- Department of Assisted Reproduction, Shanghai Ninth People’s Hospital, Shanghai Jiaotong University School of Medicine, Shanghai, China
Purpose: The purpose of this study is to assess the safety of progestin-primed ovarian stimulation (PPOS) protocol regarding the neonatal outcomes and congenital malformations in babies born after in vitro fertilization (IVF) and frozen embryo transfer (FET).
Methods: In this large retrospective cohort study, a total of 16,493 infants born between 1 September 2013 and 31 July 2021 from IVF and FET cycles after treatment with either PPOS (n = 15,245) or gonadotropin-releasing hormone antagonist (GnRH-ant) (n = 1,248) were finally enrolled. The primary outcome measure was the incidence of congenital malformations. The secondary outcome measures were rates of low birth weight (LBW), very low birth weight (VLBW), preterm birth (PTB), very preterm birth (VPTB), and early neonatal death.
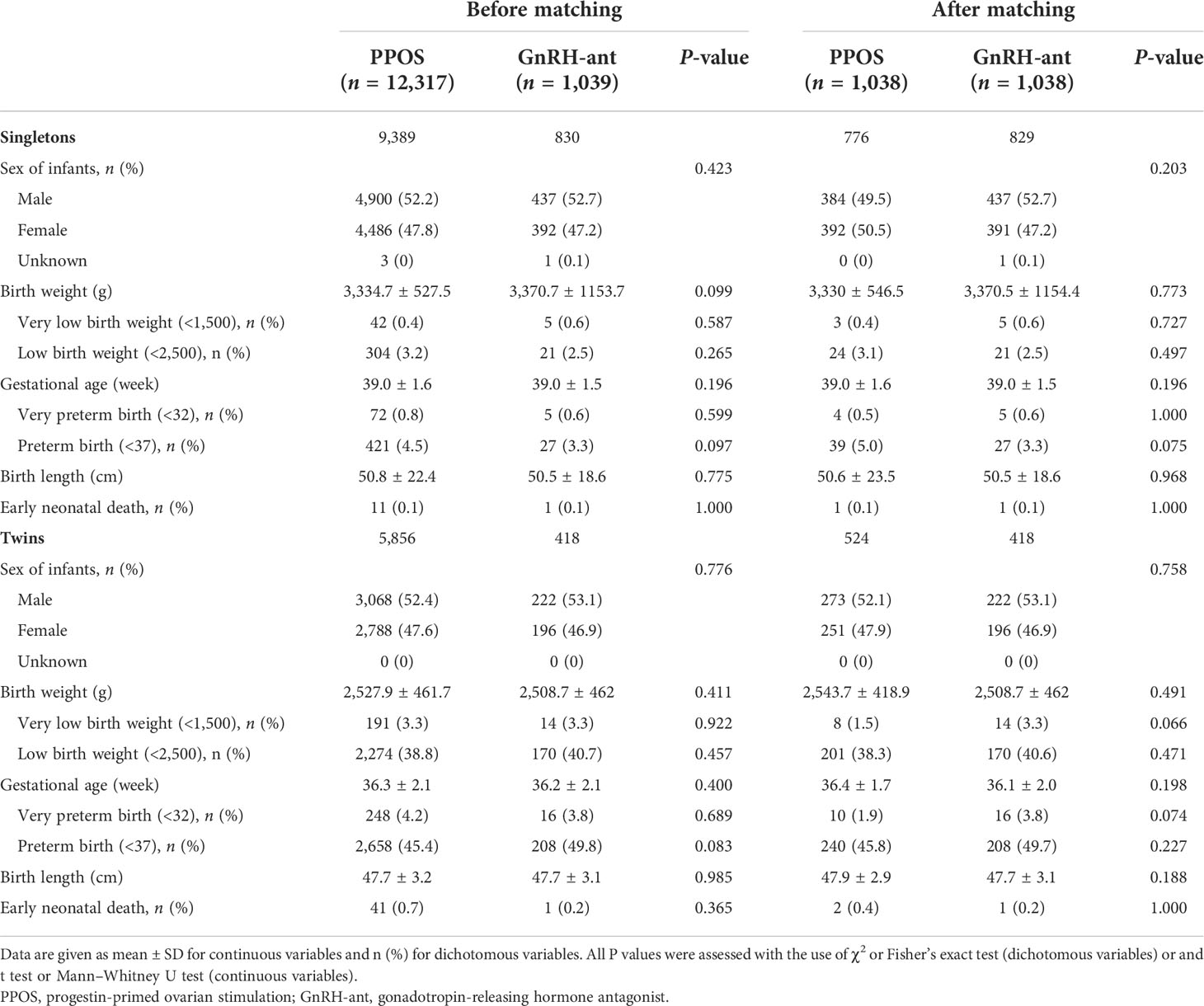
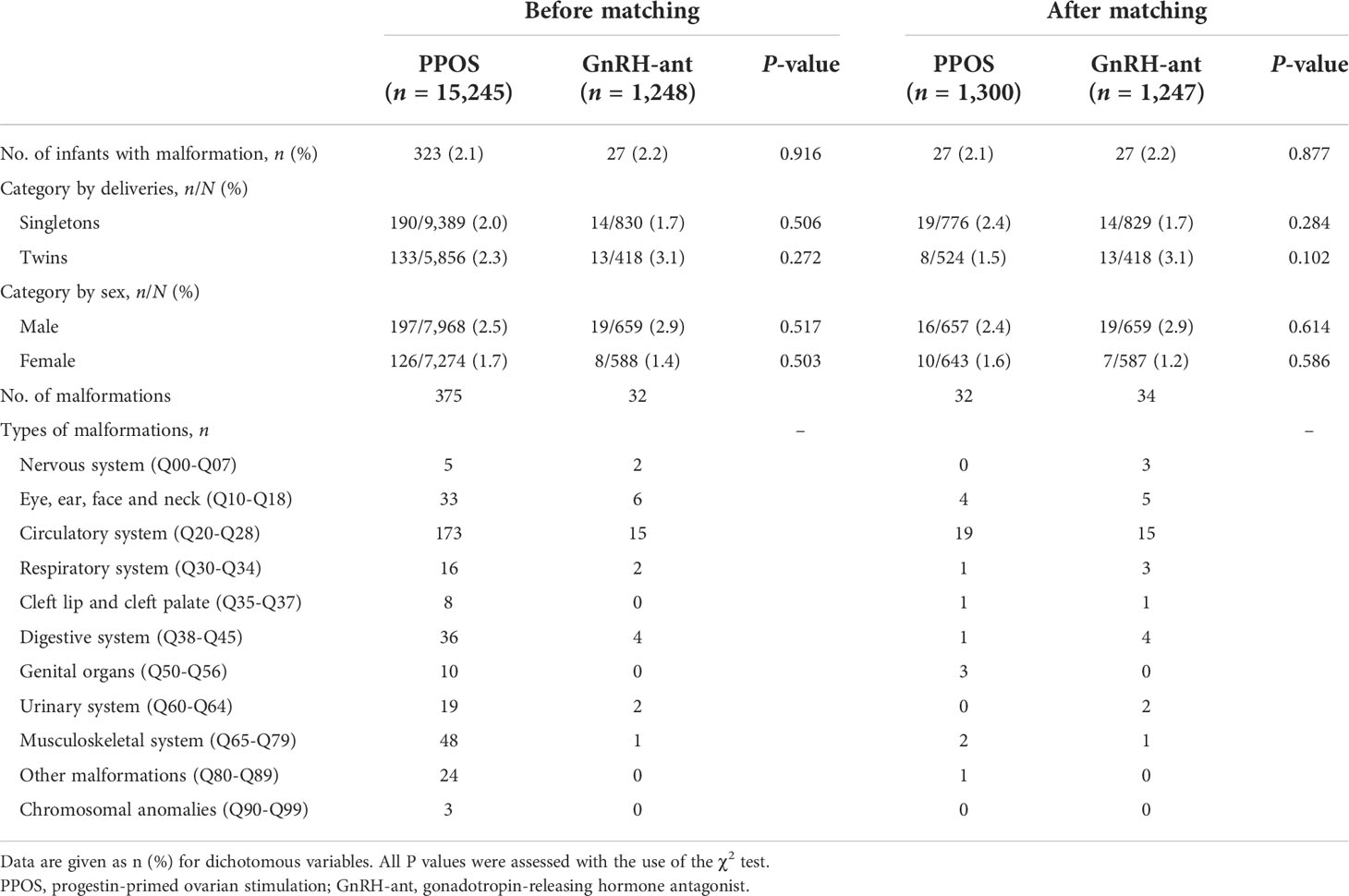
Results: Birth characteristics for both singletons and twins regarding the sex of infants, gestational age, birth weight, and birth length were comparable between the PPOS group and the GnRH-ant group. Rates of LBW, VLBW, PTB, VPTB, and early neonatal death were also similar. The reanalysis using propensity score matching (PSM) and multivariable logistic regression indicated that the PPOS protocol could not increase the risk of adverse neonatal outcomes compared with the GnRH-ant protocol. Furthermore, no significant difference was observed in the overall incidence of congenital malformations in live-born babies. After PSM and controlling for all confounders, the results remained insignificant with an adjusted odds ratio of 0.66 [95% confidence interval (CI) 0.32–1.34] and 2.43 [95% CI 0.97–6.06], respectively, for singletons and twins.
Conclusions: Our study suggests that compared with GnRH-ant treatment for IVF, the PPOS protocol could not produce a negative effect on the newborn population in terms of neonatal outcomes and congenital malformations.
Introduction
With the wide spread of assisted reproductive technology (ART), an increasing number of infertile couples have successfully conceived via in vitro fertilization/intracytoplasmic sperm injection (IVF/ICSI) over the past decades. Currently, at least five million of infants have been born as a result of ART, approximately accounting for 2% of all births in European countries (1). Notably, this proportion may be gradually increasing each year. However, the