
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Endocrinol. , 26 January 2023
Sec. Thyroid Endocrinology
Volume 14 - 2023 | https://doi.org/10.3389/fendo.2023.1083171
This article is part of the Research Topic Thyroid Hormone and Metabolites: Central Versus Peripheral Effects, Volume II View all 8 articles
Purpose: Thyroid hormones (THs) significantly affect the cardiovascular system. N-terminal pro-B-type natriuretic peptide (NT-proBNP) is a useful biomarker for diagnosing, evaluating, and predicting outcomes in heart failure (HF). This comprehensive review and meta-analysis aimed to investigate the effects of thyroid dysfunction (hypothyroidism and hyperthyroidism) on NT-proBNP levels.
Methods: Two investigators independently searched PubMed, Embase, Cochrane Library, and Web of Science databases for studies published from inception to July 31, 2022, without any restrictions on language.
Results: 21 studies were included. In participants without HF, NT-proBNP levels may be elevated in those with overt hyperthyroidism (standardized mean difference [SMD] 2.38, 95% confidence interval [CI]:1.0-3.76). Notably, among patients with preexisting HF, significantly higher NT-proBNP levels were found in patients with overt hyperthyroidism, overt hypothyroidism, or subclinical hypothyroidism than in euthyroid subjects (SMD [95%CI] = 0.31[0.01, 0.62], 0.32[0.08, 0.56], and 0.33[0.21, 0.46], respectively). Seven trials compared NT-proBNP levels in patients with thyroid dysfunction before and after therapy, and significant drops in NT-proBNP levels were observed in patients with hyperthyroidism (SMD [95%CI] = -1.53[-2.50, -0.55]) upon achieving a euthyroid state. In contrast, increased NT-proBNP levels were observed in hypothyroid patients after treatment (SMD [95%CI] = 1.07[0.28, 1.85]).
Conclusion: Thyroid dysfunction can significantly affect NT-proBNP levels, which may change upon achieving a euthyroid state. Notably, the effect of thyroid dysfunction on cardiac function may depend on the underlying cardiac status. Thus, timely recognition and effective treatment of cardiac symptoms in patients with thyroid dysfunction are mandatory because the prognosis of HF may be improved with appropriate treatment of thyroid dysfunction.
Systematic review registration: https://www.crd.york.ac.uk/prospero, identifier CRD42022353700.
Thyroid dysfunction (TD) may occur because of hypothyroidism or hyperthyroidism. Serum thyroid stimulating hormone (TSH) levels are increased with normal (subclinical hypothyroidism, SHypo) or low serum free thyroxin (FT4) (overt hypothyroidism, OHypo) levels, whereas serum TSH levels are low with normal (subclinical hyperthyroidism, SHyper) or high FT4 (overt hyperthyroidism, OHyper) levels. As thyroid hormones play an important role in regulating cardiac activities and affecting cardiovascular hemodynamics, thyroid conditions can cause metabolic and hemodynamic changes that may result in heart failure (HF) (1).
B-type natriuretic peptide (BNP) is a cardiac neurohormone generated by ventricles in response to volume expansion or pressure overload. BNP and N-terminal prohormone of brain natriuretic peptide (NT-proBNP) are two types of natriuretic peptides cleaved from Pro brain natriuretic peptide (proBNP). Compared to BNP, NT-proBNP is more stable and has a longer biological half-life. Thus, NT-proBNP is a better indicator for diagnosing or ruling out HF (2). NT-proBNP is also a good marker for assessing the severity and prognosis of this condition (3). Some studies have revealed that TD may affect serum NT-proBNP levels, but no consensus has been reached. Several studies (4–6) demonstrated that OHypo patients had significantly elevated NT-proBNP levels compared to euthyroid patients, but some studies found no correlation between them (7–9). Pakula et al. (9) observed a significant increase in NT-proBNP levels in SHyper patients, but Christ et al. (7) reported no such increase in SHyper patients compared to subjects in the control group. Furthermore, Hadzovic et al. (10) found that treating hypothyroidism resulted in a significant elevation of NT-proBNP levels, which appears inconsistent with the findings of Schultz et al. (11). Therefore, this meta-analysis aimed to investigate the effect of TD on NT-proBNP levels.
This meta-analysis followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement (12), and this systematic review was registered in the International Prospective Register of Systematic Reviews (PROSPERO), CRD42022353700.
Two investigators independently searched for studies in databases including PubMed, Embase, Cochrane Library, and Web of Science from inception to July 31, 2022, without language restrictions. The search strategies (Table S1 of the Supplementary Materials) were (hypothyroidism* OR hyperthyroidism* OR thyroid dysfunction) AND (BNP OR NT-proBNP).
Inclusion criteria: 1) studies comparing NT-proBNP levels in TD subjects and euthyroid subjects; clinical trials that compared NT-proBNP levels at pre-to-post treatment in TD; 2) studies reporting TD according to thyroid function test results; and 3) NT-proBNP levels in patients were reported as mean ± standard deviation (or calculable).
The exclusion criteria were as follows: 1) participants from a specific population (e.g., children or pregnant women); 2) studies investigating the effect of TD on BNP levels instead of NT-proBNP levels; and 3) reviews, conference abstracts, case reports, and studies with unavailable full texts.
Two researchers conducted the literature screening independently, and disagreements were resolved through discussion with a third researcher.
Two researchers independently performed data extraction, and any disagreements were settled through discussion with a third researcher. Extracted information was as follows: first author, publication year, country, sample size, sex, age, type of thyroid dysfunction, LVEF%, NT-proBNP detection method, TSH, FT3, FT4, and NT-proBNP levels in subjects with euthyroid, TD, and pre-to-post treatment in TD.
Given the types of included studies (case-control and cohort studies), the Newcastle-Ottawa Scale (NOS) was used to assess the quality of the included studies (13). The score ranges from 0 to 9; 7–9 represent high-quality scores, 4–6 represent medium scores and 1–3 represent low scores. Self-controlled trials that discussed NT-proBNP at pre-to-post treatment in TD were assessed using the JBI critical appraisal tool for quasi-experimental studies (14).
We extracted the data on NT-proBNP levels in TD and euthyroid subjects, as well as at pre-to-post treatment in TD. Continuous variables were reported as standardized mean differences (SMDs) with a 95% confidence interval (CI). The chi-squared-based Q test and the I2 test were performed to evaluate the heterogeneity across included studies, and I2 ≤50% and I2 >50% indicated low and high levels of heterogeneity, respectively. If there was a low level of heterogeneity, a fixed-effects model was used to pool data. Otherwise, a random effects model was used. Since some studies compared NT-proBNP levels in TD patients with HF and euthyroid patients with HF, subgroup analyses were performed according to whether the patients had HF. A sensitivity analysis was performed by sequentially removing each study. Publication bias was evaluated using a funnel plot, and if the included studies had an outcome of more than 10. All statistical tests were two-sided, and the significance level was set at p < 0.05. Review Manager software (Version 5.4.1, The Cochrane Collaboration, 2020) was used to conduct the meta-analysis.
The study selection process is shown in Figure 1. After duplicates were removed, titles and abstracts were screened, and 72 studies were obtained. After a comprehensive review of full texts, 48 articles were excluded for the following reasons: review articles (n=15), case reports (n=1), the outcome being BNP rather than NT-proBNP (n=9), irrelevant focuses (n=17), no control group (n=4), and unavailable data (n=2). Finally, 24 papers were included in this meta-analysis.
