- 1Medical Laboratories Techniques Department, Al-Mustaqbal University College, Babylon, Hilla, Iraq
- 2College of Science, University of Babylon, Babylon, Iraq
- 3Faculty of Sport, Universitas Sebelas Maret, Surakarta, Indonesia
- 4Department of Internal Medicine, Faculty of Medicine, Mansoura Specialized Medical Hospital, Mansoura University, Mansoura, Egypt
- 5Department of Clinical Laboratory Sciences, College of Applied Medical Sciences, King Khalid University, Abha, Saudi Arabia
- 6Faculty of Nursing, Umm al- Qura University, Makkah, Saudi Arabia
- 7Medical Laboratory Techniques Department, Al-Farahidi University, Baghdad, Iraq
- 8Medical Laboratory Techniques Department, Al-Turath University College, Baghdad, Iraq
- 9Veterinary Medicine College, Al-Qasim Green University, Al-Qasim, Iraq
- 10Department of Mathematics, Dwaraka Doss Goverdhan Doss Vaishnav College, Arumbakkam, University of Madras, Chennai, India
- 11Department of General Studies, Universidad Continental, Lima, Peru
- 12Department of Pharmaceutics, College of Pharmacy, Prince Sattam Bin Abdulaziz University, Al-kharj, Saudi Arabia
- 13Department of Pharmaceutical Chemistry, College of Pharmacy, University of Mosul, Mosul, Iraq
Background: The detrimental role of advanced glycation end products (AGEs) against cardio-metabolic health has been revealed in several previous reports. However, the results of studies regarding the association between AGEs and obesity measurements are inconsistent. In the current meta-analysis, we aimed to quantitatively summarize the results of studies that evaluated the association between circulating and dietary AGEs with obesity measurements among the adult population.
Methods: A systematic search from PubMed, Embase, and Scopus electronic databases until 30 October 2022 retrieved a total of 21,429 observational studies. After duplicate removal, title/abstract screening, and full-text reading by two independent researchers, a final number of 18 manuscripts remained to be included in the meta-analysis.
Results: Those in the highest category of circulating AGEs had ~1.5 kg/m2 reduced BMI compared with those in the lowest AGEs category [weighted mean difference (WMD): −1.485; CI: −2.459, −0.511; p = 0.003], while a nonsignificant increase in BMI was observed in the highest versus lowest category of dietary AGEs (WMD: 0.864, CI: −0.365, 2.094; p = 0.186). Also, lower amounts of circulating AGEs in individuals with obesity versus individuals without obesity were observed (WMD: −57.220, CI: −84.290, −30.149; p < 0.001). AGE type can be considered as a possible source of heterogeneity.
Conclusion: In the current meta-analysis, we observed an inverse association between circulating AGEs and body mass index among adults. Due to low study numbers, further studies are warranted to better elucidate these results.
Introduction
Advanced glycation end products (AGEs), also named glycotoxins, are a group of prooxidant, cytotoxic adducts that are involved in the pathogenesis of numerous diseases including diabetes, cardiovascular comorbidities, and obesity (1). AGEs, identified as low-molecular-weight (LMW) and high-molecular-weight (HMW) heterogeneous molecules (2), are formed by non-enzymatic glycation of proteins, amino acids, and nucleic acids; they are formed in high temperatures of foods such as during grilling, roasting, and broiling or frying (3). Numerous studies have revealed the role of AGEs in the development of diabetes complications by raising intracellular reactive oxidative species (ROS), inducing beta cell injury, and malfunction and peripheral insulin resistance (4). Some important types of AGEs include N-ϵ-carboxymethyl lysine (CML), pentosidine, pyrraline, N-ϵ-carboxyethyl lysine (CEL), and methylglyoxal (MGO)-derived hydroimidazolones (MG-H1) (5). AGEs have exogenous or endogenous sources in the body; AGEs formation in the body is through Millard reaction as part of normal metabolism; however, hyperglycemia and oxidative stress could also trigger their formation in the body (6). Dietary AGEs (dAGEs) are important contributors to the body’s AGE pool and are common in modern Western diets containing highly processed foods such as fried and smoked meats, roasted chicken, white French fires potato, broiled lamb, high-sugar and high-fat foods, or foods that are cooked at high temperatures or for long periods (7). Estimation of dietary AGE consumption is possible by measuring one of their main metabolites, carboxymethyl lysine (CML); also, CML’s circulating amounts can be measured with gas chromatography–mass spectrometry (GC-MS) and enzyme-linked immunosorbent assay (ELISA) (8, 9). The association of AGEs with adiposity and obesity risk has been reported in several previous studies; the study by Amin et al. (10), reported a significant positive association between CML and obesity measurements such as body mass index and waist-to-hip ratio. In another study by Uribarri et al. (1), N-carboxymethyl lysine, MGO levels were significantly increased in persons with obesity with more than one other metabolic syndrome criteria but not in obese without metabolic syndrome criteria. Several other studies reported lower CML, as an indicator of circulating AGE concentrations in individuals with obesity (11, 12) or lower BMI in highest versus lowest circulating AGE concentrations (13, 14). In the earliest study that was performed in this field, plasma AGE products were decreased in obese children compared with lean controls (15), whereas Mirmiran et al. (3) and Peterson et al. (8) reported higher BMI in the highest versus lowest circulating AGE concentrations. In one mechanistic study by Gaens et al. (16), it was demonstrated that CML accumulation and the expression of its receptor, RAGE, were higher in the adipose tissue of subjects with obesity compared to those without obesity. The authors concluded that reduced circulating levels of CML observed in subjects with obesity were due to the trapping of CML via RAGE in the adipose tissue, resulting in lower circulating CML levels. As mentioned in the above introduction, there are big discrepancies in the results of studies that make it impossible to infer conclusive evidence about the AGE–obesity relationship. Therefore, in the current meta-analysis, we summarized the results of observational studies that evaluated the relationships between circulating and dietary AGEs with obesity measurements among adults.
Methods and materials
The current report was written according to Preferred Reporting Items for Systematic Reviews and Meta‐Analyses (PRISMA) guidelines (Supplementary Table 1) (17). The study protocol’s registration number in the International Prospective Register of Systematic Reviews system (PROSPERO) was CRD42021243323.
