- Department of Pancreatic Surgery, West China Hospital of Sichuan University, Chengdu, China
Background: Diabetes mellitus among patients with exocrine pancreatic disorders is commonly known to be associated with chronic inflammation, including chronic pancreatitis and pancreatic ductal adenocarcinoma (PDAC). The neutrophil-to-lymphocyte ratio (NLR) is a novel marker that indicates the presence of various chronic inflammatory diseases, including type 2 diabetes (T2DM). However, no studies have examined the relationship between the NLR value and diabetes secondary to exocrine pancreatic disorders.
Aim: To determine whether the NLR value is associated with diabetes secondary to exocrine pancreatic disorders.
Methods: The medical data of subjects with confirmed pancreatic disease who were admitted to the Department of Pancreatic Surgery of our institution from August 2017 to October 2021 were obtained from the database and retrospectively analyzed. Anthropometric measures, laboratory data, including HbA1c, fasting insulin, and fasting C-peptide levels and the inflammatory index (white blood cell count, NLR, platelet-to-lymphocyte ration, monocyte-to-lymphocyte ratio) were recorded. The NLR is the ratio of neutrophils to lymphocytes. A homeostasis model (HOMA-B and HOMA-IR) was used to measure beta-cell dysfunction and insulin resistance.
Results: The NLR values of the diabetes secondary to exocrine pancreatic disorders group were significantly higher than those of the nondiabetic group (P=0.001). In multivariate logistic regression, after adjusting for covariates, high NLR values were found to be an independent risk factor for diabetes secondary to exocrine pancreatic disorders (OR: 1.37, 95% CI: 1.138-1.649, P=0.001). According to Spearman correlation analysis, the NLR was significantly correlated with fasting plasma glucose levels (P<0.0001) and HOMA2-IR values (P=0.02).
Conclusion: The NLR inflammation marker was significantly higher in subjects with diabetes secondary to exocrine pancreatic disorders and was associated with insulin resistance. NLR values may be reliable predictive markers for diabetes among patients with exocrine pancreatic disorders.
Introduction
Type 3c diabetes mellitus (T3cDM), termed pancreatic or pancreatogenic diabetes and resulting from underlying exocrine pancreatic disease, accounts for 5-10% of all diabetic patients in Western populations (1). Unlike type 1 diabetes mellitus(T1DM), which is characterized by autoimmune inflammation, or type 2 diabetes mellitus (T2DM), which is characterized by insufficiency of insulin secretion and insulin resistance, T3cDM affects all subtypes of islet β cells, leading to endocrine and exocrine dysfunction of the pancreas. T3cDM is heterogeneous and involves multifactorial underlying mechanisms consisting of the inflammation, fibrosis, and sclerosis of pancreatic tissue (2).
Prior studies have identified an association between an altered immune system and T2DM, and chronic inflammation has been implicated as a potential contributor to the development of DM and its complications (3–5). The neutrophil-to-lymphocyte ratio (NLR), a repeatable and affordable indicator of inflammation derived from peripheral blood, has been discussed as a potential biomarker to reflect the inflammatory status. Previous researchers have identified its role in the occurrence and progression of T2DM and T2DM-related complications (4, 6). Moreover, many studies found that the NLR was also an effective indicator for the severity or prognosis of pancreatic diseases, including acute pancreatitis and pancreatic cancer (7, 8). However, no studies have examined the relationship between the NLR and pancreatic diabetes. Therefore, we aimed to explore the association between the NLR and DM secondary to exocrine pancreatic disorders.
Methods
The medical database of patients with pancreatic disease who were admitted to the Department of Pancreatic Surgery, West China Hospital, during the period from August 2017 to October 2021 was retrospectively analyzed after approval of the institutional board. The inclusion criteria were as follows: patients with pancreatic disease confirmed by postoperative pathology and patients with pancreatic disease with plasma HbA1c levels and fasting plasma glucose (FPG) levels measured before surgery. Patients with acute inflammatory diseases, infectious diseases, immune disorders, glucagonomas, insulinomas, hepatic failure, previously diagnosed DM, serum creatinine levels >120 µmol/L, or those receiving hormone therapy were excluded. According to the criteria for inclusion, 291 patients were analyzed in this study, 100 of whom were diagnosed with pancreatic benign and low-grade tumors (PBLT), 45 of whom were diagnosed with chronic pancreatitis (CP), and 146 of whom were diagnosed with pancreatic ductal adenocarcinoma (PDAC).
Information on the participants’ demographics (age, sex, ethnicity), height and weight was collected from computerized records and databases. Body mass index (BMI) was calculated by dividing weight in kg by height in m2. Laboratory tests were performed within several minutes after blood samples were obtained before surgery. Plasma HbA1c, FPG, serum urea, creatinine, triglyceride, albumin, HDL and LDL cholesterol levels were obtained and recorded from the patient file system. We also recorded the hemoglobin level, white blood cell count, lymphocyte count, platelet count, neutrophil count, and monocyte count. An NLR, monocyte-to-lymphocyte ratio (MLR) or platelet-to-lymphocyte ration (PLR) value was obtained by dividing the number of neutrophils, monocytes, or platelets by the number of lymphocytes. A homeostasis model (HOMA-B and HOMA-IR) was used to measure beta-cell dysfunction and insulin resistance. DM is defined as a fasting blood glucose level (FBG) >7.0 mmol/L and a glycated hemoglobin (HbA1c) level > 6.5% or a postprandial blood glucose level (PBG) >11.1 mmol/L according to the 2010 American Diabetes Association guidelines.
