- 1West China Centre of Excellence for Pancreatitis, Institute of Integrated Traditional Chinese and Western Medicine, West China-Liverpool Biomedical Research Centre, West China Hospital, Sichuan University, Chengdu, China
- 2Department of Gastroenterology, First Affiliated Hospital of Nanchang University, Nanchang, China
- 3Department of Endocrinology and Metabolism, West China Hospital, Sichuan University, Chengdu, China
- 4Department of Guideline and Rapid Recommendation, Cochrane China Center, MAGIC China Centre, Chinese Evidence-Based Medicine Center, West China Hospital, Sichuan University, Chengdu, China
- 5Applied Surgery and Metabolism Laboratory, School of Biological Sciences, University of Auckland, Auckland, New Zealand
- 6Liverpool Pancreatitis Research Group, Liverpool University Hospitals National Health Service (NHS) Foundation Trust and Institute of Translational Medicine, University of Liverpool, Liverpool, United Kingdom
Background: To determine the impact of glucose levels at admission and during first week (early phase) on clinical outcomes in patients with acute pancreatitis (AP) and to investigate the relationship between stress hyperglycaemia (SHG) and hypertriglyceridaemia (HTG).
Methods: Two independent and prospective databases were retrospectively analysed (n = 1792). Patients admitted with pain of less than 48 hours and confirmed AP were included. SHG was defined as admission blood glucose ≥ 10.00 mmol/L (non-diabetic) or ≥ 16.67 mmol/L (diabetic). Blood glucose records for the first week were inspected to determine whether SHG lasted ≥ 48 hours (persistent) or < 48 hours (transient). Clinical outcomes were compared between designated patient groups using multivariate and trend analyses. The correlation between SHG and HTG (serum triglyceride ≥ 5.65 mmol/L) was also analysed.
Results: On admission, SHG was present in 27.8% (499/1792) patients; during the first 48 hours of admission, transient and persistent SHG was found in 31% (556/1792) and 8.0% (144/1792) patients, respectively. Admission SHG was associated with higher incidence of persistent organ failure, acute necrotic collection, major infection, and mortality as well as prolonged length of hospital stay (all P < 0.05). Duration of SHG was also associated with worsened clinical outcomes (all P < 0.05). In HTG-AP patients, more severe clinical outcomes were observed in those who concomitantly had SHG (P < 0.05).
Conclusions: Admission and persistent SHG during the first week of admission worsens clinical outcomes of AP patients. These effects are more pronounced when admission HTG co-existed.
Introduction
Acute pancreatitis (AP) is one of the leading acute gastrointestinal diseases which has no effective and targeted drug treatment (1) and causes a significant social-economic burden (2). The global incidence of AP is increasing (3) with gallstones and alcohol excess being the most common aetiologies (4). Hypertriglyceridaemia (HTG) has become more common worldwide (5) and has become one of the leading causes in China (6–8). The sequelae of AP, including diabetes mellitus (DM) has a serious impact on quality of life (9, 10). About 20% of patients with AP will develop DM within 3 years of discharge from hospital and the risk increases over time (11, 12). Early diagnosis of hyperglycaemia and optimisation of in-hospital management may help prevent AP-related DM inferred from strong evidence of critically illness (13).
It is now well known that acute illness or injury can result in hyperglycaemia, insulin resistance and glucose intolerance, collectively termed stress hyperglycaemia (SHG) (14). SHG is a key risk factor for incident DM in survivors of critical illness (13). It is plausible, however, that critical illness uncovers latent insulin resistance and/or impaired pancreatic β-cell function, such that SHG identifies patients at increased risk of subsequently developing DM (15). Evidence to date also demonstrates that prolonged severe SHG is associated with a significantly elevated risk of mortality in patients in intensive care unit (ICU) (16). While a pilot study from New Zealand suggested elevated admission fasting blood glucose (BG) might be associated with worse clinical outcomes in AP (17), the relationship between SHG and AP warrants further study. Furthermore, the relationship between SHG and HTG is uncertain. DM, dyslipidaemia and their treatment are highly linked (18, 19). Insulin treatment has been frequently used in the management of HTG-associated AP (HTG-AP), in patients both with and without diabetes (20). And while the overall clinical outcomes are worse with HTG-AP than other aetiologies (6), it is not known whether this is also attributed by SHG.
We have recently investigated the specific BG levels that define SHG in AP patients with or without pre-existing DM (21). It was found that BG ≥ 10.00 mmol/L (180 mg/dL; in non-diabetic patients) or ≥ 16.67 (300 mg/dL; in diabetic patients) were independently associated with persistent organ failure. These findings have not been validated and SHG was only investigated at the time admission without knowing the impact of SHG duration during the early phase of AP on the clinical outcomes. The aims of this study were to (1) validate and explore the impact of admission SHG and persistent SHG during first week, respectively; and (2) investigate the relationship between SHG and HTG and impact on clinical outcomes in AP patients.
Methods
Study design and patient population
The present study was based on the retrospective analysis using the STROBE guidelines (22) of two large Chinese AP prospective databases of consecutively enrolled AP patients. The two cohorts included patients admitted to the West China Hospital of Sichuan University (Chengdu) from January 2016 to August 2017 and First Affiliated Hospital of Nanchang University (Nanchang) from January 2011 to December 2018 (23, 24), respectively. The institutional review boards at both centres (database approval number: Chengdu, No. 2015[247]; Nanchang, No. 2011[001]) approved the study. Data of 1792 patients with AP were used in the study, 688 patients were contributed by Chengdu, and 1104 by Nanchang, respectively (Supplementary Figures 1A, B).
Data collection
All patients followed uniform diagnostic criteria for AP according to Revised Atlanta Criteria (RAC) (25). Comprehensive clinical data were prospectively recorded on admission, within 24 hours, 24-48 hours, day 3 and if still being hospitalised, then on days 5, 7 and once a week then as previously reported (6, 8, 21, 23, 24). The data collected included demographics of age, sex, body mass index (BMI), date of admission, time from abdominal pain onset to hospital admission, referral status, and co-morbidities. Clinical data included vital signs, haematology, biochemistry, blood gas analysis, clinical severity scores, CT pancreatic imaging. Treatment data included drugs, drainage, debridement, organ failure support. Outcome data included clinical outcomes (below), date of discharge or death. Patients were managed according to the International Association of Pancreatology/American Pancreatic Association (IAP/APA) (26).
Inclusion and exclusion criteria
The inclusion criteria were adult patients (18-80 years) who had pain for 48 hours or less prior to admission, including those referred from other hospitals. The BG levels were determined at the time of admission and during the first week of admission at a frequency of at least every 48 hours.
The exclusion criteria were admission hypoglycaemia (BG level < 3.9 mmol/L) (27); use of glucocorticoids before admission; pregnancy or lactation; AP aetiologies of trauma, chronic pancreatitis or neoplasia; advanced comorbidities (congestive heart failure 3-4 or unstable coronary heart disease, end stage lung diseases, chronic kidney disease stage 4-5, liver cirrhosis with modified Child-Pugh grade 2-3, malignancy or immune deficiency); and incomplete data.
