
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Endocrinol. , 24 May 2023
Sec. Reproduction
Volume 14 - 2023 | https://doi.org/10.3389/fendo.2023.1022272
This article is part of the Research Topic Polycystic Ovary Syndrome (PCOS): Mechanism and Management, volume II View all 16 articles
 Nirmin F. Juber1*
Nirmin F. Juber1* Abdishakur Abdulle1
Abdishakur Abdulle1 Abdulla AlJunaibi2
Abdulla AlJunaibi2 Abdulla AlNaeemi3
Abdulla AlNaeemi3 Amar Ahmad1
Amar Ahmad1 Andrea Leinberger-Jabari1
Andrea Leinberger-Jabari1 Ayesha S. Al Dhaheri4
Ayesha S. Al Dhaheri4 Eiman AlZaabi5
Eiman AlZaabi5 Fatima Mezhal1
Fatima Mezhal1 Fatma Al-Maskari6,7
Fatma Al-Maskari6,7 Fatme Alanouti8
Fatme Alanouti8 Habiba Alsafar9,10,11
Habiba Alsafar9,10,11 Juma Alkaabi12
Juma Alkaabi12 Laila Abdel Wareth13
Laila Abdel Wareth13 Mai Aljaber14
Mai Aljaber14 Marina Kazim15
Marina Kazim15 Michael Weitzman16
Michael Weitzman16 Mohammed Al-Houqani17
Mohammed Al-Houqani17 Mohammed Hag-Ali18
Mohammed Hag-Ali18 Naima Oumeziane15
Naima Oumeziane15 Omar El-Shahawy19
Omar El-Shahawy19 Scott Sherman19
Scott Sherman19 Syed M. Shah6
Syed M. Shah6 Tom Loney20
Tom Loney20 Wael Almahmeed21
Wael Almahmeed21 Youssef Idaghdour1
Youssef Idaghdour1 Raghib Ali1,22
Raghib Ali1,22Introduction: Asthma and polycystic ovarian syndrome (PCOS) are linked in several possible ways. To date, there has been no study evaluating whether pediatric asthma is an independent risk factor for adult PCOS. Our study aimed to examine the association between pediatric asthma (diagnosed at 0-19 years) and adult PCOS (diagnosed at ≥20 years). We further assessed whether the aforementioned association differed in two phenotypes of adult PCOS which were diagnosed at 20-25 years (young adult PCOS), and at >25 years (older adult PCOS). We also evaluated whether the age of asthma diagnosis (0-10 vs 11-19 years) modified the association between pediatric asthma and adult PCOS.
Material and methods: This is a retrospective cross-sectional analysis using the United Arab Emirates Healthy Future Study (UAEHFS) collected from February 2016 to April 2022 involving 1334 Emirati females aged 18-49 years. We fitted a Poisson regression model to estimate the risk ratio (RR) and its 95% confidence interval (95% CI) to assess the association between pediatric asthma and adult PCOS adjusting for age, urbanicity at birth, and parental smoking at birth.
Results: After adjusting for confounding factors and comparing to non-asthmatic counterparts, we found that females with pediatric asthma had a statistically significant association with adult PCOS diagnosed at ≥20 years (RR=1.56, 95% CI: 1.02-2.41), with a stronger magnitude of the association found in the older adult PCOS phenotype diagnosed at >25 years (RR=2.06, 95% CI: 1.16-3.65). Further, we also found females reported thinner childhood body size had a two-fold to three-fold increased risk of adult PCOS diagnosed at ≥20 years in main analysis and stratified analyses by age of asthma and PCOS diagnoses (RR=2.06, 95% CI: 1.08-3.93 in main analysis; RR=2.74, 95% CI: 1.22-6.15 among those diagnosed with PCOS > 25 years; and RR=3.50, 95% CI: 1.38-8.43 among those diagnosed with asthma at 11-19 years).
Conclusions: Pediatric asthma was found to be an independent risk factor for adult PCOS. More targeted surveillance for those at risk of adult PCOS among pediatric asthmatics may prevent or delay PCOS in this at-risk group. Future studies with robust longitudinal designs aimed to elucidate the exact mechanism between pediatric asthma and PCOS are warranted.
Asthma is a multifactorial respiratory disease defined by reversible airway hyperactivity and a wide range of symptoms (1). Inflammation is a key factor in the pathology of asthma, and cross-communication between the airways and inflammatory mediators leads to inflammation that is not only confined to the local airways but also tends to be systemic (2). Asthma is a common pediatric disease with more than 80% of first asthma episodes happening in the first six years of life (3). The main risk factors associated with pediatric asthma include genetic predisposition, viral respiratory infections, and female sex hormones (4). In addition, pediatric asthma has long-term health consequences and is known to be associated with adult non-communicable diseases such as hypertension and diabetes (5).
Polycystic Ovarian Syndrome (PCOS) is a complex endocrine disorder affecting 5-10% of females of reproductive age (6). PCOS is a multifactorial disorder and risk factors associated with PCOS include genetic predisposition, hormonal factors, as well as maternal environmental factors (e.g. metabolic disturbance during pregnancy) (7, 8). Previous studies found that females with PCOS have an increased risk of developing subsequent metabolic disorders, such as cardiovascular disease, hypertension, and diabetes (7, 9, 10). The etiology of PCOS is not exactly known, however, chronic systemic inflammation has been proposed as one of the possible mechanisms (8, 11). In addition, PCOS may have its early-life origins through exposure to excess androgens at any stage from fetal development to childhood period (7, 8).
Asthma and PCOS are linked in several possible ways. Previous studies have established the association between PCOS and subsequent asthma among reproductive-aged females (12–14). There is clinical overlap between asthma and PCOS (15), including alterations in gut microbiota (16–18), menstrual cycle abnormalities (19–21), infertility (22, 23), obesity (24–26), and insulin resistance that was found in asthmatics as well as females with PCOS (27, 28). Previous epidemiological studies have also found an association between PCOS and subsequent asthma (13, 29). However, there is a limited epidemiological study on another possible direction of the association between asthma and subsequent PCOS, including our previous work examining the association between asthma diagnosed at <25 years with subsequent PCOS diagnosed at ≥25 years (30). Asthma may be associated with subsequent PCOS as asthma and PCOS are multifactorial complex diseases and they shared pathophysiological mechanisms, including female hormonal disturbance, systemic/low-grade inflammation, as well as obesity and, metabolic syndrome (11–14, 31). We thus predict that pediatric asthma might be associated with adult PCOS, independent of other relevant risk factors found in our dataset.
Our study aimed to examine the association between pediatric asthma (diagnosed at 0-19 years of age) and adult PCOS (overall adult PCOS: diagnosed at ≥20 years, young adult PCOS: diagnosed at 20-25 years, and older adult PCOS: diagnosed at 25-49 years). We also assessed whether the age of asthma diagnosis (childhood asthma: diagnosed at 0-10 years, and adolescent asthma: diagnosed at 11-19 years) modified the association between pediatric asthma and adult PCOS. Finally, in the main analysis and stratified models, we performed restriction analysis by body size at 10 years old and health status up to 10 years old, to better address the potential mediating effect of childhood body size and childhood health on the association between pediatric asthma and adult PCOS.
