- 1Ultrasound Medical Department, China Japan Friendship Hospital, Beijing, China
- 2Department of Ultrasound, Civil Aviation General Hospital, Beijing, China
Background: This study is aimed at evaluating the diagnostic efficacy and unnecessary fine-needle aspiration (FNA) rate of ultrasound-based risk stratification for thyroid nodules in the American College of Radiology (ACR) Thyroid Imaging Reporting and Data System (TI-RADS) and the American Thyroid Association (ATA) risk stratification systems.
Methods: Children and adolescents with pathology confirmed thyroid nodules were retrospectively included in this study. A total of 217 thyroid nodules from multicenter of Union Medical College Hospital, China Japan Friendship Hospital and Civil Aviation Hospital were included, the diagnostic efficiency and unnecessary FNA rate were calculated according to ACR and ATA guidelines.
Results: Among all thyroid nodules, 139 nodules were malignant, and 78 nodules were benign. Choosing ATA high suspicion and ACR TI-RADS TR5 as benign and malignant cut-off points, the area under the curve and sensitivity of ATA were higher than ACR (AUC: 0.887 vs 0.840, p=0.0037; sensitivity 81.3% vs 71.0%, P <0.049;specificity 96.2% vs 97.4%, p=1.000;specificity both 85.9%); choosing high/intermediate suspicion in ATA and ACR TR4/5 as benign and malignant cut-off points, the two guidelines demonstrated similar diagnostic efficacy (AUC:0.890 vs 0.897, p=0.6038, sensitivity 92.1% vs 93.5%, P =0.817;specificity both 85.9%, p=1.000). The inappropriate FNA rate of ACR guideline was relatively lower (ATA 42.9% vs ACR 27.2%, P <0.001). If ACR TI-RADS TR5 nodules less than 1.0cm were included in the FNA indication, the unnecessary biopsy rate would be further reduced to 17.9%.
Conclusion: This study indicated that both ATA and ACR TI-RADS risk stratification systems could provide a feasible differential diagnosis of benign and malignant thyroid nodules, while the ACR risk stratification system demonstrates a lower rate of inappropriate FNA rate. In addition, it was necessary to further study the minimum FNA threshold of thyroid nodules in Children and adolescents in order to reduce the missed biopsy rate of malignant nodules.
1 Introduction
The incidence rate of thyroid cancer in children and adolescents is relatively low, at approximately 1.9% of all thyroid cancers (1). While thyroid cancer is still the most common endocrine malignant tumor in children and adolescents, accounting for about 11% of all cancers in children and adolescents (2), its incidence rate is increasing yearly (3, 4). The pathological composition, ultrasonic imaging manifestations and biological characteristics of thyroid cancer in children and adolescents are also different from those in adults. The probability of metastasis and recurrence is higher. The metastasis rate can be as high as 40% - 80%, and the recurrence rate is about 30% (5, 6). Therefore, early diagnosis and treatment of thyroid cancer in children and adolescents are vitally important. Ultrasound is the first-line medical imaging choice for the diagnosis of thyroid nodules. At present, there are many thyroid grading diagnostic systems. For most grading diagnostic systems the nodule’s differentiation between benign and malignant and whether the nodules need to undergo ultrasound-guided fine needle aspiration cytology (FNA) was determined according to the ultrasonic characteristics of thyroid nodules and the size of the nodules. However, the current guidelines, such as the 2015 American Thyroid Association (7) (ATA for short), and ACR Thyroid Imaging Reporting and Data System (ACR TI-RADS for short) proposed in 2017 (8), were based on the ultrasonic characteristics of the adult thyroid nodules. More research was needed to verify the application of the above guidelines in children and adolescents with thyroid nodules (9). Therefore, this study aimed to compare the diagnostic efficacy and value for guiding FNA of ATA guidelines and ACR TI-RADS guidelines in children and adolescents.
2 Materials and methods
2.1 Patients
This study was retrospective. Patients with thyroid nodules younger than 18 years old were included. 178 patients who underwent thyroid surgery in Beijing Union Medical College Hospital from January 2000 to October 2017 were selected. Excluding 15 patients with incomplete clinical data, 11 patients with unclear ultrasound images, and 8 patients with no correspondence between ultrasound and pathological results, a total of 34 patients were excluded. 144 patient with 176 nodules were finally included. And 59 patients that had pathology results in China Japan Friendship Hospital and Civil Aviation General Hospital from November 2015 to January 2022 were selected, 12 patients had unclear images and 6 patients had no precise pathological results, a total of 18 patients were excluded, 41 patients with 41 nodules were finally included. All of the above included nodules have a clear pathological diagnoses.
2.2 Thyroid US examination and retrospective evaluation
Instrument: ultrasound examinations were performed by GE Logiq 9, Philips iU22, Philips IPQ7 and other color Doppler ultrasound diagnostic instruments, and the equipped probe frequency was 5-12 MHz.
