- 1Department of Radiation Oncology, Chengdu Women’s and Children’s Central Hospital, School of Medicine, University of Electronic Science and Technology of China, Chengdu, China
- 2Department of Radiology, Chengdu Women’s and Children’s Central Hospital, School of Medicine, University of Electronic Science and Technology of China, Chengdu, China
- 3Department of Medical Oncology, Sichuan Cancer Hospital and Institute, Chengdu, China
- 4Department of Gynecology, Chengdu Women’s and Children’s Central Hospital, School of Medicine, University of Electronic Science and Technology of China, Chengdu, China
Background: This study aimed to develop a nomogram to predict the survival for stage IIIC endometrial cancer (EC) patients with adjuvant radiotherapy (ART) alone and personalize recommendations for the following adjuvant chemotherapy (ACT).
Methods: In total, 746 stage IIIC EC patients with ART alone were selected from the Surveillance, Epidemiology, and End Results (SEER) registry. Cox regression analysis was performed to identify independent risk factors. A nomogram was developed accordingly, and the area under the receiver operating characteristic curve (AUC) and C-index were implemented to assess the predictive power. The patients were divided into different risk strata based on the total points derived from the nomogram, and survival probability was compared between each risk stratus and another SEER-based cohort of stage IIIC EC patients receiving ART+ACT (cohort ART+ACT).
Results: Five independent predictors were included in the model, which had favorable discriminative power both in the training (C-index: 0.732; 95% CI: 0.704–0.760) and validation cohorts (C-index: 0.731; 95% CI: 0.709–0.753). The patients were divided into three risk strata (low risk <135, 135 ≤ middle risk ≤205, and high risk >205), where low-risk patients had survival advantages over patients from cohort ART+ACT (HR: 0.45, 95% CI: 0.33–0.61, P < 0.001). However, the middle- and high-risk patients were inferior to patients from cohort ART+ACT in survival (P < 0.001).
Conclusion: A nomogram was developed to exclusively predict the survival for stage IIIC EC patients with ART alone, based on which the low-risk patients might be perfect candidates to omit the following ACT. However, the middle- and high-risk patients would benefit from the following ACT.
Introduction
Endometrial cancer (EC) is the most prevalent gynecological malignancy in developed countries, with 65,950 new cases expected and 12,550 deaths in the United States alone in 2022 (1). EC patients with lymph node (LN) metastases, which have been relocated as stage IIIC in the revised 09 International Federation of Gynecology and Obstetrics (FIGO) staging system, still accounted for approximately 8–10% of all EC cases (2, 3), though most of the EC patients were diagnosed at a relatively early stage. The recommended primary treatment for stage IIIC patients was surgery, consisting of total hysterectomy, bilateral salpingo-oophorectomy, and systematic lymphadenectomy, and adjuvant treatment would be delivered depending on the pathological assessment. However, the optimal adjuvant treatment for these patients is still open to debate given that stage IIIC EC is a significantly heterogenous disease with a 5-year overall survival (OS) that ranges from 40 to 80% (4–6).
As one of the most prominent randomized clinical trials comparing different adjuvant treatment modalities for stage III EC patients, the PORTEC-3 trial failed to specifically address whether combined adjuvant chemotherapy with radiotherapy (ACT+ART) was a better treatment choice for stage IIIC patients over adjuvant radiotherapy (ART) alone (7). Several retrospective studies have established the advantages of ACT+ART over ART alone for post-operative stage IIIC EC patients (8–10). However, the subgroup analysis in Chapman’s research demonstrated the absence of a significant difference between ART+ACT and ART alone in stage IIIC EC patients with a grade 1/2 endometrioid disease (8), which was in tune with the study from Binder et al. showing that low-grade patients would benefit similarly from adjuvant ART+ACT or ART alone (9). Moreover, we have noticed that a study from Taiwan (China) indicated that there was no survival difference between ART+ACT and ACT alone in stage IIIC EC patients when the total positive lymph node number did not exceed 5 (11), which further highlighted the value of the lymph node status in guiding the adjuvant treatment.
The lack of a reliable model incorporating all of these pathological indices to predict which individuals would benefit from ART alone while avoiding overtreatment impelled us to construct this model to better guide the adjuvant treatment for stage IIIC EC patients.
Methods
Selection of patients
We retrieved the data of EC patients diagnosed between 2010 and 2017 from the Surveillance, Epidemiology, and End Results (SEER) database which captured approximately 34.6% of the cancer statistics in the United States (12), and a case listing was formed through the SEER*Stat software (version 8.4.0; http://seer.cancer.gov/seerstat/). Exemption for ethic review was obtained due to the de-identified feature of the information collected from this public database.
All pathologically stage IIIC EC patients treated with primary surgery—including total hysterectomy, bilateral salpingo-oophorectomy, systematic lymphadenectomy, and ART alone—were included, and the exclusion criteria were as follows: (I) T4 or M1 stage, (II) patients with neoadjuvant radiotherapy, (III) patients who received ACT alone or ACT+ART, (IV) patients who succumbed to surgery complications (survival time of less than 1 month), and (V) patients with insufficient information. It is worth noting that the detailed selection process is listed in Supplementary Table S1.
Cohort definition and study covariate
Patients’ data regarding the year of diagnosis, age, marital status, race, grade (differentiation), histology type, T stage, FIGO stage, tumor size, number of retrieved LN, number of positive LN, survival time, and survival status were collected. It is noteworthy that rare pathological histology type was not included in this study, and we presented the histology type as type I (endometrioid cancer and adenocarcinoma) and type II consisting of serous cancer, carcinosarcoma, and clear cell cancer. The log odds of positive lymph nodes (LODDS), defined as loge[(number of positive LN + 0.5)/(number of negative LN + 0.5)], was calculated to represent the LN status given that our previous studies have demonstrated that LODDS was the optimal LN index to predict the survival probability for EC patients with LN metastases (13, 14). X-tile software (version 3.6.1; Yale University, New Haven, CT, USA) was used to identify the best cutoff values for continuous variables including age, tumor size, and LODDS given the inconsistency of these cutoff values from previous research (8, 15–18). As a result, age was categorized as ≤55, 56–75, and ≥76, and tumor size was labeled as ≤3.5, 3.6–5.0, and ≥5.1 cm. Similarly, the LODDS was divided into three subgroups (LODDS1: ≤–0.96, LODDS2: -0.96 to 0.55, and LODDS3: ≥0.56). The remaining variables were also displayed as the year of diagnosis (2010–2013 and 2014–2017), race (White, Black, and Asian/Alaska Indian), marital status (married, separated, and never married or unmarried), grade/differentiation (I/well, II/moderate, and III+IV/poor+un), T stage (T1a, T1b, T2, T3a, and T3b), and FIGO stage (IIIC1 and IIIC2). Lastly, we randomly split these patients into the training and validation cohorts with a ratio of 7:3 as indicated by the previous studies (19, 20).
