
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Endocrinol., 09 August 2022
Sec. Endocrinology of Aging
Volume 13 - 2022 | https://doi.org/10.3389/fendo.2022.964069
This article is part of the Research TopicOvarian Ageing: Pathophysiology and Recent Development of Maintaining Ovarian Reserve: Volume IIView all 7 articles
 Baoyu Fu1†
Baoyu Fu1† Rui Ma1*†
Rui Ma1*† Fangbing Liu2
Fangbing Liu2 Xuenan Chen1
Xuenan Chen1 Xiaoyu Teng3
Xiaoyu Teng3 Pengdi Yang3
Pengdi Yang3 Jianzeng Liu2
Jianzeng Liu2 Daqing Zhao2,4
Daqing Zhao2,4 Liwei Sun1,4*
Liwei Sun1,4*Aging ovaries caused diminished fertility and depleted steroid hormone level. Ginsenosides, the active ingredient in ginseng, had estrogen-like hormonal effects. Although ginsenosides were well known for their ability to alleviate many age-related degenerative diseases, the effect of ginsenosides on the decline in reproductive capability caused by aging, as well as the mechanism, are unknown. We found that ginsenosides improved the quantity and quality of the offspring, prolonged life and restored muscle ability in aged female Drosophila. In addition, ginsenosides inhibited ovarian atrophy and maintained steroid hormone 20-Hydroxyecdysone (20E) and juvenile-preserving hormone (JH)) levels. Ginsenosides activated ecdysteroid receptor (ECR) and increased the expression of the early transcription genes E74 and Broad (Br), which triggered steroid signaling pathway. Meanwhile, ginsenosides promoted JH biosynthesis by increasing the expression of Hydroxyl-methylglutaryl-CoA reductase (HMGR) and juvenile hormone acid O-methyltransferase (JHAMT). Subsequently, JH was bound to Methoprene Tolerant (Met) and activated the transcription of the responsive gene Kruppel Homolog 1 (Kr-h1), which coordinated with 20E signaling to promote the reproduction of aged female Drosophila. The reproductive capacity and steroid hormone levels were not improved and the steroid signaling pathway was not activated in ginsenoside-treated ECR knockout Drosophila. This suggested that ginsenosides played a role dependent on targeted ECR. Furthermore, 17 kinds of ginsenoside monomers were identified from the total ginsenosides. Among them, Rg1, Re and Rb1 improved the reproductive capacity and steroid hormone levels of aged female Drosophila, which has similar effects to the total ginsenoside. These results indicated that ginsenosides could enhance the reproductive capacity of aged female Drosophila by activating steroid signals dependent on nuclear receptor ECR. In addition, ginsenoside monomers Rg1, Rb1 and Re are the main active components of total ginsenosides to improve reproductive ability. This will provide strong evidence that ginsenosides had the potential to alleviate age-induced reproductive degradation.
1. Ginsenosides enhanced the reproductive capacity of aged female Drosophila.
2. Ginsenosides repaired ovarian size and restored steroid hormone secretion.
3. Ginsenosides activated steroid signals dependent on nuclear receptor ECR.
4. Ginsenosides play an estrogen-like role to alleviate age-induced ovarian dysfunction.
5. Rg1, Rb1 and Re might play a major contribution to promoting reproduction.
During the past 150 years, the average lifespan of human beings has increased from 45 to 85 years, while the reproductive lifespan of women has remained constant at 50-52 years (1). The social phenomenon of late marriage and late childbearing of women has been formed with the extension of human life span and the change of life style. Therefore, it is urgent to prolong reproductive life and improve fertility. The ovaries, known as the pacemakers of reproductive aging, are the endocrine organs that control female reproduction. According to the report, women typically begin to lose ovarian function at the age of 35, manifested by reduced reproductive function and steroid deficiency, which negatively affects quality of life (2, 3). At present, most clinical treatments are based on exogenous hormone therapy, which can alleviate reproductive decline. However, when such treatments are administered long-term, they can trigger side effects such as hypertension and edema, while also increasing risks of incidence of gynecologic tumors associated with diseases such as breast and endometrial cancers (4–6). Therefore, a hormone substitute for estrogen is urgently needed that can preserve reproductive capacity without adverse side-effects.
Ginseng (Panax ginseng C. A. Meyer) is a traditional herb, which has been widely used to address aging, healthy life expectancy and ovarian dysfunction (7, 8). Ginsenosides is a steroid compound, regarded as the active ingredient with high content in ginseng, which has a variety of pharmacological activities such as relieving body fatigue, enhancing immunity, improving sleep and protecting nerves (9). Multiple pharmacological researches have confirmed that ginsenosides can enhance D-galactose-induced estrous cycle in ovarian senescent mice and help repair oxidative stress-induced damage to ovarian reproductive function (10). Ginsenosides is able to accelerate sexual maturation and promote ovulation in dinbutyl phthalate-induced reproductive impairment in mice (11). It also enhances egg production in cholesterol-deprived Caenorhabditis elegans (12). Ginsenosides can increase the steroid hormone output of adrenocortical cells in Y1 mice to help alleviate ovarian endocrine disorders (13). Ginsenoside Rg1 has been found to have estrogen-like effects in vivo by binding to estrogen receptors (14, 15). All of this implied that ginsenosides may play an estrogen-like role in improving ovarian aging due to their similar structure to steroids.
In old age, estrogen production decreases with loss of ovarian function, which leads to age-related diseases such as heart disease, osteoporosis, and Alzheimer’s disease (16, 17). Estrogen is a steroid hormone that promotes the development of the reproductive system and enhances sexual behavior. Studies had shown that steroid hormone levels in older women were restored to repair damaged ovarian function (18). The steroid hormone most similar to estrogen in Drosophila is 20-Hydroxyecdysone (20E) (19). It had been reported that 20E binds to its receptor ecdysteroid receptor (ECR) to induce expression of E74 and Broad (Br) transcription factors that regulate female accessory gland development and ovum formation (7, 20). Meanwhile, 20E indirectly influences juvenile-preserving hormone (JH) synthesis that controls sexual development, induces sexual priming, and promotes oocyte maturation (21, 22). However, there were no reports on the regulation of fertility by ginsenosides through steroidal signaling.
In recent years, Drosophila has served as a classic in vivo model for use in studying aging and reproduction (23), since Drosophila reproductive biology resembles that of mammals (24, 25). As compared to other animal models, Drosophila has a simple ovarian structure, short reproductive cycle, and easy gene editing (26). In this study, we investigated effects of ginsenosides on reproductive capacity, life energy and steroid hormone levels in natural aging female Drosophila. In addition, the regulation of steroidal signals on reproductive ability was discussed. The main contributing components of ginseng with improved reproductive ability were further identified and screened. This is of great significance for improving female reproductive ability and prolonging reproductive life.
Ginsenosides from Panax ginseng roots, ginsenoside Rg1, Re, Rf, Rg2, Rh1, Rb1, Rc, F1, Rb2, Rb3, Rd, F2, Rk3, Rg3, PPT, CK, Rh2(s), and PPD, each of > 98% purity, were purchased from Shanghai Yuanye Bio-Technology Co., Ltd. (Shanghai, China).
Drosophila were obtained from the Drosophila research repository of the Experimental Center of the Affiliated Hospital of Changchun University of Chinese Medicine. ECR knock-down mutant Drosophila were obtained from the Core Facility of Drosophila Resource and Technology, CEMCS, CAS. All the study protocols were approved by the Ethics Committee of Changchun University of Chinese Medicine of TCM (No: 2021349) and conducted according to the guidelines of the Ethics Committee of Changchun University of Chinese Medicine of TCM. Drosophila were reared on standard cornmeal-sugar-yeast agar medium that contained 8% sugar (Tianjin Xinbote, China), 0.5% Oxoid LP0021 yeast extract (Oxoid, United Kingdom), 0.5% propionic acid (Sinopharm., China), and 2% agar (Biofroxx, Germany). Ginsenosides were added in medium to final concentrations of 0.25, 0.5 and 1 mg/mL for further experiments. All Drosophila were maintained and reared under conditions of 25°C with 60% humidity and a 12-h light/12-h dark cycle.
The virgin female Drosophila aged 7 and 35 days were collected under CO2 anesthesia and assigned to randomly assigned to five subgroups that included the control group (7 days), model group (35 days), and three ginsenosides treated groups of 35-day-old female Drosophila. Female Drosophila in the untreated control group and untreated model group consumed standard medium, while 35-day-old female Drosophila in the other three groups received medium containing ginsenosides at concentrations of 0.25, 0.5 and 1 mg/mL. After 7 days, all groups were transferred to tubes containing standard medium. Next, a total of 100 mated female Drosophila in one tube each were transferred to fresh tubes containing standard medium, where they remained for 48 h while they laid eggs. Number of eggs, number of pupae, and pupation rate for each tube of female Drosophila were recorded and analyzed to determine effects of ginsenosides on female Drosophila development (27).