This is a retrospective cross-sectional study using the United Arab Emirates Healthy Future Study (UAEHFS) collected from February 2016 to April 2022. We included all 1334 females aged 18-49 years who had complete information on the age of asthma and PCOS diagnosis (Figure 1). The study design, questionnaire, and methodologies of the UAEHFS are described elsewhere (32). In brief, the UAEHFS is an ongoing population-based prospective cohort study among Emirati nationals aged 18 years or above. A convenience sample of Emirati individuals was invited to participate from across the UAE. Multiple recruitment centers were set up across the country where participants filled out the questionnaire and had some physical measurements. Due to the COVID-19 pandemic, the recruitment shifted to online-based starting in April 2020, and an online questionnaire was introduced to the new participants. Physical measurements, such as body mass index (BMI), were taken in the participating centers for new participants that filled out and returned the online questionnaire.
The study and its procedures have been reviewed and approved by the Institutional Review Board at New York University Abu Dhabi, Dubai Health Authority, Ministry of Health and Prevention in the UAE, and Health Research and Technology Committee, reference number DOH/HQD/2020/516. Written consent was obtained from participants at the centers or by filling out an online consent form before data collection started.
We analyzed self-reported physician diagnoses for asthma based on the questionnaire response to: “Has a doctor ever told you that you had asthma?”. The age at asthma diagnosis was extracted from the questionnaire response to: “What was your age when asthma was first diagnosed?”, and pediatric asthma was defined as asthma first diagnosed at 19 years of age or younger (pediatric population) (5, 33). Similarly, self-reported physician diagnosis for PCOS was used based on the questionnaire response to: “Has a doctor ever told you that you had PCOS?”. The age at PCOS diagnosis was extracted from the questionnaire response to: “How old were you when the doctor first told you that you had PCOS?”, and adult PCOS was defined as PCOS first diagnosed at 20 years of age or above (34). Overall health up to 10 years old was determined based on the questionnaire response to: “In general, how was your health in childhood (less than 10 years old)?”, we then categorized childhood health status variable into two categories: poor or fair and good or excellent based on the responses. Body size at 10 years old was determined based on the questionnaire responses to: “When you were 10 years old, compared to average, would you describe yourself as: about average, thinner, or plumper?”, we then classified body size at 10 years old into four categories of around average, average, thinner, and plumper. Age was constructed based on the questionnaire response to “What is your date of birth”, and we kept its continuous form in our analysis. Urbanicity at birth and current urbanicity were determined based on the questionnaire response to: “Where do you and your family live around the time of your birth?”, and “Where do you and your family live now?”, respectively. We then categorized the city as an urban area and non-cities (villages, deserts, islands, and others) as rural or other non-urban. Parental smoking (at birth) was constructed based on the questionnaire response to “Did your mother or father smoke regularly around the time when you were born?” (no/yes). Parental education and education attainment were constructed based on the questionnaire response to: “What level of education did your father or mother complete?”, and “What is the highest level of education that you have completed?”, respectively. We categorized education levels into three categories (≤6 years, >6 to 12 years, and 12+ years of schooling). Birthweight which was determined based on the questionnaire item: “What was your birth weight (in kg)?”, was categorized later into low birthweight (<2.5 kg) and normal birthweight (2.5+ kg). BMI was calculated using the Tanita MC 780 by nurses at the recruitment centers, and the values for each participant were recorded and its continuous form was used in our analysis.
Characteristics of the study participants were evaluated using numbers with percentage (n, %) for each categorical variable and mean with standard deviation (mean ± SD) for each continuous variable (Table 1). To establish asthma as an exposure preceding PCOS as an outcome, we relied on age at disease diagnosis and excluded those with adult asthma (diagnosed at >19 years of age) and adolescent PCOS (diagnosed at <20 years of age). We then fitted a Poisson regression model with robust variance to estimate the risk ratio (RR) and its 95% confidence interval (95% CI) to assess the association between pediatric asthma (diagnosed at 0-19 years of age) and adult PCOS (diagnosed at ≥20 years of age) (Tables 2–4) (35). We examined the RR and its 95% CI in the crude and adjusted models, adjusting for age (5, 36), urbanicity at birth (37, 38), and parental smoking (37, 39). Due to a high amount of missing values (>50%) on parental education (40, 41) and birthweight (42, 43), we excluded these potential confounding factors in the regression analysis even though these are important confounding factors in this study. In addition, the missing indicator method was used to handle uncertain values from missing, prefer not to answer (PFA), and do not know (DN) responses. Analyses were carried out using STATA 17.0 (StataCorp, TX). P values <0.05 were considered statistically significant.
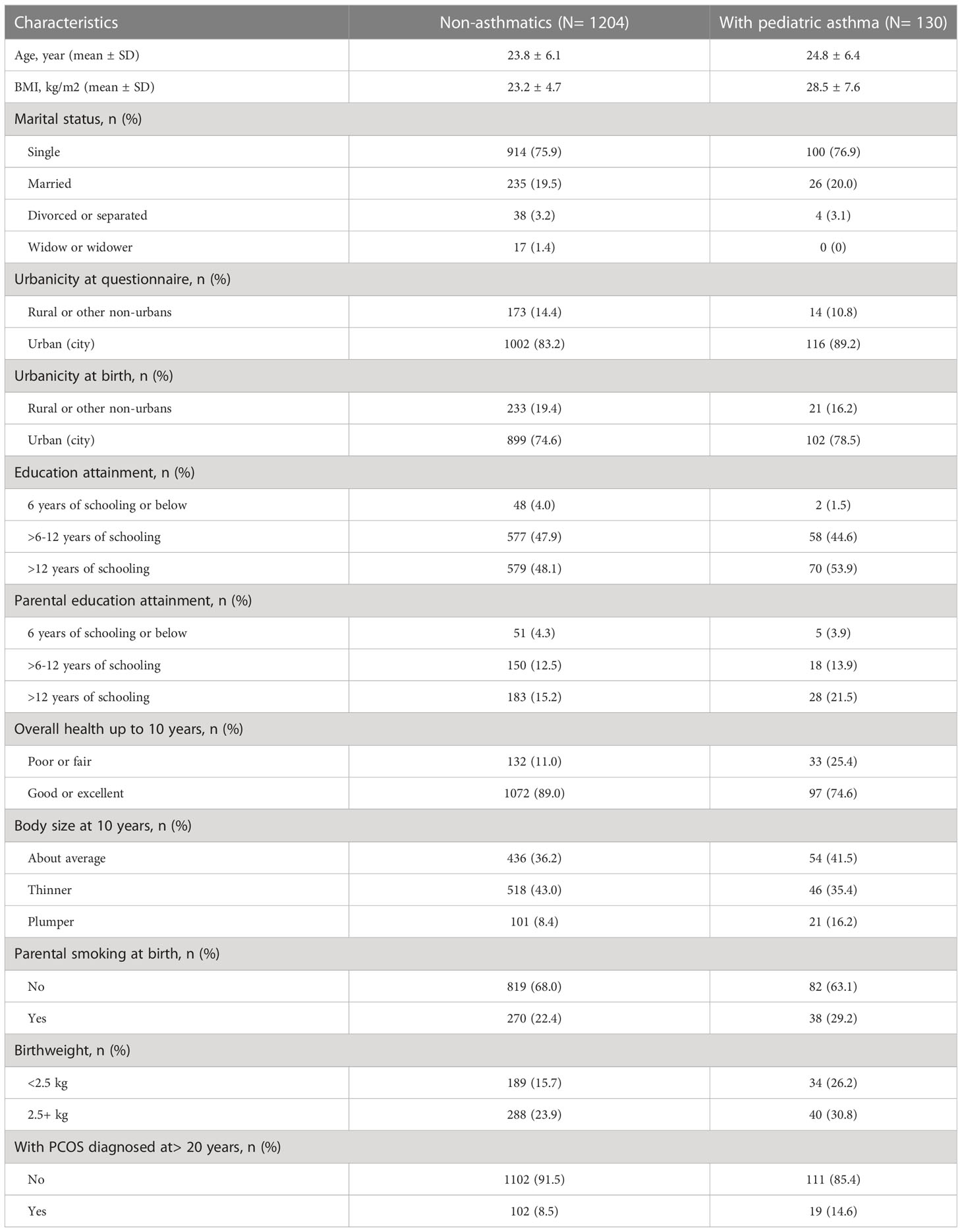
Table 1 Characteristics of female study participants based on pediatric asthma status, missing or uncertain vales are not shown (N=1334).