Statistics Analysis
All statistical analyses were carried out using Statistical Package for Social Sciences (SPSS) Version 26. Categorical variables were expressed as percentages (%) and continuous variables were expressed as the mean ± SD or median and interquartile range (IQR). A P value <0.05 was considered statistically significant. Based on the median, NLR values were divided into two groups. Differences in baseline characteristics in the diabetic and nondiabetic groups were compared using χ2 tests for categorical variables and an independent sample t test or ManneWhitney U test for continuous variables. An analysis of multivariate logistic regression was conducted to examine the association between the NLR and diabetes, and odds ratios (ORs) and 95% confidence intervals (CIs) were calculated. Correlation analysis was assessed by Spearman’s rank test.
Results
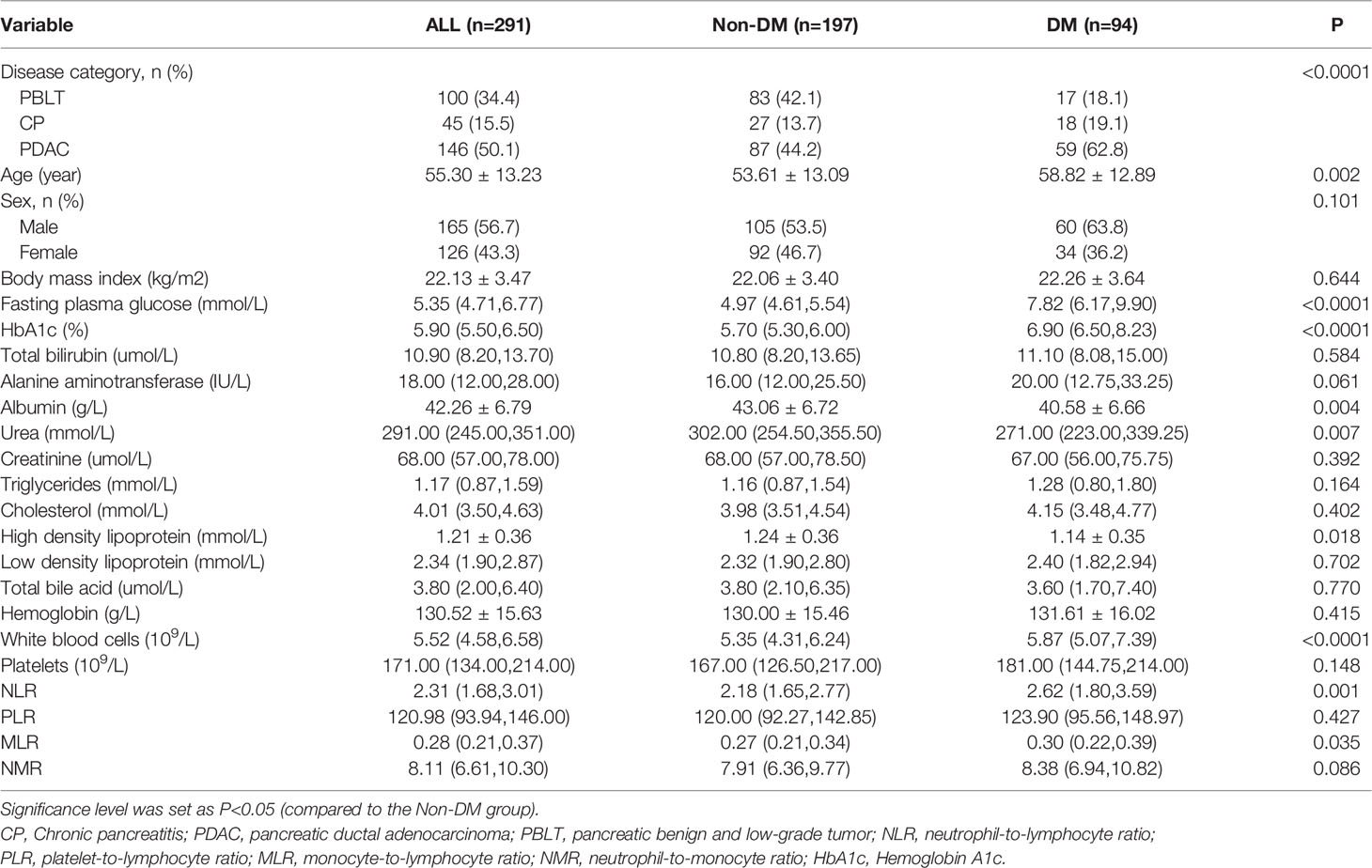
There were 291 patients with pancreatic disease in our study and 32.3% (n=94) of them were diagnosed with diabetes mellitus. Table 1 shows the biochemical and clinical characteristics of the patients. Age (P =0.002), FPG levels (P < 0.0001), HbA1C levels (P < 0.0001), white blood cell counts (P < 0.0001), NLR values (P=0.001), and MLR values (P=0.035) were higher for DM patients than for those without DM. Serum urea (P=0.007), albumin (P=0.004) and high-density lipoprotein (P=0.018) levels were significantly higher in patients with DM than in those without DM. In terms of BMI, PLR and NMR values, there were no significant differences between the groups.
