
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Endocrinol. , 08 September 2022
Sec. Obesity
Volume 13 - 2022 | https://doi.org/10.3389/fendo.2022.991902
This article is part of the Research Topic The Impact of Adipose Tissue Dysfunction on Cardiovascular and Renal Disease, Volume II View all 12 articles
 Olga V. Gruzdeva†
Olga V. Gruzdeva† Yulia A. Dyleva†*
Yulia A. Dyleva†* Ekaterina V. Belik†
Ekaterina V. Belik† Maxim Yu. Sinitsky
Maxim Yu. Sinitsky Kiril A. Kozyrin
Kiril A. Kozyrin Olga L. Barbarash
Olga L. BarbarashIn our study we investigated the relationships between adipocytokines in adipose tissue (AT) and cardiovascular disease (CVD) risk factors; (2) Methods: fat tissue biopsies were obtained from 134 patients with stable CAD undergoing coronary artery bypass grafting and 120 patients undergoing aortic or mitral valve replacement. Adipocytes were isolated from subcutaneous (SAT), epicardial (EAT), and perivascular AT (PVAT) samples, and cultured for 24 h, after which gene expression of adipocytokines in the culture medium was determined; (3) Results: men showed reduced ADIPOQ expression in EAT and PVAT, LEP expression in PVAT, and LEPR expression in SAT and PVAT compared to women. Men also exhibited higher SAT and lower PVAT IL6 than women. Meanwhile, dyslipidemia associated with decreased ADIPOQ expression in EAT and PVAT, LEPR in EAT, and IL6 in PVAT. Arterial hypertension (AH) associated with low EAT and PVAT ADIPOQ, and high EAT LEP, SAT, as well as PVAT LEPR, and IL6 in SAT and EAT. ADIPOQ expression decreased with increased AH duration over 20 years against an increased LEP background in ATs. Smoking increased ADIPOQ expression in all ATs and increased LEP in SAT and EAT, however, decreased LEPR in PVAT. Patients 51–59 years old exhibited the highest EAT and PVAT LEP, IL-6, and LEPR expression compared to other age groups; (4) Conclusions: decreased EAT ADIPOQ expression against an increased pro-inflammatory IL6 background may increase atherogenesis and contribute to CAD progression in combination with risk factors including male sex, dyslipidemia, and AH.
Morbidity and mortality from cardiovascular diseases (CVD) remain prevalent in many countries, despite ongoing prophylaxis and the introduction of new treatment methods (1). The epicardial adipose tissue (EAT) and perivascular (PVAT) are important in the pathogenesis of atherosclerosis as they are located in close proximity to the myo-cardium and coronary arteries and function as active endocrine organs, being able to synthesize and produce adipocytokines. In patients with elevated cardiovascular risk, higher pro-inflammatory adipocytokine levels are observed in EAT than subcutaneous adipose tissue (SAT) (2), and the EAT of patients with severe coronary artery disease (CAD) expresses less adiponectin (3). Moreover, EAT thickness correlates with metabolic risk factors and contributes to coronary artery atherosclerotic plaque development (4).
Adiponectin, the main protein secreted by adipocytes, exhibits cardioprotective, an-ti-diabetic, anti-atherogenic, and anti-inflammatory effects, unlike other adipokines (5). Low adiponectin levels are associated with arterial hypertension (AH), obesity, insulin resistance, type 2 diabetes mellitus, and myocardial infarction (MI) (6). Conversely, leptin has pro-inflammatory and prothrombotic effects (7), whereas pro-inflammatory inter-leukin 6 (IL-6) can elicit hypertrophy-inducing effects and is an independent predictor of CAD vessel disease (8). Recently, an increasing body of evidence has demonstrated that the expression of adipocytokines differ depending on the location of the fat depot.
Although assessing cardiovascular risk based on traditional risk factors has high prognostic value, identifying new parameters can significantly improve the stratification model for patients with cardiovascular disease. Various factors, including pro-inflammatory markers, that have demonstrated potential association with athero-genesis, are produced by AT (9), however, data regarding the association between age, sex, and other parameters, with adiponectin, leptin, and IL-6 levels in the various local fat depots, remain limited and are often contradictory. Accordingly, determining the factors that impact the course of CAD, as well as the associated prognosis, remains critical (10). Moreover, as atherosclerosis constitutes a multifactorial disease influenced by both un-modifiable (e.g., sex, age) and modifiable (smoking, dyslipidemia) factors, clarification of the pathogenetic relationships between adipocytokines and CVD risk factors is necessary. Toward this end, we evaluated the expression of adiponectin, leptin, its soluble receptor (sOB-R), and IL-6 in EAT, PVAT, SAT, and their relationships with the main CVD risk factors.
This study was performed at the Federal State Budgetary Institution’s Research In-stitute for Complex Issues of Cardiovascular Diseases. We examined 134 patients with a median age of 65.6 (49.3; 70.3) years with CAD who underwent elective coronary artery bypass grafting (CABG) and 120 patients median aged 60.47 (45.2; 63.2) years with aortic or mitral valve replacement. Exclusion criteria included: 1) > 75 years of age; 2) clinical conditions including MI, type 1 or type 2 diabetes mellitus, anemia, autoimmune diseases, liver or kidney failure, infectious or inflammatory diseases, and oncological diseases.
Traditional cardiovascular risk factors and patient treatment were recorded. AH was defined as systolic blood pressure > 140 mm Hg Art., diastolic blood pressure > 90 mm Hg. Dyslipidemia was defined as a previously detected increase in total serum cholesterol (> 200 mg/dl), triglycerides (>200 mg/dl), or low-density lipoprotein (LDL) cholesterol (> 150 mg/dl) for at least 1 year or use of lipid-lowering drugs. Smoking was classified as current or former smokers; current smoking status was defined as at least one cigarette daily over the last year.
SAT, EAT, and PVAT biopsies (3 to 5 g) were obtained during aortocoronary bypass surgery and aortic or mitral valve replacement. SAT samples were obtained from the subcutaneous tissue of the lower angle of the mediastinal wound. EAT was sourced from its largest source, from the right heart (right atrium and ventricle) and PVAT were ob-tained from the area of the right coronary artery. Adipocytes were isolated from adipose tissues under sterile conditions in a laminar flow hood (BOV-001-AMS MZMO, Millerovo, Russia), as previously described (11). Adipocytes were counted in a Goryaev chamber. Cell viability was evaluated according to the method described by Suga et al. (12). Adipocytes (20 × 105) were seeded into a 24-well plate (Greiner Bio One International GmbH, Kremsmünster, Austria), and the volume in each well was adjusted to 1 mL with culture medium, as previously described (11). Cells were incubation for 24 h at 37 ± 1°C in an atmosphere of 5% CO2 and 10% oxygen. The adipocytes were then immediately processed for RNA extraction to determine adipocytokine gene expression.
