- 1Department of Cell Biology, University of Oklahoma Health Sciences Center, Oklahoma City, OK, United States
- 2Vascular Cognitive Impairment, Neurodegeneration and Healthy Brain Aging Program, Department of Neurosurgery, University of Oklahoma Health Sciences Center, Oklahoma City, OK, United States
- 3Oklahoma Center for Geroscience and Healthy Brain Aging, University of Oklahoma Health Sciences Center, Oklahoma City, OK, United States
- 4International Training Program in Geroscience, Department of Public Health, Semmelweis University, Budapest, Hungary
- 5Institute of Biophysics, Biological Research Centre, Eötvös Lorand Research Network (ELKH), Szeged, Hungary
Age-related cerebrovascular pathologies, ranging from cerebromicrovascular functional and structural alterations to large vessel atherosclerosis, promote the genesis of vascular cognitive impairment and dementia (VCID) and exacerbate Alzheimer’s disease. Recent advances in geroscience, including results from studies on heterochronic parabiosis models, reinforce the hypothesis that cell non-autonomous mechanisms play a key role in regulating cerebrovascular aging processes. Growth hormone (GH) and insulin-like growth factor 1 (IGF-1) exert multifaceted vasoprotective effects and production of both hormones is significantly reduced in aging. This brief overview focuses on the role of age-related GH/IGF-1 deficiency in the development of cerebrovascular pathologies and VCID. It explores the mechanistic links among alterations in the somatotropic axis, specific macrovascular and microvascular pathologies (including capillary rarefaction, microhemorrhages, impaired endothelial regulation of cerebral blood flow, disruption of the blood brain barrier, decreased neurovascular coupling, and atherogenesis) and cognitive impairment. Improved understanding of cell non-autonomous mechanisms of vascular aging is crucial to identify targets for intervention to promote cerebrovascular and brain health in older adults.
1 Introduction
Age-related cognitive impairment and dementia are major public health challenges in the rapidly aging societies of the developed world. In older adults, cognitive impairment of vascular etiology [vascular cognitive impairment and dementia or VCID (1)] is the second most common cause of clinically diagnosed dementia after Alzheimer’s disease (AD) (2–4). VCID is also one of the most frequent causes of loss of independence and increased morbidity in older adults (1). Vascular pathologies are also a critical component of AD, and vascular dysfunction is one of the earliest pathologies to appear in patients with mild cognitive impairment, many of whom go on to develop dementia (5–8).
In addition to large vessel disease, age-related VCID is associated with a wide variety of microvascular pathologies (9–17). Microvascular contributions to cognitive decline and dementia include microvascular rarefaction (18–21), impaired endothelial regulation of cerebral blood flow (5, 22–30), disruption of the blood brain barrier (BBB) (19, 31–36), decreased neurovascular coupling (NVC) (37–41), cerebral microhemorrhages (CMH) (42–44), lacunar infarcts (45–49), increased pulsatility (50–53) and small vessel disease-related white matter damage (54–57), and amyloid pathologies (58–62). Critically, the severity of age-related increases in microvascular pathological alterations predict cognitive decline in aging (44, 63, 64), leading to great interest in understanding the associated cellular and molecular mechanisms. Additionally, age-related microvascular pathologies also exacerbate severity of ischemic brain injury (65–67).
There is growing preclinical evidence that interventions that promote cerebromicrovascular health and rejuvenation (68–71) have beneficial effects on cognitive health in aging. Understanding the mechanisms implicated in age-related impairment of the cerebral circulation is essential for identification of novel targets for translationally relevant interventions and development of innovative therapies to promote healthy cerebrovascular and brain aging. In this review, the effect of aging on a critical endocrine pathway, the somatotropic axis, and its role in regulating the functional and structural integrity of the cerebral circulation is considered in terms of potential mechanisms involved in age-related dysregulation of cerebral blood flow and increased susceptibility to microvascular damage.
2 Regulation of aging processes by the GH/IGF-1 axis
The neuroendocrine hypothesis of aging posits that changes in endocrine output of the hypothalamic-pituitary axis regulate the process of organismal aging in a cell non-autonomous manner (72). There is particularly strong evidence that among the related endocrine factors, the somatotropic axis, including growth hormone (GH) and insulin-like growth factor-1 (IGF-1), exerts a central role in regulation of cellular processes involved in aging.
IGF-1 is an evolutionarily highly conserved pleiotropic anabolic hormone and growth factor (73–84). It exhibits high sequence similarity to insulin and is a member of a complex intercellular signaling system. IGF-1 is secreted by the liver as a result of stimulation by GH and is also produced locally by a number of cell types where it acts in a paracrine and autocrine manner (including cardiovascular cells, astrocytes and neurons). The GH/IGF-1 axis also includes cell-surface receptors (IGF1R and IGF2R) and a family of high-affinity IGF-binding proteins (IGFBP1 to IGFBP7), as well as associated IGFBP degrading proteases (see overview in Figure 1). GH is secreted from the anterior pituitary gland in response to GH releasing hormone, and acts on the liver and other tissues to promote the secretion of IGF-1 (86). The GH/IGF-1 axis is essential for proper growth and development (87–89) and confers multifacteed pro-survival, anabolic and cellular protective effects. Levels of GH decrease by ~14%/decade after approximately the third decade of life (90, 91), and as a result levels of circulating IGF-1 also significantly decrease with age (92–95).
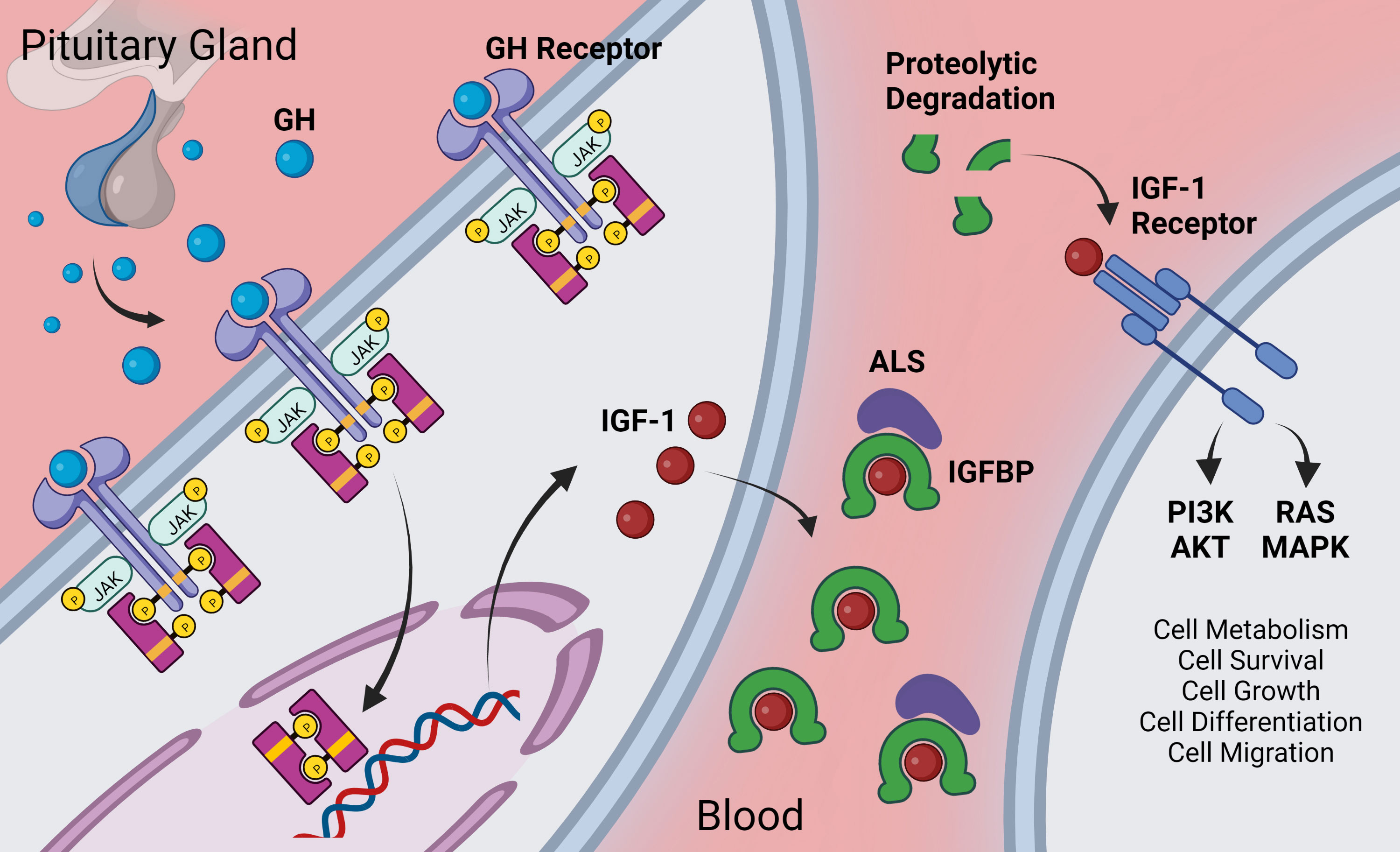
Figure 1 Overview of GH/IGF-1 signaling axis. Growth hormone is secreted from the anterior pituitary gland. It acts via the GH receptor on target cells via Janus kinase (JAK)-signal transducer and activator of transcription (STAT) –signaling to promote expression of various genes including IGF-1. Target cells include hepatocytes (for endocrine/circulating IGF-1) as well as other cells throughout the body that locally secrete IGF-1. Extracellular IGF-1 is bound to IGF-1 binding proteins (IGFBP) either in a two-part complex or in a three-part complex with the glycoprotein acid labile subunit (ALS). IGF-1 has higher affinity for IGFBPs than for the IGF-1 receptor, and IGF-1 is released from the IGFBP upon proteolytic cleavage of the IGFBP [for more on the complex role of IGFBPs, see (85)]. Freed IGF-1 interacts with the IGF-1 receptor where it signals, largely through the PI3K/AKT and the RAS/MAPK cascades to regulate many pro-survival cellular pathways. Created with BioRender.com.
The role of decreased GH and IGF-1 in aging has been extensively studied. Age-related GH/IGF-1 deficiency has been causally linked to the genesis of aging phenotypes in various organ systems, including the cardiovascular system, musculoskeletal system and the central nervous system (95–105). Patients with decreased GH/IGF-1 have an increased risk of VCID and other forms cardiovascular and cerebrovascular disease (94, 106–108), including gait and cognitive impairment (109–111), as well as diabetes mellitus (106). Older adults with low circulating IGF-1 levels have 39% higher risk of cardiovascular mortality (112). Animal models of circulating IGF-1 deficiency serve as models of accelerated aging (18, 21, 93, 113–120), mimicking many age-related cerebrovascular pathologies.
Many cytoprotective and anti-aging effects of GH and IGF-1 have been described, and the protective role of the GH/IGF-1 axis in regulation of the development of age-related diseases (e.g. via modulation of metabolism, protein synthesis, glucose metabolism, cellular proliferation and differentiation) is well-supported by the literature (10, 93, 97, 98, 102, 103, 105, 115, 116, 119, 121–134). However, the role of GH/IGF-1 in the regulation of lifespan is admittedly complex and highly controversial. There is strong experimental evidence suggesting that disruption of GH/IGF-1 signaling (or of their orthologs in lower organisms) is often associated with lifespan extension both in invertebrate model organisms (C. elegans, D. melanogaster) and laboratory rodents (135, 136), including Ames and Snell dwarf mice (135–141), the ‘little mouse’ (Ghrhrlit/lit), mice null for either GH receptor/binding protein (GHR/BP-/-) or p66(shc) (p66(shc-/-)), GHRH and GHR double-knockout mice (142), mice heterozygous for the IGF-I receptor (Igf1r+/-), and fat-specific insulin receptor knockout mice (143–152). Interestingly, GH receptor knockout (GHRKO) mice (153), which also have low IGF-1 levels, hold the Methuselah prize for the world’s longest-lived laboratory mouse. There have been several attempts to reconcile these two, apparently contradicting, aspects of the “GH/IGF-1 paradox of aging” (98, 154). A key observation is that dwarfism in murine (Ames and Snell dwarf mice and the ‘little mouse’) and rat [spontaneous dwarf rat (155)] models caused by early-onset disruption of the GH/IGF-1 axis is associated with longevity [a notable exception being the Lewis dwarf rat (156)]. Importantly, the remarkable life-span extension of hypopituitary Ames dwarf mice was shown to depend on low levels of GH in a relatively short peripubertal time-window (149, 154). These data raised the possibility that in murine models, GH/IGF-1 regulates lifespan primarily through developmental programming of aging (154). Despite the significant progress made in the field of geroscience in the past two decades, the impact of disruption of the GH/IGF-1 axis on human lifespan and longevity is vigorously debated (98, 157–163). Overall, the available epidemiological evidence has suggested that neither early-life nor late-life disruption of GH/IGF-1 signaling in humans extends lifespan (98). While the literature on the role of the GH/IGF-1 axis in modulating aging processes, lifespan and the development of specific age-related diseases is large, here we focus on links between the GH/IGF-1 axis and manifestations of cerebrovascular aging.
3 Role of GH/IGF-1 in cerebrovascular remodeling
Blood vessels undergo constant functional and structural remodeling to respond to changing tissue demands, metabolic conditions, and injury repair (164, 165). Structural remodeling consists of proangiogenic processes, vascular quiescence, and vascular regression. Angiogenesis provides increased blood supply in a long-term manner to tissues with high metabolic demand, for example exercise-mediated angiogenesis and uterine artery adaptation during pregnancy. During vascular quiescence, vascular cells stop proliferating and undergo further maturation, specialization, and stabilization. During this phase, brain endothelial cells form tight and adherens junctions, providing a physical barrier in vessels. Lastly, blood vessels can undergo vascular regression including vascular involution, in which an extended vascular network regresses, or vascular pruning in which single vessels regress. These processes are crucial for fine-tuning the hierarchical organization of blood vessels, providing highly organized networks of arteries, capillaries, and veins. The mechanisms and regulation of the processes involved in vascular remodeling have been extensively reviewed by Ouarné et al. (164). Dysregulation of vascular remodeling can lead to and exacerbate several vascular and non-vascular pathologies. For example, AD is associated with excessive microvascular pruning, vasoconstriction, and brain hypoperfusion (164). Aging is also associated with pathological vascular remodeling, predominantly characterized by changes in wall rigidity, increased fragility, and vascular rarefaction (72).
3.1 The ECM in IGF-1 mediated vascular remodeling
The extracellular matrix (ECM) plays a critical role in vascular remodeling. In addition to providing structural support to tissues, the ECM is involved in transducing biochemical and biomechanical signals, modulating adhesion of adjacent cells, and regulating differentiation, migration, and stability. The ECM constantly undergoes a qualitative and quantitative remodeling process mediated by specific enzymes such as matrix metalloproteinases (MMPs), a disintegrin and metalloproteinases (ADAMs), and meprins (166), and altered ECM remodeling contributes to pathologies such as AD and cancers (72, 167). Aging is also characterized by dysregulation of vascular ECM remodeling (72, 168, 169). With age, ECM biosynthesis decreases, and there are alterations in cell-matrix attachments, biomechanical signaling, and balance between proteases and their inhibitors. These age-mediated changes in the ECM contribute to vascular pathologies such as arterial stiffening, loss of BBB integrity, vascular fragility, and the development of CMH (72, 169, 170).
IGF-1 deficiency has been shown to lead to defects in vascular remodeling (Figure 2). One of the most widely-used models to study IGF-1 deficiency in aging is an adult-onset circulating IGF-1 knockdown model in which liver-specific IGF-1 knockdown is induced post-development (~3-6 months of age) via injection of AAV-TBG-Cre. When this model is exposed to hypertension as a cerebrovascular challenge, IGF-1 deficiency increased MMP activation and oxidative stress (93), increased vessel rigidity, promoted medial atrophy, and decreased vessel elasticity (114). Hypertension is normally associated with protective ECM remodeling (largely regulated by vascular smooth muscle cells [VSMCs]), including upregulation of ECM cross-linking genes (e.g. lox, loxl1, loxl4), elastin and elastin-associated genes (e.g. eln, fbn1), and some collagens (e.g. Col1a1, Col3a1, Col 8a1). However, this remodeling response was blunted or abolished in mice with circulating IGF-1 deficiency (114). Support for a role of IGF-1 in the maintenance of vascular stability through ECM proteins comes from the observation that across seven different mouse strains, aortic collagen levels were positively correlated with serum IGF-1 levels (171).
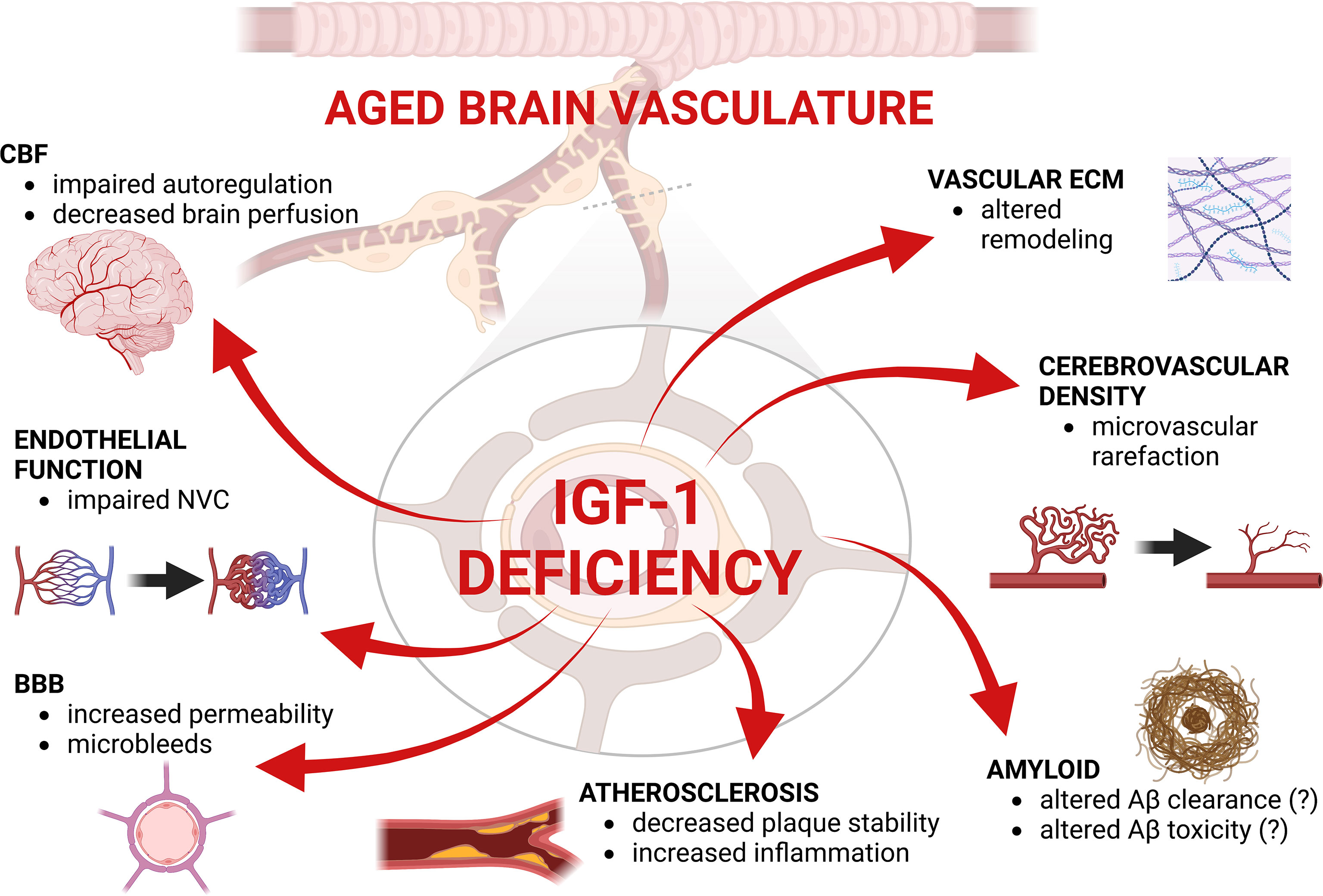
Figure 2 Summary figure highlighting the effects of IGF-1 deficiency in the aged brain vasculature. CBF, Cerebral blood flow; ECM, extracellular matrix; BB, blood-brain barrier; NVC, neurovascular coupling. Created with BioRender.com.