Among the 24 included studies, 21 (4–10, 15–28) compared NT-proBNP levels in TD and euthyroid subjects. The characteristics of the subjects in the TD and euthyroid groups are summarized in Table 1. The NOS results are presented in Table 1 and Table S2 (Supplementary Materials). The results showed that these studies were of medium-to-high quality. Table 2 shows the characteristics of self-controlled trials (7, 9–11, 19, 29, 30) that discussed NT-proBNP levels at pre-to-post treatment in TD patients. The prior cardiovascular disease in patients with HF in ten studies (6, 20–28) is summarized in Table S3. The JBI critical appraisal tool was employed for quasi-experimental studies to assess the quality of the self-controlled trials, and we found the following reasons for lower study quality: 1) all studies had no control group, and 2) results were not measured multiple times. In general, the included self-controlled trials were of high quality (Table S4 of the Supplementary Materials).
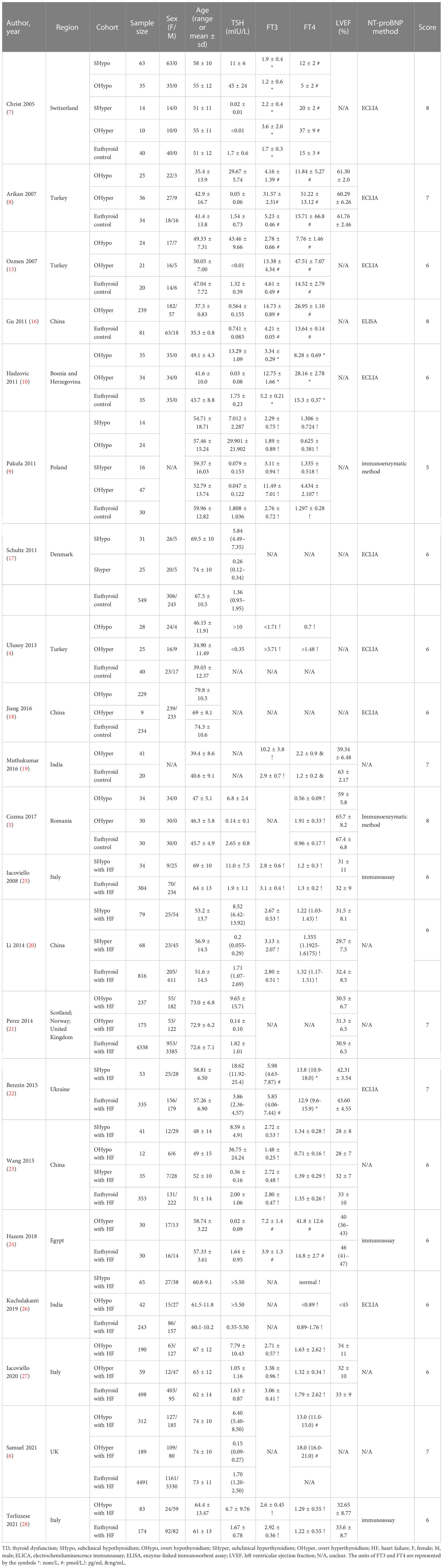
Table 1 General characteristics of the included studies discussing NT-proBNP levels in TD and euthyroid.
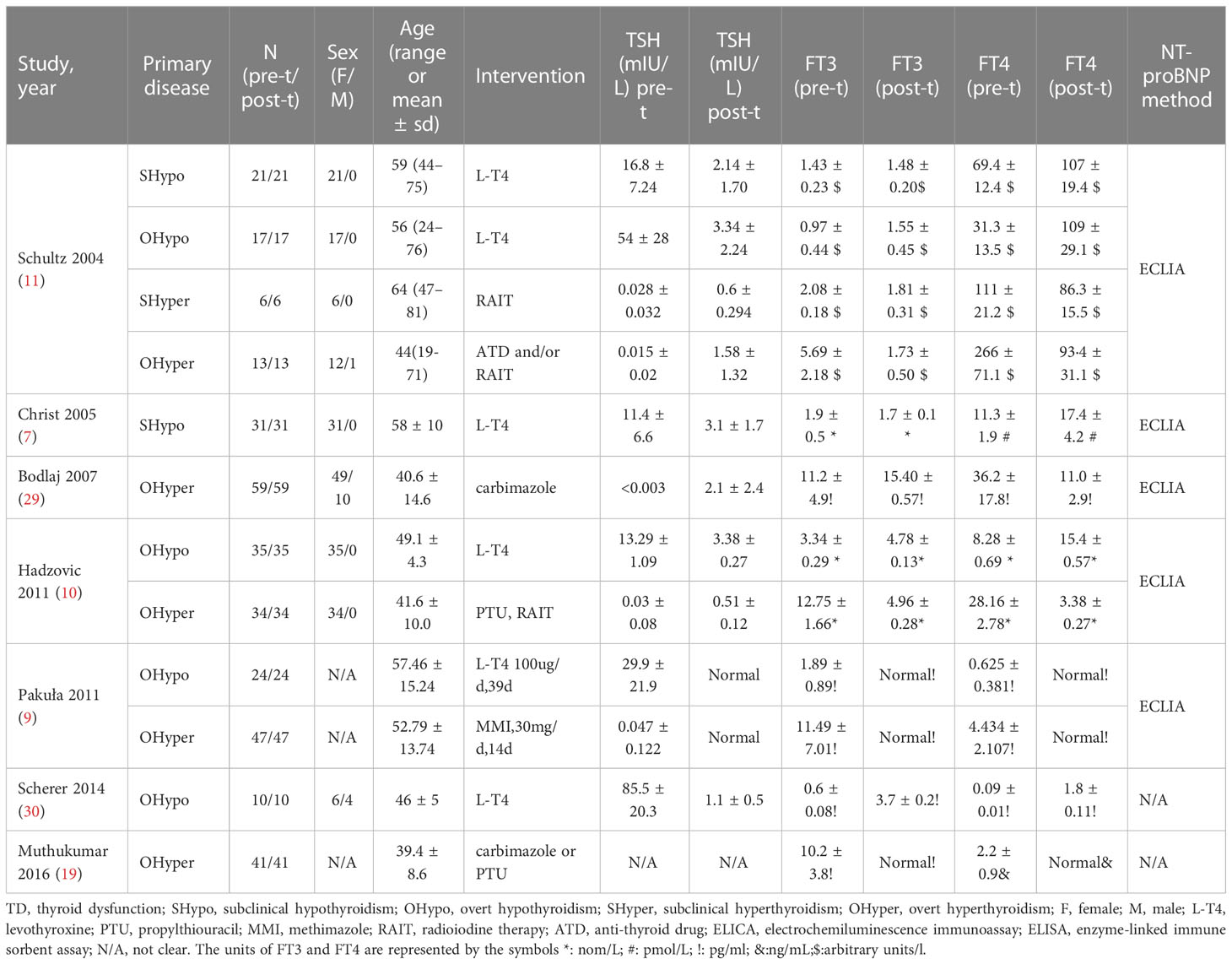
Table 2 The characteristics of the included self-controlled trials that discussed NT-proBNP levels at pre-to-post treatment in TD.
The pooled results of the 14 studies showed that NT-proBNP levels were significantly higher in subjects with OHyper than in euthyroid subjects (I2 = 98%, P <0.00001, REM; SMD [95%CI] = 1.77[1.05, 2.49], P <0.00001) (Figure 2A). Furthermore, the subgroup analysis indicated that NT-proBNP levels were significantly higher in subjects with OHyper than in euthyroid subjects, regardless of whether they had HF (Figure 2A).