Total RNA was isolated from adipocytes using the commercial RNeasy® Plus Uni-versal Mini Kit (Qiagen, Hilden, Germany), according to the manufacturer’s instructions with slight modifications, as described previously (13). The quantity and quality of puri-fied RNA were assessed using a NanoDrop 2000 Spectrophotometer (Thermo Fisher Scientific) by measuring the light absorbance at 280 nm, 260 nm, and 230 nm and calcu-lating the 260/280 (A260/280) and 260/230 (A260/230) ratios. The integrity of the RNA was determined by electrophoresis in agarose gel, followed by visualization using the Gel Doc™ XR+ System (Bio-Rad, Hercules, CA, USA). Extracted RNA was stored at –70°C.
Single-stranded cDNA was synthesized using the High-Capacity cDNA Reverse Transcription Kit (Applied Biosystems, Foster City, CA, USA) on a VeritiTM 96-Well Thermal Cycler (Applied Biosystems). Reverse transcription was performed using the program suggested by the manufacturer. The quantity and quality of synthesized cDNA were assessed using a NanoDrop 2000 Spectrophotometer. Samples were stored at –20°C.
Expression of adiponectin (ADIPOQ), leptin (LEP), soluble leptin receptor (LEPR) and IL6 genes was evaluated by quantitative real-time polymerase chain reaction (qPCR) using TaqManTM Gene Expression Assays (ADIPOQ Hs00605917_m1, LEP Hs00174877_m1, LEPR Hs00174497_m1, IL6 Hs00174131_m1, Applied Biosystems, USA) on a ViiA 7 Real-Time PCR System (Applied Biosystems). Each 20 µL reaction mix contained 10 µL of TaqManTM Gene Expression Master Mix (Applied Biosystems), 1 µL of TaqManTM Gene Expression Assay (Applied Biosystems), and 9 µL of cDNA template comprising 100 ng of cDNA + nuclease-free water). Samples were amplified under the following thermal cycling conditions: 2 min at 50°C, 10 min at 95°C, 40 cycles of 15 sec at 95°C and 1 min at 60°C. As a negative control, 20 µL of reaction mix with no cDNA template was used. For each sample and negative control, three technical replicates were prepared.
The results were normalized using reference genes HPRT1, GAPDH, and B2M. Test gene expression was calculated using the Pfaffl method and expressed on a logarithmic (log10) scale as a multiple change relative to the control samples (14).
Statistical analysis was performed using GraphPad Prism 6 (La Jolla, CA, USA) and Statistica version 9.1 (Dell Software, Inc., Round Rock, TX, USA). The Kolmogorov–Smirnov test was used to verify normal distribution of data. For non-normally distributed variables, data were presented as median (Me) and 25th and 75th quartiles (Q1; Q3). Comparison of two independent groups was carried out using the nonparametric Mann-Whitney test. Differences between three groups were compared using one-way analysis of variance (ANOVA) for continuous variables. Categorical variables are ex-pressed as percentages and compared using chi-squared test or Fisher’s exact test. P values < 0.05 were considered statistically significant.
Analysis of the clinical and anamnestic characteristics revealed that 75% of the sub-jects were men, AH was observed in 90.5% of all patients, angina pectoris in 97.63%, family history of CAD in 59.5%, previous MI in 67.86%, history of cerebrovascular acci-dent/transient ischemic attack in 7.14%, and 69.0% of patients smoked (Table 1). Patients received standard therapy with antiplatelet agents, beta-blockers, ACE inhibitors, and HMG-CoA reductase inhibitors.
Moreover, ADIPOQ expression in CAD patients was lower in EAT compared to SAT (p = 0.038) and PVAT (p = 0.027), while that of leptin was higher in EAT compared to SAT and PVAT (p = 0.003 and p = 0.002, respectively). Similarly, LEPR was more highly ex-pressed in EAT (p = 0.001) and PVAT (0.0003) compared to SAT, with that in the PVAT higher than in the EAT (p = 0.028). In addition, EAT was characterized by the highest IL-6 expression in comparison with the of SAT and PVAT samples (p = 0.001 and p = 0.025, respectively). Similar expression patterns were observed in the tissues collected from patients with heart defects. However, patients with defects were characterized by a higher ADIPOQ expression in EAT (p = 0.031), lower LEP expression in EAT (p = 0.004) and PVAT (p = 0.008), lower LEPR expression in the PVAT (p = 0.022), as well as lower IL-6 expression in EAT (p = 0.0002) and PVAT (p = 0.003) compared to patients with CAD.
Women exhibited higher ADIPOQ expression in EAT (2.5-fold, p = 0.001) and PVAT (2.8-fold, p = 0.002) compared to men, whereas expression in SAT did not differ between sexes (Figure 1).
