
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Endocrinol., 27 July 2022
Sec. Thyroid Endocrinology
Volume 13 - 2022 | https://doi.org/10.3389/fendo.2022.952113
This article is part of the Research TopicThermal Ablation of Thyroid NodulesView all 8 articles
Background: Papillary thyroid cancer (PTC) is the most common thyroid tumor, and early diagnosis and treatment can effectively improve prognosis. Many controversies surround the treatment method of T1N0M0 PTC. Recently, thermal ablation (TA) has shown some benefits in the treatment of PTC patients, but the safety and efficacy of its treatment remain controversial. This article performs a meta-analysis of TA in patients with T1aN0M0 and T1bN0M0 PTC.
Methods: The PubMed, Embase, Web of Science, and Cochrane Library databases were systematically searched for retrospective or prospective studies of TA for treating patients with T1N0M0 PTC from the database establishment to May 1, 2022. Data on volume reduction rate (VRR), disease progress, and complication rate were collected. In addition, a meta-analysis was performed using the Stata 12.0 and Review Manager 5.3.
Results: A total of 9 eligible studies were included. Our study demonstrated the effectiveness of VRR and disease progress. The VRR was reduced after 3 months (−75.90%; 95% CI [−118.46–33.34%]), 6 months (34.33%; 95% CI [15.01–53.65%]), 12 months (78.69%; 95% CI [71.69–85.68%]), and 24 months (89.97%; 95% CI [84.00–95.94%]). The disease progress was 1.9% (95% CI [1.1–3.0]). Safety is justified by the complication rate, which was 6.5% (95% CI [3.5–10.2]). Pain and hoarseness were the most common complications, and no life-threatening complications were reported. Egger’s test demonstrated that publication bias was acceptable.
Conclusions: TA is an effective and safe method for managing T1aN0M0 and T1bN0M0 papillary thyroid nodules.
Thyroid cancer currently ranks fifth among cancers affecting women worldwide, accounting for almost 5% of the whole cancer burden in female patients (1). Papillary thyroid carcinoma (PTC) is the most frequent type of thyroid cancer, accounting for up to 90% of all thyroid malignancies (2). In spite of the fact that well-differentiated PTC may remain inactive (3), distant metastases and the recurrence rates are approximately 50% and 20%, respectively (4). T1N0M0 PTC is a subgroup of PTC that is characterized by a diameter equal to or less than 2 cm (T1a: ≤1 cm; T1b: >1 cm, ≤2 cm) (5).
Thermal ablation (TA) methods such as radiofrequency ablation (RFA), microwave ablation (MWA), and laser ablation (LA) have recently been deployed for thyroid nodules (6–10). Some studies have reported that TA is a rather effective and safe treatment because of its low incidence of tumor metastasis and complications (11–13). Although TA has been considered a suggested therapy method for T1N0M0 PTC, the results are not reliable enough for the short follow-up duration and small sample size in previous studies. Accordingly, it is necessary to collect and aggregate published data regarding the performance of TA for T1N0M0 PTC and to analyze the efficacy and safety of ablation techniques.
This meta-analysis aims to evaluate the efficacy and safety of TA in treating patients with T1N0M0 PTC by variables such as tumor volume reduction rate (VRR), disease progress, and complication rate and to explore factors causing heterogeneity.
We collected our data with the articles published in PubMed, Web of Science, Embase, and Cochrane Library from the establishment of the database to May 1, 2022. The search MESH terms were as follows: [(“papillary thyroid cancer” OR “papillary thyroid carcinoma” OR “papillary thyroid microcarcinoma” OR “thyroid micropapillary carcinoma” OR “papillary thyroid micro-carcinoma”) AND (“radiofrequency ablation” OR “RFA” OR “laser ablation” OR “LA” OR “microwave ablation” OR “MWA” OR “thermal ablation”)]. Article‐related data were further evaluated to identify potential additional eligible data.
Studies according to all of the following criteria were included: (1) ultrasound-guided biopsy confirmed PTC without distant metastases; 2 cm or less in maximum diameter. (2) patients received only one modality of LA, RFA, or MWA; (3) the experimental methods used in the study were randomized controlled trials, retrospective analysis, or prospective analysis; and (4) the experimental results included VRR, disease progress, and complication rate.
Exclusion criteria were as follows: (1) patients who have not been treated with ablation; (2) other types of study: review, case reports, letters, and comments; and (3) patient and data overlapping studies.
The following characteristics were extracted from the included studies: (1) study characteristics: the first author’s family name, publication year, study period, design style, and treatment methods; (2) basic characteristics of the patients: mean age, sample size, sex ratio, and follow‐up interval; and (3) postoperative characteristics of the patients: VRR, disease progress, and postoperative complications. The data were extracted by two researchers independently, and when data are at odds, discussion was conducted by the two researchers.
Because all the studies included in the analysis here were non-randomized controlled trials, this quality assessment adopted the Newcastle–Ottawa Scale (NOS) (14). Two researchers independently conducted literature quality evaluations using the NOS for cohort study. NOS includes 4 items (4 points) for “Research Subject Selection”, 1 item (2 points) for “Comparability between Groups”, and 3 items (3 points) for “Result Measurement”. The scale is set with a full score of 9 points. When a study ≥7, it is regarded as high-quality literature. Correspondingly, a study with a score of lower than 7 is considered lower-quality literature. When the opinions are inconsistent, it is decided through discussion or consultation with the third person.
