- Department of Endocrinology, Sher-I-Kashmir Institute of Medical Sciences, Srinagar, Kashmir, India
Sheehan syndrome (SS) caused by postpartum hemorrhage leads to partial or complete pituitary hormone deficiency. In addition to lipid and glucose abnormalities, patients with SS have increased body fat, insulin resistance (IR), coagulation abnormalities, increased leptin concentration, low-grade inflammation, and endothelial dysfunction that predispose them to cardiovascular diseases. Untreated growth hormone (GH) deficiency, hypogonadism, and excess glucocorticoid use are considered risk factors for these abnormalities. Compared to other hypopituitary subjects, patients with SS are younger and have a longer duration of disease and severe GH deficiency. Replacement with GH in addition to standard hormone replacement improves their cardiometabolic profile.
Introduction
Sheehan syndrome (SS) also known as postpartum pituitary necrosis, though rare in developed countries, is one of the common causes of hypopituitarism in developing nations (1). It is primarily caused by vasospasm of hypothalamic portal vessels following massive postpartum hemorrhage (PPH), resulting in complete or partial loss of anterior pituitary gland cells. In addition, small sella turcica size, coagulation abnormalities and the presence of anti-pituitary antibodies contribute to ischemic damage to pituitary gland. Somatotroph and thyrotroph cell loss occurs in almost all patients, with preservation or recovery of gonadotroph, lactotroph, and corticotroph cell function in few (2–4). The duration from the onset of disease to diagnosis usually ranges from 7-19 years (2, 3). SS presents with long-standing non-specific symptoms of fatigue and generalized body aches. The major clinical features of typical and complete SS include lactation failure, failure of resumption of menstrual cycles after the puerperium, loss of pubic and axillary hair, symptoms of hypothyroidism, and hypocortisolism (1, 5). Less common manifestations of SS include hematological abnormalities (like anemia and pancytopenia), cardiac abnormalities (like cardiomyopathy and ventricular arrhythmias), and neuropsychiatric abnormalities (like psychosis) (6–9).
Metabolic abnormalities in patients with SS are a recent focus of attention. Patients with SS have increased body fat, insulin resistance (IR), dyslipidemia, coagulation abnormalities, increased leptin, low-grade inflammation, endothelial dysfunction (ED), and non-alcoholic fatty liver disease (NAFLD) (6, 10–14). Many of these effects are attributed to low insulin-like growth factor-1(IGF-1), hypogonadism, untreated secondary hypothyroidism, and glucocorticoid (GC) overuse (11). These conventional (age and dyslipidemia) and non-conventional (increased inflammatory markers and leptin) risk factors promote cardiovascular (CV) diseases in the general population as well as in hypopituitary patients like those with SS (15–19). Studies published in late 1900, which also recruited patients with SS, suggested that hypopituitary patients have increased mortality than the general population and predominantly died of CV diseases with an overall standardized mortality ratio (SMR) of 1.99 (15, 20, 21). Onset of hypopituitarism at a younger age and female gender were associated with higher SMR (20).
Epidemiology of SS
The prevalence of SS is variable. In a population based study from Iceland, SS was diagnosed in 5.1 individuals per 100,000 population (22), whereas the prevalence of SS among 11,700 women >20 years of age was around 3% in northern India (23). In developed nations, up to 6% of hypopituitary subjects are diagnosed with SS (15, 24) while in countries like Turkey and Pakistan, up to one out of three cases of hypopituitarism is attributed to SS (25, 26). The lower prevalence of SS in developed countries is a result of better obstetric care facilities in these countries and possibly disease unawareness and missed diagnosis.
Risk factors of CV diseases in hypopituitarism and SS
Hypopituitary patients, including persons with SS have higher mortality rates than the general population, which is attributed to increased CV disease, strokes and malignancy (15, 20, 27). The risk factors for increased mortality include younger age at diagnosis, female gender, diagnosis of craniopharyngioma, radiation therapy, transcranial surgery, diabetes insipidus, and hypogonadism. Dyslipidemia, ED and radiation induced vascular damage predispose these patients to higher risk of CV diseases (17, 28). Untreated GH deficiency (GHD) leading to IR, dyslipidemia and ED is the primary driver of increased CV mortality in hypopituitary subjects, who are adequately replaced with GC, thyroid and sex hormones (15, 28). In addition over-replacement with GC (29, 30) and sex steroid deficiency contribute to impaired metabolic parameters and atherogenesis in such patients (15). In a large study, atherosclerotic plaques in carotid arteries were present in half of the patients with hypopituitarism (31). Coronary flow reserve (CFR), as measured by transthoracic color echocardiography is a simple measurement of blood flow in coronary arteries, is impaired in subjects with GH deficiency, and corelates with serum IGF-1 concentration (32). Coronary artery calcification (CAC), a surrogate of coronary atherosclerosis was documented in around 50% of hypopituitary patients (33).