Definitions
Blood glucose: admission BG data used in this study were derived from the first blood biochemistry analysis of patients who presented at emergence department and very few were obtained at general ward. Subsequent daily BG levels during the first week were from the biochemistry analysis of blood drawn prior to any potential food intake in the morning (fasted at least for 8 hours overnight).
Stress hyperglycaemia: defined as BG ≥ 10.00 mmol/L or ≥ 16.67 mmol/L for non-diabetic and diabetic patients, respectively (21), regardless of insulin treatment status.
Persistent SHG: defined as SHG not resolved after 48 hours of treatment. Patients with early fulminant pancreatitis who died within 48 hours after admission and hence were not able to develop SHG lasting > 48 hours, were included in the group of patients with persistent SHG (28, 29).
Transient SHG: defined as SHG of less than 48 hours duration regardless of insulin treatment.
Pre-existing DM: diagnosed based on disease and medicine history, or serum glycated haemoglobin (HbA1c; ≥ 6.5% or 48 mmol/mol) as per American Diabetes Association (ADA) criteria (27).
HTG-AP: defined as AP with serum triglyceride (TG) levels ≥ 5.65 mmol/L on admission after ruling out common aetiologies (6, 21). Definitions for other aetiologies were as previously described (6, 8, 21, 23, 24).
Outcomes
The primary clinical outcome was persistent organ failure, defined as at least one of the systems (respiratory, circulatory, or renal) having modified Marshall organ failure score ≥ 2 and lasting ≥ 48 hours (25). Secondary outcome measures included multiple organ dysfunction syndrome (MODS) (2 or more systems), acute necrotic collection (25), major infection (presence of infected pancreatic necrosis, sepsis and/or pneumonia) with microbiological and/or imaging evidences (30), mortality followed up for 3 months, and length of hospital stay (LOHS).
Statistical analysis
Continuous data are displayed as medians with 25th-75th percentile and compared using Mann–Whitney U test, Kruskal-Wallis H test, or Cuzick’s trend test analyses. Categorical data are expressed as number with percentages and compared using Chi-square test (or Fisher’s test), linear trend test or proportional trend test analyses.
Multivariate logistic regression analysis was used to report categorical outcome measures and expressed as odds ratios (ORs) with 95% confidence intervals (CIs). Cox proportional hazards analysis was used to report LOHS and expressed as hazard ratios (HRs) with 95% CI (days for deceased patients were considered as truncated data). In both multivariate logistic regression and Cox proportional hazards analyses, baseline factors including age, or those of important clinical significance were adjusted. To quantify the effect of unmeasured potential confounding factors, we report the E-value, which represents the minimum strength of association on the risk ratio scale that an unmeasured confounder would need to have with both the exposure (with SHG) and the outcomes to fully explain away an association between the two (31). Survival differences between the duration of SHG were performed using the log rank test and plotted on Kaplan–Meier curve and adjusted using the methods of marginal balancing in groups. A two-sided P < 0.05 was considered statistically significant. Statistical analyses were performed using SPSS® 26.0 (IBM, Armonk, New York, USA). The E-value and 95% CI were calculated using an online calculator: https://www.evalue-calculator.com/evalue/ (31, 32).
Results
The demographics and clinical outcomes are shown in Supplementary Table 1. Of the included 1792 patients, the median age was 47 years (38–57), with 1135 (63.3%) were male and 245 (13.7%) had pre-existing DM. Persistent organ failure developed in 373 (20.8%) and 81 (4.5%) were MODS. Of 1510 patients had CT scan, 419 (27.7%) had acute necrotic collection. Major infection was diagnosed in 141 (7.9%) of all patients and 43 died (2.4%), all occurred in those who had persistent organ failure/MODS. The overall median LOHS was 9 days (6–14). We found these two cohorts had the same distribution of the primary outcome persistent organ failure and other important clinical outcomes such as MODS, acute necrotic collection, major infection and mortality (all P > 0.05) of the present study design, albeit with the demographic, aetiologies, and admission clinical severity scoring systems varied.
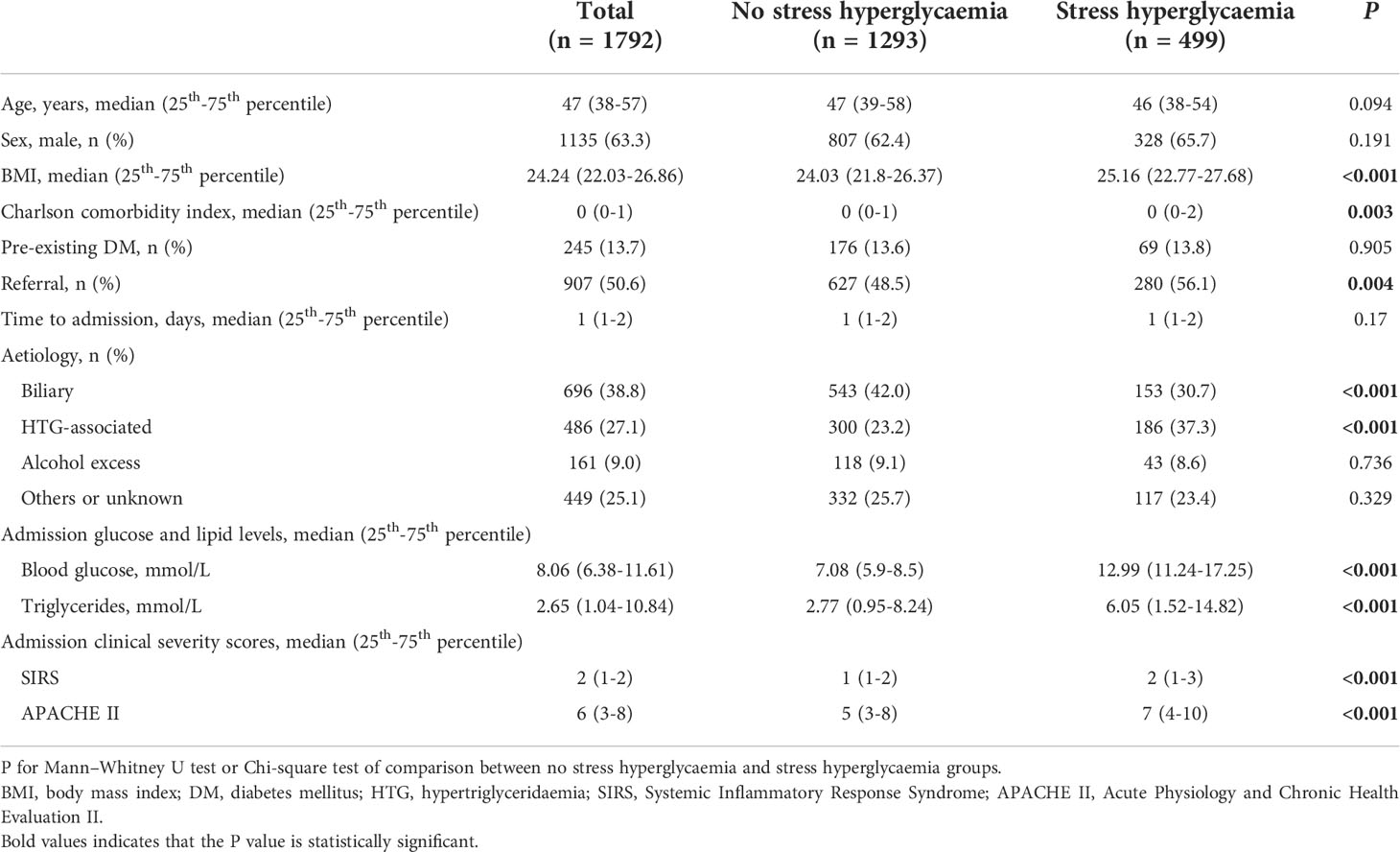
SHG on admission and impact on clinical outcomes
SHG on admission was present in 499 (27.8%) patients (Table 1). There were no statistical differences in age, gender, pre-existing DM and time to admission between those with and without SHG. There were significant differences in BMI, Charlson comorbidity index, tertiary cases, admission TG, HTG-associated aetiology (37.3% vs 23.2%), and clinical severity scores (all P < 0.05) between patients with and without SHG.
