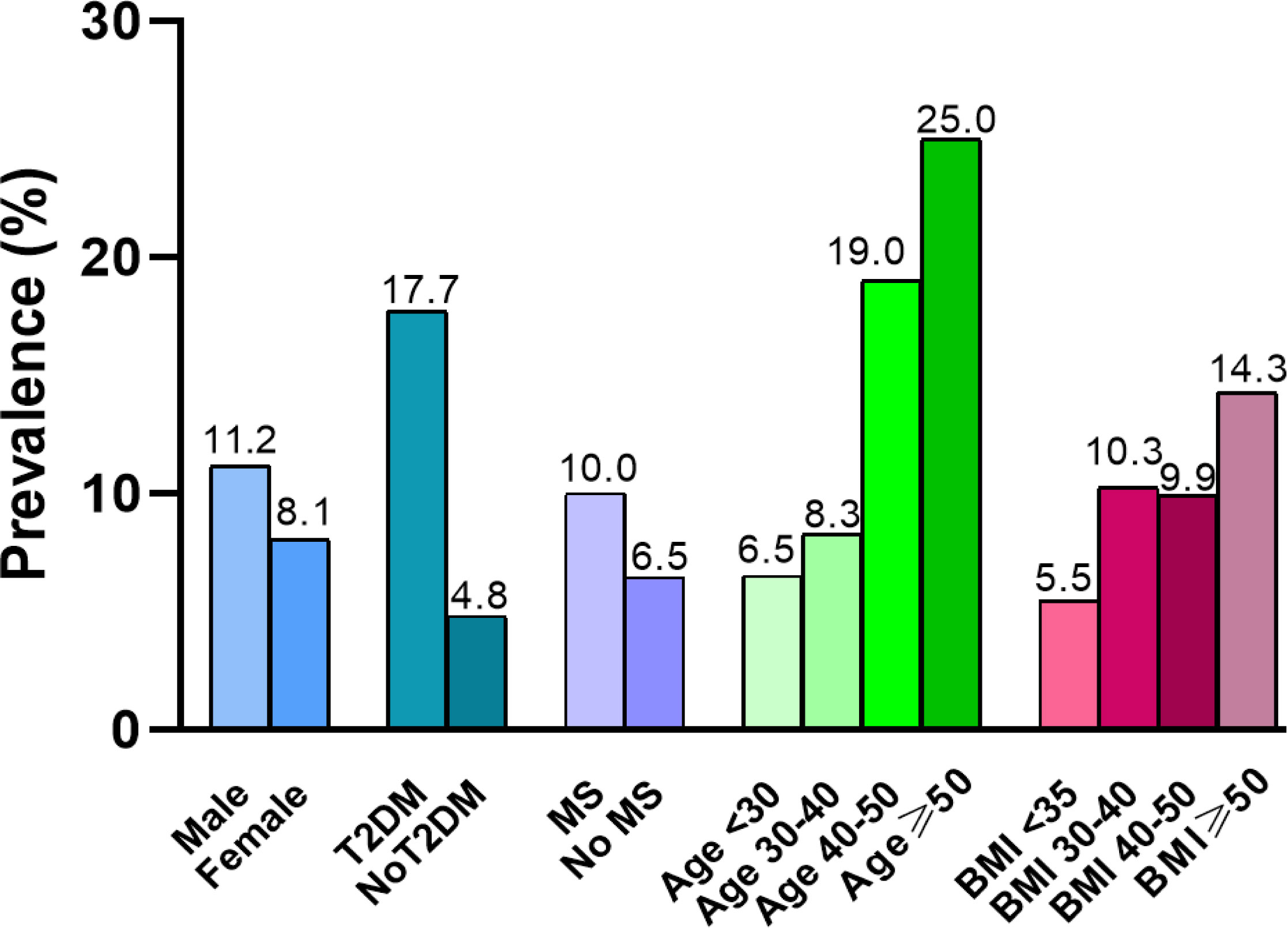
Of those 373 patients, 89.0% (332/373) of patients fulfilled the NAFLD criteria and 68.9% met the NASH criteria. The overall prevalence of significant fibrosis (F≥2) was 9.1%. Our analysis showed that patients with T2DM have a significantly higher prevalence of significant fibrosis than those without T2DM (χ2 = 13.407, p=0.003). The prevalence of significant fibrosis increased significantly as age increased. We determined the frequency of fibrosis as 7.0% in individuals with age < 30 years rising to 25% in patients with an age ≥50 years (χ2 = 10.315, p=0.016). However, when patients were stratified according to gender, MS or BMI, there was no statistically significant correlation between the occurrence of significant fibrosis and any of these factors. In addition, we observed a 4.0% prevalence of advanced fibrosis (F≥3) and a 1.6% prevalence of cirrhosis in bariatric surgery patients. (Table 2, Figure 2).