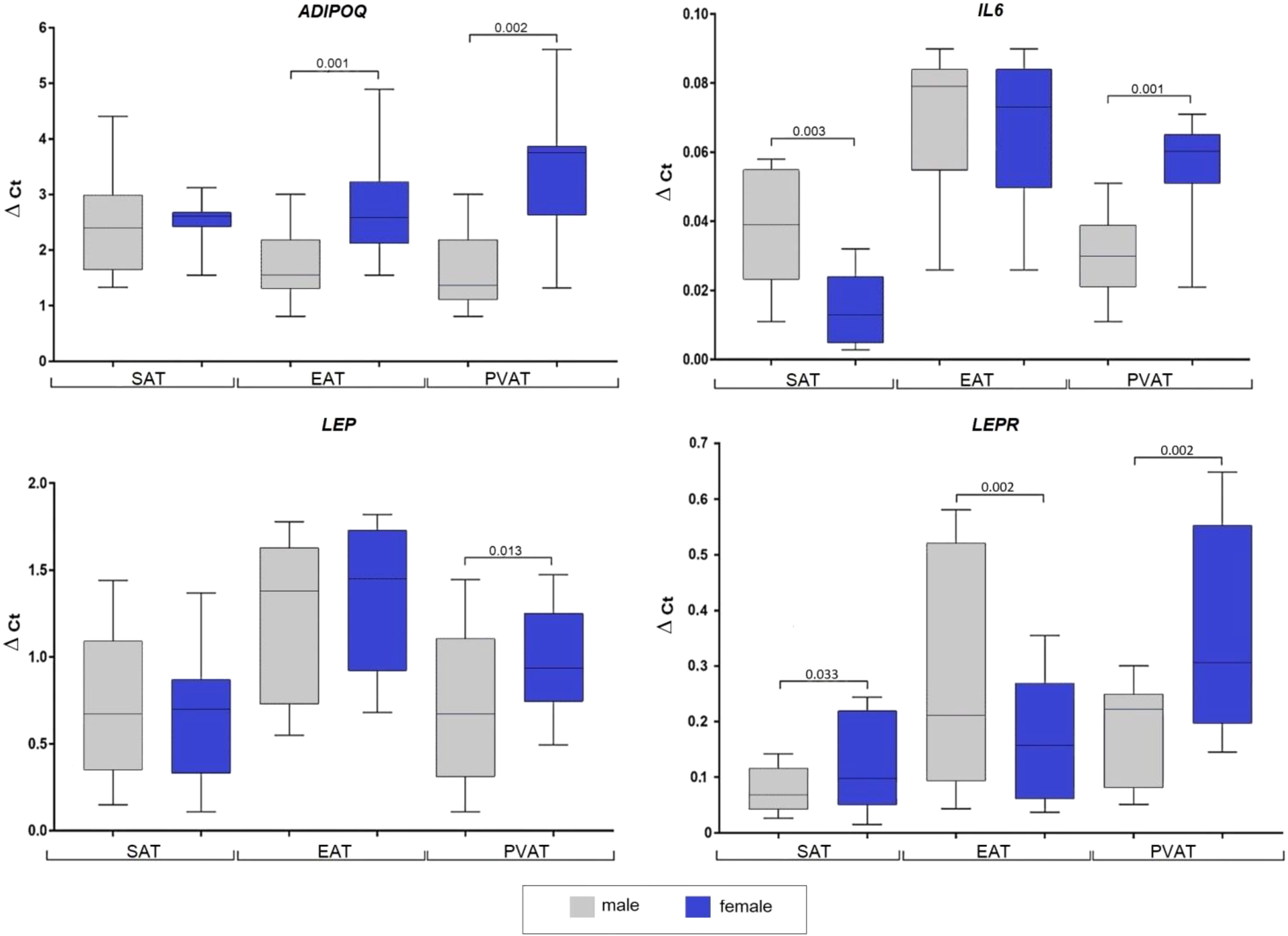
Figure 1 Adipocytokine genes expression in the subcutaneous, epicardial, and perivascular adipocytes based on the sex of patients with coronary artery disease. Data are presented in Me (Q1; Q3). SAT, subcutaneous adipose tissue; EAT, epicardial adipose tissue; PVAT, perivascular adipose tissue; p, level of statistical significance; sOB-R, soluble leptin receptor; IL6, interleukin 6.
Women also showed higher LEP mRNA expression in PVAT (1.4-fold, p = 0.013), while that in SAT and EAT did not significantly differ. In men, LEPR expression was lower in SAT (1.5-fold, p = 0.033) and PVAT (1.3-fold, p = 0.042), but higher in EAT (1.3-fold, p = 0.013). Also, the IL6 mRNA expression in men was significantly higher in SAT (3-fold, p = 0.003), however, was 2-fold lower in PVAT (p = 0.01), whereas no dif-ference was observed between sexes in EAT.
Evaluation according to patient age (≤ 50 years; younger, 51–59; mid-age, and ≥ 60; older) revealed maximum SAT ADIPOQ expression in the younger group, which was equivalent to twice that observed in the mid- age (p = 0.021) and older (p = 0.013) groups (Figure 2).
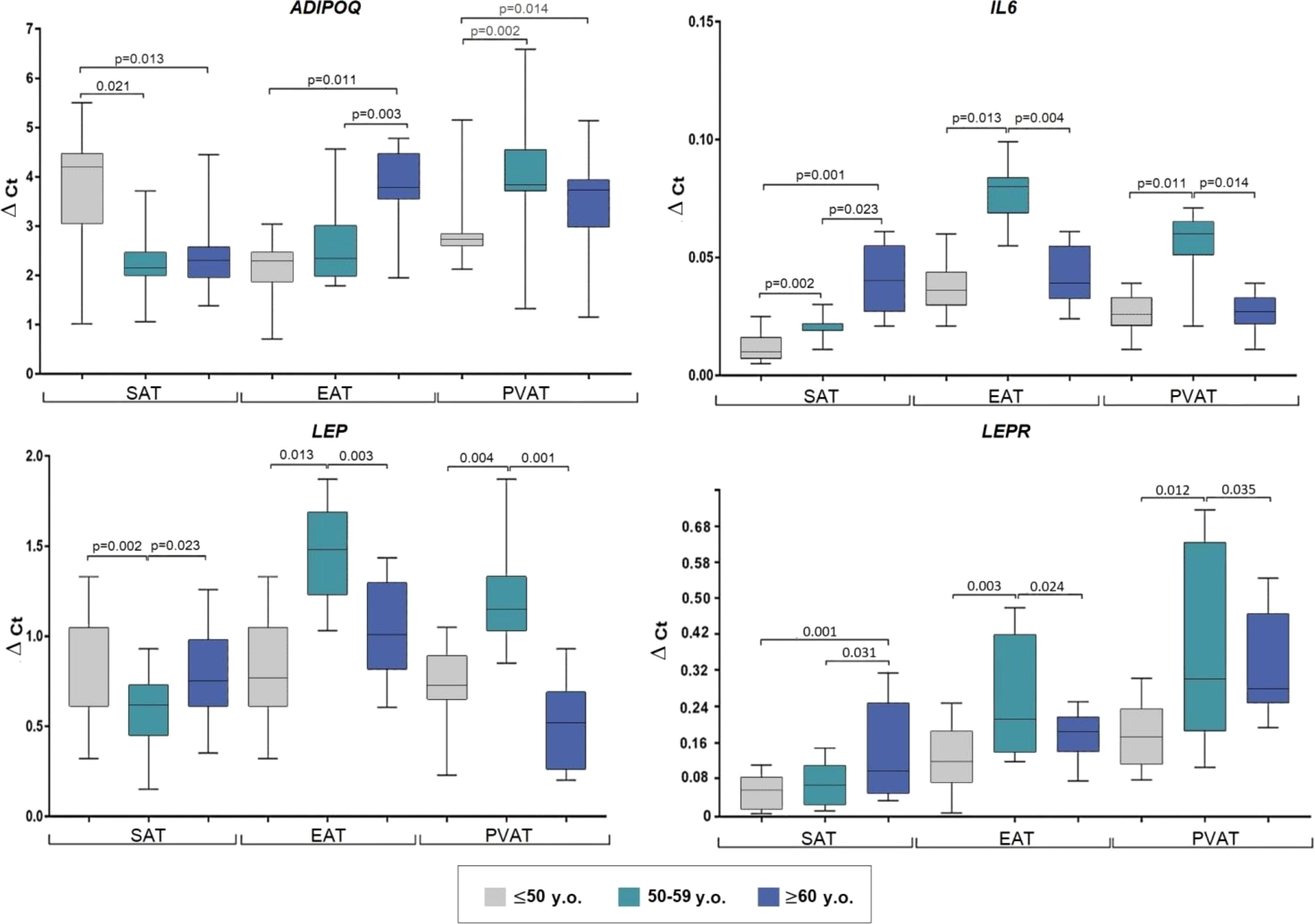
Figure 2 Adipocytokine gene expression in the adipocytes collected from adipose tissue based on the age of coronary artery patients. Data are presented in Me (Q1; Q3). SAT, subcutaneous adipose tissue; EAT, epicardial adipose tissue; PVAT, perivascular adipose tissue; p, level of statistical significance; sOB-R, soluble leptin receptor; IL6, interleukin 6.
