
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Endocrinol., 19 August 2022
Sec. Thyroid Endocrinology
Volume 13 - 2022 | https://doi.org/10.3389/fendo.2022.970682
This article is part of the Research TopicTranslational Research in Thyroid Cancer, Volume IIView all 7 articles
 Tetiana Bogdanova1,2†
Tetiana Bogdanova1,2† Serhii Chernyshov3†
Serhii Chernyshov3† Liudmyla Zurnadzhy1,2
Liudmyla Zurnadzhy1,2 Tatiana I. Rogounovitch4
Tatiana I. Rogounovitch4 Norisato Mitsutake2,4
Norisato Mitsutake2,4 Mykola Tronko5
Mykola Tronko5 Masahiro Ito6
Masahiro Ito6 Michael Bolgov3
Michael Bolgov3 Sergii Masiuk7
Sergii Masiuk7 Shunichi Yamashita8,9
Shunichi Yamashita8,9 Vladimir A. Saenko2*
Vladimir A. Saenko2*The potential overtreatment of patients with papillary thyroid microcarcinoma (MPTC) has been an important clinical problem in endocrine oncology over the past decade. At the same time, current clinical guidelines tend to consider prior radiation exposure as a contraindication to less extensive surgery, even for low-risk thyroid carcinomas, which primarily include microcarcinomas. This study aims to determine whether there are differences in the behavior of MPTC of two etiological forms (radiogenic and sporadic), including invasive properties, clinical data, and recurrence in patients aged up to 30 years. For this purpose, 136 radiogenic (from patients aged up to 18 years at the time of the Chornobyl accident) and 83 sporadic (from patients born after the Chornobyl accident) MPTCs were selected and compared using univariate and multivariate statistical methods in a whole group and in age and tumor size subgroups. No evidence of more aggressive clinical and histopathological behavior of radiogenic MPTCs as compared to sporadic tumors for basic structural, invasive characteristics, treatment options, and postoperative follow-up results was found. Moreover, radiogenic MPTCs were characterized by the lower frequencies of oncocytic changes (OR = 0.392, p = 0.004), nodal disease (OR = 0.509, p = 0.050), and more frequent complete remission (excellent response) after radioiodine therapy (OR = 9.174, p = 0.008). These results strongly suggest that internal irradiation does not affect tumor phenotype, does not associate with more pronounced invasive properties, and does not worsen prognosis in pediatric or young adult patients with MPTC, implying that radiation history may be not a pivotal factor for determining treatment strategy in such patients.
Epidemiological studies have shown a worldwide increase in thyroid cancer incidence in recent decades (1, 2). To a large extent, the increase is due to a more frequent detection of papillary thyroid carcinoma as compared to other thyroid cancer histotypes, and particularly of low-grade tumors (3–8) which intrinsically include papillary thyroid microcarcinomas (MPTCs) measuring up to 10 mm. The increase in the detection of small tumors is attributed to rapid strides in ultrasound diagnostic capabilities, improved fine-needle aspiration biopsy (FNA), introduction of screenings, and increased patient awareness about the availability of facile thyroid examination (3–5, 7).
The notions of “overdiagnosis” and “overtreatment” are actively discussed over the past decade in many areas of contemporary medicine, including management of small thyroid nodules (3–5, 9–11). Avoiding overtreatment of patients with MPTCs became a challenging clinical problem in the field of endocrine oncology. Prospects of changing treatment strategies from total thyroidectomy to organ-preserving surgery or even active surveillance instead of surgery are widely debated in the medical and scientific communities (12–20).
At the same time, clinical guidelines recommend considering prior head and neck radiation of a patient even with low-risk thyroid cancer as a potential contraindication to less extensive surgery, at least in adults (21, 22). Indeed, data obtained in previous years showed histopathological differences between the radiogenic (patients exposed to Chornobyl fallouts in childhood) and sporadic PTCs (non-exposed patients born after the Chornobyl accident) pointing at more aggressive behavior of radiogenic tumors especially in children and adolescents (23–26). Even so, whether this holds true for MPTCs in young patients exposed to radiation remains unclear since previous studies did not specifically address this question and included tumors of all sizes. The current study retrospectively investigates the differences between MPTCs of radiogenic and sporadic etiology in terms of tumor structure and invasiveness, treatment options and follow-up results in young patients aged up to 30 years treated at a single institution.
The radiogenic series included MPTCs from 136 patients aged 8.8-30.9 years at diagnosis operated on at the State Institution “VP Komisarenko Institute of Endocrinology and Metabolism of the National Academy of Medical Sciences of Ukraine” (IEM), Kyiv during the period from 1992 to 2016 when a significant increase in thyroid cancer incidence after the Chornobyl accident was documented (23, 27, 28). Given that the high thyroid cancer risk was observed in individuals who were children and adolescents at the time of the Chornobyl accident and lived in the six northern, most contaminated by 131I regions of Ukraine (23, 27–29), we defined radiogenic cases as those diagnosed in subjects aged up to 18 years in April 1986, who lived in Kyiv, Chernihiv, Zhytomyr, Rivne, Cherkasy regions, and Kyiv city in 1986. For additional analyses, patients were divided into the pediatric (≤18), and young adult (19-30 years old at diagnosis) subgroups.
The sporadic MPTCs were from 83 patients aged 9.7-30.9 years at diagnosis who were born after the Chornobyl accident (from January 1st, 1987 or later, i.e. not affected by 131I) and lived in the same six northern regions at the time of the operation. Patients were operated on from 2001 to 2018.
Inclusion criteria for both radiogenic and sporadic MPTCs were non-incidental tumor finding (to ensure the adequacy of clinical option comparisons), and the absence of screening history in tumor detection (as screening may be expected to introduce a bias toward smaller and less advanced tumors). Since the oldest patient with sporadic MPTC at the time of this study was aged 30 years, the upper limit for the age of patients with radiogenic MPTC was also set to 30 years old. None of MPTCs in the study was familial.
The study was conducted according to the guidelines of the Declaration of Helsinki and was approved by the IEM Bioethics Committee (protocols N 22-KE of April 26, 2018, and N 31-KE of February 27, 2020), the Chornobyl Tissue Bank (CTB, project N001-2020), and the Ethics Committee of Nagasaki University (protocol 20130401–7 of July 1, 2021, the latest update). Informed consent was obtained from all patients enrolled in the study or their guardians (for minors).
Histopathological examination of hematoxylin/eosin-stained paraffin sections was performed by two experienced pathologists at IEM (ТB and LZ). The pathological diagnosis was based on the 4th edition of the WHO histological classification (30). Most cases were also reviewed by the international pathology panel of the CTB project (31, 32). The diagnosis of MPTC was confirmed in all analyzed cases. TNM categories were determined according to the 8th edition of the pTNM classification (33). Tumors were also classified according to the dominant histological growth pattern into three categories: papillary, follicular, or solid-trabecular when the corresponding structural component exceeded 50% of a tumor section surface, and also evaluated for the presence of oncocytic (oxyphilic/Hurtle) cell changes in the tumor epithelium.
As in our previous works (26, 34, 35), in addition to conventional clinical-pathological features, we used an integrative variable, the “invasiveness score”, which is the arithmetic sum of every instance of multifocality, lymphatic/vascular invasion, any extrathyroidal extension (i.e., minimal to the fat or connective tissues, or extending to the muscle), pN1 and M1 (commonly detected by diagnostic imaging), either isolated or in combination with other(s), for each tumor. Thus defined, the invasiveness score ranged from 0 (no invasive feature presents) to 5 (all features present). The actual highest invasiveness score observed in the present MPTC series was 4.
Immunohistochemical (IHC) staining for BRAFV600E and Ki67 were performed in 34 and 32 radiogenic, and in 38 and 36 sporadic MPTCs, respectively, for which the additional tissue sections were available.
IHC staining for BRAFV600E was performed as described before (34, 35). In brief, a mouse monoclonal anti-BRAF (mutated V600E) antibody (VE1) ab228461(Abcam) at a 1:100 dilution and the Novolink Polymer Detection System (250T) (Leica RE7140-K) were used to detect IHC reaction product. In our hands, the BRAFV600E positivity on IHC was concordant with the presence of the BRAFV600E mutation (36).
The proliferative activity of tumors was evaluated by IHC using a Ki67 antibody (clone MIB-1; DAKO, Glostrup, Denmark, 1:100 dilution) in a Ventana BenchMark ULTRA instrument. The Ki67 labeling index (Ki67 LI) was determined with the image-analyzing software (CountσCell, Ki67 antigen Semi-Auto Counter, Seiko Tec LTD, Fukuoka, Japan) in a total of approximately 1,000 PTC cells per case (LZ). Image analysis was performed in a blind for the BRAFV600E status manner.
131I thyroid radiation doses were calculated for each patient from the radiogenic group in the Radiation Protection Laboratory of the State Institution “National Research Center for Radiation Medicine of the National Academy of Medical Sciences of Ukraine”, Kyiv using an ecological dosimetric model, which includes the system of ecological iodine transport and biokinetic models of iodine (“TD-CTB”) (29). The median (and IQR) 131I thyroid radiation dose for radiogenic MPTCs were 106 (56–203) mGy.
The Fisher’s exact test, Fisher-Freeman-Halton exact test, Cochran-Armitage test and the Kaplan-Meier test were used for univariate analysis of categorical data; the Mann-Whitney test was used to compare continuous data between any two groups. Logistic regression models were adjusted for age at operation and sex; models with very small numbers of outcomes (< 5 per cell) were conducted using Firth’s approach to bias-reducing penalized maximum likelihood fit. Multivariable linear regression models were used for continuous dependent variables. The Cox proportional hazard model was used to analyze disease-free survival. Calculations were performed using IBM SPSS Statistics Version 24 software (International Business Machines Corp., Armonk, NY, USA) and/or the 9.4 version of SAS (SAS Institute Inc., Cary, NC, USA). Correspondence analysis was performed in R with the “ca” package (37). The “colgreen” option was used to calculate biplot principal coordinates for the MPTC subgroups and contribution coordinates (the standard coordinates multiplied by the square root of the corresponding masses) for categorical clinicopathological features. All tests were two-sided; p < 0.05 was considered statistically significant. To identify the variables potentially contributing to the differences between the radiogenic and sporadic MPTCs, the adaptive lasso, best subset (maximum 20 variables), pruned forward selection and adaptive elastic net algorithms of variable selection with holdback validation method (0.3 holdback proportion) were applied using the binomial generalized regression in JMP Pro 16.2.0 (SAS Institute Inc., Cary, NC, USA). The non-zeroed variables were considered as potentially relevant. The Akaike information criterion (AIC), and areas under curve (AUC) for training and validation subsets were calculated for each algorithm result.