Clinical predictors for significant fibrosis
To explore the predictive factors of significant fibrosis, clinical variables associated with significant fibrosis were evaluated using univariate analysis. Further analysis using a multivariable logistic regression model was performed based on variables with P < 0.05 in the univariate analysis (age, WHR, T2DM, FPG, c-peptide, HbA1c, AST, GGT, platelets). The results revealed that: age (OR], 1.06; 95% CI, 1.02-1.11, p=0.003); T2DM (OR, 2.62; 95% CI, 1.17-5.88, p=0.019); c- peptide (OR, 1.26; 95% CI, 1.03-1.55, p=0.025) and AST (OR, 1.02; 95% CI, 1.01-1.03, p=0.004) were detected as independent predictors of significant fibrosis. (Table 3)
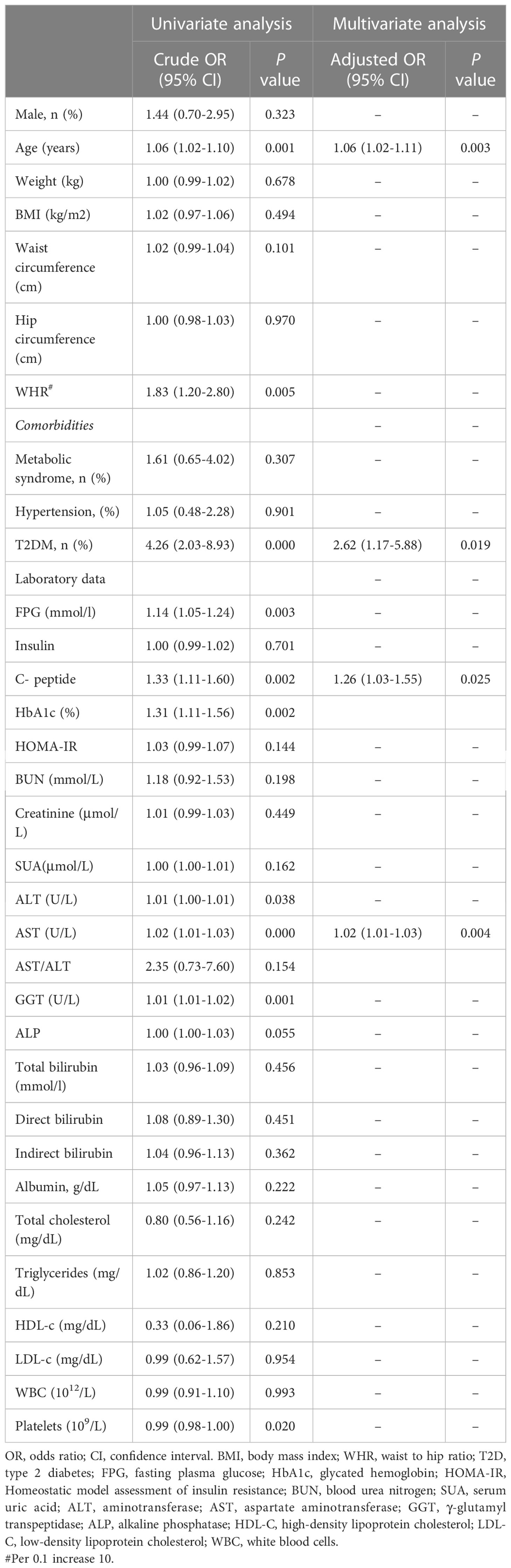
Table 3 Univariate and multivariate logistic regression analyses were used to identify independent factors associated with significant fibrosis.
Comparison of non-invasive scoring systems
To validate the reliability of non-invasive scoring algorithms for the diagnosis of significant fibrosis, we calculated the AUROC for the results of the five non-invasive scoring systems that were applied to our data. This yielded AUROC ranging from 0.652 to 0.781. The HFS had the best predictive performance, with an AUROC of 0.781, followed by the FIB-4 (0.745), APRI (0.759), NFS (0.657) and BARD (0.652) (Figure 3, Table 4). Pairwise comparison of the AUROC of different scoring systems demonstrated that there were significant differences between these non-invasive scoring systems, including APRI vs NFS, BRAD vs FIB-4, BRAD vs HFS, FIB-4 vs NFS and HFS vs NFS (all P < 0.05); while no significant differences between other non-invasive scoring systems were detected (all P > 0.05).
