
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Endocrinol., 05 October 2022
Sec. Clinical Diabetes
Volume 13 - 2022 | https://doi.org/10.3389/fendo.2022.959860
 Liang Pan1†
Liang Pan1† Qianqian Xu2†
Qianqian Xu2† Jianmin Liu3†
Jianmin Liu3† Yang Gao4
Yang Gao4 Jun Li5
Jun Li5 Hongye Peng6
Hongye Peng6 Linli Chen6
Linli Chen6 Miyuan Wang7
Miyuan Wang7 Gang Mai1*
Gang Mai1* Shuo Yang8*
Shuo Yang8*Introduction: China has the largest population of diabetic patients (about 116 million) in the world. As a novel model of the fat index for Chinese people, the Chinese visceral adiposity index (CVAI) was considered a reliable indicator to assess the dysfunction of visceral fat. This study aimed to explore the dose–response relationship between CVAI and type 2 diabetes mellitus (T2DM) in the Chinese population, considering CVAI as a continuous/categorical variable.
Method: Baseline and follow-up data were collected from waves 2011 and 2015, respectively, of the China Health and Retirement Longitudinal Study (CHARLS). Multivariate logistic regression models were used to explore the relationship between CVAI and T2DM. We built three models to adjust the possible effect of 10 factors (age, gender, education level, location, marital status, smoking status, drinking status, sleep time, systolic blood pressure (SBP), and diastolic blood pressure (DBP)) on the outcome. The restricted cubic splines were used to examine possible non-linear associations and visualize the dose–response relationship between CVAI and T2DM.
Results: A total of 5,014 participants were included, with 602 (12.00%) T2DM patients. The last CVAI quartile group (Q4) presented the highest risk of T2DM (OR, 2.17, 95% CI, 1.67–2.83), after adjusting for all covariates. There was a non-linear (U-shaped) relationship between the CVAI and the risk of T2DM (p for non-linear <0.001) in the restricted cubic spline regression model. CVAI was a risk factor of T2DM when it exceeded 92.49; every interquartile range (IQR) increment in the CVAI was associated with a 57% higher risk of developing T2DM (OR = 1.57, 95% CI = 1.36–1.83) after adjusting for potential confounders. The area under the receiver operating characteristic curve (AUC) (95% confidence interval) for CVAI was 0.623, and the optimal cutoff point was 111.2. There was a significant interaction between CVAI and gender by stratified analysis.
Conclusion: CVAI was closely associated with the risk of T2DM and might possibly be a potential marker in predicting T2DM development. The outcome suggested that it might be better to maintain CVAI within an appropriate range.
Type 2 diabetes mellitus (T2DM) is a group of metabolic diseases characterized by hyperglycemia and is closely associated with a variety of factors, such as genes, obesity, and lack of exercise. The incidence of T2DM is rising rapidly due to living standards improvement, changes in diet, sedentary lifestyle, and work-related stress. According to the International Diabetes Federation (IDF), the population with diabetes mellitus worldwide is about 537 million presently and is expected to reach 700 million by 2045 (1). Diabetes mellitus may contribute to retinopathy (2), foot ulcer and necrosis (3), cardiovascular disease (4), myocardial infarction, and stroke, which may seriously endanger human health and impose a heavy burden on society. Based on the epidemiological and economic data from 184 countries (5), the global cost associated with diabetes mellitus is about 1.31 trillion USD in 2015 (95% CI: 1.28–1.36). On average, such cost as a percentage of GDP in middle-income countries is higher than that in high-income countries. According to IDF, China has the largest population of diabetic patients (about 116 million) and spends over 109 billion USD on treatment in 2021, which is the largest expenditure in the world outside the United States (6). A large population of diabetic patients and associated substantial costs may impose a heavy burden on society in China. Over 90% of those diabetic patients are T2DM patients. Therefore, it is crucial to lower the incidence of T2DM and improve overall health by identifying high-risk populations and implementing early intervention and treatment.
According to the WHO, there are 650 million obese adults worldwide in 2021 (7). It is known that obesity, especially abdominal obesity, predisposes the development of insulin resistance and T2DM (8). However, commonly used indicators, such as body mass index (BMI), waist circumference (WC), and waist-to-height ratio (WHtR), are unable to differentiate visceral and subcutaneous fat. Visceral adiposity index (VAI), a novel fat fraction calculated with WC, BMI, and serum lipid, is closely associated with multiple chronic metabolic diseases, such as coronary heart disease (9), hypertension (10), and T2DM (11). Compared with conventional indices, VAI has a higher predictive value for obesity. It is known that the degree and susceptibility of obesity vary in different ethnicities and regions (12). Chinese people are more likely to have abdominal obesity compared with Western populations, which makes VAI an inadequate predictor for Chinese people. Based on VAI, Xia et al. proposed the Chinese visceral adiposity index (CVAI) as a novel model of the fat index for Chinese people, integrating parameters of age, BMI, WC, triglyceride (TG), and high-density lipoprotein cholesterol (HDL-C). CVAI is considered a reliable index to assess the dysfunction of visceral fat (13).
