- 1Cardiology Division of Cardiovascular Medical Center, Far Eastern Memorial Hospital, New Taipei City, Taiwan
- 2Graduate Institute of Medicine, Yuan Ze University, Taoyuan, Taiwan
- 3Department of Medical Imaging, National Taiwan University Hospital and National Taiwan University College of Medicine, Taipei, Taiwan
- 4Department of Internal Medicine, National Taiwan University Hospital Yun-Lin Branch, Yun-Lin, Taiwan
- 5Division of Cardiology, Department of Internal Medicine, National Taiwan University Hospital and National Taiwan University College of Medicine, Taipei, Taiwan
- 6Department of Medicine, National Taiwan University Cancer Center, Taipei, Taiwan
- 7Department of Obstetrics and Gynecology, National Taiwan University Hospital and National Taiwan University College of Medicine, Taipei, Taiwan
- 8Department of Nuclear Medicine, National Taiwan University Hospital, Taipei, Taiwan
- 9Division of Nephrology, Department of Internal Medicine, National Taiwan University Hospital and National Taiwan University College of Medicine, Taipei, Taiwan
- 10Cardiovascular Center, National Taiwan University Hospital, Taipei, Taiwan
Background: Primary aldosteronism (PA) is the leading cause of curable endocrine hypertension, which is associated with a higher risk of cardiovascular and metabolic insults compared to essential hypertension. Aldosterone-producing adenoma (APA) is a major cause of PA, which can be treated with adrenalectomy. Somatic mutations are the main pathogenesis of aldosterone overproduction in APA, of which KCNJ5 somatic mutations are most common, especially in Asian countries. This article aimed to review the literature on the impacts of KCNJ5 somatic mutations on systemic organ damage.
Evidence acquisition: PubMed literature research using keywords combination, including “aldosterone-producing adenoma,” “somatic mutations,” “KCNJ5,” “organ damage,” “cardiovascular,” “diastolic function,” “metabolic syndrome,” “autonomous cortisol secretion,” etc.
Results: APA patients with KCNJ5 somatic mutations are generally younger, female, have higher aldosterone levels, lower potassium levels, larger tumor size, and higher hypertension cure rate after adrenalectomy. This review focuses on the cardiovascular and metabolic aspects of KCNJ5 somatic mutations in APA patients, including left ventricular remodeling and diastolic function, abdominal aortic thickness and calcification, arterial stiffness, metabolic syndrome, abdominal adipose tissue, and correlation with autonomous cortisol secretion. Furthermore, we discuss modalities to differentiate the types of mutations before surgery.
Conclusion: KCNJ5 somatic mutations in patients with APA had higher left ventricular mass (LVM), more impaired diastolic function, thicker aortic wall, lower incidence of metabolic syndrome, and possibly a lower incidence of concurrent autonomous cortisol secretion, but better improvement in LVM, diastolic function, arterial stiffness, and aortic wall thickness after adrenalectomy compared to patients without KCNJ5 mutations.
Introduction
Primary aldosteronism and its impact on the cardiovascular system and metabolic system
Primary aldosteronism (PA) was considered to be a rare disease but is now recognized to be the most common modifiable form of secondary hypertension (1–3). The prevalence ranges from 5% to 15% in hypertensive patients, and up to 20%-30% in those with resistant and refractory hypertension (4, 5). The two most common causes of PA are aldosterone-producing adenoma (APA), accounting for about 30-35% of all PA patients (6, 7) which can be treated by adrenalectomy, and bilateral adrenal hyperplasia (BAH), accounting for approximately 60-65% of patients with PA and is often treated with medications. The aldosterone overproduction in PA patients causes both cardiac structural changes, including left ventricular hypertrophy and remodeling (8), and declines in diastolic and systolic function (9). In addition, aldosterone-induced endothelial dysfunction plays an important role in vascular fibrosis and cellular hypertrophy, resulting in increased arterial stiffness (10–12). Clinically, PA is associated with higher cardiovascular morbidity and mortality, including stroke, coronary artery disease, atrial fibrillation, and heart failure, compared with essential hypertension (EH) (13–22). Moreover, aldosterone impairs glucose-stimulated insulin secretion and insulin sensitivity in skeletal muscle and adipocytes (23), which contributes to insulin resistance in humans (24). The prevalence of metabolic syndrome is also higher in PA patients compared to EH patients (25).
Material and methods
We conducted a PubMed literature search, using a broad range of keywords combination, including “primary aldosteronism,” “aldosterone-producing adenoma,” “somatic mutations,” “pathogenesis,” “CYP11B2,” “KCNJ5,” “ATP1A1,” “ATP2B3,” “CLCN2,” “CACNA1D,” “CACNA1H,” “CTNNB1,” “organ damage,” “cardiovascular,” “cardiac,” “vascular,” “left ventricular,” “left ventricular mass,” “arterial stiffness,” “pulse wave velocity,” “diastolic function,” “metabolic syndrome,” “aortic wall,” “calcification,” “abdominal obesity,” “autonomous cortisol secretion,” “subclinical Cushing’s syndrome,” “adrenal vein sampling,” “NP59,” “steroid profiling.” We focused on various trials discussing the somatic mutations in APA and their impacts on clinical presentations published from 2011 to March 31, 2022. The retrieved articles were hand-selected according to the relevance cautiously. The reference lists of these selected articles were attentively reviewed to look for additional publications.
The pathogenesis and types of somatic mutations in APA
The primary mineralocorticoid, aldosterone, is synthesized in the outer zone of the adrenal cortex, the zona glomerulosa (ZG) (26). In the ZG, 11-deoxycorticosterone is converted sequentially to corticosterone, 18-hydroxycorticosterone, and then aldosterone, catalyzed by the enzyme aldosterone synthase (encoded by the gene CYP11B2) (27). The production of aldosterone is normally regulated by angiotensin II (Ang II), serum potassium, and adrenocorticotropic hormone (ACTH) (28). However, the autonomous excess secretion of aldosterone in PA is independent of these factors.
In 2011, Lifton et al. were the first to report a somatic mutation in patients with APA (29). Somatic mutations occur in normal somatic cells, such as the adrenal gland, so that such mutations do not pass from parents to offspring. The location of the somatic mutation discovered by Lifton et al. is in the KCNJ5 gene (Potassium Inwardly Rectifying Channel Subfamily J Member 5), which encodes G-protein-activated inward rectifier potassium channel (GIRK4). This potassium channel mediates the outward current of potassium ions to maintain hyperpolarization and stabilize resting membrane potential (30). However, mutations of the KCNJ5 gene result in the channel losing its selectivity for potassium ions and increase the entry of extracellular sodium ions into the cell, causing the cell membrane depolarization, which causes the opening of voltage-gated calcium ion channel and allowing calcium ions to enter the cell. The increase in intracellular calcium ions induces transcription of the CYP11B2 gene through the activation of calcium signaling. Activation of aldosterone synthase causes excessive production of aldosterone and PA. Many somatic mutation sites of KCNJ5 have now been identified, most of which can affect the selectivity of the GIRK4 channel (Figure 1), thus resulting in cell membrane potential depolarization, and ultimately increasing aldosterone production.

Figure 1 Somatic mutations related pathogenesis of aldosterone producing adenomas. *PMCA3: plasma membrane calcium-transporting ATPase 3.
In addition, many other somatic mutations in APAs have been found to cause an increase in intracellular calcium ion concentration and result in aldosterone overproduction via various pathways, including ATP2B3 (encoding the plasma membrane calcium-transporting ATPase 3, PMCA3) (31), CLCN2 (encoding the chloride channel 2, ClC-2) (32), CACNA1D (encoding the voltage-gated calcium channels Cav1.3) (33) and CACNA1H (encoding Cav3.2) (34) genes (Figure 1). While the somatic mutations of ATP1A1(encoding the α1 subunit of Na+/K+ ATPase) genes result in increased intracellular Na+ concentration and H+ influx, which cause cells depolarized, but without significantly increased intracellular Ca2+ activity, and intracellular acidification which stimulate aldosterone production (31, 35). Mutations of CTNNB1 gene (encoding β-catenin) have been shown to decrease the degradation of β-catenin, which causes an increase in the synthesis of aldosterone by regulating the WNT/β-catenin signaling pathway (36). In a recent study, double mutation of CTNNB1 and GNA11 (encoding the G protein subunit alpha11) or GNAQ (encoding the G protein subunit alpha q) results in upregulation of LHCGR (encoding the luteinizing hormone/choriogonadotropin receptor) and increase of aldosterone production (37).
Prevalence of somatic mutations
The incidence of somatic mutations reported in a large European multicenter study of 474 patients with APA ranged from 27.2% to 56.8%, including 38% with KCNJ5, 9.3% with CACNA1D, 5.3% with ATP1A1, and 1.7% with ATP2B3 mutations (38). In our previous study in Taiwan, we found that 128 of 219 (58.4%) patients with APA had somatic mutations, most of which were KCNJ5 mutations (52.9%), followed by CTNNB1 (3.7%), ATP1A1 (1.4%), and ATP2B3 (0.5%) (39). The expression of KCNJ5 mutation differs in different ethnic groups. In Asia (Taiwan, Japan (40)) the prevalence of KCNJ5 somatic mutations in APA patients is up to 55-75%, compared to only about 25-50% in western countries (41). These data are mainly based on adrenal specimens from random biopsy and Sanger sequencing.
In recent years, with the progress in CYP11B2 immunohistochemical (IHC) staining-guided biopsy and next-generation gene sequencing (NGS), Rainey et al. reported that the detection rate of somatic mutations was nearly 90% (42). De Sousa et al. rechecked 14 specimens which were found to be negative for somatic mutations using traditional methods (random biopsy and Sanger sequencing) with CYP11B2-IHC staining-guided biopsy, and detected 11 more somatic mutations, thereby increasing the detection rate of somatic mutations from 71% (by traditional methods) to 94% (IHC-guided biopsy and NGS) (43). Interestingly, the newly detected mutations included 8 in the CACN1D gene, 2 in the ATP1A1 gene, and 1 in the ATP2B3 gene, but no new KCNJ5 mutations were detected. In addition, they also found that KCNJ5 somatic mutations could be detected in high, low, or even non-CYP11B2 IHC stained areas. These findings show that CYP11B2 staining-guided biopsy is an important tool to detect somatic mutations in PA patients, especially non-KCNJ5 somatic mutations.