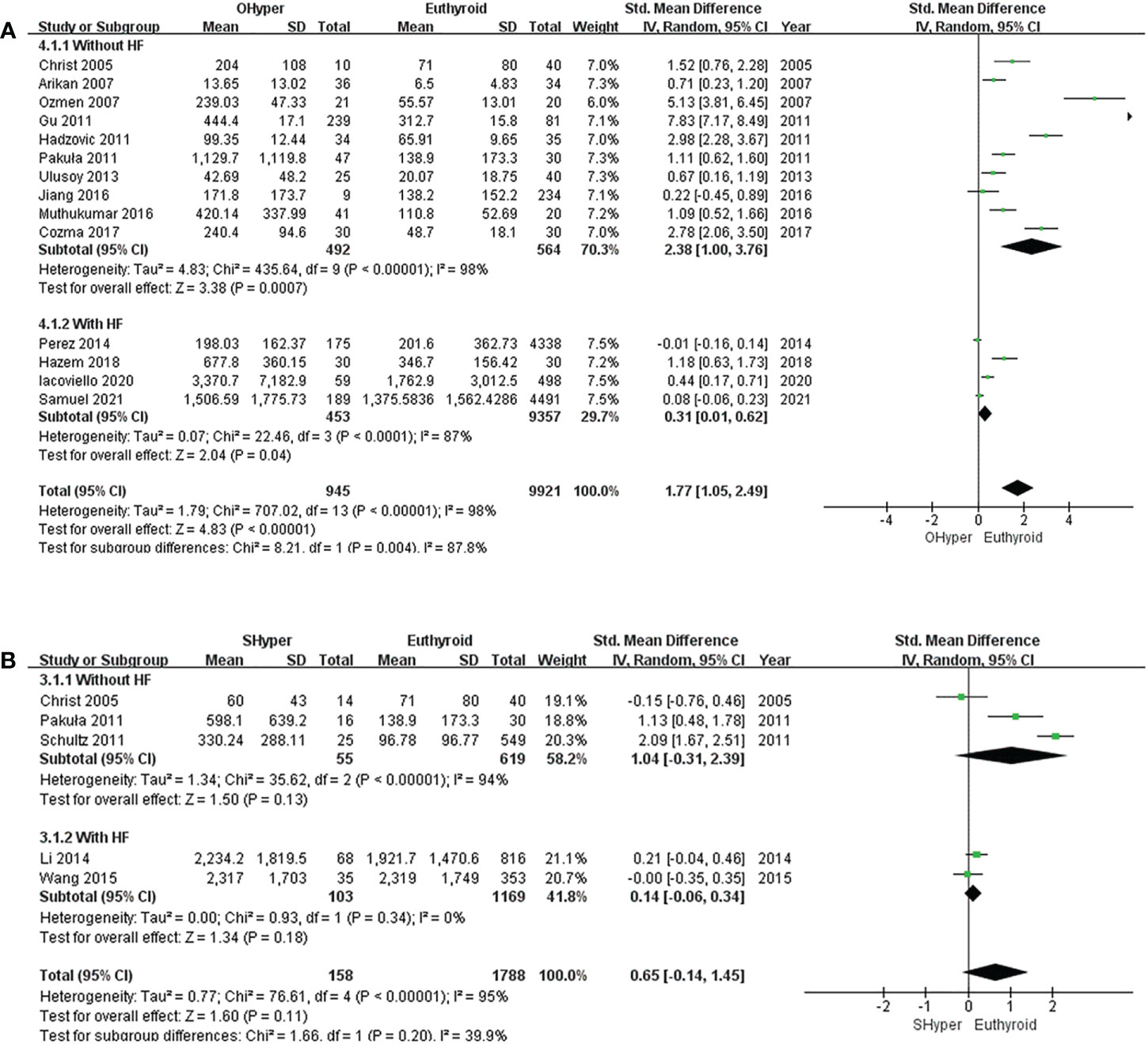
Figure 2 Forest plot of the NT-proBNP levels in patients with (A) OHyper and euthyroid subjects; (B) SHyper and euthyroid subjects.
In five studies that compared NT-proBNP levels in SHyper and euthyroid subjects, no significant difference was observed in NT-proBNP levels between SHyper and euthyroid subjects. (I2 = 95%, P <0.00001, REM; SMD [95%CI] = 0.65[-0.14, 1.45], P=0.11; Figure 2B). Then, subgroup analyses revealed that patients with SHyper did not significantly differ in NT-proBNP levels, compared with euthyroid subjects, whether participants were suffering from HF or not (Figure 2B).
The pooled estimate for the 14 studies showed that levels of NT-proBNP were significantly elevated in subjects with OHypo compared to euthyroid subjects (I2 = 89%, P <0.00001, REM; SMD [95%CI] = 0.23 [0.01, 0.46], P=0.04; Figure 3A). Subgroup analyses were conducted according to whether the participants had HF, but heterogeneity did not change significantly. No significant difference in NT-proBNP levels was found between patients with OHypo and those with euthyroidism. (I2 = 91%, P <0.00001, REM; SMD [95%CI] = 0.14 [-0.37, 0.64], P=0.59; Figure 3A). However, HF patients with OHypo had significantly higher NT-proBNP levels than those with euthyroidism (I2 = 89%, P <0.00001, REM; SMD [95%CI] = 0.32 [0.08, 0.56], P=0.01; Figure 3A).
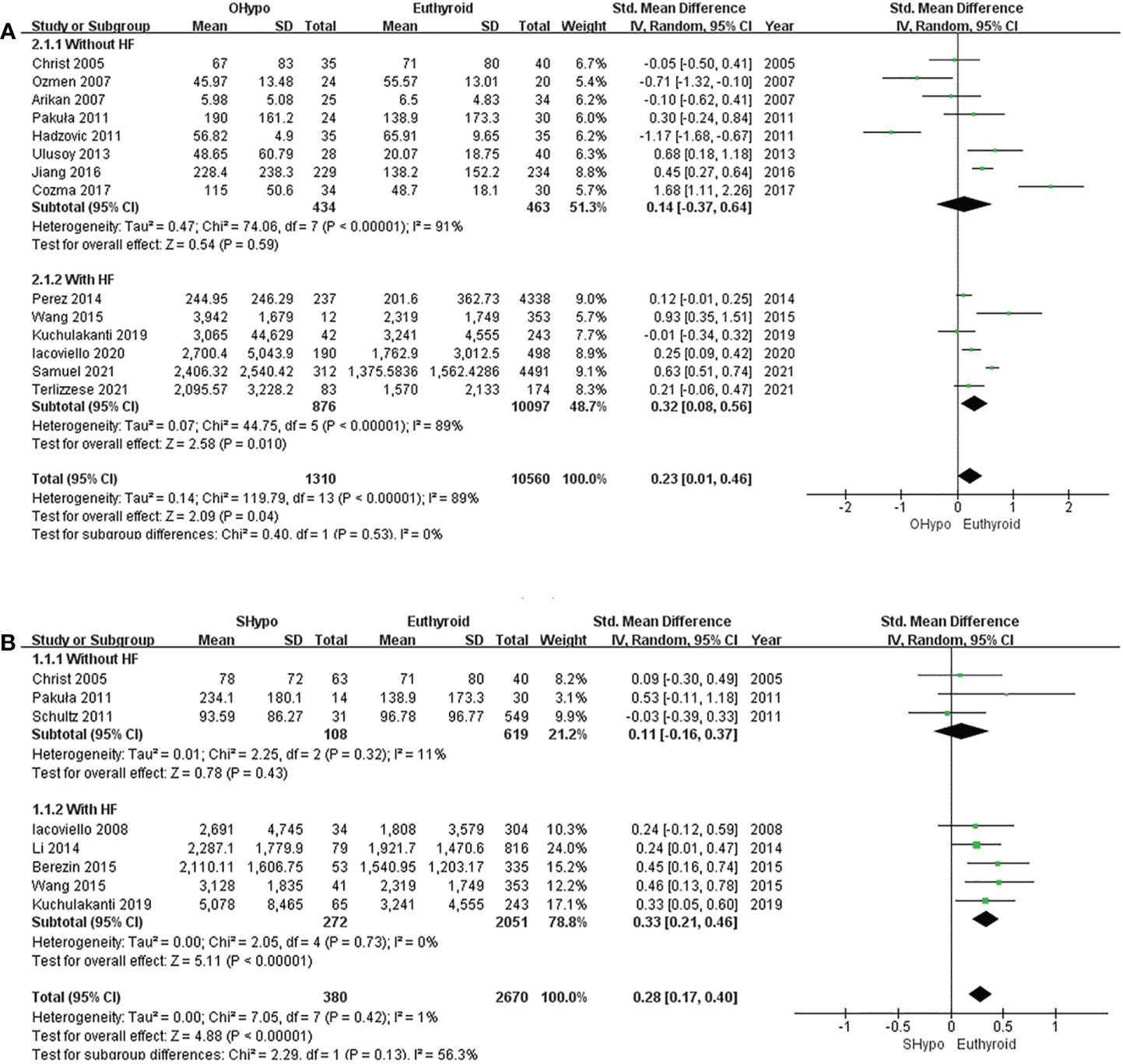
Figure 3 Forest plot of the NT-proBNP levels in patients with (A) OHypo and euthyroid subjects; (B) SHypo and euthyroid subjects.
