- 1Human Cancer Genomic Research, Research Center, King Faisal Specialist Hospital and Research Centre, Riyadh, Saudi Arabia
- 2Department of Surgery, King Faisal Specialist Hospital and Research Centre, Riyadh, Saudi Arabia
- 3Department of Pathology, King Faisal Specialist Hospital and Research Centre, Riyadh, Saudi Arabia
Background: The incidence of pediatric differentiated thyroid carcinoma (DTC) is increasing. Despite the advanced disease at presentation, the overall prognosis of DTC in children is excellent. The aim of this study is to investigate the risk stratifying factors for event free survival (EFS) of pediatric DTC from Middle Eastern ethnicity.
Methods: Eighty-eight patients aged ≤18 years with diagnosis of primary DTC were retrospectively analyzed. Cox proportional hazards model were used to calculate Hazard Ratios (HR) and Kaplan–Meier analysis were conducted to investigate EFS.
Results: Eighty-eight (23 males and 65 females) pediatric DTCs who underwent surgery and radioactive iodine therapy had been reported (median age at diagnosis 15 years; range 5.9-17.9), with lymph node metastasis (LNM) noted in 70.5% and distant metastasis in 13.6%. Mean follow-up was 8.4 years. Ten-year overall survival rate was 98.4% while 10-year EFS was 79.2%. EFS was negatively impacted by the presence of LNM, distant metastasis and tumor size >4cm. American Thyroid Association risk stratification did not impact EFS in our cohort. Multivariate analysis revealed tumor size >4cm (HR = 5.34; 95% confidence interval (CI) = 1.36 – 20.22; p = 0.0177) and distant metastasis (HR = 8.73; 95% CI = 1.48 – 60.05; p = 0.0154) as independent negative prognostic factors for EFS.
Conclusions: Primary tumor size and the presence of distant metastasis at diagnosis are the only independent prognostic risk factors for EFS in pediatric DTC in Middle Eastern ethnicity. Children with tumor size over 4cm had poor EFS, which may justify the need of more aggressive treatment and frequent follow-up.
1 Introduction
Differentiated thyroid carcinomas (DTC) are the most common endocrine malignancy, and account for more than 4% of all pediatric cancers (1). Papillary Thyroid Carcinoma (PTC) accounts for approximately 90% of pediatric DTC, whereas follicular thyroid carcinoma (FTC) accounts for about 10% (2, 3). The incidence of pediatric DTC has been increasing over the decades (4–6). Pediatric DTC differ clinically from adult DTC. At diagnosis children usually present with more advanced disease, with larger tumor size, frequent extrathyroidal extension (ETE), and high rate of lymph node metastasis and distant metastasis (7–9). Molecular differences between pediatric and adult DTC have also been established. BRAF mutation is not as common a driver mutation as in adult DTC and RAS gene mutations are rare (7, 10, 11). These molecular differences could contribute to the difference in clinical behavior between adult and pediatric DTC.
Despite the aggressive behavior, the prognosis of pediatric DTC is excellent (12, 13). The management of DTC in children can be challenging, given the excellent prognosis and the extremely low disease specific mortality, regardless of the presence of metastasis. Knowledge regarding prognostic factors for survival in pediatric patients with DTC, can be helpful for individualized therapy and follow-up. In 2015, the American Thyroid Association (ATA) published management guidelines for children with DTC, where these guidelines classify patient into low, intermediate and high risk categories (14). Recent studies have illustrated that ATA risk group is a prognostic factor for event-free survival (EFS) in pediatric DTC patients (8, 15, 16).
The ultimate aim of this retrospective study was to determine the prognostic factors for EFS in pediatric DTC from Middle Eastern ethnicity and whether ATA risk classification is a predictor of recurrence in pediatric DTC.
2 Materials and methods
2.1 Patient selection
Eighty-eight pediatric (≤ 18 years) DTC patients diagnosed between 1988 and 2018 at King Faisal Specialist Hospital and Research Centre (Riyadh, Saudi Arabia) who underwent surgery and radioactive iodine (RAI) therapy were included in the study. Cases were identified based on clinical history followed by fine needle aspiration biopsy for confirmation. The Institutional Review Board of the hospital approved this study and since only retrospective patient data were used, the Research Advisory Council (RAC) provided waiver of consent under project RAC # 221 1168 and # 2110 031. The study was conducted in accordance with Declaration of Helsinki.