potential risk of fetal adverse outcomes due to ART is always the source of debate. Several studies have reported the increasing risk of major congenital malformations of live-born babies following ART compared with natural conceptions (2–4). The increased incidence of birth defects in offspring might be mainly attributable to the infertility characteristics, ovarian stimulation regimens, or specific ART procedures (5, 6).
Controlled ovarian stimulation (COS) has always been considered to play a critical role in the development of ART. The application of exogenous gonadotropin resulting in supraphysical serum estradiol levels and the subsequently positive feedback at the pituitary body might hence cause a premature luteinizing hormone (LH) and premature luteinizaton (7). Consequently, in order to minimize the occurrence of premature LH surge, the coadministration of gonadotropin-releasing hormone agonists (GnRHa) or gonadotropin-releasing hormone antagonists (GnRH-ant) was applied into stimulation regimens and gradually accepted as routine regimens safe for maternal and neonatal health (8, 9). However, these solutions could be accompanied by the complication of ovarian hyperstimulation syndrome (OHSS) triggered by human chorionic gonadotropin (hCG) or complex stimulation (10).
Previously, studies have found that progestin (P) secreted during the luteal phase could strongly inhibit LH secretion and estradiol-induced positive feedback effects with no spontaneous LH surge (11). Moreover, considering the need for new methods with improved efficacy and safety, our center in 2015 firstly proposed to introduce oral P into the regimen for COS, namely progestin-primed ovarian stimulation (PPOS) (12, 13). P’s LH-suppression effects and popularized freeze-all protocol’s efficacy suggest that the PPOS protocol has the advantages of control over LH surge and OHSS incidence. The general concern regarding whether P used in COS is safe for the offspring has still not been resolved, and information about the safety of PPOS for live-born infants compared with GnRH analogues was limited. Prior studies have demonstrated that the neonatal outcomes and the risk of congenital malformations were comparable with the use of P, including medroxyprogesterone acetate (MPA), utrogestan, or dydrogesterone for the PPOS protocol when compared with the GnRHa short protocol (14–17), and a meta-analysis has concluded that the PPOS protocol, compared with the GnRHa protocol, was associated with a similar congenital malformation risk profile (18).