3.2 Role of GH/IGF-1 in regulation of cerebral capillary density
Cerebrovascular complexity and high vessel density are essential for healthy brain function, but decrease with age, manifesting as microvascular rarefaction in humans and animal models (172–175). Age-related microvascular rarefaction has been causally linked to cognitive decline (176–178) and other organ-specific manifestations of organismal aging (179–182).
A role for circulating/endocrine factors in age-related microvascular loss is highlighted by recent work showing that exposure to young blood via heterochronic parabiosis reversed age-related microvascular rarefaction and hypoperfusion (183–185). Analysis of upstream transcriptional regulators identified IGF-1 receptor signaling as one of the candidates responsible for this rejuvenation. IGF-1 is a potent pro-survival factor in both endothelial cells and VSMCs, and microRNA-mediated knockdown of IGF-1 receptor in endothelial cells can induce apoptosis in vitro (186). Consistent with this, decreased cerebrovascular density is observed in IGF-1 deficient models. Animals with decreased circulating IGF-1 exhibit hippocampal microvascular rarefaction (21, 178), and circulating IGF-1 has been shown to be essential for exercise induced increases in cerebral vascular density (Figure 2) (18, 187). Treatment with IGF-1 has also been shown to promote cerebrovascular angiogenesis and increase vessel density, for example in post-stroke models (188, 189) and in the normal adult mouse brain (187). Increased IGF-1 levels associated with high circulating GH also result in increased retinal microvascular density (190, 191). The dynamic balance between the processes of angiogenesis and capillary regression is essential for maintenance of the optimal network architecture of the cerebromicrovasculature. Aging is associated with a progressive deterioration of microvascular homeostasis, at least in part due to age-related impairment of angiogenic processes (173, 192–196). IGF-1 is known to confer potent and multifaceted pro-angiogenic effects, whereas IGF-1 deficiency impairs multiple aspects of angiogenesis (197–201). The pro-aging effects of endocrine GH/IGF-1 deficiency may be exacerbated by an age-related decline in other vasculoprotective growth factors, including pituitary adenylate cyclase-activating polypeptide (PACAP) (202) and vascular endothelial growth factor (VEGF) (203–205) and endothelial resistance to the effects of pro-angiogenic stimuli (206).
Because of the tight relationship between GH/IGF-1, some studies have supplemented animals with GH in order to induce endogenous production of IGF-1. In aged rats, supplementation with GH not only increased systemic levels of IGF-1 but also increased the density of microvessels within the top layer of the cerebral cortex (178). Combined these data highlight a clear role for IGF-1 deficiency in microvascular rarefaction in the brain.
3.3 Role of GH/IGF-1 in remodelling of larger vessels
In addition to microvascular rarefaction, aging is also associated with defects in remodeling in larger vessels. In parabiosis studies, the rejuvenating effects of young blood were also observed in macrovasculature. Aortas from aged mice exposed to young blood showed improved functional remodeling manifested as restored endothelium-mediated vasorelaxation and decreased oxidative stress. Additionally, upstream regulator analysis (via IPA) of the transcriptome of rejuvenated aortas suggested that rejuvenation was associated with activation of the IGF-1 pathway (185). On the other hand, young aortas exposed to aged blood also showed significant transcriptomic changes. Gene ontology analysis revealed that exposure to aged systemic factors upregulated genes associated with pathologic vascular remodeling. IPA upstream analysis showed that pro-geronic effects of aged blood might include inhibition of pathways mediated by IGF-1, serum response factor, and vascular endothelial growth factor (VEGF-A) (184). Recent human studies support the hypothesis that cell non-autonomous mechanisms contribute to age-mediated changes in vascular remodeling. Yu et al. identified a protein signature in the urine of older individuals that is associated with vascular remodeling (207). Human studies have also highlighted a role for the somatotropic axis in ECM regulation in the vasculature. Patients with uncontrolled acromegaly, a disease caused by hypersecretion of GH (and characterized by consequent increase in IGF-1 levels), exhibit baseline increases in vessel wall thickness and increased wall-to-lumen ratio in retinal arterioles compared to controls (191). This is similar to what has been observed in the GH overexpressing transgenic mouse, which exhibits increased medial layer area in the aorta and mesenteric vessels (208). This GH/IGF-1 mediated increased layer thickness is likely due to both altered ECM deposition and cell proliferation. Indeed VSMC proliferation has been reported in the aorta after perfusion with IGF-1 in rat diabetic aortic catheterization model (209). These structural changes have functional correlates, and it has been shown that noradrenaline-induced VSMC-mediated aorta contraction is increased in mice pre-treated with IGF-1 (210).
Combined these findings suggest that IGF-1 deficiency is associated with microvascular rarefaction and impaired vascular remodeling in response to stressors such as hypertension. In contrast, overexpression of IGF-1/GH can lead to excess vascular wall hypertrophy, highlighting a central role for the somatotropic axis in the regulation of vascular wall growth. Much of this regulation is tied to IGF-1 mediated changes in ECM gene expression, in particular regulation of elastin and elastin-associated genes, which is consistent with the importance of maintaining appropriate responses to mechanical stress in the vasculature.
4 Role of GH/IGF-1 in regulation of cerebrovascular function
4.1 GH/IGF-1 in regulation of cerebral blood flow and autoregulatory reactivity
The limited energy storage and high metabolic rate of the brain demands a constant flow of blood to deliver oxygen and nutrients and remove cellular, metabolic, or toxic by-products. There are several overlapping mechanisms which regulate cerebral blood flow (CBF) to maintain the baseline flow as well as mediate activity-dependent adjustment in flow to increased oxygen and nutrient demand (211).
To help maintain constant intravascular blood pressure in the brain in the face of systemic changes in blood pressure, cerebral vessels have a myogenic autoregulatory response system, where the arteries and arterioles in the pial and parenchymal circulation respond to changes in intraluminal pressure with changes in vascular tone and diameter (211). Healthy young animals exhibit structural (increased wall thickness) and functional (increased myogenic tone) adaptation of the proximal arterial branches of the cerebrovascular tree in response to permanent increases in blood pressure, thereby maintaining normal pressure and blood flow in the thin-walled, injury-prone downstream portion of the microcirculation (212). There is growing evidence that aging is associated with functional (myogenic autoregulatory dysfunction) and structural maladaptation to increased blood pressure in the cerebral circulation (213, 214), which has been causally linked to the increased susceptibility to the development of microhemorrhages and BBB disruption. Age-related arterial stiffening results in an increased pulse pressure which causes increased pulsatility in CBF in elderly individuals (215). Studies show that in aged mice, the myogenic response to static increases in pressure is intact in isolated middle cerebral arteries, but is significantly impaired in response to increases in pulsatile pressure (213, 214), while responses in aged parenchymal arterioles were impaired in response to static pressure (214). Functional maladaptation of aged cerebral arteries to hypertension is partly due to the dysregulation of the transient receptor potential canonical channel 6 (TRPC6), which is a non-selective cation channel from the transient receptor potential (TRP) ion channel superfamily. In young animals, increased blood pressure activates TRPC6, which is sensitive to wall stretch (due to increased intraluminal pressure), thus leading to the depolarization of the VSMC plasma membrane, opening of voltage-gated Ca2+ channels, increasing intracellular Ca2+ concentration, and consequent constriction of VSMCs (216). Impaired autoregulatory protection in the brain of hypertensive aged mice aggravates cerebromicrovascular injury and neuroinflammation (217) by allowing high pressure to penetrate the distal portion of the cerebral microcirculation.
Reductions in circulating IGF-1 may contribute to this age-related loss of adaptive ability (115). Circulating IGF-1-deficient mice exhibit significant impairment of cerebrovascular autoregulation compared to control mice (115) (Figure 2), mimicking the phenotype seen in aging (217). Autoregulatory impairment was most pronounced in hypertensive IGF-1 deficient mice, who also failed to exhibit the protective increase in myogenic tone and protective increases in TRPC6 channel expression that accompany hypertension in control mice (115). This functional maladaptation of cerebral arteries to hypertension in IGF-1 deficient animals correlates with the structural maladaptations described in section 3, in particular the significant reduction in hypertension-induced adaptive hypertrophy and decreased elasticity in the medial layer of vessels in IGF-1 deficient animals (114). Studies on isolated aorta preparations also suggest that IGF-1 regulates contractile function of vascular smooth muscle cells (210).
Collectively, impaired autoregulatory function and impaired protective vessel remodeling can lead to damaging increases in pressure in the cerebral microvasculature. High intraluminal pressure is a key stimulus for increased vascular production of reactive oxygen species (ROS) (218). Previous studies showed that in aging, increased oxidative stress led to matrix metalloprotease (MMP) activation (219) thereby compromising the structural integrity of the cerebral microvasculature. Similarly, hypertensive IGF-1‐deficient mice exhibit increased vascular ROS and increased vascular MMP activity compared to control mice (93).
IGF-1 may also play a role in regulating baseline CBF. Magnetic resonance imaging studies have shown that baseline mean blood flow velocity in the middle cerebral artery is decreased in aged vs. young cohorts, and that flow is significantly correlated with serum IGF-1 levels (94). Overall, IGF-1 has a central role in regulation of CBF by contributing to pressure- and flow-dependent responses of cerebral arteries, plays a role in structural adaptation to hypertension, and contributes to adaptive ECM changes and in ECM-related gene expression. IGF-1 deficiency dysregulates the myogenic response to high blood pressure, it impairs the hypertension-induced adaptive media hypertrophy and leads to dysregulation of ECM remodeling contributing to increased fragility of intracerebral arterioles and exacerbating cerebromicrovascular injury and neuroinflammation mimicking the aging phenotype.
4.2 Role of GH/IGF-1 in regulation of microvascular endothelial function and neurovascular coupling responses
Neurovascular coupling (NVC) is the ability of the neurovascular unit (NVU) to increase local blood flow based on neuronal activity/energy requirements and is critical for maintaining proper brain function. The NVU is comprised of neurons, glial cells, and the vascular subunit (brain endothelial cells, pericytes and the surrounding basement membrane) (220). NVC is the result of a tightly controlled interaction between activated neurons and astrocytes which leads to the release of vasodilator metabolites from the astrocyte end-feet and microvascular endothelial cells. These metabolites include nitric oxide (NO), potassium, adenosine, epoxyeicosatrienoic acids and prostaglandins and collectively elicit vasodilation in arterioles (221, 222).
The cellular mechanisms by which aging impairs neurovascular coupling responses primarily involve a significant reduction in endothelial production/release of NO (223–225). Neurovascular dysfunction compromises adjustment of cerebral blood flow to meet the needs of active brain regions, impairing energy and oxygen delivery to the firing neurons and hindering washout of toxic metabolic by-products (226). Cells of the neurovascular unit (including neurons, astrocytes, and endothelial cells) abundantly express the IGF-1 receptor, as IGF-1 signaling can also play a role in endothelial mediated vasodilation (227, 228). IGF-1 mediated activation of the phosphatidylinositol-3 kinase (PI3-K) pathway in endothelial cells can lead to production of NO by nitric oxide synthase, leading to paracrine signaling on VSMCs, resulting in VSMC relaxation, and subsequent vessel dilation (229, 230).
Laser doppler flowmetry has been routinely used in preclinical models to evaluate NVC. CBF is measured in the somatosensory cortex before and after whisker stimulation, and the increased CBF following stimulation is reflective of NVC response. Stimulation-induced increases in CBF were much lower in circulating IGF-1 deficient mice compared to controls (113, 116). This impairment of NVC in IGF-1 deficiency supports a protective role for IGF-1 in vascular function. Subsequent mechanistic work demonstrated that both endothelium-mediated and astrocyte-dependent NVC responses were reduced in IGF-1-deficient mice, mimicking the aged human condition (231, 232). To help further elucidate the cellular contributions to IGF-1-mediated regulation of NVC, various studies have either over-expressed or knocked out the IGF-1 receptor in specific cell types. Mice overexpressing human IGF-1 receptor in the endothelium were shown to exhibit unaltered vasorelaxation to endothelium-dependent vasodilators (233). However, disruption of endogenous mouse IGF-1 receptor signaling specifically in endothelial cells (VE-Cadherin-CreERT2/Igf1rf/f) or astrocytes (GFAP-CreERT2/Igf1rf/f) significantly impaired NVC responses (10, 234). These effects in part are mediated by decreased NO bioavailability due to increased production of ROS, analogous to the effects of circulating IGF-1 deficiency and aging.
IGF-1 also plays a significant role in blood flow changes in response to other types of stimuli such as physical activity. Exercise-mediated neuronal activity elicits changes in cerebral blood flow through both NVC and other regulatory mechanisms. These changes are part of the anti-aging effects of exercise on cerebrovascular and neuronal plasticity. However, these positive effects of exercise were abolished in the circulating IGF-1 knockdown model (235). IGF-1 is also essential for downstream results of NVC in the brain. For example, NVC is an essential component of activity-dependent neurogenesis in the hippocampus (236). However, when IGF-1 signaling in the brain is blocked, activity-dependent but not baseline neurogenesis is eliminated (236).
These preclinical studies have clinical correlates. NVC decreases in aging humans, contributing to VCID (94). In addition, recent work in aged and young study participants show that decreased serum IGF-1 levels are a significant predictor of impaired NVC responses (94). Combined, these findings highlight an essential role for IGF-1 in NVC during aging (105).
4.3 GH/IGF-1 in regulation of blood-brain barrier integrity and the development of cerebral microhemorrhages
The BBB is a functional part of the NVU and is critical for the protection of neurons, maintenance of homeostasis, and the integrity of the brain itself (19, 237, 238). BBB dysfunction is one of the hallmarks of the aging brain, in both humans and animal models (19, 33, 34, 239–241). The BBB comprises brain capillary endothelial cells with support from pericytes embedded in the basement membrane and astrocytes. Tight junctions between endothelial cells are a main component of this barrier, leading to the requirement of facilitated transport for nutrient and waste exchange through the capillary endothelial cells. This highly regulated process allows the brain to be an immune-privileged organ giving the BBB an important role as a regulator of neuroinflammation and lymphocyte migration (237, 242–244). Early evidence to support the idea of a multicellular barrier came from work showing that cultured astrocytes induced tighter junctions between endothelial cells (245). When degradation of the BBB begins (for example in inflammaging), small molecules such as cytokines can leak into the surrounding brain tissue leading to subsequent inflammation (19, 243, 244, 246).
GH has a protective role in establishment and maintenance of the BBB during development and in the neonatal brain, especially in models of hypoxia-induced injury (247–249). Recombinant human GH (rhGH) has been shown to have a protective effect in a mouse model of neonatal hypoxic brain injury. Specifically, while hypoxia significantly reduced occludin-positive cortical endothelial cells (a measure of BBB junctional integrity), their frequency was increased in the cortex in response to rhGH (248, 249).
IGF-1 also plays an important role in the maintenance of the BBB. Mice with circulating IGF-1 deficiency have increased BBB permeability (115). This disruption of the BBB can lead to hemorrhaging, neuroinflammation, and neuronal loss (250). IGF-1 also has a well-known protective role in neuroinflammatory processes (251) and has a protective effect on the BBB in other relevant models such as stroke (252–254). IGF-1 supplementation induced Akt activation, reduced blood-brain barrier permeability at 4h poststroke, and suppressed cytokine expression including TNF-α, IL-6, and IL-10 (252). Based on these data, cellular components of the blood-brain barrier may serve as targets of IGF-1 in the aging brain, and IGF-1 supplementation in aged animals and patients may be a useful post-stroke treatment. Recombinant human IGF-1 (rhIGF-1) was able to increase the expression of tight junction proteins (e.g. claudin 5 and occludin) and partially restore BBB integrity in mice with intracerebral hemorrhages (ICHs) (255). Animals treated with rhIGF-1 also showed improved performance on cognitive tests and decreased brain water content compared to ICH mice without rhIGF-1.
In contrast, there is some evidence that a more cautious approach is needed in the application of IGF-1 in the developing brain. At a low dose, IGF-1 delivered intraventricularly significantly reduced lipopolysaccharide (LPS)-induced negative effects such as loss of pre-oligodendrocytes and myelin and in a model of periventricular leukomalacia (a form of brain damage in premature infants) without altering IL-1β expression and microglia/astrocytes activation in the developing brain (256). On the other hand, this low dose of IGF-1 increased LPS-induced BBB permeability, increased polymorphonuclear cell recruitment, and caused ICHs. At higher doses, IGF-1 treatment with LPS highly enhanced mortality of the animals (256).
Penetration of increased pulsatile pressure and pressure surges (e.g. Valsalva maneuver) into the distal, vulnerable part of the cerebral microcirculation can result in rupture of small vessels and genesis of cerebral microhemorrhages (CMHs). CMH are increasingly recognized in T2* and SWI MRI sequences in the majority of older adults. Both preclinical and clinical studies show that advanced age significantly increases the prevalence of CMHs, which contribute to the development of VCID (42–44, 63, 64, 219, 257, 258). CMHs are thought to arise due to a combination of age-associated factors that lead to increased microvascular fragility including: 1) structural defects such as impaired hypertension-induced adaptive changes in the ECM and impaired protective hypertrophy in the medial layer, 2) functional defects such as impaired myogenic autoregulation, and 3) age-related cellular and molecular changes such as increased oxidative stress and MMP activation (93, 114, 115). These age-associated factors are significantly affected by IGF-1 deficiency, and IGF-1 deficiency in mouse models of accelerated aging significantly exacerbates the development of CMHs (93, 113).
5 Role of GH/IGF-1 in regulation of amyloid pathologies
Amyloid pathologies (amyloidoses) are a heterogeneous group of diseases characterized by the accumulation of plaques and fibrils made of misfolded proteins. These are formed as a consequence of excessive protein aggregation and/or impairments in the quality control systems responsible for their clearance. Amyloidoses are classified as either systemic or localized. AD is the most commonly diagnosed localized amyloidosis of the central nervous system. A major cause of AD is the aggregation of toxic amyloid-β (Aβ) peptides (259), which are formed when an amyloid precursor protein (APP) is abnormally cleaved. Under physiological conditions, APP is sequentially cleaved by α- and γ-secretases, producing nonamyloidogenic peptides which are essential for neuronal homeostasis. In pathophysiological conditions associated with AD, APP is cleaved by β-secretase and subsequently γ-secretase, generating amyloidogenic Aβ monomers: Aβ1-40 and Aβ1-42 (260, 261). Decades of studies in AD provide growing evidence that the accumulation of β-amyloid aggregates plays a central role in the pathogenesis of AD, however, mechanisms underlying genesis and regulation of this molecular hallmark of AD remain incompletely understood.