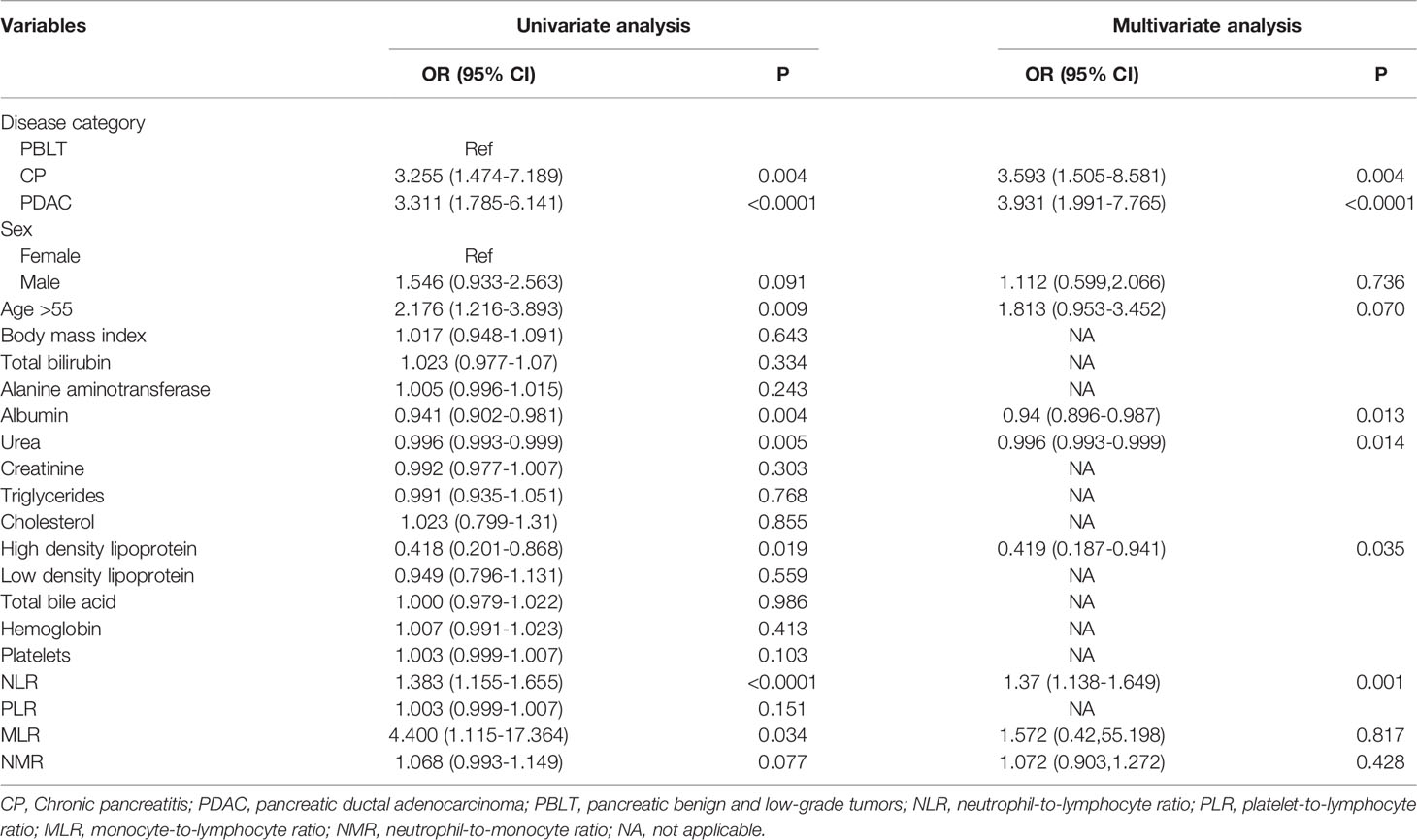
Table 2 shows the crude and adjusted correlations between the NLR and risk factors for DM. In the crude model, the unadjusted OR (95% CI) of DM was correlated with a gradual increase in the NLR value (OR: 1.383, 95% CI: 1.155-1.655, P<0.0001). In the final multivariate models, a gradual increase in the NLR value was an independent risk factor for diabetes secondary to exocrine pancreatic disorders (OR: 1.37, 95% CI: 1.138-1.649, P=0.001) after adjusting for covariates. On the other hand, higher values of albumin (OR: 0.94, 95CI%: 0.896-0.987, P=0.013), urea (OR: 0.996, 95CI%: 0.993-0.999, P=0.014 and high density lipoprotein (OR: 0.419, 95%CI: 0.187-0.941, P=0.035) were also as proved as predictors of DM among pancreatic disease.