Female Drosophila (35-day-old) were divided into four groups (100 flies/group): control group (35 days) female Drosophila were fed standard medium, while the other three groups (35 days female Drosophila) received medium containing ginsenosides concentrations of 0.25, 0.5 and 1 mg/mL, respectively. Every three days, female Drosophila in all groups were transferred to tubes containing fresh culture medium then mortality rates were recorded. Survivorships were scored regularly and eliminated the unnatural death flies, and death flies were removed out of the cage when possible. Lifespan curves, mean lifespan (average value of all values), and maximum lifespan (average values of the last 20 dead flies) were calculated for each group of female Drosophila (28).
Female Drosophila aged 7 days and 35 days were collected under CO2 anesthesia and assigned to randomly assigned to five subgroups: a 7-day-old untreated control group, a 35-day-old untreated model group, and three 35-day-old Drosophila groups treated with different concentrations of ginsenosides (0.25, 0.5, and 1 mg/mL). Drosophila in the untreated control group (7 days) and the untreated model group (35 days) ate standard food, while Drosophila in the other three groups ate food containing ginsenosides. Prior to climbing experiments, female Drosophila received food with or without ginsenosides for 7 days. Exercise ability was evaluated using a negative axis test with maximum crawl path restricted to 12 cm in length. After a 10-min acclimation period in the test tube, female Drosophila were knocked to the bottom of the tube every 1 min then this procedure was repeated 5 times followed by counting of crawling heights of female Drosophila, with assays repeated at least 3 times (29).
To explore the effect of ginsenosides on ovarian morphology, ovaries were dissected in anatomical buffer at room temperature. Briefly, 5-10 adult flies per group were anesthetized with CO2, and ovaries were isolated with the aid of forceps. The length and diameter of individual ovaries were measured under a stereomicroscope (SMZ1000 with Digital Sight DS-U3, Nikon, Japan).
After female Drosophila were pretreated with ginsenosides for 7 days, female Drosophila ovaries were collected under CO2 anesthesia, and 100 ul of RIPA lysate per 10 ovaries was added for tissue crushing. After crushing, the supernatant was centrifuged at 12,000 rpm for 5 minutes, and the supernatant was taken out for subsequent experiments. Contents of 20E and JH were measured using enzyme-linked immunosorbent assays (Sino Best Biological Technology Co., Ltd., Shanghai, China) based on methods as described by the manufacturer.
The female Drosophila ovaries pretreated with ginsenosides for 7 days were weighed and frozen in liquid nitrogen. Total RNA was extracted using a Magnetic Tissue/Cell/Blood Total RNA Kit (Invitrogen, Carlsbad, CA, USA). Next, 1 µg of RNA was reverse transcribed into cDNA using the script cDNA synthesis kit (Bio-Rad, CA, United States). Subsequently, RT-qPCR was performed in a Bio-Rad CFX96 system using the SYBR Premix Ex Taq Kit (Takara Biomedical Technology Co., Ltd, Beijing, China). Set as 95°C for 5 min, 95°C for 15 s, 60°C for 30 s, and 72°C for 30 s for 40 cycles. Steroid signal pathway genes were selected, with the β-actin gene used as the internal control. Primer sequences are shown in Table 1. Comparisons among groups were made based on gene expression levels calculated using the threshold cycle (Ct) method. Gene expression levels in all groups were expressed as ratios of values for each group to corresponding values obtained for the control group (30).
Total protein was extracted from ovaries with lysis buffer (Beyotime, Haimen, China) and Bullet Blender (NY, USA). Protein concentration was determined using bicinchoninic acid (BCA) protein assay reagent. Glyceraldehyde 3-phosphate dehydrogenase (GAPDH, BM1623; Boster, Beijing, China) was used as the loading control. Samples were separated by 12% SDS-PAGE (Bio-Rad, Hercules, CA, USA) and transferred to polyvinylidene difluoride membranes (Millipore, USA). After blocking in 5% non-fat milk, membranes were immunoblotted with the following antibodies: ECR, Br (both from Developmental Studies Hybridoma Bank, USA). Kr-h1 (Santa Cruz Biotechnology, Santa Cruz, USA). Next, the membranes were incubated with appropriate secondary antibodies (Li-Cor, Lincoln, NE, USA) for 1 h at room temperature (31). Finally, chemiluminescence-based visualization and analysis of protein bands were conducted using a FluorChem imaging system (ProteinSimple, San Jose, CA, USA).
Ginsenosides composition was analyzed and quantified via HPLC analysis using a LC-2030C Plus system equipped with a C18 column (5 µm, 4.6 × 250 mm; Kromasil, Sweden), with ultra-violet (UV) detection conducted at 203 nm. A stepwise gradient elution method was employed using solvent A (water) and solvent B (acetonitrile) that were maintained at 30°C. The flow rate was 1 mL/min and sample injection volume was 10 µL (32, 33).
All test data were expressed as means ± standard error of mean (SEM) based on data obtained for three replicate samples. Using D’Agostino-Pearson omnibus K2 testing, and Shapiro-Wilk-test to assess normality, and data are in accordance with normal distribution. After checking the normality and homogeneity, all the data with normal distribution and homogenous variances were analyzed by One-way ANOVA. Statistical analysis was performed using GraphPad Prism 6 (Version No. 6, GraphPad Software, La Jolla, CA, USA). Statistical significance was established using one-way ANOVA followed by Dunnett’s t-test except for survivorship results. Survivorship values among groups were compared and tested for significance using a log rank test. Statistical significance was set to ∗ p < 0.05, ∗∗ p < 0.01, and ∗∗∗ p < 0.001.
Ovarian function declines as a woman ages, resulting in reduced reproductive capacity. In order to determine ginsenosides effects on reproductive capacity of aged female Drosophila, 35-day-old female Drosophila were fed ginsenosides at different concentrations. For model group (untreated 35-day-old female Drosophila), numbers of eggs, pupae, and pupation rates were significantly lower than corresponding results obtained for control group (7-day-old untreated female Drosophila) (Figure 1). In contrast, the numbers of eggs of older female Drosophila fed with 0.25 mg/mL, 0.5 mg/mL and 1 mg/mL ginsenosides were increased significantly, and 1 mg/mL ginsenosides increased the number of eggs 1.7-fold. The number and rate of pupae of older female Drosophila were increased with the ginsenoside concentration, with the highest concentration increasing by 2-fold and 1.2-fold. (Figures 1A–C). These results suggested that ginsenosides could promote increased reproductive capacity when fed to elderly female Drosophila.
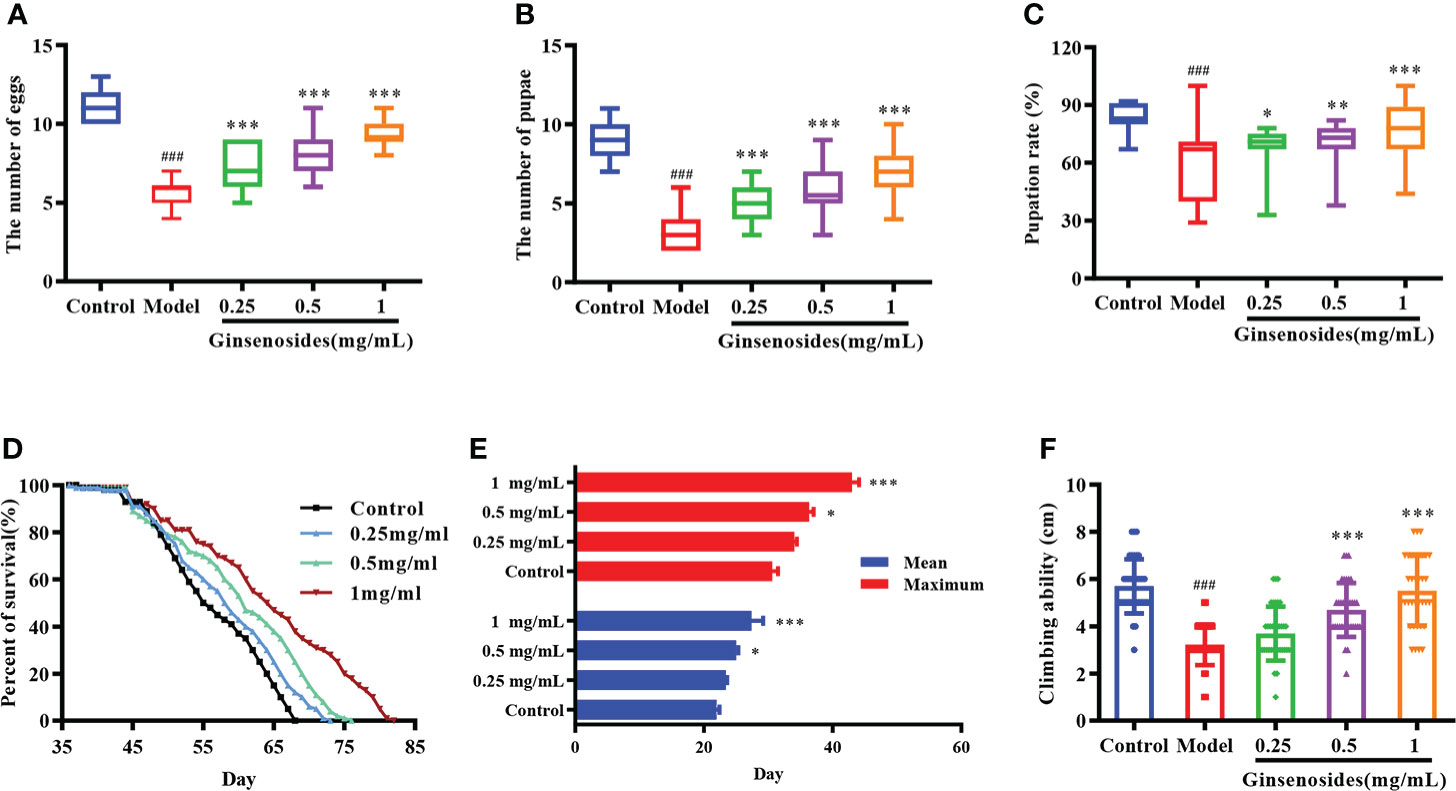
Figure 1 Effects of ginsenosides on Drosophila reproduction and aging. (A) The number of eggs; (B) The number of pupae; (C) Pupation rate; (D) Percent of survival; (E) Mean and maximum lifespans; (F) Climbing ability. ###p<0.001 compared with control (7-day-old female Drosophila); *p<0.05, **p<0.01, ***p<0.001 compared with model (35-day-old female Drosophila).
