- Department of Ultrasound, China-Japan Union Hospital, Jilin University, Changchun, China
Background: This study is a meta-analysis based on evidence-based medicine to explore the long-term (≥3 years) efficacy of thermal ablation in the treatment of papillary thyroid carcinoma (PTC).
Methods: We searched the PubMed, Embase, and Cochrane Library databases for studies published during the time between the establishment of the databases through June 2022. We included 13 non-randomized-controlled trials (non-RCTs) that reported the application of ultrasound-guided thermal ablation in PTC. We excluded studies that were repeated publications, research without full text, contained incomplete information, lacked data extraction, involved animal experiments, reviews, and systematic reviews. STATA 15.1 software was used to analyze the data.
Results: Tumor volume after thermal ablation at 3-year follow-up was significantly lower than pre-ablation (standardized mean difference [SMD] = -1.06, 95% CI: -1.32~-0.80). The pooled results indicated that the maximum diameter after thermal ablation at 3-year follow-up was significantly lower than pre-ablation (SMD = -1.93, 95% CI: -12.13~-1.73). The pooled results indicated that volume reduction rate (VRR) after thermal ablation at 3-year follow-up was 98.91% (95% CI: 97.98–99.83%), and complete disappearance rate (CDR) after thermal ablation at 3-year follow-up was 83% (95% CI: 67–94%). In addition, the incidence of newly discovered mPTC and lymph node metastases after thermal ablation was 0.3% (95% CI: 0.0–1.0%) and 0.0% (95% CI: 0.0–0.0%), respectively.
Conclusion: Overall, the long-term (≥3 years) efficacy of ultrasound-guided thermal ablation in the treatment of PTC was significant, with favorable disease progression. Ultrasound-guided thermal ablation can be considered an alternative approach for patients with PTC who refuse surgery or are unable to undergo surgery.
Introduction
Thyroid nodules are a common disease of the endocrine system; most are benign, and malignant nodules account for 5–15%. The most common type of malignant thyroid tumor is papillary thyroid carcinoma (PTC), accounting for 85% of all malignant thyroid tumors (1). It is associated with a favorable prognosis and a low mortality rate (2, 3). In PTC, if the diameter of the tumor is ≤1 cm, then it can be defined as papillary thyroid microcarcinoma (PTMC), which account for 50–60% of all PTC cases. Yu et al. reported 10- and 15-year cause-specific survival (CSS) rates for PTMC of 99.5% and 99.3%, respectively (4). Although surgery is the standard treatment for PTC, there is considerable disagreement regarding the management of PTC (5, 6). In general, the risk of PTC is very low, and the disease course is slow. Therefore, surgical thyroidectomy may be considered a very aggressive treatment option for some patients (6, 7). However, some patients are not candidates for surgery or are reluctant to undergo surgery for cosmetic reasons; therefore, exploration of other treatment options for PTC is imperative.
As a new minimally invasive treatment modality, ultrasound-guided thermal ablation, including laser ablation, radiofrequency ablation (RFA), and microwave ablation (MWA), has been reported in numerous attempts at treating PTC (8–12). In the past, surgery to treat papillary thyroid microcarcinoma did suffer from overtreatment. Therefore, many international counterparts suggested dynamic observation of PTC. Indeed, most patients with PTC did not show significant changes in dynamic observation. However, some patients withdrew from dynamic observation due to concerns or local progression and went for surgery instead (13). Thermal ablation therapy is the third option in addition to surgery and dynamic observation because it can effectively treat PTC without the huge trauma like surgery, nor will it cause obvious damage to thyroid function. Therefore, patients who are unwilling to perform dynamic observation in the past can use thermal ablation therapy, which can not only relieve the patient’s physical and psychological discomfort, but also avoid the damage caused by the operation, making thermal ablation therapy possible solution to solve the overtreatment of PTMC (13). These thermal ablation techniques all have unique advantages and have been shown to achieve favorable clinical results, with a low incidence of complications (14, 15). However, to date, there have been no evidence-based results addressing the long-term efficacy of thermal ablation applications in PTC. Therefore, we conducted a meta-analysis based on evidence-based medicine to explore the long-term (≥3 years) efficacy of thermal ablation in the treatment of PTC.
Methods
Literature inclusion and exclusion criteria
The inclusion criteria:
1) Study object: patients with PTC
2) Intervention measures: thermal ablation
3) Outcome indicators: volume of tumor, volume reduction rate (VRR), complete disappearance rate (CDR), incidence of newly discovered PTC, and lymph node metastasis (LNM)
4) Study design: non-randomized controlled trial (non-RCT)
The exclusion criteria: repeated publications, studies without full text or that could not conduct data extraction, studies using animal experiments, reviews, and systematic reviews.
Search strategy
In this meta-analysis, we searched the PubMed, Embase, and Cochrane Library databases from establishment of the database to September 2022. The search terms were: (“Thyroid Cancer, Papillary” OR “Papillary Thyroid Cancer” OR “Papillary Thyroid Carcinoma” OR “Nonmedullary Thyroid Carcinomas” OR “papillary thyroid microcarcinoma” OR “PTMC”) AND (“radiofrequency ablation” OR “RFA” OR “laser ablation” OR “LA” OR “microwave ablation” OR “MWA” OR “thermal ablation”).
Literature screening and data extraction
Two researchers independently performed the literature search, screening, and information extraction. When a question or dispute arose, we reached a consensus after discussion or negotiation. The data extraction included: author(s); article publication year; country; research type; number of patients; and outcome indicators, including tumor volume, VRR, CDR, incidence of newly discovered PTC, and LNM.
Literature quality assessment
Two independent researchers assessed the quality of evidence for each study using the methodological index for non-randomized studies (MINORS) scale (16). There were 12 items in total, each with a potential score of 0 to 2 and a total score of 24. Based on score, the studies were classified as “moderate quality” from 9 to 16 and \ “high quality” from 17 to 24.
Data synthesis and statistical analysis
We analyzed all data using STATA (version 15.1). Standardized mean difference (SMD) was used to assess differences in continuous variables. We used I2 and Q tests to evaluate heterogeneity. If the heterogeneity test was P ≥ 0.1 and I2 ≤ 50%, there was homogeneity among studies, and the fixed effects model was used for combined analysis; if P < 0.1 and I2 > 50%, there was heterogeneity, and a sensitivity analysis was conducted to identify its source. If the heterogeneity remained large, we used a random effects model or abandoned the combination of results and used descriptive analysis. We used a funnel plot and Egger’s test to assess publication bias.