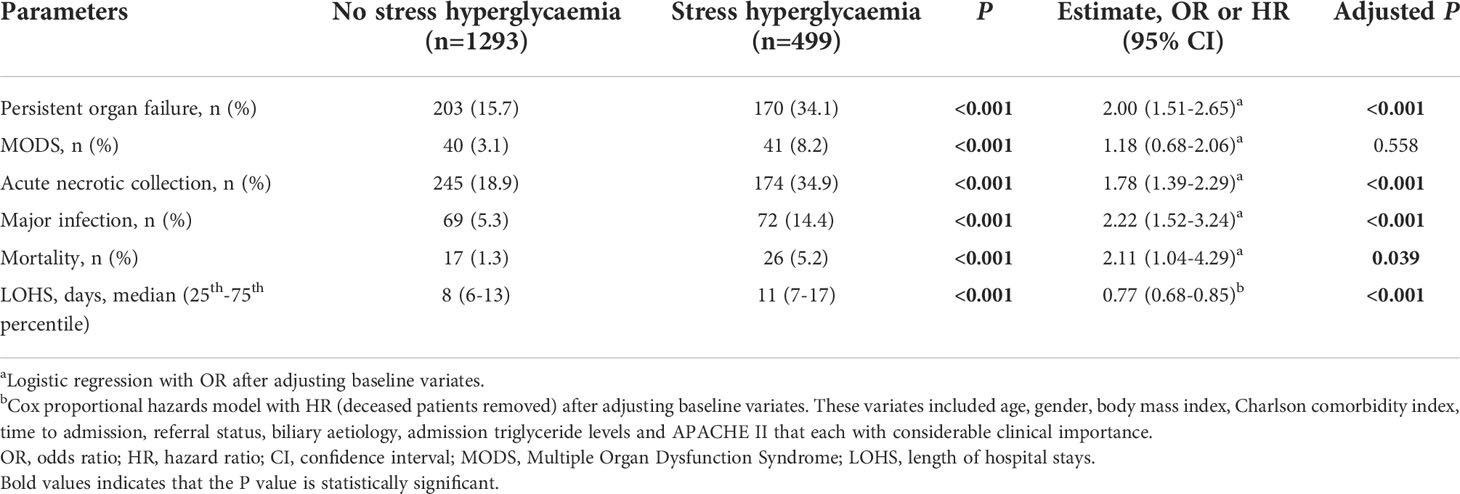
Results for comparing clinical outcomes between patients with and without admission SHG are shown in Table 2. These were adjusted for baseline parameters including age, gender, BMI, Charlson comorbidity index, time to admission, referral status, biliary aetiology, admission TG levels and APACHE II score. Patients with admission SHG had significantly worse clinical outcomes: persistent organ failure (OR 2.00, 95% CI 1.51-2.65), acute necrotic collection (OR 1.78, 95%CI 1.39-2.29), major infection (OR 2.22, 95%CI 1.52-3.24), and mortality (OR 2.11, 95%CI 1.04-4.29) (all adjusted P < 0.05), corresponded to E-values of 2.18, 2.00, 3.87 and 3.64, respectively; the respective E-values for the low 95% CIs were 1.76, 1.64, 2.41 and 1.24. Admission SHG was also significant associated with increased LOHS (HR 0.77, 95%CI 0.68-0.85) when compared with those without SHG, E-value with the high 95% CI were 1.92 and 1.63.
Duration of SHG and impact on clinical outcomes
Transient SHG was found in 556 (31%) and persistent SHG in 144 (8.0%) patients (139 were non-diabetic) and comparison between these two groups is demonstrated in Supplementary Table 2. There were no significant differences between these groups for age, gender, and alcohol aetiology (all P > 0.05). In patients with increased duration of SHG there was a significant increase in BMI, HTG aetiology, admission BG and TG levels, and clinical severity scores (all P < 0.001).
Persistent SHG was associated with more severe AP compared with transient SHG or no SHG groups (both P < 0.001). There were no significant differences between transient SHG and no SHG groups (Figure 1A). There were 10 (0.9%), 20 (3.6%) and 13 (9.0%) deaths in no, transient and persistent SHG patients, respectively, followed a significant step-wise increase with duration of hyperglycaemia (all Log rank P < 0.001; Figure 1B), even after adjusting for baseline parameters including age, gender, BMI, Charlson comorbidity index, time to admission, referral status, biliary aetiology, and admission TG levels (all P < 0.001; Figure 1C).