A scatter plot of the Spearman correlation analysis is shown in Figure 1. The NLR was significantly positively correlated with FBG (r = 0.23, P<0.0001) and HOMA2-IR (r=0.17, P=0.02).No significances were observed between NLR and HbA1c (r=0.10, P=0.10), fasting insulin (r=0.12, P=0.10), fasting C-peptide (r=0.11, P=0.15) and HOMA2-B (r=0.13, P=0.07).

Figure 1 A scatter plot of the Spearman correlation analysis between the NLR and glucose-related index.
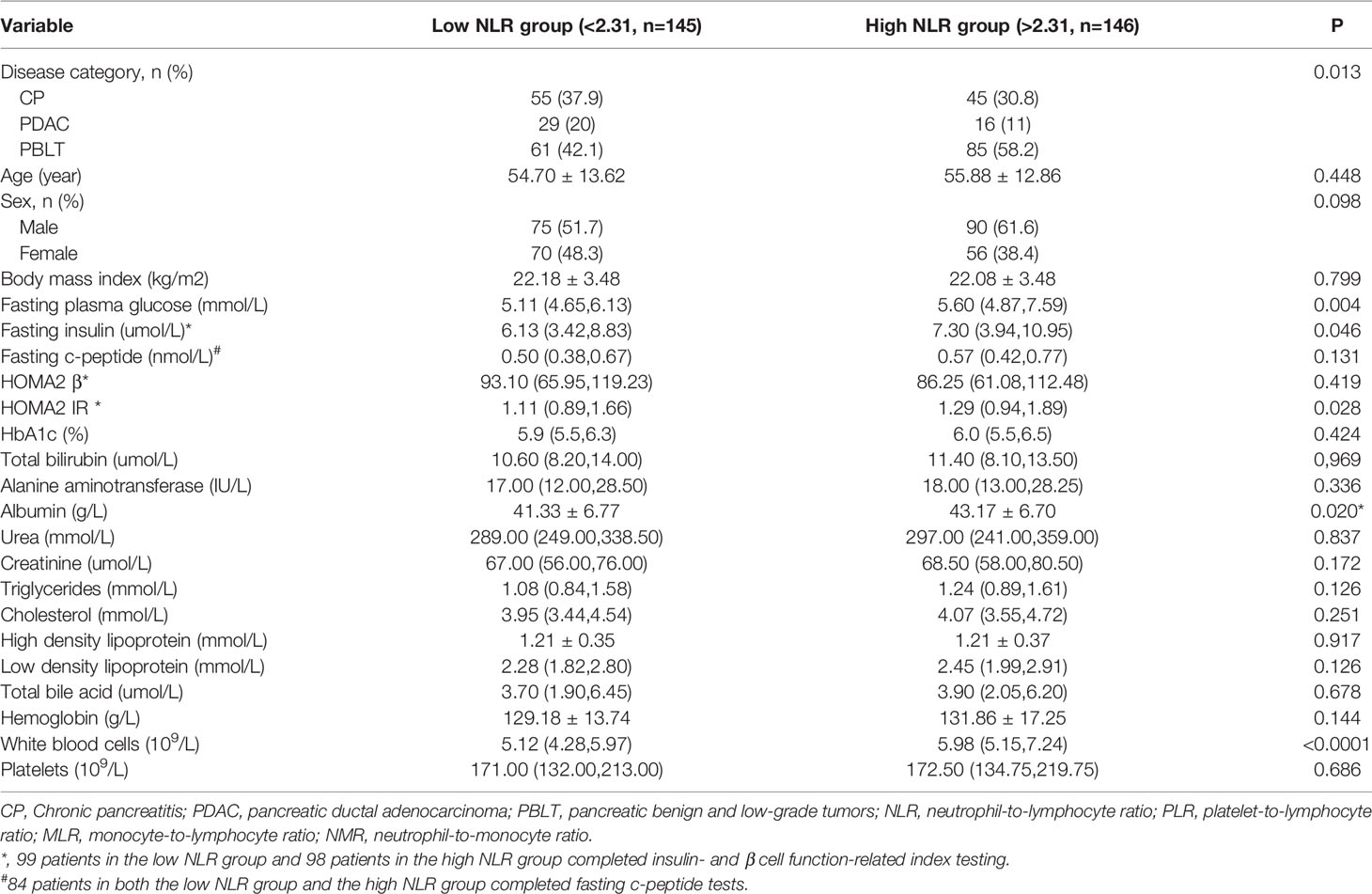
The stratified analysis of the clinical and demographic variables based on the median NLR value (2.31) is shown in Table 3. More patients with PDAC, but fewer patients with PBLT, had higher NLR values. Patients with higher NLR values had higher fasting insulin (P=0.046), FPG (P=0.004) and HOMA2-IR values (P=0.028). Patients with a higher NLR value were more likely to have higher albumin and white blood cell levels. No significant differences in age, BMI, HbA1c, HOMA2-B, fasting c-peptide or liver and kidney function parameters were observed (P > 0.05 for all).
