- 1Chronic Diseases Research Center, Endocrinology and Metabolism Population Sciences Institute, Tehran University of Medical Sciences, Tehran, Iran
- 2Non-Communicable Diseases Research Center, Alborz University of Medical Sciences, Karaj, Iran
Background and aims: Some studies have reported that the topical forms with aminophylline as the active ingredient appear to be relatively effective on local fat burning while having no/minimal side effects. This systematic review accumulates all of the data on the local fat-burning potency of aminophylline topical formulation.
Methods: Documents were retrieved from PubMed, Web of Science, and Scopus databases until Aug 2022. Data were extracted from clinical trials reporting the reduction in thigh or waist circumference as a result of using topical forms containing aminophylline. Screening of included studies was performed independently by two authors and the quality assessment of included studies was performed based on the Cochrane Collaboration’s approach.
Results: Of the 802 initial studies, 5 studies were included in the systematic review. Several concentrations of aminophylline were used in different studies. Most studies administred the topical formulation on participants’ one thigh, and the other thigh was considered to be the control for comparing the fat reduction amount. Except for one study, all other studies reported that all participants lost more fat on the treated area than the control groups. The amount of fat reduction differed in studies regarding their different aminophylline concentrations and administration routines. In the case of side effects, except for some studies reporting skin rashes, other studies reported no significant side effects at all.
Conclusions: Aminophylline topical formulation offers a safe, effective, and much less invasive alternative to cosmetic surgery for localized fat reduction. It seems that the 0.5% concentration, administered five times a week for five weeks is the most potent concentration. However, more high-quality clinical trials are needed to verify this conclusion.
Systematic review registration: https://www.crd.york.ac.uk/prospero/, identifier CRD42022353578.
Introduction
Obesity is a multifactorial chronic and progressive disease of excess adipose tissue that can occur at any age (1). According to reports (2), 46% of adults aged ≥20 are overweight or obese worldwide. Moreover, United States (US) has the highest obesity rate, with a prevalence of 34% in adults (3). Studies have also predicted that nearly 1.92 billion people worldwide will be obese or overweight by 2030 (2). On top of the physical health risks associated with obesity, negative psychological consequences are also inevitable. Depression (4–6), body image dissatisfaction (7), and stress (7–9) are all examples of this regard that can reduce one’s quality of life both directly and indirectly.
Regarding obesity management strategies, behavioral modification is one of the main approaches. However, depressed mood and anxiety reduce obese people’s functioning and their adherence to lifestyle changes (8). Medical interventions, on the other hand, are becoming more popular, and the obesity control guidelines strongly recommend medical interventions for overweight patients (10). In the case of these interventions, the most common approaches are oral/injectable anti-obesity medications (11–13) and bariatric surgeries (14). However, there are some drawbacks to these medical interventions too. The most significant disadvantages of oral anti-obesity agents (i.e., phentermine/topiramate, naltrexone/bupropion, and orlistat) are their systematic side effects, such as neuro-psychiatric, fetal, and cardiovascular side effects (11–13). Furthermore, it has been reported that the degree of weight loss provided by anti-obesity medications does not typically provide the type of cosmetic benefit that many patients seek (11, 12). In the case of injectable medications (i.e., liraglutide) and bariatric surgeries, their main disadvantage is their aggressive method of administration and their high costs (12, 14, 15). As a result, the most appropriate drug is one that is more effective, has fewer side effects, is less expensive, and is administered in a non-aggressive manner.
Topical formulations, on the other hand, are non-aggressive methods of drug administration and have significantly fewer side effects due to their low systematic absorbance. Regarding efficacy, several attempts have been made to develop a topical formulation for local fat loss (16–21). Among all, some studies have reported that the topical forms with aminophylline as the active ingredient appear to be relatively effective on local fat burning while having no/minimal side effects.
Therefore, the goal of this study was to accumulate all of the data on the local fat-burning potency of aminophylline topical formulation in order to get one step closer to developing topical and effective fat burner agents.
Methods
Prior to the start of the study, a detailed research protocol was prepared and registered in the international prospective register of systematic reviews (PROSPERO) with the CRD42022353578 ID, which was then followed throughout the process. The 27-item PRISMA (preferred reporting items for systematic reviews and meta-analyses) statement was used as the reporting model for this systematic review to ensure inclusiveness (22). Furthermore, the 12-item “PRISMA for abstract” extension was used to write the abstract (23).
Search strategy
A complete search strategy used for each database is reported in Supplementary Table 1. Two researchers independently searched until Aug 2022 for relevant articles published (with no restriction of publication year) in the following data sources: PubMed, web of science, and Scopus using the following search terms: “Aminophylline” OR “Theophylline ethylenediamine” OR “Theophylline-ethylenediamine” AND “Topical” OR “Topical administration” OR “Cream” OR “Lotion” OR “Local” OR “Subcutaneous” OR “Fat” OR “Fat burn” OR “Fat burner” OR “Fat burning” OR “Fat reduction” OR “Fat reducer” OR “Fat loss” OR “Lipolysis” OR “Contouring” OR “Body contouring” OR “Waist” OR “Thigh” OR “Skin” OR “Circumference” OR “Regional” OR “Cellulite” OR “Adipose tissue” OR “Adiposity” OR “Obesity” OR “Mesotherapy” OR “Cosmetic”. Studies with any search terms in their title or abstract were found, and their abstracts were pooled and imported into Endnote Reference Manager. After excluding duplicate studies and completing screening stages, the research team decided which studies will finally be chosen.
