- 1Division of Endocrinology and Metabolism, Tokyo Metropolitan Children’s Medical Center, Tokyo, Japan
- 2Department of Pediatrics, Keio University School of Medicine, Tokyo, Japan
- 3Department of Pediatrics, Toho University Omori Medical Center, Tokyo, Japan
- 4Clinical Research Support Center, Tokyo Metropolitan Children’s Medical Center, Tokyo, Japan
- 5Department of Pediatrics, Oita University Faculty of Medicine, Oita, Japan
- 6Department of Bone and Mineral Research, Research Institute, Osaka Women’s and Children’s Hospital, Osaka, Japan
- 7Department of Gastroenterology, Nutrition, and Endocrinology, Osaka Women’s and Children’s Hospital, Osaka, Japan
Delayed and absent puberty and infertility in Turner syndrome (TS) are caused by primary hypogonadism. A majority of patients with TS who are followed at hospitals during childhood will not experience regular menstruation. In fact, almost all patients with TS need estrogen replacement therapy (ERT) before they are young adults. ERT in TS is administered empirically. However, some practical issues concerning puberty induction in TS require clarification, such as how early to start ERT. The present monograph aims to review current pubertal induction therapies for TS without endogenous estrogen production and suggests a new therapeutic approach using a transdermal estradiol patch that mimics incremental increases in circulating, physiological estradiol. Although evidence supporting this approach is still scarce, pubertal induction with earlier, lower-dose estrogen therapy more closely approximates endogenous estradiol secretion.
Introduction
Turner syndrome (TS) is one of the most common chromosomal abnormalities and occurs in phenotypic females with one X chromosome and absence of all or part of the second X chromosome. TS is linked to a wide range of clinical features, including short stature and hypogonadism. Primary hypogonadism is an extremely common feature of this disorder. At outpatient clinics for children with TS, 80% or more of the patients require estrogen replacement therapy (ERT) (1). In this monograph, “induction” refers to the initial phase of ERT through puberty, from the initiation of estrogen therapy to the addition of progestin.
The type of ERT recently advocated worldwide for induction was originally published following the 2016 Cincinnati Meeting (2) and was endorsed by the European Society of Endocrinology, the Endocrine Society, the Pediatric Endocrine Society, the European Society for Pediatric Endocrinology, the European Society of Human Reproduction and Embryology, the American Academy of Pediatrics, and the Society of Endocrinology (United Kingdom) (3). In line with the consensus reached at the Cincinnati Meeting, a more precise, practical review of puberty and estrogen replacement was released in 2018 (3).
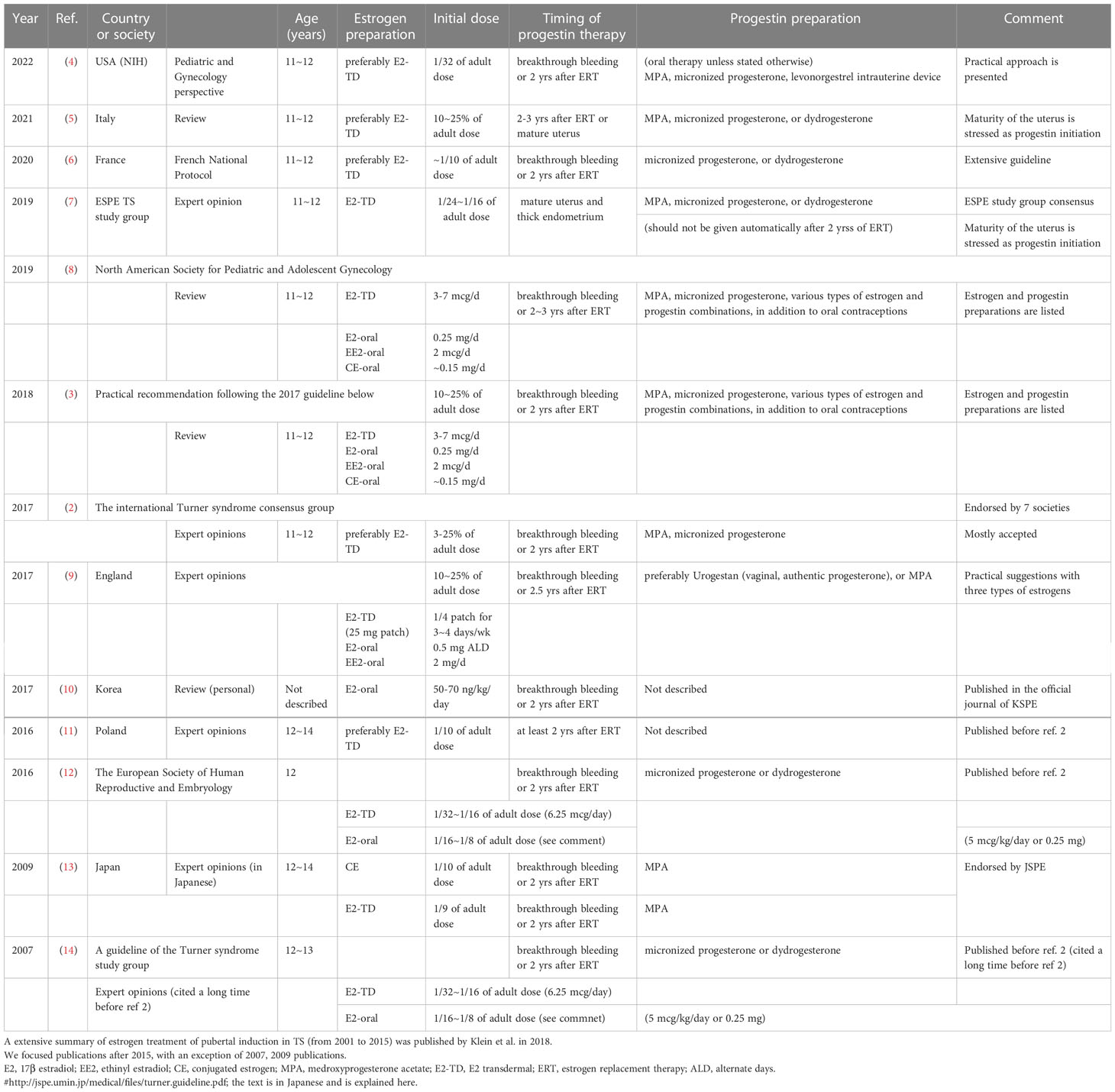
Several excellent guidelines were issued following the two previously mentioned publications (2, 3). Publications before 2016 were summarized in one of the Tables of reference 3. Table 1 in the present monograph summarizes the relevant points of studies published in 2016 or later (2–14); some of these focused on using transdermal 17β estradiol (E2), while others were more practical in their recommendations. As seen in Table 1, most expert opinions and guidelines from around the world agree on the following points regarding induction therapy in TS without endogenous estrogen production:
● Therapy should be started at age 11–12 years.
● The dosage should gradually be increased, starting at 3 to 10% of the adult dosage.
● Progestin-induced menarche should be considered between two to three years after pubertal induction.
● Progestin may also be indicated to induce menstruation in patients who have irregular menses when receiving estrogen only.
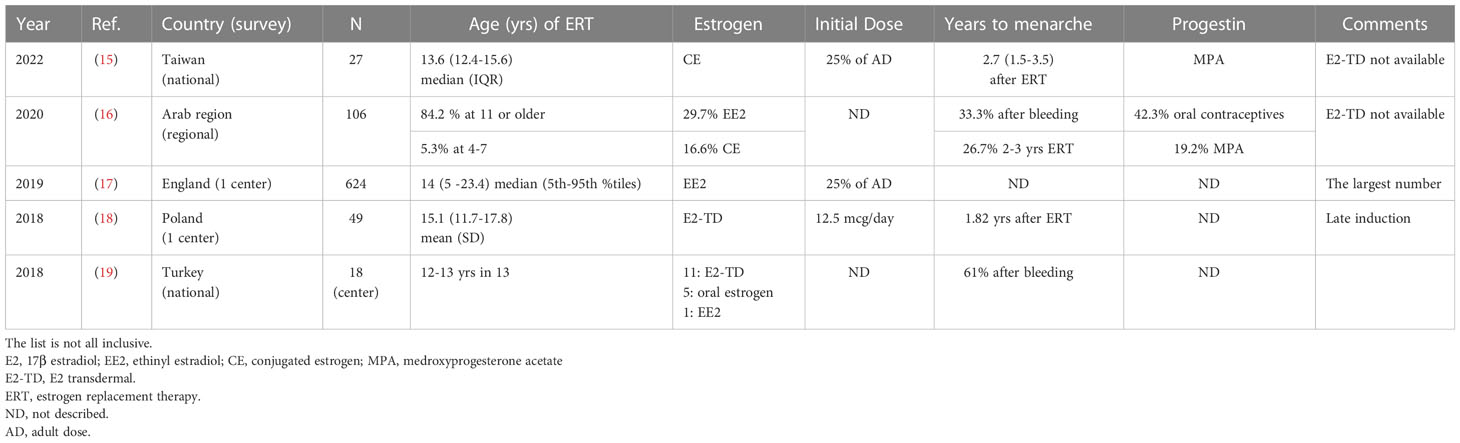
The guidelines and reviews on TS covering pubertal induction have demonstrated variable methods of starting induction. E2 is most commonly recommended for induction, preferably via transdermal administration (2, 3, Table 1). In practice, many types of E2 patch are available in different parts of the worlds, as listed by Klein et al. (3). In addition, other types of estrogen, such as oral E2, synthetic ethinyl estradiol (EE2), and conjugated estrogen (CE) produced from horse urine, are also used worldwide (15-19 in Table 2). The list in Table 2 is not exhaustive, but shows the methods used for inducing puberty in five institutions, regions, or countries after the release of the Cincinnati Meeting (2). The points outlined above can also be applied to oral E2, EE2, and CE as well as transdermal E2 application. A more extensive list of estrogen preparations was published by Klein et al. (3).
In addition to reviewing the current understanding of pubertal induction in TS, the present monograph attempts to explain the rationale for the induction therapy and suggests an ideal method of induction in patients with TS without endogenous estrogen production. This method is not evidence-based but involves “earlier and lower-dose estrogen” than current, most frequently recommended forms of induction therapy.
