- 1Department of Microbiology, Immunology and Parasitology, College of Health Sciences, Addis Ababa University, Addis Ababa, Ethiopia
- 2Department of Medical Laboratory Science, Debre Birhan College of Health Sciences, Debre Birhan, Ethiopia
- 3Department of Internal Medicine, College of Health Sciences, Addis Ababa University, Addis Ababa, Ethiopia
Introduction: Infected diabetic foot ulcer (IDFU) is a worldwide problem associated with diabetes mellitus. It could lead from soft tissue infection to bone infection and is a leading cause of lower limb amputation. Gram-negative and Gram-positive bacteria, including anaerobic bacteria and fungi, are considered potential causes of infection. The early diagnosis of DFU infection and appropriate treatment based on the identification of the pathogens and their antimicrobial susceptibility pattern is important for good prognosis. Therefore, the purpose of this study was to isolate the bacteria that infect foot ulcers in selected Hospitals and determine their antimicrobial resistance profile.
Method: An institutional-based multicenter, cross-sectional study was conducted in selected Hospitals in Addis Ababa, Ethiopia, from November 2020 to May 2021. A sterile swab was used to collect samples from the foot ulcer and a sterile needle to collect pus. Isolates were identified by culture, Gram-staining, and a series of biochemical tests. For each bacterial species identified, the antibiotic profiling was determined by the Kirby-Bauer disk diffusion method.
Results: one hundred and twenty-seven pathogenic bacteria were isolated from samples taken from 130 patients with a diabetic foot ulcer. Sixty-eight percent had growth of multiple microorganisms. Two-thirds (66.7%) of the isolates were gram-negative bacteria. The predominant bacterial species were S. aureus 25.19% (32/127), Pseudomonas species 18.89% (24/127), and Escherichia coli 16.53% (21/127). Overall, 92.9% (118/127) of the isolates were identified as multi-drug resistant. Gram-positive isolates were susceptible to chloramphenicol, clindamycin, and amikacin. Gram-negative isolates were also sensitive to chloramphenicol, aztreonam, and amikacin.
Conclusion: The majority of bacteria isolated from patients presenting with Diabetic foot ulcer infections were found to be multi-drug resistant in the study sites of the current study. The results demonstrate the importance of timely identification of infection of diabetic foot ulcers, proper sample collection for identification of the pathogens and for determining their antibiotic susceptibility pattern before initiating antimicrobial treatment
Introduction
Diabetic foot ulcer (DFU) is a common complication in diabetes and can lead to a considerable social, psychological, and economic burden on patients and the health sector (1). DFU accounts for significant morbidity and mortality. DFU patients have a 2.5 times higher risk of death compared to diabetic patients without foot ulcers (2, 3). According to the International Diabetes Federation, 9.1-26.1 billion people will develop DUFs every year (1). The prevalence of DFU has increased significantly with a recent global prevalence averaging around 6.4% (4).
Once DFU occurs, the main challenge is the increased vulnerability to different potential pathogens with potentially serious outcomes such as infection, gangrene, osteomyelitis, amputation, and even death (5). According to IDSA and other studies, infection with diabetic foot ulcers will increase the chance of amputation by 50% compared to patients with uninfected foot ulcers (6, 7).
Diabetes-related hospital admissions are most pronounced because of infected diabetic foot ulcers. Major and minor (83% and 96% respectively) amputations have been done related to DFU aggravated by infection (8). On the other hand, diabetic foot osteomyelitis development is seen in around 44-68% of patients admitted to the hospital and it is the principal reason for amputation among such patients (9).
A study from Nigeria revealed that the burden of diabetic foot ulcers accounts for 24.9% and that the majority of the ulcers are already infected with Wagner grade ≥ 3 (10).
Ethiopia is also among the countries affected by diabetic foot ulcers. A systematic meta-analysis indicated that the national prevalence of DFU in Ethiopian diabetic patients was 11.27% (11). Other related studies in Ethiopia, have shown the diverse prevalence in the country as 17.9% in Nekemte (12), 13.6% in Gondar (13), and 15% in Arbaminch (14), and 78% in Addis Ababa (15). In the study from Addis Ababa, DFU with cellulitis was 12.2%, DFU with toe gangrene at 16.3%, DFU with foot gangrene at 18.4%, and only 31.1% with foot ulcer (15). Another more recent study found that the rate of foot ulcers at Tikur Anbessa Specialized Hospital was 26% (16).
Diabetic foot ulcer infection is essentially polymicrobial. Many complications of DFUs are caused by bacterial infections and can be minimized by paying due attention to the identification and control of infections. It is necessary to detect the specific pathogens and their susceptibility pattern to initiate early treatment with the appropriate antibiotics.
Therefore, this study aimed to identify the bacteria that cause diabetic foot ulcers and the antimicrobial sensitivity patterns of these bacterial isolates.
Materials and methods
A multicenter hospital-based cross-sectional study was done in the diabetes mellitus (DM) clinics of three selected public hospitals in Addis Ababa, Ethiopia. The study sites were Tikur Anbessa Specialized Hospital (TASH), Menelik II, and Yekatit 12 Hospitals. All adult diabetic patients with diabetic foot ulcers, whose ulcers were greater than or equal to the Wagner first degree grading system, who visited the DM clinics at the study sites during the study period, and gave informed consent were included in the study. The study took place from November 2020 to May 2021.
Samples were taken from the deepest part of the ulcer using two sterile swabs, soaked in sterile glucose broth. The samples were taken using a firm circular motion with the swab. One swab was used for Gram staining and the other was used for culture. Semi-structured questionnaires were used to collect sociodemographic and other clinical data.
A Gram smear directly from the sample was examined. The samples were inoculated on blood agar, MacConkey agar, and chocolate agar. The inoculated plates were incubated at 37°C overnight and the plates were examined for growth the next day.
In this study, ulcers were classified according to the Wagner Diabetic Foot Ulcer Classification System. The classification was Grade 0-Pre-ulcerative, with no open lesion or cellulitis, Grade1-Superficial ulcer, Grade2-Deep ulcer up to tendons and joint tissue, Grade 3-Deep ulcer with abscess, osteomyelitis, and joint sepsis, Grade 4- Localized gangrene of forefoot or heel, and Grade 5-Gangrene of entire foot/global gangrene.
Antimicrobial susceptibility testing
Antimicrobial susceptibility testing (AST) was done for the isolated bacteria with 21 antibiotics on Mueller Hinton Agar (MHA) using the Kirby Bauer disk diffusion technique according to the Clinical Laboratory Standard Institute (CLSI) guidelines 2020 (17). The inoculum for each isolate was prepared by emulsifying colonies from the purified culture overnight in normally sterile saline (0.85%) in test tubes with turbidity adjusted to standard 0, 5 McFarland. The bacterial suspension was spread evenly on the MHA plate with a sterile swab, left for 3 minutes, and then the antibiotic discs were applied.