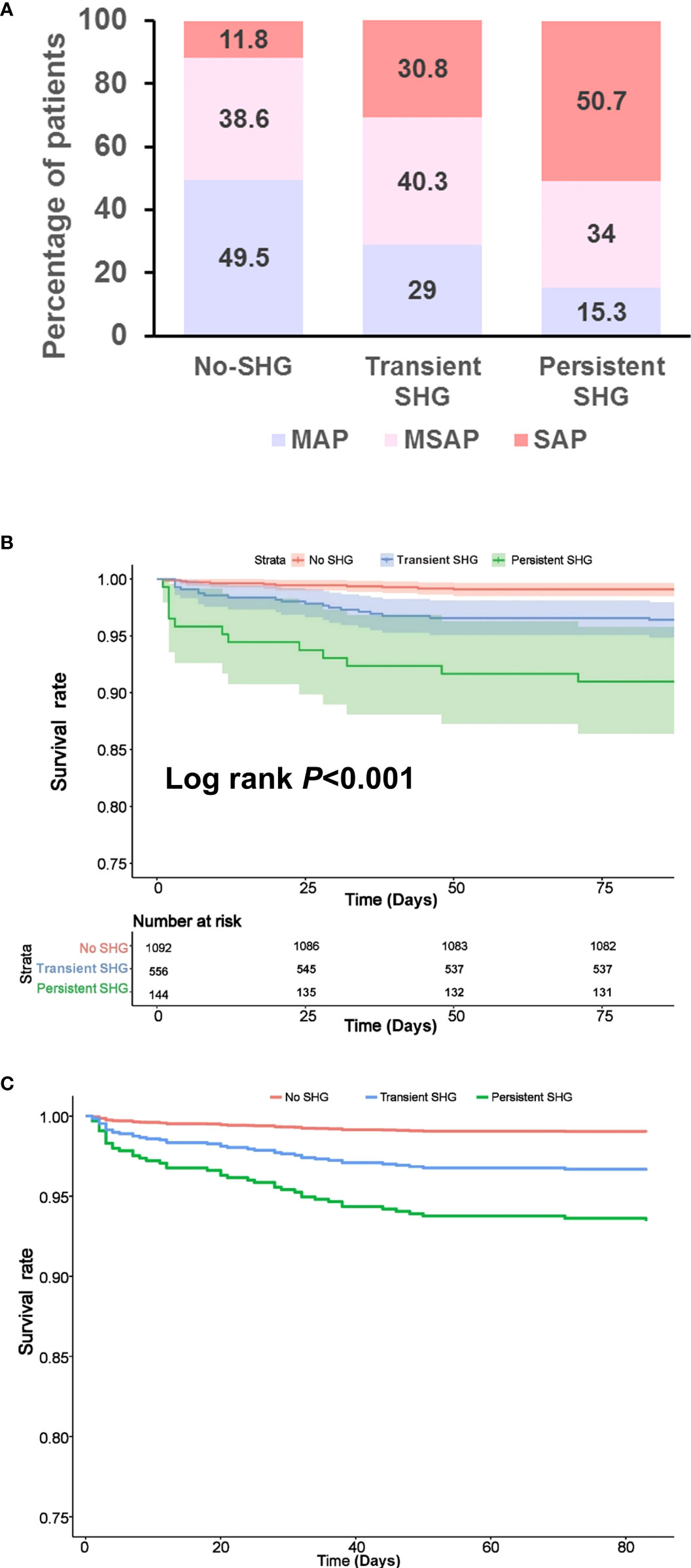
Figure 1 Features of patients stratified by duration of stress hyperglycaemia during the first week of admission. (A) Severity classification. (B) Kaplan–Meier survival curve. (C) Survival curve after adjusting for age, gender, BMI, Charlson comorbidity index, time to admission, referral status, biliary aetiology and admission TG levels. SHG, stress hyperglycaemia; MAP, mild acute pancreatitis; MSAP, moderately severe acute pancreatitis; SAP, severe acute pancreatitis.
The comparison of clinical outcomes between patients with no, transient, and persistent SHG was adjusted age, gender, BMI, Charlson comorbidity index, time to admission, referral status, biliary aetiology, admission TG levels, and APACHE II using multivariate analysis (adjusted OR or HR). Across these 3 groups there was a step-wise increase in the incidence of persistent organ failure, MODS, acute necrotic collection, major infection, and mortality with corresponding prolonged LOHS (all Ptrend < 0.05; Figure 2). In addition, the clinical outcomes were worse in patients with persistent SHG compared with those with admission SHG (Supplementary Figure 2).
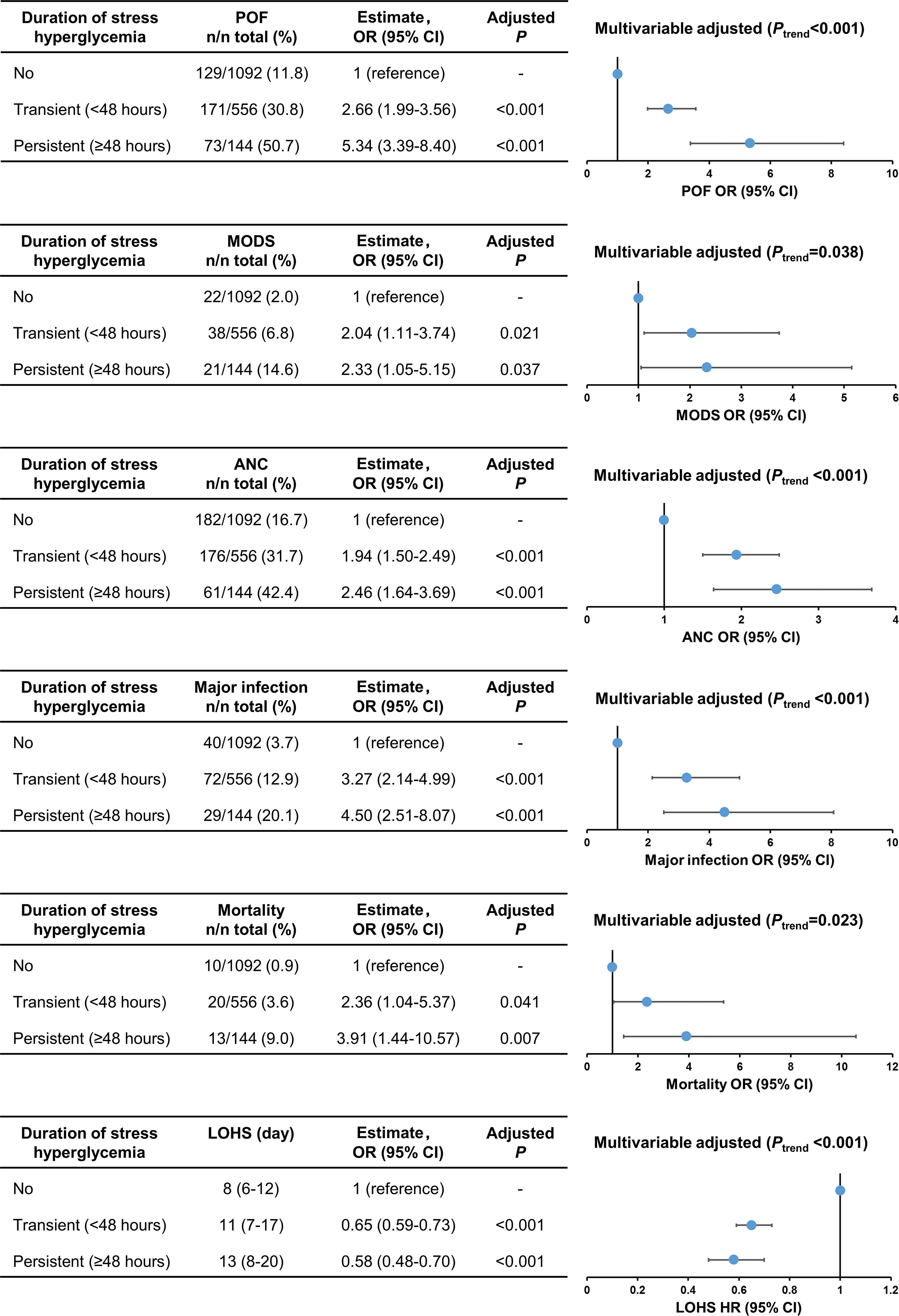
Figure 2 Trend analysis for clinical outcomes stratified by duration of stress hyperglycaemia. POF, persistent organ failure; MODS, Multiple Organ Dysfunction Syndrome; ANC, acute necrotic collection; LOHS, length of hospital stays.