Results
Results of the literature search
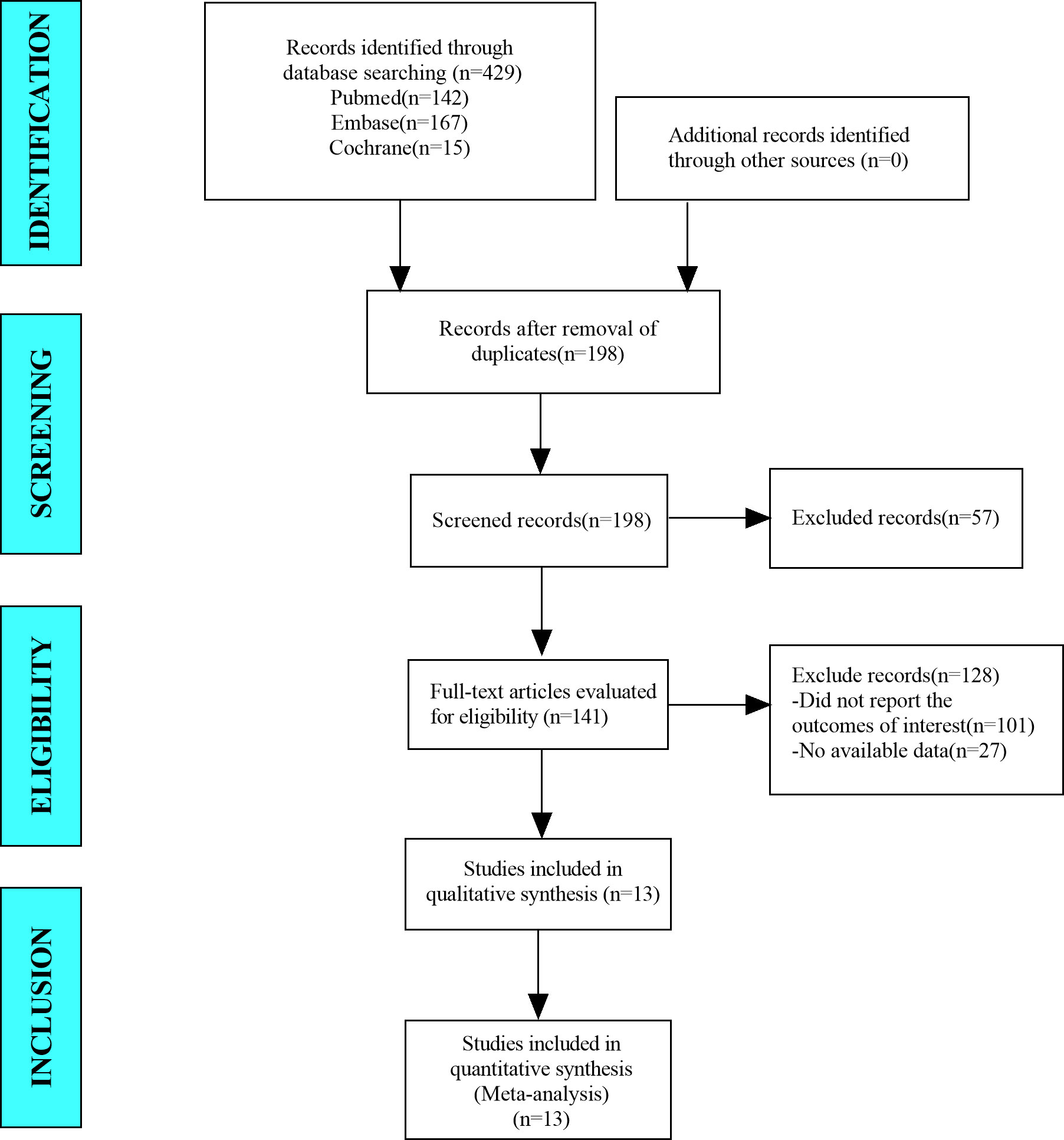
In this meta-analysis, we retrieved a total of 429 studies from the databases, which included PubMed, Embase, and Cochrane Library. After eliminating duplicate studies, 198 studies remained. After browsing titles and abstracts, 141 studies remained. After browsing full-text studies, we obtained 13 studies and excluded any that did not report the outcomes of interest or other ablation methods. Finally, we included 13 articles in the meta-analysis (Figure 1).
Baseline characteristics and quality assessment of the included studies
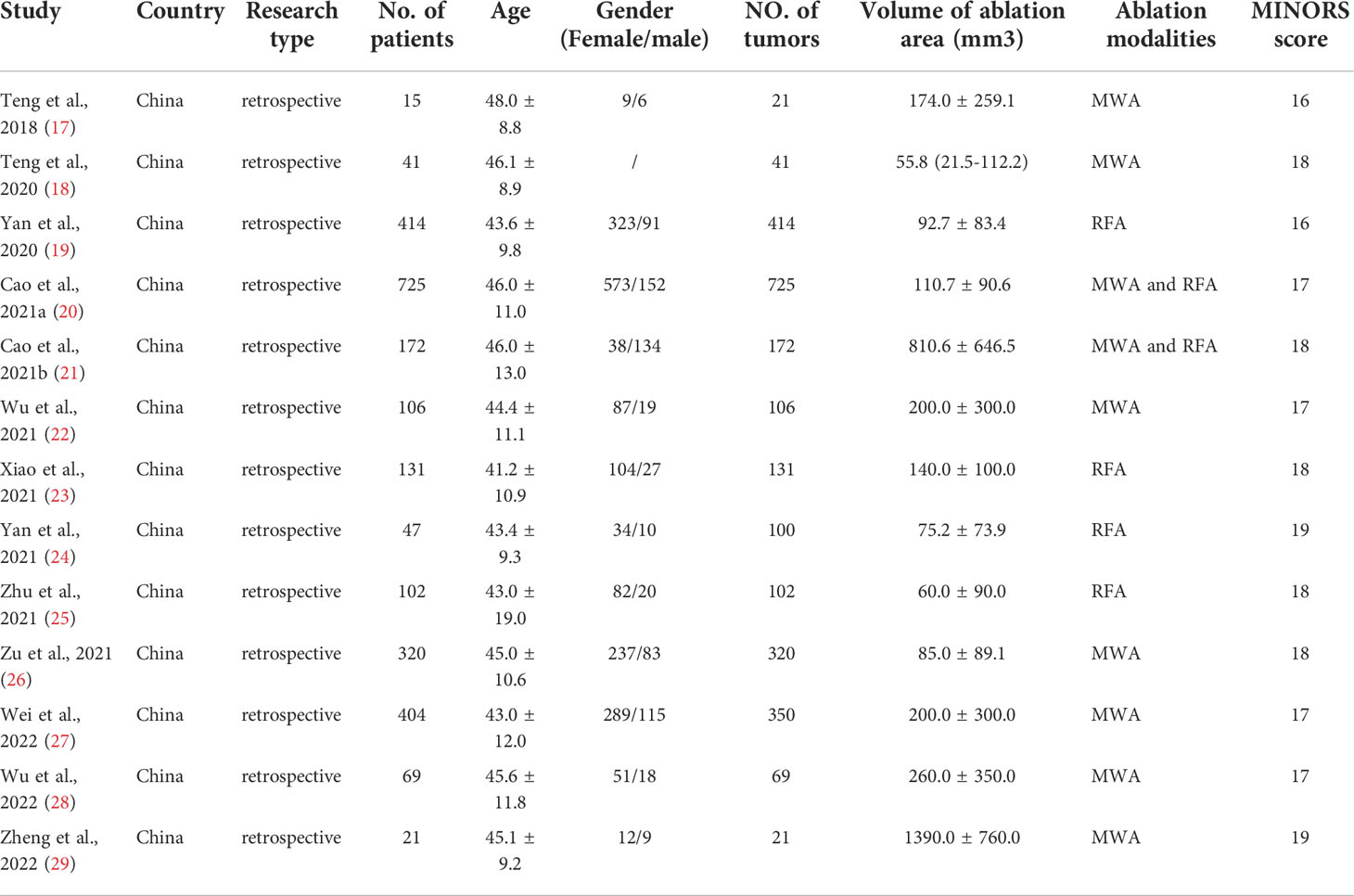
In total, we included 13 non-RCT studies in this meta-analysis, and all were retrospective studies. The sample size was 2567 patients, and the number of tumors was 2572. MINORS scores were all above 16 points. The mean age distribution of patients was between 41.2 and 48.0 years, which is comparable. Thermal ablation modalities used in selected studies included MWA and RFA. Two studies received a score of 16, four studies scored 17, five studies scored 18, and two studies scored 19, indicating that the literature included was of moderate or high quality (Table 1).
Results of the meta-analysis
Tumor volume
There were 11 studies in which 2510 patients were enrolled that reported changes in tumor volume after thermal ablation at 3-year follow-up. Because there was significant heterogeneity (I2 = 88.5%, P = 0.000), we conducted a sensitivity analysis and found that the Yan et al.’s 2020 study had a large impact on the results (Figure S1). Heterogeneity decreased after excluding this study (I2 = 82.9%, P = 0.000) (Figure 2A), and we conducted a meta-analysis using a random effects model. The pooled results showed that tumor volume after thermal ablation at 3-year follow-up was significantly lower than pre-ablation (SMD = -1.06, 95% CI: -1.32~-0.80, P = 0.000; Figure 2B).