The decline of ovarian function could accelerate aging, leading to a shortened life span and reduced body function. In order to observe the effects of ginsenosides on the life span of aged female Drosophila, 35-day-old female Drosophila were fed ginsenosides at different concentrations (0.25 mg/mL, 0.5 mg/mL, and 1 mg/mL). The life span of female Drosophila was extended with the increase ginsenosides concentration (Figure 1D). In addition, ginsenosides also extended the average and maximum life span of female Drosophila in a concentration dependent manner (Figure 1E). Climbing ability can be used to evaluate the aging level of Drosophila vitality. The climbing height of 35-day-old Drosophila was lower than that of 7-day-old Drosophila. Ginsenosides increased the climbing ability of aged female Drosophila. The climbing height of aged female Drosophila fed 1 mg/mL ginsenoside increased by 71% compared with that without ginsenosides (Figure 1F). Senile female Drosophila treated with ginsenosides alleviated aging and restored youthful vigor.
Ovarian size was dominated by age. The length and diameter of older ovaries were shortened and atrophied. Ginsenosides maintained the size (length and diameter) of the aging ovary to prevent the atrophy, with a dose-dependent pattern (Figures 2A–C). Ovarian atrophy leads to a decrease in steroid hormones secretion, reducing ovarian function. Levels of steroid hormones, including 20E and JH, were consumed with age. The levels of 20E and JH in aged female Drosophila treated with ginsenoside were significantly increased (Figures 2D, E). These results suggested that ginsenoside treatment delay ovarian dysfunction of aged female Drosophila.
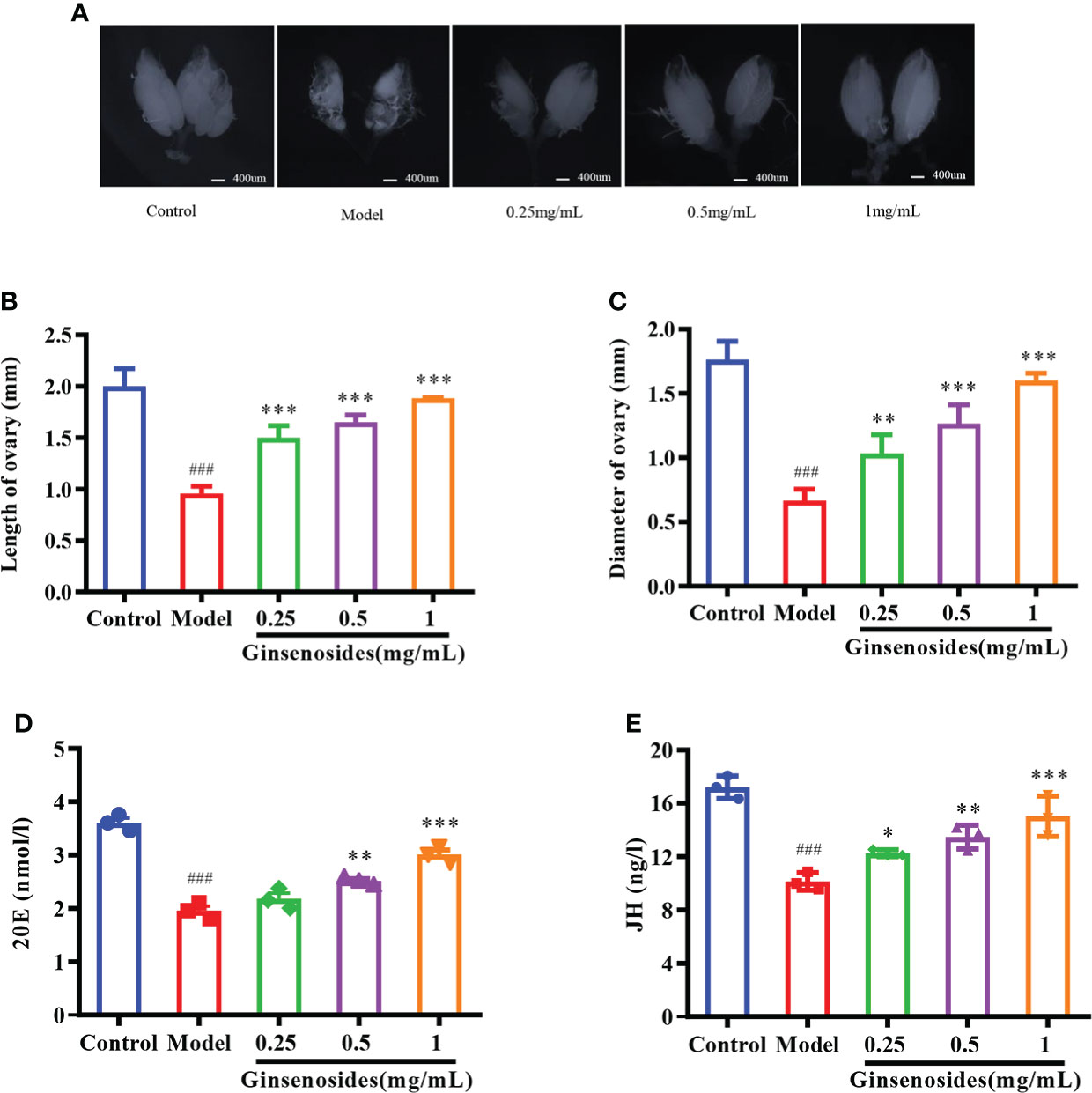
Figure 2 Effect of ginsenosides on ovarian function in Drosophila. (A) Ovary size; (B) Length of ovary; (C) Diameter of ovary; (D) 20E content; (E) JH content. *p<0.05, **p<0.01, ***p<0.001 compared with model (35-day-old female Drosophila); ###p<0.001 compared with control (7-day-old female Drosophila).
To determine whether ginsenosides enhanced reproductive capacity through triggering of steroid signals, we measured expression of genes related to steroid signaling pathways in aged female Drosophila. Expression levels of 20E receptor genes encoding proteins ECR, early transcription factor E74 and Br were reduced in model group as compared with levels in control group, but were all significantly improved with ginsenoside treatment (Figures 3A–C). In addition, 20E could promote the expression of juvenile hormone acid O-methyltransferase (JHAMT) and Hydroxyl-methylglutaryl-CoA reductase (HMGR) genes, thus promoting JH biosynthesis (34). The expression levels of JHAMT and HMGR in the model group were lower than those in the control group, and increased after ginsenosides treatment (Figures 3D, E). Meanwhile, expression levels of Kruppel Homolog 1 (Kr-h1) and Methoprene Tolerant (Met) increased in a dose-dependent manner with increasing ginsenosides concentration (Figures 3F, G). Again, we verified that the protein expression levels of 20E receptor protein ECR, early transcription factor Br and Kr-h1 protein were reduced in the model group compared with the control group, but all were significantly increased by ginsenosides treatment, in line with mRNA expression (Figure 3H). These results indicate that ginsenosides activated steroid signaling to promote the reproductive capacity of aged female Drosophila.
