
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Endocrinol. , 08 September 2022
Sec. Gut Endocrinology
Volume 13 - 2022 | https://doi.org/10.3389/fendo.2022.994930
This article is part of the Research Topic The Cross-Talk Between Gut Microbiota and Endogenous Metabolites in Endocrine Diseases View all 9 articles
The prevalence of overweight and obesity in children and adolescents is an increasing public health problem. Pediatric overweight and obesity result from multiple factors, including genetic background, diet, and lifestyle. In addition, the gut microbiota and their metabolites play crucial roles in the progression of overweight and obesity of children. Therefore, we reviewed the roles of gut microbiota in overweight/obese children. The relationship between pediatric overweight/obesity and gut metabolites, such as short-chain fatty acids, medium-chain fatty acids, amino acids, amines, and bile acids, are also summarized. Targeting gut microbiota and metabolites might be a promising strategy for interventions aimed at reducing pediatric overweight/obesity.
Currently, the epidemic of pediatric overweight/obesity is one of the most concerning public health issues. In the past three decades, the prevalence of pediatric overweight/obesity has dramatically increased (1). Overweight/obesity in childhood increases the risk of diet-related noninfectious diseases, which are closely related to cardiovascular events in adulthood (2). Pediatric overweight/obesity is also associated with metabolic diseases (3) and depression (4).
In developed countries, the rates of pediatric overweight/obesity in boys and girls were 23.8% and 22.6% while the rates were 12.9% and 13.4% in developing countries in 2013, respectively (5). In UK, 25.5% children aged 10-11 were obese while 15.4% were overweight, but not obese and the rate of world-wide pediatric obesity is of concerning (6). Epidemiological investigation indicates that the prevalence of overweight/obesity among children and adolescents was 17.8% in the United States (7). It is also estimated that the rate of pediatric overweight/obesity has increased in China from 5.3% in 1995 to 20.5% in 2014 (8), which is positively related to socioeconomic conditions (8, 9). In 2019, 11.1% and 3.6% of Chinese children under six years of age were overweight and obese, respectively, and 34.3% and 7.9% of Chinese children aged 6-17 years were overweight and obese, respectively (10), indicating that one-quarter of Chinese children are overweight/obese (11). In addition, there are significant regional and sex differences in the incidence of pediatric overweight/obesity, with the rate in boys generally higher than in girls. For eastern, southern, northern, central, and western China, Children from southern and northern had the lowest and highest prevalence of overweight and obesity, respectively (12).
Studies have demonstrated a strong correlation between childhood and adult obesity. Compared to non-obese children, obese children are five times more likely to be obese in adulthood (13). Therefore, intervention and research on pediatric overweight/obesity are of great importance.
Excessive energy intake and conversion to lipid accumulation in the body are the main causes of obesity. Therefore, a sensible diet is a key to avoiding obesity. Dietary factors, including high fat, high fructose, and other unhealthy dietary patterns (14, 15), are important obesity-causing factors in addition to genetic background, sleep, mental state, and exercise habits (16). A study found that from childhood to adolescence, the dietary quality decreases significantly in South Carolina, especially the declines in fruit, vegetable and total dairy intake followed by increasing protein intake (17). Another study also found that weight gain is positively related to the intake of potato chips, sugary beverages and red meats, and is inversely related to the intake of vegetables, fruits, nuts, and yogurt (18). In the past thirty years, the transition from a traditional low fat, high carbohydrate diet to a high fat and low carbohydrate diet has been associated with a dramatic increase in the risk of obesity, type 2 diabetes, cardiovascular diseases and colon cancer in China (19). Furthermore, Obesity is also positively correlated to fat intake, especially a diet rich in long-chain saturated fatty acids, which promotes inflammation of multiple organs (20). Children’s sugar intake, such as fructose, is of concern. Dietary fructose promotes hepatic de novo lipogenesis (DNL) via acetate from the gut microbiota (21–23). The World Health Organization recommends that the average daily sugar intake should not exceed 25 g (24, 25). Despite the decrease in the consumption of sugary beverages in children, it is still a critical source of energy intake (26) and is positively related to obesity (27). Although the restriction of carbohydrate intake may benefit short-term body weight control and improve the blood glucose levels of overweight/obese patients, long-term carbohydrate restriction may lead to a reduction in dietary fiber intake and aggravate fatigue, making it difficult to maintain (28). Therefore, further studies on the safety and effectiveness of carbohydrate restriction in children and adolescents are warranted.
Gut microbiota is regarded as an essential factor regulating the process of overweight/obesity as it participates in the energy metabolism of the host and maintains homeostasis of the internal environment. Firmicutes, Bacteroidetes, Proteobacteria, and Actinobacteria are the most dominant bacterial phyla in the human gut microbiota (29, 30). Moreover, low-abundance Verrucomicrobia shows potential benefits on metabolism. With the development of high-throughput sequencing, many studies have shown that an imbalance in gut microbiota is closely related to the progression of overweight/obesity in children (31) (Table 1). Both simple and genetic obesity (such as Prader-Willi Syndrome, PWS) can be relieved by adjusting the dietary structure, partly targeting the gut microbiota (14). Improving the imbalance of the gut microbiota may be an effective way to intervene against overweight/obesity in children (48).
Firmicutes and Bacteroidetes are the two most abundant phyla in human gut microbiota. Firmicutes are gram-positive bacteria with low GC content, including Clostridium, Lactobacillus, and Coprococcus. Bacteroidetes mainly contain Bacteroides, Prevotella, and Desulfuribacillus. It is generally believed that the abundance of Firmicutes in overweight/obese adults increases, whereas the abundance of Bacteroidetes decreases (48, 49), resulting in an increase in Firmicutes: Bacteroidetes ratio. The same result was observed in the gut microbiota of overweight/obese children (32). Compared to normal-weight children, the abundance of Firmicutes in overweight/obese children is positively correlated with body mass index (BMI) (47, 50) whereas the abundance of Bacteroidetes is negatively correlated with BMI (35, 36). Therefore, Firmicutes: Bacteroidetes ratio is positively correlated with overweight/obesity in children (31, 51).
Although Firmicutes: Bacteroidetes ratio is a common index to measure the structure of the gut microbiota, heterogeneity still exists between this ratio and overweight/obesity. Indiani et al. found that the abundance of Bacteroidetes was increased in overweight/obese children along with a decrease of Firmicutes only in some cohorts (35). Another systematic review also indicated that the Firmicutes: Bacteroidetes ratio was not related to pediatric overweight/obesity (52), which might be the result of potential heterogeneity in the roles of Firmicutes and Bacteroidetes. Members of Firmicutes show a greater variation in abundance than Bacteroidetes (31). Since the structure of the gut microbiota in children is still under development, the composition is unstable. Consequently, changes in gut microbiota in overweight/obese children can show an individualized trend (53). Compared to differences in gut microbiota abundance at the phylum level, differences at the genus level and specific metabolites may be more commonly and directly associated with pediatric overweight/obesity (31).
Further research has shown that the abundance of Firmicutes was closely related to inflammatory levels and positively correlated to serum tumor necrosis factor α (TNF-α) levels in obese children (50). The epigenetic effects of Firmicutes are also concerning. During pregnancy, a high Firmicutes abundance is associated with DNA methylation of genes related to lipid metabolism, inflammatory response, and obesity (54). Lactobacillus, a key genus of Firmicutes, is usually considered a probiotic with a long history of application (55). However, the link between Lactobacillus and pediatric overweight/obesity remains paradoxical. The abundance of Lactobacillus is positively related to the risk of pediatric overweight/obesity (33), and fecal Lactobacillus concentrations in children are associated with serum C-reactive protein (51). Lactobacillus colonization predicts a higher risk of overweight/obesity in infants and children (56). Certain members of Lactobacillus, such as Lactobacillus paracasei are protective factors against obesity in children with an unhealthy diet (57, 58). Clostridium is positively associated with BMI in children (34), and is more significant in young adults (59). Bacteroides fragilis is significantly associated with a higher BMI z-score in children, contributing to weight gain during childhood (37).
The abundance of Verrucomicrobia is relatively low in the human gut microbiota. However, recent research has shown that the abundance of Verrucomicrobia was low in obese children (38, 39, 60), and it has vital benefits. Hence, it is a potential probiotic against metabolic inflammation and obesity (61). The anaerobic bacterium Akkermansia muciniphila is the only known member of Verrucomicrobia in human’s intestinal tract (62). Overweight/obese children have lower Akkermansia muciniphila abundance (40), which is also observed in obese adults (63).
Akkermansia muciniphila is a pivotal species in the intestinal mucous layer that produces acetate and propionate (62). The decreased abundance of Akkermansia muciniphila may lead to high intestinal permeability (60), and the protection of interleukin-36 against obesity is partly realized by promoting Akkermansia muciniphila levels (64). Akkermansia muciniphila can prevent diet-induced obesity and demonstrate hepatoprotective effects by downregulating the metabolism of tyrosine, phenylalanine, tryptophan, and their intermediates, all of which have adverse effects. In addition, Akkermansia muciniphila weakens acetyl-CoA oxidation in the citric acid cycle and promotes ketogenesis (63). Surprisingly, oral vancomycin administration can lead to an increase in Akkermansia muciniphila (65). Despite the relatively low abundance of Verrucomicrobia and Akkermansia muciniphila, multiple studies have indicated that their abundance is strongly associated with pediatric and adult obesity.
Proteobacteria include purple photosynthetic bacteria and their relatives. There is a high abundance of Proteobacteria in the feces of obese children, and these bacteria have a significant positive correlation with BMI levels (41, 42). Proteobacteria are also relatively abundant among malnourished children and contain many potentially pathogenic species that induce immature intestines or potentially high inflammatory burdens (66). Of note, physical exercise significantly reduces the abundance of Proteobacteria in obese children (67).
Proteobacteria mainly include Alphaproteobacteria, Betaproteobacteria, Gammaproteobacteria, delta/epsilon subdivisions, and Zetaproteobacteria, among which Gammaproteobacteria participates in the metabolism of choline, and it has a high abundance in overweight/obese children with non-alcoholic fatty liver disease (NAFLD) (68, 69). Enterobacteriaceae, a member of Gammaproteobacteria, is commonly observed in overweight adolescents (43). The abundance of Escherichia coli is also significantly higher in obese children (44). Escherichia coli can produce alcohol as an endotoxin that can impair hepatic metabolism (60). Endogenous alcohol exaggerates the metabolic burden on the liver, which is of great importance in obese children with non-alcoholic hepatitis (NASH) (42). However, it has been shown that a decrease in Escherichia coli is related to higher lipopolysaccharide (LPS) levels (59).