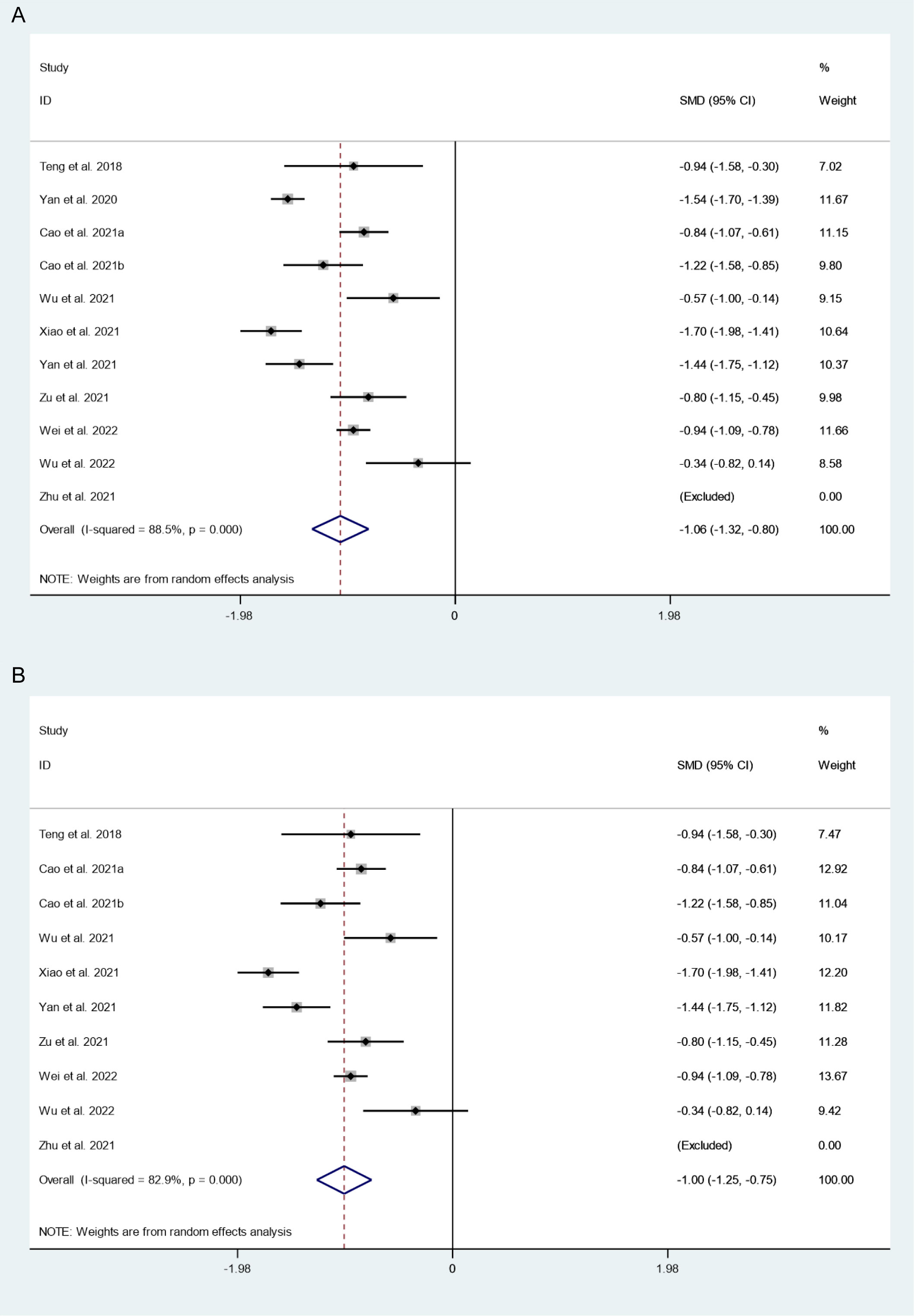
Figure 2 Changes in tumor volume after thermal ablation at 3-year follow-up (A) Before sensitivity analysis; (B) After sensitivity analysis).
Maximum diameter
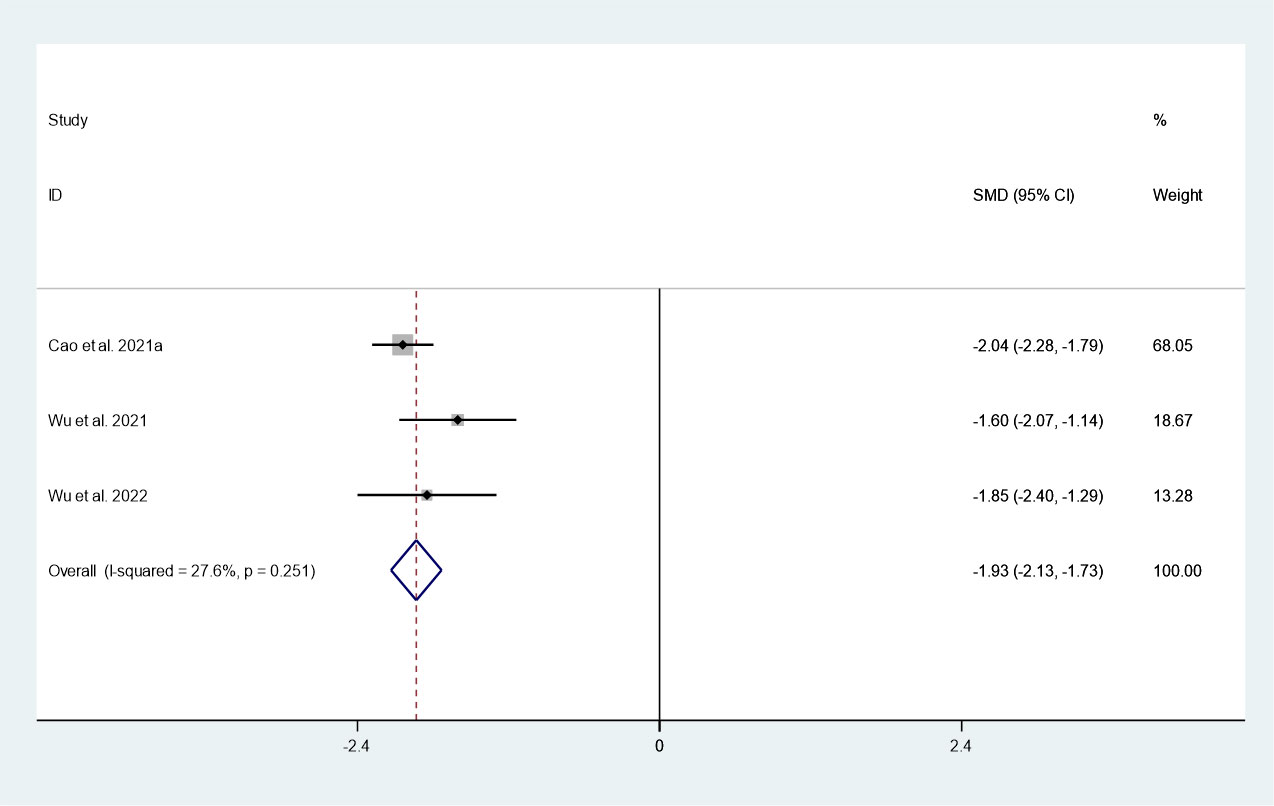
There were 3 studies in which 1036 patients were enrolled that reported changes in maximum diameter after thermal ablation at 3-year follow-up. Because there was no significant heterogeneity (I2 = 27.6%, P = 0.251), we conducted a meta-analysis using a fixed effects model. The pooled results indicated that the maximum diameter after thermal ablation at 3-year follow-up was significantly lower than pre-ablation (SMD = -1.93, 95% CI: -12.13~-1.73, P = 0.000; Figure 3).
VRR
There were 8 studies in which 794 patients were enrolled that reported VRR after thermal ablation at 3-year follow-up. Because there was significant heterogeneity (I2 = 81.0%, P = 0.000), we conducted sensitivity analysis and found that the Yan et al.’s 2021 study had a large impact on the results (Figure S3). Heterogeneity decreased after excluding this study (I2 = 77.2%, P = 0.000) (Figure 4A), and we conducted a meta-analysis using a random effects model. The pooled results indicated that VRR after thermal ablation at 3-year follow-up was 98.91% (95% CI: 97.98–99.83%; Figure 4B).