Eligibility criteria and study selection
Obesity and cellulite were designated as the study’s exposures of interest. The intervention was defined as topical aminophylline application, and the local fat reduction in the studied body area, as our primary outcome of interest, was compared between exposed and unexposed populations. After excluding duplicate studies, two independent researchers performed the first screening stage, examining the remaining articles in accordance with the study’s stated objectives. Reviews, animal studies, experimental studies, clinical trials, and editorial or other types of letters were excluded at this stage.
Through the second screening stage, clinical trials were included if:
● Aminophylline was used in topical form (cream, lotion, ointment, and gel) alone or in combination with other ingredients.
● Desired information on the participants was provided.
● Results were reported as the amount of subcutaneous fat reduced.
Studies got excluded if:
● The study was on the production process of topical formulation.
● Fat reduction was not reported at all or as a result of using topical aminophylline.
Each researcher reported the studies they had picked after the second screening stage, and the team decided which studies would ultimately be selected for data synthesis. Disagreements were settled, if necessary, by a discussion with a third research team member.
Data extraction
Data from papers were individually gathered by two reviewers and entered into Microsoft Excel.
The data that was extracted included:
● Publication details: author(s), title, journal, date, stated aims.
● Study characteristics: study location, number of participants, participants’ characteristics, pharmaceutical form of aminophylline, other ingredients of topical formulation (if applicable), and body area(s) studied.
● Critical data: Topical formulation and concentration of active pharmaceuticals, administration routine, other interventions (if applicable), adverse effects (if reported), and final results in as much detail as possible (mean ± SD or median ± SE).
Risk of bias assessment
Using the Cochrane Collaboration’s approach (24), two reviewers independently categorized studies as having a low, high, or uncertain risk of bias in various domains. Then, if necessary, disagreements were discussed with a third researcher on the research team in order to be resolved.
The risk assessment method developed by Cochrane Collaboration evaluates the probability of bias in six areas for each study. Areas are “Selection bias”, “Performance bias”, “Detection bias”, “Attrition bias “, “Reporting bias”, and “Other bias”. The “selection bias” section checks whether the study clearly describes the allocation sequence generation process so that it can be determined whether or not it should result in comparable