MPTCs detected by ultrasound and assessed by FNA/cytology as malignant or suspicious for malignancy accounted for 136 and 83 cases in the radiogenic and sporadic groups, respectively. All data collected for or generated during this study are presented in Figure 1.
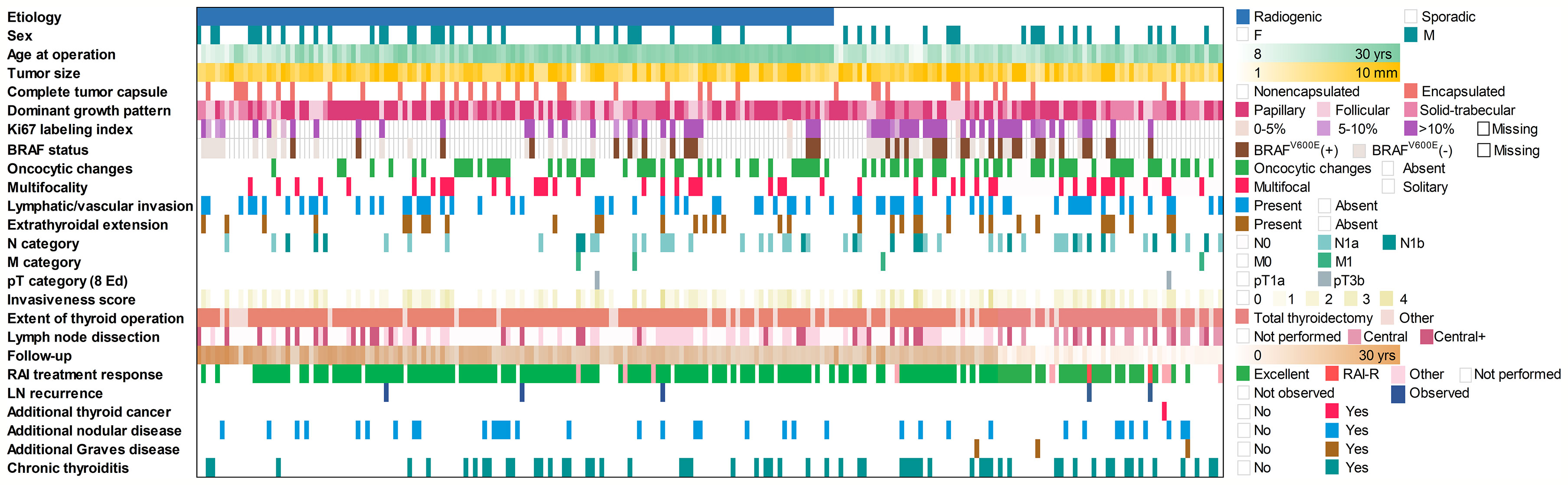
Figure 1 Baseline and clinicopathological profile of 136 radiogenic and 83 sporadic papillary microcarcinomas in the study.
Comparison of MPTCs from two etiological groups revealed significantly older age of patients in the radiogenic series (b = 26.519, p = 7.41E-08). Therefore, multivariate analyses were adjusted for this parameter and sex (Table 1). Statistically significant differences between the two groups for tumor size, architecture, frequency of the BRAFV600E mutation or proliferative activity were not observed. The radiogenic MPTCs were less likely to display oncocytic changes (OR = 0.392, p = 0.004), lymph node metastases (OR = 0.509, p = 0.050) and signs of concomitant chronic thyroiditis (OR = 0.431, p = 0.015). Of importance, the response to radioiodine therapy (RIT) was overall better among the radiogenic MPTCs (OR = 9.059, p = 0.008) with the higher frequency of excellent response (i.e., a complete remission, OR = 9.174, p = 0.008) and suggestively lower frequency (in fact, the absence) of radioiodine-refractory (RAI-R) recurrence (OR = 0.090, p = 0.092). Of note, among the recurrent metastases (5 in total), there were 0/3 RAI-R metastases in the radiogenic group and 2/2 in the sporadic group (OR = 0.024, p = 0.389; the lack of statistical significance is likely due to an extremely small number of recurrences).
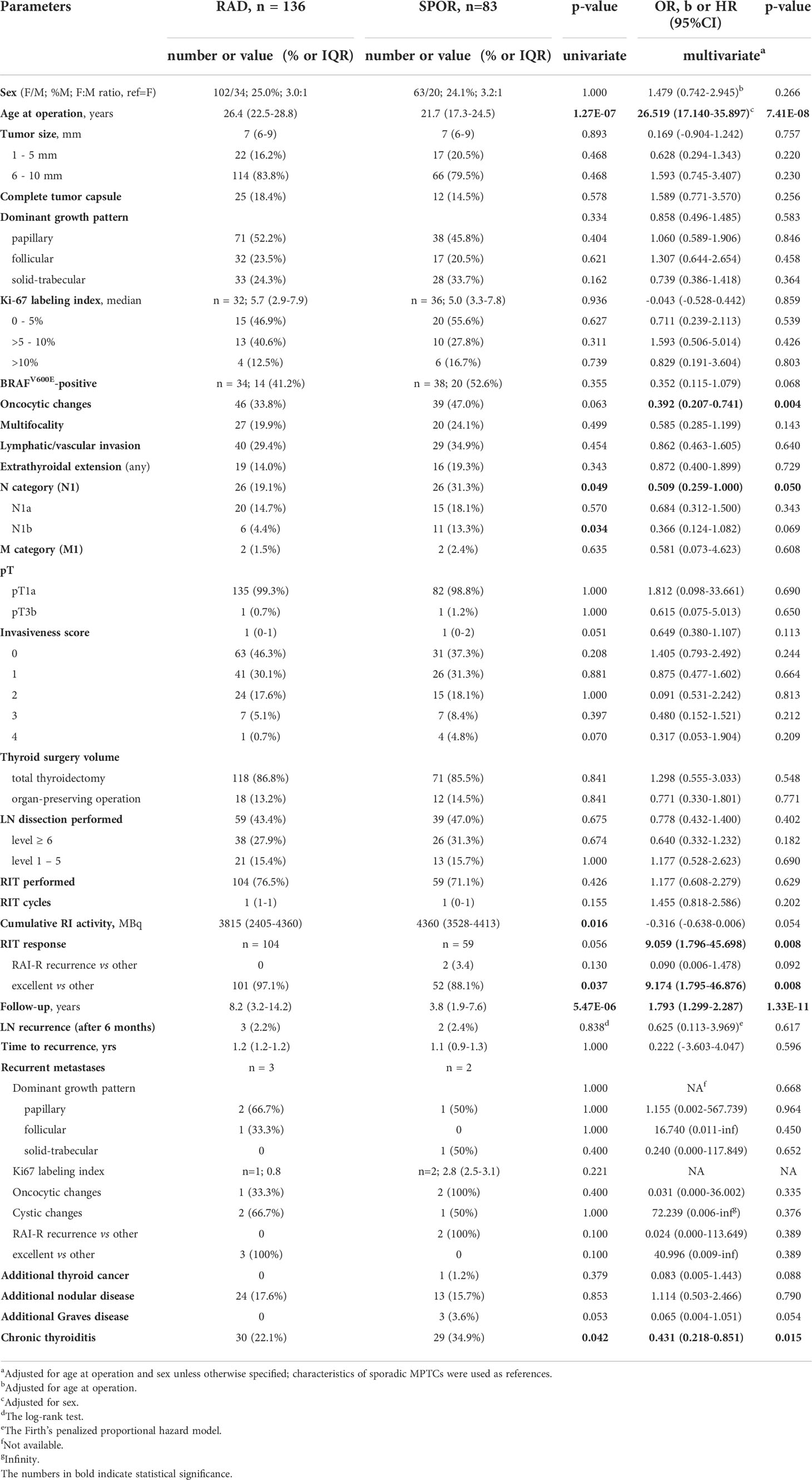
Table 1 Characteristics of the radiogenic and sporadic papillary thyroid microcarcinomas in patients aged up to 30 years.
Another parameter that was different between the groups was the longer duration of postoperative follow-up (b = 1.793, p = 1.33E-11) of radiogenic MPTCs. It is explained by the earlier calendar time of inclusion of patients with radiogenic MPTCs in the study.
In the pediatric subgroup aged ≤18years at diagnosis (Table 2), there were no structural or invasive differences between the radiogenic and sporadic tumors. Only the lower frequencies of oncocytic changes (OR = 0.188, p = 0.034), and lower cumulative 131I activity during RIT (b = -0.722, p = 0.021) were observed in the radiogenic subgroup. As in the whole series, the period of postoperative follow-up was longer for the radiogenic MPTCs (b = 2.464, p = 2.00E-06); recurrences were not documented in either etiological subgroup.