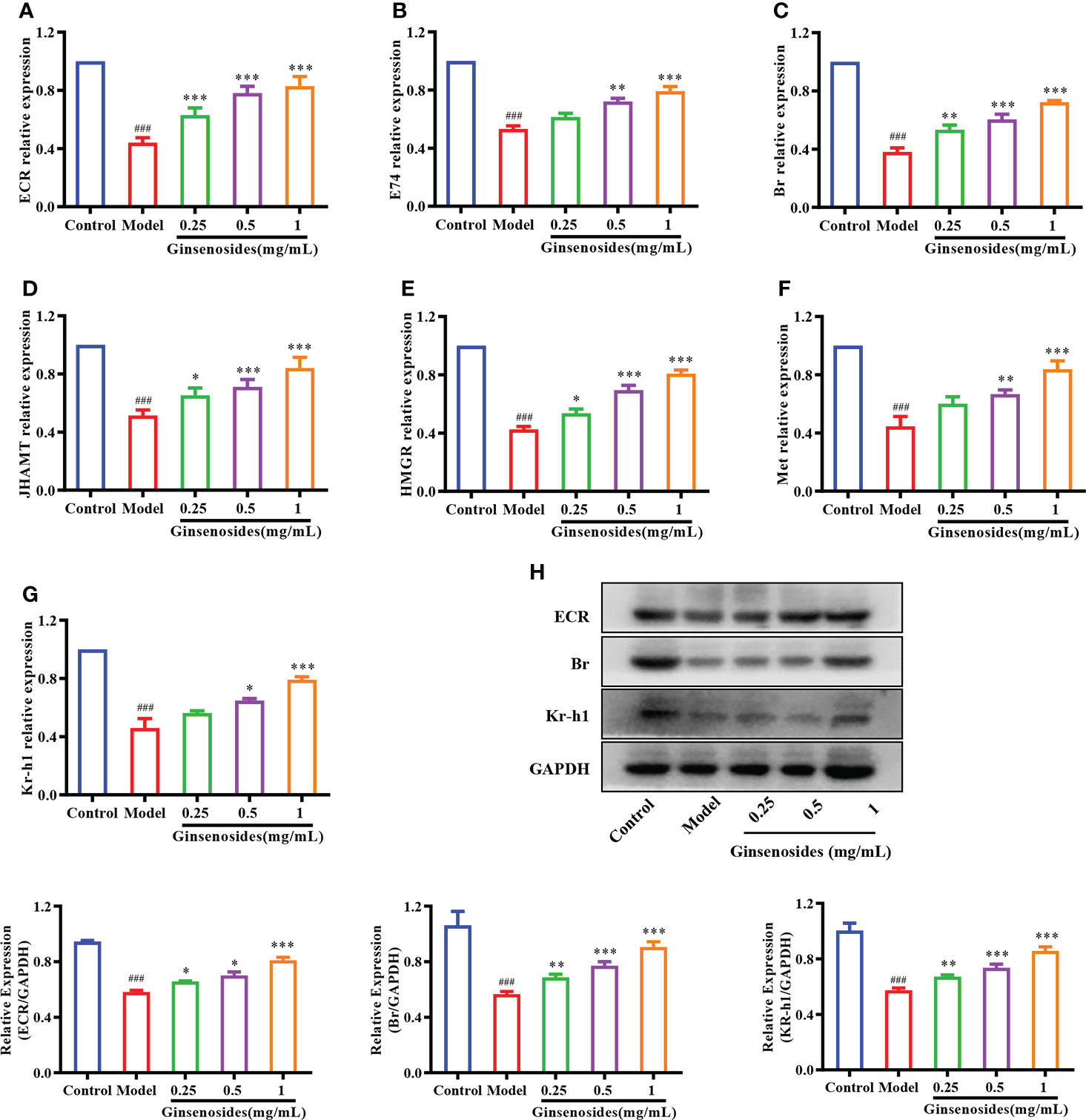
Figure 3 Effect of ginsenosides on expression of steroidal signal-related genes in Drosophila. (A) ECR; (B) E74; (C) Br; (D) JHAMT; (E) HMGR; (F) Met; (G) Kr-h1; (H) Relative expression levels of ECR, Br and Kr-h1; GAPDH antibody was used as loading control. ###p<0.001 compared with control (7-day-old female Drosophila); *p<0.05, **p<0.01, ***p<0.001 compared with model (35-day-old female Drosophila).
Ginsenosides activated steroid signaling was verified in ECR mutant female Drosophila. Older Drosophila had reduced reproductive capacity compared to younger Drosophila, and ginsenosides could increase the numbers of egg and pupal in older females. In addition, the reproductive ability of ECR mutant Drosophila was reduced compared to normal Drosophila and was not improved by feeding ginsenosides (Figures 4A, B). This indicates that ginsenosides depend on ECR to enhance the reproductive ability of older female Drosophila. Moreover, ginsenosides had no effect on the expression of steroidal signaling related genes E74 and Br in ECR knockdown mutant Drosophila (Figures 4C, D). Additionally, in the ECR knockout mutant Drosophila, ginsenosides exhibited no impact on the expression of the sterol signaling-related protein Br (Figure 4E). That is to say, ginsenosides were dependent on ECR to activate steroid signals and improve reproductive capacity.
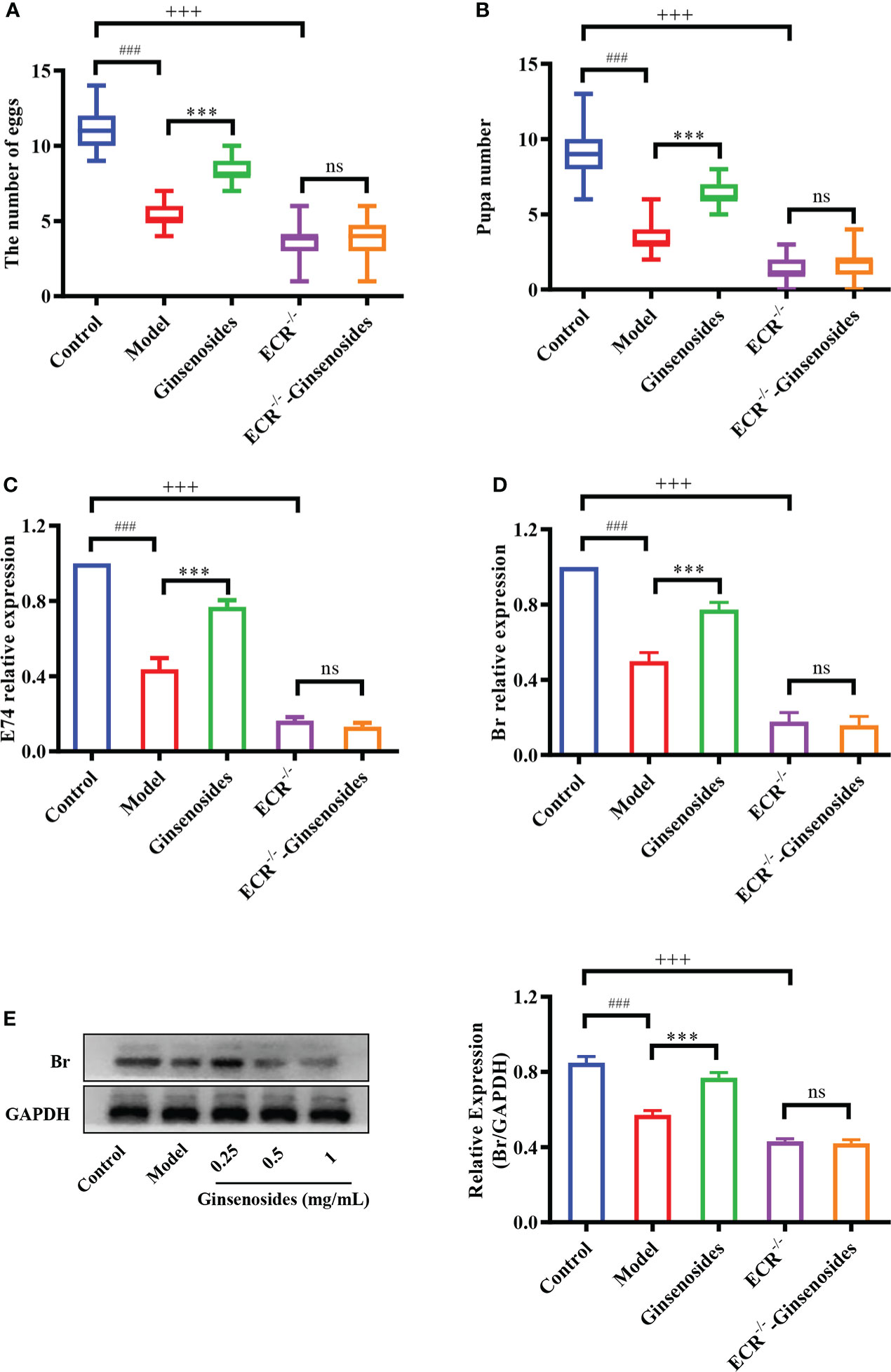
Figure 4 Effect of ginsenosides on the reproductive capacity of ECR mutant Drosophila. (A) The number of eggs; (B) The number of pupae; (C) Relative expression of gene encoding E74; (D) Relative expression of Br; (E) Relative expression levels of Br; GAPDH antibody was used as loading control. ###p<0.001 model compared with control (7-day-old female Drosophila); ***p<0.001 compared with model (35-day-old female Drosophila); +++p<0.001 ECR-/- compared with control (35-day-old female Drosophila); ns, no significance compared with ECR-/-.