Discussion
We present the first study demonstrating the link between the NLR and pancreatic diabetes and insulin resistance. In our study, we observed that patients with elevated NLR values that were associated with tumors or inflammation due to exocrine pancreatic diseases were more likely to develop diabetes. The prevalence of patients with diabetes secondary to exocrine pancreatic disease was higher in those with an NLR value > 2.31 than in those with an NLR value < 2.31. Moreover, HOMA-IR-measured insulin resistance was also positively associated with increases in the NLR. This finding indicates that a low-grade systemic inflammatory response may be considered an underlying factor that might contribute to the pathogenesis of pancreatic diabetes. While further research is needed to clarify this issue, we believe that elevated NLR values could be a significant predictor of diabetes secondary to exocrine pancreatic disorders.
Neutrophils respond to active, nonspecific inflammation as the first line of defense and can promote the chronic inflammatory state by recruiting macrophages and by interacting with antigen-presenting cells, whereas lymphocytes play a crucial role in the regulation and protection of inflammation (9). As opposed to the total WBC count, the NLR shows a dynamic aspect and has a better predictive value than the total leukocyte count (10). Although there are still many unknowns about the mechanisms behind the association between systemic inflammation and prevalent conditions, an increasing number of studies have established the utility of the NLR as a medically relevant biomarker. The NLR, as a repeatable and affordable biomarker calculated by peripheral blood, has been discussed as an innovative inflammatory biomarker to reflect the inflammatory status (11). Specifically, the NLR acts as an inflammatory factor, both by reducing the lymphocyte count and by increasing the neutrophil count.
However, the details of the mechanisms underlying the predictive value of the NLR are not entirely understood. Accumulated epidemiological evidence has identified that the inflammatory response is involved throughout the course of diabetes and cancer, and furthermore, systemic inflammation has been described as a potential pathogenic factor for the development of diabetes mellitus and its complications (3, 4). Recently, Wan et al. (12) also revealed that elevated NLR values are associated with a risk not only for cardiovascular and cerebrovascular diseases but also for diabetic kidney disease. In addition, a large number of studies have demonstrated the association between the NLR and pancreatic diseases, including PDAC and pancreatitis (7, 13, 14). A high NLR value plays an important role throughout the course of pancreatic cancer, and it could be a novel marker for survival evaluation and could help clinicians develop therapeutic strategies for pancreatic cancer patients (7, 15, 16). The NLR value alone or combined with the CA199 level could allow earlier diagnosis of PDAC in T2DM patients (16, 17). Dong et al. observed significantly higher NLR and PLR values in PDAC patients with T2DM than in patients with T2DM alone and healthy controls (17). Similarly, the NLR was used as a novel serum marker to predict the severity of acute pancreatitis and its adverse events (18, 19). Previous studies have revealed a significant association between CP and altered total leukocyte and lymphocyte counts (20, 21). Our study is in agreement with the previous results of NLR values among patients with exocrine pancreatic diseases regardless of their diabetic status, which indicate that elevated NLR values due to the existence of tumors or inflammation from exocrine pancreatic disorders were found to be associated with an increased risk for DM. The prevalence of diabetes secondary to exocrine pancreatic disease was higher in patients with an NLR value > 2.31 than in those with an NLR < 2.31. Together, growing evidence suggests that the NLR value is a significant predictor of diabetes secondary to exocrine pancreatic disorders.
T3cDM is heterogeneous and involves multifactorial underlying mechanisms consisting of the inflammation, fibrosis, and sclerosis of pancreatic tissue (2). The initial process of pancreatic disease, such as pancreatic cancer and pancreatitis, involves a multifactorial response involving the inflammation-mediated injury of endocrine and exocrine cells (18, 22–24). Previously, researchers showed a link between a high NLR value and varying degrees of glucose intolerance and insulin resistance in patients with T2DM (25–27). Shiny et al. observed that NLR values increased with the severity of T2DM and were positively related to HbA1c (r=0.411, P<0.0001), FPG (r=0.384, P<0.0001) and IR (r=0.233, P<0.0001) (26). Another study showed a significant positive correlation between the NLR and HOMA-IR (r = 0.285, P < 0.001). The IR odds ratio increased by a factor of 7.231 (95% CI, 4.277-12.223) for every one-unit increase in the NLR (26). The present study is in accordance with other reports that found that elevated NLR values were positively associated with high insulin resistance, which indicates that the NLR was significantly positively correlated with FPG levels and HOMA2-IR values. Although no significance was found between NLR and HOMA2-B, a trend that the negative correlation between the both was observed. The neutrophil effect may lead not only to insulin resistance but also to dysfunction of islet β cells and further studies should be performed to clarify it.