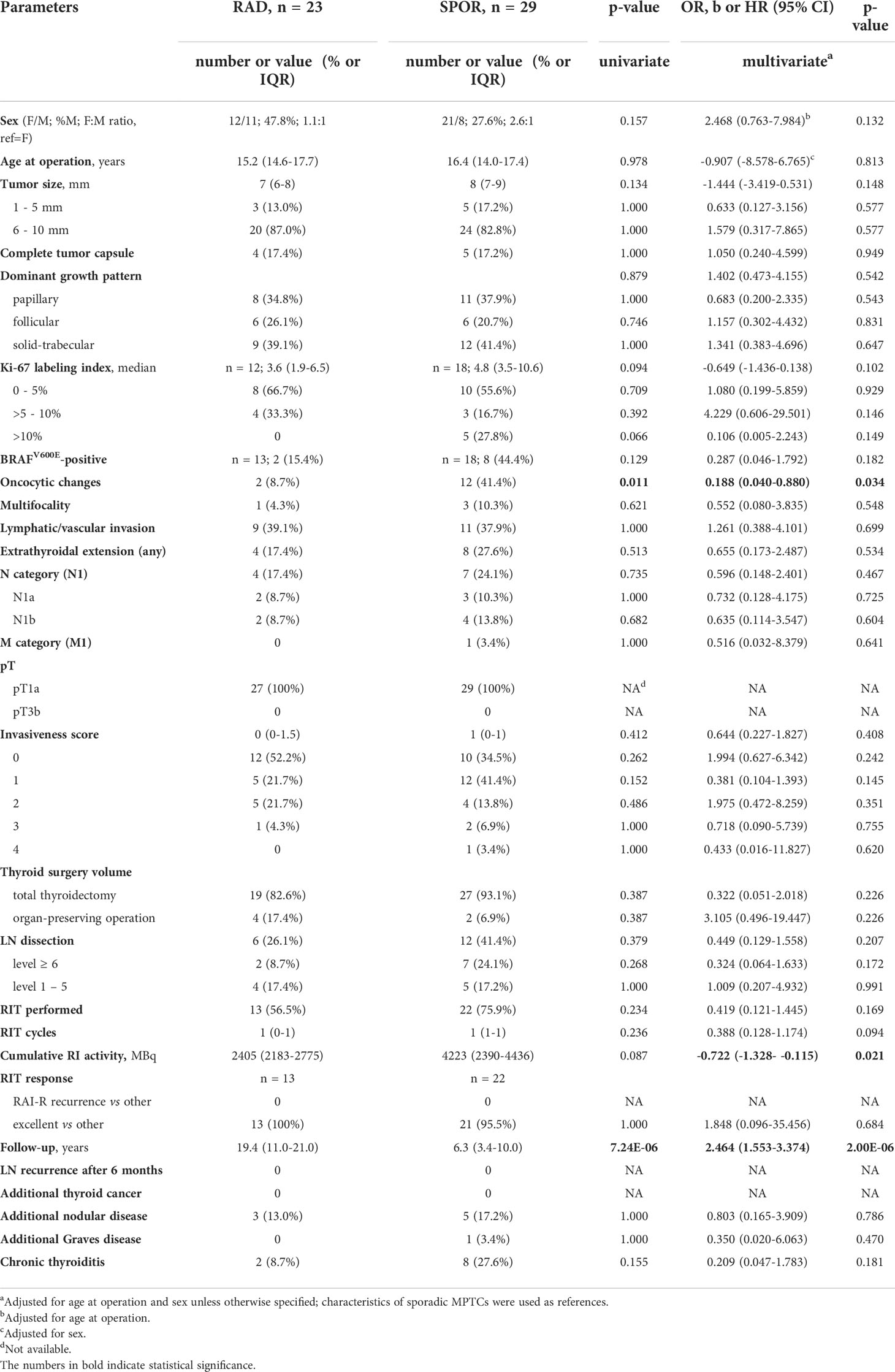
Table 2 Characteristics of the radiogenic and sporadic papillary thyroid microcarcinomas in pediatric patients aged ≤ 18 years.
In young adults aged 19-30 years at diagnosis (Table 3), statistically significant differences in the radiogenic subgroup were found for older age (b = 19.241, p = 5.27E-07), longer follow-up period (b = 1.501, p = 2.00E-06) and lower frequency of concomitant chronic thyroiditis (OR = 0.421, p = 0.030). Patients with radiogenic MPTC were more likely to receive RIT (OR = 2.391, p = 0.039) and more RIT cycles (OR = 3.583, p = 6.61E-04). As in the whole group, RIT response was more favorable in the radiogenic subgroup (OR = 6.797, p = 0.024), and the frequency of excellent response was higher (OR = 6.914, p = 0.025). Since all recurrences occurred only in this age subgroup, all observations from the whole-group analysis were reproduced: RAI-R metastases were absent among 3 recurrences in the radiogenic group, and 2/2 occurred in the sporadic group, with statistical estimates identical to those presented in Table 1. The higher frequency of encapsulated tumors (OR = 2.487, p = 0.082), and less frequent solid-trabecular growth pattern (OR = 0.459, p = 0.065), oncocytic changes (OR = 0.490, p = 0.055), N1b tumors (OR = 0.292, p = 0.080) and concomitant Graves disease (OR = 0.065, p = 0.060) for the radiogenic MPTCs were suggestive.
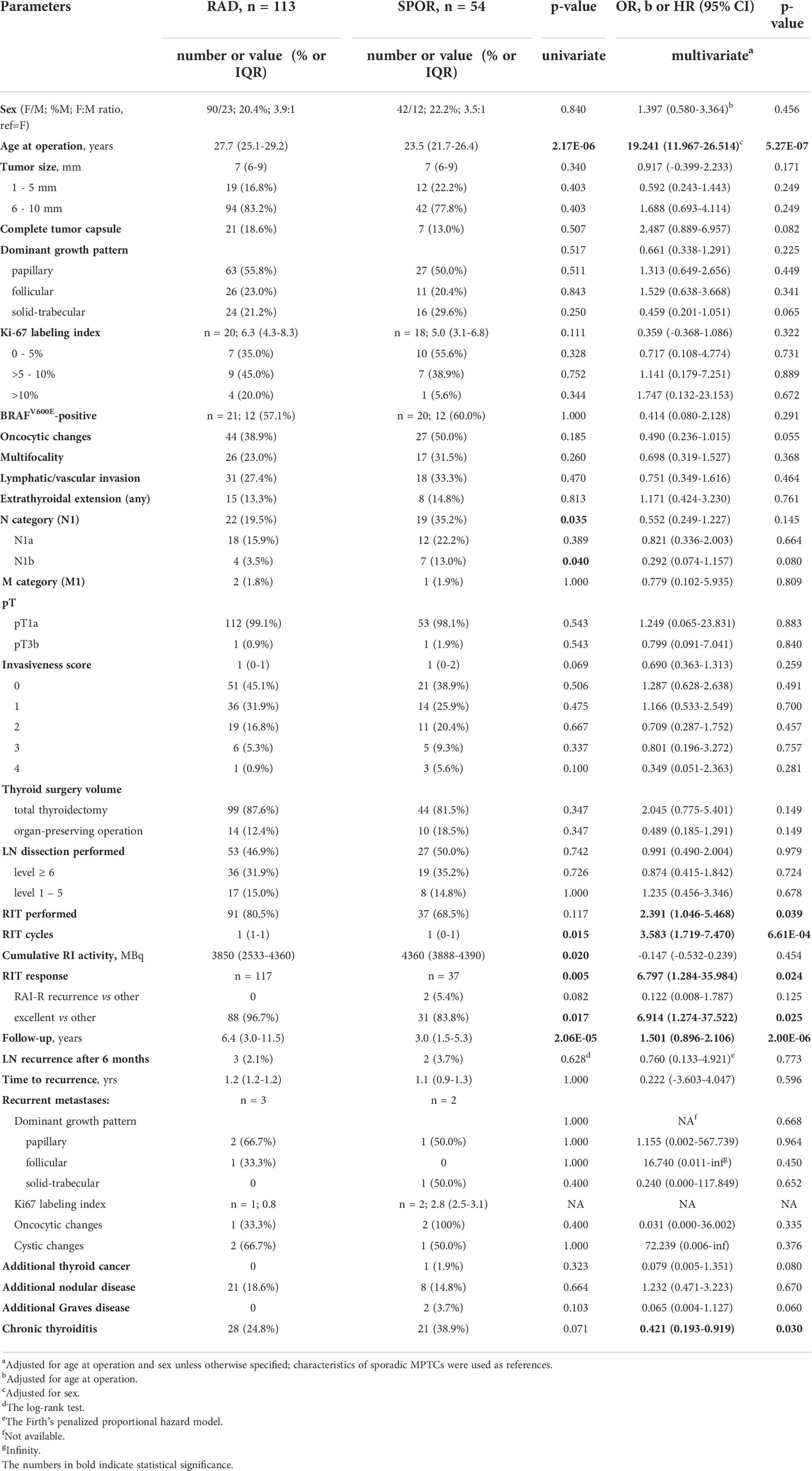
Table 3 Characteristics of the radiogenic and sporadic papillary thyroid microcarcinomas in young adult patients aged 19-30 years.
We also performed correspondence analysis to visualize the similarities or differences in structural, invasive and prognostic characteristics of MPTCs of the two etiological forms in patients of different age at diagnosis (Figure 2). The results clearly demonstrated a pronounced similarity between pediatric radiogenic and sporadic MPTCs (note the acute angle between these groups). MPTCs from young adults showed differences from pediatric tumors (the obtuse angles between any young adult vs. any pediatric subgroup). The radiogenic and sporadic MPTCs from young adult patients also looked different (note the right angle between these subgroups). In agreement with the multivariate analysis (see Tables 2, 3), no evidence in support of more aggressive tumor behavior or poorer prognosis were observed for the radiogenic MPTCs of any age subgroup: their localizations on the graph were more likely to be in the opposite directions to the invasive or prognostically less favorable parameters, in contrast to sporadic MPTCs.
