
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Endocrinol. , 14 October 2022
Sec. Obesity
Volume 13 - 2022 | https://doi.org/10.3389/fendo.2022.967102
 Xiaoli Gong1,2†
Xiaoli Gong1,2† Jiaxin Li1,2†
Jiaxin Li1,2† Yuanhui Jiang1,2
Yuanhui Jiang1,2 Pengbo Yuan1,2
Pengbo Yuan1,2 Lian Chen1,2,3
Lian Chen1,2,3 Yike Yang1,2
Yike Yang1,2 You Li1,2
You Li1,2 Mengxing Sun1,2
Mengxing Sun1,2 Yangyu Zhao1,2,3*
Yangyu Zhao1,2,3* Huifeng Shi1,2,3*
Huifeng Shi1,2,3* Yuan Wei1,2,3*
Yuan Wei1,2,3*Introduction: Despite the important clinical significance, limited data on the joint contribution of prepregnancy body mass index (BMI) and gestational weight gain (GWG) to preeclampsia, the second leading cause of maternal mortality worldwide. This study aimed to estimate the risk of preeclampsia by GWG among women with varied prepregnancy BMI.
Methods: We conducted a retrospective cohort study using data of 117 738 singleton pregnant women aged 18–49 years from 150 maternity hospitals in China between 2015 and 2018. GWG was calculated as the measured weight at the time of preeclampsia assessment minus prepregnancy weight; GWG velocity was calculated as the GWG divided by the gestational age at weighing. The non-linear associations of GWG with preeclampsia were examined by restricted cubic spline regression analysis according to prepregnancy BMI. The association of the GWG categories with preeclampsia was further examined by performing robust Poisson regression stratified by the prepregnancy BMI categories.
Results: Among participants, 2426 (2.06%) were diagnosed with preeclampsia. Compared to women with normal BMI, those who were overweight and obese had 1.92- fold (95%CI, 1.73–2.14) and 5.06- fold (95%CI, 4.43–5.78) increased risks for preeclampsia, respectively. The association of GWG velocity with preeclampsia was presented as a J-shaped curve with the varied inflexion point (where the rate of preeclampsia was 2%), which was 0.54, 0.38, and 0.25 kg/week in women with normal BMI, overweight, and obesity, respectively; a steep risk rise was observed along with GWG velocity beyond the inflexion points. The overall adjusted relative risk for preeclampsia was calculated among women with the different GWG categories of GWG.
Conclusions: The findings highlight that high prepregnancy BMI and exceed GWG contributed to increased risk of preeclampsia with a superimposed effect and underscore the need to optimize the recommendations for GWG for women with different prepregnancy BMI.
Preeclampsia, commonly defined as new-onset hypertension and proteinuria after 20 weeks of gestation (1), is the second leading cause of maternal mortality and a major contributor of maternal and perinatal morbidity worldwide (2). Preeclampsia complicates 2–8% of pregnancies (1, 3). Given no known cure for preeclampsia other than delivery, identifying the risk factors is critical for developing prevention strategies to reduce the incidence of preeclampsia and consequent adverse outcomes.
Overweight and obesity, the well documented risk factor for preeclampsia (4–10), has seen a rising trend since 1980 all over the world (11, 12). The World Health Organization (WHO) estimates that the global age-standardized mean body mass index (BMI) increased from 22 kg/m2 in 1975 to 24.6 kg/m2 in 2016 in adult women, with the global age-standardized prevalence of overweight and obesity increased from 22.7% and 6.3% to 39.2% and 15.1% in adult women, respectively (12). In China, the annual increase in age-standardized mean BMI increased 0.09 kg/m² for adult women in 14 years from 2004, leading to virtually identical age-standardized mean BMIs of 24.1 kg/m², overweight prevalence of 36.7% and obesity prevalence of 7.2% in 2018 (13). The rise in mean BMI was faster in women aged 18-29, the main fertility population (13).
Weight gain during pregnancy may vary among pregnant women with different prepregnancy BMI and excessive gestational weight gain (GWG) is more likely to occur in women who are overweight and obese (14). However, the relationship between GWG and preeclampsia is inconclusive due to limited evidence (15, 16). In clinical practice, it is more valuable to estimate the joint contribution of prepregnancy BMI and GWG to the occurrence of preeclampsia (17), but this has rarely been investigated (15, 18). This study therefore aimed to estimate the risk of preeclampsia by gestational weight gain among women with varied prepregnancy BMI, by using data from a retrospective, multicenter cohort data.
A prospective cohort study determining the factors of preeclampsia had been conducted at 180 hospitals across 23 provinces in China between 2015 and 2018. Pregnant women who had been registered for antenatal care in the hospitals were recruited to participate. Our study population was drawn from singleton pregnant women aged 18–49 years with preeclampsia assessment at 20–40 weeks in the cohort database. We excluded women with prepregnancy hypertension, diabetes mellitus, and those without available data of height, prepregnancy weight, and gestational weight measurement. Finally, 117 738 pregnant women with required data from 150 maternity hospitals were included (Figure S1).
Prepregnancy BMI was calculated as the self-reported prepregnancy weight in kilograms (kg) divided by height in meters squared measured at the first time of antenatal care. Subsequently, prepregnancy BMI was then classified as underweight [BMI: <18.5 kg/m2], normal weight [BMI: 18.5–23.9 kg/m2], overweight [BMI: 24–27.9 kg/m2], obesity [BMI: ≥ 28 kg/m2] by using the diagnostic criteria in Chinese adults (19).
