
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CLINICAL TRIAL article
Front. Endocrinol. , 23 January 2023
Sec. Clinical Diabetes
Volume 14 - 2023 | https://doi.org/10.3389/fendo.2023.1054946
This article is part of the Research Topic Practice Innovation and Outcome Evaluation in Diabetes View all 13 articles
 Yu Su1†
Yu Su1† Shuo Zhang2,3,4†
Shuo Zhang2,3,4† Zezhen Wu2,3,4†
Zezhen Wu2,3,4† Weiting Liu5
Weiting Liu5 Jingxian Chen2,4
Jingxian Chen2,4 Feiying Deng2,3,4
Feiying Deng2,3,4 Fengwu Chen3,4
Fengwu Chen3,4 Dan Zhu4
Dan Zhu4 Kaijian Hou6*
Kaijian Hou6*Aim: To evaluate the treatment effect Fand pharmacoeconomic value of Dugaglutide in women with type 2 diabetes.
Methods: Women (n=96) with type 2 diabetes recruited from June 2019 to December 2021 were randomized into two equal groups. The control group was treated with Liraglutide, and the observation group was treated with Dulaglutide, both for 24 weeks. The blood glucose levels, biochemical index, insulin resistance index (HOMA-IR), cost-effect ratio (CER), and drug safety were determined and compared between the two groups.
Results: Blood glucose levels, the biochemical index, and HOMA-IR were lower in both groups after the treatment (P < 0.05), and there was no statistical difference in the blood glucose levels, biochemical index and HOMA-IR between the two groups (P > 0.05). The CER levels did not differ statistically between the two groups (P > 0.05). Both the cost and the incidence of drug side effects during solution injection were lower in the observation group than in the control group after 24 weeks of treatment (P < 0.05).
Conclusion: Both Dulaglutide and Liraglutide can reduce blood glucose levels, improve biochemical index, and HOMA-IR levels in women with type 2 diabetes. Dulaglutide is more cost-effective and safe.
Clinical trial registration: https://www.chictr.org.cn/index.aspx, identifier ChiCTR1900026514.
Type 2 diabetes tends to occur in adults because of a continuous increase in the blood glucose level, which is caused by insufficient insulin secretion or difficulty in the use of insulin for various reasons. Among all of the type 2 diabetes patients, evidence suggests that women experience a higher excess mortality than men (1, 2). Persistent hyperglycemia can cause pathological changes in the macrovascular, microvascular, and nervous systems and, in severe cases, damage to the heart and kidney (3). The pathology of type 2 diabetes is complex, and clinical symptoms can include polyphagia, polyuria, polydipsia, and weight loss (4, 5).
In the early 1980s, glucagon-like peptide 1 (GLP-1) was found to be the glucagon-stimulating enzyme cleavage product (6)produced in intestinal L cells. GLP-1, as an intestinal peptide mainly secreted after ingestion of glucose or mixed diet, increases glucose-stimulated insulin secretion at physiological plasma concentration, meeting all standards of incretin hormone (7, 8). The insulin-promoting effect of GLP-1 in type 2 diabetes patients shows that it has a potential role in drug treatment of the disease (9, 10). The most obvious physiological effect of GLP-1 is its insulin-promoting effect (6). It is worth noting that GLP-1 only increases insulin release in the case of hyperglycemia, so it will not lead to hypoglycemia. In addition, GLP-1 inhibits the pancreas α The cells release glucagon, which may be through the islets δ Somatostatin is locally released from the cells to mediate the release of (10, 11). In addition, GLP-1 has many other functions: the central nervous system (CNS) induces satiety and satiety (12), reduces blood pressure (13), and reduces postprandial triglyceride and free fatty acid concentrations. Lilalutide is a GLP-1 receptor agonist. The standard therapeutic dose of liraglutide is 1.2mg once a day. However, if the patient has insufficient blood glucose response to the drug, it is recommended to titrate to 1.8mg once a day. In phase III clinical trial of liraglutide in patients with type 2 diabetes, HbA1c levels were reduced by 1.1 – 1.8% (14, 15).
Liraglutide is a commonly used glucagon-like peptide 1 (GLP-1) receptor agonist. In vivo, Liraglutide can bind to GLP-1 receptors on pancreatic beta cells and then stimulate the synthesis and secretion of insulin, which can increase insulin sensitivity in peripheral tissues, enhance insulin-mediated glucose utilization, inhibit hepatic glycogen callogenesis, reduce glucose uptake by intestinal cells and decrease hepatic glucose output (16). Liraglutide also increases satiety by acting on the central nervous system (17) and slowing gastric emptying time, which reduces the total energy intake (18, 19).
