- 1Department of Neuroscience, Rehabilitation, Ophthalmology, Genetics, Maternal and Child Health, University of Genoa, Genoa, Italy
- 2Department of Pediatrics, IRCCS Istituto Giannina Gaslini, Genoa, Italy
- 3Epidemiology and Biostatistics Unit, IRCCS Istituto Giannina Gaslini, Genoa, Italy
Objective: Tandem Control-IQ is an advanced hybrid closed loop (AHCL) system with a Sleep Activity Mode to intensify glycemic control overnight. The aim of the study is to evaluate the effectiveness of using Sleep Mode or not among Tandem Control-IQ users.
Research design and methods: We performed a retrospective Tandem Control-IQ data download for patients followed at IRCCS G. Gaslini Pediatric Diabetes Centre. We divided the patients into group 1 (Sleep Mode users) and group 2 (non-users) and compared their overall glycemic data, particularly during nighttime.
Results: Group 1 (n = 49) does not show better nocturnal glycemic control as expected when compared with group 2 (n = 34). Group 2 shows a nighttime TIR% of 69.50 versus 66.25 (p = 0.20). Only the patients who do not use Sleep Mode and with sensor and automatic mode use ≥90% reached TIR >70% during nighttime, as well as lower nocturnal TAR% (18.80 versus 21.78, p = 0.05).
Conclusions: This is the first study that evaluates the real-life effectiveness of the use of Sleep Mode in young patients with T1D. Control-IQ Sleep Activity Mode may not be as effective in Italian patients as in American patients due to the different habits.
Introduction
The management of type 1 diabetes (T1D) has changed substantially in the last few years. New technologies allow the improvement of glycemic control by reducing the risk of hypoglycemia and hyperglycemia and decrease the rate of diabetes complications (1–3). Since the FDA approved the first hybrid closed loop (HCL) system, further advanced devices which integrate insulin infusion with continuous glucose monitoring (CGM) have been commercialized (4–6).
The goal of advanced hybrid closed loop (AHCL) technology is to reduce the burden of managing diabetes by automatically adjusting insulin delivery based on CGM data. Using CGM data, AHCL systems predict glucose values and adjust insulin delivery in order to keep glycemic values in a target range (7, 8).
The Tandem t:slim X2 insulin pump (Tandem Inc., San Diego, CA, USA) uses a Dexcom G6 sensor (Dexcom Inc., San Diego, CA, USA) and a closed loop algorithm (Control-IQ™) that automates basal insulin delivery and correction boluses, prevents and protects against hypoglycemia, and intensifies control overnight (9).
The Control-IQ system works based on well-defined target and treatment ranges with the aim of implementing the time spent in the recommended target range. When the predicted glucose value in the following 30 min is between 112.5 and 160 mg/dl, the pump delivers the basal insulin rate based on the active personal profile. When the predicted glucose value is <112.5 mg/dl, Control-IQ technology decreases personal insulin delivery rate and completely stops basal insulin delivery when predicted glucose values are below 70 mg/dl. When the predicted glucose value is above 160 mg/dl, the pump increases basal insulin delivery and delivers an automatic correction bolus if the predicted value is greater than 180 mg/dl. The system is able to deliver a maximum of one correction bolus per hour (reduced by 60% compared with the calculated).
Control-IQ technology has two integrated modes to optimize glycemic control during the night and during exercise; these modes can be activated and deactivated manually or scheduled by the patient. The Sleep Activity Mode works on a target range of 112.5–120 mg/dl instead of 112.5–160 mg/dl. When the predicted glucose value is >120 mg/dl, the pump increases the delivery of basal insulin, but it does not deliver correction boluses.
Currently, good glycemic control is defined on the basis of CGM data by the International Consensus as follows: time in range (TIR) (70–180 mg/dl) >70%, time below range (TBR) (<70 mg/dl) <4%, TBR (<54 mg/dl) <1%, time above range (TAR) (>180 mg/dl) <25%, and TAR (>250 mg/dl) <1% (10, 11).
Data from the first studies on the Control-IQ system in children and adults with type 1 diabetes have shown encouraging results in terms of glycemic outcomes and patient satisfaction (12, 13). Several multicenter randomized trials and real-life studies in children, adolescents, and adults demonstrated the efficacy of the Control-IQ technology when compared to the sensor-augmented pump, Basal-IQ technology, and other AHCL systems (14–20). The Control-IQ technology has been shown to be effective in terms of patient satisfaction, improvement of quality of life and quality of sleep for patients and parents, ease of use, and improvement of positive emotions (21–24).
Most studies have shown that the improvement in time in range is better overnight, which is consistent with the Control-IQ algorithm design (12–16, 19). Despite the evidence on the efficacy of the overnight system, there are no clinical studies evaluating the effectiveness of using or not using Sleep Mode among Tandem Control-IQ users.
The aim of this study was to compare glycemic control (globally and overnight) between Sleep Mode users and non-users in a cohort of children and young patients with type 1 diabetes using Control-IQ technology.