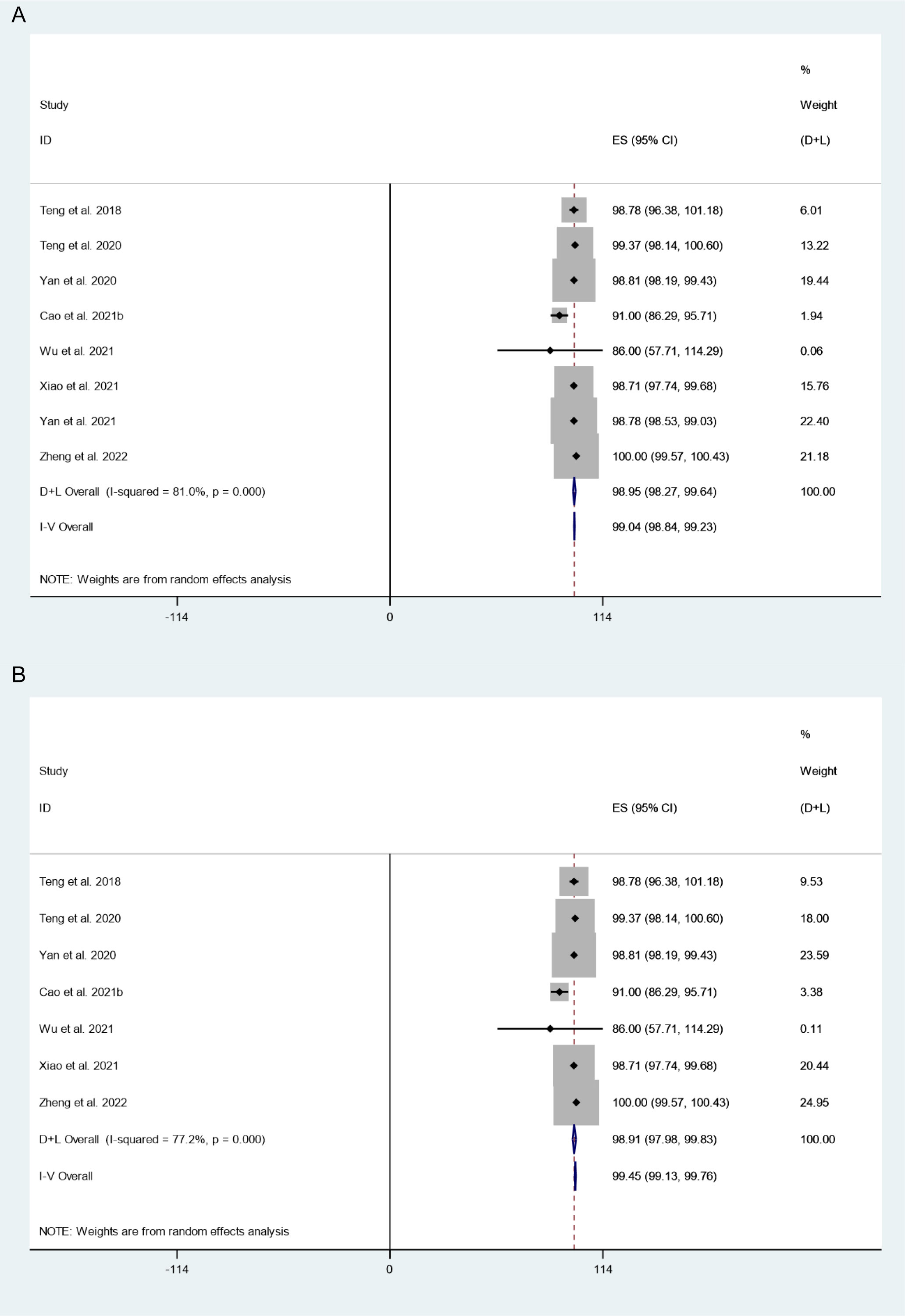
Figure 4 VRR after thermal ablation at 3-year follow-up (A) Before sensitivity analysis; (B) After sensitivity analysis).
CDR
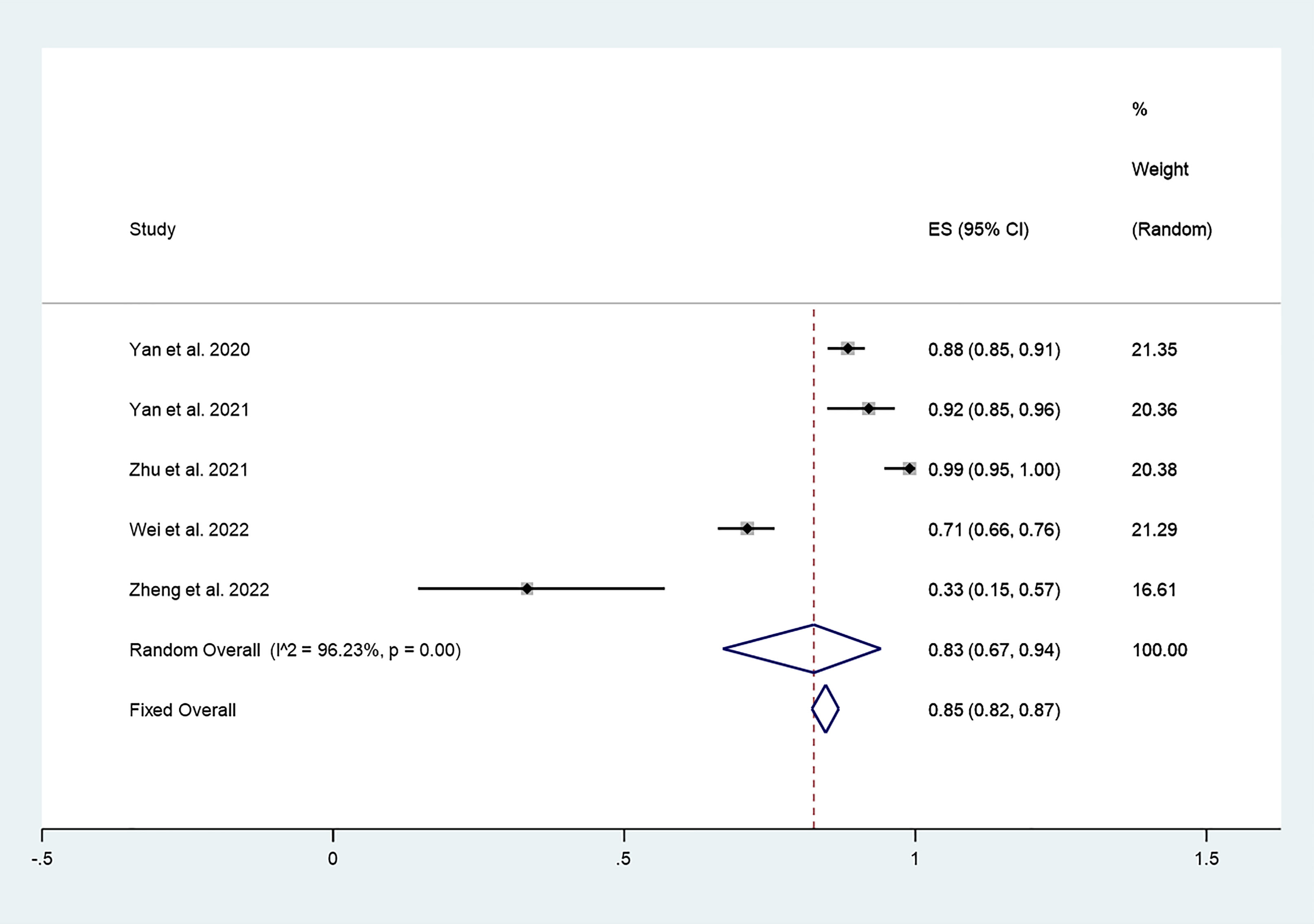
There were 5 studies in which 987 patients were enrolled that reported CDR after thermal ablation at 3-year follow-up. Because there was significant heterogeneity (I2 = 96.23%, P = 0.000), we conducted a meta-analysis using a random effects model. The pooled results indicated that CDR after thermal ablation at 3-year follow-up was 83% (95% CI: 67–94%; Figure 5).