Gonadal function in Turner syndrome
Pubertal development in healthy subjects and patients with TS
Between 1977 and 2013, the average, initial age of breast development decreased by almost three months per decade worldwide (20). The median age at Tanner Breast 2 (95% confidence interval [CI]) ranged from 9.8 to 10.8 years in Europe, 9.7 to 10.3 years in the Middle East, 8.9 to 11.5 years in Asia, 8.8 to 10.3 years in the United States, and 10.1 to 13.2 years in Africa (20). Even in patients with TS, spontaneous puberty reportedly began earlier than in the past, with numbers of such cases increasing significantly from pre-1980 to 2004 (21). The median age of menarche internationally was reportedly to be 12 to 13 years (22, 23).
Delayed or absent puberty is a common finding in TS. Menarche occurs in approximately 20% of TS patients seen in outpatient clinics worldwide (24–28). Aso et al. (24) demonstrated that, in Japan, only 5% of teenaged patients with TS who were followed up had regular, spontaneous menstruation at age 16 years. Thus, not all adult patients with TS with a history of spontaneous menarche can maintain their cycle.
Patients with TS who require pubertal induction therapy at a young age typically have no spontaneous breast development by 12 to 13 years. It is important to differentiate true thelarche from lipomastia, especially in overweight patients with TS. However, given that some patients with TS are unlikely to enter puberty or to have residual endogenous estrogen production indicated by an increased FSH level and/or streak gonads on imaging, pubertal induction therapy may be started earlier, for instance, around age 10 years, which is the average age of breast development in Europe and Asia (20, 23). On the other hand, in cases where it is unclear whether estrogen production will be absent or delayed, pubertal induction therapy should not be started until confirmation is obtained by physical examination and elevated FSH (> 40-50 mIU/mL depending on the assay used) and low estradiol (< 40-50 pg/mL) are recorded at least twice one month apart. Of course, delaying diagnosis until age 15 years, for example, in patients with no pubertal development, will inevitably entail a delay in induction as well. Furthermore, pubertal development in TS can be arrested at any stage during puberty, such as at breast development, menarche or later.
Pubertal induction in TS without gonadal function
Matters for patients and their caregivers to consider puberty induction
In pediatric medicine, the physician-patient-caregiver partnership is crucial. Physicians must understand the importance of this when treating patients with TS. Maintaining excellent rapport with patients is essential, as sensitive, psychosocial issues are often involved in patient care. Discussing concerns with patients and their guardians to support them holistically with other co-medicals is critical to managing hypogonadism in TS. Absent or delayed puberty may cause significant emotional and psychosocial morbidity [reviewed in ref. (29)]. Indeed, low self-esteem and social adjustment issues in young women with TS were reportedly influenced by the quality of pubertal management (30).
First, the patients and their parents should be made to understand healthy pubertal development. The possibility of delayed puberty should also be explained, especially in cases where it is likely to occur. Physicians should speak directly to their patients using understandable language and tailor their approach to each patient’s personality, intelligence, and developmental stage. The following information about induction treatment should be shared with the patients and their guardians:
● Estrogen replacement therapy is necessary in most patients with TS.
● One of the determinants of adult height is the timing of pubertal development, whether spontaneous or induced.
● An extreme delay in breast development may have unfavorable effects on psychosocial development and bone health.
● Various types of estrogen formulations and methods of administration are available, but transdermal administration of natural estrogen is currently recommended.
Medically speaking, the likelihood of spontaneous puberty must be considered before induction therapy. There are three markers capable of predicting pubertal outcomes in TS. The first, karyotype analysis, is a conventional method of predicting pubertal development. Patients with TS with 45,X/46,XX or 45,X/47,XXX, especially with a low mosaic ratio of 45,X, are more likely to be able to become pregnant naturally; thus, breast development and menarche are also likely to occur normally (31, 32). One limitation of karyotype analysis is that the mosaic ratio derived from blood cells, a common source, does not necessarily match that derived from other tissues, such as the ovaries (33, 34).
Second, the FSH level may serve as an index of spontaneous and cyclical menstruation in TS (24, 35, 36). Aso et al. investigated the utility of FSH level in 50 subjects aged 10–12 years with TS as an index of spontaneous and cyclical menstruation (24) and found that this occurred before age 16 in subjects with serum FSH <10 mIU/mL at age 10 to 12 years. The sensitivity and specificity of both was 90%. Similarly, Hankus et al. recommended FSH 6.7 mIU/mL as a criterion of menstruation onset in patients aged 6 to 10 years (35).
Third, Lunding et al. reported the use of the anti-Mullerian hormone (AMH) level as a predictor of pubertal development (37) and demonstrated that AMH < -2 SD during the prepubertal period predicted the failure of puberty onset. AMH was also able to predict imminent premature ovarian insufficiency (POI) in adolescent and adult patients with TS. No prepubertal patients with AMH < 4 pmol/L experienced spontaneous puberty, and adolescent patients with AMH < 5 pmol/L experienced imminent POI, consistent with the findings of several studies (38–41).
Providing adequate estrogen and progestin in TS without gonadal function
Estrogen
Appropriate estrogen dosage is essential for pubertal induction. First, estrogen should be administered systemically because the ovaries normally release estrogen into the bloodstream. A practical method of administration in children is transdermal and uses a patch or gel (3). Transdermal patches are recommended by most of the current guidelines (2-14 in Table 1) because they are easy to use. However, patches are not yet available everywhere. Klein et al. recently extensively summarized current estrogen formulations (3). Administration of E2 or physiological estrogen allows the serum estrogen levels to be measured. In particular, the serum level achieved through transdermal administration ideally corresponds to the normal value per pubertal stage.
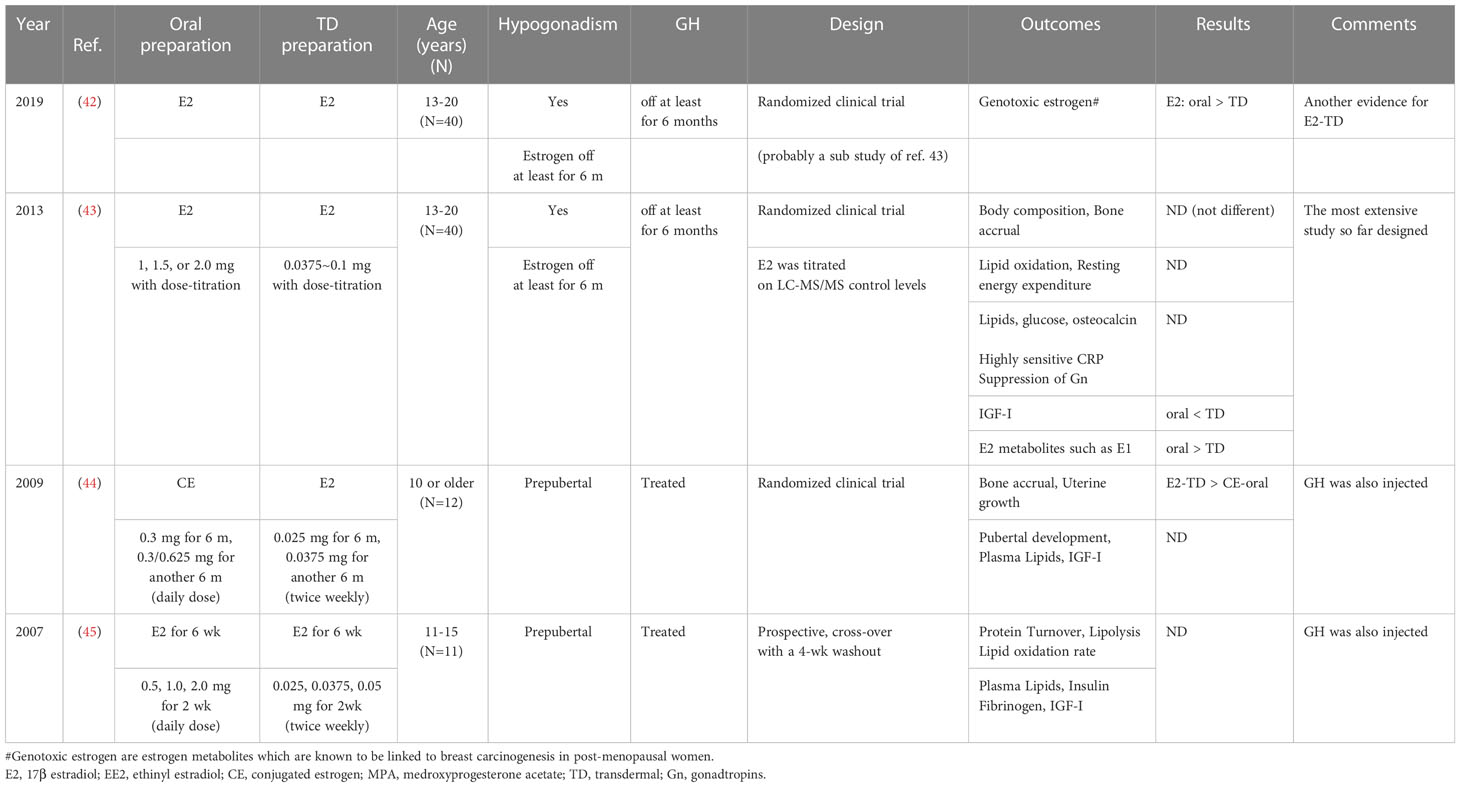
While transdermal administration of E2 has a number of the well-known, physiological advantages (Table 3), the oral administration of E2 has a rather salient disadvantage that is related to the first-pass effect. When taken orally, E2 is exposed to the liver through the portal vein after digestive absorption. However, there is little research directly comparing the transdermal and oral methods of administration during pubertal induction (42–45). Only one large study (42, 43) directly compared methods of pubertal induction with E2 in TS. Table 3 shows more favorable, short-term outcomes for transdermal administration than for oral administration, but while the former may appear to be superior, its long-term effects are still unknown.