As one of routine COS procedures, the GnRH-ant protocol could reduce the incidence of OHSS without affecting the pregnancy and neonatal outcomes compared with the GnRHa protocol (19, 20). However, no comparison of neonatal outcomes has been made between the PPOS protocol and the GnRH-ant protocol. Moreover, to date, more than 10,000 infants have been born with the use of the PPOS regimen in our center. Therefore, a larger cohort retrospective study is needed to further assess the safety for the new COS regimen. Considering these, we chose the GnRH-ant protocol as the control group to comprehensively assess the neonatal outcomes and congenital malformations for the newborn population after PPOS in a larger sample.
Materials and methods
Study design and participants
This retrospective cohort study was performed in the Department of Assisted Reproduction of the Ninth People’s Hospital of Shanghai Jiao Tong University. The study protocol was approved by the institutional ethics committee of the Ninth Hospital. All participants gave written informed consent after describing the research in detail.
All infertile patients (n = 19,385) who underwent IVF/ICSI followed by frozen-thawed embryo transfer (FET) using the PPOS or GnRH-ant protocol were recruited for the study during the period between 1 January 2013 and 31 October 2020 in our center. Considering that these factors could be associated with birth defects, women with reported pregnancy-related diseases such as hypertension (n = 522), gestational diabetes mellitus (n = 1,321), thyroid diseases (n = 35), intrahepatic cholestasis of pregnancy (n = 59), anemia (n = 58), cardiac diseases (n = 13), and inflammatory diseases in pregnancy (n = 32) and combined disorders (n = 172) were excluded (21). Furthermore, cycles receiving donor sperm due to the possible consequence on neonatal outcomes (n = 194) were excluded (22). Cycles with missing core data (n = 245) were also excluded. A total of 16,493 infants born between 1 September 2013 and 31 July 2021 from IVF and FET cycles after treatment with either PPOS (n = 15,245) or gonadotropin-releasing hormone antagonist (GnRH-ant) (n = 1,248) were finally enrolled in this study. The flow diagram of the study design and cohort selection is shown in Supplemental Figure 1.
Regimens
In the PPOS group, patients underwent daily intramuscular injection of 150 or 225 IU of human menopausal gonadotropin (hMG; Anhui Fengyuan Pharmaceutical Co., Ltd., Hefei, China) and simultaneously administered with 10 mg of MPA or 100 mg of utrogestan (Laboratories Besins International, Paris, France) or 20 mg of dydrogesterone (Duphaston, Abbott Biologicals, USA) beginning from the menstrual cycle day 2 or 3 (MC2 or 3) until the trigger day. In the GnRH-ant group, patients were injected daily with 150 or 225 IU hMG intramuscularly from MC2 or 3 onward and 0.25 mg of GnRH-ant (cetrotide; Baxter) from MC5 onward. Ovarian response was assessed according to the ultrasound monitoring and serum estradiol analysis. When the diameter of one dominant follicle reached 20 mm or at least three follicles reached diameters of 18 mm, the final oocyte maturation was triggered by 0.1 mg of triptorelin (decapeptyl, Ferring Pharmaceuticals) alone or co-triggered with 1,000–5,000 IU of hCG (Lizhu Pharmaceutical Trading Co., China).
All follicles with diameters of over 10 mm were retrieved within 32–36 h following maturation induction under transvaginal ultrasound guidance (based on per-group protocol). Then, the retrieved oocytes were fertilized in vitro by conventional IVF and/or ICSI, depending on the semen parameters. The embryos were further cultured in the Continuous Single Culture (CSC; Irvine Scientific, CA) supplemented with 10% serum substitute supplements (SSS; Irvine Scientific, CA). The cleavage-stage embryos (day 3) were graded according to the Cummins criteria (23). Grade I and II embryos regarded as top-quality were frozen by vitrification, and the remaining embryos (grades III and IV) were further cultured until the blastocyst stage. Thereafter, only blastocysts with good morphology were frozen. The freezing and thawing procedures were carried out as previously described (24).
Before embryos were thawed on the transfer of day, endometrial preparation for frozen embryo transfer (FET) could be performed in the natural cycle, artificial cycle, or mild stimulation cycle. The choice of method of endometrial preparation depends on the maternal infertile characteristics such as menstrual regularity. Then, no more than two thawed embryos were transferred for each patient. Once the pregnancy was achieved, P supplement would be continued until 10 weeks of gestations.
Follow-up of pregnancy and neonatal outcomes
All patients who obtained clinical pregnancy were followed up in the form of a telephone interview and recorded every stage of pregnancy until 1 week after delivery. Information including pregnancy-related complications, infant gender, mode of delivery, birth weight and length, birth date and locality, gestational weeks, neonatal diseases, and presence of congenital malformations was included in the standardized questionnaires. Furthermore, detailed reports on pregnancies, deliveries, and neonatal outcomes could be obtained from the gynecologists and pediatricians in charge. For live-born babies with birth defects, case information was gathered by a specially designated nurse to make it clear whether the infants met the definition of the Chinese Birth Defects Monitoring Program.
Outcome measures
The primary outcome of this study was the incidence of congenital malformations. The secondary outcome measures included low birth weight (LBW), very low birth weight (VLBW), preterm birth (PTB), very preterm birth (VPTB), and early neonatal death rates.
Congenital malformations were defined as the structural and functional anomalies as well as genetic defects occurring during pregnancy or at birth, which were classified based on the International Classification of Diseases Q Codes, 10th revision (ICD10: Q00-Q99) (25, 26). LBW and VLBW were considered as birth weight <2,500 and <1,500 g, respectively. PTB and VPTB were considered as delivery taking place before 37 and 32 completed weeks of gestation, respectively. Early neonatal death referred to the death of a live-born baby within 7 days of birth.
Statistical analysis
Sample size calculation was not performed as this was an exploratory retrospective study, and there were no prior data to guide its sample size calculation. Statistical analyses were performed using Statistical Package for Social Sciences (version 25.0; SPSS Inc., USA) and R statistical programming language (version 4.1.0; R Foundation for Statistical Computing, Austria). The normality of continuous variables was tested by the histograms and Q–Q plots as well as the Kolmogorov–Smirnov test. Data with normal distribution were presented as the mean ± standard deviation (SD) or as medians for non-normal distribution, and between-group differences were compared via Student’s t-test or the Mann–Whitney U test, as appropriate, whereas categorical variables were described as number (percentage) and were compared via the chi-squared test or Fisher’s exact test when appropriate. Statistical significance was defined as a P value <0.05.