The details of the mechanisms underlying the association between the NLR and DM are not entirely understood, especially for T3cDM. Increased neutrophil levels may mediate IR in part through increased inflammation (28, 29). Increased NLR values appear to underlie the increased levels of proinflammation, as evident from the persistent neutrophil activation, hypersecretion of inflammatory factors, and enhanced release of neutrophil elastase, and induce insulin resistance, leading to subsequent overt diabetes (30). Moreover, the inflammatory environment might partly explain the β-cell dysfunction observed in chronic pancreatitis and PDAC. A previous study demonstrated increased inflammatory cell infiltration near islet β cells and reduced beta cell identity in chronic pancreatitis and PDAC (31, 32). In contrast, the effects of hyperglycemia on neutrophil apoptosis have been studied, which result in impairments in neutrophil clearance and prolonged inflammation in mice with diabetes (33). An increase in apoptosis has been documented both in rats with diabetes and in patients with diabetes, and elevated levels of oxidative DNA damage have been found in peripheral blood lymphocytes (34). Hyperglycemia due to DM is associated with the increased activation of leukocytes and their subtypes (35). In addition, hyperglycemia promotes the suppression of cytokine signaling action, thereby impairing insulin release and signaling cascades (36). This implies that improvement in glycemic control might suppress the inflammatory response, which could support the link between glucose metabolism disorders and inflammation. Future studies should focus on this pancreatic disease due to its important foundation for the development of pancreatic diabetes.
Limitations of the present study should be noted. One of the limitations of this study was that the determination of diabetes through HbA1c and fasting blood glucose levels might have affected the definition of diabetes and the analysis results. In addition, the present study had a retrospective cross-sectional design and did not allow us to investigate causal associations between glucose intolerance and the NLR. With a prospective design and multiple measurements of the NLR, future research should be able to provide strong evidence on the NLR as a subclinical inflammatory indicator for diabetes.
Conclusions
In conclusion, the NLR is significantly increased in patients with diabetes secondary to exocrine pancreatic disorders and is associated with insulin resistance. NLR values may be reliable predictive markers for diabetes among patients with exocrine pancreatic disorders.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Ethics Statement
The study was reviewed and approved by the Medical Ethics Committee of West China Hospital at Sichuan University. The patients/participants provided their written informed consent to participate in this study. All study participants or their legal guardians provided informed written consent about personal and medical data collection prior to study enrollment.
Author Contributions
GC and CT contributed equally to this paper. YC and XL designed the research. GC, CT, YC and XL performed research and analyzed data. GC, CT and YC wrote the manuscript. All authors read and approved the final manuscript.
Funding
This study was supported by Research grants from the Key Research and Development Projects in Sichuan Province (2019YFS0043) and the 1·3·5 Project for Disciplines of Excellence, West China Hospital, Sichuan University (ZY2017302−1.3.5).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2022.957129/full#supplementary-material
References
1. Ewald N, Bretzel RG. Diabetes Mellitus Secondary to Pancreatic Diseases (Type 3c)–are We Neglecting an Important Disease? Eur J Intern Med (2013) 24:203–6. doi: 10.1016/j.ejim.2012.12.017
2. Wynne K, Devereaux B, Dornhorst A. Diabetes of the Exocrine Pancreas. J Gastroenterol Hepatol (2019) 34:346–54. doi: 10.1111/jgh.14451
3. Donath MY, Shoelson SE. Type 2 Diabetes as an Inflammatory Disease. Nat Rev Immunol (2011) 11:98–107. doi: 10.1038/nri2925