Search strategy and study selection
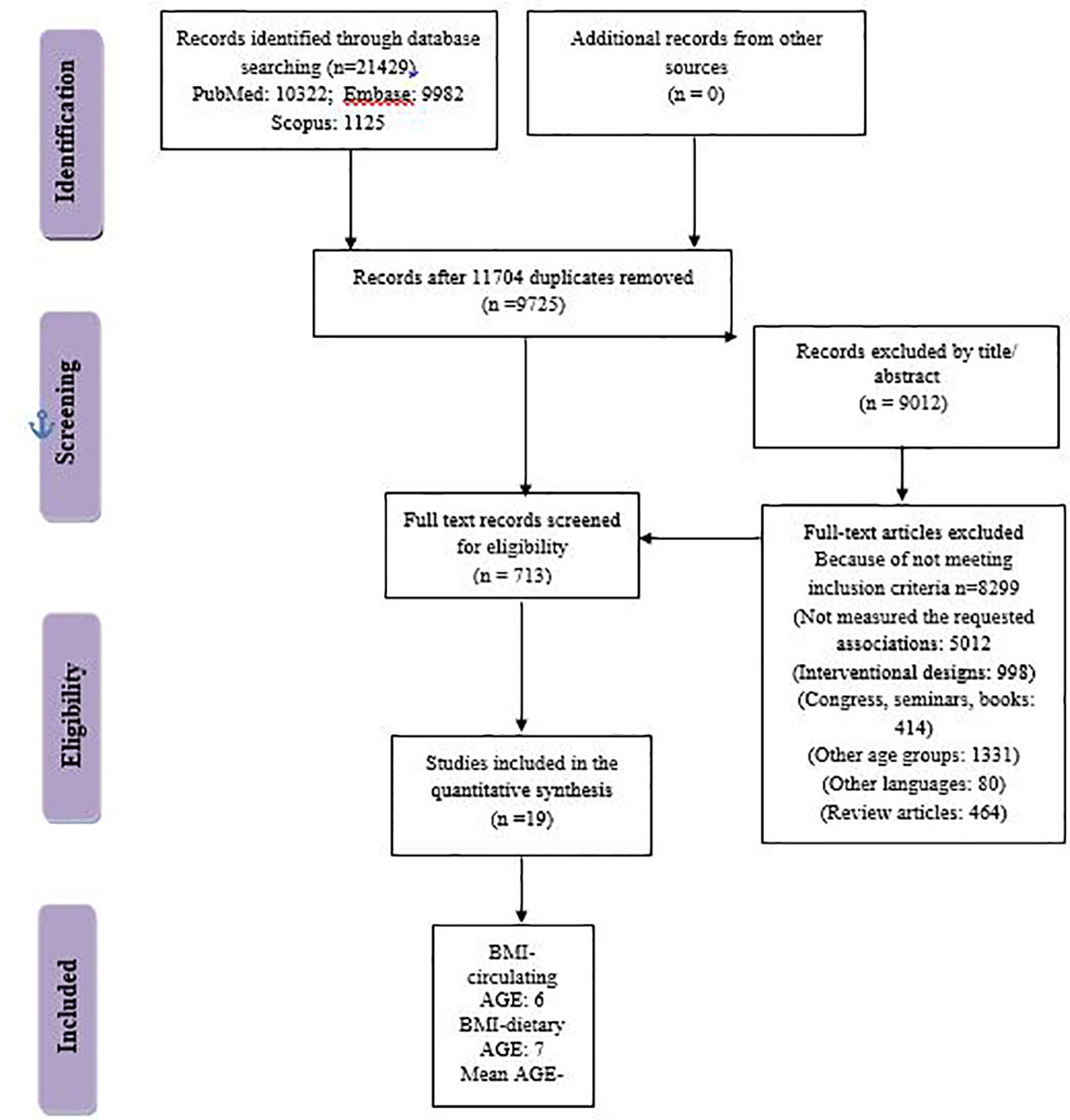
A total of 21,429 articles were obtained through a systematic search from PubMed, Embase, and Scopus electronic databases until 30 October 2022. We had no language restrictions. No missing document was found through hand-searching from reference lists of all papers. The search strategy is presented in Supplementary Table 2. To avoid missing the studies that measured obesity as a secondary or tertiary measurement variable, in the search strategy, we also included some other keywords related to obesity like waist circumference (WC), waist-to-hip ratio (WHR), abdominal obesity, and central obesity. The retrieved articles were imported into EndNote software. A total of 11,704 articles were removed because of duplication and 9,012 articles were removed according to title/abstract. As a consequence, 713 articles remained to be evaluated by two independent researchers. Finally, 18 manuscripts were included in the final meta-synthesis (Figure 1).
Inclusion and exclusion criteria
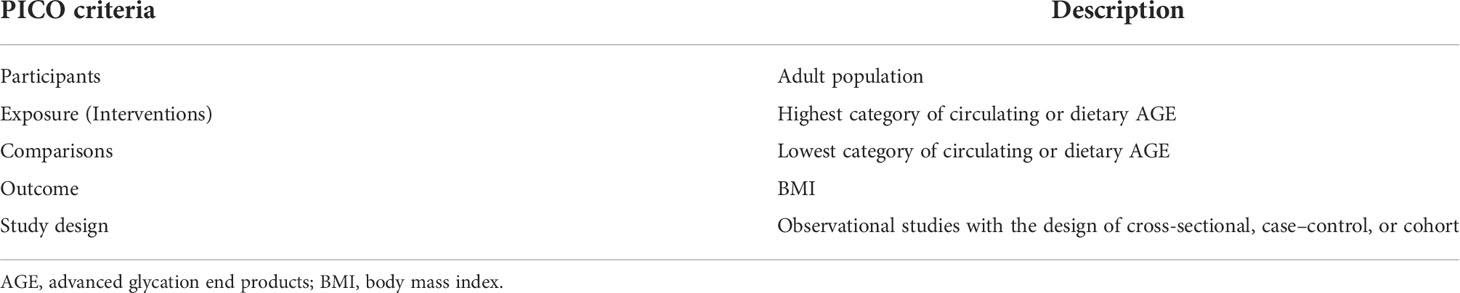
In the current systematic review and meta-analysis, included studies were observational studies with a cross-sectional design; evaluated the relationship between circulating or dietary AGEs and obesity measurements like body mass index (BMI), fat mass, waist circumference (WC), or waist-to-hip ratio (WHR); and were conducted among adults. The included studies also provided the mean ± standard deviation (SD) of BMI, fat mass, WC, or WHR of those in the lowest versus highest categories of circulating or dietary AGE and/or provided the mean ± SD of AGE in adults with or without obesity.
The studies with interventional design, case reports, and case series, experimental and in vitro studies, reviews, letters to editors, abstracts of congress or seminars, and short communications were excluded. The PICO (patients, intervention, control-comparator, and outcome) model for the studies’ selection is presented in Table 1.
Data extraction and quality assessment of included studies
The retrieved articles were extracted by three independent researchers using a standard Excel extraction datasheet. Some of the characteristics of retrieved articles include first author name, year of publication, journal name, country, age of participants, study design, the total number of participants, adjusted covariates, gender, study setting, and circulating or dietary AGE measurement tools; the main findings of the studies were extracted. The methodological quality of the included studies was assessed using the Agency for Healthcare Research and Quality (AHRQ) checklist (18) (Table 2).
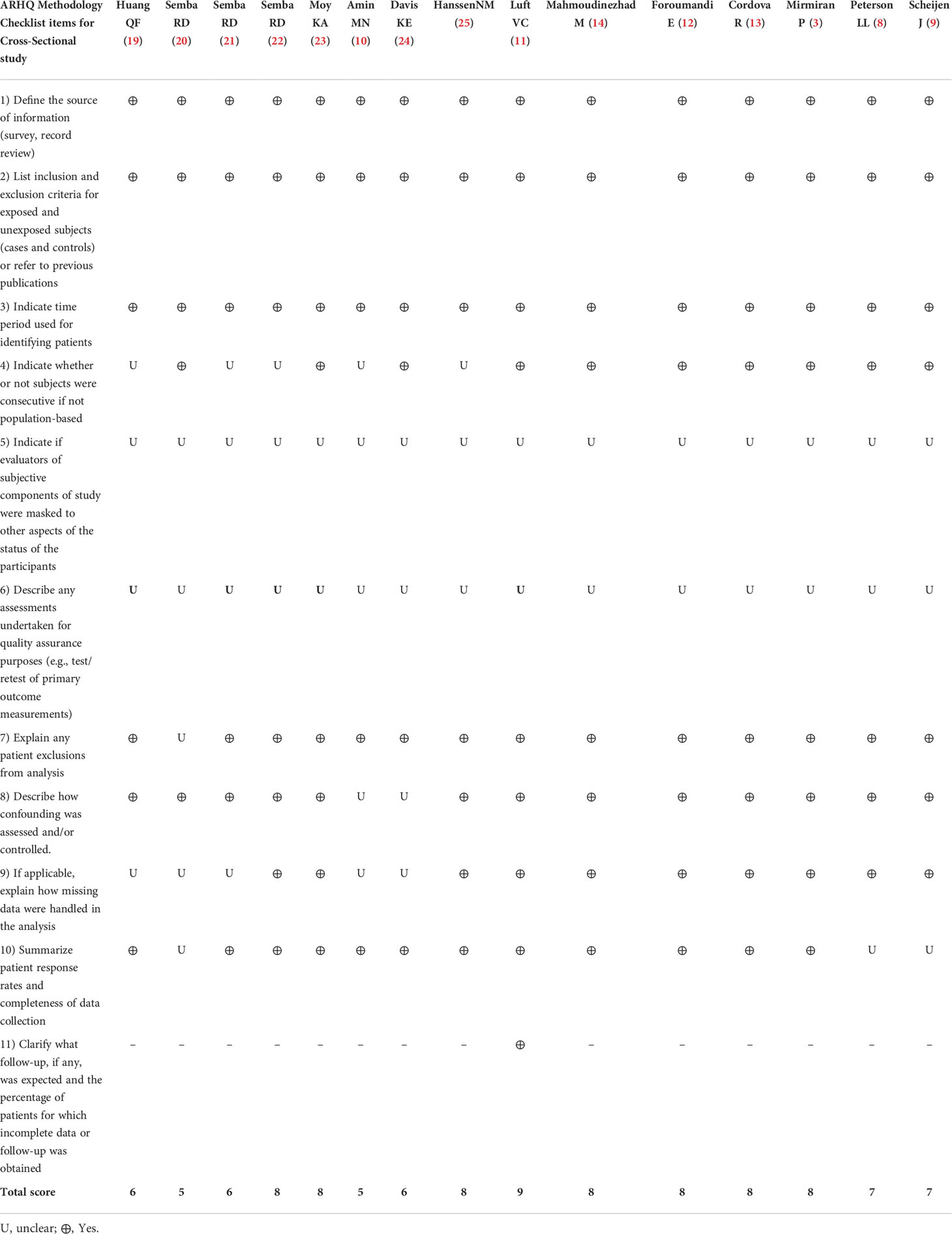
Table 2 Agency for Healthcare Research and Quality (AHRQ) checklist to assess quality of the cross-sectional studies.
