
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
PERSPECTIVE article
Front. Endocrinol. , 25 January 2023
Sec. Clinical Diabetes
Volume 14 - 2023 | https://doi.org/10.3389/fendo.2023.1083145
This article is part of the Research Topic Application of Telehealth to Diabetes Care Delivery and Medical Training: Challenges and Opportunities View all 8 articles
 Elizabeth A. Vrany1*
Elizabeth A. Vrany1* Felicia Hill-Briggs1,3
Felicia Hill-Briggs1,3 Patti L. Ephraim1
Patti L. Ephraim1 Alyson K. Myers2,3
Alyson K. Myers2,3 Patricia Garnica4
Patricia Garnica4 Stephanie L. Fitzpatrick1
Stephanie L. Fitzpatrick1Continuous glucose monitors (CGMs) have become an important tool to aid self-management of blood glucose for many patients with diabetes in the U.S., and the benefits of CGM use are well-documented. However, disparities in CGM use exist, with lower use in certain marginalized racial and ethnic groups. CGM may be an important and underutilized tool to help reduce inequities. Evidence supporting the use of CGMs as a part of virtual care is discussed, with an emphasis on designing virtual diabetes care programs to promote health equity. Recommendations for clinical practice and research are presented. In clinical practice, CGM should be an option for all people with diabetes who qualify based on clinical practice guidelines, regardless of race, ethnicity, or other individual characteristics. Future research should characterize the use of, benefit from, and preferences for CGM among individuals from racial and ethnic groups to guide interventions at the health system, clinic, provider, and patient levels to promote equitable, evidence-based, and guideline-directed CGM use in marginalized racial and ethnic groups with diabetes.
Approximately 37 million people in the U.S. had diabetes in the year 2021 (1). Decades of research have documented health disparities in diabetes, with individuals from marginalized racial and ethnic groups experiencing excess risk of diabetes incidence, prevalence, complications, and mortality (2). Improving diabetes management and outcomes in populations of health inequity is a priority for research and public health organizations (3–5). Recent studies demonstrate that, while rates of diabetes-related complications are decreasing in the U.S., rates continue to rise in Black and Hispanic persons with diabetes (6, 7). Lowering blood glucose is directly associated with lower rates of diabetes complications (8), making self-monitoring of blood glucose a key component of diabetes management (9). Continuous glucose monitoring (CGM) has emerged as an important tool to support self-monitoring of blood glucose and may be an important tool to help reduce inequities.
Well-conducted, large randomized controlled trials and prospective studies demonstrate that CGM improves A1C, reduces diabetes-related hospitalizations and emergency room visits, reduces the frequency of dysglycemia, reduces diabetes distress, and improves quality of life in people with diabetes on intensive insulin regimens (10–16). In addition to improving health and well-being, CGMs offer a simplified, automated approach to blood glucose monitoring that removes many hassles of daily diabetes self-management.
Recent reviews have summarized that CGM use is lower in Black/African American and Latinx American populations, relative to the White American population (17, 18). These same marginalized groups engage in lower rates of self-monitoring of blood glucose (19) and face challenges in traditional health care due to limited access to and quality of care, racism and bias in care, and social determinants. CGMs may be an important and underutilized tool to help reduce inequities.
In this paper, we summarize disparities in CGM use, barriers to equitable CGM use, and opportunities for using CGM in diverse populations as a part of virtual diabetes care to help reduce inequities. Additionally, we identify knowledge gaps and provide recommendations for research and clinical practice to promote equitable and guideline-directed diabetes care that leverages CGM, particularly as a part of virtual care.
Several clinical practice guidelines developed by diabetes-focused professional organizations provide recommendations for CGM use for people with diabetes (20–22). The American Diabetes Association’s (ADA) Standards of Medical Care in Diabetes recommends that CGM should be offered to adults and youth with diabetes on multiple daily injections or continuous subcutaneous insulin; they additionally recommend that CGM can be used by adults with diabetes on basal insulin (20). In consensus, the American Association of Clinical Endocrinology’s (AACE) Clinical Practice Guidelines state that CGM is recommended for all persons with diabetes treated with intensive insulin therapy, and CGM may be recommended for individuals with type 2 diabetes (T2D) who are treated with less intensive insulin therapy (21). Uniquely, AACE’s guidelines recommend CGM for individuals with problematic hypoglycemia. Although some recommendations vary across guidelines, CGM is consistently recommended for individuals with diabetes who are treated with intensive insulin regimens, with stipulation that treatment using CGM should be individualized and be offered to those who are willing and capable.
Practice guidelines are clear that CGM use is beneficial for people with T1D across the lifespan (23), and CGM adoption is increasingly common for many people with T1D (24). Practice guidelines are not definitive regarding CGM use in people with T2D. A few clinical trials in people with T2D on intensive insulin regimens have demonstrated that CGM improves hemoglobin A1C and reduces hypoglycemia (12, 14), but little is known about benefits of CGM in individuals on noninsulin or less intensive insulin regimens (25). However, The ADA’s Standards of Care state that routine glucose monitoring may be helpful for adults with T2D who are not on insulin to elucidate the impact of diet, activity, and medication on glucose levels (20).