Examination method and ultrasonic image evaluation method: The physician performed all examinations. During the examination, the patient took a supine position, fully exposed the neck, instructed the patient to breathe calmly, placed the probe gently in the thyroid region, and adjusted the gain, depth, image focus and other related parameters at any time to obtain the best imaging effect. The ultrasound physician routinely scanned the thyroid and bilateral cervical lymph nodes, and the ultrasonic examination data of all nodules were stored. Two radiologists specialized in ultrasound diagnosis with more than 5 years of experience retrospectively analyzed the ultrasound images of thyroid nodules, recorded the size, location, structure, echo, shape, edge, calcification, relationship with capsule of the nodules, and evaluated the nodules using the 2017 ACR TI-RADS (8) and the 2015 ATA (7) guideline classification diagnostic criteria. Both radiologists were blinded to final pathology and abnormal lymph nodes. If a patient had multiple nodules, each nodule should be analyzed separately. If they disagreed, the final judgment was discussed and negotiated. Ultrasound illustration of ATA and ACR TI-RADS pattern could be found in Figure 1.
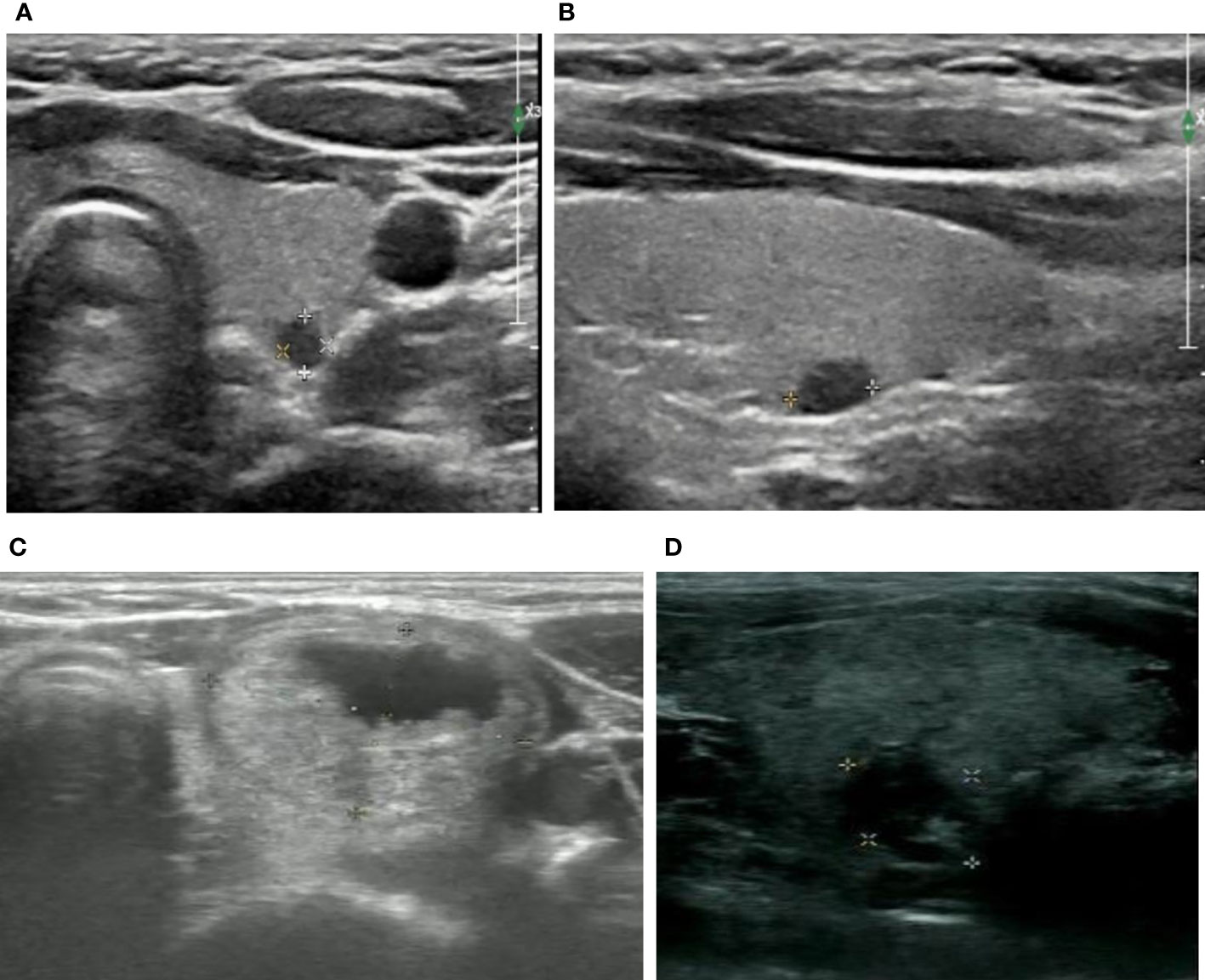
Figure 1 Ultrasound illustration of ATA and ACR TI-RADS pattern. (A, B) female, 18y, ACR TI-RADS 5 and ATA high suspicion, maximum diameter of 0.5cm, FNA and surgical pathology confirmed papillary carcinoma of thyroid (C) female, 16y, ACR TI-RADS 2 FNA not recommended;ATA low suspicion FNA recommended;maximum diameter of 2.2cm, surgical pathology confirmed nodular goiters (D) female,16y,ACR TI-RADS 4 FNA not recommended;ATA intermediate suspicion FNA recommended;maximum diameter of 1.2cm, FNA pathology confirmed inflammatory nodule.