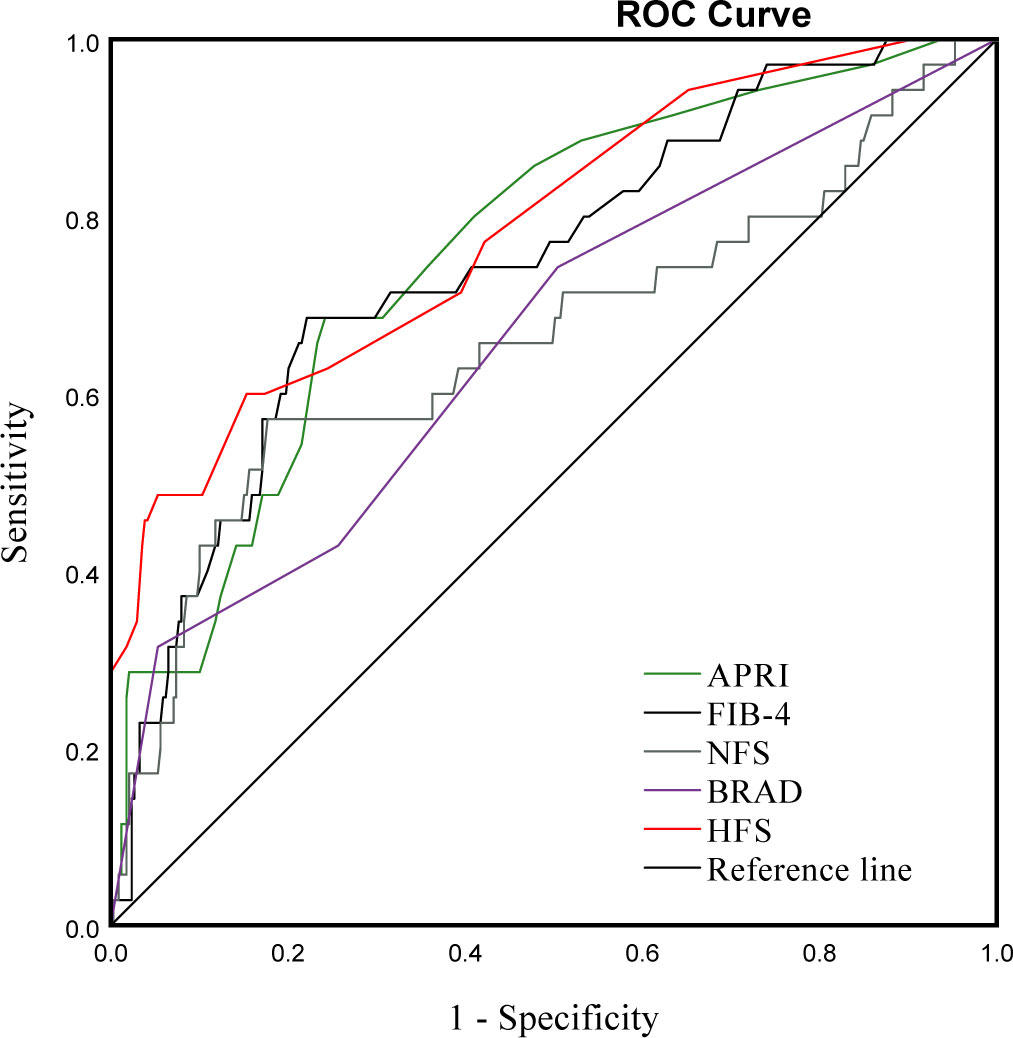
Figure 3 ROC curve for APRI, FIB-4, NFS, BARD and HFS in bariatric surgery patients with and without significant fibrosis.