groupings. Furthermore, it also verifies whether the study has indicated if intervention allocations could have been anticipated before or during enrollment by providing enough information about the technique employed to disguise the allocation sequence. The “Performance bias” section monitors if the study describes the procedures taken, if any, to prevent trial participants’ intervention status from being known to researchers. The “Detection bias” section ensures if the study describes all procedures taken, if any, to prevent participants’ particular interventions from being known during outcome evaluation. The “Attrition bias” determines whether the study describes how comprehensive the outcome data is for each primary outcome, taking attrition and analytical exclusions into account. And finally, the “Reporting bias” section examines if the study describes the methodology used to analyze selective outcome reporting.
Results
Study selection
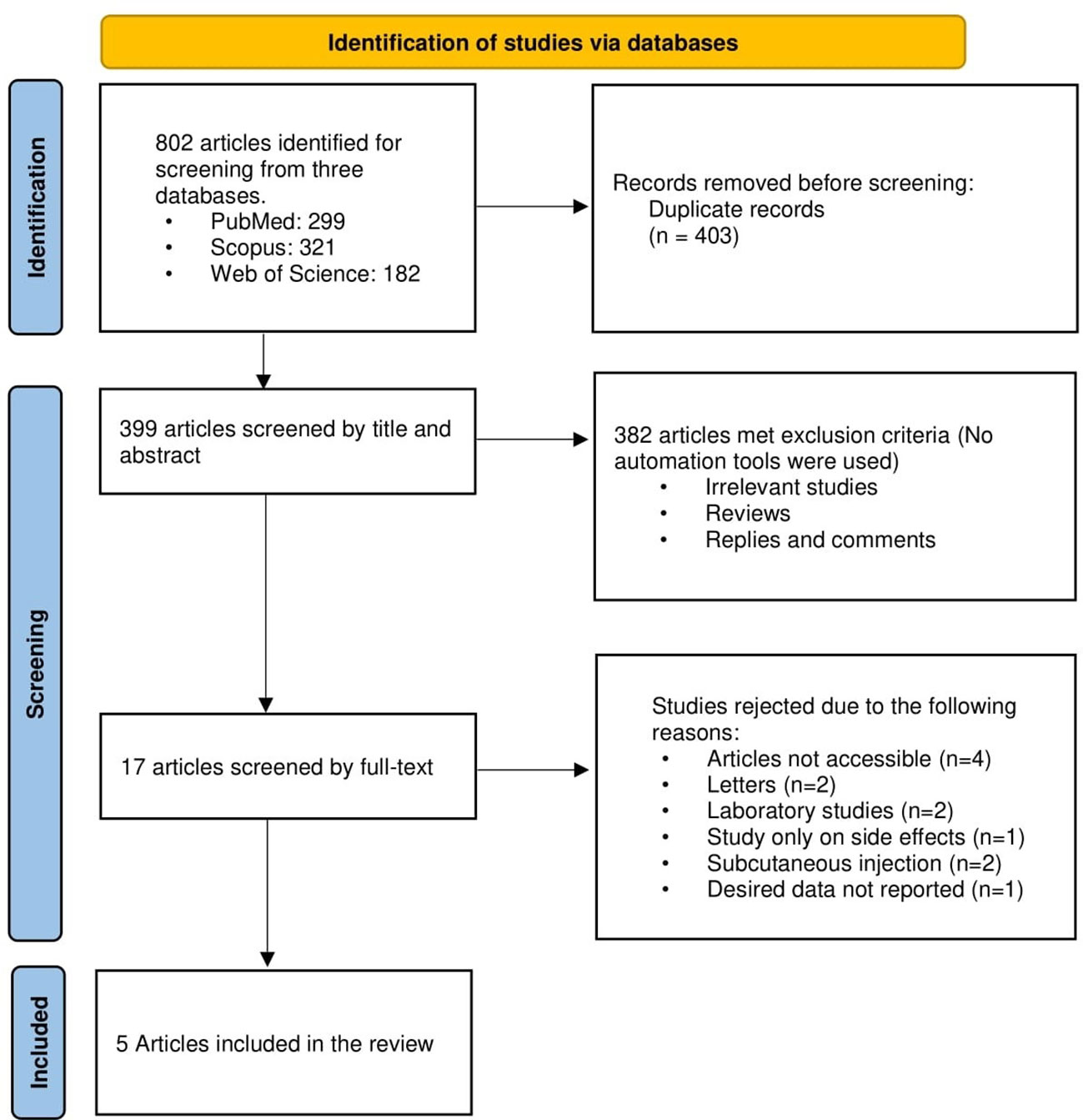
Figure 1 displays a flowchart of the study selection process’ summary. Using the aforementioned search protocol, 802 articles were initially found, including 299 from PubMed, 321 from Scopus, and 182 from Web of Science. The total number of 399 items made it to the first stage of screening after 403 duplicates were eliminated. Then, 382 articles were excluded as a consequence of the first step’s title and abstract screening because they were either unrelated to the issue or did not include the information that was sought. Twelve studies were eliminated from the 17 remaining papers that underwent full-text screening because there was insufficient information presented on the relationship between the topical use of aminophylline and local fat reduction. Finally, data from five papers (17, 18, 25–27) were used after they satisfied all inclusion criteria.
Basic characteristics of the selected studies
Table 1 shows the basic characteristics of the five studies that were finally included. All studies except one that was carried out in England (25), were done in the US. Six sub-trials were included in one of the studies (18), of which five were utilized in this study. That one study was dropped because it utilized injection administration. In general, sample sizes in studies were small but varied, ranging from five to 25 participants and totaling 117 people, which were mostly women. The majority of the research was done on participants who were obese, thought they were obese, or thought their thighs were unattractively fat. With the exception of two studies that created lotion (27) and gel (17) forms, all other studies formulated creams and ointments. Three of the trials added additional components to the formulation. Forskolin, yohimbine, caffeine, L-carnitine, and gotu kola (Centella asiatica) were added in two of the studies to boost the fat-burning impact, while glycolic acid was added in the other to increase cream penetration into the skin. Three trials used the Aquaphor® base for formulating ointment. Regarding the body area(s) tested, all studies tested on the thighs, except for one study that tested the topical formulation on the waist (26). Among all, two studies examined the buttocks as well (17, 25).