Relationship between SHG and HTG and impact on clinical outcomes
There was a significant positive association between admission BG and TG levels (rs = 0.297, P < 0.001; Supplementary Figure 3A). The incidence of SHG in AP patients with HTG was significantly higher than those with non-HTG (39.2% vs 21.3%, P < 0.001; Supplementary Figure 3B).
Subgroup analyses compared the impact of admission SHG on clinical outcomes between non-HTG and HTG-associated AP patients. The clinical outcomes were worse in non-HTG-AP patients with admission SHG than those without SHG (Table 3, upper panel). The same was true for HTG-AP patients (Table 3, lower panel). These results were confirmed after adjusting for age, gender, BMI, Charlson comorbidity index, time to admission, and referral status (Table 3).
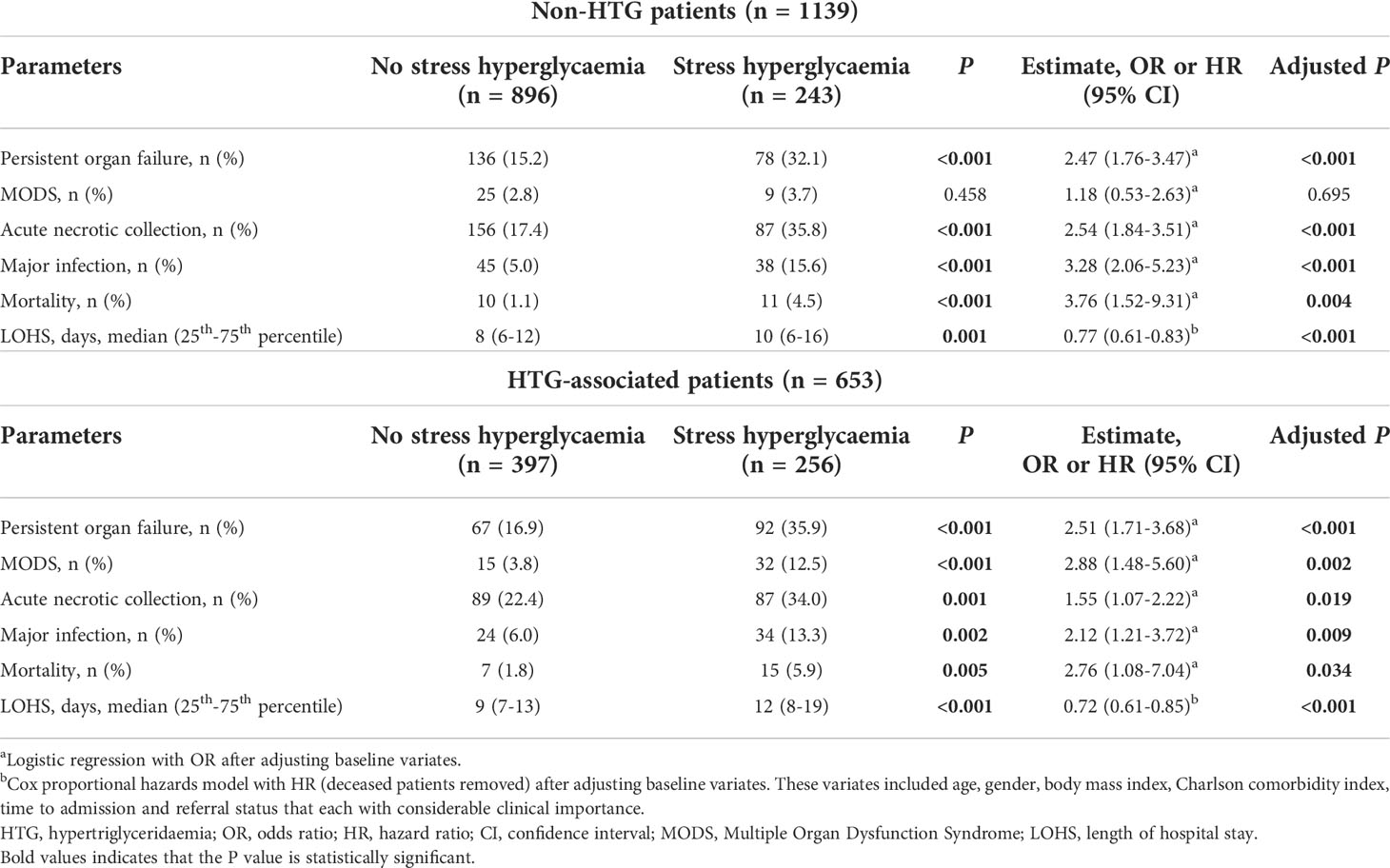
Table 3 Clinical outcomes of patients stratified by stress hyperglycaemia in non-HTG and HTG groups on admission.
Discussion
In this study, we have validated the newly defined SHG in AP patients showing a step-wise relationship between increased admission glucose levels and worsened clinical outcomes. This study also demonstrates that AP patients can be classified according to glycaemic status during the disease early phase (first week) into no, transient, and persistent SHG which were associated with escalating disease severity and adverse clinical outcomes; clinical outcomes were worse in patients with persistent SHG compared with those with admission SHG. Furthermore, the association of SHG with adverse clinical outcomes remained robust in HTG-AP patients.
A large number of studies have focused on the probability of DM after pancreatitis and its possible mechanism (11, 12, 33–35). Few have studied on BG changes under stress status and its impact on the severity or outcomes of AP patients (21). The pathogenesis of SHG during critical illness is complex, including increased release of counter-regulatory hormones, altered insulin receptor signalling due to inflammation, pancreatic beta-cell inhibition and interventions such as administration of glucocorticoids or parenteral nutrition (36, 37). How glucose metabolism is affected by derangement of adrenaline, glucagon, cortisol, and insulin remains to be elusive due to lack of comparable studies, it is relatively clearer that hyperglycaemia in AP mainly due to both impairment of beta-cells resulting a decrease in insulin secretion and the production of cytokines. This causes the appearance/worsening of insulin resistance and subsequently induces hyperglycaemia, which, in turn, may further damage beta-cells and worsen insulin resistance observed in critical illness (16). In this study, we also further demonstrate that AP patients with SHG often had higher BMI and TG levels, suggesting that well accepted risk factors for DM also contribute to the development of SHG.