In EAT, the highest ADIPOQ mRNA expression was observed in the older group, which was 1.7-fold that in the younger (p = 0.011) and mid-age (p = 0.003) groups. Lowest PVAT ADIPOQ expression was observed in the younger group which was approximately 1.4-fold lower than that in the mid-age (p = 0.002) and older (p = 0.014) groups.
The mid-age group exhibited decreased LEP in SAT (1.2-fold) compared to the younger (p = 0.002) and older (p = 0.023) groups, however, increased LEP levels were observed in EAT and PVAT of mid-age patients compared to the younger (2-fold, p = 0.013; 1.6-fold, p = 0.004, respectively) and older (1.5-fold, p = 0.003; 2.2-fold, p = 0.001, respectively) groups.
The older group exhibited the highest LEPR expression in SAT (3-fold, p = 0.001 vs. younger; 2.3-fold, p = 0.031 vs mid-age), whereas the mid-age group showed highest ex-pression in EAT and PVAT (1.6-fold, p = 0.003 and 1.8-fold, p = 0.012 vs younger; 1.1-fold, p = 0.024 and 1.2-fold, p = 0.035 vs older).
SAT IL-6 expression was higher in the older group (4-fold, p = 0.011 vs younger; 2-fold, p = 0.023 vs mid-age). Meanwhile, the mid-age individuals exhibited the highest IL6 expression in EAT (2.2-fold, p = 0.013 vs younger; 2.05-fold, p = 0.004 vs older) and PVAT (2.3-fold, p = 0. 011; 2.2-fold, p = 0.014 times, respectively) (Figure 2).
In patients with dyslipidemia, ADIPOQ mRNA was decreased in EAT (2.7-fold, p = 0.021) and PVAT (3.6-fold, p = 0.033), however, did not differ in SAT (Figure 3).

Figure 3 Adipocytokine gene expression in the adipocytes collected from adipose tissue based on the presence of dyslipidemia in coronary artery disease patients. Data are presented in Me (Q1; Q3). SAT, subcutaneous adipose tissue; EAT, epicardial adipose tissue; PVAT, perivascular adipose tissue; p, level of sta-tistical significance; sOB-R, soluble leptin receptor; IL6, interleukin 6.
Meanwhile, LEP expression was not correlated with dyslipidemia. Conversely, dyslipidemia was associated with a 2.1-fold (p = 0.014) decrease in LEPR mRNA in EAT and 2.3-fold decrease in IL6 in PVAT (p = 0.023). Alternatively, IL-6 did not differ based on dyslipidemia status in SAT and EAT.
AH in patients with CAD exhibited decreased ADIPOQ expression in EАT (2-fold, p = 0.004) and PVAT (1.8-fold, p = 0.021) coordinated with increased LEP mRNA expression in EАT (1.7-fold, p = 0.001), LEPR in SAT (3-fold, p = 0.003), and PVAT (1.7-fold, p = 0.001), but not in EAT. AH was also found to correlate with increased IL6 expression in SAT (8-fold, p = 0.013) and EAT (10.4-fold, p = 0.001) (Figure 4).
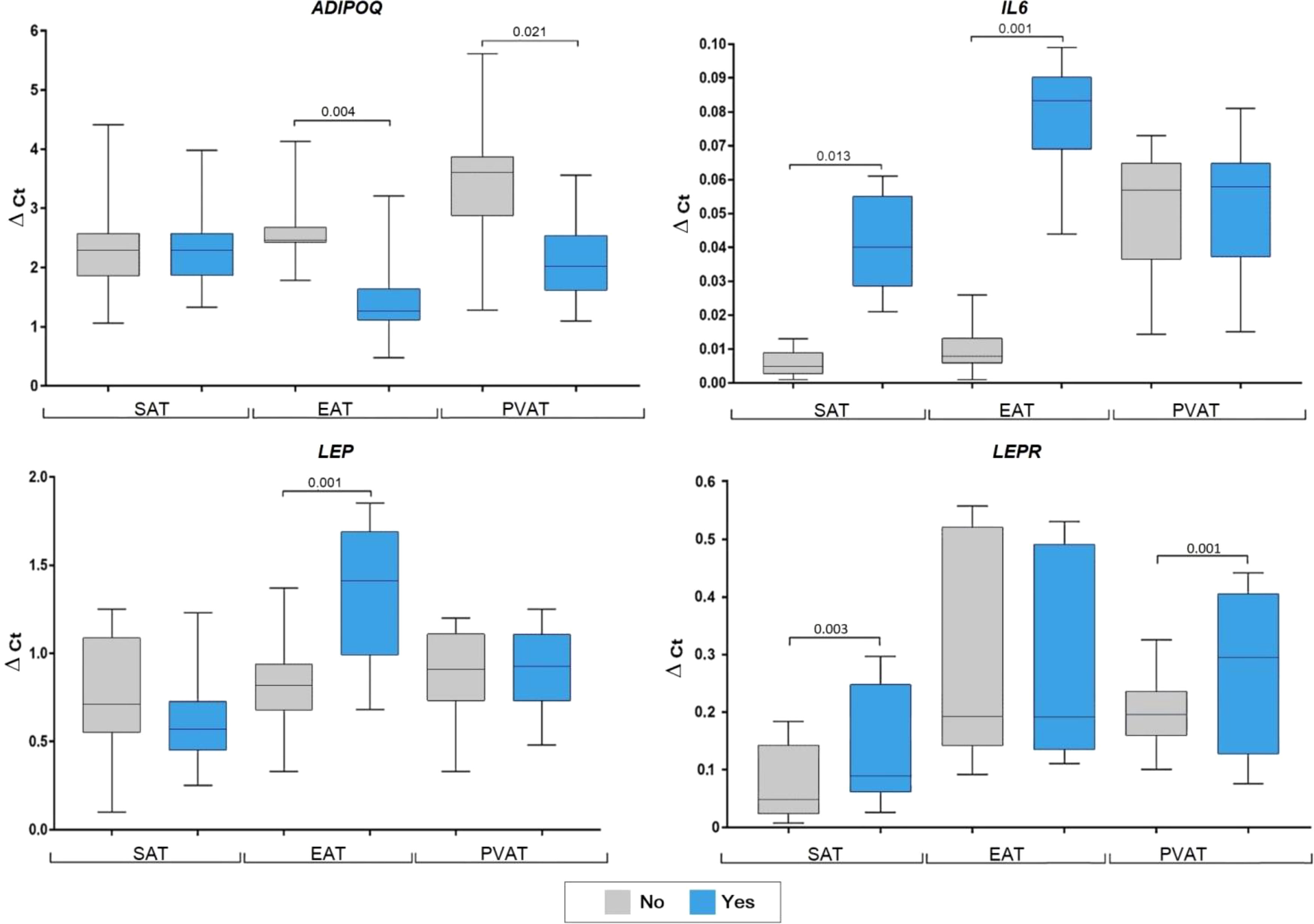
Figure 4 Adipocytokine gene expression in the adipocytes collected from adipose tissue based on arterial hypertension in coronary artery disease patients. Data are presented in Me (Q1; Q3). SAT, subcutaneous adipose tissue; EAT, epicardial adipose tissue; PVAT, perivascular adipose tissue; AH, arterial hypertension; p; level of statistical significance; sOB-R, soluble leptin receptor; IL6, in-terleukin 6.