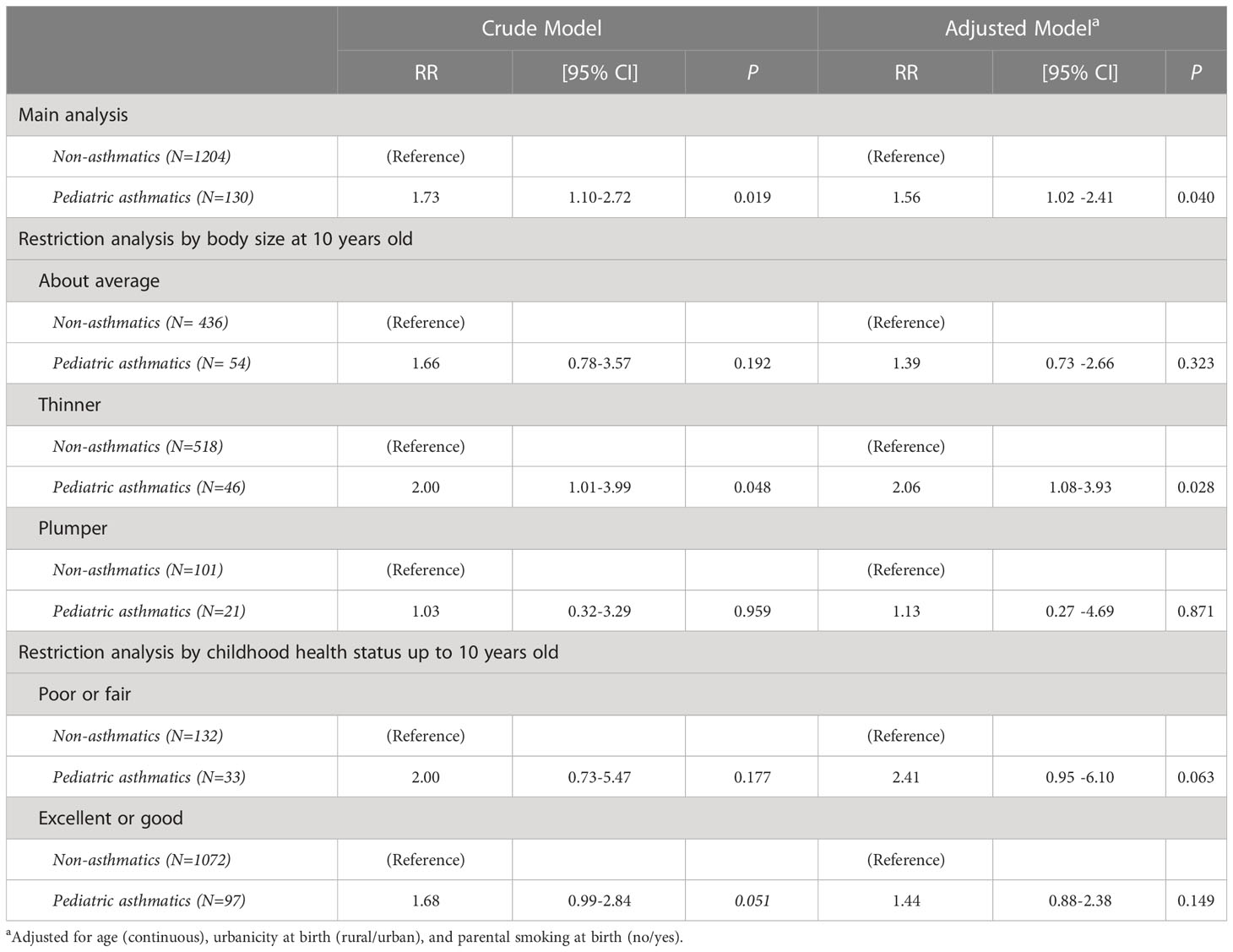
Table 2 Modified Poisson regression analysis between females with pediatric asthma history (diagnosed at 0-19 years of age) and adult PCOS (diagnosed at ≥20 years of age), in total population and restricted by potential mediators (N=1334).
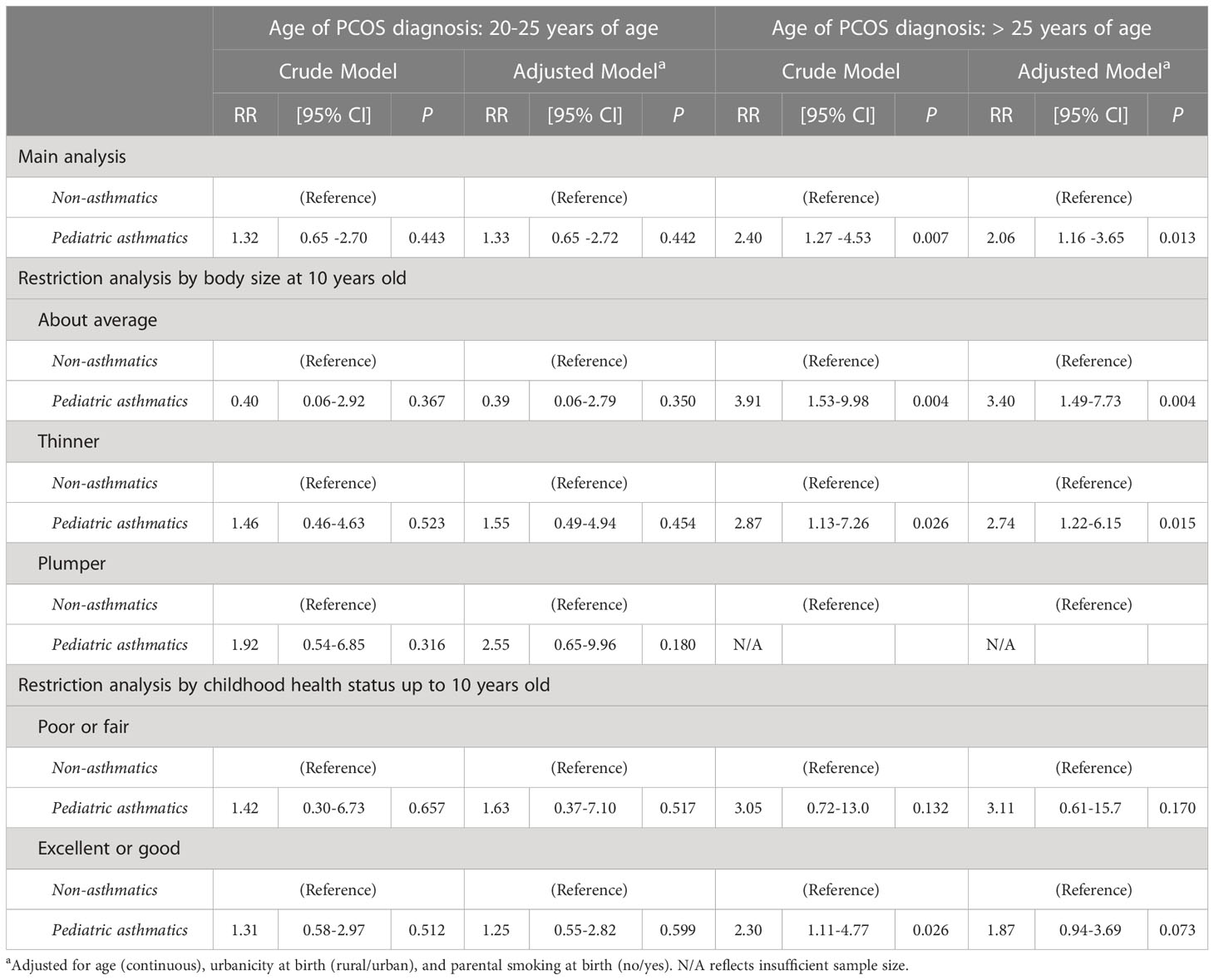
Table 3 Modified Poisson regression analysis between females with pediatric asthma and adult PCOS, stratified by the age of PCOS diagnosis (20-25 years vs >25 years), in total population and restricted by potential mediators (N=1334).
