- 1Department of Medicine, Faculty of Medicine, Universiti Kebangsaan Malaysia Medical Center (UKMMC), Kuala Lumpur, Malaysia
- 2Department of Medicine, Faculty of Medicine and Health Sciences, Universiti Malaysia Sarawak, Sarawak, Kota Samarahan, Malaysia
Both primary aldosteronism and obstructive sleep apnea are well-known causes of hypertension and contribute to increased cardiovascular morbidity and mortality independently. However, the relationship between these two entities remains unclear, with studies demonstrating contradictory results. This review aims to collate and put into perspective current available research regarding the association between primary aldosteronism and obstructive sleep apnea. The relationship between these two entities, clinical characteristics, clinical implications, outcomes of treatment, potential causal links and mechanisms are hereby presented.
Introduction
Primary aldosteronism (PA) is now recognized as one of the most common causes of secondary hypertension (1). Patients with PA were demonstrated to have higher risk of cerebrovascular and cardiovascular events, cardiovascular mortality and renal injuries compared to patients with essential hypertension, independent of blood pressure levels (2). Unfortunately, this disorder of the adrenal gland is substantially under-diagnosed (3). For decades, it is believed that the activation of renin-angiotensin-aldosterone system (RAAS) regulated the biosynthesis of aldosterone (4–7). There is now growing evidence that aldosterone secretion is not solely under RAAS regulation.
Obstructive sleep apnea (OSA) is a chronic and potentially life-threatening sleep-related breathing disorder caused by periodic narrowing and obstruction of upper airway during sleep, leading to repetitive apnea and hypopnea episodes (8). The prevalence has continued to rise over the years, mainly driven by an increase in prevalence of obesity, which is one of the major causes of OSA. It is another well-known risk factor for hypertension and is demonstrated to affect more than 80% of patients with resistant hypertension (9). In addition, OSA is associated with multiple long-term health complications, which include cardiovascular diseases and metabolic disorders (10).
Many studies have attempted to demonstrate a relationship between OSA and RAAS. Activation of RAAS, especially excess aldosterone, has been implicated to play a pathophysiological role in the relationship between OSA and hypertension, particularly resistant hypertension and PA (11). In fact, the Endocrine Society has identified OSA in the presence of hypertension as one of the groups with high prevalence of PA, and thus now recommends screening of PA among this cohort of patients (12).
In animal studies, episodic hypoxia led to elevated blood pressure which was prevented by renal artery denervation or treatment with angiotensin receptor blockers, suggesting a relationship between OSA and RAAS (13). Subsequently, this led to few human studies examining this relationship, which are illustrated below. Nevertheless, to date, the relationship between these two entities remains unclear. As both PA and OSA are known to contribute to increased cardiovascular morbidity and mortality, understanding the relationship between these two entities is essential in improving healthcare management of these patients by reducing cardiovascular-associated risks.
Hence, this review aimed to gather and put into perspective current available research regarding the association between PA and OSA. It focused on the relationship between these two entities, and presented evidence for their clinical characteristics with its implications, outcomes of treatment as well as the potential causal links and mechanisms.
OSA in PA
Prevalence
In a cohort of 207 patients with confirmed PA, 67.6% were found to have OSA (64.4% in White, 70.0% in Chinese), of which 27.1% were mild, 21.7% moderate and 18.8% severe (14). An almost similar prevalence of 55% was also observed in a retrospective analysis of 71 Japanese patients with PA (15). The prevalence of OSA seems to be much higher in PA than non-PA population (59.5% vs. 42.4%), albeit not significant (p=0.058) (16). This is probably due to the small sample size. Nevertheless, apnea hypopnea index (AHI) was demonstrated to be higher among patients with PA compared to those without PA (p=0.024) (16).
The prevalence of OSA in patients with confirmed PA is summarized in Table 1.
Clinical characteristics
Patients with PA and OSA were more likely to be males, older, with larger neck circumference, more abdominal obesity, higher body mass index (BMI) and worse metabolic profile. These individuals have higher blood glucose and triglyceride levels with lower HDL-cholesterol concentrations (14–16). The elevated plasma aldosterone level was noted to be significantly correlated with OSA severity. However, this correlation was only observed among the White, but not in the Chinese and Japanese cohort (14, 15), despite Chinese PA population demonstrated a more severe phenotype of OSA compared to the White (14). The discrepancy observed could probably be attributed to differences in craniofacial anatomy, adiposity and salt intake between Asians and Caucasians (18–20).
Outcomes of treatment
When patients with OSA and co-existent essential hypertension, resistant hypertension or PA were given mineralocorticoid receptor (MR) blockade for total duration of 8 weeks to 8 months, a significant reduction in AHI, hypoxic index and oxygen desaturation index was observed along with a decrease in body weight, neck circumference and blood pressure in all three groups of patients (17, 21, 22). Nevertheless, the reduction in AHI among patients with PA was not uniform and the difference seen could be confounded by difference in population studied, methodologies and sample sizes.