2.3 Statistical analysis
The age and nodule size of patients was expressed by x ± S and interquartile ranges (IQR). The comparison of age between benign group and malignant group was analyzed by t test, and the comparison of sex adopted chi-square test. According to the recommendations of ACR TIRADS and ATA guidelines on biopsy, the nodules were divided into two groups, with pathological results as the “gold standard”, the receiver operator characteristic (ROC) curve was established. The sensitivity, specificity, positive predictive value, negative predictive value, accuracy and 95% confidence interval were calculated to evaluate the differential diagnostic effectiveness of the two guidelines. The inappropriate biopsy for thyroid nodules was defined as the number of benign nodules requiring biopsy and the number of malignant not requiring biopsy. SPSS 25.0 (IBM, Armonk, New York, USA) and MedCalc 11.4.2.0 software (MedCalc Software, Ostend, Belgium) were used for data analysis in this experiment, P<0.05 was statistically significant.
3 Results
3.1 Comparison of general data between benign group and malignant group
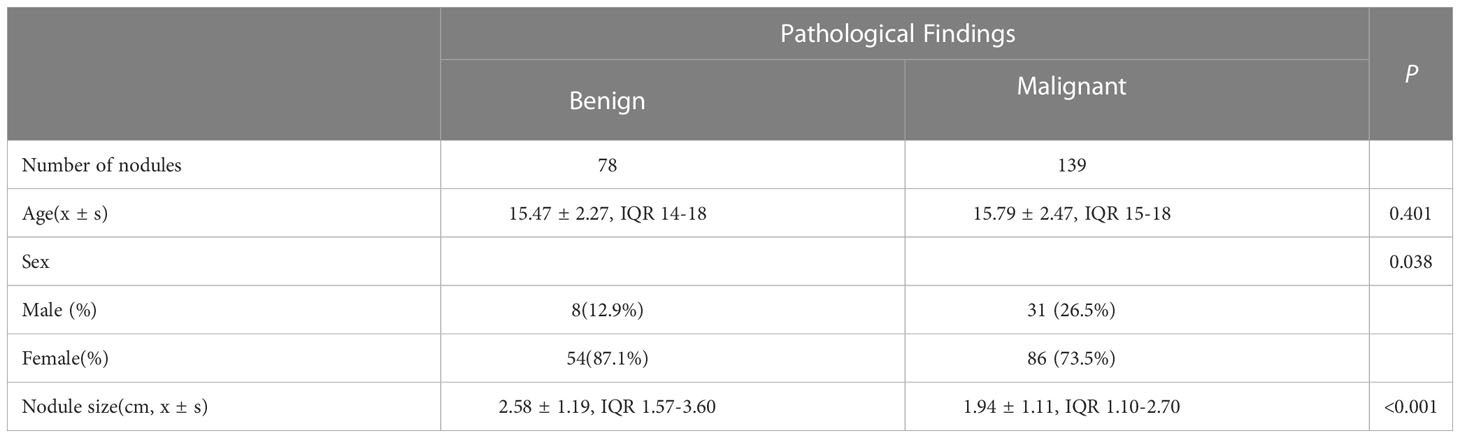
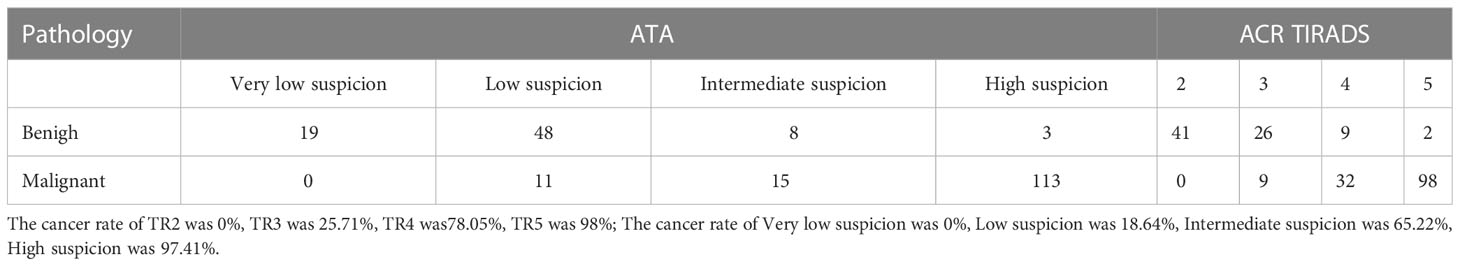
Among 217 thyroid nodules, 139 were malignant(including 131 papillary carcinoma, 7 follicular carcinoma and 1 undifferentiated carcinoma) and 78 were benign(including 53 nodular goiters, 5 inflammatory nodules, 10 adenomas, 2 hyperthyroidism changes after treatment, 1 fibrous hyperplasia and 7 FNA benign). Of all the nodules, 202 of them had surgical pathology and 15 of them had FNA pathology. The mean age of patients in malignant group was not significantly different from that in benign group [(15.47 ± 2.27) years vs (15.79 ± 2.47) years, P=0.401]. The proportion of male patients in malignant group and benign group was less than that of female patients, with a statistically significant difference [54 (87.1%) vs 86 (73.5%), P=0.038]. The size of nodules in malignant group was significantly smaller than that in benign group [(1.93 ± 1.11) cm vs (2.58 ± 1.20) cm, P<0.001] Table 1. The diagnosis performance of ATA and ACR TIRADS could be found in Table 2–8, performance as indication for FNA could be found in Table 9, 10.