Materials and methods
Study design and study population
This was a retrospective study conducted in the Regional Reference Centre for Pediatric Diabetes, Istituto Giannina Gaslini, Genoa, Italy, a tertiary care pediatric hospital of Liguria, northwest Italy.
Patients were enrolled according to the following inclusion criteria: T1D diagnosis at least 1 year prior to the study, Tandem Control-IQ use for at least 1 month, and data download from February to May 2022 during an outpatient visit or a telemedicine visit. The exclusion criteria were as follows: percentage of use of automatic mode and/or sensor less than 80%, infections, or major changes in the usual lifestyle in the 14 days prior to data download (traveling, holidays, sickness).
Data collection
During the first routine follow-up visit in which the inclusion criteria were met, the following data were collected for each patient: demographic data (sex, date of birth, age), age at clinical onset of T1D, duration of disease, time of use of the Control-IQ system, CGM data of the 14 days before the checkup, bolus time, and average consumption of carbohydrates (CHO) at dinner in the previous 14 days.
Study outcomes
We divided the patients into two groups: group 1 (users of Sleep Mode for at least 6 h a night) and group B (non-users of Sleep Mode). During the Tandem Control-IQ training at our center, the Sleep Mode function is explained to all patients; then, they independently choose whether to set Sleep Mode or not. The following parameters were compared between the two groups: TIR, TAR, TAR >250 mg/dl, TBR, TBR <54 mg/dl, coefficient of variation (CV), standard deviation (SD), mean glucose value, glucose management indicator (GMI), percentage of sensor use, percentage of time in automatic mode, and percentage of time spent in Sleep Mode. We also compared the following data relating only to the night period (from 0 a.m. to 6 a.m.) between the two groups: TIR, TAR, TAR >250 mg/dl, TBR, TBR <54 mg/dl, CV, SD, and mean glucose value.
In addition, we decided to restrict the data analysis to patients who used sensor and automatic mode for a percentage of time greater than 90%, in order to select patients with the best possible use of the Control-IQ algorithm.
Considering the retrospective nature of the study, the informed consent form already signed by parents and/or patients at disease onset and renewed yearly, in which they agree on the use of clinical data for research purposes, was used. In addition, all parents and patients provided a specific informed consent form for the collection of data. The study was conducted in accordance with the Declaration of Helsinki and the International Conference on Harmonization Good Clinical Practice.
Statistical analysis
Data are described as mean and SD or median and range for continuous variables and as absolute and relative frequencies for categorical variables.
Non-parametric analysis (Mann–Whitney U test) for continuous variables and the chi-square or Fisher’s exact test for categorical variables were used to measure differences between groups. p-values ≤0.05 were considered statistically significant, and all p-values were based on two-tailed tests. Statistical analysis was performed using SPSS for Windows (SPSS Inc., Chicago, IL, USA).
Results
Data from a total of 110 T1D patients using Tandem Control-IQ (aged 4 to 35 years) were retrospectively collected at the IRCCS G. Gaslini Pediatric Diabetes Centre. We excluded 27 patients: 13 did not perform a visit or data download in the study period, 6 were diagnosed with diabetes in the previous year, 7 had become Tandem Control-IQ users less than a month prior to the beginning of the study period, and 1 used Sleep Mode for less than 6 h. We collected data of the remaining 83 T1D patients: 49 of these patients (group 1) used Sleep Mode and 34 (group 2) did not use it. Most patients of group 1 (n = 42) had scheduled Sleep Mode between 11 p.m. and 7 a.m.; the remaining 7 patients had scheduled it at different times between 10 p.m. and 8 a.m. and always for a duration of at least 6 h.
No significant differences were found in the clinical and demographic characteristics of the patients belonging to the two groups, with the exception of the percentage of nighttime sensor use, which was greater than 95% in both groups, as well as the duration of AHCL use (328.39 ± 111.34 days in group 1 and 181.50 ± 150.83 days in group 2, p = 0.001). Particularly, in our study population, the mean time of bolus for dinner was 8:17 p.m., and the mean number of carbohydrates consumed at dinner was 69.31 g; no significant differences were observed between the two groups for these meal parameters. The characteristics of the study population are summarized in Table 1.