Compared to medical therapy, the effect of surgical treatment among patients with PA and OSA is less studied. Adrenalectomy performed among patients with co-existing PA and OSA led to reduction in AHI and neck circumference (17). Nevertheless, the small number of patients (n=7) in this study limited statistical significance and certainty. In a larger cohort of patients with PA and OSA (n=48), the probability of OSA reduced significantly (Berlin score 1.69 pre-operation vs 1.33 post-operation, p<0.001) after adrenalectomy (23). However, the absence of OSA confirmation with sleep study might have explained the non-significant difference on the reduction of OSA probability between the surgical and medical therapies.
Possible mechanisms
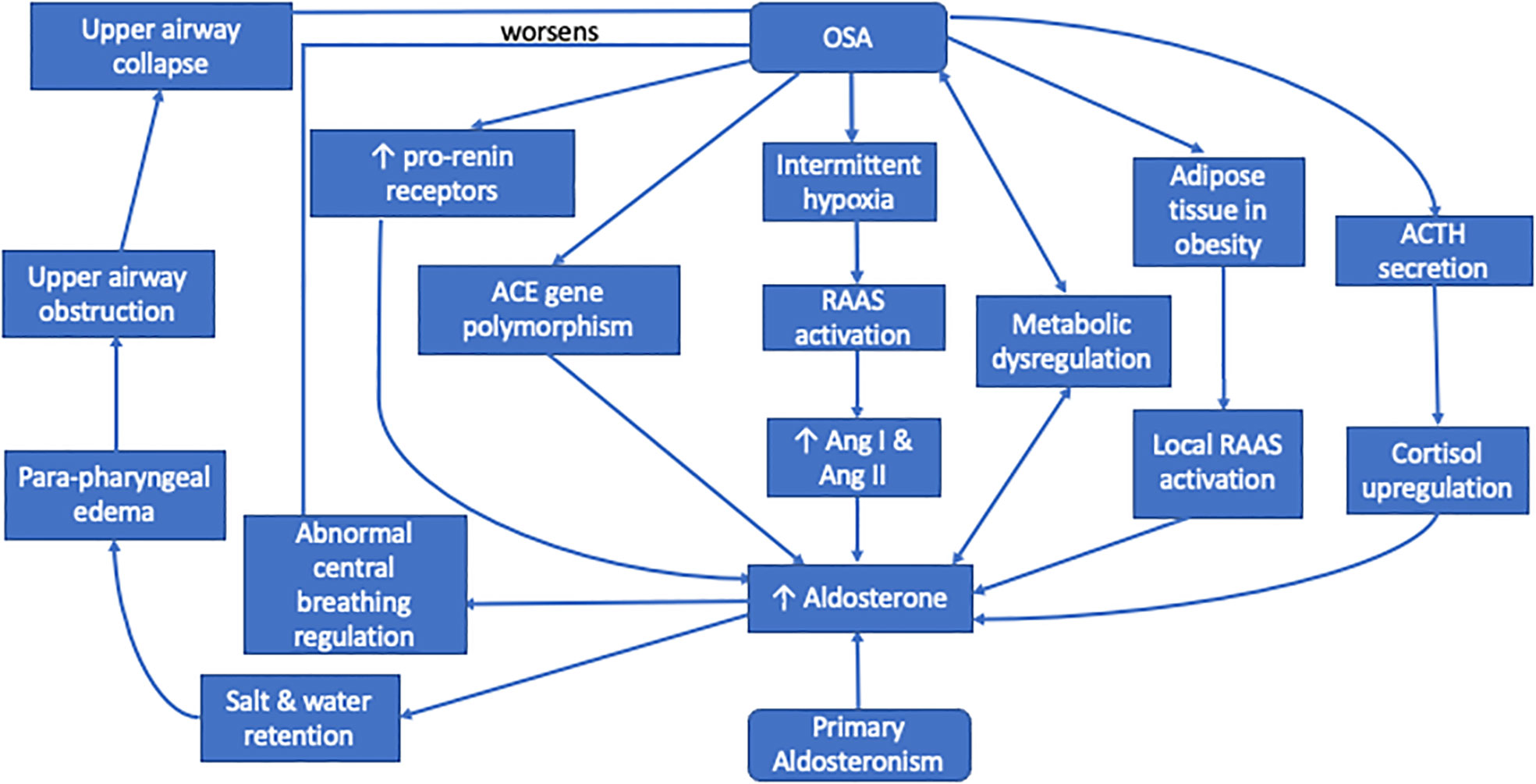
Hyperaldosteronism may worsen the clinical course of OSA in patients with PA due to aldosterone-induced fluid accumulation in the neck (24). Aldosterone excess leads to salt and water retention in the distal tubules (25) causing rostral fluid shifts and para-pharyngeal edema. With the presence of neck tissue congestion, this increases upper airway resistance and subsequently collapse, thus worsening OSA (26, 27).
Besides, in rat models, infused aldosterone acted centrally to increase brain RAAS activity, oxidative stress, and sympathetic drive (28). This aldosterone-induced activation of central receptors may also lead to abnormal regulation of central breathing mechanisms, leading to deterioration of OSA.