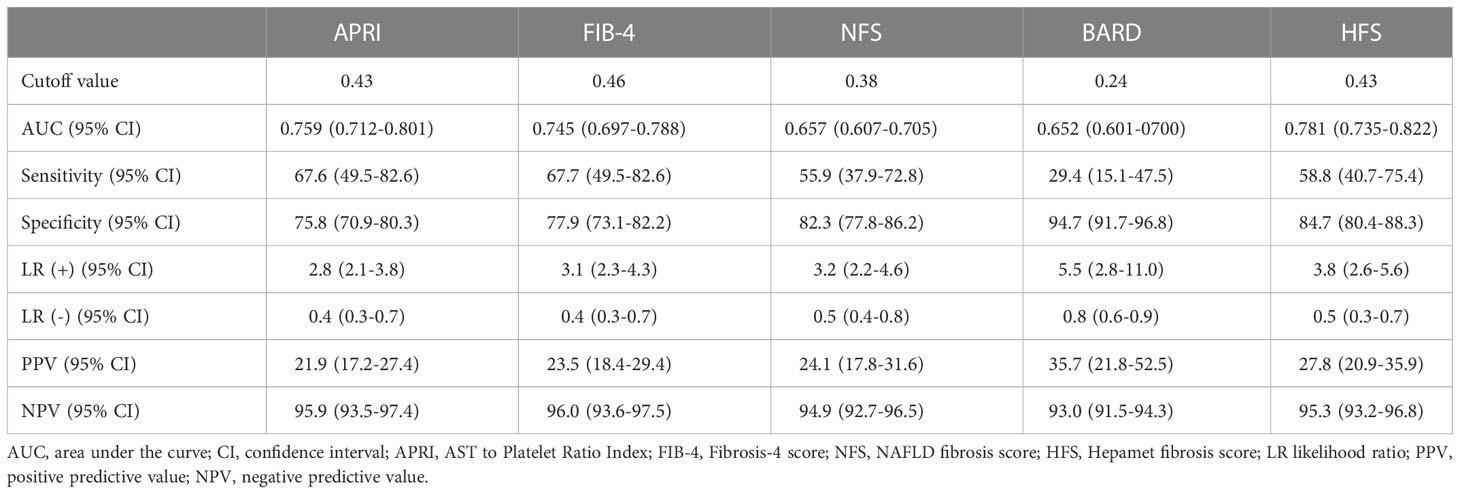
Table 4 Performance of the APRI, FIB-4, NFS, BARD and HFS for the detection of significant fibrosis.
Discussion
The presence of fibrosis in NAFLD patients affects clinical prognoses. NAFLD has got widespread attention in bariatric surgery patients, but there are still scant studies into the prevalence of significant fibrosis. For this reason, we first examined the prevalence and potential risk factors of significant fibrosis among Chinese bariatric surgery patients. Our results indicated an overall prevalence of significant fibrosis, advanced fibrosis and cirrhosis of 9.1%, 4.0% and 1.6%, respectively. Specifically, the odds of having significant fibrosis were independently associated with the presence of T2DM, increasing age, and elevated AST, c-peptide levels. Furthermore, we also validated the reliability of non-invasive scoring systems and found that APRI, FIB-4 and HFS showed appropriate AUROC (>0.70) for predicting significant fibrosis, but BRAD and NFS score revealed poorly predictive performance compared to the other scores.
Previous studies reported the prevalence of biopsy-proven NASH during bariatric surgery, ranging from 2.6% to 98% (4). Some potential explanations for the discrepancy in prevalence are different histological scoring systems, selection bias, race-based differences and variability of observations among pathologists. In this study, we observed 68.9% population had NASH and 60.9% had fibrosis, which was similar to those from Japan (77.5%) and Taiwan (71.3%) (4). In contrast, a study with 1000 patients who underwent routine liver biopsies during bariatric surgery showed the rate of NASH/fibrosis was only 14.3% (29). Another large-scale study including 2557 bariatric surgery patients also discovered that only 30.9% and 29.3% of individuals had NASH and fibrosis respectively (30). Obviously, our results were significantly higher than those from two studies (29, 30), as well as those from the USA (24.1-58.6%) and Australia (18.4-24.8%) (4). This discrepancy may be due to racial differences, as Asian populations (even individuals with relatively low BMI) have an elevated risk of metabolic disease due to differing body fat percentages and body composition (31). In addition, 9.1% of patients were found to have significant fibrosis, 4.0% had advanced fibrosis and 1.6% had cirrhosis. Our findings are in agreement with the study by Udelsman BV, which found that in a cohort of bariatric surgery patients, 7.8% had significant fibrosis and 3.6% had advanced fibrosis (30). However, another retrospective study of 330 patients undergoing routine liver biopsy during bariatric surgery showed an increased prevalence of significant fibrosis, although results for advanced fibrosis, and cirrhosis were more similar to our findings (20.9%, 4.2% and 1.5%, respectively) (32).