CVAI has been reported with predictive value in previous studies. Based on research in rural China, Han et al. (14) suggested that CVAI is positively associated with the risk of T2DM. Shang et al. (15) explored the relationship between CVAI and the risk of newly emerging T2DM in Japanese adults. However, those studies cannot reflect the characteristics of the Chinese population due to their geographical limitations. Based on the China Health and Retirement Longitudinal Study (CHARLS) database, this prospective cohort study aims to explore the value of CVAI in predicting the risk of developing T2DM in the Chinese population, considering CVAI as a continuous/categorical variable. Moreover, stratified analysis has been performed with age, gender, marital status, and education level.
In this prospective cohort study, we analyzed baseline data in wave 2011 and follow-up data for T2DM in wave 2015 from CHARLS (http://charls.pku.edu.cn/), a freely accessible database of a nationally representative sample.
As a nationwide interdisciplinary survey, CHARLS investigated participants aged 45 and above from 450 villages/communities in 28 provinces (autonomous regions and municipalities) and collected comprehensive data, such as basic information, family structure, physical condition, health care and insurance, work and pension, income and expenditure, housing, and laboratory examination. With a total of 21,097 participants in 12,241 households in 2015, CHARLS was a reliable database for research on the health status and possible contributing factors for the middle-aged and elderly population.
Venous blood samples were collected from participants after overnight fasting for over 12 h. A complete blood count was tested promptly on site. Whole blood specimens were stored at 4°C. All remaining samples were delivered to the central laboratory for further analysis. Enzymatic colorimetric analysis was performed to test glucose, total cholesterol (TC), TG, low-density lipoprotein cholesterol (LDL-C), and HDL-C levels. Boronate-affinity high-performance liquid chromatography method was used to test glycated hemoglobin (HbA1c) levels.
The program CHARLS was reviewed and approved by Ethical Review Committee at Peking University in 2008 (IRB00001052-11, 015). Our study followed all applicable specifications and guidelines of CHARLS. Informed consent forms were signed voluntarily by all participants before the investigation.
This study implemented the following inclusion criteria according to the research purpose: age ≥ 45 years; complete sociodemographic data including gender, education level, marital status, and location; and complete data of fasting blood glucose. Participants with lipid-lowering drug-taking history were excluded (n = 253). A total of 5,014 eligible participants were included finally.
VAI, an empirical-mathematical model integrating anthropometric (age, BMI, and WC) and functional (TG and HDL) parameters, indicated the fat distribution and function. The VAI score for the Chinese population was calculated with the following specific formulas:
Chinese man: CVAI = −267.93 + 0.68 × age + 0.03 × BMI + 4.00 × WC + 22.00 × log10 (TG) − 16.32 × HDL-C
Chinese woman: CVAI = −187.32 + 1.71 × age + 4.23 × BMI + 1.12 × WC + 39.76 × log10 (TG) − 11.66 × HDL-C.
According to the standards published by American Diabetes Association in 2005, this study defined T2DM as a fasting blood glucose level ≥126 mg/dl (7 mmol/L), and/or a random blood glucose level ≥200 mg/dl (11.1 mmol/L), and/or an HbA1c level ≥6.5%, and/or self-reported diagnosis with yes (“Have you ever been diagnosed with diabetes or hyperglycemia?”)
The analyses were adjusted for sociodemographic characteristics, health-related behaviors, and anthropometric measurements. We analyzed age, gender, education level (“primary school or below”, “high school”, and “college or above”), location (“city/town” and “village”), and marital status (“married” and “never-married/separated/widowed”) as demographic variables. We analyzed smoking status (“non-smoker”, “ex-smoker”, and “current smoker”), drinking status (“never”, “less than once a month”, and “more than once a month”), and sleep time as variables of health-related behavior. Those data were obtained from self-reported questionnaires and with the help of trained interviewers. Anthropometric measurements included systolic blood pressure (SBP) and diastolic blood pressure (DBP), which were the means of the three-time measurements using Omron HEM-7200 sphygmomanometer.
Continuous variables with normal distribution were expressed by means and standard deviation (SD), while those with abnormal distribution were expressed by median (interquartile range (IQR)). Categorical variables were expressed by percentages. One-way ANOVA, Kruskal–Wallis H test, or chi-square tests were employed to compare the baseline characteristics and incidence of T2DM after grouping by CVAI quartiles (Q1, Q2, Q3, and Q4). Three logistic models were employed to estimate the odds ratio (OR) with a 95% confidence interval (CI) for T2DM using CVAI as continuous variables (per IQR increment) or categorical (quartiles) variables. All three models were to explore the relationship between CVAI and T2DM, including the unadjusted crude model (Model 1); the adjusted model with age, gender, education level, location, and marital status (Model 2); and a further adjusted model with smoking status, drinking status, sleep time, SBP, and DBP (Model 3). Those results were presented with ORs and 95% CIs. The area under the receiver operating characteristic (ROC) curve (AUC) was performed to test the predictive power of CVAI for the risk of T2DM, and the “addfor” algorithm was used to determine the optimal cutoff points. Interaction analysis was performed to identify the effect modifications of sociodemographic characteristics, health-related behaviors, and anthropometric measurements in the relationship between CVAI and T2DM, using a product term in the main analysis [CVAI × (interaction term)]. Moreover, the restricted cubic splines were used to examine possible non-linear associations and visualize the dose–response association of CVAI with T2DM.