Aldosterone excess has detrimental effects on β cell function leading to hyperglycemia. Hence it is commonly associated with metabolic dysregulation including type 2 diabetes (2, 29). Moreover, hyperaldosteronism is reported to induce insulin resistance by several other mechanisms such as autonomous cortisol secretion, impairment of glucose uptake into the liver, increment of hepatic glucose release, and enhancement of insulin-like growth factor-1 signaling (2). This echoes the results from Framingham Offspring study which demonstrated that aldosterone level is positively correlated with development of metabolic syndrome and increment of systolic blood pressure (30, 31). Patients with type 2 diabetes were reported to have an almost 50% increased risk in developing OSA compared to those without diabetes, especially among insulin-treated cohort, suggesting the role of insulin resistance in development of OSA (32, 33). This could be contributed by several mechanisms, including mixed apneic events seen in patients with type 2 diabetes (34), increased oxidative stress, autonomic dysfunction (35) and weight gain secondary to anti-diabetic medications (33).
Soluble plasma pro-renin receptors, which are specific receptors for both renin and pro-renin, were found to be significantly higher in male patients with OSA compared to age matched non-OSA male (36). This might explain the higher prevalence of OSA seen in male patients with PA. These receptor levels were also demonstrated to be positively correlated with severity of OSA, but not BMI, which further supports the association between OSA and RAAS (36).
MR antagonists augment diuresis and reduce leg-to-neck fluid redistribution. This leads to reduction in pharyngeal edema and upper airway resistance, causing improvement in OSA severity (14).
PA in OSA
Prevalence
Among 203 multi-ethnic cohort of patients diagnosed with OSA and hypertension, the prevalence of PA was reported to be 8.9% (11.8% in White, 5.9% in Chinese) (14). The authors concluded that this prevalence is not significantly different compared to the prevalence of PA observed in earlier studies (8.9% vs 5.9% in general hypertensive population and 11.2% from referral centers). However, in these earlier studies, OSA was not screened for in the subjects, which may explain the comparable prevalence. Furthermore, the prevalence of PA in OSA reported in this study needs to be interpreted with caution as the study was performed in different centers, using different confirmatory tests, different kits for aldosterone and renin, with lack of single scoring center, which could have biased the results.
In another study of 94 patients with moderate-to-severe OSA and hypertension, PA was confirmed in 21.3% compared to 8% of those without OSA (37). The prevalence of other metabolic disorders was noted to be high in this studied population (diabetes and impaired fasting glucose 90%, difficult-to-treat hypertension 70%, resistant hypertension 60%). These show that PA is a common part of multi-morbidity in patients with OSA, including diabetes and resistant hypertension (38).
The prevalence of PA in patients with confirmed OSA is presented in Table 2.
Clinical characteristics
Majority of patients with OSA who were subsequently diagnosed with PA presented with uncontrolled blood pressure ≥150/100mmHg, resistant hypertension or hypokalemia (14, 37). The frequency of PA in patients who presented only with OSA symptoms is low (1/18 and 4/20 respectively) (14, 37). Plasma aldosterone level in patients with OSA and metabolic syndrome was significantly higher compared to patients with OSA without metabolic syndrome, and this level was significantly related to AHI, waist circumference, triglyceride and HDL-cholesterol levels (11). Among patients with moderate-to-severe OSA and type 2 diabetes, plasma aldosterone, plasma renin and urinary aldosterone levels were higher compared to non-OSA patients with type 2 diabetes, although no correlation was found between AHI and the RAAS components in this cohort (40).
Outcomes of treatment
The use of CPAP therapy, ranging from 1 week to 12 months, among different cohort of patients with OSA, ie presence of metabolic syndrome (11), normotension (41), essential (42–45) and resistant hypertension (46, 47), and type 2 diabetes (40), showed significant reduction in RAAS components. Several studies which demonstrated lack of reduction in the RAAS components were mostly limited by a small sample size or short duration of CPAP use (48–53). To date, there are no studies evaluating the effect of CPAP therapy on RAAS components amongst OSA and PA patients.
Possible mechanisms
Intermittent hypoxia was shown to increase plasma levels of renin and aldosterone. It also enhanced angiotensin (Ang) I expression and resulted in AngII stimulation of carotid body receptors in animal models (13, 54–57). Similarly, sleep fragmentation and repetitive arousals in patients with OSA may lead to activation of the RAAS, causing an increased secretion of AngI, AngII and subsequently aldosterone.
In animal studies, acute hypercapnia or hypoxia separately increased plasma aldosterone levels, which was independent of increases in plasma renin activity, suggesting a renin-independent pathway in aldosterone secretion (58, 59). Sleep fragmentation and repeated arousal induce stress which stimulates the release of ACTH from the pituitary (11). The persistent activation of sympathetic nerve during both sleep and wakefulness not only stimulates the RAAS (53), but also the hypothalamic-pituitary-adrenal axis in releasing cortisol (40). Both RAAS and ACTH synergistically regulate aldosterone pulse wave. While RAAS plays a major role at night when plasma cortisol concentration is low, elevated cortisol concentration controls the pulse amplitude of aldosterone during the daytime (40).