To balance maternal baseline characteristics between two groups, we established a one-to-one propensity score matching (PSM) model using the nearest-neighbor matching algorithm, and after matching, the balance between the two groups was evaluated by the standardized mean difference (<0.1). The potential confounding factors chosen for matching included maternal age, body mass index (BMI), duration of infertility, obstetrical history, cause of infertility, sperm origin, fertilization method, FET endometrial preparation, endometrial thickness, number of transferrable embryos, and embryo stage at transfer. Then, the multivariable logistic regression analysis was further performed to evaluate the possible association between the ovarian stimulation protocol (PPOS vs. GnRH-ant) and LBW, PTB, and congenital malformations after adjusting for the aforementioned confounders. The crude and adjusted odds ratios (ORs) with corresponding 95% confidence intervals (CIs) were calculated.
Results
Originally, a total of 19,385 clinical pregnancy cycles were selected from our database. According to the exclusive criteria mentioned above, 16,734 clinical pregnancy cycles were ultimately recruited for our study including 15,382 cycles from the PPOS protocol and 1,352 cycles from the GnRH-ant protocol. For the treatment with the PPOS protocol, 12,988 ongoing pregnancies could lead to the birth of 15,245 live-born infants (12,317 live-birth cycles). Moreover, for the treatment with the GnRH-ant protocol, 1,092 ongoing pregnancies could lead to the birth of 1,248 live-born infants (1,039 live birth cycles). In addition, the selective terminations of pregnancy due to fetal anomalies are listed in Supplemental Table 1. We could see a comparable prevalence of congenital malformations in fetuses between the two groups.
As shown in Table 1, before matching, some maternal and cyclic parameters, such as maternal age, BMI, the proportion of primary infertility and nulliparity, sperm origin, and embryo stage at transfer, were comparable between the two groups. However, women in the GnRH-ant group tended to undergo a notably longer duration of infertility compared with women in the PPOS group (3.28 ± 2.61 vs. 3.01 ± 2.61, P < 0.001). Moreover, the cause of infertility for women in the GnRH-ant group might have significantly contributed more to male factors, endometriosis, and unexplained factor than women in the PPOS group (P = 0.001 <0.05). Furthermore, IVF was applied more frequently in women from the PPOS group than that from the GnRH-ant group (59.0% vs. 57.1%, P = 0.004 <0.05), whereas hormone replacement therapy was more preferably used for FET endometrial preparation in women from the GnRH-ant group than that from the PPOS group (37.8% vs. 31.1%, P < 0.001). Moreover, a significantly fewer proportion of double embryos was transferred in the PPOS group compared with the GnRH-ant group (81.7% vs. 84.4%, P = 0.028 <0.05). Regarding the thickness of the endometrium, it was significantly greater in the GnRH-ant group than in the PPOS group (10.91 ± 2.35 vs. 10.64 ± 2.17, P = 0.002 <0.05). After matching, all maternal baseline characteristics of 1,038 women per group were similarly adjusted, and no significant difference was observed between the two groups concerning all confounding factors.
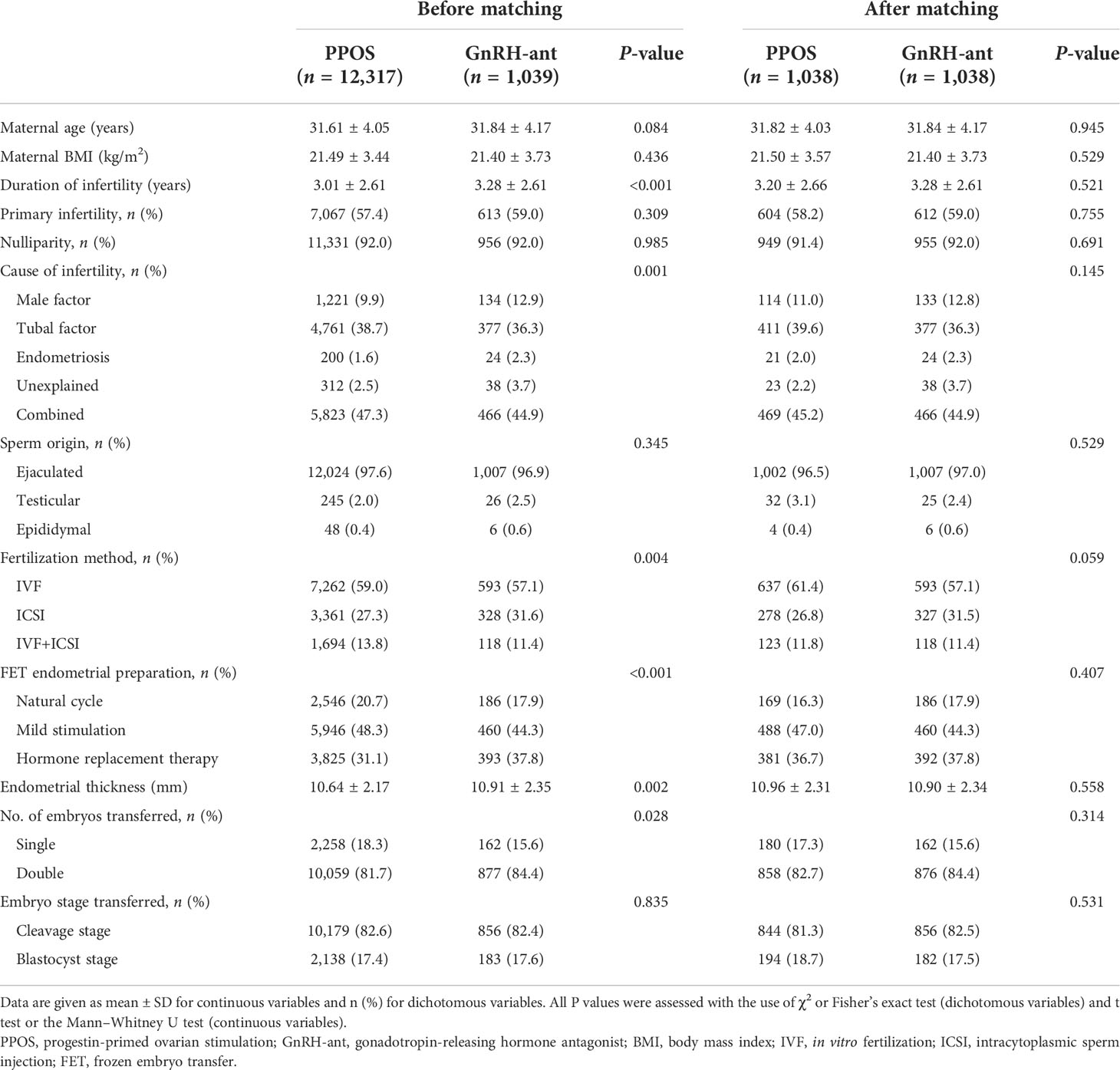
Table 1 Maternal and cyclic characteristics of the live birth cycles between the PPOS and GnRH-ant groups.
