- 1Department of Nephrology, Zhongshan Hospital, Fudan University, Shanghai, China
- 2Shanghai Institute of Kidney and Dialysis, Zhongshan Hospital, Fudan University, Shanghai, China
- 3Shanghai Medical Center of Kidney, Zhongshan Hospital, Fudan University, Shanghai, China
- 4Shanghai Key Laboratory of Kidney and Blood Purification, Zhongshan Hospital, Fudan University, Shanghai, China
- 5Department of Internal Medicine, Zhongshan Hospital, Fudan University, Shanghai, China
Background: Crescent formation indicates severe glomerular pathology, and hypothyroidism usually predicts poor prognosis for severe diseases. However, the relationship between thyroid function and the progression of chronic kidney disease (CKD) is unclear. This study analysed the prognostic predictive value of the serum free triiodothyronine (FT3) to free thyroxine (FT4) ratio and its correlation with renal function in patients with CKD with crescent formation.
Methods: This single-centre study included 162 CKD patients with glomerular crescents confirmed by renal pathology between March 2012 and December 2014. According to the first tertile (0.284) of FT3/FT4 ratio, the patients were divided into high and low FT3/FT4 ratio groups. Kaplan-Meier and Cox regression analyses were performed to evaluate the prognostic value of the FT3/FT4 ratio.
Results: The age, haemoglobin, eGFR, urinary albumin-to-creatinine ratio, cardiac troponin T, N-terminal brain natriuretic peptide precursor, FT3, FT4, percentage of total crescents in non-globally sclerotic glomeruli, prevalences of hypertension, moderate to severe renal tubulopathy and crescentic nephritis, and proportion of patients receiving glucocorticoids and immunosuppressants were significantly different between high and low FT3/FT4 ratio groups (P < 0.05). Multivariate Cox regression analysis showed that when compared with patients with a high FT3/FT4 ratio (>0.284), those with intermediate and low FT3/FT4 ratios (≤0.284) had an increased risk of the long-term composite endpoint (P < 0.05 for various adjustment models).
Conclusions: A low FT3/FT4 ratio is associated with increased mortality and worse outcome risk in CKD patients with crescent pathology.
Introduction
Glomerular crescent formation is a hallmark of severe glomerular injury and can present in patients with primary and secondary glomerulonephritis. The presence of extensive glomerular crescents (usually greater than 50%) is the main pathological feature of rapidly progressive glomerulonephritis. Several studies (1, 2) have suggested that crescent formation is an independent predictor of a poor renal prognosis. Therefore, crescent was added to the MEST score in the Oxford Classification of IgA nephropathy that was updated in 2017 (3). However, there is insufficient evidence to base treatment decisions on the presence or number of crescents in a kidney biopsy (4). Therefore, we need to explore new predictors to assess the risk of outcomes in chronic kidney disease (CKD) patients with crescent formation and identify target populations that require early aggressive intervention.
‘Non-thyroidal illness syndrome’ (NTIS) is a syndrome of abnormal thyroid hormone metabolism that is general in critically ill patients and has been also reported in patients with CKD. Thyroxine (T4) is the main secreted and transported form of thyroid hormone. Its free form can be transported into cells to be de-iodinated and converted into free triiodothyronine (FT3), which regulates energy metabolism and protein synthesis and stimulates tissue growth, maturation, and differentiation. Impaired FT4-FT3 conversion is a major characteristic of NTIS, and the decreased FT3/FT4 ratio is therefore its biomarker. NITS is associated with decreased renal function in non-dialysis CKD patients (5, 6) and also predicts adverse outcomes in renal replacement therapy, such as kidney graft failure (7) and cardiovascular events in haemodialysis (HD) patients (8). However, a retrospective study of 317 patients with nephrotic syndrome failed to demonstrate the association between renal pathological type and NITS, marked as low FT3 levels or hypothyroidism (9).
Therefore, to clarify the association between the degree of T4-T3 conversion and the progression of CKD with crescents, the severe glomerular pathological changes suggesting high disease activity and rapidly progression, our study assessed the association of the FT3/FT4 ratio with a composite endpoint in a retrospective cohort of CKD patients with crescent formation in our centre.
Materials and methods
Study participants
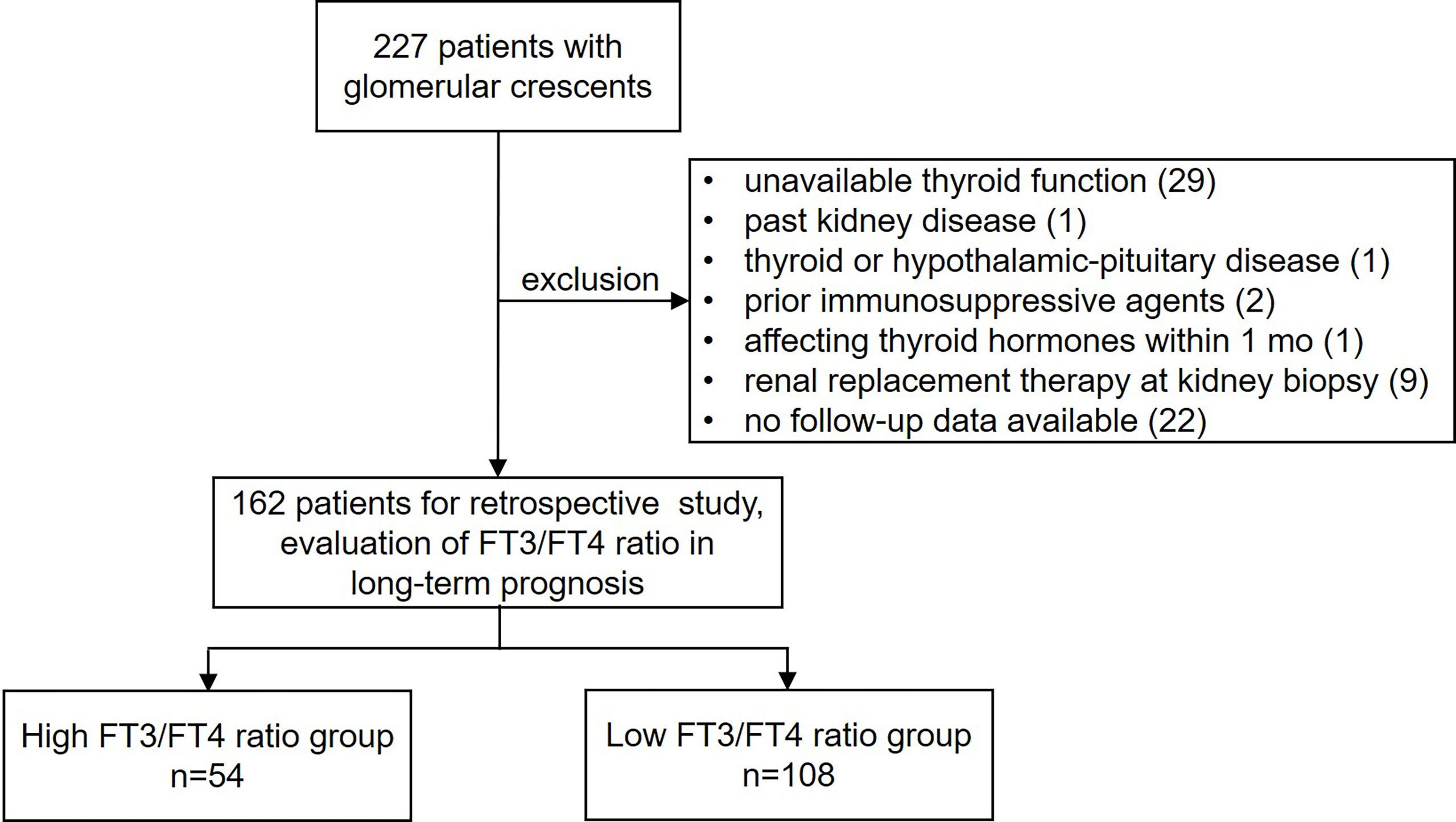