Statistical analysis was conducted with Stata 12.0. Continuous variables are presented as the means with standard error (SE). For each continuous variable comparison, the mean difference (MD) was calculated with a 95% confidence interval (CI). p < 0.05 is considered to be statistically significant. The I2 test evaluates heterogeneity, and I2 > 50% indicates significant heterogeneity. When there is significant heterogeneity, the random effect model (DerSimonian–Laird method) is used to calculate the combined effect; otherwise, the fixed model (Mantel–Haenszel method) is used instead. Egger’s test of Stata12.0 version was used to assess possible publication bias.
The study screening procedure is presented in a PRISMA flowchart (Figure 1). A total of 3,060 related articles were collected by searching the databases. After removing 2,272 duplicate articles, 386 review articles, 60 case reports, and 306 unrelated studies, only 36 articles need to be reviewed. To avoid data duplication, only one study of the same set of data from the same team was employed. Finally, 9 articles including 1,215 patients were ultimately enrolled in data synthesis and meta-analysis (15–23). Within the eligible studies, 1 study was prospectively designed while the remaining studies were retrospective. The overall quality of the studies was high based on NOS. All study characteristics are displayed in Table 1. In addition, 6 studies collected data on T1aN0M0 PTC, while other ones focused on T1bN0M0 PTC.
The overall pooled estimates for the mean difference (MD) of VRR (the unit is mm3) at 3 months, 6 months, 12 months, and 24 months after TA are −75.90% (95% CI, −118.46 to −33.34; p = 0.000; Figure 2A), 34.33% (95% CI, 15.01–53.65; p = 0.000; Figure 2B), 78.69% (95% CI, 71.69–85.68; p = 0.000; Figure 2C), and 89.97% (95% CI, 84.00–95.94; p < 0.00001; Figure 2D), respectively.
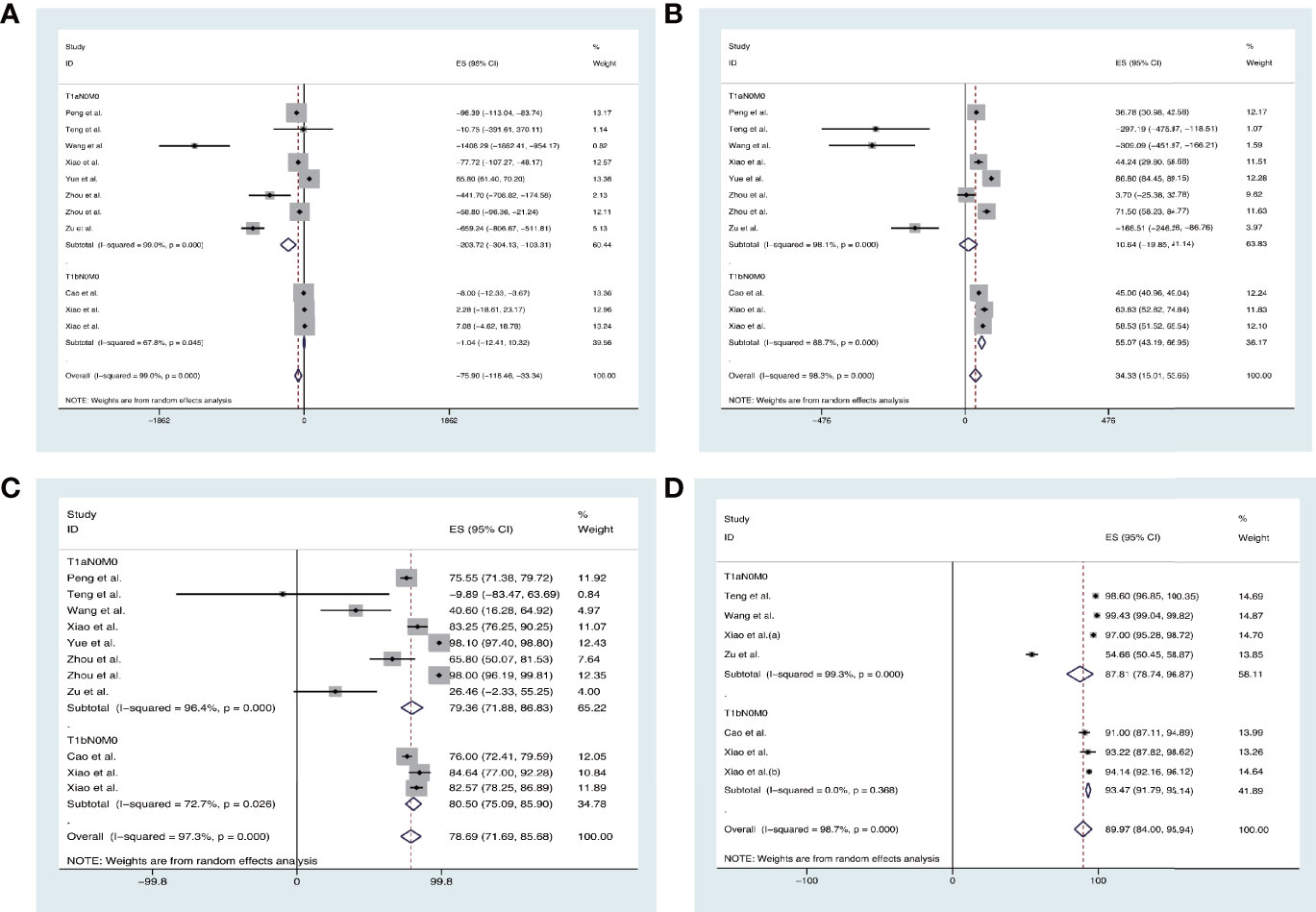
Figure 2 Forest plot of the effect of thermal ablations subgrouped by T1aN0M0 and T1bN0M0 on VAA (A) 3 months, (B) 6 months, (C) 12 months, and (D) 24 months after intervention.