Compared to other hypopituitary subjects, patients with SS are younger in age, have longer duration of hypopituitarism, have severe GH/IGF-1 deficiency and decreased lean body mass (34). Though no long term data is available on CV mortality in SS, a large series of hypopituitary patients (which also enrolled few patients of SS) observed an increased mortality and morbidity primarily related to CV diseases in such patients (20, 35, 36). Two recent studies have demonstrated high frequency of coronary calcium deposits in women with SS. In one study enrolling 30 patients and an equal number of age and BMI matched controls, CAC score of >10 was documented in 32% of women compared with age/BMI matched controls (37), while in another study enrolling 60 patients of SS and 35, age and BMI matched controls, CAC score of >10 was documented in 27% of patients against 1.6% in controls (38).
Obesity and dyslipidemia
As is true with hypopituitarism of other etiologies, patients with SS in comparison to age and gender matched population have increased body mass index (BMI) and total body fat with predominant abdominal fat deposition. Obesity and increased fat mass persisted after replacement with thyroxine and GC (10, 11). GH/IGF-1 deficiency appears to be the predominant contributor for abdominal obesity in such patients. GH impairs generation of active cortisol from inactive cortisone by inhibiting 11β-hydroxysteroid dehydrogenase type 1 (11β-HSD1). In presence of GHD, up regulation of 11β-HSD1 enzyme contributes to increased conversion of inactive cortisol to active cortisol. This phenomenon results in marked weight gain in hypopituitary patients with GHD even on small doses of GC (39). In one study, patients with SS had higher fat mass compared to other hypopituitary patients despite similar BMI, and waist circumference (WC) (34). Inspite of thyroxine and GC replacement, patients with SS have adverse lipid parameters like increased serum total cholesterol (TC), low density lipoprotein cholesterol (LDL-C) and triglycerides (TG) and low high density lipoprotein cholesterol (HDL-C). These lipid abnormalities are attributed to persistent GHD (10, 13) and replacement with GH improves these lipid abnormalities (11, 40).
Metabolic syndrome and insulin resistance
In a large population of hypopituitary patients (not on GH replacement), half had BMI ≥30 kg/m2 and 86% had central obesity (defined as WC ≥94 cms in men and ≥90 cms in women). Around one-third were treated for hypertension and dyslipidemia (16). Similarly, a high prevalence of metabolic syndrome (MS) (50%) was observed in KIMS (Pfizer International Metabolic) database of 2479 patients with severe adult-onset GHD, naïve to GH replacement (41).
The prevalence of MS in SS is around 50% with major constituents being increased WC, decreased HDL-C and increased TG. Most of these abnormalities are a consequence of GHD, GC therapy and hypogonadism, all these factors favour abdominal fat deposition and atherogenic dyslipidemia. Around one fourth of patients with SS had diabetes mellitus and fasting and post-meal glucose values were high. Homeostasis model assessment-insulin resistance (HOMA-IR), an index of IR was high in large group of women with SS compared to healthy controls (10). Contrary to beneficial effects of GH replacement on lipids and body fat, glucose tolerance may worsen transiently after GH replacement and is attributed to insulin antagonistic effect of GH. The glucose increasing tendency of GH usually is greater in females and those receiving higher doses (11, 40, 42). The effect of GH replacement for 24 months on lipid profile, carotid intimal medial thickness (CIMT), glucose metabolism and visceral fat was studied in ten patients of SS and an equal number of controls matched for age and BMI. GH treatment had a favourable effect on CIMT, lipids and visceral fat. Despite change in body composition there was a tendency towards development of abnormal glucose tolerance (40). In a study recruiting 91 patients with SS, and were treated with GH for 24 months, blood glucose increased after 1 year but returned to normal levels at 2 years of treatment (40). These studies are limited by relatively short duration of follow-up and long-term consequences are not known. In addition to GH deficiency, excess GC replacement worsens metabolic profile of hypopituitary subjects which increases their risk for CV diseases (43).