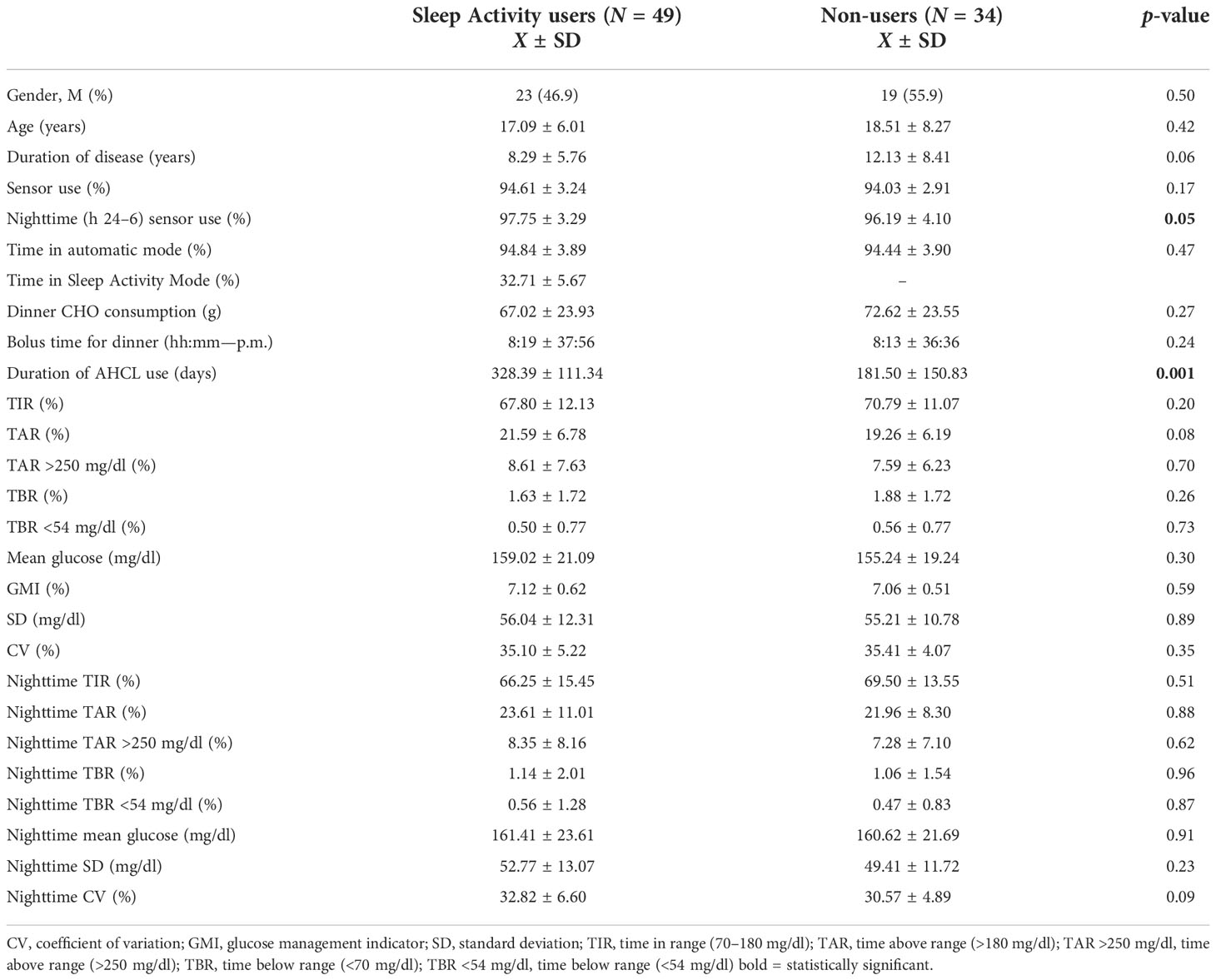
Table 1 Comparison of the overall and nighttime (h 24–6) glycemic control of Sleep Activity Mode users (group 1) and non-users (group 2); analysis included patients using sensor and automatic mode ≥80% (N = 83).
The differences in overall and nocturnal glycemic control between the two groups are shown in Table 1. Group 1 had a similar TIR% compared with group 2 both overall (67.80 ± 12.13 versus 70.79 ± 11.07, p = 0.20) and during nighttime (66.25 ± 15.45 versus 69.50 ± 13.55, p = 0.51).
Limiting the analysis to patients with percentage of time of sensor use and automatic mode use ≥90% (N of patients = 71), data confirmed a similar TIR% in group 1 compared with group 2 overall (68.00 ± 12.81 versus 71.97 ± 9.58, p = 0.20) and during nighttime (66.52 ± 15.76 versus 70.77 ± 12.46, p = 0.43). A statistically significant difference between the two groups in terms of TAR% (21.78 ± 7.10 in group 1 versus 18.80 ± 5.94 in group 2, p = 0.05) was observed (Table 2).