Results from research groups studying GH/IGF-1 signaling in AD are inconsistent, making drawing meaningful conclusions difficult (262). Meta-analysis focused on IGF-1 levels in AD patients revealed that individuals with dementia or AD had lower levels of circulating IGF-1 than healthy individuals (263). Lower levels of circulating IGF-1 were also positively correlated with a faster decline in the Mini Mental State Examination (MMSE) score in AD patients (110). Conversely, a study by Johansson et al. found that serum levels of IGF-1 and IGFBP3 were elevated in AD patients (264). The explanation for these conflicting findings is not clear, but there are many potential contributing factors. The bioactivity and bioavailability of IGF-1 are regulated by IGF-1 binding proteins (IGFBPs) (265), and the increased IGF-1 levels observed in some AD patients might be functionally suppressed by correspondingly elevated IGFBP3 levels. Support for this theory comes from a study which found a decreased ratio of IGF-1 to IGFBP3 (active/inactive) in the hippocampus of AD patients (266). Additionally, circulating IGF-1 levels change throughout the progression of AD. Several studies have reported that the early phase of AD might be associated with insulin receptor (IRs)/IGF-1R resistance, manifested as increased serum IGF-1 levels and reduced expression of IRs, IGF-1Rs, and their downstream substrates IRS-1 and IRS-2 in the brain, followed by decreased levels of serum IGF-1 at later stages of this amyloidosis (267–271).
The role of the GH/IGF-1 axis has been extensively studied in preclinical models of AD with similarly conflicting results. Many studies have suggested that IGF-1 exacerbates amyloid pathologies. Cells expressing the Swedish APP mutation treated with IGF-1 have been shown to secrete more amyloid-β peptides than untreated cells (272). In vitro and in vivo experiments demonstrated that inhibition of the IR/IGF-1 axis by NT219 (a small molecule inhibitor of scaffold proteins such as IRS1/2 that transduce IGF-1 mediated signaling) protected both cultured cells and nematodes from prion protein- or amyloid-beta-induced proteotoxicity through the formation of less toxic aggregates of higher molecular weight (273, 274). In a transgenic mouse model of AD (AβPP/PS1), treatment with picropodophyllin, a selective IGF-1R inhibitor, reduced levels of insoluble Aβ1-40 and Aβ1-42 in the temporal cortex but not in the hippocampus (275). The neuron-specific deletion of IR or IGF-1R in the mouse model of AD provided a myriad of beneficial effects, manifested by decreased APP processing, fewer amyloid plaques, less amyloid-β, improved spatial memory, and protection from premature death (276–278). Additionally, in APP/PS1 mice, GH deficiency is associated with fewer amyloid-beta plaques and lower levels of Aβ1-40 and Aβ1-42 peptides (279, 280).
However, other studies have suggested that IGF-1 may have beneficial effects in AD models. In vitro experiments have suggested that activating the IR/IGF-1R axis could confer protection from amyloid-β toxicity. In neuronal cultures, IGF-1 treatment decreased Aβ production and protected neurons from Aβ25-35- and Aβ1-42-induced toxicity (266, 281–284). Similarly, hippocampal overexpression of IGF-1 prevented Aβ1-42-induced memory loss (266). Astrocytic IGF-1 receptors prove to be crucial for the uptake of β-amyloid from neurons and the preservation of cognitive function (129). In APP/PS1 mice, treatment with IGF-1 restored levels of ADAM10; the constitutive α-secretase involved in APP processing and decreased the prevalence of Aβ1-40 in the cortex and hippocampus (285). However, work from other groups has suggested that enhancing GH/IGF-1 signaling either by administration of IGF-1 or by the GH secretagogue, CP-424391 failed to alter amyloid-beta clearance (286). Altogether, these observations highlight the complexity of the GH/IGF-1 axis in AD. Further studies are needed to develop a better understanding of the role of these hormones in the genesis and progression of various amyloidoses.
While development of effective anti-amyloid therapies is ongoing, physical exercise appears to improve several physiological outcomes in AD patients, including improved cognitive function, functional independence, reduced neuroinflammation and oxidative stress, and decreased cardiovascular risks (287). Evidence also shows that caloric restriction (CR) may improve cognitive performance as another beneficial lifestyle intervention (288). In both these interventions, IGF-1 levels were elevated, suggesting that the beneficial effects seen in these healthy lifestyle changes might be at least partially mediated by the GH/IGF-1 axis.
6 Role of GH/IGF-1 in regulation of atherogenesis
Cerebral atherogenic changes have been associated with VCID since it was first described (289), and this intracranial atherosclerosis is associated with lipid dysregulation, accumulation of cholesterol and related esters, and inflammation. Several clinical studies have found that decreased peripheral vascular health and atherosclerosis increase the risk for VCID and cognitive impairment (7, 8, 289–296). The increased risk is most robust in cases of intracranial atherosclerosis or carotid artery disease with coronary and aortic atherosclerosis having weaker or no association with VCID risk (292, 294, 297). Animal studies also support a link between atherosclerosis and VCID. When compared with control mice, LDLr-/-:hApoB+/+ mice (a model of atherosclerosis) exhibited worsened VCID pathologies including CMH, microvascular rarefaction, BBB leakage, neuroinflammation, and cognitive impairment (298). Carotid plaque thickness and low IGF-1 levels are both independent predictors of VCID (299). Studies using aged rats demonstrated that IGF-1 supplementation reversed age-related insulin resistance, reduced serum cholesterol and triglycerides as well as reduced oxidative stress in the cortex and hippocampus (300). However, very few studies have specifically evaluated the role of IGF-1 in atherogenesis in the brain.
In contrast, there is a vast literature evaluating the role of IGF-1 and the broader somatotropic axis in systemic atherogenesis [reviewed in (231, 301–304)]. The current body of evidence suggests that IGF-1 is protective in atherosclerosis, due largely to its role in VSMCs, endothelial cells and macrophages (302, 305, 306). IGF-1 levels inversely correlate with atherosclerotic burden in a variety of animal models (307–310), as one example, systemic infusion of IGF-1 in ApoE null mice on a high-fat diet reduced vascular inflammation, reduced oxidative stress, and suppressed plaque progression (307). In part, the beneficial effects of IGF-1 in atherosclerosis have been attributed to the ability of IGF-1 to stabilize plaques via VSMC-mediated effects (311–313). In ApoE knockout models, supplementation of a stable IGF-1 analog stabilized plaque development by increasing vascular smooth muscle (VSMC) cell proliferation, suppressing inflammation-induced VSMC apoptosis, and increasing the cap to core ratio in early atherosclerosis. ECM regulation plays a key role in these IGF-1 mediated benefits and many of the relevant ECM proteins overlap with those important to protective IGF-1-associated cerebrovascular remodeling (discussed above) such as Col3a1 and elastin (311). Low IGF-1 levels are also associated with increased inflammation and oxidative stress in atherosclerosis models while elevated IGF-1 is associated with improvements in those measures as well as decreased apoptosis, increased presence of VSMCs, and increased collagen (121, 307, 311, 314, 315). Studies on GH have been a little more contradictory. Overall GH is also thought to be atheroprotective based on the observation that GH deficiency is associated with premature atherosclerosis and increased prevalence of other cardiovascular risk factors (316–318). However, treatment with GH reduces only some of these risk factors, and much remains to be understood (318, 319). In addition, acromegaly patients with chronic overexpression of GH have been reported to have increased proinflammatory blood-derived cytokines, endothelial dysfunction, and increased cardiovascular mortality when compared to patients who had normal levels of GH/IGF-1 (318, 320, 321).
Recent data suggest that GH/IGF-1 may also play a role in regulating cellular senescence in the context of atherosclerosis. Senescent cells accumulate in aging, contributing to cerebrovascular pathologies and atherosclerosis (322–331). Senescent cells can cause tissue dysfunction, and IGF-1 has been shown, in vitro, to suppress the formation of oxidative-stress-induced senescent endothelial cells (314). Multiple types of senescent cells have deleterious effects throughout the timeline of atherosclerosis including endothelial cells, VSMCs, and macrophages/foam cells. These senescent cells contribute to disease progress and plaque rupture by promoting degradation of elastic tissue, thinning of the fibrous cap, increased plaque burden, adoption of a proinflammatory macrophage phenotype, and suppression of a protective migratory phenotype by VSMCs (329–331). Consistent with the previously discussed role of IGF-1 in promoting cap thickening and its importance as a regulator of elastic ECM components, it is not surprising that impaired IGF-1 signaling in VSMCs is important in the context of senescence-induced plaque progression. In addition to improving cap thickness, reducing lesion size, and promoting VSMC migration, treatment of high fat diet fed Ldlr-/- mice with senolytics depletes IGFBP3 in the atherosclerotic lesions (329). IGFBP3 sequesters IGF-1 preventing it from acting on the IGF-1 receptor, suggesting that one of the beneficial effects of senolytics is to increase the levels of available IGF-1 in the plaque. The importance of IGF-1 in plaques was highlighted by subsequent experiments showing that supplementation with an IGF-1 variant with reduced IGFBP3 binding or treatment with IGFBP3 neutralizing antibodies promoted adoption of the migratory VSMC phenotype which is thought to play a protective role in cap maintenance and repair (329). Treatment with GH, and the subsequent increases in IGF-1 have also been shown to decrease the number of senescent endothelial progenitor cells, a key part of the vascular repair process in atherosclerosis (332).
7 Sex-based differences in the GH/IGF-1 axis in the aging cerebrovasculature
Sexual dimorphism and sex-based differences in phenotypes associated with the GH/IGF-1 signaling axis are complex (333, 334). There is evidence for complicated interactions between the somatotropic and gonadotropic axes during development and during pubertal growth (334, 335). In the context of aging, studies in Ames dwarf mice (336) showed that both females and males had increased longevity compared to controls. GH receptor knockout mice also exhibited increased longevity in both females and males, although there was variation across genetic backgrounds (337). In contrast, increased longevity in Igf1r+/- mice was seen only in females (143, 338). The mechanisms underlying these sex differences are not known.
There has been some work evaluating sex-differences associated with the GH/IGF-1 axis in the cardiovascular system and brain. Several sex-specific differences in cardiac function are eliminated in liver specific IGF-1 knockouts. This has been attributed to the fact that female C57BL/6 mice have higher circulating levels of IGF-1 than male mice but the liver-specific knockout reduces IGF-1 to low levels that are similar in both males and females (339). Estrogen and IGF-1 can both exert neuroprotective effects, and there have been reports suggesting that the two pathways can act cooperatively in the context of ischemic stroke (252, 253, 335, 340). In addition, activation of estrogen (E2) receptors has been shown to increase IGF-1 uptake into brain endothelial cells, resulting in some sex-specific differences in IGF-1 bioavailability (341).
More recent work has demonstrated that post-developmental neuronal over-expression of IGF-1 rescued age-related defects in neuromuscular function in males but not females (105). Chronic overexpression of IGF-1 did not ameliorate age-related losses in cognitive function, although short-term delivery (4-weeks intranasal) of IGF-1 in aged male mice did lead to minor improvements in cognitive function (female mice were not evaluated) (105). However in most studies evaluating the effects of IGF-1 deficiency on the vasculature, including those studying neurovascular coupling, blood-brain-barrier permeability, development of CMH, vascular structure and function, progression of atherosclerosis, etc. sex-specific effects were either not evaluated or not reported, making this an area ripe for further exploration.
8 Conclusion and perspectives
There is a strong body of evidence highlighting the vasoprotective effects of IGF-1, particularly in the cerebral vasculature. IGF-1 is essential for brain vascular health, and IGF-1 deficiency in aging contributes to the development of VCID and cognitive impairment. It can be difficult to separate out the effects of GH from those of IGF-1, since one of the primary functions of GH is to induce secretion of IGF-1. However, there are also direct effects of GH in some systems which may be tied to effects of GH signaling on NO production (342). While much is known about GH/IGF-1 axis in aging and cardiovascular disease, much remains to be explored. In particular, the cellular contributions to various pathologies remain incompletely understood. It can also be hard to dissect out what the contributions of GH/IGF-1 signaling on individual cell types in complicated multicellular pathologies such as atherogenesis and cerebrovascular dysfunction. Part of the challenge lies in the fact that so many of the cells involved (endothelial cells, astrocytes, neurons, VSMCs, macrophages) can all respond to IGF-1 and contribute to disease development. In addition, while GH and IGF-1 are most often thought of as circulating systemic factors that act in a cell non-autonomous way, there is locally produced IGF-1 in many tissues, including the brain and atherosclerotic plaque. Dissecting the contributions of these different IGF-1 pools can be time-consuming and challenging with current animal models. Much remains to be explored regarding the downstream molecular mediators of GH/IGF-1 vasoprotective effects. Canonical signaling through GH/IGF-1 has been well-established for decades but it remains unclear whether there are tissue specific downstream mediators that might make good therapeutic targets without the broader effects of delivering hormones with as many pleiotropic effects as GH/IGF-1.
Author contributions
All authors listed have made a substantial, direct, and intellectual contribution to the work and approved it for publication.
Funding
This work was supported by grants from the National Institute on Aging (R01-AG070915), the NIA-supported Geroscience Training Program in Oklahoma (T32AG052363), the Cellular and Molecular GeroScience CoBRE (1P20GM125528), and the American Heart Association (AHA834339-ANT). The funding sources had no role in the study design; in the collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the article for publication.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Zlokovic BV, Gottesman RF, Bernstein KE, Seshadri S, Mckee A, Snyder H, et al. Vascular contributions to cognitive impairment and dementia (Vcid): A report from the 2018 national heart, lung, and blood institute and national institute of neurological disorders and stroke workshop. Alzheimers Dement (2020) 16(12):1714–33. doi: 10.1002/Alz.12157
2. Gorelick PB, Bowler JV. Advances in vascular cognitive impairment. Stroke (2010) 41(2):E93–8. doi: 10.1161/Strokeaha.109.569921
3. Iadecola C, Duering M, Hachinski V, Joutel A, Pendlebury ST, Schneider JA, et al. Vascular cognitive impairment and dementia: Jacc scientific expert panel. J Am Coll Cardiol (2019) 73(25):3326–44. doi: 10.1016/J.Jacc.2019.04.034
4. Dichgans M, Leys D. Vascular cognitive impairment. Circ Res (2017) 120(3):573–91. doi: 10.1161/Circresaha.116.308426
5. Iturria-Medina Y, Sotero RC, Toussaint PJ, Mateos-Perez JM, Evans AC. Alzheimer’s disease neuroimaging i. early role of vascular dysregulation on late-onset alzheimer’s disease based on multifactorial data-driven analysis. Nat Commun (2016) 7:11934. doi: 10.1038/Ncomms11934
6. Levit A, Hachinski V, Whitehead SN. Neurovascular unit dysregulation, white matter disease, and executive dysfunction: The shared triad of vascular cognitive impairment and Alzheimer disease. Geroscience (2020) 42(2):445–65. doi: 10.1007/S11357-020-00164-6
7. Tarantini S, Fulop GA, Kiss T, Farkas E, Zolei-Szenasi D, Galvan V, et al. Demonstration of impaired neurovascular coupling responses in Tg2576 mouse model of alzheimer’s disease using functional laser speckle contrast imaging. Geroscience (2017) 39(4):465–73. doi: 10.1007/S11357-017-9980-Z
8. Csiszar A, Tarantini S, Fulop GA, Kiss T, Valcarcel-Ares MN, Galvan V, et al. Hypertension impairs neurovascular coupling and promotes microvascular injury: Role in exacerbation of alzheimer’s disease. Geroscience (2017) 39(4):359–72. doi: 10.1007/S11357-017-9991-9
9. Lamar M, Leurgans S, Kapasi A, Barnes LL, Boyle PA, Bennett DA, et al. Complex profiles of cerebrovascular disease pathologies in the aging brain and their relationship with cognitive decline. Stroke (2022) 53(1):218–27. doi: 10.1161/Strokeaha.121.034814
10. Tarantini S, Nyul-Toth A, Yabluchanskiy A, Csipo T, Mukli P, Balasubramanian P, et al. Endothelial deficiency of insulin-like growth factor-1 receptor (Igf1r) impairs neurovascular coupling responses in mice, mimicking aspects of the brain aging phenotype. Geroscience (2021) 43:2387–94. doi: 10.1007/S11357-021-00405-2
11. Daulatzai MA. Cerebral hypoperfusion and glucose hypometabolism: Key pathophysiological modulators promote neurodegeneration, cognitive impairment, and alzheimer’s disease. J Neurosci Res (2016) 4:943–72. doi: 10.1002/Jnr.23777
12. Ighodaro ET, Abner EL, Fardo DW, Lin AL, Katsumata Y, Schmitt FA, et al. Risk factors and global cognitive status related to brain arteriolosclerosis in elderly individuals. J Cereb Blood Flow Metab (2016) 37:201–16. doi: 10.1177/0271678x15621574
