
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
OPINION article
Front. Endocrinol., 21 September 2022
Sec. Reproduction
Volume 13 - 2022 | https://doi.org/10.3389/fendo.2022.996531
This article is part of the Research TopicOvarian Ageing: Pathophysiology and Recent Development of Maintaining Ovarian Reserve: Volume IIView all 7 articles
Ovarian reserve depletion secondary to an intact endometrioma continues to be actively debated (1, 2) and has been recently challenged (3). Conversely, endometriotic cyst surgery, specifically endometriotic ovarian cystectomy, seems to have an irreversible damaging impact and is of concern for women and practitioners alike. Past histological studies have demonstrated inadvertent primordial follicle removal adjacent to the endometrioma, which seems inevitable even in experienced hands (4, 5). Furthermore, well-performed systematic reviews and meta-analyses have resulted in a significant irreversible reduction in serum AMH levels (3, 6), suggesting potentially lasting damage to the reproductive life span.
Recently, our group has shown a significant drop in serum AMH levels by 1.65 ng/ml (95% CI: 1.15 to 2.15) and by 2.03 ng/mL (95% CI: 1.47 to 2.58) at 9-12 months postoperatively as compared to baseline in the unilateral and bilateral ovarian endometriotic cystectomy groups, respectively, corresponding to 39% and 57% decline of the functional ovarian reserve following surgery (3). Furthermore, we have shown that AMH is a much more sensitive biomarker than AFC in this setting (7). Since our publication considered studies that employed the stripping technique (3), more conservative and less invasive modalities of endometrioma treatment impact on ovarian reserves, such as ultrasound-guided sclerotherapy or laser vaporization, ought to be further explored (8, 9).
The impact of endometrioma cystectomy on ovarian reserve argues that these women need fertility preservation counseling. However, the main question remains whether fertility preservation should be recommended to every woman with ovarian endometriosis considering surgery and planning for a future pregnancy and whether preoperative serum AMH levels are essential for adopting this strategy.
At present, many papers suggest fertility preservation, particularly oocyte vitrification, in women with intact endometrioma considering surgery and postponement of pregnancy. Interestingly, most of these publications are opinion papers and experts’ viewpoints. At the same time, only a few cohort studies of actual oocyte vitrification in women with endometriosis have been published (10–16). Although ovarian tissue cryopreservation is suggested in this setting, only a few case reports have been published so far (17). The utility of such an approach in this setting needs further assessment.
Overall, although the true benefit of fertility preservation in women with endometriosis remains unknown, the recent guidelines of the ESHRE endometriosis core group recommend discussing the pros and cons of such practice, especially in women with extensive ovarian endometriosis (18).
Among published papers on oocyte vitrification in women with endometriosis, all retrospectively conducted (Table 1), the most comprehensive so far is from Spain (12). It contains 1044 women with intact endometrioma that underwent oocyte preservation, of which 43% came back to thaw their gametes to attain pregnancy. The main findings of this study suggest oocyte preservation before 35 years of age and before endometriotic surgery to obtain significantly better oocyte yield and cumulative live birth rate (CLBR) (12). A recent report by a different group from France (n = 146) corroborates these findings and advocates integrating fertility preservation into endometriosis management (16).
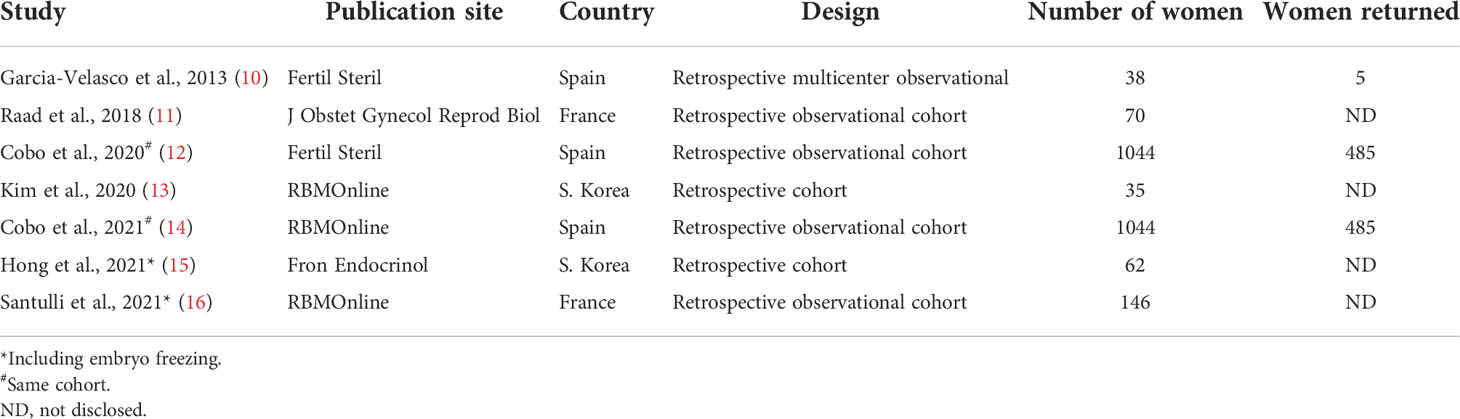
Table 1 Fertility preservation (Oocyte Vitrification) in Cases with Endometriosis: Summary of the Literature.