All statistical analyses were completed with R 4.1. Restricted cubic splines were completed with the “rms” package; optimal cutoff points were tested with the “CatPredi” package. A two-tailed p < 0.05 denoted statistical significance.
Characteristics of the study population are presented in Table 1. A total of 5,014 participants were included (median age = 58, men = 2,321 (46.29%), and women = 2,693 (53.71%)), of whom 602 (12.00%) developed T2DM. The baseline median (IQR) CVAI in all participants was 91.32 (62.17, 122.07). The characteristics of participants with T2DM were obviously different from the characteristics of those without; specifically, the former were more likely to be older and non-married, with higher SBP, DBP, BMI, WC, glucose, TG, and CVAI and lower HDL-C.
Table 2 showed the association of the CVAI with the risk of T2DM, as well as the quartiles of the CVAI. The risk of T2DM showed a gradual increase with the quartiles of the CVAI (p for trend <0.001). Compared with the first CVAI group (Q1), the last CVAI quartile group (Q4) presented the highest risk of developing T2DM (OR, 2.17, 95% CI, 1.67–2.83), after adjusting for age, gender, education level, location and marital status, smoking status, drinking status, sleep time, SBP, and DBP. Analyzed as a continuous variable, CVAI was consistently and significantly associated with T2DM (OR, 1.59, 95% CI, 1.41–1.81). The AUC (95% confidence interval) for CVAI was 0.623, and the optimal cutoff point was 111.2.