Significant fibrosis is an established risk factor for cirrhosis and overall mortality (33). Research has shown that advanced fibrosis can persist for many years despite substantial weight loss following bariatric surgery (34). Accordingly, the early identification of clinically significant fibrosis could potentially improve patient outcomes. Several independent predictors of advanced fibrosis have been reported in prior studies (9, 19, 25, 35), including increasing age, T2DM, HOMA-IR, hypertension, elevated AST, and decreased platelets. Of those predictors, T2DM is one of the most useful predictors of liver fibrosis. In this study, patients with T2DM have a higher prevalence of significant fibrosis than patients without T2DM. Glucose metabolism-related indicators, such as T2DM and c-peptide, were found to be strongly associated using multivariate logistic regression models. However, hypertension and MS were not accepted as predictors of significant fibrosis, in line with previous study (4, 36). In addition, our study found that increasing age and elevated AST were independently associated with significant fibrosis, as has been mentioned above predictors.
Current guidelines recommend utilizing non-invasive scoring systems to identify at-risk NASH or fibrosis (37). Among such non-invasive scoring systems, the APRI, FIB-4, BRAD and NFS are widely used to detect liver fibrosis (38). HFS was recently developed based on an international multicenter study with 2452 participants and provided superior performance to detect patients with advanced fibrosis with an AUROC of 0.85, the sensitivity of 74%, and specificity of 97.2%, when compared with the FIB-4 and NFS systems (25). Another international multicenter retrospective study of 379 biopsy-proven NAFLD patients showed HFS and FIB-4 had higher AUROC for identifying significant fibrosis (0.744 and 0.725, respectively) than that of the no NFS, but no statistical differences were found between HFS and FIB-4 AUROC (39). Similarly, a retrospective study including 222 patients with biopsy-proven NAFLD demonstrated that the HFS(AUROC,0.758) was marginally less superior than FIB-4(AUROC,0.796) in detecting advanced fibrosis (40). In this study, APRI, FIB-4 and HFS all showed sufficient prediction accuracy (all AUROC ≥0.70), but there were no significant differences between APRI, FIB-4 and HFS AUROC. Compared to other scoring systems, BRAD and NFS scores did not exhibit satisfactory diagnostic performance in detecting significant fibrosis. In this prospective derivation and global validation study, the accuracies of BRAD and NFS for predicting significant fibrosis were 0.58 (0.54–0.62) and 0.66 (0.62–0.70), respectively (7). In the study by Zambrano-Huailla R et al, NFS was unable to effectively detect significant fibrosis in patients with NAFLD, with an AUROC of 0.581 (39). Thus, the role of the BRAD and NFS in predicting significant fibrosis in bariatric surgery patients should be further explored. Based on the current results, we can use non-invasive scores (APRI, FIB-4 and HFS) to monitor these patients with fibrosis closely. (Reviewer #2)
The strength of our study is that it was the first to prospectively evaluate the prevalence and clinical predictors of biopsy-confirmed significant fibrosis among Chinese bariatric surgery patients. However, we acknowledged there were several limitations in the current study. Firstly, this was a single-center cross-section study, limiting our study’s generalizability. Secondly, the biopsy samples were only from the left lobe of the liver, which may lead to misclassification of liver fibrosis severity as, in terms of histology, severity varies depending on the specific area of the liver being biopsied (41). Thirdly, some drugs, such as lipid-lowering drugs, antihypertensive drugs and antidiabetic drugs, may influence the results. Finally, we could not evaluate the application of this test in bariatric patients, because our hospital lacked “FibroScan”. (Reviewer #1) Therefore, multicenter studies with larger sample sizes should be undertaken to better evaluate the prevalence of fibrosis and its predictive factors in Chinese bariatric surgery patients.
Conclusions
Our study showed more than two-thirds of bariatric surgery patients had NASH, and the prevalence of significant fibrosis was high. Risk factors for significant fibrosis include increasing age, presence of T2DM, elevated AST and c-peptide levels. Non-invasive models (including APRI, FIB-4 and HFS) can help clinicians to identify significant liver fibrosis in bariatric surgery patients. Further multicenter studies with larger sample sizes on liver fibrosis are warranted in bariatric surgery patients.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving human participants were reviewed and approved by the Ethics Research Committee of the First Affiliated Hospital of Jinan University (2019-024). The patients/participants provided their written informed consent to participate in this study.
Author contributions
YS and WC: conceptualization and writing-original draft preparation. YS, SD, and WC: methodology, data curation, and formal analysis. CW and ZD: resources, supervision, and project administration. CW, ZD, and WC: writing-review and editing. All authors contributed to the article and approved the submitted version.
