- 1Merck Research Laboratories (MRL) Global Medical Affairs, Merck Sharp & Dohme (MSD) China, Shanghai, China
- 2Hatter Cardiovascular Institute, University College London, UK and Ulster University, Coleraine, United Kingdom
- 3Merck Research Laboratories, Merck & Co., Inc., Rahway, NJ, United States
- 4Department of Endocrinology, People’s Hospital of Peking University, Beijing, China
Aims: Hyperglucagonemia occurs in the pathogenesis of type 2 diabetes mellitus (T2DM). In this meta-analysis, we summarized the effects of DPP4 inhibitors on glucagon levels in patients with T2DM.
Materials and methods: Randomized controlled trials (RCTs) comparing the influence of DPP4 inhibitors on circulating glucagon levels with placebo or other oral antidiabetic drugs (OADs) in patients with T2DM were identified by searches of Medline (PubMed), Embase (Ovid), and CENTER (Cochrane Library). Only studies reporting changes in glucagon level presented as total area under the curve (AUCglucagon) using a meal or oral glucose tolerance test were included. Results were combined using a random-effects model that incorporated potential heterogeneity among the included studies.
Results: A total of 36 RCTs with moderate to high quality were included. Overall, the numbers of T2DM patients included for the meta-analyses comparing DPP4 inhibitors with placebo and other OADs were 4266 and 1652, respectively. Compared to placebo, DPP4 inhibitors significantly reduced circulating glucagon levels (standard mean difference [SMD]: -0.32, 95% CI: -0.40 to -0.24, P<0.001; I2 = 28%). Analysis of subgroups revealed that study characteristics had no significant effect on results, such as study design (parallel group or crossover), number of patients, mean patient age, proportion of men, baseline HbA1c, duration of diabetes, background therapy, treatment duration, or methods for glucagon measurement (all P for subgroup differences >0.05). Moreover, DPP4 inhibitors significantly reduced glucagon levels compared to other OADs (SMD: -0.35, 95% CI: -0.53 to -0.16, P<0.001; I2 = 66%), and the reduction in glucagon was greater in comparison with insulin secretagogues than in comparison with non-insulin secretagogues (P for subgroup difference =0.03).
Systematic review registration: https://inplasy.com/, identifier INPLASY202280104.
Conclusions: DPP4 inhibitors are effective at reducing the circulating postprandial glucagon level in T2DM patients.
Introduction
Currently, type 2 diabetes mellitus (T2DM) is one of the key factors contributing to morbidity and mortality worldwide (1, 1). It has conventionally been believed that insulin resistance and impaired insulin secretion are the key mechanisms underlying T2DM development (2, 3). Despite this common knowledge, abnormally elevated serum levels of glucagon (hyperglucagonemia) also contribute to diabetes pathogenesis (4–6), which may reflect α-cell dysfunction within the pancreatic islets. Clinical discussions often focus on insulin, but glucagon has an equally important role to play in understanding T2DM (7). Actually, all types of poorly controlled diabetes are associated with hyperglucagonemia (8). Therefore, treatment targeting hyperglucagonemia may also become an established antidiabetic strategy (9). There are several potential mechanisms for hyperglucagonemia in T2DM, including β-cell dysfunction, disturbances in α-cell/β-cell interplay, and dysfunctional incretin effect (10–13). Knowing the role of glucagon is crucial to appreciating differences in glucose-lowering therapies’ mechanisms of action. Amongst oral anti-diabetic drugs (OADs), dipeptidyl-peptidase 4 (DPP-4) inhibitors are a well-applied class of glucose-lowering medications that function by inhibiting the DPP-4–induced degradation of incretin hormone glucagon-like peptide-1 (GLP-1) and glucose-dependent insulinotropic polypeptide (GIP). The benefits of DPP-4 inhibitors in T2DM are not only limited to their regulation of insulin secretion in a glucose-dependent manner, but also include their efficacies in attenuating β-cell loss and improving glycemic durability (14, 15). Moreover, through increasing endogenous levels of the incretin hormones (10, 16), DPP-4 inhibitors have been suggested to suppress endogenous glucagon production in T2DM.
Compared with insulin, our understanding, research and detection methods for glucagon are not so well established. To the best of our knowledge, no consensus or gold standard has been reached regarding the optimal methods for measuring the serum glucagon concentration. In spite of the fact that plasma glucagon levels are not used in clinical stratification of diabetes treatment, health care providers may gain clinical insight by understanding how to control plasma glucagon levels pharmacologically in T2DM patients. In patients with T2DM, glucagon levels typically rise during fasting and then fail to decrease appropriately or even rise during oral glucose tolerance testing (OGTT) or after ingestion of a carbohydrate-rich meal, leading to undesirably high plasma glucagon with hyperglycemia. Typically, the effects of antidiabetic treatment on the postprandial glucagon level are measured by the changes in glucagon total area under the curve (AUCglucagon) using a meal tolerance test (MTT) or a standard OGTT (17–19). Although there have been few small-scale randomized controlled trials (RCTs) evaluating the effect of DPP-4 inhibitors on plasma glucagon levels in patients with type 2 diabetes (20–55), little is known about the summarized efficacy of DPP4 inhibitors on AUC compared to placebo. There are also other oral glucose-lowering drug classes that affect glucagon secretion (positively or negatively), including sulfonylureas and sodium-glucose cotransporter 2 inhibitors (SGLT2is). Therefore, the summarized efficacy of DPP4 inhibitors on AUCglucagon compared to other OADs also seems interesting. To the best of our knowledge, no systematic review and meta-analysis has been published to date regarding the influence of DPP4 inhibitors on AUCglucagon in patients with T2DM. Accordingly, the aim of this study was to examine systematically the influence of DPP4 inhibitors on AUCglucagon in T2DM patients by performing a meta-analysis of RCTs.
Methods
This study adhered to PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) (56) and Cochrane Handbook (57) guidelines during its design and implementation. The protocol of the meta-analysis was registered at the International Platform of Registered Systematic Review and Meta-analysis Protocols (INPLASY, https://inplasy.com/) with the registration number of INPLASY202280104. The PRISMA 2020 Checklist has been provided in the Supplementary Material.
Search strategy
In order to search Medline (PubMed), Embase (Ovid), and CENTER (Cochrane Library), the following strategies were used: (1) “DPP4” OR “DPP-4” OR “dipeptidyl peptidase-4 inhibitors” OR “sitagliptin” OR “vildagliptin” OR “linagliptin” OR “saxagliptin” OR “alogliptin” OR “dutogliptin” OR “aemigliptin” OR “anagliptin” OR “teneligliptin” OR “trelagliptin” OR “omarigliptin” OR “gemigliptin” OR “evogliptin”; (2) “α cell” OR “α-cell” OR “glucagon” OR “alpha cell” OR “islet” OR “hormone” OR “hormonal” OR “meal” OR “prandial” OR “postprandial” OR “Oral Glucose Tolerance Test” OR “OGTT”; and (3) “random” OR “randomized” OR “randomised” OR “randomly”. Only studies including human subjects were considered. As part of the final database search, references to related reviews and original articles were also searched. The final database search was carried out on August 25, 2022.
Study selection
We included studies that met the following criteria: (1) English-language articles with full-length content; (2) RCTs with parallel groups or crossovers; (3) Adults with T2DM were randomly assigned to DPP4 inhibitors or placebo groups, or other OADS, for treatment; and (4) reported the changes of AUCglucagon from baseline after treatment utilizing MTT or OGTT in participants in the interventional and control arms. In this review, we included studies with patients who are drug-nave or with T2DM patients who are receiving background OAD therapy. However, studies including T2DM patients on concurrent antidiabetic injection treatment, such as insulin and GLP-1 receptor agonists (GLP-1RAs) were excluded from the current meta-analysis. Our meta-analysis did not include studies that included patients treated with single-dose or single-day DPP4 inhibitors, since we weren’t planning to evaluate the acute effects of DPP4 inhibitors on circulating glucagon. In addition, non-randomized studies, studies including non-T2DM patients, or those without a measurement of AUCglucagon during MTT or OGTT setting were also excluded.