Pregnant women were weighed at routine antenatal care visits. GWG was calculated as the measured weight at the time of preeclampsia assessment, which was represented by the measured weight at the last assessment time if all preeclampsia assessments (≥1 times) were negative or at the time of being firstly diagnosed with preeclampsia, minus prepregnancy weight. We calculated GWG velocity as the GWG divided by gestational age at weighing, in order to account for that greater weight gain may be observed at later measurement. Given no previously-published data of BMI-specific weight-gain-for-gestational-age for Chinese population, this is a reasonable approach because previous studies have shown that weight gain during pregnancy is roughly linear (20).
Preeclampsia assessment had been conducted by obstetricians in the original prospective cohort by using the criteria recommended by the Chinese Society of Obstetrics and Gynecology (21). Preeclampsia cases were identified if a woman had new onset hypertension at or after 20 weeks of gestation, accompanied by one of the following: proteinuria, other maternal organ dysfunction (including heart, lung, liver, kidney), or hematological, digestive, and neurological involvement, and/or uteroplacental dysfunction (21).
Covariates including year, geographical region, age, ethnic origin, education, Hukou (urban residents, rural residents, or rural-to-urban migrants), mode of conception, and primigravida were drawn from medical records.
Age was described as mean and standard deviation (SD) and comparisons between women with preeclampsia and those without preeclampsia were performed using the Student’s t test. Categorical variables, including year, region, ethnic origin, education, hukou, mode of conception, and primigravida, were described as counts with percentages and comparisons in the two groups were performed using Chi-squared test.
GWG (<6.5, 6.5–9.9, 10.0–13.4, 13.5–16.9, 17.0–19.9, and ≥ 20.0 kg) and GWG velocity (<0.23, 0.23–0.32, 0.33–0.42, 0.43–0.52, 0.53–0.59, ≥0.60 kg/week) were divided into six groups according to the 25th, 50th, 75th, 90th, and 95th percentiles. The incidence of preeclampsia was calculated within each combination of prepregnancy BMI categories and GWG or GWG velocity categories.
The non-linear associations of GWG and GWG velocity with preeclampsia were examined by employing logistic regression models with restricted cubic splines. GWG and GWG velocity were modelled using restricted cubic splines; knots were determined according to the principle of minimized Akaike Information Criterion (AIC) (22). Sensitivity analyses were performed by adjusting for different covariates. Model 1 adjusted for no covariate. Model 2 adjusted for the covariates. Model 3 additionally adjusted for prepregnancy BMI. Similar methods were used to determine the association of prepregnancy BMI with preeclampsia and GWG velocity were additionally adjusted for in model 3. We also determined the association of GWG and GWG velocity with preeclampsia given prepregnancy BMI categories (underweight, normal weight, overweight, and obesity) by employing logistic regression models with restricted cubic splines which adjusted for covariates as model 2. Predicted absolute probabilities of preeclampsia with 95% confidence intervals (CIs) were calculated with respect to prepregnancy BMI, GWG and GWG velocity based on these models.
The associations were further examined by performing multivariable robust Poisson regression models, in which the categorical variables of GWG/GWG velocity and prepregnancy BMI were used. We also performed a stratified analysis by the prepregnancy BMI categories. All models adjusted for the covariates. Relative risks (RRs) and the 95% CIs were calculated by these models.
All statistical analyses were performed with SAS software, version 9.0 and the R statistical software, version 3.6.2. A two-tailed P-value < 0.05 was considered statistically significant.
Among 117 738 pregnant women (mean [SD] age, 28.9 [4.4] years) included in the study, 2426 (2.06%) were diagnosed with preeclampsia (Table 1). The incidence of preeclampsia was 1.63% in women with underweight, 1.80% in women with normal weight, 2.82% in women with overweight, and 6.68% in women with obesity. The incidence of preeclampsia was 1.64%, 1.43%, 1.61%, 2.22%, 3.85% and 7.45% in women with pregnancy weight gain of < 0.23, 0.23 – 0.32, 0.33 – 0.42, 0.43 – 0.52, 0.53 – 0.59 and ≥ 0.60 kg/week. The incidence ranged from 1.33% to 45.86% for preeclampsia in all combinations of prepregnancy BMI categories and GWG velocity categories; it increased across the full range of GWG velocity categories from the group of < 0.23 kg/week to the group of ≥ 0.60 kg/week (Figure 1). Similar association were found in combinations of prepregnancy BMI categories and GWG categories (Figure S2).