2.2 Clinico-pathological and follow-up data
Baseline clinico-pathological data were collected from case records and have been summarized in Table 1. Of the 88 patients included in our study, only 2.3% (2/88) underwent lobectomy/hemithyroidectomy alone, whereas 5.7% (5/88) of patients underwent total thyroidectomy alone and 92.0% (81/88) underwent thyroidectomy with lymph node dissection (prophylactic central lymphadenectomy was done in 15.9% (14/88) and therapeutic central/lateral lymphadenectomy in 76.1% (67/88)). Extrathyroidal extension (ETE) was further classified as follows: microscopic ETE was defined as tumor extending beyond the thyroid capsule into the surrounding peri-thyroidal soft tissues of fat and/or skeletal muscle, without visual evidence of this invasion and macroscopic ETE defined as visual evidence of tumor invasion into strap muscles, subcutaneous soft tissue, larynx, trachea, esophagus, recurrent laryngeal nerve or prevertebral fascia. Staging of DTC was performed using the eighth edition of American Joint Committee on Cancer (AJCC) staging system. All the patients included in this study had undergone RAI therapy with an average dosage of 186 mCi. 34.1% (30/88) of patients had undergone multiple treatments with RAI (16 patients received two doses, 11 received three doses, two received four doses and one patient received five doses) and the average dosage for these patients was 328 mCi. Patients were stratified into low, intermediate and high risk based on 2015 American Thyroid Association (ATA) guidelines (17). Following initial surgery, low-risk DTC patients were followed up annually, intermediate risk patients were followed up at 6 months’ intervals and high risk patients were followed up at 3 months’ intervals. At each follow-up, neck ultrasound, thyroid function tests, thyroglobulin levels and thyroglobulin antibodies were performed. In addition, for high risk patients, radioiodine scan and PET CT scan were performed to identify tumor recurrence. Complete remission was defined as serum thyroglobulin (Tg) levels below the reference range and no evidence of structural disease. Partial response was defined as decreasing Tg levels and/or functional evidence of disease after surgery and RAI therapy. Persistent disease was defined as biochemical, structural or functional evidence of disease within one year of surgery. Recurrence was defined as any new biochemical or structural disease following complete remission. Response to therapy was evaluated based on criteria adapted from Tuttle et al. (18, 19), whereby excellent response was defined as complete remission achieved during the first two years of follow-up and all other cases being classified as poor response.
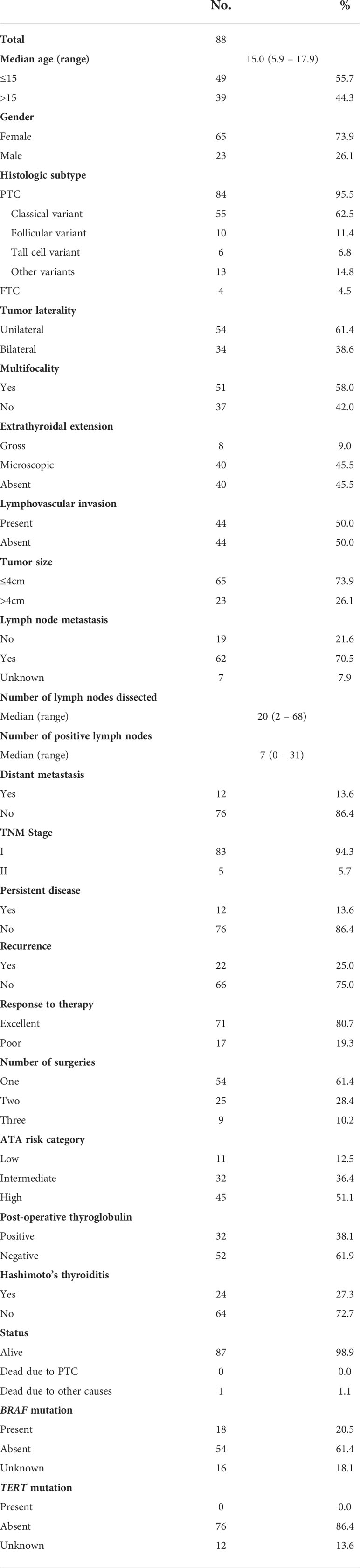
Table 1 Clinico-pathological characteristics of pediatric differentiated thyroid carcinoma who underwent surgery and RAI therapy.
2.3 BRAF and TERT mutation analysis
BRAF and TERT mutation data for the DTC cohort was available from our previous studies (20, 21).
2.4 Statistical analysis
The associations between clinico-pathological variables and patient age was performed using contingency table analysis and Chi square tests. Overall survival (OS) and event-free survival (EFS) were determined using Kaplan-Meier estimates. OS was defined as the time from diagnosis to death from any cause. EFS was defined as the time from diagnosis to the occurrence of persistent or recurrent disease. Cox proportional hazards model was used for analyzing the impact of prognostic factors on EFS in univariate and multivariate manner. Two-sided tests were used for statistical analyses with a limit of significance defined as p value < 0.05. Data analyses were performed using the JMP14.0 (SAS Institute, Inc., Cary, NC) software package.
3 Results
3.1 Patient and tumor characteristics
Median age of the entire cohort was 15 years (range: 5.9 – 17.9 years), with a male: female ratio of 1:3. Majority of the tumors were PTC (95.5%; 84/88). Regional lymph node metastasis (LNM) was noted in 70.5% (62/88) of cases and distant metastasis was present in 13.6% (12/88), of which synchronous distant metastasis was noted in 8.0% (7/88) and metachronous distant metastasis was noted in 5.6% (5/88). The site of distant metastasis in all 12 patients was the lung. Mean follow-up was 8.4 years (range 3.1 – 25.7 years). Ten-year overall survival and event-free survival (EFS) rates were 98.4% and 79.2%. Events occurred in 32.9% (29/88), including locoregional recurrence (n = 12), pulmonary metastasis (n = 5) and persistent disease (n = 12) (Table 1). Of the 12 patients with persistent disease, five had biochemical persistence and seven had structural persistence. Among the seven patients with structural persistence, three had lymph node persistence and four had distant persistent disease.