Data collection and quality evaluation
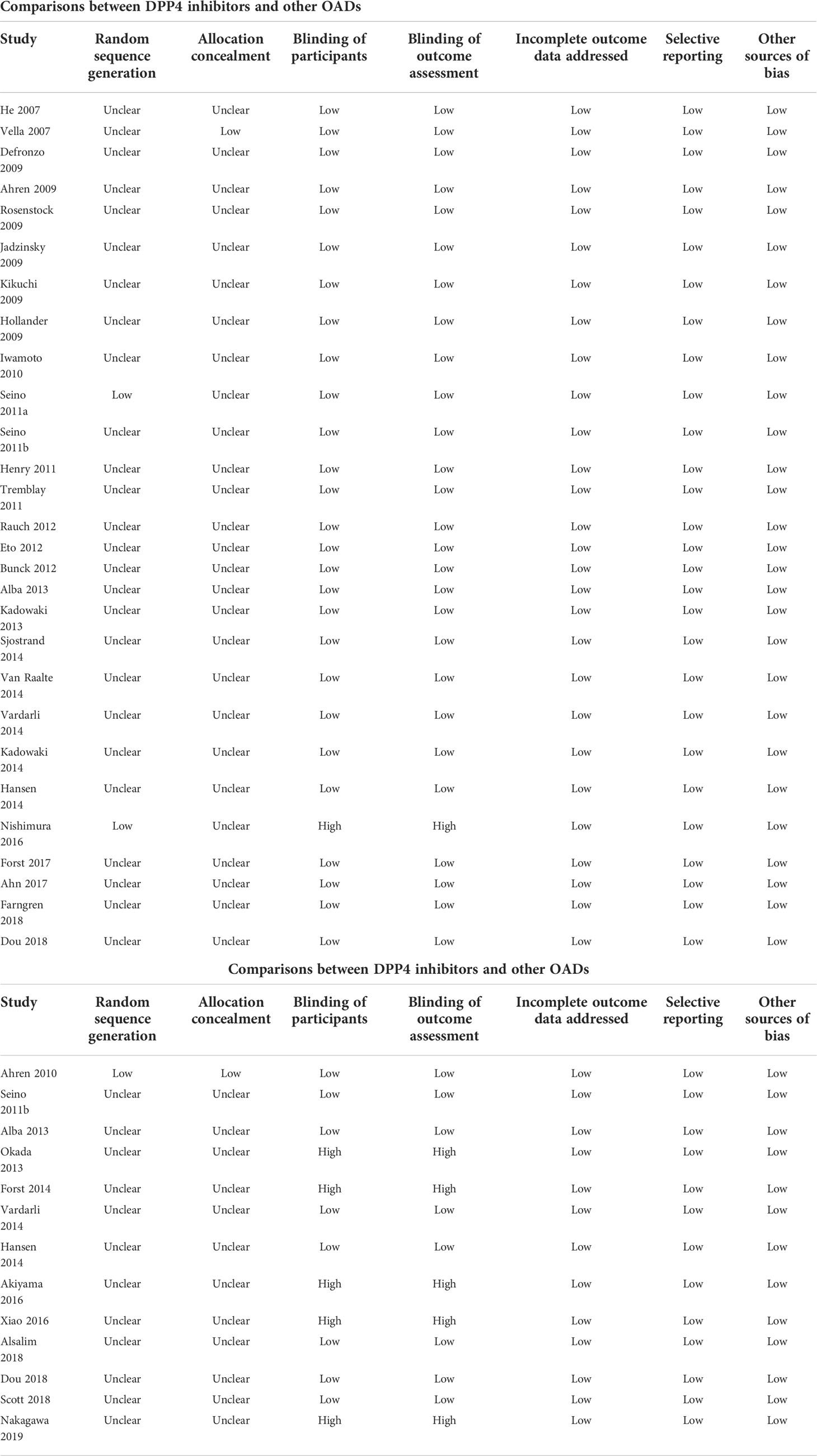
Database searches, data collection, and quality assessment were carried out by two authors independently. Discussions with the corresponding author were conducted if disagreements occurred. We collected data of study information (first author, publication year, and study country), study design (blind or open-label, crossover or parallel group), patient information (number of patients, mean age, sex, baseline hemoglobin A1c [HbA1c], and T2DM duration), details of background antidiabetic treatments, drugs and doses of DPP4 inhibitors used, regimens of controls, treatment durations, and methods for circulating glucagon measurement. The Cochrane Risk of Bias Tool was used to determine the quality of the included RCTs (57) according to the following aspects: assigning random sequences; concealing allocations; blinding participants and personnel; blinding outcomes assessors; incomplete outcomes data; and selective outcome reporting.
Statistical analysis
The effects of DPP4 inhibitors on circulating glucagon levels compared to controls in T2DM patients were presented as a standard mean difference (SMD) with 95% confidence interval (CI) because the methods and durations for measuring AUCglucagon varied among the included RCTs. Heterogeneity was assessed using Cochrane’s Q test (58). It was also calculated the I2 statistic, and an I2 >50% indicates significant heterogeneity. A random-effects model was used when calculating pooled analyses, since it incorporates potential heterogeneity and provides more generalized results (57). Analyses of sensitivity were conducted by excluding one study at a time from the meta-analysis to evaluate the effect of each study on the pooled results (57). Additionally, sensitivity analyses limited to studies with FDA-approved DPP4 inhibitors and dosages were also performed, including sitagliptin 100 mg once daily, saxagliptin 5 mg once daily, linagliptin 5 mg once daily, and alogliptin 25 mg once daily. Analysis of predefined subgroups was conducted to determine whether study characteristics could influence the results, including study characteristics such as study design (parallel group or crossover), number of patients, mean patient age, proportion of men, baseline HbA1c, duration of diabetes, background therapy, treatment duration, and methods for glucagon measurement. For continuous variables, medians were selected as cutoffs for defining of subgroups. For a meta-analysis comparing DPP4 inhibitors and other OADs, subgroup analyses were performed according to whether the OADs taken by controls were insulin secretagogues or non-insulin secretagogues. An evaluation of publication bias was conducted via visual inspection of funnel plots and Egger’s regression asymmetry test (59). For studies including multiple dose groups of DPP4 inhibitors, the shared control groups were equally split and included as independent comparisons to overcome a unit-of-analysis error, according to the instruction of Cochrane’s Handbook (57). Differences for which P<0.05 were considered statistically significant. Statistical analyses were conducted using the RevMan (Version 5.1; Cochrane, Oxford, UK) software.
Results
Literature search
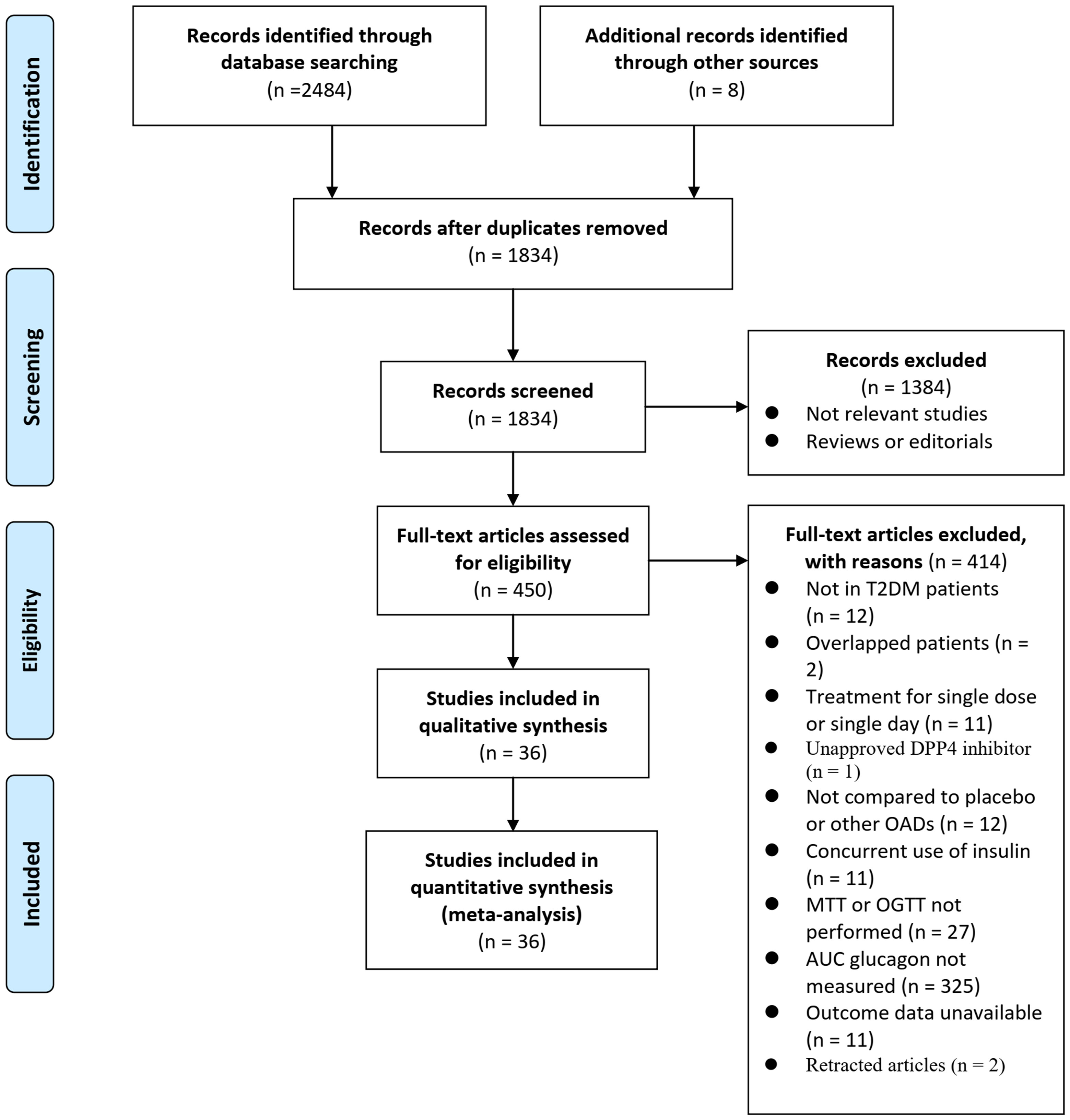
Figure 1 illustrates the process of searching databases and identifying studies. Briefly, database searches yielded 2492 articles, and 1834 were retrieved after the duplicate records were excluded. Thirteen hundred eighty-four articles were subsequently excluded based on titles and abstracts, primarily because they were unrelated to the goal of the meta-analysis. Then, 414 articles out of the 450 that received full-text reviews were further excluded for the reasons illustrated in Figure 1. Finally, 36 RCTs (20–55) were deemed to be eligible for the meta-analysis.