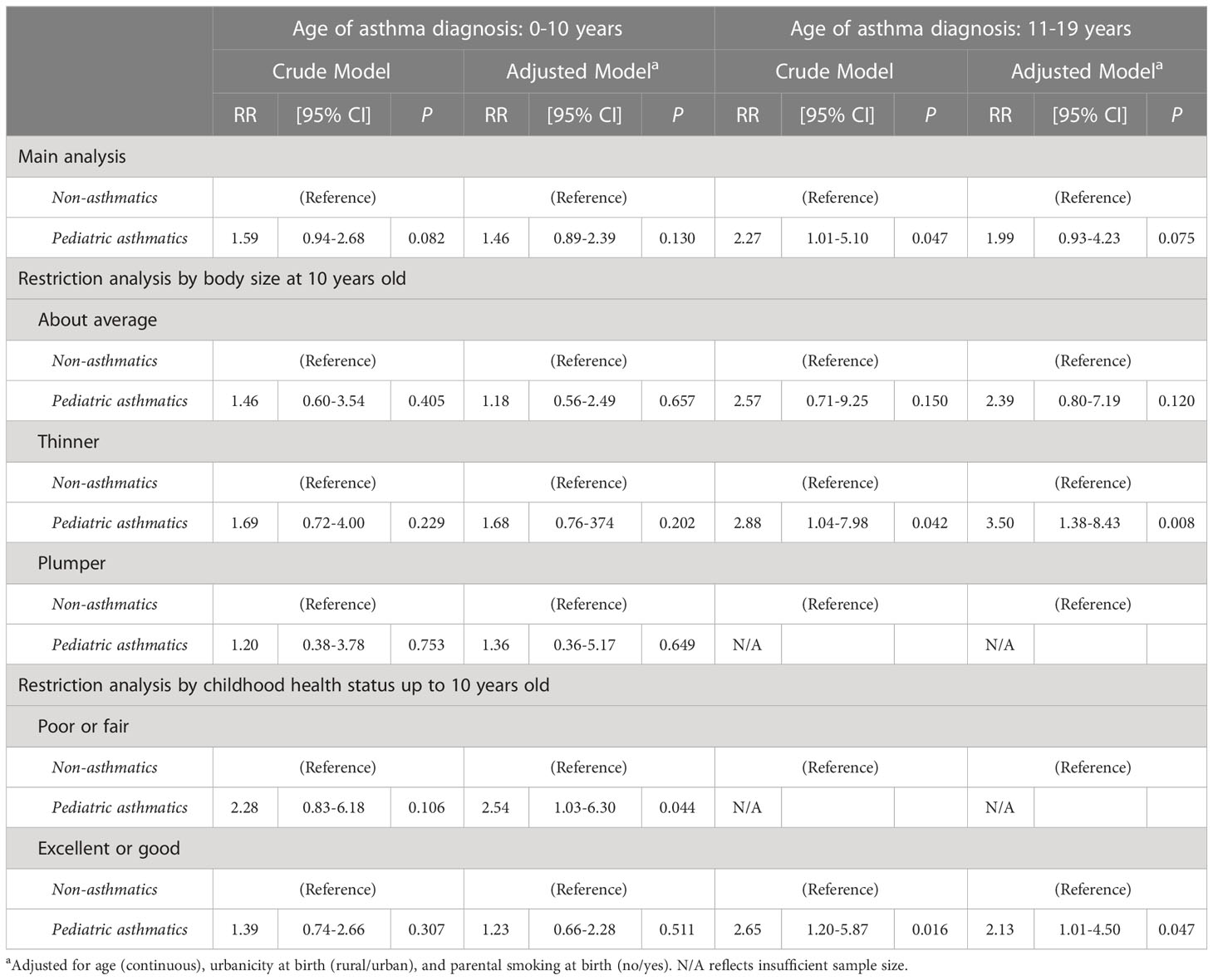
Table 4 Modified Poisson regression analysis between females with pediatric asthma and adult PCOS, stratified by the age of asthma diagnosis (0-10 vs >11-19 years), in total population and restricted by potential mediators (N=1334).
Table 1 summarizes the descriptive characteristics of study participants based on their pediatric asthma status. Out of 1334 females aged 18 to 49 years, 130 females reported ever being diagnosed with asthma when they were 0-19 years of age (pediatric asthma prevalence = 9.75%). Compared to non-asthmatics, females with pediatric asthma were older (24.8 ± 6.4 vs 23.8 ± 6.1 years), had a higher BMI (28.5 ± 7.6 vs 23.2 ± 4.7 kg/m2), a greater proportion resided in urban areas (89.2% vs 83.2%), a greater proportion had >12 years of schooling (high level of education) (53.9% vs 48.1%), and a higher proportion diagnosed with PCOS (14.6% vs 8.5%). In terms of early-life characteristics, females with pediatric asthma had a higher proportion reporting poorer overall health up to 10 years old (25.4% vs 11.0%), had a higher proportion having plumper body size at 10 years old (16.2% vs 8.4%), had a higher proportion of parental smoking at birth (29.2% vs 22.4%), and had a higher proportion of lower birthweight (26.2% vs 15.7%), compared to non-asthmatic females.