13. Cooper LL, Woodard T, Sigurdsson S, Van Buchem MA, Torjesen AA, Inker LA, et al. Cerebrovascular damage mediates relations between aortic stiffness and memory. Hypertension (2016) 67(1):176–82. doi: 10.1161/Hypertensionaha.115.06398
14. Jessen SB, Mathiesen C, Lind BL, Lauritzen M. Interneuron deficit associates attenuated network synchronization to mismatch of energy supply and demand in aging mouse brains. Cereb Cortex (2015) 27:646–59. doi: 10.1093/Cercor/Bhv261
15. Zlokovic BV. Neurovascular pathways to neurodegeneration in alzheimer’s disease and other disorders. Nat Rev Neurosci (2011) 12(12):723–38. doi: 10.1038/Nrn3114
16. Hajdu MA, Heistad DD, Siems JE, Baumbach GL. Effects of aging on mechanics and composition of cerebral arterioles in rats. Circ Res (1990) 66(6):1747–54. doi: 10.1161/01.RES.66.6.1747
17. Mayhan WG, Faraci FM, Baumbach GL, Heistad DD. Effects of aging on responses of cerebral arterioles. Am J Physiol (1990) 258(4 Pt 2):H1138–43. doi: 10.1152/ajpheart.1990.258.4.H1138
18. Norling AM, Gerstenecker AT, Buford TW, Khan B, Oparil S, Lazar RM. The role of exercise in the reversal of igf-1 deficiencies in microvascular rarefaction and hypertension. Geroscience (2020) 42(1):141–58. doi: 10.1007/S11357-019-00139-2
19. Nyul-Toth A, Tarantini S, Delfavero J, Yan F, Balasubramanian P, Yabluchanskiy A, et al. Demonstration of age-related blood-brain barrier disruption and cerebromicrovascular rarefaction in mice by longitudinal intravital two-photon microscopy and optical coherence tomography. Am J Physiol Heart Circ Physiol (2021) 320(4):H1370–H92. doi: 10.1152/ajpheart.00709.2020
20. Toth P, Tarantini S, Csiszar A, Ungvari Z. Functional vascular contributions to cognitive impairment and dementia: Mechanisms and consequences of cerebral autoregulatory dysfunction, endothelial impairment, and neurovascular uncoupling in aging. Am J Physiol Heart Circ Physiol (2017) 312(1):H1–H20. doi: 10.1152/Ajpheart.00581.2016
21. Tarantini S, Tucsek Z, Valcarcel-Ares MN, Toth P, Gautam T, Giles CB, et al. Circulating igf-1 deficiency exacerbates hypertension-induced microvascular rarefaction in the mouse hippocampus and retrosplenial cortex: Implications for cerebromicrovascular and brain aging. Age (Dordr) (2016) 38(4):273–89. doi: 10.1007/S11357-016-9931-0
22. Thomas KR, Osuna JR, Weigand AJ, Edmonds EC, Clark AL, Holmqvist S, et al. Regional hyperperfusion in older adults with objectively-defined subtle cognitive decline. J Cereb Blood Flow Metab (2021) 41(5):1001–12. doi: 10.1177/0271678x20935171
23. Tomoto T, Riley J, Turner M, Zhang R, Tarumi T. Cerebral vasomotor reactivity during hypo- and hypercapnia across the adult lifespan. J Cereb Blood Flow Metab (2020) 40(3):600–10. doi: 10.1177/0271678x19828327
24. Trigiani LJ, Bourourou M, Lacalle-Aurioles M, Lecrux C, Hynes A, Spring S, et al. A functional cerebral endothelium is necessary to protect against cognitive decline. J Cereb Blood Flow Metab (2022) 42(1):74–89. doi: 10.1177/0271678x211045438
25. Vestergaard MB, Jensen ML, Arngrim N, Lindberg U, Larsson HB. Higher physiological vulnerability to hypoxic exposure with advancing age in the human brain. J Cereb Blood Flow Metab (2020) 40(2):341–53. doi: 10.1177/0271678x18818291
26. Liu D, Ahmet I, Griess B, Tweedie D, Greig NH, Mattson MP. Age-related impairment of cerebral blood flow response to katp channel opener in alzheimer’s disease mice with presenilin-1 mutation. J Cereb Blood Flow Metab (2021) 41(7):1579–91. doi: 10.1177/0271678x20964233
27. Liu W, Chen Z, Ortega D, Liu X, Huang X, Wang L, et al. Arterial elasticity, endothelial function and intracranial vascular health: A multimodal mri study. J Cereb Blood Flow Metab (2021) 41(6):1390–7. doi: 10.1177/0271678x20956950
28. Maasakkers CM, Thijssen DH, Knight SP, Newman L, O’connor JD, Scarlett S, et al. Hemodynamic and structural brain measures in high and low sedentary older adults. J Cereb Blood Flow Metab (2021) 41(10):2607–16. doi: 10.1177/0271678x211009382
29. Milej D, He L, Abdalmalak A, Baker WB, Anazodo UC, Diop M, et al. Quantification of cerebral blood flow in adults by contrast-enhanced near-infrared spectroscopy: Validation against mri. J Cereb Blood Flow Metab (2020) 40(8):1672–84. doi: 10.1177/0271678x19872564
30. Pradillo JM, Hernandez-Jimenez M, Fernandez-Valle ME, Medina V, Ortuno JE, Allan SM, et al. Influence of metabolic syndrome on post-stroke outcome, angiogenesis and vascular function in old rats determined by dynamic contrast enhanced mri. J Cereb Blood Flow Metab (2021) 41(7):1692–706. doi: 10.1177/0271678x20976412
31. Sweeney MD, Sagare AP, Zlokovic BV. Blood-brain barrier breakdown in Alzheimer disease and other neurodegenerative disorders. Nat Rev Neurol (2018) 14(3):133–50. doi: 10.1038/Nrneurol.2017.188
32. Sweeney MD, Zhao Z, Montagne A, Nelson AR, Zlokovic BV. Blood-brain barrier: From physiology to disease and back. Physiol Rev (2019) 99(1):21–78. doi: 10.1152/Physrev.00050.2017
33. Verheggen ICM, De Jong JJA, Van Boxtel MPJ, Gronenschild E, Palm WM, Postma AA, et al. Increase in blood-brain barrier leakage in healthy, older adults. Geroscience (2020) 42(4):1183–93. doi: 10.1007/S11357-020-00211-2
34. Montagne A, Barnes SR, Nation DA, Kisler K, Toga AW, Zlokovic BV. Imaging subtle leaks in the blood-brain barrier in the aging human brain: Potential pitfalls, challenges, and possible solutions. Geroscience (2022) 44(3):1339–51. doi: 10.1007/S11357-022-00571-X
35. Li W, Lo EH. Leaky memories: Impact of Apoe4 on blood-brain barrier and dementia. J Cereb Blood Flow Metab (2020) 40(9):1912–4. doi: 10.1177/0271678x20938146
36. Moon WJ, Lim C, Ha IH, Kim Y, Moon Y, Kim HJ, et al. Hippocampal blood-brain barrier permeability is related to the Apoe4 mutation status of elderly individuals without dementia. J Cereb Blood Flow Metab (2021) 41(6):1351–61. doi: 10.1177/0271678x20952012
37. Cortes-Canteli M, Iadecola C. Alzheimer’s disease and vascular aging: Jacc focus seminar. J Am Coll Cardiol (2020) 75(8):942–51. doi: 10.1016/J.Jacc.2019.10.062
38. Iadecola C, Park L, Capone C. Threats to the mind: Aging, amyloid, and hypertension. Stroke (2009) 40(3 Suppl):S40–4. doi: 10.1161/Strokeaha.108.533638
39. Park L, Anrather J, Girouard H, Zhou P, Iadecola C. Nox2-derived reactive oxygen species mediate neurovascular dysregulation in the aging mouse brain. J Cereb Blood Flow Metab (2007) 27(12):1908–18. doi: 10.1038/Sj.Jcbfm.9600491
40. Lipecz A, Csipo T, Tarantini S, Ra H, B-Tn N, Conley S, et al. Age-related impairment of neurovascular coupling responses: A dynamic vessel analysis (Dva)-based approach to measure decreased flicker light stimulus-induced retinal arteriolar dilation in healthy older adults. Geroscience (2019) 41(3):341–9. doi: 10.1007/S11357-019-00078-Y
41. Zhang Y, Du W, Yin Y, Li H, Liu Z, Yang Y, et al. Impaired cerebral vascular and metabolic responses to parametric n-back tasks in subjective cognitive decline. J Cereb Blood Flow Metab (2021) 41(10):2743–55. doi: 10.1177/0271678x211012153
42. Poels MM, Ikram MA, van der Lugt A, Hofman A, Krestin GP, Breteler MM, et al. Incidence of cerebral microbleeds in the general population: The Rotterdam scan study. Stroke (2011) 42(3):656–61. doi: 10.1161/Strokeaha.110.607184
43. Poels MM, Ikram MA, van der Lugt A, Hofman A, Niessen WJ, Krestin GP, et al. Cerebral microbleeds are associated with worse cognitive function: The Rotterdam scan study. Neurology (2012) 78(5):326–33. doi: 10.1212/Wnl.0b013e3182452928
44. Ungvari Z, Tarantini S, Kirkpatrick AC, Csiszar A, Prodan CI. Cerebral microhemorrhages: Mechanisms, consequences, and prevention. Am J Physiol Heart Circ Physiol (2017) 312(6):H1128–H43. doi: 10.1152/Ajpheart.00780.2016
45. Wiegertjes K, Chan KS, Telgte AT, Gesierich B, Norris DG, Klijn CJ, et al. Assessing cortical cerebral microinfarcts on iron-sensitive mri in cerebral small vessel disease. J Cereb Blood Flow Metab (2021) 41(12):3391–9. doi: 10.1177/0271678x211039609
46. Ling Y, Chabriat H. Incident cerebral lacunes: A review. J Cereb Blood Flow Metab (2020) 40(5):909–21. doi: 10.1177/0271678x20908361
47. Zhang L, Biessels GJ, Hilal S, Chong JSX, Liu S, Shim HY, et al. Cerebral microinfarcts affect brain structural network topology in cognitively impaired patients. J Cereb Blood Flow Metab (2021) 41(1):105–15. doi: 10.1177/0271678x20902187
48. Zwartbol MH, Rissanen I, Ghaznawi R, De Bresser J, Kuijf HJ, Blom K, et al. Cortical cerebral microinfarcts on 7t mri: Risk factors, neuroimaging correlates and cognitive functioning - the medea-7t study. J Cereb Blood Flow Metab (2021) 41(11):3127–38. doi: 10.1177/0271678x211025447
49. Zwartbol MH, van der Kolk AG, Kuijf HJ, Witkamp TD, Ghaznawi R, Hendrikse J, et al. Intracranial vessel wall lesions on 7t mri and mri features of cerebral small vessel disease: The smart-Mr study. J Cereb Blood Flow Metab (2021) 41(6):1219–28. doi: 10.1177/0271678x20958517
50. Shi Y, Thrippleton MJ, Blair GW, Dickie DA, Marshall I, Hamilton I, et al. Small vessel disease is associated with altered cerebrovascular pulsatility but not resting cerebral blood flow. J Cereb Blood Flow Metab (2020) 40(1):85–99. doi: 10.1177/0271678x18803956
51. Vikner T, Eklund A, Karalija N, Malm J, Riklund K, Lindenberger U, et al. Cerebral arterial pulsatility is linked to hippocampal microvascular function and episodic memory in healthy older adults. J Cereb Blood Flow Metab (2021) 41(7):1778–90. doi: 10.1177/0271678x20980652
52. Vikner T, Nyberg L, Holmgren M, Malm J, Eklund A, Wahlin A. Characterizing pulsatility in distal cerebral arteries using 4d flow mri. J Cereb Blood Flow Metab (2020) 40(12):2429–40. doi: 10.1177/0271678x19886667
53. Wartolowska KA, Webb AJ. White matter damage due to pulsatile versus steady blood pressure differs by vascular territory: A cross-sectional analysis of the uk biobank cohort study. J Cereb Blood Flow Metab (2022) 42(5):802–10. doi: 10.1177/0271678x211058803
54. Pahlavian SH, Wang X, Ma S, Zheng H, Casey M, D’orazio LM, et al. Cerebroarterial pulsatility and resistivity indices are associated with cognitive impairment and white matter hyperintensity in elderly subjects: A phase-contrast mri study. J Cereb Blood Flow Metab (2021) 41(3):670–83. doi: 10.1177/0271678x20927101
55. Palhaugen L, Sudre CH, Tecelao S, Nakling A, Almdahl IS, Kalheim LF, et al. Brain amyloid and vascular risk are related to distinct white matter hyperintensity patterns. J Cereb Blood Flow Metab (2021) 41(5):1162–74. doi: 10.1177/0271678x20957604
56. Zeng W, Chen Y, Zhu Z, Gao S, Xia J, Chen X, et al. Severity of white matter hyperintensities: Lesion patterns, cognition, and microstructural changes. J Cereb Blood Flow Metab (2020) 40(12):2454–63. doi: 10.1177/0271678x19893600
57. Zhang R, Huang P, Jiaerken Y, Wang S, Hong H, Luo X, et al. Venous disruption affects white matter integrity through increased interstitial fluid in cerebral small vessel disease. J Cereb Blood Flow Metab (2021) 41(1):157–65. doi: 10.1177/0271678x20904840
58. Parodi-Rullan R, Ghiso J, Cabrera E, Rostagno A, Fossati S. Alzheimer’s amyloid beta heterogeneous species differentially affect brain endothelial cell viability, blood-brain barrier integrity, and angiogenesis. Aging Cell (2020) 19(11):E13258. doi: 10.1111/Acel.13258
59. Castillo-Carranza DL, Nilson AN, Van Skike CE, Jahrling JB, Patel K, Garach P, et al. Cerebral microvascular accumulation of tau oligomers in alzheimer’s disease and related tauopathies. Aging Dis (2017) 8(3):257–66. doi: 10.14336/Ad.2017.0112
60. Costanza A, Xekardaki A, Kovari E, Gold G, Bouras C, Giannakopoulos P. Microvascular burden and Alzheimer-type lesions across the age spectrum. J Alzheimers Dis (2012) 32(3):643–52. doi: 10.3233/Jad-2012-120835
61. Steinman J, Sun HS, Feng ZP. Microvascular alterations in alzheimer’s disease. Front Cell Neurosci (2020) 14:618986. doi: 10.3389/Fncel.2020.618986
62. Thal DR, Attems J, Ewers M. Spreading of amyloid, tau, and microvascular pathology in alzheimer’s disease: Findings from neuropathological and neuroimaging studies. J Alzheimers Dis (2014) 42 Suppl 4:S421–9. doi: 10.3233/Jad-141461
63. Vernooij MW, van der Lugt A, Ikram MA, Wielopolski PA, Niessen WJ, Hofman A, et al. Prevalence and risk factors of cerebral microbleeds: The Rotterdam scan study. . Neurol (2008) 70(14):1208–14. doi: 10.1212/01.Wnl.0000307750.41970.D9
64. Kato H, Izumiyama M, Izumiyama K, Takahashi A, Itoyama Y. Silent cerebral microbleeds on T2*-weighted mri: Correlation with stroke subtype, stroke recurrence, and leukoaraiosis. Stroke (2002) 33(6):1536–40. doi: 10.1161/01.STR.0000018012.65108.86
65. Koton S, Schneider ALC, Windham BG, Mosley TH, Gottesman RF, Coresh J. Microvascular brain disease progression and risk of stroke: The aric study. Stroke (2020) 51(11):3264–70. doi: 10.1161/Strokeaha.120.030063
66. Lin MP, Brott TG, Liebeskind DS, Meschia JF, Sam K, Gottesman RF. Collateral recruitment is impaired by cerebral small vessel disease. Stroke (2020) 51(5):1404–10. doi: 10.1161/Strokeaha.119.027661
67. Sagnier S, Catheline G, Dilharreguy B, Linck PA, Coupe P, Munsch F, et al. Normal-appearing white matter integrity is a predictor of outcome after ischemic stroke. Stroke (2020) 51(2):449–56. doi: 10.1161/Strokeaha.119.026886
68. Tarantini S, Yabluchanskiy A, Csipo T, Fulop G, Kiss T, Balasubramanian P, et al. Treatment with the Poly(Adp-ribose) polymerase inhibitor pj-34 improves cerebromicrovascular endothelial function, neurovascular coupling responses and cognitive performance in aged mice, supporting the nad+ depletion hypothesis of neurovascular aging. Geroscience (2019) 41:533–42. doi: 10.1007/S11357-019-00101-2
69. Kiss T, Nyul-Toth A, Balasubramanian P, Tarantini S, Ahire C, Yabluchanskiy A, et al. Nicotinamide mononucleotide (Nmn) supplementation promotes neurovascular rejuvenation in aged mice: Transcriptional footprint of Sirt1 activation, mitochondrial protection, anti-inflammatory, and anti-apoptotic effects. Geroscience (2020) 42(2):527–46. doi: 10.1007/S11357-020-00165-5
70. Tarantini S, Valcarcel-Ares MN, Toth P, Yabluchanskiy A, Tucsek Z, Kiss T, et al. Nicotinamide mononucleotide (Nmn) supplementation rescues cerebromicrovascular endothelial function and neurovascular coupling responses and improves cognitive function in aged mice. Redox Biol (2019) 24:101192. doi: 10.1016/J.Redox.2019.101192
71. Yabluchanskiy A, Balasubramanian P, Tarantini S. Cerebrovascular rejuvenation: Novel strategies for prevention of vascular cognitive impairment. Rejuvenation Res (2020) 23(6):451–2. doi: 10.1089/Rej.2020.2402
72. Ungvari Z, Tarantini S, Donato AJ, Galvan V, Csiszar A. Mechanisms of vascular aging. Circ Res (2018) 123(7):849–67. doi: 10.1161/Circresaha.118.311378
73. Hennebry A, Oldham J, Shavlakadze T, Grounds MD, Sheard P, Fiorotto ML, et al. Igf1 stimulates greater muscle hypertrophy in the absence of myostatin in Male mice. J Endocrinol (2017) 234(2):187–200. doi: 10.1530/Joe-17-0032
74. Kaur H, Muhlhausler BS, Roberts CT, Gatford KL. The growth hormone-insulin like growth factor axis in pregnancy. J Endocrinol (2021) 251:R23–39. doi: 10.1530/Joe-21-0087
75. Roberts RE, Cavalcante-Silva J, Kineman RD, Koh TJ. Liver is a primary source of insulin-like growth factor-1 in skin wound healing. J Endocrinol (2021) 252(1):59–70. doi: 10.1530/Joe-21-0298
76. Shuang T, Fu M, Yang G, Huang Y, Qian Z, Wu L, et al. Interaction among estrogen, igf-1, and H2s on smooth muscle cell proliferation. J Endocrinol (2021) 248(1):17–30. doi: 10.1530/Joe-20-0190
77. Wood CL, Van ‘T Hof R, Dillon S, Straub V, Wong SC, Ahmed SF, et al. Combined growth hormone and insulin-like growth factor-1 rescues growth retardation in glucocorticoid-treated mdxmice but does not prevent osteopenia. J Endocrinol (2022) 253(2):63–74. doi: 10.1530/Joe-21-0388
78. Yan JJ, Lee YC, Tsou YL, Tseng YC, Hwang PP. Insulin-like growth factor 1 triggers salt secretion machinery in fish under acute salinity stress. J Endocrinol (2020) 246(3):277–88. doi: 10.1530/Joe-20-0053
79. Eichner M, Wallaschofski H, Schminke U, Volzke H, Dorr M, Felix SB, et al. Relation of igf-I with subclinical cardiovascular markers including intima-media thickness, left ventricular mass index and nt-probnp. Eur J Endocrinol (2020) 182(1):79–90. doi: 10.1530/Eje-19-0470
80. Van Den Beld AW, Carlson OD, Doyle ME, Rizopoulos D, Ferrucci L, van der Lely AJ, et al. Igfbp-2 and aging: A 20-year longitudinal study on igfbp-2, igf-I, bmi, insulin sensitivity and mortality in an aging population. Eur J Endocrinol (2019) 180(2):109–16. doi: 10.1530/Eje-18-0422
81. Van Nieuwpoort IC, Vlot MC, Schaap LA, Lips P, Drent ML. The relationship between serum igf-1, handgrip strength, physical performance and falls in elderly men and women. Eur J Endocrinol (2018) 179(2):73–84. doi: 10.1530/Eje-18-0076
82. Ziagaki A, Blaschke D, Haverkamp W, Plockinger U. Long-term growth hormone (Gh) replacement of adult gh deficiency (Ghd) benefits the heart. Eur J Endocrinol (2019) 181(1):79–91. doi: 10.1530/Eje-19-0132
83. Smith TJ. Insulin-like growth factor pathway and the thyroid. Front Endocrinol (Lausanne) (2021) 12:653627. doi: 10.3389/Fendo.2021.653627
84. Kraemer WJ, Ratamess NA, Hymer WC, Nindl BC, Fragala MS. Growth Hormone(S), testosterone, insulin-like growth factors, and cortisol: Roles and integration for cellular development and growth with exercise. Front Endocrinol (Lausanne) (2020) 11:33. doi: 10.3389/Fendo.2020.00033
85. Allard JB, Duan C. Igf-binding proteins: Why do they exist and why are there so many? Front Endocrinol (Lausanne) (2018) 9:117. doi: 10.3389/Fendo.2018.00117
86. Reiter EO, Cohen LE, Rogol AD. Editorial: History of growth hormone: Animal to human. Front Endocrinol (Lausanne) (2021) 12:793272. doi: 10.3389/Fendo.2021.793272
87. Blum WF, Alherbish A, Alsagheir A, El Awwa A, Kaplan W, Koledova E, et al. The growth hormone-Insulin-Like growth factor-I axis in the diagnosis and treatment of growth disorders. Endocr Connect (2018) 7(6):R212–R22. doi: 10.1530/Ec-18-0099
88. Murray PG, Clayton PE, Feingold KR, Anawalt B, Boyce A, Chrousos G, et al. Disorders of growth hormone in childhood. In: Feingold KR, Anawalt B, Boyce A, Chrousos G, De Herder WW, Dhatariya K, et al Endotext, editors. South Dartmouth MA, USA (2000).