For Gram-negative bacteria the following antibiotic discs were used (in/disk): ampicillin-sulbactam (10/5), amoxicillin and clavulanic acid (10/10 μg), ceftriaxone (18), cefotaxime (18), cefoxitin (18), ceftazidime (18), cefepime (18), amikacin (18), gentamicin (19), ciprofloxacin (5), sulfamethoxazole-trimethoprim(1.25/23.75), piperacillin-tazobactam (100/10), tobramycin (18), imipenem (19), aztreonam (18), and meropenem (19). While for Gram-positive bacteria, antibiotics discs used were (in μg/disk): gentamicin (19), doxycycline (18), erythromycin (14), vancomycin (18), cefoxitin (18), sulfamethoxazole-trimethoprim (1.25/23.75), ciprofloxacin (5) penicillin (10 units), clindamycin (2), and chloramphenicol (18) (17).
The plates were incubated at 35°C for 16-18 hours, and the diameters of the zone of inhibition were measured with a Vernier caliper, and the results were interpreted according to the CLSI standards (17).
Standard operating procedures (SOPs) were used for specific purposes for all laboratory procedures. Quality control strains of Escherichia coli ATCC® 25922, Enterococcus faecalis ATCC® 29212, Pseudomonas aeruginosa ATCC® 27853, Staphylococcus aureus ATCC® 25923, K. pneumoniae ATCC®1705, and K. pneumoniae ATCC®1706 were used to confirm the result of antibiotics, media and to assess the quality of the general laboratory procedure (17). The quality of the reagents, antibiotic disk, and media used was checked regularly.
Ethical consideration
Ethical approval was obtained from the ethics and review committee of the Department of Microbiology, Immunology, and Parasitology, College of Health Sciences, Addis Ababa University (meeting DERC/0022/2020). A formal letter of support from the Department was written to the three study sites. An information sheet with the necessary information about the study was given to the potential participants and their voluntary consent was taken before enrolling them in the study. For those who were younger than 18, their parents or guardians were asked for consent and assent taken from the children. Confidentiality was maintained by omitting their personal identifiers throughout the study.
Results
Sociodemographic and clinical characteristics of participants
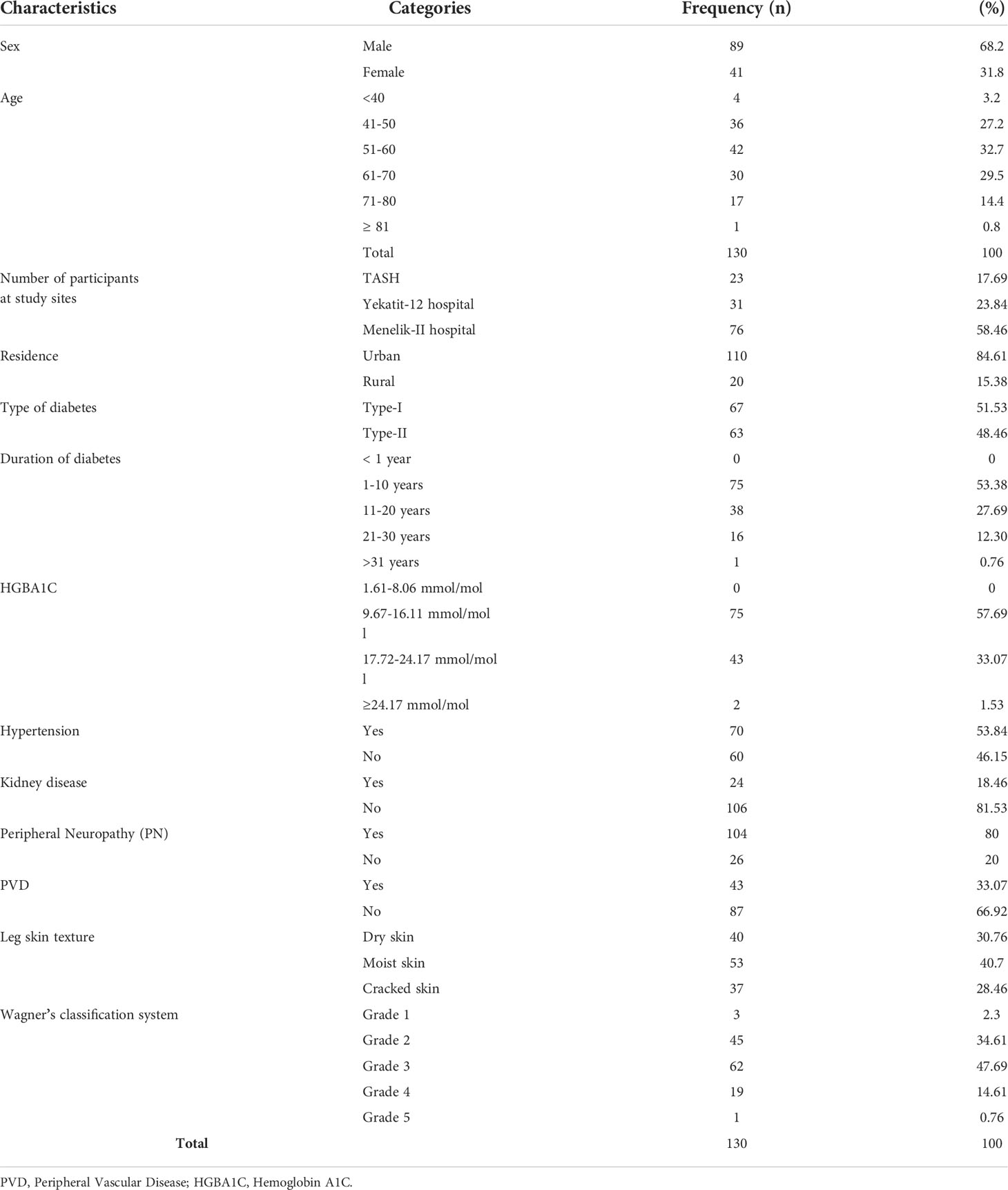
One hundred thirty (130) study participants (23 from TASH, 31 from Yekatit-12 Hospital, and 76 from Menelik II hospital) were included in the present study. Out of these, 88 (67.69%) were men and 42 (32.3%) were women. Most participants were between 50 and 75 years old and the mean age of the study, participants were 54 ± 7SD. The majority of the study participants were residents of Addis Ababa (84.5%), while only 15.5% were from rural areas The majority of the participants had type I diabetes as tabulated in Table 1.
