
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Endocrinol. , 06 October 2022
Sec. Obesity
Volume 13 - 2022 | https://doi.org/10.3389/fendo.2022.993137
This article is part of the Research Topic Bariatric and Metabolic Surgery for Obesity and Related Diseases: Effects and Mechanisms View all 14 articles
 Hui You1,2
Hui You1,2 Huihui Ma1
Huihui Ma1 Xingchun Wang1,2
Xingchun Wang1,2 Xin Wen1
Xin Wen1 Cuiling Zhu1
Cuiling Zhu1 Wangjia Mao1
Wangjia Mao1 Le Bu1
Le Bu1 Manna Zhang1
Manna Zhang1 Jiajing Yin1,2
Jiajing Yin1,2 Lei Du1
Lei Du1 Xiaoyun Cheng1
Xiaoyun Cheng1 Haibing Chen1
Haibing Chen1 Jun Zhang3,4*
Jun Zhang3,4* Shen Qu1,2*
Shen Qu1,2*Background: Liver-type fatty acid-binding protein (FABP1) contributes to metabolic disorders. However, the relationship between FABP1 and hyperuricemia remains unknown. We aimed to evaluate the correlation between serum FABP1 and hyperuricemia in patients with obesity before and after laparoscopic sleeve gastrectomy (LSG).
Methods: We enrolled 105 patients (47 men and 58 women) with obesity who underwent LSG. They were divided into two groups: normal levels of uric acid (UA) (NUA, n = 44) and high levels of UA (HUA, n = 61) with matching sexes. FABP1 levels and other biochemical parameters were measured at baseline and 3, 6, and 12 months after LSG.
Results: Serum FABP1 levels were significantly higher in the HUA group than in the NUA group (34.76 ± 22.69 ng/mL vs. 25.21 ± 21.68 ng/mL, P=0.024). FABP1 was positively correlated with UA (r=0.390, P=0.002) in the HUA group. The correlation still existed after adjusting for confounding factors. Preoperative FABP1 levels were risk factors for hyperuricemia at baseline. UA and FABP1 levels decreased at 3, 6, and 12 months postoperatively. FABP1 showed a more significant decrease in the HUA group than in the NUA group at 12 months (27.06 ± 10.98 ng/mL vs. 9.54 ± 6.52 ng/mL, P=0.003). Additionally, the change in FABP1 levels positively correlated with changes in UA levels in the HUA group 12 months postoperatively (r=0.512, P=0.011).
Conclusions: FABP1 was positively associated with UA and may be a risk factor for hyperuricemia in obesity. FABP1 levels were higher but decreased more after LSG in obese patients with hyperuricemia than in those without hyperuricemia.
Uric acid (UA) is a final enzyme product of purine nucleotide degradation and can scavenge oxygen radicals and protect the erythrocyte membrane from lipid oxidation (1). Hyperuricemia is a metabolic disease characterized by elevated serum UA (SUA) concentration (>420 μmol/L for men and >360 μmol/L for women) (2). In the past few decades, the prevalence of hyperuricemia has been increasing rapidly worldwide (2). In addition, hyperuricemia has been traditionally considered a potential risk factor for obesity, diabetes mellitus, nonalcoholic fatty liver disease (NAFLD), and other metabolic syndromes (3, 4). However, the underlying mechanisms of hyperuricemia and its risk factors remain unclear.
Liver-type fatty acid-binding protein (FABP1) (5) is a member of the fatty acid-binding protein family and is distributed mainly in the liver, kidney, lung, and gastrointestinal tract, accounting for 3% to 5% of the total cytoplasmic protein. It mainly enhances long-chain fatty acid uptake and fatty acid metabolism, and plays a central role in the hepatic β-oxidation of unesterified fatty acids, ultimately leading to various metabolic disorders (5–8). A previous study (8) has found that FABP1 can play a negative regulatory role in the formation of very-low-density lipoproteins, which can lead to lipid metabolism disorders when combined with total cholesterol, triglycerides, and apolipoprotein B, thereby accelerating NAFLD progression. Tsai et al. (9) found increasing concentrations of FABP1 were associated independently and significantly with diabetic nephropathy. Our previous research (10) also found serum FABP1 levels were correlated closely with obesity. However, the link between FABP1 and hyperuricemia has not been reported.
Recently, laparoscopic sleeve gastrectomy (LSG) (1, 11) has become a popular procedure to assist with weight loss owing to its low damaging effects on the patient’s physiology, high safety, and evident curative effect. This procedure has been shown to improve many metabolic syndromes, such as obesity, diabetes, and NAFLD, as well as hyperuricemia (12). Therefore, we aimed to evaluate the correlation between serum FABP1 and hyperuricemia in patients with obesity before and after LSG.
This retrospective, follow-up study enrolled 105 patients with obesity (body mass index [BMI], 38.39 ± 6.33 kg/m2) aged 18 to 60 years from the Shanghai Tenth People’s Hospital. Patients were divided into the following two groups according to their SUA levels (1): normal levels of UA (NUA, men ≤ 420 μmol/L, women ≤ 360 μmol/L, n = 44) and high levels of UA (HUA, men > 420 μmol/L, women > 360 μmol/L, n = 61) groups. There was no statistical difference in sex distribution between the two groups. All patients underwent LSG. Exclusion criteria were patients with: (1) a history of substance abuse or chronic mental illness; (2) psychiatric disorders; (3) previous gastrointestinal surgery; and (4) unwillingness to undergo LSG. The patients underwent follow-up at 3, 6, and 12 months after LSG (hereafter referred to as 3, 6, and 12 M post-LSG, respectively). This study was conducted in accordance with the Declaration of Helsinki and French Legislation, and all the patients provided informed consent. The study protocol was approved by the Ethics Committee of our hospital (clinical registration number ChiCTR-OCS-12002381).