To determine the optimal tumor diameter cut-off for analysis, we divided the tumor sizes into four groups (<2cm, 2-3cm, 3-4cm and >4cm) and analysed the EFS for these groups. On both univariate and multivariate analysis, we found that tumor size more than 4cm was the most robust predictor of EFS (Table 2). 26.1% (23/88) of DTCs in our cohort were larger than 4cm in largest diameter.
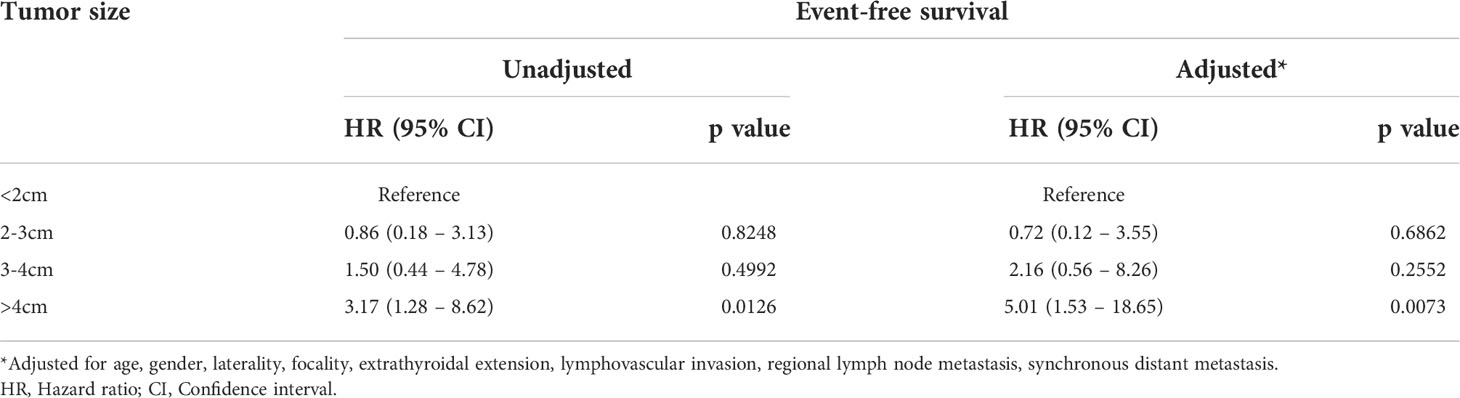
Table 2 The Association between tumor size and event-free survival in pediatric differentiated thyroid carcinoma.
3.2 Factors predicting event-free survival
On univariate analysis, EFS was impaired for patients with gross extrathyroidal extension (p = 0.0229), tumor size larger than 4cm (p = 0.0245), regional lymph node metastasis (p = 0.0132), synchronous distant metastasis (p = 0.0055) and response to therapy (p = 0.0436). However, on multivariate analysis, only tumor size larger than 4cm (Hazard ratio = 5.34; 95% confidence interval = 1.36 – 20.22; p = 0.0177) and synchronous distant metastasis (Hazard ratio = 8.73; 95% confidence interval = 1.48 – 60.05; p = 0.0154) were found to be independent negative predictors of EFS (Table 3).