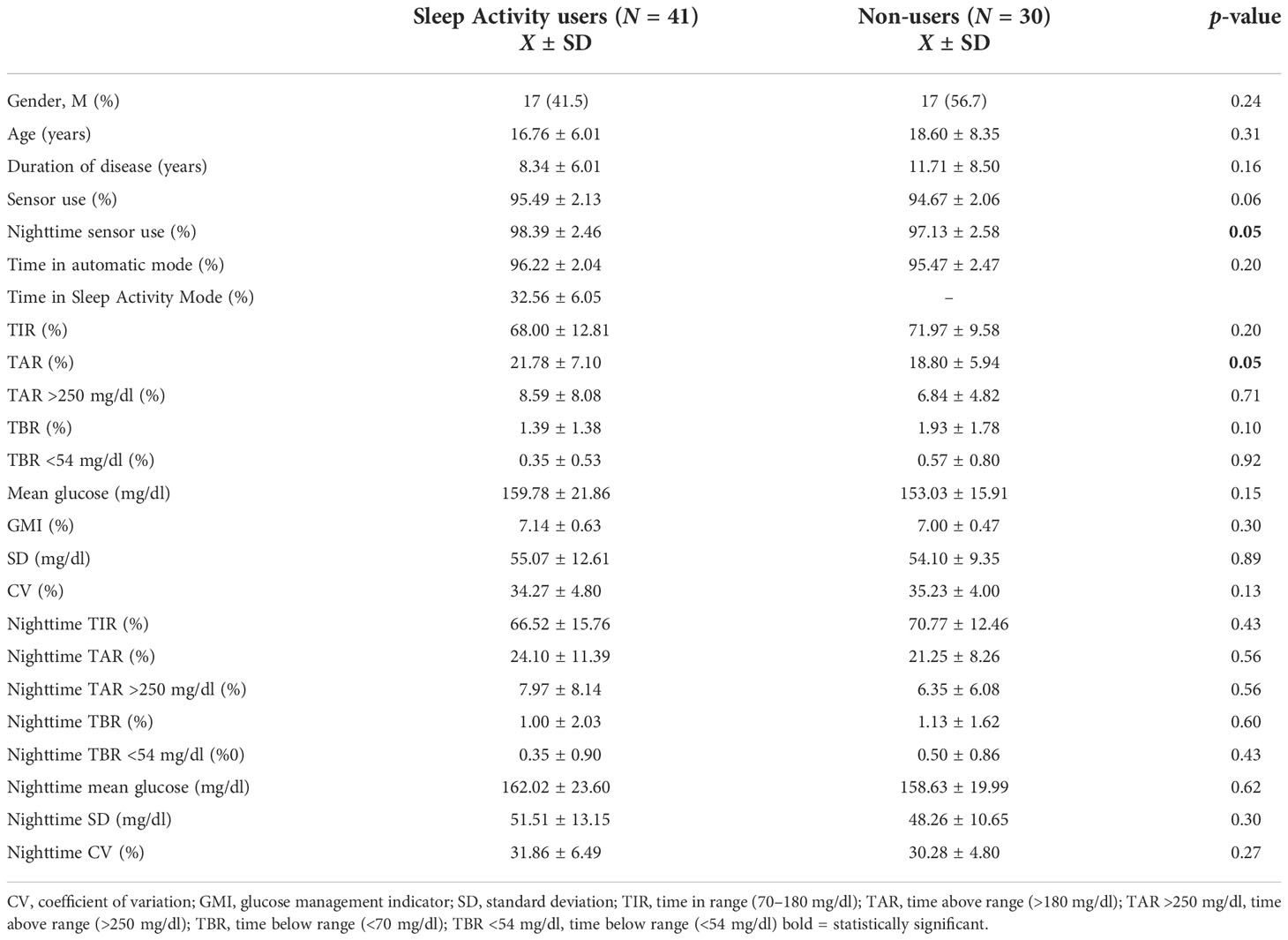
Table 2 Comparison of the overall and nighttime (h 24-6–) glycemic control of Sleep Activity Mode users (group 1) and non-users (group 2); analysis included patients using sensor and automatic mode ≥90% (N = 71).
No statistically significant differences in terms of TBR, CV, SD, mean glucose, and GMI were found between the two groups in either the original or the restricted analysis (Tables 1, 2). Further stratifying the analysis between age groups (<18 and ≥18 years), no significant differences were found for all the nocturnal parameters analyzed.
Comparing the patients’ nighttime TIR (TIR ≥70%, TIR 50%–70%, and TIR <50%), data showed that the percentage of patients that reach the recommended target of TIR ≥70% is 46.9% in group 1 and 58.8% in group 2. Patients who have a nighttime TIR lower than 50% are 20.4% of group 1 and 5.9% of group 2. Restricting the analysis to patients who used automatic mode and sensor for more than 90% of the time, 19.5% of the patients in group 1 and 3.3% in group 2 (p = 0.09) had nocturnal TIR <50% (Tables 3, 4; Figure 1).

Table 3 Comparison by category of nighttime TIR between Sleep Activity Mode users (group 1) and non-users (group 2); analysis included patients using sensor and automatic mode ≥80% (N = 83).

Table 4 Comparison by category of nighttime TIR between Sleep Activity Mode users (group 1) and non-users (group 2); analysis included patients using sensor and automatic mode ≥90% (N = 71).