The relevance of preoperative serum AMH levels in predicting the likelihood of pregnancy attainment or the need for fertility preservation is unclear since studies reporting on this topic have made inconsistent conclusions (18).
Counseling for oocyte preservation in women with ovarian endometriosis may depend on several composite and interrelated factors: age, preoperative serum AMH level, endometrioma diameter and laterality, uni-or multicystic display, pelvic anatomy and adhesions, surgical skills, techniques, and complexity, hemostasis methods, and previous surgery. While some of these factors may be readily assessed before surgery, others would not.
While the decrease in functional ovarian reserve following endometriotic cystectomy is exemplified by statistically significant serum AMH levels reduction, the range in AMH reduction among individual-operated women seems vast. Some women may exhibit a substantial serum AMH reduction, while others may show a minor decrease in serum AMH levels. In many cases, the magnitude of serum AMH decrease is unpredictable. Nowadays, there is no means to predict individual AMH reduction since many interrelated measurable and non-measurable confounders are involved.
Extreme damage to ovarian reserve, causing premature ovarian insufficiency, has been shown at an advanced reproductive age following bilateral endometriotic cystectomy and repeat surgery (19–21). However, it occurs only in 2.4% of cases (19), while the impact on the ovarian reserve is unanticipated in other women.
To quantify the wide variation of AMH reduction following endometriotic cystectomy, we have looked again into eligible studies in our recent systematic review examining the impact of endometriotic cystectomy on serum AMH levels (3). Twelve studies were eligible for meta-analysis, including 783 women: 489 and 294 in the unilateral and bilateral groups, respectively. This time, we calculated the coefficient of variation (CV) in serum AMH reduction following endometriotic cystectomy at the early (1 – 4 weeks), intermediate (6 weeks – 6 months), and late (9 – 12 months) postoperative periods. The CV is the ratio of the standard deviation to the mean difference of serum AMH values. The higher the coefficient of variation, the greater the level of dispersion around the mean, suggesting a wide variation of postoperative serum AMH reduction. The CV among unilateral and bilateral groups in all three time periods, except in the late bilateral group, was substantial, ranging from 35% to 73% (Table 2), implying a vast and unpredictable span of serum AMH reduction following surgery. The modest CV in the bilateral cases during the late postoperative period is not surprising. There is an agreement that bilateral endometriotic cystectomy is constantly associated with significant harm to the ovarian reserve (22–24). Collectively, these results imply that whatever the pre-surgical serum AMH levels, the variation in post-surgical AMH levels reduction is vast, rendering them from being an obligatory requisite for fertility preservation counseling.
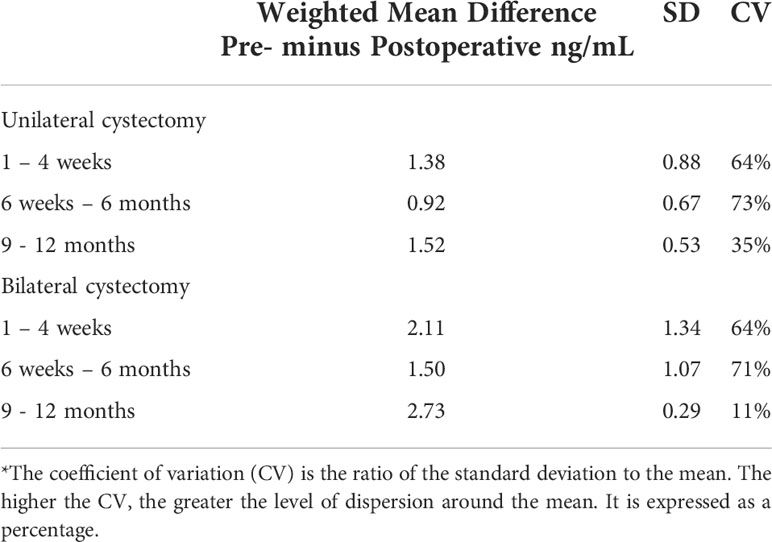
Table 2 Weighted mean difference, standard deviation (SD), and coefficient of variation (CV)* between preoperative and postoperative serum AMH levels in the unilateral and bilateral endometriotic cystectomy groups at the early, intermediate, and late postoperative periods.