To elucidate the components of the ginsenosides that might be responsible for improving reproductive effects, ginsenoside monomers were identified and quantified from the total ginsenosides by HPLC. As shown in Figure 5, ginsenoside monomers were included Rg1 (26.41%), Re (18.61%), Rg2 (12.54%), Rb3 (8.19%), Rb1 (6.10%), Rf (3.18%), Rh2S (1.84%), Rh1 (1.77%), PPT (1.48%), Rk3 (1.40%), Rd (0.71%), Rc (0.56%), F2 (0.39%), Rb2 (0.35%), CK (0.32%), F1 (0.29%), PPD (0.24%), and Rg3 (0.12%), with the concentration of 84.52%, according to the standard curve of the different ginsenosides. Subsequently, the effects of 17 ginsenoside monomers on reproductive capacity and steroid hormone levels of 35-day-old female Drosophila were analyzed. Rg1, Rb1 and Re were significantly increased the number of eggs by 1.6, 1.5 and 1.4 times, respectively. Meanwhile, Rg1, Rb1 and Re were also increased the number and pupation rate of pupae. The other 14 ginsenosides monomers had no significant effect (Figures 6A–C). In addition, Rg1, Rb1 and Re also promoted steroid hormone secretion, and the increase of steroid hormone content was more significant in older female Drosophila (Figures 6D, E). These results suggested that Rg1, Rb1 and Re might be the active components of total ginsenosides to improve the reproductive capacity of elderly female Drosophila.
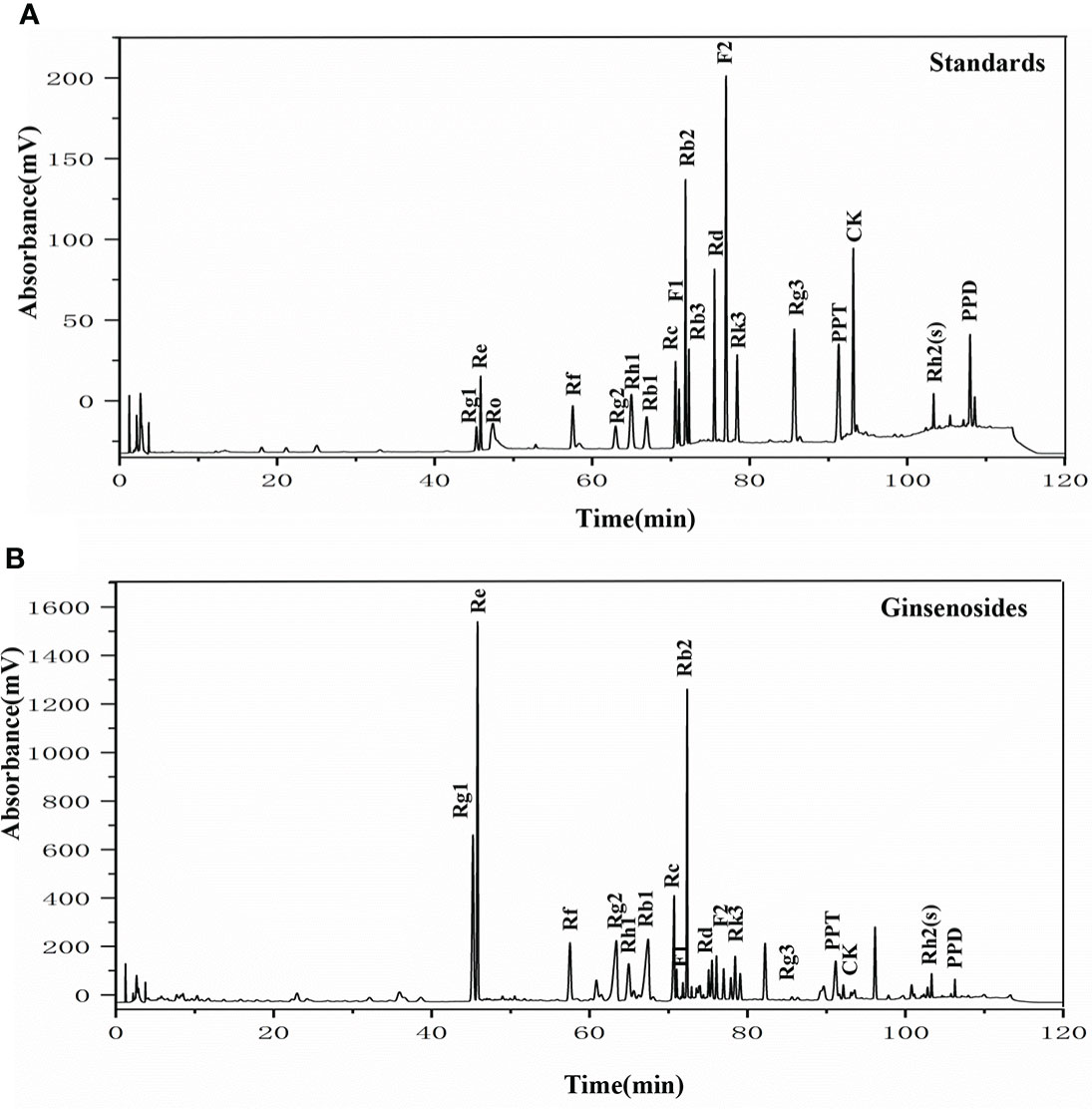
Figure 5 The HPLC chromatogram of ginsenosides. (A) HPLC analysis of the standard ginsenosides monomers including Rg1, Re, Ro, Rf, Rg2, Rh1, Rb1, Rc, F1, Rb2, Rb3, Rd, F2, Rk3, Rg3, PPT, CK, Rh2(s), and PPD; (B) HPLC chromatogram of ginsenosides test samples, with UV detection at 203 nm.
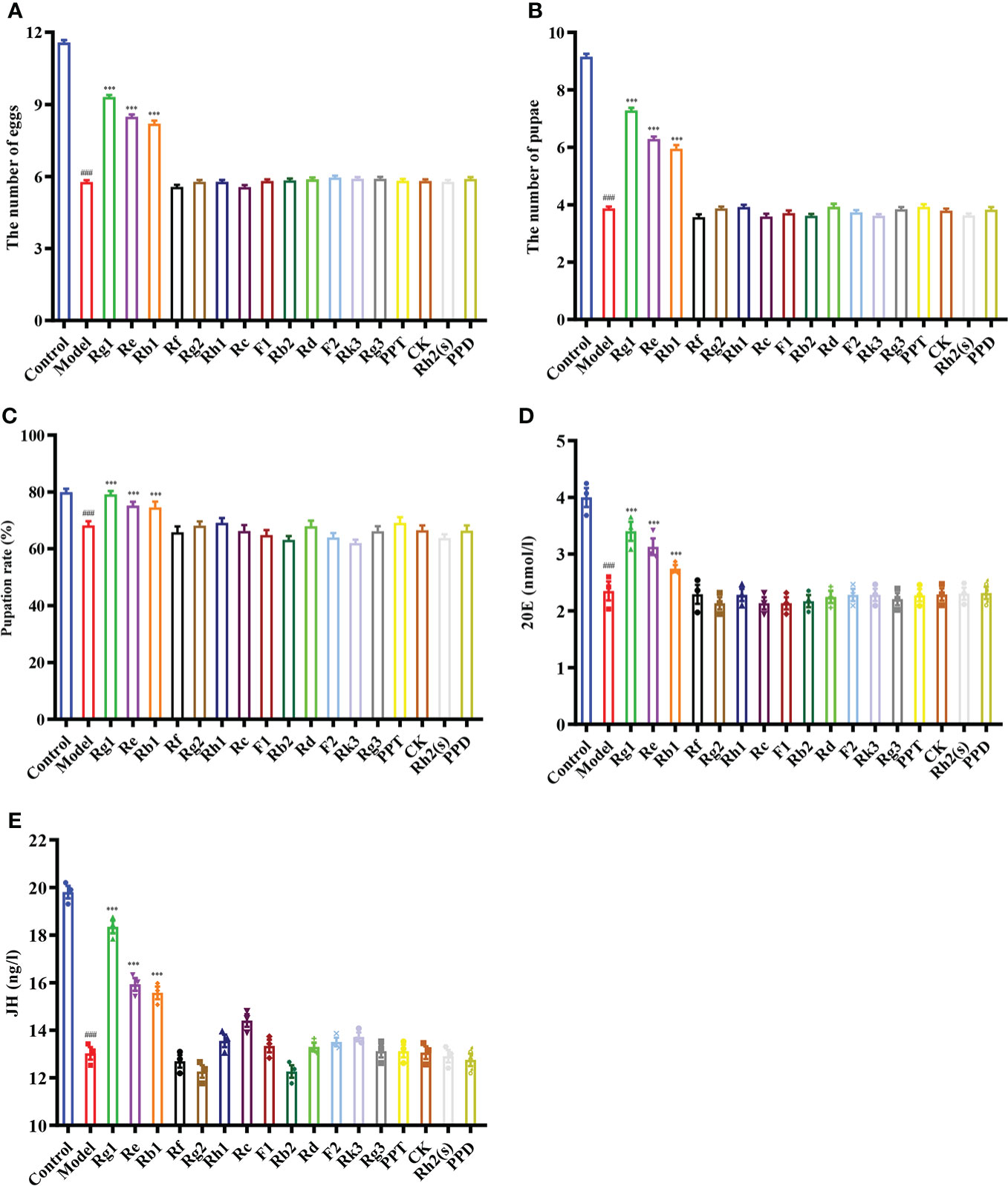
Figure 6 Effect of ginsenoside monomers on the reproductive ability and steroid hormone levels of Drosophila. (A) The number of eggs; (B) The number of pupae; (C) Pupation rate; (D) 20E content; (E) JH content; Rg1, 0.26 mg/mL; Re, 0.19 mg/mL; Rg2, 0.13 mg/mL; Rb1, 0.06 mg/mL; Rf, 0.03 mg/mL; Rh2(s), 0.02 mg/mL; Rh1, 0.02 mg/mL; PPT, 0.01 mg/mL; Rk3, 0.01 mg/mL; Rd, 0.007 mg/mL; Rc, 0.006 mg/mL; F2, 0.004 mg/mL; Rb2, 0.004 mg/mL; CK, 0.003 mg/mL; F1, 0.003 mg/mL; PPD, 0.002 mg/mL; Rg3, 0.001 mg/mL; according to the concentration ratio of ginsenosides at 1 mg/mL. ###p<0.001 compared with control (7-day-old female Drosophila); ***p<0.001 compared with model (35-day-old female Drosophila).