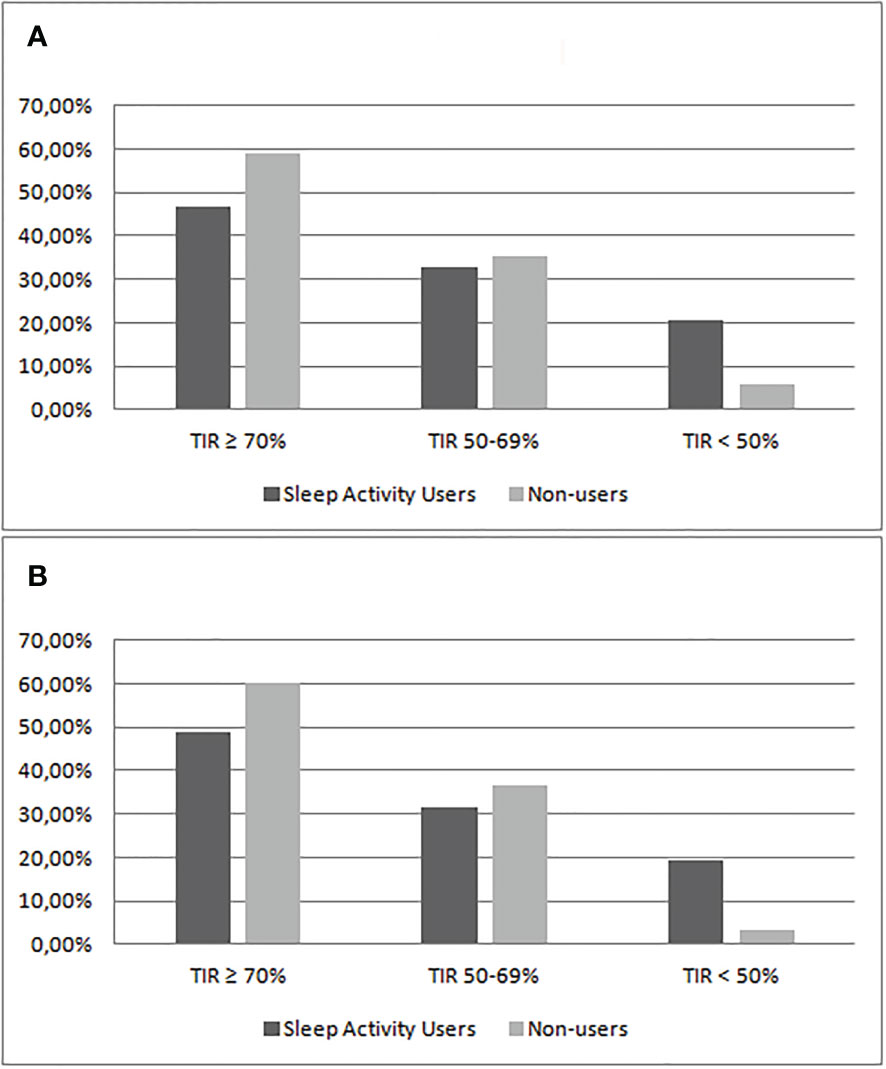
Figure 1 Comparison of the percentage of patients divided by categories of nighttime TIR between Sleep Activity Mode users (group 1) and non-users (group 2). The analysis included patients using sensor and automatic mode ≥80% (A) or ≥90% (B).
Discussion
The aim of this study was to compare real-life glycemic control data between Tandem Control-IQ Sleep Mode Users and non-users. To the best of our knowledge, this is the first study to compare the overnight effectiveness of the Tandem Control-IQ Sleep Mode compared with the Standard Control-IQ algorithm.
The Control-IQ system has been shown to be effective in glycemic control and has been appreciated by patients since the first studies on children and adults with type 1 diabetes (12, 13). Several multicenter randomized trials in children, adolescents, and adults demonstrated the efficacy of Control-IQ compared with sensor-augmented pumps, showing an improvement in TIR without increasing hypoglycemia (14–16). A recent study in children demonstrates an improvement of TIR with Control-IQ in comparison with Basal-IQ, a predictive low-glucose suspend (PLGS) algorithm (17). A single multicenter study that compared AHCL systems currently approved for the pediatric population showed the non-inferiority of the efficacy of Tandem Control-IQ in reaching glycemic targets compared with the other systems (18). Recent studies on the real-world use of the Tandem Control-IQ system confirmed the conclusions reached by the pivotal trials and previous studies. The use of Control-IQ technology increased time in range at 12 months in a sample of 7,813 patients (19) and at 6 months in a sample of 191 youth patients with type 1 diabetes (20).