The abundance of Actinobacteria is negatively correlated with children’s BMI (45, 46). Actinomycetales, an order of Actinobacteria, is positively related to hemoglobin concentration in anemic infants (70). Bifidobacterium is a well-known probiotic of Actinobacteria, which can promote the development and maturation of infant intestinal mucosa, thereby lowering the incidence of diarrhea (71). Additionally, Bifidobacterium inhibits the growth of adverse microbiota via competitive colonization, demonstrating an antagonistic relationship with Enterobacteria and Enterococci (72). The abundance of Bifidobacterium is also high in the intestines of vegetarians (45). Bifidobacterium carries genes encoding bacterial bile salt hydrolase (BSH) (73), which increases the excretion of bile acid and simultaneously inhibits the absorption of cholesterol. Furthermore, Bifidobacterium also produces short-chain fatty acids (SCFAs) (45).
The abundance of Bifidobacterium is negatively correlated with BMI in children. Studies have shown that the abundance of Bifidobacterium in overweight/obese children is significantly lower than that in children of normal weight, and it is hypothesized to participate in fat accumulation and obesity (47). During weight loss, Bifidobacterium abundance rebounds (58). Bifidobacterium infantis metabolizes human milk oligosaccharides (HMO) and suppresses HMO uptake by pathogenic microbes. Increased levels of SCFAs stimulate the immune response and regulate the function of pancreatic β cells (74). Introducing Bifidobacterium, as a dietary supplement, is one strategy that reduces pediatric obesity (75). Treatment with Bifidobacterium breve BR03 and B632 significantly improves insulin sensitivity in obese children and adolescents (76). Supplementation with Bifidobacterium pseudocatenulatum CECT 7765 can improve the inflammatory response in obese children with insulin resistance (IR) (77). Interestingly, other probiotics, such as Lactobacillus casei, can also upregulate the abundance of Bifidobacterium in obese children, exhibiting a synergistic effect (78).
The effects of the gut microbiota are mainly mediated by the absorption and distribution of their metabolites (79). The gut microbiota can produce dozens of metabolites that enter the bloodstream to have a systemic effect on the host (80). There are new evidences to support the association between obesity and these metabolites, including SCFAs, medium-chain fatty acids (MCFAs) (Figure 1), amino acids, amines, and bile acids (Figure 2). The gut microbiota and metabolites in obese people can significantly change compared to those in people with normal weight. A decrease in the abundance of Bacteroides thetaiotaomicron, which is capable of metabolizing glutamate, results in a higher risk of obesity, and the gut microbiota in obese adolescents has a stronger ability to oxidize carbohydrates (81). Therefore, it is possible to intervene in overweight/obesity by targeting the gut microbiota and its metabolites (82). Changes in the gut microbiota have been shown to be related to pediatric obesity and NAFLD. Biosynthesis of SCFAs, amino acids, and LPS is inversely correlated with IR, whereas peptidoglycan biosynthesis pathways are positively correlated with IR (83).
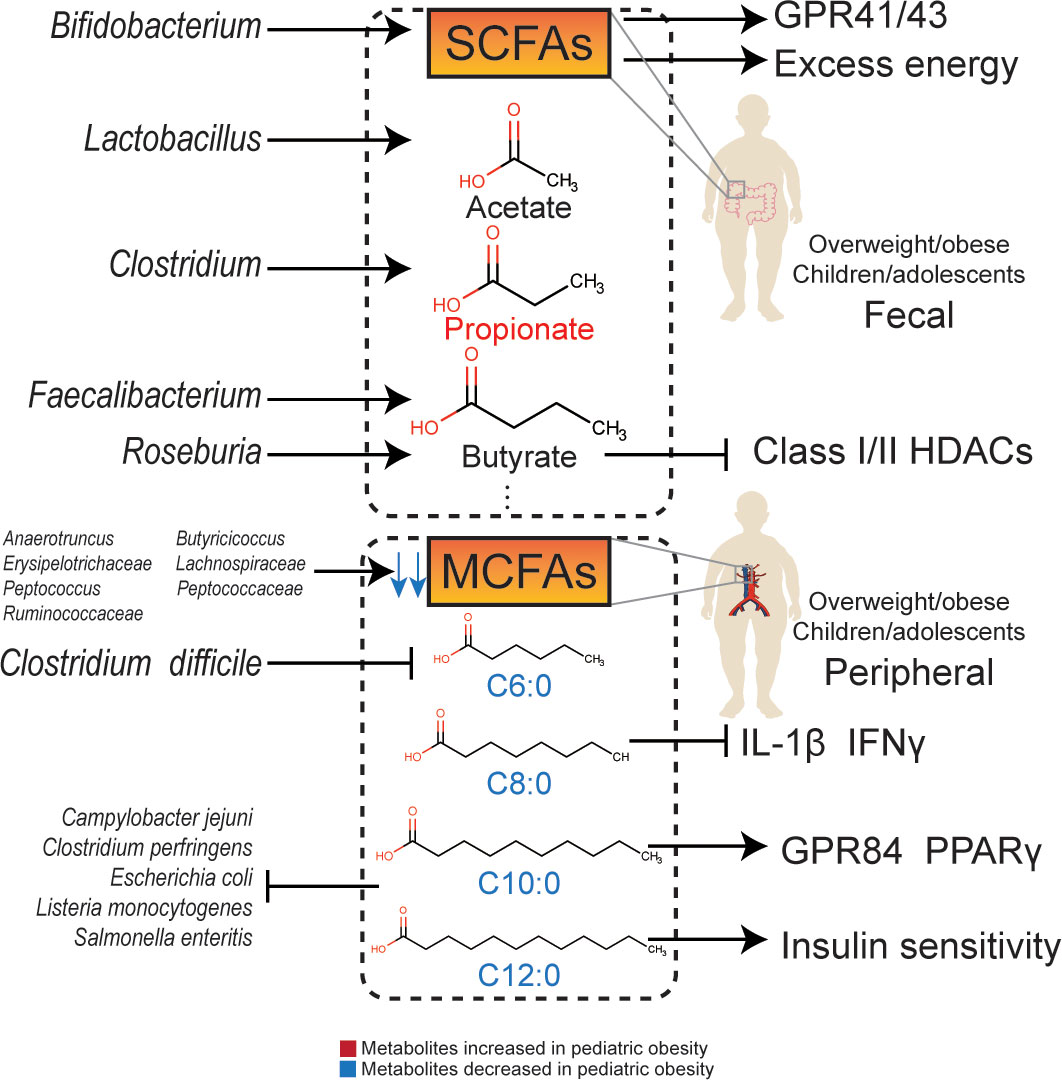
Figure 1 The relationship and molecular signaling between gut microbiota and their metabolites with obesity.
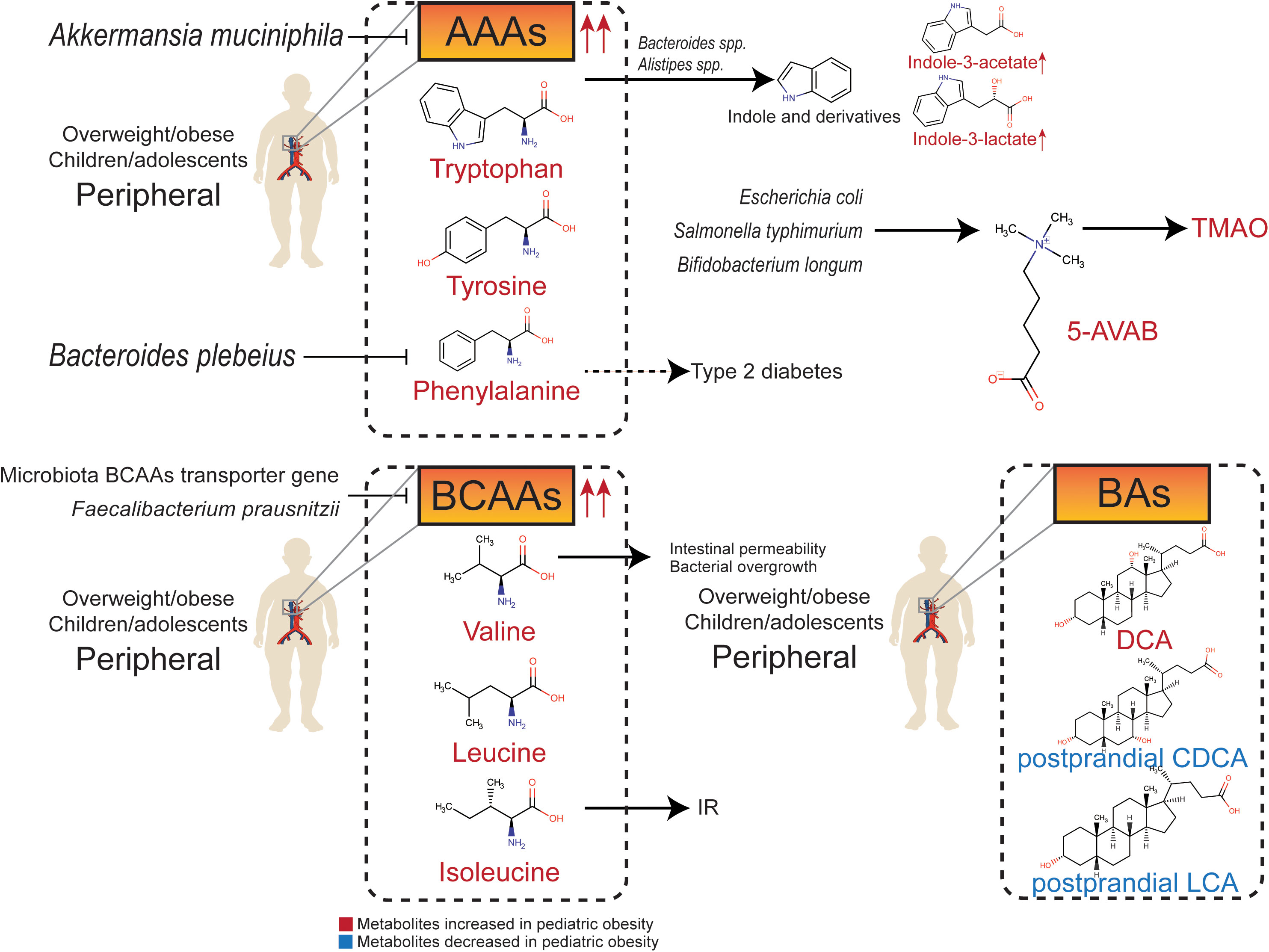
Figure 2 Relationship between gut metabolites (amino acids, amines, and bile acids) and pediatric overweight/obesity.