Figure 1 showed the dose–response relationship between CVAI and the risk of T2DM. Restricted cubic spline regression showed a non-linear (U-shaped) relationship between CVAI and risk of T2DM (p for non-linear <0.001). We found that when CVAI ≥ 92.49, the cumulative risk of developing T2DM increased gradually, so we used piecewise logistic regression to calculate the risk of T2DM (Table 3). Results showed that every IQR increment in CVAI was associated with a 57% higher risk of developing T2DM (OR, 1.57, 95% CI, 1.36–1.83) after adjusting for potential confounders.
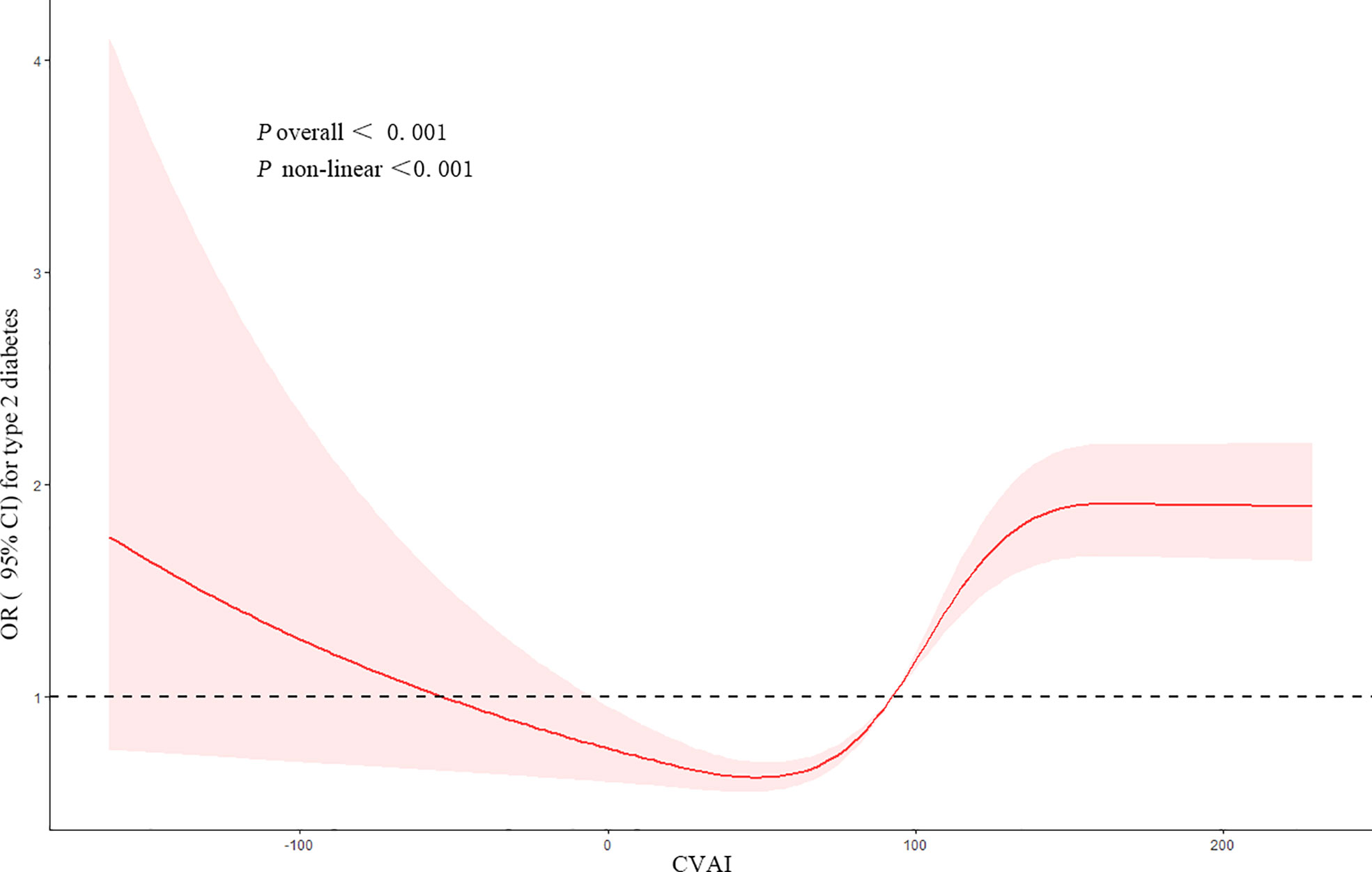
Figure 1 Restricted cubic spline of the association between CVAI and the risk of T2DM. The model was adjusted for age, gender, education level, location and marital status, smoking status, drinking status, sleep time, SBP, and DBP. The plot shows a non-linear (U-shaped) relationship between CVAI and the risk of T2DM. CVAI, Chinese visceral adiposity index; T2DM, type 2 diabetes mellitus; SBP, systolic blood pressure; DBP, diastolic blood pressure.
To find out whether CVAI would exert different influence on the risk of developing T2DM among different subgroups, we grouped participants by characteristics. Results showed that the influence of CVAI on the risk of T2DM was consistent across subgroups. There was a significant interaction between CVAI and gender (p for interaction <0.001). Each IQR increment in CVAI was associated with a 98.7% higher risk of developing T2DM (OR, 1.987, 95% CI, 1.663–2.375) for women (Figure 2).
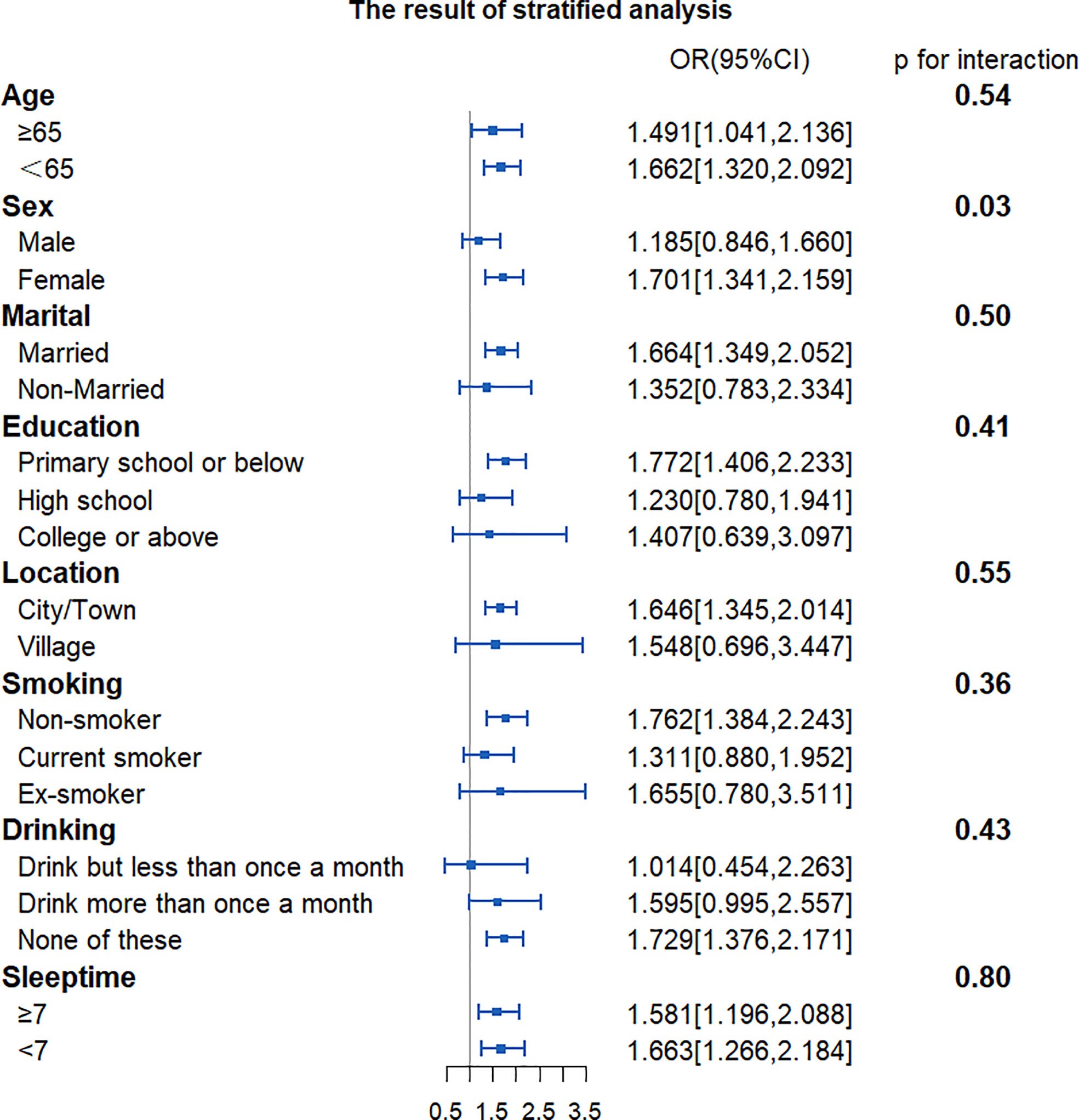
Figure 2 Forest plot of stratified analysis of the association of CVAI with the risk of T2DM. The plot shows that there were significant interactions between CVAI and sex. OR, odds ratio; CI, confidence intervals; CVAI, Chinese visceral adiposity index; T2DM, type 2 diabetes mellitus.