Demographic and clinical data, including medical history and date of birth, were analyzed. Anthropometric measurements were performed for all patients. Height and weight measurements were performed by the medical staff, with the patients clad in light clothes, using an Omron HBF-358 body fat analyzer (Q40102010L01322F, Japan). The BMI was calculated as weight in kilograms divided by height in meters squared (kg/m2). Morning venous blood was obtained from all patients after a 12h overnight fast and centrifuged thereafter with the supernatant being used in the laboratory tests. Serum FABP1 levels were measured using an enzyme-linked immunosorbent assay (ELISA; Abcam (Cambridge), ab218261) kit. As per the manufacturer’s protocol, the normal reference level of FABP1 was 6.36–19.0 ng/mL. UA, fasting blood glucose (FBG), fasting insulin (FINS), fasting C-peptide (FCP), alanine aminotransferase (ALT), aspartate aminotransferase (AST), and γ-transaminase (γ-GT) levels were assessed using an automated biochemical analyzer.
Statistical analysis was performed using SPSS 20.0 (SPSS Inc., Chicago, IL, USA) and GraphPad Prism 8 (GraphPad Software, San Diego, USA) in Windows 10. Data are presented as means ± standard deviations (SDs) for continuous variables, and count data are expressed as the number of columns (n). Comparisons between continuous or categorical variables in two groups were tested using the Student’s t test or variance analysis, whereas paired-sample t tests were used to compare the data before and after surgery. To explore the correlation between serum FABP1 levels and UA levels or other biochemical criteria, Spearman’s correlation analysis was performed. To further study the relationship between serum FABP1 levels and UA levels, we performed a multiple linear regression. We then performed a binary logistic regression for Models 1, 2, and 3 to identify potential factors related to the risk of hyperuricemia in patients with obesity. Statistical significance was set at P < 0.05.
We divided the patients with obesity (male, n = 47 and female, n = 58) into the NUA (n = 44) and HUA (n = 61) groups. There was no statistical difference in the sex distribution between the two groups (P = 0.604). The FABP1 levels were found to be significantly higher in the HUA group than in the NUA group at baseline (34.76 ± 22.69 ng/mL vs. 25.21 ± 21.68 ng/mL, P = 0.024). Furthermore, the FINS, FCP, ALT, AST and γ-GT levels were also significantly lower in the NUA group than in the HUA group (25.61 ± 20.99 mU/L vs. 37.50 ± 21.61 mU/L, P = 0.015; 3.89 ± 1.68 ng/ml vs. 4.97 ± 1.65 ng/ml, P = 0.004; 45.69 ± 44.99 U/L vs. 67.67 ± 62.02 U/L, P = 0.036; 28.67 ± 23.28 U/L vs. 44.00 ± 36.47 U/L, P = 0.013; 44.26 ± 32.47 U/L vs. 62.75 ± 48.00 U/L, P = 0.034). However, BMI and FBG levels were not statistically different between the two groups (37.58 ± 6.46 kg/m2 vs. 38.98 ± 6.23 kg/m2, P=0.679; 6.85 ± 3.09 mmol/L vs. 5.80 ± 2.08 mmol/L, P=0.054) (Table 1)
Since hyperuricemia and FABP1 are closely related to diabetes and liver-related metabolic disorders, we first assessed the correlation between serum FABP1 or UA levels with glucose metabolism and liver enzyme indices in the NUA and HUA groups (Figure 1). We found the serum FABP1 levels were related to the FBG, AST and γ -GT levels in the NUA group (r = 0.422, P = 0.004; r=0.430, P = 0.006; r = 0.311, P = 045, respectively). Moreover, the UA levels correlated with the ALT, AST, and γ-GT levels (r = 0.297, P = 0.049; r = 0.394, P = 0.013; r = 0.381, P = 0.013, respectively). Furthermore, in the HUA group, the serum FABP1 level was positively correlated with the UA level (r = 0.390, P = 0.002), and with the BMI, ALT and AST levels (r = 0.375, P = 0.002; r = 0.376, P = 0.003; r = 0.446, P < 0.001, respectively). In addition, the UA level were related to the BMI, FINS, FCP and ALT levels (r = 0.338, P = 0.008; r = 0.334, P = 0.014; r = 0.428, P = 0.01; r = 0.308, P = 0.016, respectively).
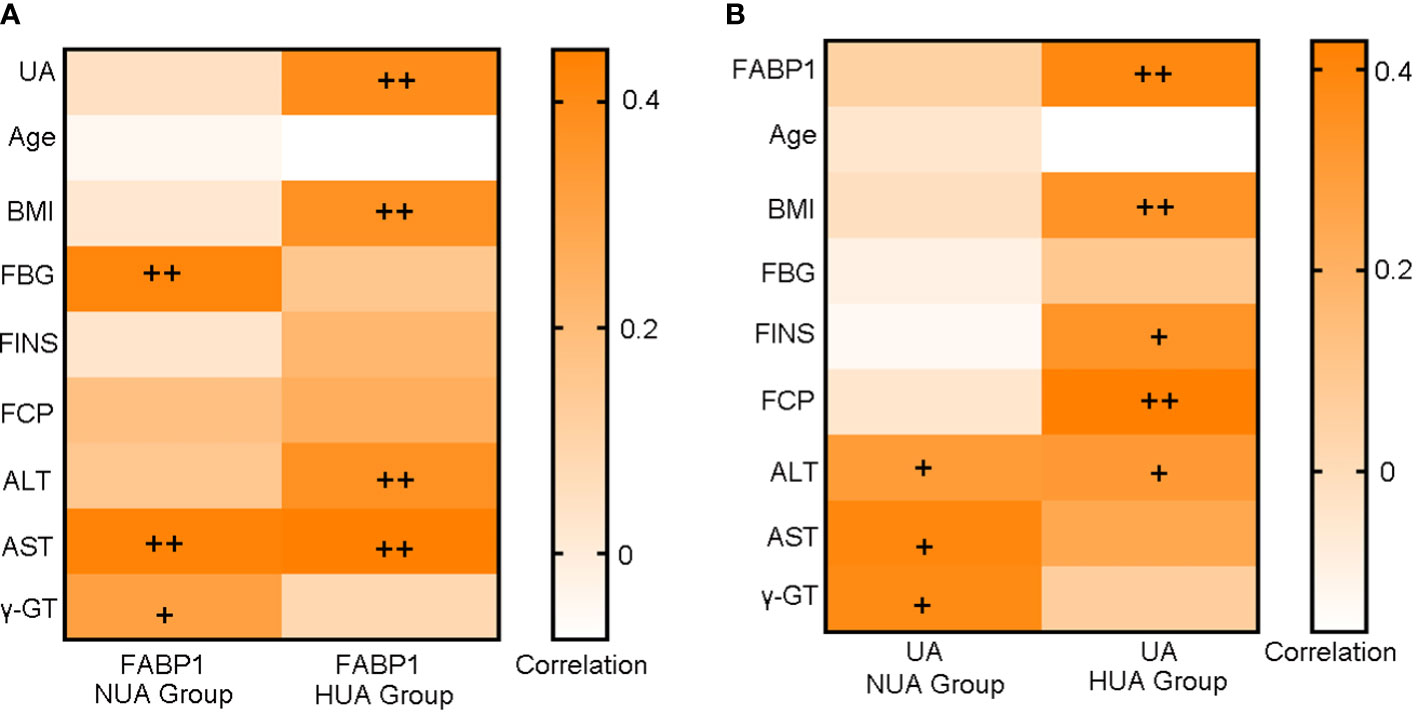
Figure 1 Heat map of the correlation analysis of serum FABP1 levels (A) or UA levels (B) with glucose metabolism and liver function-related indicators at baseline. +P<0.05; ++P<0.01.