89. Miller BS, Rogol AD, Rosenfeld RG. The history of the insulin-like growth factor system. Horm Res Paediatr (2022) 95(6):619–30. doi: 10.1159/000527123
90. Toogood AA, O’neill PA, Shalet SM. Beyond the somatopause: Growth hormone deficiency in adults over the age of 60 years. J Clin Endocrinol Metab (1996) 81(2):460–5. doi: 10.1210/Jcem.81.2.8636250
91. Iranmanesh A, Lizarralde G, Veldhuis JD. Age and relative adiposity are specific negative determinants of the frequency and amplitude of growth hormone (Gh) secretory bursts and the half-life of endogenous gh in healthy men. J Clin Endocrinol Metab (1991) 73(5):1081–8. doi: 10.1210/Jcem-73-5-1081
92. Sonntag WE, Deak F, Ashpole N, Toth P, Csiszar A, Freeman W, et al. Insulin-like growth factor-1 in cns and cerebrovascular aging. Front In Aging Neurosci (2013) 5:27. doi: 10.3389/Fnagi.2013.00027
93. Tarantini S, Valcarcel-Ares NM, Yabluchanskiy A, Springo Z, Fulop GA, Ashpole N, et al. Insulin-like growth factor 1 deficiency exacerbates hypertension-induced cerebral microhemorrhages in mice, mimicking the aging phenotype. Aging Cell (2017) 16(3):469–79. doi: 10.1111/Acel.12583
94. Toth L, Czigler A, Hegedus E, Komaromy H, Amrein K, Czeiter E, et al. Age-related decline in circulating igf-1 associates with impaired neurovascular coupling responses in older adults. Geroscience (2022) 44:2771–83. doi: 10.1007/S11357-022-00623-2
95. Ungvari Z, Csiszar A. The emerging role of igf-1 deficiency in cardiovascular aging: Recent advances. J Gerontol A Biol Sci Med Sci (2012) 67(6):599–610. doi: 10.1093/Gerona/Gls072
96. Sonntag WE, Ramsey M, Carter CS. Growth hormone and insulin-like growth factor-1 (Igf-1) and their influence on cognitive aging. Ageing Res Rev (2005) 4(2):195–212. doi: 10.1016/J.Arr.2005.02.001
97. Deak F, Sonntag WE. Aging, synaptic dysfunction, and insulin-like growth factor (Igf)-1. J Gerontol A Biol Sci Med Sci (2012) 67(6):611–25. doi: 10.1093/Gerona/Gls118
98. Sonntag WE, Csiszar A, Decabo R, Ferrucci L, Ungvari Z. Diverse roles of growth hormone and insulin-like growth factor-1 in mammalian aging: Progress and controversies. J Gerontol A Biol Sci Med Sci (2012) 67(6):587–98. doi: 10.1093/Gerona/Gls115
99. Khan AS, Sane DC, Wannenburg T, Sonntag WE. Growth hormone, insulin-like growth factor-1 and the aging cardiovascular system. Cardiovasc Res (2002) 54(1):25–35. doi: 10.1016/S0008-6363(01)00533-8
100. Ungvari Z, Tarantini S, Sorond F, Merkely B, Csiszar A. Mechanisms of vascular aging, a geroscience perspective: Jacc focus seminar. J Am Coll Cardiol (2020) 75(8):931–41. doi: 10.1016/J.Jacc.2019.11.061
101. Giovannini S, Marzetti E, Borst SE, Leeuwenburgh C. Modulation of Gh/Igf-1 axis: Potential strategies to counteract sarcopenia in older adults. Mech Ageing Dev (2008) 129(10):593–601. doi: 10.1016/J.Mad.2008.08.001
102. Ashpole NM, Herron JC, Estep PN, Logan S, Hodges EL, Yabluchanskiy A, et al. Differential effects of igf-1 deficiency during the life span on structural and biomechanical properties in the tibia of aged mice. Age (Dordr) (2016) 38(2):38. doi: 10.1007/S11357-016-9902-5
103. Ashpole NM, Herron JC, Mitschelen MC, Farley JA, Logan S, Yan H, et al. Igf-1 regulates vertebral bone aging through sex-specific and time-dependent mechanisms. J Bone Miner Res (2015) 31:443–54. doi: 10.1002/Jbmr.2689
104. Gong Z, Kennedy O, Sun H, Wu Y, Williams GA, Klein L, et al. Reductions in serum igf-1 during aging impair health span. Aging Cell (2014) 13(3):408–18. doi: 10.1111/Acel.12188
105. Farias Quipildor GE, Mao K, Hu Z, Novaj A, Cui MH, Gulinello M, et al. Central igf-1 protects against features of cognitive and sensorimotor decline with aging in Male mice. Geroscience (2019) 41(2):185–208. doi: 10.1007/S11357-019-00065-3
106. Abs R, Mattsson AF, Thunander M, Verhelst J, Goth MI, Wilton P, et al. Prevalence of diabetes mellitus in 6050 hypopituitary patients with adult-onset gh deficiency before gh replacement: A kims analysis. Eur J Endocrinol (2013) 168(3):297–305. doi: 10.1530/Eje-12-0807
107. Abs R, Feldt-Rasmussen U, Mattsson AF, Monson JP, Bengtsson BA, Goth MI, et al. Determinants of cardiovascular risk in 2589 hypopituitary gh-deficient adults - a kims database analysis. Eur J Endocrinol (2006) 155(1):79–90. doi: 10.1530/Eje.1.02179
108. Quinlan P, Horvath A, Nordlund A, Wallin A, Svensson J. Low serum insulin-like growth factor-I (Igf-I) level is associated with increased risk of vascular dementia. Psychoneuroendocrinology (2017) 86:169–75. doi: 10.1016/J.Psyneuen.2017.09.018
109. Doi T, Shimada H, Makizako H, Tsutsumimoto K, Hotta R, Nakakubo S, et al. Association of insulin-like growth factor-1 with mild cognitive impairment and slow gait speed. Neurobiol Aging (2015) 36(2):942–7. doi: 10.1016/J.Neurobiolaging.2014.10.035
110. Vidal JS, Hanon O, Funalot B, Brunel N, Viollet C, Rigaud AS, et al. Low serum insulin-like growth factor-I predicts cognitive decline in alzheimer’s disease. J Alzheimers Dis (2016) 52(2):641–9. doi: 10.3233/Jad-151162
111. Watanabe T, Yamamoto H, Idei T, Iguchi T, Katagiri T. Influence of insulin-like growth factor-1 and hepatocyte growth factor on carotid atherosclerosis and cognitive function in the elderly. Dement Geriatr Cognit Disord (2004) 18(1):67–74. doi: 10.1159/000077812
112. Xie Y, Huang C, Zhu X, Wang J, Fan X, Fu Z, et al. Association between circulating insulin-like growth factor 1 and risk of all-cause and cause-specific mortality. Eur J Endocrinol (2021) 185(5):681–9. doi: 10.1530/Eje-21-0573
113. Miller LR, Tarantini S, Nyúl-Tóth Á, Johnston MP, Martin T, Bullen EC, et al. Increased susceptibility to cerebral microhemorrhages is associated with imaging signs of microvascular degeneration in the retina in an insulin-like growth factor 1 deficient mouse model of accelerated aging. Front In Aging Neurosci (2022) 14:788296. doi: 10.3389/Fnagi.2022.788296
114. Fulop GA, Ramirez-Perez FI, Kiss T, Tarantini S, Valcarcel Ares MN, Toth P, et al. Igf-1 deficiency promotes pathological remodeling of cerebral arteries: A potential mechanism contributing to the pathogenesis of intracerebral hemorrhages in aging. J Gerontol A Biol Sci Med Sci (2019) 74(4):446–54. doi: 10.1093/Gerona/Gly144
115. Toth P, Tucsek Z, Tarantini S, Sosnowska D, Gautam T, Mitschelen M, et al. Igf-1 deficiency impairs cerebral myogenic autoregulation in hypertensive mice. J Cereb Blood Flow Metab (2014) 34(12):1887–97. doi: 10.1038/Jcbfm.2014.156
116. Toth P, Tarantini S, Ashpole NM, Tucsek Z, Milne GL, Valcarcel-Ares NM, et al. Igf-1 deficiency impairs neurovascular coupling in mice: Implications for cerebromicrovascular aging. Aging Cell (2015) 14(6):1034–44. doi: 10.1111/Acel.12372
117. Tarantini S, Giles CB, Wren JD, Ashpole NM, Valcarcel-Ares MN, Wei JY, et al. Igf-1 deficiency in a critical period early in life influences the vascular aging phenotype in mice by altering mirna-mediated post-transcriptional gene regulation: Implications for the developmental origins of health and disease hypothesis. Age (Dordr) (2016) 38(4):239–58. doi: 10.1007/S11357-016-9943-9
118. Podlutsky A, Valcarcel-Ares MN, Yancey K, Podlutskaya V, Nagykaldi E, Gautam T, et al. The Gh/Igf-1 axis in a critical period early in life determines cellular dna repair capacity by altering transcriptional regulation of dna repair-related genes: Implications for the developmental origins of cancer. Geroscience (2017) 39(2):147–60. doi: 10.1007/S11357-017-9966-X
119. Bailey-Downs LC, Sosnowska D, Toth P, Mitschelen M, Gautam T, Henthorn JC, et al. Growth hormone and igf-1 deficiency exacerbate high-fat diet-induced endothelial impairment in obese Lewis dwarf rats: Implications for vascular aging. J Gerontol A Biol Sci Med Sci (2012) 67(6):553–64. doi: 10.1093/Gerona/Glr197
120. Ashpole NM, Logan S, Yabluchanskiy A, Mitschelen MC, Yan H, Farley JA, et al. Igf-1 has sexually dimorphic, pleiotropic, and time-dependent effects on healthspan, pathology, and lifespan. Geroscience (2017) 39(2):129–45. doi: 10.1007/S11357-017-9971-0
121. Bailey-Downs LC, Mitschelen M, Sosnowska D, Toth P, Pinto JT, Ballabh P, et al. Liver-specific knockdown of igf-1 decreases vascular oxidative stress resistance by impairing the Nrf2-dependent antioxidant response: A novel model of vascular aging. J Gerontol A Biol Sci Med Sci (2012) 67(4):313–29. doi: 10.1093/Gerona/Glr164
122. D’costa AP, Xu X, Ingram RL, Sonntag WE. Insulin-like growth factor-1 stimulation of protein synthesis is attenuated in cerebral cortex of aging rats. Neuroscience (1995) 65(3):805–13. doi: 10.1016/0306-4522(94)00495-Q
123. Grill JD, Sonntag WE, Riddle DR. Dendritic stability in a model of adult-onset igf-I deficiency. Growth Horm Igf Res (2005) 15(5):337–48. doi: 10.1016/J.Ghir.2005.07.002
124. Groban L, Lin M, Kassik KA, Ingram RL, Sonntag WE. Early-onset growth hormone deficiency results in diastolic dysfunction in adult-life and is prevented by growth hormone supplementation. Growth Horm Igf Res (2011) 21(2):81–8. doi: 10.1016/J.Ghir.2011.01.003
125. Hua K, Forbes ME, Lichtenwalner RJ, Sonntag WE, Riddle DR. Adult-onset deficiency in growth hormone and insulin-like growth factor-I alters oligodendrocyte turnover in the corpus callosum. Glia (2009) 57(10):1062–71. doi: 10.1002/Glia.20829
126. Khan AS, Lynch CD, Sane DC, Willingham MC, Sonntag WE. Growth hormone increases regional coronary blood flow and capillary density in aged rats. J Gerontol A Biol Sci Med Sci (2001) 56(8):B364–71. doi: 10.1093/gerona/56.8.B364
127. Lichtenwalner RJ, Forbes ME, Bennett SA, Lynch CD, Sonntag WE, Riddle DR. Intracerebroventricular infusion of insulin-like growth factor-I ameliorates the age-related decline in hippocampal neurogenesis. Neuroscience (2001) 107(4):603–13. doi: 10.1016/S0306-4522(01)00378-5
128. Lichtenwalner RJ, Forbes ME, Sonntag WE, Riddle DR. Adult-onset deficiency in growth hormone and insulin-like growth factor-I decreases survival of dentate granule neurons: Insights into the regulation of adult hippocampal neurogenesis. J Neurosci Res (2006) 83(2):199–210. doi: 10.1002/Jnr.20719
129. Logan S, Pharaoh GA, Marlin MC, Masser DR, Matsuzaki S, Wronowski B, et al. Insulin-like growth factor receptor signaling regulates working memory, mitochondrial metabolism, and amyloid-beta uptake in astrocytes. Mol Metab (2018) 9:141–55. doi: 10.1016/J.Molmet.2018.01.013
130. Mitschelen M, Yan H, Farley JA, Warrington JP, Han S, Herenu CB, et al. Long-term deficiency of circulating and hippocampal insulin-like growth factor I induces depressive behavior in adult mice: A potential model of geriatric depression. Neuroscience (2011) 185:50–60. doi: 10.1016/J.Neuroscience.2011.04.032
131. Nieves-Martinez E, Sonntag WE, Wilson A, Donahue A, Molina DP, Brunso-Bechtold J, et al. Early-onset gh deficiency results in spatial memory impairment in mid-life and is prevented by gh supplementation. J Endocrinol (2010) 204(1):31–6. doi: 10.1677/Joe-09-0323
132. Poe BH, Linville C, Riddle DR, Sonntag WE, Brunso-Bechtold JK. Effects of age and insulin-like growth factor-1 on neuron and synapse numbers in area Ca3 of hippocampus. Neuroscience (2001) 107(2):231–8. doi: 10.1016/S0306-4522(01)00341-4
133. Ramsey MM, Weiner JL, Moore TP, Carter CS, Sonntag WE. Growth hormone treatment attenuates age-related changes in hippocampal short-term plasticity and spatial learning. Neuroscience (2004) 129(1):119–27. doi: 10.1016/J.Neuroscience.2004.08.001
134. Shi L, Linville MC, Tucker EW, Sonntag WE, Brunso-Bechtold JK. Differential effects of aging and insulin-like growth factor-1 on synapses in Ca1 of rat hippocampus. Cereb Cortex (2005) 15(5):571–7. doi: 10.1093/Cercor/Bhh158
135. Bartke A. Benefits of living without growth hormone. J Gerontol A Biol Sci Med Sci (2021) 76(10):1769–74. doi: 10.1093/Gerona/Glab147
136. Dixit M, Duran-Ortiz S, Yildirim G, Poudel SB, Louis LD, Bartke A, et al. Induction of somatopause in adult mice compromises bone morphology and exacerbates bone loss during aging. Aging Cell (2021) 20(12):E13505. doi: 10.1111/Acel.13505
137. Fang Y, Mcfadden S, Darcy J, Hascup ER, Hascup KN, Bartke A. Lifespan of long-lived growth hormone receptor knockout mice was not normalized by housing At 30 degrees c since weaning. Aging Cell (2020) 19(5):E13123. doi: 10.1111/Acel.13123
138. Saccon TD, Schneider A, Marinho CG, Nunes ADC, Noureddine S, Dhahbi J, et al. Circulating microrna profile in humans and mice with congenital gh deficiency. Aging Cell (2021) 20(7):E13420. doi: 10.1111/Acel.13420
139. Schneider A, Saccon TD, Garcia DN, Zanini BM, Isola JVV, Hense JD, et al. The interconnections between somatic and ovarian aging in murine models. J Gerontol A Biol Sci Med Sci (2021) 76(9):1579–86. doi: 10.1093/Gerona/Glaa258
140. Yuan R, Musters CJM, Zhu Y, Evans TR, Sun Y, Chesler EJ, et al. Genetic differences and longevity-related phenotypes influence lifespan and lifespan variation in a sex-specific manner in mice. Aging Cell (2020) 19(11):E13263. doi: 10.1111/Acel.13263
141. Wiesenborn DS, Galvez EJC, Spinel L, Victoria B, Allen B, Schneider A, et al. The role of Ames dwarfism and calorie restriction on gut microbiota. J Gerontol A Biol Sci Med Sci (2020) 75(7):E1–8. doi: 10.1093/Gerona/Glz236
142. Icyuz M, Zhang F, Fitch MP, Joyner MR, Challa AK, Sun LY. Physiological and metabolic characteristics of novel double-mutant female mice with targeted disruption of both growth hormone-releasing hormone and growth hormone receptor. Aging Cell (2021) 20(4):E13339. doi: 10.1111/Acel.13339
143. Holzenberger M, Dupont J, Ducos B, Leneuve P, Geloen A, Even PC, et al. Igf-1 receptor regulates lifespan and resistance to oxidative stress in mice. Nature (2003) 421(6919):182–7. doi: 10.1038/Nature01298
144. Mao K, Quipildor GF, Tabrizian T, Novaj A, Guan F, Walters RO, et al. Late-life targeting of the igf-1 receptor improves healthspan and lifespan in female mice. Nat Commun (2018) 9(1):2394. doi: 10.1038/S41467-018-04805-5
145. Flurkey K, Papaconstantinou J, Miller RA, Harrison DE. Lifespan extension and delayed immune and collagen aging in mutant mice with defects in growth hormone production. Proc Natl Acad Sci U.S.A. (2001) 98(12):6736–41. doi: 10.1073/pnas.111158898
146. Liang H, Masoro EJ, Nelson JF, Strong R, Mcmahan CA, Richardson A. Genetic mouse models of extended lifespan. Exp Gerontol (2003) 38(11-12):1353–64. doi: 10.1016/j.exger.2003.10.019
147. Hill CM, Fang Y, Miquet JG, Sun LY, Masternak MM, Bartke A. Long-lived hypopituitary Ames dwarf mice are resistant to the detrimental effects of high-fat diet on metabolic function and energy expenditure. Aging Cell (2016) 15(3):509–21. doi: 10.1111/Acel.12467
148. Gesing A, Wiesenborn D, Do A, Menon V, Schneider A, Victoria B, et al. A long-lived mouse lacking both growth hormone and growth hormone receptor: A new animal model for aging studies. J Gerontol A Biol Sci Med Sci (2016) 24:5073–9. doi: 10.1093/Gerona/Glw193
149. Panici JA, Harper JM, Miller RA, Bartke A, Spong A, Masternak MM. Early life growth hormone treatment shortens longevity and decreases cellular stress resistance in long-lived mutant mice. FASEB J (2010) 24:1–7. doi: 10.1096/Fj.10-163253
150. Bokov AF, Lindsey ML, Khodr C, Sabia MR, Richardson A. Long-lived Ames dwarf mice are resistant to chemical stressors. J Gerontol A Biol Sci Med Sci (2009) 64(8):819–27. doi: 10.1093/Gerona/Glp052
151. Bartke A, Brown-Borg H. Life extension in the dwarf mouse. Curr Top Dev Biol (2004) 63:189–225. doi: 10.1016/S0070-2153(04)63006-7
152. Mattison JA, Wright C, Bronson RT, Roth GS, Ingram DK, Bartke A. Studies of aging in Ames dwarf mice: Effects of caloric restriction. J Am Aging Assoc (2000) 23(1):9–16. doi: 10.1007/S11357-000-0002-0
153. Duran-Ortiz S, List EO, Ikeno Y, Young J, Basu R, Bell S, et al. Growth hormone receptor gene disruption in mature-adult mice improves Male insulin sensitivity and extends female lifespan. Aging Cell (2021) 20(12):E13506. doi: 10.1111/Acel.13506