Statistical considerations
The categorical variables in this study were estimated as percentages or frequencies and compared using Pearson χ2 test or Fisher’s exact test. Univariate and multivariate Cox regression analyses were applied to identify the independent risk factors for OS in the training cohort, and a predicting nomogram incorporating these risk factors was constructed accordingly. The internal validation of this model consisted of two steps. Firstly, C-index, calculated by bootstrapping, was used to evaluate the discriminative ability of this model, which varied from 0.5 to 1.0, with 0.5 indicating random chance and 1.0 representing perfect fit. Secondly, calibration curves were plotted to visualize the relationship between the predictive and observed outcomes, where the closer the predictive curve to the observed curve, the better predictive accuracy the model had (21). Moreover, the validation cohort was used to externally validate these results. The receiver operating characteristic curve was plotted to further visualize the ability of the model in predicting the 1-, 3-, and 5-year OS in both of the training and validation cohorts.
To further explore the clinical value of this model, we stratified the patients from the training cohort into three risk groups (low risk, middle risk, and high risk) based on the total points derived from the nomogram, and the OS curves in different risk strata were plotted. Subsequently, the survival probability between each risk strata and another SEER-based cohort of stage IIIC EC patients receiving both ART+ACT (cohort ART+ACT) was compared using the Kaplan–Meier method. As a result, the candidates for ART alone would be identified. Given that our prediction model was developed merely based on the patients with ART alone and that the applicability of this model for patients with ART+ACT was uncertain, the patients from cohort ART+ACT were not further stratified.
All analyses were operated via R software (version.3.6.1; http://www.r-project.org). A two-tailed P-value <0.05 was recognized as statistically significant.
Results
Characteristics of patients
After selection, we identified 746 stage IIIC EC patients with ART alone from the database, and the median follow-up time for these patients was 61 months (interquartile range: 29–93 months). The sample size was considerably enough to develop a prediction model considering that at least 10 events were needed for each variable (22). Patients aged between 56 and 75 (69.7%) and with white ethnicity (72.8%), grade III/IV (68.4%), and histological type I (64.6) accounted for a big proportion of the training cohort (N = 522), where patients characterized with tumor size ranging from 3.6 to 5.0 cm (52.7%) and patients with LODDS1 (LODDS ≤-0.96) (61.9%) were more than half of this cohort. Patients with stage IIIC1 disease also accounted for a bigger proportion than those with stage IIIC2 disease in the training cohort (57.7 vs. 42.3%) (Table 1). No significant difference was observed in terms of the baseline characteristics between the training and validation cohorts (all p >0.05) (Table 1).
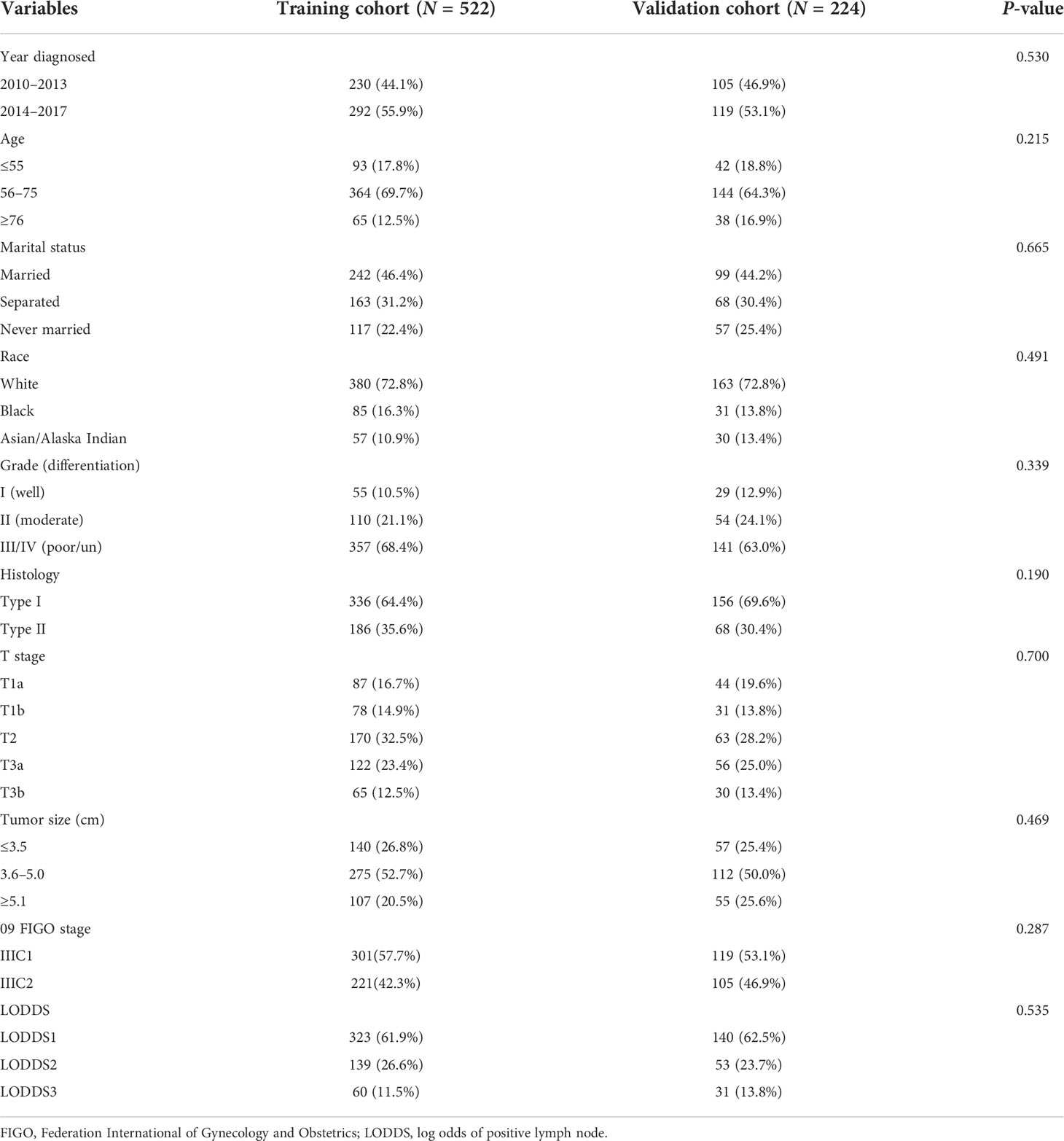
Table 1 Demographic and clinico-pathological characteristics in the training and the validation cohorts.