According to the baseline data of CHARLS, we found that participants with T2DM were more likely to be older and non-married, with higher SBP, DBP, BMI, WC, glucose, TG, and CVAI and lower HDL-C than those without. Based on the follow-up data, we found that CVAI was positively associated with the risk of T2DM. After adjusting for confounders, each IQR increment in CVAI was associated with a 59% higher risk of developing T2DM (OR, 1.59, 95% CI, 1.41–1.81). Based on the dose–response relationship analysis, we found a non-linear (U-shaped) relationship between CVAI and the risk of T2DM.
Obesity, especially abdominal obesity, is an important risk factor for T2DM. Our study used CVAI to predict the risk of T2DM in the Chinese population and suggested that they were positively associated, which was consistent with previous studies (16, 17). Han et al. (14) suggested that the risk of developing T2DM increased with each SD increment of CVAI, VAI, WHtR, WC, and BMI. Of all those five indices, CVAI presented the best performance in predicting T2DM, with the largest AUC. Wan et al. (18) explored the association of CVAI with T2DM and its complications (such as cardiovascular diseases, diabetic nephropathy, and fundus lesions) and suggested that CVAI was closely associated with the incidence of cardiovascular diseases and diabetic nephropathy. Different from the mentioned studies using CVAI only as a categorical variable, this study also used CVAI as a continuous variable to explore its dose–response relationship with the risk of T2DM. We also performed a stratified analysis.
When using CVAI as a continuous variable, we found that it was non-linearly (U-shaped) associated with the risk of T2DM. An appropriate CVAI level might lower the risk of T2DM, while a high CVAI level might increase the risk of T2DM. However, these results were not entirely unexpected. Wang et al. (19) suggested an inverted U-shaped relationship between VAI and lung function. For men, the predicted FEV1% and FVC% increased as VAI increased when VAI ≤ 14, while they decreased as VAI increased when VAI ≥ 15. Triglyceride-glucose index (TyG) is an important indicator of insulin resistance. Xuan et al. (20) suggested that TyG had a U-shaped association with incident T2DM. As a component for calculating CVAI, TG was also found to have a U-shaped association with the risk of T2DM (21). Interestingly, a 5.5-year prospective cohort study with a sample of 149,345 cases suggested that long-term overweight (BMI 25 to <30 kg/m2) subjects with T2DM had a lower risk of excess mortality compared with those age/sex-matched general subjects in the control group, whereas those with BMI ≥ 40 kg/m2 had a substantially higher risk.
The dose–response curve showed that the risk of T2DM decreased as CVAI increased when the latter fell within a lower range, which should be interpreted with caution. Most of the previous studies focused on the relationship between T2DM and obesity instead of low body fat. Adipose tissue plays an important role in energy storage and other biological processes (22), including TG storage and secretion of proteins with multiple functions. Either too little or too much adipose tissue would possibly cause metabolic disorders and changes in insulin sensitivity. Previous studies suggested that patients with adipose tissue deficiency (lipodystrophy) would possibly have limited TG storage in adipose tissue and would redistribute the fat into skeletal muscle and liver (23, 24). Ectopic fat accumulation might contribute to severe insulin resistance, fatty liver, hypertriglyceridemia, and T2DM. Kursawe et al. (25) suggested that the reduction of adipogenesis in adolescents might cause abnormal distribution of abdominal fat, hepatic steatosis, and insulin resistance. Interestingly, Kimet al. (26) found in animal experiments that adipose tissue could be a buffer against risk factors for metabolic disorders. Through appropriate overexpression of adiponectin, adipose tissue would possibly lower the TG level in the liver and muscle and redistribute those TG into subcutaneous adipose tissue, so as to reduce the expression of proinflammatory cytokine and improve insulin sensitivity. The appropriate CVAI level could possibly lower the risk of T2DM and protect the body.