To evaluate the correlation between serum UA levels and FABP1 levels, we also performed a multiple linear regression analysis on all individuals. First, Model 1 revealed a significantly positive correlation between serum UA levels and FABP1 levels (β = 0.344, P <0.001). Second, in Model 2, a correlation was also evident after adjusting for BMI, age, and sex (β = 0.257, P = 0.005). Third, in Model 3 (β = 0.280, P = 0.005), serum FABP1 levels were another determinant of the UA levels after adjusting for BMI, age, and the related indicators of glucose metabolism (FBG, FINS, and FCP). The same findings were observed in Model 4 (β = 0.283, P = 0.009) after adjusting for BMI, age, sex, and ALT, AST, and γ-GT levels (Table 2).
We also constructed a multivariable logistic model for the occurrence of hyperuricemia in the preoperative obese cohort (Table 3). In Model 1, with BMI and age as additional covariables, we observed a statistically significant association between serum FABP1 levels and the risk of hyperuricemia in patients with obesity (Odds ratio [OR], 95% confidence interval [CI] = 1.023, 1.001–1.046, P = 0.048). This association was also significant in Model 2 (OR, 95% CI = 1.045, 1.009–1.083, P = 0.016) with the same variables as in Model 1 and with FBG, FINS, and FCP levels as additional covariables. After further adjustment for age, BMI, sex, ALT, AST, and γ-GT levels in Model 3, there was no statistical difference in the association between FABP1 and the risk of hyperuricemia (OR, 95% CI = 1.022, 0.994–1.052, P = 0.125).
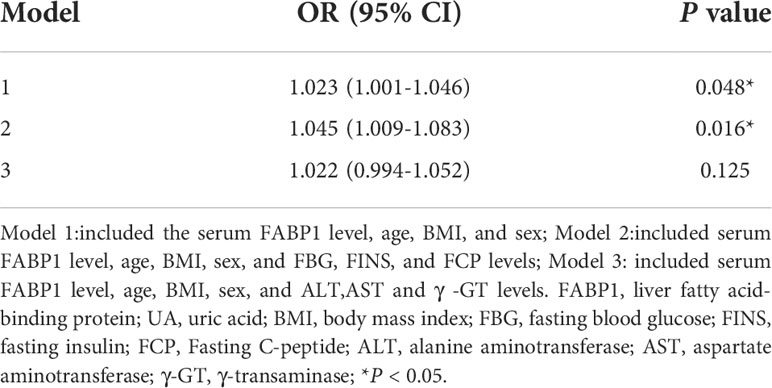
Table 3 Multivariable-adjusted association of serum FABP1 levels and hyperuricemia in patients with obesity.
The UA levels decreased significantly in the HUA group after LSG (from 479.47 ± 79.91 µmol/L to 429.98 ± 90.52 µmol/L at 3 M, P < 0.001; 396.78 ± 80.87 µmol/L at 6 M, P < 0.001; and 406.34 ± 93.90 µmol/L at 12 M, P < 0.001) (Figure 2A). Moreover, the serum FABP1 levels decreased progressively at 3, 6, and 12 months after surgery (for the NUA group: from 25.21 ± 21.68 ng/mL to 14.38 ± 11.88 ng/mL at 3M, P = 0.142 ; 11.09 ± 4.25 ng/mL at 6 M, P = 0.015; and 13.77 ± 9.94 ng/mL at 12 M, P=0.005; and for the HUA group: from 34.76 ± 22.69 ng/m to 16.43 ± 8.59 ng/mL at 3 M, P < 0.001; 20.60 ± 16.62 ng/mL at 6 M, P = 0.012; 11.77 ± 6.9 ng/mL at 12 M, P < 0.001)(Figure 2B). Furthermore, the UA levels in the HUA group showed a greater decrease than that in the NUA group (Figure 2C). Meanwhile, the serum FABP1 levels showed a more significant decrease in the HUA group than in the NUA group at 12 months (27.06 ± 10.98 ng/mL vs. 9.54 ± 6.52 ng/mL, P = 0.003) (Figure 2D). Table 4 shows the correlations of the changes (△) in the serum FABP1 levels and the metabolic factors in the NUA and HUA groups. The changes in the serum FABP1 levels at 12 months after surgery were positively correlated with the changes in the UA levels (r = 0.512, P = 0.011). Moreover, the changes in BMI (r = 0.399, P = 0.048) and ALT levels (r = 0.390, P = 0.031) were associated with the change in serum FABP1 levels at 12M post-LSG in the HUA group.