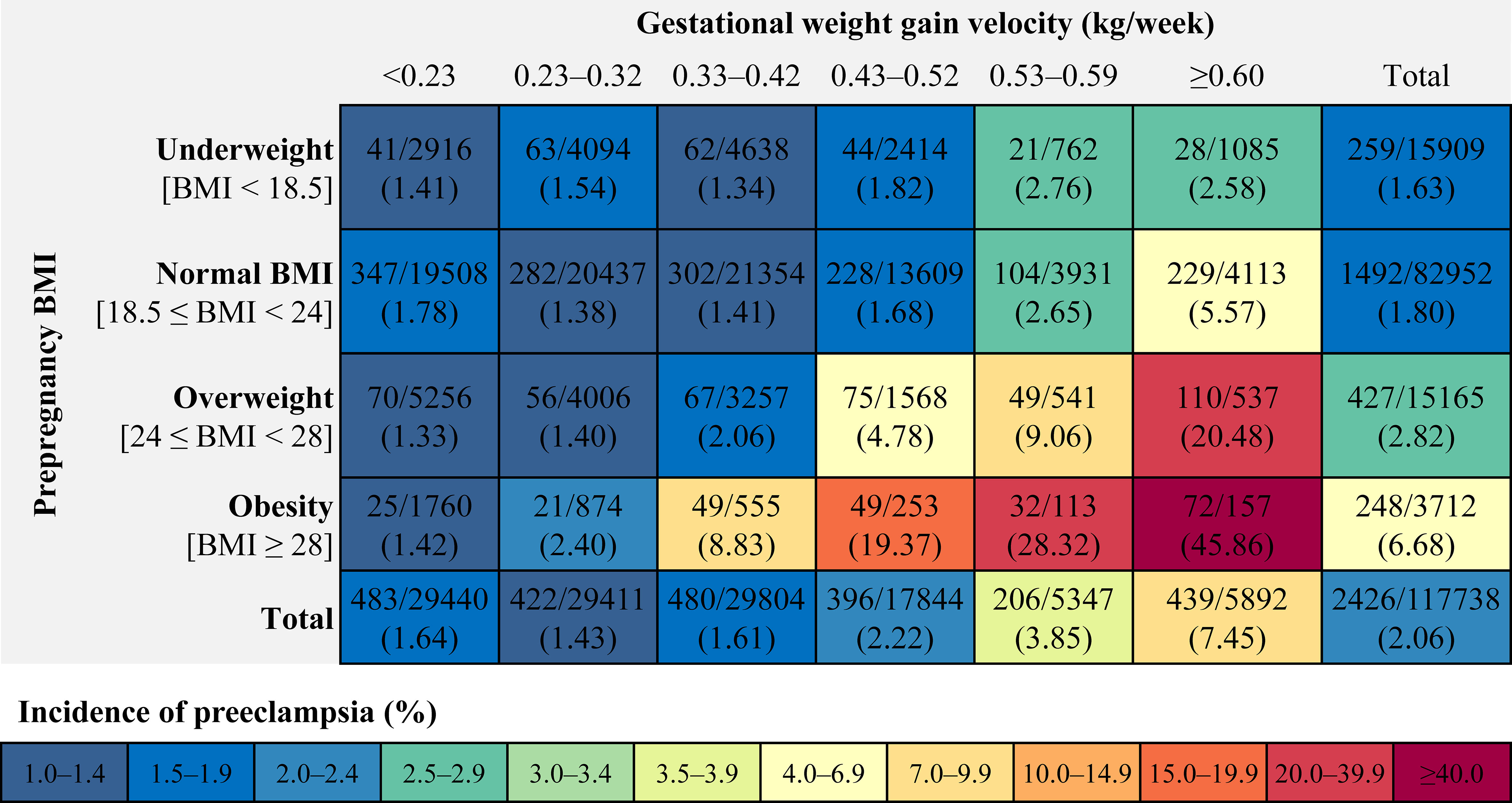
Figure 1 Incidence of preeclampsia within each combination of prepregnancy BMI categories and categories of gestational weight gain velocity. BMI, body mass index.
Using restricted cubic spline regression analysis, we found that the association of prepregnancy BMI with the risk of preeclampsia is presented as an inverse L-shaped curve with an inflexion point at 24 kg/m2; higher prepregnancy BMI was associated with increased risk of preeclampsia beyond the inflexion point (Figure 2A). Adjusting for different covariates did not substantially influence the estimates (Figure S3A). The results of multivariable adjusted robust Poisson regression show that compared with women with normal weight, those who were overweight and obese had 1.92- fold (95%CI, 1.73–2.14) and 5.06- fold (95%CI, 4.43–5.78) increased risks for preeclampsia, respectively (Table 2). There was no significant difference in the risk of preeclampsia between pregnant women with underweight and those with normal weight. Sensitivity analyses adjusting for different covariates indicate the robustness of estimates (Table 2).