Pooled data from eight studies showed significantly higher NT-proBNP levels among subjects with SHypo than those with euthyroidism. (I2 = 1%, P=0.42, REM; SMD [95%CI] =0.28 [0.17, 0.40], P<0.00001; Figure 3B). We conducted a subgroup analysis according to whether participants had HF. NT-proBNP levels in SHypo and euthyroid subjects were not significantly different. (I2 = 11%, P=0.32, REM; SMD [95%CI] = 0.11 [-0.16, 0.37], P=0.43; Figure 3B). However, patients with HF and SHypo had significantly higher NT-proBNP levels than those with euthyroid HF. (I2 =0%, P =0.73, REM; SMD [95%CI] = 0.33 [0.21, 0.46], P<0.00001; Figure 3B).
Six studies discussed the effects of treatment on NT-proBNP levels in subjects with hyperthyroidism, and significant decreases in NT-proBNP levels were observed in hyperthyroid patients upon achievement of a euthyroid state. (I2 =94%, P<0.00001, REM; SMD [95%CI] = -1.53 [-2.50, -0.55], P=0.002; Figure 4A). Six trials compared NT-proBNP levels in patients before and after levothyroxine administration. Levothyroxine therapy was related to significantly higher NT-proBNP levels in subjects with hypothyroidism (I2 =89%, P<0.00001, REM; SMD [95%CI] = 1.07 [0.28, 1.85], P=0.008; Figure 4B).
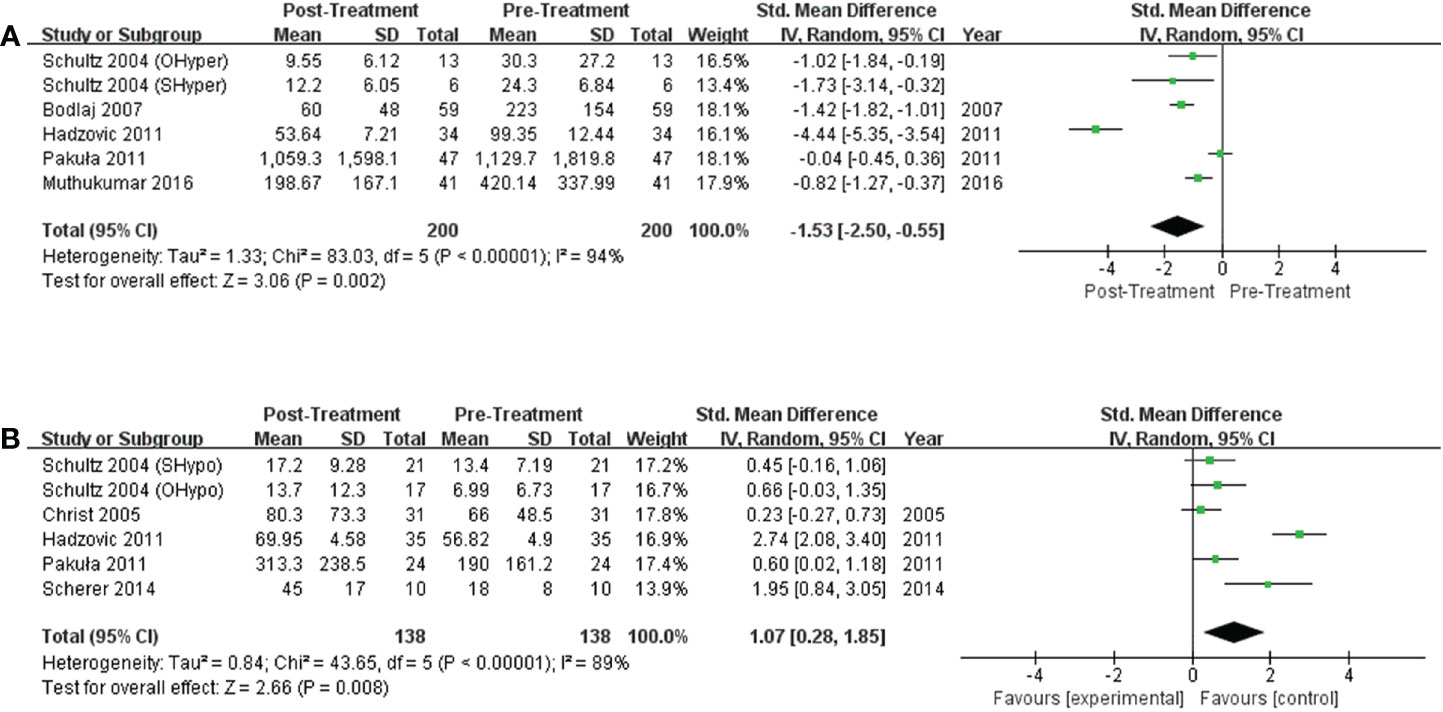
Figure 4 Forest plot of the NT-proBNP levels in subjects with (A) hyperthyroidism from pre- to post-treatment; (B) hypothyroidism from pre- to post-treatment.
The sensitivity analysis showed that the pooled data were similar before and after removing any studies, indicating that the results were relatively stable. Funnel plots were used to determine publication bias. We drew funnel plots for two outcomes (comparing NT-proBNP levels in subjects with OHyper or OHypo and euthyroid controls) that involved more than 10 studies. The funnel plots for OHyper and OHypo are shown in Figures S1 and S2. Figure S1 is basically symmetric, indicating no significant publication bias. In contrast, Figure S2 is significantly asymmetrical due to several studies. After we excluded these studies that resulted in the asymmetry of the funnel plot, the result obtained is consistent with the original result, showing that publication bias may not affect our result.