Most studies on the effectiveness of the Control-IQ System included additional analysis focusing on the overnight period; all of these studies have shown that the algorithm is more effective on TIR during nighttime (12–16, 19). Forlenza et al. observed significative improvement of TIR overnight (from 11 p.m. to 7 a.m.) in the Control-IQ group compared with the sensor-augmented pump (SAP) group (Control-IQ: 74.9%–10.1% vs. SAP: 49.6%–18.8%; p = 0.001), with an overall TIR of 71.0% in Control-IQ users (12). In the first randomized multicenter trial of closed loop control (CLC) in T1D, Brown et al. showed that TIR was 70% in the closed loop group and 59% in the control group during the daytime (6 a.m. to midnight) and was 76% and 59%, respectively, during the nighttime (midnight to 6 a.m.) (13). Comparing CLC with SAP, Breton et al. observed a daytime (6 a.m. to midnight) TIR of 63% in the closed loop group and 56% in the control group, and the corresponding values during the nighttime (midnight to 6:00 a.m.) were 80% and 54% (14). Kanapka et al. also observed a better improvement in TIR overnight (midnight to 6 a.m.), while Isganaitis et al. showed an improvement in TIR in the Control-IQ group compared with the SAP group especially between 1 a.m. and 8 a.m. (+19% of TIR at night and +11% during the day, p < 0.0001) (15, 16). Recently, Breton et al. showed a profound TIR increase at night, reaching a median >90% between 4 and 7 a.m. in T1D Control-IQ users in a 1-year real-world study (19).
Despite the evidence on the efficacy of the system overnight, to date, there are no studies that evaluate the effectiveness of using Sleep Mode or not among Tandem Control-IQ users.
We decided to download the data during the visits that took place between February and May 2022. The choice to download data only in a specific time window derives from the need to avoid as much as possible substantial differences in the life habits of patients related to the pandemic situation and to the seasonal habits and to exclude Italian prolonged periods of holidays (e.g., Christmas or summer holidays); in particular, schools, sports, and extracurricular activities were open in Italy during the study period. Patients followed at the IRCCS G. Gaslini Pediatric Diabetes Centre are both children and young adults (up to 35 years), and this is the reason for the age range of the study population. Despite the wide spectrum of the age of the patients included, stratifying the analysis by age, no significant differences emerged in the parameters analyzed compared with the entire population.
We defined nighttime as the period between midnight and 6 a.m., according to most of the studies that evaluated the effectiveness of Tandem Control-IQ overnight (13–15); all the scheduled Sleep Mode set by the group 1 patients included this time range. The study had predefined inclusion criteria of time in connectivity in closed loop control and CGM of at least 80% overall and during nighttime; a lower percentage of use of the closed loop technology would not allow to evaluate the algorithm and to compare the night mode with the standard mode adequately (8).
Data showed that the use of Sleep Mode does not significantly improve nighttime glycemic control and that the group of non-users surprisingly has a similar overnight TIR (69.50 ± 13.55 versus 66.25 ± 15.45). Further restricting the analysis to patients with an automatic insulin delivery time and sensor use greater than 90%, we also observed that only the group of non-users of Sleep Mode (TIR% 70.77 ± 12.46) reached the recommended TIR (11), and TAR% is surprisingly and significantly reduced in this group (18.80 ± 5.94 versus 21.78 ± 7.10, p = 0.05). The overnight TBR% did not significantly increase in group 2 (TBR 1.06 ± 1.54 versus 1.14 ± 2.01). The mean duration of use of AHCL was longer in patients belonging to group 1 (about 6 versus 11 months in group 2); therefore, the experience of using AHCL can be considered a factor in favor of group 1. These data demonstrate the non-inferiority and safety of non-use of the Control-IQ Sleep Mode (Tables 1, 2).
Furthermore, by stratifying the population by TIR groups, we observed that in patients who do not use the Sleep Mode, the percentage of patients who reached the recommended target of TIR ≥70% is higher compared with users (58.8% versus 46.9%), and the percentage of patients with a nighttime TIR lower than 50% is lower compared with those who use the Sleep Mode (5.9% versus 20.4%). Restricting the analysis to patients using automatic mode and sensor for more than 90% of the time, patients with nocturnal TIR <50% were less in the non-user group (3.3% versus 19.5%) (Tables 3, 4 and Figure 1). These data, despite not reaching statistical significance, once again underline the non-efficacy of the Sleep Mode in our sample of young Italian patients.
The Sleep Mode has a narrower target range (112.5–120 mg/dl) to ensure optimal glucose values during the night and has been shown to perform brilliantly in system efficacy studies and in comparison with SAP. Nevertheless, if Sleep Mode is activated, no corrective boluses are delivered; on the one hand, this feature guarantees the safety of the algorithm during the night; on the other hand, the increase in the basal rate alone may sometimes not be sufficient to quickly bring glucose values back to target values. The Sleep Mode may be more effective when bedtime glucose value is in the target range, while in the case of post-dinner hyperglycemia or the consumption of foods with high-fat content, it may have more difficulty in bringing glucose values back to the target. This particular feature assumes great relevance in a country like Italy, where dinner is served late (usually from 8 p.m. to 9 p.m.), often rich in carbohydrates, and children often go to sleep shortly after dinner consumption. These Italian habits are very different from the American ones, on the basis of which the Control-IQ standard algorithm and activity modes were probably created. All of the system efficacy studies on nocturnal glycemic control were also performed in the USA (12–16, 19).