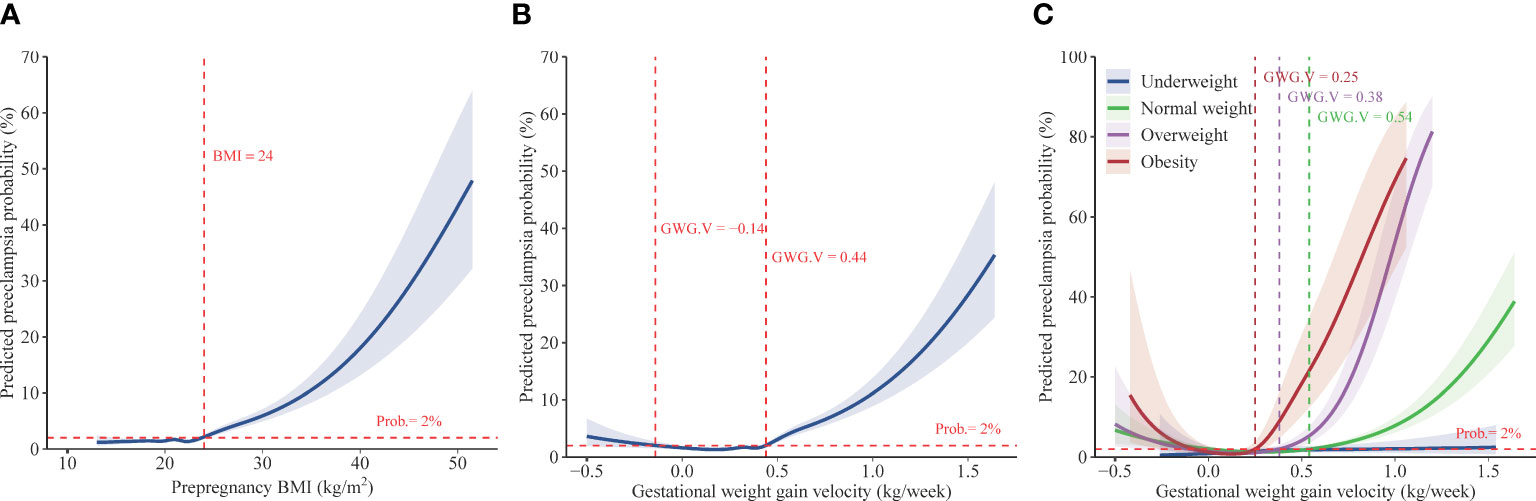
Figure 2 Predicted preeclampsia probabilities with respect to prepregnancy BMI and gestational weight gain velocity. Data are from China, 2015 to 2018. Predicted preeclampsia probabilities with 95% CIs were calculated with respect to prepregnancy BMI (A) and gestational weight gain velocity (B) by performing logistic regression models with restricted cubic splines. Models adjusted for year, age, education, ethnic origin, region, Hukou, assisted reproductive technology, primigravida, and prepregnancy BMI (in Figure 2B)/gestational weight gain velocity (in Figure 2A). Predicted preeclampsia probabilities with 95% CIs were also calculated (C) with respect to gestational weight gain velocity given varied prepregnancy BMI (underweight, normal weight, overweight, and obesity), by using performing logistic regression models with restricted cubic splines which adjusted for year, age, education, ethnic origin, region, Hukou, assisted reproductive technology, and primigravida. BMI, body mass index. GWG.V, gestational weight gain velocity. Prob., predicted preeclampsia probability.
The associations between GWG velocity and the risk of preeclampsia present a J-shaped curve with the lowest risk of less than 2% at GWG velocity of -0.14–0.44 kg/week and a sharply increased risk with GWG velocity beyond the GWG velocity range, according to restricted cubic spline regression analysis (Figure 2B). Adjusting for different covariates did not substantially influence the estimates (Figure S3B). Multivariable robust Poisson regression show that higher pregnancy weight gain velocity was associated with increased risks of preeclampsia. The adjusted relative risk was 1.20 (95%CI, 1.05–1.36), 1.55 (95%CI, 1.35–1.79), 2.73 (95%CI, 2.32–3.22), and 4.87 (95%CI, 4.27–5.56) in women with GWG velocity of 0.33–0.42, 0.43–0.52, 0.53–0.59, and ≥ 0.60 kg/week, respectively, compared with women with GWG of 0.23–0.32 kg/week (Table 3).
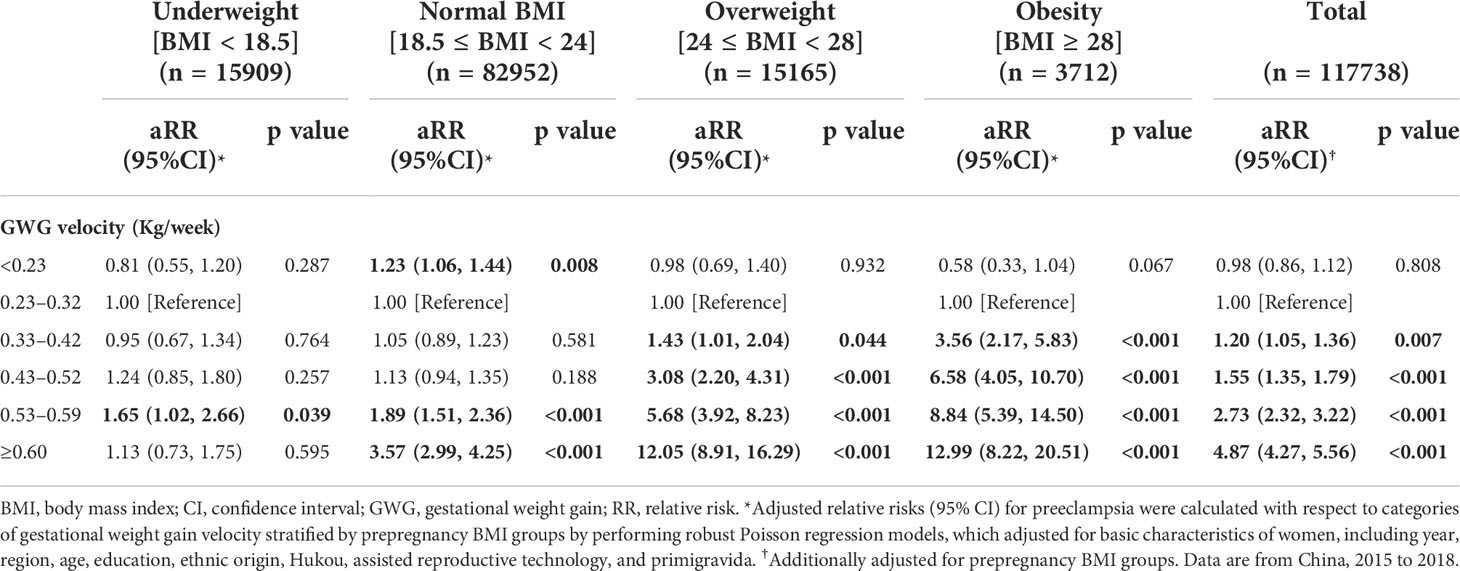
Table 3 Adjusted relative risks (95% CI) for preeclampsia according to GWG velocity stratified by prepregnancy BMI.