Despite clinical practice guidelines endorsing CGM use and strong evidence demonstrating the benefit of CGM, rates of CGM adoption remain low, particularly in marginalized groups. Recent reviews summarizing disparities in diabetes technology use conclude that rates of CGM use vary by race and ethnicity, with lower use in historically marginalized racial and ethnic populations (17, 18). To expand upon these reviews, characteristics of the extant studies examining CGM use by race and ethnicity are reported in Table 1. Only one new study has been published since the most recent review in this area (2022) (17). Kanbaour et al., 2023 conducted a retrospective clinic-based cohort study of 1,258 adults with T1D who received care between 2013-2020 (28). The authors report that, relative to non-Black adults, Black adults were less likely to use CGM at baseline and were less likely to initiate CGM over the study period. This study aligns with prior studies in this area, which demonstrate that CGM use is lower in non-Hispanic Black and Hispanic individuals with T1D across all age ranges, relative to non-Hispanic Whites (26, 27, 29, 30). As explained by Agarwal et al., 2022, these disparities persist after adjusting for socioeconomic status, education level, insurance, health literacy, numeracy, diabetes clinical outcomes and management factors, and care setting (17). Therefore, lower use in people with T1D from these marginalized racial and ethnic groups occurs independently of objective clinical decision-making factors.
Factors that have the potential to cause disparities in CGM use among people with T1D and T2D have been proposed (17, 18), including provider, health system/structural, and insurance barriers that cause people with diabetes from marginalized racial and ethnic groups to have less access to CGMs.
Healthcare providers hold an important responsibility to educate patients about their treatment options and engage with patients in shared decision-making. Bias, both implicit and explicit, may contribute to providers’ perceptions of patients’ interest, willingness, capacity, and financial ability to obtain and effectively use CGM devices. Provider implicit bias has been documented across a variety of provider and patient populations (31). Of relevance to CGM use, a few studies document provider implicit bias to recommend diabetes technology based on insurance (32, 33) and race or ethnicity (33). Relatedly, a recent clinic-based retrospective study demonstrated that, relative to non-Black adults, Black adults with T1D were less likely to discuss CGMs with their providers and be prescribed a CGM than non-Black adults (see Table 1) (28). It is plausible that providers may eliminate CGM as an option for members of marginalized groups based on biases, stereotypes, and generalizations regarding factors such as health literacy, socioeconomic status, and social contexts affecting their ability to take on new treatment regimens; however, this is an area requiring further study. Critically, these perceived barriers to using CGM are the same reasons why CGM is important to use in marginalized populations with diabetes who may benefit from automated and simplified daily diabetes routines.
People with diabetes may not be aware that CGM is an option or that insurance may cover the cost of the device. This may be especially the case among marginalized populations with limited healthcare access and suboptimal quality of care (34–38). Social determinants of health are systemic, structural barriers caused by the conditions in which people are born, grow, work, live, and age (39). Social determinants of health include socioeconomic status, neighborhood and physical environment, food environment, health care access/affordability/quality, and social contexts (40). In the U.S., these social determinants adversely affect marginalized populations and are directly associated with worse diabetes-related outcomes (40). In the setting of structural barriers to optimal diabetes management, it is even more imperative that the most effective treatment tools, including CGM, be made available.
The high cost of CGM and restrictive insurance policies are a barrier to CGM use. Based on data from the T1D Exchange, the most common barriers to CGM initiation and use are the cost of CGM and insurance coverage (41, 42). Insurance policies impose restrictions on who is eligible for CGM and require rigorous documentation from providers to demonstrate medical necessity (43, 44), requiring patients to have high-quality and consistent care by knowledgeable providers to facilitate CGM insurance coverage. Additionally, some insurance policies require patients to obtain CGMs through durable medical equipment suppliers (43), rather than through pharmacies in local communities. There is evidence demonstrating that obtaining CGM as a pharmacy benefit is faster than through durable medical equipment companies, thus reducing time-to-initiation of CGM (45). As added challenges, insurance policies for CGM coverage vary by insurance provider and evolve in response to advances in diabetes technology and most recently the COVID-19 pandemic. In response to the pandemic, the Centers for Medicare and Medicaid (CMS) updated policies to reduce barriers to CGM access by eliminating requirements for in-person visits, lab tests, and documented finger sticks (46). However, it is unclear whether these changes will persist, and challenges remain (47). Some private insurance does not cover CGM for T2D (48). Emerging evidence indicates that access to CGM varies by region within the US due, in part, to insurance coverage (49). Illustratively, Southeast states (e.g., Texas, Arkansas, Mississippi) have the lowest CGM use through Medicaid in the US (49). Variable and limited use of CGM in Medicaid beneficiaries may be due to variability in policies by state (43). As of 2022, Medicaid in 40 states covers CGM in some capacity, with variability in coverage based on diabetes-specific documentation (e.g., documentation of hypoglycemic episodes, hypoglycemia unawareness, and insulin pump use), prescriber qualifications (e.g., some states limit to endocrinologists only), need for preauthorization, coverage for people with type 2 diabetes, coverage for children, and locale of prescription fill (durable medical equipment supplier versus pharmacy). In July 2021, the requirement of documenting 4 blood glucose measurements via fingerstick per day was eliminated to increase access to CGM, particularly in the context of the COVID-19 pandemic. Medicaid policies by state are discussed comprehensively in a report from the Center for Healthcare Strategies (43). Critically, Medicaid enrollees are least likely to use a CGM, with particularly low rates of use among Black Americans and Hispanic individuals (50), highlighting the potential impact of insurance policies on CGM use disparities.
The COVID-19 pandemic precipitated an abrupt shift toward virtual care for ambulatory health services. In the post-pandemic era, there continues to be a role for telehealth and health technology, which improve care in some instances and circumvent barriers such as limited access, transportation, or time to attend medical visits (51, 52). Clinical practice guidelines for diabetes recommend visits with a provider every 3-6 months to measure hemoglobin A1C, conduct a physical exam, measure vitals, and review the treatment plan (53). It has been proposed that telehealth can reduce the frequency of in-person visits for some patients with diabetes (54). However, telehealth limits the physician’s ability to conduct physical exams and measure clinical values. To augment telehealth, there has been interest in the use of technology for remote patient monitoring.