Study characteristics and data quality
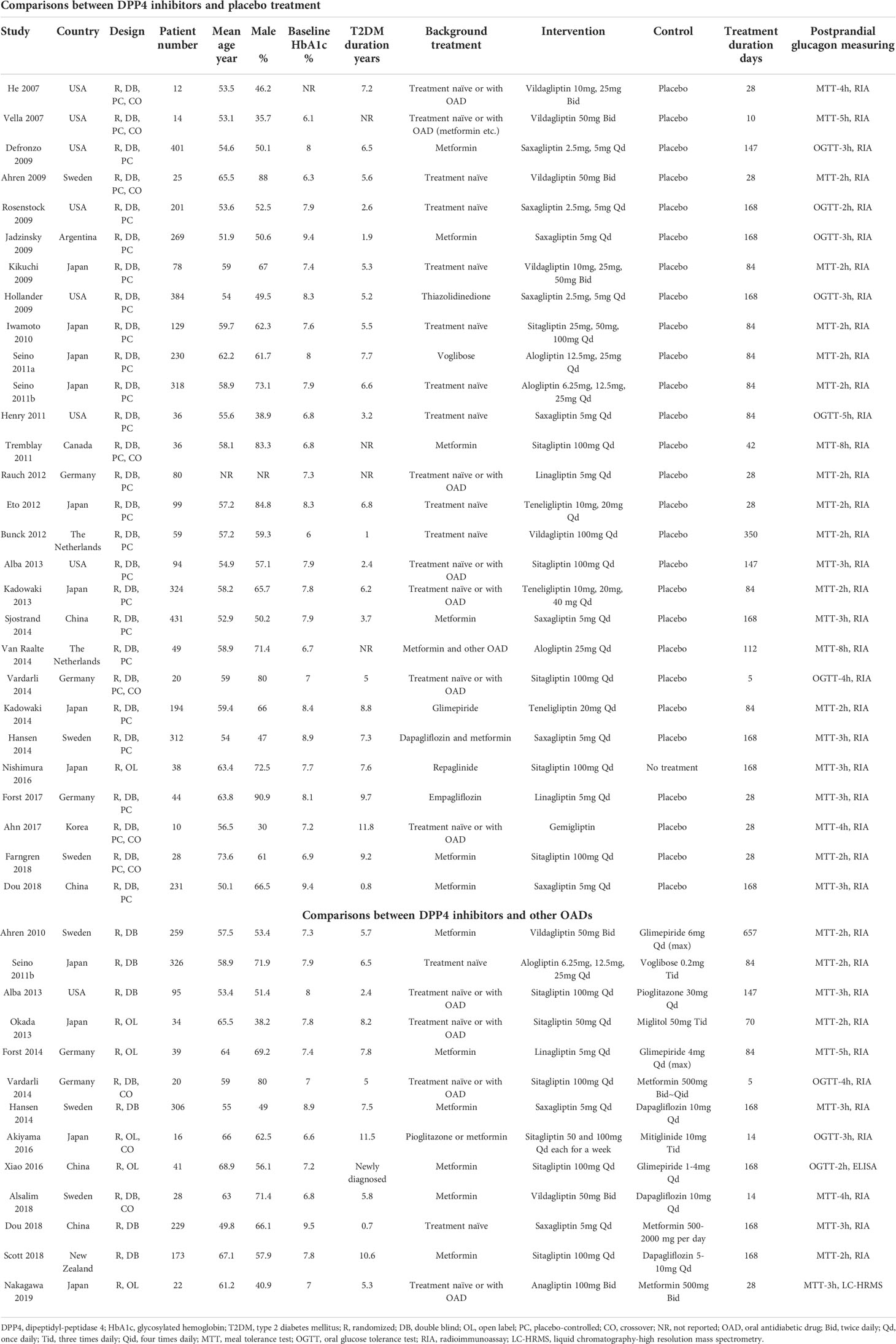
An overview of the included studies can be found in Table 1. Overall, 23 studies compared DPP4 inhibitors with placebo (20–22, 25–27, 30–42, 44–47). Eight studies compared DPP4 inhibitors with other OADs (28, 48–55), and the other five studies included both comparisons with placebo and other OADs (23, 24, 28, 29, 43).
Accordingly, a total of 28 RCTs were available for the meta-analysis comparing the influence of DPP4 inhibitors with placebo on postprandial glucagon (20–47). The characteristics of these studies are presented in the upper panel of Table 1. Briefly, these studies were all RCTs including T2DM patients which were published between 2007 and 2018. Seven of them were crossover studies (20, 24, 25, 27, 31, 37, 45), whilst the remaining studies were parallel-group RCTs. The mean ages of the patients varied between 51 and 74 years. Various DPP4 inhibitors were used among these studies, such as vildagliptin, saxagliptin, linagliptin, sitagliptin, alogliptin, teneligliptin, and gemigliptin, whilst placebo was used as the control in all of the included RCTs except one study which received no treatment (42). The treatment durations varied between 5 and 350 days, and circulating postprandial glucagon was measured with radioimmunoassay in MTT/OGTT settings. Using Cochrane’s Risk of Bias Tool, Table 2 provides a detailed analysis of the included RCTs.
A total of 13 studies compared circulating glucagon levels in T2DM patients treated with a DPP4 inhibitor or other OAD (23, 24, 28, 29, 43, 48–55). Vildagliptin, sitagliptin, linagliptin, saxagliptin, alogliptin, and anagliptin were used as treatments, while glimepiride, voglibose, pioglitazone, dapagliflozin, miglitol and metformin were used as controls. The mean ages of the patients varied between 51 and 69 years. The follow-up durations varied from 5–657 days. Circulating glucagon levels were measured by radioimmunoassay (23, 24, 28, 29, 43, 48–51, 53, 54), enzyme-linked immunosorbent assay (ELISA) (55), or liquid chromatography-high resolution mass spectrometry (52) in a MTT/OGTT setting. A detailed risk of bias assessment of the included RCTs can also be found in Table 2.
Comparisons between DPP4 inhibitors and placebo on circulating postprandial glucagon
Because 10 studies reported data according to multiple dosages of DPP4 inhibitors separately (20–23, 26, 30, 35, 38, 41, 46), these datasets were included into the meta-analysis independently. Accordingly, 42 datasets from 28 RCTs were available comparing the effects of DPP4 inhibitors and placebo on circulating glucagon levels in T2DM patients (20–47). Studies included in this review showed mild heterogeneity (P for Cochrane’s Q test =0.05, I2 = 28%). Pooled results showed that compared to placebo treatment, DPP4 inhibitors significantly reduced the circulating postprandial glucagon level in patients with T2DM (AUCglucagon: SMD=-0.32, 95% CI: -0.40 to -0.24, P<0.001; Figure 2A). The results were not significantly affected by excluding one dataset at a time during the sensitivity analysis (SMD: -0.31 ~ -0.34, all P<0.05). Sensitivity analyses limited to studies with FDA-approved DPP4 inhibitors and dosages showed consistent results (SMD = -0.34, 95% CI: -0.47 to -0.22, P<0.001; Figure 2B). Subgroup analyses showed that the results were not significantly affected by study characteristics such as study design (parallel group or crossover), number of patients, mean patient age, proportion of men, baseline HbA1c, duration of diabetes, background therapy, treatment duration, or methods for glucagon measurement (all P for subgroup difference >0.05; Table 3).