NT-proBNP levels may increase in patients with OHyper regardless of HF status. The increased NT-proBNP levels may be explained by the following reasons. First, thyroid hormone can directly affect cardiomyocytes and then increase the levels of NT-proBNP. Kohno et al. (31) found that triiodothyronine (T3) and thyroxine (T4) stimulated BNP release in cultured rat ventricular myocytes in a dose-dependent manner. Subsequently, an in vitro study by Liang et al. identified the BNP gene as a transcriptional target of thyroid hormone (32). T3 may bind to thyroid hormone receptors (TRs) in the cardiomyocyte nucleus to form a hormone-receptor complex, which regulates BNP transcription by binding to thyroid hormone response elements (TREs) located in approximately 1 kb upstream from the BNP promoter (33–35). In addition, thyroid hormone may activate β-adrenergic signaling (36, 37), and β-adrenergic activation can stimulate BNP mRNA (38–40). In patients with OHyper, specific cardiac-adrenergic receptors are upregulated, and the β-adrenergic responsiveness of cardiomyocytes is increased (36, 37). Anees et al. (2016) found that isoprenaline significantly upregulated BNP (39). In the study by Tshori et al. (2006), β agonists induced protein kinase A (PKA) activity, and PKA has been demonstrated to activate cAMP response element binding protein to increase the expression of microphthalmia transcription factor (MITF), thereby enhancing the activity of BNP promoter (40). Third, OHyper patients are generally in a hypermetabolic state (41–44) and often have various hemodynamic changes, including increased cardiac output, increased heart rate, accelerated blood flow, and increased circulating blood volume, which impacts ventricular pressure (1, 45–48). Secondary changes in blood dynamics can also lead to increased NT-proBNP levels (35, 49). Fourth, OHyper may cause ventricular myocyte structural changes in the heart that conventional echocardiography cannot detect (50), and these alterations may be responsible for NT-proBNP level elevation. In subjects without preexisting cardiac diseases, elevated NT-ProBNP levels in OHyper patients showed only levels comparable to mild HF and may instead signify volume overload than severe HF (8). Moreover, as reported by Hazem et al. (24), increased levels of NT-proBNP in OHyper patients with ischemic heart disease are attributed to the release of BNP stimulated by both OHyper and myocardial ischemic changes, which makes it necessary to check the threshold of NT-proBNP level as a serological marker for the initial diagnosis of HF in this patient group.
According to the meta-analysis, no significant difference was noted between SHyper and euthyroid subjects in NT-proBNP levels, which is inconsistent with the results of some studies. A recent study that investigated the effects of SHyper on BNP levels in 47 patients found that patients with SHyper had higher BNP levels than euthyroid subjects (51). Young adults with serum TSH concentrations below 0.1 mIU/L may experience increased left ventricular (LV) mass, systolic and diastolic LV dysfunction, increased heart rate, and arterial stiffness (52). Thus, the meta-analysis results should be interpreted with caution, given the small number of included studies, relatively small sample sizes, and significant heterogeneity (I2 =95%, P <0.00001).
The NT-proBNP levels in OHypo and euthyroid subjects without HF did not differ significantly. However, patients with HF and OHypo had significantly elevated NT-proBNP levels compared to HF patients with euthyroid. For the former outcome, Pakula et al. (9) reported that the combined opposite effects of a hypometabolic state brought on by hypothyroidism and increased production of proinflammatory cytokines and endothelins may explain the neutral effect of OHypo on NT-proBNP. Endothelins and proinflammatory cytokines are known to initiate the release of NT-proBNP (53), and there is some proof that thyroid autoimmunity and OHypo cause an inflammatory state and endothelial dysfunction (54–56). However, the underlying cardiac condition may affect the manifestation of TD. In particular, patients with HF may not have sufficient cardiac tolerance to slight changes in thyroid hormone levels. Thus, for patients with HF, small changes, including lowered heart contractility, elevated systemic vascular resistance, impaired left ventricular diastolic filling, and lowered heart output due to OHypo may worsen their preexisting HF, which explains the higher NT-proBNP level in HF patients with OHypo (1, 57–59). Several prospective cohort studies have found that OHypo is an independent risk factor for all-cause mortality and cardiac death among patients with HF (60–62). Therefore, timely and effective treatment of OHypo can improve HF prognosis.
According to the meta-analysis, SHypo was not associated with the changes in NT-proBNP levels in patients free from HF. However, Huang et al. (63) found that functional thyroid stimulating hormone receptor (TSHR) was expressed in ventricular tissue and myocytes, and TSH, by acting on TSHR in ventricular myocytes, induced ventricular HMGCR expression via the cAMP/PKA/pCREB signaling pathway and promoted BNP secretion to a certain degree. Two studies showed that the plasma BNP level was significantly and positively correlated with the TSH level (18, 64). In addition, some studies showed an inverse association between TSH levels and BNP levels (7, 11, 15, 16). Given the controversy over this point, further studies with larger samples are required. In addition, our meta-analysis demonstrated that HF patients with SHypo had significantly higher levels of NT-proBNP than HF patients with euthyroidism. SHypo may be associated with systolic and diastolic dysfunction, blood pressure alterations, endothelial and vascular dysfunction, and dyslipidemia, which contribute to the development of HF, as reflected by higher NT-proBNP levels (58, 65, 66). According to a recent study, SHypo with TSH ≥7 mIU/L and isolated low T3 levels were related to a poor prognosis over a median of 4.2 years of follow-up in 1365 patients with preexisting HF (67). Randomized controlled trials (RCTs) with placebo controls should be conducted to ascertain the clinical outcomes of treating HF patients with SHypo.
Interestingly, this meta-analysis showed that the use of levothyroxine increased NT-proBNP levels. This finding is in line with the a forementioned explanation of higher NT-proBNP levels in hyperthyroidism, which is probably associated with the direct effect of exogenous thyroid hormone on the heart. It is still unclear whether levothyroxine therapy is a predisposing factor for HF or whether it aggravates previous HF (65, 66, 68), so more research is needed in the future. In addition, for HF patients receiving levothyroxine therapy, clinicians should closely follow up and pay attention to the occurrence of cardiovascular adverse events in medical practice (69, 70). Moreover, this meta-analysis showed that antithyroid drugs restored the hyperthyroidism-induced increase in plasma NT-pro-BNP level. Therefore, aggressive treatment should be used to avoid severe cardiac complications of hyperthyroidism (atrial fibrillation, heart failure, and embolic events) and reduce the risk of cardiovascular death (57, 71, 72).
This is the first systematic review to investigate the effects of thyroid disease on NT-proBNP levels. This study has some limitations. First, the observational nature of all the included studies may have affected the validity of the overall results. Second, this systematic review has language bias due to the limited language ability of our researchers, who were unable to access the literature published in languages other than English. Third, given the small number of studies on each prior cardiovascular disease and the recruitment of HF patients with multiple etiologies in some studies, subgroup analyses could not be performed according to the etiology of HF. Patients with HF in all these studies received conventional medical therapy, and there were no significant differences in therapeutic medications. Moreover, the included studies did not further group patients according to the treatment regimens. Thus, we could not perform subgroup analyses according to whether HF patients received treatment or not. Fourth, although this meta-analysis included 24 studies, the sample size was small, considering the wide range of TD. Additionally, more studies with small heterogeneity are needed in the future, considering the significant heterogeneity of most of our outcomes, which may be because several studies did not adjust for important confounders, such as age, sex, and body mass index. In particular, there is controversy about whether gender has a significant effect on BNP (73–78). Only three of the included studies recruited single-sex participants. Based on such a small amount of evidence, we could not further assess whether gender affects BNP, and it therefore needs to be further explored in future studies.
In conclusion, TD can significantly affect NT-pro-BNP levels, which may change upon reaching a euthyroid state. Notably, the effect of TD on cardiac function may depend on the underlying cardiac status. Thus, timely recognition and effective treatment of cardiac symptoms in patients with TD are mandatory because the prognosis of HF may be improved with appropriate treatment of TD. In the future, RCTs are necessary to examine the prognosis and potential improvement in HF with appropriate treatment of TD.