AH duration also proved important for adipocytokine expression dynamics. Spe-cifically, in patients with AH for less than 10 years, LEPR was increased in SAT, whereas it was increased in the EAT of patients with AH for 11–19 years. Meanwhile, an AH du-ration of more than 20 years was associated with decreased ADIPOQ expression owing to LEP increases in all ATs, along with decreased IL6 in SAT, which was increased in PVAT (Figure 5).

Figure 5 Adipocytokine genes expression in the subcutaneous, epicardial, and perivascular adipocytes based on duration of arterial hypertension in patients with coronary artery disease. Data are presented in Me (Q1; Q3). SAT, subcutaneous adipose tissue; EAT, epicardial adipose tissue; PVAT, perivascular adipose tissue; p, level of sta-tistical significance; sOB-R, soluble leptin receptor; IL6, interleukin 6.
Smokers with CAD showed increased ADIPOQ expression in SAT (1.9-fold, p = 0.012), EAT (1.7-fold, p = 0.003), and PVAT (1.5-fold, p = 0.033), as well as increased LEP expression in SAT (1.6-fold, p = 0.024) and EAT (1.8-fold, p = 0.003). However, LEPR ex-pression was decreased (1.3- fold, p = 0.001) only in PVAT of smokers. No associations were found for smokers with CAD and IL6 expression (Figure 6).
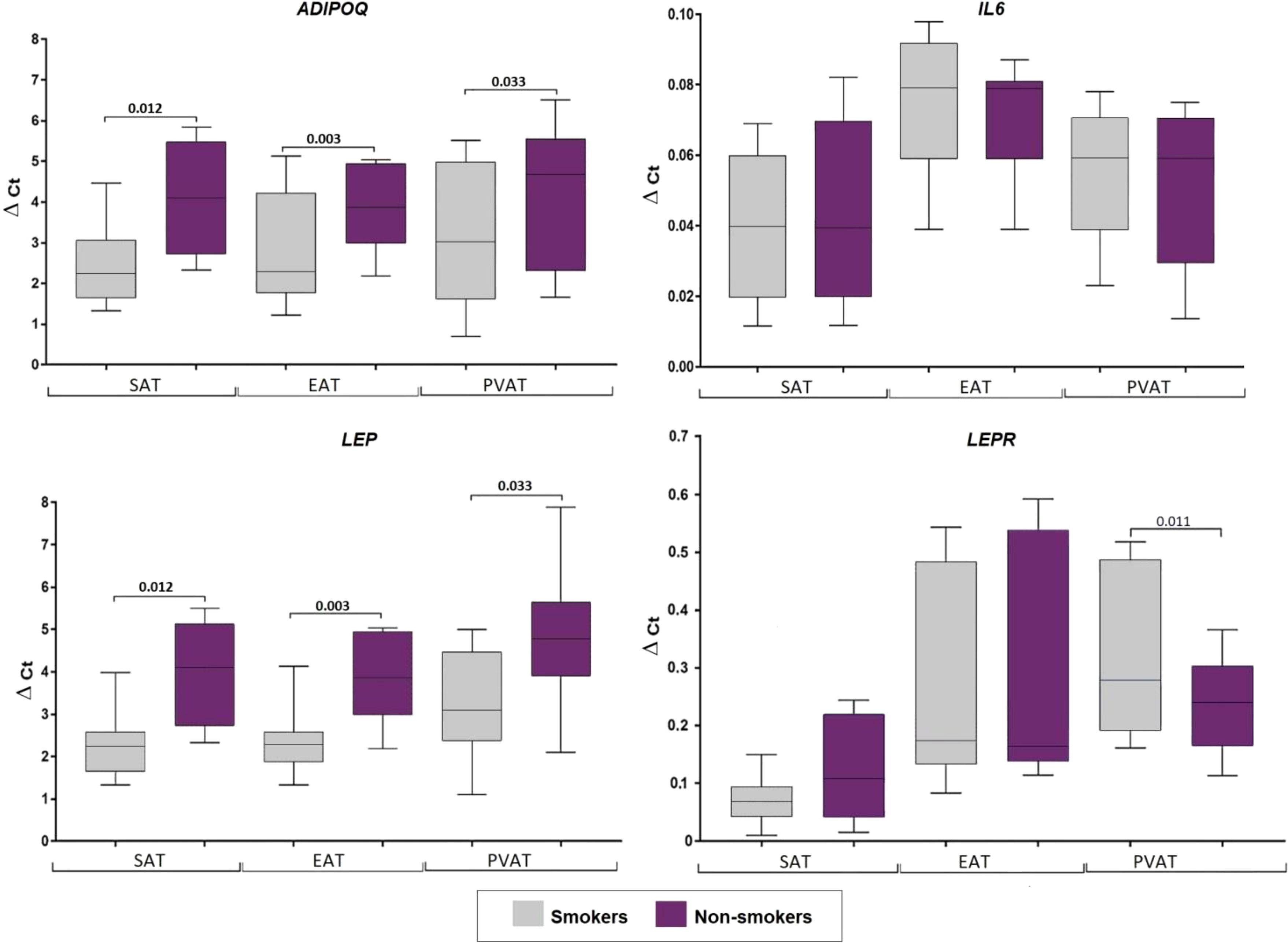
Figure 6 Adipocytokine gene expression in the culture medium of adipocytes collected from adipose tissue based on smoking status in coronary artery disease patients. Data are presented in Me (Q1; Q3). SAT, subcutaneous adi-pose tissue; EAT, epicardial adipose tissue; PVAT, perivascular adipose tissue; p, level of statistical significance; sOB-R, soluble leptin receptor; IL6, interleukin 6.