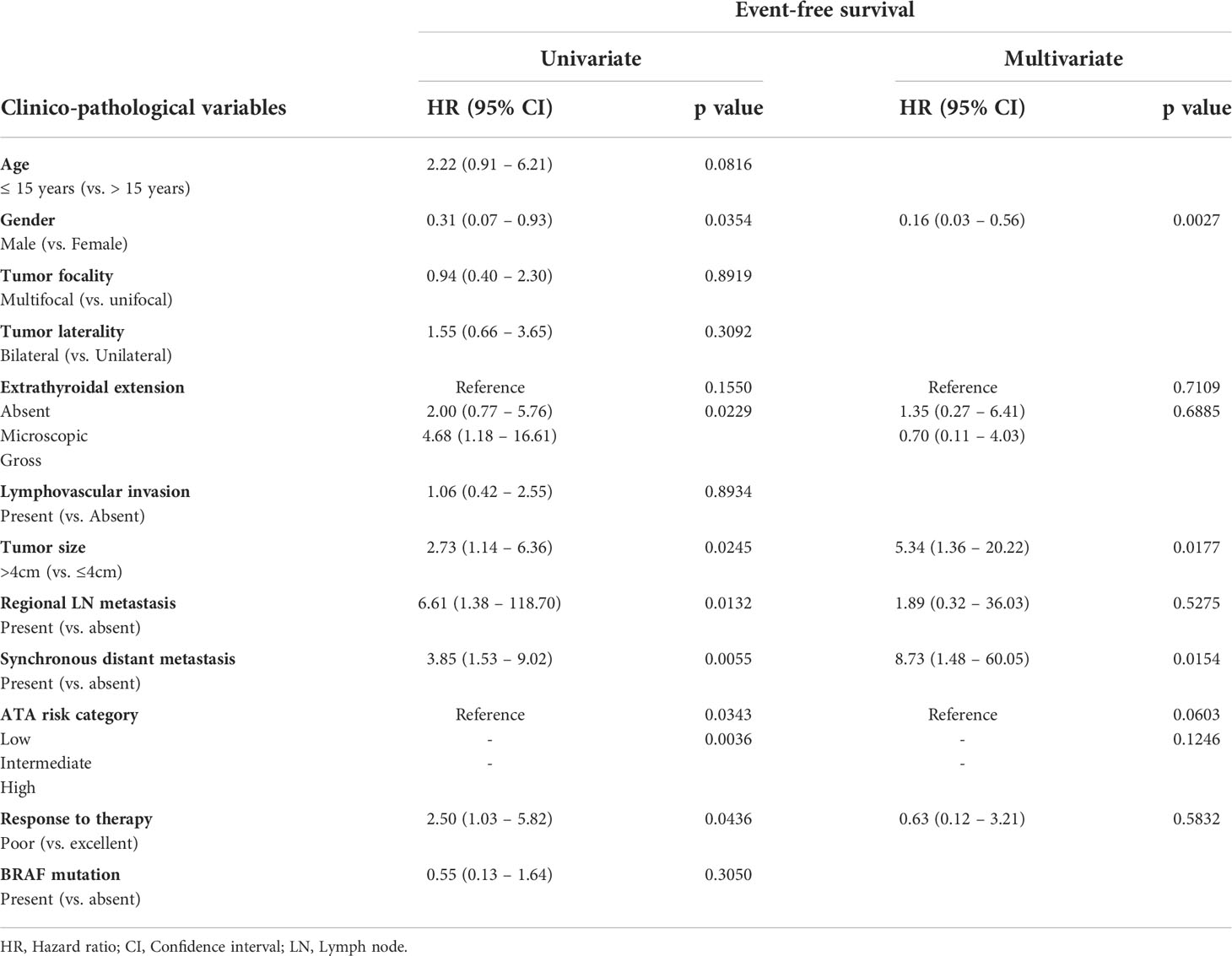
Table 3 Cox proportional hazards model for predictors of event-free survival in pediatric differentiated thyroid carcinoma.
3.3 Age and clinico-pathological associations
Since our data showed a trend towards association between age (15 years) and EFS (Hazard ratio = 2.22; 95% confidence interval = 0.91 – 6.21; p = 0.0816; Table 3), we sought to determine the clinico-pathological associations with age. Since the median age of our cohort was 15 years, we divided the patients based on this age cut-off. Younger age (≤ 15 years) was associated with adverse clinico-pathological parameters such as male gender (p = 0.0368), tumor larger than 4cm (p = 0.0368), distant metastasis (p = 0.0295) and poor response to therapy (p = 0.0102) (Table 4).
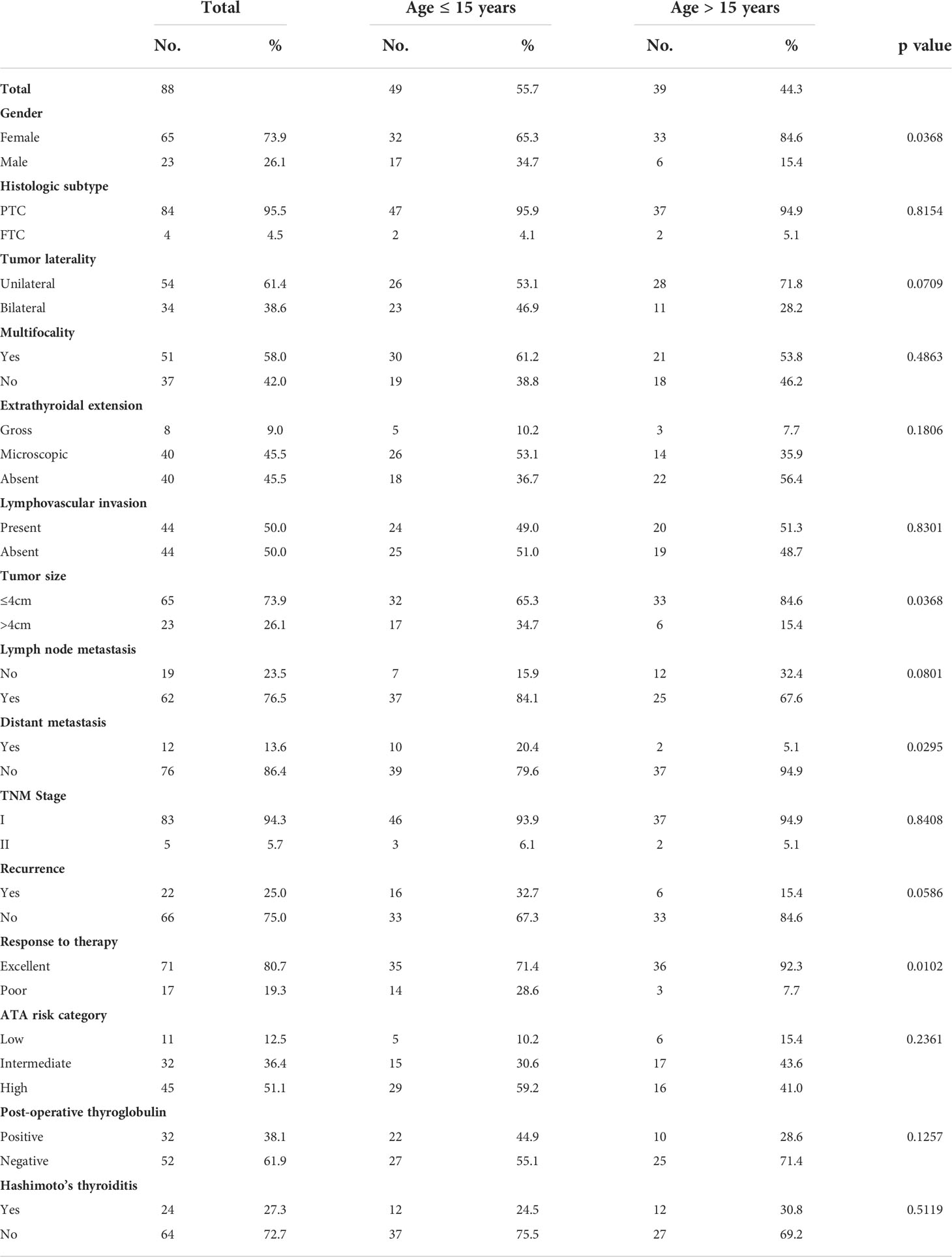
Table 4 Clinico-pathological characteristics of pediatric differentiated thyroid carcinoma who underwent surgery and RAI therapy.
3.4 Association of ATA risk categories with recurrence, persistent disease and response to therapy