Unlike short-acting compounds, long-acting GLP-1 receptor agonists do not appear to substantially affect gastric motility when taken for a long time. Long-acting GLP-1 receptor agonists lack influence on gastric emptying rate76. Dulaglutide is a GLP-1 peptide fused with IgG. Compared with natural GLP-1, Dulaglutide shows extended biological activity due to its extended half-life (~90 hours), which supports the weekly administration of the drug (15). A weekly dose of 0.05 – 8.0mg resulted in a decrease of 0.2 – 1.2% in HbA1c levels after 5 weeks. Compared with short-acting drugs that require more frequent administration, the convenience of injecting long-acting compounds once a day or once a week is an obvious advantage. Patients with frequent changes in daily activities, such as business travelers and shift workers, may prefer long-acting compounds, which can improve patient compliance (20).
Dulaglutide is one GLP-1 Fc fusion protein that activates GLP-1 receptors and promotes glucose-dependent insulin secretion, which helps reduce fasting and postprandial glucose levels (21). Dulaglutide improves the insulin secretion index and helps regulate the body’s blood glucose level (22). Growing evidence suggests that Dulaglutide has the potential to treat diabetes-related neurodegenerative diseases (23, 24). The mechanism of action for how the drug decreases blood glucose is shown in (Figure 1). However, few clinical studies have focused on the economic value of Dulaglutide injection in type 2 diabetic patients (25). This study was conducted to investigate the therapeutic effect and pharmacoeconomic value of Dulaglutide in female patients with type 2 diabetes.
Female patients (n=96) with type 2 diabetes were recruited from June 2019 to December 2021, ranging in age from 23 to 69 years, and the average age of patients was 46.14 ± 5.78 years (mean ± S.D.); the mean body mass index (BMI) was 22.62 ± 3.71 kg/m2 (the BMI levels ranged from18.31 to 29.34 kg/m2); and the disease duration was an average 5.73 ± 0.92 years (the range was 1-12 years). We randomly assigned participants at a ratio of 1:1. Using an interactive voice response system, all patients were randomly divided into two groups according to a computer-generated random sequence.
The study design included the following inclusion criteria (26): (1) The type 2 diabetes patients were diagnosed based on the American Diabetes Association criteria which specify that the FPG ≥ 126 mg/dL (7.0 mmol/L), 2-h PG ≥ 200 mg/dL (11.1 mmol/L) during OGTT (Oral Glucose Tolerance Test), an A1C level ≥ 6.5% (48 mmol/mol), or in a patient with classic symptoms of hyperglycemia or hyperglycemic crisis, a random plasma glucose ≥ 200 mg/dL (11.1 mmol/L). Fasting is defined as no caloric intake for at least 8 h. The fasting glucose test should be performed as described by WHO, using a glucose load containing the equivalent of 75 g anhydrous glucose dissolved in water, and the test should be performed in a laboratory using a method that is NGSP (National Glycohemoglobin Standardization Program) certified and standardized to the DCCT (Diabetes Control and Complications Trial) assay.(2) No history of allergy and contraindication to Dulaglutide and Liraglutide, and be able to tolerate the treatment. Exclusion criteria included: (1) Patients with other types of diabetes mellitus (DM) rather than T2DM; (2) Patients who have used weight reduction drugs within 24 weeks; (3) Patients with clinically significant hepatobiliary, renal, cardiovascular, gastrointestinal or autoimmune system disease; (4) Coagulation disorders; (5) Patients who are judged by the investigator as unlikely to comply with the protocol, or patients with serious physical or psychological illnesses that could affect the effectiveness or safety of the study.
Both groups of patients were admitted to the hospital and underwent stringent blood glucose monitoring. All patients did not use other hypoglycemic drugs. During the study period, the patients’ exercise intensity was medium to low, and they followed the diabetes diet. The control group was injected subcutaneously with Liraglutide (Novo Nordisk Pharmaceutical Co., Ltd., China; at the specification 3 mL: 18 mg/stick) at the following regimen: 0.6 mg once a day during the first week before breakfast; then 1.2 mg once a day, from the 2nd to 24th week. The observation group was treated with Dulaglutide. Patients were given a subcutaneous injection of Dulaglutide every week. The dose of Dulaglutide injected was 0.75mg in the first week, if the blood glucose is not well controlled, the dose can be increased according to the patient’s actual situation, where the range of injection was generally 0.75-1.5 mg for 24 weeks (1 course of treatment).