Acknowledgments
The authors thank the survey respondents for participating in this study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Younossi ZM. Non-alcoholic fatty liver disease - a global public health perspective. J Hepatol (2019) 70(3):531–44. doi: 10.1016/j.jhep.2018.10.033
2. Younossi ZM, Golabi P, de Avila L, Paik JM, Srishord M, Fukui N, et al. The global epidemiology of NAFLD and NASH in patients with type 2 diabetes: A systematic review and meta-analysis. J Hepatol (2019) 71(4):793–801. doi: 10.1016/j.jhep.2019.06.021
3. Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver disease-meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology (2016) 64(1):73–84. doi: 10.1002/hep.28431
4. Seki Y, Kakizaki S, Horiguchi N, Hashizume H, Tojima H, Yamazaki Y. Prevalence of nonalcoholic steatohepatitis in Japanese patients with morbid obesity undergoing bariatric surgery. J Gastroenterol (2016) 51(3):281–9. doi: 10.1007/s00535-015-1114-8
5. Powell EE, Wong VW, Rinella M. Non-alcoholic fatty liver disease. Lancet (2021) 397(10290):2212–24. doi: 10.1016/S0140-6736(20)32511-3
6. Chalasani N, Younossi Z, Lavine JE, Charlton M, Cusi K, Rinella M, et al. The diagnosis and management of nonalcoholic fatty liver disease: Practice guidance from the American association for the study of liver diseases. Hepatology (2018) 67(1):328–57. doi: 10.1002/hep.29367
7. Harrison SA, Ratziu V, Boursier J, Francque S, Bedossa P, Majd Z, et al. A blood-based biomarker panel (NIS4) for non-invasive diagnosis of non-alcoholic steatohepatitis and liver fibrosis: a prospective derivation and global validation study. Lancet Gastroenterol Hepatol (2020) 5(11):970–85. doi: 10.1016/S2468-1253(20)30252-1
8. Hagström H, Nasr P, Ekstedt M, Hammar U, Stål P, Hultcrantz R, et al. Fibrosis stage but not NASH predicts mortality and time to development of severe liver disease in biopsy-proven NAFLD. J Hepatol (2017) 67(6):1265–73. doi: 10.1016/j.jhep.2017.07.027
9. Wong RJ, Liu B, Bhuket T. Significant burden of nonalcoholic fatty liver disease with advanced fibrosis in the US: A cross-sectional analysis of 2011-2014 national health and nutrition examination survey. Aliment Pharmacol Ther (2017) 46(10):974–80. doi: 10.1111/apt.14327
10. Pelusi S, Cespiati A, Rametta R, Pennisi G, Mannisto V, Rosso C, et al. Prevalence and risk factors of significant fibrosis in patients with nonalcoholic fatty liver without steatohepatitis. Clin Gastroenterol Hepatol (2019) 17(11):2310–9.e6. doi: 10.1016/j.cgh.2019.01.027
11. Yu SJ, Kim W, Kim D, Yoon JH, Lee K, Kim JH, et al. Visceral obesity predicts significant fibrosis in patients with nonalcoholic fatty liver disease. Med (Baltimore) (2015) 94(48):e2159. doi: 10.1097/MD.0000000000002159
12. European Association for the Study of the Liver (EASL)., European Association for the Study of Diabetes (EASD) and European Association for the Study of Obesity (EASO). EASL-EASD-EASOEASL-EASD-EASO clinical practice guidelines for the management of non-alcoholic fatty liver disease. J Hepatol (2016) 64(6):1388–402. doi: 10.1007/s00125-016-3902-y
13. Wai CT, Greenson JK, Fontana RJ, Kalbfleisch JD, Marrero JA, Conjeevaram HS, et al. A simple noninvasive index can predict both significant fibrosis and cirrhosis in patients with chronic hepatitis C. Hepatology (2003) 38(2):518–26. doi: 10.1053/jhep.2003.50346
14. Harrison SA, Oliver D, Arnold HL, Gogia S, Neuschwander-Tetri BA. Development and validation of a simple NAFLD clinical scoring system for identifying patients without advanced disease. Gut (2008) 57(10):1441–7. doi: 10.1136/gut.2007.146019
15. Angulo P, Hui JM, Marchesini G, Bugianesi E, George J, Farrell GC, et al. The NAFLD fibrosis score: a noninvasive system that identifies liver fibrosis in patients with NAFLD. Hepatology (2007) 45(4):846–54. doi: 10.1002/hep.21496