Identification of independent risk factors
We chose age, race, grade, histology, T stage, tumor size, and LODDS from the univariate Cox analysis (all p <0.05). Surprisingly or not, 09 FIGO stage was not able to discern the patients with optimal overall survival from those who had not (p = 0.062), which was in accordance with the previous studies (9, 13, 14, 16, 18). Multivariate analysis was sequentially performed to identify the age, grade, T stage, tumor size, and LODDS as the independent risk factors for the survival outcomes in stage IIIC EC patients with ART alone (Table 2).
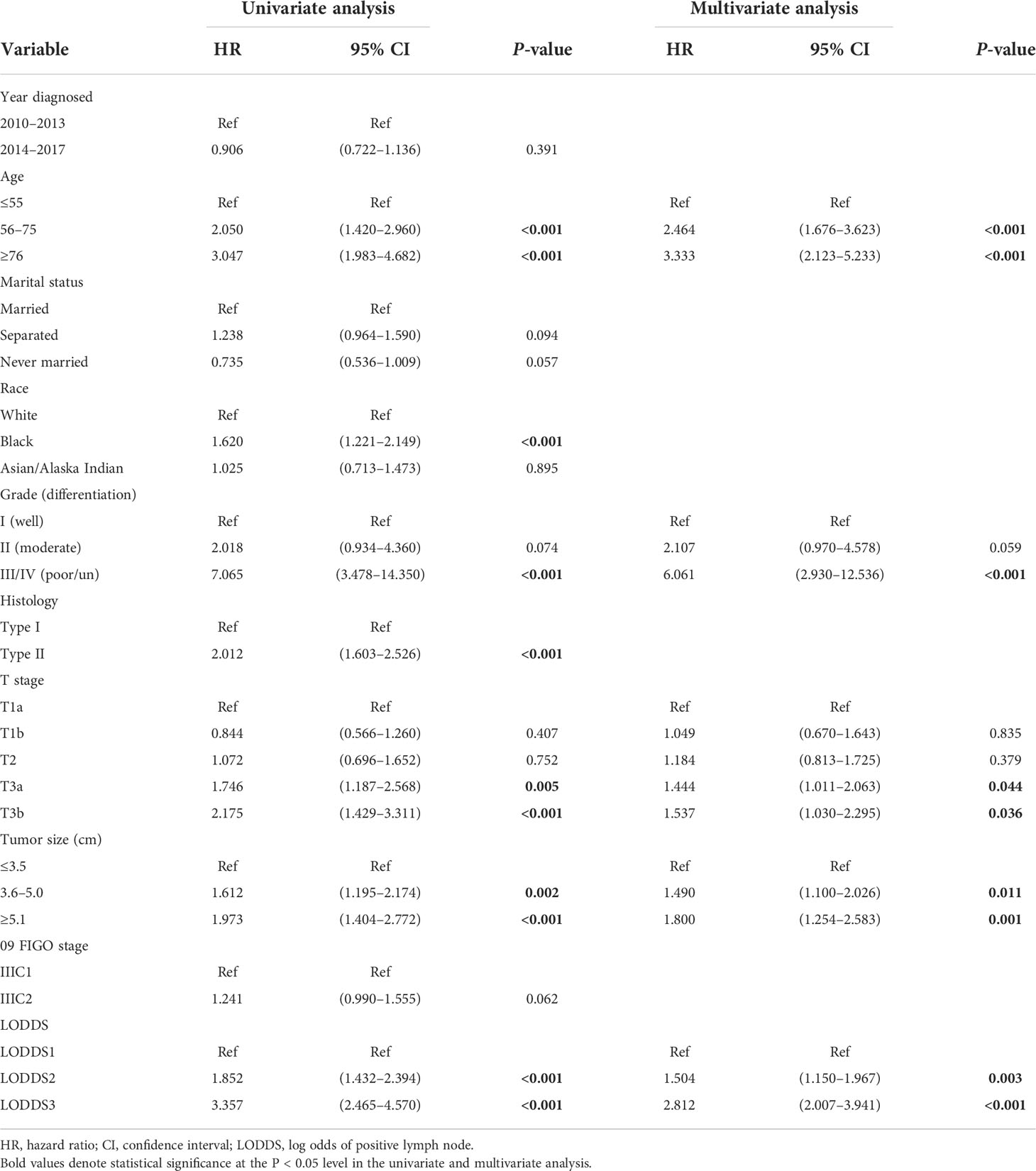
Table 2 Univariate and multivariate Cox regression analysis on variables for the prediction of overall survival in the training cohort (N = 522).
Development and validation of the nomogram
We included all of the significant predictors identified in the multivariate Cox regression analysis to build this original model (Figure 1), which yielded a Harrell’s C-index of 0.732 (95% CI: 0.704–0.760) and was able to effectively predict the 1-, 3-, and 5-year OS for stage IIIC EC patients with ART alone (all AUC values >0.700) (Figures 2A, B). The calibration curves of the model were then plotted to visualize the optimal consistency between the model-predicted and observed survival probability in the training cohort (Figures 2C–E). To validate the predictive power of this model externally, we applied the validation cohort, which turned out to possess a resembling C-index of 0.731 (95% CI: 0.709–0.753). Besides this, decent consistency was observed in the calibration curves of the validation cohort, further underpinning the predictive accuracy of this model (Figures 2F–H).