According to the results of stratified analysis using restricted cubic spline regression, the association of GWG velocity with preeclampsia was also presented as a J-shaped curve with the varied inflexion point (where the risk of preeclampsia was 2%) in women who were normal weight (0.54 kg/week), overweight (0.38 kg/week), and obese (0.25 kg/week); a steep rise in the risk of preeclampsia was observed along with GWG velocity beyond the inflexion points (Figure 2C). Multivariable robust Poisson regression show that, after adjusting for confounders, higher GWG velocity beyond the inflexion points was associated with increased risks of preeclampsia in women who were normal weight, overweight, and obese (Table 3). No significant association was observed between GWG velocity and the risk of preeclampsia in women with underweight. Similar association were found when employing the variable “GWG” (Table S1 and Figure S3C-D).
In this large cohort, we found that prepregnancy overweight and obesity was strongly associated with preeclampsia. Furthermore, the J-shaped association of GWG velocity with the risk of preeclampsia was observed in women with prepregnancy BMI of ≥ 18.5 and a sharply increased risk was observed with higher pregnancy weight gain velocity beyond the inflexion point, which varied in women who were normal weight, overweight, and obese. Our findings highlight the joint contribution of prepregnancy BMI and GWG to the occurrence of preeclampsia.
Consistent with previous findings, our study confirmed that pregnant women who were overweight and obese had a higher risk of preeclampsia while women with underweight had a lower or similar risk compared to those with normal BMI (5–7, 9, 10, 23). To date, there are few studies that have investigated the association between GWG and preeclampsia, especially given varied prepregnancy BMI (24). Our study supports evidences from previous observations (10, 15, 17, 25), and contribute to a deeper understanding of the association. We found that for women with prepregnancy BMI ≥ 18.5, the association of GWG velocity with preeclampsia was presented as J-shaped curve and the risk of preeclampsia increased along with GWG beyond a threshold. Higher prepregnancy BMI and GWG not only resulted in higher risk of preeclampsia independently, but also had superimposed effect (15, 26–30). An overweight and obese prepregnancy BMI coupled with excessive GWG resulted in a multiplicative increase in the risk of preeclampsia.
Notably, the threshold for GWG for preeclampsia morbidity was lower as prepregnancy BMI increased. In our study, the threshold of GWG, beyond which risk of preeclampsia increased, was 0.54, 0.38, and 0.25 kg/week in women who were normal weight, overweight, and obese, respectively. The thresholds are consistent with the upper limit of GWG recommended by Chinese Nutrition Society in pregnant women with corresponding prepregnancy BMI (31). As recommended in Weight Monitoring and Evaluation during Pregnancy Period of Chinese Women by Chinese Nutrition Society (31), the mean (range) of GWG was 0.46 (0.37–0.56), 0.37 (0.26–0.48), 0.30 (0.22–0.37), and 0.22 (0.15–0.30) kg/week in women with underweight, normal weight, overweight, and obesity during the second and third trimester of pregnancy, respectively. The findings further confirm the important influence of BMI and GWG on pregnancy outcome.
We found no significant association between GWG and the risk of preeclampsia in women with prepregnancy underweight BMI. There is little published data on this association and existing studies offer contradictory findings (10, 32, 33). A population-based cohort survey of 98,820 women with singleton pregnancies in Slovenia found that excessive GWG was associated with increased odds of preeclampsia in all pre-pregnancy BMI categories, especially in underweight women (33). However, in an individual participant data meta-analysis of 265 270 pregnancies from 39 cohorts in Europe, North America, and Oceania, the significant association between GWG and preeclampsia risk in pregnant women with underweight was not found (10). More research on this topic is needed. In addition, insufficient gestational weight gain was found to be associated with increased risk of preeclampsia among women with normal weight in our study. The result was supported by a retrospective study showed that low GWG could lead to severe maternal morbidity, including eclampsia postpartum (24). Unfortunately, we did not distinguish the types of preeclampsia and weight gain in different pregnancy period. A cohort study of 62705 nulliparous women from Sweden indicates that higher GWG was much more strongly associated with late-onset preeclampsia than early-onset preeclampsia (16).
The mechanism by which prepregnancy obesity and excessive GWG result in preeclampsia had not been fully clarified, but studies suggested that oxidative stress may play an important role in the pathogenesis. High prepregnancy BMI and excessive GWG might increase the level of oxidative stress, induce systemic inflammation and accelerate damage to vascular endothelial cells, resulting in preeclampsia (15, 34–36). Pregnant women with normal prepregnancy BMI and low GWG might be accompanied by insufficient intake of essential nutrients such as antioxidant vitamins and calcium, which would increase the risk of preeclampsia (37–41).