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
HZ and LT contributed to the study conception and design. Material preparation, data collection and analysis were performed by HZ, XL, and NZ. The first draft of the manuscript was written by HZ and LT, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
This work was supported by grant from the National Natural Science Foundation of China (No. 82060152)
We would like to thank the researchers and study participants for their contributions.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2023.1083171/full#supplementary-material
1. Paschou SA, Bletsa E, Stampouloglou PK, Tsigkou V, Valatsou A, Stefanaki K, et al. Thyroid disorders and cardiovascular manifestations: An update. Endocrine (2022) 75(3):672–83. doi: 10.1007/s12020-022-02982-4
2. Alcidi G, Goffredo G, Correale M, Brunetti ND, Iacoviello M. Brain natriuretic peptide biomarkers in current clinical and therapeutic scenarios of heart failure. J Clin Med (2022) 11(11). doi: 10.3390/jcm11113192
3. Cao Z, Jia Y, Zhu B. BNP and NT-proBNP as diagnostic biomarkers for cardiac dysfunction in both clinical and forensic medicine. Int J Mol Sci (2019) 20(8):1820. doi: 10.3390/ijms20081820
4. Ulusoy M, Gerek S, Yiǧit N, Ayer FA, Gürkan Y, Feyizoǧlu H, et al. N-terminal pro-b-type natriuretic peptide for detecting early cardiac dysfunction in patients with thyroid dysfunction. Haseki Tip Bulteni (2013) 51(3):102–6. doi: 10.4274/Haseki.1011
5. Cozma LCD, Szalontay AS, Cozma S, Dascalu CG, Cojocaru DC, Mitu F, et al. Uric acid and NT-proBNP as biomarkers of cardiovascular dysfunction in hyperthyroidism and hypothyroidism. Rev Chimie (2017) 68(12):2959–62. doi: 10.37358/RC.17.12.6016
6. Samuel NA, Cuthbert JJ, Brown OI, Kazmi S, Cleland JGF, Rigby AS, et al. Relation between thyroid function and mortality in patients with chronic heart failure. Am J Cardiol (2021) 139:57–63. doi: 10.1016/j.amjcard.2020.10.034
7. Christ-Crain M, Morgenthaler NG, Meier C, Müller C, Nussbaumer C, Bergmann A, et al. Pro-a-type and n-terminal pro-b-type natriuretic peptides in different thyroid function states. Swiss Med Wkly (2005) 135(37-38):549–54. doi: 10.4414/smw.2005.11119
8. Arikan S, Tuzcu A, Gokalp D, Bahceci M, Danis R. Hyperthyroidism may affect serum n-terminal pro-b-type natriuretic peptide levels independently of cardiac dysfunction. Clin Endocrinol (Oxf) (2007) 67(2):202–7. doi: 10.1111/j.1365-2265.2007.02861.x
9. Pakuła D, Marek B, Kajdaniuk D, Krysiak R, Kos-Kudła B, Pakuła P, et al. Plasma levels of NT-pro-brain natriuretic peptide in patients with overt and subclinical hyperthyroidism and hypothyroidism. Endokrynol Pol (2011) 62(6):523–8.
10. Hadzovic-Dzuvo A, Kucukalic-Selimovic E, Nakas-Icindic E, Lepara O, Valjevac A, Rasic S, et al. Amino-terminal pro-brain natriuretic peptide (NT-proBNP) serum levels in females with different thyroid function states. Turkish J Biochem (2011) 36(2):116–21. doi: 10.4238/2011.October.31.1
11. Schultz M, Faber J, Kistorp C, Jarløv A, Pedersen F, Wiinberg N, et al. N-terminal-pro-B-type natriuretic peptide (NT-pro-BNP) in different thyroid function states. Clin Endocrinol (Oxf) (2004) 60(1):54–9. doi: 10.1111/j.1365-2265.2004.01941.x
12. Moher D, Shamseer L, Clarke M, Ghersi D, Liberati A, Petticrew M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-p) 2015 statement. Syst Rev (2015) 4(1):1. doi: 10.1186/2046-4053-4-1
13. Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol (2010) 25(9):603–5. doi: 10.1007/s10654-010-9491-z
14. Tufanaru C, Munn Z, Aromataris E, Campbell J, Hopp L. Chapter 3: Systematic reviews of effectiveness. In: Aromataris E, Munn Z, editors. JBI manual for evidence synthesis. Japan: JBI (2020). Available at: https://synthesismanual.jbi.global.
15. Ozmen B, Ozmen D, Parildar Z, Mutaf I, Bayindir O. Serum n-terminal-pro-B-type natriuretic peptide (NT-pro-BNP) levels in hyperthyroidism and hypothyroidism. Endocr Res (2007) 32(1-2):1–8. doi: 10.1080/07435800701670047
16. Gu LQ, Zhao L, Zhu W, Li FY, Zhang MJ, Liu Y, et al. Relationships between serum levels of thyroid hormones and serum concentrations of asymmetric dimethylarginine (ADMA) and n-terminal-pro-B-type natriuretic peptide (NT-proBNP) in patients with graves’ disease. Endocrine (2011) 39(3):266–71. doi: 10.1007/s12020-011-9436-7
17. Schultz M, Kistorp C, Raymond I, Dimsits J, Tuxen C, Hildebrandt P, et al. Cardiovascular events in thyroid disease: A population based, prospective study. Horm Metab Res (2011) 43(9):653–9. doi: 10.1055/s-0031-1283162
18. Jiang X, Qiao C, Liu Y, Yang L, Wang L, Wang L, et al. The changes of thyroid function in the elderly and correlation of the changes with atherosclerosis and cardiac function. Chin J Geriatrics (2016) 35(10):1079–83. doi: 10.3760/cma.j.issn.0254-9026.2016.10.013
19. Muthukumar S, Sadacharan D, Ravikumar K, Mohanapriya G, Hussain Z. Suresh RV. a prospective study on cardiovascular dysfunction in patients with hyperthyroidism and its reversal after surgical cure. World J Surg (2016) 40(3):622–8. doi: 10.1007/s00268-015-3352-6
20. Li X, Yang X, Wang Y, Ding L, Wang J, Hua W. The prevalence and prognostic effects of subclinical thyroid dysfunction in dilated cardiomyopathy patients: A single-center cohort study. J Card Fail (2014) 20(7):506–12. doi: 10.1016/j.cardfail.2014.05.002
21. Perez AC, Jhund PS, Stott DJ, Gullestad L, Cleland JGF, van Veldhuisen DJ, et al. Thyroid-stimulating hormone and clinical outcomes. JACC: Heart Failure (2014) 2(1):35–40. doi: 10.1016/j.jchf.2013.07.008
22. Berezin AE, Kremzer AA, Martovitskaya YV, Samura TA, Berezina TA. The association of subclinical hypothyroidism and pattern of circulating endothelial-derived microparticles among chronic heart failure patients. Res Cardiovasc Med (2015) 4(4):e29094. doi: 10.5812/cardiovascmed.29094
23. Wang W, Guan H, Gerdes AM, Iervasi G, Yang Y, Tang YD. Thyroid status, cardiac function, and mortality in patients with idiopathic dilated cardiomyopathy. J Clin Endocrinol Metab (2015) 100(8):3210–8. doi: 10.1210/jc.2014-4159
24. El-Ashmawy HM, Hussein EM, Ahmed AM. Brain natriuretic peptide as a diagnostic marker for heart failure in hyperthyroid patients with ischemic heart disease. Egyptian J Internal Med (2018) 30(2):63–7. doi: 10.4103/ejim.ejim_81_17
25. Iacoviello M, Guida P, Guastamacchia E, Triggiani V, Forleo C, Catanzaro R, et al. Prognostic role of sub-clinical hypothyroidism in chronic heart failure outpatients. Curr Pharm Des (2008) 14(26):2686–92. doi: 10.2174/138161208786264142
26. Kuchulakanti PK, Bandaru VS, Kuchulakanti A, Lakshumaiah T, Rathod M, Khare R, et al. Association of subclinical hypothyroidism in heart failure: A study from south India. India: Br J Cardiol (2019).