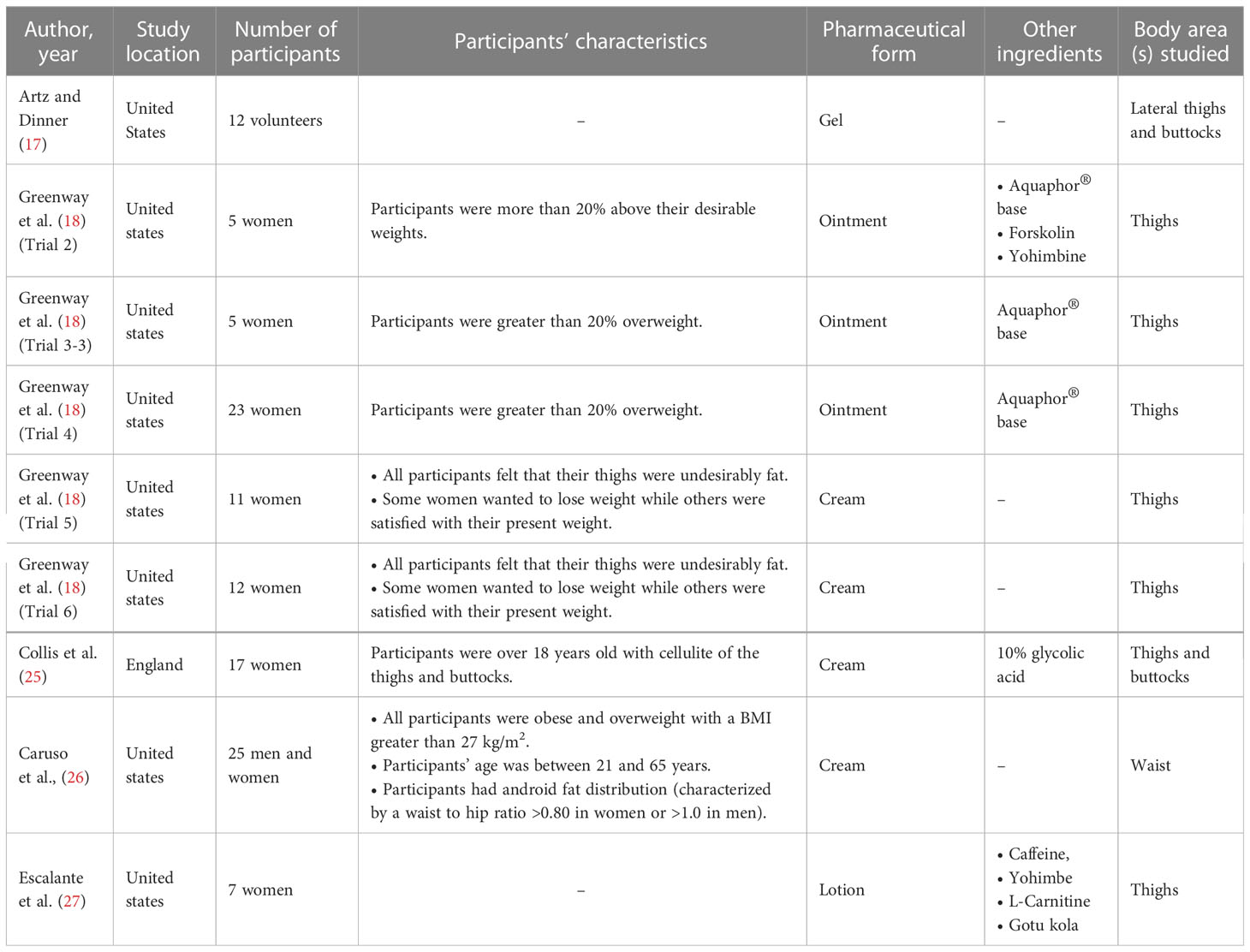
Table 1 Basic characteristics of clinical trials evaluating the effect of topical aminophylline in local fat reduction.
Outcomes
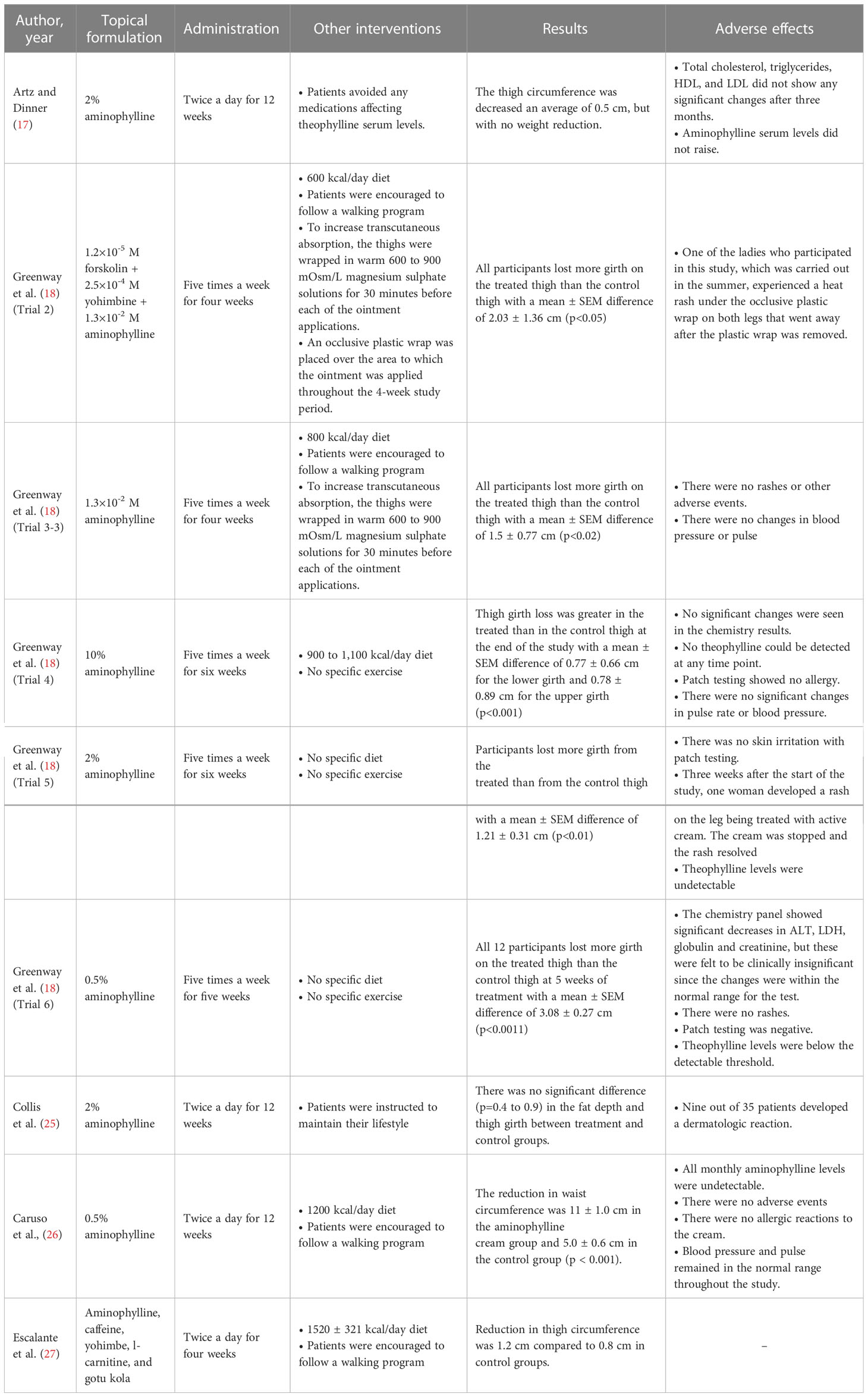
A detailed summary of studies evaluating the effect of topical aminophylline in local fat reduction is provided in Table 2. As can be seen, several concentrations of aminophylline were used in different studies. Among all, the highest concentration of aminophylline used was 10%, with the administration of five times a week on participants’ one thigh (18). The other thigh was considered to be the control for comparing the fat reduction amount. Although participants were suggested to stick to a 900 to 1,100 kcal/day diet, they were not suggested to do any exercises. After six weeks, there had been a higher loss in participants’ thigh girth in the treated thigh than in the control thigh, with a mean ± SEM difference of 0.77 ± 0.66 cm for the lower girth and 0.78 ± 0.89 cm for the upper girth (p < 0.001). Heart rate, blood pressure, and blood chemistry all remained unchanged. Additionally, no theophylline could ever be found in blood samples, and no allergic reactions were documented.
In the case of 2% aminophylline concentration, there were three experiments. None of the three studies’ participants maintained a regular diet or exercise routine. In the first two studies (17, 25), using twice-daily treatment, one of them indicated a mean 0.5 cm reduction in thigh fat after 12 weeks, and the other found no significant difference in the fat reduction after 12 weeks. The third study (18), applying the cream five times a week, reported that participants lost more girth from the treated than from the control thigh with a mean ± SEM difference of 1.21 ± 0.31 cm (p < 0.01) after six weeks. While one of the studies reported no side effects at all, two other studies reported skin rashes in nine out of 35, and one out of 11 participants.
Regarding 0.5% aminophylline concentration, there existed two experiments. In one of them (18), participants applied the topical formulation five times per week for five weeks without engaging in any physical activity or dietary changes. As a result, the treated thigh lost more circumference than the control thigh, with a mean ± SEM difference of 3.08 ± 0.27 cm (p < 0.001) in all 12 participants. Neither any dermatologic rashes nor any theophylline serum