- The Reproductive Center, The Third Affiliated Hospital of Zhengzhou University, Zhengzhou, China
Objective: The present study analyzed the effect of hCG trigger day progesterone (P) levels on the live birth rate (LBR) in the gonadotropin-releasing hormone (GnRH) antagonist protocol.
Materials and methods: This study was a single-center retrospective study. In vitro fertilization (IVF)/intracytoplasmic sperm injection (ICSI) cycles performed from January 2017 to December 2020 were included in the analysis. This study included people with a normal ovarian response to fresh embryo transfer of GnRH antagonist protocols. All cycles were divided into 2 groups by P level on the day of human chorionic gonadotropin (hCG) trigger, P<1.0 ng/ml and P≥1.0 ng/ml. The primary outcome measure was LBR.
Result: A total of 867 cycles with P<1.0 ng/ml and 362 cycles with P≥1.0 ng/ml were included in the analysis. The clinical pregnancy rate (CPR) was higher in the P<1.0 ng/ml group than the P≥1.0 ng/ml group (44.9% vs. 37.6%, P=0.02). The early spontaneous abortion rate was comparable between the groups (14.4% vs. 14.7%, P=0.93). For live birth, the rate for the P<1.0 ng/ml group was 35.3%, which was significantly higher than the 29.0% in the P≥1.0 ng/ml group (P=0.03). After binary logistic regression analysis, the P level on the hCG trigger day (adjusted odds ratio=0.74, 95% CI=0.55-0.99, P=0.04) was an independent risk factor for LBR. For the P level on the hCG trigger day, the LBR was lower in the P≥1.0 ng/ml group compared to the P<1.0 ng/ml group.
Conclusion: For normal ovarian response patients using the GnRH antagonist protocol, serum P≥1.0 ng/ml on the hCG trigger day resulted in a lower LBR than the P<1.0 ng/ml group. When P≥1.0 ng/ml, whole embryo freezing may be considered.
Introduction
Ovarian stimulation (OS) is a critical step for intracytoplasmic sperm injection (ICSI)/in vitro fertilization (IVF) (1). The rationale for OS is to achieve more follicle development using exogenous follicle-stimulating hormone (FSH), which stimulates the growth of multiple follicles in a single cycle (2, 3). However, the increase in estrogen (E2) caused by the development of multiple follicles increases luteinizing hormone (LH) levels before the follicles mature, which leads to earlier ovulation. Therefore, the key to OS is to prevent premature luteinization in advance. The most commonly used controls for endogenous LH peaks are gonadotropin-releasing hormone (GnRH) analogs, including GnRH agonists and GnRH antagonists (4, 5). A GnRH agonist protocol has been used in assisted reproductive technology since 1984 (6), and it is one of the most widely used OS protocols. GnRH agonists effectively inhibit the LH level and the occurrence of an early-onset LH surge, which improve the uniformity of follicle development. However, prolonged stimulation increases the gonadotropin (Gn) dose, which increases the risk of ovarian hyperstimulation syndrome (OHSS). GnRH antagonist protocols have been gradually used in the clinic since 2001. These protocols use a relatively short duration of stimulation with a lower Gn dose, which reduces the risk of OHSS (7, 8). Controversy exists in the use of GnRH agonists and GnRH antagonists (4, 9, 10). Based on the safety of OS, the GnRH antagonist protocol is more recommended for normal or high ovarian responders (1). However, the GnRH agonist protocol was more advantageous based on the live birth rate (LBR) of fresh embryo transfer, but there was no difference in the cumulative LBR between the two protocols (11).
Optimization of the LBR of fresh embryo transfer with GnRH antagonist protocols is the focus of much research. Serum progesterone (P) level is an important indicator in the pregnancy rate of the fresh cycle, and elevated P levels on the day of human chorionic gonadotropin (hCG) administration negatively influence clinical outcomes (12–14). Many studies examined the effect of serum P on clinical outcomes by measuring serum P levels on the day of the hCG trigger. The main reason for the controversy is that the threshold value of the serum P level is different between studies and ranges from 0.8 to 2.0 ng/ml, and there are differences in the determination methods (12, 15–17). The mechanisms of elevated P primarily include increased doses of gonadotropins (Gn), higher FSH levels, higher oocyte retrieval numbers and higher E2 levels on the trigger day (18). Therefore, the effect of elevated P levels on clinical outcomes may vary in different ovarian responders. Due to differences in populations, races, protocols, etc., the currently reported elevated P values are not uniform (13, 19, 20).
People with a normal ovarian response have a low risk of OHSS and a relatively stable number of oocytes retrieved and available embryos are the main population for fresh embryo transfer. Therefore, the present study analyzed the effect of P levels on hCG trigger day on LBR in a population with a normal ovarian response in a GnRH antagonist protocol.
Methods
This study was a single-center, retrospective, observational, cohort study. This study was performed in the Reproductive Center of the Third Affiliated Hospital of Zhengzhou University. Ethical approval was obtained from the Ethics Committee of Third Affiliated Hospital of Zhengzhou University. IVF/ICSI cycles performed from January 2017 to December 2020 were included in the analysis.