In our cohort, ATA low-, intermediate- and high-risk categories were noted in 12.5% (11/88), 36.4% (32/88) and 51.1% (45/88), respectively. ATA high-risk group was found to be significantly associated with tumor recurrence (p = 0.0031) and poor response to therapy (p < 0.0001), but not with persistent disease (p = 0.8316) (Table 5).
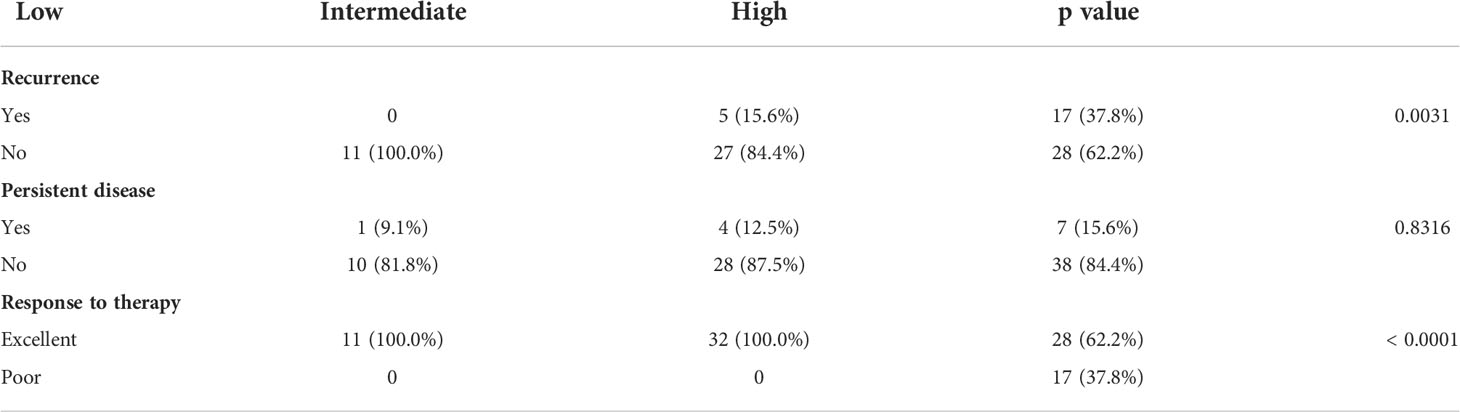
Table 5 Association of ATA risk category with recurrence, persistence and response to therapy in pediatric DTC.
4 Discussion
In this retrospective study of pediatric DTC patients, we have confirmed that DTC in children and adolescents present with advanced and more aggressive disease. In this cohort, pediatric DTC had a high incidence of LNM and distant metastasis (70.5% and 13.6%, respectively) as well as high rate of events (10 years EFS of 79.2%). This is consistent with several previous studies (8, 12, 22–25). Despite the presence of advanced disease in children with DTC in this cohort, long-term prognosis was excellent and 10 years overall survival was 98.4%, which is also in line with previous reports on pediatric DTC from different ethnicities (8, 13, 24, 26, 27).