We analysed 227 consecutive patients who underwent kidney biopsy and showed glomerular crescent pathology at the Department of Nephrology, Zhongshan Hospital, Fudan University, between March 2012 and December 2014. Demographic variables (age and sex), clinical variables, laboratory tests results, kidney pathology results, and pharmacological treatment were obtained from electronic medical records. The inclusion criteria were as follows: (i) age ≥18 years at biopsy and (ii) renal biopsy-proven crescent formation (including cellular/fibrocellular/fibrous crescents). The exclusion criteria were as follows: (i) unavailable thyroid functional test results within 1 week before kidney biopsy; (ii) past history of kidney disease; (iii) past history of thyroid disease or hypothalamic-pituitary disease; (iv) immunosuppressive therapy before kidney biopsy; (v) administration of medication that may affect thyroid hormone secretion and metabolism within 1 month of the thyroid function test; (vi) renal replacement therapy (RRT) at kidney biopsy; and (vii) no follow-up data available. This study was approved by the ethics review committee of Zhongshan Hospital, Fudan University. The overall design of this study is shown in Figure 1.
Measurements and definitions
Hypertension was defined as systolic blood pressure ≥140 mmHg and/or diastolic blood pressure ≥90 mmHg at repeated examinations on different days in the hospital, self-reported history of hypertension, or use of antihypertensive medications (10).
The estimated glomerular filtration rate (eGFR) was calculated using the 2009 CKD-EPI creatinine formula (11).
Kidney biopsy reports were based on independent grading by two histopathologists. Glomerular crescents were defined as hyperplastic lesions involving >10% of the circumference of the Bowman’s capsule, including cellular, fibrocellular, and fibrous crescents. A ‘cellular crescent’ was defined as extracapillary hypercellularity of >2 cell layers composed of >75% cells with or without fibrin and <25% fibrous matrix. A composition of 25–75% cells with or without fibrin and the remaining fibrous matrix was defined as a ‘fibrocellular crescent’, and a composition of >75% fibrous matrix and <25% cells with or without fibrin was defined as a ‘fibrous crescent’ (12). The extent of interstitial fibrosis and tubular atrophy (IFTA) was graded on the estimated percentage of IFTA in the cortical area as mild (10%–25%), moderate (26%–50%), or severe (>50%) (13).
In this cohort, normal FT4 levels ranged from 12 to 22 pmol/L, and thyrotropin (TSH) from 0.27 to 4.20 μIU/mL. Thyroid function status was defined using these cut-off values, as follows: (i) overt hypothyroidism was defined as elevated TSH levels in combination with reduced FT4 levels; (ii) subclinical hypothyroidism was defined as elevated serum TSH levels in combination with normal FT4 levels; (iii) euthyroidism was defined as TSH levels within the specific reference range; (iv) overt hyperthyroidism was defined as decreased TSH levels with elevated FT4 levels; and (v) subclinical hyperthyroidism was defined as decreased TSH levels without elevated FT4 levels.
Renin-angiotensin-aldosterone system (RAAS) blocker use was defined as treatment with angiotensin-converting enzyme inhibitors, angiotensin receptor blockers, spironolactone, or any combination. Immunosuppressive treatments, including glucocorticoids and other immunosuppressants, were recorded based on the intention to treat.
Follow-up
Serum creatinine levels were examined at least every 3 months. The primary composite endpoint included a ≥50% reduction in the eGFR, RRT initiation, or death from any cause. The eGFR and time to initiation of RRT or death were retrospectively obtained from electronic medical records.
Statistical analysis
Continuous variables were described as mean with standard deviation (SD) and median with interquartile ranges (IQR) according to normal and non-normal distributions, respectively. Categorical variables were expressed as frequencies and constituent ratios. Depending on the distribution type of values, continuous variables were compared between groups using the Student’s t−test or Mann-Whitney U-test; categorical variables were compared using the chi-square test or the Fisher exact test. Kaplan-Meier curves were used to assess event-free survival, and the log-rank test was used to evaluate differences between groups. Univariate and multivariate Cox regression models were used to assess the association of the FT3/FT4 ratio with the composite endpoint, and crude and adjusted hazard ratios (HRs) with 95% confidence intervals (CIs) were reported. All variables in the univariate analysis were entered into the forward conditional Cox regression model for the multivariate analysis. Statistical analyses were performed using SPSS Statistics (version 25.0; IBM, Armonk, NY, USA) and GraphPad Prism 8.00 (GraphPad Software, San Diego, CA, USA). P-values less than 0.05 were considered statistically significant.