4. Lorenzo C, Hanley AJ, Haffner SM. Differential White Cell Count and Incident Type 2 Diabetes: The Insulin Resistance Atherosclerosis Study. Diabetologia (2014) 57:83–92. doi: 10.1007/s00125-013-3080-0
5. Ferrucci L, Fabbri E. Inflammageing: Chronic Inflammation in Ageing, Cardiovascular Disease, and Frailty. Nature Reviews. Cardiology (2018) 15:505–22. doi: 10.1038/s41569-018-0064-2
6. Huang W, Huang J, Liu Q, Lin F, He Z, Zeng Z, et al. Neutrophil-Lymphocyte Ratio is a Reliable Predictive Marker for Early-Stage Diabetic Nephropathy. Clin Endocrinol (2015) 82:229–33. doi: 10.1111/cen.12576
7. Yang JJ, Hu ZG, Shi WX, Deng T, He SQ, Yuan SG. Prognostic Significance of Neutrophil to Lymphocyte Ratio in Pancreatic Cancer: A Meta-Analysis. World J Gastroenterol (2015) 21:2807–15. doi: 10.3748/wjg.v21.i9.2807
8. Wang Y, Fuentes HE, Attar BM, Jaiswal P, Demetria M. Evaluation of the Prognostic Value of Neutrophil to Lymphocyte Ratio in Patients With Hypertriglyceridemia-Induced Acute Pancreatitis. Pancreatology (2017) 17:893–7. doi: 10.1016/j.pan.2017.10.001
9. Bhutta H, Agha R, Wong J, Tang TY, Wilson YG, Walsh SR. Neutrophil-Lymphocyte Ratio Predicts Medium-Term Survival Following Elective Major Vascular Surgery: A Cross-Sectional Study. Vasc Endovascular Surg (2011) 45:227–31. doi: 10.1177/1538574410396590
10. Sun T, Meng F, Zhao H, Yang M, Zhang R, Yu Z, et al. Elevated First-Trimester Neutrophil Count Is Closely Associated With the Development of Maternal Gestational Diabetes Mellitus and Adverse Pregnancy Outcomes. Diabetes (2020) 69:1401–10. doi: 10.2337/db19-0976
11. Hashemi Moghanjoughi P, Neshat S, Rezaei A, Heshmat-Ghahdarijani K. Is the Neutrophil-To-Lymphocyte Ratio an Exceptional Indicator for Metabolic Syndrome Disease and Outcomes? Endocrine Pract (2022) 28:342–8. doi: 10.1016/j.eprac.2021.11.083
12. Wan H, Wang Y, Fang S, Chen Y, Zhang W, Xia F, et al. Associations Between the Neutrophil-To-Lymphocyte Ratio and Diabetic Complications in Adults With Diabetes: A Cross-Sectional Study. J Diabetes Res (2020) 2020:6219545. doi: 10.1155/2020/6219545
13. Sugiura T, Uesaka K, Kanemoto H, Mizuno T, Okamura Y. Elevated Preoperative Neutrophil-to-Lymphocyte Ratio as a Predictor of Survival After Gastroenterostomy in Patients With Advanced Pancreatic Adenocarcinoma. Ann Surg Oncol (2013) 20:4330–7. doi: 10.1245/s10434-013-3227-8
14. McLellan P, Henriques J, Ksontini F, Doat S, Hammel P, Desrame J, et al. Prognostic Value of the Early Change in Neutrophil-to-Lymphocyte Ratio in Metastatic Pancreatic Adenocarcinoma. Clinics Res Hepatol Gastroenterol (2021) 45:101541. doi: 10.1016/j.clinre.2020.08.016
15. Shusterman M, Jou E, Kaubisch A, Chuy JW, Rajdev L, Aparo S, et al. The Neutrophil-To-Lymphocyte Ratio is a Prognostic Biomarker in An Ethnically Diverse Patient Population With Advanced Pancreatic Cancer. J Gastrointest Cancer (2020) 51:868–76. doi: 10.1007/s12029-019-00316-8
16. Qin S, Lu Y, Chen S, Hu Z, Chen H, Zhong J, et al. The Relationship of Neutrophil-To-Lymphocyte Ratio or Platelet-To-Lymphocyte Ratio and Pancreatic Cancer in Patients With Type 2 Diabetes. Clin Lab (2019) 65:81226. doi: 10.7754/Clin.Lab.2019.181226
17. Dong B, Wu RR. Neutrophil-To-Lymphocyte Ratio or Platelet-to-Lymphocyte Ratio is a Predictive Factor of Pancreatic Cancer Patients With Type 2 Diabetes. Hepatobil Pancreatic Dis International: HBPD Int (2022) 21:202–4. doi: 10.1016/j.hbpd.2020.08.010
18. Kong W, He Y, Bao H, Zhang W, Wang X. Diagnostic Value of Neutrophil-Lymphocyte Ratio for Predicting the Severity of Acute Pancreatitis: A Meta-Analysis. Dis Markers (2020) 2020:9731854. doi: 10.1155/2020/9731854
19. Suppiah A, Malde D, Arab T, Hamed M, Allgar V, Smith AM, et al. The Prognostic Value of the Neutrophil-Lymphocyte Ratio (NLR) in Acute Pancreatitis: Identification of an Optimal NLR. J Gastrointest Surg (2013) 17:675–81. doi: 10.1007/s11605-012-2121-1
20. Ockenga J, Jacobs R, Kemper A, Benschop RJ, Schmidt RE, Manns MP. Lymphocyte Subsets and Cellular Immunity in Patients With Chronic Pancreatitis. Digestion (2000) 62:14–21. doi: 10.1159/000007772
21. Antal L, Kávai M, Szabó G, Sonkoly I, Pálóczi K, Szegedi G, et al. Immunological Investigations in Acute and Chronic Human Pancreatitis. Digestion (1980) 20:100–5. doi: 10.1159/000198425