(1) Glucose metabolism indices. Fasting postprandial glucose(FPG) and 2-hour postprandial glucose(2HPG) levels were measured using a glucose meter before treatment and after 24 weeks of treatment in both groups. Patients’ glycosylated hemoglobin (HbAlc) levels were measured using an automatic biochemical analyzer (27).
(2) Biochemical index and insulin resistance index (Homa-IR). The levels of visceral adiponectin were measured by enzyme-linked immunosorbent assay (2). Leptin (Lp) levels were measured by radioimmunoassay. Fasting-insulin (FINS) was measured by a fully automated immunoluminescence analyzer and HOMA-IR (HOMA-IR=FPG*FINS∕22.5) levels were calculated (17).
(3) Cost-Effectiveness Ratio (CER). The economic value analysis contained two aspects: cost determination and efficacy analysis. A cost-effectiveness ratio (CER) was performed, where the lower CER indicates the better economic value (28). Safety was assessed based on adverse events: the incidence of nausea and vomiting, hypoglycemia, cholecystitis, allergic reactions, and liver and kidney abnormalities (29).
Unpaired Student’s t-test for categorical variables was applied for comparison between observation and controlgroups. Results with a two-tailed p-value of <0.05 were considered significant (19 31). IBM SPSS Statistics 25 was used for thedata analyses.
There was no statistical difference in glucose metabolism indices between the two groups before treatment (P> 0.05), though FPG, 2hPG, and HbAlc levels were lower in both groups after 24 weeks of treatment (P< 0.05). No statistical difference was found in glucose metabolism indices between the observation and control groups after 24 weeks of treatment (P> 0.05) (Table 1).
There was no statistical difference in the biochemical indices and HOMA-IR index between the two groups before treatment (P> 0.05). However, the levels of visceral adiponectin, Lipoprotein (LP), FINS. and HOMA-IR were lower than those before treatment in both groups after 24 weeks of treatment (P< 0.05). We found no statistical difference in biochemical indices and HOMA-IR levels between the observation and control groups after 24 weeks of treatment (P> 0.05) (Table 2).
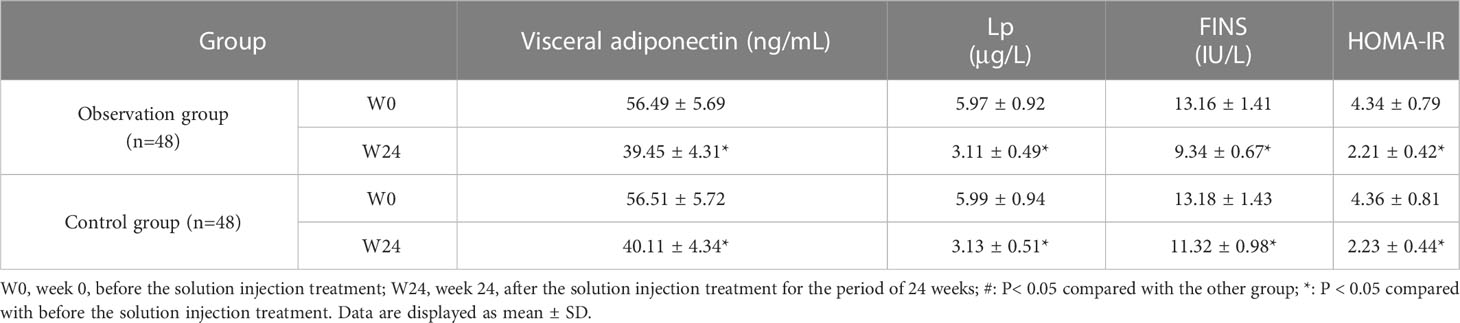
Table 2 Comparison of biochemical indices and Homa-IR index between control and observation groups .
Both groups completed the continuous treatment over 24 weeks and the clinical application value of the different drugs was assessed from an economic point of view. No statistical difference in CER levels was found between the two groups (P> 0.05); however, the cost was lower in the observation group than in the control group after 24 weeks of treatment (P< 0.05) (Table 3).
The incidence of nausea and vomiting, hypoglycemia, cholecystitis, allergic reactions, and liver and kidney abnormalities was much lower in observation group compared to the control group (Table 4).
Type 2 diabetic patients account for more than 90% of all diabetic patients. Many patients with type 2 diabetes might not have a complete loss of insulin secretion, and some might have excessive insulin secretion (30). However, type 2 diabetic patients are poor users of insulin, and the persistent hyperglycemic condition will have a negative impact on the ability of the body to metabolize glucose, leading to chronic elevation of blood glucose in patients. Liraglutide is one of the human glucagon plasmin-1 analogues, which belongs to a family of glucose-lowering drugs with a strong hypoglycemic effect. Liraglutide is an injection solution but not insulin, and it can promote insulin secretion and inhibits the secretion of hyperglycemic hormone and the feeding center in the brain.