After the age of 30, the female enters the decline period of ovarian function, which is the fundamental cause of a series of aging phenomena. Delaying ovarian senescence can essentially solve age-related fertility disorders and prolong healthy life. Ginseng is viewed as a healthy energy tonic which can delay aging and prolong life (35). It has been shown that ginsenosides increased the viability of older female Drosophila (36). Furthermore, it has been reported that ginsenosides can prolong the estrus cycle of mature females and increase the egg-laying capacity of queen bees (37). Ginsenosides being added to the culture medium encouraged the growth of chicken ovarian germ cells in vitro (38). In vitro experiments showed that ginsenosides could enhance follicular cell development in mice (39). In our study, the reproductive ability of aged female Drosophila was significantly lower than that of young females. Ginsenosides could increase the number of eggs and pupae in aged females. It suggests that ginsenosides improved the quantity and quality of the offspring, prolonged life and restored muscle ability to the young state in aged female Drosophila, thus played the role of protecting the reproductive and delaying the aging.
Ovarian function deteriorates as women age, resulting in a lack of steroid hormones and infertility (40, 41). Ginsenosides can promote the release of steroid hormones to play an estrogen-like role (42). During the larval stage, 20E starts to induce larval pupation, while JH keeps Drosophila in the immature state by inhibiting the metamorphosis of 20E, thus ensuring that the metamorphosis time is reasonable. In the adult stage, the decrease of 20E or JH content in the ovary also reduces the reproductive capacity. 20E and JH promote reproduction through multiple pathways, such as oogenesis, oocyte formation and chorionic vitellogenesis (43, 44). The ovarian size was dominated by age. The length and diameter of older ovaries were shortened and atrophied. We found that ginsenosides maintained the size of the aging ovary to prevent atrophy, with a dose-dependent pattern. Ovarian atrophy leads to a decrease in steroid hormones secretion, reducing ovarian function. Levels of steroid hormones, including 20E and JH, were consumed with age. The levels of 20E and JH in aged female Drosophila treated with ginsenosides were significantly increased. Our results further demonstrate that ginsenosides treatment delay ovarian dysfunction of aged female Drosophila. 20E has been reported to affect gonadal development and oogenesis by regulating mitosis and meiosis (44). In adult Drosophila, 20E affects courtship behavior, yolk production and egg laying, reproductive lag, innate immunity, and resistance and longevity (34, 45). Several studies have shown that ginsenosides by binding the intracellular nuclear hormone receptors, such as the proliferator-activated receptor, androgen receptor (AR), estrogen receptor (ER), and progesterone receptor (PR), can activate the genomic pathway (38). 20E acts through a heterodimeric receptor consisting of ECR and ultraspiracle protein (USP), leading to transcriptional activation of early 20E-inducible genes, including the downstream genes E74 and Br (46). 20E is similar to human estrogen, which also indicates that ginsenosides have estrogen-like effects that bind to their receptors to perform their functions. In this study, ginsenosides promoted the expression of ECR and induced the transcription of downstream genes E74 and Br to activate 20E signaling. Moreover, 20E promoted JH biosynthesis by promoting the expression of HMGR and JHAMT, thus increasing the level of JH. Studies had shown that reproductive capacity could be maintained by regulating the expression of JHAMT and HMGR (47). JH could bind to Met receptor to form an active complex that promoted the transcription of response genes Kr-h1 and Br and facilitates oogenesis (48). We found that 20E promoted JH biosynthesis by enhancing HMGR and JHAMT expression. Ginsenosides enhanced the synergistic effect of JH signal and 20E signal to promote the reproductive capacity of aged female Drosophila. However, alterations in ECR function can affect the formation of mating memory and the selection of courtship partners in Drosophila (49). In our study, ginsenosides did not improve steroid signaling-related genes and reproductive capacity in female Drosophila with ECR knockdown. These results suggested that ginsenosides depended on the activation of nuclear receptor ECR to turn on steroid signaling pathway, thus improving the fertility of older female Drosophila (Figure 7).
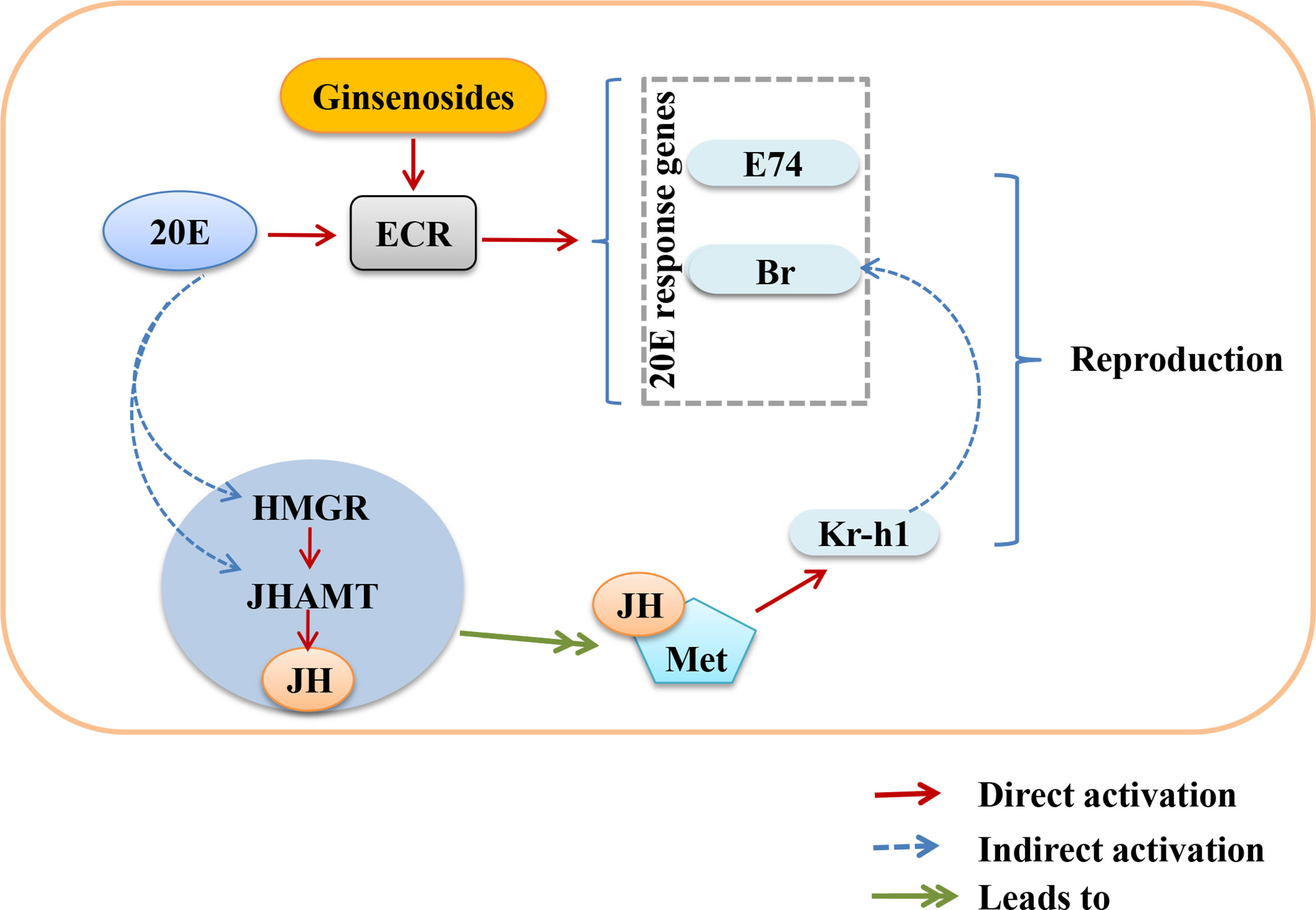
Figure 7 Overview showing the relationship between activation of nuclear receptor ECR by ginsenosides turns on steroid hormone signaling to improve reproductive capacity in aged female Drosophila.