Oral estrogen administration is thought to be associated with two, potentially deleterious long-term effects. One is the increased risk of cardiovascular outcomes. Although this risk increases after menopause in the general population (46, 47), whether patients with TS also have an increased risk is unclear. The other, potentially deleterious effect is the higher level of genotoxic estrogens in adolescent TS patients (at the age of 13-20 years) receiving oral E2 instead of transdermal E2 (42), which may lead to an increased risk of estrogen-dependent malignancies, such as some forms of breast cancer. However, there are as of yet no epidemiological data demonstrating an actual link between genotoxic estrogens and cancer. Indeed, in their epidemiological study of well over 1000 patients with TS, Viuff et al. demonstrated that the risk of breast cancer was actually lower in TS, even during hormone replacement therapy (48).
There are pressing reasons not to wait long before initiating estrogen therapy, although pubertal induction may limit the ability to achieve adult height. The major, clinical targets of estrogen replacement therapy also should be considered. Breast development has been a focus of research in TS for over a decade (13, 49). For physical as well as psychosocial reasons, healthy breast development is desirable. A recent study of this topic (50) found that the results of breast development in TS were not adequate even with intervention. Furthermore, some patients respond poorly to treatment in practice. The poor responders may have severe webbed neck and lymphoedema or they may be clinically emaciated.
Although uterine growth is another target of therapy, only a few studies on this topic have been published (44, 49, 51). Recently, researchers used a three-dimensional approach, which is more accurate than a two-dimensional approach, to demonstrate that the recommended induction method increased uterine volume significantly. However, the uterine volume was still smaller than the value in age-matched, healthy teenagers and adults (52).
Another critical effect of estrogen replacement therapy relates to bone health (53). Epidemiological studies have reported an increased prevalence of bone fractures in TS (54–56), although some controversy still surrounds this issue (57, 58). Decreased bone mineral density is considered one of the causes of the fractures in TS. Estrogen replacement increases BMD (59), making early induction essential to bone mineral accrual (57, 59–61). More advanced technologies, such as peripheral quantitative computed tomography, are needed to assess bone density and geometry (51, 62).
Other significant effects that may be likely related to pubertal induction are psychosocial outcomes and cardiovascular health. The impact of induction therapy on psychosocial wellbeing is not negligible since the period during which induction is performed is when children typically begin to acquire their sense of autonomy (reviewed in 29) and coincides with the period of physical development in patients with TS. Another potential reason for timely pubertal induction in patients with TS is the implications for neurocognitive development (63, 64) although this topic is beyond the scope of the present monograph.
Finally, epidemiological data suggest that estrogen is related to cardiovascular heath in the long run, as extensively illustrated in the most recent and widely recognized guideline (2). However, to the best of our knowledge, the impact of estrogen during pubertal induction remains unknown as discussed later.
Progestin
Progestin must be used in addition to estrogen when breakthrough bleeding occurs or after 2-3 years of pubertal induction with estrogen (1–19, 65). However, recent two guidelines emphasized that progestin should be added after confirming uterine size and endometrial thickness on ultrasonography (5, 7).
Adding progestin reduces the risk of endometrial cancer associated with unopposed estrogen (66, 67) and minimizes irregular bleeding and endometrial hyperplasia (3). The many formulations currently available have been summarized by Klein et al. (3, 7). Micronized progesterone or dydrogesterone (3, 7, 65) is recommended.
When beginning pubertal induction in patients with TS, cyclic progestin administration, typically for 12–14 days once a month or in a cycle, is the preferred method. Pubertal induction should induce menstruation as this is important for the patients’ mental and emotional health. Furthermore, because cyclic therapy promotes normal cyclical endometrial repair, it is more beneficial in patients attempting to conceive through oocyte donation (65).
Far less research has been conducted on progestin therapy than on estrogen therapy. For example, although most guidelines for TS recommend oral progestin, they rarely discuss other types of progestin administration, such as vaginal administration, which may mimic the physiological uterine tissue concentration more closely.
Estrogen and progestin combination therapy, including oral contraceptives (OCs)
Several types of patches and pills are available for estrogen and progestin combination replacement therapy in addition to OC pills. A recent review has listed these products as being available worldwide (3, 7). OC pills are used in North America and Europe by healthy, young girls. As a result, late-adolescent patients with TS after menarche may want to use these products to feel more like their peers. However, the long-term consequences of using these products, such as their effects on cardiovascular and bone health, still need to be clarified. Furthermore, it is worth noting that most of these products are not recommended for the initial phase of pubertal induction (2, 3).
Current recommendations for ERT for pubertal induction (Current E2-TD therapy)
The most recent and extensive guideline for TS, published in 2017 (2), recommends that ‘[low-dose, transdermal estradiol therapy]’ be initiated between age 11 and 12 years in patients with TS [without pubertal development]. If arrested pubertal development is predicted, the guidelines recommend increasing [to the adult dosage] for two to three years. Adding progestin is also advised once breakthrough bleeding occurs or after two years of estrogen treatment. These recommendations are mainly for patients with poor endogenous estrogen production, and the guideline offers no concrete methods of performing [‘low-dose transdermal estradiol therapy’]. For example, they do not explain how to calibrate the dosage during induction precisely. However, a concrete method of increasing the induction dosage using transdermal E2 is described in a follow-up study (3).
Recently, a group of experts with the European Society for Pediatric Endocrinology published a physiological method of pubertal induction using transdermal estradiol therapy in patients with TS without endogenous estrogen production (7). The recommended age for initiating this therapy is 11 to 12 years, and the recommended initial dosage is 1/16-1/24 of the adult dosage, mainly to be administered at night to mimic the nighttime elevation of physiological estradiol (7). The dosage and rationale for mimicking the normal physiological E2 serum concentration was explained in detail in previous studies (68, 69). While mimicking the nighttime E2 levels results in a close approximation of the physiological serum estradiol concentration profile, the consent of the patient’s’ parents is required before it is used, and their cooperation in encouraging adherence is required during the treatment course.
The previously cited recommendations (2, 3, 7) are currently widely accepted as a proposed form of induction therapy for TS (abbreviated as “current E2-TD therapy” in this monograph) (Table 4). Table 4 shows the advantages and disadvantages of this therapy in comparison to other therapies.
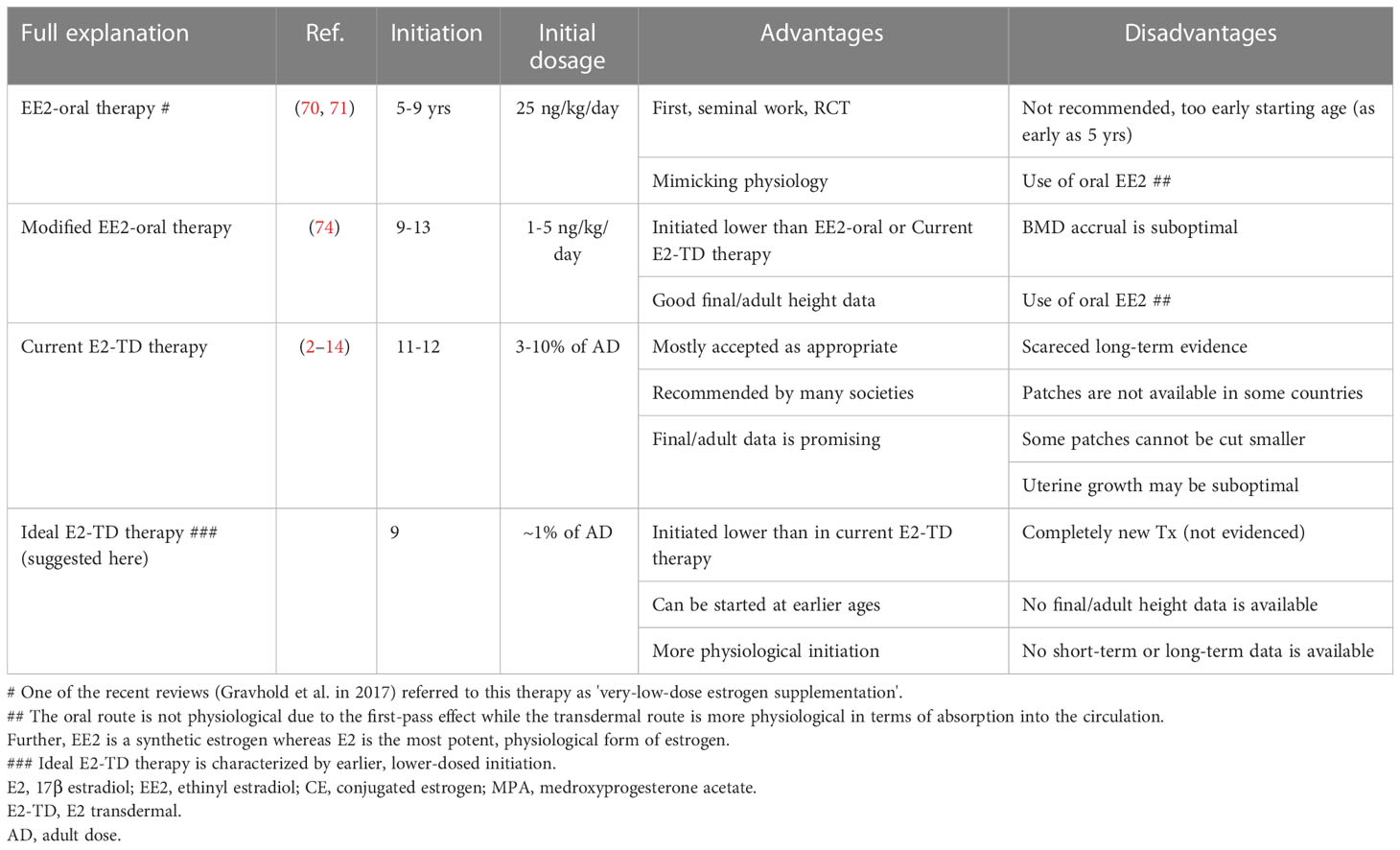
Table 4 Advantages and disadvantages of EE2-O therapy, modified EE2-O therapy, current E2-TD therapy, and ideal E2-TD therapy.
Few studies have examined adult height or bone mineral density accrual in TS patients receiving current E2-TD therapy. A recent study found that uterine development in women with TS receiving current E2-TD therapy (52) was suboptimal compared with that of healthy women.