Incidence of newly discovered PTC
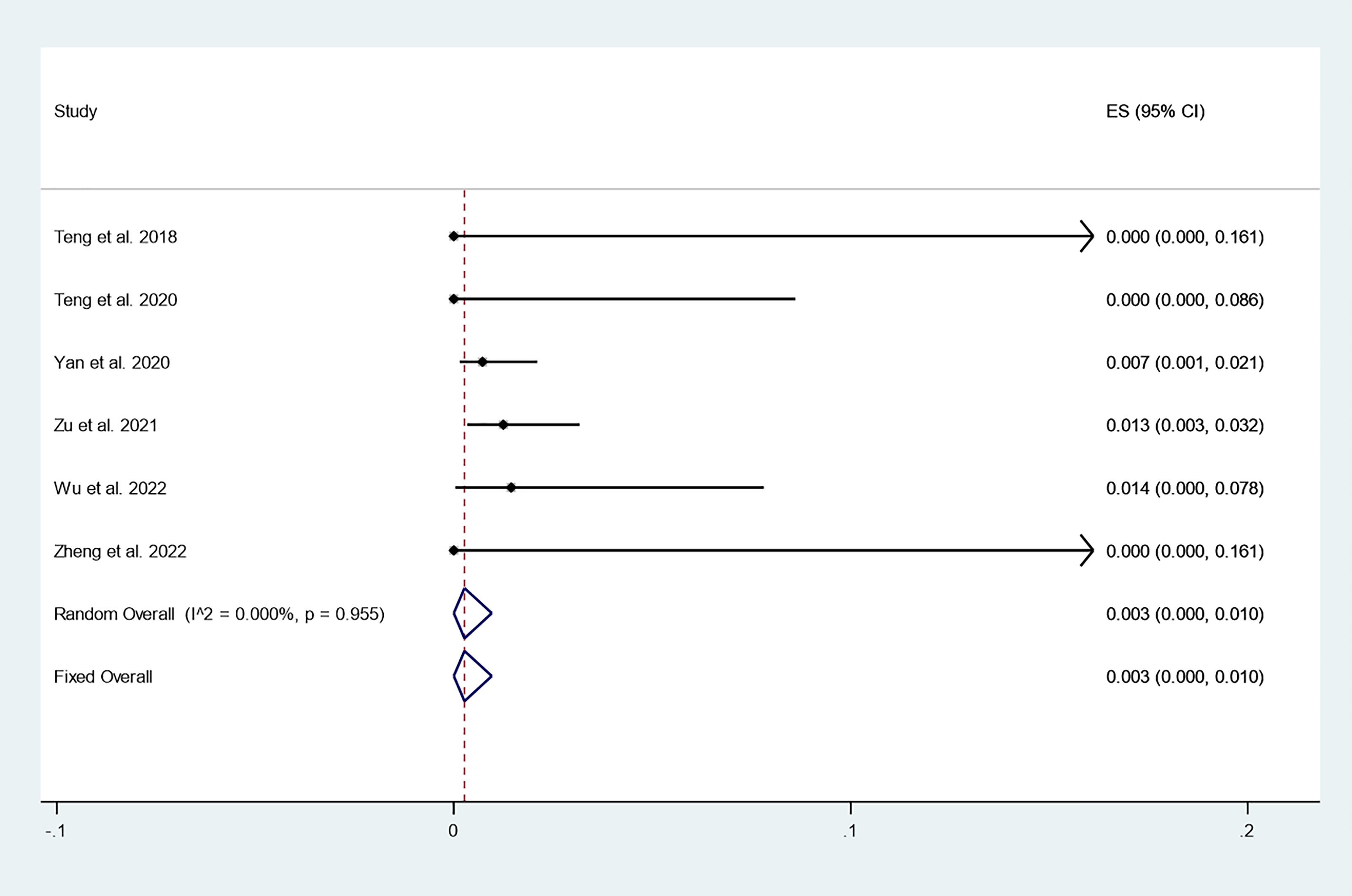
There were 6 studies in which 886 patients were enrolled that reported the incidence of newly discovered PTC after thermal ablation at 3-year follow-up. Because there was no significant heterogeneity (I2 = 0.000%, P = 0.955), we conducted a meta-analysis using a fixed effects model. The pooled results indicated that the incidence of newly discovered PTC after thermal ablation at 3-year follow-up was 0.3% (95% CI: 0.0–1.0%; Figure 6).
Incidence of LNM
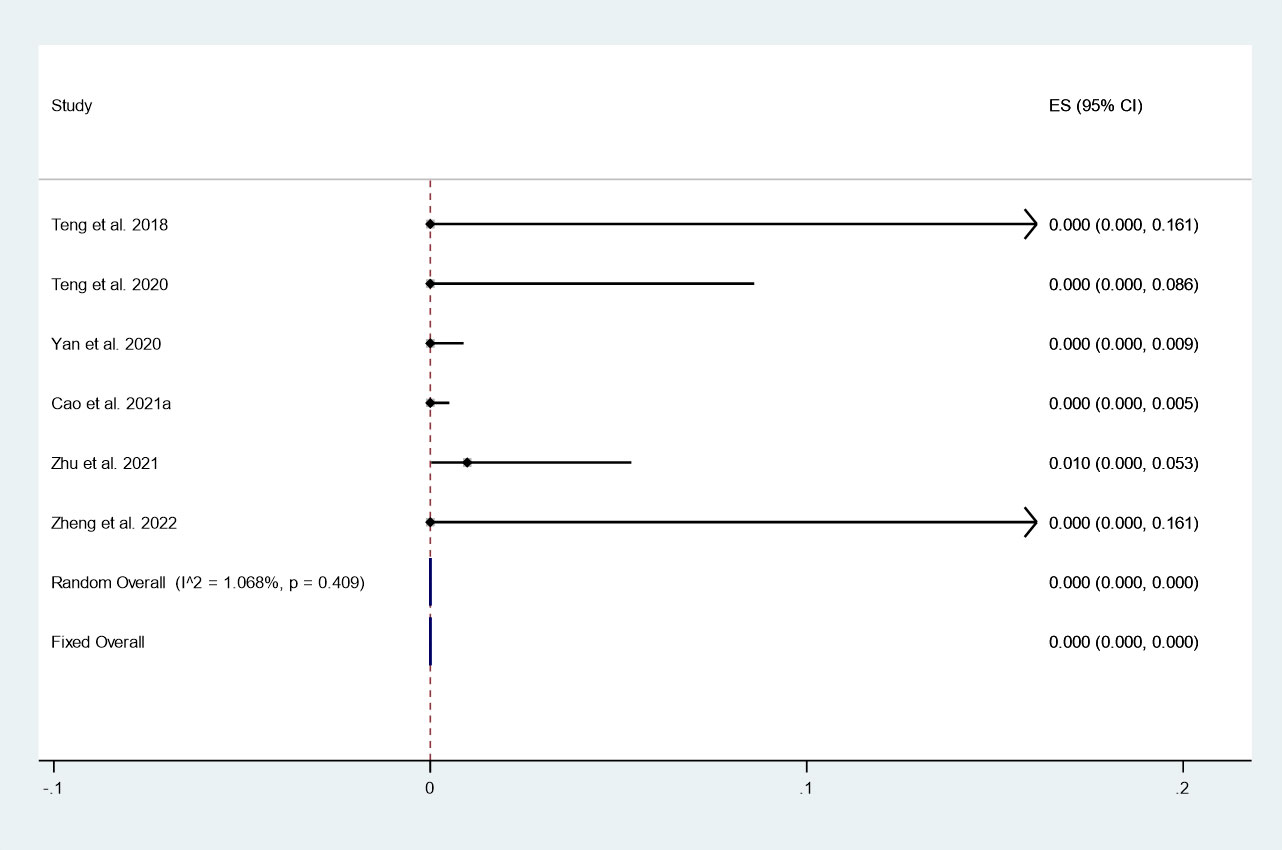
There were 6 studies in which 1324 patients were enrolled that reported the incidence of LNM after thermal ablation at 3-year follow-up. Because there was no significant heterogeneity (I2 = 1.068%, P = 0.409), we conducted a meta-analysis using a fixed effects model. The pooled results indicated that the incidence of LNM after thermal ablation at 3-year follow-up was 0.0% (95% CI: 0.0–0.0%; Figure 7).