While the definition of SHG varied among studies (36, 38), the strength of the association between each glucose parameter and outcome emphasises the significance of this relationship. SHG is associated with a significantly elevated risk of mortality in ICU patients (16). A recently meta-analysis demonstrated that COVID-19 patients with hyperglycaemia (BG ≥ 7, 7.7, 10, or 11 mmol/L) also had a higher risk of developing severe or critical illness compared with normoglycaemia patients regardless of prior DM conditions (39). Similarly, we optimised SHG as BG ≥ 10.00 mmol/L and ≥ 16.67 mmol/L for AP patients without pre-existing diabetes and those with pre-existing diabetes, respectively, according to multivariate logistic regression and ROC curves) on admission (21). Here, we further verified these admission BG cut-off values and confirmed a step-wise relationship between increased glucose levels and worsened clinical outcomes in AP patients, consistent with our previous findings. Sensitivity analyses revealed that it would take very strong confounding to negate the associations observed in this study. These observations are the same when analysing the two composition cohorts of varied baseline characteristics and aetiologies separately (data not shown).
Persistent hyperglycaemia is a common parameter used to evaluate blood glucose fluctuations, and our finding of their association with adverse clinical outcomes in AP patients is similar to that in acute ischaemic stroke, acute myocardial infarction, intracerebral haemorrhage, and other critical illness (28, 29, 40, 41). On the other hand, the early phase (first week) in severe AP patients is often accompanied by persistent SIRS which develops to persistent organ failure/MODS, serving as the predominate cause of death (8, 42). Duration of organ failure during the first week had proved to be strongly associated with the risk of local complications and death (8, 43). Therefore, in the current study, we also studied dynamic nature of glucose changes and their association with clinical outcomes during the first week in AP patients. Persistent SHG was defined as the SHG persisted over 48 hours as other disease definitions (28, 29), and we newly defined transient SHG for those SHG presented but less than 48 hours referred to the definition time of whether the organ failure in AP persists or not (25). There was a stepwise increase of adverse clinical outcomes in patients with no, transient, and persistent SHG. And the increase was more pronounced with persistent SHG than with admission SHG. This means that once SHG occurred during the first week of admission, the risk of all adverse clinical outcomes increased, and the longer the duration of SHG, the more serious the clinical outcome would be. However, we cannot directly establish the cause-effect relationship claiming that SHG aggravated the severity of AP in the current study. Whether SHG worsens clinical outcomes of AP patients warrant further basic and clinical research.
HTG has been reported as the aetiology of AP in more than a third of cases in some large Chinese AP cohorts (6–8). This may be due to an increasing prevalence of central obesity (44) and/or metabolic syndrome in Chinese populations (45). We and others have previously shown that admission TG levels are associated with worse clinical outcomes in AP patients (6, 46), but these analyses did not account for co-existing SHG. We took a subgroup analysis for HTG-AP and found the similar adverse effect of SHG on the outcomes as for the whole cohort of AP. These findings highlight a close interaction between glucose haemostasis and lipid metabolism (47, 48). This is an important finding because it provides justification for strategies to lower both glucose and TG in the acute management of AP. A recent meta-analysis of observational studies supported the use of insulin for the early management of HTG-AP patients (49). Recently, a compelling experimental study suggests that endogenous insulin protected pancreatic acinar cells during AP by preserving glycolytic ATP supply to calcium pumps (50). Therefore, insulin can protect against AP by both acting on acinar cells and hormone-sensitive lipase (preventing release of free fatty acids from TG) and this warrants clinical trials.
Our study has several limitations. Firstly, as the nature of post hoc analysis, the BG data were not available for every day (data were mainly missing on day 4 and day 6), which may cause the lower proportion of transient SHG than it actually was. Secondly, only more than half of the patients had HbA1c measured on admission and thus we most likely underestimate the prevalence of pre-existing DM in out cohorts. Thirdly, we could not determine from both databases what the timing of and which treatment strategies affected the glucose levels. Therefore, we did not try to perform an analysis of glycaemic variability. Further research with individual-level data on treatment type and timing may help clarify these questions. Finally, we did not investigate the impact of admission and duration of SHG on the probability of developing post-pancreatitis DM, which will comprise a separate study.
Conclusion
In conclusion, we demonstrate that the admission and duration of SHG had important impact on development adverse clinical outcomes in AP patients. Screening, monitoring, and targeting AP patients with high-risk of developing SHG may have beneficial clinical implication.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
Author contributions
XY and NS contributed equally to this study. XY, NS, WHe, LL, YL, LY, LD, TJ, NL, and YZ: acquisition of data. XY, NS, and WHu: drafting of manuscript. XY, NS, WHe, PZ, TL, LD, TJ, and WHu: analysis and interpretation of data. SheL and PZ: statistical analysis supervision. ShiL, RS, and JW: important intelligence input. RS, JW, YZ, and QX: critical revision of the manuscript. WHu, QX, and YZ: study concept and design, obtained funding, study supervision. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by National Natural Science Foundation of China (No. 81973632, No. 82270672, WHu; No. 81774120, QX; No. 81960128, YZ; No. 82100682, NS); Key Research and Development Programme of Science and Technology Department of Sichuan Province (2022YFS0406, XY; 2020YFS0235, NS); China Postdoctoral Science Foundation (2022M712285, XY; 2022T150453, XY); NIHR Senior Investigator Award (RS).
Acknowledgments
These authors thank all the staff from the pancreas multidisciplinary teams at West China Hospital of Sichuan University and First Affiliated Hospital of Nanchang University for their continuous support.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2022.998499/full#supplementary-material
Supplementary Figure 1 | Patient selection flow chart. (A) Chengdu cohort. (B) Nanchang cohort. AP, acute pancreatitis; CP, chronic pancreatitis.
Supplementary Figure 2 | Adverse clinical outcomes in patients with admission and persistent stress hyperglycaemia. SHG, stress hyperglycaemia; POF, persistent organ failure; MODS, multiple organ dysfunction syndrome; ANC, acute necrotic collection.
Supplementary Figure 3 | Glucose and lipid metabolism disorder on admission in AP patients. (A) Correlation between glucose and triglycerides. (B) Admission stress hyperglycaemia in HTG and non-HTG AP patients. TG, triglycerides; MAP, mild acute pancreatitis; MSAP, moderately severe acute pancreatitis; SAP, severe acute pancreatitis; No-SHG, no stress hyperglycaemia; SHG, stress hyperglycaemia.