Statistical analysis
Data analysis was performed by STATA version 13 (STATA Corp, College Station, TX, USA). p-values less than 0.05 were considered statistically significant. The mean and SDs of the variables were used to calculate the unstandardized effect size calculated by weighted mean difference (WMD) with a 95% confidence interval (CI). When, the median and range were reported instead of mean and SD, the method of Hozo et al. (26) was used for mean and SD estimation. Walter and Yao’s method was also used for calculating missing SDs as an improved “range” method (27, 28). An equal number of participants in each category was assumed if the number of participants in categories was not provided. Cochran’s Q and I2 tests were used considering the following heterogeneity measurements: no heterogeneity for I2 < 25%, moderate heterogeneity for I2 = 25%–50%, and large heterogeneity for I2 > 50% (29). For significant heterogeneities of either the Q statistic with p < 0.1 or I2 > 50%, the random-effects model was used (30). Subgrouping approaches were also performed to identify the source of heterogeneity. Begg’s Funnel plots followed by Begg’s adjusted rank correlation and Egger’s regression asymmetry tests were used for the assessment of publication bias.
Results
Study characteristics
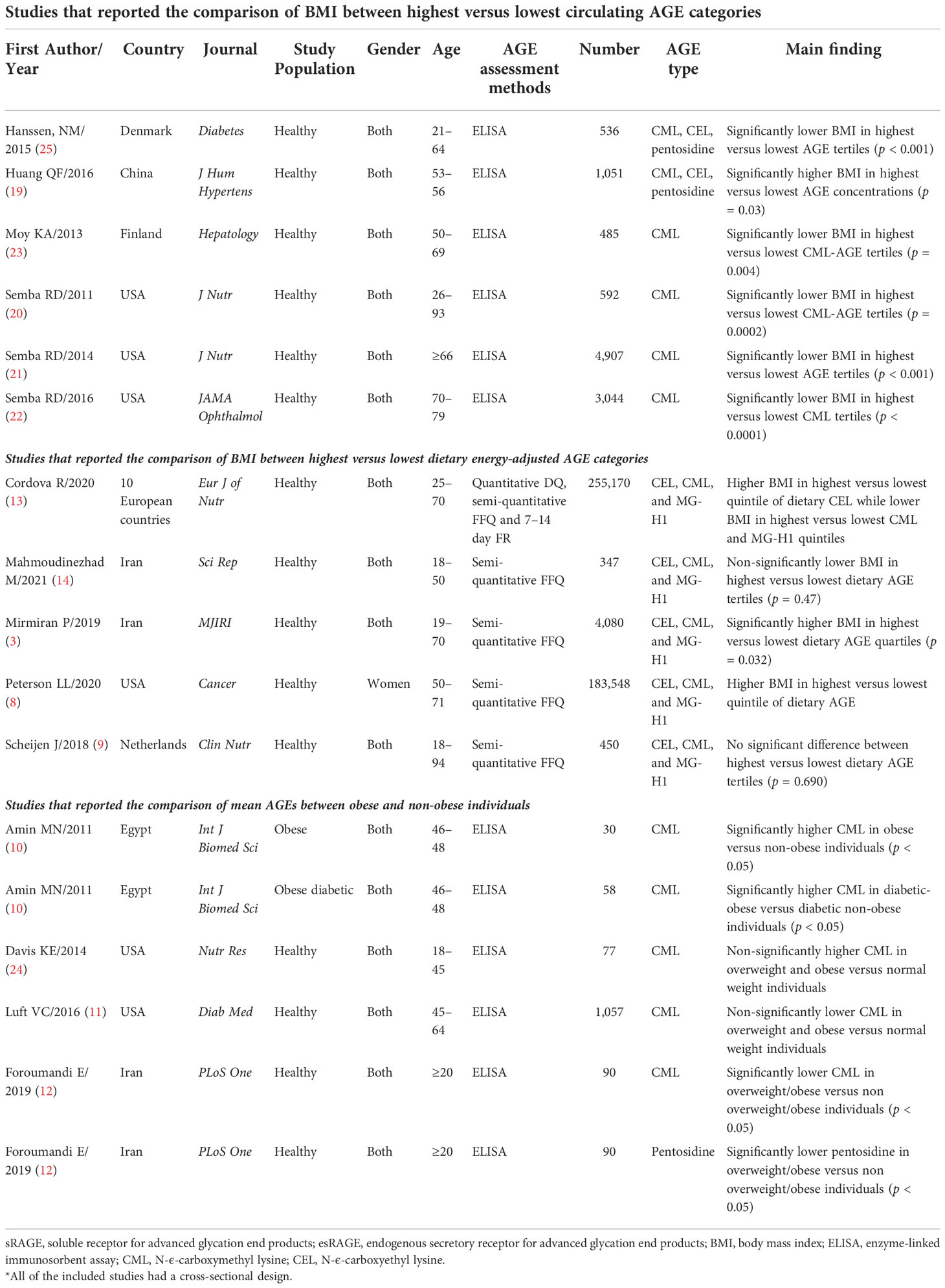
In the two-class meta-analysis of the comparison of BMI between the highest versus lowest circulating AGE categories, six studies with 10,615 total participants were included (19–23, 25). The general characteristics of included studies are presented in Table 3. Only in the study by Huang et al. (19) was BMI higher in the highest versus lowest circulating AGE categories. In the other five studies, those with higher circulating AGE concentrations had significantly lower BMI. The main AGE that was measured in most of the studies was CML, which was measured by enzyme-linked immunosorbent assay (ELISA). In the two-class meta-analysis of the comparison of BMI between the highest versus lowest dietary AGE values, seven studies with 343,595 individuals were included. The study by Cordova et al. (13), reported the comparison of BMI between categories of three different types of dietary AGE, namely, CML, CEL, and pentosidine. Therefore, it was included as three independent studies. Most of the studies reported higher BMI in the highest versus lowest categories of dietary AGE values (Table 3). In the two-class meta-analysis of the comparison of mean circulating AGE in individuals with or without obesity, five individual studies were included with 895 participants. In the study by Amin et al. (10), CML was compared in both obese diabetic patients and obese nondiabetic patients with their corresponding controls. However, we excluded diabetes and just the results of the comparison of CML between obese versus non-obese individuals were reported. Therefore, their study was included as two independent studies, and accordingly, significantly higher CML was observed in diabetic patients with obesity compared with others. Similarly, the study by Foroumandi et al. (12) measured two different types of AGEs (e.g., CML and pentosidine); they observed significantly lower CML and pentosidine in individuals with overweight/obesity versus individuals without overweight/obesity.