Population
A total of 1229 cycles of the GnRH antagonist protocol were included in the study analysis, all of which underwent the first IVF/ICSI cycle with fresh embryo transfer. This study included people with a normal ovarian response (age: 20-40 years old, baseline FSH<10 IU/L, anti-Mullerian hormone (AMH)≥1.1 ng/ml, antral follicle count (AFC)≥6). Patients with polycystic ovary syndrome were excluded from the analysis. Women with a history of uterine malformation (e.g., bicornuate uterus, unicornuate uterus or septate uterus), hydrosalpinx, history of ovarian surgery, adenomyosis or intrauterine adhesion were excluded from the analysis. Patients with recurrent spontaneous abortion were also excluded. All of the couples were screened via karyotyping, and couples with an abnormal karyotype were excluded.
GnRH antagonist protocol and IVF/ICSI-embryo transfer
A routine flexible GnRH antagonist protocol was performed in our reproductive center as described in previous studies (21). OS was initiated on the second or third day of the menstrual cycle, and the appropriate Gn starting dose (100-300 IU) was chosen based on maternal age, weight, body mass index (BMI) and AMH. Vaginal ultrasonography was performed and serum LH and E2 levels were determined 3-5 days later. A GnRH antagonist (0.25 mg/day) was added once the diameter of the dominant follicle reached 12-14 mm and was continued up to the trigger day. The GnRH antagonist was injected at approximately 5 pm each day. If the LH peak occurred during the process of ovarian stimulation, the GnRH antagonist was also injected in time. When there were 3 follicles > 17 mm or 2 follicles > 18 mm, and patients were undergoing fresh embryo transfer, 250 μg recombinant hCG was applied for follicle maturation. Oocyte retrieval was performed 36 hours later. Based on sperm quality, conventional IVF or ICSI was performed, as appropriate. Luteal support was started on the day of oocyte retrieval using oral dydrogesterone (DYG; 10 mg, 2 times daily) (Abbott Co. America). Intravaginal progesterone sustained-release vaginal gel (90 mg, Merck Co. Germany) was given. One or two cleavage stage embryos were transferred 3 days after oocyte retrieval, or 1 blastocyst was transferred 5 days after oocyte retrieval. If pregnancy occurred, corpus luteum support was continued for at least 55 days after embryo transfer.
Serum hormone level measurement and grouping
Serum hormone levels, including FSH, LH, E2 and P, were analyzed using the Roche Cobas immunoassay (Roche Diagnostics, Germany). The preparation, setup, dilution, adjustment, assay and quality control procedures were performed according to the manufacturer’s instructions, and the intra-assay and inter-assay coefficients of variation were less than 10%. On the hCG trigger day, whole blood was collected between 7:00 and 9 a.m. We routinely measured serum LH, E2 and P levels. Fresh embryo transfer was cancelled when the serum P level was >2 ng/ml, and whole embryo freezing was performed. We divided all cycles into 2 groups by the P level on the day of hCG trigger, P<1.0 ng/ml and P≥1.0 ng/ml. This grouping was primarily based on data distribution characteristics and reference to current related research (13, 22, 23).
Outcome measures and definition
The primary outcome measure was LBR after fresh embryo transfer. Live birth was defined as any viable neonate ≥ 28 gestational weeks. The secondary outcome measure was clinical pregnancy rate (CPR), which was defined as a pregnancy diagnosed via ultrasonographic visualization of one or more gestational sacs and included intrauterine pregnancy and a clinically documented ectopic pregnancy (24). Early spontaneous abortion was defined as a loss of clinical pregnancy before 12 gestational weeks and was included as an outcome measure of this study.
Statistical analysis
All data were obtained from retrospective review of our reproductive center’s medical records. All statistical management and analyses were performed using SPSS software, version 22.0.
For continuous variables, the one-sample Kolmogorov–Smirnov test was performed to check for normality. Continuous variables with abnormal distributions are expressed as medians (P25, P75), and the Wilcoxon rank sum test was used to assess between-group differences. Categorical variables are represented as the number of cases (n) and percentage (%). The between-group differences were assessed using chi-squared analyses with Fisher’s exact test when necessary. Binary logistic regression was performed to adjust for potential confounding factors for the main outcome, LBR. Adjusted odds ratios (AORs) with 95% confidence intervals (CIs) were calculated. Statistical significance was set at p value < 0.05.