levels were detected. Moreover, although alanine transaminase (ALT), lactate dehydrogenase (LDH), globulin, and creatinine levels in the chemistry panel all significantly decreased, the changes were deemed to be clinically unimportant because they were within the test’s normal range. In the second research on the 5% aminophylline concentration (26), the topical formulation was administered twice daily on the participants’ waist, the subjects consumed 1200 kcal per day, and walking programs were suggested. After 12 weeks, the mean reduction in waist circumference was 11 ± 1.0 cm in the aminophylline cream group and 5.0 ± 0.6 cm in the control group (p < 0.001). Blood pressure and heart rate remained unchanged throughout the study, no allergic reactions were reported, and all monthly aminophylline levels were undetectable.
There existed one study using 1.3×10-2 M aminophylline concentration in the topical form five times a week (18). Participants were encouraged to stick to a 800 kcal/day diet and to follow a walking program. For 30 minutes before each application of ointment, the thighs were wrapped in heated 600 to 900 mOsm/L magnesium sulphate solutions to increase absorption. After four weeks, on the treated thigh, more girth was decreased than on the untreated thigh with a mean ± SEM difference of 1.5 ± 0.77 cm (p <0.02). Regarding side effects, no rashes and no changes in blood pressure and heart rate were reported.
Finally, two studies added other active ingredients to their topical formulation. In the first one (18), the formulation was 1.2×10-5 M forskolin plus 2.5×10-4 M yohimbine plus 1.3×10-2 M aminophylline and was administered five times a week. Participants were encouraged to stick to a 600 kcal/day diet and follow a walking program. To increase transcutaneous absorption, the thighs were wrapped in warm 600 to 900 mOsm/L magnesium sulphate solutions for 30 minutes before each of the ointment applications. Moreover, the region to which the ointment was applied was covered with an occlusive plastic wrap. After four weeks, the treated thigh of each subject shrunk more than the control thigh with a mean ± SEM difference of 2.03 ± 1.36 cm (p <0.05). In the case of side effects, one of the women who took part in this trial, which was conducted in the summer, developed a heat rash on both legs under the occlusive plastic wrap, which disappeared once the plastic wrap was taken off. Another study (27), which supplemented the topical formulation with other active components, combined the effects of aminophylline, caffeine, yohimbe, l-carnitine, and gotu kola to reduce localized fat. The formulation was used twice a day along with a 1520 ± 321 kcal/day diet and walking program. After four weeks, the reduction in thigh circumference was 1.2 cm compared to 0.8 cm in the control groups.