Results
Baseline characteristics
Between March 2012 and December 2014, 227 patients underwent renal biopsy at our centre and had pathological findings of glomerular crescents. After excluding 65 patients (Figure 1), 162 patients were included in this study. Supplementary Table 1 summarizes the leading etiologies of background glomerular diseases based on renal biopsy and clinical information. At baseline, overt hypothyroidism, subclinical hypothyroidism, euthyroidism, subclinical hyperthyroidism and overt hyperthyroidism were present in 8, 27, 126, 1 and 0 patients, respectively. Over a median follow-up period of 47.9 (IQR 16.1–103.9) months, the primary outcome was observed in 33 patients (20.4%), including 18 patients with ≥50% reduction in eGFR, 13 patients who underwent RRT, 1 patient who died of heart failure, and 1 patient with anti-neutrophil cytoplasmic antibody-associated vasculitis that died of intracerebral haemorrhage. The distribution of the FT3/FT4 ratio is presented in Supplementary Figure 1, and the FT3/FT4 ratio in patients free of the primary composite endpoint was significantly higher than that in patients with the primary composite endpoint (0.269 [0.227–0.294] vs 0.247 [0.207–0.278], P=0.036). The incidence of the events composing the composite endpoint based on the FT3/FT4 ratio, separated by tertiles, is shown in Supplementary Figure 2. The incidence of events composing the primary composite endpoint was significantly lower among patients with a high FT3/FT4 ratio than among those with intermediate or low FT3/FT4 ratios (both P < 0.01); it was not significantly different between patients with intermediate and low FT3/FT4 ratios (P = 0.828). Therefore, patients were divided into high (>0.284) and low (≤0.284) FT3/FT4 ratio groups according to the first tertile.
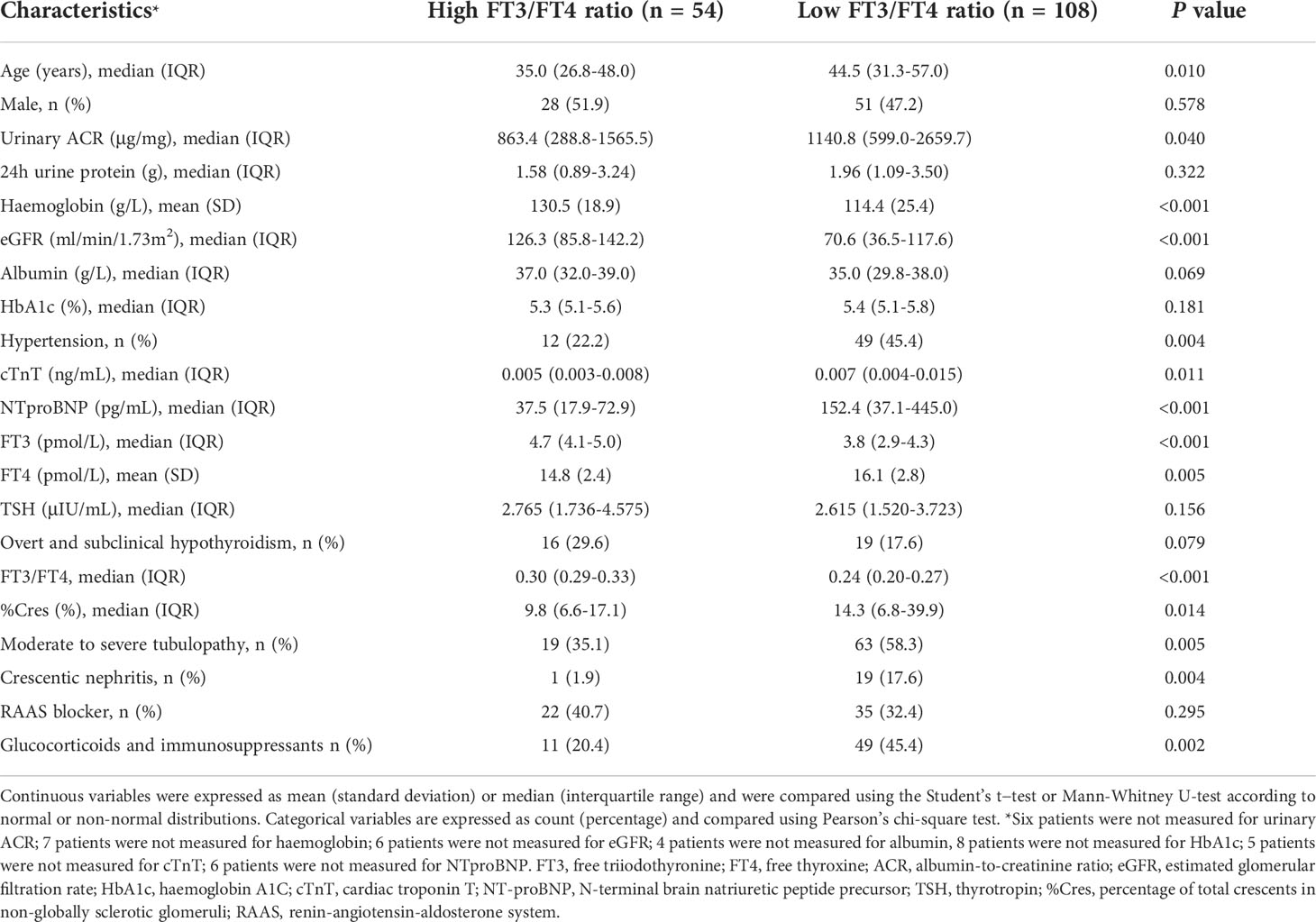
Table 1 summarises the baseline characteristics of the patients based on the FT3/FT4 ratio. Compared with the high FT3/FT4 ratio group, the low FT3/FT4 ratio group was older; had significantly higher urinary albumin-to-creatinine ratio (ACR), cardiac troponin T (cTnT), N-terminal brain natriuretic peptide precursor (NTproBNP), and percentage of total crescents in non-globally sclerotic glomeruli (%Cres); had higher prevalences of hypertension, moderate to severe renal tubulopathy, and crescentic nephritis; and had a higher proportion of patients receiving glucocorticoids and immunosuppressants. The low FT3/FT4 ratio group had lower haemoglobin and eGFR than the high FT3/FT4 ratio group. With regard to thyroid function tests, the low FT3/FT4 ratio group had higher FT4 levels and lower FT3 levels than the high FT3/FT4 ratio group (both P < 0.01); however, there were no significant differences in the TSH level or the prevalence of overt or subclinical hypothyroidism between the two groups. There were also no significant differences in sex, 24-hour urine protein, albumin, haemoglobin A1C (HbA1c), or the proportion of RAAS blocker application between the low and high FT3/FT4 ratio groups.