In diabetes virtual care, CGM devices allow for remote monitoring of blood glucose. Blood glucose values can automatically be collected, uploaded, and accessible to providers, allowing for real-time monitoring between visits and providing a wealth of data to guide treatment decision-making. Moreover, time spent interpreting CGM data is billable through insurance, promoting the sustainability of provider review of blood glucose records (44). For people with diabetes, CGM as a part of diabetes virtual care has the potential to empower patients to leverage their blood glucose data to guide daily decisions about diabetes self-management behaviors between visits.
Evidence suggests that it is feasible and acceptable to implement CGM remotely via telehealth without the need for in-office visits. A qualitative study among parents of youth with T1D demonstrated that telehealth CGM initiation was well-accepted (55). Another study in a small sample (n=34) of predominantly White (85%) adults with T1D and T2D using insulin demonstrated that the telehealth CGM initiation, delivered by a diabetes educator, was feasible and improved A1C and diabetes distress (56). Additionally, a study among adults with T2D found that a virtual diabetes clinic that incorporated a mobile application, telehealth visits with an endocrinologist, and CGM use improved A1C and reduced hyperglycemia and diabetes distress (57, 58). These findings suggest that virtual models of diabetes care leveraging CGM can work, although larger trials should be conducted in more representative samples. It is the case, however, that in practice CGM initiation is frequently done via self-initiation with online video instruction and education provided by the device manufacturers.
Although CGMs can operate without a smartphone or internet access (i.e., by using a reader to obtain glucose data from the CGM sensor), CGM use is optimal when people can view their glucose data on their smartphones and share their blood glucose data with their providers using an internet connection. Therefore, the use of continuous glucose monitors relies, in great part, on access to and proficiency with using smartphones and the internet. Rates of smartphone ownership and internet access in the US are increasing, but unique trends that vary by race and ethnicity and location warrant attention (see Table 2). Within the last decade, rates of smartphone ownership increased from 56% in 2013 (59) to 81% in 2019 (60). In 2019, rates of smartphone use were generally similar across race and ethnicity groups, but use appeared lower among rural relative to urban and suburban locations. In contrast, broadband internet access has remained relatively stable over time, with only slight increases between 2013 (61) and 2019 (60). Notably, internet access at home appears lower in Black or African American and Hispanic individuals relative to White individuals and lower in rural relative to urban and suburban locations. There has been a stark increase over time in “Smartphone Only” internet use. Between 2013 (61) and 2019 (60), rates of accessing the internet at home with only a smartphone increased from 8% to 17% among US adults, with higher rates in Black or African American and Hispanic individuals relative to White individuals and higher rates in rural and urban relative to suburban locations. In sum, rates of smartphone use are on the rise. Although members of racial and ethnic minoritized groups continue to have limited broadband internet access, they are emergingly accessing smartphones and relying on their smartphones for internet access from home.
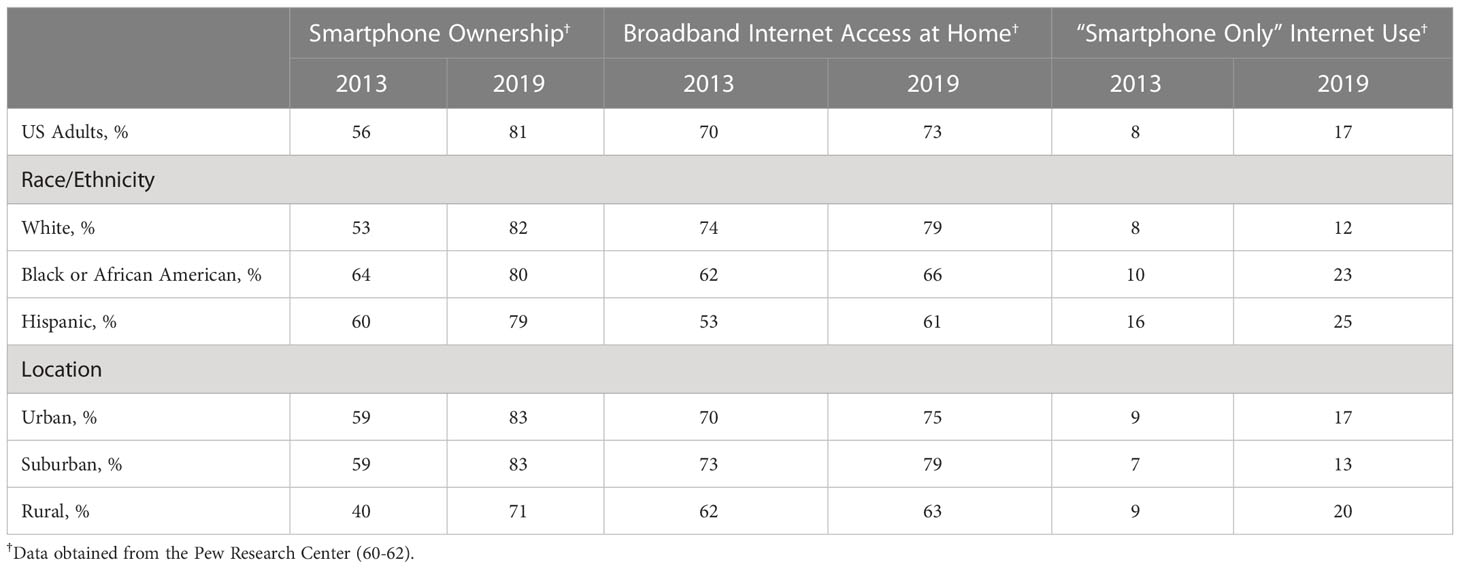
Table 2 Smartphone ownership and internet access patterns in the U.S. by race and ethnicity and by location, 2013-2019.