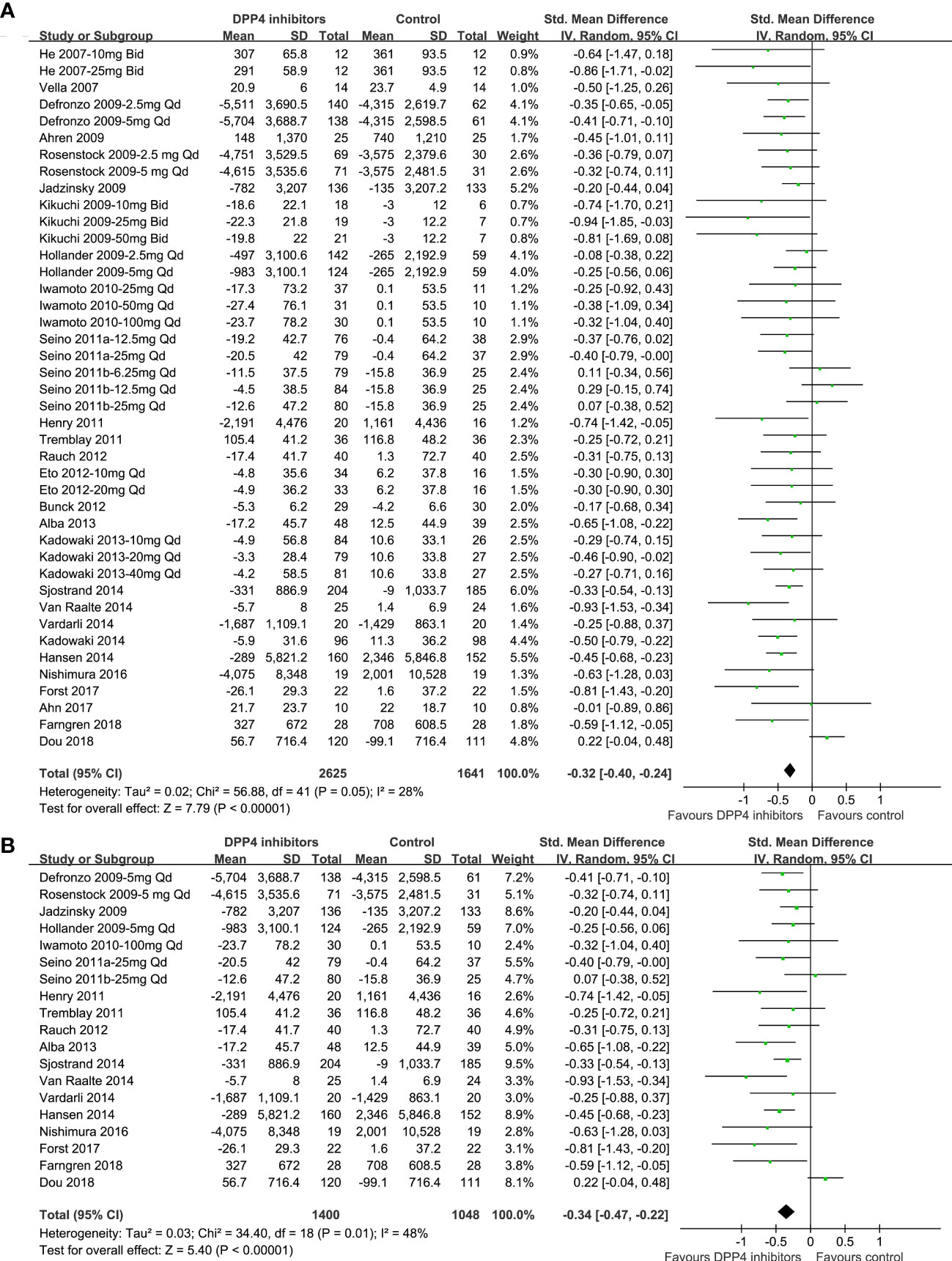
Figure 2 Forest plots for the meta-analysis comparing the effects of DPP4 inhibitors and placebo on circulating glucagon levels in T2DM patients. (A) Forest plots for the overall meta-analysis; and (B) forest plots for the sensitivity analyses limited to studies with FDA-approved DPP4 inhibitors and dosages.
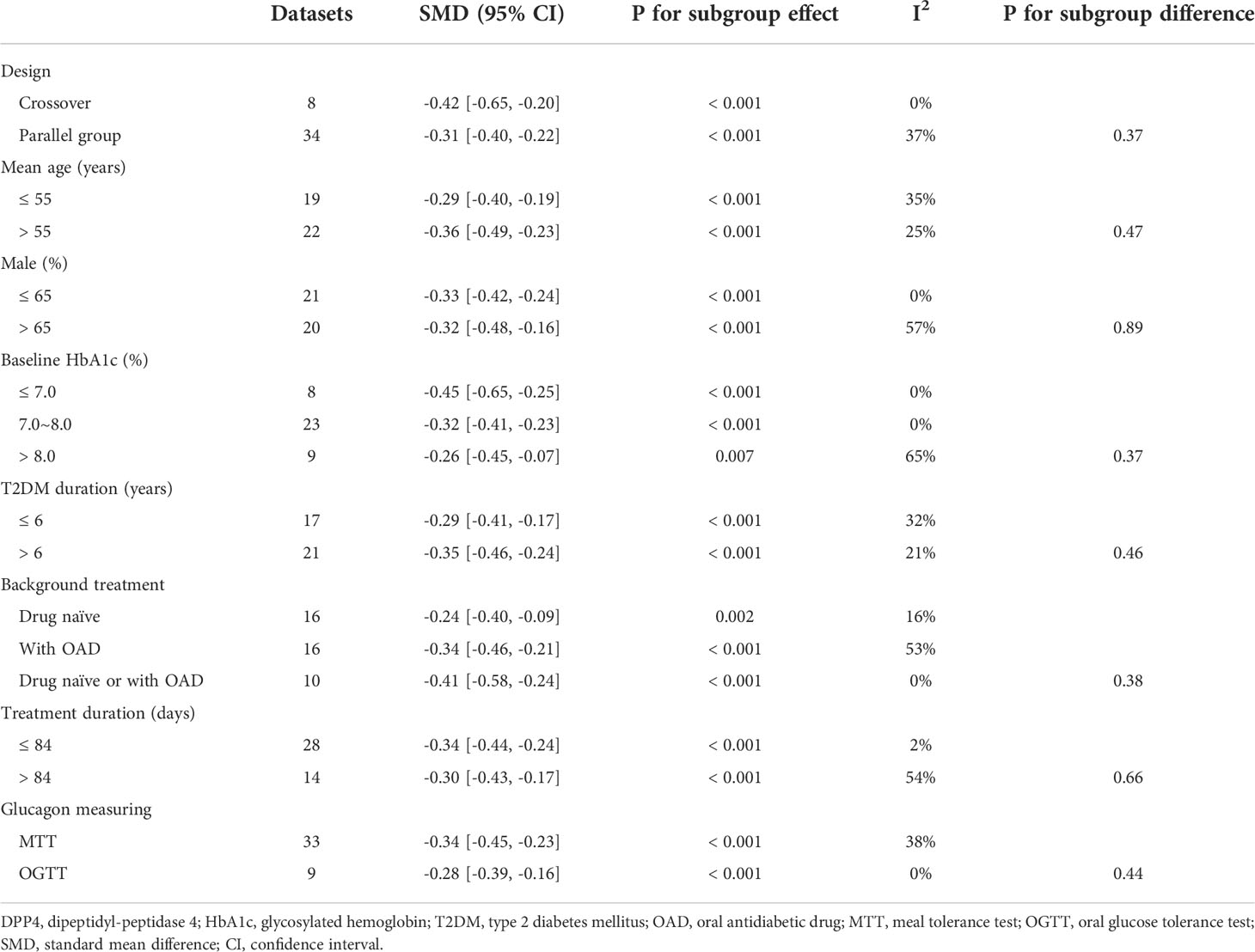
Table 3 Subgroup analysis for comparing DPP4 inhibitors with placebo treatment on circulating glucagon.
Comparisons between DPP4 inhibitors and other OADs on circulating postprandial glucagon
One study reported data according to multiple dosages of DPP4 inhibitors separately (23), and these three datasets were included into the meta-analysis independently. Overall, the pooled results of 15 datasets from 13 RCTs (23, 24, 28, 29, 43, 48–55) showed that compared with other OADs, DPP4 inhibitors significantly reduced the circulating postprandial glucagon level in patients with T2DM (AUCglucagon: SMD=-0.35, 95% CI: -0.53 to -0.16, P<0.001; I2 = 66%; Figure 3A). Sensitivity analysis by excluding one dataset at a time did not significantly change the results (SMD: -0.30 ~ -0.39, all P<0.05). Subgroup analyses showed that compared with either insulin secretagogues or non-insulin secretagogues, DPP4 inhibitors still significantly reduce glucagon levels in T2DM patients, and the reduction of glucagon was more remarkable compared with insulin secretagogues (SMD: -0.76, 95% CI: -1.21 to -0.31, P<0.001) than with non-insulin secretagogues (SMD: -0.22, 95% CI: -0.42 to -0.02, P=0.03; P for subgroup difference =0.03; Figure 3B).