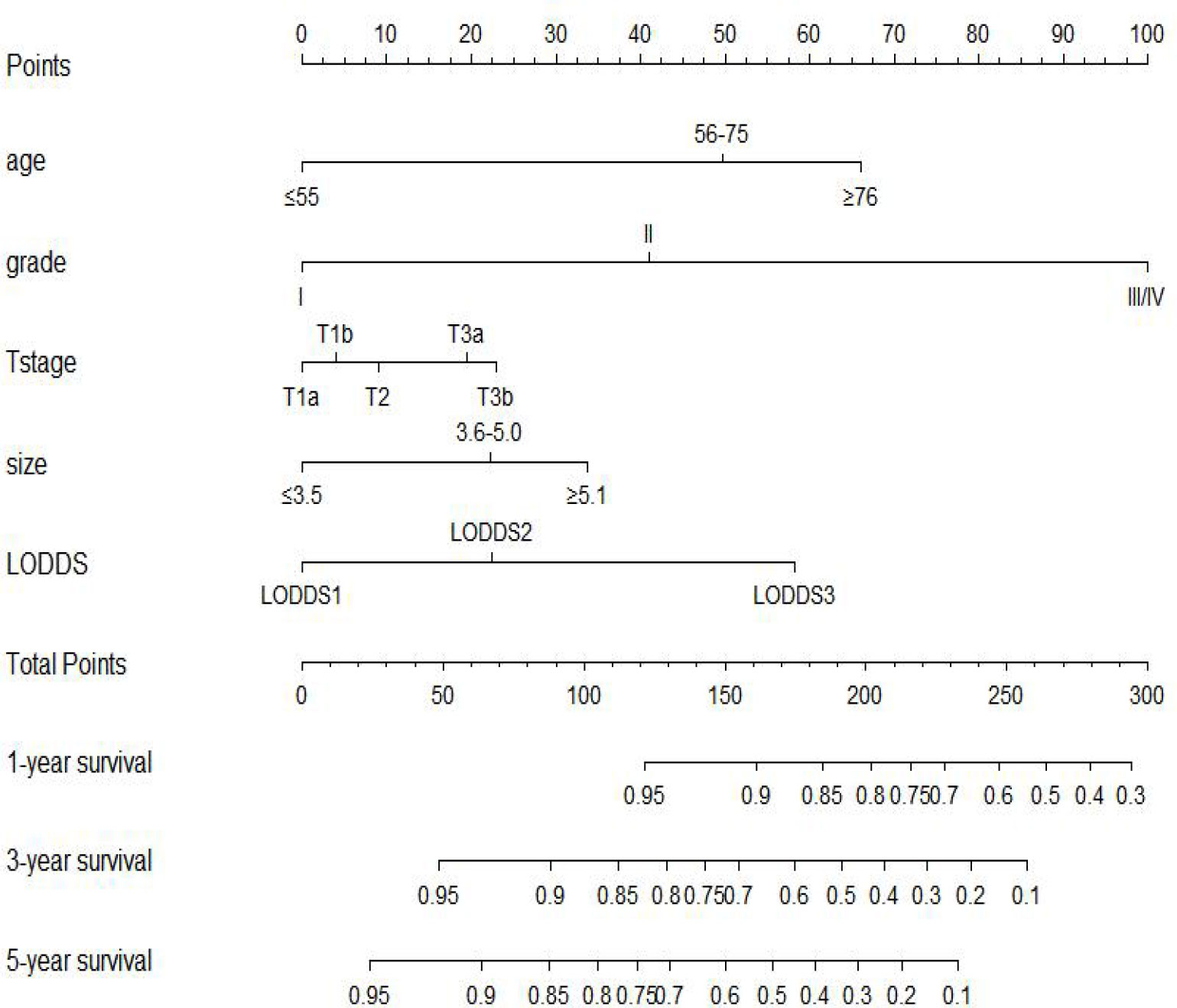
Figure 1 Prognostic nomogram to predict 1-, 3-, and 5-year overall survival in stage IIIC endometrial cancer patients with adjuvant radiotherapy alone.
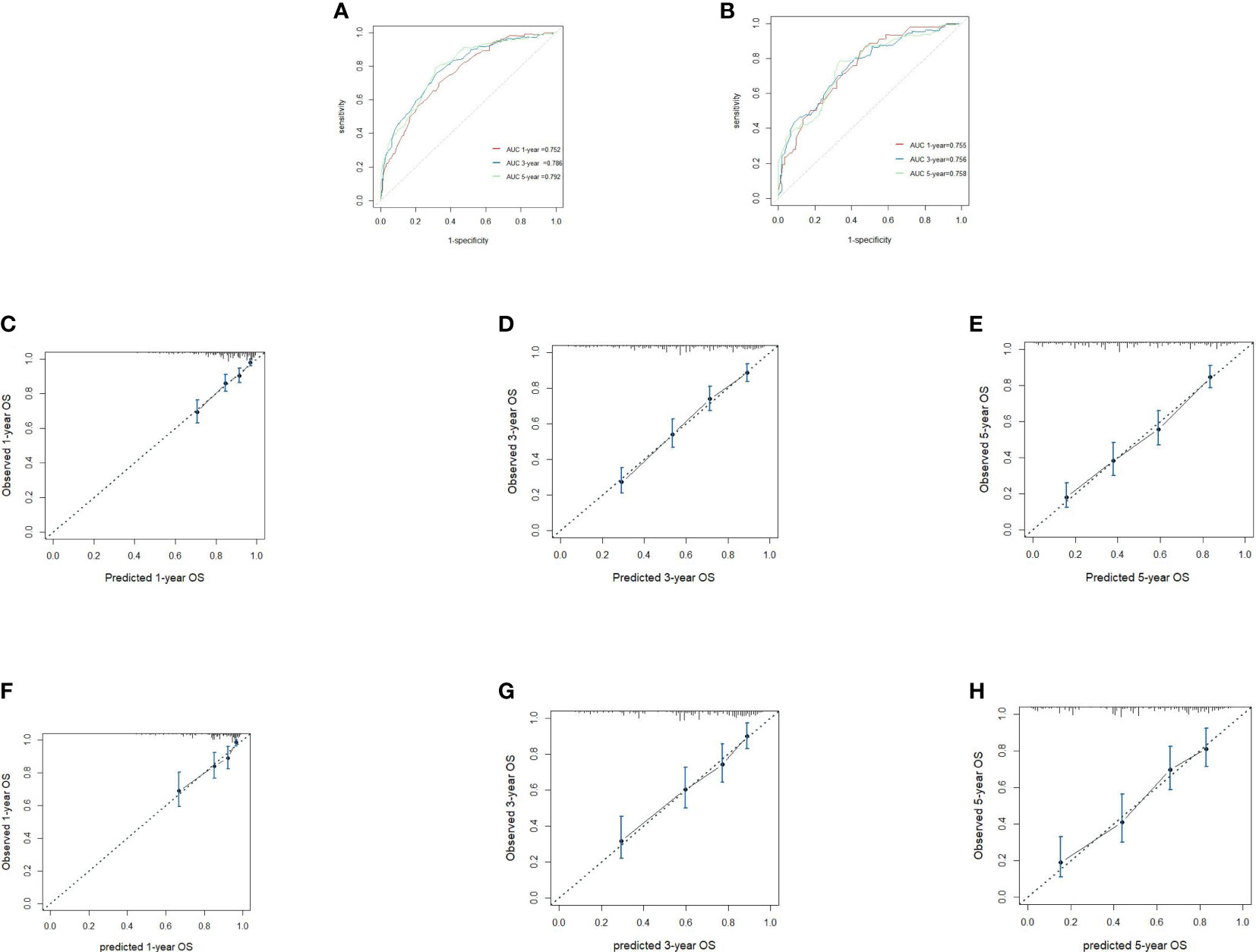
Figure 2 Receiver operating characteristics to describe the predictive power of the model in the training cohort (A) and the validation cohort (B). Calibration curves of 1-year (C), 3-year (D) and 5-year overall survival (OS) (E) for stage IIIC endometrial cancer patients with adjuvant radiotherapy alone in the training cohort. Calibration curves of 1-year (F), 3-year (G), and 5-year OS (H) in the validation cohort.
Clinical utility of the model
Apart from the ability to project the survival outcomes for stage IIIC EC patients with ART alone, we stratified the patients into three distinct risk strata according to the total points derived from the nomogram to further explore the clinical utility of this model. Overt separation among the OS curves belonging to each risk group (low risk <135, 135 ≤ middle risk ≤205, and high risk >205) was presented in both the training and validation cohorts (p < 0.0001) (Figures 3A, B). Furthermore, we compared the survival probability between the patients from each risk group and the patients from