SCFAs are endpoint products of indigestible complex carbohydrates, such as dietary fiber and resistant starch, fermented by intestinal flora (84). These mainly include organic acids whose carbon chains have less than six carbon atoms, such as acetate, propionate, butyrate, isobutyrate, valerate and isovalerate. Among them, acetate, propionate, and butyrate have the highest concentrations, with an approximate proportion of 3:1:1. SCFAs can regulate the function of adipose tissue and be used as substrates for gluconeogenesis and DNL in the liver (85). Plasma acetate, propionate, and butyrate levels are associated with BMI and visceral fat in adolescents (81). Increased levels of SCFAs may prevent gastrointestinal dysfunction, obesity, and type 2 diabetes (86).
Acetate can combine with G-protein-coupled receptor 41 (GPR41) and G-protein-coupled receptor 43 (GPR43) to regulate metabolism (87). In addition, acetate can be converted into acetyl-CoA, which enters the tricarboxylic acid cycle and participates in energy metabolism. Acetate may also affect metabolism by regulating AMP-activated protein kinase (AMPK) phosphorylation, and increase fatty acid synthesis through epigenetic mechanisms. Serum acetate levels are negatively correlated with fasting insulin levels (88). The concentration of acetate in the feces of obese children is low and is normalized after dietary intervention (14) or treatment with Lactobacillus casei, an acetate-producing bacterium (78). In contrast, the concentration of propionate in feces is positively correlated with the waistline of female adolescents, indicating an adverse metabolic effect (89). In obese adolescents, the rate of acetate in peripheral circulation is lower than that in lean individuals (90). However, after intervention with Bifidobacterium breve BR03 and B632, the concentration of fecal acetate in obese children was lower than that in a placebo group. Since Bifidobacterium breve is an acetate-producing strain, the possibility of acetate being absorbed and utilized by other acetate-dependent species needs to be considered (76).
Propionate is a metabolite partly from Clostridium, which is positively related to pediatric overweight/obesity (91). The concentration of propionate in feces is positively related to fasting blood glucose and glycosylated hemoglobin levels (92), and also related to an increased risk of type 2 diabetes (93). Compared to breastfed children, children who underwent no exposure to human milk have higher concentrations of propionate in feces (94). The serum propionate level is positively associated with BMI in obese children (81), and the fecal propionate concentration is also significantly increased in overweight/obese children (95, 96), which is consistent with that in overweight/obese adult (97). Moreover, concentrations of lactate, as an substrate of propionate metabolism, (98) are decreased in obese children, indicating that propionate metabolism was activated by gut microbiota (99).
Butyrate is an effective regulator of energy metabolism and immune functions (100). Faecalibacterium prausnitzii and Roseburia hominis are important butyrate-producing strains (101). The butyrate-producing ability of Faecalibacterium prausnitzii is essential for gastrointestinal and metabolic health (98). A decrease in butyrate-producing strains in obese children has been observed (83), and fecal butyrate concentration is negatively related to gut microbiota diversity, which also affects intestinal permeability (102). In addition to GPR41/43, butyrate can regulate metabolism through β-oxidation (103) and inhibit class I/II histone deacetylases (HDACs) (104). Butyrate can also promote the secretion of glucagon-like peptide-1 (GLP-1), thereby enhancing insulin sensitivity (105).
The concentration of SCFAs in obese children is low (106), however, some studies have shown high levels of acetate, propionate, butyrate, and isovalerate in obese children (31, 38, 99), which are positively correlated with BMI z-scores (31). Despite the potential benefits of SCFAs, the overall function of SCFAs in pediatric overweight/obesity remains unclear. SCFAs can be double-edged swords: on the one hand, excess SCFAs are an extra energy source, participating in the process of pediatric overweight/obesity; while, on the other hand, SCFAs promote insulin excretion via the GPR41/43 pathway (107).
The relationship between pediatric overweight/obesity and MCFAs were verified by epidemiological evidences. MCFAs seem to be a protective factor for pediatric overweight/obesity. A study has shown that the concentration of caproic acid (C6:0) in serum, which is also known as hexanoic acid, was significantly decreased in obese children (108). Caproic acid is also significantly decreased in patients suffering from Clostridium difficile infection, which is positively related to pediatric overweight/obesity (109). The capric acid (C10:0) level in obese children is significantly lower than that in normal-weight children (110). Compared to neonates with low adiposity, the dodecanoic acid (C12:0) level in neonates with high adiposity is significantly lower (100).
It is difficult for MCFAs from food to reach the cecum completely due to the presence of gut microbiota. Recently, an animal experiment conducted by Gregor et al. (111) observed MCFAs production by gut microbiota. MCFAs not only metabolize quickly to produce energy in the colon but can also enter the liver or adipose tissue. Gut microbiota, including Erysipelotrichaceae, Peptococcaceae, Anaerotruncus, Butyricicoccus, Lachnospiraceae, Peptococcus, and Ruminococcaceae are positively correlated with the concentrations of MCFAs in the cecum. In addition, studies have shown that Caproiciproducens, Pseudoramibacter, norank_f_Eubacteriaceae, and Oscillibacter catabolize lactate into MCFAs (112).
Zhao et al. found that caprylic acid (C8:0) downregulated the serum levels of interleukin-1β (IL-1β) and interferon γ (IFNγ) and increased the abundance of Lachnoclostridium, Roseburia, and Prevotella_9, activating GPR43 and improving intestinal permeability (113). Capric acid (C10:0) can upregulate GPR84 and peroxisome proliferator-activated receptor γ (PPARγ) (114). Dodecanoic acid (C12:0), also known as lauric acid, can improve insulin sensitivity (100). Although the difference between SCFAs and MCFAs is only a few carbon atoms, they demonstrate different immune regulatory mechanisms. SCFAs downregulate levels of IL-1β, IL-6, and TNFα via toll-like receptor 4 (TLR4), whereas MCFAs may enhance the inflammatory response through TLR2 (114).
Apart from immune regulation, MCFAs also inhibit various intestinal pathogenic microorganisms. Both capric acid and dodecanoic acid inhibit the growth of Listeria monocytogenes, Clostridium perfringens, Escherichia coli, Salmonella enteritis, and Campylobacter jejuni (115). Dodecanoic acid can also inhibit the growth of Staphylococcus aureus (116).
MCFAs have potential therapeutic effects on genetic obesity during the childhood. Dietary supplementation with MCFAs can alleviate adipose accumulation in mice (117). More importantly, patients with PWS diagnosed in early infancy responded with the improvement of motor development and nutritional conditions after dietary supplementation with MCFAs (118).
In addition to SCFAs and MCFAs, metabolites of the gut microbiota include nitrogenous compounds produced from amino acids. There are two main pathways of amino acid metabolism: deamination to carboxylic acid and ammonia, and decarboxylation to CO2 and amine. The most abundant end products of catabolism are SCFAs (119). Other metabolites, such as amino acids and amines, also affect host health.
Methionine and cysteine are sulfur-containing amino acids that are catabolized into H2S and methyl mercaptan by Bacillus and Bifidobacterium (119). Cysteine exhibits anti-diabetic properties. Parasutterella sp. is one of the main consumers of cysteine. The abundance of Parasutterella sp. is negatively related to the serum concentration of cysteine, and is significantly downregulated in weight-loss-obesity patients (120). Methionine biosynthesis mediated by gut microbiota is associated with atherosclerosis in obese children (121). The ability to utilize sulfur-containing compounds is essential for gut microbiota. Sulfatases and radical S-adenosyl-L-methionine synthetase play vital roles in the colonization of microorganisms in the intestinal tract (122).
The ability of intestinal flora to metabolize aromatic amino acids (AAAs) and branched-chain amino acids (BCAAs) is significantly increased in obese individuals (123). AAAs, BCAAs, and their downstream metabolites produced by the gut microbiota can interfere with glucose homeostasis and contribute to IR (124).
The AAAs mainly include tryptophan, phenylalanine, and tyrosine. They are essential to the intestinal tract and greatly contribute to gut dysfunction. The abundance of tryptophan derivative metabolites in plasma is significantly altered in obese children (125). Indole is produced by gut microbiota-mediated fermentation of tryptophan, which is an obesity-promoting metabolite that targets the brain-gut axis (126). Harmful tryptophan metabolites, such as indole derivatives, are produced partly by the tryptophanase of Bacteroides spp. and Alistipes spp. (14). The serum concentrations of indole-3-lactate and indole-3-acetate in obese children are increased (127). Furthermore, obese children have higher levels of serum phenylalanine, which is positive associations with the risk of type 2 diabetes (128), whereas, Bacteroides plebeius is negatively related to serum phenylalanine concentration in children (129). Both tryptophan metabolism and phenylalanine metabolism regulated by gut microbiota are significantly downregulated after dietary intervention in both simple and genetic obese children (14). The serum concentration of tyrosine is significantly decreased in obese children with substantial weight reduction (130). Furthermore, the potential probiotic Akkermansia muciniphila can decrease serum levels of diverse intermediates in tryptophan, phenylalanine, and tyrosine metabolism in obese individuals (63).
BCAAs include leucine, isoleucine, and valine. The proportion of isoleucine in total protein intake is positively correlated with BMI (21), so dietary restriction of BCAAs may help prevent childhood obesity and IR (131). Plasma BCAAs levels are significantly higher in obese children and adolescents, and are consistent in children from multiple ethnic backgrounds (132–136), which can predict the risk of IR and metabolic syndrome independently (133, 134). Metabolic analysis based on BCAAs can predict hepatic steatosis grading in high-risk children and adolescents (137). Valine metabolites are associated with increased intestinal permeability and bacterial overgrowth in the small intestine in children (138). The transporter gene of BCAAs is negatively correlated with serum levels of BCAAs in adolescents, suggesting that the gut microbiota may reduce the level of BCAAs in the peripheral circulation. In contrast, Faecalibacterium prausnitzii regulates IR through BCAAs metabolism (139).