We also conducted the analysis subgrouped by the different ablation methods. The pooled estimates for the mean difference (MD) of VRR (the unit is mm3) at 3 months, 6 months, 12 months, and 24 months after MWA were −225.96% (95% CI, −271.58 to −180.33), −99.98% (95% CI, −145.60–54.35), 55.01% (95% CI, 9.39–100.63), and 98.34% (95% CI, 31.38–165.31), respectively. The pooled estimates for MD of VRR at 3 months, 6 months, 12 months, and 24 months after RFA were −9.01% (95% CI, −104.75–86.73), 58.85% (95% CI, −36.89–154.59), 83.46% (95% CI, −12.28–179.20), and 93.69% (95% CI, −2.05–189.43), respectively (Figure 3).
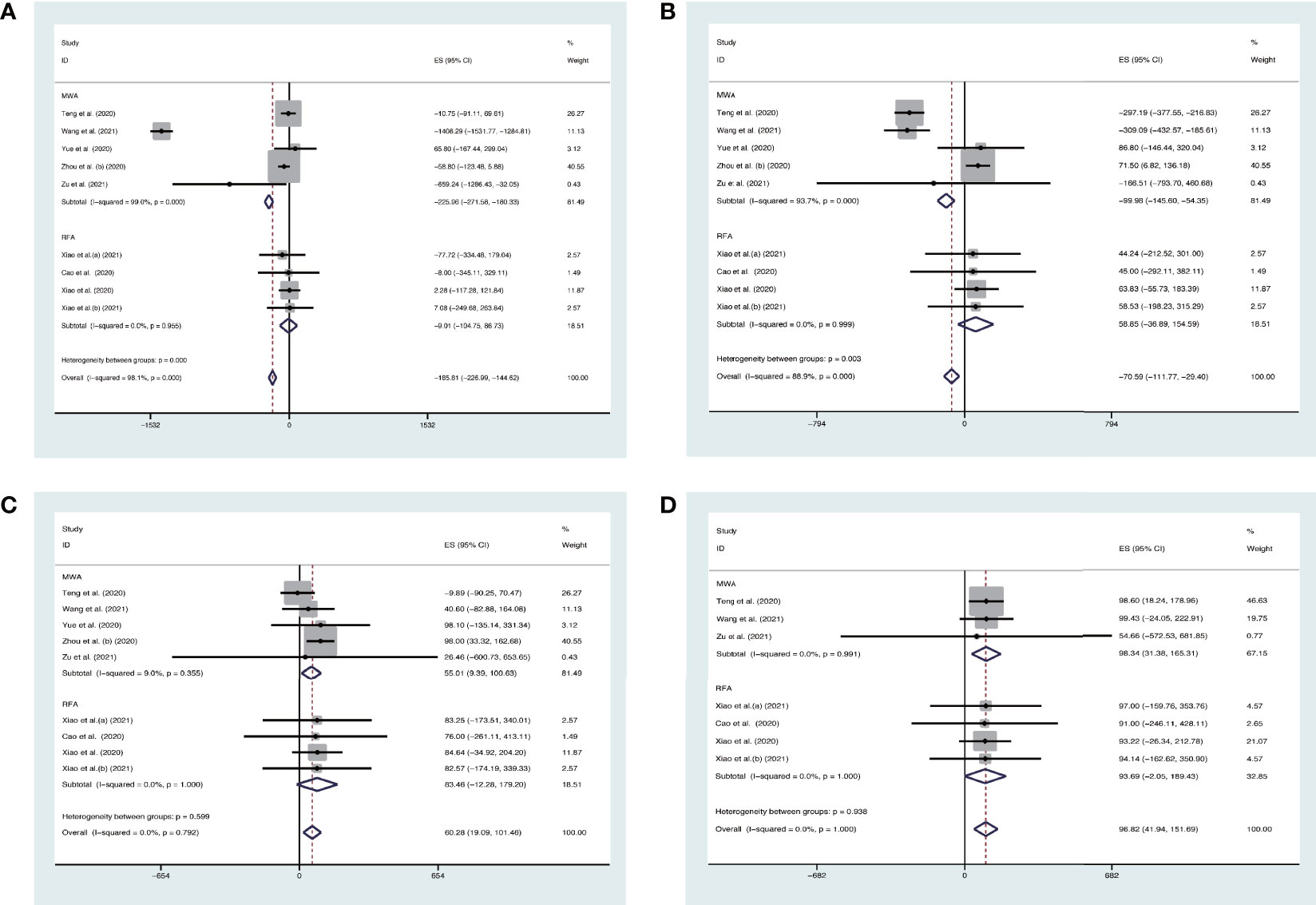
Figure 3 Forest plot of the effect of thermal ablations subgrouped by MWA and RFA on VAA (A) 3 months, (B) 6 months, (C) 12 months, and (D) 24 months after intervention.
Disease progress covered in these articles includes new tumor, tumor recurrence, and lymph node metastasis. At the end of the follow-up, the overall disease progress rate was 1.9% (95% CI, 1.1–3.0%, Figure 4A). Specifically, it was 1.5% (95% CI, 0.6–2.8) in T1aN0M0 PTC and 2.8% (95% CI, 1.2–5.1%) in T1bN0M0 PTC. At the end of follow-up, the disease progress rate was 1.0% (95% CI, 0.1–2.5%) in MWA and 3.1% (95% CI, 1.6–4.9%) in RFA (Figure 4B). Egger’s test confirmed that the result may have publication offset in both T1N0M0 studies in the analysis (p < 0.05, Figure 5A) and the studies that were involved in MWA and RFA as the ablation methods (p < 0.05, Figure 5B).