Table 2 shows the risk ratios (RRs) of the associations between pediatric asthma (diagnosed at 0-19 years of age) and adult PCOS (diagnosed at ≥20 years of age). In the crude and fully adjusted model, compared to non-asthmatics, pediatric asthma was significantly associated with adult PCOS (RR=1.73, 95% CI: 1.10-2.72 in the crude model; and RR=1.56, 95% CI: 1.02-2.41 in the adjusted model). In the restriction analysis by early-life risk factors, namely, body size at 10 years old and childhood health status up to 10 years old, the significance diminished among those who had about average and plumper body size, and those who reported excellent or good childhood health. Meanwhile, marginal significance was observed among those who reported poor or fair childhood health. Lastly, the statistical significance persisted among those with thinner body size at 10 years old (RR=2.00, 95% CI: 1.01-3.99 in the crude model; and RR=2.06, 95% CI: 1.08-3.93 in the adjusted model).
Table 3 shows the risk ratios (RRs) of the associations between pediatric asthma (diagnosed at 0-19 years of age) and adult PCOS (diagnosed at ≥20 years of age), stratified by the age of adult PCOS diagnosis (20-25 years vs >25 years at PCOS diagnosis), in the main analysis and restricted by early-life risk factors, namely, body size at 10 years old and childhood health status up to 10 years old. In the young adult PCOS diagnosis stratum (diagnosed at 20-25 years), pediatric asthma was not associated with adult PCOS. On the contrary, in the older adult PCOS diagnosis stratum (diagnosed at >25 years) and after adjusting for confounding, we found that pediatric asthma had a significantly increased risk for adult PCOS (RR=2.06, 95% CI: 1.16-3.65). The statistical significance was still observed among those with thinner and about average body size at 10 years old in the older adult PCOS group even after adjusting for confounding factors (RR=2.74, 95% CI: 1.22-6.15 among thinner childhood body size category; and RR=3.40, 95% CI: 1.49-7.73 among about average body size category).
Table 4 presents the risk ratios (RRs) of the associations between pediatric asthma (diagnosed at 0-19 years of age) and adult PCOS (diagnosed at ≥20 years of age), stratified by the age of asthma diagnosis (0-10 vs 11-19 years at asthma diagnosis), in main analysis and restricted by early-life risk factors, namely, body size at 10 years old and childhood health status up to 10 years old. We did not find any statistically significant associations in the main analysis for both strata. However, in the 0-10 years of age of asthma diagnosis and after adjusting for confounding, the significance was observed among those with poor or fair childhood health status (RR=2.54, 95% CI: 1.03-6.30). Meanwhile, in the 11-19 years of age of asthma diagnosis stratum and after adjusting for confounding factors, we found that those diagnosed with asthma at 11-19 years had a significantly increased risk of adult PCOS among those reported thinner childhood body size and those reported excellent or good childhood health status (RR=3.50, 95% CI: 1.38-8.43 among those reported thinner childhood body size, and RR=2.13, 95% CI: 1.01-4.50 among those reported excellent or good childhood health status).
To our knowledge, this is the first population-based study evaluating the association between pediatric asthma diagnosed at 0-19 years and adult PCOS diagnosed at 20 years of age or above. We are able to demonstrate temporality as there is a long interval time between pediatric asthma and adult PCOS diagnoses in this analysis. In both crude and adjusted models and compared to non-asthmatic females, we found that pediatric asthma was significantly positively associated with adult PCOS (Table 2). Previous studies have found that PCOS was an independent risk factor for asthma among reproductive-aged females and suggested a strong correlation between PCOS and chronic systemic inflammation such as asthma (12–14, 31). However, our study provided a new perspective on how asthma and PCOS might be linked in the opposite direction. Our recent bi-directional study examining asthma and PCOS found a significant association between asthma diagnosed at <25 years and adult PCOS diagnosed at ≥25 years, independent of age and BMI (30). Female hormonal disturbance, metabolic syndrome, and obesity have been suggested as overlapping mechanisms that link asthma and PCOS (11–14, 31). A previous study has shown that obesity may worsen hormone dysregulation (25), and although there is no exact explanation linking asthma and metabolic syndrome, there were various known risk factors, including obesity or high BMI and dyslipidemia (44). Compared to non-asthmatics, those with pediatric asthma are known to be more susceptible to subsequent chronic diseases due to immune system impairment and persistent systemic inflammation (45, 46). In addition, compared to their healthy counterparts, pediatric asthmatics are known to have lower sympathetic nervous activity, and thereby a lower metabolic rate which may subsequently affect their vital biological process such as growth and reproduction (47–49). A previous study that linked pediatric asthma and reproductive health found pediatric asthma to be significantly associated with an earlier age at menarche (37). Therefore, we believe there are possible mechanisms involving biological factors in the association between pediatric asthma and adult PCOS since PCOS has been recognized as a chronic metabolic condition beyond a merely reproductive disorder (50).
We stratified by the age of PCOS diagnosis (Table 3) to better understand the association between pediatric asthma and adult PCOS phenotypes; young adult PCOS (diagnosed at 20-25 years), and older adult PCOS (diagnosed at >25 years). Compared to non-asthmatics, we found pediatric asthma was significantly associated with older adult PCOS. Most PCOS-related studies have involved adult populations (mean age > 25 years), however, PCOS can also be present in adolescence and young adulthood (aged ≤25 years) (51, 52). To our knowledge, there is no study on the association between pediatric asthma and any PCOS phenotypes (young adult PCOS or older adult PCOS) to compare to our study findings, however, several possible mechanisms may explain the observed findings. The expression of PCOS in early adulthood may differ from and does not necessarily resemble that of clinical and endocrinological features observed in later adulthood (52). Elevated sex hormone of adrenal androgen was observed in females with PCOS (8), and a previous study found that a greater decrease in adrenal androgen secretion happens between the ages of 20 to 25 years (53). In addition, inflammation is known to be a key factor in the pathology of asthma (2), and a previous study has shown that inflammation affects the level of female sex hormones (11). We believe possible mechanisms involving chronic inflammation and endocrinological features or sex hormones may explain the observed association between pediatric asthma and older adult PCOS phenotype in our study. Future studies to elucidate the exact mechanism of the association between pediatric asthma and older adult PCOS are warranted.
We further stratified the analysis by the age of asthma diagnosis (Table 4) to separate two distinct asthma phenotypes of childhood asthma (diagnosed at 0-10 years of age) and adolescent asthma (diagnosed at 11-19 years of age) and its association with adult PCOS. Childhood asthma (asthma diagnosed at 0-10 years) and adolescent asthma (asthma diagnosed at 11-19 years) were shown to be not significantly associated with adult PCOS in the crude and adjusted models. Asthma is known to be a uniquely diverse disorder with many clinical expressions throughout childhood and adolescence period (3). Childhood asthma and adolescent asthma are shown to be distinct in several ways, including their risk factors. The main risk factor for childhood asthma is a genetic predisposition (4), whereas the main risk factor for adolescent asthma is related to sex hormones (3). Our significant association between pediatric asthma and adult PCOS was only observed among those diagnosed with asthma at 11-19 years in the crude analysis, but the significant association disappeared after adjusting for confounding factors. Adolescence is a transitional stage of physical and psychological development, which is marked by the puberty period in which changes in reproductive hormones occur, and body weight gain following menarche is suggested to mediate the association between pediatric asthma and adult PCOS (54). Compared to healthier counterparts, pediatric asthmatics tend to have a lower sympathetic activity, hence a lower metabolic rate that may affect fat storage and may lead to overweight or obesity (47, 48). In addition, weight gain at puberty has been shown to be a significant risk factor for adult PCOS (52). The involvement of weight gain during puberty in the association between adolescent asthma and adult PCOS may be worth further investigation.