This is the first study to evaluate the real-life effectiveness of the use of Sleep Mode compared with the Control-IQ standard mode in young patients with T1D, and the results are certainly interesting and challenging. The Control-IQ Sleep Mode may not be effective in Italian patients due to the different habits compared with American patients. The limitations of the study included the low number of patients, the retrospective model of the study, and the real-life nature of the study, which allow us to evaluate the effectiveness of the system in the daily life of patients but can give less uniformity in lifestyle habits. Further studies with a greater number of patients in uniform settings such as school camps or group activities that analyze the effectiveness of Sleep Mode in relation to the consumption of certain foods are certainly needed.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding authors.
Ethics statement
Ethical review and approval was not required for the study on human participants in accordance with the local legislation and institutional requirements. Written informed consent to participate in this study was provided by the participants’ legal guardian/next of kin.
Author contributions
MB designed the study and wrote the manuscript. MS researched the data and wrote the manuscript. VA researched the data. MC did the statistical analysis. Gd’A reviewed the manuscript and contributed to the discussion. MM reviewed the manuscript and contributed to the discussion. NM designed the study and contributed to the discussion. All authors contributed to the article and approved the submitted version.
Acknowledgments
We are grateful to the Department of Neuroscience, Rehabilitation, Ophthalmology, Genetics, Maternal and Child Health (DINOGMI), University of Genova—a Department of Excellence—for the support in the several steps leading to the approval and publishing of the study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Diabetes Control and Complications Trial Research Group, Nathan DM, Genuth S, Lachin J, Cleary P, Crofford O, et al. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med (1993) 329(14):977–86. doi: 10.1056/NEJM199309303291401
2. Nathan DM, Cleary PA, Backlund JY, Genuth SM, Lachin JM, Orchard TJ, et al. Diabetes control and complications Trial/Epidemiology of diabetes interventions and complications (DCCT/EDIC) study research group. Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. . N Engl J Med (2005) 353(25):2643–53. doi: 10.1056/NEJMoa052187
3. Cherubini V, Bonfanti R, Casertano A, De Nitto E, Iannilli A, Lombardo F, et al. Time in range in children with type 1 diabetes using treatment strategies based on nonautomated insulin delivery systems in the real world. Diabetes Technol Ther (2020) 22(7):509–15. doi: 10.1089/dia.2020.0031
4. Commissioner O of the. FDA Approves First Automated Insulin Delivery. Device for Type 1 Diabetes. FDA (2020). Available at: https://www.fda.gov/news-events/press-announcements/fda-approves-first-automated-insulindelivery-device-type-1-diabetes. (Accessed 2021 Aug 17).
5. Bergenstal RM, Garg S, Weinzimer SA, Buckingham BA, Bode BW, Tamborlane WV, et al. Safety of a hybrid closed-loop insulin delivery system in patients with type 1 diabetes. JAMA. (2016) 316(13):1407–8. doi: 10.1001/jama.2016.11708
6. Dos Santos TJ, Rodrigues TC, Puñales M, Arrais RF, Kopacek C. Newest diabetes-related technologies for pediatric type 1 diabetes and its impact on routine care: a narrative synthesis of the literature. Curr Pediatr Rep (2021) 20:1–12. doi: 10.1007/s40124-021-00248-7
7. Boughton CK, Hovorka R. New closed-loop insulin systems. Diabetologia. (2021) 64(5):1007–15. doi: 10.1007/s00125-021-05391-w
8. Leelarathna L, Choudhary P, Wilmot EG, Lumb A, Street T, Kar P, et al. Hybrid closed-loop therapy: Where are we in 2021? Diabetes Obes Metab (2021) 23(3):655–60. doi: 10.1111/dom.14273
9. Brown S, Raghinaru D, Emory E, Kovatchev B. First look at control-IQ: A new-generation automated insulin delivery system. Diabetes Care (2018) 41(12):2634–6. doi: 10.2337/dc18-1249
10. Vigersky RA, McMahon C. The relationship of hemoglobin A1C to time-in-Range in patients with diabetes. Diabetes Technol Ther (2019) 21(2):81–5. doi: 10.1089/dia.2018.0310
11. Battelino T, Danne T, Bergenstal RM, Amiel SA, Beck R, Biester T, et al. Clinical targets for continuous glucose monitoring data interpretation: Recommendations from the international consensus on time in range. Diabetes Care (2019) 42(8):1593–603. doi: 10.2337/dci19-0028