As shown in Table 1, the incidence of KCNJ5 mutations in Asian (54, 55) populations is much higher than that in European (43) and American populations (42, 53), regardless of detection by traditional random biopsy with Sanger sequencing or by CYP11B2 IHC staining-guided biopsy and NGS. We previously found that KCNJ5 somatic mutations were predominant in patients with APA harboring somatic mutations [KCNJ5 accounts for 90.6% of APA patients with somatic mutations (39) detected by traditional methods, 81.2% of APA patients with somatic mutations by sequencing combined with Sanger and IHC-guided biopsy and NGS (55)]. Besides, the majority of studies investigating the clinical impacts of somatic mutations in APA patients are almost limited to comparing “KCNJ5 mutations” with “non- KCNJ5 mutations” (40, 41, 46, 48, 57–60). The clinical data of other somatic mutations in APA patients is rare. Therefore, in this article, we focus on the clinical impacts of KCNJ5 somatic mutations in patients with APA.
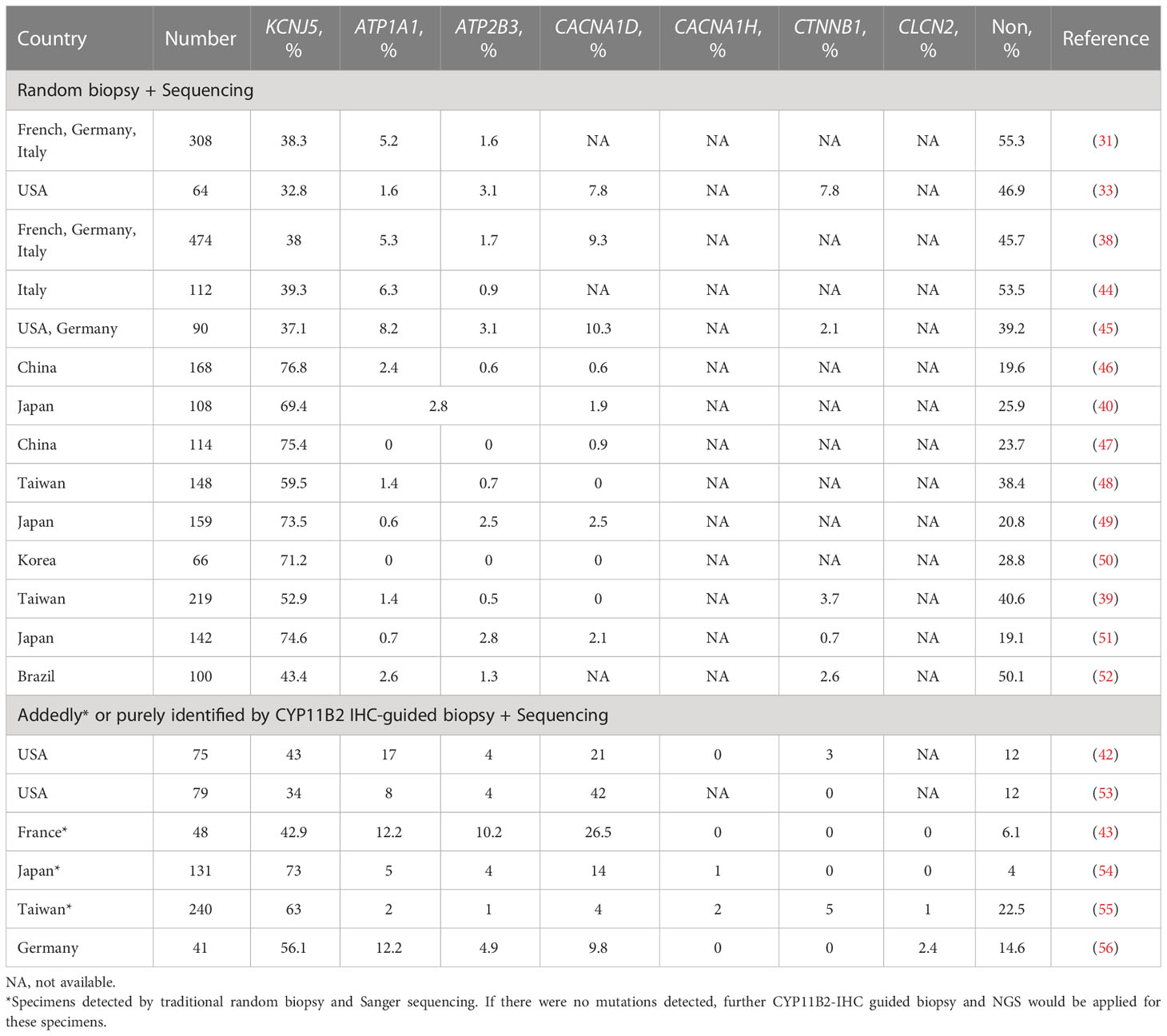
Table 1 The detection rats of various somatic mutations in different countries dependent on traditional random biopsy or CYP11B2-IHC staining guided biopsy.
KCNJ5 somatic mutations
Mutation sites of KCNJ5
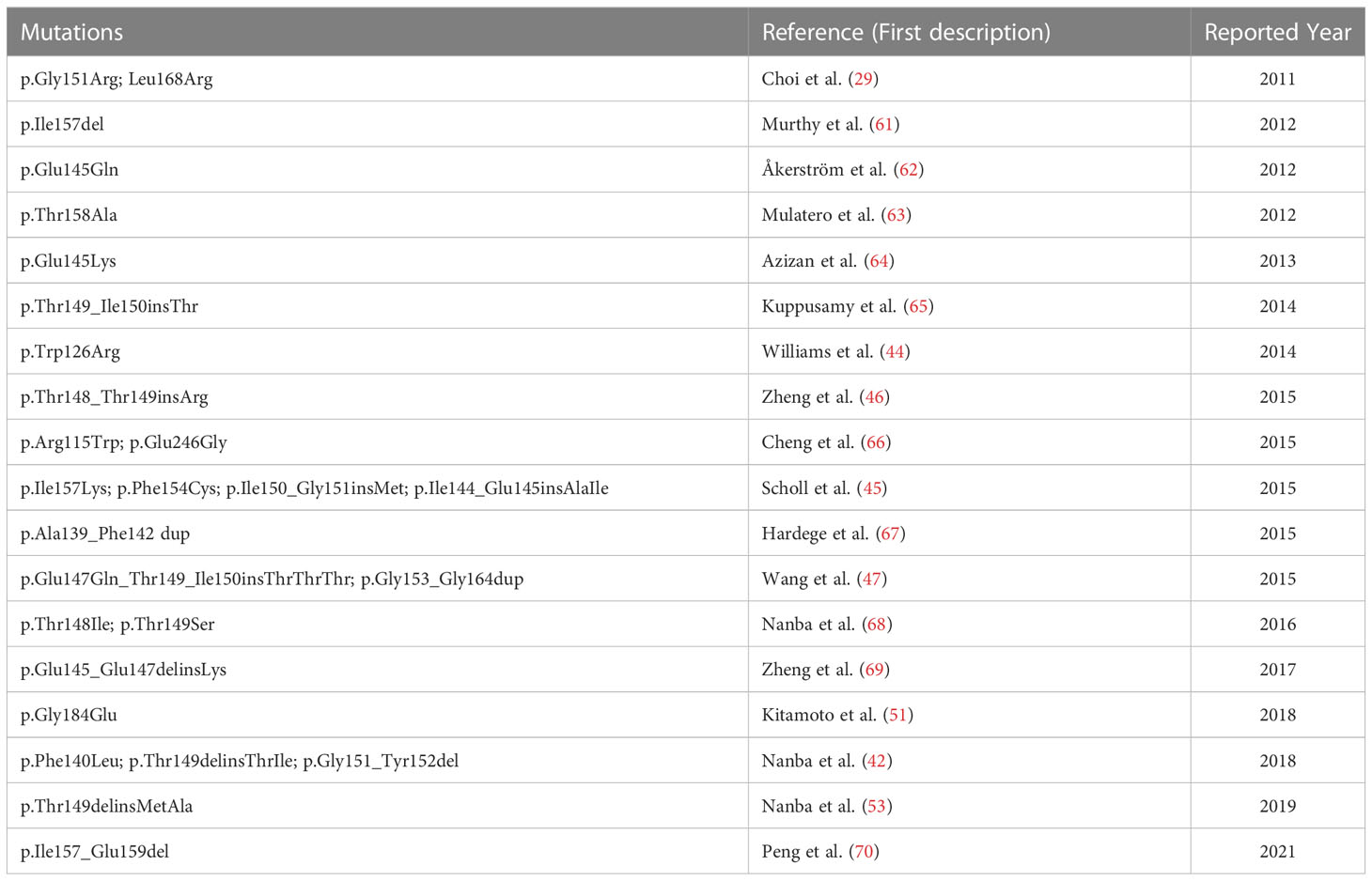
The locations of most KCNJ5 somatic mutations are within or near the potassium selectivity filter (among T149-G153) of the GIRK4 protein. The previously reported mutation sites in the literature are shown in Table 2.
Basic clinical characteristics
In a meta-analysis study including 13 studies and 1636 patients, the patients with KCNJ5 somatic mutations were significantly younger (45 ± 3 vs 52 ± 5 years), predominantly female (67% vs 44%), and had higher aldosterone level (42 ± 8 vs 33 ± 8 ng/dl), and larger tumor size (16.1 ± 6.4 versus 14.9 ± 7.4 mm) (71). In addition, lower potassium levels (38, 40, 48) and higher cure rates after adrenalectomy (48, 57) have also been reported in patients with KCNJ5 somatic mutations in other studies compared to patients without KCNJ5 mutations. Asian patients with KCNJ5 somatic mutations have similar characteristics (48) but without differences in sex and tumor size compared to patients without KCNJ5 somatic mutations (39, 46, 50, 72). Due to differences in the incidence of KCNJ5 somatic mutations between Eastern and Western populations, the cure rate of hypertension post-adrenalectomy may also be affected by ethnicity. Investigations of the impacts of KCNJ5 mutations on symptoms, prognosis, and even systemic target organ damage, including cardiovascular structure and function, and metabolic disorders, may affect the treatment strategy of patients.
Relationships of KCNJ5 somatic mutations with cardiac structure and function
Left ventricular mass
In animal studies, excessive aldosterone with salt intake has been shown to increase bilateral ventricular fibrosis (73–75) and left ventricular hypertrophy (73, 75). Clinical studies have also revealed that patients with PA have higher rates of cardiac fibrosis and left ventricular hypertrophy than patients with EH (76–78). LVM can be roughly divided into predicted LVM (pLVM) (79–81) and inappropriately excessive LVM (ieLVM) (80, 82–84), denoting the hemodynamic and non-hemodynamic contributions to LVM, respectively. Inappropriate LVM has been shown to be higher in APA patients compared with EH patients and to decrease after adrenalectomy in APA patients (82).