Results
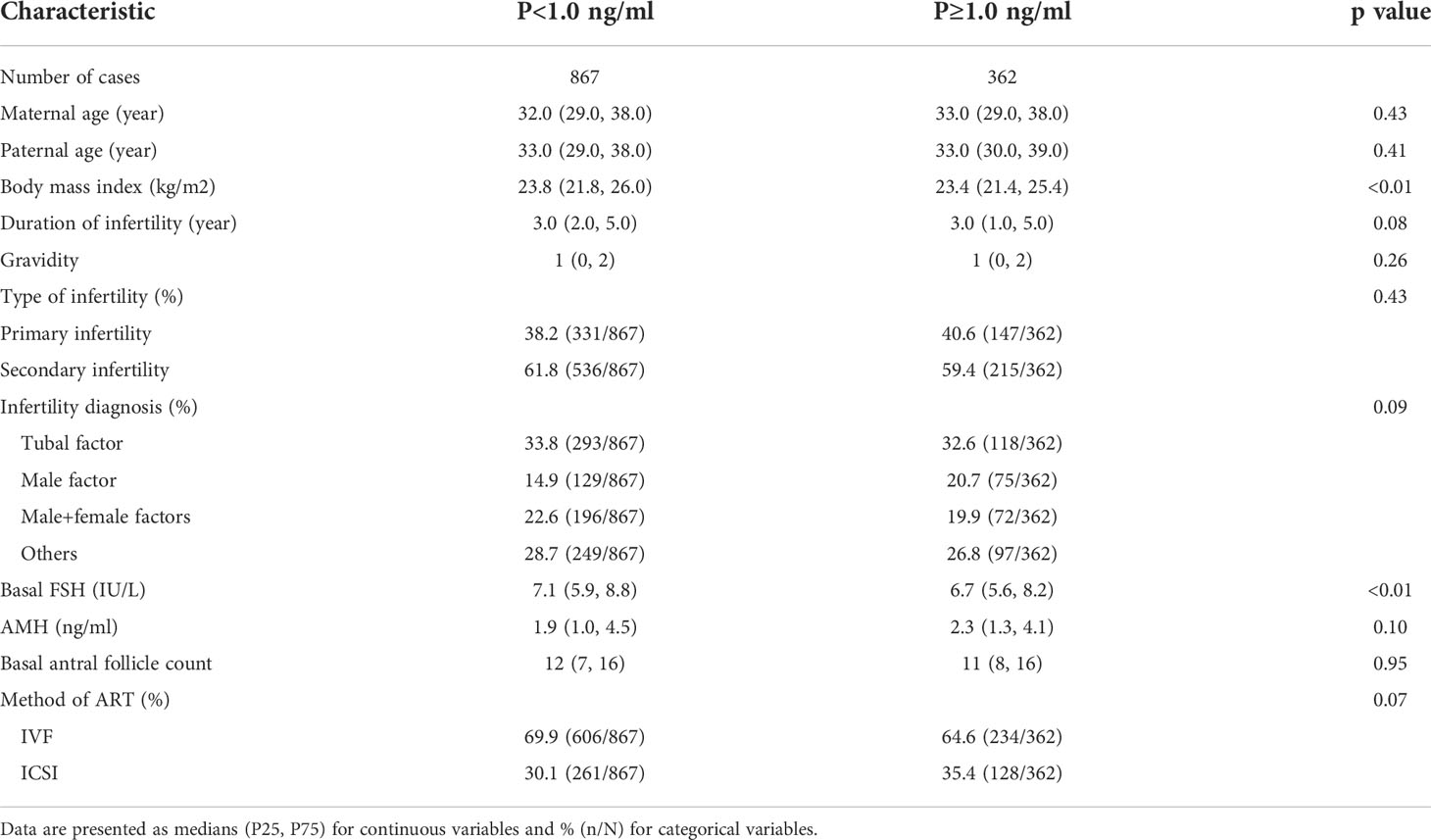
Study population
A total of 867 cycles with P<1.0 ng/ml and 362 cycles with P≥1.0 ng/ml were included for analysis. There were no statistically significant differences in maternal age, paternal age, duration of infertility, gravidity, type of infertility, infertility diagnosis, AMH, basal AFC or method of assisted reproductive technology (ART) (all p>0.05) between groups. The BMI in the P<1.0 ng/ml group was 23.8 (21.8, 26.0), which was significantly different than the P≥1.0 ng/ml group at 23.4 (21.4, 25.4) (p<0.01). Basal FSH was higher in the P<1.0 ng/ml group than the P≥1.0 ng/ml group (p<0.01). The detailed characteristics of the participants at baseline between the two groups are described in Table 1.
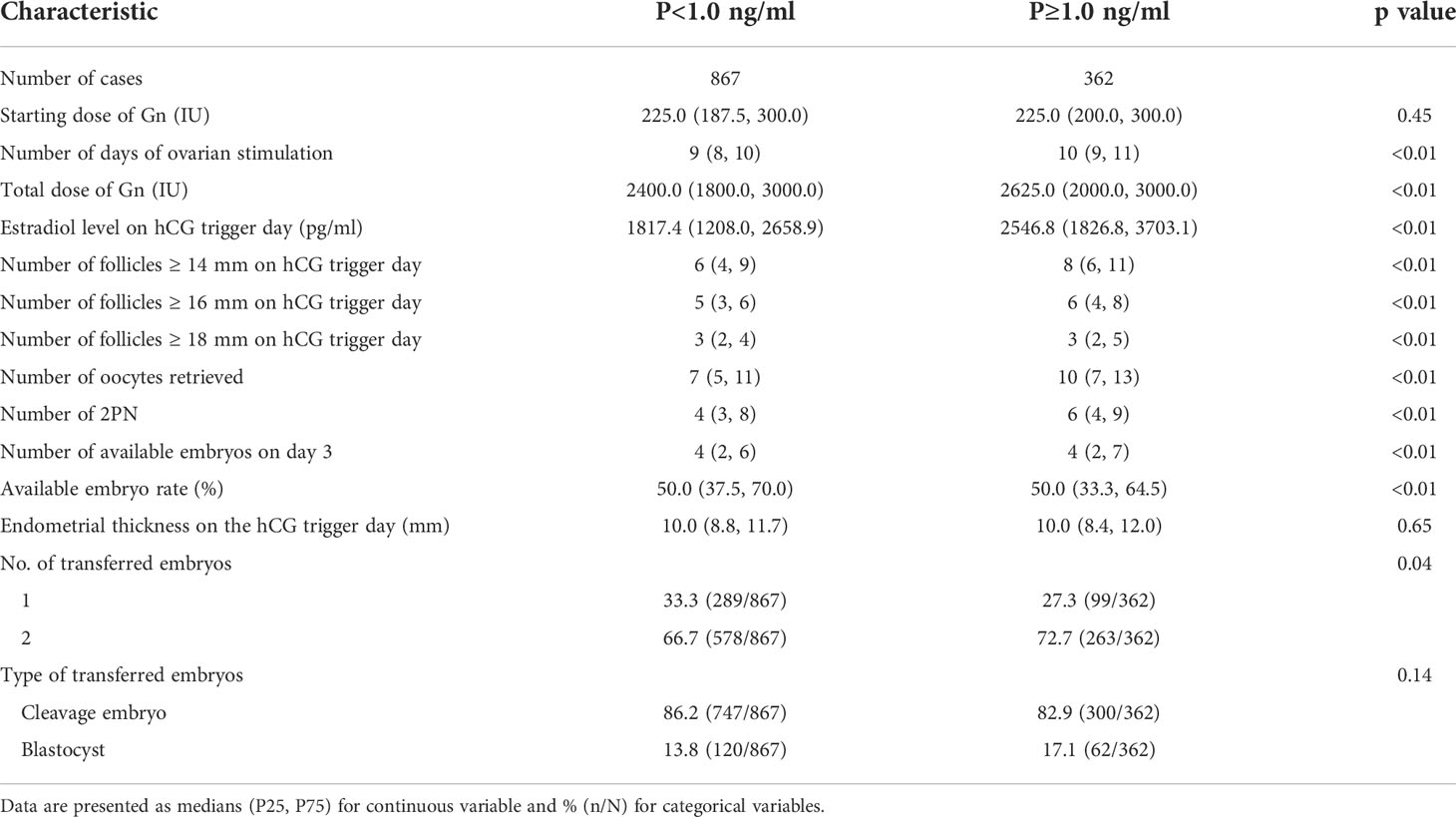
Characteristics of controlled ovarian hyperstimulation cycles
The starting dose of Gn, endometrial thickness on the hCG trigger day and type of transferred embryos (cleavage embryo/blastocyst) were comparable between the two groups (all p>0.05). There were statistically significant differences in the number of days of ovarian stimulation, total dose of Gn, estradiol level on hCG trigger day, number of follicles ≥14 mm, 16 mm and 18 mm on hCG trigger day, oocytes retrieved, two distinct pronuclei (2PN), available embryos on day 3 and available embryo rate between the groups. The number of transferred embryos was higher in the P<1.0 ng/ml group than the P≥1.0 ng/ml group (p=0.04). The detailed characteristics of the cycles between the two groups are described in Table 2.