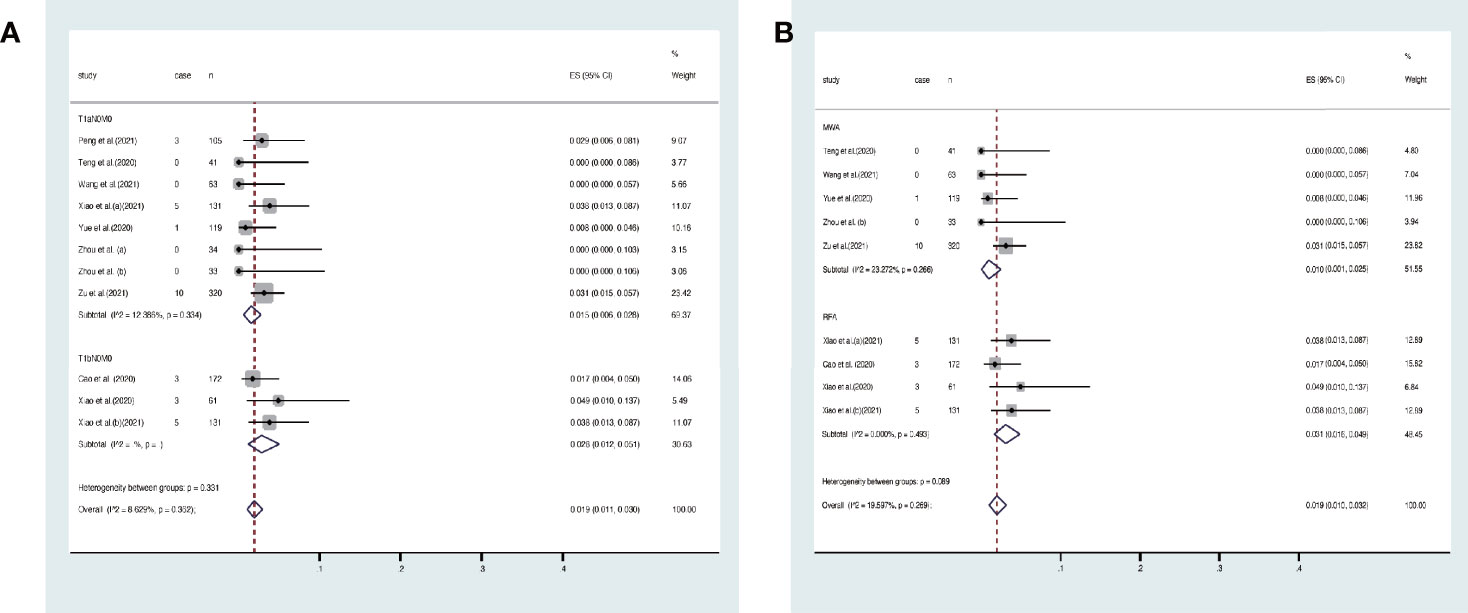
Figure 4 Forest plot of the overall disease progress rate after thermal ablations: (A) T1aN0M0 and T1bN0M0 studies, (B) MWA and RFA studies.
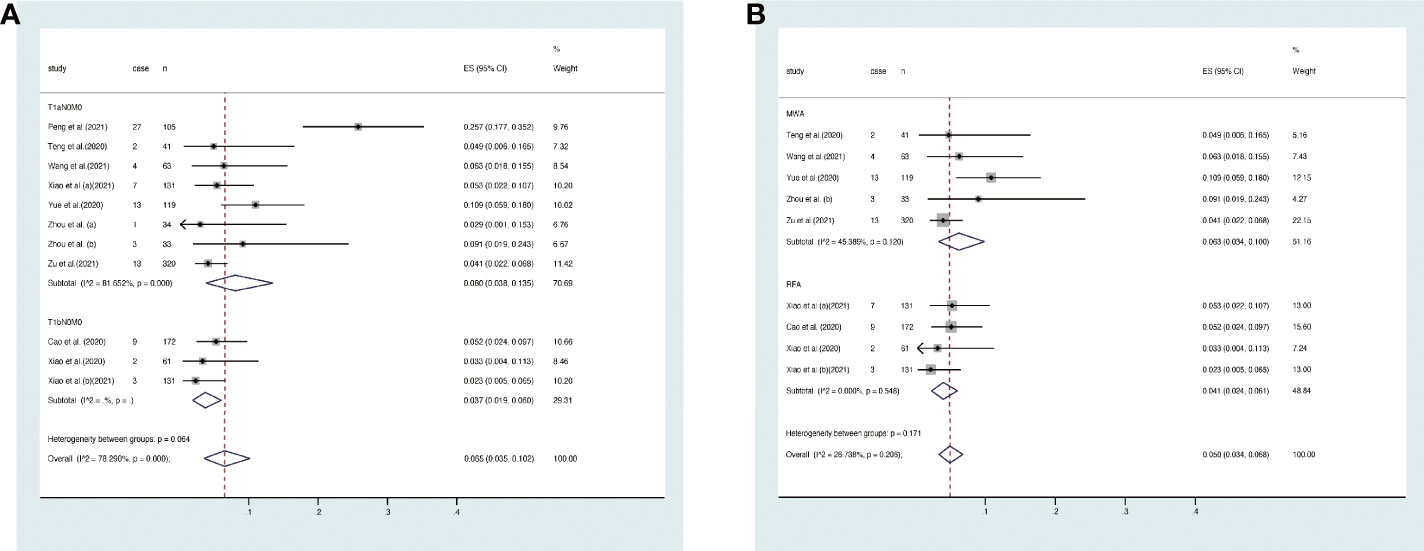
Figure 5 Egger’s funnel plot of the overall disease progress rate: (A) T1aN0M0 and T1bN0M0 studies, (B) MWA and RFA studies.