This finding has important implications for developing weight management strategies before and during pregnancy. Controlling excessive GWG was recommended to reduce the risk of preeclampsia for women with prepregnancy BMI of ≥ 18.5, although the idea needs confirmation by RCTs. Our finding that the association of GWG velocity with preeclampsia was presented as a J-shaped curve with the varied inflexion point in women with normal weight, overweight, and obesity underscores the need to optimize the recommendations for weight gain during pregnancy for different BMI groups to reduce the risk of preeclampsia. The recommendation is consistent with the GWG recommendation of the Chinese Nutrition Society (31). For women who are overweight and obese, reducing weight before pregnancy can help reduce the incidence of preeclampsia, which was supported by a cohort study of 436,414 women with singleton gestations (42).
Our study has several limitations. The prepregnancy weight and height was self-reported by pregnant women, which would lead to exposure misclassification, However, previous findings indicate that self-reported prepregnancy weight can be used for calculation of BMI and GWG when an early measurement of weight during pregnancy is not available (43). Additionally, because there are not pregnant weight gain z-score charts for Chinese women, we used GWG velocity to induce bias caused by the inherent correlation between weight gain and gestational duration (longer pregnancies have more opportunity to gain weight). However, it may do not eliminate the bias to an adequate extent. Finally, we failed to collect information of other risk factors of preeclampsia, such as smoking which has an estimated prevalence of 2.2% in Chinese women (44); and also failed to collect the indicators of preeclampsia, such as blood pressure.
In conclusion, we found that high prepregnancy BMI and exceed GWG contributed to increased risk of preeclampsia with a superimposed effect. Prepregnancy overweight and obesity was strongly associated with preeclampsia. Furthermore, the J-shaped association of GWG velocity with the risk of preeclampsia was observed in women with prepregnancy BMI of ≥ 18.5 and a steep rise in the risk of preeclampsia was observed along with higher GWG velocity beyond the inflexion point, which was 0.54, 0.38, and 0.25 kg per week in women with normal weight, overweight, and obesity, respectively. Reducing weight before pregnancy and controlling excessive GWG are recommended for women who are overweight and obese to reduce the risk of preeclampsia. Our findings also underscore the need to optimize the recommendations for GWG for women with different prepregnancy BMI.
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
The studies involving human participants were reviewed and approved by the Peking University Third Hospital Medical Science Research Ethics Committee. The patients/participants provided their written informed consent to participate in this study.
Conceptualization, HS, XG, and JL; methodology, HS, XG, and JL; validation, YJ, PY, LC, YY, YL, and MS; formal analysis, HS, XG, and JL; investigation, YJ, PY, LC, and YY; resources, YW; data curation, YL and MS; writing—original draft preparation, HS, XG, and JL; writing—review and editing, PY, LC, YY, YL, MS, YW, and YZ; supervision, YW, HS, and YZ; project administration, YW and YZ; funding acquisition, YW and YZ. All authors have read and approved the final manuscript.
This research was funded by grants from National Key Research and Development Program (grant number 2021YFC2700700 [YW]) and Peking University Third Hospital Cohort Building Program (BYSYDL2019001 [YZ]). The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
We thank all hospitals and health workers who contributed to the data collection and management, as well as the pregnant women who participated in our study.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2022.967102/full#supplementary-material
1. Mol BWJ, Roberts CT, Thangaratinam S, Magee LA, de Groot CJM, Hofmeyr GJ. Pre-eclampsia. Lancet (2016) 387(10022):999–1011. doi: 10.1016/s0140-6736(15)00070-7
2. Conti-Ramsden F, Knight M, Green M, Shennan AH, Chappell LC. Reducing maternal deaths from hypertensive disorders: learning from confidential inquiries. BMJ (2019) 364:l230. doi: 10.1136/bmj.l230
3. Abalos E, Cuesta C, Grosso AL, Chou D, Say L. Global and regional estimates of preeclampsia and eclampsia: a systematic review. Eur J Obstet. Gynecol. Reprod Biol (2013) 170(1):1–7. doi: 10.1016/j.ejogrb.2013.05.005
4. Young OM, Twedt R, Catov JM. Pre-pregnancy maternal obesity and the risk of preterm preeclampsia in the American primigravida. Obesity (2016) 24(6):1226–9. doi: 10.1002/oby.21412
5. Owens S, Thayer SM, Garg B, Caughey AB. Incidence of preeclampsia by maternal body mass index and diabetes status. Am J Obstet. Gynecol. (2021) 224(2):S193–S. doi: 10.1016/j.ajog.2020.12.317
6. Mrema D, Lie RT, Ostbye T, Mahande MJ, Daltveit AK. The association between pre pregnancy body mass index and risk of preeclampsia: a registry based study from Tanzania. BMC Pregnancy Childbirth (2018) 18(1):56. doi: 10.1186/s12884-018-1687-3
7. Mostello D, Chang JJ, Allen J, Luehr L, Shyken J, Leet T. Recurrent preeclampsia the effect of weight change between pregnancies. Obstet. Gynecol. (2010) 116(3):667–72. doi: 10.1097/AOG.0b013e3181ed74ea
8. O'Brien TE, Ray JG, Chan WS. Maternal body mass index and the risk of preeclampsia: A systematic overview. Epidemiology (2003) 14(3):368–74. doi: 10.1097/00001648-200305000-00020
9. He X-J, R-x D, Hu C-L. Maternal prepregnancy overweight and obesity and the risk of preeclampsia: A meta-analysis of cohort studies. Obes Res Clin Pract (2020) 14(1):27–33. doi: 10.1016/j.orcp.2020.01.004
10. Santos S, Voerman E, Amiano P, Barros H, Beilin LJ, Bergström A, et al. Impact of maternal body mass index and gestational weight gain on pregnancy complications: an individual participant data meta-analysis of European, north American and Australian cohorts. BJOG (2019) 126(8):984–95. doi: 10.1111/1471-0528.15661
11. Black RE, Victora CG, Walker SP, Bhutta ZA, Christian P, de Onis M, et al. Maternal and child undernutrition and overweight in low-income and middle-income countries. Lancet (2013) 382(9890):427–51. doi: 10.1016/s0140-6736(13)60937-x
12. World Health Organization. Indicator groups: Body mass index among adults. Available at: https://www.who.int/data/gho/data/themes/topics/indicator-groups/indicator-group-details/GHO/bmi-among-adults. Accessed October 2, 2022.