Risk of bias assessment
Supplementary Table 2 shows the results of Cochrane Collaboration’s tool for assessing the risk of bias in the final retrieved studies. As can be observed, in the “Random sequence generation” area all research had an uncertain risk of bias, except for one study (27) that had a low risk of bias. The results in the “Allocation concealment” domain were exactly the same. In the “Blinding of participants and personnel” area, all research had a low risk of bias, except for one study that had a high risk of bias (17). Results in the “Blinding of outcome assessment” section were varied, with two, one, and two studies having high, unclear, and low risk of bias, respectively. Regarding the “Incomplete outcome data” domain, studies had one unclear, three lows, and one high risk of bias. In the case of the “Selective reporting” area, all studies had a low risk of bias. Finally, no studies had other types of biases.
Discussion
This research established the possibility for topical formulations with aminophylline as their active component to be employed as local fat burners. Furthermore, even though it was demonstrated that these topical treatments alone can reduce subcutaneous fat, it seems that adding even a slight exercise and diet may increase their effectiveness.
Aminophylline is a bronchodilator agent that is FDA-approved for managing acute asthma and is available in the form of oral capsules, oral tablets, and intravenous solutions. Although it is not FDA-approved for local fat reduction, some aminophylline-containing creams are currently available in the store and are advertised as cellulite removers. Whether or not they are obese, many women worry about how their thighs, buttocks, waist, and double chin look. Women who turn to surgical treatments as a kind of therapy because they are so upset with the way their body fat looks benefit from topical forms of fat burning the most. Topical lipolysis is less dangerous, with avoiding the associated dangers of surgery, such as scarring and infection hazards, as well as anesthesia risks. Moreover, most of the ladies who participated in trials evaluating the potency of topical aminophylline in local fat reduction reported having a better sense of how their body looked, which may have actually enhanced how they felt about themselves. However, although the topical formulation of aminophylline seemed to be helpful in reducing localized fat, trials that did not take into account diets and exercise routines for participants did not report many cases of weight loss. Therefore, the difference in subcutaneous fat and lack of difference in weight reduction between the two groups point to a superficial alteration in fat distribution.
Side effects
Regarding side effects, all trials that tracked participant blood chemistry, blood pressure, blood levels of aminophylline and theophylline, and pulse rate found no significant changes, therefore it appears that this formulation is safe in terms of adverse effects. The only unfavorable consequence that was noted occasionally was skin rashes. Regarding why rashes happened, some studies suggested possible mechanisms. It was recognized that aminophylline, which is made up of two theophylline molecules linked by an ethylenediamine molecule, is a skin irritant (26). In studies using a simple base cream, it was reported that a chemical reaction with aminophylline causes a common cream base to become yellow. This yellow cream fails to work and gave some test subjects rashes (26). As a result, it was proven that utilizing a specially created cream base that stabilizes the aminophylline decreases skin rashes in addition to assisting in adjusting its skin penetration.
Possible mechanism of action
Xanthenes are thought to increase collagen production, lipolysis, microcirculation, and thermogenesis along with decreasing adipogenesis (28–31). In the case of their lipolytic actions, publications have shown that beta-adrenergic stimulation directly increases the cyclic adenosine monophosphate (cAMP) concentrations of fat cells in adipocytes, which is believed to be the underlying mechanism (28, 30, 32, 33). The increased cAMP levels cause protein kinase A to phosphorylate the activating hormone-sensitive lipase (HSL). Now, triglycerides are hydrolyzed by phosphorylated HSL into free fatty acids, glycerol, diglycerides, and monoglycerides (34). Parallelly, since xanthines are thought to inhibit phosphodiesterase (PDE), an enzyme involved in the degradation of cAMP, as well, the inhibition of PDE further raises cAMP activity. Therefore, the researchers believe that by applying aminophylline topically to suppress PDE, local cAMP concentrations and local lipolysis would both rise (28, 30, 35–37).
Strength and limitations
This study was one of the first systematic reviews about local fat burning by topical formulations. To ensure comprehensiveness, we reported the outcomes of studies using various aminophylline concentrations in their topical formulations, as well as formulations using substances other than aminophylline alone. However, there are some limitations to this study. First of all, despite a comprehensive search strategy, there were few recent research on our subject, and the sources used for this study are relatively old. One possible reason for this oldness may be that most of the studies concentrate on the intravenous administration of aminophylline, and the topical use is not well known. Second, there isn’t enough information accessible for each concentration to allow for useful comparison because there aren’t many references, and each reference employed different concentrations. Third, some references exhibited a high risk of bias in particular areas after utilizing the Cochrane Collaboration’s methodology to assess the risk of bias.
Conclusion
Aminophylline topical formulation offers a safe, effective, and much less invasive alternative to cosmetic surgery for the localized reduction of fat. Conducting further trials to compare the efficacy of different concentration of aminophylline is suggested for future works, as well as evaluating the efficacy of other xanthines in local fat burning.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Author contributions
RA and AH: Writing the manuscript, Collecting the data. ED and MQ: Designing the study, Checking and revising, Supervision. All authors contributed to the article and approved the submitted version.