The overall complication rate was 6% (95% CI, 4–9%, Figure 6A). Specifically, it was 8% (95% CI, 4–12%) in T1aN0M0 PTC and 3% (95% CI, 1–5%) in T1bN0M0 PTC. The result of Egger’s test showed an acceptable publication bias (p > 0.05, Figure 7) in the included studies of T1aN0M0 and T1aN0M0 PTC. When we subgrouped the studies with different ablation methods, the complication rate was 6.3% (95% CI, 3.4–10.0%) in MWA and 4.1% (95% CI, 2.4–6.1%) in RFA (Figure 6B). It is confirmed by Egger’s test (Figure 7) that the result may have publication offset in the studies of MWA and RFA (p < 0.05).
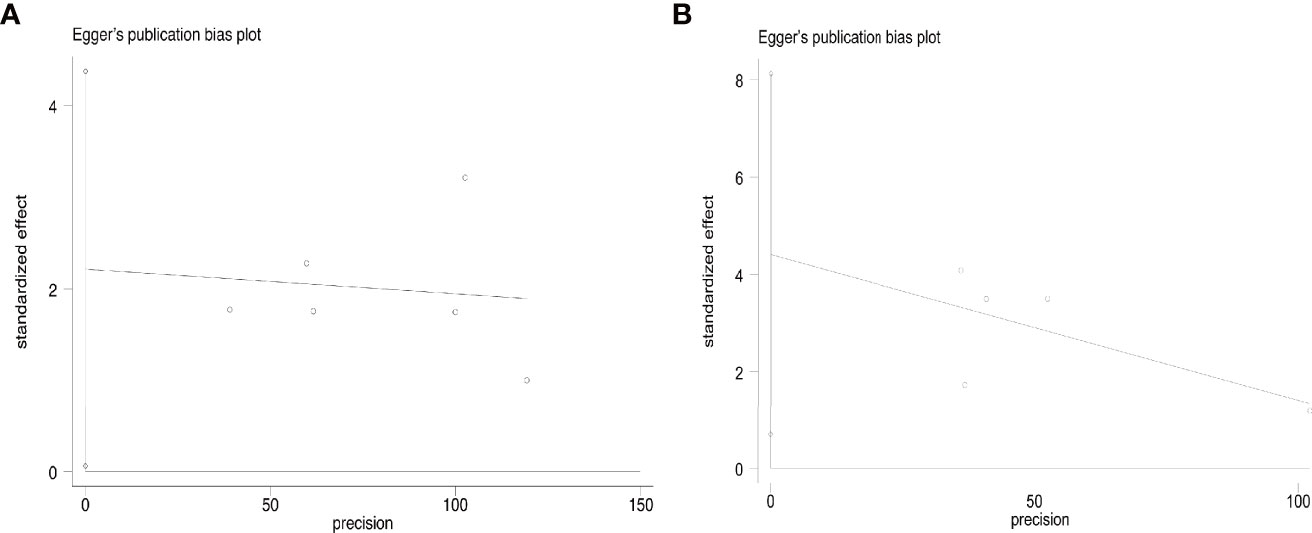
Figure 6 Forest plot of complication rate after thermal ablations: (A) T1aN0M0 and T1bN0M0 studies, (B) MWA and RFA studies.
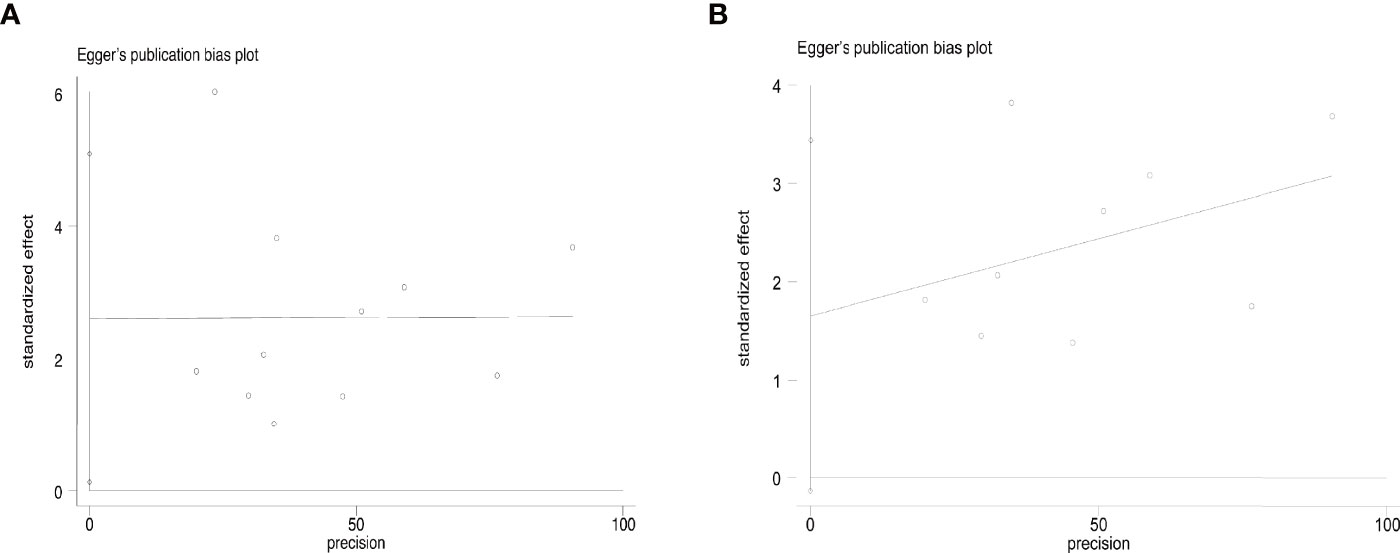
Figure 7 Egger’s funnel plot of the complication rate: (A) T1aN0M0 and T1bN0M0 studies, (B) MWA and RFA studies.