Clinical outcomes
The CPR was higher in the P<1.0 ng/ml group than the P≥1.0 ng/ml group (44.9% vs. 37.6%, p=0.02). The early spontaneous abortion rate was comparable between the groups (14.4% vs. 14.7%, p=0.93). The LBR of the P<1.0 ng/ml group was 35.3%, which was significantly higher than the 29.0% in the P≥1.0 ng/ml group (p=0.03). A binary logistic regression model was performed to adjust the influence of confounding factors, including maternal age (continuous variable), maternal BMI (continuous variable), type of infertility (primary/secondary), infertility diagnosis (tubal/male/male+female/others), basal AFC (continuous variable), basal FSH (continuous variable), method of ART (IVF/ICSI), total dose of Gn, endometrial thickness on the hCG trigger day (continuous variable), number of oocytes retrieved (continuous variable), number of transferred embryos (1/2), type of transferred embryos (cleavage embryo/blastocyst) and P level on hCG trigger day (<1.0 ng/ml/≥1.0 ng/ml). Binary logistic regression analysis revealed that maternal age (AOR=0.91, 95% CI=0.88-0.93, p<0.01), endometrial thickness on the hCG trigger day (AOR=1.13, 95% CI=1.06-1.20, p<0.01), P level on hCG trigger day (AOR=0.74, 95% CI=0.55-0.99, p=0.04), number of transferred embryos (AOR=2.01, 95% CI=1.37-2.95, p<0.01) and type of transferred embryos (AOR=2.20, 95% CI=1.28-3.79, p<0.01) were independent risk factors for LBR. For the P level on the hCG trigger day, the LBR was lower in the P≥1.0 ng/ml group compared to the P<1 ng/ml group. The specific data are described in Tables 3, 4.