The magnitude of bacterial isolates from diabetic foot ulcer infections
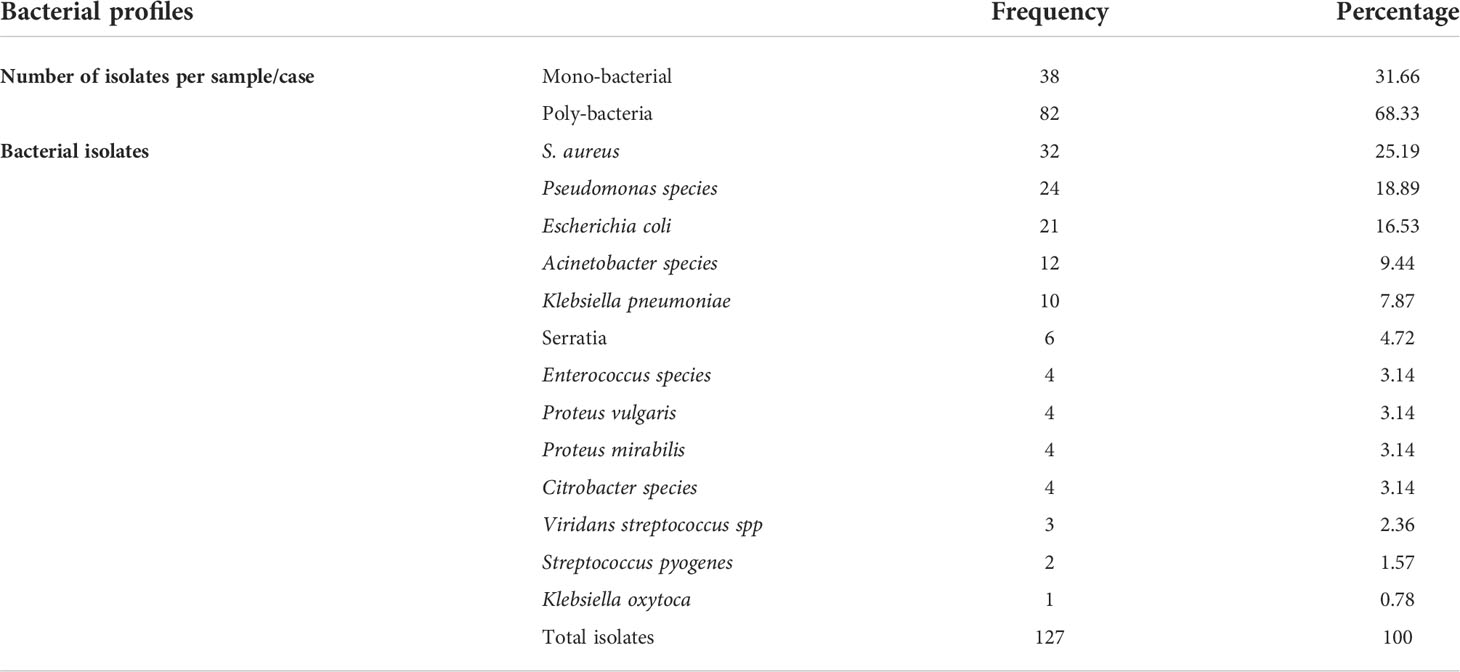
One hundred and twenty-seven bacterial isolates were identified from 130 patients with diabetic foot ulcers. Of these, 41 (32%) were Gram-positive, and 86 (68%) were Gram-negative isolates. Sixty-eight percent (82/120) of the samples had poly-bacterial growth. The percentage of Gram-negative bacteria 86 (68%) was greater than Gram-positive bacteria 41 (32%). Among the isolated bacteria, the most predominant bacteria were Staphylococcus aureus 25.19% (32/127), followed by Pseudomonas species 18.89% (24/127), and Escherichia coli 16.53% (21/127). Other isolates included Acinetobacter species (9.4%), Klebsiella pneumoniae (7.9%), Serratia species (4.7%), Enterococcus species (3.1%), Proteus vulgaris (3.1%), and Proteus mirabilis (3.1%) (Table 2).
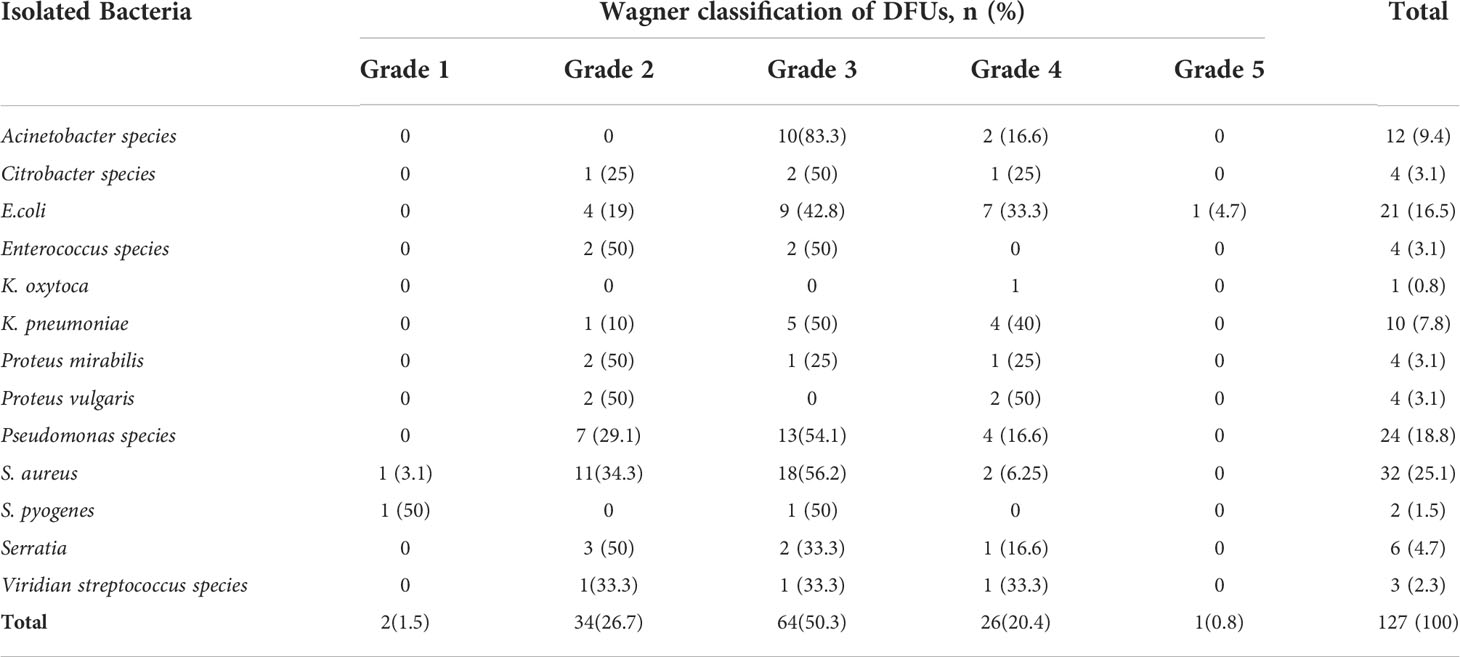
The highest number of culture-positive cases 50.3% (64/127), was found in Wagner grade 3 of diabetic foot ulcers, followed by Wagner grade 2 26.7% (34/127). With an increase in Wagner’s score, the rate of infections caused by gram-negative bacteria increased. Staphylococcus aureus 32 (25.1%), Pseudomonas species 24 (18.8%), and E. coli (16.5%) were the most common isolates in each of Wagner’s rating scales. Overall, infection was caused by one bacteria in 31.66% (38/120) and polymicrobial in 68.33% (82/120) samples. In general, as the degree of ulcers increases, the percentage of ulcers with polymicrobial growth increases (Table 3).