Funding
The present manuscript was supported by Alborz University of Medical Sciences (ID: 103-5410).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2023.1087614/full#supplementary-material
References
1. Jastreboff AM, Kotz CM, Kahan S, Kelly AS, Heymsfield SB. Obesity as a disease: The obesity society 2018 position statement. Obesity (2019) 27(1):7–95. doi: 10.1002/oby.22378
2. Orukwowu U. Epidemiology of adult obesity, measurements, global prevalence and risk factors. IPS Intelligentsia Multidiscip J (2022) 1(1):1–6. doi: 10.54117/iimj.v1i1.1
3. Ward ZJ, Long MW, Resch SC, Gortmaker SL, Cradock AL, Giles C, et al. Redrawing the US obesity landscape: Bias- corrected estimates of state-specific adult obesity prevalence. PloS One (2016) 11(3):1–135. doi: 10.1371/journal.pone.0150735
4. Patsalos O, Keeler J, Schmidt U, Penninx BWJH, Young AH, Himmerich H. Diet, obesity, and depression: A systematic review. J Personalized Med (2021) 11(3):1–195. doi: 10.3390/jpm11030176
5. Pistis G, Milaneschi Y, Vandeleur CL, Lasserre AM, Penninx BWJH, Lamers F, et al. Obesity and atypical depression symptoms: Findings from mendelian randomization in two European cohorts. Trans Psychiatry (2021) 11(1). doi: 10.1038/s41398-021-01236-7
6. Frank P, Jokela M, Batty GD, Lassale C, Steptoe A, Kivimäki M. Overweight, obesity, and individual symptoms of depression: A multicohort study with replication in UK biobank. Brain Behavior Immun (2022) 105:192–200. doi: 10.1016/j.bbi.2022.07.009
7. Chu DT, Nguyet NTM, Nga VuT, Lien NVuT, Vo DD, Lien N, et al. An update on obesity: Mental consequences and psychological interventions. Diabetes Metab Syndrome: Clin Res Rev (2019) 13(1):155–60. doi: 10.1016/j.dsx.2018.07.015
8. Finer N. Medical consequences of obesity. Med (United Kingdom) (2015) 43(2):88–93. doi: 10.1016/j.mpmed.2014.11.003
9. Fulton S, Décarie-Spain L, Fioramonti X, Guiard B, Nakajima S. The menace of obesity to depression and anxiety prevalence. Trends Endocrinol Metab (2022) 33(1):18–35. doi: 10.1016/j.tem.2021.10.005
10. Tak YJ, Lee SY. Long-term efficacy and safety of anti-obesity treatment: Where do we stand? Curr Obes Rep (2021) 10(1):14–30. doi: 10.1007/s13679-020-00422-w
11. Kang JG, Park CY. Anti-obesity drugs: A review about their effects and safety. Diabetes Metab J (2012) 36(1):13–255. doi: 10.4093/dmj.2012.36.1.13
12. Bessesen DH, Van Gaal LF. Progress and challenges in anti-obesity pharmacotherapy. Lancet Diabetes Endocrinol (2018) 6(3):237–48. doi: 10.1016/S2213-8587(17)30236-X
13. Singh AK, Singh R. Pharmacotherapy in obesity: A systematic review and meta-analysis of randomized controlled trials of anti-obesity drugs. Expert Rev Clin Pharmacol (2020) 13(1):53–64. doi: 10.1080/17512433.2020.1698291
14. Arterburn DE, Telem DA, Kushner RF, Courcoulas AP. Benefits and risks of bariatric surgery in adults: A review. JAMA - J Am Med Assoc (2020) 324(9):879–8875. doi: 10.1001/jama.2020.12567
15. Lin CH, Shao L, Zhang Y, Tu YJ, Zhang Y, Tomlinson B, et al. An evaluation of liraglutide including its efficacy and safety for the treatment of obesity. Expert Opin Pharmacother. (2020) 21(3):275–2855. doi: 10.1080/14656566.2019.1695779
16. Greenway FL, Bray GA. Regional fat loss from the thigh in obese women after adrenergic modulation. Clin Ther (1987) 9(6):663–9.
17. Artz JS, Dinner MI. Treatment of cellulite deformities of the thighs with topical aminophylline gel. Plast Surg (1995) 3(4). doi: 10.4172/plastic-surgery.1000129
18. Greenway FL, Bray GA, Heber D. Obesity research. Torrance, CA: Harbor-UCLA Medical Center, Department of Medicine, Division of Endocrinology, UCLA School of Medicine (1995). 3:S561–8. doi: 10.1002/j.1550-8528.1995.tb00228.x