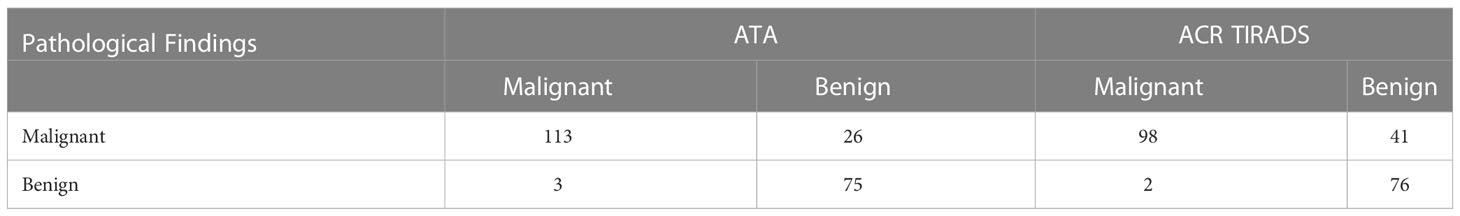
Table 3 Comparison of diagnostic efficacy between the diagnostic criteria of ACR TI-RADS TR5 and ATA high suspicion as malignant.
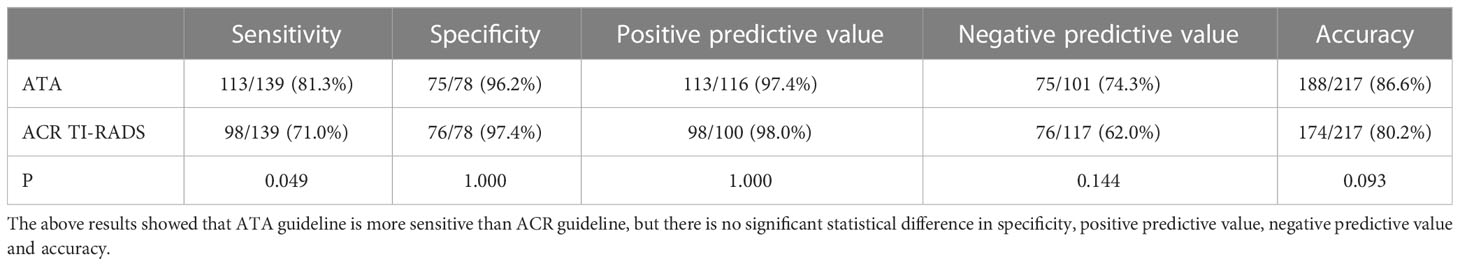
Table 4 Comparison of sensitivity, specificity, positive predictive value, negative predictive value, and accuracy of malignant tumors according to ATA guideline high suspicion and ACR TI-RADS TR5.
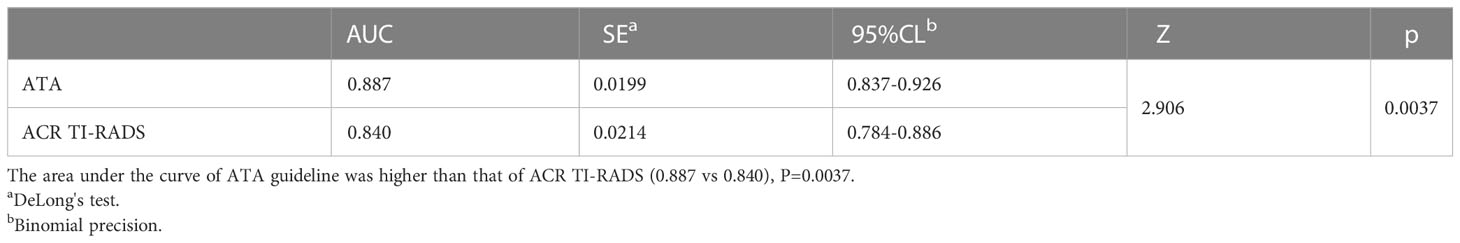
Table 5 Comparison of area under curve of malignant tumors according to ATA guideline high suspicion and ACR TIRADS TR5.
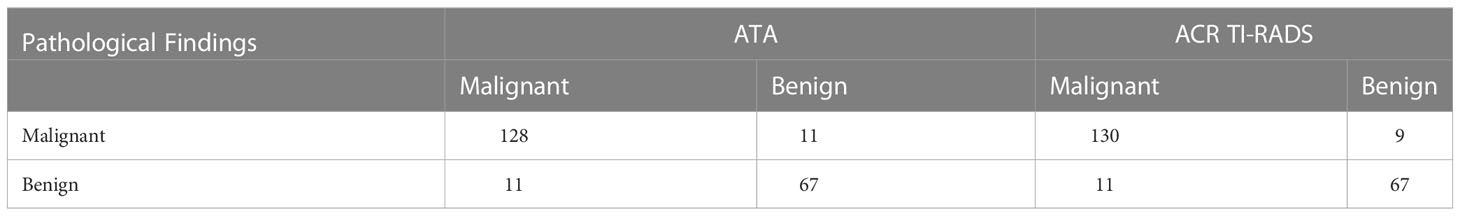
Table 6 Comparison of diagnostic efficacy between the diagnostic criteria of ACR TI-RADS TR4/5 and ATA high/intermediate suspicion as malignant.