To further determine potential independent factors for patients’ outcome, we included the clinico-pathological factors in multivariate Cox regression analysis. In order to determine the exact threshold for tumor size, we divided the patients by tumor size into different subgroups (<2cm, 2-3cm, 3-4cm and >4cm). Higher risk of DTC event free survival was observed in tumors larger than 4cm in both univariate and multivariate analysis. Hence, this size threshold was used for further analysis. Our results indicated that two predictive factors significantly and negatively impacted EFS: tumor size >4cm and the presence of initial distant metastasis. There are several reports where large tumor size and initial distant metastasis are found to have adverse impact on the recurrence and prognosis of pediatric DTC (14, 24, 28, 29). Although several other factors have been previously reported to be negatively associated with EFS, such as age, extent of resection and serum thyroglobulin levels (8, 25, 30), in our study, age, ATA risk stratification and other clinico-pathological parameters (extrathyroidal extension, bilaterality, multifocality, lymphovascular invasion and response to therapy) were not independent risk factors for EFS.
Our data shows trend towards association of younger age (<15 years) and EFS but this did not reach statistical significance (p = 0.0816), while several previous reports showed positive correlation between younger patients and worse prognosis (24, 30–35). Despite the lack of significant correlation between age and EFS in this cohort, younger patients did present with adverse clinico-pathological characteristics such as male gender, large tumor size, distant metastasis and poor response to therapy. Interestingly, only 71% of patients aged <15 years achieved complete remission whereas 92% patient aged >15 achieved complete remission (p = 0.0102).
In the recent ATA guidelines for adult DTC patients, risk stratification is clearly defined (17). However, whether ATA risk stratification effectively defines pediatric patients at risk of recurrent or persistent disease is unclear. Our data from pediatric DTC who underwent surgery and RAI shows that ATA risk stratification system effectively defines patient at risk of recurrence and response to therapy (Table 5) but does not correlate with persistent disease nor predicts EFS. This is contrary to recently published data (8, 36), where ATA risk group were identified as prognostic factor for EFS (15, 16). Whether this inconsistency is due to sample size or ethnic differences between the cohorts needs to be clarified by larger studies of pediatric DTC.
To explore if there are known genetic alteration that could affect the course of the disease in our cohort, we have analyzed BRAF and TERT mutation and found that BRAF mutation had no effect on patient’s outcome, whereas no TERT mutations were detected in our cohort.
Being retrospective in nature, this study has its inherent limitation, such as selection bias. Another limitation was that all patients included in this study underwent surgery and RAI. Our results should be interrupted with caution due to limited sample size and unique ethnicity.
In conclusion, our results suggest evaluating tumor size as a prognostic factor and risk stratification marker in pediatric DTC. Future studies are needed to confirm the impact of tumor size to define the likelihood of poor EFS and to guide risk adapted therapy and follow-up.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving human participants were reviewed and approved by Research Advisory Council, King Faisal Specialist Hospital and Research Centre. Written informed consent for participation was not provided by the participants’ legal guardians/next of kin because: Since only retrospective patient data were used, waiver of written consent was granted.
Author contributions
Study concept and design: KA-K, SP, AS. Executed the study: SP, AS, PA, NS, WH, SA-S, FA-D. Statistical analysis: SP. Drafting the article: KA-K, AS, SP. Critical revision of the article for important intellectual content, writing of the article, and approval of the final version: KA-K, SP, AS, PA, NS, WH, SA-S, FA-D. All authors contributed to the article and approved the submitted version.
Acknowledgments
The authors would like to thank Felisa DeVera for and Kaleem Iqbal for their technical assistance.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Howlader N, Noone A, Krapcho ME, Miller D, Brest A, Yu M, et al. SEER cancer statistics review, 1975–2016. Natl Cancer Inst (2019) 1.
2. Bernier MO, Withrow DR, Berrington de Gonzalez A, Lam CJ, Linet MS, Kitahara CM, et al. Trends in pediatric thyroid cancer incidence in the united states, 1998-2013. Cancer. (2019) 125(14):2497–505. doi: 10.1002/cncr.32125