The results of this study revealed sexual dimorphism in the expression of adipo-cytokines in local fat depots, with primary EAT and PVAT localization. Specifically, men with CAD had lower ADIPOQ expression in EAT and PVAT, and lower LEP expression in PVAT compared to women. These results agreed with previously obtained data on lower LEP expression in the SAT of men relative to women (15). Our results also show a decrease in ADIPOQ and LEP expression in the EAT of male patients with CAD, while no difference was observed in the SAT (16). However, currently, a unanimous opinion has not been reached regarding the relationship between sex and the level of ADIPOQ in the PVAT. Thus, some researchers believe that sex affects both ADIPOQ expression and secretion, while others note differences only in the level of adiponectin secretion (17).
The revealed differences in adipokine expression may be due to the influence of sex hormones. For example, androgens, including testosterone, can cause dysfunction of AT through repression of ADIPOQ and LEP mRNA transcription via blocking of RNA polymerase and formation of a transcriptional complex (18). Moreover, Machinal-Quélin, F. at al. investigated the effects of in vitro 24-hour exposure to androgens and estrogens on LEP expression in the SAT of men and women. In men, only high concentration di-hydrotestosterone (100 nM) caused a decrease in the level of LEP expression, while in women 17-estradiol (10–100 nM) increased the expression of LEP. The authors suggested that the sexual dimorphism of LEP expression in humans is due to estrogen recep-tor-dependent stimulation of LEP expression in the AT by estrogens and estrogen pre-cursors in women (19). Further, a portion of testosterone becomes converted to estrogen through aromatization. McTernan P.G. at al. showed that the level of LEP expression in the adipocytes of women did not change in the presence of testosterone due to the low expression of aromatase in human adipocytes compared to pre-adipocytes (20).
When determining the level of IL6 expression, taking into account patient sex, men were found to have increased IL6 in SAT and decreased expression in PVAT, whereas no differences were observed in the EAT, compared to women. These results are consistent with our previous study, which demonstrated an increase of IL-6 expression in SAT during cardiac surgery (21).
It was hypothesized that the expression of adipocytokines changes with age, which is inextricably linked to CVD risk, an increase in the number and degree of coronary arteries, and the incidence of CAD. Our results demonstrate a clear association between age and adipocytokine mRNA levels. For instance, patients aged 50–59 were characterized by a low level of ADIPOQ in EAT, as well as high levels of LEP and IL-6 in EAT and PVAT. These expression patterns agree with the generally accepted opinion regarding increased pro-inflammatory activity in AT with age and, thus highlights the vulnerability of this patient population. The increase in IL-6 expression is likely caused by AT aging, which represents the main source of this cytokine. This was demonstrated in an in vitro study that treated visceral AT of C57BL/6 mice with lipopolysaccharides and found that IL-6 production was significantly higher in adipocyte cultures of mice aged 24 months com-pared to young mice (4 months). The authors also showed that IL-6 overproduction is regulated by the autocrine/paracrine action of IL-1β, which initiates inflammatory pro-cesses in old age (22).
Age-related AT dysfunction is believed to be associated with dyslipidemia, metabolic dysfunction, and mild chronic systemic inflammation, which affect the quality and duration of life (23). In the current study, the presence of dyslipidemia in CAD patients correlated with decreased ADIPOQ expression in EАT and PVАT. Similarly, EAT LEPR expression was lower in individuals with dyslipidemia, as was PVAT IL6 expression.
However, it was previously shown that ADIPOQ expression increases in the PVAT of men with CAD and a BMI above 30 kg/m2 compared to patients with a lower BMI (24), which, according to the authors, is reflective of the “obesity paradox.” Adiponectin affects the accumulation of LDL-C in the vascular wall, inhibiting its oxidation, as well as the transformation of macrophages into foam cells, and proliferation of smooth muscle cell neointima, while stimulating expression of the cholesterol ABCA1 ATP-binding transporter in the liver, thereby enhancing the biogenesis and reverse transport of HDL cholesterol exhibiting antiatherogenic properties (25). The observed decrease in ADIPOQ expression within patients with dyslipidemia, therefore, indicates negation of the above protective effects and contributes to the progression of atherosclerosis and vascular damage.
Furthermore, the decreased IL6 expression observed in the PVAT of CAD patients may result in increased accumulation of lipids in adipocytes, causing their hypertrophy. IL-6 is known to inhibit the expression of lipoprotein lipase (LPL), the most abundant of which is the cells of AT, heart and skeletal muscles. Normally, LPL is exported from ad-ipocytes to the endothelial lining of AT capillaries, where it cleaves the triglycerides of chylomicrons and VLDL, thereby regulating the concentration of triglycerides (26).
Analysis of adipocytokine expression based on the presence of AH demonstrated a decrease in ADIPOQ expression in EAT against the background of increased LEP and IL-6 expression. Moreover, the presence of hypertension for more than 20 years was found to be associated with a decrease in the level of ADIPOQ and increase in LEP within all AT types. Our results are consistent with those of a previous study that reported reduced expression of ADIPOQ and its receptors (AdipoR1 and AdipoR2) within the perivascular adipocytes of mice with angiotensin II-induced hypertension (27). Similarly, Teijeira-Fernandez et al. reported a decrease in ADIPOQ expression in the EAT of AH patients. The authors concluded that of ADIPOQ expression in EAT may be associated with AH status regardless of CAD or other concomitant diseases, which confirms the hypothesis regarding the effect of EAT on CVD (28).
In hypertension, the expression of LEP increases in EAT, which, given the possible proliferative effect of leptin and the effect on vascular permeability, may contribute to the progression of this disease. Research by Nepomuceno at al. demonstrated the presence of a direct correlation between LEP expression and blood pressure (29). The simultaneous decrease of ADIPOQ expression in the EAT and PVAT may also have an unfavorable affect as this adipokine attenuates vascular damage in hypertension.