Trimethylamine N-oxide (TMAO) is a hazardous metabolite that originates from trimethylamine, which is produced in the liver. The urine concentration of TMAO is significantly higher in children with simple and genetic obesity (14). 5-amino valeric acid betaine (5-AVAB), also known as δ-Valerobetaine, is a precursor of TMAO produced by Escherichia coli, Salmonella typhimurium, and Bifidobacterium longum (140). 5-AVAB affects the carnitine shuttle system of mitochondria, lowering the ability of hepatocytes to oxidize fatty acids, and resulting in lipid accumulation. Obese individuals have higher plasma 5-AVAB concentrations, which are also positively related to hepatic steatosis in children with NAFLD (141).
Cholesterol is oxidized to primary bile acid in hepatocytes through classical and alternative pathways to produce cholic acid (CA) and chenodeoxycholic acid (CDCA), respectively. CA and CDCA combine with glycine and taurine to generate glycocholic acid (GCA), taurocholic acid (TCA), glycochenodeoxycholic acid (GCDCA), and taurochenodeoxycholic acid (TCDCA), respectively. Primary bile acids enter the intestinal tract via the biliary system. Glycine and taurine are dissociated under the catalysis of gut microbiota BSH, and a series of secondary bile acids are formed, such as lithocholic acid (LCA), hyocholic acid (HCA), hyodeoxycholic acid (HDCA), deoxycholic acid (DCA), and ursodeoxycholic acid (UDCA). Bile acids are released into the small intestine to promote the intake of lipids and fat-soluble vitamins. The imbalance in the composition and spectrum of bile acids is related to obesity. Therefore, the regulation of bile acids may be a potential strategy to intervene against obesity (142).
Most of the secondary bile acids produced by intestinal microflora can enter the liver through the portal vein, and only a few of them are excreted through feces. Therefore, secondary bile acids can regulate hepatic steatosis and NAFLD, which is highly associated with obesity. A sex-specific study in mice exposed to high fructose showed that both primary and secondary bile acids decreased in female mice, but there was no similar trend in male mice (143). In overweight children with NAFLD, serum total bile acids, especially glycine-binding bile acids such as GCDCA, GCA, glycodeoxycholic acid (GDCA), and glycoursodeoxycholic acid (GUDCA), are lower than those in cohorts with normal BMI (144). Compared to obese children without NAFLD, obese children with NAFLD have higher levels of serum total bile acids and glycine-bound bile acids (145).
Secondary bile acids have complex and unelucidated biological functions. Studies have illustrated that secondary bile acids regulated by high fat diet has adverse metabolic effects (146). DCA tends to increase in diet-related or genetic childhood obesity (147). Contrary to previous study in obese adults (148), levels of postprandial bile acids are significantly lower in obese adolescents. Serum levels of non-12-OH bile acids, including CDCA and LCA, and intermediates in bile acids synthesis are lower in adolescents with obesity (149). UDCA is a drug used for the treatment of NAFLD, while low DCA is considered to be characteristic of a healthy metabolic spectrum of bile acids (73). UDCA supplementation can control diet-induced obesity in prenatally malnourished mice (150). In obese mice, the abundance of Lactobacillaceae and Lachnospiraceae producing secondary bile acids is higher and leads to higher levels of LCA and DCA (151). The proportion of non-12-OH bile acids, including HCA, HDCA, glycohyodeoxycholic acid (GHDCA), UDCA, GUDCA, and CDCA, in total bile acid, is significantly lower in people with high BMI, indicating that non-12-OH bile acids may contribute to the process of obesity. In addition, the ratio of CA and DCA is significantly higher, and the ratio of CDCA and UDCA is significantly lower in the population with high BMI, while fecal total bile acid is not significantly different in those with high BMI group compared with healthy people (152).
The gut microbiota of obese patients differs from that in healthy people. Abnormalities in the gut microbiota and their metabolites are involved in the occurrence of obesity. An early difference in fecal microbiota in children may predict the occurrence of overweight, as this has a profound impact on the function of the digestive system (153). However, a single acquired factor or genetic factor is not sufficient to fully explain the changes in gut microbiota and metabolites in overweight/obese children. The adaptive changes in the structure of gut microbiota in early life are also related to long-term health problems, such as mucosal immune development and height retardation (154). Factors such as mode of delivery, diet, and breastfeeding can cause changes in the gut microbiota, which in turn can increase the risk of pediatric overweight/obesity (155). Therefore, gut microbiota in the early stages of life should play an important role in overweight and obese children.
Maternal gut microbiota has a long-lasting effect on the gut microbiota and metabolites of offspring. One of the key determinants is that Bacteroides abundance is reduced and delays colonization (156), which results in lower microbial genes associated with amino acids and nucleic acids metabolism, and higher genes associated with fatty acid metabolism and amino acid degradation (157). In addition, despite the low abundance of fungi in the gut microbiota, fungal host phenotypes can be transferred from parents to offspring, and fungal diversity and species composition in offspring may develop towards the fungal community of parents (158).
Breastfeeding is independently associated with infant gut microbiota diversity, which benefits the infant’s immune system by specifically providing nutrients to the microbes to form healthier immune-microbe relationships (159). For instance, deficiency of Bifidobacterium and its HMO-utilizing gene is related to systemic inflammation and immune dysregulation in early life. Feces from Bifidobacterium infantis EVC001-supplemented infants are rich in indole lactate and indole-3-lactate, which upregulate Galectin1 expression during Th2 and Th17 cell polarization (160). Supplementation with Lactobacillus rhamnosus HN001 in mothers during pregnancy or breastfeeding can reduce eczema and allergy rates in babies (161). Maternal obesity may also affect the ability of offspring gut microbiota to metabolize BCAAs (135).
While the structural characteristics of gut microbiota in obese children have been described in detail, there is still variation in the abundance of some bacteria. Changes in metabolic function caused by these variations need to be considered. Although probiotics are generally believed to be beneficial to the metabolism of children, current studies have not reached a consensus. An Iranian study found that probiotics could improve liver function in children with obesity. However, other studies have found that probiotics seem to increase obesity in Hispanic adolescents (162). Therefore, it is necessary to screen for probiotics in the treatment of childhood obesity.
Numerous mechanisms that associate intestinal flora with the risk of obesity and metabolic disorders are based on the findings of rodent models, but the structure of intestinal flora in rodents is quite different from that in humans (163). On the other hand, at present, research on gut microbiota and metabolites is still mainly focused on obese adults, while the research on obese children is relatively deficient. Due to the large differences in the structure of the gut microbiota between adults and children, future cohort studies on gut microbiota and metabolites in obese children are required.
YD conceived, designed, and supervised the manuscript. SZ wrote the paper. YD edited and revised the paper. All authors reviewed and approved the final manuscript. All authors contributed to the article and approved the submitted version.
This work was supported by the Shanghai Rising-Star Program (21QA1409000), and the Shanghai Frontier Research Base of Disease and Syndrome Biology of Inflammatory cancer transformation (2021KJ03-12).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Lobstein T, Jackson-Leach R, Moodie ML, Hall KD, Gortmaker SL, Swinburn BA, et al. Child and adolescent obesity: Part of a bigger picture. Lancet (2015) 385:2510–20. doi: 10.1016/S0140-6736(14)61746-3
2. Jacobs DR, Woo JG, Sinaiko AR, Daniels SR, Ikonen J, Juonala M, et al. Childhood cardiovascular risk factors and adult cardiovascular events. N Engl J Med. (2022) 386:1877–88. doi: 10.1056/NEJMoa2109191
3. Kelly AS, Barlow SE, Rao G, Inge TH, Hayman LL, Steinberger J, et al. Severe obesity in children and adolescents: Identification, associated health risks, and treatment approaches: A scientific statement from the American heart association. Circulation (2013) 128:1689–712. doi: 10.1161/CIR.0b013e3182a5cfb3
4. Sutaria S, Devakumar D, Yasuda SS, Das S, Saxena S. Is obesity associated with depression in children? systematic review and meta-analysis. Arch Dis Child. (2019) 104:64–74. doi: 10.1136/archdischild-2017-314608