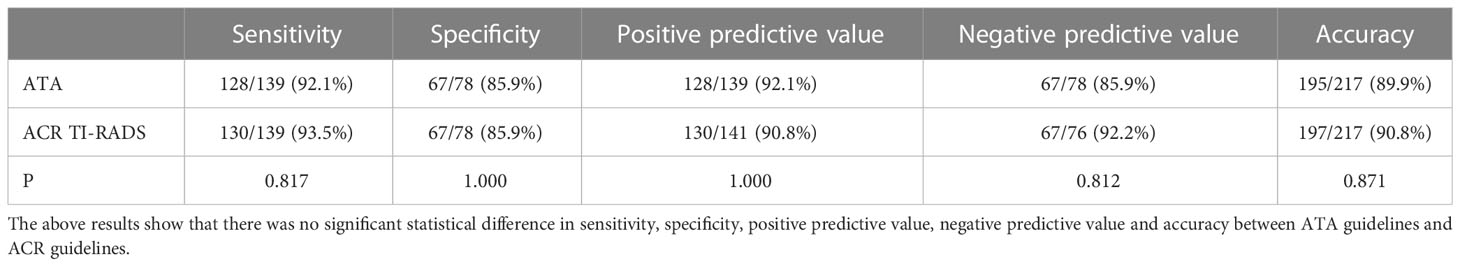
Table 7 Comparison of sensitivity, specificity, positive predictive value, negative predictive value, and accuracy according to ACR TI-RADS TR4/5 and ATA high/intermediate suspicion as malignant.
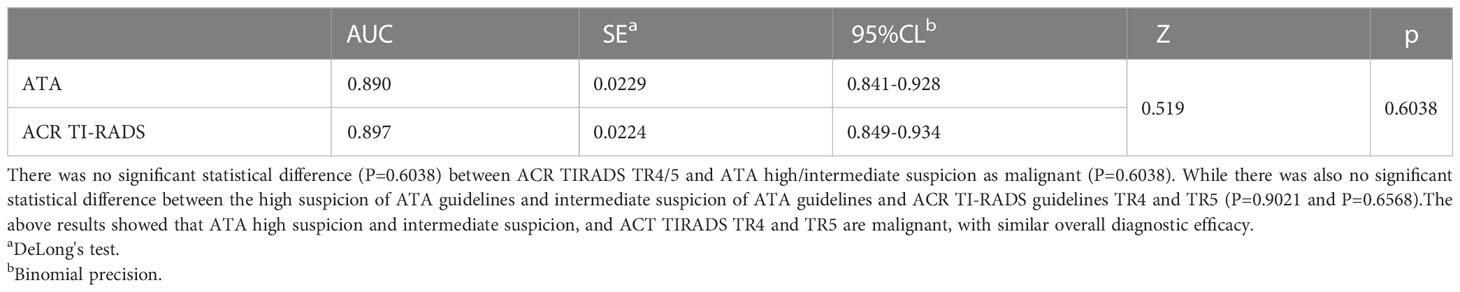
Table 8 Comparison of area under curve of malignant tumors according to ACR TI-RADS TR4/5 and ATA high/intermediate suspicion as malignant.
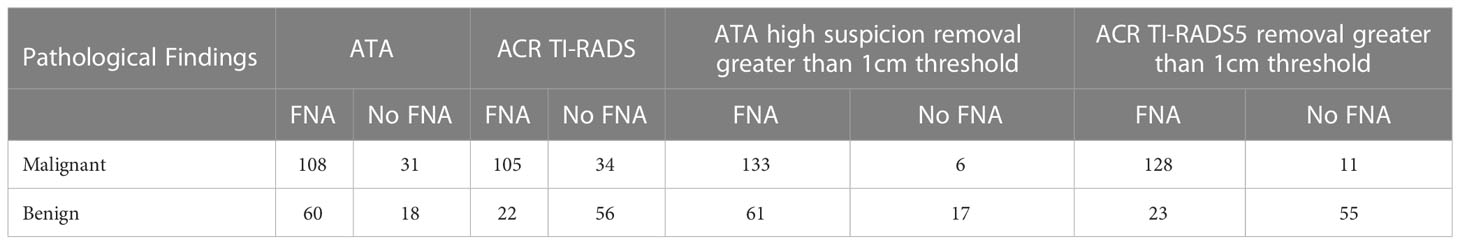
Table 9 The application guidelines recommend threshold, ATA high suspicion/TR5 nodule nodule removal greater than 1cm threshold, and compare the value of the above guidelines in guiding FNA.