Our suggestions for ideal pubertal induction
Our initial study: modified EE2-O therapy
The benefits of low-dose estrogen therapy for women with TS were initially discussed in 2011 by Ross et al. (70) and in 2014 by Quigley et al. (71). These seminal studies examined the effects of oral ethinyl estradiol (EE2) starting at 25 ng/kg/day; the adult dosage for complete hypogonadism is 100–200 ng/kg/day. The studies attempted to mimic the physiological increase in estrogen by starting treatment at a low dosage, then gradually increasing it with maturation in a form of treatment, described as EE2 oral (EE2-O) therapy (Table 4).
Although the concept of EE2-O therapy is physiologically sound, there are some concerns. First, the therapy reportedly begins as early as age 5 years although the 95% CI for the onset of breast development in the US ranges from 8.8 to 10.3 years (20). The study was also designed to analyze the impact of this therapy on neurocognition in TS (72, 73). Second, the initial EE2 dosage of 25 ng/kg is not particularly low, in comparison to the adult dosage of this compound. Indeed, some of the subjects in this study (the exact proportion was not described) showed breast development at this dosage (70). EE2-O therapy is not recommended in the most recent guideline (2), which state, “We suggest not routinely [adding] very-low-dose estrogen supplementation in the prepubertal years to further promote growth.” The observation regarding “very-low-dose estrogen supplementation” in ref. 2 implicates the historical studies using EE2 discussed above (70, 71), although this is not explicitly stated in the reference. Our previous work (74) using an even lower EE2 dosage for induction is not mentioned in the guideline in question (2).
We recently published a study examining the effects of induction using lower-dose estradiol therapy (74) (modified EE2-O therapy in Table 4), which begins with an oral dosage of 1–5 (mostly 1–2) ng/kg/day. The lower starting dosage was assumed to approximate more closely the increase in physiological estrogen. Our study demonstrated that the initial EE2 dosage did not induce breast development and recorded final/adult height (height velocity < 2 cm/year) of 152.4 +/- 3.4 cm (+2.02 +/- 0.62 SD for TS) (N=17), which was significantly greater than that of a control group who received a different treatment (148.5 +/- 3.0 cm; +1.30 +/- 0.55 SD). The mean, female, Japanese, adult height was reportedly about 158 cm with no secular trend (75, 76). Our data on adult height in the study, which are among those of the highest quality published thus far (1), suggested that the modified EE2-O therapy did not compromise height acquisition despite early initiation. The findings of our first study (74) led to the formation of the ideal, estradiol, transdermal therapy (ideal E2-TD therapy) explained below.
In hindsight, there are a number of issues related to our study. EE2 is a synthetic estrogen whereas estradiol is the most potent physiological form of estrogen. Owing to the first-pass effect (Table 4), the oral route cannot replicate the natural secretion of estrogen. Additionally, starting estrogen therapy earlier and increasing the dosage more slowly than in our study may be necessary from a physiological point of view (see below) and would likely have led to a better BMD outcome than observed in our initial study (74).
Background knowledge and concept of ideal E2-TD therapy with earlier, lower-dose transdermal E2 administration
This section discusses the rationale for ideal E2-TD therapy in greater detail than our previous publications on the topic [(77, 78) as an erratum of 79]. The rationale of pubertal induction for TS without endogenous ovarian function includes the following:
● Use of E2 as a physiological form of estrogen
● Use of the transdermal route
● Gradual dose escalation with age as consistent with serum E2 concentration (physiological age at the induction)
● Introduction of menarche at the appropriate age
To further improve outcomes observed in our modified EE2-O therapy, a couple of issues should be considered. First, the recent guidelines clearly recommend transdermal E2 administration (2–12). Oral estrogens are reportedly associated with an increased risk of poor cardiovascular outcomes in the general, post-menopausal population although there is some controversy about the issue (46, 47, 79, 80) and this finding has not yet been established in patients with TS. It is obvious that transdermal administration is more physiological as discussed above.
Furthermore, earlier, lower-dose estrogen therapy approximates more closely to physiology, which may lead to improved height prognosis and bone mineral acquisition. As shown, final/adult height and BMD data were still suboptimal in our previous study (74). Moreover, ideal pubertal induction might achieve more appropriate uterine growth. Even in TS patients with the current E2-TD therapy, the uterine growth was less than ideal (52).
There are fundamental observations necessary for the understanding of ideal pubertal induction. First, breast development seems to be related to the amount of estrogen exposure over time, and the initial release of estrogen probably starts before breast development. Indeed, on a longitudinal growth velocity curve, the annual growth rate (cm/year) at each age reaches a nadir before the onset of breast development. This minimum value and the ensuing increase in the annual growth rate are referred to as ‘nadir’ in the present review. This change in growth velocity may reflect earlier estrogen production before breast 2 Tanner stage; estrogen is sufficient for growth acceleration but not for breast development. Tanner et al. reported the same phenomenon in their landmark study (81).
Second, bone maturation during childhood as assessed in terms of bone age occurs earlier in female. Figure 1 show the results of an analysis of bone age (BA) with sex-related differences in bone maturation. The analysis in Figure 1 used a bone age atlas for female Japanese children (82) as a reference. When radiographs in the atlas for female, Japanese were evaluated using a scoring system designed for male, Japanese children, the latter demonstrated a BA increase at all ages, suggesting that bones develop more quickly in females (Figure 1). The radiographs in the atlas for female, Japanese children were also evaluated using a scoring system designed for female and male British children based on the TW2-Radius Ulna Short bones (RUS) method (82, 83). Faster bone maturation was also observed in the female, British subjects (Figure 1).
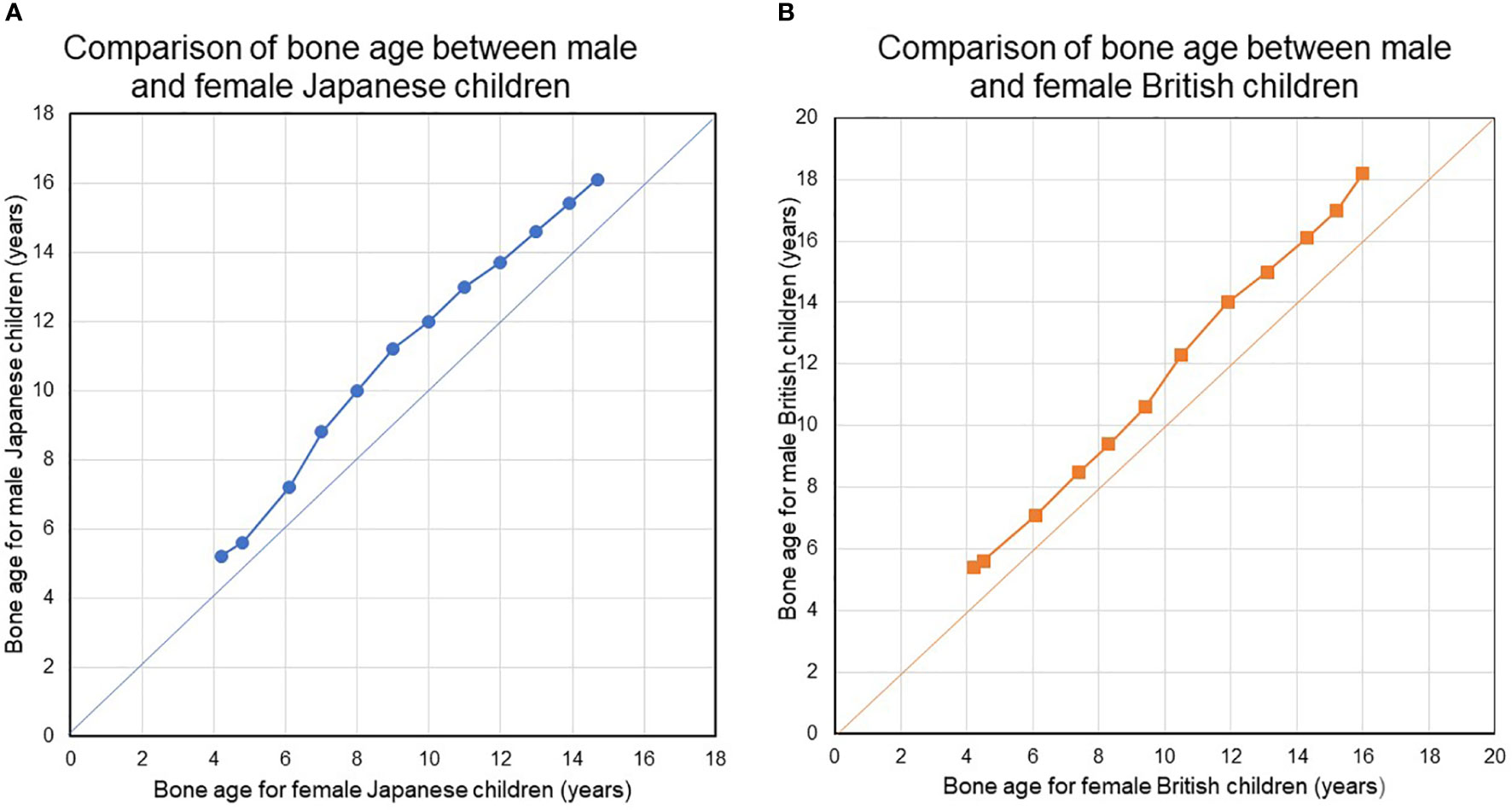
Figure 1 (A, B) Compare bone ages between females and males in Japanese and British children. During childhood and adolescence, bone maturation advances faster in girls than in boys at all ages. During childhood and adolescence, bone maturation advances faster in girls than in boys at all ages. BA was evaluated using the original TW2-Radius-Ulna-Short bones (RUS) method and the TW2-RUS method standardized for Japanese children, for British children, and for Japanese children, respectively.