Antimicrobial susceptibility profiles of gram-positive isolates
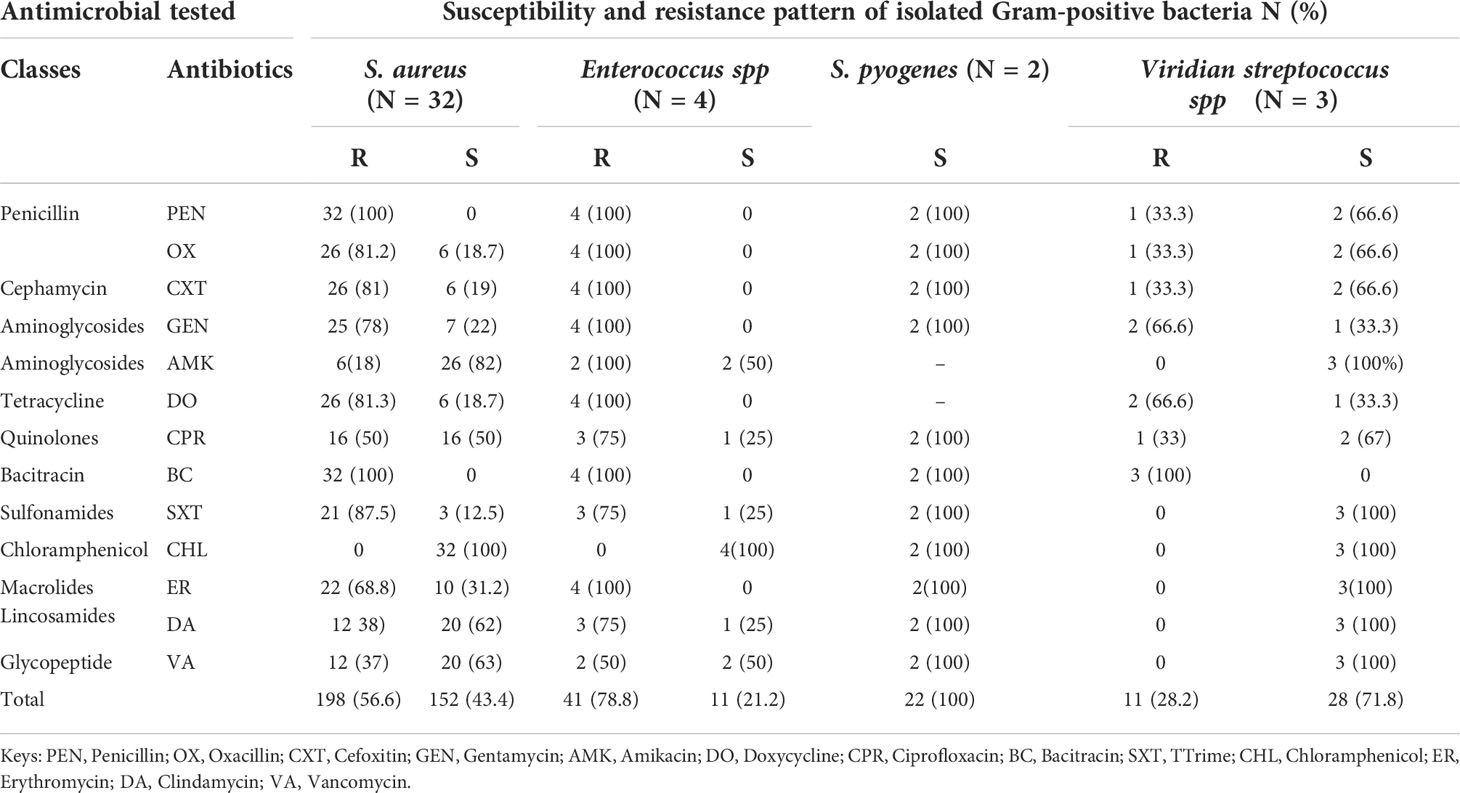
As shown in Table 4, among 41/127 (32.2%) Gram-positive bacteria, all S. aureus and Enterococcus species were resistant to oxacillin, penicillin, cefoxitin, and bacitracin. However, a lower level of 18.7% (6/32) susceptibility rates for S. aureus isolates were documented for oxacillin. A high level of resistance was also observed among the majority of the isolates of S. aureus and all Enterococcus species, which were resistant to gentamycin, doxycycline, erythromycin, and cotrimoxazole. However, all S. pyogenes and Viridans streptococcus species were sensitive to the majority of antimicrobial agents.
The majority of S. aureus isolates were sensitive to amikacin 81.25% (26/32), ciprofloxacin 50% (16/32), clindamycin 62.5% (20/32), and vancomycin 62.5% (20/32). All isolated S. aureus and Enterococcus species were sensitive to chloramphenicol (100%). In this study, 50% (2/4) of Enterococcus species were resistant to vancomycin. Overall, 56.4% of S. aureus isolates, 78.8% of Enterococcus species isolates, and 72% of Viridans streptococcus species were resistant to the tested antibiotics (Table 4).