There are about 40 ginsenoside monomers with definite structures, all of which contain sterane steroid nuclei arranged in four rings by 30 carbon atoms. The multiple pharmacological activities of various ginsenoside monomers have been reported, but there are relatively few studies on improving the fertility decline caused by aging. To excavate the ginsenoside monomers that might be responsible for improving reproductive effects, various monomers were identified and quantified by HPLC. It was found that Rg1, Re, and Rb1 exhibited similar effects as the total ginsenosides. Ginsenoside Rg1 has been shown to improve fertility and repair ovarian damage in a mouse with premature ovarian failure (11). Meanwhile, ginsenoside Rb1 has been shown to protect porcine oocytes from damage, thus improving parthenogenesis and embryo quality after in vitro fertilization (50). These studies are consistent with our conclusions. However, individual treatments with Rg1, Re, and Rb1 were less effective than that of ginsenosides. Therefore, it was speculated that there were synergistic and synergistic effects among various ginsenosides monomers, which should be the subject of further research.
In conclusion, ginsenosides depend on ECR to activate steroid signals to improve the reproductive capacity of aged female Drosophila, which play an estrogen-like role. It is noteworthy that this effect may be commonly performed by ginsenoside monomers such as Rg1, Re and Rb1. This study provides new insights for the further development of ginsenosides as active components for alleviating female reproductive aging. However, multiple ginsenoside monomers may exert synergistic effect through different drug therapy approaches. We will continue to investigate in depth the complex interactions between different ginsenosides naturally present in ginseng roots. This will provide an experimental basis for the development of natural drugs for the treatment and prevention of diseases related to reproductive aging to benefit more women with fertility needs.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The animal study was reviewed and approved by the Ethics Committee of Changchun University of Chinese Medicine of TCM (No: 2021349).
BF and RM designed the study and drafted, revised the manuscript, conducted experiments, extracted and analyzed the experimental data. BF, FL, and XC performed the experiments. XT, PY, and JL analyzed the data and performed the statistical analysis. DZ and LS evaluated the quality of the whole study and drafted the manuscript. RM and LS provided funding support. All authors contributed to the article and approved the submitted version.
This work was supported by the National Natural Science Foundation of China (No. U20A20402) and the Science and Technology Development of Jilin Province (No. 20210304002YY and YDZJ202201ZYTS167).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2022.964069/full#supplementary-material
1. Schwartz K, Martin C, Hipp H, Kawwass J. Pregnancy and fertility concerns: A survey of United States obstetrics and gynecology residents. Matern Child Hlth J (2021) 25(1):172–9. doi: 10.1007/s10995-020-03027-w
2. Carson S, Kallen A. Diagnosis and management of infertility: A review. JAMA (2021) 326(1):65–76. doi: 10.1001/jama.2021.4788
3. Li C, Lin L, Tsai H, Chern C, Wen Z, Wang P, et al. The molecular regulation in the pathophysiology in ovarian aging. Aging Dis (2021) 12(3):934–49. doi: 10.14336/ad.2020.1113
4. Zu Y, Yang J, Zhang C, Liu D. The pathological mechanisms of estrogen-induced cholestasis: Current perspectives. Front Pharmacol (2021) 12:761255. doi: 10.3389/fphar.2021.761255
5. Kumar G, Du B, Chen J. Effects and mechanisms of dietary bioactive compounds on breast cancer prevention. Pharmacol Res (2021) 178:105974. doi: 10.1016/j.phrs.2021.105974
6. Mastorakos G, Iatrakis G, Zervoudis S, Syropoulou S. Progestins and the risk of breast cancer. Acta Endocrinol-Buch (2021) 17(1):90–100. doi: 10.4183/aeb.2021.90
7. Ghamari K, Kashani L, Jafarinia M, Tadayon Najafabadi B, Shokraee K, Esalatmanesh S, et al. Vitamin e and ginseng supplementation to enhance female sexual function: A randomized, double-blind, placebo-controlled, clinical trial. Women Health (2020) 60(10):1164–73. doi: 10.1080/03630242.2020.1803465
8. Ma S, Ma R, Xia T, Afnan M, Song X, Xu F, et al. Efficacy and safety of ding-Kun-Dan for female infertility patients with predicted poor ovarian response undergoing in vitro fertilization/intracytoplasmic sperm injection: Study protocol for a randomized controlled trial. Trials (2018) 19(1):124. doi: 10.1186/s13063-018-2511-0
9. Lee JH, Choi SH, Kwon OS, Shin TJ, Lee JH, Lee BH, et al. Effects of ginsenosides, active ingredients of panax ginseng, on development, growth, and life span of caenorhabditis elegans. Biol Pharm Bull (2007) 30(11):2126–34. doi: 10.1248/bpb.30.2126
10. He L, Ling L, Wei T, Wang Y, Xiong Z. Ginsenoside Rg1 improves fertility and reduces ovarian pathological damages in premature ovarian failure model of mice. Exp Biol Med (2017) 242(7):683–91. doi: 10.1177/1535370217693323
11. Xu X, Qu Z, Qian H, Li Z, Sun X, Zhao X, et al. Ginsenoside Rg1 ameliorates reproductive function injury in C57BL/6J mice induced by di-n-butyl-phthalate. Environ Toxicol (2021) 36(5):789–99. doi: 10.1002/tox.23081
12. Lee JH, Ahn JY, Shin TJ, Choi SH, Lee BH, Hwang SH, et al. Effects of minor ginsenosides, ginsenoside metabolites, and ginsenoside epimers on the growth of caenorhabditis elegans. J Ginseng Res (2011) 35(3):375–83. doi: 10.5142/jgr.2011.35.3.375
13. Jin W, Ma R, Zhai L, Xu X, Lou T, Huang Q, et al. Ginsenoside Rd attenuates ACTH-induced corticosterone secretion by blocking the MC2R-cAMP/PKA/CREB pathway in Y1 mouse adrenocortical cells. Life Sci (2020) 245:117337. doi: 10.1016/j.lfs.2020.117337
14. Du J, Cheng B, Zhu X, Ling C. Ginsenoside Rg1, a novel glucocorticoid receptor agonist of plant origin, maintains glucocorticoid efficacy with reduced side effects. J Immunol (2011) 187(2):942–50. doi: 10.4049/jimmunol.1002579
15. Hao K, Gong P, Sun S, Hao H, Wang G, Dai Y, et al. Beneficial estrogen-like effects of ginsenoside Rb1, an active component of panax ginseng, on neural 5-HT disposition and behavioral tasks in ovariectomized mice. Eur J Pharmacol (2011) 659(1):15–25. doi: 10.1016/j.ejphar.2011.03.005
16. Colella M, Cuomo D, Peluso T, Falanga I, Mallardo M, De Felice M, et al. Ovarian aging: Role of pituitary-ovarian axis hormones and ncRNAs in regulating ovarian mitochondrial activity. Front Endocrinol (Lausanne) (2021) 12:791071. doi: 10.3389/fendo.2021.791071
17. Ikeda K, Horie-Inoue K, Inoue S. Functions of estrogen and estrogen receptor signaling on skeletal muscle. J Steroid Biochem Mol Biol (2019) 191:105375. doi: 10.1016/j.jsbmb.2019.105375
18. Lin XM, Chen M, Wang QL, Ye XM, Chen HF. Clinical observation of kuntai capsule combined with fenmotong in treatment of decline of ovarian reserve function. World J Clin cases (2021) 9(28):8349–57. doi: 10.12998/wjcc.v9.i28.8349
19. Ahmed S, Maldera J, Krunic D, Paiva-Silva G, Pénalva C, Teleman A, et al. Fitness trade-offs incurred by ovary-to-gut steroid signalling in Drosophila. Nature (2020) 584(7821):415–9. doi: 10.1038/s41586-020-2462-y
20. Sharma V, Pandey A, Kumar A, Misra S, Gupta H, Gupta S, et al. Functional male accessory glands and fertility in Drosophila require novel ecdysone receptor. PloS Genet (2017) 13(5):e1006788. doi: 10.1371/journal.pgen.1006788