OSA is commonly found in patients who are obese. The adipose tissue present in obesity is an important source of RAAS hormone secretion, which is independent from the classical RAAS activation (60, 61). This is demonstrated in experimental studies which showed that adipocytes release adipokines and free fatty acids that could stimulate aldosterone secretion from the adrenocortical cells (59, 62, 63). The finding of renin-binding protein gene in adipocytes, which acts as renin inhibitor, might be involved in modulation of renin activity. AngI and AngII receptors were obtained in rodent and human adipocytes with increased expression of AngI gene, especially in visceral adipocytes (64).
The angiotensin converting enzyme (ACE) is a vital enzyme in RAAS, playing a major role in development of cardiovascular diseases with I/D polymorphism of the ACE gene. Interaction between OSA and the ACE gene I/D polymorphism was significantly associated with presence of hypertension among Swedish patients (65) but not in the Turkish cohort (66). Furthermore, it is shown that Caucasian individuals with DD genotype are more prone to develop hypertension (66), in contrast to II genotype in Asians (67). Hence, ethnic differences in the genotype distribution for ACE gene I/D polymorphism may explain the differences seen in RAAS dysregulation in patients with OSA of different ethnicity.
As intermittent hypoxia is closely related to activation of RAAS, the resolution of intermittent hypoxia by CPAP may decrease the activity of RAAS leading to a reduction in aldosterone level (45). Additionally, CPAP improves ventilation, reduces sleep interruption, reduces sympathetic excitability, and increases insulin sensitivity, which in turn reduces aldosterone level (40).
The proposed mechanisms of this bi-directional relationship which are understood so far are summarized in Figure 1.
Research gap and future direction
Despite the studies summarized above depicting the relationship between PA, RAAS and OSA, there remains gaps in knowledge with regards to the associations among these entities. Given that clear relationships between PA and OSA have yet to be established, there is a need for further larger prospective studies to examine these links, especially among patients with hypertension, to determine if OSA is truly more prevalent among patients with PA. Likewise, large scale studies to screen the RAAS hormones among patients with OSA are needed to elucidate the true prevalence of PA among this cohort of patients. Risk factors for these patients to have co-existent PA and OSA should be determined to enable clinicians to screen more effectively for the presence of these disorders in high risk patients.
Furthermore, as data of a few studies point toward a likelihood of adipocyte-derived factor releasing adipokines and free fatty acids that could stimulate aldosterone secretion from the adrenocortical cells, independent from the systemic RAAS circulation, studies which examine this local RAAS effect on patients with OSA can further contribute to the knowledge of the relationship between PA and OSA.
It is shown that ACE enzyme is involved in RAAS regulation and ACE gene I/D polymorphism may play a role in hypertension development. As genotype distribution could be contributed by ethnic differences, studies examining ethnic factors can further explain the mechanisms of association between PA and OSA. This might lead to exploration of the role of ethnicity in the complex relationship between PA and OSA, including diagnosis and response to treatment. For example, those with ACE gene polymorphism may respond better to anti-aldosterone treatment for hypertension or blockade of different aspects of the RAAS, hence may benefit more from this class of treatment. Some of the studies have also demonstrated the association between PA and OSA to be more prevalent in specific phenotype with male predominance. Hence studies examining gender differences of this relationship may provide further insights in this field.
Data on the effect of treatment, whether CPAP on RAAS or PA-directed treatment on OSA severity, is substantially insufficient to date. This is especially true for long term outcomes of these treatment, not only on the disease per se, but on cardiovascular outcomes and reduction of target organ damage. This is an important aspect to be explored as it can lead to targeted therapy, which can be beneficial in this cohort of patients. The use of MR antagonists, ACE inhibitor or angiotensin receptor blockers remains low and clinical trials exploring the effect of these treatment are essential towards optimal blood pressure control.
In clinical context, for patients who are confirmed to have PA, OSA screening needs to be considered especially among males and those who are older, with larger neck circumference, greater abdominal obesity, higher BMI and worse metabolic profile. On the other hand, among patients with confirmed OSA, PA should be screened especially if blood pressure is ≥150/100mmHg, or in the presence of resistant hypertension or hypokalemia.
The evidence presented herein further underscores the necessity of early recognition and diagnosis of PA in patients with OSA and hypertension, as well as OSA in patients with PA, in line with the current Endocrine Society Guideline recommendations.
Conclusion
Current evidence suggests a bi-directional relationship between PA and OSA via aldosterone-induced worsening of OSA and OSA-associated dysregulation of RAAS. The beneficial effect seen with treatment of OSA on RAAS as well as PA-directed treatment on OSA severity further supports the role of RAAS-driven pathogenesis in worsening of OSA, as well as the role of OSA-driven pathogenesis in worsening of PA. Nevertheless, given that clear relationship between PA and OSA has yet to be established, there is a need for further studies to examine this link, particularly the role of genotypic and phenotypic relationship, as well as long term beneficial effects of PA-directed therapy in OSA and vice versa.
Author contributions
HL conceived, designed and drafted the work. NS revised it critically for important intellectual content. All authors contributed to the article and approved the submitted version.