Primary composite endpoint in high and low FT3/FT4 ratio groups
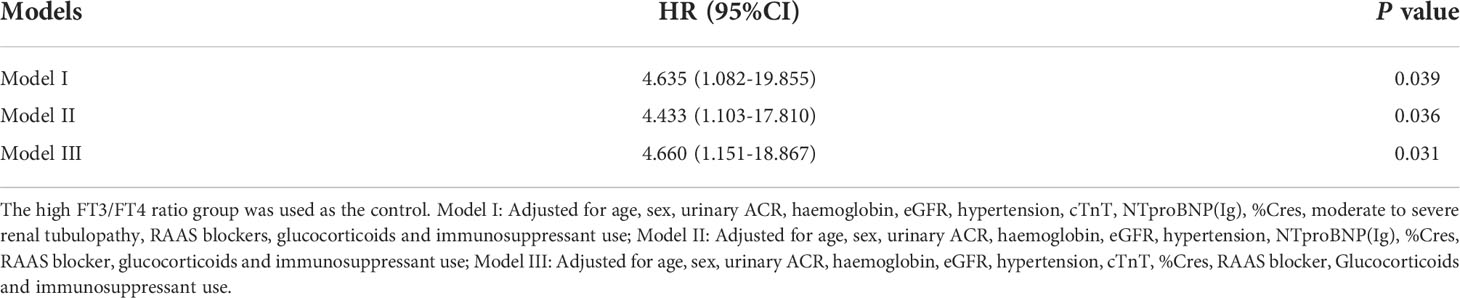
The mean event-free survival was higher in the high FT3/FT4 ratio group than in the low FT3/FT4 ratio group (mean survival 99.4 months [95% CI 91.3–107.6] versus 78.3 months [95% CI 69.8–86.8], P = 0.001) (Figure 2). In the univariate Cox regression analysis, the FT3/FT4 ratio was associated with the primary composite endpoint (per 0.1-unit increase; HR = 0.549 [95% CI 0.326–0.922], P = 0.024). The HR of the primary endpoint increased significantly with lower haemoglobin, eGFR, and FT3; higher cTnT, NTproBNP (Ig), and %Cres; hypertension; and moderate to severe tubulopathy (all P < 0.01) (Table 2). With regard to therapy, the HR of the primary endpoint was significantly lower in patients treated with RAAS blocker (P < 0.05, Table 2). Compared to the high FT3/FT4 ratio group, the low FT3/FT4 ratio group had a higher risk of the primary composite endpoint (HR = 4.896 [95% CI 1.709–14.024], P = 0.003, Table 2). All variables in the univariate analysis were included in the forward multivariate Cox proportional hazard model. A low FT3/FT4 ratio was independently associated with the primary composite endpoint (HR = 3.079 [95% CI 1.022–9.276], P = 0.046, Table 2). In addition, hypertension was an independent risk factor for the primary outcome (HR = 3.627 [95% CI, 1.655–7.950], P = 0.001), whereas an elevated eGFR-EPI was an independent protective factor (per 10-unit increase; HR = 0.788 [95% CI, 0.709–0.876], P < 0.001) (Table 2). We also assessed the association of a low FT3/FT4 ratio with the composite endpoint in three multivariate Cox regression models. Model I adjusted for all variables with P < 0.1 in the univariate Cox regression analysis plus sex. Model II included all of the variables in Model I except cTnT and moderate to severe renal tubulopathy, which closely related to NTproBNP and eGFR, respectively. Model III included all of the variables from Model I except NTproBNP and moderate to severe renal tubulopathy, which closely related to cTnT and eGFR, respectively. Compared to the high FT3/FT4 ratio group, the low FT3/FT4 ratio group had an increased risk of the composite endpoint in all three models (P < 0.05) (Table 3).