In our study, both short-acting and long-acting GLP-1 receptor agonists can reduce the levels of FPG, 2hPG, and HbAlc, which is consistent with previous studies (31). Kapodistria’s study (32) showed that Liraglutide could promote enterocytes to secrete insulin by elevating endogenous GLP-1 levels from a physiological dose to a pharmacological dose. Actually, long-acting GLP-1 receptor agonists can provide better blood glucose control than short-acting ones because patients with long-acting receptor agonists have higher fasting insulin levels (possibly at night) (33, 34). Persistent high plasma levels of long-acting GLP-1 receptor agonists lead to a decrease in plasma HbA1c levels, which is greater than the decrease observed in intermittent activation of GLP-1 receptor caused by the administration of short-acting compounds (13, 35). Moreover, long-acting GLP-1 receptor agonists have no substantial effect on gastric motility, 76 which may be due to rapid immune response, which means that the effect of these compounds on gastric emptying decreases rapidly over time because they continuously activate GLP-1 receptor (36). In addition, long-acting GLP-1 receptor agonists do not reduce postprandial blood glucose fluctuations like short-acting compounds (37). by comparison, the clinical application of Liraglutide requires patients to inject the solution once a day. The drug is expensive with relatively low-cost performance, which limits its clinical use and makes it difficult to promote its application in primary hospitals.
In response to the expensive price and relatively low-cost performance of Liraglutide, Dulaglutide has begun to be used clinically (38). In our study, the levels of FPG, 2hPG, and HbAlc were decreased in both groups after the treatment for the period of 24 weeks. There was no statistical difference in blood glucose levels between the observation group and the control group after the treatment with Dulaglutide for the period of 24 weeks. However, the levels of visceral adiponectin, LP, FINS, and HOMA-IR were lower in both groups after treatment for 24 weeks than before the treatment. Cardiovascular disease caused by diabetes is one of the common complications of T2DM. Lipoprotein rich in cholesterol is an important risk factor for atherosclerosis, including coronary heart disease, myocardial infarction, stroke and peripheral vascular disease. Low density lipoprotein (LDL) and lipoprotein (a) Lp (a) are important components of cholesterol ester rich lipoproteins (39, 40). Kotani et al. found that endothelial dysfunction may be related to oxidized Lp (a) in T2DM patients (41). Saeed et al. studied the relationship between elevated Lp (a) and CVD risk in nearly 10000 male and female participants, including 1543 people with diabetes or pre diabetes (42). No statistical difference was determined in biochemical indices and HOMA-IR index levels between the observation group and the control group after the treatment for the period of 24 weeks. Dulaglutide can be applied to control blood glucose in type 2 diabetic patients. Because of the relatively high molecular weight of the injection solution, it is generally not easily absorbed and degraded by the body, thus the duration of drug activity is relatively long. Therefore, Dulaglutide can be used once a week to meet the clinical requirements. In addition, the solution can promote the release of insulin, delay gastric emptying, and control the total daily energy intake in a certain range by reducing the intake of food, to achieve a good hypoglycemic effect (43).
Pharmacoeconomics is the specific application of economic principles and methods in pharmaceuticals (44). By a broad generalized definition, pharmacoeconomics focuses on the study of the economic behavior of the supply and demand of drugs, the interaction between supply and demand of drug market pricing, and the measures of various intervention policies in the field of drugs (45). In a narrow sense, however, pharmacoeconomics is the application of the basic principles, methods, and analytical techniques of economics in the clinical treatment process of the drug, using the pharmacoepidemiological population as a guide and based on a society-wide perspective to seek maximum rational utilization (46). To analyze further the pharmacoeconomic value of Dulaglutide, we evaluated its use in this study from the perspective of cost and CER. We found no statistical difference between the two groups in terms of CER levels; the cost of the observation group was lower than that of the control group after the treatment for the period of 24 weeks.