16. Sterling RK, Lissen E, Clumeck N, Sola R, Correa MC, Montaner J, et al. Development of a simple noninvasive index to predict significant fibrosis in patients with HIV/HCV coinfection. Hepatology (2006) 43(6):1317–25. doi: 10.1002/hep.21178
17. Drolz A, Wolter S, Wehmeyer MH, Piecha F, Horvatits T, Schulze Zur Wiesch J, et al. Performance of non-invasive fibrosis scores in non-alcoholic fatty liver disease with and without morbid obesity. Int J Obes (Lond) (2021) 45(10):2197–204. doi: 10.1038/s41366-021-00881-8
18. Alkayyali T, Qutranji L, Kaya E, Bakir A, Yilmaz Y. Clinical utility of noninvasive scores in assessing advanced hepatic fibrosis in patients with type 2 diabetes mellitus: A study in biopsy-proven non-alcoholic fatty liver disease. Acta Diabetol (2020) 57(5):613–8. doi: 10.1007/s00592-019-01467-7
19. Labenz C, Huber Y, Kalliga E, Nagel M, Ruckes C, Straub BK, et al. Predictors of advanced fibrosis in non-cirrhotic non-alcoholic fatty liver disease in Germany. Aliment Pharmacol Ther (2018) 48(10):1109–16. doi: 10.1111/apt.14976
20. Xanthakos SA, Jenkins TM, Kleiner DE, Boyce TW, Mourya R, Karns R, et al. High prevalence of nonalcoholic fatty liver disease in adolescents undergoing bariatric surgery. Gastroenterology (2015) 149(3):623–34.e8. doi: 10.1053/j.gastro.2015.05.039
21. NCD Risk Factor Collaboration (NCD-RisC). Trends in adult body-mass index in 200 countries from 1975 to 2014: A pooled analysis of 1698 population-based measurement studies with 19·2 million participants. Lancet (2016) 387(10026):1377–96. doi: 10.1016/S0140-6736(16)30054-X
22. Yang W, Wang C. Metabolic surgery needs stronger endorsement in Asian T2DM patients with low BMI. Obes Surg (2022) 32(1):212–3. doi: 10.1007/s11695-021-05636-y
23. Alberti KG, Eckel RH, Grundy SM, Zimmet PZ, Cleeman JI, Donato KA, et al. Harmonizing the metabolic syndrome: a joint interim statement of the international diabetes federation task force on epidemiology and prevention; national heart, lung, and blood institute; American heart association; world heart federation; international atherosclerosis society; and international association for the study of obesity. Circulation (2009) 120(16):1640–5. doi: 10.1161/CIRCULATIONAHA.109.192644
24. American Diabetes Association. (2) classification and diagnosis of diabetes. Diabetes Care (2015) 38 Suppl:S8–S16. doi: 10.2337/dc15-S005
25. Ampuero J, Pais R, Aller R, Gallego-Durán R, Crespo J, García-Monzón C, et al. Development and validation of hepamet fibrosis scoring system-a simple, noninvasive test to identify patients with nonalcoholic fatty liver disease with advanced fibrosis. Clin Gastroenterol Hepatol (2020) 18(1):216–25.e5. doi: 10.1016/j.cgh.2019.05.051
26. Bedossa P, Poitou C, Veyrie N, Bouillot JL, Basdevant A, Paradis V, et al. Histopathological algorithm and scoring system for evaluation of liver lesions in morbidly obese patients. Hepatology (2012) 56(5):1751–9. doi: 10.1002/hep.25889
27. Kleiner DE, Brunt EM, Van Natta M, Behling C, Contos MJ, Cummings OW, et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology (2005) 41(6):1313–21. doi: 10.1002/hep.20701
28. Hanley JA, McNeil BJ. A method of comparing the areas under receiver operating characteristic curves derived from the same cases. Radiology (1983) 148(3):839–43. doi: 10.1148/radiology.148.3.6878708
29. Subichin M, Clanton J, Makuszewski M, Bohon A, Zografakis JG, Dan A. Liver disease in the morbidly obese: A review of 1000 consecutive patients undergoing weight loss surgery. Surg Obes Relat Dis (2015) 11(1):137–41. doi: 10.1016/j.soard.2014.06.015