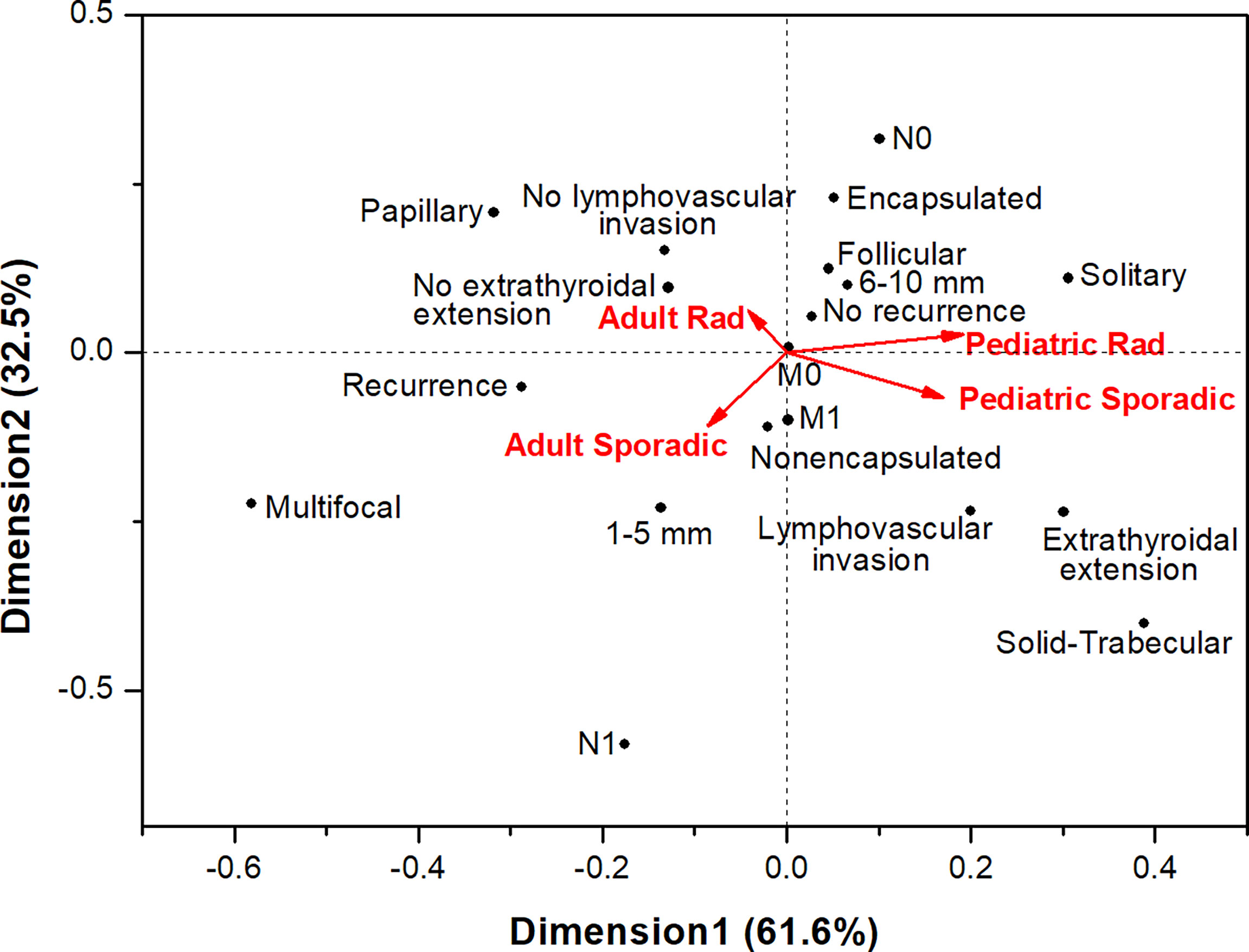
Figure 2 Correspondence analysis of the associations of papillary thyroid microcarcinomas of different etiology from pediatric ( ≤ 18-year-old.) and young adult (19-30-year-old) patients with the major histopathological characteristics, tumor invasive features and recurrence.
The radiogenic and sporadic tumors sized up to 5 mm (Table 4) displayed practically no differences between the baseline, histopathological and clinical characteristics except for the less frequent central lymph node dissection (OR = 0.163, p = 0.031) in, and longer follow-up period (OR = 1.935, p = 0.001) of patients with radiogenic MPTC.
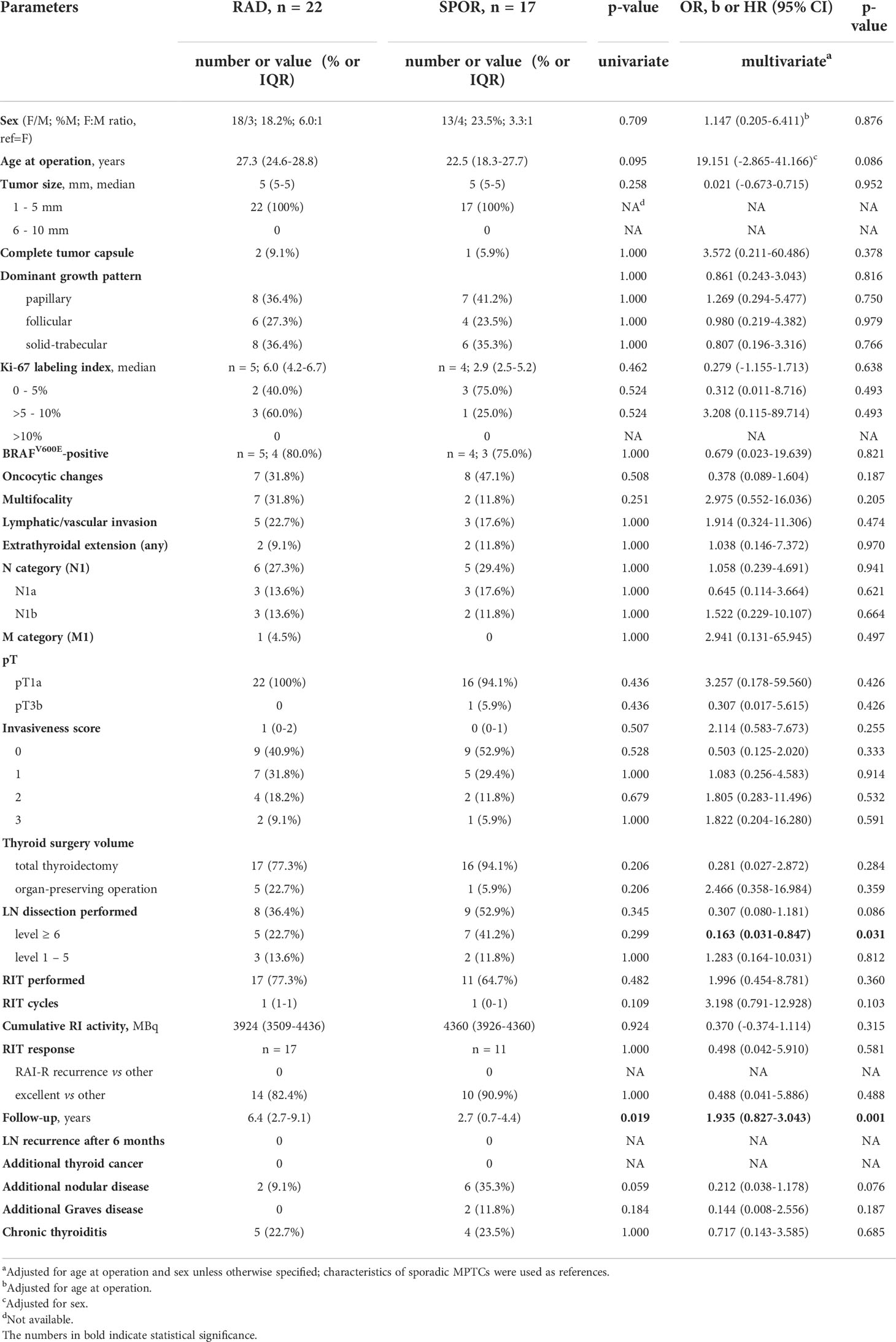
Table 4 Characteristics of the radiogenic and sporadic papillary thyroid microcarcinomas sized up to 5 mm.
In MPTCs of larger size (6-10 mm), statistically significant differences between the radiogenic and sporadic series were found for a number of parameters (Table 5). Those included the older age of patients with radiogenic MPTCs (b = 28.445, p = 2.62E-07), less frequent oncocytic changes (OR = 0.390, p = 0.010), multifocality (OR = 0.365, p = 0.017), nodal disease (OR = 0.419, p = 0.028; for N1b, OR = 0.229, p = 0.025), lower invasiveness score (OR = 0.474, p = 0.015; and more frequent invasiveness score 0, OR = 2.095, p = 0.035), and less frequent chronic thyroiditis (OR = 0.373, p = 0.011). The radiogenic MPTCs were also associated with lower cumulative RI activity (b = -0.384, p = 0.021), yet better excellent RIT response (OR = 94.032, p = 0.003), suggestively less frequent RAI-R (OR = 0.066, p = 0.073), and longer follow-up (b = 1.717, p = 8.23E-09). Again, all two RAI-R recurrences measuring 6 and 8 mm were among the sporadic MPTCs. In Figure 3 we present one of these tumors harboring the BRAFV600E mutation and featuring oncocytic changes in both primary tumor and recurrent metastasis epithelial cells.
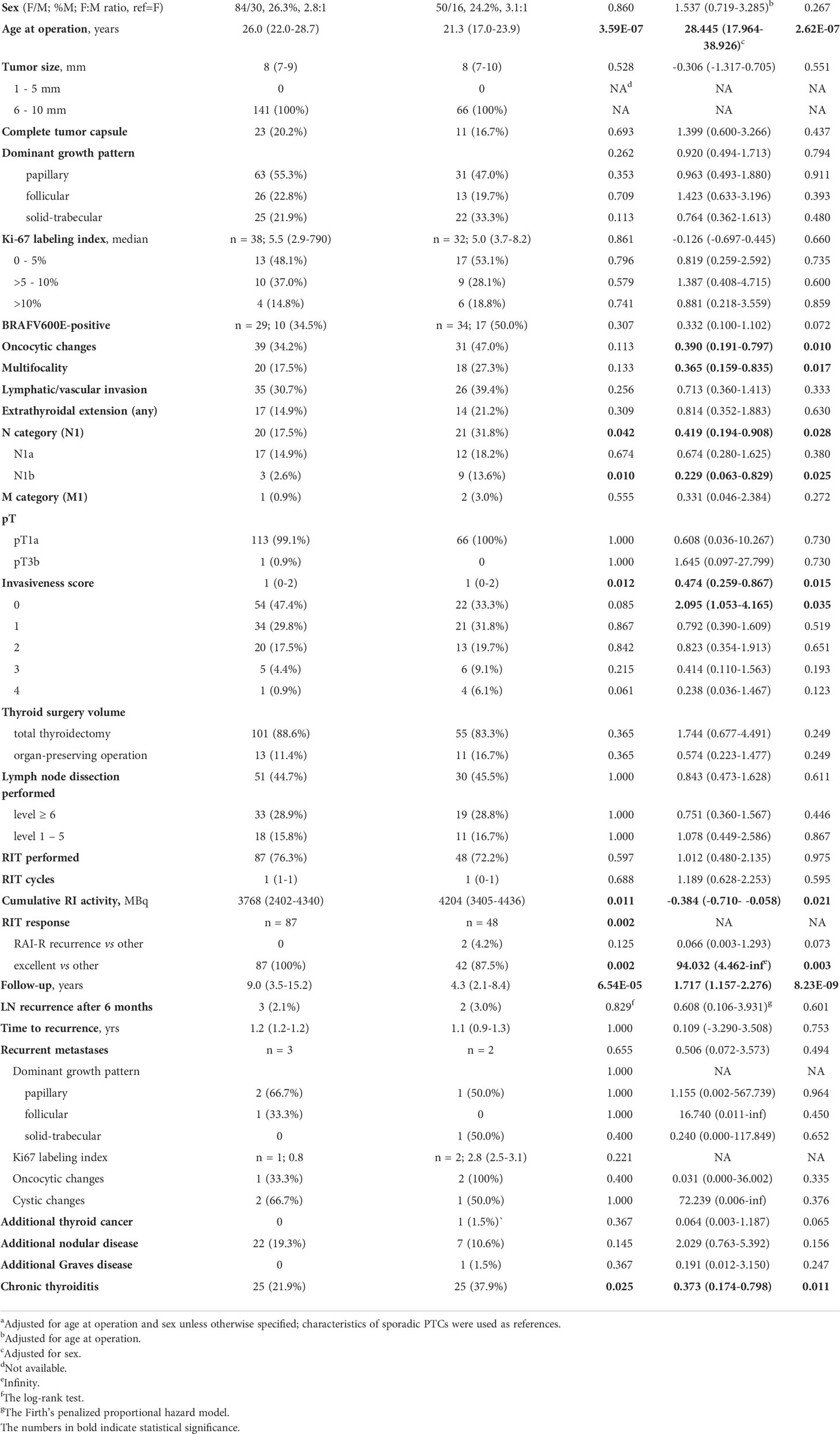
Table 5 Characteristics of the radiogenic and sporadic papillary thyroid microcarcinomas sized 6-10 mm.