Only some studies have discussed the impact of KCNJ5 somatic mutations on the left ventricular structure. Rossi et al. (41) were the first to report that APA patients with KCNJ5 mutations had a higher LVM index (LVMI) than patients without mutations, even with a higher ratio of female patients. A similar trend albeit without statistical significance was also noted in another study in China, in which patients with KCNJ5 mutations had higher baseline systolic blood pressure, which may have led to an increase in LVMI (47). Interestingly, other studies have not shown a difference in LVMI between patients with and without mutations (40, 46, 48). This discrepancy may be due to differences in age, sex, hypertension duration, or the number of antihypertensive drugs between the studies, which may have influenced LVM.
In our previous study, after matching for age, sex, body mass index (BMI), and hypertension status, KCNJ5 mutation carriers had a higher aldosterone level, LVMI, and inappropriately excessive LVMI (ieLVMI) than non-carriers (57). We also found that the increased LVMI in KCNJ5 mutation carriers was mainly attributable to ieLVMI via a non-hemodynamic pathway, thus probably caused by a higher aldosterone level (57). Furthermore, the decreases in LVMI and ieLVMI after adrenalectomy were higher in KCNJ5 mutation carriers than in non-carriers (57).
In short, the impact of KCNJ5 somatic mutations on LVMI seems to be discrepant in different studies, which may relate to different patients’ baseline characteristics. However, after matching age, sex, and blood pressure status, the APA patients with KCNJ5 somatic mutations seem to have higher LVMI, ieLVMI, and greater improvement after adrenalectomy.
Diastolic function
Excessive aldosterone results in cardiac fibrosis and left ventricular hypertrophy (76–78), which contribute to impaired left ventricular relaxation in patients with PA (85–87). However, few studies have discussed left ventricular diastolic function in patients with KCNJ5 mutations. Rossi et al. (41) reported no difference between patients with and without KCNJ5 mutations in atrial contribution to left ventricular filling (ACLVF) or E/e’, and only ACLVF was significantly lower in the patients with KCNJ5 mutations, but not in those without mutations after adrenalectomy. In another study (57), only e’ was significantly higher in KCNJ5 mutation carriers compared to non-carriers before surgery, even after matching for age, sex, and hypertension status between both groups. After adrenalectomy, a significant decrease in E/e’ and a borderline increase in e’ were noted in KCNJ5 mutation carriers, but not in non-carriers (57).
In short, patients with KCNJ5 mutations benefit from adrenalectomy, not only in the left ventricular structure but also in diastolic function.
Relationships of KCNJ5 somatic mutations with vascular structure and function
Thickness and calcification of the aorta
PA is associated with increased intima-media thickness of the carotid artery (88, 89). A recent study demonstrated that APA patients with KCNJ5 mutations had a thicker abdominal aorta, but less abdominal aorta calcification compared to patients without mutations on abdominal CT (58). Moreover, patients harboring KCNJ5 mutations had greater improvements in abdominal aorta thickness compared to those without mutations after adrenalectomy (58).
Arterial stiffness
Aldosterone infusion accompanied by a salty diet was shown to increase arterial stiffness and fibronectin accumulation in an animal study, which was independent of normotensive controls and reversed by an aldosterone antagonist (90). Clinically, arterial stiffness can be evaluated by pulse wave velocity (PWV). Previous studies have reported higher PWV in PA patients compared with EH patients (91). In addition, APA patients with KCNJ5 mutations have been shown to have lower PWV compared to those without mutations (40, 48). However, the patients with mutations were younger in these studies, which may have resulted in a lower PWV and interfered with the effect of KCNJ5 mutations. In another study comparing patients with and without KCNJ5 mutations matched for age, sex, and BMI, there was no difference in PWV between the two groups. However, there was a trend of a greater decrease in PWV after adrenalectomy in the patients with KCNJ5 mutations (59).
A recent study (60) revealed lower brachial-ankle PWV (baPWV) in patients with KCNJ5 mutations compared to those without mutations before propensity score matching (PSM), but similar baPWV in both patients with and without mutations after matching for age, sex, BMI and hypertension status. After adrenalectomy, the decrease in baPWV in APA patients with KCNJ5 mutations was greater than that in those without mutations both before and after PSM. Furthermore, only the APA patients with KCNJ5 mutations had a significant decrease in baPWV after adrenalectomy, which was not found in the patients without mutations either before or after PSM. The patients with KCNJ5 mutations were correlated with a change in baPWV even after adjusting for age, sex, and hypertension status both before and after PSM. The possible causes of the greater decrease in PWV after surgery in KCNJ5 mutation carriers may be due to higher baseline aldosterone levels, less residual hypertension, and lower incidence of autonomous cortisol secretion (92) compared to patients without KCNJ5 mutations.
In short, with comparable age, sex, and hypertension status, there is no difference in arterial stiffness between patients with and without KCNJ5 mutations, but patients with KCNJ5 mutations have a greater improvement in arterial stiffness after adrenalectomy.
Relationships of KCNJ5 somatic mutations with metabolic disorder and abdominal obesity
Metabolic syndrome (MetS) is a combination of metabolic abnormalities, including obesity, diabetes, dyslipidemia, and hypertension (93). In spite of the considerable amount of previous studies that have revealed that excessive aldosterone is related to the development of MetS (94, 95), a large controlled cross-sectional study has shown no significant difference in metabolic profiles between PA and EH patients (96). Various diagnosis criteria of MetS and ratios of unilateral or bilateral PA in different studies may cause heterogeneous prevalence of MetS in patients with PA (97).
Although APA leads to higher aldosterone secretion compared to BAH (98), several reports have revealed higher prevalence rates of MetS, obesity, and dyslipidemia in patients with BAH compared to those with APA (97, 99–101). Youichi et al. found that after adjusting background characteristics, including PAC, patients with BAH still have a higher prevalence of obesity than patients with APA (100). These findings suggest PAC may be not the only contributor to metabolic disorders in PA patients. Therefore, obesity itself may be the potential contributor to the higher prevalence of MetS in BAH. The relevance between aldosterone overproduction and obesity is vague in patients with APA and BAH and further investigations were needed.
CT is a well-established imaging tool to quantify abdominal adipose tissue, utilizing the Hounsfield Units (HU) range to measure the subcutaneous and visceral fat areas (102). Concerning the effect of KCNJ5 somatic mutations on MetS, Chen et al. first reported that APA patients with KCNJ5 mutations had fewer MetS, lower triglyceride (TG) levels, waist circumference, and subcutaneous adipose tissue (SAT) and visceral adipose tissue (VAT) area than those without KCNJ5 mutations even after matching for age (103). APA patients with KCNJ5 mutations also had fewer MetS and lower triglyceride levels compared to patients with BAH. Furthermore, APA patients with KCNJ5 mutations have been reported to have significantly increased abdominal adipose tissue after adrenalectomy, but not in patients without mutations (103). A previous study has reported that aldosterone and mineralocorticoid receptors play an important role in adipose tissue development (104). An increase in adipose tissue area after adrenalectomy was reported in APA patients (105). However, the mechanism of these findings is still uncertain and may be intertwined with many factors in addition to excessive aldosterone.
The evidence about the direct effect of aldosterone on lipid metabolism has not been confirmed. Some studies reported lipid disorders were similar in patients with PA and EH (25, 106), but others showed a positive correlation between aldosterone and TG and low-density lipoprotein (107, 108). Interestingly, deterioration in lipid metabolism has been found in APA patients after adrenalectomy (105, 109). This change may be driven by the decline of renal function after treatment of PA, which would lead to a decrease of lipoprotein lipase and hepatic triglyceride lipase and interfere with the metabolism of lipids (110). As for KCNJ5 mutation, the previous study revealed only patients with mutations had a significant increase of TG after adrenalectomy, but not in patients without mutations (103).
In short, APA patients appear lean, especially those with KCNJ5 mutations, but physicians should be aware of the high risk of cardiovascular diseases related to aldosterone toxicity and worsening dyslipidemia after adrenalectomy (103). However, considering the complex interaction of aldosterone and MetS, and the only study discussing the impacts of KCNJ5 mutations on MetS, further investigation is necessary.
Relationship of KCNJ5 somatic mutations with subclinical hypercortisolism
Autonomous cortisol secretion (ACS), formerly known as subclinical Cushing syndrome or subclinical hypercortisolism, is characterized by autonomous cortisol hypersecretion from adrenal adenomas or hyperplasia, but the absence of clinical symptoms of overt Cushing’s syndrome (111–113). The prevalence of ACS has been reported to be around 30% in patients with adrenal incidentalomas (113), and 12.8% to 32% in patients with concurrent ACS and PA (92, 114–116). Patients with ACS have a higher risk of hypertension, obesity, dyslipidemia, hyperglycemia, adverse cardiovascular events, and mortality compared to patients with nonfunctional adrenal tumors (113, 117). PA patients with concurrent ACS have also been reported to have a higher incidence of cardiovascular and metabolic complications than patients with pure PA (118–122).
Recently, KCNJ5 mutations have been identified in some aldosterone- and cortisol-co-secreting adrenal adenomas (123). Interestingly, results from the TAIPAI study group indicated that ACS (1 mg dexamethasone suppression test > 1.5 µg/dL) is more common in APA patients without KCNJ5 mutations than in patients with KCNJ5 mutations (92). Furthermore, APA patients without KCNJ5 mutations and concurrent ACS were shown to have a lower clinical success rate (36.8%) after adrenalectomy compared to patients with KCNJ5 mutations and concurrent ACS (42.9%) (92).
The IHC examination of CYP11B2 and CYP11B1, key enzymes in aldosterone and cortisol biosynthesis, in adrenal slices is commonly used to identify the source of aberrant secretions of aldosterone and cortisol (124, 125). A recent study demonstrated that the immunoreactivity of CYP11B1 was higher in adrenal adenomas of PA patients with concurrent ACS compared to PA patients without ACS (126). Interestingly, some studies have reported that the immunoreactivity of CYP11B1 was higher in adenomas without KCNJ5 mutations compared with adenomas harboring mutant KCNJ5 (92, 127). These results seem to support that patients without KCNJ5 mutations are more likely to have concurrent ACS than patients with adenomas harboring mutant KCNJ5. However, other studies showed that the immunoreactivity of CYP11B1 was relatively low in adenomas without KCNJ5 mutations (43, 128). Therefore, further research is still needed to explore whether KCNJ5 mutations can directly or indirectly affect the occurrence of ACS.