5. Ng M, Fleming T, Robinson M, Thomson B, Graetz N, Margono C, et al. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980-2013: A systematic analysis for the global burden of disease study 2013. Lancet (2014) 384:766–81. doi: 10.1016/S0140-6736(14)60460-8
6. Jebeile H, Kelly AS, O’Malley G, Baur LA. Obesity in children and adolescents: epidemiology, causes, assessment, and management. Lancet Diabetes Endocrinol (2022) 10:351–65. doi: 10.1016/S2213-8587(22)00047-X
7. Ogden CL, Fryar CD, Hales CM, Carroll MD, Aoki Y, Freedman DS. Differences in obesity prevalence by demographics and urbanization in US children and adolescent2013-2016. JAMA (2018) 319:2410–8. doi: 10.1001/jama.2018.5158
8. Dong Y, Jan C, Ma Y, Dong B, Zou Z, Yang Y, et al. Economic development and the nutritional status of Chinese school-aged children and adolescents from 1995 to 2014: An analysis of five successive national surveys. Lancet Diabetes Endocrinol (2019) 7:288–99. doi: 10.1016/S2213-8587(19)30075-0
9. Jia P, Xue H, Zhang J, Wang Y. Time trend and demographic and geographic disparities in childhood obesity prevalence in China-evidence from twenty years of longitudinal data. Int J Environ Res Public Health (2017) 14:E369. doi: 10.3390/ijerph14040369
10. Pan XF, Wang L, Pan A. Epidemiology and determinants of obesity in China. Lancet Diabetes Endocrinol (2021) 9:373–92. doi: 10.1016/S2213-8587(21)00045-0
11. Luo D, Song Y. Socio-economic inequalities in child growth: identifying orientation and forward-looking layout. Lancet Reg Health West. Pac. (2022) 21:100412. doi: 10.1016/j.lanwpc.2022.100412
12. Zhang L, Chen J, Zhang J, Wu W, Huang K, Chen R, et al. Regional disparities in obesity among a heterogeneous population of Chinese children and adolescents. JAMA Netw Open (2021) 4:e2131040. doi: 10.1001/jamanetworkopen.2021.31040
13. Simmonds M, Llewellyn A, Owen CG, Woolacott N. Predicting adult obesity from childhood obesity: a systematic review and meta-analysis. Obes Rev (2016) 17:95–107. doi: 10.1111/obr.12334
14. Zhang C, Yin A, Li H, Wang R, Wu G, Shen J, et al. Dietary modulation of gut microbiota contributes to alleviation of both genetic and simple obesity in children. EBiomedicine (2015) 2:968–84. doi: 10.1016/j.ebiom.2015.07.007
15. Zhou P, Li R, Liu K. The neighborhood food environment and the onset of child-hood obesity: A retrospective time-trend study in a mid-sized city in China. Front Public Health (2021) 9:688767. doi: 10.3389/fpubh.2021.688767
16. Zhang Y, Zhang X, Li J, Zhong H, Pan CW. Associations of outdoor activity and screen time with adiposity: findings from rural Chinese adolescents with relatively low adiposity risks. BMC Public Health (2020) 20:1769. doi: 10.1186/s12889-020-09897-7
17. Ross SET, Militello G, Dowda M, Pate RR. Changes in diet quality in youth living in south Carolina from 5th to 11th grade. J Nutr Educ Behav (2020) 52:928. doi: 10.1016/j.jneb.2020.03.001
18. Mozaffarian D, Hao T, Rimm EB, Willett WC, Hu FB. Changes in diet and lifestyle and long-term weight gain in women and men. N Engl J Med (2011) 364:2392–404. doi: 10.1056/NEJMoa1014296
19. Wilson AS, Koller KR, Ramaboli MC, Nesengani LT, Ocvirk S, Chen C, et al. Diet and the human gut microbiome: An international review. Dig Dis Sci (2020) 65:723–40. doi: 10.1007/s10620-020-06112-w
20. Basson AR, Chen C, Sagl F, Trotter A, Bederman I, Gomez-Nguyen A, et al. Regulation of intestinal inflammation by dietary fats. Front Immunol (2020) 11:604989. doi: 10.3389/fimmu.2020.604989
21. Yu D, Richardson NE, Green CL, Spicer AB, Murphy ME, Flores V, et al. The adverse metabolic effects of branched-chain amino acids are mediated by isoleucine and valine. Cell Metab (2021) 33:905–922.e6. doi: 10.1016/j.cmet.2021.03.025
22. Zhao S, Jang C, Liu J, Uehara K, Gilbert M, Izzo L, et al. Dietary fructose feeds hepatic lipogenesis via microbiota-derived acetate. Nature (2020) 579:586–91. doi: 10.1038/s41586-020-2101-7
23. Yu S, Li C, Ji G, Zhang L. The contribution of dietary fructose to non-alcoholic fatty liver disease. Front Pharmacol (2021) 12:783393. doi: 10.3389/fphar.2021.783393
24. Jin C, Lin L, Li C, Peng Y, MacGregor GA, He F, et al. The sugar and energy in non-carbonated sugar-sweetened beverages: A cross-sectional study. BMC Public Health (2019) 19:1141. doi: 10.1186/s12889-019-7486-6
25. Lin L, Li C, Jin C, Peng Y, Hashem KM, MacGregor GA, et al. Sugar and energy content of carbonated sugar-sweetened beverages in haidian district, Beijing: a cross-sectional study. BMJ (Open). (2018) 8:e022048. doi: 10.1136/bmjopen-2018-022048
26. Dai J, Soto MJ, Dunn CG, Bleich SN. Trends and patterns in sugar-sweetened beverage consumption among children and adults by race and/or ethnicity, 2003–2018. Public Health Nutr (2021) 24:2405–10. doi: 10.1017/S1368980021001580
27. Hwang SB, Park S, Jin GR, Jung JH, Park HJ, Lee SH, et al. Trends in beverage consumption and related demographic factors and obesity among Korean children and adolescents. Nutrients (2020) 12:E2651. doi: 10.3390/nu12092651
28. Gow ML, Garnett SP, Baur LA, Lister NB. The effectiveness of different diet strategies to reduce type 2 diabetes risk in youth. Nutrients (2016) 8:486. doi: 10.3390/nu8080486
29. Arumugam M, Raes J, Pelletier E, Le Paslier D, Yamada T, Mende DR, et al. Enterotypes of the human gut microbiome. Nature (2011) 473:174–80. doi: 10.1038/nature09944
30. Gomaa EZ. Human gut microbiota/microbiome in health and diseases: A review. Antonie Van Leeuwenhoek (2020) 113:2019–40. doi: 10.1007/s10482-020-01474-7
31. Riva A, Borgo F, Lassandro C, Verduci E, Morace G, Borghi E, et al. Pediatric obesity is associated with an altered gut microbiota and discordant shifts in f irmicutes populations. Environ Microbiol (2017) 19:95–105. doi: 10.1111/1462-2920.13463
32. Gomes AC, Hoffmann C, Mota JF. The human gut microbiota: Metabolism and perspective in obesity. Gut Microbes (2018) 9:308–25. doi: 10.1080/19490976.2018.1465157
33. Ignacio A, Fernandes MR, Rodrigues VA, Groppo FC, Cardoso AL, Avila-Campos MJ, et al. Correlation between body mass index and faecal microbiota from children. Clin Microbiol Infect (2016) 22:258.e1–8. doi: 10.1016/j.cmi.2015.10.031
34. Nirmalkar K, Murugesan S, Pizano-Zárate ML, Villalobos-Flores LE, García-González C, Morales-Hernández RM, et al. Gut microbiota and endothelial dysfunction markers in obese Mexican children and adolescents. Nutrients (2018) 10:2009. doi: 10.3390/nu10122009
35. Indiani C. M. D. S. P., Rizzardi KF, Castelo PM, Ferraz LFC, Darrieux M, Parisotto TM. Childhood obesity and Firmicutes/Bacteroidetes ratio in the gut microbiota: A systematic review. Child. Obes (2018) 14:501–9. doi: 10.1089/chi.2018.0040
36. Sepp E, Lõivukene K, Julge K, Voor T, Mikelsaar M. The association of gut microbiota with body weight and body mass index in preschool children of Estonia. Microb Ecol Health Dis (2013) 24:19231. doi: 10.3402/mehd.v24i0.19231
37. Scheepers LEJM, Penders J, Mbakwa CA, Thijs C, Mommers M, Arts IC. The intestinal microbiota composition and weight development in children: the KOALA birth cohort study. Int J Obes (Lond). (2015) 39:16–25. doi: 10.1038/ijo.2014.178
38. Gyarmati P, Song Y, Dotimas J, Yoshiba G, Christison A. Cross-sectional comparisons of gut microbiome and short-chain fatty acid levels among children with varied weight classifications. Pediatr Obes (2021) 16:e12750. doi: 10.1111/ijpo.12750
39. Vazquez-Moreno M, Perez-Herrera A, Locia-Morales D, Dizzel S, Meyre D, Stearns JC, et al. Association of gut microbiome with fasting triglycerides, fasting insulin and obesity status in Mexican children. Pediatr Obes (2021) 16:e12748. doi: 10.1111/ijpo.12748
40. Karlsson CLJ, Onnerfält J, Xu J, Molin G, Ahrné S, Thorngren-Jerneck K. The microbiota of the gut in preschool children with normal and excessive body weight. Obes (Silver Spring). (2012) 20:2257–61. doi: 10.1038/oby.2012.110
41. Méndez-Salazar EO, Ortiz-López MG, Granados-Silvestre MLÁ, Palacios-González B, Menjivar M. Altered gut microbiota and compositional changes in firmicutes and proteobacteria in Mexican undernourished and obese children. Front Microbiol (2018) 9:2494. doi: 10.3389/fmicb.2018.02494
42. Zhu L, Baker SS, Gill C, Liu W, Alkhouri R, Baker RD, et al. Characterization of gut microbiomes in nonalcoholic steatohepatitis (NASH) patients: A connection between endogenous alcohol and NASH. Hepatology (2013) 57:601–9. doi: 10.1002/hep.26093
43. Grigorova EV, Belkova NL, Nemchenko UM, Klimenko ES, Pogodina AV, Romanitsa AI, et al. Metasequencing of V3-V4 variable regions of 16S rRNA gene in opportunistic microbiota and gut biocenosis in obese adolescents. Bull Exp Biol Med (2021) 170:321–5. doi: 10.1007/s10517-021-05060-3
44. Gao X, Jia R, Xie L, Kuang L, Feng L, Wan C. Obesity in school-aged children and its correlation with gut e.coli and bifidobacteria: a case-control study. BMC Pediatr (2015) 15:64. doi: 10.1186/s12887-015-0384-x
45. Bai J, Hu Y, Bruner DW. Composition of gut microbiota and its association with body mass index and lifestyle factors in a cohort of 7–18 years old children from the American gut project. Pediatr Obes (2019) 14:e12480. doi: 10.1111/ijpo.12480
46. Del Chierico F, Nobili V, Vernocchi P, Russo A, De Stefanis C, Gnani D, et al. Gut microbiota profiling of pediatric nonalcoholic fatty liver disease and obese patients unveiled by an integrated meta-omics-based approach. Hepatology (2017) 65:451–64. doi: 10.1002/hep.28572