Although serum AMH level is a robust biomarker of ovarian reserve, its reliability in live-birth prediction in the ART setting is poor (25). Furthermore, age was considered a better predictor than AMH for live-birth achievement (26). Recently, in a nationwide high-order study with a validated database, serum AMH levels were highly correlated with the CLBR in women with low ovarian reserve (AMH < 1 ng/mL) (27). This was mainly related to its association with the quantitative ART cycle outcomes, primarily the number of oocytes retrieved. Furthermore, AMH and female age each independently provided prognostic information regarding the probability of CLBR. Higher AMH levels were associated with greater CLBR for each age category. In women < 35 years of age, the CLBR ranged between 22.1% and 41.2%, stratified with AMH levels in-between < 0.1 and 0.91-1.0 ng/mL, respectively. In contrast, in women 41-42 years of age, the CLBR ranged between 6.1% and 11.1%, stratified with the same AMH levels (27). Accordingly, for women with intact endometrioma with serum AMH levels < 1.0 ng/mL, considering ovarian surgery and planning for future pregnancy, fertility preservation counseling should not be precluded, especially if they are < 35 years of age.
There are no guidelines for fertility preservation in women with endometriosis, especially in women with intact endometrioma, planning for a future pregnancy. Furthermore, the true benefit of fertility preservation in young women with endometriosis remains unknown. Nonetheless, the recent guidelines of the ESHRE endometriosis group recommend discussing the pros and cons of such practice. The topic of fertility preservation in women with endometriosis is evolving, and only a few cohort studies have been published. Although there is a consensus on the harmful impact of endometriotic cystectomy on ovarian reserve, the effect of endometrioma per se on the quantitative and qualitative aspects of the ovarian reserve is still debated (1, 2, 28) and has been recently challenged (3, 29). The present opinion focuses on preoperative AMH levels and whether to be included in counseling women undergoing endometriotic cystectomy, specifically in those with low serum AMH levels.
There is a general agreement that age, endometrioma bilaterality, and previous ovarian surgery are key risk factors for significant serum AMH reduction and the threat of premature ovarian insufficiency in women undergoing endometriotic cystectomy. In most other cases, the degree of AMH reduction following surgery is unpredictable.
Serum AMH levels are reliable measures of functional ovarian reserve, essential for the patient’s counseling, and a good reference for future management, particularly in women postponing pregnancy. However, for women undergoing endometriotic cystectomy, preoperative AMH values do not predict surgical damage to the ovarian reserve. They should not be considered a critical measure to counsel fertility preservation before surgery. After surgery, serum AMH drop has a wide variation through a composite occurrence with numerous case-by-case interrelated factors that are largely unmeasurable and unpredictable. Until developing a reliable measure or score capable of predicting individual postoperative serum AMH reduction, discussing the pros and cons of fertility preservation with every woman planning a pregnancy regardless of her preoperative AMH levels seems plausible. Even in cases with low preoperative serum AMH levels (< 1 ng/mL), practitioners should not avert fertility preservation counseling, since reasonable CLBR may be achieved, notably in women below 35. Such a policy’s economic and psychological implications need further evaluation.