cohort ART+ACT (the baseline characteristics of these patients are documented in Supplementary Table S2). Compared with the patients from cohort ART+ACT, patients in the low-risk group were associated with a decreased risk (HR = 0.45, 95% CI: 0.33–0.61, P < 0.001) (Figure 3C). However, the patients from cohort ART+ACT possessed survival advantages over the patients in the middle-risk group (HR = 1.87, 95% CI: 1.58–2.22, P < 0.001) and the high-risk group (HR = 5.40, 95% CI: 4.47–6.53, P < 0.001) (Figures 3D, E). In summary, the patients benefitting most from ART alone were the nomogram-generated low-risk patients, who might be exempted from ART+ACT without compromising the survival probability. On the contrary, more intensive adjuvant treatment was required for the middle- and high-risk patients.
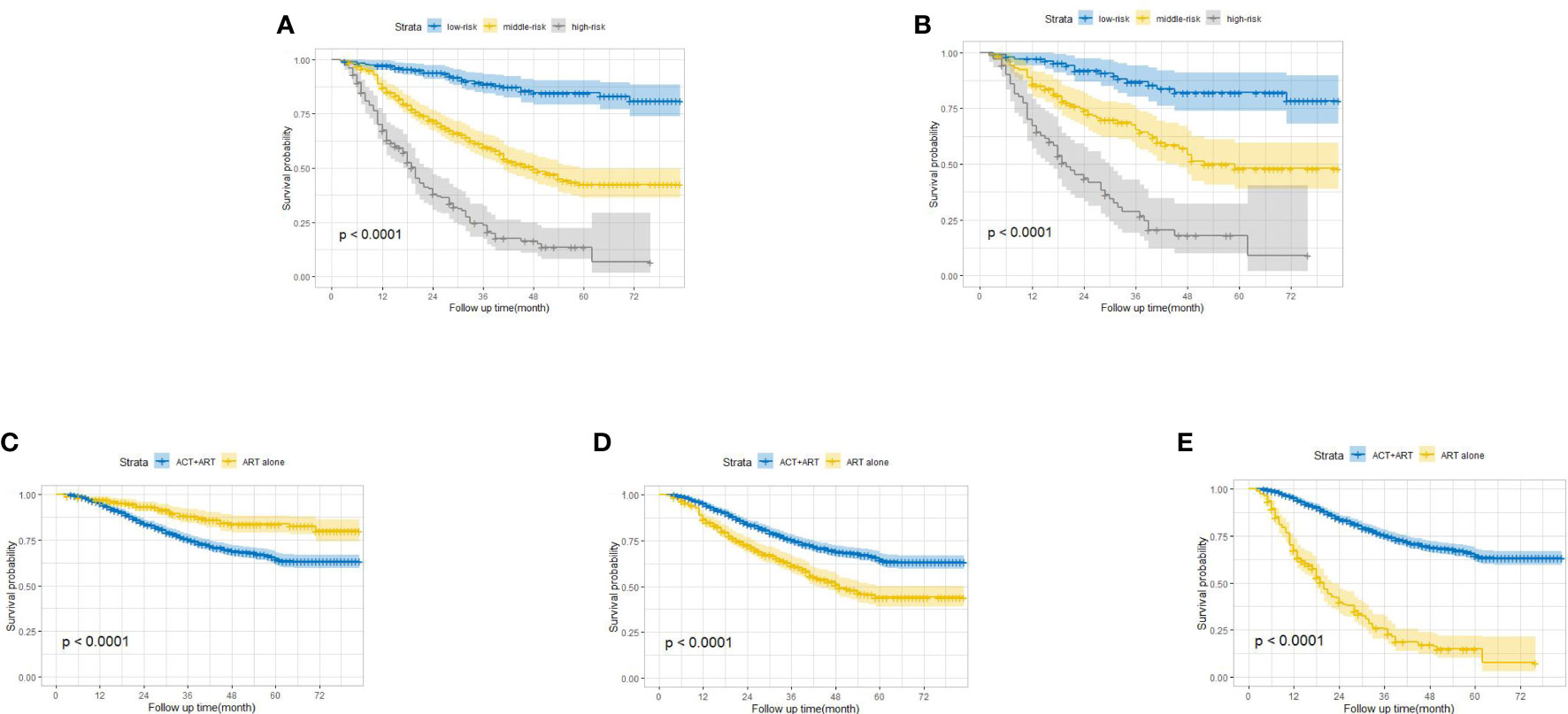
Figure 3 Kaplan–Meier overall survival curves for stage IIIC endometrial cancer patients with adjuvant radiotherapy alone stratified by different risks in the training cohort (A) and the validation cohort (B). Comparison of survival probability between low-risk patients and patients from cohort ART+ACT (C), middle-risk patients and patients from cohort ART+ACT (D), and high-risk patients and patients from cohort ART+ACT (E).