47. Da Silva CC, Monteil MA, Davis EM. Overweight and obesity in children are associated with an abundance of firmicutes and reduction of bifidobacterium in their gastrointestinal microbiota. Child. Obes (2020) 16:204–10. doi: 10.1089/chi.2019.0280
48. Ley RE, Turnbaugh PJ, Klein S, Gordon JI. Microbial ecology: Human gut microbes associated with obesity. Nature (2006) 444:1022–3. doi: 10.1038/4441022a
49. Turnbaugh PJ, Ley RE, Mahowald MA, Magrini V, Mardis ER, Gordon JI. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature (2006) 444:1027–31. doi: 10.1038/nature05414
50. Orbe-Orihuela YC, Lagunas-Martínez A, Bahena-Román M, Madrid-Marina V, Torres-Poveda K, Flores-Alfaro E, et al. High relative abundance of firmicutes and increased TNF-α levels correlate with obesity in children. Salud Publica Mex. (2018) 60:5–11. doi: 10.21149/8133
51. Bervoets L, Van Hoorenbeeck K, Kortleven I, Van Noten C, Hens N, Vael C, et al. Differences in gut microbiota composition between obese and lean children: A cross-sectional study. Gut Pathog (2013) 5:10. doi: 10.1186/1757-4749-5-10
52. Vander Wyst KB, Ortega-Santos CP, Toffoli SN, Lahti CE, Whisner CM. Diet, adiposity, and the gut microbiota from infancy to adolescence: A systematic review. Obes Rev (2021) 22:e13175. doi: 10.1111/obr.13175
53. Hollister EB, Foster BA, Dahdouli M, Ramirez J, Lai Z. Characterization of the stool microbiome in Hispanic preschool children by weight status and time. Child. Obes (2018) 14:122–30. doi: 10.1089/chi.2017.0122
54. Kumar H, Lund R, Laiho A, Lundelin K, Ley RE, Isolauri E, et al. Gut microbiota as an epigenetic regulator: pilot study based on whole-genome methylation analysis. mBio. (2014) 5:e02113–14. doi: 10.1128/mBio.02113-14
55. Doron S, Snydman DR. Risk and safety of probiotics. Clin Infect Dis (2015) 60 Supplement 2:S129–34. doi: 10.1093/cid/civ085
56. Kozyrskyj AL, Kalu R, Koleva PT, Bridgman SL. Fetal programming of overweight through the microbiome: Boys are disproportionately affected. J Dev Orig. Health Dis (2016) 7:25–34. doi: 10.1017/S2040174415001269
57. Castañeda-Márquez AC, Díaz-Benítez CE, Bahena-Roman M, Campuzano-Benítez GE, Galván-Portillo M, Campuzano-Rincón JC, et al. Lactobacillus paracasei as a protective factor of obesity induced by an unhealthy diet in children. Obes Res Clin Pract (2020) 14:271–8. doi: 10.1016/j.orcp.2020.04.005
58. Hou YP, He QQ, Ouyang HM, Peng HS, Wang Q, Li J, et al. Human gut microbiota associated with obesity in Chinese children and adolescents. BioMed Res Int (2017) 2017:7585989. doi: 10.1155/2017/7585989
59. Radilla-Vázquez RB, Parra-Rojas I, Martínez-Hernández NE, Márquez-Sandoval YF, Illades-Aguiar B, Castro-Alarcón N. Gut microbiota and metabolic endotoxemia in young obese Mexican subjects. Obes Facts. (2016) 9:1–11. doi: 10.1159/000442479
60. Miura K, Ohnishi H. Role of gut microbiota and toll-like receptors in nonalcoholic fatty liver disease. World J Gastroenterol (2014) 20:7381–91. doi: 10.3748/wjg.v20.i23.7381
61. Edwards PT, Kashyap PC, Preidis GA. Microbiota on biotics: probiotics, prebiotics, and synbiotics to optimize growth and metabolism. Am J Physiol Gastrointest. Liver Physiol (2020) 319:G382–90. doi: 10.1152/ajpgi.00028.2020
62. Becken B, Davey L, Middleton DR, Mueller KD, Sharma A, Holmes ZC, et al. Genotypic and phenotypic diversity among human isolates of akkermansia muciniphila. mBio. (2021) 12:e00478–21. doi: 10.1128/mBio.00478-21
63. Depommier C, Everard A, Druart C, Maiter D, Thissen JP, Loumaye A, et al. Serum metabolite profiling yields insights into health promoting effect of a. muciniphila in human volunteers with a metabolic syndrome. Gut Microbes (2021) 13:1994270. doi: 10.1080/19490976.2021.1994270
64. Giannoudaki E, Hernandez-Santana YE, Mulfaul K, Doyle SL, Hams E, Fallon PG, et al. Interleukin-36 cytokines alter the intestinal microbiome and can protect against obesity and metabolic dysfunction. Nat Commun (2019) 10:4003. doi: 10.1038/s41467-019-11944-w
65. Basolo A, Hohenadel M, Ang QY, Piaggi P, Heinitz S, Walter M, et al. Effects of underfeeding and oral vancomycin on gut microbiome and nutrient absorption in humans. Nat Med (2020) 26:589–98. doi: 10.1038/s41591-020-0801-z
66. Huey SL, Jiang L, Fedarko MW, McDonald D, Martino C, Ali F, et al. Nutrition and the gut microbiota in 10- to 18-month-old children living in urban slums of Mumbai, India. mSphere. (2020) 5:e00731–20. doi: 10.1128/mSphere.00731-20
67. Quiroga R, Nistal E, Estébanez B, Porras D, Juárez-Fernández M, Martínez-Flórez S, et al. Exercise training modulates the gut microbiota profile and impairs inflammatory signaling pathways in obese children. Exp Mol Med (2020) 52:1048–61. doi: 10.1038/s12276-020-0459-0
68. Zhao Y, Zhou J, Liu J, Wang Z, Chen M, Zhou S. Metagenome of gut microbiota of children with nonalcoholic fatty liver disease. Front Pediatr (2019) 7:518. doi: 10.3389/fped.2019.00518
69. Michail S, Lin M, Frey MR, Fanter R, Paliy O, Hilbush B, et al. Altered gut microbial energy and metabolism in children with non-alcoholic fatty liver disease. FEMS Microbiol Ecol (2015) 91:1–9. doi: 10.1093/femsec/fiu002
70. Jaeggi T, Kortman GA, Moretti D, Chassard C, Holding P, Dostal A, et al. Iron fortification adversely affects the gut microbiome, increases pathogen abundance and induces intestinal inflammation in Kenyan infants. Gut. (2015) 64:731–42. doi: 10.1136/gutjnl-2014-307720
71. Bozzi Cionci N, Baffoni L, Gaggìa F, Di Gioia D. Therapeutic microbiology: the role of bifidobacterium breve as food supplement for the prevention/treatment of paediatric diseases. Nutrients (2018) 10:E1723. doi: 10.3390/nu10111723
72. Nagpal R, Kurakawa T, Tsuji H, Takahashi T, Kawashima K, Nagata S, et al. Evolution of gut bifidobacterium population in healthy Japanese infants over the first three years of life: A quantitative assessment. Sci Rep (2017) 7:10097. doi: 10.1038/s41598-017-10711-5
73. Li R, Andreu-Sánchez S, Kuipers F, Fu J. Gut microbiome and bile acids in obesity-related diseases. Best Pract Res Clin Endocrinol Metab (2021) 35:101493. doi: 10.1016/j.beem.2021.101493
74. Insel R, Knip M. Prospects for primary prevention of type 1 diabetes by restoring a disappearing microbe. Pediatr Diabetes. (2018) 19:1400–6. doi: 10.1111/pedi.12756
75. Salgaço MK, Perina NP, Tomé TM, Mosquera EMB, Lazarini T, Sartoratto A, et al. Probiotic infant cereal improves children’s gut microbiota: Insights using the simulator of human intestinal microbial ecosystem (SHIME®). Food Res Int (2021) 143:110292. doi: 10.1016/j.foodres.2021.110292
76. Solito A, Bozzi Cionci N, Calgaro M, Caputo M, Vannini L, Hasballa I, et al. Supplementation with bifidobacterium breve BR03 and B632 strains improved insulin sensitivity in children and adolescents with obesity in a cross-over, randomized double-blind placebo-controlled trial. Clin Nutr (2021) 40:4585–94. doi: 10.1016/j.clnu.2021.06.002
77. Sanchis-Chordà J, Del Pulgar EMG, Carrasco-Luna J, Benítez-Páez A, Sanz Y, Codoñer-Franch P. Bifidobacterium pseudocatenulatum CECT 7765 supplementation improves inflammatory status in insulin-resistant obese children. Eur J Nutr (2019) 58:2789–800. doi: 10.1007/s00394-018-1828-5
78. Nagata S, Chiba Y, Wang C, Yamashiro Y. The effects of the lactobacillus casei strain on obesity in children: A pilot study. Benef. Microbes (2017) 8:535–43. doi: 10.3920/BM2016.0170
79. Thaiss CA. Microbiome dynamics in obesity. Science (2018) 362:700058. doi: 10.3389/fnut.2021.700058
80. Dodd D, Spitzer MH, Van Treuren W, Merrill BD, Hryckowian AJ, Higginbottom SK, et al. A gut bacterial pathway metabolizes aromatic amino acids into nine circulating metabolites. Nature (2017) 551:648–52. doi: 10.1038/nature24661
81. Goffredo M, Mass K, Parks EJ, Wagner DA, McClure EA, Graf J, et al. Role of gut microbiota and short chain fatty acids in modulating energy harvest and fat partitioning in youth. J Clin Endocrinol Metab (2016) 101:4367–76. doi: 10.1210/jc.2016-1797
82. Liu R, Hong J, Xu X, Feng Q, Zhang D, Gu Y, et al. Gut microbiome and serum metabolome alterations in obesity and after weight-loss intervention. Nat Med (2017) 23:859–68. doi: 10.1038/nm.4358
83. Orsso CE, Peng Y, Deehan EC, Tan Q, Field CJ, Madsen KL, et al. Composition and functions of the gut microbiome in pediatric obesity: relationships with markers of insulin resistance. Microorganisms (2021) 9:1490. doi: 10.3390/microorganisms9071490
84. Macfarlane GT, Macfarlane S. Bacteria, colonic fermentation, and gastrointestinal health. J AOAC Int (2012) 95:50–60. doi: 10.5740/jaoacint.sge_macfarlane
85. Canfora EE, Jocken JW, Blaak EE. Short-chain fatty acids in control of body weight and insulin sensitivity. Nat Rev Endocrinol (2015) 11:577–91. doi: 10.1038/nrendo.2015.128
86. Blaak EE, Canfora EE, Theis S, Frost G, Groen AK, Mithieux G, et al. Short chain fatty acids in human gut and metabolic health. Benef. Microbes (2020) 11:411–55. doi: 10.3920/BM2020.0057
87. Hernández MAG, Canfora EE, Jocken JWE, Blaak EE. The short-chain fatty acid acetate in body weight control and insulin sensitivity. Nutrients (2019) 11:1943. doi: 10.3390/nu11081943