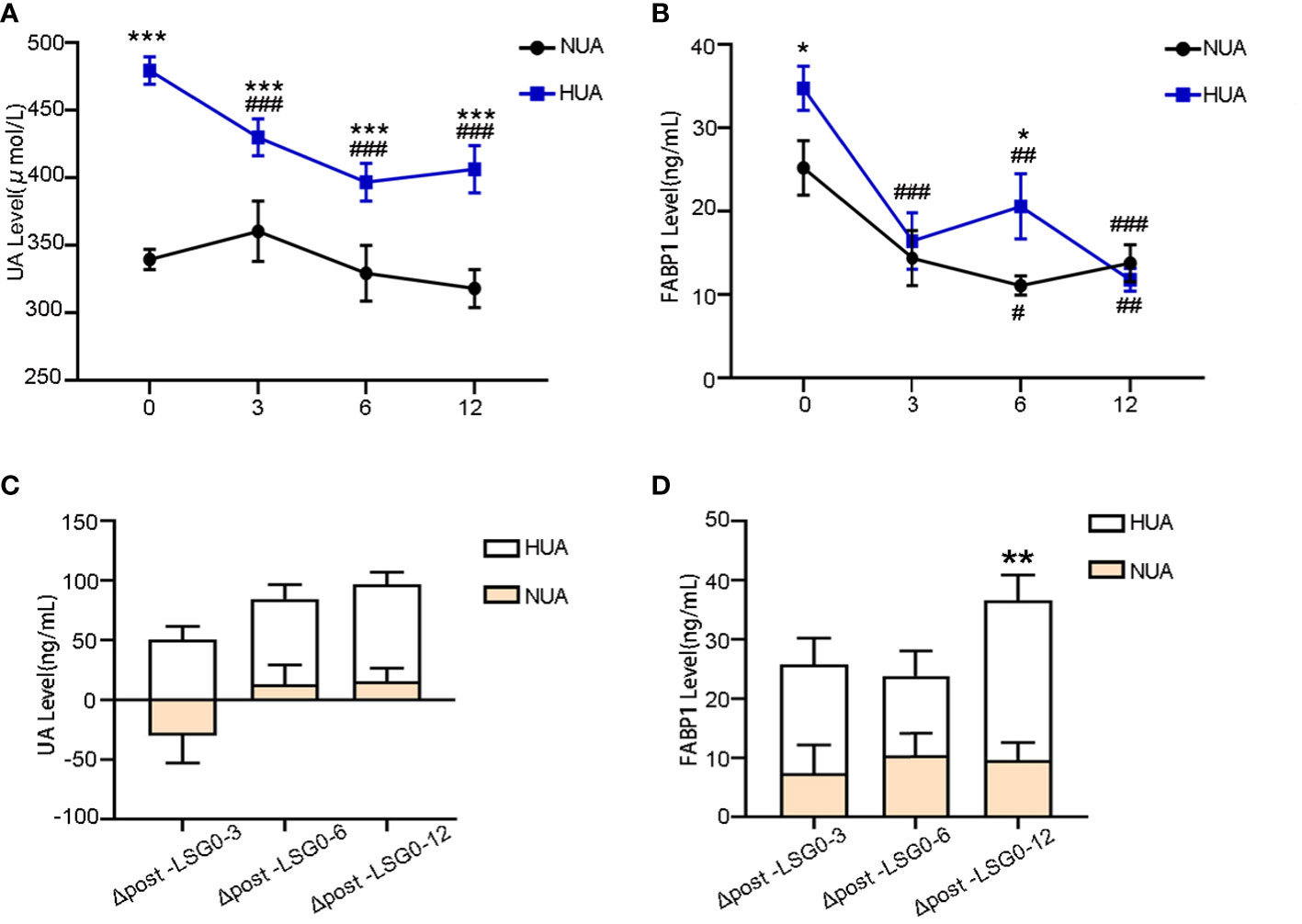
Figure 2 Changes in the serum FABP1 and UA levels between the NUA and HUA groups after LSG. (A, B) Decrease in the UA and FABP1 levels after LSG. (C, D) Comparison of the magnitude of the change in the UA and FABP1 levels at 3, 6, and 12 M post-LSG and baseline; Comparison of the variables between 3, 6, and 12 M post-LSG and baseline, ##P < 0.01, ###P < 0.001 Comparison of the variables between the NUA and HUA groups, *P < 0.05, **P < 0.01, ***P < 0.001.