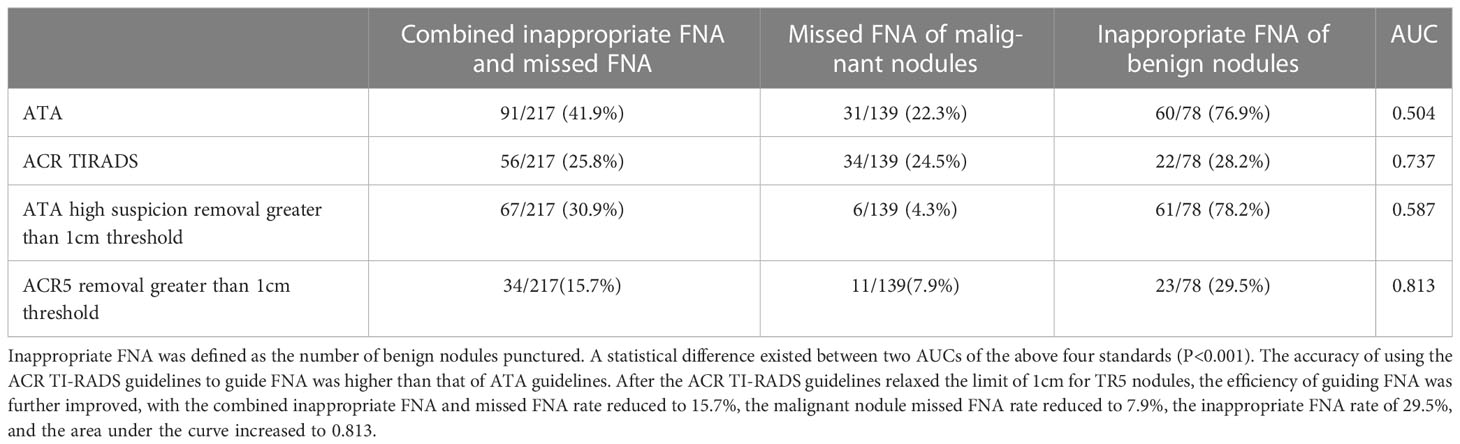
Table 10 Inappropriate FNA rate, missed FNA rate of malignant nodules, FNA rate of benign nodules and area under the curve of recommended threshold and optimized threshold in the application guideline.
4 Discussion
Ultrasound is the first-line imaging method for thyroid diseases. The grading system can be used to evaluate the benign and malignant nodules by standard methods, reduce the difference between observers, and guide whether the nodules have indications of FNA (10). The most widely used ATA and ACR TI-RADS guidelines are based on ultrasonic image characteristics and nodule size to determine the risk stratification of nodules and whether FNA is recommended. However, these two the guidelines are based on adult ultrasound data. There were still controversy about the diagnostic efficacy of the above guidelines in adults’ and children’s thyroid nodules. There were also large differences in the sensitivity and specificity data obtained from different studies (11–13 ), the sensitivies of different reports were between 0.37-1.00, and the specificities were between 0.24-0.90 (9, 14, 15). Therefore, applying the diagnostic grading diagnostic system in children and adolescents’ thyroid nodules deserves further research.
Different studies chose different cutoff points for differentiating benign and malignant tumors. This study showed that the specificity was relatively higher when selecting high suspicion in the ATA and TR5 as malignant compared to those choosing high/intermediate suspicion in the ATA and TR4/5 in ACR TI-RADS, the specificity was relatively higher (96.2% and 97.4% vs. 85.9% and 85.9%). The sensitivity was relatively lower (92.1% and 93.5% vs. 81.3% and 71.0%). Nevertheless, there was no significant difference in the area under the curve between ATA high suspicion, ACR TR5 and ACR TR4/5(0.887 vs 0.890 vs 0.897). It showed that the above two guidelines had similar overall diagnostic efficacy, which was similar to some previous studies (13, 16, 17). Although these two guidelines had similar overall diagnostic efficacy, appropriate diagnostic thresholds could be selected in our daily work to obtain higher diagnostic sensitivity or specificity.
ATA guidelines and ACR TI-RADS guidelines would not propose to recommend FNA for most thyroid nodules<1cm (8, 9), but previous studies had shown that different nodule FNA thresholds had a great impact on the guidance of nodules (18–20). Different guidelines differed in terms of the minimum nodule FNA size. For example, the French thyroid guidelines set the minimum FNA size as 7mm (21). Currently, studies have shown a high rate of missed FNA of malignant nodules and inappropriate FNA of benign nodules in children and adolescents using ACR and ATA guidelines for thyroid nodules (22). As thyroid cancer in children and adolescents was more malignant than that in adults, with a higher probability of local invasion and metastasis, early diagnosis was crucial to managing of nodules (23). In the 2015 ATA Guidelines for Children’s Thyroid, it was pointed out that it is difficult to deal with thyroid nodules smaller than 1cm in children’s thyroid, because the volume of children’s thyroid increases with age, and the size of individual nodules had little significance in predicting benign and malignant tumors. Therefore, when determining FNA criteria, we should refer to the ultrasonic characteristics of nodules and whether there are high-risk factors in patients (24). In this study, thyroid nodules smaller than 1cm accounted for 21.2% (46/217) of all nodules, so the minimum FNA size of TR5 and ATA high suspicion nodules with the highest risk classification was reduced in this study because these nodules had the most apparent ultrasonic malignant characteristics. After reducing the minimum size of nodule FNA, the rate of missed FNA of malignant nodules was significantly reduced, and the area under the curve was further improved.