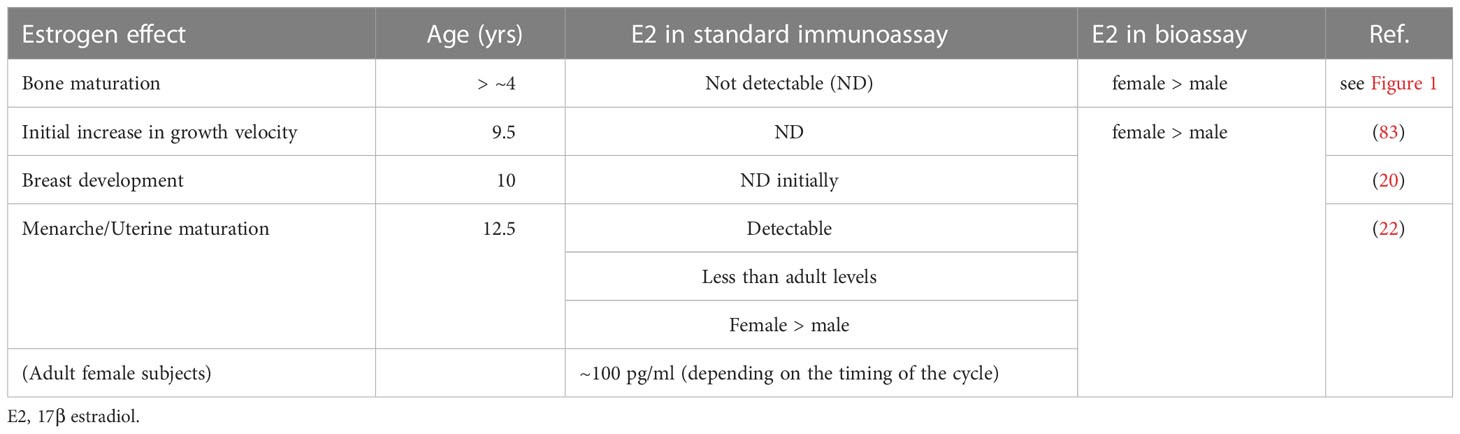
BA is a surrogate marker of bone maturation and is at least partially dependent on estrogen function in both sexes, as exemplified by BA retardation in a 46,XY patient with estrogen receptor α defect (84) and in male and female patients with aromatase deficiency (85, 86). Estradiol levels may gradually increase before breast development. Indeed, bioassays have found a higher serum estradiol level in healthy, prepubertal female subjects as young as 4-5 years than in male subjects (87, 88). This early, slow rise in estradiol suggests that ideal E2-TD therapy may best be started before the average age at the nadir and the start of breast development. Table 5 shows the estimated serum E2 concentration for each pre-pubertal or pubertal stage. Pubertal induction therapy in TS without gonadal function ideally mimic the E2 levels shown in Table 5 via earlier, lower-dose, transdermal E2 administration.
Theoretically, ideal pubertal induction therapy has the following targets:
● Appropriate final/adult height, not compromised by estrogen replacement
● Appropriate quality of life for pubertal female, including the achievement of healthy pubertal development
● Appropriate acquisition of bone mineral density at final/adult height
Of course, ideal E2-TD therapy suggested here have never addressed any of these points.
Our practical proposal for an ideal induction therapy: Suggestions for a more physiological approach
Tables 6, 7 shows our proposal for the clinical application of ‘ideal E2-TD therapy’ with earlier, lower-dose E2 initiation, together with current, widely accepted estrogen therapy (current E2-TD therapy, see also Table 4). Table 6 shows the initial dosage required for pubertal induction (89) and Table 7 shows the precise method of calibrating the increases in estrogen dosage.
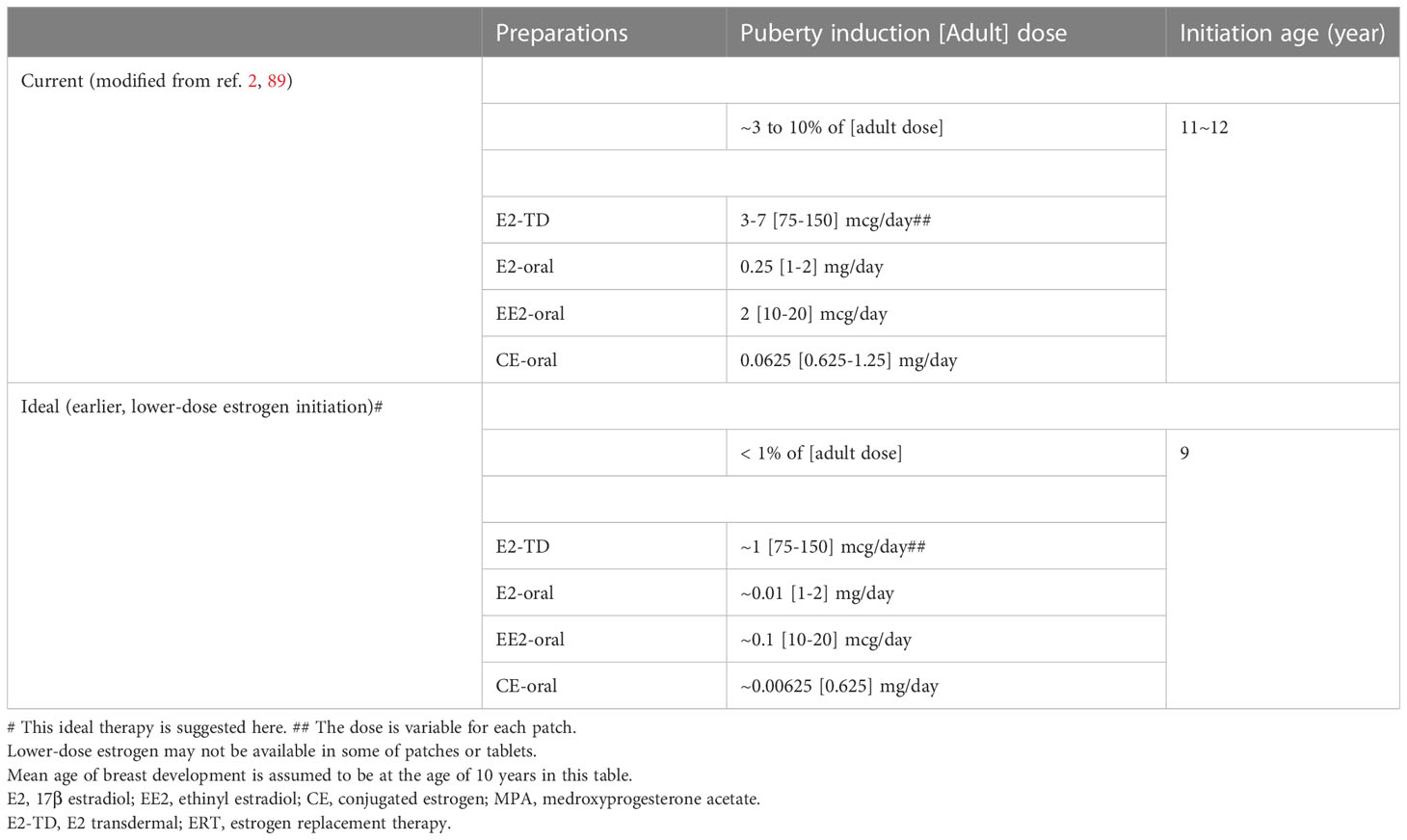
Table 6 Comparisons between current and ideal# pubertal induction using estrogen in TS without endogenous estrogen.
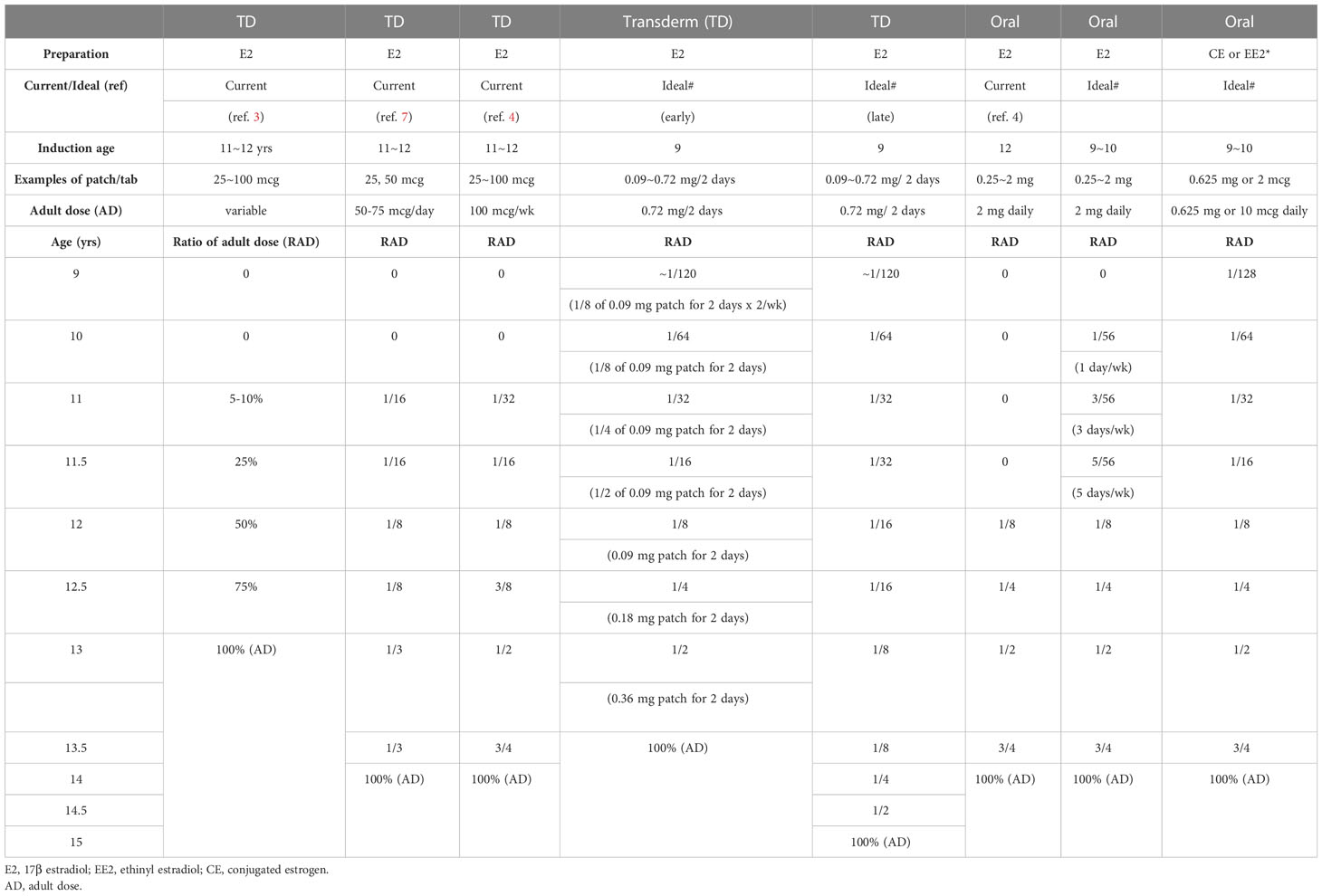
Table 7 Examples of dose escalation of Current and Ideal (earlier, lower-dosed)#, estrogen pubertal induction using estrogen in TS without endogenous estrogen.