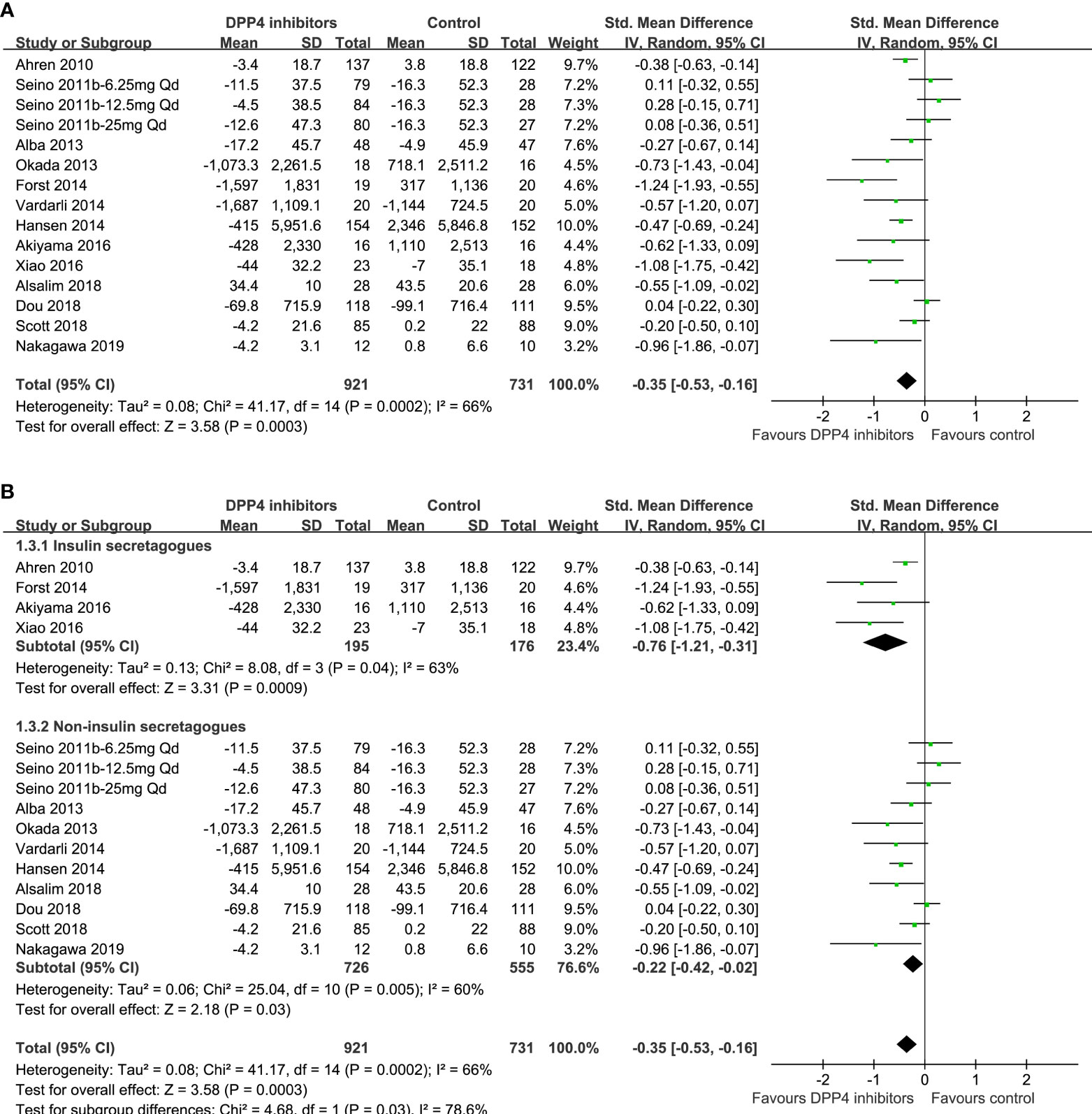
Figure 3 Forest plots for the meta-analysis comparing the effects of DPP4 inhibitors and other OADs on circulating glucagon levels in T2DM patients. (A) Forest plots for the overall meta-analysis; and (B) forest plots for the subgroup analyses according to the OADs given to controls.
Publication bias
The funnel plots for the meta-analyses comparing DPP4 inhibitors with placebo and other OADs were symmetrical, suggesting low-risk of publication biases (Figures 4A, B). Egger’s regression tests also suggested low risk of publication biases (P=0.167 and 0.156, respectively).
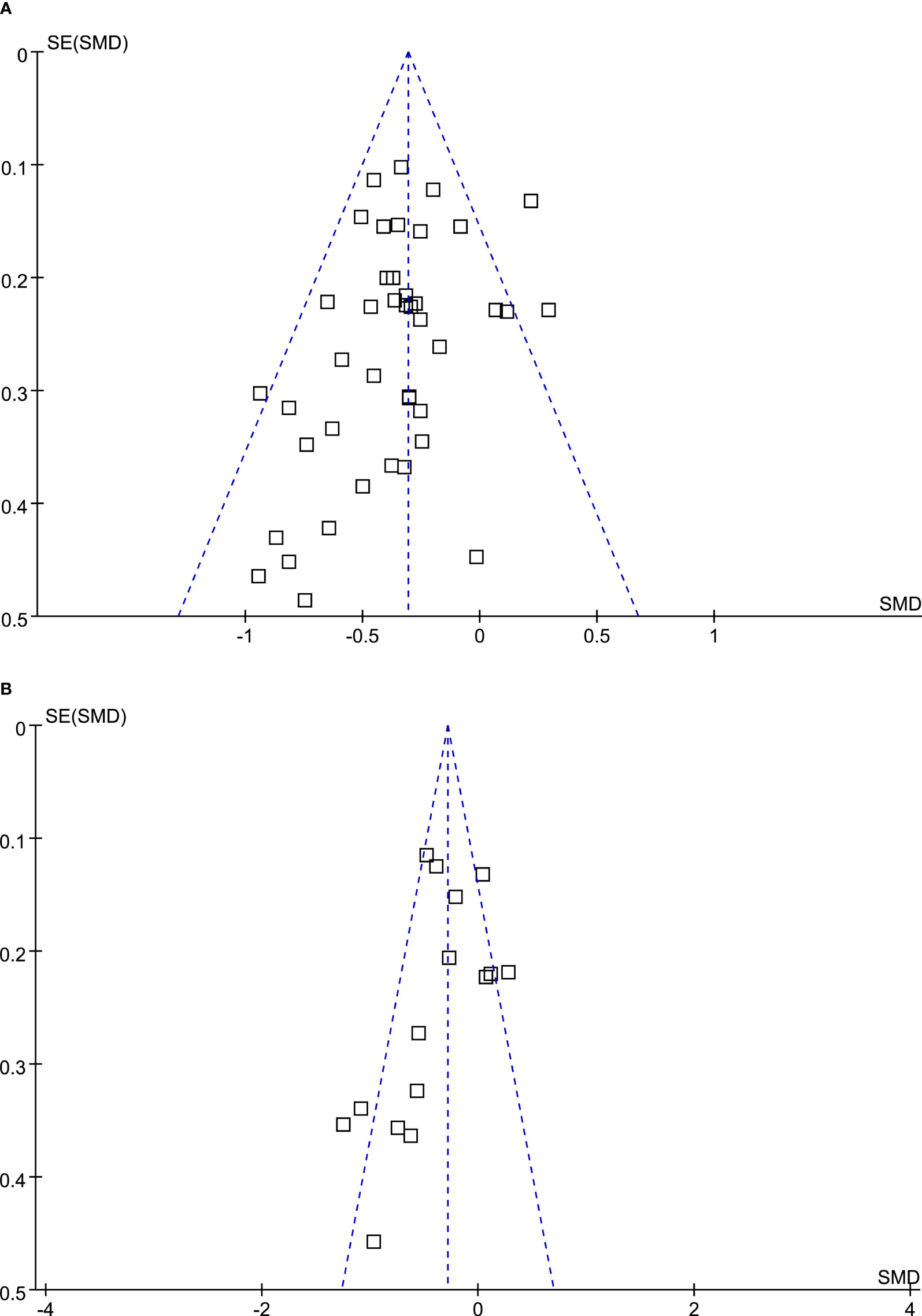
Figure 4 Funnel plots for the meta-analysis evaluating the influences of DPP4 inhibitors on circulating glucagon in T2DM patients. (A) Funnel plots for the meta-analysis comparing the effects of DPP4 inhibitors and placebo; and (B) funnel plots for the meta-analysis comparing the effects of DPP4 inhibitors and other OADs.
Discussion
Based on a pooled analysis of RCT results, this meta-analysis concluded that DPP4 inhibitors are effective at reducing postprandial glucagon levels in T2DM patients compared to placebo, as evidenced by a significantly reduced AUCglucagon in MTT/OGTT settings in patients allocated to the DPP4 inhibitor arm. Moreover, multiple sensitivity and subgroup analyses confirmed the stability of the findings, which were not significantly driven by any one of the included studies or significantly affected by predefined study characteristics, such as study design (parallel group or crossover), number of patients, mean patient age, proportion of men, baseline HbA1c, duration of diabetes, background therapy, treatment duration, or methods for glucagon measurement. In addition, the pooled results of 13 eligible RCTs showed that DPP4 inhibitors may also significantly reduce the circulating postprandial glucagon level in T2DM patients as compared to other OADs. Researchers have suggested that factors originating from nutrient stimulation of the digestive tract may play a key role in this process, namely, that postprandial glucagon production may be gut-derived, not originating from the pancreas (60, 61). Therefore, we divided other OADs into two groups for subgroup analysis. The results of this subgroup analysis showed that DPP4 inhibitors significantly reduced glucagon levels in T2DM patients compared to both the insulin and non-insulin secretagogues, separately, and the reduction in glucagon level was more noteworthy compared to that achieved with insulin secretagogues than with non-insulin secretagogues. Taken together, these results indicate that DPP4 inhibitors are effective at reducing postprandial glucagon levels in T2DM patients, which may be an additional mechanism underlying their benefits in patients with T2DM.