19. Pekarovics S, Raum WJ, Greenway FL. Obesity drugs: Xanthines in regional fat reduction. J Invest Med (1999) 47(2).
20. Armanini D, Nacamulli D, Francini-Pesenti F, Battagin G, Ragazzi E, Fiore C. Glycyrrhetinic acid, the active principle of licorice, can reduce the thickness of subcutaneous thigh fat through topical application. Steroids (2005) 70(8):538–5425. doi: 10.1016/j.steroids.2005.01.007
21. Lee G-R, Shin MK, Yoon D-J, Kim A-R, Yu R, Park N-H, et al. Topical application of capsaicin reduces visceral adipose fat by affecting adipokine levels in high-fat diet-induced obese mice. Obesity (2013) 21(1):115–22. doi: 10.1002/oby.20246
22. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. BMJ (2009) 339(7716):332–6. doi: 10.1136/bmj.b2535
23. Beller EM, Glasziou PP, Altman DG, Hopewell S, Bastian H, Chalmers I, et al. PRISMA for abstracts: Reporting systematic reviews in journal and conference abstracts. PloS Med (2013) 10(4). doi: 10.1371/journal.pmed.1001419
24. Higgins JPT, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, et al. The cochrane collaboration’s tool for assessing risk of bias in randomised trials. BMJ (Online) (2011) 343(7829):1–9. doi: 10.1136/bmj.d5928
25. Collis N, Elliot LA, Sharpe C, Sharpe DT. Cellulite treatment: A myth or reality: A prospective randomized, controlled trial of two therapies, endermologie and aminophylline cream. Plast Reconstructive Surg. (1999) 104:1110–4. doi: 10.1097/00006534-199909020-00037
26. Caruso MK, Pekarovic S, Raum WJ, Greenway F. Topical fat reduction from the waist. Diabetes Obes Metab (2007) 9(3):300–3. doi: 10.1111/j.1463-1326.2006.00600.x
27. Escalante G, Bryan P, Rodriguez J. Effects of a topical lotion containing aminophylline, caffeine, yohimbe, l-carnitine, and gotu kola on thigh circumference, skinfold thickness, and fat mass in sedentary females. J Cosmetic Dermatol (2019) 18(4):1037–10435. doi: 10.1111/jocd.12801
28. Article, Review. Cellulite and its treatment. Int J Cosmetic Sci (2006) 28:175–90. doi: 10.1111/j.1467-2494.2006.00318.x
29. Dulloo AG. The search for compounds that stimulate thermogenesis in obesity management: From pharmaceuticals to functional food ingredients. Obes Rev (2011) 12(10):866–83. doi: 10.1111/j.1467-789X.2011.00909.x
30. Byun SY, Kwon SH, Heo SuH, Shim JS, Du MH, Na JI. Efficacy of slimming cream containing 3.5% water-soluble caffeine and xanthenes for the treatment of cellulite: Clinical study and literature review. Ann Dermatol (2015) 27(3):243–2495. doi: 10.5021/ad.2015.27.3.243
31. Carrageta DF, Dias TR, Alves MG, Oliveira PF, Monteiro MP, Silva BM. Anti-obesity potential of natural methylxanthines. J Funct Foods (2018) 43:84–94. doi: 10.1016/j.jff.2018.02.001
32. Epstein E, Young VL, Schorr M, Young AE, Weisenborn S. Prospective and randomized determination of the efficacy of topical lipolytic agents. Aesthetic Surg J (1997) 17(5):304–3075. doi: 10.1016/S1090-820X(97)80019-2
33. Eisenstein A, Ravid K. G Protein-coupled receptors and adipogenesis: A focus on adenosine receptors. J Cell Physiol (2014) 229(4):414–4215. doi: 10.1002/jcp.24473
34. Diepvens K, Westerterp KR, Westerterp-Plantenga MS. Obesity and thermogenesis related to the consumption of caffeine, ephedrine, capsaicin, and green tea. Am J Physiol - Regul Integr Comp Physiol (2007) 292(1):77–85. doi: 10.1152/ajpregu.00832.2005
35. Dickinson BI, GoraHarper ML. Aminophylline for cellulite removal. Ann OF PHARMACOTHERAPY (1996) 30(3):292–3. doi: 10.1177/106002809603000315
36. Arnold R, Beer D, Bhalay G, Baettig U, Collingwood SP, Craig S, et al. 8-aryl xanthines potent inhibitors of phosphodiesterase 5. Bioorganic Medicinal Chem Lett (2002) 12(18):2587–90. doi: 10.1016/S0960-894X(02)00480-8
Keywords: aminophylline, topical, fat reduction, thigh, lipolysis
Citation: Abdi Dezfouli R, Hosseinpour A, Qorbani M and Daneshzad E (2023) The efficacy of topical aminophylline in local fat reduction: A systematic review. Front. Endocrinol. 14:1087614. doi: 10.3389/fendo.2023.1087614
Received: 24 November 2022; Accepted: 06 February 2023;
Published: 16 February 2023.
Edited by:
Claire Joanne Stocker, Aston University, United KingdomReviewed by:
Sathya Sethuraman, Alagappa University, IndiaNebojsa Petar Stilinovic, University of Novi Sad, Serbia
Copyright © 2023 Abdi Dezfouli, Hosseinpour, Qorbani and Daneshzad. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Elnaz Daneshzad, ZGFuZXNoemFkQGdtYWlsLmNvbQ==
 Ramin Abdi Dezfouli
Ramin Abdi Dezfouli Ali Hosseinpour2
Ali Hosseinpour2 Mostafa Qorbani
Mostafa Qorbani Elnaz Daneshzad
Elnaz Daneshzad