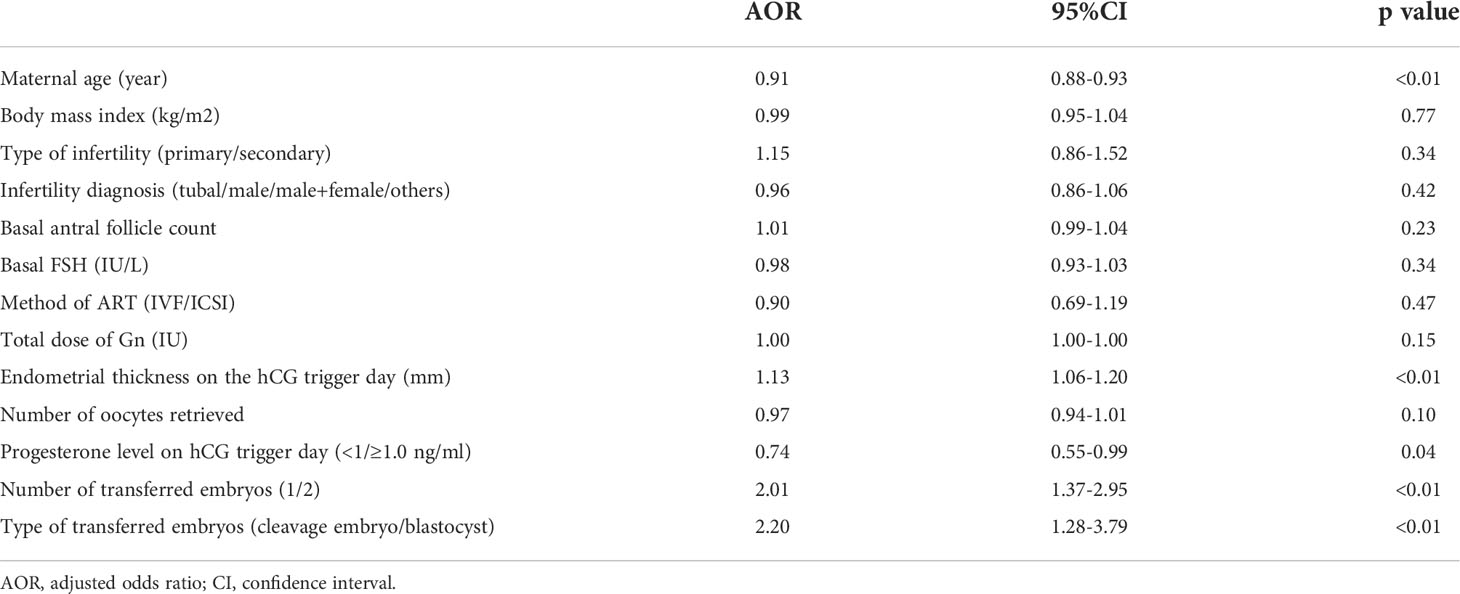
Table 4 Binary logistic regression analysis to account for confounding variables of live birth rate.
Discussion
Our single-center, retrospective cohort study involving normal ovarian response patients with the GnRH antagonist protocol found that the LBR for patients with P≥1.0 ng/ml was lower than patients with P<1.0 ng/ml. The risk of early spontaneous abortion did not differ significantly between the two groups.
There are many studies on the effects of P level on pregnancy. The synergistic effect of P and E2 is a necessary factor for embryo implantation in the natural state. Under the physiological state, when the dominant follicle is close to maturity and before ovulation, the follicle slightly increases the secretion of P to coordinate the positive feedback effect of E2 and induce the appearance of the peak of FSH and LH during ovulation. A large amount of P is secreted after ovulation (25). The increase in P level completely changes the endometrial state and endometrial receptivity (26). With the application of GnRH agonists and antagonists in OS cycles, the occurrence of endogenous LH peaks may be effectively prevented, but some patients still exhibit elevated serum P levels in the late follicular development stage (27). Schoolcraft et al. (28) first reported the phenomenon of elevated serum P levels on the day of hCG injection in some populations during the IVF treatment cycle with GnRH agonist protocols.
Subsequent reports of elevated P gradually appeared, with the overall incidence ranging from 5% to 38%. The incidence in GnRH agonist protocols ranged from 5% to 35%, and the incidence in GnRH antagonist protocols ranged from 9% to 38% (29, 30). Due to differences in study populations, laboratory testing methods, and groupings, the relationship between elevated serum P levels and IVF pregnancy outcomes remains controversial, and there are differences in the definition of elevated P. A large retrospective cohort study by Xu et al. (12) included populations with different ovarian responses and defined different serum P levels on the hCG trigger day. The defined values of serum P in patients with low ovarian response, normal response and high response were 1.5 ng/ml, 1.75 ng/ml and 2.25 ng/ml, respectively. Bosch et al. (31) and Van Vaerenbergh et al. (32) set the limit of the hCG daily P level to 1.5 ng/ml, which is widely used in clinical practice. However, studies show that when P>1 ng/ml, the CPR or LBR decreases (13, 19, 23). Only people with normal ovarian response with GnRH antagonist protocols were included for analysis in our study, and P>1 ng/ml affected the LBR of fresh embryo transfer. Fresh embryo transfer should be canceled, and whole embryo freezing should be performed when P is high.
Although many clinical studies showed that elevated serum P levels had a negative impact on CPR and LBR, the specific endocrine mechanisms are not clear. Major mechanistic studies focused on the effects of elevated serum P on endometrial receptivity and oocyte and embryo quality. Elevated serum P levels reduced CPR in fresh embryo transfer cycles but did not affect clinical outcomes in frozen-thawed embryo transfer cycles (33). Chen et al. (34) showed that high serum P does not affect embryo quality, and most studies believe that elevated serum P levels had no effect on oocyte quality, fertilization rate, or embryo quality. Santos-Ribeiro et al. (35) reported that IVF fertilization rates were similar between different P levels (≤0.50 ng/ml, 0.5–1.5 ng/ml and >1.5 ng/ml), which confirmed that serum P levels did not affect IVF fertilization rates. A recent study using the 90th percentile of the distribution of serum P levels as a basis for grouping also showed that P levels had no negative effects on oocyte or embryo quality (36). Embryo implantation theory suggests a specific implantation window for embryo implantation. When the endometrium and embryonic development are out of sync for more than 3 days, the pregnancy rate is extremely low (37). Premature elevation of serum P levels affects endometrial receptivity by altering the expression of endometrial-specific genes and promoting endometrial transition from early secretory to late secretory (38). The increase in serum P levels has a specific effect on the gene expression profile of the endometrium (32). Different serum P levels induce different gene expression in the endometrium, and the expression of specific genes is related to embryo adhesion, the implantation process, and the immune system, which affect CPR in fresh cycles (39).