In previous studies, it was found that the economic benefit of dulaglutide is higher than that of liraglutide in the short term, but the long-term economic benefit is still unclear. Our research results extend the previous results (12).Moreover, the economic value of Dulaglutide was higher, and the drug was relatively cost-effective compared with Liraglutide. Some researchers (47) gave Dulaglutide and Liraglutide to patients with type 2 diabetes and then evaluated the effect from the perspective of economics. Our research conclusion is consistent with the previous study. Their results also found that Dulaglutide has a price advantage for type 2 diabetic patients because Dulaglutide has a relatively low cost coupled with the fact that the solution is injected once a week and, therefore, is suitable for promotion in primary hospitals. In this study, the incidence of nausea and vomiting, hypoglycemia, cholecystitis, allergic reactions, and hepatic and renal abnormalities was lower in the observation group than in the control group during the treatment period, the complications of diabetes and its high hospitalization rate are important factors for the increase in treatment costs of diabetes (48). The gastrointestinal reaction of dulaglutide is significantly reduced. The weekly injection rate can improve the compliance of patients, reduce the incidence of complications of diabetes (20), and reduce the cost of consumables such as injection needles and diabetes management (48).
Therefore, patients with type 2 diabetes should be treated to improve relevant glucose and insulin indices then, appropriate hypoglycemic drugs should be selected in combination with their economic status and family background, to improve the pertinent treatment.
Both Liraglutide and Dulaglutide can reduce blood glucose level and improve visceral adiponectin, Lp, and the HOMA-IR index level in type 2 diabetic patients. The effects of Liraglutide and Dulaglutide are similar. Because Dulaglutide is more cost-effective and safer with fewer adverse reactions, the application of Dulaglutide deserves further promotion.
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
This study was approved by the Ethics Committee of Ethics Committee of Longhu Hospital, First Affiliated Hospital of Medical College of Shantou University (the registration number is: ChiCTR1900026514). The patients/participants provided their written informed consent to participate in this study.
Conceptualization, KH and YS; methodology, KH; formal analysis, FWC and JC; investigation, ZZW and FYD; resources, DZ; data curation, SZ; writing-original draft preparation, SZ and KH; writing-review and editing, YS, ZZW, WL; supervision, WL, FWC; funding acquisition, KH; All authors contributed to the article and approved the submitted version.
This study was supported by Shantou science and technology project (No: 200812225264260).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Hou K, Zhang S, Wu Z, Zhu D, Chen F, Lei ZN, et al. Reconstruction of intestinal microecology of type 2 diabetes by fecal microbiota transplantation: Why and how. Bosn J Basic Med Sci (2021) 22(3):315–25. doi: 10.17305/bjbms.2021.6323
2. Tönnies T, Brinks R, Hoyer A. Mortalität bei typ-2-Diabetes in deutschland. Der Diabetologe. (2019) 15(3):223–9. doi: 10.1007/s11428-018-0436-6
3. Chen F, He L, Li J, Yang S, Zhang B, Zhu D, et al. Polyethylene glycol loxenatide injection (GLP-1) protects vascular endothelial cell function in middle-aged and elderly patients with type 2 diabetes by regulating gut microbiota. Front Mol Biosci (2022) 9:879294. doi: 10.3389/fmolb.2022.879294
4. Hou K, Wu ZX, Chen XY, Wang JQ, Zhang D, Xiao C, et al. Microbiota in health and diseases. Signal Transduct Target Ther (2022) 7(1):135. doi: 10.1038/s41392-022-00974-4
5. Que Y, Cao M, He J, Zhang Q, Chen Q, Yan C, et al. Gut bacterial characteristics of patients with type 2 diabetes mellitus and the application potential. Front Immunol (2021) 12:722206. doi: 10.3389/fimmu.2021.722206