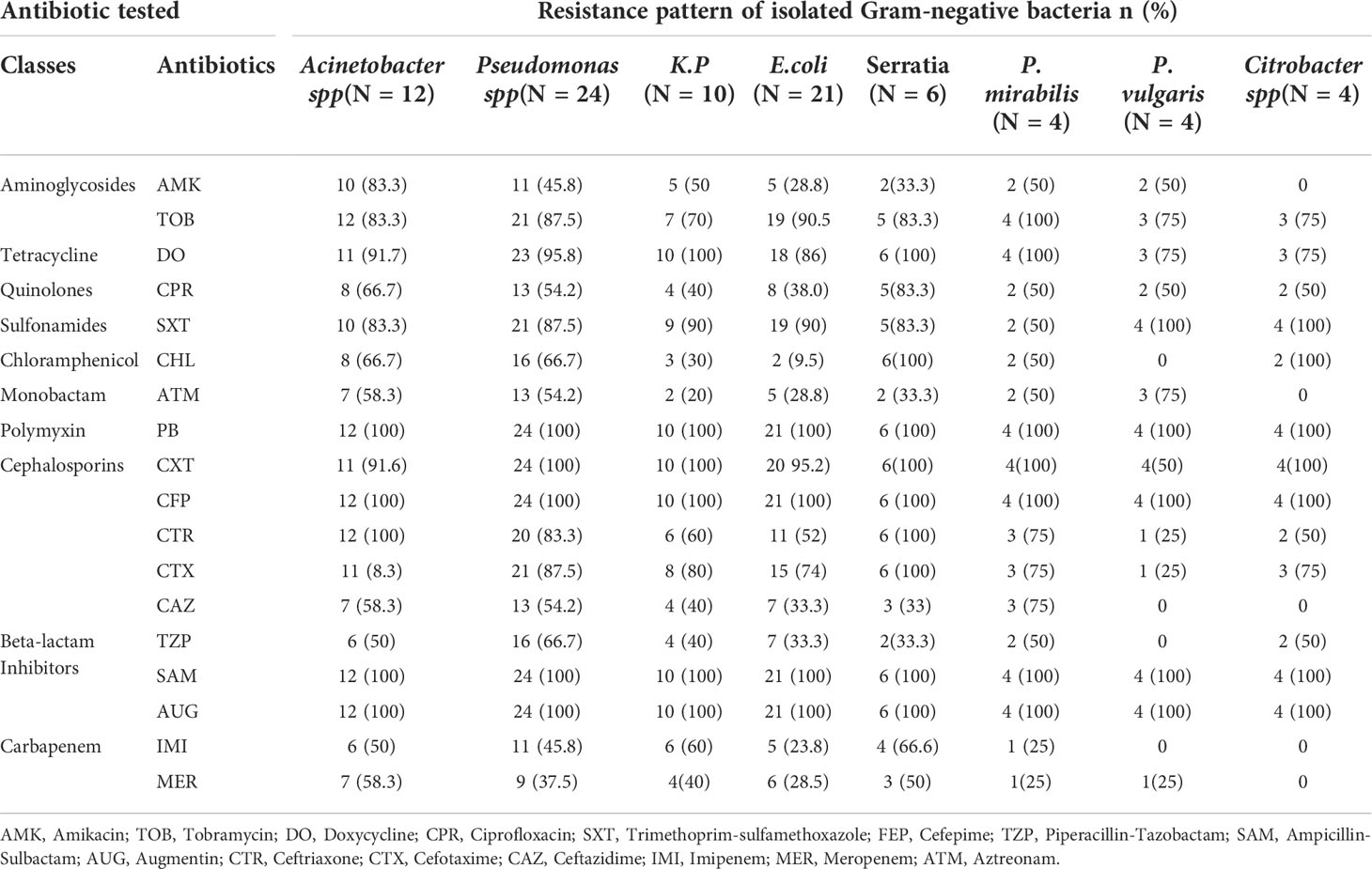
Antimicrobial-resistance profiles of the gram-negative isolates
All the gram-negative isolates were resistant to cefoxitin, ampicillin-sulbactam, tobramycin, polymyxin b, cefepime, and augmentin. Acinetobacter and Pseudomonas species were the most resistant to most antibacterial drugs, including amikacin, chloramphenicol, aztreonam, ceftriaxone, ceftazidime, imipenem, and meropenem. More than half of gram-negative bacterial isolates were resistant to doxycycline, trimethoprim, piperacillin, tazobactam, ceftriaxone, cefotaxime, imipenem, and meropenem.
The majority of Escherichia coli, Klebsiella pneumoniae, Serratia, Proteus vulgaris, Proteus mirabilis, Citrobacter species, and Klebsiella oxytoca, were sensitive to Chloramphenicol, Amikacin, and Ceftazidime. Amikacin was found to be the best drug for Citrobacter species (Table 5).
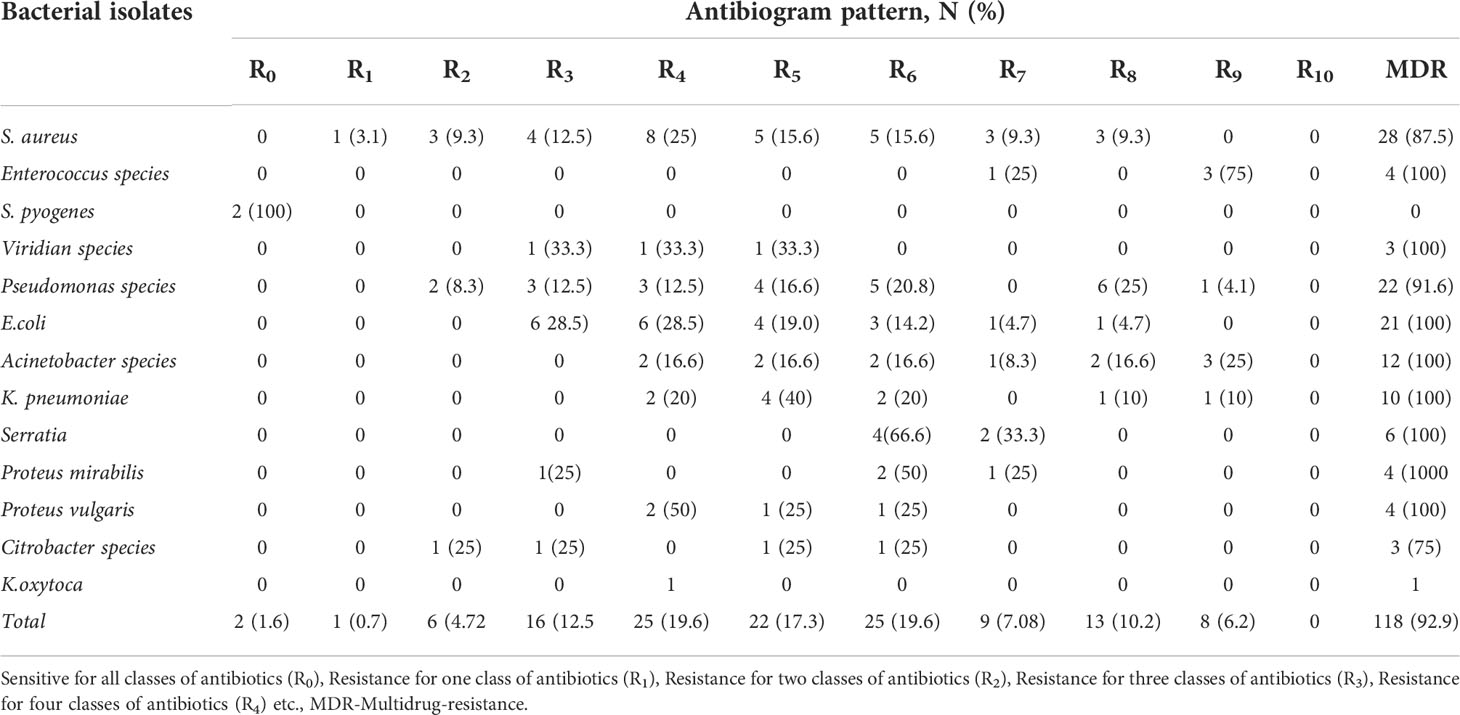
The magnitude of multidrug-resistant isolates
Multidrug-resistance profiles of the organisms showed that of the 127 bacterial isolates, 92.9% (118/127) were MDR, that is resistant to more than two agents of antibiotic classes, whereas 7.08%(9/127) were non-MDR. Twenty-eight (88%) of Staphylococcus aureus isolates were MDR. Acinetobacter and Pseudomonas pathogens were resistant to all types of antibiotics. However, the MDR profiles within species vary (Table 6).