We also performed restriction analysis by body size at 10 years old and childhood health status up to 10 years old in all analyses (Table 2-4) to further explore the role of obesity in the association between pediatric asthma and adult PCOS since we could not rule out the possibility that childhood obesity and childhood health status may mediate the association between pediatric asthma and adult PCOS. Further analysis showed that childhood obesity or childhood health status alone was unlikely to explain the mechanism between pediatric asthma and adult PCOS, as the observed significance disappeared when the analysis was restricted to those with average or plumper body size at 10 years old, as well as to those with poorer/fair or excellent/good childhood health status up to 10 years old (Table 2). Childhood body size (obesity or overweight) may be related to pediatric asthma and adult PCOS through the following mechanisms. In addition to their lower metabolic rate as previously mentioned (47, 48), pediatric asthmatics are known to have poorer childhood health and may limit their physical activities due to their asthma conditions, hence, more susceptible to subsequent childhood overweight or obesity, compared to their healthy counterparts (37, 55). However, due to the design of this study, we could not further evaluate whether childhood obesity or childhood health status indeed mediated the association between pediatric asthma and adult PCOS.
To our knowledge, our study is unique because it is the first population-based study examining the association between pediatric asthma and adult PCOS. We were able to assess the association with clear temporality and were able to control for relevant confounding factors. In addition, the large sample size in this study was suitable to perform stratification and/or restriction analysis to better assess and address the potential mediating effect on the association between pediatric asthma and adult PCOS. Epidemiological study of PCOS during different life stages is still limited and this study might guide future research.
Despite the strength of our study, we acknowledge some limitations. One major limitation pertains to the self-reported diagnosis of asthma and PCOS which might raise the concern about disease ascertainment accuracy. However, previous studies have shown self-reported asthma and PCOS diagnoses to be reliable (56–58). Self-reported age of asthma diagnosis was found to be accurate and had a low variability across categories of demographic and health-related characteristics (56), and self-reported PCOS was found to have high sensitivity in predicting PCOS (78%) compared to the sensitivity of PCOS diagnosis using the Rotterdam criteria (89%) (59). We have also adjusted for age in our adjusted analysis to further address the recall error for all self-reported variables. Furthermore, our study was also prone to misclassification, especially regarding childhood variables such as childhood body size, childhood health status, and parental smoking. However, we believe asthmatics and non-asthmatics in this study reported childhood variables in a similar fashion, resulting in non-differential misclassification. With regards to the convenience sampling design employed in our study, we have attempted to improve the representativeness of the sample by inviting the entire eligible population of the UAE to participate and operating multiple recruitment centers in different regions across the country to ensure ease of access. Another limitation is that we did not have information on the severity of asthma and PCOS to examine the associations involving disease severity (31). Our study may be prone to survivorship bias since we only included those who were alive at the questionnaire time; however, childhood mortality rates are low in the UAE and this is likely to have had a minimal effect on the findings (60). Our study also a had low sample size and statistical power in certain stratification analyses, such as in certain strata in the childhood body size and childhood health status (with N/A reflecting insufficient sample size). Lastly, our study may have been subjected to unmeasured confounding factors, including those factors with significant missing values such as parental education (40, 41) and birthweight (42, 43). However, our sensitivity analysis revealed similar results with or without the involvement of parental education and birthweight in the adjusted model (data not shown).
Our data demonstrated that pediatric asthma was an independent risk factor for adult PCOS. More targeted surveillance for those at risk of adult PCOS among pediatric asthmatics, may prevent or delay adult PCOS occurrence in this at-risk group. Future population-based studies with robust longitudinal designs aimed to elucidate the exact mechanism between pediatric asthma and PCOS are warranted.
The study and its procedures have been reviewed and approved by the Institutional Review Board at New York University Abu Dhabi, Dubai Health Authority, Ministry of Health and Prevention in the UAE, and Health Research and Technology Committee, reference number DOH/HQD/2020/516.
Written consent was obtained from participants at the centers or by filling out an online consent form before data collection started.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by The study and its procedures have been reviewed and approved by the Institutional Review Board at New York University Abu Dhabi, Dubai Health Authority, Ministry of Health and Prevention in the UAE, and Health Research and Technology Committee, reference number DOH/HQD/2020/516. The patients/participants provided their written informed consent to participate in this study.
Conceptualization, NJ, RA. Formal analysis, NJ. Data curation, NJ. Writing—original draft preparation, NJ. Writing—review and editing, AAb, AAh, AL-J, ASA., EA, FM, FA-M, FA, HA, JA, LW, MA, MK, MW, MA-H, MH-A, NO, OE-S, SS, SMS, TL, WA, YI, RA. Funding acquisition, RA All authors contributed to the article and approved the submitted version.
This publication is supported by Tamkeen under Research Institute Grant No. G1206.
The authors would like to thank all members of the Public Health Research Center at New York University Abu Dhabi and those who are actively involved in the UAE Healthy Future Study (UAEHFS). This study would not have been possible without their collective effort.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Miranda C, Busacker A, Balzar S, Trudeau J, Wenzel SE. Distinguishing severe asthma phenotypes: role of age at onset and eosinophilic inflammation. J Allergy Clin Immunol (2004) 113(1):101–8. doi: 10.1016/j.jaci.2003.10.041