88. Mueller NT, Differding MK, Zhang M, Maruthur NM, Juraschek SP, Miller ER, et al. Metformin affects gut microbiome composition and function and circulating short-chain fatty acids: A randomized trial. Diabetes Care (2021) 44:1462–71. doi: 10.2337/dc20-2257
89. Miranda VPN, Dos Santos Amorim PR, Bastos RR, de Faria ER, de Castro Moreira ME, do Carmo Castro Franceschini S, et al. Abundance of gut microbiota, concentration of short-chain fatty acids, and inflammatory markers associated with elevated body fat, overweight, and obesity in female adolescents. Mediators Inflamm (2019) 2019:7346863. doi: 10.1155/2019/7346863
90. Galuppo B, Cline G, Van Name M, Shabanova V, Wagner D, Kien CL, et al. Colonic fermentation and acetate production in youth with and without obesity. J Nutr (2021) 151:3292–8. doi: 10.1093/jn/nxab277
91. Gonzalez-Garcia RA, McCubbin T, Navone L, Stowers C, Nielsen LK, Marcellin E. Microbial propionic acid production. Fermentation (2017) 3:21. doi: 10.3390/fermentation3020021
92. Salazar N, Dewulf EM, Neyrinck AM, Bindels LB, Cani PD, Mahillon J, et al. Inulin-type fructans modulate intestinal bifidobacterium species populations and decrease fecal short-chain fatty acids in obese women. Clin Nutr (2015) 34:501–7. doi: 10.1016/j.clnu.2014.06.001
93. Sanna S, van Zuydam NR, Mahajan A, Kurilshikov A, Vich Vila A, Võsa U, et al. Causal relationships among the gut microbiome, short-chain fatty acids and metabolic diseases. Nat Genet (2019) 51:600–5. doi: 10.1038/s41588-019-0350-x
94. Bridgman SL, Azad MB, Field CJ, Haqq AM, Becker AB, Mandhane PJ, et al. Fecal short-chain fatty acid variations by breastfeeding status in infants at 4 Months: Differences in relative versus absolute concentrations. Front Nutr (2017) 4:11. doi: 10.3389/fnut.2017.00011
95. Murugesan S, Ulloa-Martínez M, Martínez-Rojano H, Galván-Rodríguez FM, Miranda-Brito C, Romano MC, et al. Study of the diversity and short-chain fatty acids production by the bacterial community in overweight and obese Mexican children. Eur J Clin Microbiol Infect Dis (2015) 34:1337–46. doi: 10.1007/s10096-015-2355-4
96. Barczynska R, Slizewska K, Litwin M, Szalecki M, Kapusniak J. Effects of dietary fiber preparations made from maize starch on the growth and activity of selected bacteria from the firmicutes, bacteroidetes, and actinobacteria phyla in fecal samples from obese children. Acta Biochim Pol (2016) 63:261–6. doi: 10.18388/abp.2015_1068
97. Schwiertz A, Taras D, Schäfer K, Beijer S, Bos NA, Donus C, et al. Microbiota and SCFA in lean and overweight healthy subjects. Obesity (2010) 18:190–5. doi: 10.1038/oby.2009.167
98. Louis P, Flint HJ. Formation of propionate and butyrate by the human colonic microbiota. Environ Microbiol (2017) 19:29–41. doi: 10.1111/1462-2920.13589
99. Payne AN, Chassard C, Zimmermann M, Müller P, Stinca S, Lacroix C. The metabolic activity of gut microbiota in obese children is increased compared with normal-weight children and exhibits more exhaustive substrate utilization. Nutr Diabetes. (2011) 1:e12–2. doi: 10.1038/nutd.2011.8
100. Aydogan Mathyk B, Piccolo BD, Alvarado F, Shankar K, O’Tierney-Ginn PF. Metabolomic signatures of low and high adiposity neonates differ based on maternal BMI. Am J Physiol Endocrinol Metab (2022) 322:E540–50. doi: 10.1152/ajpendo.00356.2021
101. Leylabadlo HE, Ghotaslou R, Feizabadi MM, Farajnia S, Moaddab SY, Ganbarov K, et al. The critical role of faecalibacterium prausnitzii in human health: An overview. Microb Pathog (2020) 149:104344. doi: 10.1016/j.micpath.2020.104344
102. Cuesta-Zuluaga J, Mueller NT, Álvarez-Quintero R, Velásquez-Mejía EP, Sierra JA, Corrales-Agudelo V, et al. Higher fecal short-chain fatty acid levels are associated with gut microbiome dysbiosis, obesity, hypertension and cardiometabolic disease risk factors. Nutrients (2018) 11:51. doi: 10.3390/nu11010051
103. Stilling RM, van de Wouw M, Clarke G, Stanton C, Dinan TG, Cryan JF. The neuropharmacology of butyrate: The bread and butter of the microbiota-gut-brain axis? Neurochem Int (2016) 99:110–32. doi: 10.1016/j.neuint.2016.06.011
104. Davie JR. Inhibition of histone deacetylase activity by butyrate. J Nutr (2003) 133 Supplement:2485S–93S. doi: 10.1093/jn/133.7.2485S
105. Coppola S, Avagliano C, Calignano A, Berni Canani R. The protective role of butyrate against obesity and obesity-related diseases. Molecules (2021) 26:682. doi: 10.3390/molecules26030682
106. Barczyńska R, Litwin M, Sliżewska K, Szalecki M, Berdowska A, Bandurska K, et al. Bacterial microbiota and fatty acids in the faeces of overweight and obese children. Pol J Microbiol (2018) 67:339–45. doi: 10.21307/pjm-2018-041
107. Murugesan S, Nirmalkar K, Hoyo-Vadillo C, García-Espitia M, Ramírez-Sánchez D, García-Mena J. Gut microbiome production of short-chain fatty acids and obesity in children. Eur J Clin Microbiol Infect Dis (2018) 37:621–5. doi: 10.1007/s10096-017-3143-0
108. Papierkowski A, Kowalczyk J, Trocewicz T, Gundlach A. Monocarboxylic short-chain fatty acids C2-C6 in serum of obese children. Acta Paediatr Acad Sci Hung (1975) 16:277–80.
109. Patel M, Fowler D, Sizer J, Walton C. Faecal volatile biomarkers of clostridium difficile infection. PloS One (2019) 14:e0215256. doi: 10.1371/journal.pone.0215256
110. Liu X, Liu X, Shi Q, Fan X, Qi K. Association of telomere length and telomerase methylation with n-3 fatty acids in preschool children with obesity. BMC Pediatr (2021) 21:24. doi: 10.1186/s12887-020-02487-x
111. Gregor A, Auernigg-Haselmaier S, Trajanoski S, König J, Duszka K. Colonic medium-chain fatty acids act as a source of energy and for colon maintenance but are not utilized to acylate ghrelin. Nutrients (2021) 13:3807. doi: 10.3390/nu13113807
112. Ren W, Wu Q, Deng L, Hu Y, Guo W, Ren N. Simultaneous medium-chain fatty acids production and process carbon emissions reduction in a continuous-flow reactor: re-understanding of carbon flow distribution. Environ Res. (2022) 212:113294. doi: 10.1016/j.envres.2022.113294
113. Zhao J, Hu J, Ma X. Sodium caprylate improves intestinal mucosal barrier function and antioxidant capacity by altering gut microbial metabolism. Food Funct (2021) 12:9750–62. doi: 10.1039/d1fo01975a
114. Sam QH, Ling H, Yew WS, Tan Z, Ravikumar S, Chang MW, et al. The divergent immunomodulatory effects of short chain fatty acids and medium chain fatty acids. Int J Mol Sci (2021) 22:6453. doi: 10.3390/ijms22126453
115. Szterk A, Ofiara K, Strus B, Abdullaev I, Ferenc K, Sady M, et al. Content of health-promoting fatty acids in commercial sheep, cow and goat cheeses. Foods (2022) 11:1116. doi: 10.3390/foods11081116
116. Kitahara T, Koyama N, Matsuda J, Aoyama Y, Hirakata Y, Kamihira S, et al. Antimicrobial activity of saturated fatty acids and fatty amines against methicillin-resistant staphylococcus aureus. Biol Pharm Bull (2004) 27:1321–6. doi: 10.1248/bpb.27.1321
117. van de Heijning BJM, Oosting A, Kegler D, van der Beek EM. An increased dietary supply of medium-chain fatty acids during early weaning in rodents prevents excessive fat accumulation in adulthood. Nutrients (2017) 9:E631. doi: 10.3390/nu9060631
118. Ma Y, Wu T, Liu Y, Wang Q, Song J, Song F, et al. Nutritional and metabolic findings in patients with prader-willi syndrome diagnosed in early infancy. J Pediatr Endocrinol metabolism (2012) JPEM 25:1103–9. doi: 10.1515/jpem-2012-0167
119. Yao CK, Muir JG, Gibson PR. Review article: insights into colonic protein fermentation, its modulation and potential health implications. Aliment. Pharmacol Ther (2016) 43:181–96. doi: 10.1111/apt.13456
120. Henneke L, Schlicht K, Andreani NA, Hollstein T, Demetrowitsch T, Knappe C, et al. A dietary carbohydrate – gut parasutterella – human fatty acid biosynthesis metabolic axis in obesity and type 2 diabetes. Gut Microbes (2022) 14:2057778. doi: 10.1080/19490976.2022.2057778
121. Kurilshikov A, van den Munckhof ICL, Chen L, Bonder MJ, Schraa K, Rutten JHW, et al. Gut microbial associations to plasma metabolites linked to cardiovascular phenotypes and risk. Circ Res (2019) 124:1808–20. doi: 10.1161/CIRCRESAHA.118.314642
122. Benjdia A, Berteau O. Sulfatases and radical SAM enzymes: Emerging themes in glycosaminoglycan metabolism and the human microbiota. Biochem Soc Trans (2016) 44:109–15. doi: 10.1042/BST20150191
123. Hoyles L, Fernández-Real JM, Federici M, Serino M, Abbott J, Charpentier J, et al. Molecular phenomics and metagenomics of hepatic steatosis in non-diabetic obese women. Nat Med (2018) 24:1070–80. doi: 10.1038/s41591-018-0061-3
124. Hung TKW, Dong TS, Chen Z, Elashoff D, Sinsheimer JS, Jacobs JP, et al. Understanding the heterogeneity of obesity and the relationship to the brain-gut axis. Nutrients (2020) 12:3701. doi: 10.3390/nu12123701
125. Virtue AT, McCright SJ, Wright JM, Jimenez MT, Mowel WK, Kotzin JJ, et al. The gut microbiota regulates white adipose tissue inflammation and obesity via a family of microRNAs. Sci Transl Med (2019) 11:eaav1892. doi: 10.1126/scitranslmed.aav1892
126. Osadchiy V, Labus JS, Gupta A, Jacobs J, Ashe-McNalley C, Hsiao EY, et al. Correlation of tryptophan metabolites with connectivity of extended central reward network in healthy subjects. PloS One (2018) 13:e0201772. doi: 10.1371/journal.pone.0201772
127. Shestopalov AV, Shatova OP, Gaponov AM, Moskaleva NE, Appolonova SA, Tutelyan AV, et al. The study of tryptophan metabolite concentrations in blood serum and fecal extracts from obese children. BioMed Khim (2020) 66:494–501. doi: 10.18097/PBMC20206606494