Pre-operative differentiation of mutations
Somatic mutations in APA patients can only be detected using adrenal gland specimens after surgery. However, this is not helpful to predict the cure rate or long-term prognosis before surgery. Although adrenalectomy currently is preferred for patients with PA concerning the risk of all-cause mortality and major adverse cardiovascular events compared to medical treatment (129), predicting the types of mutations with simple and safe methods before surgery would possibly allow physicians to make a comprehensive treatment strategy for patients. Future studies are needed to explore the most appropriate diagnostic methods.
18-oxocortisol, 18-hydroxycortisol, and other steroid fingerprints to predict KCNJ5 mutations
Steroid profiling using tandem mass spectrometry has shown promising results for the pre-operative differentiation of somatic mutations. APA patients with KCNJ5 mutations have been shown to have a distinct steroid signature, with the highest concentrations of 18-hydroxycortisol and 18-oxocortisol in the plasma from both adrenal and peripheral veins (130). A comprehensive mass spectrometry imaging study also reported elevated intensities of 18-hydroxycortisol and 18-oxocortisol in KCNJ5-mutated APA specimens (127). A study utilizing a steroid panel consisting of aldosterone, 18-hydroxycortisol, 18-oxocortisol, 11-deoxycorticosterone, corticosterone, cortisol, and 21-deoxycortisol in plasma from peripheral veins showed that 92% of APAs could be classified according to their underlying somatic mutations (130).
Adrenal vein sampling to predict KCNJ5 mutations
Adrenal vein sampling is the gold-standard diagnostic procedure for the identification of surgically curable patients with PA. Conflicting results have been reported regarding the relationship between somatic mutations of PA and adrenal vein sampling results. Seccia et al. reported a higher lateralization index in PA patients with KCNJ5 mutations, probably due to higher aldosterone secretion (131). In contrast, another study showed that the lateralization index between mutation non-carriers, ATPase-mutated, and KCNJ5 mutated patients was not significantly different (132). These discrepant findings may be due to the different lateralization indexes used (2 and 4 respectively) impeding direct comparisons of the two studies.
NP-59 to predict KCNJ5 mutations
NP-59 adrenal scintigraphy is a functional study used to evaluate adrenal cortical activity. Lu et al. reported using semiquantitative NP-59 adrenal scintigraphy as an imaging biomarker to predict KCNJ5 mutations in PA patients (133). Among 62 PA patients who underwent NP-59 adrenal scintigraphy with available KCNJ5 mutation status, adrenal-to-liver ratio (ALR) and maximal count ratio between two adrenal glands (contrast, CON) derived from NP-59 adrenal scintigraphy were used to differentiate patients with and without KCNJ5 mutations. The results showed that the patients with KCNJ5 mutations had significantly higher ALR and CON compared to those without KCNJ5 mutations. Using optimal cutoff values of ALR and CON, NP-59 adrenal scintigraphy could predict KCNJ5 mutations with sensitivity and specificity of 85% and 57%, respectively. This is the first study using single photon emission computed tomography (SPECT) to predict somatic mutation status in PA patients.
Limitations
There are two major limitations in this review. First, in most of the studies discussing the cardiovascular or metabolic impacts of KCNJ5 somatic mutations in APA patients, the mutation detection was made by traditional methods (random biopsy and Sanger sequencing). The group called “non-KCNJ5 mutations” were not homogeneous, that might induce a bias in the interpretation of the results. As the advance of genotype detection improved, further studies discussing the difference of cardiovascular or metabolic impacts among various somatic mutations are expected. Second, the majority of the studies analyzing the impacts of KCNJ5 somatic mutations on cardiovascular or metabolism were performed in Asian cohorts. Since the prevalence of KCNJ5 somatic mutations in Asia is higher than in other western countries, the ethnic and environmental factors may involve in the difference observed among APA patients with or without KCNJ5 mutations. We look forward to the clinical outcome data of KCNJ5 somatic mutations from other countries other than Asia.
Prospects
KCNJ5 somatic mutations have been shown to be a good prognostic predictor for the remission of hypertension after unilateral adrenalectomy in APA patients (52), and steroid profiling to predict mutation status may be of value to make a comprehensive plan of treatments. However, more studies are still needed to validate the diagnostic value of steroid profiling in APA patients of different ethnicity.
Mutated potassium channel GIRK4, coded by a mutated KCNJ5 gene, has been demonstrated to have different pharmacological characteristics to the wild-type channel. The calcium channel blocker verapamil and macrolides such as amiloride have shown particularly strong inhibitive abilities for mutant channels (134, 135). Targeted blockade of mutated GIRK4 may offer new therapeutic strategies for APA patients with KCNJ5 mutations who are unsuitable for surgery.
In conclusion, KCNJ5 somatic mutations in patients with APA play an important role in cardiovascular outcomes (Figure 2), including higher LVM, more impaired diastolic function, thicker aortic wall, lower incidence of MetS, and possibly a lower incidence of concurrent ACS, but better improvement in LVM, diastolic function, arterial stiffness, and aortic wall thickness after adrenalectomy compared to patients without KCNJ5 mutations.
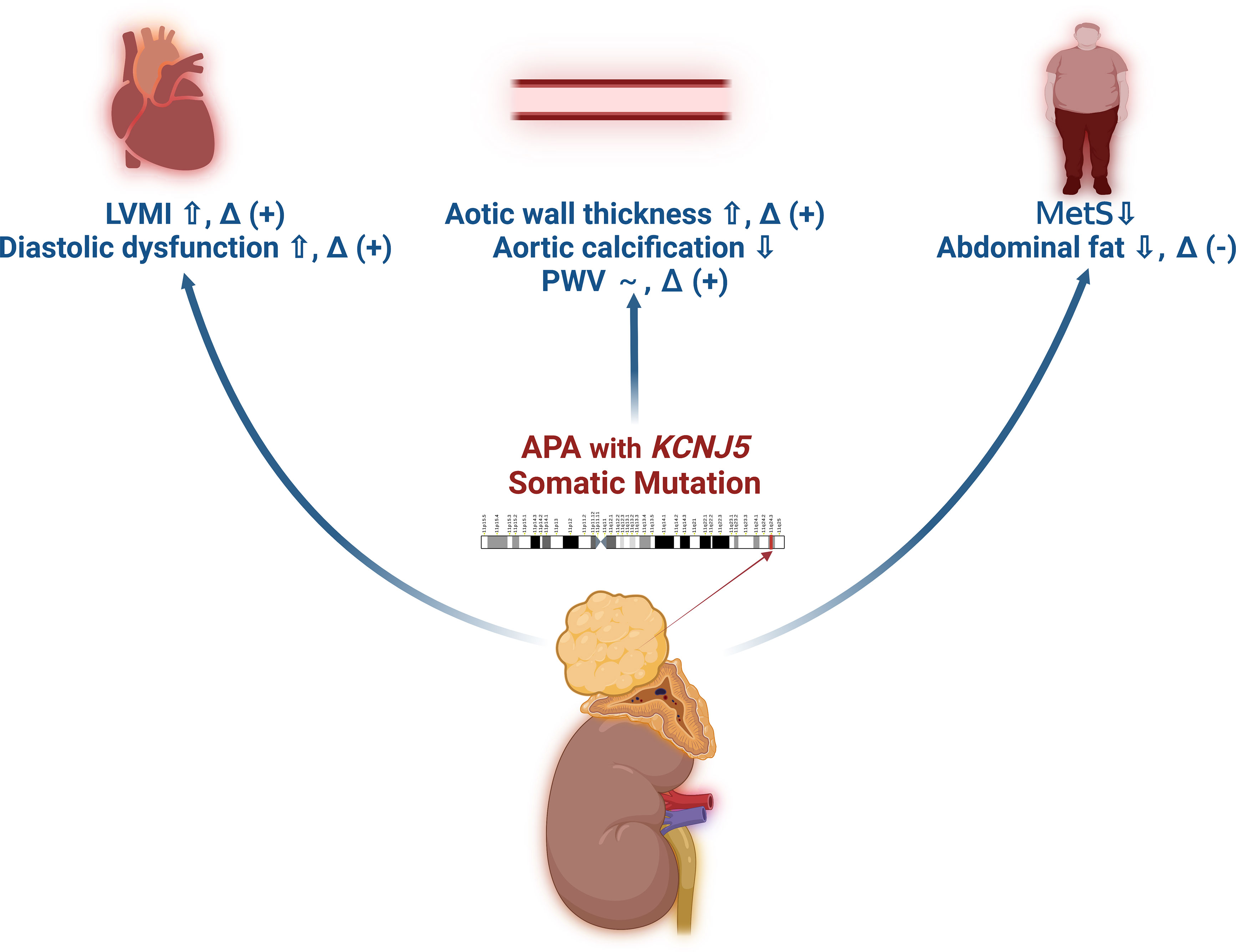
Figure 2 The schematic diagram of the effect of KCNJ5 somatic mutations on target organ damage in patients with aldosterone producing adenoma. *~: similar results between patients with or without KCNJ5 somatic mutations. *Δ (+): Improvement after adrenalectomy. *Δ (-): Deterioration after adrenalectomy.
Author contributions
Y-YC: Project concept and design, studies review (CV outcomes), initial draft writing. B-CL: Data collection and editing (aorta aspects). Z-WC: Data collection and editing (background). C-HT: Data collection and editing (mechanism). C-CC: Data collection and editing (metabolic aspects). C-WL: Critical revision. C-TP: Critical revision. K-YP: Critical revision and data collection and editing (gene detection). C-HC: Critical revision. C-CL: Data collection and editing (nuclear medicine). V-CW: Project concept and data collection. C-SH: Critical revision. Y-HL: Project concept and design, data collection, and critical revision. All authors contributed to the article and approved the submitted version.