JSY contributed to the conception and design of the manuscript and drafted the article. All authors contributed to data acquisition, analyses, and interpretation and approved the paper.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Benaglia L, Castiglioni M, Paffoni A, Sarais V, Vercellini P, Somigliana E. Eur is endometrioma-associated damage to ovarian reserve progressive? Insights from IVF cycles. J Obstet Gynecol Reprod Biol (2017) 217:101–5. doi: 10.1016/j.ejogrb.2017.08.034
2. Kasapoglu I, Ata B, Uyaniklar O, Seyhan A, Orhan A, Yildiz Oguz S, et al. Endometrioma-related reduction in ovarian reserve (ERROR): A prospective longitudinal study. Fertil Steril (2018) 110:122–7. doi: 10.1016/j.fertnstert.2018.03.015
3. Younis JS, Shapso N, Fleming R, Ben-Shlomo I, Izhaki I. Impact of unilateral versus bilateral ovarian endometriotic cystectomy on ovarian reserve: A systematic review and meta-analysis. Hum Reprod Update (2019) 25:375–91. doi: 10.1093/humupd/dmy049
4. Roman H, Tarta O, Pura I, Opris I, Bourdel N, Marpeau L, et al. Direct proportional relationship between endometrioma size and ovarian parenchyma inadvertently removed during cystectomy, and its implication on the management of enlarged endometriomas. Hum Reprod (2010) 25:1428–32. doi: 10.1093/humrep/deq069
5. Muzii L, Marana R, Angioli R, Bianchi A, Cucinella G, Vignali M, et al. Histologic analysis of specimens from laparascopic endometrioma excision performed by different surgeons: Does the surgeon matter? Fertil Steril (2011) 95:2116–9. doi: 10.1016/j.fertnstert.2011.02.034
6. Raffi F, Metwally M, Amer S. The impact of excision of ovarian endometrioma on ovarian reserve: A systematic review and meta-analysis. J Clin Endocrinol Metab (2012) 97:3146–54. doi: 10.1210/jc.2012-1558
7. Younis JS, Shapso N, Ben-Sira Y, Nelson SM, Izhaki I. Endometrioma surgery-a systematic review and meta-analysis of the effect on antral follicle count and anti-müllerian hormone. Am J Obstet Gynecol (2022) 226:33–51.e7. doi: 10.1016/j.ajog.2021.06.102
8. Candiani M, Ottolina J, Posadzka E, Ferrari S, Castellano LM, Tandoi I, et al. Assessment of ovarian reserve after cystectomy versus ‘one-step’ laser vaporization in the treatment of ovarian endometrioma: a small randomized clinical trial. Hum Reprod (2018) 33:2205–11. doi: 10.1093/humrep/dey305
9. Kim GH, Kim PH, Shin JH, Nam IC, Chu HH, Ko HK. Ultrasound-guided sclerotherapy for the treatment of ovarian endometrioma: An updated systematic review and meta-analysis. Eur Radiol (2022) 32:1726–37. doi: 10.1007/s00330-021-08270-5
10. Garcia-Velasco JA, Domingo J, Cobo A, Martínez M, Carmona L, Pellicer A. Five years’ experience using oocyte vitrification to preserve fertility for medical and nonmedical indications. Fertil Steril (2013) 99:1994–9. doi: 10.1016/j.fertnstert.2013.02.004
11. Raad J, Sonigo C, Tran C, Sifer C, Durnerin IC, Grynberg M. Oocyte vitrification for preserving fertility in patients with endometriosis: first observational cohort study and many unresolved questions. Letter to Editor Eur J Obstet Gynecol Reprod Biol (2018) 220:140–1. doi: 10.1016/j.ejogrb.2017.12.001
12. Cobo A, Giles J, Paolelli S, Pellicer A, Remohí J, García-Velasco JA. Oocyte vitrification for fertility preservation in women with endometriosis: an observational study. Fertil Steril (2020) 113:836–44. doi: 10.1016/j.fertnstert.2019.11.017
13. Kim SJ, Kim SK, Lee JR, Suh CS, Kim SH. Oocyte cryopreservation for fertility preservation in women with ovarian endometriosis. Reprod BioMed Online (2020) 40:827–34. doi: 10.1016/j.rbmo.2020.01.028
14. Cobo A, Coello A, de Los Santos MJ, Giles J, Pellicer A, Remohí J, et al. Number needed to freeze: Cumulative live birth rate after fertility preservation in women with endometriosis. Reprod BioMed Online (2021) 42:725–32. doi: 10.1016/j.rbmo.2020.12.013
15. Hong YH, Lee HK, Kim SK, Lee JR, Suh CS. The significance of planned fertility preservation for women with endometrioma before an expected ovarian cystectomy. Front Endocrinol (Lausanne) (2021) 12:794117. doi: 10.3389/fendo.2021.794117