Funding
The authors received funding from Malaysian Ministry of Higher Education Fundamental Research Grant Scheme (FRGS/1/2021/SKK01/UNIMAS/02/1) and the National University of Malaysia (UKM) grant (FF-2022-066).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Reincke M, Bancos I, Mulatero P, Scholl UI, Stowasser M, Williams TA. Diagnosis and treatment of primary aldosteronism. Lancet Diabetes Endocrinol (2021) 9(12):876–92. doi: 10.1016/S2213-8587(21)00210-2
2. Loh HH, Sukor N. Associations between primary aldosteronism and diabetes, poor bone health, and sleep apnea-what do we know so far? J Hum Hypertens (2020) 34(1):5–15. doi: 10.1038/s41371-019-0294-8
3. Yozamp N, Vaidya A. The prevalence of primary aldosteronism and evolving approaches for treatment. Curr Opin Endocr Metab Res (2019) 8:30–9. doi: 10.1016/j.coemr.2019.07.001
4. Cooper SA, Whaley-Connell A, Habibi J, Wei Y, Lastra G, Manrique C, et al. Renin-angiotensin-aldosterone system and oxidative stress in cardiovascular insulin resistance. Am J Physiol Heart Circulatory Physiol (2007) 293(4):H2009–23. doi: 10.1152/ajpheart.00522.2007
5. Epstein M. Aldosterone blockade: an emerging strategy for abrogating progressive renal disease. Am J Med (2006) 119(11):912–9. doi: 10.1016/j.amjmed.2006.03.038
6. Williams JS, Williams GH. 50th anniversary of aldosterone. J Clin Endocrinol Metab (2003) 88(6):2364–72. doi: 10.1210/jc.2003-030490
7. Brown NJ. Aldosterone and vascular inflammation. Hypertension (2008) 51(2):161–7. doi: 10.1161/HYPERTENSIONAHA.107.095489
8. Rundo JV. Obstructive sleep apnea basics. Cleve Clin J Med (2019) 86(9 Suppl 1):2–9. doi: 10.3949/ccjm.86.s1.02
9. Valaiyapathi B, Calhoun DA. Role of mineralocorticoid receptors in obstructive sleep apnea and metabolic syndrome. Curr Hypertens Rep (2018) 20(3):23. doi: 10.1007/s11906-018-0819-5
10. Surani SR. Diabetes, sleep apnea, obesity and cardiovascular disease: Why not address them together? World J Diabetes (2014) 5(3):381–4. doi: 10.4239/wjd.v5.i3.381
11. Barceló A, Piérola J, Esquinas C, de la Peña M, Arqué M, Alonso-Fernández A, et al. Relationship between aldosterone and the metabolic syndrome in patients with obstructive sleep apnea hypopnea syndrome: effect of continuous positive airway pressure treatment. PloS One (2014) 9(1):e84362. doi: 10.1371/journal.pone.0084362
12. Funder JW, Carey RM, Mantero F, Murad MH, Reincke M, Shibata H, et al. The management of primary aldosteronism: Case detection, diagnosis, and treatment: An endocrine society clinical practice guideline. J Clin Endocrinol Metab (2016) 101(5):1889–916. doi: 10.1210/jc.2015-4061
13. Fletcher EC, Bao G, Li R. Renin activity and blood pressure in response to chronic episodic hypoxia. Hypertension (1999) 34(2):309–14. doi: 10.1161/01.HYP.34.2.309
14. Buffolo F, Li Q, Monticone S, Heinrich DA, Mattei A, Pieroni J, et al. Primary aldosteronism and obstructive sleep apnea: A cross-sectional multi-ethnic study. Hypertension (2019) 74(6):1532–40. doi: 10.1161/HYPERTENSIONAHA.119.13833
15. Nakamura Y, Kobayashi H, Tanaka S, Hatanaka Y, Fuke Y, Fukuda N, et al. Primary aldosteronism and obstructive sleep apnea: A single-center cross-sectional study of the Japanese population. Med (Baltimore) (2021) 100(11):e25049. doi: 10.1097/MD.0000000000025049
16. Prejbisz A, Florczak E, Klisiewicz A, Dobrowolski P, Janaszek-Sitkowska H, Bielen P, et al. Relationship between primary aldosteronism and obstructive sleep apnoea, metabolic abnormalities and cardiac structure in patients with resistant hypertension. Endokrynol Pol (2013) 64(5):363–7. doi: 10.5603/EP.2013.0019
17. Wolley MJ, Pimenta E, Calhoun D, Gordon RD, Cowley D, Stowasser M. Treatment of primary aldosteronism is associated with a reduction in the severity of obstructive sleep apnoea. J Hum hypertension (2017) 31(9):561–7. doi: 10.1038/jhh.2017.28
18. Li KK, Powell NB, Kushida C, Riley RW, Adornato B, Guilleminault C. A comparison of Asian and white patients with obstructive sleep apnea syndrome. Laryngoscope (1999) 109(12):1937–40. doi: 10.1097/00005537-199912000-00007