Subgroup analysis
To further explore the differences in the efficacy of different thermal ablation modalities, we conducted subgroup analysis.
Tumor volume
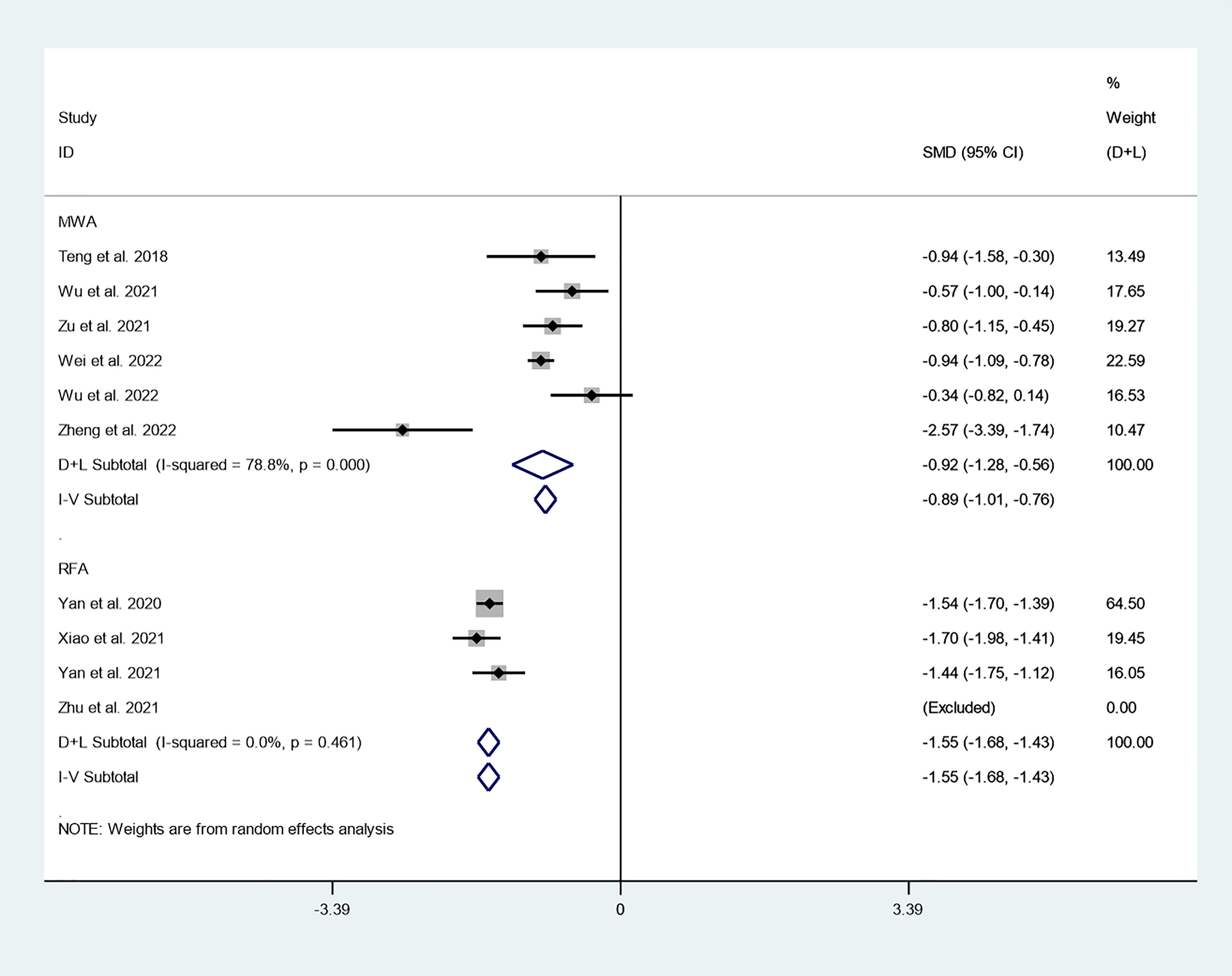
We first explored differences in the effect of different thermal ablation modalities on changes in tumor volume through subgroup analysis. There were 6 studies that reported changes in tumor volume after MWA at 3-year follow-up. Because there was significant heterogeneity (I2 = 78.8%, P = 0.000), we conducted a meta-analysis using a random effects model. The pooled results indicated that tumor volume after MWA at 3-year follow-up was significantly lower than pre-ablation (SMD = -0.92, 95% CI: -1.28~-0.56, P = 0.000; Figure 8). There were six studies that reported changes in tumor volume after RFA at 3-year follow-up. Because there was no significant heterogeneity (I2 = 0.0%, P = 0.461), we conducted a meta-analysis using a fixed effects model. The pooled results indicated that tumor volume after RFA at 3-year follow-up was significantly lower than pre-ablation (SMD = -1.55, 95% CI: -1.68~-1.43, P = 0.000; Figure 8).
VRR
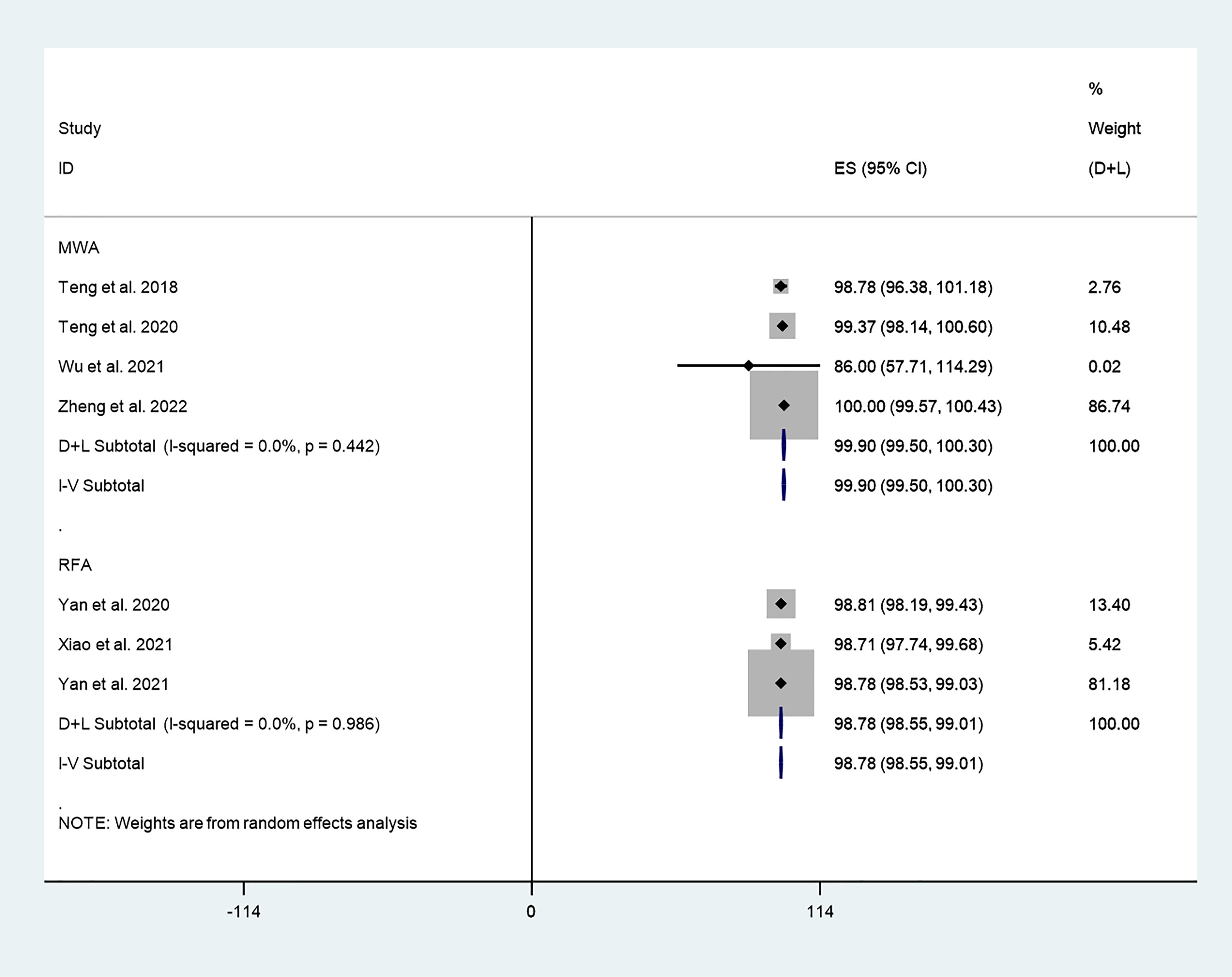
We explored differences in the effect of different thermal ablation modalities on VRR through subgroup analysis. The pooled results indicated that VRR after MWA at 3-year follow-up was 99.90% (95% CI: 99.50–100.30%; I2 = 0.0%, P = 0.442; enrolling 4 studies; Figure 8). In addition, the pooled results indicated that VRR after RFA at 3-year follow-up was 98.78% (95% CI: 98.55–99.01%; I2 = 0.0%, P = 0.986; enrolling 3 studies; Figure 9).