6. Bell GI, Sanchez-Pescador R, Laybourn PJ, Najarian RC. Exon duplication and divergence in the human preproglucagon gene. Nature. (1983) 304(5924):368–71. doi: 10.1038/304368a0
7. Gutniak M, Orskov C, Holst JJ, Ahren B, Efendic S. Antidiabetogenic effect of glucagon-like peptide-1 (7-36)amide in normal subjects and patients with diabetes mellitus. N Engl J Med (1992) 326(20):1316–22. doi: 10.1056/NEJM199205143262003
8. Nauck MA, Bartels E, Orskov C, Ebert R, Creutzfeldt W. Additive insulinotropic effects of exogenous synthetic human gastric inhibitory polypeptide and glucagon-like peptide-1-(7-36) amide infused at near-physiological insulinotropic hormone and glucose concentrations. J Clin Endocrinol Metab (1993) 76(4):912–7. doi: 10.1210/jcem.76.4.8473405
9. Nauck MA, Heimesaat MM, Orskov C, Holst JJ, Ebert R, Creutzfeldt W. Preserved incretin activity of glucagon-like peptide 1 [7-36 amide] but not of synthetic human gastric inhibitory polypeptide in patients with type-2 diabetes mellitus. J Clin Invest. (1993) 91(1):301–7. doi: 10.1172/JCI116186
10. Nauck MA, Kleine N, Orskov C, Holst JJ, Willms B, Creutzfeldt W. Normalization of fasting hyperglycaemia by exogenous glucagon-like peptide 1 (7-36 amide) in type 2 (non-insulin-dependent) diabetic patients. Diabetologia. (1993) 36(8):741–4. doi: 10.1007/BF00401145
11. Hansen L, Hartmann B, Bisgaard T, Mineo H, Jorgensen PN, Holst JJ. Somatostatin restrains the secretion of glucagon-like peptide-1 and -2 from isolated perfused porcine ileum. Am J Physiol Endocrinol Metab (2000) 278(6):E1010–8. doi: 10.1152/ajpendo.2000.278.6.E1010
12. Meier JJ, Gethmann A, Gotze O, Gallwitz B, Holst JJ, Schmidt WE, et al. Glucagon-like peptide 1 abolishes the postprandial rise in triglyceride concentrations and lowers levels of non-esterified fatty acids in humans. Diabetologia. (2006) 49(3):452–8. doi: 10.1007/s00125-005-0126-y
13. Buse JB, Rosenstock J, Sesti G, Schmidt WE, Montanya E, Brett JH, et al. Liraglutide once a day versus exenatide twice a day for type 2 diabetes: a 26-week randomised, parallel-group, multinational, open-label trial (LEAD-6). Lancet (London England). (2009) 374(9683):39–47. doi: 10.1016/S0140-6736(09)60659-0
14. Zinman B, Gerich J, Buse JB, Lewin A, Schwartz S, Raskin P, et al. Efficacy and safety of the human glucagon-like peptide-1 analog liraglutide in combination with metformin and thiazolidinedione in patients with type 2 diabetes (LEAD-4 Met+TZD). Diabetes Care (2009) 32(7):1224–30. doi: 10.2337/dc08-2124
15. Degn KB, Juhl CB, Sturis J, Jakobsen G, Brock B, Chandramouli V, et al. One week's treatment with the long-acting glucagon-like peptide 1 derivative liraglutide (NN2211) markedly improves 24-h glycemia and alpha- and beta-cell function and reduces endogenous glucose release in patients with type 2 diabetes. Diabetes. (2004) 53(5):1187–94. doi: 10.2337/diabetes.53.5.1187
16. Pechenov S, Bhattacharjee H, Yin D, Mittal S, Subramony JA. Improving drug-like properties of insulin and GLP-1 via molecule design and formulation and improving diabetes management with device & drug delivery. Adv Drug Delivery Rev (2017) 112:106–22. doi: 10.1016/j.addr.2017.01.006
17. Trapp S, Brierley DI. Brain GLP-1 and the regulation of food intake: GLP-1 action in the brain and its implications for GLP-1 receptor agonists in obesity treatment. Br J Pharmacol (2022) 179(4):557–70. doi: 10.1111/bph.15638
18. Faerch K, Torekov SS, Vistisen D, Johansen NB, Witte DR, Jonsson A, et al. GLP-1 response to oral glucose is reduced in prediabetes, screen-detected type 2 diabetes, and obesity and influenced by sex: The ADDITION-PRO study. Diabetes. (2015) 64(7):2513–25. doi: 10.2337/db14-1751
19. Inagaki N, Takeuchi M, Oura T, Imaoka T, Seino Y. Efficacy and safety of tirzepatide monotherapy compared with dulaglutide in Japanese patients with type 2 diabetes (SURPASS J-mono): a double-blind, multicentre, randomised, phase 3 trial. Lancet Diabetes Endocrinol (2022) 10(9):623–33. doi: 10.1016/S2213-8587(22)00188-7
20. Meier JJ. GLP-1 receptor agonists for individualized treatment of type 2 diabetes mellitus. Nat Rev Endocrinology. (2012) 8(12):728–42. doi: 10.1038/nrendo.2012.140