References
1. Moggia E, Koti R, Belgaumkar AP, Fazio F, Pereira SP, Davidson BR, et al. Pharmacological interventions for acute pancreatitis. Cochrane Database Syst Rev (2017) 4:CD011384. doi: 10.1002/14651858.CD011384.pub2
2. Peery AF, Crockett SD, Murphy CC, Lund JL, Dellon ES, Williams JL, et al. Burden and cost of gastrointestinal, liver, and pancreatic diseases in the united states: Update 2018. Gastroenterology (2019) 156(1):254–72 e11. doi: 10.1053/j.gastro.2018.08.063
3. Cho J, Petrov MS. Pancreatitis, pancreatic cancer, and their metabolic sequelae: Projected burden to 2050. Clin Transl Gastroenterol (2020) 11(11):e00251. doi: 10.14309/ctg.0000000000000251
4. Boxhoorn L, Voermans RP, Bouwense SA, Bruno MJ, Verdonk RC, Boermeester MA, et al. Acute pancreatitis. Lancet (2020) 396(10252):726–34. doi: 10.1016/S0140-6736(20)31310-6
5. Hines OJ, Pandol SJ. Management of severe acute pancreatitis. BMJ (2019) 367:l6227. doi: 10.1136/bmj.l6227
6. Zhang R, Deng L, Jin T, Zhu P, Shi N, Jiang K, et al. Hypertriglyceridaemia-associated acute pancreatitis: Diagnosis and impact on severity. HPB (Oxford) (2019) 21(9):1240–9. doi: 10.1016/j.hpb.2019.01.015
7. Ding Y, Zhang M, Wang L, Yin T, Wang N, Wu J, et al. Association of the hypertriglyceridemic waist phenotype and severity of acute pancreatitis. Lipids Health Dis (2019) 18(1):93. doi: 10.1186/s12944-019-1019-2
8. Shi N, Liu T, de la Iglesia-Garcia D, Deng L, Jin T, Lan L, et al. Duration of organ failure impacts mortality in acute pancreatitis. Gut (2020) 69(3):604–5. doi: 10.1136/gutjnl-2019-318241
9. Petrov MS, Yadav D. Global epidemiology and holistic prevention of pancreatitis. Nat Rev Gastroenterol Hepatol (2019) 16(3):175–84. doi: 10.1038/s41575-018-0087-5
10. Hart PA, Bradley D, Conwell DL, Dungan K, Krishna SG, Wyne K, et al. Diabetes following acute pancreatitis. Lancet Gastroenterol Hepatol (2021) 6(8):668–75. doi: 10.1016/S2468-1253(21)00019-4
11. Das SL, Singh PP, Phillips AR, Murphy R, Windsor JA, Petrov MS. Newly diagnosed diabetes mellitus after acute pancreatitis: A systematic review and meta-analysis. Gut (2014) 63(5):818–31. doi: 10.1136/gutjnl-2013-305062
12. Zhi M, Zhu X, Lugea A, Waldron RT, Pandol SJ, Li L. Incidence of new onset diabetes mellitus secondary to acute pancreatitis: A systematic review and meta-analysis. Front Physiol (2019) 10:637. doi: 10.3389/fphys.2019.00637
13. Ali Abdelhamid Y, Kar P, Finnis ME, Phillips LK, Plummer MP, Shaw JE, et al. Stress hyperglycaemia in critically ill patients and the subsequent risk of diabetes: A systematic review and meta-analysis. Crit Care (2016) 20(1):301. doi: 10.1186/s13054-016-1471-6
14. Marik PE, Bellomo R. Stress hyperglycemia: An essential survival response! Crit Care Med (2013) 41(6):e93–4. doi: 10.1097/CCM.0b013e318283d124
15. Smith FG, Sheehy AM, Vincent JL, Coursin DB. Critical illness-induced dysglycaemia: Diabetes and beyond. Crit Care (2010) 14(6):327. doi: 10.1186/cc9266
16. Badawi O, Waite MD, Fuhrman SA, Zuckerman IH. Association between intensive care unit-acquired dysglycemia and in-hospital mortality. Crit Care Med (2012) 40(12):3180–8. doi: 10.1097/CCM.0b013e3182656ae5
17. Kennedy JI, Askelund KJ, Premkumar R, Phillips AR, Murphy R, Windsor JA, et al. Leptin is associated with persistence of hyperglycemia in acute pancreatitis: A prospective clinical study. Med (Baltimore) (2016) 95(6):e2382. doi: 10.1097/MD.0000000000002382
18. Berglund L, Brunzell JD, Goldberg AC, Goldberg IJ, Sacks F, Murad MH, et al. Evaluation and treatment of hypertriglyceridemia: An endocrine society clinical practice guideline. J Clin Endocrinol Metab (2012) 97(9):2969–89. doi: 10.1210/jc.2011-3213
19. Li J, Du H, Wang Y, Aertgeerts B, Guyatt G, Hao Q, et al. Safety of proprotein convertase subtilisin/kexin 9 inhibitors: A systematic review and meta-analysis. Heart (2022) 108(16):1296–302. doi: 10.1136/heartjnl-2021-320556
21. Yang X, Zhang R, Jin T, Zhu P, Yao L, Li L, et al. Stress hyperglycemia is independently associated with persistent organ failure in acute pancreatitis. Dig Dis Sci (2021) 67(5):1879–89. doi: 10.1007/s10620-021-06982-8
22. von Elm E, Altman DG, Egger M, Pocock SJ, Gotzsche PC, Vandenbroucke JP, et al. Strengthening the reporting of observational studies in epidemiology (Strobe) statement: Guidelines for reporting observational studies. BMJ (2007) 335(7624):806–8. doi: 10.1136/bmj.39335.541782.AD
23. Li L, Jin T, Wen S, Shi N, Zhang R, Zhu P, et al. Early rapid fluid therapy is associated with increased rate of noninvasive positive-pressure ventilation in hemoconcentrated patients with severe acute pancreatitis. Dig Dis Sci (2020) 65(9):2700–11. doi: 10.1007/s10620-019-05985-w
24. Yu B, He WH, Lu N. Risk factors for acute kidney injury in acute pancreatitis: A 7-year retrospective analysis of patients in a Large tertiary hospital. Pancreas (2020) 49(8):1057–62. doi: 10.1097/MPA.0000000000001613
25. Banks PA, Bollen TL, Dervenis C, Gooszen HG, Johnson CD, Sarr MG, et al. Classification of acute pancreatitis–2012: Revision of the Atlanta classification and definitions by international consensus. Gut (2013) 62(1):102–11. doi: 10.1136/gutjnl-2012-302779
26. Iap/Apa evidence-based guidelines for the management of acute pancreatitis. Pancreatology (2013) 13(4 Suppl 2):e1–15. doi: 10.1016/j.pan.2013.07.063
27. American Diabetes A. 15. diabetes care in the hospital: Standards of medical care in diabetes-2019. Diabetes Care (2019) 42(Suppl 1):S173–S81. doi: 10.2337/dc19-S015
28. van der Horst IC, Nijsten MW, Vogelzang M, Zijlstra F. Persistent hyperglycemia is an independent predictor of outcome in acute myocardial infarction. Cardiovasc Diabetol (2007) 6:2. doi: 10.1186/1475-2840-6-2