Trends in smartphone ownership and internet access should be considered as efforts are taken to promote equitable CGM use and diabetes technology use. Health care team members should discuss smartphone and internet access with patients when collaboratively evaluating the option of using CGM. Researchers using CGM in their studies should confirm smartphone ownership and internet access for their participants and, in cases where access is limited, provide connected devices to circumvent selective recruitment based on access. Health systems and policymakers should attend to these trends and disparities in the use of and access to devices and the internet, particularly as technology and telehealth continue to become an important part of healthcare delivery.
It is a common assumption that virtual care models have the potential to address barriers faced by marginalized populations. For instance, virtual care has the capacity to improve access to health care providers and clinics, eliminate transportation barriers, and allow appointments to be conducted where people live and work, thus reducing conflicts due to work schedules and personal/family responsibilities. Yet, it has been documented that virtual care can increase healthcare disparities (62–64). Commonly discussed is the “digital divide,” a term that describes disparities in access to digital devices and internet connection (65). Even among those with access to devices, there are further disparities in digital literacy (i.e., knowledge and skills to use technology effectively) (66–68) that may contribute to disparities in technology use outcomes. Moreover, accessing and using CGM technology may be limited by language barriers and device compatibility, as some CGM applications are available in English only and are compatible with a limited range of smartphone devices and operating systems (69).
To prevent disparities in access to, use of, and outcomes of virtual care, telehealth and health technology should be intentionally designed to promote equity. Weiss et al. report that the impact of health technology on health disparities depends on a particular community’s context and pathways through which they use and access the technology (70). Additionally, African American individuals expressed that past abuses by the U.S. medical system affect their views on new and innovative medical care (71). Shaw et al. provide recommendations to improve health equity in virtual care in the context of COVID-19 (64). Key recommendations were to engage marginalized community members in the planning and evaluation of virtual care programs, simplify complex interfaces and workflows, and leverage supportive intermediaries to help patients engage with virtual care. These recommendations are applicable to integrating CGM use in a virtual care environment with marginalized groups.
In order to design virtual care models using CGM that are effective and meaningful for people with diabetes from marginalized racial and ethnic groups, we must first characterize rates of CGM use, benefits of CGM use, and patient preferences around CGMs and diabetes virtual care, within each race and ethnic group. Research funding should be directed specifically to supporting research in marginalized populations. Consistent with these needs, research recommendations are summarized in Table 3 and described below.

Table 3 Research and clinical recommendations for CGM use in marginalized populations as a component of diabetes virtual care.
First, research is needed to examine the rates of CGM use within marginalized groups with T1D and T2D, including Black/African American, Native American, Latinx American, and Asian American groups. Although studies of disparities in CGM use provide a signal of low use in some groups with T1D, no study has reported rates of use in Native American and Asian American groups with T1D, and no study has reported rates of use in people with T2D by race and ethnicity.
Second, research is needed to characterize the effect of CGM use on diabetes-related clinical, behavioral, and psychosocial outcomes within marginalized populations, including Black or African American, Native American, Latinx American, and Asian American groups. It is well-established that CGM improves clinical and behavioral outcomes on the aggregate, but, to our knowledge, no study has reported the benefits of CGM in each racial and ethnic group. This represents a critical gap in our understanding of the potential benefit of CGM in diverse communities with diabetes.
Third, there is a need to conduct qualitative research to understand diverse patient perspectives on CGM use and diabetes virtual care. Soliciting patient perspectives will elucidate the preferences, barriers, and needs of diverse communities related to the use of diabetes technology and telehealth, which will guide the development of interventions and clinical operations at the health system, provider, and patient levels to promote equitable, guideline-directed care using CGMs. Illustratively, in a qualitative analysis of Black and Latinx individuals who dropped out of a diabetes telehealth study, themes emerged around disinterest, inconvenience, and lack of perceived benefit (72). In the broader diabetes literature, qualitative studies document patient preferences and perspectives. A study among African American adults with diabetes identified that shared decision-making was affected by providers’ bias, discrimination, and cultural discordance as well as patients’ mistrust of White physicians and internalized racism (73). A study among predominantly Mexican American people with diabetes reported that the telephone-based intervention approach may be impersonal and may impede the establishment of a trusting bond (74). Additionally, providers’ cultural and linguistic competence is essential to develop a trusting patient-provider relationship for Hispanic adults with diabetes (75). Another qualitative study reported that African American and Latino individuals share concerns about confidentiality and the physical absence of the provider in telemedicine (71). This collection of findings provides insights, but future qualitative research should directly examine preferences related to CGM use and diabetes virtual care.
Finally, preliminary evidence demonstrates that CGM can be initiated via telehealth (56) and that diabetes virtual care that incorporates CGM is feasible and improves outcomes (57, 58). However, there is a need to design, evaluate, and implement culturally relevant and meaningful interventions for CGM use as a component of virtual care, based on the formative research, above, and in alignment with clinical practice guidelines.
In clinical practice, increasing CGM access and use in diverse populations will require widespread changes for health systems, clinics, and providers. Fundamentally, CGM should be offered to all patients who may qualify based on clinical practice guidelines, regardless of race, ethnicity, or other individual characteristics. Implicit bias and discrimination in health care may impact providers’ prescribing practices for diabetes technology (32, 33), even among qualified and well-meaning providers. Interventions to reduce bias in care increase provider awareness but do not result in sustained behavior change (76). To circumvent provider bias in CGM prescription, population-based approaches can be developed to systematically provide education about the option of CGM to all people with diabetes and identify the population of patients who may qualify for CGM based on clinical practice guidelines. For instance, patient registries can be developed from the electronic medical record to identify patient populations (e.g., diagnosed with T1D or T2D and on intensive insulin regimens). Members of the health care team can engage with every patient with diabetes to provide education on the option of CGM and its benefits/limitations to empower patients with knowledge to effectively engage with providers in shared decision-making.