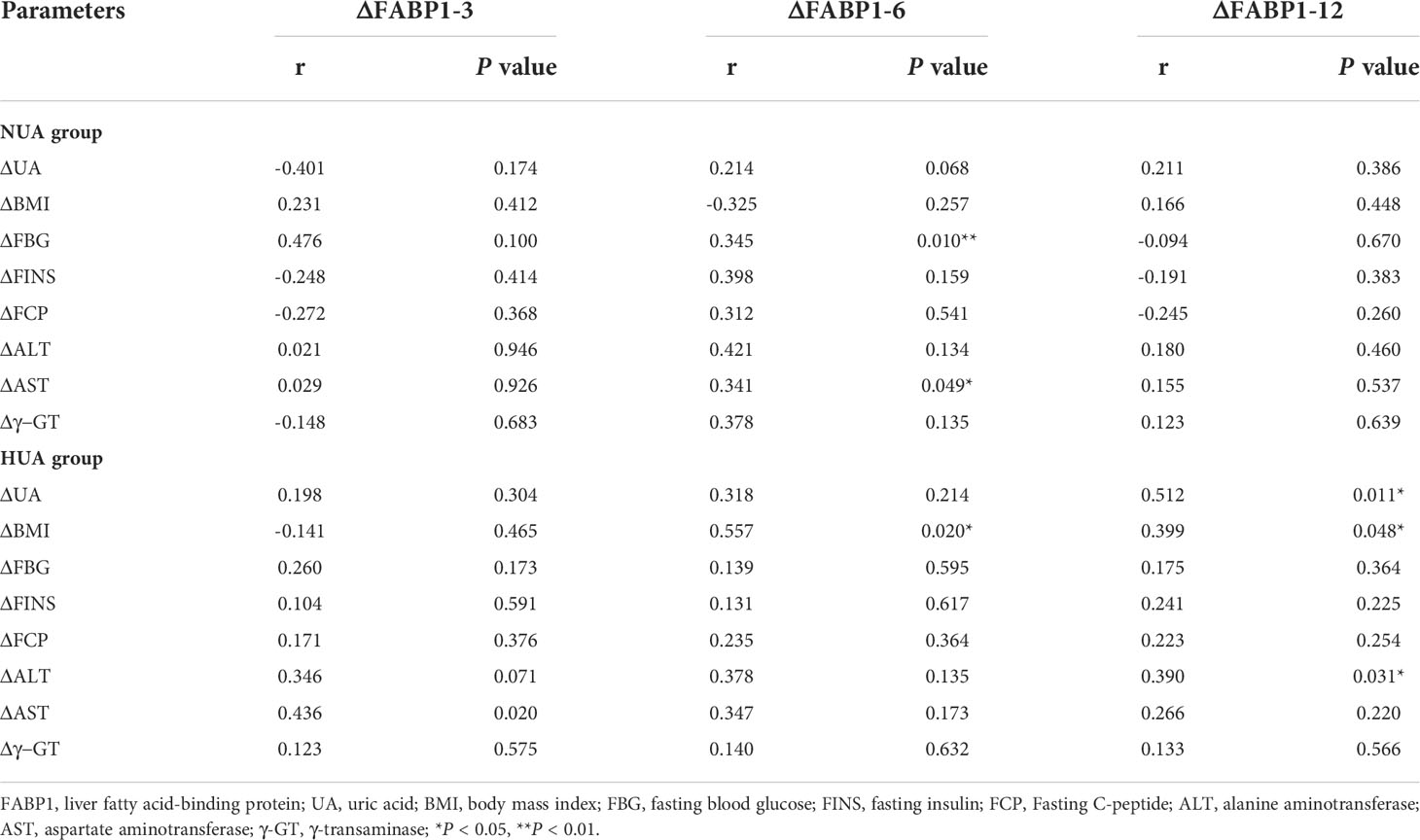
Table 4 Correlation analysis between the improvement in the serum FABP1 levels and the clinical indices between the different UA groups after LSG.
To evaluate the contribution of FABP1 and other metabolic factors toward UA, a multiple linear regression analysis was also performed in Table 5. After adjusting the △BMI, age, and sex, △FBG, △FINS, △FCP, △ALT, △AST and △γ-GT levels, ΔUA (at 12M post-LSG) was also significantly correlated with ΔFABP1.
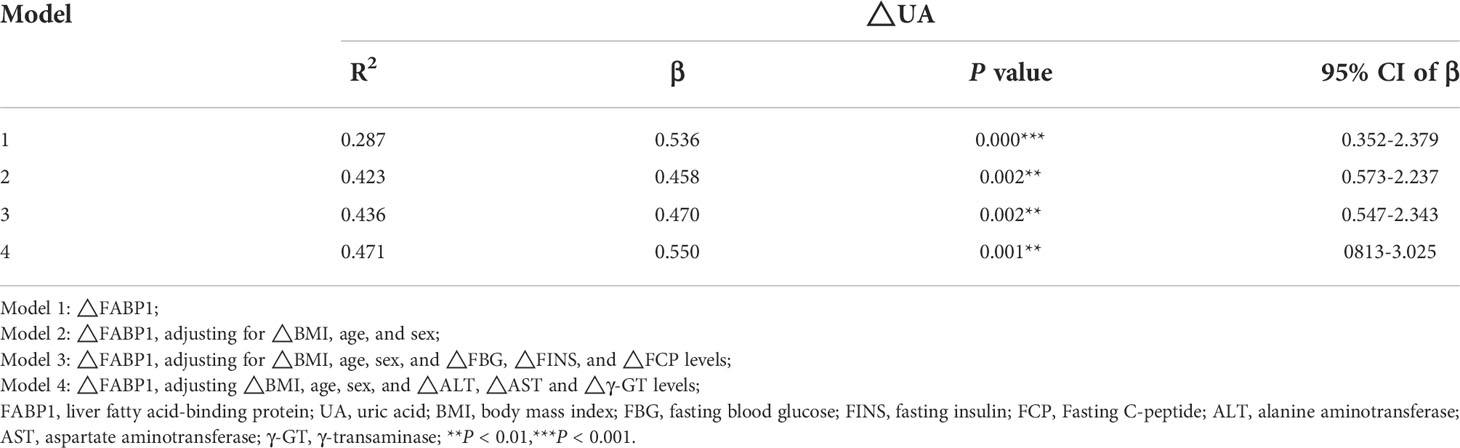
Table 5 Multiple linear analysis of the correlation between changes in UA and FABP1 levels at 12M post-LSG.
Currently, FABP1 is commonly used as a specific biomarker for liver disease and type 1 diabetes mellitus in clinical practice (13–15). However, hyperuricemia as a common metabolic disease is closely related to liver and pancreas islet function, and its relationship with FABP1 has not been reported. Our study demonstrated that serum FABP1 levels were significantly higher in the HUA group than in the NUA group and the positive correlativity between the change in FABP1 and UA levels at 3, 6, and 12 months after LSG. To the best of our knowledge, this is the first study to provide clinical evidence confirming the relationship between serum FABP1 levels and hyperuricemia in patients with obesity before and after LSG.