Table 2 presents the neonatal outcomes of live-born singletons and twins before and after the analysis of PSM. As the results show, no significant differences regarding the sex of infants, gestational age, birth weight, and birth length were observed between the GnRH-ant and PPOS groups in both singletons and twins. Moreover, the overall incidence of early neonatal death was comparable between the two groups among singletons and twins. Singletons and twins born after treatment with PPOS exhibited similar rates of LBT, VLBT, PTB, and VPTB compared with that born after treatment with GnRH-ant.
Congenital malformations were observed in 323 of 15,245 (2.1%) in the PPOS group and 27 of 1,248 (2.2%), with a non-significant difference (P = 0.916 <0.05) as Table 3 summarizes. When categorized by deliveries and sex, no between-group comparison of birth defects was found. In addition, apart from the overall analysis of the prevalence of congenital malformations, a detailed breakdown of congenital malformations was further analyzed according to the various organ systems, which was statistically insignificant. Consistently, the reanalysis of PSM demonstrated that the incidence of congenital malformations remained insignificant.
Just as Table 4 presents, after adjusting for a variety of confounders, the results for PTB and LBT of singletons and twins could not be changed. Also, no evidently elevated risk of congenital malformations was seen in infants born after PPOS treatment in comparison with the GnRH-ant treatment in both singletons (adjusted OR 0.81, 95% CI 0.47–1.41) and twins (adjusted OR 1.32, 95% CI 0.74–2.37). The results were still invariable after reanalysis using PSM (Table 4).
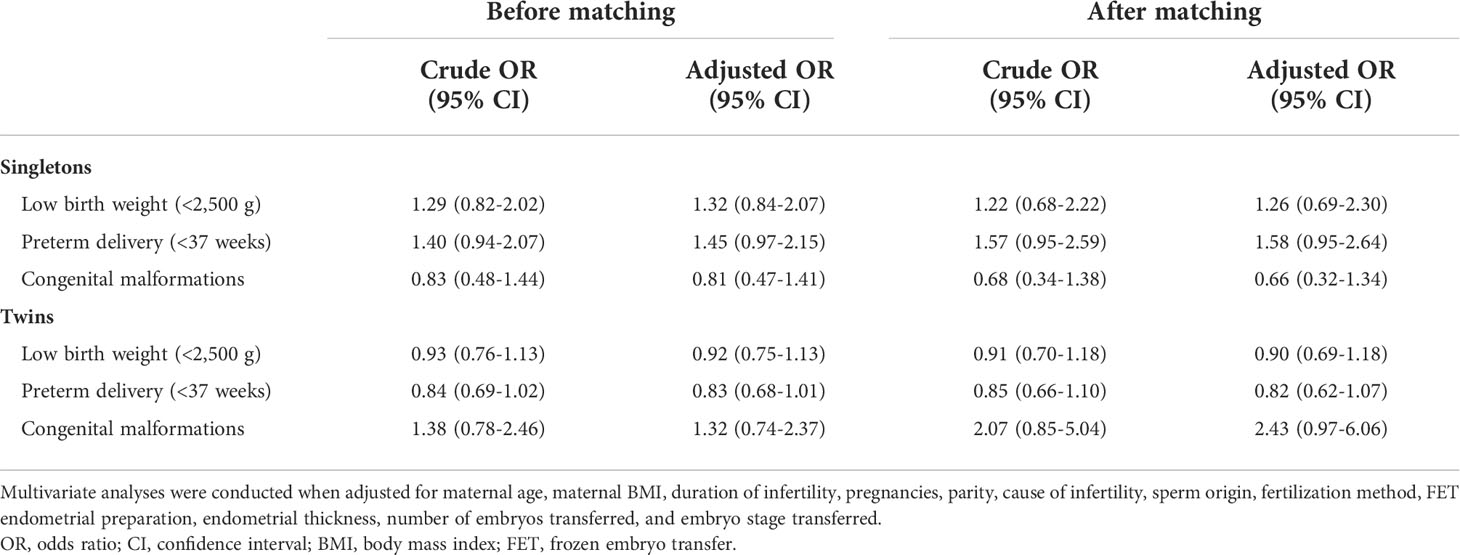
Table 4 Crude and adjusted odds ratios (ORs) of neonatal outcomes in live-born singletons and twins between the PPOS and GnRH-ant groups.
Discussion
Recently, the PPOS protocol has been suggested to reduce the incidence of OHSS and produce similar pregnancy outcomes compared with conventional protocols. However, the safety about the novel protocol needs to be well settled. As far as we know, our study is the largest retrospective cohort study to investigate the neonatal outcomes and congenital malformations after PPOS treatment compared with GnRH-ant treatment considering and matching the possible influence factors as much as possible to date. We found that PPOS was a safe choice of ovarian stimulation for offspring without compromised neonatal outcomes or increasing the risk of congenital malformations.