Results of meta-analysis
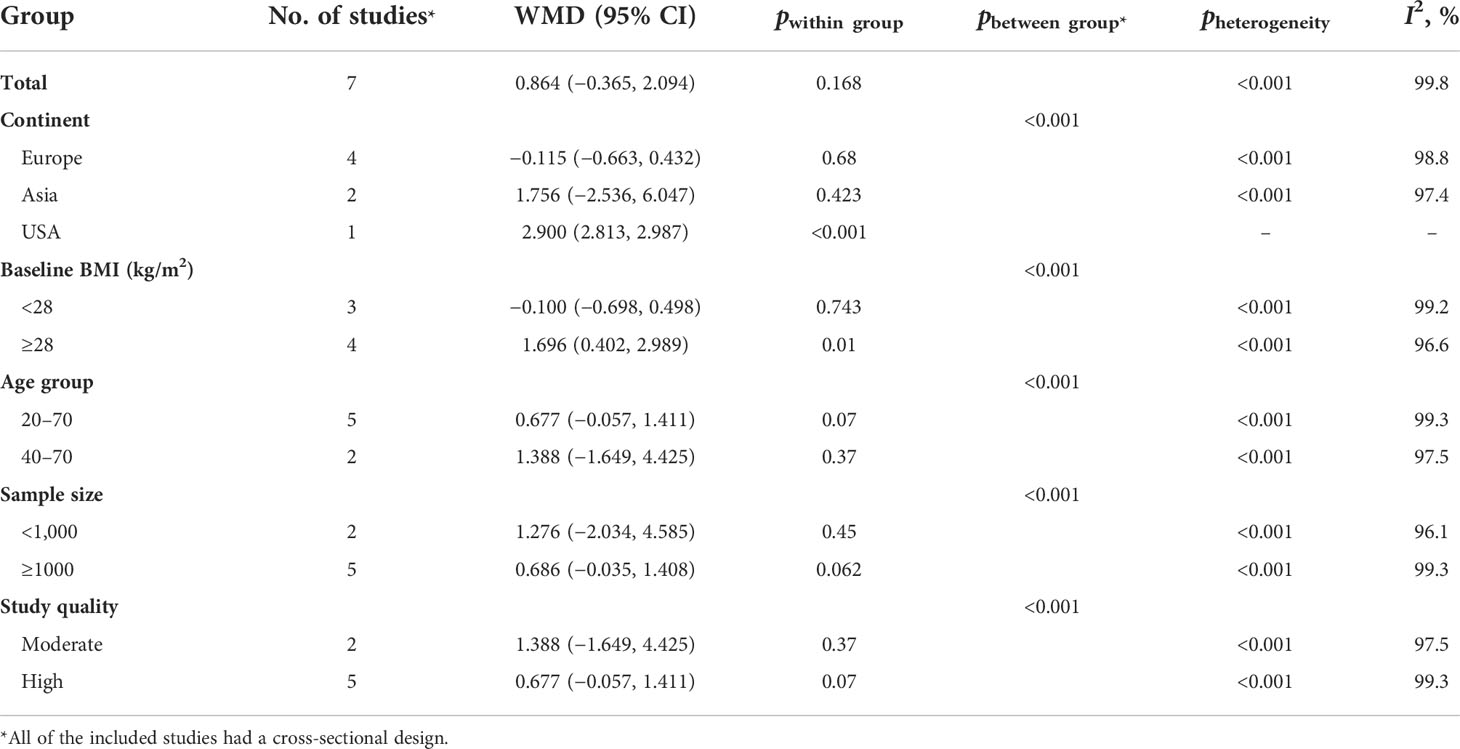
The results of the two-class meta-analysis are presented in Figures 2–4. As shown in Figure 2, being in the highest category of circulating AGEs was accompanied by ~1.5 kg/m2 reduced BMI in apparently healthy adults (WMD: −1.485; CI: −2.459, −0.511; p = 0.003). However, in the comparison of BMI in different categories of dietary AGEs (Figure 3), the result was a nonsignificant increased BMI in the highest versus lowest category of dietary AGEs (WMD: 0.864, CI: −0.365, 2.094; p = 0.186). The comparison of circulating AGEs in individuals with or without obesity, and lower amounts of circulating AGEs in individuals with obesity versus those without obesity were reported (WMD: −57.220, CI: −84.290, −30.149; p < 0.001; Figure 4). Because of the high heterogeneity values in these meta-analyses, we performed a subgroup analysis, and the results are shown in Tables 4–6. AGE type can be considered as a possible source of heterogeneity in the comparison of BMI in the highest versus lowest AGE categories. However, other parameters could not reduce heterogeneity in a meaningful way and, therefore, possibly are not heterogeneity sources.
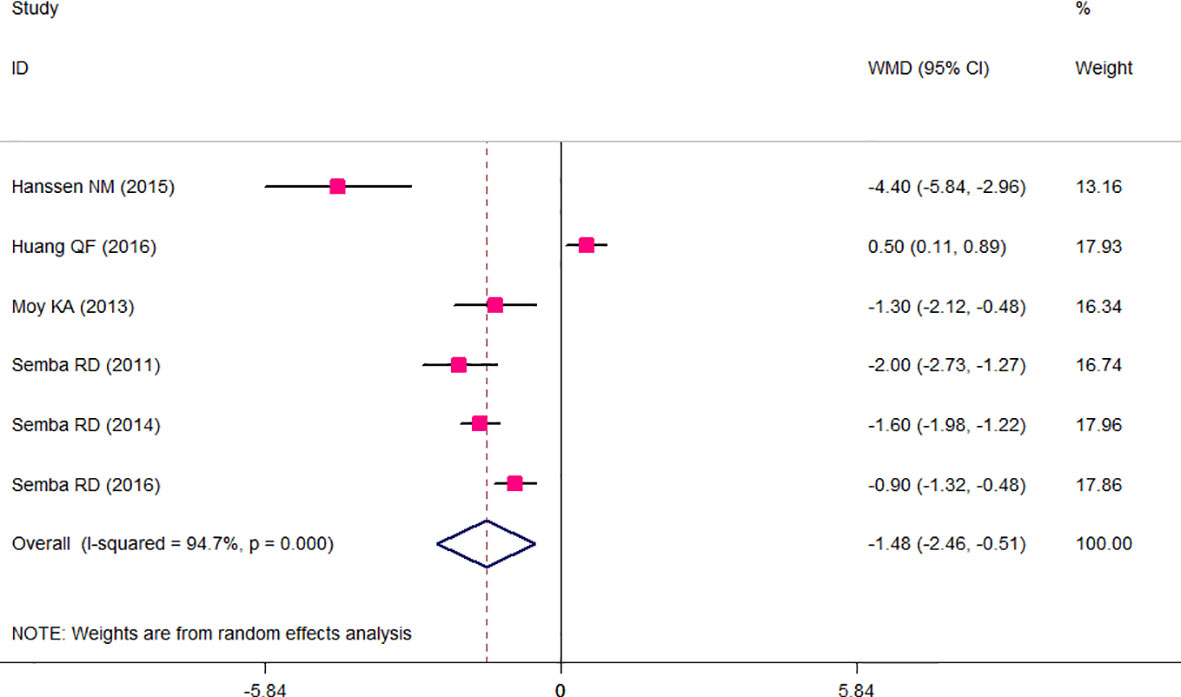
Figure 2 Weighted mean difference (WMD) with 95% confidence interval (CI) of body mass index (BMI) in highest versus lowest categories of circulating advanced glycation end products (AGEs). I2 represents the degree of heterogeneity.
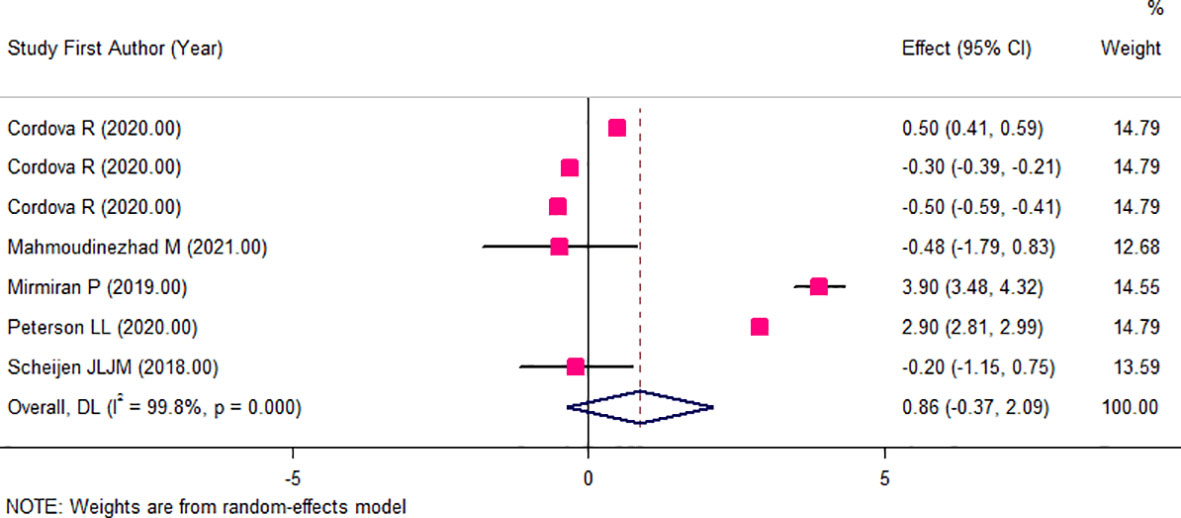
Figure 3 Weighted mean difference (WMD) with 95% confidence interval (CI) of body mass index (BMI) in highest versus lowest categories of dietary advanced glycation end products (AGEs). I2 represents the degree of heterogeneity.