According to the previous data of the center and other studies, fresh embryo transfer was cancelled when the serum P level on hCG day >2 ng/ml, and whole embryo freezing was performed in our reproductive center. However, current data at our center show that the LBR of fresh embryo transfer with GnRH antagonist remains lower than a GnRH agonist. Therefore, the present study investigated whether slightly elevated P levels also affected LBR. First, this study only included GnRH antagonist regimens with normal ovarian response to minimize the influence of confounding factors. Second, the observational endpoint of this study was the LBR, which is more clinically valuable than comparisons of only the CPR. However, the current study is limited by its retrospective cohort nature. Second, this study did not further analyze the impact of the quality of different embryos or blastocysts transferred on clinical outcomes, and there may be confounding factors. A retrospective cohort study revealed that a slight increase in P levels (0.85 ng/mL) affected the CPR of cleavage-stage embryo transfers, but it did not affect clinical outcomes after blastocyst transfer (40). Therefore, for patients with mildly elevated P, whether blastocyst transfer improves clinical outcomes is a direction of further research. The current study only included people with normal ovarian response, and further research is needed in populations with high and low ovarian responses.
Conclusion
For normal ovarian response patients with the GnRH antagonist protocol, serum P≥1.0 ng/ml on the hCG trigger day resulted in a lower LBR compared to P<1.0 ng/ml. When serum P≥1.0, whole embryo freezing may be considered, followed by frozen-thawed embryo transfer.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Ethics statement
This study was approved by the Ethics Committee of the Third Affiliated Hospital of Zhengzhou University. Study reference number: 2022-198-01. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Author contributions
JZ, MD and YG designed the study and selected the population to be included and excluded. YW and ZW were involved in the data extraction and analyses. MD reviewed the data. JZ was involved in drafting this article. All authors approved the final version of the manuscript. JZ and MD contributed equally to this article. All authors contributed to the article and approved the submitted version.
Funding
The study was supported by 2021 Henan Province Medical Science and Technology Research and Joint Construction Project (LHGJ20210441, LHGJ20210451).
Acknowledgments
We thank the patients who participated in the study and cooperated with the follow-up. We thank the medical staff at our reproductive center who participated in data entry and follow-up. We also thank American Journal Experts for their professional manuscript editing services.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. T.E.G.G.O.O. Stimulation, Bosch E, Broer S, Griesinger G, Broekmans F. ESHRE guideline: ovarian stimulation for IVF/ICSI. Hum Reprod Open (2020) 2020(2) hoaa009. doi: 10.1093/hropen/hoaa009
2. Alper MM, Fauser BC. Ovarian stimulation protocols for IVF: is more better than less? Reprod Biomed Online (2017) 34:345–53. doi: 10.1016/j.rbmo.2017.01.010
3. Ata B, Capuzzo M, Turkgeldi E, Yildiz S, La Marca A. Progestins for pituitary suppression during ovarian stimulation for ART: A comprehensive and systematic review including meta-analyses. Hum Reprod Update (2021) 27:48–66. doi: 10.1093/humupd/dmaa040
4. Lambalk CB, Banga FR, Huirne JA, Toftager M, Pinborg A, Homburg R, et al. GnRH antagonist versus long agonist protocols in IVF: A systematic review and meta-analysis accounting for patient type. Hum Reprod Update (2017) 23:560–79. doi: 10.1093/humupd/dmx017
5. Check ML, Check JH, Choel JK, Davies E, Kiefer D. Effect of antagonists vs agonists on in vitro fertilization outcome. Clin Exp Obstet Gynecol (2004) 31:257–9.
6. Porter RN, Smith W, Craft IL, Abdulwahid NA, Jacobs HS. Induction of ovulation for in-vitro fertilisation using buserelin and gonadotropins. Lancet (1984) 324:1284–5. doi: 10.1016/S0140-6736(84)92840-X
7. Xing W, Lin H, Li Y, Yang D, Wang W, Zhang Q. Is the GnRH antagonist protocol effective at preventing OHSS for potentially high responders undergoing IVF/ICSI? PloS One (2015) 10:e0140286. doi: 10.1371/journal.pone.0140286
8. Lainas TG, Sfontouris IA, Zorzovilis IZ, Petsas GK, Lainas GT, Alexopoulou E, et al. Flexible GnRH antagonist protocol versus GnRH agonist long protocol in patients with polycystic ovary syndrome treated for IVF: A prospective randomised controlled trial (RCT). Hum Reprod (Oxf Engl) (2010) 25:683–9. doi: 10.1093/humrep/dep436
9. Al-Inany HG, Youssef MA, Ayeleke RO, Brown J, Lam WS, Broekmans FJ. Gonadotrophin-releasing hormone antagonists for assisted reproductive technology. Cochrane Database System Rev (2016) 4:Cd001750. doi: 10.1002/14651858.CD001750.pub4