Discussion
Diabetic foot ulcers are the main complication of Diabetes mellitus. Diabetic foot ulcers, if left untreated, can become infected and cause other complications, such as gangrene, osteomyelitis, and amputation. Surgery and antibiotic therapy are the options used to control this infection. This study was conducted to determine the main pathogenic bacterial infections associated with DFU and their antimicrobial susceptibility patterns to commonly used antibiotics at the study sites.
A male predominance in the study participants was noted in this study, in line with previous studies in Indonesia and India (20, 21). This may be explained by the more active role of men in outdoor activities leading to injuries and exposure to the development of ulcers. Similarly, the majority of participants with DFU infections were found to be within the age range of 51-60 years in agreement with the same studies in India and Indonesia (20, 22).
In this study, ulcers were classified according to the Wagner Diabetic Foot Ulcer Classification System. The most common was grade 3 at 47.69% (62/130) followed by grade 2 at 34.61% (45/130), which is consistent with the research conducted in Egypt, where grade 3 was found in 50% (60/120) followed by grade 2 in 25% (30/120) of participants (23). Contrary to these findings, a study from India showed that grade 2 (69.2%) is higher than grade 3 (5.1%).
In this study, a high growth rate (92.3%) of the bacteria was found, comparable with an earlier study in Ethiopia with a growth rate of 77.3% (92/119), compared to no growth at 22.7% (27/1190) (15). A recent study also reported, that the growth rate was 81.7% (98/120), and no growth of 22% (18.34%), respectively (23).
Overall, gram-negative bacteria (71.6%) were predominantly isolated (86/120) compared to gram-positive isolates (34.16%). This finding is in agreement with an earlier study done in the same study site (out of three included in the current study) Tikur Anbessa Specialized Hospital, where gram-negative bacteria were isolated in 88.55% (54/61) versus 7% (11.47%) gram-positive bacteria (15). Similarly, a study from Egypt reported 56% gram-negative and 27.7% gram positives, while 79% gram-positive and 21% gram negatives were isolated in northeast India (23, 24).
The rate of bacterial isolation and the type of bacteria in the ulcer increased as the severity of the ulcer increased. This shows the extent to which organisms influence the DFU healing process, which is supported by various papers published elsewhere, such as in Nigeria (18), China (25), and India (21, 26).
In the present study, S. aureus was the predominant isolate 25.19% (32/127), unlike a previous study in Ethiopia which reported, Klebsiella species 23.9% (22/92) as the predominant bacteria followed by Proteus species 18.47% (17/92) (15). In Egypt, P. mirabilis (16.8%) is the most common isolate (24), in Saudi Arabia Pseudomonas species 15.6% (n = 134) (27), and in South America Pseudomonas species (18.8%) was the most common isolate (28). Similarly in agreement with studies in Kenya 17.5% (14/80 (29), Nigeria 32.9% (32/97 (30), India 24.42% (32/131) (31), 25% (18/85) (21), China 65.2% (n=232) (25), and in Iran 28% (n=92) (32). This shows that the predominant bacteria causing DFU infections could vary in different settings.
The current study found that the most common gram-negative isolates were Pseudomonas species (18.89%), followed by Escherichia coli, comparable with other studies conducted in Libya 17.5% (21/120) (33), India 23.2% (n = 85) (26) and 23.6% (n = 85) (21).
On the other hand, a previous study in Ethiopia reported that no Pseudomonas species were isolated from 92 cultured samples, whereas E.coli was isolated in 5.43% (5/92) (15). Similarly in Pakistan, the most common gram-negative bacteria was E.coli 15.72% (n=671) (34). This variation may be due to the sample size differences of the different studies and other unique characteristics of each study site.
A very high rate of multidrug resistance (92.9%) was found in the present study, consistent with the findings of a study in India (26), and Nigeria (35).
In this study, the majority of isolated Staphylococcus aureus were resistant to gentamicin; doxycycline, erythromycin, and trimethoprim, while the majority are sensitive to amikacin, oxacillin, ciprofloxacin, clindamycin, and vancomycin. Similarly, all enterococci are resistant to gentamicin, doxycycline, erythromycin, and trimethoprim. While all Staphylococcus aureus and Enterococci were found to be susceptible to chloramphenicol (100%) (36). The possible reasons for this high level of resistance could be multifactorial. It could be explained by inappropriate antibiotic use, self -medication, repeated courses of antibiotics associated with the chronic nature of the DFU, and the patients encounters with the Hospital environment during their frequent follow up visits.
The current study confirmed that both gram-positive and gram-negative aerobic pathogenic bacteria cause DFU infection in the study sites and because of their antimicrobial resistance profile can cause challenges for the management of patients and can lead to more complications such as osteomyelitis, and possibly amputation of the limbs.