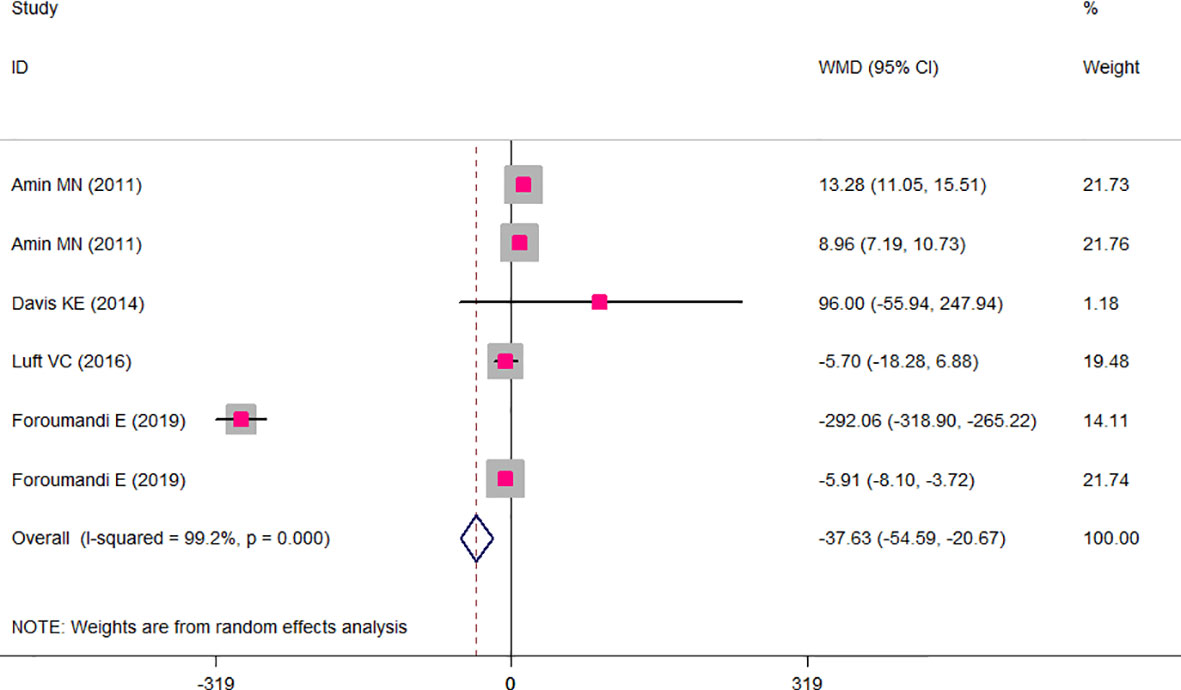
Figure 4 Weighted mean difference (WMD) with 95% confidence interval (CI) of mean circulating advanced glycation end products (AGEs) in obese versus non-obese individuals. I2 represents the degree of heterogeneity.
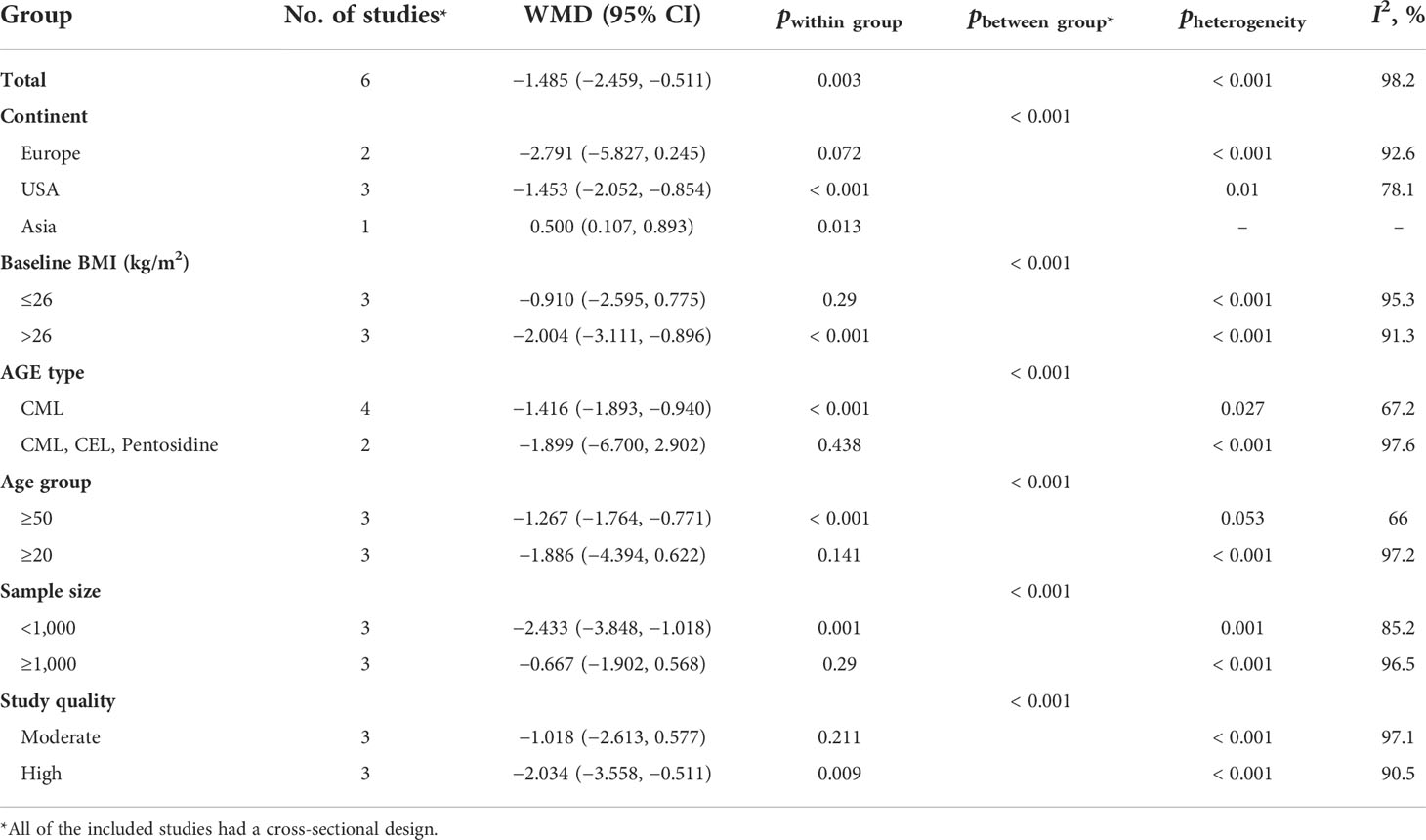
Table 4 Subgroup analysis for the comparison of BMI between highest versus lowest category of circulating AGE.