19. Yamagishi K, Ohira T, Nakano H, Bielinski SJ, Sakurai S, Imano H, et al. Cross-cultural comparison of the sleep-disordered breathing prevalence among americans and Japanese. Eur Respir J (2010) 36(2):379–84. doi: 10.1183/09031936.00118609
20. Graudal N, Jurgens G. The blood pressure sensitivity to changes in sodium intake is similar in asians, blacks and whites. an analysis of 92 randomized controlled trials. Front Physiol (2015) 6:157. doi: 10.3389/fphys.2015.00157
21. Gaddam K, Pimenta E, Thomas SJ, Cofield SS, Oparil S, Harding SM, et al. Spironolactone reduces severity of obstructive sleep apnoea in patients with resistant hypertension: a preliminary report. J Hum hypertension (2010) 24(8):532–7. doi: 10.1038/jhh.2009.96
22. Yang L, Zhang H, Cai M, Zou Y, Jiang X, Song L, et al. Effect of spironolactone on patients with resistant hypertension and obstructive sleep apnea. Clin Exp Hypertens (2016) 38(5):464–8. doi: 10.3109/10641963.2015.1131290
23. Wang E, Chomsky-Higgins K, Chen Y, Nwaogu I, Seib CD, Shen WT, et al. Treatment of primary aldosteronism reduces the probability of obstructive sleep apnea. J Surg Res (2019) 236:37–43. doi: 10.1016/j.jss.2018.10.040
24. Dudenbostel T, Calhoun DA. Resistant hypertension, obstructive sleep apnoea and aldosterone. J Hum Hypertens (2012) 26(5):281–7. doi: 10.1038/jhh.2011.47
25. Laragh J. Laragh's lessons in pathophysiology and clinical pearls for treating hypertension. Am J Hypertens (2001) 14(9 Pt 1):837–54. doi: 10.1016/S0895-7061(01)02222-1
26. Pratt-Ubunama MN, Nishizaka MK, Boedefeld RL, Cofield SS, Harding SM, Calhoun DA. Plasma aldosterone is related to severity of obstructive sleep apnea in subjects with resistant hypertension. Chest (2007) 131(2):453–9. doi: 10.1378/chest.06-1442
27. Gonzaga CC, Gaddam KK, Ahmed MI, Pimenta E, Thomas SJ, Harding SM, et al. Severity of obstructive sleep apnea is related to aldosterone status in subjects with resistant hypertension. J Clin sleep Med JCSM Off Publ Am Acad Sleep Med (2010) 6(4):363–8. doi: 10.5664/jcsm.27878
28. Zhang ZH, Yu Y, Kang YM, Wei SG, Felder RB. Aldosterone acts centrally to increase brain renin-angiotensin system activity and oxidative stress in normal rats. Am J Physiol Heart Circ Physiol (2008) 294(2):H1067–74. doi: 10.1152/ajpheart.01131.2007
29. Reutrakul S, Mokhlesi B. Obstructive sleep apnea and diabetes: A state of the art review. Chest (2017) 152(5):1070–86. doi: 10.1016/j.chest.2017.05.009
30. Vasan RS, Evans JC, Larson MG, Wilson PW, Meigs JB, Rifai N, et al. Serum aldosterone and the incidence of hypertension in nonhypertensive persons. N Engl J Med (2004) 351(1):33–41. doi: 10.1056/NEJMoa033263
31. Ingelsson E, Pencina MJ, Tofler GH, Benjamin EJ, Lanier KJ, Jacques PF, et al. Multimarker approach to evaluate the incidence of the metabolic syndrome and longitudinal changes in metabolic risk factors: the framingham offspring study. Circulation (2007) 116(9):984–92. doi: 10.1161/CIRCULATIONAHA.107.708537
32. Huang T, Lin BM, Stampfer MJ, Tworoger SS, Hu FB, Redline S. A population-based study of the bidirectional association between obstructive sleep apnea and type 2 diabetes in three prospective U.S. cohorts. Diabetes Care (2018) 41(10):2111–9. doi: 10.2337/dc18-0675
33. Subramanian A, Adderley NJ, Tracy A, Taverner T, Hanif W, Toulis KA, et al. Risk of incident obstructive sleep apnea among patients with type 2 diabetes. Diabetes Care (2019) 42(5):954–63. doi: 10.2337/dc18-2004
34. Iftikhar IH, Hoyos CM, Phillips CL, Magalang UJ. Meta-analyses of the association of sleep apnea with insulin resistance, and the effects of CPAP on HOMA-IR, adiponectin, and visceral adipose fat. J Clin sleep Med JCSM Off Publ Am Acad Sleep Med (2015) 11(4):475–85. doi: 10.5664/jcsm.4610
35. Tantucci C, Scionti L, Bottini P, Dottorini ML, Puxeddu E, Casucci G, et al. Influence of autonomic neuropathy of different severities on the hypercapnic drive to breathing in diabetic patients. Chest (1997) 112(1):145–53. doi: 10.1378/chest.112.1.145