Limitation of the study
The current study, which has been done in a resource constrained environment has tried to address the problem of resistance to commonly used antibiotics in the study sites, but has its limitations. The study was done during the COVID-19 pandemic, which has affected the health seeking behavior of potential study participants. The study focused on anaerobic bacteria, even though naerobic bacteria are known to be important etiologic agents in DFU infections, including fungi. We were not able to do further analysis of the study using molecular techniques to get more information about the profile of the organisms isolated from these patients, because of resource constraints.
Conclusion
Diabetic foot ulcers can be infected with a wide variety of pathogens and a large number of multi-drug resistant bacteria. In this study, Staphylococcus aureus was the dominant isolate followed by other gram-negative bacteria. In the current study, a high level of resistance to commonly used antibiotics was found highlighting the need for cautious care in the use of antibiotics for the treatment of infections. Some isolates in the current study were more sensitive to chloramphenicol, aztreonam, amikacin, clindamycin, and vancomycin, which can be used as first-line treatment for these infections. The results showed an overall increase in the resistance of bacteria to antimicrobial agents and emphasizes the importance of microbiological analysis and antimicrobial susceptibility testing before initiating antibiotics treatment for diabetic foot ulcer infections.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving human participants were reviewed and approved by the Review and Ethics Committee of the Department of Microbiology, Immunology & Parasitology, College of Health Sciences, Addis Ababa University. The patients/participants provided their written informed consent to participate in this study.
Author contributions
AA collected the primary data, conducted the analyses, and drafted the manuscript. HK and YW contributed to conceptualization, collection of data and data analysis. AA, YW, HK, and AAb have read, reviewed, and approved the article.
Funding
The study was supported by funds from Addis Ababa University, Ethiopia.
Acknowledgments
The authors would like to acknowledge the data collectors, particularly health extension workers. In addition, we are grateful to the study participants for their voluntary participation in the study. Moreover, the authors would like to acknowledge the following individuals, Shemse Sebre, Aminu Seman, and Shambel Araya for their assistance.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
DFU, Diabetic foot ulcer; DM, Diabetic Mellitus; ESBL,Extended spectrum; MDR,Multidrug resistance.
References
1. Armstrong D, Boulton M, Bus S. Diabetic foot ulcers and their recurrence. N Engl J Med (2017) 376(24):2367–75. doi: 10.1056/NEJMra1615439
2. Chammas NK, Hill RL, Edmonds ME. Increased mortality in diabetic foot ulcer patients: The significance of ulcer type. J Diabetes Res (2016) 7. doi: 10.1155/2016/2879809
3. Walsh JW, Hoffstad OJ, Sullivan MO, Margolis DJ. Association of diabetic foot ulcer and death in a population-based cohort from the united kingdom. Diabetes Med (2016) 33(11):1493–8. doi: 10.1111/dme.13054
4. Zhang P, Lu J, Jing Y, Tang S, Zhu D, Bi Y. Global epidemiology of diabetic foot ulceration: a systematic review and meta-analysis †. Ann Med (2017) 49(2):106–16. doi: 10.1080/07853890.2016.1231932
5. Hitam SAS, Hassan SA, Maning N. The significant association between polymicrobial diabetic foot infection and its severity and outcomes. Malays J Med Sci (2019) 26(1):107–14. doi: 10.21315/mjms2019.26.1.10
6. Lipsky BA, Berendt AR, Cornia PB, Pile JC, Peters EJ, Armstrong DG, et al. Infectious diseases society of America clinical practice guideline for the diagnosis and treatment of diabetic foot infections. Clin Infect Dis (2012) 54(12):e132–73. doi: 10.1093/cid/cis346
7. Noor S, Zubair M, Ahmad J. Diabetic foot ulcer–a review on pathophysiology, classification and microbial aetiology. Diabetes Metab Syndr (2015) 9(3):192–9. doi: 10.1016/j.dsx.2015.04.007
8. Hicks CW, Selvarajah S, Mathioudakis N, Sherman RE, Hines KF, Black JH 3rd, et al. The burden of infected diabetic foot ulcers on hospital admissions and costs. Ann Vasc Surg (2016) 33:149–58. doi: 10.1016/j.avsg.2015.11.025
9. van Asten SA, La Fontaine J, Peters EJ, Bhavan K, Kim PJ, Lavery LA. The microbiome of diabetic foot osteomyelitis. Eur J Clin Microbiol Infect Dis (2016) 35(2):293–8. doi: 10.1007/s10096-015-2544-1
10. Ugwu E, Adeleye O, Gezawa I, Okpe I, Enamino M, Ezeani I. Burden of diabetic foot ulcer in Nigeria: Current evidence from the multicenter evaluation of diabetic foot ulcer in Nigeria. World J Diabetes (2019) 10(3):200–11. doi: 10.4239/wjd.v10.i3.200
11. Mulugeta H, Wagnew F, Zeleke H, Tesfaye B, Amha H, Leshargie CT, et al. Prevalence of diabetic foot ulcer and its association with duration of illness and residence in Ethiopia: a systematic review and meta-analysis. medRxiv (2019), 19003061. doi: 10.1101/19003061
12. Bekele F, Fekadu G, Bekele K, Dugassa D. Incidence of diabetic foot ulcer among diabetes mellitus patients admitted to nekemte referral hospital, Western Ethiopia: a prospective observational study. Endocrinol Metab Syndr (2019) 8(2):1–5.
13. Mariam TG, Alemayehu A, Tesfaye E, Mequannt W, Temesgen K, Yetwale F, et al. Prevalence of diabetic foot ulcer and associated factors among adult diabetic patients who attend the diabetic follow-up clinic at the university of gondar referral hospital, north West Ethiopia, 2016: Institutional-based cross-sectional study. J Diabetes Res (2017), 2879249. doi: 10.1155/2017/2879249
14. Deribe B, Woldemichael K, Nemera G. Prevalence and factors influencing diabetic foot ulcer among diabetic patients attending arbaminch hospital, south Ethiopia. J Diabetes Metab (2014) 5(1):1–7. Available at: doi: 10.4172/2155-6156.1000322
15. Amogne W, Reja A, Amare A. Diabetic foot disease in Ethiopian patients: a hospital-based study. Ethiopian J Health Dev (2011) 25(1):17–21. doi: 10.4314/ejhd.v25i1.69841
16. Yimam A. Assessment of prevalence of diabetic foot ulcer and associated factors among diabetic patient attending tikur anbesa specialized hospital diabetic clinic, Addis Ababa, Ethiopia. (2017) 25:17–21. Doctoral dissertation: http://etd.aau.edu.et/handle/123456789/6551?show=full
17. Clinical and laboratory standard institutes (CLSI). Performance standards for antimicrobial susceptibility testing. 29th ed. CLSI supplement M100 Vol. 39. Wayne, PA: Clinical Laboratory Standards Institute (2020).