2. Bjermer L. Time for a paradigm shift in asthma treatment: from relieving bronchospasm to controlling systemic inflammation. J Allergy Clin Immunol (2007) 120(6):1269–75. doi: 10.1016/j.jaci.2007.09.017
3. Trivedi M, Denton E. Asthma in children and adults-what are the differences and what can they tell us about asthma? Front Pediatr (2019) 7:256. doi: 10.3389/fped.2019.00256
4. Bisgaard H, Bønnelykke K. Long-term studies of the natural history of asthma in childhood. J Allergy Clin Immunol (2010) 126(2):187–97. doi: 10.1016/j.jaci.2010.07.011
5. Juber NF, Lee CC, Pan WC, Liu JJ. Associations between pediatric asthma and adult non-communicable diseases. Pediatr Allergy Immunol (2021) 32(2):314–21. doi: 10.1111/pai.13395
6. De Leo V, Musacchio M, Cappelli V, Massaro M, Morgante G, Petraglia F. Genetic, hormonal and metabolic aspects of PCOS: an update. Reprod Biol Endocrinol (2016) 14(1):1–17. doi: 10.1186/s12958-016-0173-x
7. Franks S, McCarthy MI, Hardy K. Development of polycystic ovary syndrome: involvement of genetic and environmental factors. Int J Androl (2006) 29(1):278–85. doi: 10.1111/j.1365-2605.2005.00623.x
8. Sanchez-Garrido MA, Tena-Sempere M. Metabolic dysfunction in polycystic ovary syndrome: pathogenic role of androgen excess and potential therapeutic strategies. Mol Metab (2020) 35:100937. doi: 10.1016/j.molmet.2020.01.001
9. Talbott EO, Zborowski JV, Rager JR, Kip KE, Xu X, Orchard TJ. Polycystic ovarian syndrome (PCOS): a significant contributor to the overall burden of type 2 diabetes in women. J Womens Health (2007) 16(2):191–7. doi: 10.1089/jwh.2006.0098
10. Bednarska S, Siejka A. The pathogenesis and treatment of polycystic ovary syndrome: what’s new? Adv Clin Exp Med (2017) 26(2):359–67. doi: 10.17219/acem/59380
11. Zierau L, Gade EJ, Lindenberg S, Backer V, Thomsen SF. Coexistence of asthma and polycystic ovary syndrome: a concise review. Respir Med (2016) 119:155–9. doi: 10.1016/j.rmed.2016.08.025
12. Htet TD, Teede HJ, De Courten B, Loxton D, Real FG, Moran LJ, et al. Asthma in reproductive-aged women with polycystic ovary syndrome and association with obesity. Eur Respir J (2017) 49(5):1601334. doi: 10.1183/13993003.01334-2016
13. Sun H, Li D, Jiao J, Liu Q, Bian J, Wang X. A potential link between polycystic ovary syndrome and ssthma: a meta-analysis. Reprod Sci (2022) 29(1):312–9. doi: 10.1007/s43032-021-00662-8
14. Grieger JA, Hodge A, Mishra G, Joham AE, Moran LJ. The association between dietary intake, asthma, and PCOS in women from the Australian longitudinal study on women’s health. J Clin Med (2020) 9(1):233. doi: 10.3390/jcm9010233
15. Guarnieri G, Iervolino M, Cavallone S, Unfer V, Vianello A. The “Asthma-polycystic ovary overlap syndrome” and the therapeutic role of yo-inositol. Int J Mol Sci (2023) 24(8):6959. doi: 10.3390/ijms24086959
16. Tremellen K, Pearce K. Dysbiosis of gut microbiota (DOGMA)–a novel theory for the development of polycystic ovarian syndrome. Med Hypotheses (2012) 79(1):104–12. doi: 10.1016/j.mehy.2012.04.016
17. Molyneaux PL, Cox MJ, Wells AU, Kim HC, Ji W, Cookson WO, et al. Changes in the respiratory microbiome during acute exacerbations of idiopathic pulmonary fibrosis. Respir Res (2017) 18:1–6. doi: 10.1186/s12931-017-0511-3
18. Ramírez-Labrada AG, Isla D, Artal A, Arias M, Rezusta A, Pardo J, et al. The influence of lung microbiota on lung carcinogenesis, immunity, and immunotherapy. Trends Cancer (2020) 6(2):86–97. doi: 10.1016/j.trecan.2019.12.007
19. Hart R, Doherty DA. The potential implications of a PCOS diagnosis on a woman’s long-term health using data linkage. J Clin Endocrinol Metab (2015) 100(3):911–9. doi: 10.1210/jc.2014-3886
20. Sánchez-Ramos JL, Pereira-Vega AR, Alvarado-Gómez F, Maldonado-Pérez JA, Svanes C, Gómez-Real F. Risk factors for premenstrual asthma: a systematic review and meta-analysis. Expert Rev Respir Med (2017) 11(1):57–72. doi: 10.1080/17476348.2017.1270762
21. Becerra-Diaz M, Song M, Heller N. Androgen and androgen receptors as regulators of monocyte and macrophage biology in the healthy and diseased lung. Front Immunol (2020) 11:1698. doi: 10.3389/fimmu.2020.01698
22. Collée J, Mawet M, Tebache L, Nisolle M, Brichant G. Polycystic ovarian syndrome and infertility: overview and insights of the putative treatments. Gynecol Endocrinol (2021) 37(10):869–74. doi: 10.1080/09513590.2021.1958310
23. Gade E, Thomsen S, Lindenberg S, Backer V. Female asthma has a negative effect on fertility: what is the connection? Allergy (2014) 2014:131092. doi: 10.1155/2014/131092
24. Assad N, Qualls C, Smith LJ, Arynchyn A, Thyagarajan B, Schuyler M, et al. Body mass index is a stronger predictor than the metabolic syndrome for future asthma in women. the longitudinal CARDIA study. Am J Respir Crit Care Med (2013) 188(3):319–26. doi: 10.1164/rccm.201303-0457OC
25. Glueck CJ, Goldenberg N. Characteristics of obesity in polycystic ovary syndrome: etiology, treatment, and genetics. Metab (2019) 92:108–20. doi: 10.1016/j.metabol.2018.11.002
26. Naderpoor N, Shorakae S, Joham A, Boyle J, De Courten B, Teede HJ. Obesity and polycystic ovary syndrome. Minerva Endocrinol (2014) 40(1):37–51.
27. Barber TM, Franks S. Obesity and polycystic ovary syndrome. Clin Endocrinol (2021) 95(4):531–41. doi: 10.1111/cen.14421
28. Carpaij OA, Van den Berge M. The asthma–obesity relationship: underlying mechanisms and treatment implications. Curr Opin Pulm Med (2018) 24(1):42–9. doi: 10.1097/MCP.0000000000000446
29. Zierau L, Meteran H, Backer V, Lindenberg S, Skytthe A, Thomsen SF. The risk of asthma is increased among women with polycystic ovary syndrome: a twin study. ERJ Open Res (2019) 5(3):00018-2018. doi: 10.1183/23120541.00018-2018
30. Juber NF, Abdulle A, AlJunaibi A, AlNaeemi A, Ahmad A, Leinberger-Jabari A, et al. Association between self-reported polycystic ovary syndrome with chronic diseases among emiratis: a cross-sectional analysis from the UAE healthy future study. Int J Women’s Health (2023) 15:289–98. doi: 10.2147/IJWH.S398651
31. Xu Y, Zhou Z-Y, Pan J-X, Huang H-F. Associations between asthma and polycystic ovary syndrome: current perspectives. Front Endocrinol (2022) 13:936948. doi: 10.3389/fendo.2022.936948
32. Abdulle A, Alnaeemi A, Aljunaibi A, Al Ali A, Al Saedi K, Al Zaabi E, et al. The UAE healthy future study: a pilot for a prospective cohort study of 20,000 united Arab Emirates nationals. BMC Public Health (2018) 18(1):1–9. doi: 10.1186/s12889-017-5012-2
33. Furu K, Skurtveit S, Langhammer A, Nafstad P. Use of anti-asthmatic medications as a proxy for prevalence of asthma in children and adolescents in Norway: a nationwide prescription database analysis. Eur J Clin Pharmacol (2007) 63(7):693–8. doi: 10.1007/s00228-007-0301-9