154. Sun LY, Fang Y, Patki A, Koopman JJ, Allison DB, Hill CM, et al. Longevity is impacted by growth hormone action during early postnatal period. Elife (2017) 6:e24059. doi: 10.7554/Elife.24059
155. Kuramoto K, Tahara S, Sasaki T, Matsumoto S, Kaneko T, Kondo H, et al. Spontaneous dwarf rat: a novel model for aging research. Geriatr Gerontol Int (2010) 10(1):94–101. doi: 10.1111/J.1447-0594.2009.00559.X
156. Ungvari Z, Gautam T, Koncz P, Henthorn JC, Pinto JT, Ballabh P, et al. Vasoprotective effects of life span-extending peripubertal gh replacement in Lewis dwarf rats. J Gerontol A Biol Sci Med Sci (2010) 65(11):1145–56. doi: 10.1093/Gerona/Glq147
157. Zhang WB, Ye K, Barzilai N, Milman S. The antagonistic pleiotropy of insulin-like growth factor 1. Aging Cell (2021) 20(9):E13443. doi: 10.1111/Acel.13443
158. Milman S, Atzmon G, Huffman DM, Wan J, Crandall JP, Cohen P, et al. Low insulin-like growth factor-1 level predicts survival in humans with exceptional longevity. Aging Cell (2014) 13(4):769–71. doi: 10.1111/Acel.12213
159. Zhang WB, Aleksic S, Gao T, Weiss EF, Demetriou E, Verghese J, et al. Insulin-like growth factor-1 and igf binding proteins predict all-cause mortality and morbidity in older adults. Cells (2020) 9(6):1368. doi: 10.3390/Cells9061368
160. Bartke A, Sun LY, Longo V. Somatotropic signaling: Trade-offs between growth, reproductive development, and longevity. Physiol Rev (2013) 93(2):571–98. doi: 10.1152/Physrev.00006.2012
161. Tatar M, Bartke A, Antebi A. The endocrine regulation of aging by insulin-like signals. Sci (New York Ny (2003) 299(5611):1346–51. doi: 10.1126/science.1081447
162. Colon G, Saccon T, Schneider A, Cavalcante MB, Huffman DM, Berryman D, et al. The enigmatic role of growth hormone in age-related diseases, cognition, and longevity. Geroscience (2019) 41(6):759–74. doi: 10.1007/S11357-019-00096-W
163. Fang Y, Mcfadden S, Darcy J, Hill CM, Huber JA, Verhulst S, et al. Differential effects of early-life nutrient restriction in long-lived ghr-ko and normal mice. Geroscience (2017) 39(3):347–56. doi: 10.1007/S11357-017-9978-6
164. Ouarné M, Pena A, Franco CA. From remodeling to quiescence: The transformation of the vascular network. Cells Dev (2021) 168:203735. doi: 10.1016/J.Cdev.2021.203735
165. Silpanisong J, Pearce WJ. Vasotrophic regulation of age-dependent hypoxic cerebrovascular remodeling. Curr Vasc Pharmacol (2013) 11(5):544–63. doi: 10.2174/1570161111311050002
166. Bonnans C, Chou J, Werb Z. Remodelling the extracellular matrix in development and disease. Nat Rev Mol Cell Biol (2014) 15(12):786–801. doi: 10.1038/Nrm3904
167. Sonbol HS. Extracellular matrix remodeling in human disease. J Microsc Ultrastruct (2018) 6(3):123–8. doi: 10.4103/Jmau.Jmau_4_18
168. Phillip JM, Aifuwa I, Walston J, Wirtz D. The mechanobiology of aging. Annu Rev BioMed Eng (2015) 17:113–41. doi: 10.1146/Annurev-Bioeng-071114-040829
169. Tarantini S, Yabluchanskiy A, Lindsey ML, Csiszar A, Ungvari Z. Effect of genetic depletion of mmp-9 on neurological manifestations of hypertension-induced intracerebral hemorrhages in aged mice. Geroscience (2021) 43(5):2611–9. doi: 10.1007/S11357-021-00402-5
170. Reed MJ, Damodarasamy M, Banks WA. The extracellular matrix of the blood-brain barrier: Structural and functional roles in health, aging, and alzheimer’s disease. Tissue Barriers (2019) 7(4):1651157. doi: 10.1080/21688370.2019.1651157
171. Behmoaras J, Osborne-Pellegrin M, Gauguier D, Jacob MP. Characteristics of the aortic elastic network and related phenotypes in seven inbred rat strains. Am J Physiol Heart Circ Physiol (2005) 288(2):H769–77. doi: 10.1152/Ajpheart.00544.2004
172. Kiss T, Balasubramanian P, Valcarcel-Ares MN, Tarantini S, Yabluchanskiy A, Csipo T, et al. Nicotinamide mononucleotide (Nmn) treatment attenuates oxidative stress and rescues angiogenic capacity in aged cerebromicrovascular endothelial cells: A potential mechanism for the prevention of vascular cognitive impairment. Geroscience (2019) 41(5):619–30. doi: 10.1007/S11357-019-00074-2
173. Ungvari Z, Kaley G, De Cabo R, Sonntag WE, Csiszar A. Mechanisms of vascular aging: New perspectives. J Gerontol A Biol Sci Med Sci (2010) 65(10):1028–41. doi: 10.1093/Gerona/Glq113
174. Feihl F, Liaudet L, Waeber B, Levy BI. Hypertension: A disease of the microcirculation? Hypertension (2006) 48(6):1012–7. doi: 10.1161/01.Hyp.0000249510.20326.72
175. Schager B, Brown CE. Susceptibility to capillary plugging can predict brain region specific vessel loss with aging. J Cereb Blood Flow Metab (2020) 40(12):2475–90. doi: 10.1177/0271678x19895245
176. Riddle DR, Sonntag WE, Lichtenwalner RJ. Microvascular plasticity in aging. Ageing Res Rev (2003) 2(2):149–68. doi: 10.1016/S1568-1637(02)00064-8
177. Ingraham JP, Forbes ME, Riddle DR, Sonntag WE. Aging reduces hypoxia-induced microvascular growth in the rodent hippocampus. J Gerontol A Biol Sci Med Sci (2008) 63(1):12–20. doi: 10.1093/gerona/63.1.12
178. Sonntag WE, Lynch CD, Cooney PT, Hutchins PM. Decreases in cerebral microvasculature with age are associated with the decline in growth hormone and insulin-like growth factor 1. Endocrinology (1997) 138(8):3515–20. doi: 10.1210/endo.138.8.5330
179. Sardone R, Sborgia G, Niro A, Giuliani G, Pascale A, Puzo P, et al. Retinal vascular density on optical coherence tomography angiography and age-related central and peripheral hearing loss in a southern Italian older population. J Gerontol A Biol Sci Med Sci (2021) 76(12):2169–77. doi: 10.1093/Gerona/Glaa269
180. Anversa P, Li P, Sonnenblick EH, Olivetti G. Effects of aging on quantitative structural properties of coronary vasculature and microvasculature in rats. Am J Physiol (1994) 267(3 Pt 2):H1062–73. doi: 10.1152/ajpheart.1994.267.3.H1062
181. Faber JE, Zhang H, Lassance-Soares RM, Prabhakar P, Najafi AH, Burnett MS, et al. Aging causes collateral rarefaction and increased severity of ischemic injury in multiple tissues. Arterioscler Thromb Vac Biol (2011) 31(8):1748–56. doi: 10.1161/Atvbaha.111.227314
182. Kang DH, Anderson S, Kim YG, Mazzalli M, Suga S, Jefferson JA, et al. Impaired angiogenesis in the aging kidney: Vascular endothelial growth factor and thrombospondin-1 in renal disease. Am J Kidney Dis (2001) 37(3):601–11. doi: 10.1053/ajkd.2001.22087
183. Katsimpardi L, Litterman NK, Schein PA, Miller CM, Loffredo FS, Wojtkiewicz GR, et al. Vascular and neurogenic rejuvenation of the aging mouse brain by young systemic factors. Science (2014) 344(6184):630–4. doi: 10.1126/Science.1251141
184. Kiss T, Nyúl-Tóth Á, Gulej R, Tarantini S, Csipo T, Mukli P, et al. Old blood from heterochronic parabionts accelerates vascular aging in young mice: Transcriptomic signature of pathologic smooth muscle remodeling. Geroscience (2022) 44(2):953–81. doi: 10.1007/S11357-022-00519-1
185. Kiss T, Tarantini S, Csipo T, Balasubramanian P, Nyúl-Tóth Á, Yabluchanskiy A, et al. Circulating anti-geronic factors from heterochonic parabionts promote vascular rejuvenation in aged mice: Transcriptional footprint of mitochondrial protection, attenuation of oxidative stress, and rescue of endothelial function by young blood. Geroscience (2020) 42(2):727–48. doi: 10.1007/S11357-020-00180-6
186. Pan Y, Liang H, Liu H, Li D, Chen X, Li L, et al. Platelet-secreted microrna-223 promotes endothelial cell apoptosis induced by advanced glycation end products Via targeting the insulin-like growth factor 1 receptor. J Of Immunol (Baltimore Md: 1950) (2013) 192:437–46. doi: 10.4049/Jimmunol.1301790
187. Lopez-Lopez C, Leroith D, Torres-Aleman I. Insulin-like growth factor I is required for vessel remodeling in the adult brain. Proc Natl Acad Sci U.S.A. (2004) 101(26):9833–8. doi: 10.1073/Pnas.0400337101
188. Zhu W, Fan Y, Hao Q, Shen F, Hashimoto T, Yang GY, et al. Postischemic igf-1 gene transfer promotes neurovascular regeneration after experimental stroke. J Cereb Blood Flow Metab (2009) 29(9):1528–37. doi: 10.1038/Jcbfm.2009.75
189. Hayes CA, Valcarcel-Ares MN, Ashpole NM. Preclinical and clinical evidence of igf-1 as a prognostic marker and acute intervention with ischemic stroke. J Cereb Blood Flow Metab (2021) 41(10):2475–91. doi: 10.1177/0271678x211000894
190. Fuchtbauer L, Olsson DS, Coopmans EC, Bengtsson BA, Norrman LL, Neggers S, et al. Increased number of retinal vessels in acromegaly. Eur J Endocrinol (2020) 182(3):293–302. doi: 10.1530/Eje-19-0778
191. Gallo A, Chaigneau E, Jublanc C, Rosenbaum D, Mattina A, Paques M, et al. Igf-1 is an independent predictor of retinal arterioles remodeling in subjects with uncontrolled acromegaly. Eur J Endocrinol (2020) 182(3):375–83. doi: 10.1530/Eje-19-0390
192. Tarnawski AS, Pai R, Tanigawa T, Matysiak-Budnik T, Ahluwalia A. Pten silencing reverses aging-related impairment of angiogenesis in microvascular endothelial cells. Biochem Biophys Res Commun (2010) 394(2):291–6. doi: 10.1016/J.Bbrc.2010.02.161
193. Bach MH, Sadoun E, Reed MJ. Defects in activation of nitric oxide synthases occur during delayed angiogenesis in aging. Mech Ageing Dev (2005) 126(4):467–73. doi: 10.1016/J.Mad.2004.10.005
194. Sadoun E, Reed MJ. Impaired angiogenesis in aging is associated with alterations in vessel density, matrix composition, inflammatory response, and growth factor expression. J Histochem Cytochem (2003) 51(9):1119–30. doi: 10.1177/002215540305100902
195. Ahluwalia A, Tarnawski AS. Activation of the metabolic sensor - amp activated protein kinase reverses impairment of angiogenesis in aging myocardial microvascular endothelial cells. implications for the aging heart. J Physiol Pharmacol (2011) 62(5):583–7.
196. Lahteenvuo J, Rosenzweig A. Effects of aging on angiogenesis. Circ Res (2012) 110(9):1252–64. doi: 10.1161/Circresaha.111.246116
197. Delafontaine P, Song YH, Li Y. Expression, regulation, and function of igf-1, igf-1r, and igf-1 binding proteins in blood vessels. Arterioscler Thromb Vac Biol (2004) 24(3):435–44. doi: 10.1161/01.Atv.0000105902.89459.09
198. Humpert PM, Djuric Z, Zeuge U, Oikonomou D, Seregin Y, Laine K, et al. Insulin stimulates the clonogenic potential of angiogenic endothelial progenitor cells by igf-1 receptor-dependent signaling. Mol Med (2008) 14(5-6):301–8. doi: 10.2119/2007-00052.Humpert
199. Menu E, Kooijman R, Van Valckenborgh E, Asosingh K, Bakkus M, Van Camp B, et al. Specific roles for the Pi3k and the mek-erk pathway in igf-1-Stimulated chemotaxis, vegf secretion and proliferation of multiple myeloma cells: Study in the 5t33mm model. Br J Cancer (2004) 90(5):1076–83. doi: 10.1038/Sj.Bjc.6601613
200. Oomen PH, Beentjes JA, Bosma E, Smit AJ, Reitsma WD, Dullaart RP. Reduced capillary permeability and capillary density in the skin of gh-deficient adults: Improvement after 12 months gh replacement. Clin Endocrinol (Oxf) (2002) 56(4):519–24. doi: 10.1046/J.1365-2265.2002.01517.X
201. Viana IM, De Almeida ME, Lins MP, Dos Santos Reis MD, De Araujo Vieira LF, Smaniotto S. Combined effect of insulin-like growth factor-1 and cc chemokine ligand 2 on angiogenic events in endothelial cells. PloS One (2015) 10(4):E0121249. doi: 10.1371/Journal.Pone.0121249
202. Banki E, Sosnowska D, Tucsek Z, Gautam T, Toth P, Tarantini S, et al. Age-related decline of autocrine pituitary adenylate cyclase-activating polypeptide impairs angiogenic capacity of rat cerebromicrovascular endothelial cells. J Gerontol A Biol Sci Med Sci (2015) 70(6):665–74. doi: 10.1093/Gerona/Glu116
203. Wagatsuma A. Effect of aging on expression of angiogenesis-related factors in mouse skeletal muscle. Exp Gerontol (2006) 41(1):49–54. doi: 10.1016/J.Exger.2005.10.003
204. Ryan NA, Zwetsloot KA, Westerkamp LM, Hickner RC, Pofahl WE, Gavin TP. Lower skeletal muscle capillarization and vegf expression in aged vs. young men. J Appl Physiol (1985) (2006) 100(1):178–85. doi: 10.1152/Japplphysiol.00827.2005
205. Iemitsu M, Maeda S, Jesmin S, Otsuki T, Miyauchi T. Exercise training improves aging-induced downregulation of vegf angiogenic signaling cascade in hearts. Am J Physiol Heart Circ Physiol (2006) 291(3):H1290–8. doi: 10.1152/Ajpheart.00820.2005
206. Mieno S, Boodhwani M, Clements RT, Ramlawi B, Sodha NR, Li J, et al. Aging is associated with an impaired coronary microvascular response to vascular endothelial growth factor in patients. J Thorac Cardiovasc Surg (2006) 132(6):1348–55. doi: 10.1016/J.Jtcvs.2006.08.043
207. Yu Y, Singh H, Kwon K, Tsitrin T, Petrini J, Nelson KE, et al. Protein signatures from blood plasma and urine suggest changes in vascular function and il-12 signaling in elderly with a history of chronic diseases compared with an age-matched healthy cohort. Geroscience (2021) 43(2):593–606. doi: 10.1007/S11357-020-00269-Y
208. Dilley RJ, Schwartz SM. Vascular remodeling in the growth hormone transgenic mouse. Circ Res (1989) 65(5):1233–40. doi: 10.1161/01.Res.65.5.1233
209. Chen Y, Capron L, Magnusson JO, Wallby LA, Arnqvist HJ. Insulin-like growth factor-1 stimulates vascular smooth muscle cell proliferation in rat aorta In vivo. Growth Horm Igf Res (1998) 8(4):299–303. doi: 10.1016/S1096-6374(98)80125-1
210. Kobayashi T, Kaneda A, Kamata K. Possible involvement of igf-1 receptor and igf-binding protein in insulin-induced enhancement of noradrenaline response in diabetic rat aorta. Br J Pharmacol (2003) 140(2):285–94. doi: 10.1038/Sj.Bjp.0705438
211. Claassen J, Thijssen DHJ, Panerai RB, Faraci FM. Regulation of cerebral blood flow in humans: Physiology and clinical implications of autoregulation. Physiol Rev (2021) 101(4):1487–559. doi: 10.1152/Physrev.00022.2020
212. Linde CI, Karashima E, Raina H, Zulian A, Wier WG, Hamlyn JM, et al. Increased arterial smooth muscle Ca2+ signaling, vasoconstriction, and myogenic reactivity in Milan hypertensive rats. Am J Physiol Heart Circ Physiol (2012) 302(3):H611–20. doi: 10.1152/Ajpheart.00950.2011
213. Springo Z, Toth P, Tarantini S, Ashpole NM, Tucsek Z, Sonntag WE, et al. Aging impairs myogenic adaptation to pulsatile pressure in mouse cerebral arteries. J Cereb Blood Flow Metab (2015) 35(4):527–30. doi: 10.1038/Jcbfm.2014.256
214. Wang S, Lv W, Zhang H, Liu Y, Li L, Jefferson JR, et al. Aging exacerbates impairments of cerebral blood flow autoregulation and cognition in diabetic rats. Geroscience (2020) 42(5):1387–410. doi: 10.1007/S11357-020-00233-W
215. Tarumi T, Ayaz Khan M, Liu J, Tseng BY, Parker R, Riley J, et al. Cerebral hemodynamics in normal aging: Central artery stiffness, wave reflection, and pressure pulsatility. J Cereb Blood Flow Metab (2014) 34(6):971–8. doi: 10.1038/Jcbfm.2014.44
216. Brayden JE, Earley S, Nelson MT, Reading S. Transient receptor potential (Trp) channels, vascular tone and autoregulation of cerebral blood flow. Clin Exp Pharmacol Physiol (2008) 35(9):1116–20. doi: 10.1111/J.1440-1681.2007.04855.X
217. Toth P, Tucsek Z, Sosnowska D, Gautam T, Mitschelen M, Tarantini S, et al. Age-related autoregulatory dysfunction and cerebromicrovascular injury in mice with angiotensin ii-induced hypertension. J Cereb Blood Flow Metab (2013) 33(11):1732–42. doi: 10.1038/Jcbfm.2013.143
218. Ungvari Z, Csiszar A, Huang A, Kaminski PM, Wolin MS, Koller A. High pressure induces superoxide production in isolated arteries Via protein kinase c-dependent activation of Nad(P)H oxidase. Circulation (2003) 108(10):1253–8. doi: 10.1161/01.Cir.0000079165.84309.4d
219. Toth P, Tarantini S, Springo Z, Tucsek Z, Gautam T, Giles CB, et al. Aging exacerbates hypertension-induced cerebral microhemorrhages in mice: Role of resveratrol treatment in vasoprotection. Aging Cell (2015) 14(3):400–8. doi: 10.1111/Acel.12315
220. Yu X, Ji C, Shao A. Neurovascular unit dysfunction and neurodegenerative disorders. Front Neurosci (2020) 14:334. doi: 10.3389/Fnins.2020.00334
221. Hendrikx D, Smits A, Lavanga M, De Wel O, Thewissen L, Jansen K, et al. Measurement of neurovascular coupling in neonates. Front Physiol (2019) 10:65. doi: 10.3389/Fphys.2019.00065
222. Toth P, Tarantini S, Davila A, Valcarcel-Ares MN, Tucsek Z, Varamini B, et al. Purinergic glio-endothelial coupling during neuronal activity: Role of P2y1 receptors and enos in functional hyperemia in the mouse somatosensory cortex. Am J Physiol Heart Circ Physiol (2015) 309(11):H1837–45. doi: 10.1152/Ajpheart.00463.2015