Chronic inflammation and endothelial dysfunction
Chronic low-grade inflammation, documented by increased high sensitive C-reactive protein (hsCRP), considered as coronary artery disease (CAD) risk enhancer is a better predictor of risk of CV events than LDL-C (44). In a cross-sectional study that enrolled 53 women with hypopituitarism and 111 healthy control women, interleukin-6 (IL-6) and CRP concentration were significantly higher in women with hypopituitarism than in healthy controls and were attributed to GH and estrogen deficiency (45). In another study enrolling 47 hypopituitary patients and 37 age, gender and BMI matched controls had higher hs-CRP levels despite lower levels of blood glucose and insulin resistance (46).
Likewise, in a study enrolling 30 patients with SS, on thyroxine and GC replacement but GH naïve, had higher hsCRP concentration compared to the healthy controls which correlated to insulin, HOMA-IR, HDL-C and IGF-1 (10). This inflammation in hypopituitary states like SS is attributed to GHD, hypogonadism, obesity and excess GC use (45). It is hypothesised that GH directly stimulates anti-inflammatory cells by cytokine receptors and indirectly through central redistribution of fat as visceral adipocytes which in turn release pro inflammatory markers like interleukin-6 in circulation (47). Replacement of GH in women with SS decreases hsCRP independent of improvement in serum lipids and lean body mass (48).
Endothelial dysfunction defined as change in vascular endothelium from antithrombotic to pro thrombotic state is considered an early marker for atherosclerosis which is detected before structural changes in the vessel wall are apparent on angiography or ultrasound (49). ED can be quantified by measuring flow mediated dilation (FMD) of brachial artery, CIMT or by quantifying serum adhesion molecules like Intercellular adhesion molecule-1 (ICAM-1), vascular cell adhesion molecule-1 (VCAM-1) and fibrinogen (50). Smith et al. studied 32 patients with hypopituitarism and observed that FMD of brachial artery was decreased compared to healthy subjects and GH replacement for six months improved the FMD along with other parameters of arterial stiffness (51). Also serum ICAM-1 and E-selectin were higher in GH deficient hypopituitary subjects (n=52) than healthy controls (n=54) which negatively correlated with IGF-1 concentrations (52). In another study enrolling GH naïve 30 patients with SS and equal number of matched healthy controls, serum ICAM-1 and VCAM-1 were increased in SS patients compared to age and BMI matched controls (10).
Non-alcoholic fatty liver disease