When CVAI fell within a higher range, the cumulative risk of T2DM increased gradually as CVAI increased. It suggested that a higher risk of developing T2DM was associated with higher content of visceral fat when the latter exceeded a specific cutoff point. Too much visceral fat was one of the recognized risk factors for T2DM. Adipose tissue, an important endocrine organ, was a source of multiple proinflammatory cytokines including IL-6 and TNF-α (27). Excessive accumulation of adipose tissue would upregulate the expression of inflammatory factors, resulting in the development of T2DM. Moreover, as hormones expressed in adipose tissue, adiponectin, leptin, and resistin were all closely associated with insulin resistance.
According to the results of the subgroup analysis, there were gender differences in the relationship between CVAI and the risk of T2DM. A higher quartile in CVAI was associated with a higher risk of T2DM for women, but not for men. There were some possible explanations. First, estrogen plays an important role in regulating insulin sensitivity and metabolic homeostasis. It can lower the inflammation levels in white adipose tissue and reduce the levels of immune cell infiltration and oxidative stress in adipose tissue, which reduce possible ectopic lipids in the liver and skeletal muscle (28, 29). Middle-aged and elderly women are losing the protection of estrogen and are more susceptible to insulin resistance than men. Second, Koutsari (30) suggested that women had greater rates of non-oxidative free fatty acid (FFA) disposal than men. The non-oxidative disposal of FFA could possibly increase very low-density lipoprotein (VLDL) and TG and also cause insulin resistance, resulting in metabolic disorders (31–33). Last, previous studies suggested that there are significant differences in the size of adipocytes, basal lipolysis, and fatty acid oxidation rate between women and men. Compared with men, women have lower basal fatty acid oxidation levels and are more susceptible to metabolic disorders (34).
According to our findings, the U-shaped relationship between CVAI and T2DM risk might implicate that it was important to maintain proper visceral fat content. The lowest visceral fat content might not guarantee the best health state. A reduction in visceral fat content was recommended if the baseline CVAI was high, whereas an appropriate increase in visceral fat content might contribute to lowering the risk of T2DM if the baseline CVAI was low. A prospective cohort study (35) suggested that the mortality of diabetic patients decreased as BMI increased, independent of confounders such as smoking, cardiovascular disease, cancer, and other potential factors. Therefore, we suggested that CVAI should be maintained within an appropriate range, which could possibly lower the risk of developing T2DM and reduce the mortality risk associated with T2DM. CVAI at either a very high or a very low level might be detrimental.
As a nationwide population-based prospective cohort study, this work covered a wide range of participants with good sample representativeness. We used CVAI for the first time as a continuous variable to explore its dose–response relationship with the risk of T2DM. We built three models to adjust the possible effect of 10 factors (age, gender, education level, location, marital status, smoking status, drinking status, sleep time, SBP, and DBP) on the outcome, which made the study results reliable and convincing. We performed a stratified analysis to explore the differences in the association of CVAI with T2DM risk in multiple subgroups.
First, although multiple covariates were adjusted in our study, there were still other potential confounders, such as diet and exercise time. Second, a 2-h oral glucose tolerance test was not performed to detect newly developed T2DM cases, which might underestimate the incidence. Last, we only included participants aged 45 years and above, so further research would be required to generalize our results among younger populations.
CVAI was closely associated with the risk of T2DM and could possibly be a potential marker in predicting T2DM development. The results might indicate that it would be better to maintain CVAI within an appropriate range.
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
GM, SY, LP, and QX: Conception and design of the study, acquisition and interpretation of data, drafting the article. JML: Conception and design of the study, formal analysis and Methodology. YG, JL, HP, LC, and MW: Draw Figures and tables, drafting the article. All authors contributed to and have approved the final manuscript.