For patients who will initiate CGM, the healthcare team should deliver evidence-based, meaningful education and support programs for CGM initiation and maintenance that are tailored to the needs, preferences, and challenges of that individual. Members of the healthcare team who engage patients in these conversations should be culturally aware and knowledgeable about CGM. Social determinants of health should be assessed and incorporated into interventions, as they influence many facets of diabetes treatment and decision-making.
Marginalized populations face barriers to obtaining high-quality care. The shift to virtual care in the wake of the COVID-19 pandemic presented an opportunity to address these barriers through telehealth and technology. Diabetes virtual care should be designed to promote equity by involving marginalized community members in planning and evaluation to ensure the programs align with the community’s needs and preferences. Virtual care should consider device access and digital literacy and should engender a trusting relationship in the absence of in-person interaction. CGM devices can be incorporated into diabetes virtual care to augment remote monitoring of blood glucose for providers and patients to leverage as a part of shared decision-making and diabetes management.
Disparities in access to and use of CGM in historically marginalized racial and ethnic populations contribute to widening of, rather than reduction in, long-standing disparities in diabetes outcomes in the U.S. It is well-established that CGM use improves the health and well-being of many patients with diabetes (11, 15, 16). However, there is a need to increase access to CGM and to characterize the use and potential benefit of CGM use in diverse populations.
The causes of disparities in CGM use are complex and multifactorial, and strategies to address these disparities will require widespread changes, including policy changes, with multilevel interventions at the health system, provider, and patient levels. Yet, CGMs may be particularly beneficial for marginalized populations with diabetes, who stand to benefit the most from improved blood sugar management and simplified, automated approaches to daily diabetes management. CGMs may be an important and underutilized tool to help reduce inequities in diabetes care and outcomes, particularly when used in virtual diabetes care.
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
EV contributed to the conceptualization, literature review, synthesis of literature, and draft writing. FH-B, PE, AM, PG, and SF contributed to the conceptualization, article identification, and review of drafts. SF and FH-B additionally contributed to the identification and refinement of recommendations. All authors contributed to the article and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. National diabetes statistics report. estimates of diabetes and its burden in the united states (2021). Available at: https://www.cdc.gov/diabetes/data/statistics-report/index.html.
2. Golden SH, Brown A, Cauley JA, Chin MH, Gary-Webb TL, Kim C, et al. Health disparities in endocrine disorders: biological, clinical, and nonclinical factors–an endocrine society scientific statement. J Clin Endocrinol Metab (2012) 97(9):E1579–639. doi: 10.1210/jc.2012-2043
3. Thornton PL, Kumanyika SK, Gregg EW, Araneta MR, Baskin ML, Chin MH, et al. New research directions on disparities in obesity and type 2 diabetes. Ann N Y Acad Sci (2020) 1461(1):5–24. doi: 10.1111/nyas.14270
4. National Institute of Diabetes and Digestive and Kidney Diseases. Developing the inaugural NIDDK health disparities and health equity research implementation plan . Available at: https://www.niddk.nih.gov/about-niddk/strategic-plans-reports/developing-inaugural-niddk-health-disparities-health-equity-research-implementation-plan.
5. Centers for Disease Control and Prevention. Advancing health equity (2022). Available at: https://www.cdc.gov/diabetes/health-equity/index.html.
6. Haw JS, Shah M, Turbow S, Egeolu M, Umpierrez G. Diabetes complications in racial and ethnic minority populations in the USA. Curr Diabetes Rep (2021) 21(1):2. doi: 10.1007/s11892-020-01369-x
7. Chiou T, Tsugawa Y, Goldman D, Myerson R, Kahn M, Romley JA. Trends in racial and ethnic disparities in diabetes-related complications, 1997-2017. J Gen Intern Med (2020) 35(3):950–1. doi: 10.1007/s11606-019-05308-9
8. Gaster B, Hirsch IB. The effects of improved glycemic control on complications in type 2 diabetes. Arch Intern Med (1998) 158(2):134–40. doi: 10.1001/archinte.158.2.134
9. American Diabetes Association Professional Practice C, Draznin B, Aroda VR, Bakris G, Benson G, Brown FM, et al. 6. glycemic targets: Standards of medical care in diabetes-2022. Diabetes Care (2022) 45(Suppl 1):S83–96. doi: 10.2337/dc22-S006
10. Bergenstal RM, Kerr MSD, Roberts GJ, Souto D, Nabutovsky Y, Hirsch IB. Flash CGM is associated with reduced diabetes events and hospitalizations in insulin-treated type 2 diabetes. J Endocr Soc (2021) 5(4):bvab013. doi: 10.1210/jendso/bvab013
11. Charleer S, Mathieu C, Nobels F, De Block C, Radermecker RP, Hermans MP, et al. Effect of continuous glucose monitoring on glycemic control, acute admissions, and quality of life: A real-world study. J Clin Endocrinol Metab (2018) 103(3):1224–32. doi: 10.1210/jc.2017-02498
12. Dicembrini I, Mannucci E, Monami M, Pala L. Impact of technology on glycaemic control in type 2 diabetes: A meta-analysis of randomized trials on continuous glucose monitoring and continuous subcutaneous insulin infusion. Diabetes Obes Metab (2019) 21(12):2619–25. doi: 10.1111/dom.13845
13. Fokkert M, van Dijk P, Edens M, Barents E, Mollema J, Slingerland R, et al. Improved well-being and decreased disease burden after 1-year use of flash glucose monitoring (FLARE-NL4). BMJ Open Diabetes Res Care (2019) 7(1):e000809. doi: 10.1136/bmjdrc-2019-000809
14. Ida S, Kaneko R, Murata K. Utility of real-time and retrospective continuous glucose monitoring in patients with type 2 diabetes mellitus: A meta-analysis of randomized controlled trials. J Diabetes Res (2019) 2019:4684815. doi: 10.1155/2019/4684815
15. Maiorino MI, Signoriello S, Maio A, Chiodini P, Bellastella G, Scappaticcio L, et al. Effects of continuous glucose monitoring on metrics of glycemic control in diabetes: A systematic review with meta-analysis of randomized controlled trials. Diabetes Care (2020) 43(5):1146–56. doi: 10.2337/dc19-1459
16. Polonsky WH, Hessler D, Ruedy KJ, Beck RW, Group DS. The impact of continuous glucose monitoring on markers of quality of life in adults with type 1 diabetes: Further findings from the DIAMOND randomized clinical trial. Diabetes Care (2017) 40(6):736–41. doi: 10.2337/dc17-0133
17. Agarwal S, Simmonds I, Myers AK. The use of diabetes technology to address inequity in health outcomes: Limitations and opportunities. Curr Diabetes Rep (2022) 22(7):275–81. doi: 10.1007/s11892-022-01470-3
18. Isaacs D, Bellini NJ, Biba U, Cai A, Close KL. Health care disparities in use of continuous glucose monitoring. Diabetes Technol Ther (2021) 23(S3):S81–S7. doi: 10.1089/dia.2021.0268
19. Levine DA, Allison JJ, Cherrington A, Richman J, Scarinci IC, Houston TK. Disparities in self-monitoring of blood glucose among low-income ethnic minority populations with diabetes, united states. Ethn Dis (2009) 19(2):97–103.