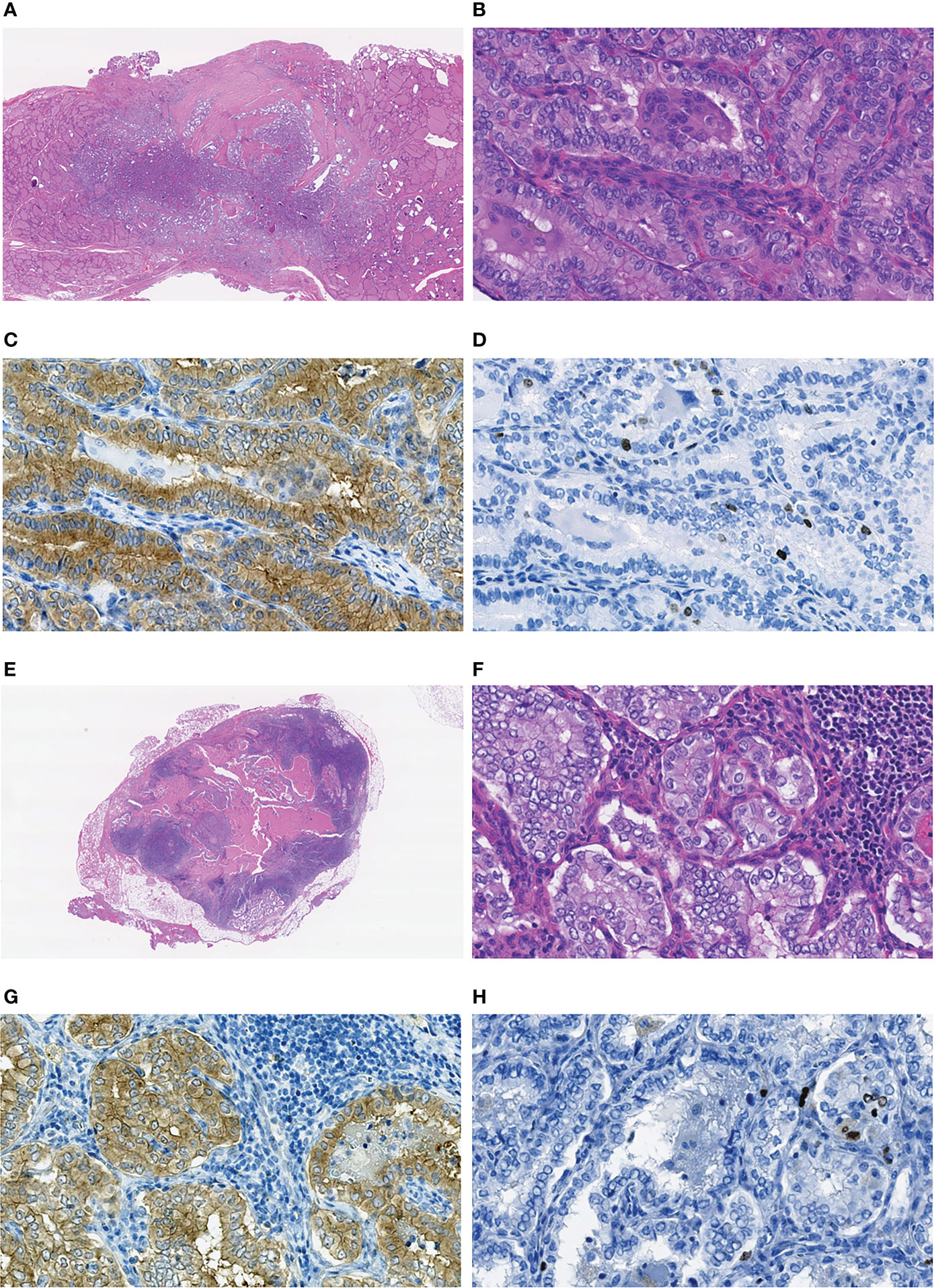
Figure 3 Sporadic recurrent MPTC sized 8 mm (pT1aN0M0 at the first operation) removed from a 22-year-old male patient: (A–D) primary tumor, (E–H) the RAI-R recurrent metastasis. (A) Nonencapsulated primary tumor with follicular-papillary (conventional) growth pattern with extrathyroidal extension to the connective tissue, H&E, 15X magnification. (B) Fragment of the primary tumor with papillary structures featuring tall cell areas and oncocytic changes, H&E, 400X magnification. (C) Primary tumor: positive IHC reaction with the anti-BRAF (mutated V600E) antibody, 400X magnification. (D) Primary tumor: IHC reaction with Ki67 (Clone MIB-1) antibody (Ki67 LI 3.1%), 400X magnification. (E) RAI-R recurrent metastasis with cystic changes removed 1.3 years after the first surgery, H&E, 15X magnification. (F) Fragment of the RAI-R recurrent metastasis with solid structure and oncocytic changes, H&E, 400X magnification. (G) fragment of the RAI-R recurrent metastasis: positive IHC reaction with anti-BRAF (mutated V600E) antibody, 400X magnification. (H) RAI-R recurrent metastasis: IHC reaction with Ki67 (Clone MIB-1) antibody (Ki67 LI 3.3%), 400X magnification.
Correspondence analysis of MPTCs of different etiology and size subgroups (Figure 4) confirmed that these tumors did not display aggressive phenotype overall (note the upper right quadrant, where the aggressive characteristics are located). The radiogenic MPTCs measuring 6-10 mm appeared different from other subgroups (note the obtuse angles between this and other subgroups), yet without signs of association with aggressive features. The subgroups of radiogenic and sporadic MPTCs measuring 1-5 mm were rather similar in their properties (note the acute angle between those) without evidence of tumor aggressiveness or poorer prognosis. The sporadic MPTCs sized 6-10 mm, but not the radiogenic ones, weakly tended to associate with more aggressive tumor phenotype.
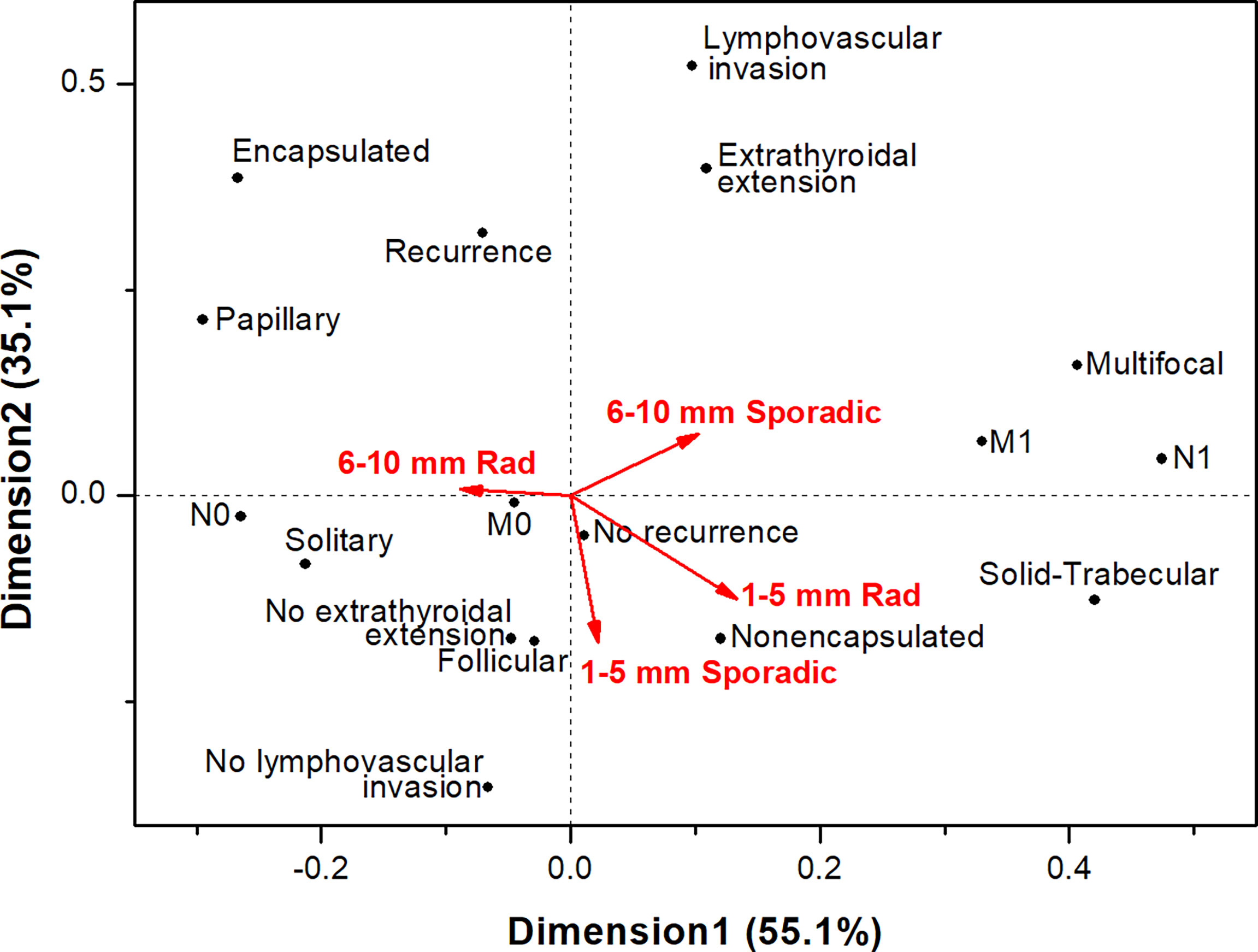
Figure 4 Correspondence analysis of the associations of papillary thyroid microcarcinomas of different etiology sized 1-5 mm and 6-10 mm with the major histopathological characteristics, tumor invasive features and recurrence.