Overall recent trends suggest that hypopituitary patients have higher prevalence of NAFLD owing to GHD, low thyroid hormones and low sex steroids (53). The prevalence of NAFLD varies from 54-70% on ultrasonography (54, 55). Similarly, patients with SS have higher prevalence of NAFLD compared with age and BMI matched controls. In a recent case control study, Das et al. studied 60 patients with SS for steatosis and liver stiffness, as measured by transient elastography. Hepatic steatosis of different grades was seen in 63% (with 50% having severe steatosis compared to 30% of controls). The mean-controlled attenuation parameter (CAP) score, a measure of hepatic steatosis, was significantly higher in patients with SS compared with age and BMI-matched controls. BMI and GH deficiency were two strong predictors of steatosis. In two such patients with biopsy-proven severe hepatic steatosis, the GH replacement resulted in complete resolution of steatosis (14).
Effect of treatment on CV risk factors
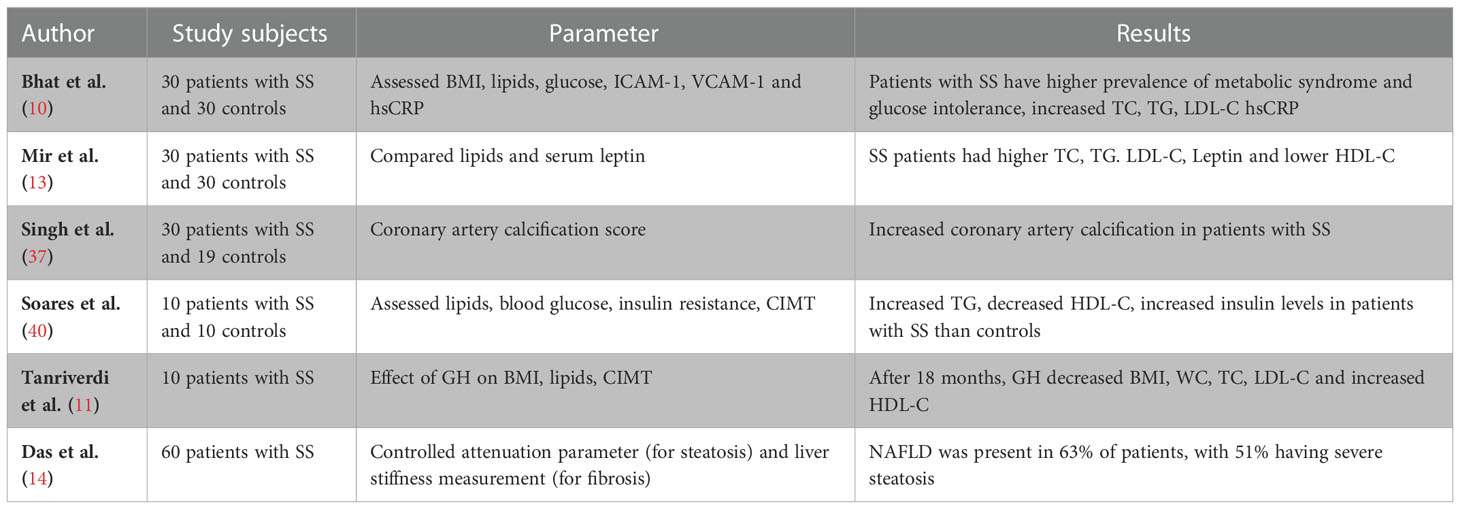
Like other patients with hypopituitarism, treatment of SS patients with GH results in significant improvement in lipid parameters, lean body mass, endothelial function, arterial stiffness and exercise capacity (18, 50, 56–58) resulting in improved mortality benefits (20). In one study, 14 patients of SS treated with GH for 18 months, resulted in improvement in body composition (like decrease WC and waist to hip ratio) and lipid abnormalities (like decrease in TC, LDL-C, TG and increase in HDL-C) (11). Similarly in another study enrolling 10 patients with SS, replacement with GH for 24 months lead to reduction in the relation ApoB/ApoA ratio, increase in HDL-C, decrease in CIMT and visceral fat (40). In another large study enrolling 91 patients with SS, GH replacement for 24 months (after adequate thyroxine/GC replacement), improved lean body mass, TC and LDL-C but no improvement in WC and waist hip ratio was detected (34). Duration of treatment for less than 12 months in patients with hypopituitarism suggest that GH replacement has significant benefits on lean body mass and lipid parameters, at the cost of modestly decreased insulin sensitivity, predominantly in men (42). However, studies with longer duration of GH replacement suggest that insulin sensitivity does not decrease after GH replacement (34, 59) and the acute worsening of IR may be related to higher dose of GH prescribed (60). Likewise, it was observed that GH replacement for 24 months improved liver enzymes and markers of fibrosis in patients with hypopituitarism and NAFLD (61). It has also been observed that sympathetic tone is decreased in patients with SS and GH replacement in these patients improves sympathetic tone and normalizes sympathovagal balance after 6 and 12 months of treatment (62). Table 1 summarises the studies on metabolic abnormalities in SS.
Conclusion
In addition to presence of conventional risk factors for CV diseases, SS patients have increased body fat, higher inflammatory and ED markers, which are directly correlated to GH/IGF-1 deficiency. Replacement of GH may ameliorate some of these risks, though at the cost of increased glucose tolerance. However, GH replacement may not be easily affordable in developing nations. But efforts should be made by government with the help of other non-governmental organizations, to provide GH at reasonable cost.