20. American Diabetes Association Professional Practice Committee, Draznin B, VR A, Bakris G, Benson G, FM B, et al. 7. diabetes technology: Standards of medical care in diabetes-2022. Diabetes Care (2022) 45(Suppl 1):S97–S112. doi: 10.2337/dc22-S007
21. Grunberger G, Sherr J, Allende M, Blevins T, Bode B, Handelsman Y, et al. American Association of clinical endocrinology clinical practice guideline: The use of advanced technology in the management of persons with diabetes mellitus. Endocr Pract (2021) 27(6):505–37. doi: 10.1016/j.eprac.2021.04.008
22. Peters AL, Ahmann AJ, Battelino T, Evert A, Hirsch IB, Murad MH, et al. Diabetes technology-continuous subcutaneous insulin infusion therapy and continuous glucose monitoring in adults: An endocrine society clinical practice guideline. J Clin Endocrinol Metab (2016) 101(11):3922–37. doi: 10.1210/jc.2016-2534
23. Mauras N, Fox L, Englert K, Beck RW. Continuous glucose monitoring in type 1 diabetes. Endocrine. (2013) 43(1):41–50. doi: 10.1007/s12020-012-9765-1
24. Galindo RJ, Aleppo G. Continuous glucose monitoring: The achievement of 100 years of innovation in diabetes technology. Diabetes Res Clin Pract (2020) 170:108502. doi: 10.1016/j.diabres.2020.108502
25. Dabbagh Z, McKee MD, Pirraglia PA, Clements KM, Liu F, Amante DJ, et al. The expanding use of continuous glucose monitoring in type 2 diabetes. Diabetes Technol Ther (2022) 24(7):510–5. doi: 10.1089/dia.2021.0536
26. Agarwal S, Schechter C, Gonzalez J, Long JA. Racial-ethnic disparities in diabetes technology use among young adults with type 1 diabetes. Diabetes Technol Ther (2021) 23(4):306–13. doi: 10.1089/dia.2020.0338
27. Foster NC, Beck RW, Miller KM, Clements MA, Rickels MR, DiMeglio LA, et al. State of type 1 diabetes management and outcomes from the T1D exchange in 2016-2018. Diabetes Technol Ther (2019) 21(2):66–72. doi: 10.1089/dia.2018.0384
28. Kanbour S, Jones M, Abusamaan MS, Nass C, Everett E, Wolf RM, et al. Racial disparities in access and use of diabetes technology among adult patients with type 1 diabetes in a U.S. academic medical center. Diabetes Care (2023) 46(1):56–64. doi: 10.2337/dc22-1055
29. Lai CW, Lipman TH, Willi SM, Hawkes CP. Racial and ethnic disparities in rates of continuous glucose monitor initiation and continued use in children with type 1 diabetes. Diabetes Care (2021) 44(1):255–7. doi: 10.2337/dc20-1663
30. Fantasia KL, Wirunsawanya K, Lee C, Rizo I. Racial disparities in diabetes technology use and outcomes in type 1 diabetes in a safety-net hospital. J Diabetes Sci Technol (2021) 15(5):1010–7. doi: 10.1177/1932296821995810
31. FitzGerald C, Hurst S. Implicit bias in healthcare professionals: A systematic review. BMC Med Ethics. (2017) 18(1):19. doi: 10.1186/s12910-017-0179-8
32. Addala A, Hanes S, Naranjo D, Maahs DM, Hood KK. Provider implicit bias impacts pediatric type 1 diabetes technology recommendations in the united states: Findings from the gatekeeper study. J Diabetes Sci Technol (2021) 15(5):1027–33. doi: 10.1177/19322968211006476
33. Odugbesan O, Addala A, Nelson G, Hopkins R, Cossen K, Schmitt J, et al. Implicit racial-ethnic and insurance-mediated bias to recommending diabetes technology: Insights from T1D exchange multicenter pediatric and adult diabetes provider cohort. Diabetes Technol Ther (2022) 24(9):619–27. doi: 10.1089/dia.2022.0042
34. Rudd RE. Health literacy skills of U.S. adults. Am J Health Behav (2007) 31 Suppl 1:S8–18. doi: 10.5993/AJHB.31.s1.3
35. Benkert R, Cuevas A, Thompson HS, Dove-Meadows E, Knuckles D. Ubiquitous yet unclear: A systematic review of medical mistrust. Behav Med (2019) 45(2):86–101. doi: 10.1080/08964289.2019.1588220
36. Boulware LE, Cooper LA, Ratner LE, LaVeist TA, Powe NR. Race and trust in the health care system. Public Health Rep (2003) 118(4):358–65. doi: 10.1016/S0033-3549(04)50262-5
37. Guadagnolo BA, Cina K, Helbig P, Molloy K, Reiner M, Cook EF, et al. Medical mistrust and less satisfaction with health care among native americans presenting for cancer treatment. J Health Care Poor Underserved. (2009) 20(1):210–26. doi: 10.1353/hpu.0.0108
38. Lopez-Cevallos DF, Harvey SM, Warren JT. Medical mistrust, perceived discrimination, and satisfaction with health care among young-adult rural latinos. J Rural Health (2014) 30(4):344–51. doi: 10.1111/jrh.12063
39. Solar O, Irwin A. A conceptual framework for action on the social determinants of health. social determinants of health discussion paper 2 (Policy and practice). Geneva: World Health Organization (2010). Available at: https://apps.who.int/iris/bitstream/handle/10665/44489/?sequence=1.