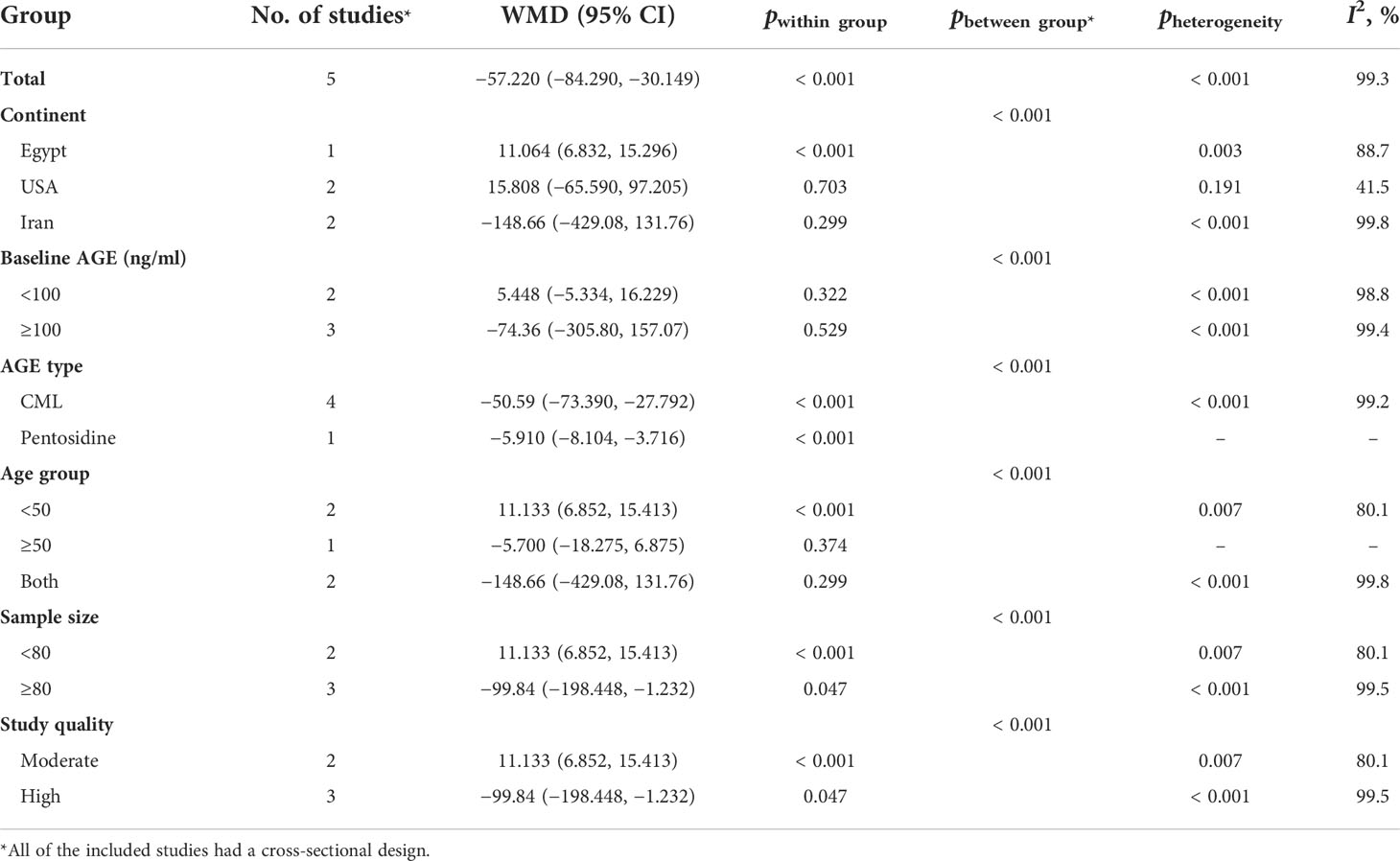
Table 5 Subgroup analysis for the comparison of mean circulating AGE between obese and non-obese participants.
Discussion
In the current meta-analysis, we observed an inverse association between BMI and circulating AGE concentrations in a healthy adult population in two meta-analyses with 11,510 participants. At first glance, these findings seem to be in contrast with the pre-established detrimental effects of AGE in promoting obesity and its related disorders; in the previous meta-analysis of interventional studies by Sohouli et al. (31), low-AGE diets significantly reduced weight and BMI in subjects with obesity, polycystic ovary syndrome, or diabetes. They did not evaluate circulating AGEs; however, we observed a nonsignificant higher BMI in the highest versus lowest dietary AGEs categories. Numerous previous studies reported higher BMI in those with high dietary AGE consumption (3, 8, 13). High dietary AGE content promotes chronic inflammation and insulin resistance in subjects with obesity and helps the transition from healthy obesity to unhealthy obesity with susceptibility to metabolic syndrome; in other words, the inverse association between circulating AGEs and obesity is the concept of healthy versus non-healthy or metabolic syndrome-prone obesity and circulating AGEs that was first proposed by Uribarri et al. (1); they observed significantly higher circulating AGEs (CML and MG-H1) only in subjects with obesity with at least one component of metabolic syndrome versus normal weight individuals, whereas no significant difference was observed among AGE concentrations of individuals with obesity without any metabolic syndrome components versus normal weight individuals. The authors suggested that in individuals with obesity with one metabolic syndrome component, there were also higher circulating amounts of inflammatory factors including leptin, tumor necrosis factor (TNF)-α, and receptor for the advanced glycation end product (RAGE) that promotes deteriorating effects of AGE in metabolic syndrome-prone patients with obesity. AGEs are potent inducers of inflammation and oxidative stress, and this is mostly done via the activation of the AGE/RAGE axis, excessive stimulation of the PI3K-PKB-IKK pathway, and NF-κB binding on the RAGE promoter (32). Also, higher CML concentrations and RAGE expression in the adipose tissue of subjects with obesity lead to the assumption of trapping of CML-AGE via RAGE in the adipose tissue, which causes reduced circulating levels of CML in subjects with obesity (16). However, in the experimental model conducted by Tessier et al. (33), mice were exposed to chronic oral CML administration and showed an accumulation of CML in all tissues except fat. The rate of deposition was high in the kidneys, intestine, and lungs, and low (<5 μg/g) in the heart, muscle, and liver, and they reported that this accumulation was not RAGE dependent. It was an animal model and not the usual dietary intake of AGEs; therefore, the results might not be distributed to human models and further studies should be performed. It is mostly known as “dicarbonyl stress in obesity”, which suggests that the increased MGO formation from glyceroneogenesis on adipose tissue and liver and the decreased glyoxalase-1 activity in obesity possibly cause dicarbonyl stress in white adipose tissue with increased dicarbonyl proteome and, as a consequence, increased transcapillary escape rate of albumin and total body interstitial fluid volume in obesity; this phenomenon will make the levels of glycation of plasma protein (e.g., AGEs) become unreliable indicators of glycation status in obesity (34). Also, another important issue is the type of AGEs that are measured in different studies. As shown in our study, most of the studies that reported an inverse association between circulating AGEs and BMI measured CML as the main AGEs. However, it is well-known that serum CML concentration is strongly affected by body fat; actually, CML is preferentially deposited in fat tissue and adipocytes affect the metabolism of AGE (31). In this situation, circulating CML does not reflect the total body content of CML, and the most important AGE that will be a more reliable indicator of the body’s AGE status is MG-H1, which, in most of the studies, is only measured in the dietary assessment of AGE and not its circulating amounts (35). MGO leads mainly to the formation of MGO-derived cyclic hydroimidazolones (MG-Hs) in three well-known isoforms of MG-H1, MG-H2, and MG-H3. MG-H1, the most abundant and important MGO-derived AGE, accounts for more than 90% of all MGO adducts (36, 37). Both free MG-Hs and MG-H-modified proteins serve as ligands for the receptor of AGEs (RAGE) (38). MG-H1 is strongly associated with increased oxidative stress and even very small increases of MG-H1 modifications of mitochondrial proteins have been linked to a two- to threefold increase in oxidative stress that has a great role in the pathogenesis of chronic diseases like diabetes, chronic kidney disease, and atherosclerosis (39, 40). MG-H1 increases the generation of superoxide anion radicals and serves as an early indicator of the progression of diabetic nephropathy lesions and glomerular basement membrane increase (41). A summary of these mechanistic pathways is illustrated in Figure 5. In the current meta-analysis, all of the included studies had moderate or high study quality and no study had poor quality. In the subgrouping, we demonstrated the possible role of the AGE type as a source of heterogeneity. However, because of the low number of studies in each subgroup, making a reliable conclusion will be limited. In conclusion, in the current systematic review and meta-analysis, for the first time, we summarized the studies that evaluated the association between obesity with circulating and dietary AGE values, and we found a negative association between BMI and circulating AGE concentrations among adults. Further observational studies are warranted to make this conclusion more reliable. Also, it is suggested that, in other studies, MG hydroimidazolone-1 must be measured instead of CML as the main AGE to better elucidate the body’s AGE status.