Discussion
It has been widely acknowledged that the primary treatment for the EC patients with LN metastases was radical surgery, while the optimal adjuvant treatment modality after operation for these patients remained controversial, which led to the ambiguous recommendation for post-operative stage IIIC EC patients as external beam radiotherapy ± vaginal brachytherapy ± systemic therapy in the National Comprehensive Cancer Network guidelines (23). Two well-known prospective randomized trials have tried to compare the adjuvant treatment efficacy between single modality and combined modality in stage III EC patients (7, 24). However, the PORTEC-3 trial failed to draw an exclusive conclusion on whether combined modality was better than single modality in stage IIIC EC patients, and the GOG-258 trial appeared to conflict with some real-world reports where the single modality seemed to be inferior to the combined modality (8–10). Therefore, a tailored adjuvant treatment on the basis of the characteristics of each individual was required.
In the process of selecting the predictors, the role of histology type drew much of our attention, which showed a significant impact on overall survival in the univariate analysis (P < 0.001) but did not hold its position in the multivariate analysis (P = 0.287). Despite the consensus that type II EC was a more aggressive disease than type I EC (25), this index indeed loses the ability to differentiate the survival outcomes in stage IIIC EC patients with ART alone. The same phenomenon was also observed by previous investigators who found that stage IIIC EC patients with the same grade would have an equivalent survival probability from different treatment modalities despite the histology type (8, 9), which implied that grade clearly had a much bigger role to play compared with histology type in differentiating the survival outcomes for stage IIIC EC patients. Besides this, grade had the greatest length in our nomogram (Figure 1), indicating the strongest prediction ability, which further proved that the predictive power of histology type would be diminished when it was adjusted together with the grade. Other emerging predictive biomarkers including molecular subtypes were not under evaluation in our study given the lack of large-scale randomized clinical trials to establish the role of these subtypes in treatment decision-making (8, 26, 27). The five predictors incorporated in our model did not exceed our initial anticipation, which were consistent with some previous studies (11, 17, 28, 29). However, none of the previous studies had evaluated the predictive power of the combination of these indices in stage IIIC EC patients with ART alone, let alone guide the clinical decision-making using this combination.
As one nascent calculation method integrating pivotal clinical–pathological risk factors to predict oncological outcomes, nomogram has been widely used in EC patients (14, 30–32). however, few of these had focused exclusively on stage IIIC EC patients (14). Actually, we have developed a pretty solid prediction model for post-operative stage IIIC EC patients receiving different kinds of adjuvant treatment in previous research (14), which was able to individually predict the oncological outcomes for stage IIIC EC regardless of the adjuvant treatment modality, but it was not suitable for adjuvant treatment decision-making. Therefore, we further constructed this prediction model where only stage IIIC EC patients with ART alone were included. Beyond prediction for individuals’ outcome, the most novel feature of this model was that it possessed the potential to guide the adjuvant treatment for post-operative stage IIIC EC patients, which further expanded the scope of its clinical utility. Several previous studies have explored the optimal sequence of the ART and ACT. However, a consensus has not been reached (33–35), where concurrent chemoradiation, ART sequenced with ACT, or upfront ACT did not seem to impact the survival outcomes for stage IIIC EC patients. Despite this, ART delivered within 8 weeks after surgery has been strongly recommended (36, 37). Therefore, few of the cancer institutions would prefer upfront ACT before ART, which further reinforced the clinical applicability of our model—that is, all of the stage IIIC EC patients after ART could be assessed by the model to determine whether sequential CT should be delivered or not. There was a beneficial trend for nomogram-generated low-risk patients with ART alone rather than ART+ACT, although we just simply compared the survival difference between the low-risk group and patients receiving ART+ACT.
Some limitations in this study had to be noted. Selection bias in terms of diagnostic methods, censorship, or follow-up arrangement was hard to avoid given the retrospective nature of this study. With the well-known limitation of the SEER database, we were unable to retrieve some important information such as the lympho-vascular space invasion status and the sequence of ACT and ART, which might impair the discriminative ability of our model in some ways. Our prediction model would surely be tested in different datasets across all sorts of institutions in the future, with which some revisions might be imparted to make this model become more accurate. However, our design philosophy for this model might be extrapolated to some other malignancies with uncertain recommendations for the treatment choices.
Conclusion
A nomogram was constructed and validated to individually predict the survival probability for stage IIIC EC patients with ART alone and potentially tailor the adjuvant treatment for these patients. In this study, nomogram-generated low-risk patients were candidates for omitting of the sequential ACT; however, middle- and high-risk patients might benefit from ACT following the ART.
Data availability statement
Publicly available datasets were analyzed in this study. This data can be found here: https://seer.cancer.gov/data/.