29. Fuentes B, Ortega-Casarrubios MA, Sanjose B, Castillo J, Leira R, Serena J, et al. Persistent hyperglycemia >155 Mg/Dl in acute ischemic stroke patients: How well are we correcting it?: implications for outcome. Stroke (2010) 41(10):2362–5. doi: 10.1161/STROKEAHA.110.591529
30. Bakker OJ, van Brunschot S, van Santvoort HC, Besselink MG, Bollen TL, Boermeester MA, et al. Early versus on-demand nasoenteric tube feeding in acute pancreatitis. N Engl J Med (2014) 371(21):1983–93. doi: 10.1056/NEJMoa1404393
31. VanderWeele TJ, Ding P. Sensitivity analysis in observational research: Introducing the e-value. Ann Intern Med (2017) 167(4):268–74. doi: 10.7326/M16-2607
32. Mathur MB, Ding P, Riddell CA, VanderWeele TJ. Web site and r package for computing e-values. Epidemiology (2018) 29(5):e45–e7. doi: 10.1097/EDE.0000000000000864
33. Shen HN, Yang CC, Chang YH, Lu CL, Li CY. Risk of diabetes mellitus after first-attack acute pancreatitis: A national population-based study. Am J Gastroenterol (2015) 110(12):1698–706. doi: 10.1038/ajg.2015.356
34. Bharmal SH, Cho J, Alarcon Ramos GC, Ko J, Stuart CE, Modesto AE, et al. Trajectories of glycaemia following acute pancreatitis: A prospective longitudinal cohort study with 24 months follow-up. J Gastroenterol (2020) 55(8):775–88. doi: 10.1007/s00535-020-01682-y
35. Pendharkar SA, Singh RG, Bharmal SH, Drury M, Petrov MS. Pancreatic hormone responses to mixed meal test in new-onset Prediabetes/Diabetes after non-necrotizing acute pancreatitis. J Clin Gastroenterol (2020) 54(2):e11–20. doi: 10.1097/MCG.0000000000001145
36. Dungan KM, Braithwaite SS, Preiser JC. Stress hyperglycaemia. Lancet (2009) 373(9677):1798–807. doi: 10.1016/S0140-6736(09)60553-5
37. Deane AM, Horowitz M. Dysglycaemia in the critically ill - significance and management. Diabetes Obes Metab (2013) 15(9):792–801. doi: 10.1111/dom.12078
38. Inzucchi SE. Clinical practice. management of hyperglycemia in the hospital setting. N Engl J Med (2006) 355(18):1903–11. doi: 10.1056/NEJMcp060094
39. Lee MH, Wong C, Ng CH, Yuen DCW, Lim AYL, Khoo CM. Effects of hyperglycaemia on complications of covid-19: A meta-analysis of observational studies. Diabetes Obes Metab (2021) 23(1):287–9. doi: 10.1111/dom.14184
40. Faustino EV, Apkon M. Persistent hyperglycemia in critically ill children. J Pediatr (2005) 146(1):30–4. doi: 10.1016/j.jpeds.2004.08.076
41. Wu TY, Putaala J, Sharma G, Strbian D, Tatlisumak T, Davis SM, et al. Persistent hyperglycemia is associated with increased mortality after intracerebral hemorrhage. J Am Heart Assoc (2017) 6(8):e005760. doi: 10.1161/JAHA.117.005760
42. Garg PK, Singh VP. Organ failure due to systemic injury in acute pancreatitis. Gastroenterology (2019) 156(7):2008–23. doi: 10.1053/j.gastro.2018.12.041
43. Johnson CD, Abu-Hilal M. Persistent organ failure during the first week as a marker of fatal outcome in acute pancreatitis. Gut (2004) 53(9):1340–4. doi: 10.1136/gut.2004.039883
44. Zhang L, Wang Z, Wang X, Chen Z, Shao L, Tian Y, et al. Prevalence of abdominal obesity in China: Results from a cross-sectional study of nearly half a million participants. Obes (Silver Spring) (2019) 27(11):1898–905. doi: 10.1002/oby.22620
45. Li R, Li W, Lun Z, Zhang H, Sun Z, Kanu JS, et al. Prevalence of metabolic syndrome in mainland China: A meta-analysis of published studies. BMC Public Health (2016) 16:296. doi: 10.1186/s12889-016-2870-y
46. Nawaz H, O'Connell M, Papachristou GI, Yadav D. Severity and natural history of acute pancreatitis in diabetic patients. Pancreatology (2015) 15(3):247–52. doi: 10.1016/j.pan.2015.03.013
47. Guilherme A, Henriques F, Bedard AH, Czech MP. Molecular pathways linking adipose innervation to insulin action in obesity and diabetes mellitus. Nat Rev Endocrinol (2019) 15(4):207–25. doi: 10.1038/s41574-019-0165-y
48. Kopchick JJ, Berryman DE, Puri V, Lee KY, Jorgensen JOL. The effects of growth hormone on adipose tissue: Old observations, new mechanisms. Nat Rev Endocrinol (2020) 16(3):135–46. doi: 10.1038/s41574-019-0280-9
49. He W, Cai W, Camilleri G, Yao L. Insulin versus blood purification in the early management of hypertriglyceridaemia-associated acute pancreatitis: Systemic review and meta-analysis. 2020 EPC/IAP S1424-3903(22):00469-0 abstract. doi: 10.1016/j.pan.2020.07.108
Keywords: acute pancreatitis, blood glucose, stress hyperglycaemia, hypertriglyceridaemia, clinical outcomes
Citation: Yang X, Shi N, Yao L, He W, Zhu P, Li S, Li L, Li Y, Liu S, Deng L, Jin T, Liu T, Lu N, Windsor JA, Sutton R, Zhu Y, Xia Q and Huang W (2022) Impact of admission and early persistent stress hyperglycaemia on clinical outcomes in acute pancreatitis. Front. Endocrinol. 13:998499. doi: 10.3389/fendo.2022.998499
Received: 20 July 2022; Accepted: 20 September 2022;
Published: 07 October 2022.
Edited by:
Sonia Michael Najjar, Ohio University, United StatesReviewed by:
Jason I. E. Bruce, The University of Manchester, United KingdomMaryam Zahedi, Shahid Beheshti University of Medical Sciences, Iran
Copyright © 2022 Yang, Shi, Yao, He, Zhu, Li, Li, Li, Liu, Deng, Jin, Liu, Lu, Windsor, Sutton, Zhu, Xia and Huang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wei Huang, ZHJfd2VpX2h1YW5nQHNjdS5lZHUuY24=; Qing Xia, eGlhcWluZ0BtZWRtYWlsLmNvbS5jbg==; Yin Zhu, bmR5ZnkwMTk3N0BuY3UuZWR1LmNu
†These authors have contributed equally to this work and share first authorship
 Xinmin Yang1†
Xinmin Yang1† Wenhua He
Wenhua He Sheyu Li
Sheyu Li Tingting Liu
Tingting Liu Nonghua Lu
Nonghua Lu John A. Windsor
John A. Windsor Robert Sutton
Robert Sutton Wei Huang
Wei Huang