As far as we know, this is the first meta-analysis examining how DPP4 inhibitors affect circulating postprandial glucagon levels in people with T2DM. Unlike pharmacological studies in healthy volunteers with a single dose of the medication, we only included studies with T2DM patients who were treated for a stable period with DPP4 inhibitors, aiming to show that the possible glucagon-lowering efficacy of DPP4 inhibitors is clinically relevant. Because the relevant data on glucagon levels after fasting is limited and the circulating level of glucagon is variable according to feeding status, we chose AUCglucagon using standard MTT/OGTT as the measurement rather than plasma glucagon level at a certain time to comprehensively reflect the dynamic status of postprandial glucagon (62). Studies including patients with concurrent insulin, GLP-1RAs or pramlintide injections were also excluded, because the imbalance of these treatments between groups may significantly affect the circulating glucagon levels (63, 64). These methodological considerations minimized the possible confounding effects of other clinical factors on the level of glucagon. The glucagon-suppressing effect of DPP4 inhibitors in T2DM patients observed in this meta-analysis could be explained by the pharmacological mechanisms of the drugs and confirmed that DPP4 inhibitors improve glycemic control at least partially via influencing glucagon levels. As part of its biological function, GLP-1 stimulates insulin secretion and inhibits glucagon action when secreted by the small intestine’s L-cells. By inhibiting the DPP-4–induced degradation of GLP-1, DPP4 inhibitors enhance the inhibitory effect of GLP-1 on glucagon, which may be more remarkable in a hyperglucagonaemic state in T2DM patients (64). Interestingly, glucose-dependent insulinotropic polypeptide also increases glucagon secretion under hypoglycemic and euglycemic conditions, but not under hyperglycemic conditions (65–67). This specific mechanism is rarely reported for other OADs, which is also consistent with our findings that DPP4 inhibitors significantly reduced circulating postprandial glucagon levels in T2DM patients as compared to other OADs. In view of the importance of hyperglucagonemia in the pathogenesis of T2DM, the results of this study highlight the additional benefits of glucagon suppression by DPP4 inhibitors compared to other commonly used OADs. Interestingly, accumulating evidence from clinical studies has suggested that similar to DPP-4 inhibitors, GLP-1RAs substantially lower glucagon concentrations in both the fasting state and after a meal, thus reducing the hyperglucagonemia in patients with T2DM (68, 69). However, nearly all cardiovascular outcomes trials conducted with DPP4 inhibitors in T2DM patients so far have demonstrated a neutral effect on major adverse cardiovascular events (70, 71).
This meta-analysis has several strengths, including a rigorous literature review, strict inclusion and exclusion criteria, and robust results, comprehensive full-text review to include available related RCTs, and performance of multiple sensitivity analyses to confirm the stability and robustness of the findings. However, this study also has limitations. First, the optimal assessment method for circulating glucagon remains to be developed (72). Therefore, differences in the measurement methods for glucagon among the included studies may have contributed to the clinical heterogeneity of the meta-analysis, such as the different durations for MTT/OGTT and assays for plasma glucagon. In addition, the data used for this meta-analysis were study-level rather than individual patient-level. Subgroup analyses, therefore, should be interpreted cautiously. Large-scale RCTs or meta-analyses based on individual patient data may be considered to validate whether patient characteristics or concurrent medications may influence the potential glucagon-suppressing effect of DPP4 inhibitors. Besides, although hyperglucagonemia has been known to be involved in the pathogenesis and progression of T2DM, studies evaluating the significance of hyperglucagonemia in determination of glycemic control and prognosis in patients with T2DM are rare, at least partly because of the lack of standard methods for glucagon measuring in clinical practice. Future studies are also needed in this regard.
Overall, the results of this meta-analysis showed that DPP4 inhibitors are effective at reducing circulating postprandial glucagon levels in T2DM patients. In view of the importance of hyperglucagonemia in the pathogenesis of T2DM, these results highlight the additional effect of DPP4 inhibitors in patients with T2DM.
Conclusions
In conclusion, this meta-analysis revealed the effectiveness of DPP4 inhibitors for reducing circulating postprandial glucagon levels in T2DM patients in comparison with placebo or other OADs. The results confirmed that DPP4 inhibitors improve glycemic control, in part, by affecting glucagon levels.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Author contributions
SC, YZ, and RC conceived, designed, or planned the study; SC collected data and conducted research; RZ and LJ performed or supervised analyses; RZ, SR and YMZ interpreted the results; SC wrote the initial paper; All authors provided substantive suggestions for revision or critically reviewed subsequent iterations of the manuscript, reviewed and approved final version of the paper, and for all aspects of the work in ensuring that questions related to the accuracy.
Funding
This study received funding from MSD China. The funder had the following involvement in the study: study design, data collection and analysis, and preparation of the manuscript.
Acknowledgments
Jingya Chen of MSD China Holding Co., Ltd., Shanghai, China, assisted literature research of the manuscript. Administrative assistance was provided by Li Qi of MSD China Holding Co., Ltd., Shanghai, China. Medical writing and editorial assistance were provided by Medjaden, Inc. This assistance was funded by MSD China.
Conflict of interest
SC, RZ, YZ and YMZ are employees of MSD China. SR is an employee of Merck Sharp & Dohme LLC., a subsidiary of Merck & Co., Inc., Rahway, NJ, US.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2022.994944/full#supplementary-material
Abbreviations
CI, confidence interval; DPP-4, dipeptidyl-peptidase 4; GIP, glucose-dependent insulinotropic polypeptide; GLP-1, glucagon-like peptide-1; MTT, meal tolerance test; OADs, oral anti-diabetic drugs; OGTT, oral glucose tolerance test; RCTs, randomized controlled trials; SGLT2is, sodium-glucose cotransporter 2 inhibitors; SMD, standard mean difference; T2DM, type 2 diabetes mellitus.
References
1. Jia W, Weng J, Zhu D, Ji L, Lu J, Zhou Z, et al. Standards of medical care for type 2 diabetes in China 2019. Diabetes Metab Res Rev (2019) 35:e3158. doi: 10.1002/dmrr.3158
2. Petersen MC, Shulman GI. Mechanisms of insulin action and insulin resistance. Physiol Rev (2018) 98:2133–223. doi: 10.1152/physrev.00063.2017
3. Taylor R, Al-Mrabeh A, Sattar N. Understanding the mechanisms of reversal of type 2 diabetes. Lancet Diabetes Endocrinol (2019) 7:726–36. doi: 10.1016/S2213-8587(19)30076-2
4. Moon JS, Won KC. Pancreatic alpha-cell dysfunction in type 2 diabetes: Old kids on the block. Diabetes Metab J (2015) 39:1–9. doi: 10.4093/dmj.2015.39.1.1
5. Gilon P. The role of alpha-cells in islet function and glucose homeostasis in health and type 2 diabetes. J Mol Biol (2020) 432:1367–94. doi: 10.1016/j.jmb.2020.01.004
6. Wewer Albrechtsen NJ, Kuhre RE, Pedersen J, Knop FK, Holst JJ. The biology of glucagon and the consequences of hyperglucagonemia. biomark Med (2016) 10:1141–51. doi: 10.2217/bmm-2016-0090
7. Haedersdal S, Lund A, Knop FK, Vilsboll T. The role of glucagon in the pathophysiology and treatment of type 2 diabetes. Mayo Clin Proc (2018) 93:217–39. doi: 10.1016/j.mayocp.2017.12.003
8. Gosmain Y, Masson MH, Philippe J. Glucagon: the renewal of an old hormone in the pathophysiology of diabetes. J Diabetes (2013) 5:102–9. doi: 10.1111/1753-0407.12022
9. Grondahl MF, Keating DJ, Vilsboll T, Knop FK. Current therapies that modify glucagon secretion: What is the therapeutic effect of such modifications? Curr Diabetes Rep (2017) 17:128. doi: 10.1007/s11892-017-0967-z