Author contributions
Conception/design: X-LY, CX, and D-JW. Provision of study materials or patients: X-LY. Collection and/or assembly of data: X-LY, L-NK, and F-LY. Data analysis and interpretation: X-LY, L-NK, and F-LY. Manuscript writing: X-LY. Manuscript revision: F-LY, CX, and D-JW. Final approval of the manuscript: all authors. All authors contributed to the article and approved the submitted version.
Acknowledgments
We would like to thank those who have been involved in the establishment of the SEER database and making this database available online.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2022.989063/full#supplementary-material
References
1. Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA Cancer J Clin (2022) 72:7–33. doi: 10.3322/caac.21708
2. Lewin SN, Herzog TJ, Barrena MN, Deutsch I, Burke WM, Sun X, et al. Comparative performance of the 2009 international federation of gynecology and obstetrics' staging system for uterine corpus cancer. Obstet Gynecol (2010) 116:1141–9. doi: 10.1097/AOG.0b013e3181f39849
3. McMeekin DS, Lashbrook D, Gold M, Johnson G, Walker JL, Mannel R. Analysis of FIGO stage IIIc endometrial cancer patients. Gynecol Oncol (2001) 81:273–8. doi: 10.1006/gyno.2001.6157
4. Mariani A, Webb MJ, Keeney GL, Haddock MG, Aletti G, Podratz KC. Stage IIIC endometrioid corpus cancer includes distinct subgroups. Gynecol Oncol (2002) 87:112–7. doi: 10.1006/gyno.2002.6789
5. Onda T, Yoshikawa H, Mizutani K, Mishima M, Yokota H, Nagano H, et al. Treatment of node-positive endometrial cancer with complete node dissection, chemotherapy and radiation therapy. Br J Cancer (1997) 75:1836–41. doi: 10.1038/bjc.1997.313
6. Nelson G, Randall M, Sutton G, Moore D, Hurteau J, Look K. FIGO stage IIIC endometrial carcinoma with metastases confined to pelvic lymph nodes: analysis of treatment outcomes, prognostic variables, and failure patterns following adjuvant radiation therapy. Gynecol Oncol (1999) 75:211–4. doi: 10.1006/gyno.1999.5569
7. de Boer SM, Powell ME, Mileshkin L, Katsaros D, Bessette P, Haie-Meder C, et al. Adjuvant chemoradiotherapy versus radiotherapy alone in women with high-risk endometrial cancer (PORTEC-3): patterns of recurrence and post-hoc survival analysis of a randomised phase 3 trial. Lancet Oncol (2019) 20:1273–85. doi: 10.1016/S1470-2045(19)30395-X
8. Chapman BV, Swanick CW, Ning MS, Allen PK, Soliman PT, Westin SN, et al. Adjuvant combined-modality therapy for stage IIIC endometrioid and non-endometrioid endometrial cancer. Gynecol Oncol (2019) 154:22–8. doi: 10.1016/j.ygyno.2019.05.002
9. Binder PS, Kuroki LM, Zhao P, Cusworth S, Divine LM, Hagemann AR, et al. Benefit of combination chemotherapy and radiation stratified by grade of stage IIIC endometrial cancer. Gynecol Oncol (2017) 147:309–14. doi: 10.1016/j.ygyno.2017.08.031
10. van Weelden WJ, Reijnen C, Eggink FA, Boll D, van der berg H, van der Aa M, et al. Impact of different adjuvant treatment approaches on survival in stage III endometrial cancer: A population-based study. Eur J Cancer (2020) 133:104–11. doi: 10.1016/j.ejca.2020.04.012
11. Lee J, Yu T, Tsai MH. Lymph node number predicts the efficacy of adjuvant chemoradiotherapy in node-positive endometrial cancer patients. Diagn (Basel) (2020) 10. doi: 10.3390/diagnostics10060373
12. Cronin KA, Ries LA, Edwards BK. The surveillance, epidemiology, and end results (SEER) program of the national cancer institute. Cancer (2014) 120 Suppl 23:3755–7. doi: 10.1002/cncr.29049
13. Yang XL, Huang N, Wang MM, Lai H, Wu DJ. Comparison of different lymph node staging schemes for predicting survival outcomes in node-positive endometrioid endometrial cancer patients. Front Med (Lausanne) (2021) 8:688535. doi: 10.3389/fmed.2021.688535
14. Yang XL, Huang H, Kou LN, Lai H, Chen XP, Wu DJ. Construction and validation of a prognostic model for stage IIIC endometrial cancer patients after surgery. Eur J Surg Oncol (2022) 48:1173–80. doi: 10.1016/j.ejso.2021.12.462
15. Wright JD, Lewin SN, Barrena Medel NI, Sun X, Burke WM, Deutsch I, et al. Endometrial cancer in the oldest old: Tumor characteristics, patterns of care, and outcome. Gynecol Oncol (2011) 122:69–74. doi: 10.1016/j.ygyno.2011.02.040
16. Onal C, Sari SY, Yavas G, Guler OC, Yigit E, Oymak E, et al. Impact of lymph node ratio in patients with stage IIIC endometrial carcinoma treated with postoperative radiotherapy. Future Oncol (2021) 17:3321–30. doi: 10.2217/fon-2020-1308
17. Narasimhulu DM, Block MS, Weaver AL, McGree M, Kumar A, Langstraat C, et al. Sequencing chemotherapy before radiotherapy for women with stage IIIC endometrial cancer. Int J Gynecol Cancer (2021) 31:702–8. doi: 10.1136/ijgc-2020-002158
18. Nasioudis D, Byrne M, Ko EM, Giuntoli Ii RL, Haggerty AF, Cory L, et al. The impact of sentinel lymph node sampling versus traditional lymphadenectomy on the survival of patients with stage IIIC endometrial cancer. Int J Gynecol Cancer (2021) 31:840–5. doi: 10.1136/ijgc-2021-002450
19. Liu R, Xiao Z, Hu D, Luo H, Yin G, Min Y, et al. Cancer-specific survival outcome in early-stage young breast cancer: Evidence from the SEER database analysis. Front Endocrinol (Lausanne) (2021) 12:811878. doi: 10.3389/fendo.2021.811878
20. Yang XL, Zhang YE, Kou LN, Yang FL, Wu DJ. A population-based risk scoring system to individualize adjuvant treatment for stage IIIC endometrial cancer patients after surgery. Eur J Surg Oncol (2022). doi: 10.1016/j.ejso.2022.09.004
21. Zuo ZC, Zhang GC, Song P, Yang J, Li ST, Zhong Z, et al. Survival nomogram for stage IB non-Small-Cell lung cancer patients, based on the SEER database and an external validation cohort. Ann Surg Oncol (2021) 28:3941–50. doi: 10.1245/s10434-020-09362-0
22. Riley RD, Ensor J, Snell KIE, Harrell FE, Martin GP, Reitsma JB, et al. Calculating the sample size required for developing a clinical prediction model. BMJ (2020) 368:m441. doi: 10.1136/bmj.m441
23. National Comprehensive Cancer Network (NCCN). NCCN clinical practice guidelines in oncology. uterine neoplasms version 1. 2022. (The United States of American: National Comprehensive Cancer Network) (2021). Available at: http://www.nccn.org/professionals/physician_gls/pdf/uterine.pdf.