27. Iacoviello M, Parisi G, Gioia MI, Grande D, Rizzo C, Guida P, et al. Thyroid disorders and prognosis in chronic heart failure: A long-term follow-up study. Endocr Metab Immune Disord Drug Targets (2020) 20(3):437–45. doi: 10.2174/1871530319666191018134524
28. Terlizzese P, Albanese M, Grande D, Parisi G, Gioia MI, Brunetti ND, et al. TSH variations in chronic heart failure outpatients: Clinical correlates and outcomes. Endocr Metab Immune Disord Drug Targets (2021) 21(10):1935–42. doi: 10.2174/1871530321666210430131510
29. Bodlaj G, Pichler R, Brandstätter W, Hatzl-Griesenhofer M, Maschek W, Biesenbach G, et al. Hyperthyroidism affects arterial stiffness, plasma NT-pro-B-type natriuretic peptide levels, and subendocardial perfusion in patients with graves’ disease. Ann Med (2007) 39(8):608–16. doi: 10.1080/07853890701528579
30. Scherer T, Wolf P, Winhofer Y, Duan H, Einwallner E, Gessl A, et al. Levothyroxine replacement in hypothyroid humans reduces myocardial lipid load and improves cardiac function. J Clin Endocrinol Metab (2014) 99(11):E2341–6. doi: 10.1210/jc.2014-2112
31. Kohno M, Horio T, Yasunari K, Yokokawa K, Ikeda M, Kurihara N, et al. Stimulation of brain natriuretic peptide release from the heart by thyroid hormone. Metabolism (1993) 42(8):1059–64. doi: 10.1016/0026-0495(93)90023-H
32. Liang F, Webb P, Marimuthu A, Zhang S, Gardner DG. Triiodothyronine increases brain natriuretic peptide (BNP) gene transcription and amplifies endothelin-dependent BNP gene transcription and hypertrophy in neonatal rat ventricular myocytes. J Biol Chem (2003) 278(17):15073–83. doi: 10.1074/jbc.M207593200
33. Bassett JD, Harvey CB, Williams GR. Mechanisms of thyroid hormone receptor-specific nuclear and extra nuclear actions. Mol Cell Endocinol (2003) 213(1):1–11. doi: 10.1016/j.mce.2003.10.033
34. Dan G-A. Thyroid hormones and the heart. Heart failure Rev (2016) 21(4):357–9. doi: 10.1007/s10741-016-9555-6
35. Nakagawa Y, Nishikimi T, Kuwahara K. Atrial and brain natriuretic peptides: Hormones secreted from the heart. Peptides (2019) 111:18–25. doi: 10.1016/j.peptides.2018.05.012
36. Kim B, Carvalho-Bianco SD, Larsen PR. Thyroid hormone and adrenergic signaling in the heart. Arquivos Brasileiros Endocrinologia Metabologia (2004) 48:171–5. doi: 10.1590/S0004-27302004000100019
37. Carvalho-Bianco SD, Kim BW, Zhang JX, Harney JW, RrS R, Gereben B, et al. Chronic cardiac-specific thyrotoxicosis increases myocardial β-adrenergic responsiveness. Mol Endocinol (2004) 18(7):1840–9. doi: 10.1210/me.2003-0125
38. He Q, Wu G, Lapointe MC. Isoproterenol and cAMP regulation of the human brain natriuretic peptide gene involves src and rac. Am J Physiology-Endocinol And Metab (2000) 278(6):E1115–23. doi: 10.1152/ajpendo.2000.278.6.E1115
39. Syed AA, Lahiri S, Mohan D, Valicherla GR, Gupta AP, Kumar S, et al. Cardioprotective effect of ulmus wallichiana planchon in β-adrenergic agonist induced cardiac hypertrophy. Front Pharmacol (2016) 7:510. doi: 10.3389/fphar.2016.00510
40. Tshori S, Gilon D, Beeri R, Nechushtan H, Kaluzhny D, Pikarsky E, et al. Transcription factor MITF regulates cardiac growth and hypertrophy. J Clin Invest (2006) 116(10):2673–81. doi: 10.1172/JCI27643.
41. Teixeira P, Dos Santos PB, Pazos-Moura CC. The role of thyroid hormone in metabolism and metabolic syndrome. Ther Adv Endocrinol Metab (2020) 11:2042018820917869. doi: 10.1177/2042018820917869
42. Riis ALD, Jørgensen JOL, Gjedde S, Nørrelund H, Jurik AG, Nair K, et al. Whole body and forearm substrate metabolism in hyperthyroidism: evidence of increased basal muscle protein breakdown. Am J Physiology-Endocinol Metab (2005) 288(6):E1067–E73. doi: 10.1152/ajpendo.00253.2004
43. Abdel-Moneim A, Gaber AM, Gouda S, Osama A, Othman SI, Allam G. Relationship of thyroid dysfunction with cardiovascular diseases: updated review on heart failure progression. Hormones (2020) 19(3):301–9. doi: 10.1007/s42000-020-00208-8
44. Cai Z, Dai M, Zhang Y, Zhong H, Tan T, Bao M. Imbalance of cardiac autonomic nervous activity and increase of ventricular repolarization dynamicity induced by thyroid hormones in hyperthyroidism. Autonomic Neurosci (2018) 213:86–91. doi: 10.1016/j.autneu.2018.06.006
45. Yamakawa H, Kato TS, Noh JY, Yuasa S, Kawamura A, Fukuda K, et al. Thyroid hormone plays an important role in cardiac function: From bench to bedside. Front Physiol (2021) 12:606931. doi: 10.3389/fphys.2021.606931
46. Navarro-Navajas A, Cruz JD, Ariza-Ordoñez N, Giral H, Palmezano J, Bolívar-Mejía A, et al. Cardiac manifestations in hyperthyroidism. Rev Cardiovasc Med (2022) 23(4):136. doi: 10.31083/j.rcm2304136
47. Faber J, Wiinberg N, Schifter S, Mehlsen J. Haemodynamic changes following treatment of subclinical and overt hyperthyroidism. Eur J Endocinol (2001) 145(4):391–6. doi: 10.1530/eje.0.1450391
48. Petretta M, Bonaduce D, Spinelli L, Vicario ML, Nuzzo V, Marciano F, et al. Cardiovascular haemodynamics and cardiac autonomic control in patients with subclinical and overt hyperthyroidism. Eur J Endocrinol (2001) 145(6):691–6. doi: 10.1530/eje.0.1450691
49. Balion CM, Santaguida P, McKelvie R, Hill SA, McQueen MJ, Worster A, et al. Physiological, pathological, pharmacological, biochemical and hematological factors affecting BNP and NT-proBNP. Clin Biochem (2008) 41(4-5):231–9. doi: 10.1016/j.clinbiochem.2007.10.005
50. Wei T, Zeng C, Tian Y, Chen Q, Wang L. B-type natriuretic peptide in patients with clinical hyperthyroidism. J Endocrinol Invest (2005) 28(1):8–11. doi: 10.1007/BF03345522
51. Ertugrul DT, Gursoy A, Sahin M, Unal AD, Pamuk B, Berberoglu Z, et al. Evaluation of brain natriuretic peptide levels in hyperthyroidism and hypothyroidism. J Natl Med Assoc (2008) 100(4):401–5. doi: 10.1016/S0027-9684(15)31272-4
52. Biondi B, Palmieri EA, Fazio S, Cosco C, Nocera M, Saccà L, et al. Endogenous subclinical hyperthyroidism affects quality of life and cardiac morphology and function in young and middle-aged patients. J Clin Endocrinol Metab (2000) 85(12):4701–5. doi: 10.1210/jcem.85.12.7085
53. Pandhi P, Ter Maaten JM, Anker SD, Ng LL, Metra M, Samani NJ, et al. Pathophysiologic processes and novel biomarkers associated with congestion in heart failure. JACC Heart Fail (2022) 10(9):623–32. doi: 10.1016/j.jchf.2022.05.013
54. Talebi S, Karimifar M, Heidari Z, Mohammadi H, Askari G. The effects of synbiotic supplementation on thyroid function and inflammation in hypothyroid patients: A randomized, double−blind, placebo−controlled trial. Complement Ther Med (2020) 48:102234. doi: 10.1016/j.ctim.2019.102234
55. Naseem Z, Iqbal MA, Ahmad S, Roohi N. Inflammatory markers as prognosticators of cardiovascular dysfunction in hypothyroid patients. J Biol Regul Homeost Agents (2019) 33(6):1891–5. doi: 10.23812/19-334-L
56. Saif A, Mousa S, Assem M, Tharwat N, Abdelhamid A. Endothelial dysfunction and the risk of atherosclerosis in overt and subclinical hypothyroidism. Endocr Connect (2018) 7(10):1075–80. doi: 10.1530/EC-18-0194
57. Biondi B. Mechanisms in endocrinology: Heart failure and thyroid dysfunction. Eur J Endocrinol (2012) 167(5):609–18. doi: 10.1530/EJE-12-0627
58. Chaker L, Razvi S, Bensenor IM, Azizi F, Pearce EN, Peeters RP. Hypothyroidism. Nat Rev Dis Primers (2022) 8(1):30. doi: 10.1038/s41572-022-00357-7
59. Biondi B. Levothyroxine and the heart. In: Kahaly GJ, editor. 70 years of levothyroxine. Cham (CH: Springer Copyright 2021 (2021). p. 85–96. The Author(s).