Author contributions
BL and MB designed the study and contributed to the manuscript. BL and MB wrote the manuscript and reviewed the pertinent literature. Both authors contributed to the article and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Karaca Z, Laway BA, Dokmetas HS, Atmaca H, Kelestimur F. Sheehan syndrome. Nat Rev Dis Primers (2016) 2(1):16092. doi: 10.1038/nrdp.2016.92
2. Laway BA, Misgar RA, Mir SA, Wani AI. Clinical, hormonal and radiological features of partial sheehan’s syndrome: An Indian experience. Arch Endocrinol Metab (2016) 60(2):125–9. doi: 10.1590/2359-3997000000137
3. Laway BA, Mir SA, Gojwari TA, Shah TR, Zargar AH. Selective preservation of anterior pituitary functions in patients with sheehan’s syndrome. Ind J Endocrinol Metab (2011) 15(3):238–41. doi: 10.4103/2230-8210.84874
4. Gei-Guardia O, Soto-Herrera E, Gei-Brealey A, Chen-Ku CH. Sheehan syndrome in Costa Rica: clinical experience with 60 cases. Endocr Pract (2011) 17(3):337–44. doi: 10.4158/EP10145.OR
5. Du GL, Liu ZH, Chen M, Ma R, Jiang S, Shayiti M, et al. Sheehan’s syndrome in xinjiang: Clinical characteristics and laboratory evaluation of 97 patients. Hormones (2015) 14(4):660–7. doi: 10.14310/horm.2002.1624
6. Laway BA, Mir SA, Bhat JR, Lone MI, Samoon J, Zargar AH. Hematological response of pancytopenia to glucocorticoids in patients with sheehan’s syndrome. Pituitary (2012) 15(2):184–7. doi: 10.1007/s11102-011-0304-5
7. Laway BA, Ramzan M, Allai MS, Wani AI, Misgar RA. Cardiac structural and functional abnormalities in females with untreated hypopituitarism due to sheehan syndrome: response to hormone replacement therapy. Endocr Pract (2016) 22(9):1096–103. doi: 10.4158/EP161262.OR
8. Laway BA, Alai MS, Gojwari T, Ganie MA, Zargar AH. Sheehan syndrome with reversible dilated cardiomyopathy. Ann Saudi Med (2010) 30(4):321–4. doi: 10.4103/0256-4947.65269
9. Laway BA, Shah TR, Bashir MI, Hussain A, Zargar AH. Acute onset psychosis following steroid replacement in sheehan syndrome. Acta Endo (Buc) (2010) 6(4):533–8. doi: 10.4183/aeb.2010.533
10. Bhat MA, Laway BA, Shah ZA, Wani AI, Mubarik I. Insulin resistance, metabolic syndrome and chronic low grade inflammation in sheehan’s syndrome on standard replacement therapy: A case control study. Pituitary (2015) 18(3):312–28. doi: 10.1007/s11102-014-0575-8
11. Tanriverdi F, Unluhizarci K, Kula M, Guven M, Bayram F, Kelestimur F. Effects of 18-month of growth hormone (GH) replacement therapy in patients with sheehan’s syndrome. Growth Horm IGF Res (2005) 15(3):231–7. doi: 10.1016/j.ghir.2005.03.005
12. Pasa S, Altintas A, Tumer C, Demircin M, Cil T, Bayan K, et al. Prothrombin time, activated thromboplastin time, fibrinogen and d-dimer levels and von-willebrand activity of patients with sheehan’s syndrome and the effect of hormone replacement therapy on these factors. Int J Hematol Oncol (2010) 20:212–9.
13. Mir SA, Shah T, Singh H, Shabir I, Laway BA. Serum lipid and leptin concentrations in patients with sheehan syndrome. Ind J Endocrinol Metab (2018) 22(4):466–8. doi: 10.4103/ijem.IJEM_23_18
14. Das L, Sahoo J, Dahiya N, Taneja S, Bhadada SK, Bhat MH, et al. Long-term hepatic and cardiac health in patients diagnosed with sheehan’s syndrome. Pituitary (2022) 25(6):971–81. doi: 10.1007/s11102-022-01282-4
15. Tomlinson JW, Holden N, Hills RK, Wheatley K, Clayton RN, Bates AS, et al. Association between premature mortality and hypopituitarism. West Midlands prospective hypopituitary study group. Lancet (2001) 357(9254):425–31. doi: 10.1016/S0140-6736(00)04006-X
16. Deepak D, Furlong NJ, Wilding JPH, MacFarlane IA. Cardiovascular disease, hypertension, dyslipidemia and obesity in patients with hypothalamic-pituitary disease. Postgrad Med J (2007) 83(978):277–80. doi: 10.1136/pgmj.2006.052241
17. Verhelst J, Abs R. Cardiovascular risk factors in hypopituitary GH-deficient adults. Eur J Endocrinol (2009) 161(1):41–9. doi: 10.1530/EJE-09-0291