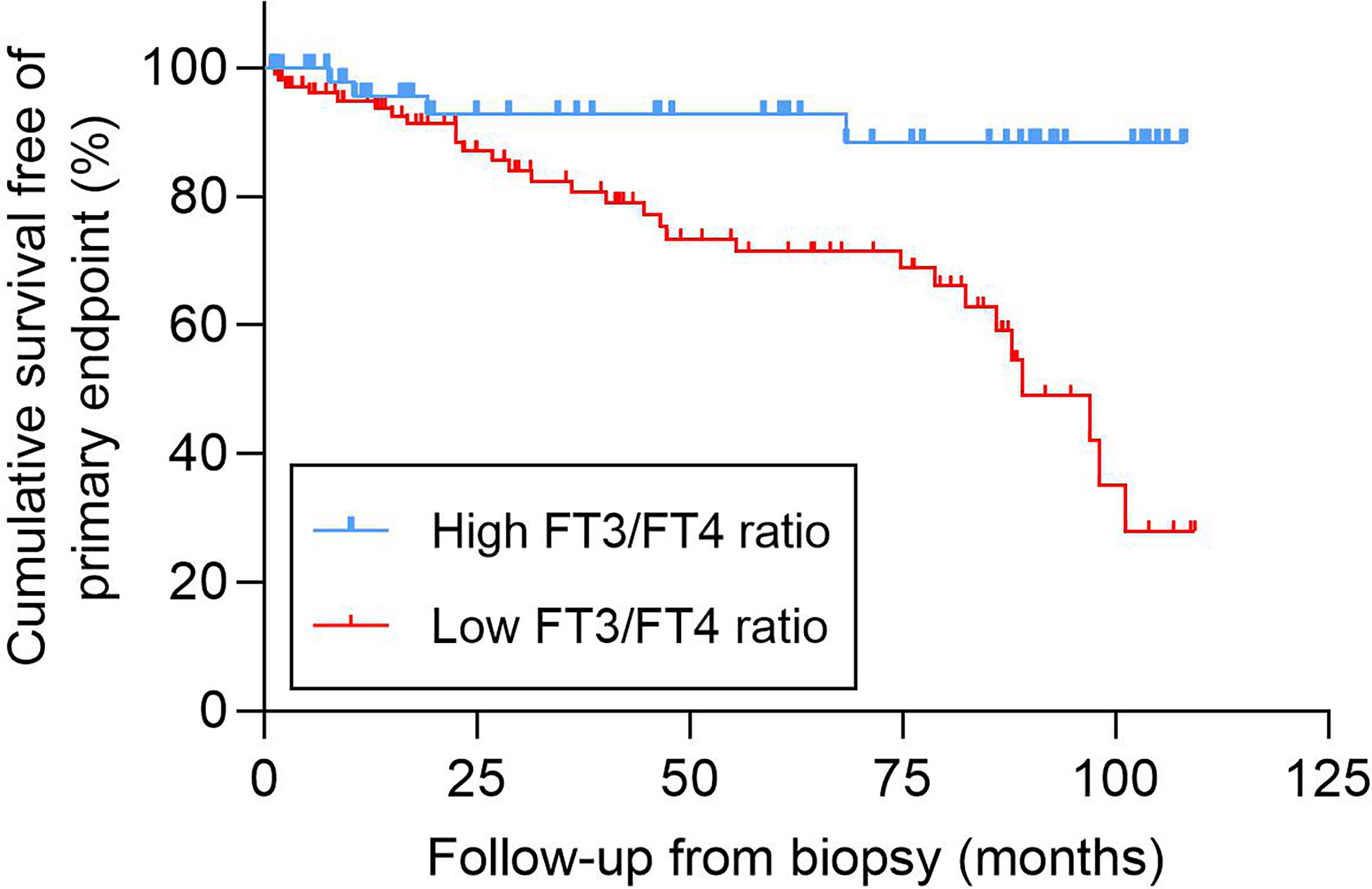
Figure 2 Kaplan-Meier analysis of the cumulative event-free survival according to the FT3/FT4 ratio. FT3, free triiodothyronine; FT4, free thyroxine.
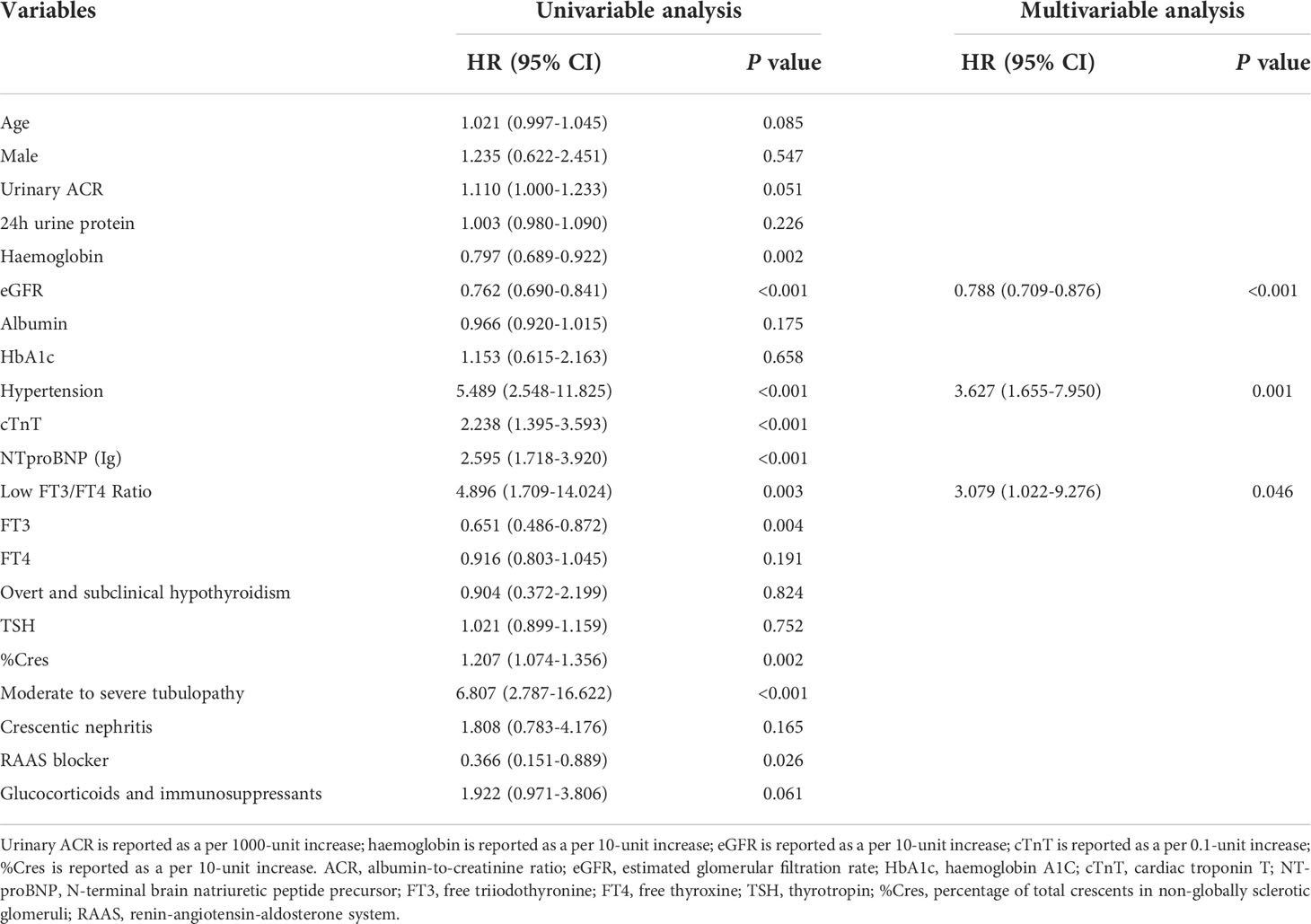
Table 2 Associations between clinical, laboratory and pathological parameters and primary combined endpoint.