13. Wang L, Zhou B, Zhao Z, Yang L, Zhang M, Jiang Y, et al. Body-mass index and obesity in urban and rural China: findings from consecutive nationally representative surveys during 2004-18. Lancet (2021) 398(10294):53–63. doi: 10.1016/s0140-6736(21)00798-4
14. Santos S, Eekhout I, Voerman E, Gaillard R, Barros H, Charles MA, et al. Gestational weight gain charts for different body mass index groups for women in Europe, north America, and Oceania. BMC Med (2018) 16(1):201. doi: 10.1186/s12916-018-1189-1
15. Shao Y, Qiu J, Huang H, Mao B, Dai W, He X, et al. Gestational weight gain and risk of preeclampsia: a birth cohort study in lanzhou, China. BMC Pregnancy Childbirth (2017) 17(1):400. doi: 10.1186/s12884-017-1567-2
16. Hutcheon JA, Stephansson O, Cnattingius S, Bodnar LM, Wikstrom A-K, Johansson K. Pregnancy weight gain before diagnosis and risk of preeclampsia: A population-based cohort study in nulliparous women. Hypertension (2018) 72(2):433–41. doi: 10.1161/hypertensionaha.118.10999
17. Premru-Srsen T, Kocic Z, Vodusek VF, Gersak K, Verdenik I. Total gestational weight gain and the risk of preeclampsia by pre-pregnancy body mass index categories: a population-based cohort study from 2013 to 2017. J Perinat. Med (2019) 47(6):585–91. doi: 10.1515/jpm-2019-0008
18. Bodnar LM, Himes KP, Abrams B, Parisi SM, Hutcheon JA. Early-pregnancy weight gain and the risk of preeclampsia: A case-cohort study. Pregnancy Hypertens (2018) 14:205–12. doi: 10.1016/j.preghy.2018.10.005
19. Working Group on Obesity in China Guidelines for prevention and control of overweight and obesity in Chinese adults. Acta Nutrimenta. Sin (2004) 26(1):1–4. doi: 10.3321/j.issn:0512-7955.2004.01.001
20. Santos S, Eekhout I, Voerman E, Gaillard R, Barros H, Charles MA, et al. Gestational weight gain charts for different body mass index groups for women in Europe, north America, and Oceania. BMC Med (2018) 16(1):201. doi: 10.1186/s12916-018-1189-1
21. Chinese Society of Obstetrics and Gynecology Diagnosis and treatment of hypertension and pre-eclampsia in pregnancy: a clinical practice guideline in China (2020). Chin J Obstet. Gynecol. (2020) 55(4):227–38. doi: 10.3760/cma.j.cn112141-20200114-00039
22. Harrell F. Regression modeling strategies with applications to linear models, logistic regression, and survival analysis. New York: Springer (2001).
23. Wu LL, Chen YX, Guan XN, Tong JN, Wu XX, Niu JM. Associations between pre-pregnancy body mass index and occurrence and clinical features of preeclampsia. Chin J Obstet. Gynecol. (2021) 56(2):96–101. doi: 10.3760/cma.j.cn112141-20200904-00691
24. Leonard SA, Abrams B, Main EK, Lyell DJ, Carmichael SL. Weight gain during pregnancy and the risk of severe maternal morbidity by prepregnancy BMI. Am J Clin Nutr (2020) 111(4):845–53. doi: 10.1093/ajcn/nqaa033
25. Belogolovkin V, Engel S, Savitz D, Chelimo C, Siega-Riz AM, Sperling R. Weight gain velocity in relation to the development of gestational hypertension and or preeclampsia. Am J Obstet. Gynecol. (2006) 195(6):S127–S. doi: 10.1016/j.ajog.2006.10.434
26. Mutsaerts MA, Groen H, Buiter-Van der Meer A, Sijtsma A, Sauer PJ, Land JA, et al. Effects of paternal and maternal lifestyle factors on pregnancy complications and perinatal outcome. a population-based birth-cohort study: the GECKO drenthe cohort. Hum Reprod (2014) 29(4):824–34. doi: 10.1093/humrep/deu006
27. Vinturache A, Moledina N, McDonald S, Slater D, Tough S. Pre-pregnancy body mass index (BMI) and delivery outcomes in a Canadian population. BMC Pregnancy Childbirth (2014) 14:422. doi: 10.1186/s12884-014-0422-y
28. Barton JR, Joy SD, Rhea DJ, Sibai AJ, Sibai BM. The influence of gestational weight gain on the development of gestational hypertension in obese women. Am J Perinatol. (2015) 32(7):615–20. doi: 10.1055/s-0034-1386634
29. Cedergren M. Effects of gestational weight gain and body mass index on obstetric outcome in Sweden. Int J Gynecol. Obstet. (2006) 93(3):269–74. doi: 10.1016/j.ijgo.2006.03.002