First, it should be borne in mind that the therapy proposed here as ‘ideal’ is a preliminary version and is not based as of yet on any evidence. The idea awaits testing in a future study. In Tables 6 and 7, breast development is assumed to start roughly at age 10 years world-wide for the sake of the simplicity (20). The initial dosage at age 9 years is roughly 1/100th of the adult dosage. Not only the transdermal E2 regimen but also the oral EE2 and CE regimens are shown, because transdermal E2 patch is still unavailable in some regions (15, 16). There are two protocols using transdermal E2 patch for patients aged 9 years or older, either of which can be used depending upon patients’ psychological age, height, BA, BMD, and the consent of the patients and their guardians (Table 7).
Several clinical considerations must be made before administrating this ideal E2-TD therapy in patients with TS. First, if patients do not have a high FSH level (> 10 mIU/mL at age10 years or older, > 6.7 mIU/mL at age 6 to 9 years) (24, 35), treatment should await confirmation of the absence of pubertal development, or an increase in FSH. The FSH level (as discussed above) is particularly helpful in regions where AMH cannot be measured. Second, treatment initiation depends on patients’ racial background; for instance, patients of African descent mature earlier than their European counterparts (20).
Last but not least, initiation of estrogen treatment at age 9 years as shown in Tables 6, 7, is tentative. Given the size of E2 patches, the minimum dosage shown in those Tables is the lowest possible dosage available for each formulation. If a smaller transdermal estradiol patch were used, the ideal age at ERT initiation in patients with TS without endogenous estrogen production might be around 5 years, as discussed above (87, 88).
Prospects
Undoubtedly, further research is needed to gather evidence to determine whether the ideal E2-TD therapy is viable. First, the final/adult height data obtained with the ideal E2-TD therapy are unclear. Our first study of modified EE2-O therapy produced some of the best adult height outcomes (final/adult height; roughly -1.1 SD for reference Japanese female adult) (1, 74). Our recent experience in outpatient clinic, the outlook for the ideal E2-TD therapy is promising.
Second, QOL data, including satisfaction with breast size (50), should be evaluated in patients receiving the ideal regimen. The age of menarche in Table 7 is 13 to 14 years, which does not represent a significant delay, suggesting that the deterioration in QOL may not be caused by the delay in menarche.
Third, further research is needed to improve BMD outcomes. The BMD values obtained in our initial study of modified EE2-O therapy (74) were suboptimal as discussed. Our research team (90) and others (91) have published further, prepubertal data on TS indicating that BMD begins to decrease at around age 7–10 years. Therefore, future studies of therapeutic approaches should aim at achieving better BMD outcomes. Similarly, uterine volume in patients treated with the current E2-TD therapy was also suboptimal as above explained (52). To attain better BMD and uterine development, estrogen therapy may be started earlier in patients with TS than indicated in Table 6 or 7. Furthermore, LC-MS/MS may be preferable for monitoring the serum E2 level in patients receiving earlier, lower-dose, transdermal E2 because it has a much lower sensitivity than immunoassays. Monitoring the E2 level and calibrating the dosage using LC-MS/MS measurements were done in one of the most elegant studies to date (42, 43).
Of course, a balance needs to be struck in practical medicine between the scientific ideal and the realities of clinical practice. For example, an RCT comparing two treatment plans through pubertal induction to adulthood is not feasible or patient-oriented; on the other hand, a single-arm trial using transdermal E2 may be practical for assessing the quality of the long-term prognosis. An international, collaborative study would be ideal for reaching a consensus on clinically relevant matters.
Author contributions
YH planned the review, searched for previous articles, and wrote the first draft of the manuscript. MS analyzed the bone age data. All authors contributed to the article and approved the submitted version.
Funding
YH received a grant from Japan for Medical Research and Development (AMED 22ek01099464s0403).
Acknowledgments
We are indebted to Mr. James R. Valera for his assistance with editing this manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Nordenström A, Ahmed SF, van den Akker E, Blair J, Bonomi M, Brachet C, et al. Pubertal induction and transition to adult sex hormone replacement in patients with congenital pituitary or gonadal reproductive hormone deficiency: ERN clinical practice guideline. Eur J Endocrinol (2022) 186:G9–G49. doi: 10.1530/EJE-22-0073
2. Gravholt CH, Andersen NH, Conway GS, Dekkers OM, Geffner ME, Klein KO, et al. Clinical practice guidelines for the care of girls and women with turner syndrome: Proceedings from the 2016 Cincinnati international turner syndrome meeting. Eur J Endocrinol (2017) 177:G1–70. doi: 10.1530/EJE-17-0430
3. Klein KO, Rosenfield RL, Santen RJ, Gawlik AM, Backeljauw PF, Gravholt CH, et al. Estrogen replacement in turner syndrome: Literature review and practical considerations. J Clin Endocrinol Metab (2018) 103:1790–803. doi: 10.1210/jc.2017-02183.
4. Dowlut-McElroy T, Shankar RK. The care of adolescents and young adults with turner syndrome: A pediatric and adolescent gynecology perspective. J Pediatr Adolesc gynecol (2022) 35:429–34. doi: 10.1016/j.jpag.2022.02.002
5. Aversa T, Corica D, Pepe G, Pajno GB, Valenzise M, Messina MF, et al. Pubertal induction in girls with turner syndrome. Minerva Endocrinol (Torino) (2021) 46:469–80. doi: 10.23736/S2724-6507.20.03285-X
6. Fiot E, Alauze B, Donadille B, Samara-Boustani D, Houang M, De Filippo G, et al. Turner syndrome: French national diagnosis and care protocol (NDCP; national diagnosis and care protocol). Orphanet J Rare Dis (2022) 17(Suppl 1):261. doi: 10.1186/s13023-022-02423-5
7. Donaldson M, Kriström B, Ankarberg-Lindgren C, Verlinde S, van Alfen-van der Velden J, Gawlik A, et al. Optimal pubertal induction in girls with turner syndrome using either oral or transdermal estradiol: A proposed modern strategy. Horm Res Paediatr (2019) 91:153–63. doi: 10.1159/000500050
8. Klein KO, Phillips SA. Review of hormone replacement therapy in girls and adolescents with hypogonadism. J Pediatr Adolesc gynecol (2019) 32:460–8. doi: 10.1016/j.jpag.2019.04.010
9. Matthews D, Bath L, Högler W, Mason A, Smyth A, Skae M. Hormone supplementation for pubertal induction in girls. Arch Dis childhood (2017) 102:975–80. doi: 10.1136/archdischild-2016-311372
10. Yang S. Diagnostic and therapeutic considerations in turner syndrome. Ann Pediatr Endocrinol Metab (2017) 22:226–30. doi: 10.6065/apem.2017.22.4.226
11. Gawlik A, Hankus M, Such K, Drosdzol-Cop A, Madej P, Borkowska M, et al. Hypogonadism and sex steroid replacement therapy in girls with turner syndrome. J Pediatr Adolesc gynecol (2016) . 29:542–50. doi: 10.1016/j.jpag.2016.03.005
12. European Society for Human Reproduction and Embryology (ESHRE) Guideline Group on POI, Webber L, Davies M, Anderson R, Bartlett J, Braat D, et al. ESHRE guideline: management of. England) (2016) 31:926–37. doi: 10.1093/humrep/dew027
13. Tanaka T, Yokoya S, Hasegawa T, Kanzaki S, Sugihara S, Tanaka H, et al. A guideline of pubertal induction therapy in turner syndrome. Available at: http://jspe.umin.jp/medical/files/turner.guideline.pdf.
14. Bondy CA, Turner Syndrome Study Group. Care of girls and women with turner syndrome: A guideline of the turner syndrome study group. J Clin Endocrinol Metab (2007) 92:10–25. doi: 10.1210/jc.2006-1374
15. Lee YC, Huang CY, Lin CH, Cheng BW, Huang SK, Yeh SN, et al. The effects of estrogen induction therapy on pubertal presentations in turner syndrome patients. Taiwanese J obstet gynecol (2022) 61:788–93. doi: 10.1016/j.tjog.2022.05.014
16. Hamza RT, Deeb A, Al Saffar H, Alani SH, Habeb A. Timing and regimen of puberty induction in children with hypogonadism: A survey on the practice in Arab countries. J Pediatr Endocrinol Metab: JPEM (2020) 33:1197–202. doi: 10.1515/jpem-2020-0157
17. Cameron-Pimblett A, Davies MC, Burt E, Talaulikar VS, La Rosa C, King TFJ, et al. Effects of estrogen therapies on outcomes in turner syndrome: Assessment of induction of puberty and adult estrogen use. J Clin Endocrinol Metab (2019) 104:2820–6. doi: 10.1210/jc.2018-02137
18. Gawlik AM, Hankus M, Szeliga K, Antosz A, Gawlik T, Soltysik K, et al. Late-onset puberty induction by transdermal estrogen in turner syndrome girls-a longitudinal study. Front Endocrinol (2018) 9:23. doi: 10.3389/fendo.2018.00023
19. Uçar A, Abacı A, Pirgon Ö, Dündar B, Tütüncüler F, Çatlı G, et al. A synopsis of current practice in the diagnosis and management of patients with turner syndrome in Turkey: A survey of 18 pediatric endocrinology centers. J Clin Res Pediatr Endocrinol (2018) 10:230–8. doi: 10.4274/jcrpe.0003
20. Eckert-Lind C, Busch AS, Petersen JH, Biro FM, Butler G, Brauner EV, et al. Worldwide secular trends in age at pubertal onset assessed by breast development among girls. JAMA Pediatr (2020) 174:e195881. doi: 10.1001/jamapediatrics.2019.5881
21. Woelfle J, Lindberg A, Aydin F, Ong KK, Camacho-Hubner C, Gohlke B. Secular trends on birth parameters, growth, and pubertal timing in girls with turner syndrome. Front Endocrinol (2018) 9:54. doi: 10.3389/fendo.2018.00054
22. InterLace study team. Variations in reproductive events across life: A pooled analysis of data from 505147 women across 10 countries. Hum Prod (2019) 34:881–93. doi: 10.1093/humrep/dez015
23. Matsuo N. Skeletal and sexual maturation in Japanese children. Clin Pediatr Endocrinol (1993) 2(Sup 1):1–4. doi: 10.1297/cpe.2.Supple1_1
24. Aso K, Koto S, Higuchi A, Ariyasu D, Izawa M, Miyamoto-Igaki J, et al. Serum FSH level below 10 mIU/mL at twelve years old is an index of spontaneous and cyclical menstruation in turner syndrome. Endocr J (2010) 57:909–13. doi: 10.1507/endocrj.k10e-092
25. Tanka T, Igarashi Y, Ozono K, Ohyama K, Ogawa M, Osada H, et al. Frequencies of spontaneous breast development and spontaneous menarche in turner syndrome in Japan. Clin Pediatr Endocrinol (2015) 24:167–73. doi: 10.1297/cpe.24.167
26. Pasquino AM, Passeri F, Pucarelli I, Segni M, Municchi G. Spontaneous pubertal development in turner's syndrome. Italian study group for turner's syndrome. J Clin Endocrinol Metab (1997) 82:1810–3. doi: 10.1210/jcem.82.6.3970
27. Borgström B, Hreinsson J, Rasmussen C, Sheikhi M, Fried G, Keros V, et al. Fertility preservation in girls with turner syndrome: Prognostic signs of the presence of ovarian follicles. J Clin Endocrinol Metab (2009) 94:74–80. doi: 10.1210/jc.2008-0708
28. Hibi I, Tanae A. Final height in turner syndrome with and without gonadal function. In: Rosenfeld R, Grumbach MM, editors. Turner syndrome. New York and Basel: Marcel Dekker (1990). p. 163–74.