40. Hill-Briggs F, Adler NE, Berkowitz SA, Chin MH, Gary-Webb TL, Navas-Acien A, et al. Social determinants of health and diabetes: A scientific review. Diabetes Care (2020) 44(1), 258–279. doi: 10.2337/dci20-0053
41. Engler R, Routh TL, Lucisano JY. Adoption barriers for continuous glucose monitoring and their potential reduction with a fully implanted system: Results from patient preference surveys. Clin Diabetes. (2018) 36(1):50–8. doi: 10.2337/cd17-0053
42. Tanenbaum ML, Hanes SJ, Miller KM, Naranjo D, Bensen R, Hood KK. Diabetes device use in adults with type 1 diabetes: Barriers to uptake and potential intervention targets. Diabetes Care (2017) 40(2):181–7. doi: 10.2337/dc16-1536
43. Howe G, Chavis J. Expanding Medicaid access to continuous glucose monitors. Center for Health Care Strategies (2022). Available at: https://www.chcs.org/media/Expanding-Medicaid-Access-to-Continuous-Glucose-Monitors_011222.pdf.
44. Miller EM. Using continuous glucose monitoring in clinical practice. Clin Diabetes. (2020) 38(5):429–38. doi: 10.2337/cd20-0043
45. Modzelewski KL, Murati J, Charoenngam N, Rehm C, Steenkamp DW. Delays in continuous glucose monitoring device initiation: A single center experience and a call to change. Diabetes Technol Ther (2022) 24(6):390–5. doi: 10.1089/dia.2021.0557
46. McAdam-Marx C. Addressing healthcare disparities and managed care considerations with continuous glucose monitoring. Am J Manag Care (2022) 28(4 Suppl):S76–s84. doi: 10.37765/ajmc.2022.89215
47. Hughes A, Vela A, Tynan WD, Fitzpatrick SL. Position statement: Expand U.S. health plan guidelines for coverage of diabetes-related medications and supplies (2021). Available at: https://www.sbm.org/UserFiles/image/Diabetes-brief21_FINAL.pdf.
48. Kruger DF, Anderson JE. Continuous glucose monitoring (CGM) is a tool, not a reward: Unjustified insurance coverage criteria limit access to CGM. Diabetes Technol Ther (2021) 23(S3):S45–55. doi: 10.1089/dia.2021.0193
49. American Diabetes Association. Health equity and diabetes technology: A study of access to continuous glucose monitors by payer, geography and race executive summary (2022). Available at: https://diabetes.org/sites/default/files/2022-10/ADA-CGM-Utilization-White-Paper-Oct-2022.pdf.
50. American Diabetes Association. Health equity and diabetes technology: a study of access to continuous glucose monitors by payer and race executive summary (2021). Available at: https://diabetes.org/sites/default/files/2021-10/ADA%20CGM%20Utilization%20White%20Paper.pdf.
51. Herzer KR, Pronovost PJ. Ensuring quality in the era of virtual care. JAMA. (2021) 325(5):429–30. doi: 10.1001/jama.2020.24955
52. Saeed SA, Masters RM. Disparities in health care and the digital divide. Curr Psychiatry Rep (2021) 23(9):61. doi: 10.1007/s11920-021-01274-4
53. American Diabetes Association Professional Practice Committee, Draznin B, VR A, Bakris G, Benson G, FM B, et al. 4. comprehensive medical evaluation and assessment of comorbidities: Standards of medical care in diabetes-2022. Diabetes Care (2022) 45(Suppl 1):S46–s59. doi: 10.2337/dc22-S004
54. Aberer F, Hochfellner DA, Mader JK. Application of telemedicine in diabetes care: The time is now. Diabetes Ther (2021) 12(3):629–39. doi: 10.1007/s13300-020-00996-7
55. Tanenbaum ML, Zaharieva DP, Addala A, Prahalad P, Hooper JA, Leverenz B, et al. 'Much more convenient, just as effective': Experiences of starting continuous glucose monitoring remotely following type 1 diabetes diagnosis. Diabetes Med (2022) 39(11):e14923. doi: 10.1111/dme.14923
56. Gal RL, Cohen NJ, Kruger D, Beck RW, Bergenstal RM, Calhoun P, et al. Diabetes telehealth solutions: Improving self-management through remote initiation of continuous glucose monitoring. J Endocr Soc (2020) 4(9):bvaa076. doi: 10.1210/jendso/bvaa076
57. Majithia AR, Kusiak CM, Armento Lee A, Colangelo FR, Romanelli RJ, Robertson S, et al. Glycemic outcomes in adults with type 2 diabetes participating in a continuous glucose monitor-driven virtual diabetes clinic: Prospective trial. J Med Internet Res (2020) 22(8):e21778. doi: 10.2196/21778
58. Polonsky WH, Layne JE, Parkin CG, Kusiak CM, Barleen NA, Miller DP, et al. Impact of participation in a virtual diabetes clinic on diabetes-related distress in individuals with type 2 diabetes. Clin Diabetes. (2020) 38(4):357–62. doi: 10.2337/cd19-0105
59. Pew Research Center. Smartphone ownership 2013 (2013). Available at: https://www.pewresearch.org/internet/2013/06/05/smartphone-ownership-2013/.