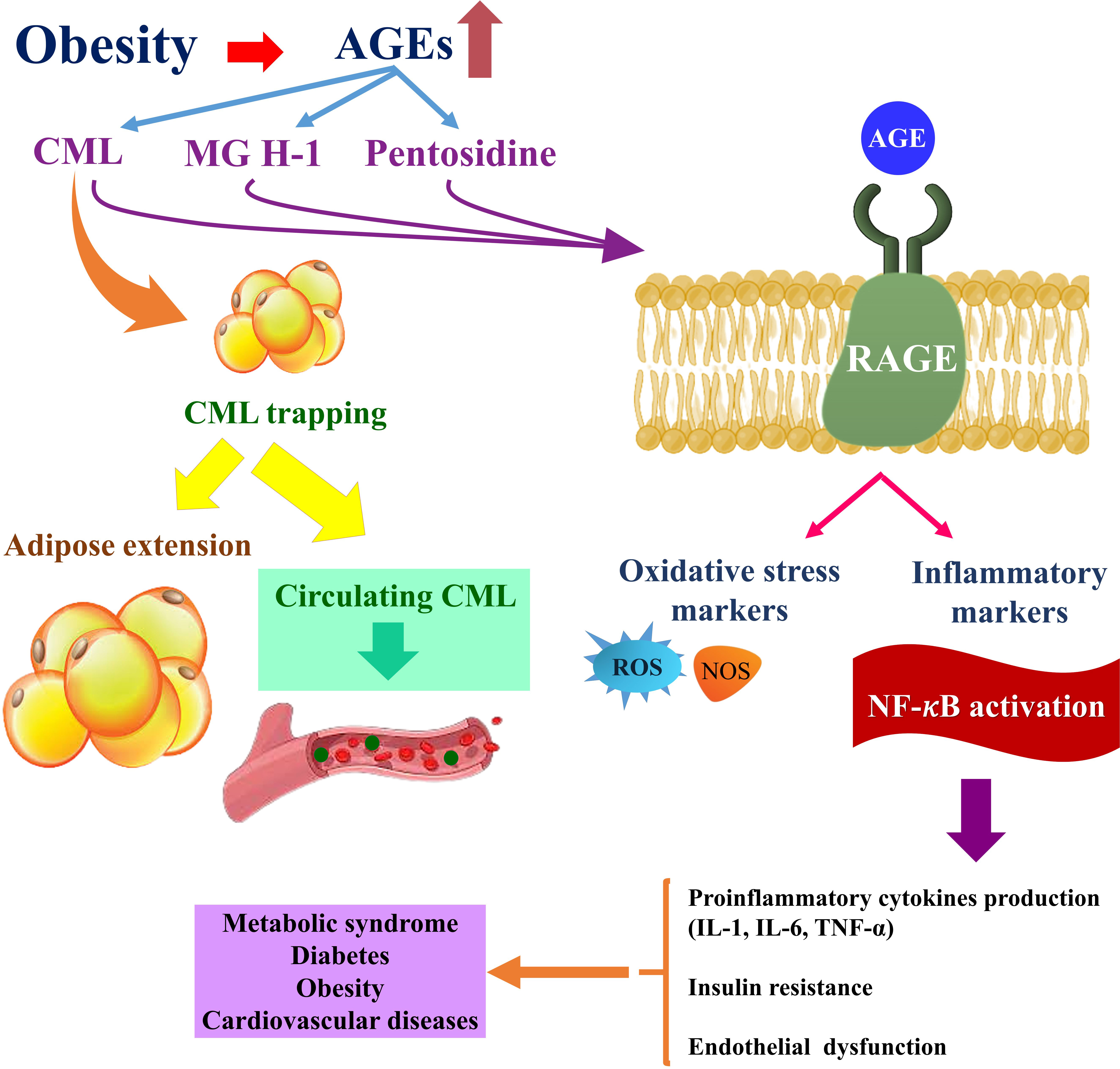
Figure 5 Obesity-related complications are exacerbated by the accumulation of AGEs in adipose tissue. Obesity-related CML accumulation and CML-RAGE-mediated activation of intracellular signaling pathways in adipocytes may promote inflammatory signaling in adipose tissue. The dysregulation of pro-inflammatory and anti-inflammatory cytokines that results may then contribute to the development of obesity-related complications such as metabolic syndrome, diabetes, and cardiovascular diseases (42). On the other hand, trapping CML in adipose tissue reduces serum levels of CML in the bloodstream and expands adipose tissue. Because of the negative relationship of fat mass with serum CML concentrations, serum CML levels may be lower among the obese compared with those who are lean despite a higher intake of AGE-rich foods (20). AGE, advanced glycation end product; CML, carboxymethyl-lysine; MG H1, methylglyoxal-derived hydroimidazolone; RAGE, receptor for advanced glycation end products; NF-κB, nuclear factor κB; ROS, reactive oxygen species; NOS, nitric oxide; IL, interleukin; TNF-α, tumor necrosis factor-alpha.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author contributions
ATJ and AAE supervised the project and were involved in hypothesis generation, searching and conceptualization. AAA and RID were involved in extraction and data analysis. IA, PR, MMK and HHK were all involved in data analysis, extraction and searching through different search engines, RMRP and MJA were involved in manuscript writing and edition. YFM was involved in manuscript revision and English edition. All of the authors have read and approved the final draft of the article to be published.
Acknowledgments
The authors are grateful to Scientific Research Deanship at King Khalid University, Abha, Saudi Arabia for their financial support through the Large Research Group Projectunder grant number (RGP.02-219-43).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2022.966590/full#supplementary-material
References
1. Uribarri J, Cai W, Woodward M, Tripp E, Goldberg L, Pyzik R, et al. Elevated serum advanced glycation endproducts in obese indicate risk for the metabolic syndrome: A link between healthy and unhealthy obesity? J Clin Endocrinol Metab (2015) 100(5):1957–66. doi: 10.1210/jc.2014-3925
2. Deluyker D, Evens L, Bito V. Advanced glycation end products (AGEs) and cardiovascular dysfunction: focus on high molecular weight AGEs. Amino Acids (2017) 49(9):1535–41. doi: 10.1007/s00726-017-2464-8
3. Mirmiran P, Hadavi H, Mottaghi A, Azizi F. Advanced glycation end products and risk of general and abdominal obesity in Iranian adults: Tehran lipid and glucose study. Med J Islam Repub Iran (2019) 33:21. doi: 10.47176/mjiri.33.21
4. Vlassara H, Uribarri J. Advanced glycation end products (AGE) and diabetes: cause, effect, or both? Curr Diabetes Rep (2014) 14(1):453. doi: 10.1007/s11892-013-0453-1
5. Ruiz HH, Ramasamy R, Schmidt AM. Advanced glycation end products: Building on the concept of the "Common soil" in metabolic disease. Endocrinology (2020) 161(1):bqz006. doi: 10.1210/endocr/bqz006
6. Luévano-Contreras C, Gómez-Ojeda A, Macías-Cervantes MH, Garay-Sevilla ME. Dietary advanced glycation end products and cardiometabolic risk. Curr Diabetes Rep (2017) 17(8):63. doi: 10.1007/s11892-017-0891-2
7. Uribarri J, del Castillo MD, de la Maza MP, Filip R, Gugliucci A, Luevano-Contreras C, et al. Dietary advanced glycation end products and their role in health and disease. Adv Nutr (2015) 6(4):461–73. doi: 10.3945/an.115.008433
8. Peterson LL, Park S, Park Y, Colditz GA, Anbardar N, Turner DP. Dietary advanced glycation end products and the risk of postmenopausal breast cancer in the national institutes of health-AARP diet and health study. Cancer (2020) 126(11):2648–57. doi: 10.1002/cncr.32798
9. Scheijen J, Hanssen NMJ, van Greevenbroek MM, van der Kallen CJ, Feskens EJM, Stehouwer CDA, et al. Dietary intake of advanced glycation endproducts is associated with higher levels of advanced glycation endproducts in plasma and urine: The CODAM study. Clin Nutr (2018) 37(3):919–25. doi: 10.1016/j.clnu.2017.03.019
10. Amin MN, Mosa AA, El-Shishtawy MM. Clinical study of advanced glycation end products in egyptian diabetic obese and non-obese patients. Int J BioMed Sci (2011) 7(3):191–200.