36. Nishijima T, Tajima K, Takahashi K, Sakurai S. Elevated plasma levels of soluble (pro)renin receptor in patients with obstructive sleep apnea syndrome: association with polysomnographic parameters. Peptides (2014) 56:14–21. doi: 10.1016/j.peptides.2014.03.008
37. Dobrowolski P, Kolodziejczyk-Kruk S, Warchol-Celinska E, Kabat M, Ambroziak U, Wrobel A, et al. Primary aldosteronism is highly prevalent in patients with hypertension and moderate to severe obstructive sleep apnea. J Clin Sleep Med (2021) 17(4):629–37. doi: 10.5664/jcsm.8960
38. Umpierrez GE, Cantey P, Smiley D, Palacio A, Temponi D, Luster K, et al. Primary aldosteronism in diabetic subjects with resistant hypertension. Diabetes Care (2007) 30(7):1699–703. doi: 10.2337/dc07-0031
39. Di Murro A, Petramala L, Cotesta D, Zinnamosca L, Crescenzi E, Marinelli C, et al. Renin-angiotensin-aldosterone system in patients with sleep apnoea: prevalence of primary aldosteronism. J Renin Angiotensin Aldosterone Syst (2010) 11(3):165–72. doi: 10.1177/1470320310366581
40. Zhang J, Tian L, Guo L. Changes of aldosterone levels in patients with type 2 diabetes complicated by moderate to severe obstructive sleep apnea-hypopnea syndrome before and after treatment with continuous positive airway pressure. J Int Med Res (2019) 47(10):4723–33. doi: 10.1177/0300060519868337
41. Nicholl DD, Hanly PJ, Poulin MJ, Handley GB, Hemmelgarn BR, Sola DY, et al. Evaluation of continuous positive airway pressure therapy on renin-angiotensin system activity in obstructive sleep apnea. Am J Respir Crit Care Med (2014) 190(5):572–80. doi: 10.1164/rccm.201403-0526OC
42. Wang HL, Wang Y, Zhang Y, Chen YD, Wang XC, Liu ZX, et al. Changes in plasma angiotensin II and circadian rhythm of blood pressure in hypertensive patients with sleep apnea syndrome before and after treatment. Chin Med Sci J (2011) 26(1):9–13. doi: 10.1016/S1001-9294(11)60013-8
43. Casitas R, Martínez-Cerón E, Galera R, Cubillos-Zapata C, González-Villalba MJ, Fernández-Navarro I, et al. The effect of treatment for sleep apnoea on determinants of blood pressure control. Eur Respir J (2017) 50(5):1701261. doi: 10.1183/13993003.01261-2017
44. Saarelainen S, Hasan J, Siitonen S, Seppälä E. Effect of nasal CPAP treatment on plasma volume, aldosterone and 24-h blood pressure in obstructive sleep apnoea. J sleep Res (1996) 5(3):181–5. doi: 10.1046/j.1365-2869.1996.t01-1-00007.x
45. Lacedonia D, Tamisier R, Roche F, Monneret D, Baguet JP, Lévy P, et al. Respective effects of OSA treatment and angiotensin receptor blocker on aldosterone in hypertensive OSA patients: a randomized cross-over controlled trial. Int J Cardiol (2014) 177(2):629–31. doi: 10.1016/j.ijcard.2014.09.123
46. de Souza F, Muxfeldt ES, Margallo V, Cortez AF, Cavalcanti AH, Salles GF. Effects of continuous positive airway pressure treatment on aldosterone excretion in patients with obstructive sleep apnoea and resistant hypertension: a randomized controlled trial. J hypertension (2017) 35(4):837–44. doi: 10.1097/HJH.0000000000001254
47. Sanchez-de-la-Torre M, Khalyfa A, Sanchez-de-la-Torre A, Martinez-Alonso M, Martinez-Garcia MA, Barcelo A, et al. Precision medicine in patients with resistant hypertension and obstructive sleep apnea: Blood pressure response to continuous positive airway pressure treatment. J Am Coll Cardiol (2015) 66(9):1023–32. doi: 10.1016/j.jacc.2015.06.1315
48. Joyeux-Faure M, Baguet JP, Barone-Rochette G, Faure P, Sosner P, Mounier-Vehier C, et al. Continuous positive airway pressure reduces night-time blood pressure and heart rate in patients with obstructive sleep apnea and resistant hypertension: The RHOOSAS randomized controlled trial. Front Neurol (2018) 9:318. doi: 10.3389/fneur.2018.00318
49. Meston N, Davies RJ, Mullins R, Jenkinson C, Wass JA, Stradling JR. Endocrine effects of nasal continuous positive airway pressure in male patients with obstructive sleep apnoea. J Internal Med (2003) 254(5):447–54. doi: 10.1046/j.1365-2796.2003.01212.x
50. Moller DS, Lind P, Strunge B, Pedersen EB. Abnormal vasoactive hormones and 24-hour blood pressure in obstructive sleep apnea. Am J Hypertens (2003) 16(4):274–80. doi: 10.1016/S0895-7061(02)03267-3