Discussion
In this observational retrospective cohort study of patients with glomerular crescents, a low baseline FT3/FT4 ratio was associated with higher renal function exacerbation and a greater risk of mortality. This prognostic association remained after multivariate adjustment for confounding factors, such as age, concomitant disease, laboratory and pathological parameters, and treatment strategy. In addition to the FT3/FT4 ratio, the presence or absence of hypertension and the eGFR at kidney biopsy were independent risk factors for the long-term composite endpoint, suggesting that baseline hypertensive comorbidity and renal function may be valuable for assessing the prognosis of CKD patients with crescent formation.
Previous studies on thyroid function in CKD patients mainly focus on the relationship between hypothyroidism and renal function and its role in renal prognosis (14–16). Thyroid hormone replacement therapy may improve renal function in patients with CKD and subclinical or overt hypothyroidism (17, 18). However, a meta-analysis of 72,856 participants with varying thyroid function found that hypothyroidism has no relationship with a faster decline in kidney function (19). Therefore, alterations in thyroid hormone metabolism in CKD may not be fully recapitulated by hypothyroidism. This was also confirmed by findings in our study that TSH level, the most sensitive biomarker of hypothyroidism, was not associated with the primary composite endpoint in univariate Cox regression analysis. And similar TSH levels between the high and low FT3/FT4 ratio groups suggested that the altered thyroid hormone metabolism in CKD with crescents was not related to hypothyroidism but rather dependent on inappropriate T4-T3 conversion.
NTIS is highly prevalent in patients with CKD, and it has value as a predictor of CKD exacerbation (20) and poor outcomes in patients with ESRD (21). In NTIS, serum FT3 is decreased relative to serum FT4 without dysregulated TSH because of decreased T4 to T3 de-iodination caused by various factors, such as inflammation and malnutrition (22). This process occurs mainly in the skeletal muscle (23). The rate of T4 to T3 de-iodination is represented by the serum FT3/FT4 ratio (24, 25), which is positively associated with muscle mass in aged, obese, and HD individuals (24–26); a lower ratio indicates impaired physical performance. Moreover, recent evidences suggest that a low FT3/FT4 ratio may be related to unfavourable prognosis in specific clinical settings. A prospective observational cohort study in a British population found that a low FT3/FT4 ratio was associated with frailty and long-term death in older hospitalised patients (27). Another study revealed that, in patients with dilated cardiomyopathy, the FT3/FT4 ratio was an independent risk factor for 1-year mortality and was associated with deteriorative heart function (28). In the context of ACS, the FT3/FT4 ratio is inversely associated with an increased risk of adverse outcomes, including all-cause and cardiac death and major adverse cardiac and cerebrovascular events (29–31). In addition, decreased FT3 and elevated FT4 levels were independent predictors of long-term mortality risk in a retrospective cohort study of hospitalised patients with chronic NTIS (32). However, few studies have focused on the relationship between the degree of T4-T3 conversion and clinical outcomes in patients with CKD. Therefore, this study provides a novel and reasonable index for predicting worsening renal function and poor prognosis in CKD patients with crescent formation, as well as a powerful supplement to the relationship between thyroid function and CKD prognosis. In addition, serum FT3 and FT4 levels are less affected by thyroid-binding globulin and serum albumin and had good stability; no additional testing is required for this ratio.
There is still no consensus on the optimal cut-off point to define a high and low FT3/FT4 ratio. Most studies categorise patients into tertiles (24, 29), as we did in our study. In studies of coronary artery disease and elderly individuals, the average values for the high tertile of the FT3/FT4 ratio ranged between 2.92 and 3.33 [(pg/mL)/(ng/dL)]; our median also fell in this range.
Age, comorbid hypertension, haemoglobin, eGFR, cTnT, NTproBNP, %Cres, moderate-to-severe tubulopathy, and administration of glucocorticoids combined with other immunosuppressive therapies are classical prognostic factors for CKD; the results of our univariate Cox analysis also showed these factors to be related to the composite endpoint. These prognostic indexes were significantly different between the high and low FT3/FT4 ratio groups. The eGFR is usually inversely associated with the severity of tubulointerstitial injury (33), and simultaneous elevation of cTnT and NTproBNP is associated with a rapid decline in kidney function and incident CKD (34). In our multivariate Cox regression models adjusted for these classic prognostic factors, a low FT3/FT4 ratio was an independent risk factor for poor prognosis in CKD patients with crescent formation, regardless of whether cTnT, NTproBNP, or moderate to severe tubulopathy were excluded.
In conclusion, we found that a low FT3/FT4 ratio was associated with poor long-term outcomes in patients with CKD with glomerular crescents. This finding suggests that the FT3/FT4 ratio is a risk factor in these patients. However, there are some unresolved questions. The mechanism by which the metabolic variation in thyroid hormones affects the progression of CKD remains to be elucidated by further basic and clinical studies. Another question is whether patients with a low FT3/FT4 ratio would benefit from a more aggressive etiological treatment or thyroid hormone replacement. Additionally, our study had several limitations. First, there were only baseline data on the FT3/FT4 ratio, and the effect of variations in this ratio during follow-up on prognosis could not be assessed. Second, it should be noted that the participants in this study were patients with CKD with crescent formation; therefore, the conclusions of this study may not be generalizable to all CKD populations. Third, information on some potential confounders, such as established cardiovascular disease or obesity, was not documented in this study; however, we had a large number of other relevant confounding variables for adjusting multivariate models.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving human participants were reviewed and approved by Ethics Committee, Zhongshan Hospital, Fudan University. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Author contributions
LZ and YW were responsible for the study design. LZ, YW, YN and WL were responsible for data collection. LZ, YL and BZ were responsible for analysis and interpretation of data. LZ, SJ, ZS, FL and HL were responsible for writing the manuscript. YF and XD were responsible for supervision or mentorship. All authors were involved in writing the paper and had final approval of the submitted and published versions.