11. Luft VC, Duncan BB, Schmidt MI, Chambless LE, Pankow JS, Hoogeveen RC, et al. Carboxymethyl lysine, an advanced glycation end product, and incident diabetes: A case-cohort analysis of the ARIC study. Diabetes Med (2016) 33(10):1392–8. doi: 10.1111/dme.12963
12. Foroumandi E, Alizadeh M, Kheirouri S, Asghari Jafarabadi M. Exploring the role of body mass index in relationship of serum nitric oxide and advanced glycation end products in apparently healthy subjects. PloS One (2019) 14(3):e0213307. doi: 10.1371/journal.pone.0213307
13. Cordova R, Knaze V, Viallon V, Rust P, Schalkwijk CG, Weiderpass E, et al. Dietary intake of advanced glycation end products (AGEs) and changes in body weight in European adults. Eur J Nutr (2020) 59(7):2893–904. doi: 10.1007/s00394-019-02129-8
14. Mahmoudinezhad M, Farhangi MA, Kahroba H, Dehghan P. Personalized diet study of dietary advanced glycation end products (AGEs) and fatty acid desaturase 2 (FADS(2)) genotypes in obesity. Sci Rep (2021) 11(1):19725. doi: 10.1038/s41598-021-99077-3
15. Sebeková K, Somoza V, Jarcusková M, Heidland A, Podracká L. Plasma advanced glycation end products are decreased in obese children compared with lean controls. Int J Pediatr Obes (2009) 4(2):112–8. doi: 10.1080/17477160802248039
16. Gaens KH, Goossens GH, Niessen PM, Van-Greevenbroek MM, Van-der Kallen CJ, Niessen HW, et al. Nepsilon-(carboxymethyl)lysine-receptor for advanced glycation end product axis is a key modulator of obesity-induced dysregulation of adipokine expression and insulin resistance. Arterioscler Thromb Vasc Biol (2014) 34:1199–208. doi: 10.1161/ATVBAHA.113.302281
17. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Internal Med (2009) 151(4):264–9. doi: 10.7326/0003-4819-151-4-200908180-00135
18. Cho CE, Taesuwan S, Malysheva OV, Bender E, Tulchinsky NF, Yan J. Trimethylamine-n-oxide (TMAO) response to animal source foods varies among healthy young men and is influenced by their gut microbiota composition: A randomized controlled trial. Mol Nutr Food Res (2017) 61:1600324. doi: 10.1002/mnfr.201600324
19. Huang QF, Sheng CS, Kang YY, Zhang L, Wang S, Li FK, et al. Central and peripheral blood pressures in relation to plasma advanced glycation end products in a Chinese population. J Hum Hypertens (2016) 30(7):430–5. doi: 10.1038/jhh.2015.60
20. Semba RD, Arab L, Sun K, Nicklett EJ, Ferrucci L. Fat mass is inversely associated with serum carboxymethyl-lysine, an advanced glycation end product, in adults. J Nutr (2011) 141(9):1726–30. doi: 10.3945/jn.111.143172
21. Semba RD, Cotch MF, Gudnason V, Eiríksdottir G, Harris TB, Sun K, et al. Serum carboxymethyllysine, an advanced glycation end product, and age-related macular degeneration: the age, Gene/Environment susceptibility-Reykjavik study. JAMA Ophthalmol (2014) 132(4):464–70. doi: 10.1001/jamaophthalmol.2013.7664
22. Semba RD, Sun K, Schwartz AV, Varadhan R, Harris TB, Satterfield S, et al. Serum carboxymethyl-lysine, an advanced glycation end product, is associated with arterial stiffness in older adults. J Hypertens (2015) 33(4):797–803. doi: 10.1097/HJH.0000000000000460
23. Moy KA, Jiao L, Freedman ND, Weinstein SJ, Sinha R, Virtamo J, et al. Soluble receptor for advanced glycation end products and risk of liver cancer. Hepatology (2013) 57(6):2338–45. doi: 10.1002/hep.26264
24. Davis KE, Prasad C, Vijayagopal P, Juma S, Imrhan V. Serum soluble receptor for advanced glycation end products correlates inversely with measures of adiposity in young adults. Nutr Res (2014) 34(6):478–85. doi: 10.1016/j.nutres.2014.04.012
25. Hanssen NM, Beulens JW, van Dieren S, Scheijen JL, van der AD, Spijkerman AM, et al. Plasma advanced glycation end products are associated with incident cardiovascular events in individuals with type 2 diabetes: A case-cohort study with a median follow-up of 10 years (EPIC-NL). Diabetes (2015) 64(1):257–65. doi: 10.2337/db13-1864
26. Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Method (2005) 5(1):13. doi: 10.1186/1471-2288-5-13
27. Weir CJ, Butcher I, Assi V, Lewis SC, Murray GD, Langhorne P, et al. Dealing with missing standard deviation and mean values in meta-analysis of continuous outcomes: A systematic review. BMC Med Res Method (2018) 18(1):25. doi: 10.1186/s12874-018-0483-0
28. Walter S, Yao X. Effect sizes can be calculated for studies reporting ranges for outcome variables in systematic reviews. J Clin Epidemiol (2007) 60(8):849–52. doi: 10.1016/j.jclinepi.2006.11.003
29. Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med (2002) 21:1539–58. doi: 10.1002/sim.1186
30. Riley RD, Higgins JP, Deeks JJ. Interpretation of random effects meta-analyses. BMJ (2011) 342:d549. doi: 10.1136/bmj.d549
31. Sohouli MH, Sharifi-Zahabi E, Lari A, Fatahi S, Shidfar F. The impact of low advanced glycation end products diet on obesity and related hormones: A systematic review and meta-analysis. Sci Rep (2020) 10(1):22194. doi: 10.1038/s41598-020-79216-y
32. Li J, Schmidt AM. Characterization and functional analysis of the promoter of RAGE, the receptor for advanced glycation end products. J Biol Chem (1997) 272:16498–506. doi: 10.1074/jbc.272.26.16498
33. Tessier FJ, Niquet-Léridon C, Jacolot P, Jouquand C, Genin M, Schmidt AM, et al. Quantitative assessment of organ distribution of dietary protein-bound (13) c-labeled n(ε) -carboxymethyllysine after a chronic oral exposure in mice. Mol Nutr Food Res (2016) 60(11):2446–56. doi: 10.1002/mnfr.201600140
34. Masania J, Malczewska-Malec M, Razny U, Goralska J, Zdzienicka A, Kiec-Wilk B, et al. Dicarbonyl stress in clinical obesity. Glycoconj J (2016) 33(4):581–9. doi: 10.1007/s10719-016-9692-0
35. Rodríguez-Mortera R, Luevano-Contreras C, Solorio-Meza S, Gómez-Ojeda A, Caccavello R, Bains Y, et al. Soluble receptor for advanced glycation end products and its correlation with vascular damage in adolescents with obesity. Horm Res Paediatr (2019) 92(1):28–35. doi: 10.1159/000501718
36. Ahmed N, Thornalley PJ, Dawczynski J, Franke S, Strobel J, Stein G, et al. Methylglyoxal-derived hydroimidazolone advanced glycation end-products of human lens proteins. Invest Ophthalmol Vis Sci (2003) 44:5287–92. doi: 10.1167/iovs.03-0573
37. Rabbani N, Thornalley PJ. Methylglyoxal, glyoxalase 1 and the dicarbonyl proteome. Amino Acids (2012) 42(4):1133–42. doi: 10.1007/s00726-010-0783-0
38. Xue J, Ray R, Singer D, Boühme D, Burz DS, Rai V, et al. The receptor for advanced glycation end products (RAGE) specifically recognizes methylglyoxal-derived AGEs. Biochemistry (2014) 53(20):3327–35. doi: 10.1021/bi500046t
39. Giacco F, Du X, D’Agati VD, Milne R, Sui G, Geoffrion M, et al. Knockdown of glyoxalase 1 mimics diabetic nephropathy in nondiabetic mice. Diabetes (2014) 63(1):291–9. doi: 10.2337/db13-0316
40. Morcos M, Du X, Pfisterer F, Hutter H, Sayed AA, Thornalley P, et al. Glyoxalase-1 prevents mitochondrial protein modification and enhances lifespan in caenorhabditis elegans. Aging Cell (2008) 7(2):260–9. doi: 10.1111/j.1474-9726.2008.00371.x
41. Beisswenger PJ, Howell SK, Russell GB, Miller ME, Rich SS, Mauer M. Early progression of diabetic nephropathy correlates with methylglyoxal-derived advanced glycation end products. Diabetes Care (2013) 36(10):3234–9. doi: 10.2337/dc12-2689
Keywords: AGEs, obesity, BMI - body mass index, sRAGE level, cardiovascular disease
Citation: Turki Jalil A, Alameri AA, Iqbal Doewes R, El-Sehrawy AA, Ahmad I, Ramaiah P, Kadhim MM, Kzar HH, Sivaraman R, Romero-Parra RM, Ansari MJ and Fakri Mustafa Y (2022) Circulating and dietary advanced glycation end products and obesity in an adult population: A paradox of their detrimental effects in obesity. Front. Endocrinol. 13:966590. doi: 10.3389/fendo.2022.966590
Received: 11 June 2022; Accepted: 19 October 2022;
Published: 01 December 2022.
Edited by:
Paulo Matafome, University of Coimbra, PortugalReviewed by:
Fatima O. Martins, New University of Lisbon, PortugalMa. Eugenia Garay-Sevilla, University of Guanajuato, Mexico
Copyright © 2022 Turki Jalil, Alameri, Iqbal Doewes, El-Sehrawy, Ahmad, Ramaiah, Kadhim, Kzar, Sivaraman, Romero-Parra, Ansari and Fakri Mustafa. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Abduladheem Turki Jalil, YWJlZGFsYXplZW03OTlAZ21haWwuY29t; Amr A. El-Sehrawy, c2VocmF3eWFtckBnbWFpbC5jb20=
 Abduladheem Turki Jalil
Abduladheem Turki Jalil Ameer A. Alameri2
Ameer A. Alameri2