18. Ratku B, Sebestyén V, Erdei A, Nagy EV, Szabó Z, Somodi S. Effects of adult growth hormone deficiency and replacement therapy on the cardiometabolic risk profile. Pituitary (2022) 25(2):211–28. doi: 10.1007/s11102-022-01207-1
19. Abdu TAM, Neary R, Elhadd TA, Akber M, Clayton RN. Coronary risk in growth hormone deficient hypopituitary adults: increased predicted risk is due largely to lipid profile abnormalities. Clin Endocrinol (Oxf) (2001) 55(2):209–16. doi: 10.1046/j.1365-2265.2001.01320.x
20. Pappachan JM, Raskauskiene D, Kutty VR, Clayton RN. Excess mortality associated with hypopituitarism in adults: a meta-analysis of observational studies. J Clin Endocrinol Metab (2015) 100(4):1405–11. doi: 10.1210/jc.2014-3787
21. McCallum RW, Petrie JR, Dominiczak AF, Connell JMC. Growth hormone deficiency and vascular risk. Clin Endocrinol (Oxf) (2002) 57(1):11–24. doi: 10.1046/j.1365-2265.2002.01559.x
22. Kristjansdottir HL, Bodvarsdottir SP, Sigurjonsdottir HA. Sheehan’s syndrome in modern times: a nationwide retrospective study in Iceland. Eur J Endocrinol (2011) 164(3):349–54. doi: 10.1530/EJE-10-1004
23. Zargar AH, Singh B, Laway BA, Masoodi SR, Wani AI, Bashir MI. Epidemiologic aspects of postpartum pituitary hypofunction (Sheehan’s syndrome). Fertil Steril (2005) 84(2):523–8. doi: 10.1016/j.fertnstert.2005.02.022
24. Regal M, Páramo C, Sierra JM, García-Mayor RV. Prevalence and incidence of hypopituitarism in an adult Caucasian population in northwestern Spain. Clin Endocrinol (Oxf) (2001) 55(6):735–40. doi: 10.1046/j.1365-2265.2001.01406.x
25. Tanriverdi F, Dokmetas HS, Kebapcı N, Kilicli F, Atmaca H, Yarman S, et al. Etiology of hypopituitarism in tertiary care institutions in Turkish population: Analysis of 773 patients from pituitary study group database. Endocrine (2014) 47(1):198–205. doi: 10.1007/s12020-013-0127-4
26. Malik S, Kiran Z, Rashid MO, Mawani M, Gulab A, Masood MQ, et al. Hypopituitarism other than sellar and parasellar tumors or traumatic brain injury assessed in a tertiary hospital. Pak J Med Sci (2019) 35(4):1149–54. doi: 10.12669/pjms.35.4.174
27. Rosen T, Bengtsson BA. Premature mortality due to cardiovascular disease in hypopituitarism. Lancet (1990) 336(8710):285–8. doi: 10.1016/0140-6736(90)91812-O
28. Jasim S, Alahdab F, Ahmed AT, Tamhane S, Prokop LJ, Nippoldt TB, et al. Mortality in adults with hypopituitarism: A systematic review and meta-analysis. Endocrine (2017) 56(1):33–42. doi: 10.1007/s12020-016-1159-3
29. Peacey SR, Guo CY, Robinson AM, Price A, Giles MA, Eastell R, et al. Glucocorticoid replacement therapy: Are patients over treated and does it matter? Clin Endocrinol (Oxf) (1997) 46(3):255–61. doi: 10.1046/j.1365-2265.1997.780907.x
30. Behan LA, Carmody D, Rogers B, Hannon MJ, Davenport C, Tormey W, et al. Low-dose hydrocortisone replacement is associated with improved arterial stiffness index and blood pressure dynamics in severely adrenocorticotrophin-deficient hypopituitary male patients. Eur J Endocrinol (2016) 174(6):791–9. doi: 10.1530/EJE-15-1187
31. Kim JY, Hong JW, Rhee SY, Kim CS, Kim DJ, Lee EJ. Carotid atheromatic plaque is commonly associated with hypopituitary men. Pituitary (2011) 14(2):105–11. doi: 10.1007/s11102-010-0265-0
32. Oflaz H, Sen F, Elitok A, Cimen AO, Onur I, Kasikcioglu E, et al. Coronary flow reserve is impaired in patients with adult growth hormone deficiency. Clin Endocrinol (Oxf) (2007) 66(4):524–9. doi: 10.1111/j.1365-2265.2007.02767.x
33. Cannavo S, Marini F, Curto L, Torre ML, de Gregorio C, Salamone I, et al. High prevalence of coronary calcifications and increased risk for coronary heart disease in adults with growth hormone deficiency. J Endocrinol Invest (2011) 34(1):32–7. doi: 10.1007/BF03346692