223. Chen BR, Kozberg MG, Bouchard MB, Shaik MA, Hillman EM. A critical role for the vascular endothelium in functional neurovascular coupling in the brain. J Am Heart Assoc (2014) 3(3):E000787. doi: 10.1161/Jaha.114.000787
224. Stobart JL, Lu L, Anderson HD, Mori H, Anderson CM. Astrocyte-induced cortical vasodilation is mediated by d-serine and endothelial nitric oxide synthase. Proc Natl Acad Sci U.S.A. (2013) 110(8):3149–54. doi: 10.1073/Pnas.1215929110
225. Csipo T, Mukli P, Lipecz A, Tarantini S, Bahadli D, Abdulhussein O, et al. Assessment of age-related decline of neurovascular coupling responses by functional near-infrared spectroscopy (Fnirs) in humans. Geroscience (2019) 41(5):495–509. doi: 10.1007/S11357-019-00122-X
226. Tarantini S, Tran CHT, Gordon GR, Ungvari Z, Csiszar A. Impaired neurovascular coupling in aging and alzheimer’s disease: Contribution of astrocyte dysfunction and endothelial impairment to cognitive decline. Exp Gerontol (2017) 94:52–8. doi: 10.1016/J.Exger.2016.11.004
227. Duffy KR, Pardridge WM, Rosenfeld RG. Human blood-brain barrier insulin-like growth factor receptor. Metabolism (1988) 37(2):136–40. doi: 10.1016/S0026-0495(98)90007-5
228. Rosenfeld RG, Pham H, Keller BT, Borchardt RT, Pardridge WM. Demonstration and structural comparison of receptors for insulin-like growth factor-I and -ii (Igf-I and -ii) in brain and blood-brain barrier. Biochem Biophys Res Commun (1987) 149(1):159–66. doi: 10.1016/0006-291x(87)91618-4
229. Conti E, Musumeci MB, De Giusti M, Dito E, Mastromarino V, Autore C, et al. Igf-1 and atherothrombosis: Relevance to pathophysiology and therapy. Clin Sci (Lond) (2011) 120(9):377–402. doi: 10.1042/Cs20100400
230. Conti E, Carrozza C, Capoluongo E, Volpe M, Crea F, Zuppi C, et al. Insulin-like growth factor-1 as a vascular protective factor. Circulation (2004) 110(15):2260–5. doi: 10.1161/01.Cir.0000144309.87183.Fb
231. Higashi Y, Sukhanov S, Anwar A, Shai SY, Delafontaine P. Aging, atherosclerosis, and igf-1. J Gerontol A Biol Sci Med Sci (2012) 67(6):626–39. doi: 10.1093/Gerona/Gls102
232. Tarantini S, Valcarcel-Ares NM, Yabluchanskiy A, Fulop GA, Hertelendy P, Gautam T, et al. Treatment with the mitochondrial-targeted antioxidant peptide ss-31 rescues neurovascular coupling responses and cerebrovascular endothelial function and improves cognition in aged mice. Aging Cell (2018) 17(2):e12731. doi: 10.1111/Acel.12731
233. Imrie H, Viswambharan H, Sukumar P, Abbas A, Cubbon RM, Yuldasheva N, et al. Novel role of the igf-1 receptor in endothelial function and repair: Studies in endothelium-targeted igf-1 receptor transgenic mice. Diabetes (2012) 61(9):2359–68. doi: 10.2337/Db11-1494
234. Tarantini S, Balasubramanian P, Yabluchanskiy A, Ashpole NM, Logan S, Kiss T, et al. Igf1r signaling regulates astrocyte-mediated neurovascular coupling in mice: Implications for brain aging. Geroscience (2021) 43(2):901–11. doi: 10.1007/S11357-021-00350-0
235. Nishijima T, Torres-Aleman I, Soya H. Exercise and cerebrovascular plasticity. Prog Brain Res (2016) 225:243–68. doi: 10.1016/Bs.Pbr.2016.03.010
236. Shen J, Wang D, Wang X, Gupta S, Ayloo B, Wu S, et al. Neurovascular coupling in the dentate gyrus regulates adult hippocampal neurogenesis. Neuron (2019) 103(5):878–90 E3. doi: 10.1016/J.Neuron.2019.05.045
237. Wilhelm I, Nyul-Toth A, Kozma M, AE F, Krizbai IA. Role of pattern recognition receptors of the neurovascular unit in inflamm-aging. Am J Physiol Heart Circ Physiol (2017) 313(5):H1000–12. doi: 10.1152/Ajpheart.00106.2017
238. Krizbai IA, Nyul-Toth A, Bauer HC, Farkas AE, Traweger A, Hasko J, et al. Pharmaceutical targeting of the brain. Curr Pharm Des (2016) 22(35):5442–62. doi: 10.2174/1381612822666160726144203
239. Braun DJ, Bachstetter AD, Sudduth TL, Wilcock DM, Watterson DM, Van Eldik LJ. Genetic knockout of myosin light chain kinase (Mlck210) prevents cerebral microhemorrhages and attenuates neuroinflammation in a mouse model of vascular cognitive impairment and dementia. Geroscience (2019) 41(5):671–9. doi: 10.1007/S11357-019-00072-4
240. Verheggen ICM, De Jong JJA, Van Boxtel MPJ, Postma AA, Jansen JFA, Verhey FRJ, et al. Imaging the role of blood-brain barrier disruption in normal cognitive ageing. Geroscience (2020) 42(6):1751–64. doi: 10.1007/S11357-020-00282-1
241. Towner RA, Saunders D, Smith N, Gulej R, Mckenzie T, Lawrence B, et al. Anti-inflammatory agent, okn-007, reverses long-term neuroinflammatory responses in a rat encephalopathy model as assessed by multi-parametric mri: Implications for aging-associated neuroinflammation. Geroscience (2019) 41(4):483–94. doi: 10.1007/S11357-019-00094-Y
242. Nagyoszi P, Nyul-Toth A, Fazakas C, Wilhelm I, Kozma M, Molnar J, et al. Regulation of nod-like receptors and inflammasome activation in cerebral endothelial cells. J Neurochem (2015) 135(3):551–64. doi: 10.1111/Jnc.13197
243. Nyul-Toth A, Kozma M, Nagyoszi P, Nagy K, Fazakas C, Hasko J, et al. Expression of pattern recognition receptors and activation of the non-canonical inflammasome pathway in brain pericytes. Brain Behav Immun (2017) 64:220–31. doi: 10.1016/J.Bbi.2017.04.010
244. Kozma M, Meszaros A, Nyul-Toth A, Molnar K, Costea L, Hernadi Z, et al. Cerebral pericytes and endothelial cells communicate through inflammasome-dependent signals. Int J Mol Sci (2021) 22(11):6122. doi: 10.3390/Ijms22116122
245. Bernard-Patrzynski F, Lécuyer MA, Puscas I, Boukhatem I, Charabati M, Bourbonnière L, et al. Isolation of endothelial cells, pericytes and astrocytes from mouse brain. . PloS One (2019) 14(12):E0226302. doi: 10.1371/Journal.Pone.0226302
246. Towner RA, Gulej R, Zalles M, Saunders D, Smith N, Lerner M, et al. Rapamycin restores brain vasculature, metabolism, and blood-brain barrier in an inflammaging model. Geroscience (2021) 43(2):563–78. doi: 10.1007/S11357-021-00363-9
247. Baltazar-Lara R, Zenil JM, Carranza M, Avila-Mendoza J, Martinez-Moreno CG, Aramburo C, et al. Growth hormone (Gh) crosses the blood-brain barrier (Bbb) and induces neuroprotective effects in the embryonic chicken cerebellum after a hypoxic injury. Int J Mol Sci (2022)23(19):11546. doi: 10.3390/Ijms231911546
248. Jung S, Terorde K, Dorr HG, Trollmann R. Recombinant human growth hormone activates neuroprotective growth factors in hypoxic brain injury in neonatal mice. Endocrinology (2021) 162(3):bqab008. doi: 10.1210/Endocr/Bqab008
249. Klepper S, Jung S, Dittmann L, Geppert CI, Hartmann A, Beier N, et al. Further evidence of neuroprotective effects of recombinant human erythropoietin and growth hormone in hypoxic brain injury in neonatal mice. Int J Mol Sci (2022) 23(15):8693. doi: 10.3390/Ijms23158693
250. Obermeier B, Daneman R, Ransohoff RM. Development, maintenance and disruption of the blood-brain barrier. Nat Med (2013) 19(12):1584–96. doi: 10.1038/Nm.3407
251. Labandeira-Garcia JL, Costa-Besada MA, Labandeira CM, Villar-Cheda B, Rodriguez-Perez AI. Insulin-like growth factor-1 and neuroinflammation. Front Aging Neurosci (2017) 9:365. doi: 10.3389/Fnagi.2017.00365
252. Bake S, Selvamani A, Cherry J, Sohrabji F. Blood brain barrier and neuroinflammation are critical targets of igf-1-Mediated neuroprotection in stroke for middle-aged female rats. PloS One (2014) 9(3):E91427. doi: 10.1371/Journal.Pone.0091427
253. Bake S, Okoreeh A, Khosravian H, Sohrabji F. Insulin-like growth factor (Igf)-1 treatment stabilizes the microvascular cytoskeleton under ischemic conditions. Exp Neurol (2019) 311:162–72. doi: 10.1016/J.Expneurol.2018.09.016
254. Okoreeh AK, Bake S, Sohrabji F. Astrocyte-specific insulin-like growth factor-1 gene transfer in aging female rats improves stroke outcomes. Glia (2017) 65(7):1043–58. doi: 10.1002/Glia.23142
255. Nowrangi DS, Mcbride D, Manaenko A, Dixon B, Tang J, Zhang JH. Rhigf-1 reduces the permeability of the blood-brain barrier following intracerebral hemorrhage in mice. Exp Neurol (2019) 312:72–81. doi: 10.1016/J.Expneurol.2018.11.009
256. Pang Y, Zheng B, Campbell LR, Fan LW, Cai Z, Rhodes PG. Igf-1 can either protect against or increase lps-induced damage in the developing rat brain. Pediatr Res (2010) 67(6):579–84. doi: 10.1203/Pdr.0b013e3181dc240f
257. Nyul-Toth A, Fulop GA, Tarantini S, Kiss T, Ahire C, Faakye JA, et al. Cerebral venous congestion exacerbates cerebral microhemorrhages in mice. Geroscience (2022) 44(2):805–16. doi: 10.1007/S11357-021-00504-0
258. Ungvari Z, Yabluchanskiy A, Tarantini S, Toth P, Kirkpatrick AC, Csiszar A, et al. Repeated valsalva maneuvers promote symptomatic manifestations of cerebral microhemorrhages: Implications for the pathogenesis of vascular cognitive impairment in older adults. Geroscience (2018) 40(5-6):485–96. doi: 10.1007/S11357-018-0044-9
259. Picken MM. The pathology of amyloidosis in classification: A review. Acta Haematol (2020) 143(4):322–34. doi: 10.1159/000506696
260. Guo T, Zhang D, Zeng Y, Huang TY, Xu H, Zhao Y. Molecular and cellular mechanisms underlying the pathogenesis of alzheimer’s disease. Mol Neurodegener (2020) 15(1):40. doi: 10.1186/S13024-020-00391-7
261. Siddappaji KK, Gopal S. Molecular mechanisms in alzheimer’s disease and the impact of physical exercise with advancements in therapeutic approaches. Aims Neurosci (2021) 8(3):357–89. doi: 10.3934/Neuroscience.2021020
262. Piriz J, Muller A, Trejo JL, Torres-Aleman I. Igf-I and the aging mammalian brain. Exp Gerontol (2011) 46(2-3):96–9. doi: 10.1016/J.Exger.2010.08.022
263. Hu X, Yang Y, Gong D. Circulating insulin-like growth factor 1 and insulin-like growth factor binding protein-3 level in alzheimer’s disease: A meta-analysis. Neurol Sci (2016) 37(10):1671–7. doi: 10.1007/S10072-016-2655-1
264. Johansson P, Åberg D, Johansson JO, Mattsson N, Hansson O, Ahrén B, et al. Serum but not cerebrospinal fluid levels of insulin-like growth factor-I (Igf-I) and igf-binding protein-3 (Igfbp-3) are increased in alzheimer’s disease. Psychoneuroendocrinology (2013) 38(9):1729–37. doi: 10.1016/J.Psyneuen.2013.02.006
265. Haywood NJ, Slater TA, Matthews CJ, Wheatcroft SB. The insulin like growth factor and binding protein family: Novel therapeutic targets in obesity & diabetes. Mol Metab (2019) 19:86–96. doi: 10.1016/J.Molmet.2018.10.008
266. Selles MC, Fortuna JTS, Zappa-Villar MF, De Faria YPR, Souza AS, Suemoto CK, et al. Adenovirus-mediated transduction of insulin-like growth factor 1 protects hippocampal neurons from the toxicity of aβ oligomers and prevents memory loss in an Alzheimer mouse model. Mol Neurobiol (2020) 57(3):1473–83. doi: 10.1007/S12035-019-01827-Y
267. Watanabe T, Miyazaki A, Katagiri T, Yamamoto H, Idei T, Iguchi T. Relationship between serum insulin-like growth factor-1 levels and alzheimer’s disease and vascular dementia. J Am Geriatr Soc (2005) 53(10):1748–53. doi: 10.1111/J.1532-5415.2005.53524.X
268. Vardy ER, Rice PJ, Bowie PC, Holmes JD, Grant PJ, Hooper NM. Increased circulating insulin-like growth factor-1 in late-onset alzheimer’s disease. J Alzheimers Dis (2007) 12(4):285–90. doi: 10.3233/Jad-2007-12401
269. Freude S, Schilbach K, Schubert M. The role of igf-1 receptor and insulin receptor signaling for the pathogenesis of alzheimer’s disease: From model organisms to human disease. Curr Alzheimer Res (2009) 6(3):213–23. doi: 10.2174/156720509788486527
270. Moloney AM, Griffin RJ, Timmons S, O’connor R, Ravid R, O’neill C. Defects in igf-1 receptor, insulin receptor and irs-1/2 in alzheimer’s disease indicate possible resistance to igf-1 and insulin signalling. Neurobiol Aging (2010) 31(2):224–43. doi: 10.1016/J.Neurobiolaging.2008.04.002
271. Steen E, Terry BM, Rivera EJ, Cannon JL, Neely TR, Tavares R, et al. Impaired insulin and insulin-like growth factor expression and signaling mechanisms in alzheimer’s disease–is this type 3 diabetes? J Alzheimers Dis (2005) 7(1):63–80. doi: 10.3233/Jad-2005-7107
272. Araki W, Kume H, Oda A, Tamaoka A, Kametani F. Igf-1 promotes beta-amyloid production by a secretase-independent mechanism. Biochem Biophys Res Commun (2009) 380(1):111–4. doi: 10.1016/J.Bbrc.2009.01.044
273. Moll L, Ben-Gedalya T, Reuveni H, Cohen E. The inhibition of igf-1 signaling promotes proteostasis by enhancing protein aggregation and deposition. FASEB J (2016) 30(4):1656–69. doi: 10.1096/Fj.15-281675
274. Cohen E, Bieschke J, Perciavalle RM, Kelly JW, Dillin A. Opposing activities protect against age-onset proteotoxicity. Science (2006) 313(5793):1604–10. doi: 10.1126/Science.1124646
275. Sohrabi M, Floden AM, Manocha GD, Klug MG, Combs CK. Igf-1r inhibitor ameliorates neuroinflammation in an alzheimer’s disease transgenic mouse model. Front Cell Neurosci (2020) 14:200. doi: 10.3389/Fncel.2020.00200
276. Stöhr O, Schilbach K, Moll L, Hettich MM, Freude S, Wunderlich FT, et al. Insulin receptor signaling mediates app processing and B-amyloid accumulation without altering survival in a transgenic mouse model of alzheimer’s disease. Age (Dordr) (2013) 35(1):83–101. doi: 10.1007/S11357-011-9333-2
277. Freude S, Hettich MM, Schumann C, Stöhr O, Koch L, Köhler C, et al. Neuronal igf-1 resistance reduces abeta accumulation and protects against premature death in a model of alzheimer’s disease. FASEB J (2009) 23(10):3315–24. doi: 10.1096/Fj.09-132043
278. Gontier G, George C, Chaker Z, Holzenberger M, Aïd S. Blocking igf signaling in adult neurons alleviates alzheimer’s disease pathology through amyloid-B clearance. J Neurosci (2015) 35(33):11500–13. doi: 10.1523/Jneurosci.0343-15.2015
279. Noureddine S, Saccon T, Rudeski-Rohr T, Gesing A, Mason JB, Schneider A, et al. Gh deficiency confers protective advantages against alzheimer’s disease through rescued mirna expression profile in App/Ps1 mice. Geroscience (2022) 44:2885–93. doi: 10.1007/S11357-022-00633-0
280. Puig KL, Kulas JA, Franklin W, Rakoczy SG, Taglialatela G, Brown-Borg HM, et al. The Ames dwarf mutation attenuates alzheimer’s disease phenotype of App/Ps1 mice. Neurobiol Aging (2016) 40:22–40. doi: 10.1016/J.Neurobiolaging.2015.12.021
281. Kitiyanant N, Kitiyanant Y, Svendsen CN, Thangnipon W. Bdnf-, igf-1- and gdnf-secreting human neural progenitor cells rescue amyloid B-induced toxicity in cultured rat septal neurons. Neurochem Res (2012) 37(1):143–52. doi: 10.1007/S11064-011-0592-1
282. Hou X, Jin Y, Chen J, Hong Y, Luo D, Yin Q, et al. Igf-1 protects against Aβ(25-35)-Induced neuronal cell death Via inhibition of puma expression and bax activation. Neurosci Lett (2017) 637:188–94. doi: 10.1016/J.Neulet.2016.11.012
283. Zhang H, Gao Y, Dai Z, Meng T, Tu S, Yan Y. Igf-1 reduces bace-1 expression in Pc12 cells Via activation of Pi3-K/Akt and Mapk/Erk1/2 signaling pathways. Neurochem Res (2011) 36(1):49–57. doi: 10.1007/S11064-010-0260-X
284. Wang Z, Xiong L, Wang G, Wan W, Zhong C, Zu H. Insulin-like growth factor-1 protects sh-Sy5y cells against B-Amyloid-Induced apoptosis Via the Pi3k/Akt-Nrf2 pathway. Exp Gerontol (2017) 87(Pt A):23–32. doi: 10.1016/J.Exger.2016.11.009
285. Song F, Liu T, Meng S, Li F, Zhang Y, Jiang L. Insulin-like growth factor-1 alleviates expression of Aβ(1-40) and A-, B-, and Γ-secretases in the cortex and hippocampus of App/Ps1 double transgenic mice. J Mol Neurosci (2018) 66(4):595–603. doi: 10.1007/S12031-018-1201-4
286. Lanz TA, Salatto CT, Semproni AR, Marconi M, Brown TM, Richter KE, et al. Peripheral elevation of igf-1 fails to alter abeta clearance in multiple In vivo models. Biochem Pharmacol (2008) 75(5):1093–103. doi: 10.1016/J.Bcp.2007.11.001
287. López-Ortiz S, Valenzuela PL, Seisdedos MM, Morales JS, Vega T, Castillo-García A, et al. Exercise interventions in alzheimer’s disease: A systematic review and meta-analysis of randomized controlled trials. Ageing Res Rev (2021) 72:101479. doi: 10.1016/J.Arr.2021.101479
288. Xu BL, Wang R, Ma LN, Dong W, Zhao ZW, Zhang JS, et al. Comparison of the effects of resveratrol and caloric restriction on learning and memory in juvenile C57bl/6j mice. Iran J Basic Med Sci (2015) 18(11):1118–23.