Funding
This study was supported by Ministry of Science and Technology (109-2314-B-002 -247 -MY3, 110-2314-B-002-134-MY3, 111-2314-B-002-044), National Taiwan University Hospital (NTUH 110-A141, 110-S5120, 111-N0092, 111UN-0039, 112-UN0077, VN110-14, VN111-11, VN112-03) and Far Eastern Memorial Hospital (FEMH-2023-C-003). The funders had no role in the study design, data collection, analysis, decision to publish, or preparation of the manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Mulatero P, Monticone S, Deinum J, Amar L, Prejbisz A, Zennaro MC, et al. Genetics, prevalence, screening and confirmation of primary aldosteronism: A position statement and consensus of the working group on endocrine hypertension of the European society of hypertension. J Hypertens (2020) 38(10):1919–28. doi: 10.1097/hjh.0000000000002510
2. Hundemer GL, Kline GA, Leung AA. How common is primary aldosteronism? Curr Opin Nephrol Hypertens (2021) 30(3):353–60. doi: 10.1097/mnh.0000000000000702
3. Chang CC, Chen YY, Lai TS, Zeng YH, Chen CK, Tu KH, et al. Taiwan Mini-frontier of primary aldosteronism: Updating detection and diagnosis. J Formos Med Assoc (2021) 120(1 Pt 1):121–9. doi: 10.1016/j.jfma.2020.08.001
4. Brown JM, Siddiqui M, Calhoun DA, Carey RM, Hopkins PN, Williams GH, et al. The unrecognized prevalence of primary aldosteronism: A cross-sectional study. Ann Intern Med (2020) 173(1):10–20. doi: 10.7326/m20-0065
5. Parasiliti-Caprino M, Lopez C, Prencipe N, Lucatello B, Settanni F, Giraudo G, et al. Prevalence of primary aldosteronism and association with cardiovascular complications in patients with resistant and refractory hypertension. J hypertens (2020) 38(9):1841–8. doi: 10.1097/hjh.0000000000002441
6. Young WF. Primary aldosteronism: renaissance of a syndrome. Clin Endocrinol (2007) 66(5):607–18. doi: 10.1111/j.1365-2265.2007.02775.x
7. Yang Y, Reincke M, Williams TA. Prevalence, diagnosis and outcomes of treatment for primary aldosteronism. Best Pract Res Clin Endocrinol Metab (2020) 34(2):101365. doi: 10.1016/j.beem.2019.101365
8. Tsai CH, Pan CT, Chang YY, Chen ZW, Wu VC, Hung CS, et al. Left ventricular remodeling and dysfunction in primary aldosteronism. J Hum hypertens (2021) 35(2):131–47. doi: 10.1038/s41371-020-00426-y
9. Chen ZW, Huang KC, Lee JK, Lin LC, Chen CW, Chang YY, et al. Aldosterone induces left ventricular subclinical systolic dysfunction: A strain imaging study. J hypertens (2018) 36(2):353–60. doi: 10.1097/hjh.0000000000001534
10. Chen ZW, Tsai CH, Pan CT, Chou CH, Liao CW, Hung CS, et al. Endothelial dysfunction in primary aldosteronism. Int J Mol Sci (2019) 20(20):5214. doi: 10.3390/ijms20205214
11. Hung CS, Sung SH, Liao CW, Pan CT, Chang CC, Chen ZW, et al. Aldosterone induces vascular damage. Hypertension (2019) 74(3):623–9. doi: 10.1161/HYPERTENSIONAHA.118.12342
12. Chen Z-W, Pan C-T, Tsai C-H, Chang Y-Y, Chang C-C, Lee B-C, et al. Heart-ankle pulse wave velocity is superior to brachial-ankle pulse wave velocity in detecting aldosterone-induced arterial stiffness. Biomedicines (2021) 9(10):1285. doi: 10.3390/biomedicines9101285
13. Milliez P, Girerd X, Plouin PF, Blacher J, Safar ME, Mourad JJ. Evidence for an increased rate of cardiovascular events in patients with primary aldosteronism. J Am Coll Cardiol (2005) 45(8):1243–8. doi: 10.1016/j.jacc.2005.01.015
14. Catena C, Colussi G, Nadalini E, Chiuch A, Baroselli S, Lapenna R, et al. Cardiovascular outcomes in patients with primary aldosteronism after treatment. Arch Internal Med (2008) 168(1):80–5. doi: 10.1001/archinternmed.2007.33
15. Reincke M, Fischer E, Gerum S, Merkle K, Schulz S, Pallauf A, et al. Observational study mortality in treated primary aldosteronism: The German conn's registry. Hypertension (2012) 60(3):618–24. doi: 10.1161/hypertensionaha.112.197111
16. Savard S, Amar L, Plouin PF, Steichen O. Cardiovascular complications associated with primary aldosteronism: A controlled cross-sectional study. Hypertension (2013) 62(2):331–6. doi: 10.1161/HYPERTENSIONAHA.113.01060
17. Mulatero P, Monticone S, Bertello C, Viola A, Tizzani D, Iannaccone A, et al. Long-term cardio- and cerebrovascular events in patients with primary aldosteronism. J Clin Endocrinol Metab (2013) 98(12):4826–33. doi: 10.1210/jc.2013-2805
18. Wu VC, Wang SM, Chang CH, Hu YH, Lin LY, Lin YH, et al. Long term outcome of aldosteronism after target treatments. Sci Rep (2016) 6:32103. doi: 10.1038/srep32103
19. Monticone S, D'Ascenzo F, Moretti C, Williams TA, Veglio F, Gaita F, et al. Cardiovascular events and target organ damage in primary aldosteronism compared with essential hypertension: A systematic review and meta-analysis. Lancet Diabetes Endocrinol (2018) 6(1):41–50. doi: 10.1016/S2213-8587(17)30319-4
20. Wu X, Yu J, Tian H. Cardiovascular risk in primary aldosteronism: A systematic review and meta-analysis. Medicine (2019) 98(26):e15985. doi: 10.1097/md.0000000000015985
21. Chen ZW, Hung CS, Wu VC, Lin YH. Primary aldosteronism and cerebrovascular diseases. Endocrinol Metab (2018) 33(4):429–34. doi: 10.3803/EnM.2018.33.4.429
22. Pan CT, Tsai CH, Chen ZW, Chang YY, Wu VC, Hung CS, et al. Atrial fibrillation in primary aldosteronism. Horm Metab Res (2020) 52(6):357–65. doi: 10.1055/a-1141-5989
23. Luther JM. Effects of aldosterone on insulin sensitivity and secretion. Steroids (2014) 91:54–60. doi: 10.1016/j.steroids.2014.08.016
24. Catena C, Lapenna R, Baroselli S, Nadalini E, Colussi G, Novello M, et al. Insulin sensitivity in patients with primary aldosteronism: A follow-up study. J Clin Endocrinol Metab (2006) 91(9):3457–63. doi: 10.1210/jc.2006-0736
25. Fallo F, Veglio F, Bertello C, Sonino N, Della Mea P, Ermani M, et al. Prevalence and characteristics of the metabolic syndrome in primary aldosteronism. J Clin Endocrinol Metab (2006) 91(2):454–9. doi: 10.1210/jc.2005-1733
26. Nanba K, Vaidya A, Rainey WE. Aging and adrenal aldosterone production. Hypertension (2018) 71(2):218–23. doi: 10.1161/HYPERTENSIONAHA.117.10391
27. Nakamura Y, Yamazaki Y, Konosu-Fukaya S, Ise K, Satoh F, Sasano H. Aldosterone biosynthesis in the human adrenal cortex and associated disorders. J Steroid Biochem Mol Biol (2015) 153:57–62. doi: 10.1016/j.jsbmb.2015.05.008
28. Hattangady NG, Olala LO, Bollag WB, Rainey WE. Acute and chronic regulation of aldosterone production. Mol Cell Endocrinol (2012) 350(2):151–62. doi: 10.1016/j.mce.2011.07.034
29. Choi M, Scholl UI, Yue P, Bjorklund P, Zhao B, Nelson-Williams C, et al. K+ channel mutations in adrenal aldosterone-producing adenomas and hereditary hypertension. Science (2011) 331(6018):768–72. doi: 10.1126/science.1198785
30. Bandulik S. Of channels and pumps: Different ways to boost the aldosterone? Acta physiol (2017) 220(3):332–60. doi: 10.1111/apha.12832
31. Beuschlein F, Boulkroun S, Osswald A, Wieland T, Nielsen HN, Lichtenauer UD, et al. Somatic mutations in ATP1A1 and ATP2B3 lead to aldosterone-producing adenomas and secondary hypertension. Nat Genet (2013) 45(4):440–4, 4e1-2. doi: 10.1038/ng.2550
32. Dutta RK, Arnesen T, Heie A, Walz M, Alesina P, Söderkvist P, et al. A somatic mutation in CLCN2 identified in a sporadic aldosterone-producing adenoma. Eur J Endocrinol (2019) 181(5):K37–k41. doi: 10.1530/eje-19-0377
33. Scholl UI, Goh G, Stolting G, de Oliveira RC, Choi M, Overton JD, et al. Somatic and germline CACNA1D calcium channel mutations in aldosterone-producing adenomas and primary aldosteronism. Nat Genet (2013) 45(9):1050–4. doi: 10.1038/ng.2695
34. Scholl UI, Stolting G, Nelson-Williams C, Vichot AA, Choi M, Loring E, et al. Recurrent gain of function mutation in calcium channel CACNA1H causes early-onset hypertension with primary aldosteronism. Elife (2015) 4:e06315. doi: 10.7554/eLife.06315
35. Stindl J, Tauber P, Sterner C, Tegtmeier I, Warth R, Bandulik S. Pathogenesis of adrenal aldosterone-producing adenomas carrying mutations of the Na(+)/K(+)-ATPase. Endocrinology (2015) 156(12):4582–91. doi: 10.1210/en.2015-1466
36. Berthon A, Drelon C, Ragazzon B, Boulkroun S, Tissier F, Amar L, et al. WNT/beta-catenin signalling is activated in aldosterone-producing adenomas and controls aldosterone production. Hum Mol Genet (2014) 23(4):889–905. doi: 10.1093/hmg/ddt484
37. Zhou J, Azizan EAB, Cabrera CP, Fernandes-Rosa FL, Boulkroun S, Argentesi G, et al. Somatic mutations of GNA11 and GNAQ in CTNNB1-mutant aldosterone-producing adenomas presenting in puberty, pregnancy or menopause. Nat Genet (2021) 53(9):1360–72. doi: 10.1038/s41588-021-00906-y
38. Fernandes-Rosa FL, Williams TA, Riester A, Steichen O, Beuschlein F, Boulkroun S, et al. Genetic spectrum and clinical correlates of somatic mutations in aldosterone-producing adenoma. Hypertension (2014) 64(2):354–61. doi: 10.1161/HYPERTENSIONAHA.114.03419