34. Kelestimur F, Jonsson P, Molvalilar S, Gomez JM, Auernhammer CJ, Colak R, et al. Sheehan’s syndrome: Baseline characteristics and effect of 2 years of growth hormone replacement therapy in 91 patients in KIMS - pfizer international metabolic database. Eur J Endocrinol (2005) 152(4):581–7. doi: 10.1530/eje.1.01881
35. Bülow B, Hagmar L, Eskilsson J, Erfurth EM. Hypopituitary females have a high incidence of cardiovascular morbidity and an increased prevalence of cardiovascular risk factors. J Clin Endocrinol Metab (2000) 85(2):574–84. doi: 10.1210/jcem.85.2.6346
36. Colao A, Di Somma C, Savanelli MC, De Leo M, Lombardi G. Beginning to end: cardiovascular implications of growth hormone (GH) deficiency and GH therapy. Growth Horm IGF Res (2006) 16:41–8. doi: 10.1016/j.ghir.2006.03.006
37. Singh H, Afroze M, Shafi N, Bhat JA, Kawa IA, Laway BA, et al. Prevalence of coronary calcium deposits in sheehan’s syndrome patients on long term replacement treatment. Pituitary (2022) 25(1):92–9. doi: 10.1007/s11102-021-01174-z
38. Laway BA, Rasool A, Baba MS, Misgar RA, Bashir MI, Wani AI, et al. High prevalence of coronary artery calcification and increased risk for coronary artery disease in patients with sheehan syndrome- a case control study. Clin Endocrinol (Oxf) (2022) 25. doi: 10.1111/cen.14871
39. Toogood AA, Taylor NF, Shalet SM, Monson JP. Modulation of cortisol metabolism by low-dose growth hormone replacement in elderly hypopituitary patients. J Clin Endocrinol Metab (2000) 85(4):1727–30. doi: 10.1210/jc.85.4.1727
40. Soares DV, Spina LD, de Lima Oliveira Brasil RR, Lobo PM, Salles E, Coeli CM, et al. Two years of growth hormone replacement therapy in a group of patients with sheehan’s syndrome. Pituitary (2006) 9(2):127–35. doi: 10.1007/s11102-006-9990-9
41. Verhelst J, Mattsson AF, Luger A, Thunander M, Góth MI, Koltowska-Häggström M, et al. Prevalence and characteristics of the metabolic syndrome in 2479 hypopituitary patients with adult-onset GH deficiency before GH replacement: A KIMS analysis. Eur J Endocrinol (2011) 165(6):881–9. doi: 10.1530/EJE-11-0599
42. Maison P, Griffin S, Nicoue-Beglah M, Haddad N, Balkau B, Chanson P, et al. Impact of growth hormone (GH) treatment on cardiovascular risk factors in GH-deficient adults: A metaanalysis of blinded, randomized, placebo-controlled trials. J Clin Endocrinol Metab (2004) 89(5):2192–219. doi: 10.1210/jc.2003-030840
43. Filipsson H, Monson JP, Koltowska-Häggström M, Mattsson A, Johannsson G. The impact of glucocorticoid replacement regimens on metabolic outcome and comorbidity in hypopituitary patients. J Clin Endocrinol Metab (2006) 91(10):3954–61. doi: 10.1210/jc.2006-0524
44. Ridker PM, Koenig W, Kastelein JJ, Mach F, Lüscher TF. Has the time finally come to measure hsCRP universally in primary and secondary cardiovascular prevention? Eur Heart J (2018) 39(46):4109–11. doi: 10.1093/eurheartj/ehy723
45. Sesmilo G, Miller KK, Hayden D, Klibanski A. Inflammatory cardiovascular risk markers in women with hypopituitarism. J Clin Endocrinol Metab (2001) 86(12):5774–81. doi: 10.1210/jcem.86.12.8087
46. Castillo AR, Zantut-Wittmann DE, Neto AM, Jales RM, Garmes HM. Panhypopituitarism without GH replacement: About insulin sensitivity, CRP levels, and metabolic syndrome. Horm Metab Res (2018) 50(9):690–5. doi: 10.1055/a-0649-8010
47. Auernhammer CJ, Strasburger CJ. Effects of growth hormone and insulin-like growth factor I on the immune system. Eur J Endocrinol (1995) 133(6):635–45. doi: 10.1530/eje.0.1330635
48. Bollerslev J, Ueland T, Jørgensen AP, Fougner KJ, Wergeland R, Schreiner T, et al. Positive effects of a physiological dose of GH on markers of atherogenesis: A placebo-controlled study in patients with adult-onset GH deficiency. Eur J Endocrinol (2006) 154(4):537–43. doi: 10.1530/eje.1.02125
49. Davignon J, Ganz P. Role of endothelial dysfunction in atherosclerosis. Circulation (2004) 109(23):111–27. doi: 10.1161/01.CIR.0000131515.03336.f8