Funding
This study was supported by the National Natural Science Foundation of China (grant 81900699), Shanghai Municipal Key Clinical Specialty (shslczdzk02501), Shanghai Municipal Hospital Frontier Technology Project supported by Shanghai ShenKang Hospital Development Center (SHDC12018127), Shanghai "science and technology innovation plan" technical standard project (19DZ2205600) and Shanghai "science and technology innovation plan" Yangtze River Delta scientific and technological Innovation Community project (21002411500).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2022.977355/full#supplementary-material
References
1. Edström Halling S, Söderberg MP, Berg UB. Predictors of outcome in paediatric IgA nephropathy with regard to clinical and histopathological variables (Oxford classification). Nephrol Dial Transplant (2012) 27:715–22. doi: 10.1093/ndt/gfr339
2. Shima Y, Nakanishi K, Hama T, Mukaiyama H, Togawa H, Hashimura Y, et al. Validity of the Oxford classification of IgA nephropathy in children. Pediatr Nephrol (2012) 27:783–92. doi: 10.1007/s00467-011-2061-0
3. Trimarchi H, Barratt J, Cattran DC, Cook HT, Coppo R, Haas M, et al. Oxford Classification of IgA nephropathy 2016: an update from the IgA nephropathy classification working group. Kidney Int (2017) 91:1014–21. doi: 10.1016/j.kint.2017.02.003
4. Kidney disease: Improving global outcomes (KDIGO) glomerular diseases work group. KDIGO 2021 clinical practice guideline for the management of glomerular diseases. Kidney Int (2021) 100:S1–276. doi: 10.1016/j.kint.2021.05.021
5. Meuwese CL, Dekkers OM, Stenvinkel P, Dekker FW, Carrero JJ. Nonthyroidal illness and the cardiorenal syndrome. Nat Rev Nephrol (2013) 9:599–609. doi: 10.1038/nrneph.2013.170
6. Zoccali C, Tripepi G, Cutrupi S, Pizzini P, Mallamaci F. Low triiodothyronine: a new facet of inflammation in end-stage renal disease. J Am Soc Nephrol (2005) 16:2789–95. doi: 10.1681/ASN.2005040356
7. Rotondi M, Netti GS, Rosati A, Pizzini P, Mallamaci F. Pretransplant serum FT3 levels in kidney graft recipients are useful for identifying patients with higher risk for graft failure. Clin Endocrinol (Oxf) (2008) 68:220–5. doi: 10.1111/j.1365-2265.2007.03022.x
8. Netti GS, Rotondi M, Di Lorenzo A, Papantonio D, Teri A, Schirone M, et al. Nocturnal haemodialysis is associated with a reduced occurrence of low triiodothyronine serum levels in haemodialysed patients. Clin Kidney J (2020) 13:450–60. doi: 10.1093/ckj/sfaa003
9. Li LZ, Hu Y, Ai SL, Cheng L, Liu J, Morris E, et al. The relationship between thyroid dysfunction and nephrotic syndrome: a clinicopathological study. Sci Rep (2019) 9:6421. doi: 10.1038/s41598-019-42905-4
10. Unger T, Borghi C, Charchar F, Khan NA, Poulter NR, Prabhakaran D, et al. 2020 International society of hypertension global hypertension practice guidelines. Hypertension (2020) 75:1334–57. doi: 10.1161/HYPERTENSIONAHA.120.15026
11. Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF 3rd, Feldman HI, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med (2009) 150:604–12. doi: 10.7326/0003-4819-150-9-200905050-00006
12. Haas M, Seshan SV, Barisoni L, Amann K, Bajema IM, Becker JU, et al. Consensus definitions for glomerular lesions by light and electron microscopy: recommendations from a working group of the renal pathology society. Kidney Int (2020) 98:1120–34. doi: 10.1016/j.kint.2020.08.006
13. Sethi S, Haas M, Markowitz GS, D'Agati VD, Rennke HG, Jennette JC, et al. Mayo Clinic/Renal pathology society consensus report on pathologic classification, diagnosis, and reporting of GN. J Am Soc Nephrol (2016) 27:1278–87. doi: 10.1681/ASN.2015060612
14. Lo JC, Chertow GM, Go AS, Hsu CY. Increased prevalence of subclinical and clinical hypothyroidism in persons with chronic kidney disease. Kidney Int (2005) 67:1047–52. doi: 10.1111/j.1523-1755.2005.00169.x
15. Patil VP, Shilpasree AS, Patil VS, Pravinchandra KR, Ingleshwar DG, Vani AC. Evaluation of renal function in subclinical hypothyroidism. J Lab Physicians (2018) 10:50–5. doi: 10.4103/JLP.JLP_67_17
16. Chang YC, Chang CH, Yeh YC, Chuang LM, Tu YK. Subclinical and overt hypothyroidism is associated with reduced glomerular filtration rate and proteinuria: a large cross-sectional population study. Sci Rep (2018) 8:2031. doi: 10.1038/s41598-018-19693-4
17. Bulur O, Dal K, Ertugrul DT, Eser M, Kaplan Efe F, Karakaya S, et al. Renal function improves with the treatment of hypothyroidism. Endocr Res (2017) 42:246–51. doi: 10.1080/07435800.2017.1293686
18. Shin DH, Lee MJ, Kim SJ, Oh HJ, Kim HR, Han JH, et al. Preservation of renal function by thyroid hormone replacement therapy in chronic kidney disease patients with subclinical hypothyroidism. J Clin Endocrinol Metab (2012) 97:2732–40. doi: 10.1210/jc.2012-1663