10. Siristatidis CS, Gibreel A, Basios G, Maheshwari A, Bhattacharya S. Gonadotrophin-releasing hormone agonist protocols for pituitary suppression in assisted reproduction. Cochrane Database System Rev (2015) 11:Cd006919. doi: 10.1002/14651858.CD006919.pub4
11. Xu B, Geerts D, Hu S, Yue J, Li Z, Zhu G, et al. The depot GnRH agonist protocol improves the live birth rate per fresh embryo transfer cycle, but not the cumulative live birth rate in normal responders: a randomized controlled trial and molecular mechanism study. Hum Reprod (2020) 35:1306–18. doi: 10.1093/humrep/deaa086
12. Xu B, Li Z, Zhang H, Jin L, Li Y, Ai J, et al. Serum progesterone level effects on the outcome of in vitro fertilization in patients with different ovarian response: An analysis of more than 10,000 cycles. Fertil Steril (2012) 97:1321–7. doi: 10.1016/j.fertnstert.2012.03.014
13. Zhao J, Hao J, Xu B, Wang Y, Li Y. Effect of slightly elevated progesterone on hCG trigger day on clinical pregnancy rate in GnRH-ant IVF/ICSI cycles. Reprod Health (2022) 19(1):66. doi: 10.1186/s12978-022-01371-4
14. Wu Z, Li R, Ma Y, Deng B, Zhang X, Meng Y, et al. Effect of HCG-day serum progesterone and oestradiol concentrations on pregnancy outcomes in GnRH agonist cycles. Reprod Biomed Online (2012) 24:511–20. doi: 10.1016/j.rbmo.2012.02.003
15. Hill MJ, Royster GD, Healy MW, Richter KS, Levy G, Decherney AH, et al. Are good patient and embryo characteristics protective against the negative effect of elevated progesterone level on the day of oocyte maturation? Fertil Steril (2015) 103:1477–1484.e5. doi: 10.1016/j.fertnstert.2015.02.038
16. Singh N, Malik N, Malhotra N, Vanamail P, Gupta M. Impact of progesterone (on hCG day)/oocyte ratio on pregnancy outcome in long agonist non donor fresh IVF/ICSI cycles. Taiwanese J Obstet Gynecol (2016) 55:503–6. doi: 10.1016/j.tjog.2015.09.005
17. Griesinger G, Mannaerts B, Andersen CY, Witjes H, Kolibianakis EM, Gordon K. Progesterone elevation does not compromise pregnancy rates in high responders: A pooled analysis of in vitro fertilization patients treated with recombinant follicle-stimulating hormone/gonadotropin-releasing hormone antagonist in six trials. Fertil Steril (2013) 100:1622–8.e3. doi: 10.1016/j.fertnstert.2013.08.045
18. Oktem O, Akin N, Bildik G, Yakin K, Alper E, Balaban B, et al. FSH stimulation promotes progesterone synthesis and output from human granulosa cells without luteinization. Hum Reprod (2017) 32:643–52. doi: 10.1093/humrep/dex010
19. Mahran A, Khairy M, Elkhateeb R, Hegazy AR, Abdelmeged A, Batiha GE, et al. The value of serum progesterone level on day of human chorionic gonadotrophin administration / metaphase II oocyte ratio in predicting IVF/ICSI outcome in patients with normal ovarian reserve. J Ovarian Res (2021) 14:52. doi: 10.1186/s13048-021-00800-5
20. Golbasi H, Ince O, Golbasi C, Ozer M, Demir M, Yilmaz B. Effect of progesterone/estradiol ratio on pregnancy outcome of patients with high trigger-day progesterone levels undergoing gonadotropin-releasing hormone antagonist intracytoplasmic sperm injection cycles: A retrospective cohort study. J Obstet Gynaecol J Institute Obstet Gynaecol (2019) 39:157–63. doi: 10.1080/01443615.2018.1504204
21. Du M, Zhang J, Li Z, Liu X, Li J, Liu W, et al. Comparison of the cumulative live birth rates of progestin-primed ovarian stimulation and flexible GnRH antagonist protocols in patients with low prognosis. Front Endocrinol (2021) 12:705264. doi: 10.3389/fendo.2021.705264
22. Ozturk M, Fidan U, Ceyhan T, Ozturk O, Korkmaz C. Double daily doses of cetrorelix may raise follicular phase progesterone more compared to single doses in poor ovarian response patients. J Gynecol Obstet Hum Reproduct (2021) 50(10): 102223. doi: 10.1016/j.jogoh.2021.102223
23. Hu L, Xiong Y. Effect of progesterone on hCG day-to-basal progesterone ratio on live birth rate in long agonist fresh IVF/ICSI cycles: A 5-year, single-center study of more than 10,000 cycles. Gynecol Endocrinol (2021) 37:706–10. doi: 10.1080/09513590.2020.1832067
24. Zegers-Hochschild F, Adamson GD, Dyer S, Racowsky C, de Mouzon J, Sokol R, et al. The international glossary on infertility and fertility care, 2017. Fertil Steril (2017) 108:393–406. doi: 10.1016/j.fertnstert.2017.06.005
25. Baerwald AR, Adams GP, Pierson RA. Ovarian antral folliculogenesis during the human menstrual cycle: A review. Hum Reprod Update (2012) 18:73–91. doi: 10.1093/humupd/dmr039
26. Hall JE. Endocrinology of the menopause. Endocrinol Metab Clinics North Am (2015) 44:485–96. doi: 10.1016/j.ecl.2015.05.010
27. Singh N, Kaur SD, Malik N, Malhotra N, Vanamail P. Do increased levels of progesterone and progesterone/estradiol ratio on the day of human chorionic gonadotropin affects pregnancy outcome in long agonist protocol in fresh in vitro fertilization/intracytoplasmic sperm injection cycles? J Hum Reprod Sci (2015) 8:80–5. doi: 10.4103/0974-1208.158606