This meta-analysis revealed that US-guided TA is a valid therapeutic method for T1N0M0 PTC. At 24 months after ablation, we observed that VRR was 87.81% in T1aN0M0 PTC and 93.47% in T1bN0M0 PTC. The VRR of T1bN0M0 PTC was preferable than that of T1aN0M0 PTC at 24 months of follow-up, probably because the initial volume of the nodules in T1bN0M0 was greater than that of T1aN0M0, so the relative VRR after TA treatment was more considerable. Though the tumor VRR was not 100% in both subgroups, this may be caused by the following two reasons. First, the absorption of residual tumors by the body is an immune process that takes a considerable long time. The final follow-up period selected for our study was 24 months after TA, which may not be sufficient for adequate absorption of some tumors. Teng et al. (21) showed that the volume significantly decreased from a median of 55.78 (quartile: 21.50, 112.20) mm3 to 0 (0, 0) mm3 (p < 0.001), with a VRR of 99.37 ± 4.02% at 60 months after TA. Second, residual calcification and fibrous scarring within the tumor may be another reason why the tumor volume does not completely disappear. These residues appear as no enhancement zones on contrast-enhanced ultrasound and necrotic material confirmed by fine-needle aspiration biopsies (18).
In the eligible studies, disease progress includes new discovered tumor, tumor recurrence, and lymph node metastasis. No significant differences were shown by disease progress rate between T1aN0M0 [1.5% (95% CI, 0.6–2.8%)] and T1bN0M0 [2.8% (95% CI, 1.2–5.1%)]. For newly observed or recurrent tumors, all patients received secondary TA, while patients with lymph node metastases mostly undergo open surgery. Xiao et al. (16) reported that ablation was performed in a patient with lymph node metastases at ipsilateral level IV, which was successfully treated. TA was shown in our eligible studies to be an effective treatment for T1N0M0 PTC for all cases of tumors and metastatic lymph nodes being cured with no recurrence.
In terms of safety, the main complications were pain (38 patients) and hoarseness (20 patients), with other complications: hematomas (4 patients), slight fever (1 patient), labored breathing, coughing (4 patients), and reactive hyperplastic lymphadenectasis (4 patients). The complication rate in T1aN0M0 PTC and T1bN0M0 PTC was 8.0% and 3.7%, respectively. Although the complication rate was lower in T1bN0M0 PTC, this may be due to the error caused by the smaller sample size in T1bN0M0 PTC, so we do not consider the difference between T1aN0M0 PTC and T1bN0M0 PTC to be statistically significant. Most symptoms were relieved within several months without any specific therapy. Only one person with T1aN0M0 PTC had a permanent recurrent laryngeal nerve injury, showing mild hoarseness without dysphagia and coughing after drinking water (22). In addition, in this meta-analysis, no life-threatening complications were identified; thus, TA is a safe as well as a suitable regimen for treating T1N0M0 PTC.
In our practice, we applied T1bN0M0 PTC, which was not reported in previous studies. Based on our results, TA could be considered as a very promising method to treat T1N0M0 PTC and improve patient outcomes. Compared to surgery, patients treated with TA had shorter operation times and cost less (24). The main reason was that the ablation can be performed in the outpatient clinic through local injection of lidocaine and the procedures are completed rapidly without hospital bills. In terms of complications, those caused by surgery should not be ignored. The incidence of hypothyroidism, as reported, is as high as 75%, whereas that of permanent hypocalcemia is up to 3.1% (25, 26). During the execution of TA therapy, isolation fluid can be injected around the thyroid nodules to prevent thermal injury to important anatomy such as peripheral blood vessels and nerves (27, 28). This also ensures the effectiveness and safety of the TA treatment. In addition, TA is a flexible and repeatable procedure; it can be performed many times for recurrent tumors or metastatic lymph nodes without increased technical difficulties due to the previous treatments (29). This is undoubtedly a gratifying gospel for patients with multiple recurrences of thyroid and lymph nodes. Although good results were seen in our study, the heterogenicity of the results cannot be ignored. Some studies have not been followed up long enough to observe outcomes, which may have affected the accuracy of these results. For example, 3 studies did not follow up for 24 months, which could lead to some tumor recurrences or new tumors not being detected, affecting the accuracy of disease progression. In addition, the data from the same research team may overlap (15, 17), which will also have an impact on the accuracy of our findings.
According to the results of subgroup analysis in the MWA and RFA groups, we discovered that the “tumor volume” of the MWA group was greater than that of the RFA group in the short term after treatment, but after a long-term follow-up, both subgroups showed good VRR, and the difference was not statistically significant (p > 0.05). The difference in the short-term increase of the “tumor volume” of the ablation zone is primarily due to the different ablation principles. RFA causes cell death via thermal coagulation necrosis, whereas MWA relies on dielectric heating. MWA’s higher power and larger active heating area produced by MW energy allow it to produce more uniform necrosis in the target lesion, making it more suitable for the treatment of larger nodules than RFA. Furthermore, in our results on disease progression, the disease progression rate in the MWA group was significantly lower than that in the RFA group, which may be related to the MWA’s higher power and energy. As a result, both MWA and RFA are considered to be treatment modalities for T1N0M0 PTC, with MWA possibly being more advantageous in the treatment of larger nodules and thorough treatment.