As for the limitations of this study, first of all this study was a retrospective study, which inevitably had a particular selection bias. Radiologists assessed nodules and reached consensus, which could lead to higher accuracy than “real world”. A larger sample of clinical studies could be conducted to provide more information for diagnosis and the revision of the guideline FNA threshold. Second, the proportion of benign nodules in the included subjects was relatively small, while the malignant rate was high. That was mainly because the inclusion criteria of this study was that nodules with precise pathological results, so the diameter of the included benign nodules was larger, and the average diameter of benign nodules was larger than that of malignant nodules. In addition, only the nodules with definite pathology were included in this stud. This study did not include many benign nodules requiring follow-up but no need for FNA. Third, although this study showed that the rate of inappropriate FNA and the rate of missed FNA of malignant nodules had been reduced after the high suspicion nodule less than 1cm included, further research was still needed on the minimum threshold, FNA accuracy of nodules less than 1cm and whether the prognosis of patients after FNA could be improved,.
5 Conclusion
In conclusion, ATA and ACR TI-RADS guidelines were similar in diagnosing benign and malignant nodules in children and adolescents. ACR TI-RADS guidelines had higher accuracy in guiding FNA. In addition, further research was needed to determine the FNA threshold of thyroid nodules in children and adolescents to improve the overall accuracy of FNA.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving human participants were reviewed and approved by China-Japan Friendship Hospital Ethics Committee. Written informed consent to participate in this study was provided by the participants’ legal guardian/next of kin. Written informed consent was obtained from the individual(s), and minor(s)’ legal guardian/next of kin, for the publication of any potentially identifiable images or data included in this article.
Author contributions
GL and BZ participated in the study design, performed the statistical analysis and drafted the manuscript. JL and YX participated in its design and collection of samples. BZ participated in review of manuscript. All authors read and approved the final manuscript. All authors contributed to the article and approved the submitted version.
Funding
This study was supported by a grant from Beijing Union Medical Foundation Donation Project(2022-HX-68-JZ) and China-Japan Friendship Hospital Talent Introduction Project(2019-RC-2).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Karapanou O, Tzanela M, Vlassopoulou B, Kanaka-Gantenbein C. Differentiated thyroid cancer in childhood: a literature update. Hormones (Athens). (2017) 16:381–7. doi: 10.14310/horm.2002.1758
2. Zhao L, Pang P, Zang L, Luo Y, Wang F, Yang G, et al. Features and trends of thyroid cancer in patients with thyroidectomies in Beijing, China between 1994 and 2015: a retrospective study. BMJ Open (2019) 9(1):e023334. doi: 10.1136/bmjopen-2018-023334
3. Qian ZJ, MC J, KD M, Megwalu UC. Pediatric thyroid cancer incidence and mortality trends in the united states, 1973-2013. JAMA Otolaryngol Head Neck Surg (2019) 145:617–23. doi: 10.1001/jamaoto.2019.0898
4. Ward E, DeSantis C, Robbins A, Kohler B, Jemal A. Childhood and adolescent cancer statistics, 2014. CA Cancer J Clin (2014) 64:83–103. doi: 10.3322/caac.21219
5. Rivkees SA, Mazzaferri EL, Verburg FA, Reiners C, Luster M, Breuer CK, et al. The treatment of differentiated thyroidcancer in children: emphasis on surgical approach and radioactive iodine therapy. Endocrine Rev (2011) 32(6):798–826. doi: 10.1210/er.2011-0011
6. Zhang X, Liu L, Chen Y, Huang R, Liu B. Prognostic value of post-ablation (131) I scintigraphy in children with thyroid cancer. Head Neck (2020) 42:1738–45. doi: 10.1002/hed.26088
7. Haugen BR, Alexander EK, Bible KC, Doherty GM, Mandel SJ, Nikiforov YE, et al. American Thyroid association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: The American thyroid association guidelines task force on thyroid nodules and differentiated thyroid cancer. Thyroid (2015) 26(1):1–133. doi: 10.1089/thy.2015.0020
8. Tessler FN, WD M, Grant EG, Hoang JK, Berland LL, Teefey SA, et al. ACR thyroid imaging, reporting and data system (TI-RADS): White paper of the ACR TI-RADS committee. J Am Coll Radiol (2017) 14(5):587–95. doi: 10.1016/j.jacr.2017.01.046
9. Uner C, Aydin S, Ucan B. Thyroid image reporting and data system categorization: effectiveness in pediatric thyroid nodule assessment. Ultrasound Q (2020) 36:15–9. doi: 10.1097/RUQ.0000000000000476