10. Lund A, Vilsboll T, Bagger JI, Holst JJ, Knop FK. The separate and combined impact of the intestinal hormones, GIP, GLP-1, and GLP-2, on glucagon secretion in type 2 diabetes. Am J Physiol Endocrinol Metab (2011) 300:E1038–46. doi: 10.1152/ajpendo.00665.2010
11. Unger RH, Aguilar-Parada E, Muller WA, Eisentraut AM. Studies of pancreatic alpha cell function in normal and diabetic subjects. J Clin Invest (1970) 49:837–48. doi: 10.1172/JCI106297
12. Muller WA, Faloona GR, Aguilar-Parada E, Unger RH. Abnormal alpha-cell function in diabetes. Response Carbohydr Protein Ingestion N Engl J Med (1970) 283:109–15. doi: 10.1056/NEJM197007162830301
13. Menge BA, Gruber L, Jorgensen SM, Deacon CF, Schmidt WE, Veldhuis JD, et al. Loss of inverse relationship between pulsatile insulin and glucagon secretion in patients with type 2 diabetes. Diabetes (2011) 60:2160–8. doi: 10.2337/db11-0251
14. Deacon CF. Dipeptidyl peptidase 4 inhibitors in the treatment of type 2 diabetes mellitus. Nat Rev Endocrinol (2020) 16:642–53. doi: 10.1038/s41574-020-0399-8
15. Chen K, Kang D, Yu M, Zhang R, Zhang Y, Chen G, et al. Direct head-to-head comparison of glycaemic durability of dipeptidyl peptidase-4 inhibitors and sulphonylureas in patients with type 2 diabetes mellitus: A meta-analysis of long-term randomized controlled trials. Diabetes Obes Metab (2018) 20:1029–33. doi: 10.1111/dom.13147
16. Ahren B, Foley JE. Improved glucose regulation in type 2 diabetic patients with DPP-4 inhibitors: focus on alpha and beta cell function and lipid metabolism. Diabetologia (2016) 59:907–17. doi: 10.1007/s00125-016-3899-2
17. Miyachi A, Kobayashi M, Mieno E, Goto M, Furusawa K, Inagaki T, et al. Accurate analytical method for human plasma glucagon levels using liquid chromatography-high resolution mass spectrometry: comparison with commercially available immunoassays. Anal Bioanal Chem (2017) 409:5911–8. doi: 10.1007/s00216-017-0534-0
18. Rodriguez-Diaz R, Tamayo A, Hara M, Caicedo A. The local paracrine actions of the pancreatic alpha-cell. Diabetes (2020) 69:550–8. doi: 10.2337/dbi19-0002
19. Gromada J, Chabosseau P, Rutter GA. The alpha-cell in diabetes mellitus. Nat Rev Endocrinol (2018) 14:694–704. doi: 10.1038/s41574-018-0097-y
20. He YL, Serra D, Wang Y, Campestrini J, Riviere GJ, Deacon CF, et al. Pharmacokinetics and pharmacodynamics of vildagliptin in patients with type 2 diabetes mellitus. Clin Pharmacokinet (2007) 46:577–88. doi: 10.2165/00003088-200746070-00003
21. Rosenstock J, Aguilar-Salinas C, Klein E, Nepal S, List J, Chen R. Effect of saxagliptin monotherapy in treatment-naive patients with type 2 diabetes. Curr Med Res Opin (2009) 25:2401–11. doi: 10.1185/03007990903178735
22. Seino Y, Fujita T, Hiroi S, Hirayama M, Kaku K. Alogliptin plus voglibose in Japanese patients with type 2 diabetes: a randomized, double-blind, placebo-controlled trial with an open-label, long-term extension. Curr Med Res Opin (2011) 27 Suppl 3:21–9. doi: 10.1185/03007995.2011.614936
23. Seino Y, Fujita T, Hiroi S, Hirayama M, Kaku K. Efficacy and safety of alogliptin in Japanese patients with type 2 diabetes mellitus: a randomized, double-blind, dose-ranging comparison with placebo, followed by a long-term extension study. Curr Med Res Opin (2011) 27:1781–92. doi: 10.1185/03007995.2011.599371
24. Vardarli I, Arndt E, Deacon CF, Holst JJ, Nauck MA. Effects of sitagliptin and metformin treatment on incretin hormone and insulin secretory responses to oral and "isoglycemic" intravenous glucose. Diabetes (2014) 63:663–74. doi: 10.2337/db13-0805
25. Vella A, Bock G, Giesler PD, Burton DB, Serra DB, Saylan ML, et al. Effects of dipeptidyl peptidase-4 inhibition on gastrointestinal function, meal appearance, and glucose metabolism in type 2 diabetes. Diabetes (2007) 56:1475–80. doi: 10.2337/db07-0136
26. DeFronzo RA, Hissa MN, Garber AJ, Luiz Gross J, Yuyan Duan R, Ravichandran S, et al. The efficacy and safety of saxagliptin when added to metformin therapy in patients with inadequately controlled type 2 diabetes with metformin alone. Diabetes Care (2009) 32:1649–55. doi: 10.2337/dc08-1984
27. Ahn CH, Kim EK, Min SH, Oh TJ, Cho YM. Effects of gemigliptin, a dipeptidyl peptidase-4 inhibitor, on lipid metabolism and endotoxemia after a high-fat meal in patients with type 2 diabetes. Diabetes Obes Metab (2017) 19:457–62. doi: 10.1111/dom.12831
28. Alba M, Ahren B, Inzucchi SE, Guan Y, Mallick M, Xu L, et al. Sitagliptin and pioglitazone provide complementary effects on postprandial glucose and pancreatic islet cell function. Diabetes Obes Metab (2013) 15:1101–10. doi: 10.1111/dom.12145
29. Dou J, Ma J, Liu J, Wang C, Johnsson E, Yao H, et al. Efficacy and safety of saxagliptin in combination with metformin as initial therapy in Chinese patients with type 2 diabetes: Results from the START study, a multicentre, randomized, double-blind, active-controlled, phase 3 trial. Diabetes Obes Metab (2018) 20:590–8. doi: 10.1111/dom.13117
30. Eto T, Inoue S, Kadowaki T. Effects of once-daily teneligliptin on 24-h blood glucose control and safety in Japanese patients with type 2 diabetes mellitus: A 4-week, randomized, double-blind, placebo-controlled trial. Diabetes Obes Metab (2012) 14:1040–6. doi: 10.1111/j.1463-1326.2012.01662.x
31. Farngren J, Persson M, Ahren B. Effects on the glucagon response to hypoglycaemia during DPP-4 inhibition in elderly subjects with type 2 diabetes: A randomized, placebo-controlled study. Diabetes Obes Metab (2018) 20:1911–20. doi: 10.1111/dom.13316
32. Forst T, Falk A, Andersen G, Fischer A, Weber MM, Voswinkel S, et al. Effects on alpha- and beta-cell function of sequentially adding empagliflozin and linagliptin to therapy in people with type 2 diabetes previously receiving metformin: An exploratory mechanistic study. Diabetes Obes Metab (2017) 19:489–95. doi: 10.1111/dom.12838
33. Henry RR, Smith SR, Schwartz SL, Mudaliar SR, Deacon CF, Holst JJ, et al. Effects of saxagliptin on beta-cell stimulation and insulin secretion in patients with type 2 diabetes. Diabetes Obes Metab (2011) 13:850–8. doi: 10.1111/j.1463-1326.2011.01417.x
34. Jadzinsky M, Pfutzner A, Paz-Pacheco E, Xu Z, Allen E, Chen R. Saxagliptin given in combination with metformin as initial therapy improves glycaemic control in patients with type 2 diabetes compared with either monotherapy: A randomized controlled trial. Diabetes Obes Metab (2009) 11:611–22. doi: 10.1111/j.1463-1326.2009.01056.x
35. Kadowaki T, Kondo K. Efficacy, safety and dose-response relationship of teneligliptin, a dipeptidyl peptidase-4 inhibitor, in Japanese patients with type 2 diabetes mellitus. Diabetes Obes Metab (2013) 15:810–8. doi: 10.1111/dom.12092
36. Kadowaki T, Kondo K. Efficacy and safety of teneligliptin added to glimepiride in Japanese patients with type 2 diabetes mellitus: A randomized, double-blind, placebo-controlled study with an open-label, long-term extension. Diabetes Obes Metab (2014) 16:418–25. doi: 10.1111/dom.12235
37. Tremblay AJ, Lamarche B, Deacon CF, Weisnagel SJ, Couture P. Effect of sitagliptin therapy on postprandial lipoprotein levels in patients with type 2 diabetes. Diabetes Obes Metab (2011) 13:366–73. doi: 10.1111/j.1463-1326.2011.01362.x
38. Kikuchi M, Abe N, Kato M, Terao S, Mimori N, Tachibana H. Vildagliptin dose-dependently improves glycemic control in Japanese patients with type 2 diabetes mellitus. Diabetes Res Clin Pract (2009) 83:233–40. doi: 10.1016/j.diabres.2008.10.006
39. Sjostrand M, Iqbal N, Lu J, Hirshberg B. Saxagliptin improves glycemic control by modulating postprandial glucagon and c-peptide levels in Chinese patients with type 2 diabetes. Diabetes Res Clin Pract (2014) 105:185–91. doi: 10.1016/j.diabres.2014.05.006
40. Rauch T, Graefe-Mody U, Deacon CF, Ring A, Holst JJ, Woerle HJ, et al. Linagliptin increases incretin levels, lowers glucagon, and improves glycemic control in type 2 diabetes mellitus. Diabetes Ther (2012) 3:10. doi: 10.1007/s13300-012-0010-y
41. Iwamoto Y, Taniguchi T, Nonaka K, Okamoto T, Okuyama K, Arjona Ferreira JC, et al. Dose-ranging efficacy of sitagliptin, a dipeptidyl peptidase-4 inhibitor, in Japanese patients with type 2 diabetes mellitus. Endocr J (2010) 57:383–94. doi: 10.1507/endocrj.k09e-272
42. Nishimura A, Usui S, Kumashiro N, Uchino H, Yamato A, Yasuda D, et al. Efficacy and safety of repaglinide added to sitagliptin in Japanese patients with type 2 diabetes: A randomized 24-week open-label clinical trial. Endocr J (2016) 63:1087–98. doi: 10.1507/endocrj.EJ16-0291