In terms of treatment safety, the complication rates were comparable between the two treatment modalities. The most common complications were pain and hoarseness, which all resolved spontaneously within a few days or weeks with the exception of one case in the MWA group, which had permanent hoarseness without choking. Furthermore, no life-threatening complications have been reported. As a result, we believe that MWA and RFA are safe treatments for T1N0M0 PTC.
The conducted meta-analysis still has several limitations. First, as TA is a new procedure developed in the past 15–20 years, the sample size of the studies was limited, especially in the subgroup of T1bN0M0 PTC and the LA group where we did not conduct the analysis because of insufficient data. Second, all the included studies were regionally concentrated from the same country; thus, it may produce some bias due to race. Third, most of the studies included were retrospective cohorts and all the studies were not randomized controlled trials. Fourth, the research is not compared with the current standard surgical treatment, which may make the results relatively less convincing.
In conclusion, our study is currently the first meta-analysis that covers the safety and effectiveness of TA in the treatment of T1bN0M0 PTC. We discovered that the three ablation modalities are reliable and powerful for the treatment of T1aN0M0 PTC and T1bN0M0 PTC by analyzing the results of subgroup analysis. Meanwhile, MWA is better suited for the ablation of larger nodules than RFA. Considering these results, TA has a strong chance of becoming the primary treatment method for the clinical treatment of T1N0M0 PTC in the future.
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
M-HW and QW contributed to the conception and design of the study. M-HW performed the statistical analysis. M-HW wrote the first draft of the manuscript. XL wrote the method section of the manuscript. H-WZ revised the manuscript. All authors contributed to manuscript revision, read, and approved the submitted version.
The work was supported by the Natural Science Foundation of Shandong Province (grant number ZR2021QH047 and ZR2021MH309) and the Clinical Science and Technology Innovation Development Program of Jinan (grant number 202134036).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: Globocan estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin (2021) 71(3):209–49. doi: 10.3322/caac.21660
2. Miranda-Filho A, Lortet-Tieulent J, Bray F, Cao B, Franceschi S, Vaccarella S, et al. Thyroid cancer incidence trends by histology in 25 countries: A population-based study. Lancet Diabetes Endocrinol (2021) 9(4):225–34. doi: 10.1016/s2213-8587(21)00027-9
3. Raue F, Frank-Raue K. Thyroid cancer: Risk-stratified management and individualized therapy. Clin Cancer Res (2016) 22(20):5012–21. doi: 10.1158/1078-0432.CCR-16-0484
4. Ruiz EML, Niu T, Zerfaoui M, Kunnimalaiyaan M, Friedlander PL, Abdel-Mageed AB, et al. A novel gene panel for prediction of lymph-node metastasis and recurrence in patients with thyroid cancer. Surgery (2020) 167(1):73–9. doi: 10.1016/j.surg.2019.06.058
5. Tuttle RM, Haugen B, Perrier ND. Updated American joint committee on Cancer/Tumor-Node-Metastasis staging system for differentiated and anaplastic thyroid cancer (Eighth edition): What changed and why? Thyroid (2017) 27(6):751–6. doi: 10.1089/thy.2017.0102
6. Zhou W, Jiang S, Zhan W, Zhou J, Xu S, Zhang L. Ultrasound-guided percutaneous laser ablation of unifocal T1n0m0 papillary thyroid microcarcinoma: Preliminary results. Eur Radiol (2017) 27(7):2934–40. doi: 10.1007/s00330-016-4610-1
7. Zhang M, Tufano RP, Russell JO, Zhang Y, Zhang Y, Qiao Z, et al. Ultrasound-guided radiofrequency ablation versus surgery for low-risk papillary thyroid microcarcinoma: Results of over 5 years' follow-up. Thyroid (2020) 30(3):408–17. doi: 10.1089/thy.2019.0147
8. Lu C, Li X, Chu X, Li R, Li J, Wang J, et al. Clinical effects of microwave ablation in the treatment of low-risk papillary thyroid microcarcinomas and related histopathological changes. Front Endocrinol (Lausanne) (2021) 12:751213. doi: 10.3389/fendo.2021.751213
9. Li J, Liu Y, Liu J, Yang P, Hu X, Qian L. A comparative study of short-term efficacy and safety for thyroid micropapillary carcinoma patients after microwave ablation or surgery. Int J Hyperthermia (2019) 36(1):640–6. doi: 10.1080/02656736.2019.1626492
10. Wu R, Luo Y, Tang J, Yang M, Li J, Zhang Y, et al. Ultrasound-guided radiofrequency ablation for papillary thyroid microcarcinoma: A retrospective analysis of 198 patients. Int J Hyperthermia (2020) 37(1):168–74. doi: 10.1080/02656736.2019.1708480
11. Cao XJ, Wang SR, Che Y, Liu J, Cong ZB, He JF, et al. Efficacy and safety of thermal ablation for treatment of solitary T1n0m0 papillary thyroid carcinoma: A multicenter retrospective study. Radiology (2021) 300(1):209–16. doi: 10.1148/radiol.2021202735
12. Zhang C, Yin J, Hu C, Ye Q, Wang P, Huang P. Comparison of ultrasound guided percutaneous radiofrequency ablation and open thyroidectomy in the treatment of low-risk papillary thyroid microcarcinoma: A propensity score matching study. Clin Hemorheol Microcirc (2022) 80(2):73–81. doi: 10.3233/CH-201087