24. Matei D, Filiaci V, Randall ME, Mutch D, Steinhoff MM, DiSilvestro PA, et al. Adjuvant chemotherapy plus radiation for locally advanced endometrial cancer. N Engl J Med (2019) 380:2317–26. doi: 10.1056/NEJMoa1813181
25. Wright JD, Barrena Medel NI, Sehouli J, Fujiwara K, Herzog TJ. Contemporary management of endometrial cancer. Lancet (2012) 379:1352–60. doi: 10.1016/S0140-6736(12)60442-5
26. Cosgrove CM, Cohn DE, Goodfellow PJ. Primum non nocere: Are we ready for POLE testing in endometrial cancer? Gynecol Oncol (2017) 147:240–2. doi: 10.1016/j.ygyno.2017.09.015
27. Cancer Genome Atlas Research Network, Albert Einstein College of Medicine, Analytical Biological Services, Barretos Cancer Hospital, Baylor College of Medicine, Beckman Research Institute of City of Hope, et al. Integrated genomic characterization of endometrial carcinoma. Nature (2013) 497:67–73. doi: 10.1038/nature12113
28. Wen L, Zhang YZ, Chen SY, Wang J, Hu W, Zhong G. Subdivision of IIIC stage for endometrioid carcinoma to better predict prognosis and treatment guidance. Front Oncol (2020) 10:1175. doi: 10.3389/fonc.2020.01175
29. Guo JB, Qian HL, Ma F, Zhang Y, Cui X, Duan H. The characteristics of isolated para-aortic lymph node metastases in endometrial cancer and their prognostic significance. Ther Adv Med Oncol (2020) 12:1758835920933036. doi: 10.1177/1758835920933036
30. Li X, Cheng Y, Dong Y, Zhou J, Wang Z, Li X, et al. Development and validation of predictive model for lymph node metastasis in endometrial cancer: a SEER analysis. Ann Transl Med (2021) 9:538. doi: 10.21037/atm-20-5034
31. Li X, Yang X, Fan Y, Cheng Y, Dong Y, Zhou J, et al. A ten-gene methylation signature as a novel biomarker for improving prediction of prognosis and indicating gene targets in endometrial cancer. Genomics (2021) 113:2032–44. doi: 10.1016/j.ygeno.2021.04.035
32. Xu HY, Zou RY, Liu JY, Zhu L. A risk signature with nine stemness index-associated genes for predicting survival of patients with uterine corpus endometrial carcinoma. J Oncol (2021) 2021:6653247. doi: 10.1155/2021/6653247
33. Latham AH, Chen L, Hou JY, Tergas AI, Khoury-Collado F, St Clair CM, et al. Sequencing of therapy in women with stage III endometrial carcinoma receiving adjuvant combination chemotherapy and radiation. Gynecol Oncol (2019) 155:13–20. doi: 10.1016/j.ygyno.2019.07.021
34. Hathout L, Wang Y, Wang Q, Vergalasova I, Elshaikh MA, Dimitrova I, et al. A multi-institutional analysis of adjuvant chemotherapy and radiation sequence in women with stage IIIC endometrial cancer. Int J Radiat Oncol Biol Phys (2021) 110:1423–31. doi: 10.1016/j.ijrobp.2021.02.055
35. Onal C, Sari SY, Yildirim BA, Yavas G, Gultekin M, Guler OC, et al. A multi-institutional analysis of sequential versus 'sandwich' adjuvant chemotherapy and radiotherapy for stage IIIC endometrial carcinoma. J Gynecol Oncol (2019) 30:e28. doi: 10.3802/jgo.2019.30.e28
36. Zhu S, Khalil R, Altairy O, Burmeister C, Dimitrova I, Elshaikh M. Increased risk of recurrence in early-stage endometrial carcinoma after delays in adjuvant radiation treatment. Int J Gynecol Cancer (2021) 31:73–7. doi: 10.1136/ijgc-2020-001937
Keywords: endometrial cancer, stage IIIC, nomogram, adjuvant radiotherapy, adjuvant chemotherapy
Citation: Yang X-L, Yang F-L, Kou L-N, Wu D-J and Xie C (2022) Prognostic model for the exemption of adjuvant chemotherapy in stage IIIC endometrial cancer patients. Front. Endocrinol. 13:989063. doi: 10.3389/fendo.2022.989063
Received: 08 July 2022; Accepted: 05 October 2022;
Published: 26 October 2022.
Edited by:
Erika Di Zazzo, University of Molise, ItalyReviewed by:
Octavian Sabin Tataru, George Emil Palade University of Medicine, Pharmacy, Sciences and Technology of Târgu Mureş, RomaniaMałgorzata Pawlikowska, Nicolaus Copernicus University, Poland
Copyright © 2022 Yang, Yang, Kou, Wu and Xie. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Cong Xie, ZHJ4aWVjQDE2My5jb20=; Da-Jun Wu, ZHJ3dWRqQDEyNi5jb20=
†These authors have contributed equally to this work and share first authorship
 Xi-Lin Yang
Xi-Lin Yang Feng-Leng Yang
Feng-Leng Yang Ling-Na Kou
Ling-Na Kou Da-Jun Wu
Da-Jun Wu Cong Xie
Cong Xie