30. Udelsman BV, Corey KE, Lindvall C, Gee DW, Meireles OR, Hutter MM, et al. Risk factors and prevalence of liver disease in review of 2557 routine liver biopsies performed during bariatric surgery. Surg Obes Relat Dis (2019) 15(6):843–9. doi: 10.1016/j.soard.2019.01.035
31. Kasama K, Mui W, Lee WJ, Lakdawala M, Naitoh T, Seki Y, et al. IFSO-APC consensus statements 2011. Obes Surg (2012) 22(5):677–84. doi: 10.1007/s11695-012-0610-7
32. Tseng J, Korman J, Noureddin M, Shouhed D, Miller JP, Feng X, et al. Routine versus selective liver biopsy during bariatric surgery: Postoperative outcomes and preoperative predictors of NASH. Obes Surg (2022) 32(2):463–71. doi: 10.1007/s11695-021-05797-w
33. Lomonaco R, Godinez Leiva E, Bril F, Shrestha S, Mansour L, Budd J, et al. Advanced liver fibrosis is common in patients with type 2 diabetes followed in the outpatient setting: The need for systematic screening. Diabetes Care (2021) 44(2):399–406. doi: 10.2337/dc20-1997
34. Pais R, Aron-Wisnewsky J, Bedossa P, Ponnaiah M, Oppert JM, Siksik JM, et al. Persistence of severe liver fibrosis despite substantial weight loss with bariatric surgery. Hepatology (2022) 76(2):456–68. doi: 10.1002/hep.32358
35. Ciardullo S, Monti T, Perseghin G. High prevalence of advanced liver fibrosis assessed by transient elastography among U.S. adults with type 2 diabetes. Diabetes Care (2021) 44(2):519–25. doi: 10.2337/dc20-1778
36. Yang S, Cheng J, Zhang R, Sun H, Zhang H, Lyu S, et al. Metabolic dysfunction-associated fatty liver disease and liver fibrosis: Prevalence and associated factors in the middle-aged and older US population. Hepatol Res (2022) 52(2):176–86. doi: 10.1111/hepr.13728
37. Castera L, Friedrich-Rust M, Loomba R. Noninvasive assessment of liver disease in patients with nonalcoholic fatty liver disease. Gastroenterology (2019) 156(5):1264–81.e4. doi: 10.1053/j.gastro.2018.12.036
38. Vilar-Gomez E, Chalasani N. Non-invasive assessment of non-alcoholic fatty liver disease: Clinical prediction rules and blood-based biomarkers. J Hepatol (2018) 68(2):305–15. doi: 10.1016/j.jhep.2017.11.013
39. Zambrano-Huailla R, Guedes L, Stefano JT, de Souza AAA, Marciano S, Yvamoto E, et al. Diagnostic performance of three non-invasive fibrosis scores (Hepamet, FIB-4, NAFLD fibrosis score) in NAFLD patients from a mixed Latin American population. Ann Hepatol (2020) 19(6):622–6. doi: 10.1016/j.aohep.2020.08.066
40. Higuera-de-la-Tijera F, Córdova-Gallardo J, Buganza-Torio E, Barranco-Fragoso B, Torre A, Parraguirre-Martínez S, et al. Hepamet fibrosis score in nonalcoholic fatty liver disease patients in Mexico: Lower than expected positive predictive value. Dig Dis Sci (2021) 66(12):4501–7. doi: 10.1007/s10620-020-06821-2
Keywords: non-alcoholic fatty liver disease, non-alcoholic steatohepatitis, fibrosis, bariatric surgery, obesity
Citation: Huang Y, Dong S, Wang C, Dong Z and Chen W (2023) Significant fibrosis assessed by liver biopsy among Chinese bariatric surgery patients: A prospective cross-sectional study. Front. Endocrinol. 14:1090598. doi: 10.3389/fendo.2023.1090598
Received: 10 November 2022; Accepted: 04 January 2023;
Published: 30 January 2023.
Edited by:
Amanda Brandon, The University of Sydney, AustraliaReviewed by:
Xintian Cai, People’s Hospital of Xinjiang Uygur Autonomous Region, ChinaYanlong Shi, The Second Affiliated Hospital of Nanjing Medical University, China
Wenle Li, Xiamen University, China
Hongzhu Yu, First Affiliated Hospital of Anhui Medical University, China
Copyright © 2023 Huang, Dong, Wang, Dong and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wenhui Chen, am51Y3doMjAxOUAxNjMuY29t
†These authors have contributed equally to this work
 Yongsheng Huang1†
Yongsheng Huang1† Cunchuan Wang
Cunchuan Wang Wenhui Chen
Wenhui Chen