The work was supported by the Sichuan Provincial Science & Technology Program (2022JDKP0040), Sichuan Provincial Health Commission Program (21PJ168), and Deyang Municipal Science & Technology Program (2021SZZ068, 2022SCZ089 and 2022SCZ130). The funding sources had no role in the study design, data collection, analysis and interpretation of data, decision to publish, or preparation of the manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2022.959860/full#supplementary-material
1. Yu L, Liu W, Wang X, Ye Z, Tan Q, Qiu W, et al. A review of practical statistical methods used in epidemiological studies to estimate the health effects of multi-pollutant mixture. Environ pollut (Barking Essex 1987) (2022) 306:119356. doi: 10.1016/j.envpol.2022.119356
2. Matuszewski W, Baranowska-Jurkun A, Stefanowicz-Rutkowska MM, Modzelewski R, Pieczyński J, Bandurska-Stankiewicz E. Prevalence of diabetic retinopathy in type 1 and type 2 diabetes mellitus patients in north-East Poland. Medicina (Kaunas Lithuania) (2020) 56(4):164. doi: 10.3390/medicina56040164
3. van Netten JJ, Price PE, Lavery LA, Monteiro-Soares M, Rasmussen A, Jubiz Y, et al. Prevention of foot ulcers in the at-risk patient with diabetes: A systematic review. Diabetes/metabolism Res Rev (2016) 32 Suppl 1:84–98. doi: 10.1002/dmrr.2701
4. Einarson TR, Acs A, Ludwig C, Panton UH. Prevalence of cardiovascular disease in type 2 diabetes: A systematic literature review of scientific evidence from across the world in 2007-2017. Cardiovasc Diabetol (2018) 17(1):83. doi: 10.1186/s12933-018-0728-6
5. Bommer C, Heesemann E, Sagalova V, Manne-Goehler J, Atun R, Bärnighausen T, et al. The global economic burden of diabetes in adults aged 20-79 years: A cost-of-Illness study. Lancet Diabetes Endocrinol (2017) 5(6):423–30. doi: 10.1016/s2213-8587(17)30097-9
6. Xiao X, Gao M, Zhou Y, Xu SL, Knibbs LD, Heinrich J, et al. Is greener better? associations between greenness and birth outcomes in both urban and non-urban settings. Int J Epidemiol (2022) 51(1):88–98. doi: 10.1093/ije/dyab164
7. Xiao ML, Zhong HL, Lin HR, Liu CY, Yan Y, Ke YB, et al. Higher serum vitamin a is associated with a worsened progression of non-alcoholic fatty liver disease in adults: A prospective study. Food Funct (2022) 13(2):970–7. doi: 10.1039/d1fo03119h
8. Piché ME, Tchernof A, Després JP. Obesity phenotypes, diabetes, and cardiovascular diseases. Circ Res (2020) 126(11):1477–500. doi: 10.1161/circresaha.120.316101
9. Bagyura Z, Kiss L, Lux Á, Csobay-Novák C, Jermendy ÁL, Polgár L, et al. Association between coronary atherosclerosis and visceral adiposity index. Nutr Metab Cardiovasc Dis (2020) 30(5):796–803. doi: 10.1016/j.numecd.2020.01.013
10. Leite NN, Cota BC, Gotine A, Rocha D, Pereira PF, Hermsdorff HHM. Visceral adiposity index is positively associated with blood pressure: A systematic review. Obes Res Clin Pract (2021) 15(6):546–56. doi: 10.1016/j.orcp.2021.10.001
11. Nusrianto R, Tahapary DL, Soewondo P. Visceral adiposity index as a predictor for type 2 diabetes mellitus in Asian population: A systematic review. Diabetes Metab Syndr (2019) 13(2):1231–5. doi: 10.1016/j.dsx.2019.01.056
12. Liu B, Du Y, Wu Y, Snetselaar LG, Wallace RB, Bao W. Trends in obesity and adiposity measures by race or ethnicity among adults in the united states 2011-18: Population based study. BMJ (Clinical Res ed) (2021) 372:n365. doi: 10.1136/bmj.n365
13. Xia MF, Chen Y, Lin HD, Ma H, Li XM, Aleteng Q, et al. A indicator of visceral adipose dysfunction to evaluate metabolic health in adult Chinese. Sci Rep (2016) 6:38214. doi: 10.1038/srep38214
14. Han M, Qin P, Li Q, Qie R, Liu L, Zhao Y, et al. Chinese Visceral adiposity index: A reliable indicator of visceral fat function associated with risk of type 2 diabetes. Diabetes/metabolism Res Rev (2021) 37(2):e3370. doi: 10.1002/dmrr.3370
15. Shang L, Li R, Zhao Y, Sun H, Tang B, Hou Y. Association between Chinese visceral adiposity index and incident type 2 diabetes mellitus in Japanese adults. Diabetes Metab syndrome Obes Targets Ther (2021) 14:3743–51. doi: 10.2147/dmso.S322935
16. Xia MF, Lin HD, Chen LY, Wu L, Ma H, Li Q, et al. Association of visceral adiposity and its longitudinal increase with the risk of diabetes in Chinese adults: A prospective cohort study. Diabetes/metabolism Res Rev (2018) 34(7):e3048. doi: 10.1002/dmrr.3048
17. Wei J, Liu X, Xue H, Wang Y, Shi Z. Comparisons of visceral adiposity index, body shape index, body mass index and waist circumference and their associations with diabetes mellitus in adults. Nutrients (2019) 11(7):1580. doi: 10.3390/nu11071580