21. Luo Q, Wei R, Cai Y, Zhao Q, Liu Y, Liu WJ. Efficacy of off-label therapy for non-alcoholic fatty liver disease in improving non-invasive and invasive biomarkers: A systematic review and network meta-analysis of randomized controlled trials. Front Med (Lausanne). (2022) 9:793203. doi: 10.3389/fmed.2022.793203
22. Ja'arah D, Al Zoubi MS, Abdelhady G, Rabi F, Tambuwala MM. Role of glucagon-like peptide-1 (GLP-1) receptor agonists in hypoglycemia. Clin Med Insights Endocrinol Diabetes. (2021) 14:11795514211051697. doi: 10.1177/11795514211051697
23. Cheng D, Yang S, Zhao X, Wang G. The role of glucagon-like peptide-1 receptor agonists (GLP-1 RA) in diabetes-related neurodegenerative diseases. Drug Des Devel Ther (2022) 16:665–84. doi: 10.2147/DDDT.S348055
24. Wang Y, Han B. Dulaglutide alleviates alzheimer's disease by regulating microglial polarization and neurogenic activity. Comb Chem High Throughput Screen (2022). doi: 10.2174/1386207325666220726163514
25. Bagger JI, Grondahl MFG, Lund A, Holst JJ, Vilsboll T, Knop FK. Glucagonostatic potency of GLP-1 in patients with type 2 diabetes, patients with type 1 diabetes, and healthy control subjects. Diabetes. (2021) 70(6):1347–56. doi: 10.2337/db20-0998
26. American Diabetes Association Professional Practice C. 2. Classification and diagnosis of diabetes: Standards of medical care in diabetes-2022. Diabetes Care (2022) 45(Suppl 1):S17–38. doi: 10.2337/dc22-S002
27. Yanay O, Moralejo D, Kernan K, Brzezinski M, Fuller JM, Barton RW, et al. Prolonged survival and improved glycemia in BioBreeding diabetic rats after early sustained exposure to glucagon-like peptide 1. J Gene Med (2010) 12(6):538–44. doi: 10.1002/jgm.1466
28. Saab S, Choi Y, Rahal H, Li K, Tong M. Trends in viral hepatitis cost-effectiveness studies. Am J Manag Care (2012) 18(12):790–8.
29. de Luis DA, Aller R, Izaola O, Bachiller R. Role of rs6923761 gene variant in glucagon-like peptide 1 receptor in basal GLP-1 levels, cardiovascular risk factor and serum adipokine levels in naive type 2 diabetic patients. J Endocrinol Invest. (2015) 38(2):143–7. doi: 10.1007/s40618-014-0161-y
30. Chasens ER, Korytkowski M, Burke LE, Strollo PJ, Stansbury R, Bizhanova Z, et al. Effect of treatment of OSA with CPAP on glycemic control in adults with type 2 diabetes: The diabetes sleep treatment trial (DSTT). Endocr Pract (2022) 28(4):364–71. doi: 10.1016/j.eprac.2022.01.015
31. Bunck MC, Corner A, Eliasson B, Heine RJ, Shaginian RM, Taskinen MR, et al. Effects of exenatide on measures of beta-cell function after 3 years in metformin-treated patients with type 2 diabetes. Diabetes Care (2011) 34(9):2041–7. doi: 10.2337/dc11-0291
32. Kapodistria K, Tsilibary EP, Kotsopoulou E, Moustardas P, Kitsiou P. Liraglutide, a human glucagon-like peptide-1 analogue, stimulates AKT-dependent survival signalling and inhibits pancreatic beta-cell apoptosis. J Cell Mol Med (2018) 22(6):2970–80. doi: 10.1111/jcmm.13259
33. Madsen K, Knudsen LB, Agersoe H, Nielsen PF, Thøgersen H, Wilken M, et al. Structure-activity and protraction relationship of long-acting glucagon-like peptide-1 derivatives: importance of fatty acid length, polarity, and bulkiness. J medicinal Chem (2007) 50(24):6126–32. doi: 10.1021/jm070861j
34. Buse JB, Nauck MA, Forst T, Sheu W, Schernthaner G eds. Efficacy and safety of exenatide once weekly versus liraglutide in subjects with type 2 diabetes (DURATION-6): a randomised, open-label study. 47th annual meeting of the the Lancet (2011). doi: 10.1016/S0140-6736(12)61267-7
35. Drucker DJ, Buse JB, Taylor K, Kendall DM, Trautmann M, Zhuang D, et al. Exenatide once weekly versus twice daily for the treatment of type 2 diabetes: a randomised, open-label, non-inferiority study. Lancet (London England). (2008) 372(9645):1240–50. doi: 10.1016/S0140-6736(08)61206-4
36. Nauck MA, Kemmeries G, Holst JJ, Meier JJ. Rapid tachyphylaxis of the glucagon-like peptide 1-induced deceleration of gastric emptying in humans. Diabetes. (2011) 60(5):1561–5. doi: 10.2337/db10-0474