60. De Matteis G, Covino M, Burzo ML, Della Polla DA, Petti A, Bruno C, et al. Prognostic role of hypothyroidism and low free-triiodothyronine levels in patients hospitalized with acute heart failure. Intern Emerg Med (2021) 16(6):1477–86. doi: 10.1007/s11739-020-02582-y
61. Ning N, Gao D, Triggiani V, Iacoviello M, Mitchell JE, Ma R, et al. Prognostic role of hypothyroidism in heart failure: A meta-analysis. Med (Baltimore) (2015) 94(30):e1159. doi: 10.1097/MD.0000000000001159
62. Seo SM, Koh YS, Park HJ, Kim DB, Her SH, Lee JM, et al. Thyroid stimulating hormone elevation as a predictor of long-term mortality in patients with acute myocardial infarction. Clin Cardiol (2018) 41(10):1367–73. doi: 10.1002/clc.23062
63. Huang W, Xu J, Jing F, Chen W-B, Gao L, Yuan H-T, et al. Functional thyrotropin receptor expression in the ventricle and the effects on ventricular BNP secretion. Endocrine (2014) 46(2):328–39. doi: 10.1007/s12020-013-0052-6
64. Takahashi H, Kashiwagi Y, Nagoshi T, Tanaka Y, Oi Y, Kimura H, et al. Low triiodothyronine levels correlate with high b-type natriuretic peptide levels in patients with heart failure. Sci Rep (2021) 11(1):21865. doi: 10.1038/s41598-021-01454-5
65. Manolis AA, Manolis TA, Melita H, Manolis AS. Subclinical thyroid dysfunction and cardiovascular consequences: An alarming wake-up call? Trends Cardiovasc Med (2020) 30(2):57–69. doi: 10.1016/j.tcm.2019.02.011
66. Biondi B, Cappola AR, Cooper DS. Subclinical hypothyroidism: A review. Jama (2019) 322(2):153–60. doi: 10.1001/jama.2019.9052
67. Kannan L, Shaw PA, Morley MP, Brandimarto J, Fang JC, Sweitzer NK, et al. Thyroid dysfunction in heart failure and cardiovascular outcomes. Circ Heart Fail (2018) 11(12):e005266. doi: 10.1161/CIRCHEARTFAILURE.118.005266
68. Panday P, Arcia Franchini AP, Iskander B, Anwer F, Oliveri F, Kakargias F, et al. Subclinical hypothyroidism in geriatric population and its association with heart failure. Cureus (2021) 13(4):e14296. doi: 10.7759/cureus.14296
69. Garber JR, Cobin RH, Gharib H, Hennessey JV, Klein I, Mechanick JI, et al. Clinical practice guidelines for hypothyroidism in adults: cosponsored by the American association of clinical endocrinologists and the American thyroid association. Thyroid (2012) 22(12):1200–35. doi: 10.1089/thy.2012.0205
70. Stamatouli A, Bedoya P, Yavuz S. Hypothyroidism: Cardiovascular endpoints of thyroid hormone replacement. Front Endocrinol (Lausanne) (2019) 10:888. doi: 10.3389/fendo.2019.00888
71. Bekiaridou A, Kartas A, Moysidis DV, Papazoglou AS, Baroutidou A, Papanastasiou A, et al. The bidirectional relationship of thyroid disease and atrial fibrillation: Established knowledge and future considerations. Rev Endocr Metab Disord (2022) 23(3):621–30. doi: 10.1007/s11154-022-09713-0
72. Elbers LPB, Fliers E, Cannegieter SC. The influence of thyroid function on the coagulation system and its clinical consequences. J Thromb Haemost (2018) 16(4):634–45. doi: 10.1111/jth.13970
73. Keyzer JM, Hoffmann JJ, Ringoir L, Nabbe KC, Widdershoven JW, Pop VJ. Age- and gender-specific brain natriuretic peptide (BNP) reference ranges in primary care. Clin Chem Lab Med (2014) 52(9):1341–6. doi: 10.1515/cclm-2013-0791
74. Redfield MM, Rodeheffer RJ, Jacobsen SJ, Mahoney DW, Bailey KR, Burnett JC Jr. Plasma brain natriuretic peptide concentration: impact of age and gender. J Am Coll Cardiol (2002) 40(5):976–82. doi: 10.1016/S0735-1097(02)02059-4
75. Alibay Y, Schmitt C, Beauchet A, Dubourg O, Alexandre JA, Boileau C, et al. [Non-radioimmunometric NT-ProBNP and BNP assays: impact of diluent, age, gender, BMI]. Ann Biol Clin (Paris) (2005) 63(1):43–9.
76. Knudsen CW, Riis JS, Finsen AV, Eikvar L, Müller C, Westheim A, et al. Diagnostic value of a rapid test for b-type natriuretic peptide in patients presenting with acute dyspnoe: Effect of age and gender. Eur J Heart Fail (2004) 6(1):55–62. doi: 10.1016/j.ejheart.2003.10.006
77. Fuat A, Murphy JJ, Hungin AP, Curry J, Mehrzad AA, Hetherington A, et al. The diagnostic accuracy and utility of a b-type natriuretic peptide test in a community population of patients with suspected heart failure. Br J Gen Pract (2006) 56(526):327–33.
78. Zaphiriou A, Robb S, Murray-Thomas T, Mendez G, Fox K, McDonagh T, et al. The diagnostic accuracy of plasma BNP and NTproBNP in patients referred from primary care with suspected heart failure: Results of the UK natriuretic peptide study. Eur J Heart Fail (2005) 7(4):537–41. doi: 10.1016/j.ejheart.2005.01.022
Keywords: NT-ProBNP, thyroid dysfunction, heart failure, systematic review, meta-analysis
Citation: Zhang H, Li X, Zhang N and Tian L (2023) Effect of thyroid dysfunction on N-terminal pro-B-type natriuretic peptide levels: A systematic review and meta-analysis. Front. Endocrinol. 14:1083171. doi: 10.3389/fendo.2023.1083171
Received: 28 October 2022; Accepted: 10 January 2023;
Published: 26 January 2023.
Edited by:
Federica Cioffi, University of Sannio, ItalyReviewed by:
Pieter de Lange, University of Campania Luigi Vanvitelli, ItalyCopyright © 2023 Zhang, Li, Zhang and Tian. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Limin Tian, dGxtNzA2NkBzaW5hLmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.