Hyperuricemia refers to a disorder of purine metabolism or decreased UA excretion, leading to increased SUA levels. Studies (16, 17) have shown that this disorder forms not only the biochemical basis for gout but can also induce major diseases, such as myocardial infarction, diabetes, coronary heart disease, metabolic syndrome, and other diseases, which ultimately deprive patients’ lives. The meta-analysis (18) suggested that the OR value of hypertension risk in HUA patients was 1.48, especially in younger patients with early-onset hypertension. Besides, clinical studies (19, 20) have found that elevated SUA at baseline can predict the incidence of diabetes and insulin resistance (IR) status, suggesting that elevated SUA is an independent risk factor for IR and diabetes. Our study also found that compared with the NUA group, patients in the HUA group had relatively higher BMI and poorer pancreatic islet and liver function. These results suggest the danger of hyperuricemia and the urgency of treatment. Thus, there was an urgent need for further studies to identify the pathogenesis of hyperuricemia.
FABPs (21–23) belong to the lipid-binding protein superfamily. To date, nine types of FABPs have been identified based on tissue-specific distributions: L (liver), I (intestinal), H (muscle and heart), A (adipocyte), E (epidermal), Il (ileal), B (brain), M (myelin), and T (testis) (24). The first described FABP, liver-FABP (L-FABP or FABP1), is expressed highly in the liver, as well as in the intestines and kidneys. FABP1 comprises 127 amino acids with a molecular weight of approximately 14–15 kDa, can regulate the expression of some essential genes involved in lipid metabolism, and is related closely to a variety of metabolic syndromes, such as obesity, NAFLD, and IR (25, 26). In our previous study, we also found that serum FABP1 levels positively correlated with BMI, and after performing a multiple linear regression adjusted for sex, age, ALT, and other factors, the serum FABP1 levels remained strongly correlated with BMI (10). Newberry (27) found that, compared with wild-type mice, the FABP1-gene knockout mice could significantly inhibit diet-induced obesity and reduce the development of fatty liver when fed a diet rich in fatty acids. This was related to the reduced absorption of fatty acids and their esterification in the intestines of mice with this gene deletion, which affects the synthesis rate of chylomicrons. Therefore, serum FABP1 levels can change a variety of metabolic factors and regulate the pathogenesis of obesity.
Furthermore, FABP1 is closely related to the occurrence and development of other metabolic syndromes. Studies have found that FABP1 plays an important role in lipid transport and cholesterol metabolism in the liver. FABP1 expression increased significantly with the formation of fatty liver and was correlated with the degree of hepatic steatosis, proving that serum FABP1 levels play an important role in NAFLD using a mouse model of NAFLD (28). Moreover, the serum FABP1 levels are related to glucose metabolism. Shi Juan et al. (29) found that serum FABP1 levels increased in adolescents with abnormal glucose metabolism and were related to glucose and lipid metabolism but not to the function of pancreatic islet β cells. Furthermore, a previous study suggested that the serum FABP1 level in the renal tubules is associated with glomerular disease and FABP1 plays a protective role in renal tubulointerstitial injury and glomerular injury of diabetic nephropathy (30). However, the relationship between FABP1 and hyperuricemia is unknown. In this study, we also found that serum FABP1 levels were closely related to UA, liver enzyme and glucose metabolism parameters; moreover, it is worth highlighting that after adjusting for confounding factors, preoperative FABP1 level was a risk factor for hyperuricemia at baseline.
Bariatric surgery has been found to regulate BMI safely and effectively and improve metabolic markers such as blood glucose and liver function levels. In recent years, multiple pieces of evidence (31–33) have shown that bariatric surgery is effective in reducing the incidence of gout attacks and serum urate concentration in patients with hyperuricemia or gout up to 12 months of follow-up. Our results also indicated UA and FABP1 levels decreased at 3, 6, and 12 months postoperatively, and the decrease was greater in the HUA group than in the NUA group at 3, 6, and 12 months after LSG; Moreover, the changes in the serum FABP1 levels at 12 months after LSG showed a positive correlation with changes of SUA levels in the HUA group.
The current study applied a variety of statistical methods to elucidate the relationship between serum FABP1 levels and hyperuricemia before and after LSG for the first time. However, our study had certain limitations. First, owing to the observational nature of this study, we could not determine any causal relationship between the UA levels and elevated serum FABP1 levels. Moreover, this study did not investigate the mechanism in depth. In binomial logistic regression, we found that the association between serum FABP1 levels and the risk of hyperuricemia had no statistical effect after adjusting for liver enzymes. Now, a large number of studies have shown that UA levels are closely related to liver diseases, such as NAFLD (34–36), and are also positively correlated with liver enzyme indicators (37). And there is no doubt that IR must play a crucial role in this connection. FABP1 is also mainly expressed in the liver. Many studies have also proved the association between FABP1 and liver diseases (15, 38). Silencing of FABP1 can ameliorate hepatic steatosis, inflammation, and oxidative stress (38). And FABP1 can be used as a marker of liver damage after medication (15). Meanwhile, FABP1 is also positively correlated with liver enzymes levels. So we speculated that FABP1 and hyperuricemia may interact through liver function-related indicators. We will conduct further research using animal and cell experiments in the future. Second, the study had a small sample size and short follow-up durations. There was a sampling error in this study. Future studies with larger numbers of patients and longer follow-up periods are warranted to validate our findings.
Serum FABP1 levels were significantly higher in the HUA group than in the NUA group. In addition, serum FABP1 levels positively correlated with UA levels, and the preoperative serum FABP1 levels may be a risk factor for hyperuricemia. Moreover, the serum FABP1 levels decreased postoperatively and with a greater reduction in the HUA group than in the NUA group at 3, 6, and 12 M after LSG. Furthermore, changes in the serum FABP1 levels at 12 M after LSG showed a positive correlation with changes in the UA levels in the HUA group. Our findings may provide a basis for encouraging researchers and clinicians working on metabolic diseases to adopt a new perspective on the role of FABP1, and endorse FABP1 as an important indicator of hyperuricemia.
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding authors.
The studies involving human participants were reviewed and approved by ClinicalTrial.gov ID: ChiCTR-OCS-12002381. The patients/participants provided their written informed consent to participate in this study.