To further delineate the characteristics (parameters) that might be associated with the radiogenic or sporadic etiology of MPTCs in the current series, we applied several statistical algorithms of variable selection (Table 6). The older age at surgery, the less frequent nodal disease, oncocytic changes, multifocality, and male sex were the most likely characteristics that were associated with radiogenic MPTC. Other potentially relevant variables might be excellent RIT response and less frequent lymphovascular invasion. Hence, for any algorithm, no evidence of association of the radiogenic MPTCs with aggressive tumor behavior or poorer prognosis, as compared to sporadic MPTCs, was found.
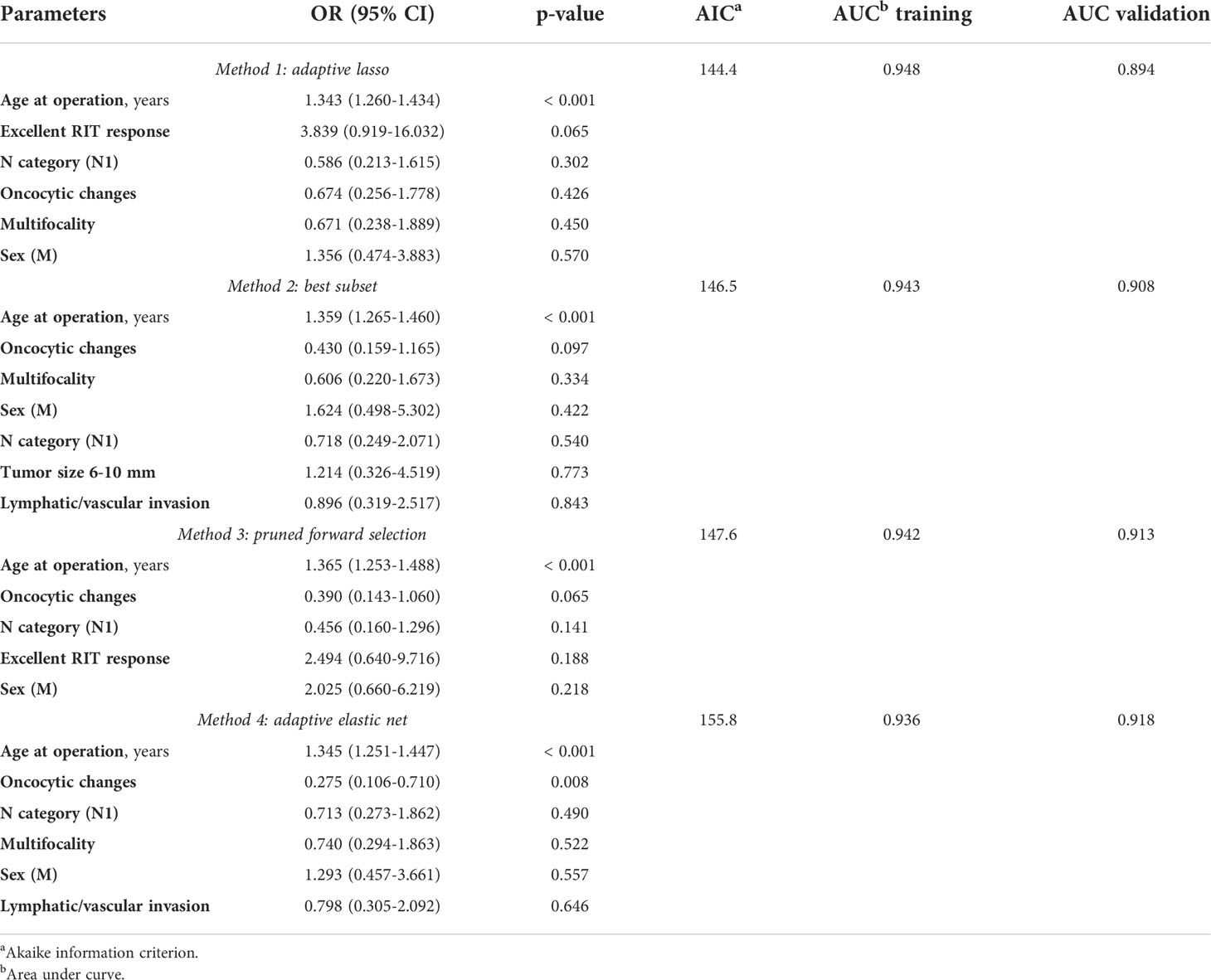
Table 6 The variables potentially contributing to the differences between the non-incidental radiogenic and sporadic papillary thyroid microcarcinomas selected by different statistical methods.
A link between previous radiation exposure and MPTC has been established in atomic bomb victims, in whom an increased dose-dependent risk for developing tumors has been observed (38). However, despite the 2015 ATA Guidelines mention of radiation history as a potential contraindication to less extensive thyroid surgery for patients with low-risk PTC (22), there is little evidence from clinical and histopathological studies that would justify such warning. In part, this may be due to the relative infrequency of patients with thyroid cancer who had been irradiated at a young age. We therefore considered it important to perform a study of MPTCs from patients of comparable age with proven previous internal radiation exposure at the age below 18 or without such. The radiogenic MPTCs in our work were from patients from the high-risk group of subjects who lived in the northern, most radiocontaminated by the Chornobyl fallouts, regions of Ukraine. The individual radiation doses to the thyroid were calculated and confirmed for all of them.
The rationale for focusing the study on young patients (here, aged up to 30 years) was that our previous investigation found higher aggressiveness of radiogenic PTCs, especially in children and adolescents (25, 26). However, the mentioned studies did not specifically address the effect of tumor size, and therefore whether these findings are applicable to MPTCs remained unclear. It is also important that more than 35 years after the Chornobyl accident, a sufficient control group of patients with MPTCs who lived in the same regions but had not been exposed to radiation became available.
Our analysis of MPTCs from two etiological groups detected several statistically significant differences including older age of patients in the radiogenic series, less frequent oncocytic changes, lymph node metastases and concomitant chronic thyroiditis (see Table 1). RIT results were better in patients with radiogenic MPTCs, excellent response was more frequent in this group, and no RAI-R metastases were observed. No signs of higher aggressiveness or poorer prognosis in the radiogenic MPTCs were found.
Comparison of MPTCs in the pediatric subgroup did not reveal significant differences in the clinical and histopathological characteristics between the radiogenic and sporadic pediatric MPTCs (see Table 2 and Figure 2), except for the lower frequency of oncocytic changes in the radiogenic MPTCs, which is in line with data obtained earlier for PTCs of any size (34). It is noteworthy that with median sizes of 7 and 8 mm of the radiogenic and sporadic MPTCs, respectively, and the presence of lymph node metastases in about 20% of patients (see Table 2), which is associated with worse prognosis according to the literature (39–41), no one pediatric patient in both groups developed recurrence during the 19.4 and 6.3 years, respectively, of median follow-up.
In young adult patients aged 19-30 years (see Table 3), no evidence in support of higher aggressiveness of the radiogenic MPTCs was found. Regional recurrences occurred in isolated cases (2.1% radiogenic and 3.7% sporadic), but in the sporadic subgroup, in contrast to the radiogenic subgroup, these were BRAFV600E-positive and RAI-R (shown in Figure 3). As in the whole group, young adult patients with radiogenic MPTCs were more likely to achieve complete remission (excellent response) after RIT (see Table 3).
In the analysis by tumor size, a number of differences between the radiogenic and sporadic MPTCs were observed for the 6-10 mm subgroups (see Tables 4, 5, Figure 4). Again, these differences were rather suggestive of a less aggressive phenotype of the radiogenic MPTC as compared to sporadic. Virtually no difference was seen between the radiogenic and sporadic MPTCs measuring 1-5 mm.
Thus, in young patients in the whole group or age or size subgroups, no clinical and histopathological parameters pointing at more aggressive behavior of radiogenic MPTCs were found. With regard to clinical implications, it would be pertinent to suggest that the extent of surgery for MPTC in internally irradiated patients should not be principally different from that in non-exposed patients aged up to 30 years; in other words, etiological considerations may be omitted. Furthermore, patients with radiogenic MPTCs were more likely to achieve complete remission and did not develop RAI-R recurrent metastases.
Notwithstanding the overall indolent clinical presentation of MPTCs, in both etiological groups there were tumors with signs of morphological aggressiveness: multifocal growth, lymphatic/vascular invasion, extrathyroidal extension (almost always minimal), lymph node metastases and even distant metastases (see Table 1). These morphological characteristics have been identified as negative prognostic factors for MPTCs in many studies (42–47), and, in turn, have been correlated with a larger MPTC size (48–51). Recurrences were observed in isolated cases of our study too, and occurred in the subgroups of patients of older age (here, the young adults) with MPTCs measuring 6-10 mm. Hence, regardless of MPTC etiology, our study additionally confirmed that the “small tumors” should be stratified into the low-, intermediate-, and high-risk ones (40, 47, 52) both in pediatric and adult patients in line with existing guidelines (22, 53).