39. Wu VC, Wang SM, Chueh SJ, Yang SY, Huang KH, Lin YH, et al. The prevalence of CTNNB1 mutations in primary aldosteronism and consequences for clinical outcomes. Sci Rep (2017) 7:39121. doi: 10.1038/srep39121
40. Kitamoto T, Suematsu S, Matsuzawa Y, Saito J, Omura M, Nishikawa T. Comparison of cardiovascular complications in patients with and without KCNJ5 gene mutations harboring aldosterone-producing adenomas. J Atheroscler Thromb (2015) 22(2):191–200. doi: 10.5551/jat.24455
41. Rossi GP, Cesari M, Letizia C, Seccia TM, Cicala MV, Zinnamosca L, et al. KCNJ5 gene somatic mutations affect cardiac remodelling but do not preclude cure of high blood pressure and regression of left ventricular hypertrophy in primary aldosteronism. J Hypertens (2014) 32(7):1514–21; discussion 22. doi: 10.1097/HJH.0000000000000186
42. Nanba K, Omata K, Else T, Beck PCC, Nanba AT, Turcu AF, et al. Targeted molecular characterization of aldosterone-producing adenomas in white americans. J Clin Endocrinol Metab (2018) 103(10):3869–76. doi: 10.1210/jc.2018-01004
43. De Sousa K, Boulkroun S, Baron S, Nanba K, Wack M, Rainey WE, et al. Genetic, cellular, and molecular heterogeneity in adrenals with aldosterone-producing adenoma. Hypertension (2020) 75(4):1034–44. doi: 10.1161/HYPERTENSIONAHA.119.14177
44. Williams TA, Monticone S, Schack VR, Stindl J, Burrello J, Buffolo F, et al. Somatic ATP1A1, ATP2B3, and KCNJ5 mutations in aldosterone-producing adenomas. Hypertension (2014) 63(1):188–95. doi: 10.1161/hypertensionaha.113.01733
45. Scholl UI, Healy JM, Thiel A, Fonseca AL, Brown TC, Kunstman JW, et al. Novel somatic mutations in primary hyperaldosteronism are related to the clinical, radiological and pathological phenotype. Clin Endocrinol (2015) 83(6):779–89. doi: 10.1111/cen.12873
46. Zheng F-F, Zhu L-M, Nie A-F, Li X-Y, Lin J-R, Zhang K, et al. Clinical characteristics of somatic mutations in Chinese patients with aldosterone-producing adenoma. Hypertension (2015) 65(3):622–8. doi: 10.1161/HYPERTENSIONAHA.114.03346
47. Wang B, Li X, Zhang X, Ma X, Chen L, Zhang Y, et al. Prevalence and characterization of somatic mutations in Chinese aldosterone-producing adenoma patients. Medicine (2015) 94(16):e708–e. doi: 10.1097/MD.0000000000000708
48. Wu VC, Huang KH, Peng KY, Tsai YC, Wu CH, Wang SM, et al. Prevalence and clinical correlates of somatic mutation in aldosterone producing adenoma-Taiwanese population. Sci Rep (2015) 5:11396. doi: 10.1038/srep11396
49. Kitamoto T, Suematsu S, Yamazaki Y, Nakamura Y, Sasano H, Matsuzawa Y, et al. Clinical and steroidogenic characteristics of aldosterone-producing adenomas with ATPase or CACNA1D gene mutations. J Clin Endocrinol Metab (2016) 101(2):494–503. doi: 10.1210/jc.2015-3284
50. Hong AR, Kim JH, Song YS, Lee KE, Seo SH, Seong M-W, et al. Genetics of aldosterone-producing adenoma in Korean patients. PloS One (2016) 11(1):e0147590–e. doi: 10.1371/journal.pone.0147590
51. Kitamoto T, Omura M, Suematsu S, Saito J, Nishikawa T. KCNJ5 mutation as a predictor for resolution of hypertension after surgical treatment of aldosterone-producing adenoma. J Hypertens (2018) 36(3):619–27. doi: 10.1097/HJH.0000000000001578
52. Vilela LAP, Rassi-Cruz M, Guimaraes AG, Moises CCS, Freitas TC, Alencar NP, et al. KCNJ5 somatic mutation is a predictor of hypertension remission after adrenalectomy for unilateral primary aldosteronism. J Clin Endocrinol Metab (2019) 104(10):4695–702. doi: 10.1210/jc.2019-00531
53. Nanba K, Omata K, Gomez-Sanchez CE, Stratakis CA, Demidowich AP, Suzuki M, et al. Genetic characteristics of aldosterone-producing adenomas in blacks. Hypertension (2019) 73(4):885–92. doi: 10.1161/HYPERTENSIONAHA.118.12070
54. Nanba K, Yamazaki Y, Bick N, Onodera K, Tezuka Y, Omata K, et al. Prevalence of somatic mutations in aldosterone-producing adenomas in Japanese patients. J Clin Endocrinol Metab (2020) 105(11):e4066–73. doi: 10.1210/clinem/dgaa595
55. Wu CH, Peng KY, Hwang DY, Lin YH, Wu VC, Chueh JS. Novel mutations detection with next-generation sequencing and its association with clinical outcome in unilateral primary aldosteronism. Biomedicines (2021) 9(9):1167. doi: 10.3390/biomedicines9091167
56. Meyer LS, Handgriff L, Lim JS, Udager AM, Kinker IS, Ladurner R, et al. Single-center prospective cohort study on the histopathology, genotype, and postsurgical outcomes of patients with primary aldosteronism. Hypertension (2021) 78(3):738–46. doi: 10.1161/hypertensionaha.121.17348
57. Chang YY, Tsai CH, Peng SY, Chen ZW, Chang CC, Lee BC, et al. KCNJ5 somatic mutations in aldosterone-producing adenoma are associated with a worse baseline status and better recovery of left ventricular remodeling and diastolic function. Hypertension (2021) 77(1):114–25. doi: 10.1161/hypertensionaha.120.15679
58. Lee BC, Kang VJ, Pan CT, Huang JZ, Lin YL, Chang YY, et al. KCNJ5 somatic mutation is associated with higher aortic wall thickness and less calcification in patients with aldosterone-producing adenoma. Front Endocrinol (2022) 13:830130. doi: 10.3389/fendo.2022.830130
59. Chang CH, Hu YH, Tsai YC, Wu CH, Wang SM, Lin LY, et al. Arterial stiffness and blood pressure improvement in aldosterone-producing adenoma harboring KCNJ5 mutations after adrenalectomy. Oncotarget (2017) 8(18):29984–95. doi: 10.18632/oncotarget.16269
60. Chang YY, Pan CT, Chen ZW, Tsai CH, Peng SY, Chang CC, et al. KCNJ5 somatic mutations in aldosterone-producing adenoma are associated with a greater recovery of arterial stiffness. Cancers (2021) 13(17):4313. doi: 10.3390/cancers13174313
61. Murthy M, Azizan EA, Brown MJ, O'Shaughnessy KM. Characterization of a novel somatic KCNJ5 mutation delI157 in an aldosterone-producing adenoma. J hypertens (2012) 30(9):1827–33. doi: 10.1097/HJH.0b013e328356139f
62. Akerstrom T, Crona J, Delgado Verdugo A, Starker LF, Cupisti K, Willenberg HS, et al. Comprehensive re-sequencing of adrenal aldosterone producing lesions reveal three somatic mutations near the KCNJ5 potassium channel selectivity filter. PloS One (2012) 7(7):e41926. doi: 10.1371/journal.pone.0041926
63. Mulatero P, Tauber P, Zennaro MC, Monticone S, Lang K, Beuschlein F, et al. KCNJ5 mutations in European families with nonglucocorticoid remediable familial hyperaldosteronism. Hypertension (2012) 59(2):235–40. doi: 10.1161/hypertensionaha.111.183996
64. Azizan EA, Poulsen H, Tuluc P, Zhou J, Clausen MV, Lieb A, et al. Somatic mutations in ATP1A1 and CACNA1D underlie a common subtype of adrenal hypertension. Nat Genet (2013) 45(9):1055–60. doi: 10.1038/ng.2716
65. Kuppusamy M, Caroccia B, Stindl J, Bandulik S, Lenzini L, Gioco F, et al. A novel KCNJ5-insT149 somatic mutation close to, but outside, the selectivity filter causes resistant hypertension by loss of selectivity for potassium. J Clin Endocrinol Metab (2014) 99(9):E1765–73. doi: 10.1210/jc.2014-1927
66. Cheng CJ, Sung CC, Wu ST, Lin YC, Sytwu HK, Huang CL, et al. Novel KCNJ5 mutations in sporadic aldosterone-producing adenoma reduce Kir3.4 membrane abundance. J Clin Endocrinol Metab (2015) 100(1):E155–63. doi: 10.1210/jc.2014-3009
67. Hardege I, Xu S, Gordon RD, Thompson AJ, Figg N, Stowasser M, et al. Novel insertion mutation in KCNJ5 channel produces constitutive aldosterone release from H295R cells. Mol Endocrinol (2015) 29(10):1522–30. doi: 10.1210/me.2015-1195
68. Nanba K, Omata K, Tomlins SA, Giordano TJ, Hammer GD, Rainey WE, et al. Double adrenocortical adenomas harboring independent KCNJ5 and PRKACA somatic mutations. Eur J Endocrinol (2016) 175(2):K1–6. doi: 10.1530/EJE-16-0262
69. Zheng FF, Zhu LM, Zhou WL, Zhang Y, Li MY, Zhu YC, et al. A novel somatic mutation 145-147delETEinsK in KCNJ5 increases aldosterone production. J Hum hypertens (2017) 31(11):756–9. doi: 10.1038/jhh.2017.50
70. Peng KY, Liao HW, Chueh JS, Pan CY, Lin YH, Chen YM, et al. Pathophysiological and pharmacological characteristics of KCNJ5 157-159delITE somatic mutation in aldosterone-producing adenomas. Biomedicines (2021) 9(8):1026. doi: 10.3390/biomedicines9081026
71. Lenzini L, Rossitto G, Maiolino G, Letizia C, Funder JW, Rossi GP. A meta-analysis of somatic KCNJ5 k(+) channel mutations in 1636 patients with an aldosterone-producing adenoma. J Clin Endocrinol Metab (2015) 100(8):E1089–95. doi: 10.1210/jc.2015-2149
72. Taguchi R, Yamada M, Nakajima Y, Satoh T, Hashimoto K, Shibusawa N, et al. Expression and mutations of KCNJ5 mRNA in Japanese patients with aldosterone-producing adenomas. J Clin Endocrinol Metab (2012) 97(4):1311–9. doi: 10.1210/jc.2011-2885
73. Brilla CG, Weber KT. Reactive and reparative myocardial fibrosis in arterial hypertension in the rat. Cardiovasc Res (1992) 26(7):671–7.
74. Sun Y, Ramires FJ, Weber KT. Fibrosis of atria and great vessels in response to angiotensin II or aldosterone infusion. Cardiovasc Res (1997) 35(1):138–47.