JZ and SQ conceived and supervised the overall study. XW, CZ, WM, LB, MZ, JY, and LD performed the literature review and collected the epidemiological and clinical data. XC and HC contributed to the statistical analysis. HY, HM, and XCW drafted the manuscript. All authors contributed to the article and approved the submitted version.
This work was supported by the National Natural Science Foundation of China (grant numbers 81700752, 81970677, 82170861, 82170904), Traditional Chinese Medicine Scientific Research Project of Shanghai Municipal Health Commission (grant number 2020_JP013), and Climbing Talent Program of the 10th People's Hospital affiliated to Tongji University (2021SYPDRC059,2021SYPDRC050).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Menenakos E, Doulami G, Tzanetakou IP, Natoudi M, Kokoroskos N, Almpanopoulos K, et al. The use of serum uric acid concentration as an indicator of laparoscopic sleeve gastrectomy success. International Surgery. (2015) 100(1):173–9. doi: 10.9738/INTSURG-D-13-00186.1
2. Li L, Zhang Y, Zeng C. Update on the epidemiology, genetics, and therapeutic options of hyperuricemia. Am J Trans Res (2020) 12(7):3167–81.
3. Li C, Hsieh M-C, Chang S-J. Metabolic syndrome, diabetes, and hyperuricemia. Curr Opin In Rheumatol (2013) 25(2):210–6. doi: 10.1097/BOR.0b013e32835d951e
4. Dehlin M, Jacobsson L, Roddy E. Global epidemiology of gout: Prevalence, incidence, treatment patterns and risk factors. Nat Rev Rheumatol (2020) 16(7):380–90. doi: 10.1038/s41584-020-0441-1
5. Wang G, Bonkovsky HL, de Lemos A, Burczynski FJ. Recent insights into the biological functions of liver fatty acid binding protein 1. J Lipid Res (2015) 56(12):2238–47. doi: 10.1194/jlr.R056705
6. Huang H, McIntosh AL, Martin GG, Landrock D, Chung S, Landrock KK, et al. FABP1: A novel hepatic endocannabinoid and cannabinoid binding protein. Biochemistry (2016) 55(37):5243–55. doi: 10.1021/acs.biochem.6b00446
7. Pi H, Liu M, Xi Y, Chen M, Tian L, Xie J, et al. Long-term exercise prevents hepatic steatosis: A novel role of FABP1 in regulation of autophagy-lysosomal machinery. FASEB J (2019) 33(11):11870–83. doi: 10.1096/fj.201900812R
8. Eguchi A, Iwasa M. The role of elevated liver-type fatty acid-binding proteins in liver diseases. Pharm Res (2021) 38(1):89–95. doi: 10.1007/s11095-021-02998-x
9. Tsai IT, Wu C-C, Hung W-C, Lee T-L, Hsuan C-F, Wei C-T, et al. FABP1 and FABP2 as markers of diabetic nephropathy. Int J Med Sci (2020) 17(15):2338–45. doi: 10.7150/ijms.49078
10. You H, Wen X, Zhu C, Chen M, Dong L, Zhu Y, et al. Serum FABP1 levels correlate positively with obesity in Chinese patients after laparoscopic sleeve gastrectomy: a 12-month follow-up study. Obes Surg (2020) 30(3):931–40. doi: 10.1007/s11695-019-04307-3
11. de Jonge C, Rensen SS, Koek GH, Joosten MF, Buurman WA, Bouvy ND, et al. Endoscopic duodenal-jejunal bypass liner rapidly improves plasma parameters of nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol (2013) 11(11):1517–20. doi: 10.1016/j.cgh.2013.07.029
12. Chang X, Cai H, Yin K. The regulations and mechanisms of laparoscopic sleeve gastrectomy (LSG) for obesity and type 2 diabetes: A systematic review. Surg Laparoscopy Endosc Percutaneous Techniques (2017) 27(6):e122–6. doi: 10.1097/SLE.0000000000000468
13. Pelsers MM, Hermens WT, Glatz JF. Fatty acid-binding proteins as plasma markers of tissue injury. Clin Chim Acta (2005) 352(1-2):15–35. doi: 10.1016/j.cccn.2004.09.001
14. Nakamura T, Sugaya T, Kawagoe Y, Ueda Y, Osada S, Koide H. Effect of pitavastatin on urinary liver-type fatty acid-binding protein levels in patients with early diabetic nephropathy. Diabetes Care (2005) 28(11):2728–32. doi: 10.2337/diacare.28.11.2728
15. Karvellas CJ, Speiser JL, Tremblay M, Lee WM, Rose CF. Elevated FABP1 serum levels are associated with poorer survival in acetaminophen-induced acute liver failure. Hepatology (2017) 65(3):938–49. doi: 10.1002/hep.28945
16. Mandal AK, Mount DB. The molecular physiology of uric acid homeostasis. Annu Rev Physiol (2015) 77:323–45. doi: 10.1146/annurev-physiol-021113-170343
17. Joosten LAB, Crişan TO, Bjornstad P, Johnson RJ. Asymptomatic hyperuricaemia: A silent activator of the innate immune system. Nat Rev Rheumatol (2020) 16(2):75–86. doi: 10.1038/s41584-019-0334-3
18. Kim SY, Guevara JP, Kim KM, Choi HK, Heitjan DF, Albert DA. Hyperuricemia and coronary heart disease: a systematic review and meta-analysis. Arthritis Care Res (Hoboken) (2010) 62(2):170–80. doi: 10.1002/acr.20065
19. Spatola L, Ferraro PM, Gambaro G, Badalamenti S, Dauriz M. Metabolic syndrome and uric acid nephrolithiasis: Insulin resistance in focus. Metabolism (2018) 83:225–33. doi: 10.1016/j.metabol.2018.02.008