34. Carmina E, Campagna AM, Lobo RA. A 20-year follow-up of young women with polycystic ovary syndrome. Obstet Gynecol (2012) 119(2):263–9. doi: 10.1097/AOG.0b013e31823f7135
35. Barros AJ, Hirakata VN. Alternatives for logistic regression in cross-sectional studies: an empirical comparison of models that directly estimate the prevalence ratio. BMC Med Res Methodol (2003) 3(1):1–13. doi: 10.1186/1471-2288-3-21
36. Bennett CJ, Mansfield DR, Mo L, Joham AE, Cain SW, Blumfield ML, et al. Sleep disturbances may influence lifestyle behaviours in women with self-reported polycystic ovary syndrome. Br J Nutr (2022) 127(9):1395–403. doi: 10.1017/S0007114521002361
37. Juber NF, Waits A, Dlamini LP, Nguyen T, Masango BZ. Associations between pediatric asthma and age at menarche: evidence from the Indonesian family life survey. J Asthma (2022) 60:1–10. doi: 10.1080/02770903.2022.2030750
38. Balaji S, Amadi C, Prasad S, Bala Kasav J, Upadhyay V, Singh AK, et al. Urban rural comparisons of polycystic ovary syndrome burden among adolescent girls in a hospital setting in India. BioMed Res Int (2015) 2015:158951. doi: 10.1155/2015/158951
39. Gollenberg AL, Addo OY, Zhang Z, Hediger ML, Himes JH, Lee PA. In utero exposure to cigarette smoking, environmental tobacco smoke and reproductive hormones in US girls approaching puberty. Horm Res Paediatr (2015) 83(1):36–44. doi: 10.1159/000369168
40. Celedón JC, Soto-Quiros ME, Silverman EK, Hanson LA, Weiss ST. Risk factors for childhood asthma in Costa Rica. Chest (2001) 120(3):785–90. doi: 10.1378/chest.120.3.785
41. Merkin SS, Azziz R, Seeman T, Calderon-Margalit R, Daviglus M, Kiefe C, et al. Socioeconomic status and polycystic ovary syndrome. J Womens Health (2011) 20(3):413–9. doi: 10.1089/jwh.2010.2303
42. Xu X-F, Li Y-J, Sheng Y-J, Liu J-L, Tang L-F, Chen Z-M. Effect of low birth weight on childhood asthma: a meta-analysis. BMC Pediatr (2014) 14(1):1–8. doi: 10.1186/1471-2431-14-275
43. Sadrzadeh S, Hui E, Schoonmade L, Painter R, Lambalk C. Birthweight and PCOS: systematic review and meta-analysis. Hum Reprod Open (2017) 2:10. doi: 10.1093/hropen/hox010
44. Serafino-Agrusa L, Spatafora M, Scichilone N. Asthma and metabolic syndrome: current knowledge and future perspectives. World J Clin cases (2015) 3(3):285. doi: 10.12998/wjcc.v3.i3.285
45. Juhn YJ. Influence of asthma epidemiology on the risk for other diseases. Allergy Asthma Immunol Res (2012) 4(3):122–31. doi: 10.4168/aair.2012.4.3.122
46. Sumino K, O’Brian K, Bartle B, Au DH, Castro M, Lee TA. Coexisting chronic conditions associated with mortality and morbidity in adult patients with asthma. J Asthma (2014) 51(3):306–14. doi: 10.3109/02770903.2013.879881
47. Wolf JM, Nicholls E, Chen E. Chronic stress, salivary cortisol, and α-amylase in children with asthma and healthy children. Biol Psychol (2008) 78(1):20–8. doi: 10.1016/j.biopsycho.2007.12.004
48. Spraul M, Ravussin E, Fontvieille AM, Rising R, Larson DE, Anderson EA. Reduced sympathetic nervous activity. a potential mechanism predisposing to body weight gain. J Clin Investig (1993) 92(4):1730–5. doi: 10.1172/JCI116760
49. Glazier DS. Is metabolic rate a universal ‘pacemaker’for biological processes. Biol Rev (2015) 90(2):377–407. doi: 10.1111/brv.12115
50. El Hayek S, Bitar L, Hamdar LH, Mirza FG, Daoud G. Polycystic ovarian syndrome: an updated overview. Front Physiol (2016) 7:124. doi: 10.3389/fphys.2016.00124
51. Jena SK, Mishra L, Naik SS, Khan S. Awareness and opinion about polycystic ovarian syndrome (PCOS) among young women: a developing country perspective. Int J Adolesc Med Health (2021) 33(3):123–6. doi: 10.1515/ijamh-2018-0166
52. Michelmore KF. Polycystic ovary syndrome in adolescence and early adulthood. Hum Fertil (2000) 3(2):96–100. doi: 10.1080/1464727002000198771
53. Davison SL, Bell R, Donath S, Montalto JG, Davis SR. Androgen levels in adult females: changes with age, menopause, and oophorectomy. J Clin Endocrinol Metab (2005) 90(7):3847–53. doi: 10.1210/jc.2005-0212
54. Abraham S, Boyd C, Lal M, Luscombe G, Taylor A. Time since menarche, weight gain and body image awareness among adolescent girls: onset of eating disorders? J Psychosom Obstet Gynaecol (2009) 30(2):89–94. doi: 10.1080/01674820902950553
55. Alqahtani N, Scott J, Ullah S. Physical activity and sedentary behaviors as risk factors of obesity among rural adolescents. J Child Adolesc Behav (2015) 3:1. doi: 10.4172/2375-4494.1000185
56. Mirabelli MC, Beavers SF, Flanders WD, Chatterjee AB. Reliability in reporting asthma history and age at asthma onset. J Asthma (2014) 51(9):956–63. doi: 10.3109/02770903.2014.930480
57. Day F, Karaderi T, Jones MR, Meun C, He C, Drong A, et al. Large-Scale genome-wide meta-analysis of polycystic ovary syndrome suggests shared genetic architecture for different diagnosis criteria. PloS Genet (2018) 14(12):e1007813. doi: 10.1371/journal.pgen.1007813
58. Toren K, Brisman J, Järvholm B. Asthma and asthma-like symptoms in adults assessed by questionnaires: a literature review. Chest (1993) 104(2):600–8. doi: 10.1378/chest.104.2.600
59. Elting MW, Korsen TJ, Rekers-Mombarg LT, Schoemaker J. Women with polycystic ovary syndrome gain regular menstrual cycles when ageing. Hum Reprod (2000) 15(1):24–8. doi: 10.1093/humrep/15.1.24
Keywords: asthma, pediatric asthma, polycystic ovarian syndrome, PCOS, epidemiology, risk factors, public health
Citation: Juber NF, Abdulle A, AlJunaibi A, AlNaeemi A, Ahmad A, Leinberger-Jabari A, Al Dhaheri AS, AlZaabi E, Mezhal F, Al-Maskari F, Alanouti F, Alsafar H, Alkaabi J, Wareth LA, Aljaber M, Kazim M, Weitzman M, Al-Houqani M, Hag-Ali M, Oumeziane N, El-Shahawy O, Sherman S, Shah SM, Loney T, Almahmeed W, Idaghdour Y and Ali R (2023) Association between pediatric asthma and adult polycystic ovarian syndrome (PCOS): a cross-sectional analysis of the UAE healthy future Study (UAEHFS). Front. Endocrinol. 14:1022272. doi: 10.3389/fendo.2023.1022272
Received: 18 August 2022; Accepted: 10 May 2023;
Published: 24 May 2023.
Edited by:
Yanting Wu, Fudan University, ChinaReviewed by:
Jiangfeng Ye, Agency for Science, Technology and Research (A*STAR), SingaporeCopyright © 2023 Juber, Abdulle, AlJunaibi, AlNaeemi, Ahmad, Leinberger-Jabari, Al Dhaheri, AlZaabi, Mezhal, Al-Maskari, Alanouti, Alsafar, Alkaabi, Wareth, Aljaber, Kazim, Weitzman, Al-Houqani, Hag-Ali, Oumeziane, El-Shahawy, Sherman, Shah, Loney, Almahmeed, Idaghdour and Ali. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Nirmin F. Juber, bmlybWluLmp1YmVyQG55dS5lZHU=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.