51. Pedrosa RP, Drager LF, de Paula LKG, Amaro ACS, Bortolotto LA, Lorenzi-Filho G. Effects of OSA treatment on BP in patients with resistant hypertension: A randomized trial. Chest (2013) 144(5):1487–94. doi: 10.1378/chest.13-0085
52. Rodenstein DO, D'Odemont JP, Pieters T, Aubert-Tulkens G. Diurnal and nocturnal diuresis and natriuresis in obstructive sleep apnea. effects of nasal continuous positive airway pressure therapy. Am Rev Respir Dis (1992) 145(6):1367–71. doi: 10.1164/ajrccm/145.6.1367
53. Svatikova A, Olson LJ, Wolk R, Phillips BG, Adachi T, Schwartz GL, et al. Obstructive sleep apnea and aldosterone. Sleep (2009) 32(12):1589–92. doi: 10.1093/sleep/32.12.1589
54. May AM, Mehra R. Obstructive sleep apnea: role of intermittent hypoxia and inflammation. Semin Respir Crit Care Med (2014) 35(5):531–44. doi: 10.1055/s-0034-1390023
55. Fletcher EC, Orolinova N, Bader M. Blood pressure response to chronic episodic hypoxia: the renin-angiotensin system. J Appl Physiol (Bethesda Md 1985) (2002) 92(2):627–33. doi: 10.1152/japplphysiol.00152.2001
56. Fung ML, Tipoe GL, Leung PS. Mechanisms of maladaptive responses of peripheral chemoreceptors to intermittent hypoxia in sleep-disordered breathing. Sheng Li Xue Bao (2014) 66(1):23–9. doi: 10.13294/j.aps.2014.0004
57. Lam SY, Liu Y, Ng KM, Liong EC, Tipoe GL, Leung PS, et al. Upregulation of a local renin-angiotensin system in the rat carotid body during chronic intermittent hypoxia. Exp Physiol (2014) 99(1):220–31. doi: 10.1113/expphysiol.2013.074591
58. Raff H, Roarty TP. Renin, ACTH, and aldosterone during acute hypercapnia and hypoxia in conscious rats. Am J Physiol (1988) 254(3 Pt 2):R431–5. doi: 10.1152/ajpregu.1988.254.3.R431
59. Marzolla V, Armani A, Zennaro MC, Cinti F, Mammi C, Fabbri A, et al. The role of the mineralocorticoid receptor in adipocyte biology and fat metabolism. Mol Cell Endocrinol (2012) 350(2):281–8. doi: 10.1016/j.mce.2011.09.011
60. Vecchiola A, Lagos CF, Carvajal CA, Baudrand R, Fardella CE. Aldosterone production and signaling dysregulation in obesity. Curr Hypertens Rep (2016) 18(3):20. doi: 10.1007/s11906-016-0626-9
61. Goodfriend TL, Calhoun DA. Resistant hypertension, obesity, sleep apnea, and aldosterone: theory and therapy. Hypertension (2004) 43(3):518–24. doi: 10.1161/01.HYP.0000116223.97436.e5
62. Goodfriend TL, Egan BM, Kelley DE. Plasma aldosterone, plasma lipoproteins, obesity and insulin resistance in humans. Prostaglandins Leukot Essent Fatty Acids (1999) 60(5-6):401–5. doi: 10.1016/S0952-3278(99)80020-9
63. Hall JE. The kidney, hypertension, and obesity. Hypertension (2003) 41(3 Pt 2):625–33. doi: 10.1161/01.HYP.0000052314.95497.78
64. Engeli S, Negrel R, Sharma AM. Physiology and pathophysiology of the adipose tissue renin-angiotensin system. Hypertension (2000) 35(6):1270–7. doi: 10.1161/01.HYP.35.6.1270
65. Boström KB, Hedner J, Melander O, Grote L, Gullberg B, Råstam L, et al. Interaction between the angiotensin-converting enzyme gene insertion/deletion polymorphism and obstructive sleep apnoea as a mechanism for hypertension. J hypertension (2007) 25(4):779–83. doi: 10.1097/HJH.0b013e328017f6d5
66. Yakut T, Karkucak M, Ursavas A, Gulten T, Burgazlioglu B, Gorukmez O, et al. Lack of association of ACE gene I/D polymorphism with obstructive sleep apnea syndrome in Turkish patients. Genet Mol Res GMR. (2010) 9(2):734–8. doi: 10.4238/vol9-2gmr755
Keywords: renin, angiotensin, aldosterone, sleep disorders, obesity, hypertension, RAAS
Citation: Loh HH and Sukor N (2022) Primary aldosteronism and obstructive sleep apnea: What do we know thus far? Front. Endocrinol. 13:976979. doi: 10.3389/fendo.2022.976979
Received: 24 June 2022; Accepted: 20 September 2022;
Published: 29 September 2022.
Edited by:
Silvia Monticone, University of Turin, ItalyReviewed by:
Aleksander Prejbisz, National Institute of Cardiology, PolandElisa Sconfienza, University of Turin, Italy
Copyright © 2022 Loh and Sukor. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Norlela Sukor, drlela2020@yahoo.com
 Huai Heng Loh
Huai Heng Loh