20. Han Y, Han X, Yin Y, Cao Y, Di H, Wu J, et al. Dose-response relationship of uric acid with fasting glucose, insulin, and insulin resistance in a united states cohort of 5,148 non-diabetic people. Front Med (Lausanne) (2022) 9:905085. doi: 10.3389/fmed.2022.905085
21. Binas B, Erol E. FABPs as determinants of myocellular and hepatic fuel metabolism. Mol Cell Biochem (2007) 299(1-2):75–84. doi: 10.1007/s11010-005-9043-0
22. Prinetti A, Mitro N. FABP1 in wonderland. J Neurochem (2016) 138(3):371–3. doi: 10.1111/jnc.13685
23. Atshaves BP, Martin GG, Hostetler HA, McIntosh AL, Kier AB, Schroeder F. Liver fatty acid-binding protein and obesity. J Nutr Biochem (2010) 21(11):1015–32. doi: 10.1016/j.jnutbio.2010.01.005
24. Gajda AM, Storch J. Enterocyte fatty acid-binding proteins (FABPs): Different functions of liver and intestinal FABPs in the intestine. Prostaglandins Leukot Essent Fatty Acids (2015) 93:9–16. doi: 10.1016/j.plefa.2014.10.001
25. Hostetler HA, McIntosh AL, Atshaves BP, Storey SM, Payne HR, Kier AB, et al. L-FABP directly interacts with PPARalpha in cultured primary hepatocytes. J Lipid Res (2009) 50(8):1663–75. doi: 10.1194/jlr.M900058-JLR200
26. Thumser AE, Moore JB, Plant NJ. Fatty acid binding proteins: tissue-specific functions in health and disease. Curr Opin Clin Nutr Metab Care (2014) 17(2):124–9. doi: 10.1097/mco.0000000000000031
27. Newberry EP, Kennedy S, Xie Y, Luo J, Jiang H, Ory DS, et al. Phenotypic divergence in two lines of l-fabp-/- mice reflects substrain differences and environmental modifiers. Am J Physiol Gastrointest Liver Physiol (2015) 309(8):G648–61. doi: 10.1152/ajpgi.00170.2015
28. Newberry EP, Xie Y, Kennedy SM, Luo J, Davidson NO. Protection against Western diet-induced obesity and hepatic steatosis in liver fatty acid-binding protein knockout mice. Hepatology (2006) 44(5):1191–205. doi: 10.1002/hep.21369
29. Shi J, Zhang Y, Gu W, Cui B, Xu M, Yan Q, et al. Serum liver fatty acid binding protein levels correlate positively with obesity and insulin resistance in Chinese young adults. PloS One (2012) 7(11):e48777. doi: 10.1371/journal.pone.0048777
30. Iguchi N, Uchiyama A, Ueta K, Sawa Y, Fujino Y. Neutrophil gelatinase-associated lipocalin and liver-type fatty acid-binding protein as biomarkers for acute kidney injury after organ transplantation. J Anesth (2015) 29(2):249–55. doi: 10.1007/s00540-014-1909-4
31. Golomb I, Ben David M, Glass A, Kolitz T, Keidar A. Long-term metabolic effects of laparoscopic sleeve gastrectomy. JAMA Surg (2015) 150(11):1051–7. doi: 10.1001/jamasurg.2015.2202
32. Romero-Talamás H, Daigle CR, Aminian A, Corcelles R, Brethauer SA, Schauer PR. The effect of bariatric surgery on gout: a comparative study. Surg Obes Relat Dis (2014) 10(6):1161–5. doi: 10.1016/j.soard.2014.02.025
33. Antozzi P, Soto F, Arias F, Carrodeguas L, Ropos T, Zundel N, et al. Development of acute gouty attack in the morbidly obese population after bariatric surgery. Obes Surg (2005) 15(3):405–7. doi: 10.1381/0960892053576802
34. Wan X, Xu C, Lin Y, Lu C, Li D, Sang J, et al. Uric acid regulates hepatic steatosis and insulin resistance through the NLRP3 inflammasome-dependent mechanism. J Hepatol (2016) 64(4):925–32. doi: 10.1016/j.jhep.2015.11.022
35. Iracheta-Vellve A, Petrasek J, Satishchandran A, Gyongyosi B, Saha B, Kodys K, et al. Inhibition of sterile danger signals, uric acid and ATP, prevents inflammasome activation and protects from alcoholic steatohepatitis in mice. J Hepatol (2015) 63(5):1147–55. doi: 10.1016/j.jhep.2015.06.013
36. Xu C, Wan X, Xu L, Weng H, Yan M, Miao M, et al. Xanthine oxidase in non-alcoholic fatty liver disease and hyperuricemia: One stone hits two birds. J Hepatol (2015) 62(6):1412–9. doi: 10.1016/j.jhep.2015.01.019
37. Molla NH, Kathak RR, Sumon AH, Barman Z, Mou AD, Hasan A, et al. Assessment of the relationship between serum uric acid levels and liver enzymes activity in Bangladeshi adults. Sci Rep (2021) 11(1):20114. doi: 10.1038/s41598-021-99623-z
Keywords: liver-type fatty acid-binding protein, laparoscopic sleeve gastrectomy, hyperuricemia, obesity, uric acid
Citation: You H, Ma H, Wang X, Wen X, Zhu C, Mao W, Bu L, Zhang M, Yin J, Du L, Cheng X, Chen H, Zhang J and Qu S (2022) Association between liver-type fatty acid-binding protein and hyperuricemia before and after laparoscopic sleeve gastrectomy. Front. Endocrinol. 13:993137. doi: 10.3389/fendo.2022.993137
Received: 13 July 2022; Accepted: 22 September 2022;
Published: 06 October 2022.
Edited by:
Sanyuan Hu, Qianfoshan Hospital, Shandong University, ChinaReviewed by:
Shiri Li, Cornell University, United StatesCopyright © 2022 You, Ma, Wang, Wen, Zhu, Mao, Bu, Zhang, Yin, Du, Cheng, Chen, Zhang and Qu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jun Zhang, anVuemhhbmdAdG9uZ2ppLmVkdS5jbg==; Shen Qu, cXVzaGVuY25AaG90bWFpbC5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.