18. Oates PJ. Polyol pathway and diabetic peripheral neuropathy. Int Rev Neurobiol (2002) 50:325–92. doi: 10.1016/s0074-7742(02)50082-9
19. Kwon KT, Armstrong DG. Microbiology and antimicrobial therapy for diabetic foot infections. Infect Chemother (2018) 50(1):11–20. doi: 10.3947/ic.2018.50.1.11
20. Murshed M. Bacteriological profile of diabetic foot infection and its effect on limb salvation. J Surg Sci (2020) 24(1):21–5. doi: 10.3329/jss.v24i1.52213
21. Shah P, Eswarawaka M, Anne D, Shah P, Srivastava N. Bacteriological profile of diabetic foot. Int Surg J (2021) 8(2):704–9. doi: 10.18203/2349-2902.isj20210389
22. Van Netten JJ, Bus SA, Apelqvist J, Lipsky BA, Hinchliffe RJ, Game F, et al. Definitions and criteria for diabetic foot disease. Diabetes/metabolism Res Rev (2020) 36:e3268. doi: 10.1002/dmrr.3268
23. Ismail AA, Meheissen MA, Elaaty TAA, Abd-Allatif NE, Kassab HS. Microbial profile, antimicrobial resistance, and molecular characterization of diabetic foot infections in a university hospital. Germs (2021) 11(1):39–51. doi: 10.18683/germs.2021.1239
24. Dwedar R, Ismail D, Abdulbaky A. Diabetic foot infection: Microbiological causes with special reference to their antibiotic resistance pattern. Egyptian J Med Microbiol (2015) 24:95–102. doi: 10.12816/0024935
25. Xie X, Bao Y, Ni L, Liu D, Niu S, Lin H, et al. Bacterial profile and antibiotic resistance in patients with diabetic foot ulcer in guangzhou, southern China: Focus on the differences among different wagner’s grades, IDSA/IWGDF grades, and ulcer types. Int J Endocrinol (2017), 8694903. doi: 10.1155/2017/8694903
26. Thanganadar Appapalam S, Muniyan A, Vasanthi Mohan K, Panchamoorthy R. A study on isolation, characterization, and exploration of multiantibiotic-resistant bacteria in the wound site of diabetic foot ulcer patients. Int J Low Extrem Wounds (2021) 20(1):6–14. doi: 10.1177/1534734619884430
27. Al Ayed MY, Ababneh M, Alwin Robert A, Alzaid A, Ahmed RA, Salman A, et al. Common pathogens and antibiotic sensitivity profiles of infected diabetic foot ulcers in Saudi Arabia. Int J Low Extrem Wounds (2018) 17(3):161–8. doi: 10.1177/1534734618793557
28. Ponce de Leon A, Merchant S, Raman G, Avendano E, Chan J, Tepichin Hernandez G, et al. Pseudomonas infections among hospitalized adults in Latin America: a systematic review and meta-analysis. BMC Infect Dis (2020) 20(1):250. doi: 10.1186/s12879-020-04973-0
29. Mutonga DM, Mureithi MW, Ngugi NN, Otieno FCF. Bacterial isolation and antibiotic susceptibility from diabetic foot ulcers in Kenya using microbiological tests and comparison with RT-PCR in the detection of s. aureus and MRSA. BMC Res Notes (2019) 12(1):244. doi: 10.1186/s13104-019-4278-0
30. Ogba OM, Nisan E, Eyam ES. Aerobic bacteria associated with diabetic foot ulcers and their susceptibility pattern. BioMed Dermatol (2019) 3:1. doi: 10.1186/s41702-019-0039-x
31. Aleem S, Multani H, Bashir H. Bacteriological profile and antimicrobial sensitivity pattern of isolates from the diabetic foot of patients attending a teaching hospital in northern India. Asian J Med Sci (2021) 12(5):83–7. doi: 10.3126/ajms.v12i5.34415
32. Akhi MT, Ghotaslou R, Asgharzadeh M, Varshochi M, Pirzadeh T, Memar MY, et al. Bacterial aetiology and antibiotic susceptibility pattern of diabetic foot infections in tabriz, Iran. GMS hygiene infection control (2015) 10:Doc02. doi: 10.3205/dgkh000245
33. Elbaz A, Dhawan A, Elramalli AK, Algondi IA, Anan A, Elazomi A, et al. Antimicrobial sensitivity patterns of pseudomonas aeruginosa isolates obtained from foot ulcer diabetes patients in Tripoli, Libya. 2018, Libyan Journal of Medical Research (LJMR), Vol : 12 (2)
34. Miyan Z, Fawwad A, Sabir R, Basit A. Microbiological pattern of diabetic foot infections at a tertiary care centre in a developing country. J Pak Med Assoc (2017) 67(5):665–9. ISSN: 0030-9982
35. Adeyemo AT, Kolawole B, Rotimi VO, Aboderin AO. Multicentre study of the burden of multidrug-resistant bacteria in the aetiology of infected diabetic foot ulcers. Afr J Lab Med (2021) 10(1):1261. doi: 10.4102/ajlm.v10i1.1261
Keywords: diabetic Mellitus, diabetic foot ulcer, antimicrobial susceptibility testing, multidrug resistance, addis Ababa, ethiopia
Citation: Atlaw A, Kebede HB, Abdela AA and Woldeamanuel Y (2022) Bacterial isolates from diabetic foot ulcers and their antimicrobial resistance profile from selected hospitals in Addis Ababa, Ethiopia. Front. Endocrinol. 13:987487. doi: 10.3389/fendo.2022.987487
Received: 06 July 2022; Accepted: 15 August 2022;
Published: 31 August 2022.
Edited by:
Sathish Thirunavukkarasu, Emory University, United StatesReviewed by:
Pramod Kumar Ta, Madras Diabetes Research Foundation, IndiaMary Chandrika Anton, Sree Balaji Medical College and Hospital, India
Copyright © 2022 Atlaw, Kebede, Abdela and Woldeamanuel. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yimtubezinash Woldeamanuel, eWltdHViZXplbmFzaC53YW1hbnVlbEBhYXUuZWR1LmV0
 Asegdew Atlaw1,2
Asegdew Atlaw1,2 Habtamu Biazin Kebede
Habtamu Biazin Kebede Yimtubezinash Woldeamanuel
Yimtubezinash Woldeamanuel