19. Meuwese CL, van Diepen M, Cappola AR, Sarnak MJ, Shlipak MG, Bauer DC, et al. Low thyroid function is not associated with an accelerated deterioration in renal function. Nephrol Dial Transplant (2019) 34:650–9. doi: 10.1093/ndt/gfy071
20. Fan J, Yan P, Wang Y, Shen B, Ding F, Liu Y. Prevalence and clinical significance of low T3 syndrome in non-dialysis patients with chronic kidney disease. Med Sci Monit (2016) 22:1171–9. doi: 10.12659/MSM.895953
21. Xu LC, Zhou FF, Li M, Dai ZW, Cai KD, Zhu BX, et al. The correlation between low serum T3 levels and all-cause and cardiovascular mortality in peritoneal dialysis patients. Ther Clin Risk Manag (2021) 17:851–61. doi: 10.2147/TCRM.S324672
22. Rhee CM. Low-T3 syndrome in peritoneal dialysis: Metabolic adaptation, marker of illness, or mortality mediator? Clin J Am Soc Nephrol (2015) 10:917–9. doi: 10.2215/CJN.04310415
23. Salvatore D, Simonides WS, Dentice M, Zavacki AM, Larsen PR. Thyroid hormones and skeletal muscle–new insights and potential implications. Nat Rev Endocrinol (2014) 10:206–14. doi: 10.1038/nrendo.2013.238
24. Kong SH, Kim JH, Park YJ, Lee JH, Hong AR, Shin CS, et al. Low free T3 to free T4 ratio was associated with low muscle mass and impaired physical performance in community-dwelling aged population. Osteoporos Int (2020) 31:525–31. doi: 10.1007/s00198-019-05137-w
25. Zupo R, Castellana F, Sardone R, Lampignano L, Paradiso S, Giagulli VA, et al. Higher muscle mass implies increased free-thyroxine to free-triiodothyronine ratio in subjects with overweight and obesity. Front Endocrinol (Lausanne) (2020) 11:565065. doi: 10.3389/fendo.2020.565065
26. Inaba M, Mori K, Tsujimoto Y, Yamada S, Yamazaki Y, Emoto M, et al. Association of reduced free T3 to free T4 ratio with lower serum creatinine in Japanese hemodialysis patients. Nutrients (2021) 13:4537. doi: 10.3390/nu13124537
27. Pasqualetti G, Calsolaro V, Bernardini S, Linsalata G, Bigazzi R, Caraccio N, et al. Degree of peripheral thyroxin deiodination, frailty, and long-term survival in hospitalized older patients. J Clin Endocrinol Metab (2018) 103:1867–76. doi: 10.1210/jc.2017-02149
28. Kozdag G, Ural D, Vural A, Agacdiken A, Kahraman G, Sahin T, et al. Relation between free triiodothyronine/free thyroxine ratio, echocardiographic parameters and mortality in dilated cardiomyopathy. Eur J Heart Fail (2005) 7:113–8. doi: 10.1016/j.ejheart.2004.04.016
29. Yuan D, Zhang C, Jia S, Liu Y, Jiang L, Xu L, et al. Predictive value of free triiodothyronine (FT3) to free thyroxine (FT4) ratio in long-term outcomes of euthyroid patients with three-vessel coronary artery disease. Nutr Metab Cardiovasc Dis (2021) 31:579–86. doi: 10.1016/j.numecd.2020.10.011
30. Yu T, Tian C, Song J, He D, Wu J, Wen Z, et al. Value of the fT3/fT4 ratio and its combination with the GRACE risk score in predicting the prognosis in euthyroid patients with acute myocardial infarction undergoing percutaneous coronary intervention: a prospective cohort study. BMC Cardiovasc Disord (2018) 18:181. doi: 10.1186/s12872-018-0916-z
31. Brozaitiene J, Mickuviene N, Podlipskyte A, Burkauskas J, Bunevicius R. Relationship and prognostic importance of thyroid hormone and n-terminal pro-B-Type natriuretic peptide for patients after acute coronary syndromes: a longitudinal observational study. BMC Cardiovasc Disord (2016) 16:45. doi: 10.1186/s12872-016-0226-2
32. Ataoğlu HE, Ahbab S, Serez MK, Yamak M, Kayaş D, Canbaz ET, et al. Prognostic significance of high free T4 and low free T3 levels in non-thyroidal illness syndrome. Eur J Intern Med (2018) 57:91–5. doi: 10.1016/j.ejim.2018.07.018
33. Hodgkins KS, Schnaper HW. Tubulointerstitial injury and the progression of chronic kidney disease. Pediatr Nephrol (2012) 27:901–9. doi: 10.1007/s00467-011-1992-9
Keywords: glomerular crescents, chronic kidney disease, FT3/FT4 ratio, prognosis, thyroid function
Citation: Zhang L, Wu Y, Nie Y, Lv W, Li Y, Zhu B, Jin S, Shen Z, Li F, Liu H, Fang Y and Ding X (2022) The serum free triiodothyronine to free thyroxine ratio as a potential prognostic biomarker of chronic kidney disease in patients with glomerular crescents: A retrospective study. Front. Endocrinol. 13:977355. doi: 10.3389/fendo.2022.977355
Received: 24 June 2022; Accepted: 12 September 2022;
Published: 29 September 2022.
Edited by:
Loredana Pagano, University of Turin, ItalyReviewed by:
Laura Croce, University of Pavia, ItalyGiuseppe Lisco, University of Bari Aldo Moro, Italy
Copyright © 2022 Zhang, Wu, Nie, Lv, Li, Zhu, Jin, Shen, Li, Liu, Fang and Ding. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yi Fang, ZmFuZy55aUB6cy1ob3NwaXRhbC5zaC5jbg==; Xiaoqiang Ding, ZGluZy54aWFvcWlhbmdAenMtaG9zcGl0YWwuc2guY24=
†These authors have contributed equally to this work
 Liwen Zhang
Liwen Zhang Yuxiao Wu5†
Yuxiao Wu5† Yang Li
Yang Li Bowen Zhu
Bowen Zhu Shi Jin
Shi Jin Ziyan Shen
Ziyan Shen Yi Fang
Yi Fang