In the prior decades, the effects of P on oocyte and embryo development both in vivo and in vitro remained controversial. Fukui et al. also found that adding P to the in vitro culture system could decrease the rate of bovine oocyte maturation (27). Moreover, a study conducted by Silva et al. showed that P could have a negative effect on blastocyst yield for in vitro matured bovine oocytes, which could be partially reversed by mifepristone, namely P antagonist (28). By contrast, Bezerra et al. observed that in the medium-sized bovine antral follicles, P could promote the growth of oocytes after prematuration in vitro (29). For rhesus monkeys, the oocyte maturation induced by P could also be observed (30). Carter et al. demonstrated that elevated P did not affect the proportion of in vitro fertilized embryos developing to the blastocyst stage (31). Overall, the consensus has not been reached, and the inconformity of data might be contributed to the difference in study design or the species-specific difference. Additionally, several retrospective studies suggested that the cumulative live birth rate or ongoing pregnancy rate was inversely associated with serum P concentration on the day of hCG triggering in fresh embryo transfer cycles, indicating that the serum P level could reduce endometrial receptivity (32, 33). This could be avoided with the wide popularization of FET nowadays. However, concerns about long-time exposure to P of oocytes and embryos have been raised by physicians, and relatively few studies have been involved in this field.
Kuang et al. proposed the first PPOS protocol in 2015 based on the prior study that confirmed the use of exogenous gonadotropin in the luteal phase, which was luteal-phase ovarian stimulation (LPS) (12, 34). Moreover, advanced vitrification techniques and the freeze-all embryo strategy made the protocol widely used. In some prospective and retrospective cohort studies, comparable embryological and clinical outcomes were observed between the PPOS protocol and the conventional ovarian stimulation protocol, which implied that the embryo development potential could not be impaired (35, 36). A previous study has demonstrated no significantly elevated rate of congenital anomalies for infants after the treatment with LPS compared with the conventional ovarian stimulation protocol (37). Apart from these findings, the impact of P on newborn babies has been investigated in the aspects of P supplement before and during pregnancy. Carmichael et al. found that the maternal use of oral contraceptives during early pregnancy was associated with an increased odd of hypospadias (38), whereas Zhang et al. proposed that the use of emergency contraception could not have adverse effects on pregnancy outcomes (39). Therefore, the safety of P for oocytes and embryos still needs to be examined further.
To further address this issue, a detailed analysis using a retrospective study containing a large amount of samples was run to investigate the safety for the PPOS protocol with regard to the neonatal outcomes and the occurrence of birth defects. In our study, we demonstrated that the neonatal outcomes and the incidence of congenital malformations were always comparable between the PPOS protocol and the GnRH-ant protocol after matching and adjusting for various confounding factors, suggesting that P could not have an adverse effect on the safety for offspring, which was in line with previous studies (14–17). Our study also found that the cardiovascular malformations were the most common defects at birth among all types of congenital malformations in both the PPOS and GnRH-ant groups, as studies previously reported. Furthermore, the overall incidence of congenital malformations in live-born infants was 2.1% in our present study, nearly in accordance with a data-linkage cohort study of IVF newborns in China (2.0%) (40).
The greatest strength of our study is that we analyze the data with more than 10,000 newborn samples to make the conclusion reliable. However, the minor differences were easy to be significant for the baseline characteristics due to the large sample size of the study, so we have adopted PSM analysis to balance the between-group differences of baseline characteristics, which could reduce the systematic bias. Moreover, the results were confirmed after using multivariable regression analysis controlling for vital confounding factors. In addition, the data of the study were collected in a unified way, and its analysis was performed in a single IVF center including applying for the same IVF procedures and vitrification or thawing procedures to avoid possible errors. Nevertheless, the study also has its limitations. The retrospective cohort study has its inherent defects easily leading to selection bias; therefore, a more rigorous prospective randomized controlled study is required to increase the strength of our study. Furthermore, although we have analyzed the results considering the influential factors as much as possible, there were still some unknown confounders not accounted into the study.
In conclusion, our study suggests that the PPOS protocol with a comparison of the GnRH-ant protocol is safe for offspring in terms of newborn outcomes and congenital malformations. In the near future, PPOS treatment might become an appealing option for infertile women. Moreover, the long-term follow-up for the health of babies born after treatment with PPOS is still further needed to testify the safety of this protocol.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving human participants were reviewed and approved by the institutional ethics committee of the ninth hospital. The patients/participants provided their written informed consent to participate in this study.
Author contributions
XL, YK, and RC conceived of and designed the study. DL collected the data, performed the statistical analysis, and drafted the first version of the manuscript. ZH, QC, and WC helped making interpretation for the data. All authors contributed to the manuscript revision and read and approved the submission of the final version of the manuscript.
Funding
This research was supported by grants from the National Natural Science Foundation of China (no.81873856), the “Two-hundred Talent” (20191814), Shanghai Health and Family Planning Commission (201940287), and the Shanghai Science and Technology Innovation Fund (22Y11906000; 22Y21900800).