18. Wan H, Wang Y, Xiang Q, Fang S, Chen Y, Chen C, et al. Associations between abdominal obesity indices and diabetic complications: Chinese visceral adiposity index and neck circumference. Cardiovasc Diabetol (2020) 19(1):118. doi: 10.1186/s12933-020-01095-4
19. Wang Y, Li Z, Li F. Nonlinear relationship between visceral adiposity index and lung function: A population-based study. Respir Res (2021) 22(1):161. doi: 10.1186/s12931-021-01751-7
20. Xuan X, Hamaguchi M, Cao Q, Okamura T, Hashimoto Y, Obora A, et al. U-Shaped association between the triglyceride-glucose index and the risk of incident diabetes in people with normal glycemic level: A population-base longitudinal cohort study. Clin Nutr (Edinburgh Scotland) (2021) 40(4):1555–61. doi: 10.1016/j.clnu.2021.02.037
21. Liu L, Shen G, Huang JY, Yu YL, Chen CL, Huang YQ, et al. U-Shaped association between low-density lipid cholesterol and diabetes mellitus in patients with hypertension. Lipids Health Dis (2019) 18(1):163. doi: 10.1186/s12944-019-1105-5
22. Scherer PE. Adipose tissue: From lipid storage compartment to endocrine organ. Diabetes (2006) 55(6):1537–45. doi: 10.2337/db06-0263
23. Decaudain A, Vantyghem MC, Guerci B, Hécart AC, Auclair M, Reznik Y, et al. New metabolic phenotypes in laminopathies: Lmna mutations in patients with severe metabolic syndrome. J Clin Endocrinol Metab (2007) 92(12):4835–44. doi: 10.1210/jc.2007-0654
24. Garg A. Acquired and inherited lipodystrophies. New Engl J Med (2004) 350(12):1220–34. doi: 10.1056/NEJMra025261
25. Kursawe R, Eszlinger M, Narayan D, Liu T, Bazuine M, Cali AM, et al. Cellularity and adipogenic profile of the abdominal subcutaneous adipose tissue from obese adolescents: Association with insulin resistance and hepatic steatosis. Diabetes (2010) 59(9):2288–96. doi: 10.2337/db10-0113
26. Kim JY, van de Wall E, Laplante M, Azzara A, Trujillo ME, Hofmann SM, et al. Obesity-associated improvements in metabolic profile through expansion of adipose tissue. J Clin Invest (2007) 117(9):2621–37. doi: 10.1172/jci31021
27. Qatanani M, Lazar MA. Mechanisms of obesity-associated insulin resistance: Many choices on the menu. Genes Dev (2007) 21(12):1443–55. doi: 10.1101/gad.1550907
28. Camporez JP, Lyu K, Goldberg EL, Zhang D, Cline GW, Jurczak MJ, et al. Anti-inflammatory effects of oestrogen mediate the sexual dimorphic response to lipid-induced insulin resistance. J Physiol (2019) 597(15):3885–903. doi: 10.1113/jp277270
29. Nickelson KJ, Stromsdorfer KL, Pickering RT, Liu TW, Ortinau LC, Keating AF, et al. A comparison of inflammatory and oxidative stress markers in adipose tissue from weight-matched obese Male and female mice. Exp Diabetes Res (2012) 2012:859395. doi: 10.1155/2012/859395
30. Koutsari C, Basu R, Rizza RA, Nair KS, Khosla S, Jensen MD. Nonoxidative free fatty acid disposal is greater in young women than men. J Clin Endocrinol Metab (2011) 96(2):541–7. doi: 10.1210/jc.2010-1651
31. Lewis GF, Uffelman KD, Szeto LW, Weller B, Steiner G. Interaction between free fatty acids and insulin in the acute control of very low density lipoprotein production in humans. J Clin Invest (1995) 95(1):158–66. doi: 10.1172/jci117633
32. Boden G, Jadali F, White J, Liang Y, Mozzoli M, Chen X, et al. Effects of fat on insulin-stimulated carbohydrate metabolism in normal men. J Clin Invest (1991) 88(3):960–6. doi: 10.1172/jci115399
33. Boden G, Chen X, Ruiz J, White JV, Rossetti L. Mechanisms of fatty acid-induced inhibition of glucose uptake. J Clin Invest (1994) 93(6):2438–46. doi: 10.1172/jci117252
34. Blaak E. Gender differences in fat metabolism. Curr Opin Clin Nutr Metab Care (2001) 4(6):499–502. doi: 10.1097/00075197-200111000-00006
Keywords: chinese visceral adiposity index, type 2 diabetes mellitus, dose–response relationship, restricted cubic spline regression, prospective cohort study
Citation: Pan L, Xu Q, Liu J, Gao Y, Li J, Peng H, Chen L, Wang M, Mai G and Yang S (2022) Dose–response relationship between Chinese visceral adiposity index and type 2 diabetes mellitus among middle-aged and elderly Chinese. Front. Endocrinol. 13:959860. doi: 10.3389/fendo.2022.959860
Received: 02 June 2022; Accepted: 14 September 2022;
Published: 05 October 2022.
Edited by:
Bert B. Little, University of Louisville, United StatesReviewed by:
Zala Jenko Pražnikar, University of Primorska, SloveniaCopyright © 2022 Pan, Xu, Liu, Gao, Li, Peng, Chen, Wang, Mai and Yang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Gang Mai, NTA5NTUzMzNAcXEuY29t; Shuo Yang, NzM0OTE2MjU2QHFxLmNvbQ==
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.