43. Hansen L, Iqbal N, Ekholm E, Cook W, Hirshberg B. Postprandial dynamics of plasma glucose, insulin, and glucagon in patients with type 2 diabetes treated with saxagliptin plus dapagliflozin add-on to metformin therapy. Endocr Pract (2014) 20:1187–97. doi: 10.4158/EP14489.OR
44. Van Raalte DH, van Genugten RE, Eliasson B, Moller-Goede DL, Mari A, Tura A, et al. The effect of alogliptin and pioglitazone combination therapy on various aspects of beta-cell function in patients with recent-onset type 2 diabetes. Eur J Endocrinol (2014) 170:565–74. doi: 10.1530/EJE-13-0639
45. Ahren B, Schweizer A, Dejager S, Dunning BE, Nilsson PM, Persson M, et al. Vildagliptin enhances islet responsiveness to both hyper- and hypoglycemia in patients with type 2 diabetes. J Clin Endocrinol Metab (2009) 94:1236–43. doi: 10.1210/jc.2008-2152
46. Hollander P, Li J, Allen E, Chen R. Saxagliptin added to a thiazolidinedione improves glycemic control in patients with type 2 diabetes and inadequate control on thiazolidinedione alone. J Clin Endocrinol Metab (2009) 94:4810–9. doi: 10.1210/jc.2009-0550
47. Bunck MC, Poelma M, Eekhoff EM, Schweizer A, Heine RJ, Nijpels G, et al. Effects of vildagliptin on postprandial markers of bone resorption and calcium homeostasis in recently diagnosed, well-controlled type 2 diabetes patients. J Diabetes (2012) 4:181–5. doi: 10.1111/j.1753-0407.2011.00168.x
48. Ahren B, Foley JE, Ferrannini E, Matthews DR, Zinman B, Dejager S, et al. Changes in prandial glucagon levels after a 2-year treatment with vildagliptin or glimepiride in patients with type 2 diabetes inadequately controlled with metformin monotherapy. Diabetes Care (2010) 33:730–2. doi: 10.2337/dc09-1867
49. Forst T, Anastassiadis E, Diessel S, Loffler A, Pfutzner A. Effect of linagliptin compared with glimepiride on postprandial glucose metabolism, islet cell function and vascular function parameters in patients with type 2 diabetes mellitus receiving ongoing metformin treatment. Diabetes Metab Res Rev (2014) 30:582–9. doi: 10.1002/dmrr.2525
50. Alsalim W, Persson M, Ahren B. Different glucagon effects during DPP-4 inhibition versus SGLT-2 inhibition in metformin-treated type 2 diabetes patients. Diabetes Obes Metab (2018) 20:1652–8. doi: 10.1111/dom.13276
51. Scott R, Morgan J, Zimmer Z, Lam RLH, O'Neill EA, Kaufman KD, et al. A randomized clinical trial of the efficacy and safety of sitagliptin compared with dapagliflozin in patients with type 2 diabetes mellitus and mild renal insufficiency: The CompoSIT-r study. Diabetes Obes Metab (2018) 20:2876–84. doi: 10.1111/dom.13473
52. Nakagawa T, Nagai Y, Yamamoto Y, Miyachi A, Hamajima H, Mieno E, et al. Effects of anagliptin on plasma glucagon levels and gastric emptying in patients with type 2 diabetes: An exploratory randomized controlled trial versus metformin. Diabetes Res Clin Pract (2019) 158:107892. doi: 10.1016/j.diabres.2019.107892
53. Akiyama Y, Morita-Ohkubo T, Oshitani N, Ohno Y, Aso Y, Inukai T, et al. Decreased glucagon levels and decreased insulin secretion after sitagliptin versus mitiglinide administration with similar glycemic levels following an oral glucose load: a randomized crossover pharmaceutical mechanistic study. Diabetol Int (2016) 7:25–33. doi: 10.1007/s13340-015-0207-1
54. Okada K, Yagyu H, Kotani K, Yamazaki H, Ozaki K, Takahashi M, et al. Effects of miglitol versus sitagliptin on postprandial glucose and lipoprotein metabolism in patients with type 2 diabetes mellitus. Endocr J (2013) 60:913–22. doi: 10.1507/endocrj.ej13-0019
55. Xiao X, Cui X, Zhang J, Han Z, Xiao Y, Chen N, et al. Effects of sitagliptin as initial therapy in newly diagnosed elderly type 2 diabetics: A randomized controlled study. Exp Ther Med (2016) 12:3002–8. doi: 10.3892/etm.2016.3729
56. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ (2009) 339::b2535. doi: 10.1136/bmj.b2535
57. Higgins J, Green S. Cochrane handbook for systematic reviews of interventions version 5.1.0. In: The cochrane collaboration. London: Wiley Press, (2011). Available at: www.cochranehandbook.org.
58. Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med (2002) 21:1539–58. doi: 10.1002/sim.1186
59. Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ (1997) 315:629–34. doi: 10.1136/bmj.315.7109.629
60. Knop FK, Vilsboll T, Madsbad S, Holst JJ, Krarup T. Inappropriate suppression of glucagon during OGTT but not during isoglycaemic i.v. glucose infusion contributes to the reduced incretin effect in type 2 diabetes mellitus. Diabetologia (2007) 50:797–805. doi: 10.1007/s00125-006-0566-z
61. Bagger JI, Knop FK, Lund A, Holst JJ, Vilsboll T. Glucagon responses to increasing oral loads of glucose and corresponding isoglycaemic intravenous glucose infusions in patients with type 2 diabetes and healthy individuals. Diabetologia (2014) 57:1720–5. doi: 10.1007/s00125-014-3264-2
62. Dunning BE, Foley JE, Ahren B. Alpha cell function in health and disease: influence of glucagon-like peptide-1. Diabetologia (2005) 48:1700–13. doi: 10.1007/s00125-005-1878-0
63. Mathiesen DS, Bagger JI, Bergmann NC, Lund A, Christensen MB, Vilsboll T, et al. The effects of dual GLP-1/GIP receptor agonism on glucagon secretion-a review. Int J Mol Sci (2019) 20(17):4092. doi: 10.3390/ijms20174092
64. Abbas G, Haq QMI, Hamaed A, Al-Sibani M, Hussain H. Glucagon and glucagon-like peptide-1 receptors: Promising therapeutic targets for an effective management of diabetes mellitus. Curr Pharm Des (2020) 26:501–8. doi: 10.2174/1381612826666200131143231
65. Christensen MB, Calanna S, Holst JJ, Vilsboll T, Knop FK. Glucose-dependent insulinotropic polypeptide: blood glucose stabilizing effects in patients with type 2 diabetes. J Clin Endocrinol Metab (2014) 99:E418–26. doi: 10.1210/jc.2013-3644
66. Christensen M, Calanna S, Sparre-Ulrich AH, Kristensen PL, Rosenkilde MM, Faber J, et al. Glucose-dependent insulinotropic polypeptide augments glucagon responses to hypoglycemia in type 1 diabetes. Diabetes (2015) 64:72–8. doi: 10.2337/db14-0440
67. Christensen M, Vedtofte L, Holst JJ, Vilsboll T, Knop FK. Glucose-dependent insulinotropic polypeptide: A bifunctional glucose-dependent regulator of glucagon and insulin secretion in humans. Diabetes (2011) 60:3103–9. doi: 10.2337/db11-0979
68. Degn KB, Juhl CB, Sturis J, Jakobsen G, Brock B, Chandramouli V, et al. One week's treatment with the long-acting glucagon-like peptide 1 derivative liraglutide (NN2211) markedly improves 24-h glycemia and alpha- and beta-cell function and reduces endogenous glucose release in patients with type 2 diabetes. Diabetes (2004) 53:1187–94. doi: 10.2337/diabetes.53.5.1187
69. Garber A, Henry R, Ratner R, Garcia-Hernandez PA, Rodriguez-Pattzi H, Olvera-Alvarez I, et al. Liraglutide versus glimepiride monotherapy for type 2 diabetes (LEAD-3 mono): A randomised, 52-week, phase III, double-blind, parallel-treatment trial. Lancet (2009) 373:473–81. doi: 10.1016/S0140-6736(08)61246-5
70. Rosenstock J, Perkovic V, Johansen OE, Cooper ME, Kahn SE, Marx N, et al. Effect of linagliptin vs placebo on major cardiovascular events in adults with type 2 diabetes and high cardiovascular and renal risk: The CARMELINA randomized clinical trial. JAMA (2019) 321:69–79. doi: 10.1001/jama.2018.18269
71. Green JB, Bethel MA, Armstrong PW, Buse JB, Engel SS, Garg J, et al. Effect of sitagliptin on cardiovascular outcomes in type 2 diabetes. N Engl J Med (2015) 373:232–42. doi: 10.1056/NEJMoa1501352
Keywords: dipeptidyl peptidase-4 inhibitor, glucagon, hyperglucagonemia, randomized controlled trials, meta-analysis
Citation: Chai S, Zhang R, Zhang Y, Carr RD, Zheng Y, Rajpathak S and Ji L (2022) Effect of dipeptidyl peptidase-4 inhibitors on postprandial glucagon level in patients with type 2 diabetes mellitus: A systemic review and meta-analysis. Front. Endocrinol. 13:994944. doi: 10.3389/fendo.2022.994944
Received: 15 July 2022; Accepted: 21 September 2022;
Published: 12 October 2022.
Edited by:
Francesco Prattichizzo, MultiMedica Holding SpA (IRCCS), ItalyReviewed by:
Phiwayinkosi V. Dludla, South African Medical Research Council, South AfricaTheocharis Koufakis, Aristotle University of Thessaloniki, Greece
Copyright © 2022 Chai, Zhang, Zhang, Carr, Zheng, Rajpathak and Ji. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Linong Ji, amlsbkBiam11LmVkdS5jbg==
 Shangyu Chai
Shangyu Chai Ruya Zhang
Ruya Zhang Ye Zhang
Ye Zhang Richard David Carr2
Richard David Carr2 Linong Ji
Linong Ji