When determining if smoking affects the expression of adipocytokines in CAD pa-tients, it was found that smoking is associated with an increase in ADIPOQ (in all types of AT), and LEP expression (in SAT and EAT), however, does not impact IL-6 expression. Similarly, a previous study sought to examine the effect of tobacco smoke in vitro and in vivo on the intracellular and extracellular distribution of adiponectin and its high mo-lecular weight form. Results showed that the total secretion of adiponectin was sup-pressed, while administration of tobacco smoke extract to mice reduced the adiponectin concentration in culture medium and the plasma of wild-type mice against the back-ground of its intracellular accumulation in cultured adipocytes. They further reported an enhancement of the adiponectin-retaining chaperone ERp44, localized in the endoplasmic reticulum, as well as suppression of the adiponectin secretion factor DsbA-L, following exposure to tobacco smoke. These results can help explain hypoadiponectinemia and the increased risk of developing T2DM in smokers due to its intracellular delay in the AT when exposed to tobacco smoke (30).
Moreover, the observed results regarding LEP expression agree with those of a pre-vious study that reported the effect of nicotine on the expression and secretion of leptin in vitro. They found that LEP expression did not differ significantly during the first 6 h of incubation with nicotine in cultured 3T3-L1 mouse adipocytes and AT explants from healthy women who underwent mammoplasty surgery. Meanwhile, LEP expression in 3T3-L1 mouse cells increased in the first hour and subsequently decreased by 45% after a 6-hour incubation with 0.5 μg/ml nicotine. However, low dose nicotine (0.05 μg/ml) did not affect LEP expression in 3T3-L1 cells. The observed change in LEP expression in cul-tured cells incubated with nicotine, and subsequent sharp decrease in plasma leptin concentration when smoking cigarettes suggested that the decrease in plasma leptin concentration in smokers is not associated with the direct effect of nicotine on LEP ex-pression and secretion, but rather with indirect exposure to catecholamines (31).
Certain limitations were noted in this study. First, it was a single-centered study and, second, the sample size was small.
Data cannot be provided upon request due to the fact that the Local Ethics Committee cannot approve the transfer of any data on the patients participating in scientific and clinical researches which was conducted on the basis of the NII KPSSZ, since this is contrary to ethical standards and Federal Law of the Russian Federation “On Personal Data” No. 152-FZ of July 27, 2006.
The study protocol was approved by the institutional Local Ethics Committee, and was performed in ac-cordance with the World Medical Association’s Declaration of Helsinki on Ethical Principles for Medical Research Involving Human Subjects, 2000 edition, and the “GCP Principles in the Russian Federation”, approved by the Russian Ministry of Health. The patients/participants provided their written informed consent to participate in this study.
Conceptualization, OG; data curation, OG, formal analysis YD, EB; investigation YD, EB, MS; methodology, OG, YD, administration, OB; resources, KK; supervision, OB; validation, OG; writing—original draft preparation, OG, YD, EB; writ-ing—review and editing, OG, YD. All authors contributed to the article and approved the submitted version.
This research is conducted within the grant of the Russian Science Foundation project No. 22-15-20007 “Ceramide profile of local heart fat depots: clinical and pathogenetic significance and therapeutic potential”.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Samorodskaya IV, Kondrikova NV, Kazachek YA, Barbarash OL, Boytsov SA. The nosological structure of mortality from circulatory system diseases in 2006 and 2013. Kompleksnye problemy serdečno-sosudistyh zabolevanij (2015) 4:67–72. doi: 10.17802/2306-1278-2015-4-67-72
2. Mazurek T, Zhang L, Zalewski A, Mannion JD, Diehl JT, Arafat H, et al. Human epicardial adipose tissue is a source of inflammatory mediators. Circulation (2003) 108:2460–6. doi: 10.1161/01.CIR.0000099542.57313.C5
3. Iacobellis G, Pistilli D, Gucciardo M, Leonetti F, Miraldi F, Brancaccio G, et al. Adiponectin expression in human epicardial adipose tissue in vivo is lower in patients with coronary artery disease. Cytokine (2005) 29:251–5. doi: 10.1016/j.cyto.2004.11.002
4. Picard FA, Gueret P, Laissy JP, Champagne S, Leclercq F, Carrie D, et al. Epicardial adipose tissue thickness correlates with the presence and severity of angiographic coronary artery disease in stable patients with chest pain. PloS One (2014) 9:e110005. doi: 10.1371/journal.pone.0110005
5. Nakamura K, Fuster JJ, Walsh K. Adipokines: A link between obesity and cardiovascular disease. J Cardiol (2014) 63:250–9. doi: 10.1016/j.jjcc.2013.11.006
6. Liberale L, Carbone F, Bertolotto M, Bonaventura A, Vecchie A, Mach F, et al. Serum adiponectin levels predict acute coronary syndrome (ACS) in patients with severe carotid stenosis. Vasc Pharmacol (2018) 102:37–43. doi: 10.1016/j.vph.2017.12.066
7. Sypniewska G. Pro-inflammatory and prothrombotic factors and metabolic syndrome. EJIFCC (2007) 18:39–46.
8. Gotsman I, Stabholz A, Planer D, Pugatsch T, Lapidus L, Novikov Y, et al. Serum cytokine tumor necrosis factor-alpha and interleukin- 6 associated with the severity of coronary artery disease: indicators of an active inflammatory burden? Isr. Med Assoc J (2008) 10:494–8.