37. Buse JB, Garber A, Rosenstock J, Schmidt WE, Brett JH, Videbaek N, et al. Liraglutide treatment is associated with a low frequency and magnitude of antibody formation with no apparent impact on glycemic response or increased frequency of adverse events: results from the liraglutide effect and action in diabetes (LEAD) trials. J Clin Endocrinol Metab (2011) 96(6):1695–702. doi: 10.1210/jc.2010-2822
38. Alatorre C, Fernandez Lando L, Yu M, Brown K, Montejano L, Juneau P, et al. Treatment patterns in patients with type 2 diabetes mellitus treated with glucagon-like peptide-1 receptor agonists: Higher adherence and persistence with dulaglutide compared with once-weekly exenatide and liraglutide. Diabetes Obes Metab (2017) 19(7):953–61. doi: 10.1111/dom.12902
39. Kostner KM, Kostner GM. Lipoprotein (a): a historical appraisal. J Lipid Res (2017) 58(1):1–14. doi: 10.1194/jlr.R071571
40. Jenner JL, Seman LJ, Millar JS, Lamon-Fava S, Welty FK, Dolnikowski GG, et al. The metabolism of apolipoproteins (a) and b-100 within plasma lipoprotein (a) in human beings. Metabolism: Clin experimental. (2005) 54(3):361–9. doi: 10.1016/j.metabol.2004.10.001
41. Kotani K, Yamada S, Takahashi H, Iwazu Y, Yamada T. The ratio of oxidized lipoprotein(a) to native lipoprotein(a) and the endothelial function in patients with type 2 diabetes mellitus. Int J Mol Sci (2019) 20(19). doi: 10.3390/ijms20194909
42. Saeed A, Sun W, Agarwala A, Virani SS, Nambi V, Coresh J, et al. Lipoprotein(a) levels and risk of cardiovascular disease events in individuals with diabetes mellitus or prediabetes: The atherosclerosis risk in communities study. Atherosclerosis. (2019) 282:52–6. doi: 10.1016/j.atherosclerosis.2018.12.022
43. Mody R, Huang Q, Yu M, Zhao R, Patel H, Grabner M, et al. Adherence, persistence, glycaemic control and costs among patients with type 2 diabetes initiating dulaglutide compared with liraglutide or exenatide once weekly at 12-month follow-up in a real-world setting in the united states. Diabetes Obes Metab (2019) 21(4):920–9. doi: 10.1111/dom.13603
44. Zueger PM, Schultz NM, Lee TA. Cost effectiveness of liraglutide in type II diabetes: a systematic review. Pharmacoeconomics. (2014) 32(11):1079–91. doi: 10.1007/s40273-014-0192-4
45. Willis M, Johansen P, Nilsson A, Asseburg C. Validation of the economic and health outcomes model of type 2 diabetes mellitus (ECHO-T2DM). Pharmacoeconomics. (2017) 35(3):375–96. doi: 10.1007/s40273-016-0471-3
46. Piao C, Zhang Q, Jin, Shao M, Bi C, Wang L, et al. Treatment of type 2 diabetes with tianqi jiangtang capsule: A systematic review and meta-analysis of randomized controlled trials. Med (Baltimore). (2020) 99(21):e19702. doi: 10.1097/MD.0000000000019702
47. Lasalvia P, Baquero L, Otalora-Esteban M, Castaneda-Cardona C, Rosselli D. Cost effectiveness of dulaglutide compared with liraglutide and glargine in type 2 diabetes mellitus patients in Colombia. Value Health Reg Issues. (2017) 14:35–40. doi: 10.1016/j.vhri.2016.10.006
48. Mata-Cases M, Casajuana M, Franch-Nadal J, Casellas A, Castell C, Vinagre I, et al. Direct medical costs attributable to type 2 diabetes mellitus: a population-based study in Catalonia, Spain. The European journal of health economics: HEPAC: health economics in prevention and care (2016) 17(8):1001–10. doi: 10.1007/s10198-015-0742-5
Keywords: Dulaglutide, type 2 diabetes, treatment effect, pharmacoeconomics, insulin resistance index, cost-effectiveness ratio
Citation: Su Y, Zhang S, Wu Z, Liu W, Chen J, Deng F, Chen F, Zhu D and Hou K (2023) Pharmacoeconomic analysis (CER) of Dulaglutide and Liraglutide in the treatment of patients with type 2 diabetes. Front. Endocrinol. 14:1054946. doi: 10.3389/fendo.2023.1054946
Received: 01 October 2022; Accepted: 09 January 2023;
Published: 23 January 2023.
Edited by:
Chunjiang Wang, Department of Pharmacy, Central South University, ChinaReviewed by:
Wei Wu, Guangdong Provincial Center for Disease Control and Prevention, ChinaCopyright © 2023 Su, Zhang, Wu, Liu, Chen, Deng, Chen, Zhu and Hou. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Kaijian Hou, S2FpamlhbmhvdUAxMjYuY29t
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.