3. Hogan AR, Zhuge Y, Perez EA, Koniaris LG, Lew JI, Sola JE. Pediatric thyroid carcinoma: incidence and outcomes in 1753 patients. J Surg Res (2009) 156(1):167–72. doi: 10.1016/j.jss.2009.03.098
4. Siegel DA, King J, Tai E, Buchanan N, Ajani UA, Li J. Cancer incidence rates and trends among children and adolescents in the united states, 2001–2009. Pediatrics (2014) 134(4):e945–e55. doi: 10.1542/peds.2013-3926
5. Cho YY, Jang HW, Joung JY, Park S-M, Jeong DJ, Kim SW, et al. Trends in thyroid cancer incidence in Korean children (1999-2012) based on palpation and nonpalpation detection methods. Eur Thyroid J (2015) 4(4):252–9. doi: 10.1159/000442047
6. Qian ZJ, Jin MC, Meister KD, Megwalu UC. Pediatric thyroid cancer incidence and mortality trends in the United States, 1973-2013. JAMA Otolaryngology–Head Neck Surg (2019) 145(7):617–23. doi: 10.1001/jamaoto.2019.0898
7. Paulson VA, Rudzinski ER, Hawkins DS. Thyroid cancer in the pediatric population. Genes (2019) 10(9):723. doi: 10.3390/genes10090723
8. Redlich A, Luster M, Lorenz K, Lessel L, Rohrer TR, Schmid KW, et al. Age, American thyroid association risk group, and response to therapy are prognostic factors in children with differentiated thyroid cancer. J Clin Endocrinol Metab (2022) 107(1):e165–e77. doi: 10.1210/clinem/dgab622
9. Galuppini F, Vianello F, Censi S, Barollo S, Bertazza L, Carducci S, et al. Differentiated thyroid carcinoma in pediatric age: Genetic and clinical scenario. Front Endocrinol (2019) 552. doi: 10.3389/fendo.2019.00552
10. Gertz RJ, Nikiforov Y, Rehrauer W, McDaniel L, Lloyd RV. Mutation in BRAF and other members of the MAPK pathway in papillary thyroid carcinoma in the pediatric population. Arch Pathol Lab Med (2016) 140(2):134–9. doi: 10.5858/arpa.2014-0612-OA
11. Pekova B, Sykorova V, Dvorakova S, Vaclavikova E, Moravcova J, Katra R, et al. RET, NTRK, ALK, BRAF, and MET fusions in a large cohort of pediatric papillary thyroid carcinomas. Thyroid (2020) 30(12):1771–80. doi: 10.1089/thy.2019.0802
12. Hay ID, Johnson TR, Kaggal S, Reinalda MS, Iniguez-Ariza NM, Grant CS, et al. Papillary thyroid carcinoma (PTC) in children and adults: comparison of initial presentation and long-term postoperative outcome in 4432 patients consecutively treated at the Mayo clinic during eight decades (1936–2015). World J Surg (2018) 42(2):329–42. doi: 10.1007/s00268-017-4279-x
13. Klein Hesselink MS, Nies M, Bocca G, Brouwers AH, Burgerhof JG, Van Dam EW, et al. Pediatric differentiated thyroid carcinoma in the Netherlands: A nationwide follow-up study. J Clin Endocrinol Metab (2016) 101(5):2031–9. doi: 10.1210/jc.2015-3290
14. Francis GL, Waguespack SG, Bauer AJ, Angelos P, Benvenga S, Cerutti JM, et al. Management guidelines for children with thyroid nodules and differentiated thyroid cancer: The American thyroid association guidelines task force on pediatric thyroid cancer. Thyroid (2015) 25(7):716–59. doi: 10.1089/thy.2014.0460
15. Sapuppo G, Hartl D, Fresneau B, Hadoux J, Breuskin I, Baudin E, et al. Differentiated thyroid cancer in children and adolescents: Long term outcome and risk factors for persistent disease. Cancers (Basel) (2021) 13(15):3732. doi: 10.3390/cancers13153732
16. Karapanou O, Tzanela M, Rondogianni P, Dacou-Voutetakis C, Chiotis D, Vlassopoulou B, et al. Long-term outcome of differentiated thyroid cancer in children and young adults: Risk stratification by ATA criteria and assessment of pre-ablation stimulated thyroglobulin as predictors of disease persistence. Endocrine (2020) 70(3):566–74. doi: 10.1007/s12020-020-02378-2
17. Haugen BR, Alexander EK, Bible KC, Doherty GM, Mandel SJ, Nikiforov YE, et al. American Thyroid association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: The American thyroid association guidelines task force on thyroid nodules and differentiated thyroid cancer. Thyroid (2015) 26(1):1–133. doi: 10.1089/thy.2015.0020
18. Tuttle RM, Tala H, Shah J, Leboeuf R, Ghossein R, Gonen M, et al. Estimating risk of recurrence in differentiated thyroid cancer after total thyroidectomy and radioactive iodine remnant ablation: Using response to therapy variables to modify the initial risk estimates predicted by the new American thyroid association staging system. Thyroid (2010) 20(12):1341–9. doi: 10.1089/thy.2010.0178
19. Tuttle RM. Risk-adapted management of thyroid cancer. Endocrine Practice (2008) 14(6):764–74. doi: 10.4158/EP.14.6.764
20. Siraj AK, Parvathareddy SK, Pratheeshkumar P, Divya SP, Al-Sobhi SS, Al-Dayel F, et al. PD-L1 is an independent prognostic marker in middle Eastern PTC and its expression is upregulated by BRAFV600E mutation. Cancers. (2021) 13(3):555. doi: 10.3390/cancers13030555