30. Shao Y, Qiu J, Huang H, Mao B, Dai W, He X, et al. Pre-pregnancy BMI, gestational weight gain and risk of preeclampsia: a birth cohort study in lanzhou, China. BMC Pregnancy Childbirth (2017) 17(1):400. doi: 10.1186/s12884-017-1567-2
31. Chinese Nutrition Society. Weight monitoring and evaluation during pregnancy period of Chinese women. (2021). Availale at: http://www.mcnutri.cn/weiyuan/702101201.html. Accessed October 2, 2022
32. Kyozuka H, Jin T, Fujimori M, Nomura S, Suzuki D, Fukuda T, et al. Effect of gestational weight gain on preeclampsia among underweight women: A single tertiary referral center study in Japanese women. J Obstet. Gynaecol. Res (2022) 48(5):1141–8. doi: 10.1111/jog.15200
33. Premru-Srsen T, Kocic Z, Fabjan Vodusek V, Geršak K, Verdenik I. Total gestational weight gain and the risk of preeclampsia by pre-pregnancy body mass index categories: a population-based cohort study from 2013 to 2017. J Perinat. Med (2019) 47(6):585–91. doi: 10.1515/jpm-2019-0008
34. Wolf M, Kettyle E, Sandler L, Ecker JL, Roberts J, Thadhani R. Obesity and preeclampsia: The potential role of inflammation. Obstet. Gynecol. (2001) 98(5):757–62. doi: 10.1016/s0029-7844(01)01551-4
35. Reilly MP, Rader DJ. The metabolic syndrome: more than the sum of its parts? Circulation (2003) 108(13):1546–51. doi: 10.1161/01.Cir.0000088846.10655.E0
36. Roberts JM, Lain KY. Recent insights into the pathogenesis of pre-eclampsia. Placenta (2002) 23(5):359–72. doi: 10.1053/plac.2002.0819
37. Endresen MJ, Morris JM, Nobrega AC, Buckley D, Linton EA, Redman CW. Serum from preeclamptic women induces vascular cell adhesion molecule-1 expression on human endothelial cells in vitro: a possible role of increased circulating levels of free fatty acids. Am J Obstet. Gynecol. (1998) 179(3 Pt 1):665–70. doi: 10.1016/s0002-9378(98)70061-4
38. Ryu S, Huppmann AR, Sambangi N, Takacs P, Kauma SW. Increased leukocyte adhesion to vascular endothelium in preeclampsia is inhibited by antioxidants. Am J Obstet. Gynecol. (2007) 196(4):400. doi: 10.1016/j.ajog.2006.12.023
39. Nash P, Eriksson UJ. Suramin-restricted blood volume in the placenta of normal and diabetic rats is normalized by vitamin e treatment. Placenta (2007) 28(5-6):505–15. doi: 10.1016/j.placenta.2006.06.015
40. Blaner WS, Shmarakov IO, Traber MG. Vitamin a and vitamin e: will the real antioxidant please stand up? Annu Rev Nutr (2021) 41:105–31. doi: 10.1146/annurev-nutr-082018-124228
41. Khaing W, Vallibhakara SA, Tantrakul V, Vallibhakara O, Rattanasiri S, McEvoy M, et al. Calcium and vitamin d supplementation for prevention of preeclampsia: A systematic review and network meta-analysis. Nutrients (2017) 9(10):1141. doi: 10.3390/nu9101141
42. Swank M, Caughey A, Farinelli C, Main E, Melsop K, Gilbert W, et al. The impact of change in pregnancy body mass index on gestational hypertension/preeclampsia. Am J Obstet. Gynecol. (2013) 208(1):S274–S. doi: 10.1016/j.ajog.2012.10.814
43. Rangel Bousquet Carrilho T, MR K, Rodrigues Farias D, Freitas Costa NC, Araújo Batalha M, ER M, et al. Agreement between self-reported pre-pregnancy weight and measured first-trimester weight in Brazilian women. BMC Pregnancy Childbirth (2020) 20(1):734. doi: 10.1186/s12884-020-03354-4
Keywords: pregnancy, preeclampsia, body mass index, gestational weight gain, cohort study
Citation: Gong X, Li J, Jiang Y, Yuan P, Chen L, Yang Y, Li Y, Sun M, Zhao Y, Shi H and Wei Y (2022) Risk of preeclampsia by gestational weight gain in women with varied prepregnancy BMI: A retrospective cohort study. Front. Endocrinol. 13:967102. doi: 10.3389/fendo.2022.967102
Received: 12 June 2022; Accepted: 26 September 2022;
Published: 14 October 2022.
Edited by:
Shan Gao, Capital Medical University, ChinaReviewed by:
Buyun Liu, University of Science and Technology of China, ChinaCopyright © 2022 Gong, Li, Jiang, Yuan, Chen, Yang, Li, Sun, Zhao, Shi and Wei. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yuan Wei, d2VpeXVhbmJ5c3lAMTYzLmNvbQ==; Huifeng Shi, bnN4bUBwa3UuZWR1LmNu; Yangyu Zhao, emhhb3lhbmd5dUBiam11LmVkdS5jbg==
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.