13. Kim HJ, Chung SM, Kim H, Jang JY, Yang JH, Moon JS, et al. Long-term efficacy of ultrasound-guided laser ablation for papillary thyroid microcarcinoma: Results of a 10-year retrospective study. Thyroid (2021) 31(11):1723–9. doi: 10.1089/thy.2021.0151
14. Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol (2010) 25(9):603–5. doi: 10.1007/s10654-010-9491-z
15. Xiao J, Zhang Y, Yan L, Zhang M, Li X, Tang J, et al. Ultrasonography-guided radiofrequency ablation for solitary T1an0m0 and T1bn0m0 papillary thyroid carcinoma: A retrospective comparative study. Eur J Endocrinol (2021) 186(1):105–13. doi: 10.1530/EJE-21-0580
16. Xiao J, Zhang M, Zhang Y, Yan L, Lan Y, Zhu Y, et al. Efficacy and safety of ultrasonography-guided radiofrequency ablation for the treatment of T1bn0m0 papillary thyroid carcinoma: A retrospective study. Int J Hyperthermia (2020) 37(1):392–8. doi: 10.1080/02656736.2020.1752945
17. Cao XJ, Liu J, Zhu YL, Qi L, Liu G, Wang HL, et al. Efficacy and safety of thermal ablation for solitary T1bn0m0 papillary thyroid carcinoma: A multicenter study J Clin Endocrinol Metab (2020) 106(2):e573–81. doi: 10.1210/clinem/dgaa776/5940804
18. Zhou W, Ni X, Xu S, Zhang L, Chen Y, Zhan W. Ultrasound-guided laser ablation versus microwave ablation for patients with unifocal papillary thyroid microcarcinoma: A retrospective study. Lasers Surg Med (2020) 52(9):855–62. doi: 10.1002/lsm.23238
19. Peng K, Zhou P, Liu W. Long-term efficacy of ultrasound-guided percutaneous laser ablation for low-risk papillary thyroid microcarcinoma: A 5-year follow-up study. BioMed Res Int (2021) 2021:6616826. doi: 10.1155/2021/6616826
20. Wang X, Niu X, Mu S, Zhang M, Jiang W, Zhai L, et al. Analysis and evaluation of the efficacy of ultrasound-guided microwave ablation for papillary thyroid microcarcinoma. Int J Hyperthermia (2021) 38(1):1476–85. doi: 10.1080/02656736.2021.1988152
21. Teng DK, Li WH, Du JR, Wang H, Yang DY, Wu XL. Effects of microwave ablation on papillary thyroid microcarcinoma: A five-year follow-up report. Thyroid (2020) 30(12):1752–8. doi: 10.1089/thy.2020.0049
22. Zu Y, Liu Y, Zhao J, Yang P, Li J, Qian L. A cohort study of microwave ablation and surgery for low-risk papillary thyroid microcarcinoma. Int J Hyperthermia (2021) 38(1):1548–57. doi: 10.1080/02656736.2021.1996643
23. Yue WW, Qi L, Wang DD, Yu SJ, Wang XJ, Xu HX, et al. Us-guided microwave ablation of low-risk papillary thyroid microcarcinoma: Longer-term results of a prospective study. J Clin Endocrinol Metab (2020) 105(6):1791–800. doi: 10.1210/clinem/dgaa128
24. Shen K, Xue S, Xie Y, Wang H, Li J, Sun Y, et al. Comparison of thermal ablation and routine surgery for the treatment of papillary thyroid microcarcinoma: A systematic review and meta-analysis. Int J Hyperthermia (2020) 37(1):913–24. doi: 10.1080/02656736.2020.1777331
25. Che Y, Jin S, Shi C, Wang L, Zhang X, Li Y, et al. Treatment of benign thyroid nodules: Comparison of surgery with radiofrequency ablation. AJNR Am J Neuroradiol (2015) 36(7):1321–5. doi: 10.3174/ajnr.A4276
26. Lang BH, Wong CK, Tsang JS, Wong KP, Wan KY. A systematic review and meta-analysis comparing surgically-related complications between robotic-assisted thyroidectomy and conventional open thyroidectomy. Ann Surg Oncol (2014) 21(3):850–61. doi: 10.1245/s10434-013-3406-7
27. Guo Y, Li Z, Wang S, Liao X, Li C. Single-fiber laser ablation in treating selected metastatic lymph nodes of papillary thyroid carcinoma and benign cold thyroid nodules-preliminary results. Lasers Surg Med (2020) 52(5):408–18. doi: 10.1002/lsm.23150
28. Ding Z, Chen J, Chen Z, Zeng X, Zheng P, Wang X, et al. Efficacy and safety of thermal ablation for treating lymph node metastasis from papillary thyroid carcinoma: A systematic review and meta-analysis. Front Oncol (2022) 12:738299. doi: 10.3389/fonc.2022.738299
29. Choi Y, Jung SL, Bae JS, Lee SH, Jung CK, Jang J, et al. Comparison of efficacy and complications between radiofrequency ablation and repeat surgery in the treatment of locally recurrent thyroid cancers: a single-center propensity score matching study. Int J Hyperthermia (2019) 36(1):359–67. doi: 10.1080/02656736.2019.1571248
Keywords: papillary thyroid carcinoma, thyroid nodule, thermal ablation, radiofrequency ablation, microwave ablation, laser ablation, meta‐analysis
Citation: Wang M-H, Liu X, Wang Q and Zhang H-W (2022) Safety and efficacy of ultrasound-guided thermal ablation in treating T1aN0M0 and T1bN0M0 papillary thyroid carcinoma: A meta-analysis. Front. Endocrinol. 13:952113. doi: 10.3389/fendo.2022.952113
Received: 24 May 2022; Accepted: 21 June 2022;
Published: 27 July 2022.
Edited by:
Hui Wang, Jilin University, ChinaReviewed by:
Chunxiao Yu, University of Kansas, United StatesCopyright © 2022 Wang, Liu, Wang and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hua-Wei Zhang, c2x5eXpod0AxNjMuY29t; Qian Wang, d2FuZ3FpYW4xMjI0MTFAMTI2LmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.