CDR
Furthermore, we explored differences in the effect of different thermal ablation modalities on CDR through subgroup analysis. The pooled results indicated that CDR after RFA at 3-year follow-up was 94% (95% CI: 86–99%; enrolling 3 studies; Figure 9). In addition, the pooled results indicated that CDR after MWA at 3-year follow-up was 70% (95% CI: 65–74%; enrolling 2 studies; Figure 10), and the CDR after 3 years of RFA treatment of PTC was significantly higher than MWA (P=0.000; Figure 10).
Sensitivity analysis
We conducted sensitivity analysis by eliminating each included study one by one and performing a summary analysis of the remaining studies. The results of the sensitivity analysis are presented in Figures S1–S5.
Publication bias
The funnel plot is shown in Figure 11. The funnel plot was symmetrical, and the P value of Egger’s test was 0.352, indicating that there was no obvious publication bias in this study.
Discussion
This meta-analysis analyzed the efficacy of thermal ablation for PTC by pooling 13 studies, including 2567 patients, that reported on the application of ultrasound-guided thermal ablation in PTC to provide guidance for clinical treatment.
Because PTC develops gradually, long-term follow-up is necessary to assess the effectiveness of thermal ablation. Our pooled results indicated that RFA treatment significantly reduced tumor volume and maximum diameter, with a VRR and CDR of 98.91% (95% CI: 97.98–99.83%) and 83% (95% CI: 67–94%) at 3 years after thermal ablation, respectively. These results suggest that thermal ablation can be considered an alternative approach in patients with PTC who refuse surgery or are unable to undergo surgery. All included studies involved ultrasound, which may be due to its ability to accurately assess the extent of the ablation area and the treatment effect (30).
To further explore the differences in the efficacy of different thermal ablation modalities, we conducted subgroup analysis. First, we found that within-group heterogeneity still existed in the MWA subgroup (I2 = 78.8%, P = 0.000); however, the heterogeneity was lower than in the analysis of all thermal ablation modalities (I2 = 88.5%, P = 0.000). In addition, there was no significant heterogeneity in the RFA subgroup (I2 = 0.0%, P = 0.461). This suggests that differences in ablation methods may be one of the reasons for the heterogeneity. The pooled results showed that tumor volume after MWA and RFA at 3-year follow-up was significantly lower than pre-ablation. These results suggest that either MWA or RFA can be used as an alternative to surgery. We then explored differences in the effect of different thermal ablation modalities on VRR via subgroup analysis. Likewise, we found no significant heterogeneity in either MWA or RFA subgroups. The pooled results indicated that VRR after MWA at 3-year follow-up was 99.90% (95% CI: 99.50–100.30%), and VRR after RFA at 3-year follow-up was 98.78% (95% CI: 98.55–99.01%). VRR in both cases was close to 100%. Despite the lack of statistical analysis, it is clear that the VRRs of the two treatments are very similar. In addition, the pooled results showed that CDR after RFA and MWA at 3-year follow-up was 94% (95% CI: 86–99%) and 70% (95% CI: 65–74%), respectively. Furthermore, CDR after 3 years of RFA treatment of PTC was significantly higher than that of MWA (P=0.000). This suggests that RFA may have better long-term efficacy than MWA.
In addition, we focused on tumor progression, including newly discovered PTC and LNM. The pooled results indicated that the incidence of newly discovered PTC after thermal ablation at 3-year follow-up was 0.3% (95% CI: 0.0–1.0%). In addition, the pooled data indicated that the incidence of LNM after thermal ablation at 3-year follow-up was 0.0% (95% CI: 0.0–0.0%). The reported incidence of newly discovered PTC and LNM after surgery was 3.2% and 0.7%, respectively (27). In addition, Zu et al. (26) reported that the incidence of LNM after surgery for PTC was 2.5%. This suggests that long-term disease progression after thermal ablation for PTC may have better prospects than surgery.
This study has several limitations. First, in the analysis of tumor volume, although our subgroup analysis for ablation modalities determined that differences in ablation modalities may be one of the reasons for the heterogeneity, we could not completely rule out the intragroup heterogeneity of the MWA subgroup. This should be explored further in the future. In addition, different teams used different ablation modalities, and the technical proficiency of operators may also have been a source of heterogeneity. Second, the studies included in this meta-analysis were all retrospective studies, which is due to the limitations of the current research. More prospective studies are needed to corroborate our findings in the future.
Conclusion
Overall, the long-term efficacy (≥3 years) of ultrasound-guided thermal ablation in the treatment of PTC was significant, with favorable disease progression. Ultrasound-guided thermal ablation can be considered an alternative approach for patients with PTC who refuse surgery or are unable to undergo surgery.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
Author contributions
JX collected data and wrote the manuscript, DT and HW conceived the manuscript. All authors have read and approved the final manuscript.