29. Dwyer AA, Phan-Hug F, Hauschild M, Elowe-Gruau E, Pitteloud N. TRANSITION IN ENDOCRINOLOGY: Hypogonadism in adolescence. Eur J Endocrinol (2015) 173:R15–24. doi: 10.1530/EJE-14-0947
30. Carel JC, Elie C, Ecosse E, Tauber M, Léger J, Cabrol S, et al. Self-esteem and social adjustment in young women with turner syndrome–influence of pubertal management and sexuality: Population-based cohort study. J Clin Endocrinol Metab (2006) 91:2972–9. doi: 10.1210/jc.2005-2652
31. Sybert VP, McCauley E. Phenotypic effects of mosaicism for a 47,XXX cell line in turner syndrome. J Med Genet (2002) 39:217–20. doi: 10.1136/jmg.39.3.217
33. Takahashi I, Miyamoto J, Hasegawa Y. Limitations of G-banding karyotype analysis with peripheral lymphocytes in diagnosing mixed gonadal dysgenesis. Clin Pediatr Endocr (2006) 15:109–15. doi: 10.1297/cpe.15.109
34. Nomura R, Miyai K, Okada M, Kajiwara M, Ono M, Ogata T, et al. A 45,X/46,XY DSD (Disorder of sexual development) case with an extremely uneven distribution of 46,XY cells between lymphocytes and gonads. Clin Pediatr Endocrinol (2015) 24:11–4. doi: 10.1297/cpe.24.11
35. Hankus M, Soltysik K, Szeliga K, Antosz A, Drosdzol-Cop A, Wilk K, et al. Prediction of spontaneous puberty in turner syndrome based on mid-childhood gonadotropin concentrations, karyotype, and ovary visualization: A longitudinal study. Horm Res Paediatr (2018) 89:90–7. doi: 10.1159/000485321
36. Hagen CP, Main KM, Kjaergaard S, Juul A. FSH, LH, inhibin b and estradiol levels in turner syndrome depend on age and karyotype: Longitudinal study of 70 turner girls with or without spontaneous puberty. Hum Reprod (2010) 25:3134–4. doi: 10.1093/humrep/deq291
37. Lunding SA, Aksglaede L, Anderson RA, Main KM, Juul A, Hagen CP, et al. AMH as predictor of premature ovarian insufficiency: A longitudinal study of 120 turner syndrome women. J Clin Endocrinol Metab (2015) 100:E1030–8. doi: 10.1210/jc.2015-1621
38. Visser JA, Hokken-Koelega AC, Zandwijken GR, Limacher A, Ranke MB, Flück CE. Anti-müllerian hormone levels in girls and adolescents with turner syndrome are related to karyotype, pubertal development and growth hormone treatment. Hum Reprod (2013) 28:1899–907. doi: 10.1093/humrep/det089
39. Hagen CP, Aksglaede L, Sørensen K, Main KM, Boas M, Cleemann L, et al. Serum levels of anti-müllerian hormone as a marker of ovarian function in 926 healthy females from birth to adulthood and in 172 turner syndrome patients. J Clin Endocrinol Metab (2010) 95:5003–10. doi: 10.1210/jc.2010-0930
40. Hamza RT, Mira MF, Hamed AI, Ezzat T, Sallam MT. Anti-müllerian hormone levels in patients with turner syndrome: Relation to karyotype, spontaneous puberty, and replacement therapy. Am J Med Genet A (2018) 176:1929–34. doi: 10.1002/ajmg.a.40473
41. Weintraub A, Eldar-Geva T. Anti-mullerian hormone (AMH) determinations in the pediatric and adolescent endocrine practice. Pediatr Endocrinol Rev (2017) 14:364–70. doi: 10.17458/per.vol14.2017.WG.Mullerian
42. Mauras N, Torres-Santiago L, Santen R, Mericq V, Ross J, Colon-Otero G, et al. Impact of route of administration on genotoxic oestrogens concentrations using oral vs. transdermal oestradiol in girls with turner syndrome. Clin Endocrinol (2019) 90:155–61. doi: 10.1111/cen.13869
43. Torres-Santiago L, Mericq V, Taboada M, Unanue N, Klein KO, Singh R, et al. Metabolic effects of oral versus transdermal 17β-estradiol (E2): A randomized clinical trial in girls with turner syndrome. J Clin Endocrinol Metab (2013) 98:2716–24. doi: 10.1210/jc.2012-4243
44. Nabhan ZM, Dimeglio LA, Qi R, Perkins SM, Eugster EA. Conjugated oral versus transdermal estrogen replacement in girls with turner syndrome: A pilot comparative study. J Clin Endocrinol Metab (2009) 94:2009–14. doi: 10.1210/jc.2008-2123
45. Mauras N, Shulman D, Hsiang HY, Balagopal P, Welch S. Metabolic effects of oral versus transdermal estrogen in growth hormone-treated girls with turner syndrome. J Clin Endocrinol Metab (2007) 92:4154–60. doi: 10.1210/jc.2007-0671
46. Marjoribanks J, Farquhar C, Roberts H, Lethaby A, Lee J. Long-term hormone therapy for perimenopausal and postmenopausal women. Cochrane Database syst Rev (2017) 1:CD004143. doi: 10.1002/14651858.CD004143.pub5
47. Oliver-Williams C, Glisic M, Shahzad S, Brown E, Pellegrino Baena C, Chadni M, et al. The route of administration, timing, duration and dose of postmenopausal hormone therapy and cardiovascular outcomes in women: A systematic review. Hum Reprod Update (2019) 25:257–71. doi: 10.1093/humupd/dmy039
48. Viuff MH, Stochholm K, Lin A, Berglund A, Juul S, Gravhold CH. Cancer occurrence in turner syndrome and the effect of sex hormone substitution therapy. Eur J Endocrinol (2021) 185:79–88. doi: 10.1530/EJE-20-0702
49. Bannink EM, van Sassen C, van Buuren S, de Jong FH, Lequin M, Mulder PG, et al. Puberty induction in turner syndrome: Results of oestrogen treatment on development of secondary sexual characteristics, uterine dimensions and serum hormone levels. Clin Endocrinol (2009) 70:265–73. doi: 10.1111/j.1365-2265.2008.03446.x
50. Idkowiak J, Smyth A, Mundy L, Wanaguru A, Gleeson H, Högler W. Breast satisfaction in adult women with turner syndrome-an international survey employing the BREAST-q questionnaire. Clin Endocrinol (2022). doi: 10.1111/cen.14755
51. Piippo S, Lenko H, Kainulainen P, Sipilä I. Use of percutaneous estrogen gel for induction of puberty in girls with turner syndrome. J Clin Endocrinol Metab (2004) 89:3241–7. doi: 10.1210/jc.2003-032069
52. Obara-Moszynska M, Dzialach L, Rabska-Pietrzak B, Niedziela M, Kapczuk K. Uterine development during induced puberty in girls with turner syndrome. Front Endocrinol (Lausanne) (2021) 12:707031. doi: 10.3389/fendo.2021.707031
53. Ikegawa K, Hasegawa Y. Fracture risk, underlying pathophysiology, and bone quality assessment in patients with turner syndrome. Front Endocrinol (2022) 13:967857. doi: 10.3389/fendo.2022.967857
54. Gravholt CH, Juul S, Naeraa RW, Hansen J. Morbidity in turner syndrome. J Clin Epidemiol (1998) 51:147–58. doi: 10.1046/j.1365-2265.2003.01807.x
55. Gravholt CH, Vestergaard P, Hermann AP, Mosekilde L, Brixen K, Christiansen JS. Increased fracture rates in turner's syndrome: A nationwide questionnaire survey. Clin Endocrinol (2003) 59:89–96. doi: 10.1046/j.1365-2265.2003.01807.x
56. Bercu BB, Kramer SS, Bode HH. A useful radiologic sign for the diagnosis of turner's syndrome. Pediatrics (1976) 58:737–9. doi: 10.1542/peds.58.5.737
57. Cardona Attard C, Cameron-Pimblett A, Puri D, Elliot J, Wilson JC, Talaulikar VS, et al. Fracture rate in women with oestrogen deficiency - comparison of turner syndrome and premature ovarian insufficiency. Clin Endocrinol (2019) 91:743–9. doi: 10.1111/cen.14110
58. Ross JL, Long LM, Feuillan P, Cassorla F, Cutler GB Jr. Normal bone density of the wrist and spine and increased wrist fractures in girls with turner's syndrome. J Clin Endocrinol Metab (1991) 73:355–9. doi: 10.1210/jcem-73-2-355
59. Itonaga T, Koga E, Nishigaki S, Kawai M, Sakakibara H, Hasegawa Y. A retrospective multicenter study of bone mineral density in adolescents and adults with turner syndrome in Japan. Endocr J (2020) 67:1023–8. doi: 10.1507/endocrj.EJ20-0083
60. Kodama M, Komura H, Kodama T, Nishio Y, Kimura T. Estrogen therapy initiated at an early age increases bone mineral density in turner syndrome patients. Endocr J (2012) 59:153–9. doi: 10.1507/endocrj.ej11-0267