Acknowledgments
We sincerely appreciate the support and cooperation from all staff members of the Department of Assisted Reproduction in Shanghai Ninth People’s Hospital.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2022.965863/full#supplementary-material
References
1. Iacusso C, Iacobelli BD, Morini F, Totonelli G, Viggiano M, Caforio L, et al. Assisted reproductive technology and anorectal malformation: A single-center experience. Front Pediatr (2021) 9:705385. doi: 10.3389/fped.2021.705385
2. Lv H, Diao F, Du J, Chen T, Meng Q, Ling X, et al. Assisted reproductive technology and birth defects in a Chinese birth cohort study. Lancet Reg Heal West Pacific (2021) 7:100090. doi: 10.1016/j.lanwpc.2020.100090
3. Reefhuis J, Honein MA, Schieve LA, Correa A, Hobbs CA, Rasmussen SA. Assisted reproductive technology and major structural birth defects in the united states. Hum Reprod (2009) 24:360–6. doi: 10.1093/humrep/den387
4. Galdini A, Fesslova VME, Gaeta G, Candiani M, Pozzoni M, Chiarello C, et al. Prevalence of congenital heart defects in pregnancies conceived by assisted reproductive technology: A cohort study. J Clin Med (2021) 10(22):5363. doi: 10.3390/jcm10225363
5. Pinborg A, Henningsen A-KA, Malchau SS, Loft A. Congenital anomalies after assisted reproductive technology. Fertil Steril (2013) 99:327–32. doi: 10.1016/j.fertnstert.2012.12.001
6. Hansen M, Kurinczuk JJ, Milne E, de Klerk N, Bower C. Assisted reproductive technology and birth defects: A systematic review and meta-analysis. Hum Reprod Update (2013) 19:330–53. doi: 10.1093/humupd/dmt006
7. Santos MA, Kuijk EW, Macklon NS. The impact of ovarian stimulation for IVF on the developing embryo. Reproduction (2010) 139:23–34. doi: 10.1530/REP-09-0187
8. Bergh T, Ericson A, Hillensjö T, Nygren KG, Wennerholm UB. Deliveries and children born after in-vitro fertilisation in Sweden 1982-95: A retrospective cohort study. Lancet (London England) (1999) 354:1579–85. doi: 10.1016/S0140-6736(99)04345-7
9. Boerrigter PJ, de Bie JJ, Mannaerts BMJL, van Leeuwen BP, Passier-Timmermans DPJ. Obstetrical and neonatal outcome after controlled ovarian stimulation for IVF using the GnRH antagonist ganirelix. Hum Reprod (2002) 17:2027–34. doi: 10.1093/humrep/17.8.2027
10. Gonen Y, Balakier H, Powell W, Casper RF. Use of gonadotropin-releasing hormone agonist to trigger follicular maturation for in vitro fertilization. J Clin Endocrinol Metab (1990) 71:918–22. doi: 10.1210/jcem-71-4-918
11. Richter TA, Robinson JE, Evans NP. Progesterone blocks the estradiol-stimulated luteinizing hormone surge by disrupting activation in response to a stimulatory estradiol signal in the ewe. Biol Reprod (2002) 67:119–25. doi: 10.1095/biolreprod67.1.119
12. Kuang Y, Chen Q, Fu Y, Wang Y, Hong Q, Lyu Q, et al. Medroxyprogesterone acetate is an effective oral alternative for preventing premature luteinizing hormone surges in women undergoing controlled ovarian hyperstimulation for in vitro fertilization. Fertil Steril (2015) 104:62–70.e3. doi: 10.1016/j.fertnstert.2015.03.022
13. Zhu X, Zhang X, Fu Y. Utrogestan as an effective oral alternative for preventing premature luteinizing hormone surges in women undergoing controlled ovarian hyperstimulation for in vitro fertilization. Med (Baltimore) (2015) 94:e909. doi: 10.1097/MD.0000000000000909
14. Wang N, Lin J, Zhu Q, Fan Y, Wang Y, Fu Y, et al. Comparison of neonatal outcomes and live-birth defects after progestin-primed ovarian stimulation versus conventional ovarian stimulation for in vitro fertilization: A large retrospective cohort study. Med (Baltimore) (2018) 97:e11906. doi: 10.1097/MD.0000000000011906
15. Huang J, Xie Q, Lin J, Lu X, Wang N, Gao H, et al. Neonatal outcomes and congenital malformations in children born after dydrogesterone application in progestin-primed ovarian stimulation protocol for IVF: a retrospective cohort study. Drug Des Devel Ther (2019) 13:2553–63. doi: 10.2147/DDDT.S210228
16. Zhang J, Mao X, Wang Y, Chen Q, Lu X, Hong Q, et al. Neonatal outcomes and congenital malformations in children born after human menopausal gonadotropin and medroxyprogesterone acetate treatment cycles. Arch Gynecol Obstet (2017) 296:1207–17. doi: 10.1007/s00404-017-4537-z
17. Zhu X, Ye H, Fu Y. Comparison of neonatal outcomes following progesterone use during ovarian stimulation with frozen-thawed embryo transfer. Sci Rep (2017) 7:7835. doi: 10.1038/s41598-017-08472-2
18. Zolfaroli I, Ferriol GA, Mora J-JH, Cano A. Impact of progestin ovarian stimulation on newborn outcomes: a meta-analysis. J Assist Reprod Genet (2020) 37:1203–12. doi: 10.1007/s10815-020-01755-0
19. Bonduelle M, Oberyé J, Mannaerts B, Devroey P. Large Prospective, pregnancy and infant follow-up trial assures the health of 1000 fetuses conceived after treatment with the GnRH antagonist ganirelix during controlled ovarian stimulation. Hum Reprod (2010) 25:1433–40. doi: 10.1093/humrep/deq072
20. Al-Inany HG, Youssef MA, Aboulghar M, Broekmans F, Sterrenburg M, Smit J, et al. Gonadotrophin-releasing hormone antagonists for assisted reproductive technology. Obstet Gynecol (2011) 118:706–7. doi: 10.1097/AOG.0b013e31822bbbb2
21. Harris BS, Bishop KC, Kemeny HR, Walker JS, Rhee E, Kuller JA. Risk factors for birth defects. Obstet Gynecol Surv (2017) 72:123–35. doi: 10.1097/OGX.0000000000000405
22. Kamath MS, Antonisamy B, Selliah HY, La Marca A, Sunkara SK. Perinatal outcomes following IVF with use of donor versus partner sperm. Reprod BioMed Online (2018) 36:705–10. doi: 10.1016/j.rbmo.2018.03.016