Funding
The study was supported by Jilin Provincial Health and Family Planning Commission (2019SCZ027) and (2020SCZ08).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2022.971038/full#supplementary-material
References
1. Lloyd RV, Buehler D, Khanafshar E. Papillary thyroid carcinoma variants. Head Neck Pathol (2011) 5:51–6. doi: 10.1007/s12105-010-0236-9
2. Cabanillas ME, McFadden DG, Durante C. Thyroid cancer. Lancet (2016) 388:2783–95. doi: 10.1016/S0140-6736(16)30172-6
3. Davies L, Welch HG. Current thyroid cancer trends in the united states. JAMA Otolaryngol Head Neck Surg (2014) 140:317–22. doi: 10.1001/jamaoto.2014.1
4. Sugitani I. Active surveillance of low-risk papillary thyroid microcarcinoma. Best Pract Res Clin Endocrinol Metab (2022) 24:101630. doi: 10.1016/j.beem.2022.101630
5. Tuttle RM. Controversial issues in thyroid cancer management. J Nucl Med (2018) 59:1187–94. doi: 10.2967/jnumed.117.192559
6. Haugen BR, Alexander EK, Bible KC, Doherty GM, Mandel SJ, Nikiforov YE, et al. 2015 American Thyroid association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: The American thyroid association guidelines task force on thyroid nodules and differentiated thyroid cancer. Thyroid (2016) 26:1–133. doi: 10.1089/thy.2015.0020
7. Rogers H. Do low grade thyroid cancers really require thyroidectomy? BMJ (2013) 347: f5734. doi: 10.1136/bmj.f5734
8. Mauri G, Gennaro N, Lee MK, Baek JH. Laser and radiofrequency ablations for benign and malignant thyroid tumors. Int J Hyperthermia (2019) 36:13–20. doi: 10.1080/02656736.2019.1622795
9. Lim HK, Cho SJ, Baek JH, Lee KD, Son CW, Son JM, et al. US-Guided radiofrequency ablation for low-risk papillary thyroid microcarcinoma: Efficacy and safety in a Large population. Korean J Radiol (2019) 20:1653–61. doi: 10.3348/kjr.2019.0192
10. Choi Y, Jung SL. Efficacy and safety of thermal ablation techniques for the treatment of primary papillary thyroid microcarcinoma: A systematic review and meta-analysis. Thyroid (2020) 30:720–31. doi: 10.1089/thy.2019.0707
11. Tong M, Li S, Li Y, Li Y, Feng Y, Che Y. Efficacy and safety of radiofrequency, microwave and laser ablation for treating papillary thyroid microcarcinoma: a systematic review and meta-analysis. Int J Hyperthermia (2019) 36:1278–86. doi: 10.1080/02656736.2019.1700559
12. Suh CH, Baek JH, Choi YJ, Lee JH. Efficacy and safety of radiofrequency and ethanol ablation for treating locally recurrent thyroid cancer: A systematic review and meta-analysis. Thyroid (2016) 26:420–8. doi: 10.1089/thy.2015.0545
13. Ou D, Chen C, Jiang T, Xu D. Research review of thermal ablation in the treatment of papillary thyroid carcinoma. Front Oncol (2022) 12:859396. doi: 10.3389/fonc.2022.859396
14. Mauri G, Nicosia L, Della Vigna P, Varano GM, Maiettini D, Bonomo G, et al. Percutaneous laser ablation for benign and malignant thyroid diseases. Ultrasonography (2019) 38:25–36. doi: 10.14366/usg.18034
15. Zhuo L, Zhang L, Peng LL, Yang Y, Lu HT, Chen DP, et al. Microwave ablation of hyperplastic parathyroid glands is a treatment option for end-stage renal disease patients ineligible for surgical resection. Int J Hyperthermia (2019) 36:29–35. doi: 10.1080/02656736.2018.1528392
16. Slim K, Nini E, Forestier D, Kwiatkowski F, Panis Y, Chipponi J. Methodological index for non-randomized studies (minors): development and validation of a new instrument. ANZ J Surg (2003) 73:712–6. doi: 10.1046/j.1445-2197.2003.02748.x
17. Teng D, Sui G, Liu C, Wang Y, Xia Y, Wang H. Long-term efficacy of ultrasound-guided low power microwave ablation for the treatment of primary papillary thyroid microcarcinoma: A 3-year follow-up study. J Cancer Res Clin Oncol (2018) 144:771–9. doi: 10.1007/s00432-018-2607-7
18. Bronchud MH, Potter MR, Morgenstern G, Blasco MJ, Scarffe JH, Thatcher N, et al. In vitro and in vivo analysis of the effects of recombinant human granulocyte colony-stimulating factor in patients. Br J Cancer (1988) 58:64–9. doi: 10.1038/bjc.1988.163
19. Yan L, Lan Y, Xiao J, Lin L, Jiang B, Luo Y. Long-term outcomes of radiofrequency ablation for unifocal low-risk papillary thyroid microcarcinoma: A large cohort study of 414 patients. Eur Radiol (2021) 31:685–94. doi: 10.1007/s00330-020-07128-6
20. Cao XJ, Yu MA, Zhu YL, Qi L, Cong ZB, Yan GZ, et al. Ultrasound-guided thermal ablation for papillary thyroid microcarcinoma: A multicenter retrospective study. Int J Hyperthermia (2021) 38:916–22. doi: 10.1080/02656736.2021.1936218
21. Cao XJ, Liu J, Zhu YL, Qi L, Liu G, Wang HL, et al. Efficacy and safety of thermal ablation for solitary T1bN0M0 papillary thyroid carcinoma: A multicenter study. J Clin Endocrinol Metab (2021) 106:e573–81. doi: 10.1210/clinem/dgaa776
22. Wu J, Zhao ZL, Cao XJ, Wei Y, Peng LL, Li Y, et al. A feasibility study of microwave ablation for papillary thyroid cancer close to the thyroid capsule. Int J Hyperthermia (2021) 38:1217–24. doi: 10.1080/02656736.2021.1962549
23. Xiao J, Zhang Y, Yan L, Zhang M, Li X, Tang J, et al. Ultrasonography-guided radiofrequency ablation for solitary T1aN0M0 and T1bN0M0 papillary thyroid carcinoma: a retrospective comparative study. Eur J Endocrinol (2021) 186:105–13. doi: 10.1530/EJE-21-0580
24. Yan L, Zhang M, Song Q, Xiao J, Zhang Y, Luo Y. The efficacy and safety of radiofrequency ablation for bilateral papillary thyroid microcarcinoma. Front Endocrinol (Lausanne) (2021) 12:663636. doi: 10.3389/fendo.2021.663636
25. Zhu Y, Che Y, Gao S, Ren S, Tong M, Wang L, et al. Long-term follow-up results of PTMC treated by ultrasound-guided radiofrequency ablation: A retrospective study. Int J Hyperthermia (2021) 38:1225–32. doi: 10.1080/02656736.2021.1963850
26. Zu Y, Liu Y, Zhao J, Yang P, Li J, Qian L. A cohort study of microwave ablation and surgery for low-risk papillary thyroid microcarcinoma. Int J Hyperthermia (2021) 38:1548–57. doi: 10.1080/02656736.2021.1996643
27. Wei Y, Niu WQ, Zhao ZL, Wu J, Peng LL, Li Y, et al. Microwave ablation versus surgical resection for solitary T1N0M0 papillary thyroid carcinoma. Radiology (2022) 304(3): 704–13. doi: 10.1148/radiol.212313
28. Wu J, Wei Y, Zhao ZL, Peng LL, Li Y, Lu NC, et al. A preliminary study of microwave ablation for solitary T1N0M0 papillary thyroid carcinoma with capsular invasion. Int J Hyperthermia (2022) 39:372–8. doi: 10.1080/02656736.2022.2040607
29. Zheng L, Liu FY, Yu J, Cheng ZG, Yu XL, Dong XC, et al. Thermal ablation for papillary thyroid microcarcinoma located in the isthmus: A study with 3 years of follow-up. Future Oncol (2022) 18:471–80. doi: 10.2217/fon-2021-0463
Keywords: thermal ablation, papillary thyroid carcinoma, long-term efficacy, systematic review and meta-analysis, MWA, RFA
Citation: Xue J, Teng D and Wang H (2022) Over than three-year follow-up results of thermal ablation for papillary thyroid carcinoma: A systematic review and meta-analysis. Front. Endocrinol. 13:971038. doi: 10.3389/fendo.2022.971038
Received: 16 June 2022; Accepted: 20 September 2022;
Published: 24 October 2022.
Edited by:
Carlo Cappelli, University of Brescia, ItalyReviewed by:
Erivelto Martinho Volpi, Centro de referencia no ensino do diagnóstico por imagem (CETRUS), BrazilDi Ou, Zhejiang Cancer Hospital, University of Chinese Academy of Sciences, China
Xu Zhang, Zhejiang University, China
Zhiguang Chen, Capital Medical University, China
Copyright © 2022 Xue, Teng and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: DengKe Teng, dGVuZ2RrQGpsdS5lZHUuY24=; Hui Wang, d2h1aTY2QGpsdS5lZHUuY24=
 JiaNan Xue
JiaNan Xue DengKe Teng
DengKe Teng Hui Wang
Hui Wang