61. Nishigaki S, Itonaga T, Hasegawa Y, Kawai M. Starting age of oestrogen-progestin therapy is negatively associated with bone mineral density in young adults with turner syndrome independent of age and body mass index. Clin Endocrinol (Oxf) (2021) 95:84–91. doi: 10.1111/cen.14484
62. Kawai M, Hasegawa Y. Skeltal characteristics of children and adolescents with turner syndrome. Endocrines (2022) 3:476–87. doi: 10.3390/endocrines3030038
63. Ross JL, McCauley E, Roeltgen D, Long L, Kushner H, Feuillan P, et al. Self-concept and behavior in adolescent girls with turner syndrome: Potential estrogen effects. J Clin Endocrinol Metab (1996) 81:926–31. doi: 10.1210/jcem.81.3.8772552
64. Ross JL, Mazzocco MM, Kushner H, Kowal K, Cutler GB Jr, Roeltgen D. Effects of treatment with oxandrolone for 4 years on the frequency of severe arithmetic learning disability in girls with turner syndrome. J Pediatr (2009) 155:714–20. doi: 10.1016/j.jpeds.2009.05.031
65. Jivraj S, Stillwell S. Turner syndrome through the lens of a gynecologist. Post Reprod Health (2021) 27:98–108. doi: 10.1117/2053369120958593
66. Shifren JL, Gass ML NAMS, Recommendations for Clinical Care of Midlife Women Working Group. The north American menopause society recommendations for clinical care of midlife women. Menopause (New York N.Y.) (2014) 21:1038–62. doi: 10.1097/GME.0000000000000319
67. Fournier A, Berrino F, Clavel-Chapelon F. Unequal risks for breast cancer associated with different hormone replacement therapies: Results from the E3N cohort study. Breast Cancer Res Treat (2008) 107:103–11. doi: 10.1007/s10549-007-9523-x
68. Ankarberg-Lindgren C, Elfving M, Wikland KA, Norjavaara E. Nocturnal application of transdermal estradiol patches produces levels of estradiol that mimic those seen at the onset of spontaneous puberty in girls. J Clin Endocrinol Metab (2001) 86:3039–44. doi: 10.1210/jcem.86.7.7667
69. Norjavaara E, Ankarberg-Lindgren C, Kriström B. Sex steroid replacement therapy in female hypogonadism from childhood to young adulthood. Endocr Dev (2016) 29:198–213. doi: 10.1159/000438892
70. Ross JL, Quigley CA, Cao D, Feuillan P, Kowal K, et al. Growth hormone plus childhood low-dose estrogen in turner's syndrome. N Engl J Med (2011) 364:1230–42. doi: 10.1056/NEJMoa1005669
71. Quigley CA, Wan X, Garg S, Kowal K, Cutler GB Jr, et al. Effects of low-dose estrogen replacement during childhood on pubertal development and gonadotropin concentrations in women with turner syndrome: Results of a randomized, double-blind, placebo-controlled clinical trial. J Clin Endocrinol Metab (2014) 99:E1754–1764. doi: 10.1210/jc.2013-4518
72. Ross JL, Roeltgen D, Feuillan P, Kushner H, Cutler GB Jr. Effects of estrogen on nonverbal processing speed and motor function in girls with turner's syndrome. J Clin Endocrinol Metab (1998) 83:3198–204. doi: 10.1210/jcem.83.9.5087
73. Ross JL, Roeltgen D, Feuillan P, Kushner H, Cutler GB Jr. Use of estrogen in young girls with turner syndrome: Effects on memory. Neurology (2000) 54:164–70. doi: 10.1212/wnl.54.1.164
74. Hasegawa Y, Ariyasu D, Izawa M, Igaki-Miyamoto J, Fukuma M, Hatano M, et al. Gradually increasing ethinylestradiol for turner syndrome may produce good final height but not ideal BMD. Endocr J (2017) 64:221–7. doi: 10.1507/endocrj.EJ16-0170
75. Suwa S, Tachibana K. Standard growth charts for height and weight of Japanese children from birth to 17 years based on a cross-sectional survey of national data. Clin Pediatr Endocrinol (1993) 2:87–97. doi: 10.1297/cpe.2.87
76. Morisaki N, Urayama KY, Yoshii K, Subramanian SV, Yokoya S. Ecological analysis of secular trends in low birth weight births and adult height in Japan. J Epidemiol Community Health (2017) 71:1014–8. doi: 10.1136/jech-2017-209266
77. Hasegawa Y, Itonaga T, Ikegawa K, Nishigaki S, Kawai M, Koga E, et al. Ultra-low-dose estrogen therapy for female hypogonadism. Clin Pediatr Endocrinol (2020) 29:49–53. doi: 10.1297/cpe.29.49
78. Hasegawa Y, Itonaga T, Ikegawa K, Nishigaki S, Kawai M, Koga E, et al. Errata to "Ultra-low-dose estrogen therapy for female hypogonadism". Clin Pediatr Endocrinol (2021) 30:119. doi: 10.1297/cpe.30.119
79. Sweetland S, Beral V, Balkwill A, Liu B, Benson VS, Canonico M, et al. Venous thromboembolism risk in relation to use of different types of postmenopausal hormone therapy in a large prospective study. J Thromb haemost: JTH (2012) 10:2277–86. doi: 10.1111/j.1538-7836.2012.04919.x
80. Zhang GQ, Chen JL, Luo Y, Mathur MB, Anagnostis P, Nurmatov U, et al. Menopausal hormone therapy and women's health: An umbrella review. PloS Med (2021) 18:e1003731. doi: 10.1371/journal.pmed.1003731
81. Tanner JM, Davies PS. Clinical longitudinal standards for height and height velosity for north American children. J Pediatr (1985) 107:317–29. doi: 10.1016/s0022-3476(85)80501-1
82. Murata M. Japanese Specific bone age standard on the TW2. Clin Pediatr Endocrinol (1993) 2(Suppl 3):35–41. doi: 10.1297/cpe.2.Supple3_35
83. Tanner JM, Whitehouse RH, Cameron N, Marshall WA, Healy MJ, Goldstein H. Assessment of skeletal maturity and prediction of adult height (TW2 method). London: Academic Press (1983).
84. Smith EP, Boyd J, Frank GR, Takahashi H, Cohen RM, Specker B, et al. Estrogen resistance caused by a mutation in the estrogen-receptor gene in a man. N Engl J Med (1994) 331:1056–61. doi: 10.1056/NEJM199410203311604
85. Morishima A, Grumbach MM, Simpson ER, Fisher C, Qin K. Aromatase deficiency in male and female siblings caused by a novel mutation and the physiological role of estrogens. J Clin Endocrinol Metab (1995) 80:3689–98. doi: 10.1210/jcem.80.12.8530621
86. Bulun SE. Aromatase and estrogen receptor alpha deficiency. Fertil Steril (2014) 101:323–9. doi: 10.1016/j.fertnstert.2013.12.022
87. Klein KO, Baron J, Colli MJ, McDonnell DP, Cutler GB Jr. Estrogen levels in childhood determined by an ultrasensitive recombinant cell bioassay. J Clin Invest (1994) 94:2475–80. doi: 10.1172/JCI117616
88. Paris F, Servant N, Térouanne B, Balaguer P, Nicolas JC, Sultan C. A new recombinant cell bioassay for ultrasensitive determination of serum estrogenic bioactivity in children. J Clin Endocrinol Metab (2002) 87:791–7. doi: 10.1210/jcem.87.2.8269
89. Backeljauw P, Klein K. Sex hormone replacement therapy for individuals with turner syndrome. Am J Med Genet Part C Semin Med Genet (2019) 181:13–7. doi: 10.1002/ajmg.c.31685
90. Nanao K, Tsuchiya Y, Kotoh S, Hasegawa Y. Low vertebral cancellous bone density in peripubertal girls with turner's syndrome and boys with hypogonadism. J Pediatr Endocrinol Metab (2002) 15:1537–42. doi: 10.1515/jpem.2002.15.9.1537
Keywords: Turner syndrome, hypogonadism, estrogen, induction, puberty
Citation: Hasegawa Y, Hasegawa T, Satoh M, Ikegawa K, Itonaga T, Mitani-Konno M and Kawai M (2023) Pubertal induction in Turner syndrome without gonadal function: A possibility of earlier, lower-dose estrogen therapy. Front. Endocrinol. 14:1051695. doi: 10.3389/fendo.2023.1051695
Received: 23 September 2022; Accepted: 06 February 2023;
Published: 28 March 2023.
Edited by:
Rodolfo A. Rey, Hospital de Niños Ricardo Gutiérrez, ArgentinaReviewed by:
Cheri Deal, University of Montreal, CanadaToshiaki Tanaka, Tanaka Growth Clinic Setagaya-ku, Japan
Copyright © 2023 Hasegawa, Hasegawa, Satoh, Ikegawa, Itonaga, Mitani-Konno and Kawai. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yukihiro Hasegawa, eWhhc2V0QGdtYWlsLmNvbQ==
 Yukihiro Hasegawa
Yukihiro Hasegawa Tomonobu Hasegawa
Tomonobu Hasegawa Mari Satoh
Mari Satoh Kento Ikegawa1,4
Kento Ikegawa1,4 Tomoyo Itonaga
Tomoyo Itonaga Masanobu Kawai
Masanobu Kawai