28. Schoolcraft W, Sinton E, Schlenker T, Huynh D, Hamilton F, Meldrum DR. Lower pregnancy rate with premature luteinization during pituitary suppression with leuprolide acetate. Fertil Steril (1991) 55:563–6. doi: 10.1016/S0015-0282(16)54186-7
29. Ochsenkühn R, Arzberger A, von Schönfeldt V, Gallwas J, Rogenhofer N, Crispin A, et al. Subtle progesterone rise on the day of human chorionic gonadotropin administration is associated with lower live birth rates in women undergoing assisted reproductive technology: a retrospective study with 2,555 fresh embryo transfers. Fertil Steril (2012) 98:347–54. doi: 10.1016/j.fertnstert.2012.04.041
30. Venetis CA, Kolibianakis EM, Papanikolaou E, Bontis J, Devroey P, Tarlatzis BC. Is progesterone elevation on the day of human chorionic gonadotrophin administration associated with the probability of pregnancy in in vitro fertilization? A System Rev Meta Analysis Hum Reprod Update (2007) 13:343–55. doi: 10.1093/humupd/dmm007
31. Bosch E, Labarta E, Crespo J, Simón C, Remohí J, Jenkins J, et al. Circulating progesterone levels and ongoing pregnancy rates in controlled ovarian stimulation cycles for in vitro fertilization: Analysis of over 4000 cycles. Hum Reprod (2010) 25:2092–100. doi: 10.1093/humrep/deq125
32. Vaerenbergh IV, Fatemi HM, Blockeel C, Lommel LV, I.t. Veld P, Schuit F, et al. Progesterone rise on HCG day in GnRH antagonist/rFSH stimulated cycles affects endometrial gene expression. Reprod Biomed Online (2011) 22:263–71. doi: 10.1016/j.rbmo.2010.11.002
33. Venetis CA, Kolibianakis EM, Bosdou JK, Lainas GT, Sfontouris IA, Tarlatzis BC, et al. Estimating the net effect of progesterone elevation on the day of hCG on live birth rates after IVF: a cohort analysis of 3296 IVF cycles. Hum Reprod (2015) 30:684. doi: 10.1093/humrep/deu362
34. Chen Y, Ma L, Wang S. The impact of an increased progesterone-to-follicle number ratio on live delivery rates in women with normal ovarian reserve. Int J Gynecol Obstet (2017) 139:84–9. doi: 10.1002/ijgo.12256
35. Santos-Ribeiro S, Polyzos NP, Haentjens P, Smitz J, Camus M, Tournaye H, et al. Live birth rates after IVF are reduced by both low and high progesterone levels on the day of human chorionic gonadotrophin administration. Hum Reprod (2014) 29:1698–705. doi: 10.1093/humrep/deu151
36. Roque Fernandez MA, Alvarez Lleo C, Gonzalez Mirasol E, Resta Serra M, Garcia Garrido C, Sanchez Toledo M, et al. Progesterone elevation on the day of oocyte retrieval and live birth rate after in vitro fertilisation treatment. J Obstet Gynaecol J Institute Obstet Gynaecol (2022) 42:1396–400. doi: 10.1080/01443615.2021.1983780
37. Kolibianakis EM, Carola A, Michel C, Herman T, André CVS, Paul D. Prolongation of the follicular phase in in vitro fertilization results in a lower ongoing pregnancy rate in cycles stimulated with recombinant follicle-stimulating hormone and gonadotropin-releasing hormone antagonists. Dkgest World Latest Med Inf (2004) 82:102–7. doi: 10.1016/j.fertnstert.2004.01.027
38. Haouzi D, Bissonnette L, Gala A, Assou S, Entezami F, Perrochia H, et al. Endometrial receptivity profile in patients with premature progesterone elevation on the day of hCG administration. BioMed Res Int (2014) 2014:951937. doi: 10.1155/2014/951937
39. Labarta E, Martínez-Conejero J, Alamá P, Horcajadas JA, Pellicer A, Simón C, et al. Endometrial receptivity is affected in women with high circulating progesterone levels at the end of the follicular phase: A functional genomics analysis. Obstet Gynecol Survey (2011) 66:1813–25. doi: 10.1097/OGX.0b013e3182402567
40. Tokgoz VY, Tekin AB. Serum progesterone level above 0.85 ng/mL and progesterone/estradiol ratio may be useful predictors for replacing cleavage-stage with blastocyst-stage embryo transfer in fresh IVF/ICSI cycles without premature progesterone elevation. Arch Gynecol Obstet (2022) 305:1011–9. doi: 10.1007/s00404-021-06304-3
Keywords: progesterone, GnRH antagonist, live birth rate, clinical pregnancy rate, in vitro fertilization
Citation: Zhang J, Du M, Wu Y, Wei Z and Guan Y (2022) Effect of serum progesterone levels on hCG trigger day on pregnancy outcomes in GnRH antagonist cycles. Front. Endocrinol. 13:982830. doi: 10.3389/fendo.2022.982830
Received: 26 July 2022; Accepted: 09 September 2022;
Published: 28 September 2022.
Edited by:
Bianca Bianco, Faculdade de Medicina do ABC, BrazilReviewed by:
Renato De Oliveira, Faculdade de Medicina do ABC, BrazilYavuz Tokgöz, Eskişehir Osmangazi University, Turkey
Copyright © 2022 Zhang, Du, Wu, Wei and Guan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yichun Guan, bGlzYW1heWd1YW5AMTYzLmNvbQ==
†These authors have contributed equally to this work and share first authorship
 Junwei Zhang†
Junwei Zhang† Mingze Du
Mingze Du Yichun Guan
Yichun Guan