16. Santulli P, Bourdon M, Koutchinsky S, Maignien C, Marcellin L, Maitrot-Mantelet L, et al. Fertility preservation for patients affected by endometriosis should ideally be carried out before surgery. Reprod BioMed Online (2021) 43:853–63. doi: 10.1016/j.rbmo.2021.08.023
17. Calagna G, Della Corte L, Giampaolino P, Maranto M, Perino A. Endometriosis and strategies of fertility preservation: A systematic review of the literature. Eur J Obstet Gynecol Reprod Biol (2020) 254:218–25. doi: 10.1016/j.ejogrb.2020.09.045
18. Becker CM, Bokor A, Heikinheimo O, Horne A, Jansen F, Kiesel L, King K, Kvaskoff M, Nap A, Petersen K, Saridogan E, Tomassetti C, van Hanegem N, Vulliemoz N, Vermeulen N, et al. ESHRE Endometriosis Guideline Group. Hum Reprod Open. (2022), (2):hoac009. doi: 10.1093/hropen/hoac009
19. Busacca M, Riparini J, Somigliana E, Oggioni G, Izzo S, Vignali M, et al. Postsurgical ovarian failure after laparoscopic excision of bilateral endometriomas. Am J Obstet Gynecol (2006) 195:421–5. doi: 10.1016/j.ajog.2006.03.064
20. Coccia ME, Rizzello F, Mariani G, Bulletti C, Palagiano A, Scarselli G. Ovarian surgery for bilateral endometriomas influences age at menopause. Hum Reprod (2011) 26:3000–7. doi: 10.1093/humrep/der286
21. Takae S, Kawamura K, Sato Y, Nishijima C, Yoshioka N, Sugishita Y, et al. Analysis of late-onset ovarian insufficiency after ovarian surgery: Retrospective study with 75 patients of post-surgical ovarian insufficiency. PloS One (2014) 9:e98174. doi: 10.1371/journal.pone.0098174
22. Alborzi S, Keramati P, Younesi M, Samsami A, Dadras N. The impact of laparoscopic cystectomy on ovarian reserve in patients with unilateral and bilateral endometriomas. Fertil Steril (2014) 101:427–34. doi: 10.1016/j.fertnstert.2013.10.019
23. Shao MJ, Hu M, He YQ, Xu XJ. AMH trend after laparoscopic cystectomy and ovarian suturing in patients with endometriomas. Arch Gynecol Obstet (2016) 293:1049–52. doi: 10.1007/s00404-015-3926-4
24. Kovačević VM, Anđelić LM, Mitrović Jovanović A. Changes in serum antimüllerian hormone levels in patients 6 and 12 months after endometrioma stripping surgery. Fertil Steril (2018) 110:1173–80. doi: 10.1016/j.fertnstert.2018.07.019
25. Iliodromiti S, Kelsey TW, Wu O, Anderson RA, Nelson SM. The predictive accuracy of anti-müllerian hormone for live birth after assisted conception: a systematic review and meta-analysis of the literature. Hum Reprod Update (2014) 20:560–70. doi: 10.1093/humupd/dmu003
26. Broer SL, Broekmans FJ, Laven JS, Fauser BC. Anti-müllerian hormone: Ovarian reserve testing and its potential clinical implications. Hum Reprod Update (2014) 20:688–701. doi: 10.1093/humupd/dmu020
27. Tal R, Seifer DB, Tal R, Granger E, Wantman E, Tal O. AMH highly correlates with cumulative live birth rate in women with diminished ovarian reserve independent of age. J Clin Endocrinol Metab (2021) 106:2754–66. doi: 10.1210/clinem/dgab168
28. Sanchez AM, Viganò P, Somigliana E, Panina-Bordignon P, Vercellini P, Candiani M. The distinguishing cellular and molecular features of the endometriotic ovarian cyst: From pathophysiology to the potential endometrioma-mediated damage to the ovary. Hum Reprod Update (2014) 20:217–30. doi: 10.1093/humupd/dmt053
Keywords: endometriosis, endometrioma, fertility preservation, anti-Müllerian hormone, endometriotic cystectomy
Citation: Younis JS, Shapso N and Izhaki I (2022) Is ovarian reserve reduction following endometriotic cystectomy predicted? The implication for fertility preservation counseling. Front. Endocrinol. 13:996531. doi: 10.3389/fendo.2022.996531
Received: 17 July 2022; Accepted: 07 September 2022;
Published: 21 September 2022.
Edited by:
Qi Chen, The University of Auckland, New ZealandReviewed by:
Fatih Aktoz, Hacettepe University, TurkeyCopyright © 2022 Younis, Shapso and Izhaki. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Johnny S. Younis, anlvdW5pc0Bwb3JpYS5oZWFsdGguZ292Lmls
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.