22. Xiang ZJ, Hu T, Wang Y, Wang H, Xu L, Cui N. Neutrophil-Lymphocyte Ratio (NLR) was Associated With Prognosis and Immunomodulatory in Patients With Pancreatic Ductal Adenocarcinoma (PDAC). Biosci Rep (2020) 40:BSR20201190. doi: 10.1042/BSR20201190
23. Jayedi A, Emadi A, Shab-Bidar S. Dietary Inflammatory Index and Site-Specific Cancer Risk: A Systematic Review and Dose-Response Meta-Analysis. Adv Nutr (Bethesda Md.) (2018) 9:388–403. doi: 10.1093/advances/nmy015
24. Roxburgh CS, McMillan DC. Role of Systemic Inflammatory Response in Predicting Survival in Patients With Primary Operable Cancer. Future Oncol (London England) (2010) 6:149–63. doi: 10.2217/fon.09.136
25. Lee CT, Harris SB, Retnakaran R, Gerstein HC, Perkins BA, Zinman B, et al. White Blood Cell Subtypes, Insulin Resistance and β-Cell Dysfunction in High-Risk Individuals–the PROMISE Cohort. Clin Endocrinol (2014) 81:536–41. doi: 10.1111/cen.12390
26. Shiny A, Bibin YS, Shanthirani CS, Regin BS, Anjana RM, Balasubramanyam M, et al. Association of Neutrophil-Lymphocyte Ratio With Glucose Intolerance: An Indicator of Systemic Inflammation in Patients With Type 2 Diabetes. Diabetes Technol Ther (2014) 16:524–30. doi: 10.1089/dia.2013.0264
27. Lou M, Luo P, Tang R, Peng Y, Yu S, Huang W, et al. Relationship Between Neutrophil-Lymphocyte Ratio and Insulin Resistance in Newly Diagnosed Type 2 Diabetes Mellitus Patients. BMC Endocrine Disord (2015) 15:9. doi: 10.1186/s12902-015-0002-9
28. Zanetti M, Barazzoni R, Guarnieri G. Inflammation and Insulin Resistance in Uremia. J Renal Nutr (2008) 18:70–5. doi: 10.1053/j.jrn.2007.10.015
29. Shim WS, Kim HJ, Kang ES, Ahn CW, Lim SK, Lee HC, et al. The Association of Total and Differential White Blood Cell Count With Metabolic Syndrome in Type 2 Diabetic Patients. Diabetes Res Clin Pract (2006) 73:284–91. doi: 10.1016/j.diabres.2006.02.001
30. Buyukkaya E, Karakas MF, Karakas E, Akçay AB, Tanboga IH, Kurt M, et al. Correlation of Neutrophil to Lymphocyte Ratio With the Presence and Severity of Metabolic Syndrome. Clin Appl Thrombosis/hemostasis (2014) 20:159–63. doi: 10.1177/1076029612459675
31. Sun J, Ni Q, Xie J, Xu M, Zhang J, Kuang J, et al. β-Cell Dedifferentiation in Patients With T2D With Adequate Glucose Control and Nondiabetic Chronic Pancreatitis. J Clin Endocrinol Metab (2019) 104:83–94. doi: 10.1210/jc.2018-00968
32. Wang Y, Ni Q, Sun J, Xu M, Xie J, Zhang J, et al. Paraneoplastic β Cell Dedifferentiation in Nondiabetic Patients With Pancreatic Cancer. J Clin Endocrinol Metab (2020) 105:1489–1503. doi: 10.1210/clinem/dgz224
33. Alba-Loureiro C, Munhoz CD, Martins JO, Cerchiaro GA, Scavone C, Curi R, et al. Neutrophil Function and Metabolism in Individuals With Diabetes Mellitus. Braz. J Med Biol Res (2007) 40:1037–44. doi: 10.1590/s0100-879x2006005000143
34. Adaikalakoteswari A, Rema M, Mohan V, Balasubramanyam M. Oxidative DNA Damage and Augmentation of Poly(ADP-Ribose) Polymerase/Nuclear Factor-Kappa B Signaling in Patients With Type 2 Diabetes and Microangiopathy. Int J Biochem Cell Biol (2007) 39:1673–84. doi: 10.1016/j.biocel.2007.04.013
35. de Vries MA, Alipour A, Klop B, van de Geijn GJ, Janssen HW, Njo TL, et al. Glucose-Dependent Leukocyte Activation in Patients With Type 2 Diabetes Mellitus, Familial Combined Hyperlipidemia and Healthy Controls. Metabol: Clin Exp (2015) 64:213–7. doi: 10.1016/j.metabol.2014.10.011
Keywords: diabetes mellitus, neutrophil-to-lymphocyte ratio, exocrine pancreatic disorders, inflammation index, pancreatic adenocarcinoma
Citation: Chen G, Tan C, Liu X and Chen Y (2022) Association Between the Neutrophil-To-Lymphocyte Ratio and Diabetes Secondary to Exocrine Pancreatic Disorders. Front. Endocrinol. 13:957129. doi: 10.3389/fendo.2022.957129
Received: 30 May 2022; Accepted: 22 June 2022;
Published: 22 July 2022.
Edited by:
Alok Raghav, Ganesh Shankar Vidyarthi Memorial Medical College, IndiaCopyright © 2022 Chen, Tan, Liu and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yonghua Chen, Y2hlbnlvbmdoaXVhMjAwN0AxNjMuY29t
†These authors have contributed equally to this work and share first authorship
 Guanhua Chen
Guanhua Chen Chunlu Tan†
Chunlu Tan† Xubao Liu
Xubao Liu Yonghua Chen
Yonghua Chen