289. Duong MT, Nasrallah IM, Wolk DA, Chang CCY, Chang TY. Cholesterol, atherosclerosis, and apoe in vascular contributions to cognitive impairment and dementia (Vcid): Potential mechanisms and therapy. Front Aging Neurosci (2021) 13:647990. doi: 10.3389/Fnagi.2021.647990
290. Solomon A, Kivipelto M, Wolozin B, Zhou J, Whitmer RA. Midlife serum cholesterol and increased risk of alzheimer’s and vascular dementia three decades later. Dement Geriatr Cognit Disord (2009) 28(1):75–80. doi: 10.1159/000231980
291. Hofman A, Ott A, Breteler MM, Bots ML, Slooter AJ, Van Harskamp F, et al. Atherosclerosis, apolipoprotein e, and prevalence of dementia and alzheimer’s disease in the Rotterdam study. Lancet (1997) 349(9046):151–4. doi: 10.1016/S0140-6736(96)09328-2
292. Dolan H, Crain B, Troncoso J, Resnick SM, Zonderman AB, Obrien RJ. Atherosclerosis, dementia, and Alzheimer disease in the Baltimore longitudinal study of aging cohort. Ann Neurol (2010) 68(2):231–40. doi: 10.1002/Ana.22055
293. Beach TG, Wilson JR, Sue LI, Newell A, Poston M, Cisneros R, et al. Circle of Willis atherosclerosis: Association with alzheimer’s disease, neuritic plaques and neurofibrillary tangles. Acta Neuropathol (2007) 113(1):13–21. doi: 10.1007/S00401-006-0136-Y
294. Haan MN, Shemanski L, Jagust WJ, Manolio TA, Kuller L. The role of apoe Epsilon4 in modulating effects of other risk factors for cognitive decline in elderly persons. Jama (1999) 282(1):40–6. doi: 10.1001/Jama.282.1.40
295. Csipo T, Lipecz A, Fulop GA, Hand RA, Ngo BN, Dzialendzik M, et al. Age-related decline in peripheral vascular health predicts cognitive impairment. Geroscience (2019) 41(2):125–36. doi: 10.1007/S11357-019-00063-5
296. Gardner AW, Montgomery PS, Wang M, Shen B, Casanegra AI, Silva-Palacios F, et al. Cognitive decrement in older adults with symptomatic peripheral artery disease. Geroscience (2021) 43(5):2455–65. doi: 10.1007/S11357-021-00437-8
297. Istvan L, Czako C, Elo A, Mihaly Z, Sotonyi P, Varga A, et al. Imaging retinal microvascular manifestations of carotid artery disease in older adults: From diagnosis of ocular complications to understanding microvascular contributions to cognitive impairment. Geroscience (2021) 43:1703–23. doi: 10.1007/S11357-021-00392-4
298. De Montgolfier O, Pouliot P, Gillis MA, Ferland G, Lesage F, Thorin-Trescases N, et al. Systolic hypertension-induced neurovascular unit disruption magnifies vascular cognitive impairment in middle-age atherosclerotic ldlr(-/-):Hapob(+/+) mice. Geroscience (2019) 41(5):511–32. doi: 10.1007/S11357-019-00070-6
299. Ban Y, Watanabe T, Miyazaki A, Nakano Y, Tobe T, Idei T, et al. Impact of increased plasma serotonin levels and carotid atherosclerosis on vascular dementia. Atherosclerosis (2007) 195(1):153–9. doi: 10.1016/J.Atherosclerosis.2006.09.005
300. Garcia-Fernandez M, Delgado G, Puche JE, Gonzalez-Baron S, Castilla Cortazar I. Low doses of insulin-like growth factor I improve insulin resistance, lipid metabolism, and oxidative damage in aging rats. Endocrinology (2008) 149(5):2433–42. doi: 10.1210/En.2007-1190
301. Steffensen LB, Conover CA, Oxvig C. Papp-a and the igf system in atherosclerosis: What’s up, what’s down? Am J Physiol Heart Circ Physiol (2019) 317(5):H1039–H49. doi: 10.1152/Ajpheart.00395.2019
302. Higashi Y, Gautam S, Delafontaine P, Sukhanov S. Igf-1 and cardiovascular disease. Growth Horm Igf Res (2019) 45:6–16. doi: 10.1016/J.Ghir.2019.01.002
303. Caicedo D, Diaz O, Devesa P, Devesa J. Growth hormone (Gh) and cardiovascular system. Int J Mol Sci (2018) 19(1):290. doi: 10.3390/Ijms19010290
304. Aguirre GA, Gonzalez-Guerra JL, Espinosa L, Castilla-Cortazar I. Insulin-like growth factor 1 in the cardiovascular system. Rev Physiol Biochem Pharmacol (2018) 175:1–45. doi: 10.1007/112_2017_8
305. Ruidavets JB, Luc G, Machez E, Genoux AL, Kee F, Arveiler D, et al. Effects of insulin-like growth factor 1 in preventing acute coronary syndromes: The prime study. Atherosclerosis (2011) 218(2):464–9. doi: 10.1016/J.Atherosclerosis.2011.05.034
306. De Lorenzo A, Moreira AS, Souza EG, Oliveira GM. Insulin-like growth factor-1 in early-onset coronary artery disease: Insights into the pathophysiology of atherosclerosis. Int J Cardiol (2016) 202:1–2. doi: 10.1016/J.Ijcard.2015.04.032
307. Sukhanov S, Higashi Y, Shai SY, Vaughn C, Mohler J, Li Y, et al. Igf-1 reduces inflammatory responses, suppresses oxidative stress, and decreases atherosclerosis progression in apoe-deficient mice. Arterioscler Thromb Vac Biol (2007) 27(12):2684–90. doi: 10.1161/Atvbaha.107.156257
308. Svensson J, Sjogren K, Levin M, Boren J, Tivesten A, Ohlsson C. Increased diet-induced fatty streak formation in female mice with deficiency of liver-derived insulin-like growth factor-I. Endocrine (2016) 52(3):550–60. doi: 10.1007/S12020-015-0809-1
309. Sivasubramaniyam T, Schroer SA, Li A, Luk CT, Shi SY, Besla R, et al. Hepatic Jak2 protects against atherosclerosis through circulating igf-1. JCI Insight (2017) 2(14):e93735. doi: 10.1172/Jci.Insight.93735
310. Hers I. Insulin-like growth factor-1 potentiates platelet activation Via the Irs/Pi3kalpha pathway. Blood (2007) 110(13):4243–52. doi: 10.1182/Blood-2006-10-050633
311. Von Der Thusen JH, Borensztajn KS, Moimas S, Van Heiningen S, Teeling P, Van Berkel TJ, et al. Igf-1 has plaque-stabilizing effects in atherosclerosis by altering vascular smooth muscle cell phenotype. Am J Pathol (2011) 178(2):924–34. doi: 10.1016/J.Ajpath.2010.10.007
312. Shai SY, Sukhanov S, Higashi Y, Vaughn C, Kelly J, Delafontaine P. Smooth muscle cell-specific insulin-like growth factor-1 overexpression in apoe-/- mice does not alter atherosclerotic plaque burden but increases features of plaque stability. Arterioscler Thromb Vac Biol (2010) 30(10):1916–24. doi: 10.1161/Atvbaha.110.210831
313. Sukhanov S, Higashi Y, Shai SY, Snarski P, Danchuk S, D’ambra V, et al. Sm22alpha (Smooth muscle protein 22-alpha) promoter-driven Igf1r (Insulin-like growth factor 1 receptor) deficiency promotes atherosclerosis. Arterioscler Thromb Vac Biol (2018) 38(10):2306–17. doi: 10.1161/Atvbaha.118.311134
314. Higashi Y, Pandey A, Goodwin B, Delafontaine P. Insulin-like growth factor-1 regulates glutathione peroxidase expression and activity in vascular endothelial cells: Implications for atheroprotective actions of insulin-like growth factor-1. Biochim Biophys Acta (2013) 1832(3):391–9. doi: 10.1016/J.Bbadis.2012.12.005
315. Shai SY, Sukhanov S, Higashi Y, Vaughn C, Rosen CJ, Delafontaine P. Low circulating insulin-like growth factor I increases atherosclerosis in apoe-deficient mice. Am J Physiol Heart Circ Physiol (2011) 300(5):H1898–906. doi: 10.1152/Ajpheart.01081.2010
316. Devesa J, Almenglo C, Devesa P. Multiple effects of growth hormone in the body: Is it really the hormone for growth? Clin Med Insights Endocrinol Diabetes (2016) 9:47–71. doi: 10.4137/Cmed.S38201
317. Isgaard J, Arcopinto M, Karason K, Cittadini A. Gh and the cardiovascular system: An update on a topic At heart. Endocrine (2015) 48(1):25–35. doi: 10.1007/S12020-014-0327-6
318. Colao A. The gh-Igf-I axis and the cardiovascular system: Clinical implications. Clin Endocrinol (Oxf) (2008) 69(3):347–58. doi: 10.1111/J.1365-2265.2008.03292.X
319. Setola E, Monti LD, Lanzi R, Lucotti P, Losa M, Gatti E, et al. Effects of growth hormone treatment on arginine to asymmetric dimethylarginine ratio and endothelial function in patients with growth hormone deficiency. Metabolism (2008) 57(12):1685–90. doi: 10.1016/J.Metabol.2008.07.024
320. Wolters TLC, Netea MG, Hermus A, Smit JWA, Netea-Maier RT. Igf1 potentiates the pro-inflammatory response in human peripheral blood mononuclear cells Via mapk. J Mol Endocrinol (2017) 59(2):129–39. doi: 10.1530/Jme-17-0062
321. Kirilov G, Zacharieva S, Alexandrov AS, Lozanov V, Mitev V. Increased plasma endothelin level as an endothelial marker of cardiovascular risk in patients with active acromegaly: A comparison with plasma homocysteine. Methods Find Exp Clin Pharmacol (2009) 31(7):457–61. doi: 10.1358/Mf.2009.31.7.1406701
322. Fulop GA, Kiss T, Tarantini S, Balasubramanian P, Yabluchanskiy A, Farkas E. Et al. Nrf2 deficiency in aged mice exacerbates cellular senescence promoting cerebrovascular inflammation. Geroscience (2018) 40(5-6):513–21. doi: 10.1007/S11357-018-0047-6
323. Yousefzadeh MJ, Wilkinson JE, Hughes B, Gadela N, Ladiges WC, Vo N, et al. Heterochronic parabiosis regulates the extent of cellular senescence in multiple tissues. Geroscience (2020) 42(3):951–61. doi: 10.1007/S11357-020-00185-1
324. Dorigatti AO, Riordan R, Yu Z, Ross G, Wang R, Reynolds-Lallement N, et al. Brain cellular senescence in mouse models of alzheimer’s disease. Geroscience (2022) 44(2):1157–68. doi: 10.1007/S11357-022-00531-5
325. Csipo T, Lipecz A, Ashpole NM, Balasubramanian P, Tarantini S. Astrocyte senescence contributes to cognitive decline. Geroscience (2020) 42(1):51–5. doi: 10.1007/S11357-019-00140-9
326. Ungvari Z, Tarantini S, Nyul-Toth A, Kiss T, Yabluchanskiy A, Csipo T, et al. Nrf2 dysfunction and impaired cellular resilience to oxidative stressors in the aged vasculature: From increased cellular senescence to the pathogenesis of age-related vascular diseases. Geroscience (2019) 41(6):727–38. doi: 10.1007/S11357-019-00107-W
327. Kiss T, Nyul-Toth A, Delfavero J, Balasubramanian P, Tarantini S, Faakye J, et al. Spatial transcriptomic analysis reveals inflammatory foci defined by senescent cells in the white matter, hippocampi and cortical grey matter in the aged mouse brain. Geroscience (2022) 44(2):661–81. doi: 10.1007/S11357-022-00521-7
328. Kiss T, Nyul-Toth A, Balasubramanian P, Tarantini S, Ahire C, Delfavero J, et al. Single-cell rna sequencing identifies senescent cerebromicrovascular endothelial cells in the aged mouse brain. Geroscience (2020) 42(2):429–44. doi: 10.1007/S11357-020-00177-1
329. Childs BG, Zhang C, Shuja F, Sturmlechner I, Trewartha S, Fierro Velasco R, et al. Senescent cells suppress innate smooth muscle cell repair functions in atherosclerosis. Nat Aging (2021) 1(8):698–714. doi: 10.1038/S43587-021-00089-5
330. Childs BG, Baker DJ, Wijshake T, Conover CA, Campisi J, Van Deursen JM. Senescent intimal foam cells are deleterious At all stages of atherosclerosis. Science (2016) 354(6311):472–7. doi: 10.1126/Science.Aaf6659
331. Gluchowska A, Cysewski D, Baj-Krzyworzeka M, Szatanek R, Weglarczyk K, Podszywalow-Bartnicka P, et al. Unbiased proteomic analysis of extracellular vesicles secreted by senescent human vascular smooth muscle cells reveals their ability to modulate immune cell functions. Geroscience (2022) 44:2863–84. doi: 10.1007/S11357-022-00625-0
332. Thum T, Hoeber S, Froese S, Klink I, Stichtenoth DO, Galuppo P, et al. Age-dependent impairment of endothelial progenitor cells is corrected by growth-Hormone-Mediated increase of insulin-like growth-Factor-1. Circ Res (2007) 100(3):434–43. doi: 10.1161/01.Res.0000257912.78915.Af
333. Dal J, Skov BG, Andersen M, Feldt-Rasmussen U, Feltoft CL, Karmisholt J, et al. Sex differences in acromegaly At diagnosis: A nationwide cohort study and meta-analysis of the literature. Clin Endocrinol (Oxf) (2021) 94(4):625–35. doi: 10.1111/Cen.14392
334. Liu Z, Mohan S, Yakar S. Does the Gh/Igf-1 axis contribute to skeletal sexual dimorphism? evidence from mouse studies. Growth Horm Igf Res (2016) 27:7–17. doi: 10.1016/J.Ghir.2015.12.004
335. Sohrabji F. Estrogen-Igf-1 interactions in neuroprotection: Ischemic stroke as a case study. Front Neuroendocrinol (2015) 36:1–14. doi: 10.1016/J.Yfrne.2014.05.003
336. Brown-Borg HM, Borg KE, Meliska CJ, Bartke A. Dwarf mice and the ageing process. Nature (1996) 384(6604):33. doi: 10.1038/384033a0
337. Coschigano KT, Holland AN, Riders ME, List EO, Flyvbjerg A, Kopchick JJ. Deletion, but not antagonism, of the mouse growth hormone receptor results in severely decreased body weights, insulin, and insulin-like growth factor I levels and increased life span. Endocrinology (2003) 144(9):3799–810. doi: 10.1210/En.2003-0374
338. Bokov AF, Garg N, Ikeno Y, Thakur S, Musi N, Defronzo RA, et al. Does reduced igf-1r signaling in Igf1r+/- mice alter aging? PloS One (2011) 6(11):E26891. doi: 10.1371/Journal.Pone.0026891
339. Ceylan-Isik AF, Li Q, Ren J. Insulin-like growth factor I (Igf-1) deficiency ameliorates sex difference in cardiac contractile function and intracellular Ca(2+) homeostasis. Toxicol Lett (2011) 206(2):130–8. doi: 10.1016/J.Toxlet.2011.07.001
340. Selvamani A, Sohrabji F. The neurotoxic effects of estrogen on ischemic stroke in older female rats is associated with age-dependent loss of insulin-like growth factor-1. J Neurosci (2010) 30(20):6852–61. doi: 10.1523/Jneurosci.0761-10.2010
341. Munive V, Santi A. Torres-Aleman i. a concerted action of estradiol and insulin like growth factor I underlies sex differences in mood regulation by exercise. Sci Rep (2016) 6:25969. doi: 10.1038/Srep25969
Keywords: dementia, microbleed, hormonal, humoral, ageing
Citation: Bickel MA, Csik B, Gulej R, Ungvari A, Nyul-Toth A and Conley SM (2023) Cell non-autonomous regulation of cerebrovascular aging processes by the somatotropic axis. Front. Endocrinol. 14:1087053. doi: 10.3389/fendo.2023.1087053
Received: 01 November 2022; Accepted: 04 January 2023;
Published: 23 January 2023.
Edited by:
Michal Masternak, University of Central Florida, United StatesReviewed by:
Dora Reglodi, University of Pécs, HungaryJoshua Thomas Butcher, Oklahoma State University, United States
Fan Fan, University of Mississippi Medical Center, United States
Copyright © 2023 Bickel, Csik, Gulej, Ungvari, Nyul-Toth and Conley. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shannon M. Conley, U2hhbm5vbi1jb25sZXlAb3Voc2MuZWR1
†These authors have contributed equally to this work
 Marisa A. Bickel
Marisa A. Bickel Boglarka Csik
Boglarka Csik Rafal Gulej
Rafal Gulej Anna Ungvari3,4
Anna Ungvari3,4 Adam Nyul-Toth
Adam Nyul-Toth Shannon M. Conley
Shannon M. Conley