21. Bu R, Siraj AK, Divya SP, Kong Y, Parvathareddy SK, Al-Rasheed M, et al. Telomerase reverse transcriptase mutations are independent predictor of disease-free survival in m iddle e astern papillary thyroid cancer. Int J cancer (2018) 142(10):2028–39. doi: 10.1002/ijc.31225
22. O'Gorman CS, Hamilton J, Rachmiel M, Gupta A, Ngan BY, Daneman D. Thyroid cancer in childhood: A retrospective review of childhood course. Thyroid (2010) 20(4):375–80. doi: 10.1089/thy.2009.0386
23. Tamam M, Uyanik E, Edís N, Mulazimoglu M, Ozpacaci T. Differentiated thyroid carcinoma in children: Clinical characteristics and long-term follow-up. World J Nucl Med (2020) 19(1):28. doi: 10.4103/wjnm.WJNM_15_19
24. Mihailovic J, Nikoletic K, Srbovan D. Recurrent disease in juvenile differentiated thyroid carcinoma: Prognostic factors, treatments, and outcomes. J Nucl Med (2014) 55(5):710–7. doi: 10.2967/jnumed.113.130450
25. Cistaro A, Quartuccio N, Garganese MC, Villani MF, Altini C, Pizzoferro M, et al. Prognostic factors in children and adolescents with differentiated thyroid carcinoma treated with total thyroidectomy and RAI: A real-life multicentric study. (2022) European Journal of Nuclear Medicine and Molecular Imaging 49(4):1374–85. doi: 10.1007/s00259-021-05586-8
26. Rangel-Pozzo A, Sisdelli L, Cordioli MIV, Vaisman F, Caria P, Mai S, et al. Genetic landscape of papillary thyroid carcinoma and nuclear architecture: An overview comparing pediatric and adult populations. Cancers. (2020) 12(11):3146. doi: 10.3390/cancers12113146
27. Ștefan A-I, Piciu A, Căinap SS, Gabora K, Piciu D. Differentiated thyroid cancer in children in the last 20 years: a regional study in Romania. J Clin Med (2020) 9(11):3617. doi: 10.3390/jcm9113617
28. Enomoto Y, Enomoto K, Uchino S, Shibuya H, Watanabe S, Noguchi S. Clinical features, treatment, and long-term outcome of papillary thyroid cancer in children and adolescents without radiation exposure. World J surg (2012) 36(6):1241–6. doi: 10.1007/s00268-012-1558-4
29. Balachandar S, La Quaglia M, Tuttle RM, Heller G, Ghossein RA, Sklar CA. Pediatric differentiated thyroid carcinoma of follicular cell origin: prognostic significance of histologic subtypes. Thyroid (2016) 26(2):219–26. doi: 10.1089/thy.2015.0287
30. Jarząb B, Junak DH, Włoch J, Kalemba B, Roskosz J, Kukulska A, et al. Multivariate analysis of prognostic factors for differentiated thyroid carcinoma in children. Eur J Nucl Med (2000) 27(7):833–41. doi: 10.1007/s002590000271
31. Lazar L, Lebenthal Y, Steinmetz A, Yackobovitch-Gavan M, Phillip M. Differentiated thyroid carcinoma in pediatric patients: comparison of presentation and course between pre-pubertal children and adolescents. J Pediatrics (2009) 154(5):708–14. doi: 10.1016/j.jpeds.2008.11.059
32. Pawelczak M, David R, Franklin B, Kessler M, Lam L, Shah B. Outcomes of children and adolescents with well-differentiated thyroid carcinoma and pulmonary metastases following 131I treatment: a systematic review. Thyroid (2010) 20(10):1095–101. doi: 10.1089/thy.2009.0446
33. Landau D, Vini L, A'Hern R, Harmer C. Thyroid cancer in children: the royal Marsden hospital experience. Eur J Cancer (2000) 36(2):214–20. doi: 10.1016/S0959-8049(99)00281-6
34. Hung W, Sarlis NJ. Current controversies in the management of pediatric patients with well-differentiated nonmedullary thyroid cancer: a review. Thyroid (2002) 12(8):683–702. doi: 10.1089/105072502760258668
35. Alessandri AJ, Goddard KJ, Blair GK, Fryer CJ, Schultz KR. Age is the major determinant of recurrence in pediatric differentiated thyroid carcinoma. Med Pediatr Oncol: Off J SIOP—International Soc Pediatr Oncol (Societé Internationale d'Oncologie Pédiatrique (2000) 35(1):41–6. doi: 10.1002/1096-911X(200007)35:1<41::AID-MPO7>3.0.CO;2-7
Keywords: differentiated thyroid carcinoma, children, tumor size, event-free survival, distant metastasis
Citation: Parvathareddy SK, Siraj AK, Annaiyappanaidu P, Siraj N, Haqawi W, Al-Sobhi SS, Al-Dayel F and Al-Kuraya KS (2022) Tumor size is an independent negative prognostic factor for event free survival in children with differentiated thyroid cancer. Front. Endocrinol. 13:979054. doi: 10.3389/fendo.2022.979054
Received: 27 June 2022; Accepted: 08 August 2022;
Published: 25 August 2022.
Edited by:
Loredana Pagano, University of Turin, ItalyReviewed by:
Giulia Sapuppo, University of Catania, ItalyLaura Giacomelli, Sapienza University of Rome, Italy
Copyright © 2022 Parvathareddy, Siraj, Annaiyappanaidu, Siraj, Haqawi, Al-Sobhi, Al-Dayel and Al-Kuraya. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Khawla S. Al-Kuraya, a2t1cmF5YUBrZnNocmMuZWR1LnNh
†These authors have contributed equally to this work
 Sandeep Kumar Parvathareddy1†
Sandeep Kumar Parvathareddy1† Abdul K. Siraj
Abdul K. Siraj Khawla S. Al-Kuraya
Khawla S. Al-Kuraya