12. Forlenza GP, Ekhlaspour L, Breton M, Maahs DM, Wadwa RP, DeBoer M, et al. Successful At-home use of the tandem control-IQ artificial pancreas system in young children during a randomized controlled trial. Diabetes Technol Ther (2019) 21(4):159–69. doi: 10.1089/dia.2019.0011
13. Brown SA, Kovatchev BP, Raghinaru D, Lum JW, Buckingham BA, Kudva YC, et al. iDCL trial research group. Six-month randomized, multicenter trial of closed-loop control in type 1 diabetes. N Engl J Med (2019) 381(18):1707–17. doi: 10.1056/NEJMoa1907863
14. Breton MD, Kanapka LG, Beck RW, Ekhlaspour L, Forlenza GP, Cengiz E, et al. iDCL trial research group. A randomized trial of closed-loop control in children with type 1 diabetes. N Engl J Med (2020) 383(9):836–45. doi: 10.1056/NEJMoa2004736
15. Kanapka LG, Wadwa RP, Breton MD, Ruedy KJ, Ekhlaspour L, Forlenza G, et al. iDCL trial research group. Extended use of the control-IQ closed-loop control system in children with type 1 diabetes. Diabetes Care (2021) 44(2):473–8. doi: 10.2337/dc20-1729
16. Isganaitis E, Raghinaru D, Ambler-Osborn L, Pinsker JE, Buckingham BA, Wadwa R, et al. iDCL trial research group. closed-loop insulin therapy improves glycemic control in adolescents and young adults: Outcomes from the international diabetes closed-loop trial. Diabetes Technol Ther (2021) 23(5):342–9. doi: 10.1089/dia.2020.0572
17. Cherubini V, Rabbone I, Berioli MG, Giorda S, Lo Presti D, Maltoni G, et al. Effectiveness of a closed-loop control system and a virtual educational camp for children and adolescents with type 1 diabetes: A prospective, multicentre, real-life study. Diabetes Obes Metab (2021) 23(11):2484–91. doi: 10.1111/dom.14491
18. Bassi M, Teliti M, Lezzi M, Iosca A, Strati Marina F, Carmisciano L, et al. A comparison of two hybrid closed-loop systems in Italian children and adults with type 1 diabetes. Front Endocrinol (Lausanne). (2022) 12:802419. doi: 10.3389/fendo.2021.802419
19. Breton MD, Kovatchev BP. One year real-world use of the control-IQ advanced hybrid closed-loop technology. Diabetes Technol Ther (2021) 23(9):601–8. doi: 10.1089/dia.2021.0097
20. Messer LH, Berget C, Pyle L, Vigers T, Cobry E, Driscoll KA, et al. Real-world use of a new hybrid closed loop improves glycemic control in youth with type 1 diabetes. Diabetes Technol Ther (2021) 23(12). doi: 10.1089/dia.2021.0165
21. Cobry EC, Kanapka LG, Cengiz E, Carria L, Ekhlaspour L, Buckingham BA, et al. iDCL trial research group. health-related quality of life and treatment satisfaction in parents and children with type 1 diabetes using closed-loop control. Diabetes Technol Ther (2021) 23(6):401–9. doi: 10.1089/dia.2020.0532
22. Pinsker JE, Müller L, Constantin A, Leas S, Manning M, McElwee Malloy M, et al. Real-world patient-reported outcomes and glycemic results with initiation of control-IQ technology. Diabetes Technol Ther (2021) 23(2):120–7. doi: 10.1089/dia.2020.0388
23. Cobry EC, Bisio A, Wadwa RP, Breton MD. Improvements in parental sleep, fear of hypoglycemia, and diabetes distress with use of an advanced hybrid closed loop system. Diabetes Care (2022) 45(5):1292–95. doi: 10.2337/dc21-1778
24. Rabbone I, Savastio S, Pigniatiello C, Carducci C, Berioli MG, Cherubini V, et al. vEC study group. significant and persistent improvements in time in range and positive emotions in children and adolescents with type 1 diabetes using a closed-loop control system after attending a virtual educational camp. Acta Diabetol (2022) 21:1–6. doi: 10.1007/s00592-022-01878-z
Keywords: TIR (time in range), CGM (continuous glucose monitoring), Type 1 diabetes (T1D), tandem control-IQ, sleep, AHCL (Advanced Hybrid Closed Loop)
Citation: Bassi M, Strati MF, Andreottola V, Calevo MG, d’Annunzio G, Maghnie M and Minuto N (2022) To sleep or not to sleep: An Italian Control-IQ-uestion. Front. Endocrinol. 13:996453. doi: 10.3389/fendo.2022.996453
Received: 17 July 2022; Accepted: 18 November 2022;
Published: 12 December 2022.
Edited by:
Davide Tinti, AOU Città della Salute e della Scienza di Torino, ItalyReviewed by:
Peter Fasching, Vienna Health Association, AustriaAlfonso Soto Moreno, Virgen del Rocío University Hospital, Spain
Copyright © 2022 Bassi, Strati, Andreottola, Calevo, d’Annunzio, Maghnie and Minuto. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Nicola Minuto, bmljb2xhbWludXRvQGdhc2xpbmkub3Jn
 Marta Bassi
Marta Bassi Marina Francesca Strati
Marina Francesca Strati Valentina Andreottola1,2
Valentina Andreottola1,2 Maria Grazia Calevo
Maria Grazia Calevo Giuseppe d’Annunzio
Giuseppe d’Annunzio Mohamad Maghnie
Mohamad Maghnie Nicola Minuto
Nicola Minuto