50. Carrero JJ, Kyriazis J, Sonmez A, Tzanakis I, Qureshi AR, Stenvinkel P, et al. Prolactin levels, endothelial dysfunction, and the risk of cardiovascular events and mortality in patients with CKD. Clin J Am Soc Nephrol (2012) 7(2):207–15. doi: 10.2215/CJN.06840711
51. Smith JC, Evans LM, Wilkinson I, Goodfellow J, Cockcroft JR, Scanlon MF, et al. Effects of GH replacement on endothelial function and large-artery stiffness in GH-deficient adults: A randomized, double-blind, placebo-controlled study. Clin Endocrinol (Oxf) (2002) 56(4):493–501. doi: 10.1046/j.1365-2265.2002.01514.x
52. Elhadd TA, Abdu TA, Oxtoby J, Kennedy G, McLaren M, Neary R, et al. Biochemical and biophysical markers of endothelial dysfunction in adults with hypopituitarism and severe GH deficiency. J Clin Endocrinol Metab (2001) 86(9):4223–32. doi: 10.1210/jcem.86.9.7813
53. Zhang X, Tian H, Li Y. The pathophysiological mechanism between hypopituitarism and nonalcoholic fatty liver disease. iLIVER (2022) 1(1):65–71. doi: 10.1016/j.iliver.2022.02.004
54. Yuan XX, Zhu HJ, Pan H, Chen S, Liu ZY, Li Y, et al. Clinical characteristics of non-alcoholic fatty liver disease in Chinese adult hypopituitary patients. World J Gastroenterol (2019) 25(14):1741–52. doi: 10.3748/wjg.v25.i14.1741
55. Hong JW, Kim JY, Kim YE, Lee EJ. Metabolic parameters and nonalcoholic fatty liver disease in hypopituitary men. Horm Metab Res (2011) 43(1):48–54. doi: 10.1055/s-0030-1265217
56. Gonzalez S, Sathyapalan T, Javed Z, Atkin SL. Effects of growth hormone replacement on peripheral muscle and exercise capacity in severe growth hormone deficiency. Front Endocrinol (Lausanne) (2018) 9:56. doi: 10.3389/fendo.2018.00056
57. Beauregard C, Utz AL, Schaub AE, Nachtigall L, Biller BMK, Miller KK, et al. Growth hormone decreases visceral fat and improves cardiovascular risk markers in women with hypopituitarism: A randomized, placebo-controlled study. J Clin Endocrinol Metab (2008) 93(6):2063–71. doi: 10.1210/jc.2007-2371
58. Scarano E, Riccio E, Somma T, Arianna R, Romano F, Di Benedetto E, et al. Impact of long-term growth hormone replacement therapy on metabolic and cardiovascular parameters in adult growth hormone deficiency: Comparison between adult and elderly patients. Front Endocrinol (Lausanne) (2021) 12:635983. doi: 10.3389/fendo.2021.635983
59. Zhou H, Sun L, Zhang S, Wang Y, Wang G. Effect of long-term growth hormone replacement on glucose metabolism in adults with growth hormone deficiency: A systematic review and meta-analysis. Pituitary (2021) 24(1):130–42. doi: 10.1007/s11102-020-01079-3
60. Yuen K, Cook D, Ong K, Chatelain P, Fryklund L, Gluckman P, et al. The metabolic effects of short-term administration of physiological versus high doses of GH therapy in GH deficient adults. Clin Endocrinol (Oxf) (2002) 57(3):333–41. doi: 10.1046/j.1365-2265.2002.01601.x
61. Matsumoto R, Fukuoka H, Iguchi G, Nishizawa H, Bando H, Suda K, et al. Long-term effects of growth hormone replacement therapy on liver function in adult patients with growth hormone deficiency. Growth Horm IGF Res (2014) 24(5):174–9. doi: 10.1016/j.ghir.2014.07.002
Keywords: Sheehan syndrome, cardiovascular disease, obesity, metabolic syndrome, insulin resistance
Citation: Laway BA and Baba MS (2023) Sheehan syndrome: Cardiovascular and metabolic comorbidities. Front. Endocrinol. 14:1086731. doi: 10.3389/fendo.2023.1086731
Received: 01 November 2022; Accepted: 09 January 2023;
Published: 20 January 2023.
Edited by:
Przemyslaw Witek, Warsaw Medical University, PolandReviewed by:
Kursad Unluhizarci, Erciyes University, TurkeyHimanshu Jindal, G.S.V.M. Medical College, India
Copyright © 2023 Laway and Baba. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Bashir Ahmad Laway, ZHJsYXdheUBnbWFpbC5jb20=
 Bashir Ahmad Laway
Bashir Ahmad Laway Mohammad Salem Baba
Mohammad Salem Baba