75. Brilla CG, Pick R, Tan LB, Janicki JS, Weber KT. Remodeling of the rat right and left ventricles in experimental hypertension. Circ Res (1990) 67(6):1355–64.
76. Tanabe A, Naruse M, Naruse K, Hase M, Yoshimoto T, Tanaka M, et al. Left ventricular hypertrophy is more prominent in patients with primary aldosteronism than in patients with other types of secondary hypertension. Hypertens Res (1997) 20(2):85–90.
77. Rossi GP, Di Bello V, Ganzaroli C, Sacchetto A, Cesari M, Bertini A, et al. Excess ldosterone is associated with alterations of myocardial texture in primary aldosteronism. Hypertension (2002) 40(1):23–7. doi: 10.1161/01.Hyp.0000023182.68420.Eb
78. Matsumura K, Fujii K, Oniki H, Oka M, Iida M. Role of aldosterone in left ventricular hypertrophy in hypertension. Am J Hypertens (2006) 19(1):13–8. doi: 10.1016/j.amjhyper.2005.05.013
79. de Simone G, Devereux RB, Kimball TR, Mureddu GF, Roman MJ, Contaldo F, et al. Interaction between body size and cardiac workload: influence on left ventricular mass during body growth and adulthood. Hypertension (1998) 31(5):1077–82. doi: 10.1161/01.hyp.31.5.1077
80. Palmieri V, de Simone G, Roman MJ, Schwartz JE, Pickering TG, Devereux RB. Ambulatory blood pressure and metabolic abnormalities in hypertensive subjects with inappropriately high left ventricular mass. Hypertension (1999) 34(5):1032–40. doi: 10.1161/01.hyp.34.5.1032
81. Muiesan ML, Salvetti M, Paini A, Agabiti-Rosei C, Monteduro C, Galbassini G, et al. Inappropriate left ventricular mass in patients with primary aldosteronism. Hypertension (2008) 52(3):529–34. doi: 10.1161/HYPERTENSIONAHA.108.114140
82. Rossi GP, Cesari M, Cuspidi C, Maiolino G, Cicala MV, Bisogni V, et al. Long-term control of arterial hypertension and regression of left ventricular hypertrophy with treatment of primary aldosteronism. Hypertension (2013) 62(1):62–9. doi: 10.1161/hypertensionaha.113.01316
83. Hung C-S, Ho Y-L, Chang Y-Y, Wu V-C, Wu X-M, Lee J-K, et al. Twenty-four-hour urinary aldosterone predicts inappropriate left ventricular mass index in patients with primary aldosteronism. ScientificWorldJournal (2013) 2013:294594–. doi: 10.1155/2013/294594
84. Hung CS, Chou CH, Wu XM, Chang YY, Wu VC, Chen YH, et al. Circulating tissue inhibitor of matrix metalloproteinase-1 is associated with aldosterone-induced diastolic dysfunction. J Hypertens (2015) 33(9):1922–30. doi: 10.1097/HJH.0000000000000619
85. Tsioufis C, Tsiachris D, Dimitriadis K, Stougiannos P, Missovoulos P, Kakkavas A, et al. Myocardial and aortic stiffening in the early course of primary aldosteronism. Clin Cardiol (2008) 31(9):431–6. doi: 10.1002/clc.20270
86. Galetta F, Bernini G, Franzoni F, Bacca A, Fivizzani I, Tocchini L, et al. Cardiac remodeling in patients with primary aldosteronism. J Endocrinol Invest (2009) 32(9):739–45.
87. Chang YY, Liao CW, Tsai CH, Chen CW, Pan CT, Chen ZW, et al. Left ventricular dysfunction in patients with primary aldosteronism: A propensity score-matching follow-up study with tissue Doppler imaging. J Am Heart Assoc (2019) 8(22):e013263. doi: 10.1161/JAHA.119.013263
88. Holaj R, Zelinka T, Wichterle D, Petrak O, Strauch B, Widimsky J Jr. Increased intima-media thickness of the common carotid artery in primary aldosteronism in comparison with essential hypertension J hypertens (2007) 25(7):1451–7 doi: 10.1097/HJH.0b013e3281268532
89. Holaj R, Rosa J, Zelinka T, Strauch B, Petrak O, Indra T, et al. Long-term effect of specific treatment of primary aldosteronism on carotid intima-media thickness. J hypertens (2015) 33(4):874–82. doi: 10.1097/HJH.0000000000000464
90. Lacolley P, Labat C, Pujol A, Delcayre C, Benetos A, Safar M. Increased carotid wall elastic modulus and fibronectin in aldosterone-salt-treated rats: effects of eplerenone. Circulation (2002) 106(22):2848–53. doi: 10.1161/01.cir.0000039328.33137.6c
91. Strauch B, Petrák O, Wichterle D, Zelinka T, Holaj R, Widimský J Jr. Increased arterial wall stiffness in primary aldosteronism in comparison with essential hypertension Am J Hypertens (2006) 19(9):909–14 doi: 10.1016/j.amjhyper.2006.02.002
92. Peng KY, Liao HW, Chan CK, Lin WC, Yang SY, Tsai YC, et al. Presence of subclinical hypercortisolism in clinical aldosterone-producing adenomas predicts lower clinical success. Hypertension (2020) 76(5):1537–44. doi: 10.1161/hypertensionaha.120.15328
93. Rochlani Y, Pothineni NV, Kovelamudi S, Mehta JL. Metabolic syndrome: pathophysiology, management, and modulation by natural compounds. Ther Adv Cardiovasc Dis (2017) 11(8):215–25. doi: 10.1177/1753944717711379
94. Ingelsson E, Pencina MJ, Tofler GH, Benjamin EJ, Lanier KJ, Jacques PF, et al. Multimarker approach to evaluate the incidence of the metabolic syndrome and longitudinal changes in metabolic risk factors: the framingham offspring study. Circulation (2007) 116(9):984–92. doi: 10.1161/circulationaha.107.708537
95. Hannemann A, Meisinger C, Bidlingmaier M, Doring A, Thorand B, Heier M, et al. Association of plasma aldosterone with the metabolic syndrome in two German populations. Eur J Endocrinol (2011) 164(5):751–8. doi: 10.1530/EJE-10-1074
96. Matrozova J, Steichen O, Amar L, Zacharieva S, Jeunemaitre X, Plouin PF. Fasting plasma glucose and serum lipids in patients with primary aldosteronism: a controlled cross-sectional study. Hypertension (2009) 53(4):605–10. doi: 10.1161/hypertensionaha.108.122002
97. Zhang Z, Luo Q, Tuersun T, Wang G, Wu T, Zhang D, et al. Higher prevalence of metabolic disorders in patients with bilateral primary aldosteronism than unilateral primary aldosteronism. Clin Endocrinol (2021) 94(1):3–11. doi: 10.1111/cen.14318
98. Young WF Jr. Diagnosis and treatment of primary aldosteronism: practical clinical perspectives J Internal Med (2019) 285(2):126–48 doi: 10.1111/joim.12831
99. Somloova Z, Widimsky J Jr., Rosa J, Wichterle D, Strauch B, Petrak O, et al. The prevalence of metabolic syndrome and its components in two main types of primary aldosteronism. J Hum hypertens (2010) 24(10):625–30. doi: 10.1038/jhh.2010.65
100. Ohno Y, Sone M, Inagaki N, Yamasaki T, Ogawa O, Takeda Y, et al. Obesity as a key factor underlying idiopathic hyperaldosteronism. J Clin Endocrinol Metab (2018) 103(12):4456–64. doi: 10.1210/jc.2018-00866
101. Chen KM, Lee BC, Chen PT, Liu KL, Lin KH, Chang CC, et al. Evaluation of abdominal computed tomography scans for differentiating the discrepancies in abdominal adipose tissue between two major subtypes of primary aldosteronism. Front Endocrinol (2021) 12:647184. doi: 10.3389/fendo.2021.647184
102. Aubrey J, Esfandiari N, Baracos VE, Buteau FA, Frenette J, Putman CT, et al. Measurement of skeletal muscle radiation attenuation and basis of its biological variation. Acta physiol (2014) 210(3):489–97. doi: 10.1111/apha.12224
103. Chen KM, Chang YL, Wu TH, Lee BC, Chen PT, Liu KL, et al. Aldosterone-producing adenoma-harbouring KCNJ5 mutations is associated with lower prevalence of metabolic disorders and abdominal obesity. J hypertens (2021) 39(12):2353–60. doi: 10.1097/HJH.0000000000002948
104. Caprio M, Fève B, Claës A, Viengchareun S, Lombès M, Zennaro MC. Pivotal role of the mineralocorticoid receptor in corticosteroid-induced adipogenesis. FASEB J (2007) 21(9):2185–94. doi: 10.1096/fj.06-7970com
105. Er LK, Lin MC, Tsai YC, Hsiao JK, Yang CY, Chang CC, et al. Association of visceral adiposity and clinical outcome among patients with aldosterone producing adenoma. BMJ Open Diabetes Res Care (2020) 8(1):e001153. doi: 10.1136/bmjdrc-2019-001153
106. Iacobellis G, Petramala L, Cotesta D, Pergolini M, Zinnamosca L, Cianci R, et al. Adipokines and cardiometabolic profile in primary hyperaldosteronism. J Clin Endocrinol Metab (2010) 95(5):2391–8. doi: 10.1210/jc.2009-2204
107. Bochud M, Nussberger J, Bovet P, Maillard MR, Elston RC, Paccaud F, et al. Plasma aldosterone is independently associated with the metabolic syndrome. Hypertension (2006) 48(2):239–45. doi: 10.1161/01.HYP.0000231338.41548.fc
108. Goodfriend TL, Egan B, Stepniakowski K, Ball DL. Relationships among plasma aldosterone, high-density lipoprotein cholesterol, and insulin in humans. Hypertension (1995) 25(1):30–6. doi: 10.1161/01.hyp.25.1.30
109. Adolf C, Asbach E, Dietz AS, Lang K, Hahner S, Quinkler M, et al. Worsening of lipid metabolism after successful treatment of primary aldosteronism. Endocrine (2016) 54(1):198–205. doi: 10.1007/s12020-016-0983-9