It is worth noting that to draw more attention to the problem of treatment of microcarcinoma, a somewhat obscure and not necessarily correct – in our opinion – term “overdiagnosis” has been introduced and extensively used (3–5, 10, 11). Indeed, the improvement of ultrasound diagnostic methods has led to “overdetection”, i.e. an ability to clearly visualize even a small lesion sized a few millimeters, perform FNA, obtain a “suspicious” cytological conclusion and make a decision on surgical treatment; in fact, this may possibly be “overtreatment”. However, the postoperative histological conclusion “papillary thyroid microcarcinoma” is by no means “overdiagnosis” since it is a completely correct final pathological diagnosis fully corresponding to the current WHO Histological Classification of thyroid tumors (33).
Our study has certain strengths and limitations. Radiogenic and sporadic cases were selected from a single institution and were well matched by the place of residence. Although patients were not matched by age at surgery and sex, multivariate models were appropriately adjusted, and therefore confounding by these parameters is unlikely. Neither radiogenic, nor sporadic cases in the study were detected by active screening, implying that selection bias did not play a noticeable role. Some birth period or cohort effects might be expected stemming from the inclusion criterion for patients with sporadic MPTCs, which required that they were born since 1987 to ensure the lack of exposure to fallout. This led to the difference in calendar time of the first patient entry in the study between the radiogenic and sporadic groups (1992 and 2001, respectively; the proportion of patients with the radiogenic MPTCs diagnosed from 1992 to 2000 accounted for 18.4%). A rather limited number of patients in the pediatric and MPTCs measuring 1-5 mm subgroups might provide insufficient statistical power to detect the effects. Similarly, a very small number of recurrent tumors in the study and a relatively short follow-up period did not allow assessment of etiology-specific prognostic factors, pointing at the need for further research.
In conclusion, our study did not find evidence in support of important effects of internal irradiation on histopathological characteristics, more pronounced invasive properties and prognosis worsening for MPTCs in pediatric or young adult patients. These results imply that young MPTC patients with a radiation history (at least of internal exposure to radioiodine) may not require treatment strategies different from those for non-exposed MPTC patients. Of importance, some MPTCs in young patients may be locally advanced or develop distant metastasis indicating a need for risk stratification and tailored treatment, regardless of tumor etiology.
The original contributions presented in the study are included in the article. Further inquiries can be directed to the corresponding author.
The studies involving human participants were reviewed and approved by Bioethics Committee, State Institution V.P. Komisarenko Institute of Endocrinology and Metabolism of the National Academy of Medical Sciences of Ukraine, the Chornobyl Tissue Bank, Ethics Committee of Nagasaki University. Written informed consent to participate in this study was provided by the participants’ legal guardian/next of kin.
TB, SC, LZ, NM, MT, SY, and VAS: study design and methodology. TB, SC, LZ, and MB: clinical and pathological data. LZ, TIR, and MI: investigation and formal analysis. SM: thyroid dosimetry. TB and VAS: statistical analysis, data interpretation, and writing of the manuscript. TB, SC, LZ, TIR, NM, MI, MT, MB, SM, SY, and VAS: revision of the manuscript. All authors have reviewed the manuscript and approved the final version.
This research was supported in part by the Program of the Network-Type Joint Usage/Research Center for Radiation Disaster Medical Science, intramurally by the Atomic Bomb Disease Institute, Nagasaki University, and the Japan Society for the Promotion of Science (JSPS), KAKENHI Grant Numbers 19K07471, 19KK02670001, and 20KK0217.
We gratefully acknowledge the commitment of the staff of the Laboratory of Morphology of Endocrine System and of the Department of Surgery of Endocrine Glands of IEM, who prepared all pathological material and operated on the patients, respectively. The authors gratefully acknowledge the confirmation of Ukrainian diagnoses by the International Pathology Panel of the Chornobyl Tissue Bank, which was supported by NCI grant number U24CA082102: A. Abrosimov, TB, G. Fadda, J. Hunt, MI, V. Livolsi, J. Rosai, E. D. Williams, N. Dvinskyh, and LZ.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Genere N, El Kawkgi OM, Giblon RE, Vaccarella S, Morris JC, Hay ID, et al. Incidence of clinically relevant thyroid cancers remains stable for almost a century: A population-based study. Mayo Clin Proc (2021) 96:2823–30. doi: 10.1016/j.mayocp.2021.04.028
2. Pizzato M, Li M, Vignat J, Laversanne M, Singh D, La Vecchia C, et al. The epidemiological landscape of thyroid cancer worldwide: Globocan estimates for incidence and mortality rates in 2020. Lancet Diabetes Endocrinol (2022) 10:264–72. doi: 10.1016/S2213-8587(22)00035-3
3. Ahn HS, Kim HJ, Welch HG. Korea's thyroid-cancer "Epidemic" – screening and overdiagnosis. N Engl J Med (2014) 371:1765–7. doi: 10.1056/NEJMp1409841
4. Ahn HS, Welch HG. South korea's thyroid-cancer "Epidemic" – turning the tide. N Engl J Med (2015) 373:2389–90. doi: 10.1056/NEJMc1507622
5. Ahn HS, Kim HJ, Kim KH, Lee YS, Han SJ, Kim Y, et al. Thyroid cancer screening in south Korea increases detection of papillary cancers with no impact on other subtypes or thyroid cancer mortality. Thyroid (2016) 26:1535–40. doi: 10.1089/thy.2016.0075
6. Doubi A, Al-Qannass A, Al-Angari SS, Al-Qahtani KH, Alessa M, Al-Dhahri S. Trends in thyroid carcinoma among thyroidectomy patients: A 12-year multicenter study. Ann Saudi Med (2019) 39:345–9. doi: 10.5144/0256-4947.2019.345
7. Kang HY, Kim I, Kim YY, Bahk J, Khang YH. Income differences in screening, incidence, postoperative complications, and mortality of thyroid cancer in south Korea: A national population-based time trend study. BMC Cancer (2020) 20:1096. doi: 10.1186/s12885-020-07597-4
8. Miranda-Filho A, Lortet-Tieulent J, Bray F, Cao B, Franceschi S, Vaccarella S, et al. Thyroid cancer incidence trends by histology in 25 countries: A population-based study. Lancet Diabetes Endocrinol (2021) 9:225–34. doi: 10.1016/S2213-8587(21)00027-9
9. Takano T. Natural history of thyroid cancer. Endocr J (2017) 64:237–44. doi: 10.1507/endocrj.EJ17-0026
10. Aliyev E, Ladra-Gonzalez MJ, Sanchez-Ares M, Abdulkader-Nallib I, Piso-Neira M, Rodriguez-Carnero G, et al. Thyroid papillary microtumor: Validation of the (Updated) Porto proposal assessing sex hormone receptor expression and mutational braf gene status. Am J Surg Pathol (2020) 44:1161–72. doi: 10.1097/PAS.0000000000001522
11. Dedhia PH, Saucke MC, Long KL, Doherty GM, Pitt SC. Physician perspectives of overdiagnosis and overtreatment of low-risk papillary thyroid cancer in the us. JAMA Netw Open (2022) 5:e228722. doi: 10.1001/jamanetworkopen.2022.8722
12. Ito Y, Miyauchi A, Inoue H, Fukushima M, Kihara M, Higashiyama T, et al. An observational trial for papillary thyroid microcarcinoma in Japanese patients. World J Surg (2010) 34:28–35. doi: 10.1007/s00268-009-0303-0
13. Tuttle RM, Fagin JA, Minkowitz G, Wong RJ, Roman B, Patel S, et al. Natural history and tumor volume kinetics of papillary thyroid cancers during active surveillance. JAMA Otolaryngol Head Neck Surg (2017) 143:1015–20. doi: 10.1001/jamaoto.2017.1442
14. Jeon MJ, Kim WG, Chung KW, Baek JH, Kim WB, Shong YK. Active surveillance of papillary thyroid microcarcinoma: Where do we stand? Eur Thyroid J (2019) 8:298–306. doi: 10.1159/000503064
15. Sugitani I, Ito Y, Takeuchi D, Nakayama H, Masaki C, Shindo H, et al. Indications and strategy for active surveillance of adult low-risk papillary thyroid microcarcinoma: Consensus statements from the Japan association of endocrine surgery task force on management for papillary thyroid microcarcinoma. Thyroid (2021) 31:183–92. doi: 10.1089/thy.2020.0330
16. Sutherland R, Tsang V, Clifton-Bligh RJ, Gild ML. Papillary thyroid microcarcinoma: Is active surveillance always enough? Clin Endocrinol (Oxf) (2021) 95:811–7. doi: 10.1111/cen.14529
17. Chen W, Li J, Peng S, Hong S, Xu H, Lin B, et al. Association of total thyroidectomy or thyroid lobectomy with the quality of life in patients with differentiated thyroid cancer with low to intermediate risk of recurrence. JAMA Surg (2022) 157:200–9. doi: 10.1001/jamasurg.2021.6442
18. Paluskievicz CM, Chang DR, Blackburn KW, Turner DJ, Munir KM, Mullins CD, et al. Low-risk papillary thyroid cancer: Treatment de-escalation and cost implications. J Surg Res (2022) 275:273–80. doi: 10.1016/j.jss.2022.01.019
19. Chou R, Dana T, Haymart M, Leung AM, Tufano RP, Sosa JA, et al. Active surveillance versus thyroid surgery for differentiated thyroid cancer: A systematic review. Thyroid (2022) 32:351–67. doi: 10.1089/thy.2021.0539
20. Orlando G, Scerrino G, Corigliano A, Vitale I, Tutino R, Radellini S, et al. Papillary thyroid microcarcinoma: Active surveillance against surgery. considerations of an Italian working group from a systematic review. Front Oncol (2022) 12:859461. doi: 10.3389/fonc.2022.859461
21. Merdad M, Eskander A, De Almeida J, Freeman J, Rotstein L, Ezzat S, et al. Current management of papillary thyroid microcarcinoma in Canada. J Otolaryngol Head Neck Surg (2014) 43:32. doi: 10.1186/s40463-014-0032-8
22. Haugen BR, Alexander EK, Bible KC, Doherty GM, Mandel SJ, Nikiforov YE, et al. 2015 American Thyroid association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: The American thyroid association guidelines task force on thyroid nodules and differentiated thyroid cancer. Thyroid (2016) 26:1–133. doi: 10.1089/thy.2015.0020
23. Tronko M, Shpak V, Bogdanova T, Saenko V, Yamashita S. Epidemiology of thyroid cancer in Ukraine after Chernobyl. In: Tronko M, Bogdanova T, Saenko V, Thomas GA, Likhtarov I, Yamashita S, editors. Thyroid cancer in Ukraine after Chernobyl: Dosimetry, epidemiology, pathology, molecular biology. Nagasaki: IN-TEX (2014). p. 39–64.