21. Luo W, Liu S, Zhang W, Yang L, Huang J, Zhou S, et al. Juvenile hormone signaling promotes ovulation and maintains egg shape by inducing expression of extracellular matrix genes. P Natl Acad Sci USA (2021) 118(39):2104461118. doi: 10.1073/pnas.2104461118
22. Khalid M, Ahmad S, Ngegba P, Zhong G. Role of endocrine system in the regulation of female insect reproduction. Biology (2021) 10(7):614. doi: 10.3390/biology10070614
23. Hou W, Pei J. Drosophila melanogasterProteomic analysis of red ginseng on prolonging the life span of Male. Front Pharmacol (2021) 12:618123. doi: 10.3389/fphar.2021.618123
24. Beachum A, Whitehead K, McDonald S, Phipps D, Berghout H, Ables E. Orphan nuclear receptor ftz-f1 (NR5A3) promotes egg chamber survival in the Drosophila ovary. G3 (Bethesda) (2021) 11(2):kab003. doi: 10.1093/g3journal/jkab003
25. Mukherjee N, Mukherjee C. Germ cell ribonucleoprotein granules in different clades of life: From insects to mammals. Wirs RNA RNA (2021) 12(4):e1642. doi: 10.1002/wrna.1642
26. Teseo S, Houot B, Yang K, Monnier V, Liu G, Tricoire HG. Sinense and extracts improve healthspan of aging flies and provide protection in a huntington disease model. Aging Dis (2021) 12(2):425–40. doi: 10.14336/ad.2020.0714-1
27. Moadeli T, Mainali B, Ponton F, Taylor PW. Effects of fatty acids and vitamin e in larval diets on development and performance of Queensland fruit fly. J Insect Physiol (2020) 125:104058. doi: 10.1016/j.jinsphys.2020.104058
28. Beghelli D, Zallocco L, Barbalace MC, Paglia S, Strocchi S, Cirilli I, et al. Pterostilbene promotes mean lifespan in both Male and female Drosophila melanogaster modulating different proteins in the two sexes. Oxid Med Cell Longev (2022) 2022:1744408. doi: 10.1155/2022/1744408
29. Baenas N, Wagner AE. Drosophila melanogaster as a model organism for obesity and type-2 diabetes mellitus by applying high-sugar and high-fat diets. Biomolecules (2022) 12(2):307. doi: 10.3390/biom12020307
30. Lu J, Huang Q, Zhang D, Lan T, Zhang Y, Tang X, et al. The protective effect of DiDang tang against AlCl3-induced oxidative stress and apoptosis in PC12 cells through the activation of SIRT1-mediated Akt/Nrf2/HO-1 pathway. Front Pharmacol (2020) 11:466. doi: 10.3389/fphar.2020.00466
31. Zhai L, Xu X, Liu J, Jing C, Yang X, Zhao D, et al. A novel biochemical study of anti-dermal fibroblast replicative senescence potential of panax notoginseng oligosaccharides. Front Pharmacol (2021) 12:690538. doi: 10.3389/fphar.2021.690538
32. Li Z, Jiang R, Wang M, Zhai L, Liu J, Xu X, et al. Ginsenosides repair UVB-induced skin barrier damage in BALB/c hairless mice and HaCaT keratinocytes. J Ginseng Res (2022) 46(1):115–25. doi: 10.1016/j.jgr.2021.05.001
33. Wang H, Zhang S, Zhai L, Sun L, Zhao D, Wang Z, et al. Ginsenoside extract from ginseng extends lifespan and health span in caenorhabditis elegans. Food Funct (2021) 12(15):6793–808. doi: 10.1039/d1fo00576f
34. Song J, Zhou S. Post-transcriptional regulation of insect metamorphosis and oogenesis. Cell Mol Life Sci (2020) 77(10):1893–909. doi: 10.1007/s00018-019-03361-5
35. Yu X, Li H, Lin D, Guo W, Xu Z, Wang L, et al. Ginsenoside prolongs the lifespan of c. elegans via lipid metabolism and activating the stress response signaling pathway. Int J Mol Sci (2021) 22(18):9668. doi: 10.3390/ijms22189668
36. Liu QX, Zhang W, Wang J, Hou W, Wang YP. A proteomic approach reveals the differential protein expression in Drosophila melanogaster treated with red ginseng extract (Panax ginseng). J Ginseng Res (2018) 42(3):343–51. doi: 10.1016/j.jgr.2017.04.006
37. Lin H, Liu Z, Pi Z, Men L, Chen W, Liu Z. Urinary metabolomic study of the antagonistic effect of p. ginseng in rats with estrogen decline using ultra performance liquid chromatography coupled with quadrupole time-of-flight mass spectrometry. Food Funct (2018) 9(3):1444–53. doi: 10.1039/c7fo01680h
38. Park J, Song H, Kim SK, Lee MS, Rhee DK, Lee Y. Effects of ginseng on two main sex steroid hormone receptors: Estrogen and androgen receptors. J Ginseng Res (2017) 41(2):215–21. doi: 10.1016/j.jgr.2016.08.005
39. Majdi Seghinsara A, Shoorei H, Hassanzadeh Taheri MM, Khaki A, Shokoohi M, Tahmasebi M, et al. Panax ginseng extract improves follicular development after mouse preantral follicle 3D culture. Cell J (2019) 21(2):210–9. doi: 10.22074/cellj.2019.5733
40. Gurvich C, Le J, Thomas N, Thomas E, Kulkarni J. Sex hormones and cognition in aging. Vitam Horm (2021) 115:511–33. doi: 10.1016/bs.vh.2020.12.020
41. Björkgren I, Chung D, Mendoza S, Gabelev-Khasin L, Petersen N, Modzelewski A, et al. Alpha/Beta hydrolase domain-containing protein 2 regulates the rhythm of follicular maturation and estrous stages of the female reproductive cycle. Front Cell Dev Biol (2021) 9:710864. doi: 10.3389/fcell.2021.710864
42. Tian M, Li L, Zheng R, Yang L, Wang Z. Advances on hormone-like activity of panax ginseng and ginsenosides. Chin J Nat Medicines (2020) 18(7):526–35. doi: 10.1016/s1875-5364(20)30063-7
43. Al Baki M, Lee D, Jung J, Kim Y. Insulin signaling mediates previtellogenic development and enhances juvenile hormone-mediated vitellogenesis in a lepidopteran insect, maruca vitrata. BMC Dev Bio (2019) 19(1):14. doi: 10.1186/s12861-019-0194-8
44. Zhu Z, Tong C, Qiu B, Yang H, Xu J, Zheng S, et al. 20E-mediated regulation of BmKr-h1 by BmKRP promotes oocyte maturation. BMC Biol (2021) 19(1):39. doi: 10.1186/s12915-021-00952-2
45. Tricoire H, Battisti V, Trannoy S, Lasbleiz C, Pret AM, Monnier V. The steroid hormone receptor EcR finely modulates drosophila lifespan during adulthood in a sex-specific manner. Mech Ageing Dev (2009) 130(8):547–52. doi: 10.1016/j.mad.2009.05.004
46. Uyehara CM, McKay DJ. Direct and widespread role for the nuclear receptor EcR in mediating the response to ecdysone in drosophila. Proc Natl Acad Sci USA (2019) 116(20):9893–902. doi: 10.1073/pnas.1900343116
47. Sheng Z, Ma L, Cao M, Jiang R, Li S. Juvenile hormone acid methyl transferase is a key regulatory enzyme for juvenile hormone synthesis in the eri silkworm, samia cynthica ricini. Arch Insect Biochem (2008) 69(3):143–54. doi: 10.1002/arch.20268
48. Gu S, Chen C, Lin P. Changes in expressions of ecdysteroidogenic enzyme and ecdysteroid signaling genes in relation to bombyx embryonic development. J Exp Zool Part A (2021) 335(5):477–88. doi: 10.1002/jez.2466
49. Carney GE, Bender M. The drosophila ecdysone receptor (EcR) gene is required maternally for normal oogenesis. Genetics (2000) 154(3):1203–11. doi: 10.1093/genetics/154.3.1203
Keywords: ginsenosides, aged female Drosophila, reproductive capacity, ECR, steroid signaling pathway
Citation: Fu B, Ma R, Liu F, Chen X, Teng X, Yang P, Liu J, Zhao D and Sun L (2022) Ginsenosides improve reproductive capability of aged female Drosophila through mechanism dependent on ecdysteroid receptor (ECR) and steroid signaling pathway. Front. Endocrinol. 13:964069. doi: 10.3389/fendo.2022.964069
Received: 08 June 2022; Accepted: 18 July 2022;
Published: 09 August 2022.
Edited by:
Antonio Simone Laganà, University of Palermo, ItalyReviewed by:
Sok Kuan Wong, National University of Malaysia, MalaysiaCopyright © 2022 Fu, Ma, Liu, Chen, Teng, Yang, Liu, Zhao and Sun. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Liwei Sun, c3VubnlsaWx3ZWlAMTYzLmNvbQ==; Rui Ma, bWFydWlqaWxpbkAxNjMuY29t
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.