23. Cummins JM, Breen TM, Harrison KL, Shaw JM, Wilson LM, Hennessey JF. A formula for scoring human embryo growth rates in in vitro fertilization: Its value in predicting pregnancy and in comparison with visual estimates of embryo quality. J In Vitro Fert Embryo Transf (1986) 3:284–95. doi: 10.1007/BF01133388
24. Li D, Khor S, Huang J, Chen Q, Lyu Q, Cai R, et al. Frozen embryo transfer in mildly stimulated cycle with letrozole compared to natural cycle in ovulatory women: A Large retrospective study. Front Endocrinol (Lausanne) (2021) 12:677689. doi: 10.3389/fendo.2021.677689
25. Zegers-Hochschild F, Adamson GD, de Mouzon J, Ishihara O, Mansour R, Nygren K, et al. International committee for monitoring assisted reproductive technology (ICMART) and the world health organization (WHO) revised glossary of ART terminology, 2009. Fertil Steril (2009) 92:1520–4. doi: 10.1016/j.fertnstert.2009.09.009
26. Wu J, Li D, Liu X, Li Q, He X, Wei J, et al. IDDB: a comprehensive resource featuring genes, variants and characteristics associated with infertility. Nucleic Acids Res (2021) 49:D1218–24. doi: 10.1093/nar/gkaa753
27. Fukui Y, Fukushima M, Terawaki Y, Ono H. Effect of gonadotropins, steroids and culture media on bovine oocyte maturation in vitro. Theriogenology (1982) 18:161–75. doi: 10.1016/0093-691x(82)90100-5
28. Silva CC, Knight PG. Effects of androgens, progesterone and their antagonists on the developmental competence of in vitro matured bovine oocytes. J Reprod Fertil (2000) 119:261–9. doi: 10.1530/reprod/119.2.261
29. Bezerra FTG, Paulino LRFM, Silva BR, Silva AWB, Souza Batista ALP, Silva JRV. Effects of epidermal growth factor and progesterone on oocyte meiotic resumption and the expression of maturation-related transcripts during prematuration of oocytes from small and medium-sized bovine antral follicles. Reprod Fertil Dev (2020) 32:1190–9. doi: 10.1071/RD20099
30. Borman SM, Chaffin CL, Schwinof KM, Stouffer RL, Zelinski-Wooten MB. Progesterone promotes oocyte maturation, but not ovulation, in nonhuman primate follicles without a gonadotropin surge. Biol Reprod (2004) 71:366–73. doi: 10.1095/biolreprod.103.023390
31. Carter F, Rings F, Mamo S, Holker M, Kuzmany A, Besenfelder U, et al. Effect of elevated circulating progesterone concentration on bovine blastocyst development and global transcriptome following endoscopic transfer of in vitro produced embryos to the bovine oviduct. Biol Reprod (2010) 83:707–19. doi: 10.1095/biolreprod.109.082354
32. Bu Z, Zhao F, Wang K, Guo Y, Su Y, Zhai J, et al. Serum progesterone elevation adversely affects cumulative live birth rate in different ovarian responders during in vitro fertilization and embryo transfer: A large retrospective study. PloS One (2014) 9:e100011. doi: 10.1371/journal.pone.0100011
33. Xu B, Li Z, Zhang H, Jin L, Li Y, Ai J, et al. Serum progesterone level effects on the outcome of in vitro fertilization in patients with different ovarian response: An analysis of more than 10,000 cycles. Fertil Steril (2012) 97:1321–4. doi: 10.1016/j.fertnstert.2012.03.014
34. Kuang Y, Hong Q, Chen Q, Lyu Q, Ai A, Fu Y, et al. Luteal-phase ovarian stimulation is feasible for producing competent oocytes in women undergoing in vitro fertilization/intracytoplasmic sperm injection treatment, with optimal pregnancy outcomes in frozen-thawed embryo transfer cycles. Fertil Steril (2014) 101:105–11. doi: 10.1016/j.fertnstert.2013.09.007
35. Xi Q, Tao Y, Qiu M, Wang Y, Kuang Y. Comparison between PPOS and GnRHa-long protocol in clinical outcome with the first IVF/ICSI cycle: A randomized clinical trial. Clin Epidemiol (2020) 12:261–72. doi: 10.2147/CLEP.S226414
36. Guan S, Feng Y, Huang Y, Huang J. Progestin-primed ovarian stimulation protocol for patients in assisted reproductive technology: A meta-analysis of randomized controlled trials. Front Endocrinol (Lausanne) (2021) 12:702558. doi: 10.3389/fendo.2021.702558
37. Chen H, Wang Y, Lyu Q, Ai A, Fu Y, Tian H, et al. Comparison of live-birth defects after luteal-phase ovarian stimulation vs. conventional ovarian stimulation for in vitro fertilization and vitrified embryo transfer cycles. Fertil Steril (2015) 103:1194–1201.e2. doi: 10.1016/j.fertnstert.2015.02.020
38. Carmichael SL, Shaw GM, Laurent C, Croughan MS, Olney RS, Lammer EJ. Maternal progestin intake and risk of hypospadias. Arch Pediatr Adolesc Med (2005) 159:957–62. doi: 10.1001/archpedi.159.10.957
39. Zhang L, Chen J, Wang Y, Ren F, Yu W, Cheng L. Pregnancy outcome after levonorgestrel-only emergency contraception failure: A prospective cohort study. Hum Reprod (2009) 24:1605–11. doi: 10.1093/humrep/dep076
Keywords: progestin-primed ovarian stimulation, in vitro fertilization, live birth, congenital malformation, neonatal outcome
Citation: Li D, Hu Z, Chen Q, Chai W, Cai R, Kuang Y and Lu X (2022) Neonatal outcomes and congenital malformations in children born after progestin-primed ovarian stimulation protocol. Front. Endocrinol. 13:965863. doi: 10.3389/fendo.2022.965863
Received: 10 June 2022; Accepted: 20 October 2022;
Published: 09 November 2022.
Edited by:
Eytan R. Barnea, BioIncept, United StatesReviewed by:
Simonetta Costa, Agostino Gemelli University Polyclinic (IRCCS), ItalyLaura Melado, ART Fertility Clinics LLC, United Arab Emirates
Copyright © 2022 Li, Hu, Chen, Chai, Cai, Kuang and Lu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xuefeng Lu, eHVlZmVuZ2x1MTYzQDE2My5jb20=; Yanping Kuang, a3Vhbmd5YW5wQDEyNi5jb20=; Renfei Cai, Y2FpcmVuZmVpMDcwQHNpbmEuY29t
 Danjun Li
Danjun Li Zhijie Hu
Zhijie Hu Qiuju Chen
Qiuju Chen Weiran Chai
Weiran Chai Renfei Cai
Renfei Cai Yanping Kuang
Yanping Kuang Xuefeng Lu
Xuefeng Lu