9. Kershaw EE, Flier JS. Adipose tissue as an endocrine organ. J Clin Endocrinol Metab (2004) 89:2548–56. doi: 10.1210/jc.2004-0395
10. Sachdev M, Sun JL, Tsiatis AA, Nelson CL, Mark DB, Jollis JG, et al. The prognostic importance of comorbidity for mortality in patients with stable coronary artery disease. J Am Coll Cardiol (2004) 43:576–82. doi: 10.1016/j.jacc.2003.10.031
11. Gruzdeva OV, Dyleva YA, Antonova LV, Antonova LV, Matveeva VG, Uchasova EG, et al. Adipokine and cytokine profiles of epicardial and subcutaneous at in patients with coronary heart disease. Bull Exp Biol Med (2017) 163(5):608–11. doi: 10.1007/s10517-017-3860-5
12. Suga H, Matsumoto D, Inoue K, Shigeura T, Eto H, Aoi N, et al. Numerical measurement of viable and nonviable adipocytes and other cellular components in aspirated fat tissue. Plast Reconstr Surg (2008) 122.1:103–14. doi: 10.1097/PRS.0b013e31817742ed
13. Sinitsky MY, Matveeva VG, Asanov MA, Ponasenko AV. Modifications in routine protocol of RNA isolation can improve quality of RNA purified from adipocytes. Anal Biochem (2018) 543:128–31. doi: 10.1016/j.ab.2017.12.020
14. Pfaffl MW. A new mathematical model for relative quantification in real-time RT–PCR. Nucleic Acids Res (2001) 29:e45. doi: 10.1093/nar/29.9.e45
15. Pericas J, Oliver P, Guitard R, Pico C, Palou A. Sexual dimorphism in age-related changes in UCP2 and leptin gene expression in subcutaneous adipose tissue in humans. J Nutr Biochem (2001) 12:444–9. doi: 10.1016/s0955-2863(01)00158-9
16. Iglesias MJ, Eiras S, Pineiro R, Lopez-Otero D, Gallego R, Fernandez AL, et al. Gender differences in adiponectin and leptin expression in epicardial and subcutaneous adipose tissue. findings in patients undergoing cardiac surgery. Rev Esp. Cardiol (2006) 59:1252–60.
17. Sade LE, Eroglu S, Bozbas H, Ozbicer S, Hayran M, Haberal A, et al. Relation between epicardial fat thickness and coronary flow reserve in women with chest pain and angiographically normal coronary arteries. Atherosclerosis (2009) 204:580–5. doi: 10.1016/j.atherosclerosis.2008.09.038
18. Wang Y, Zhu W, Zhang H. Effects of rosiglitazone on adiponectin expression in 3T3-L1 adipocytes at high levels of both testosterone and insulin In vitro culture. J Reprod Contraception (2014) 25(4):199–206. doi: 10.7669/j.issn.1001-7844.2014.04.0199
19. Machinal-Quelin F, Dieudonne MN, Pecquery R, Leneveu MC, Giudicelli Y. Direct in vitro effects of androgens and estrogens on ob gene expression and leptin secretion in human adipose tissue. Endocrine (2002) 18:179–84. doi: 10.1385/ENDO:18:2:179
20. McTernan PG, Anderson LA, Anwar AJ, Eggo MC, Crocker J, Barnett AH, et al. Glucocorticoid regulation of p450 aromatase activity in human adipose tissue: gender and site differences. J Clin Endocrinol Metab (2002) 87:1327–36. doi: 10.1210/jcem.87.3.8288
21. Kremen J, Dolinkova M, Krajickova J, Blaha J, Anderlova K, Lacinova Z, et al. Increased subcutaneous and epicardial adipose tissue production of proinflammatory cytokines in cardiac surgery patients: possible role in postoperative insulin resistance. J Clin Endocrinol Metab (2006) 91:4620–7. doi: 10.1210/jc.2006-1044
22. Starr ME, Saito M, Evers BM, Saito H. Age-associated increase in cytokine production during systemic inflammation-ii: The role of il-1β in age-dependent il-6 upregulation in adipose tissue. J Gerontol. A Biol Sci Med Sci (2015) 70:1508–15. doi: 10.1093/gerona/glu197
23. Mohler MJ, Fain MJ, Wertheimer AM, Najafi B, Nikolich-Zugich J. The frailty syndrome: clinical measurements and basic underpinnings in humans and animals. Exp Gerontol (2014) 54:6–13. doi: 10.1016/j.exger.2014.01.024
24. Cybularz M, Langbein H, Zatschler B, Brunssen C, Deussen A, Matschke K, et al. Endothelial function and gene expression in perivascular adipose tissue from internal mammary arteries of obese patients with coronary artery disease. Atheroscl Suppl (2017) 30:149–58. doi: 10.1016/j.atherosclerosissup.2017.05.042
25. Wang Y, Wang X, Guo Y, Bian Y, Bai R, Liang B, et al. Effect of adiponectin on macrophage reverse cholesterol transport in adiponectin-/- mice and its mechanism. Exp Ther Med (2017) 13:2757–62. doi: 10.3892/etm.2017.4321
26. Kern PA, Ranganathan S, Li C, Wood L, Ranganathan G. Adipose tissue tumor necrosis factor and interleukin-6 expression in human obesity and insulin resistance. Am J Physiol (2001) 280(5):745–51. doi: 10.1152/ajpendo.2001.280.5.E745
27. Guo R, Han M, Song J, Liu J, Sun Y. Adiponectin and its receptors are involved in hypertensive vascular injury. Mol Med Rep (2018) 17:209–15. doi: 10.3892/mmr.2017.7878
28. Teijeira-Fernandez E, Eiras S, Grigorian-Shamagian L, Fernandez A, Adrio B, Gonzalez-Juanatey JR. Epicardial adipose tissue expression of adiponectin is lower in patients with hypertension. J Hum Hypertens (2008) 22:856–63. doi: 10.1038/jhh.2008.75
29. Nepomuceno R, Vallerini BF, da Silva RL, Corbi SCT, de S Bastos A, Dos Santos RA, et al. Systemic expression of genes related to inflammation and lipid metabolism in patients with dyslipidemia, type 2 diabetes mellitus and chronic periodontitis. Diabetes Metab Syndr (2019) 13:2715–22. doi: 10.1016/j.dsx.2019.07.003
30. Li M, Li C, Liu Y, Chen Y, Wu X, Yu D, et al. Decreased secretion of adiponectin through its intracellular accumulation in adipose tissue during tobacco smoke exposure. Nutr Metab (Lond) (2015) 12:15. doi: 10.1186/s12986-015-0011-8
Keywords: adiponectin, leptin, leptin receptor, IL-6, adipose tissue, risk factors cardiovascular diseases
Citation: Gruzdeva OV, Dyleva YA, Belik EV, Sinitsky MY, Kozyrin KA and Barbarash OL (2022) Characteristics of adipocytokine expression by local fat depots of the heart: Relationship with the main risk factors for cardio-vascular diseases. Front. Endocrinol. 13:991902. doi: 10.3389/fendo.2022.991902
Received: 12 July 2022; Accepted: 18 August 2022;
Published: 08 September 2022.
Edited by:
Cheng-Chao Ruan, Fudan University, ChinaReviewed by:
Maoqing Ye, Fudan University, ChinaCopyright © 2022 Gruzdeva, Dyleva, Belik, Sinitsky, Kozyrin and Barbarash. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yulia A. Dyleva, ZHlsZXZhODdAeWFuZGV4LnJ1
†These authors contributed equally to this work and share senior authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.