24. Fridman M, Lam AK, Krasko O, Schmid KW, Branovan DI, Demidchik Y. Morphological and clinical presentation of papillary thyroid carcinoma in children and adolescents of Belarus: The influence of radiation exposure and the source of irradiation. Exp Mol Pathol (2015) 98:527–31. doi: 10.1016/j.yexmp.2015.03.039
25. Bogdanova TI, Saenko VA, Brenner AV, Zurnadzhy LY, Rogounovitch TI, Likhtarov IA, et al. Comparative histopathologic analysis of "Radiogenic" and "Sporadic" papillary thyroid carcinoma: Patients born before and after the Chernobyl accident. Thyroid (2018) 28:880–90. doi: 10.1089/thy.2017.0594
26. Bogdanova TI, Saenko VA, Hashimoto Y, Hirokawa M, Zurnadzhy LY, Hayashi T, et al. Papillary thyroid carcinoma in Ukraine after Chernobyl and in Japan after fukushima: Different histopathological scenarios. Thyroid (2021) 31:1322–34. doi: 10.1089/thy.2020.0308
27. Tronko MD, Bogdanova TI, Komissarenko IV, Epstein OV, Oliynyk V, Kovalenko A, et al. Thyroid carcinoma in children and adolescents in Ukraine after the Chernobyl nuclear accident: Statistical data and clinicomorphologic characteristics. Cancer (1999) 86:149–56. doi: 10.1002/(sici)1097-0142(19990701)86:1<149::aid-cncr21>3.0.co;2-a
28. Saenko V, Ivanov V, Tsyb A, Bogdanova T, Tronko M, Demidchik Y, et al. The Chernobyl accident and its consequences. Clin Oncol (R Coll Radiol) (2011) 23:234–43. doi: 10.1016/j.clon.2011.01.502
29. Likhtarov I, Thomas G, Kovgan L, Masiuk S, Chepurny M, Ivanova O, et al. Reconstruction of individual thyroid doses to the Ukrainian subjects enrolled in the Chernobyl tissue bank. Radiat Prot Dosimetry (2013) 156:407–23. doi: 10.1093/rpd/nct096
30. Lloyd RV, Osamura RY, Kloppel G, Rosai J. Who classification of tumours of endocrine organs. 4 ed. Lyon: IARC Press (2017).
31. Thomas GA, Williams ED, Becker DV, Bogdanova TI, Demidchik EP, Lushnikov E, et al. Chernobyl Tumor bank. Thyroid (2000) 10:1126–7. doi: 10.1089/thy.2000.10.1126a
32. Thomas GA. The Chernobyl tissue bank: Integrating research on radiation-induced thyroid cancer. J Radiol Prot (2012) 32:N77–80. doi: 10.1088/0952-4746/32/1/N77
33. Brierley JD, Gospodarowich MK, Wittekind C. TNM classification of malignant tumours. 8 ed. Oxford: Wiley-Blackwell (2017).
34. Zurnadzhy L, Bogdanova T, Rogounovitch TI, Ito M, Tronko M, Yamashita S, et al. The BRAFV600E mutation is not a risk factor for more aggressive tumor behavior in radiogenic and sporadic papillary thyroid carcinoma at a young age. Cancers (Basel) (2021) 13:6038. doi: 10.3390/cancers13236038
35. Zurnadzhy L, Bogdanova T, Rogounovitch TI, Ito M, Tronko M, Yamashita S, et al. Clinicopathological implications of the BRAFV600E mutation in papillary thyroid carcinoma of Ukrainian patients exposed to the Chernobyl radiation in childhood: A study for 30 years after the accident. Front Med (Lausanne) (2022) 9:882727. doi: 10.3389/fmed.2022.882727
36. Nakao T, Matsuse M, Saenko V, Rogounovitch T, Tanaka A, Suzuki K, et al. Preoperative detection of the tert promoter mutations in papillary thyroid carcinomas. Clin Endocrinol (Oxf) (2021) 95:790–9. doi: 10.1111/cen.14567
37. Nenadic O, Greenacre M. Correspondence analysis in r, with two- and three-dimensional graphics: The Ca package. J Stat Softw (2007) 20:1–13. doi: 10.18637/jss.v020.i03
38. Hayashi Y, Lagarde F, Tsuda N, Funamoto S, Preston DL, Koyama K, et al. Papillary microcarcinoma of the thyroid among atomic bomb survivors: Tumor characteristics and radiation risk. Cancer (2010) 116:1646–55. doi: 10.1002/cncr.24872
39. Kim WY, Kim HY, Son GS, Bae JW, Lee JB. Clinicopathological, immunohistochemical factors and recurrence associated with extrathyroidal extension in papillary thyroid microcarcinoma. J Cancer Res Ther (2014) 10:50–5. doi: 10.4103/0973-1482.131366
40. Ciobanu Apostol D, Giusca SE, Caruntu ID, Lozneanu L, Andriescu EC, Moscalu M. Relationships between clinicopathological prognostic factors in papillary thyroid microcarcinoma: A refined analysis based on 428 cases. Int J Clin Exp Pathol (2017) 10:8944–56.
41. Carvalho AY, Kohler HF, Gomes CC, Vartanian JG, Kowalski LP. Predictive factors of recurrence of papillary thyroid microcarcinomas: Analysis of 2,538 patients. Int Arch Otorhinolaryngol (2021) 25:e585–e93. doi: 10.1055/s-0040-1722253
42. Sun W, Lan X, Zhang H, Dong W, Wang Z, He L, et al. Risk factors for central lymph node metastasis in Cn0 papillary thyroid carcinoma: A systematic review and meta-analysis. PloS One (2015) 10:e0139021. doi: 10.1371/journal.pone.0139021
43. Gui CY, Qiu SL, Peng ZH, Wang M. Clinical and pathologic predictors of central lymph node metastasis in papillary thyroid microcarcinoma: A retrospective cohort study. J Endocrinol Invest (2018) 41:403–9. doi: 10.1007/s40618-017-0759-y
44. Perez-Soto RH, Velazquez-Fernandez D, Arellano-Gutierrez G, Chapa-Ibarguengoitia M, Trolle-Silva AM, Iniguez-Ariza N, et al. Preoperative and postoperative risk stratification of thyroid papillary microcarcinoma: A comparative study between kuma criteria and 2015 American thyroid association guidelines risk stratification. Thyroid (2020) 30:857–62. doi: 10.1089/thy.2019.0698
45. Zhao L, Sun X, Luo Y, Wang F, Lyu Z. Clinical and pathologic predictors of lymph node metastasis in papillary thyroid microcarcinomas. Ann Diagn Pathol (2020) 49:151647. doi: 10.1016/j.anndiagpath.2020.151647
46. Dirikoc A, Tam AA, Ince N, Ozdemir D, Topaloglu O, Alkan A, et al. Papillary thyroid microcarcinomas that metastasize to lymph nodes. Am J Otolaryngol (2021) 42:103023. doi: 10.1016/j.amjoto.2021.103023
47. Horiguchi K, Yoshida Y, Iwaku K, Emoto N, Kasahara T, Sato J, et al. Position paper from the Japan thyroid association task force on the management of low-risk papillary thyroid microcarcinoma (T1an0m0) in adults. Endocr J (2021) 68:763–80. doi: 10.1507/endocrj.EJ20-0692
48. Gu JH, Zhao YN, Xie RL, Xu WJ, You DL, Zhao ZF, et al. Analysis of risk factors for cervical lymph node metastasis of papillary thyroid microcarcinoma: A study of 268 patients. BMC Endocr Disord (2019) 19:124. doi: 10.1186/s12902-019-0450-8
49. Medas F, Canu GL, Cappellacci F, Boi F, Lai ML, Erdas E, et al. Predictive factors of lymph node metastasis in patients with papillary microcarcinoma of the thyroid: Retrospective analysis on 293 cases. Front Endocrinol (Lausanne) (2020) 11:551. doi: 10.3389/fendo.2020.00551
50. Taskin OC, Armutlu A, Agcaoglu O, Peker O, Terzioglu T, Demirkol MO, et al. Tumor border pattern and size help predict lymph node status in papillary microcarcinoma: A clinicopathologic study. Ann Diagn Pathol (2020) 48:151592. doi: 10.1016/j.anndiagpath.2020.151592
51. Huang Y, Yin Y, Zhou W. Risk factors for central and lateral lymph node metastases in patients with papillary thyroid micro-carcinoma: Retrospective analysis on 484 cases. Front Endocrinol (Lausanne) (2021) 12:640565. doi: 10.3389/fendo.2021.640565
52. Lu ZZ, Zhang Y, Wei SF, Li DS, Zhu QH, Sun SJ, et al. Outcome of papillary thyroid microcarcinoma: Study of 1,990 cases. Mol Clin Oncol (2015) 3:672–6. doi: 10.3892/mco.2015.495
Keywords: radiogenic papillary thyroid microcarcinoma, sporadic papillary thyroid microcarcinoma, invasiveness, treatment, BRAFV600E mutation, Ki67 labeling index
Citation: Bogdanova T, Chernyshov S, Zurnadzhy L, Rogounovitch TI, Mitsutake N, Tronko M, Ito M, Bolgov M, Masiuk S, Yamashita S and Saenko VA (2022) The high degree of similarity in histopathological and clinical characteristics between radiogenic and sporadic papillary thyroid microcarcinomas in young patients. Front. Endocrinol. 13:970682. doi: 10.3389/fendo.2022.970682
Received: 16 June 2022; Accepted: 02 August 2022;
Published: 19 August 2022.
Edited by:
Kirk Ernest Jensen, Uniformed Services University of the Health Sciences, United StatesReviewed by:
Laura Giacomelli, Sapienza University of Rome, ItalyCopyright © 2022 Bogdanova, Chernyshov, Zurnadzhy, Rogounovitch, Mitsutake, Tronko, Ito, Bolgov, Masiuk, Yamashita and Saenko. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Vladimir A. Saenko, c2FlbmtvQG5hZ2FzYWtpLXUuYWMuanA=
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.