128. Li R, Huang X, Liang X, Su M, Lai KP, Chen J. Integrated omics analysis reveals the alteration of gut microbe-metabolites in obese adults. Brief Bioinform (2021) 22:bbaa165. doi: 10.1093/bib/bbaa165
129. López-Contreras BE, Morán-Ramos S, Villarruel-Vázquez R, Macías-Kauffer L, Villamil-Ramírez H, León-Mimila P, et al. Composition of gut microbiota in obese and normal-weight Mexican school-age children and its association with metabolic traits. Pediatr Obes (2018) 13:381–8. doi: 10.1111/ijpo.12262
130. Wahl S, Holzapfel C, Yu Z, Breier M, Kondofersky I, Fuchs C, et al. Metabolomics reveals determinants of weight loss during lifestyle intervention in obese children. Metabolomics (2013) 9:1157–67. doi: 10.1007/s11306-013-0550-9
131. Lu J, Gu Y, Liu H, Wang L, Li W, Li W, et al. Daily branched-chain amino acid intake and risks of obesity and insulin resistance in children: A cross-sectional study. Obes (Silver Spring). (2020) 28:1310–6. doi: 10.1002/oby.22834
132. Cosentino RG, Churilla JR, Josephson S, Molle-Rios Z, Hossain MJ, Prado WL, et al. Branched-chain amino acids and relationship with inflammation in youth with obesity: A randomized controlled intervention study. J Clin Endocrinol Metab (2021) 106:3129–39. doi: 10.1210/clinem/dgab538
133. McCormack SE, Shaham O, McCarthy MA, Deik AA, Wang TJ, Gerszten RE, et al. Circulating branched-chain amino acid concentrations are associated with obesity and future insulin resistance in children and adolescents. Pediatr Obes (2013) 8:52–61. doi: 10.1111/j.2047-6310.2012.00087.x
134. Lee A, Jang HB, Ra M, Choi Y, Lee HJ, Park JY, et al. Prediction of future risk of insulin resistance and metabolic syndrome based on Korean boy’s metabolite profiling. Obes Res Clin Pract (2015) 9:336–45. doi: 10.1016/j.orcp.2014.10.220
135. Perng W, Gillman MW, Fleisch AF, Michalek RD, Watkins SM, Isganaitis E, et al. Metabolomic profiles and childhood obesity. Obes (Silver Spring). (2014) 22:2570–8. doi: 10.1002/oby.20901
136. Butte NF, Liu Y, Zakeri IF, Mohney RP, Mehta N, Voruganti VS, et al. Global metabolomic profiling targeting childhood obesity in the Hispanic population. Am J Clin Nutr (2015) 102:256–67. doi: 10.3945/ajcn.115.111872
137. Lischka J, Schanzer A, Hojreh A, Ba Ssalamah A, Item CB, de Gier C, et al. A branched-chain amino acid-based metabolic score can predict liver fat in children and adolescents with severe obesity. Pediatr Obes (2021) 16:e12739. doi: 10.1111/ijpo.12739
138. Troisi J, Pierri L, Landolfi A, Marciano F, Bisogno A, Belmonte F, et al. Urinary metabolomics in pediatric obesity and NAFLD identifies metabolic pathways/metabolites related to dietary habits and gut-liver axis perturbations. Nutrients (2017) 9:E485. doi: 10.3390/nu9050485
139. Moran-Ramos S, Macias-Kauffer L, López-Contreras BE, Villamil-Ramírez H, Ocampo-Medina E, León-Mimila P, et al. A higher bacterial inward BCAA transport driven by faecalibacterium prausnitzii is associated with lower serum levels of BCAA in early adolescents. Mol Med (2021) 27:108. doi: 10.1186/s10020-021-00371-7
140. Liu J, Lai L, Lin J, Zheng J, Nie X, Zhu X, et al. Ranitidine and finasteride inhibit the synthesis and release of trimethylamine n-oxide and mitigates its cardiovascular and renal damage through modulating gut microbiota. Int J Biol Sci (2020) 16:790–802. doi: 10.7150/ijbs.40934
141. Liu KH, Owens JA, Saeedi B, Cohen CE, Bellissimo MP, Naudin C, et al. Microbial metabolite delta-valerobetaine is a diet-dependent obesogen. Nat Metab (2021) 3:1694–705. doi: 10.1038/s42255-021-00502-8
142. Ma H, Patti ME. Bile acids, obesity, and the metabolic syndrome. Best Pract Res Clin Gastroenterol (2014) 28:573–83. doi: 10.1016/j.bpg.2014.07.004
143. Bhat SF, Pinney SE, Kennedy KM, McCourt CR, Mundy MA, Surette MG, et al. Exposure to high fructose corn syrup during adolescence in the mouse alters hepatic metabolism and the microbiome in a sex-specific manner. J Physiol (2021) 599:1487–511. doi: 10.1113/JP280034
144. Jahnel J, Zöhrer E, Alisi A, Ferrari F, Ceccarelli S, De Vito R, et al. Serum bile acid levels in children with nonalcoholic fatty liver disease. J Pediatr Gastroenterol Nutr (2015) 61:85–90. doi: 10.1097/MPG.0000000000000774
145. Montagnana M, Danese E, Giontella A, Bonafini S, Benati M, Tagetti A, et al. Circulating bile acids profiles in obese children and adolescents: A possible role of sex, puberty and liver steatosis. Diagn. (Basel). (2020) 10:977. doi: 10.3390/diagnostics10110977
146. Sholl J, Mailing LJ, Wood TR. Reframing nutritional microbiota studies to reflect an inherent metabolic flexibility of the human gut: A narrative review focusing on high-fat diets. mBio (2021) 12:e00579–21. doi: 10.1128/mBio.00579-21
147. Hara E. Relationship between obesity, gut microbiome and hepatocellular carcinoma development. Dig. Dis (2015) 33:346–50. doi: 10.1159/000371679
148. Chávez-Talavera O, Haas J, Grzych G, Tailleux A, Staels B. Bile acid alterations in nonalcoholic fatty liver disease, obesity, insulin resistance and type 2 diabetes: what do the human studies tell? Curr Opin Lipidol (2019) 30:244–54. doi: 10.1097/MOL.0000000000000597
149. Higgins V, Asgari S, Hamilton JK, Wolska A, Remaley AT, Hartmann B, et al. Postprandial dyslipidemia, hyperinsulinemia, and impaired gut Peptides/Bile acids in adolescents with obesity. J Clin Endocrinol Metab (2019) 105:1228–41. doi: 10.1210/clinem/dgz261
150. Ma H, Sales VM, Wolf AR, Subramanian S, Matthews TJ, Chen M, et al. Attenuated effects of bile acids on glucose metabolism and insulin sensitivity in a male mouse model of prenatal undernutrition. Endocrinology (2017) 158:2441–52. doi: 10.1210/en.2017-00288
151. Zeng H, Larson KJ, Cheng WH, Bukowski MR, Safratowich BD, Liu Z, et al. Advanced liver steatosis accompanies an increase in hepatic inflammation, colonic, secondary bile acids and Lactobacillaceae/Lachnospiraceae bacteria in C57BL/6 mice fed a high-fat diet. J Nutr Biochem (2020) 78:108336. doi: 10.1016/j.jnutbio.2019.108336
152. Wei M, Huang F, Zhao L, Zhang Y, Yang W, Wang S, et al. A dysregulated bile acid-gut microbiota axis contributes to obesity susceptibility. EBiomedicine (2020) 55:102766. doi: 10.1016/j.ebiom.2020.102766
153. Kalliomäki M, Collado MC, Salminen S, Isolauri E. Early differences in fecal microbiota composition in children may predict overweight. Am J Clin Nutr (2008) 87:534–8. doi: 10.1093/ajcn/87.3.534
154. Olszak T, An D, Zeissig S, Vera MP, Richter J, Franke A, et al. Microbial exposure during early life has persistent effects on natural killer T cell function. Science (2012) 336:489–93. doi: 10.1126/science.1219328
155. Marrs T, Jo JH, Perkin MR, Rivett DW, Witney AA, Bruce KD, et al. Gut microbiota development during infancy: Impact of introducing allergenic foods. J Allergy Clin Immunol (2021) 147:613–621.e9. doi: 10.1016/j.jaci.2020.09.042
156. Tun HM, Bridgman SL, Chari R, Field CJ, Guttman DS, Becker AB, et al. Roles of birth mode and infant gut microbiota in intergenerational transmission of overweight and obesity from mother to offspring. JAMA Pediatr (2018) 172:368–77. doi: 10.1001/jamapediatrics.2017.5535
157. Mueller NT, Shin H, Pizoni A, Werlang IC, Matte U, Goldani MZ, et al. Delivery mode and the transition of pioneering gut-microbiota structure, composition and predicted metabolic function. Genes (Basel). (2017) 8:E364. doi: 10.3390/genes8120364
158. Schei K, Avershina E, Øien T, Rudi K, Follestad T, Salamati S, et al. Early gut mycobiota and mother-offspring transfer. Microbiome. (2017) 5:107. doi: 10.1186/s40168-017-0319-x
159. Savage JH, Lee-Sarwar KA, Sordillo JE, Lange NE, Zhou Y, O’Connor GT, et al. Diet during pregnancy and infancy and the infant intestinal microbiome. J Pediatr (2018) 203:47–54.e4. doi: 10.1016/j.jpeds.2018.07.066
160. Henrick BM, Rodriguez L, Lakshmikanth T, Pou C, Henckel E, Arzoomand A, et al. Bifidobacteria-mediated immune system imprinting early in life. Cell (2021) 184:3884–3898.e11. doi: 10.1016/j.cell.2021.05.030
161. Barthow C, Wickens K, Stanley T, Mitchell EA, Maude R, Abels P, et al. The probiotics in pregnancy study (PiP study): Rationale and design of a double-blind randomised controlled trial to improve maternal health during pregnancy and prevent infant eczema and allergy. BMC Pregnancy Childbirth. (2016) 16:133. doi: 10.1186/s12884-016-0923-y
162. Tokuhara D (2021). Role of the gut microbiota in regulating non-alcoholic fatty liver disease in children and adolescents. Fron Nutr 8:100058. doi: 10.3389/fnut.2021.700058.
Keywords: children, overweight, obesity, gut microbiota, metabolites
Citation: Zhang S and Dang Y (2022) Roles of gut microbiota and metabolites in overweight and obesity of children. Front. Endocrinol. 13:994930. doi: 10.3389/fendo.2022.994930
Received: 15 July 2022; Accepted: 17 August 2022;
Published: 08 September 2022.
Edited by:
Peiyuan Yin, Dalian Medical University, ChinaReviewed by:
Zhengfeng Fang, Sichuan Agricultural University, ChinaCopyright © 2022 Zhang and Dang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yanqi Dang, ZGFuZ3lhbnFpOTAyMkAxMjYuY29t, eXFfZGFuZ0BzaHV0Y20uZWR1LmNu
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.