10. Seifert P, Görges R, Zimny M, Kreissl MC, Schenke S. Interobserver agreement and efficacy of consensus reading in kwak-, EU-, and ACR-thyroid imaging recording and data systems and ATA guidelines for the ultrasound risk stratification of thyroid nodules. Endocrine. (2020) 67(1):143–54. doi: 10.1007/s12020-019-02134-1
11. Middleton WD, Teefey SA, Reading CC, Langer JE, Beland MD, Szabunio MM, et al. Comparison of performance characteristics of American college of radiology TI-RADS, Korean society of thyroid radiology TIRADS, and American thyroid association guidelines. AJR Am J Roentgenol. (2018) 210(5):1148–54. doi: 10.2214/AJR.17.18822
12. Kim PH, HM Y, Hwang J, Lee JS, Jung AY, Cho YA, et al. Diagnostic performance of adult-based ATA and ACR-TIRADS ultrasound risk stratification systems in pediatric thyroid nodules: a systematic review and meta-analysis. Eur Radiol (2021) 31(10):7450–63. doi: 10.1007/s00330-021-07908-8
13. Zhang WB, HX Xu, Zhang YF, Guo LH, Xu SH, Zhao CK, et al. Comparisons of ACR TI-RADS, ATA guidelines, kwak TI-RADS, and KTA/KSThR guidelines in malignancy risk stratification of thyroid nodules. Clin Hemorheol Microcirc (2020) 75(2):219–32. doi: 10.3233/CH-190778
14. Shapira-Zaltsberg G, Miller E, Martinez-Rios C, Bass J, Goldbloom EB, Tang K, et al. Comparison of the diagnostic performance of the 2017 ACR TIRADSguideline to the kwak guideline in children with thyroidnodules. Pediatr Radiol (2019) 49:862–8. doi: 10.1007/s00247-019-04385-6
15. Lim-Dunham JE, Erdem Toslak I, Alsabban K, Aziz A, Martin B, Okur G, et al. Ultrasound risk stratification for malignancy using the 2015 American thyroid association management guidelines for children with thyroid nodules and differentiated thyroid cancer. Pediatr Radiol (2017) 47:429–36. doi: 10.1007/s00247-017-3780-6
16. Chen F, Sun Y, Chen G, Luo Y, Xue G, Luo K, et al. The diagnostic efficacy of the American college of radiology (ACR) thyroid imaging report and data system (TI-RADS) and the American thyroid association (ATA) risk stratification systems for thyroid nodules. Comput Math Methods Med (2022) 2022:9995962. doi: 10.1155/2022/9995962
17. Ha EJ, Na DG, Moon WJ, Lee YH, Choi N. Diagnostic performance of ultrasound-based risk-stratification systems for thyroid nodules: Comparison of the 2015 American thyroid association guidelines with the 2016 Korean thyroid Association/Korean society of thyroid radiology and 2017 American college of radiology guidelines. Thyroid. (2018) 28(11):1532–7. doi: 10.1089/thy.2018.0094
18. Ha EJ, Na DG, Moon WJ, Lee YH, Choi N. Diagnostic performance of practice guidelines for thyroid nodules: Thyroid nodule size versus biopsy rates. Radiology (2019) 291:92–9. doi: 10.1148/radiol.2019181723
19. Richman DM, Benson CB, Doubilet PM, Wassner AJ, Asch E, Cherella CE, et al. Assessment of American college of radiology thyroid imaging reporting and data system (TI-RADS) for pediatric thyroid nodules. Radiology. (2020) 294(2):415–20. doi: 10.1148/radiol.2019191326
20. Kim PH, Yoon HM, Baek JH, Chung SR, Choi YJ, Lee JH, et al. Diagnostic performance of five adult-based US risk stratification systems in pediatric thyroid nodules. Radiology. (2022) 305(1):190–8. doi: 10.1148/radiol.212762
21. Wémeau JL, Sadoul JL, d'Herbomez M, Monpeyssen H, Tramalloni J, Leteurtre E, et al. Guidelines of the French society of endocrinology for the management of thyroid nodules. Ann Endocrinol (Paris). (2011) 72(4):251–81. doi: 10.1016/j.ando.2011.05.003
22. Scappaticcio L, Maiorino MI, Iorio S, Docimo G, Longo M, Grandone A, et al. Exploring the performance of ultrasound risk stratification systems in thyroid nodules of pediatric patients. Cancers (Basel). (2021) 13(21):5304. doi: 10.3390/cancers13215304
23. Corrias A, Mussa A, Baronio F, Arrigo T, Salerno M, Segni M, et al. Diagnostic features of thyroid nodules in pediatrics. Arch Pediatr Adolesc Med (2010) 164:714–9. doi: 10.1001/archpediatrics.2010.114
Keywords: ultrasound, thyroid nodule, fine needle aspiration, American College of radiology thyroid imaging reporting and data system, American thyroid association guidelines
Citation: Li G, Zhang B, Liu J and Xiong Y (2023) The diagnostic efficacy and inappropriate biopsy rate of ACR TI-RADS and ATA guidelines for thyroid nodules in children and adolescents. Front. Endocrinol. 14:1052945. doi: 10.3389/fendo.2023.1052945
Received: 24 September 2022; Accepted: 14 March 2023;
Published: 27 March 2023.
Edited by:
Tommaso Aversa, University of Messina, ItalyReviewed by:
Lorenzo Scappaticcio, University Hospital “Luigi Vanvitelli”, ItalyLaura C. Page, Duke University Health System, United States
Copyright © 2023 Li, Zhang, Liu and Xiong. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Bo Zhang, VGh5cmlvZHVzQDE2My5jb20=
 Guanghan Li
Guanghan Li Bo Zhang
Bo Zhang Jia Liu2
Jia Liu2