60. Pew Research Center. Mobile technology and home broadband 2019 (2019). Available at: https://www.pewresearch.org/internet/2019/06/13/mobile-technology-and-home-broadband-2019/.
61. Pew Research Center. Home broadband 2013 (2013). Available at: https://www.pewresearch.org/internet/2013/08/26/home-broadband-2013/.
62. Thronson LR, Jackson SL, Chew LD. The pandemic of health care inequity. JAMA Netw Open (2020) 3(10):e2021767. doi: 10.1001/jamanetworkopen.2020.21767
63. VanderBeek BL. Telemedicine and the exacerbation of health care disparities. JAMA Ophthalmol (2021) 139(11):1182–3. doi: 10.1001/jamaophthalmol.2021.3735
64. Shaw J, Brewer LC, Veinot T. Recommendations for health equity and virtual care arising from the COVID-19 pandemic: Narrative review. JMIR Form Res (2021) 5(4):e23233. doi: 10.2196/23233
65. Litchfield I, Shukla D, Greenfield S. Impact of COVID-19 on the digital divide: a rapid review. BMJ Open (2021) 11(10):e053440. doi: 10.1136/bmjopen-2021-053440
66. Smith B, Magnani JW. New technologies, new disparities: The intersection of electronic health and digital health literacy. Int J Cardiol (2019) 292:280–2. doi: 10.1016/j.ijcard.2019.05.066
67. Campos-Castillo C, Mayberry LS. Chapter 21 - disparities in digital health in underserved populations. In: Klonoff DC, Kerr D, Weitzman ER, editors. Diabetes digital health and telehealth. Academic Press (2022). p. 269–80. https://www.sciencedirect.com/science/article/pii/B9780323905572120011
68. van Dijk JAGM. Digital divide research, achievements and shortcomings. Poetics. (2006) 34(4):221–35. doi: 10.1016/j.poetic.2006.05.004
69. Jaiswal R, Zhang M, Zuniga S, Myers AK. Integration of flash glucose monitoring during the transition of care from inpatient to outpatient settings in patients with type 2 diabetes. J Endocr Soc (2021) 5:A427–A428. doi: 10.1210/jendso/bvab048.872
70. Weiss D, Rydland HT, Øversveen E, Jensen MR, Solhaug S, Krokstad S. Innovative technologies and social inequalities in health: A scoping review of the literature. PloS One (2018) 13(4):e0195447. doi: 10.1371/journal.pone.0195447
71. George S, Hamilton A, Baker RS. How do low-income urban African americans and latinos feel about telemedicine? a diffusion of innovation analysis. Int J Telemed Appl (2012) 2012:715194. doi: 10.1155/2012/715194
72. Tong T, Myers AK, Bissoonauth AA, Pekmezaris R, Kozikowski A. Identifying the barriers and perceptions of non-Hispanic black and Hispanic/Latino persons with uncontrolled type 2 diabetes for participation in a home telemonitoring feasibility study: a quantitative analysis of those who declined participation, withdrew or were non-adherent. Ethn Health (2020) 25(4):485–94. doi: 10.1080/13557858.2019.1566520
73. Peek ME, Odoms-Young A, Quinn MT, Gorawara-Bhat R, Wilson SC, Chin MH. Race and shared decision-making: perspectives of African-americans with diabetes. Soc Sci Med (2010) 71(1):1–9. doi: 10.1016/j.socscimed.2010.03.014
74. Baig AA, Locklin CA, Wilkes AE, Oborski DD, Acevedo JC, Gorawara-Bhat R, et al. "One can learn from other people's experiences": Latino adults' preferences for peer-based diabetes interventions. Diabetes Educ (2012) 38(5):733–41. doi: 10.1177/0145721712455700
75. Rosal MC, Goins KV, Carbone ET, Cortes DE. Views and preferences of low-literate hispanics regarding diabetes education: results of formative research. Health Educ Behav (2004) 31(3):388–405. doi: 10.1177/1090198104263360
Keywords: diabetes, continuous glucose monitor (CGM), disparities, virtual care, race & ethnicity
Citation: Vrany EA, Hill-Briggs F, Ephraim PL, Myers AK, Garnica P and Fitzpatrick SL (2023) Continuous glucose monitors and virtual care in high-risk, racial and ethnic minority populations: Toward promoting health equity. Front. Endocrinol. 14:1083145. doi: 10.3389/fendo.2023.1083145
Received: 28 October 2022; Accepted: 11 January 2023;
Published: 25 January 2023.
Edited by:
Roeland Middelbeek, Joslin Diabetes Center and Harvard Medical School, United StatesReviewed by:
Prasanth Surampudi, University of California, Davis, United StatesCopyright © 2023 Vrany, Hill-Briggs, Ephraim, Myers, Garnica and Fitzpatrick. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Elizabeth A. Vrany, ZXZyYW55QG5vcnRod2VsbC5lZHU=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.