110. Goldberg A, Sherrard DJ, Brunzell JD. Adipose tissue lipoprotein lipase in chronic hemodialysis: role in plasma triglyceride metabolism. J Clin Endocrinol Metab (1978) 47(6):1173–82. doi: 10.1210/jcem-47-6-1173
111. Fassnacht M, Arlt W, Bancos I, Dralle H, Newell-Price J, Sahdev A, et al. Management of adrenal incidentalomas: European society of endocrinology clinical practice guideline in collaboration with the European network for the study of adrenal tumors. Eur J Endocrinol (2016) 175(2):G1–g34. doi: 10.1530/eje-16-0467
112. Rossi R, Tauchmanova L, Luciano A, Di Martino M, Battista C, Del Viscovo L, et al. Subclinical cushing's syndrome in patients with adrenal incidentaloma: clinical and biochemical features. J Clin Endocrinol Metab (2000) 85(4):1440–8. doi: 10.1210/jcem.85.4.6515
113. Chiodini I. Clinical review: Diagnosis and treatment of subclinical hypercortisolism. J Clin Endocrinol Metab (2011) 96(5):1223–36. doi: 10.1210/jc.2010-2722
114. Fujimoto K, Honjo S, Tatsuoka H, Hamamoto Y, Kawasaki Y, Matsuoka A, et al. Primary aldosteronism associated with subclinical cushing syndrome. J Endocrinol Invest (2013) 36(8):564–7. doi: 10.3275/8818
115. Lin JH, Peng KY, Kuo YP, Liu H, Tan CB, Lin YF, et al. Aldosterone-producing nodules and CYP11B1 signaling correlate in primary aldosteronism. Endocr Relat Cancer (2022) 29(2):59–69. doi: 10.1530/ERC-21-0287
116. Hiraishi K, Yoshimoto T, Tsuchiya K, Minami I, Doi M, Izumiyama H, et al. Clinicopathological features of primary aldosteronism associated with subclinical cushing's syndrome. Endocrine J (2011) 58(7):543–51. doi: 10.1507/endocrj.k10e-402
117. Yozamp N, Vaidya A. Assessment of mild autonomous cortisol secretion among incidentally discovered adrenal masses. Best Pract Res Clin Endocrinol Metab (2021) 35(1):101491. doi: 10.1016/j.beem.2021.101491
118. Tang L, Li X, Wang B, Ma X, Li H, Gao Y, et al. Clinical characteristics of aldosterone- and cortisol-coproducing adrenal adenoma in primary aldosteronism. Int J Endocrinol (2018) 2018:4920841. doi: 10.1155/2018/4920841
119. Yasuda S, Hikima Y, Kabeya Y, Iida S, Oikawa Y, Isshiki M, et al. Clinical characterization of patients with primary aldosteronism plus subclinical cushing's syndrome. BMC Endocr Disord (2020) 20(1):9. doi: 10.1186/s12902-020-0490-0
120. Gerards J, Heinrich DA, Adolf C, Meisinger C, Rathmann W, Sturm L, et al. Impaired glucose metabolism in primary aldosteronism is associated with cortisol cosecretion. J Clin Endocrinol Metab (2019) 104(8):3192–202. doi: 10.1210/jc.2019-00299
121. Nakajima Y, Yamada M, Taguchi R, Satoh T, Hashimoto K, Ozawa A, et al. Cardiovascular complications of patients with aldosteronism associated with autonomous cortisol secretion. J Clin Endocrinol Metab (2011) 96(8):2512–8. doi: 10.1210/jc.2010-2743
122. Arlt W, Lang K, Sitch AJ, Dietz AS, Rhayem Y, Bancos I, et al. Steroid metabolome analysis reveals prevalent glucocorticoid excess in primary aldosteronism. JCI Insight (2017) 2(8):e93136. doi: 10.1172/jci.insight.93136
123. Yamada M, Nakajima Y, Taguchi R, Okamura T, Ishii S, Tomaru T, et al. KCNJ5 mutations in aldosterone- and cortisol-co-secreting adrenal adenomas. Endocrine J (2012) 59(8):735–41. doi: 10.1507/endocrj.ej12-0247
124. Gomez-Sanchez CE, Qi X, Velarde-Miranda C, Plonczynski MW, Parker CR, Rainey W, et al. Development of monoclonal antibodies against human CYP11B1 and CYP11B2. Mol Cell Endocrinol (2014) 383(1-2):111–7. doi: 10.1016/j.mce.2013.11.022
125. Nakamura Y, Kitada M, Satoh F, Maekawa T, Morimoto R, Yamazaki Y, et al. Intratumoral heterogeneity of steroidogenesis in aldosterone-producing adenoma revealed by intensive double- and triple-immunostaining for CYP11B2/B1 and CYP17. Mol Cell Endocrinol (2016) 422:57–63. doi: 10.1016/j.mce.2015.11.014
126. Ahn CH, Na HY, Park SY, Yu HW, Kim SJ, Choi JY, et al. Expression of CYP11B1 and CYP11B2 in adrenal adenoma correlates with clinical characteristics of primary aldosteronism. Clin Endocrinol (2022) 96(1):30–9. doi: 10.1111/cen.14628
127. Murakami M, Rhayem Y, Kunzke T, Sun N, Feuchtinger A, Ludwig P, et al. In situ metabolomics of aldosterone-producing adenomas. JCI Insight (2019) 4(17):e130356. doi: 10.1172/jci.insight.130356
128. Inoue K, Yamazaki Y, Kitamoto T, Hirose R, Saito J, Omura M, et al. Aldosterone suppression by dexamethasone in patients with KCNJ5-mutated aldosterone-producing adenoma. J Clin Endocrinol Metab (2018) 103(9):3477–85. doi: 10.1210/jc.2018-00738
129. Chen SY, Chen JY, Huang WC, Puar THK, Chin Kek P, Chueh JS, et al. Cardiovascular outcomes and all-cause mortality in primary aldosteronism after adrenalectomy or mineralocorticoid receptor antagonist treatment: a meta-analysis. Eur J Endocrinol (2022) 187(6):S47–s58. doi: 10.1530/eje-22-0375
130. Williams TA, Peitzsch M, Dietz AS, Dekkers T, Bidlingmaier M, Riester A, et al. Genotype-specific steroid profiles associated with aldosterone-producing adenomas. Hypertension (2016) 67(1):139–45. doi: 10.1161/hypertensionaha.115.06186
131. Seccia TM, Mantero F, Letizia C, Kuppusamy M, Caroccia B, Barisa M, et al. Somatic mutations in the KCNJ5 gene raise the lateralization index: implications for the diagnosis of primary aldosteronism by adrenal vein sampling. J Clin Endocrinol Metab (2012) 97(12):E2307–13. doi: 10.1210/jc.2012-2342
132. Osswald A, Fischer E, Degenhart C, Quinkler M, Bidlingmaier M, Pallauf A, et al. Lack of influence of somatic mutations on steroid gradients during adrenal vein sampling in aldosterone-producing adenoma patients. Eur J Endocrinol (2013) 169(5):657–63. doi: 10.1530/EJE-13-0551
133. Lu CC, Yen RF, Peng KY, Huang JY, Wu KD, Chueh JS, et al. NP-59 adrenal scintigraphy as an imaging biomarker to predict KCNJ5 mutation in primary aldosteronism patients. Front Endocrinol (2021) 12:644927. doi: 10.3389/fendo.2021.644927
134. Tauber P, Penton D, Stindl J, Humberg E, Tegtmeier I, Sterner C, et al. Pharmacology and pathophysiology of mutated KCNJ5 found in adrenal aldosterone-producing adenomas. Endocrinology (2014) 155(4):1353–62. doi: 10.1210/en.2013-1944
135. Scholl UI, Abriola L, Zhang C, Reimer EN, Plummer M, Kazmierczak BI, et al. Macrolides selectively inhibit mutant KCNJ5 potassium channels that cause aldosterone-producing adenoma. J Clin Invest (2017) 127(7):2739–50. doi: 10.1172/JCI91733
Appendix
Membership of the Taiwan Primary Aldosteronism Investigation (TAIPAI) Study Group: Che-Hsiung Wu, MD (Chi-Taz Hospital,PI of Committee); V-CW, MD (NTUH, PI of Committee); Y-HL, MD (NTUH, PI of Committee); Hung-Wei Chang, MD, PhD (Far Eastern Clinics, PI of Committee); Lian-Yu Lin MD, PhD (NTUH, PI of Committee); Fu-Chang Hu, MS, ScD, (Harvard Statistics, Site Investigator); Kao-Lang Liu, MD (NTUH, PI of Committee); Shuo-Meng Wang, MD (NTUH, PI of Committee); Kuo-How Huang, MD (NTUH, PI of Committee); Yung-Ming Chen, MD (NTUH,PI of Committee); C-CC, MD (NTUH, PI of Committee); Shih-Cheng Liao, MD (NTUH, PI of Committee); Ruoh-Fang Yen, MD,PhD (NTUH, PI of Committee); and Kwan-Dun Wu, MD, PhD (NTUH, Director of Coordinating Center).
Keywords: somatic mutation, KCNJ5, autonomous cortisol secretion (ACS), adrenocortical adenoma, cardiovascular system, metabolic syndrome
Citation: Chang Y-Y, Lee B-C, Chen Z-W, Tsai C-H, Chang C-C, Liao C-W, Pan C-T, Peng K-Y, Chou C-H, Lu C-C, Wu V-C, Hung C-S, Lin Y-H and TAIPAI study group (2023) Cardiovascular and metabolic characters of KCNJ5 somatic mutations in primary aldosteronism. Front. Endocrinol. 14:1061704. doi: 10.3389/fendo.2023.1061704
Received: 04 October 2022; Accepted: 22 February 2023;
Published: 06 March 2023.
Edited by:
Iacopo Chiodini, University of Milan, ItalyReviewed by:
Fabio Fernandes Rosa, INSERM U970 Paris Centre de Recherche Cardiovasculaire (PARCC), FranceJung Soo Lim, Yonsei University, Republic of Korea
Copyright © 2023 Chang, Lee, Chen, Tsai, Chang, Liao, Pan, Peng, Chou, Lu, Wu, Hung, Lin and TAIPAI study group. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yen-Hung Lin, YXVzdGlucjM0QGdtYWlsLmNvbQ==
 Yi-Yao Chang1,2
Yi-Yao Chang1,2 Bo-Ching Lee
Bo-Ching Lee Zheng-Wei Chen
Zheng-Wei Chen Cheng-Hsuan Tsai
Cheng-Hsuan Tsai Chin-Chen Chang
Chin-Chen Chang Che-Wei Liao
Che-Wei Liao Chien-Ting Pan
Chien-Ting Pan Kang-Yung Peng
Kang-Yung Peng Chia-Hung Chou
Chia-Hung Chou Vin-Cent Wu
Vin-Cent Wu Chi-Sheng Hung
Chi-Sheng Hung Yen-Hung Lin
Yen-Hung Lin