- 1Department of Endocrinology, Beijing Chao-Yang Hospital, Capital Medical University, Beijing, China
- 2Physical Examination Center, Beijing Chao-Yang Hospital, Capital Medical University, Beijing, China
Background and aims: Glucose and lipoprotein(a) [Lp(a)] have been recognized risk factors for atherosclerosis. The impact of both factors on fatty liver patients has not been studied. The aim of this study is to explore the role of high-level Lp(a) and different glucose metabolism statuses on carotid plaques in fatty liver patients.
Methods: We selected 4,335 fatty liver patients in this cross-sectional study. The diagnosis of fatty liver disease and carotid plaques was made by ultrasound. Participants were divided into four groups based on glucose metabolism status (normal glucose regulation [NGR], lower bound of impaired fasting glucose [IFG-L], higher bound of impaired fasting glucose [IFG-H], diabetes mellitus [DM]) and then categorized into 12 subgroups according to Lp(a) concentrations. The association between variables was estimated by odds ratio (OR).
Results: Carotid plaques were present in 1,613 (37.2%) fatty liver patients. Lp(a)≥30 mg/dL was associated with high risk of carotid plaques in those patients with IFG-L, IFG-H and DM (OR 1.934 [95% CI 1.033-3.618], 2.667 [1.378-5.162], 4.000 [2.219-7.210], respectively; p<0.05). Fatty liver patients with DM plus Lp(a)<10 mg/dL and 10≤Lp(a)<30 mg/dL were more vulnerable to carotid plaques (OR 1.563 [95% CI 1.090-2.241], 1.930 [1.279-2.914]), respectively, p<0.05).
Conclusions: Our study first suggested that high-level Lp(a) may raise the risk of carotid plaques in fatty liver patients with not only diabetes but also IFG, manifesting that Lp(a) may be helpful for the early discovery of subclinical atherosclerosis in fatty liver patients with impaired glucose metabolism.
Introduction
The disease with the highest morbidity and mortality is still cardiovascular disease (CVD) (1). The subclinical vascular disease represents a variety of pathological vascular changes that occur before the clinical signs of CVD, providing momentous etiological insights into the early detection of CVD progression (2). The atherosclerotic plaque is a great indicator of subclinical atherosclerosis by the ultrasound, further assessing cardiovascular risk (3).
When the ectopic fat accumulates (≥5%) in liver cells, it is considered the fatty liver disease (FLD), which becomes the major contributor to chronic liver disease (4). There is a growing prevalence of 2%-44% in ordinary people (5). Its pathological classification included simple steatosis, steatohepatitis, cirrhosis and even hepatocellular carcinoma (6). Several studies have indicated that FLD had a close relationship with CVD and subclinical atherosclerosis (7, 8).
The primary public health crisis includes type 2 diabetes, and China has the world’s largest diabetic population (9). The principal causes of morbidity and mortality among patients with type 2 diabetes are cardiovascular complications (10). Moreover, the great prevalence of pre-diabetes is expected to transform into an enormous burden of diabetes and relevant CVD in the future (9).
Lipoprotein(a) [Lp(a)] is produced in the liver. It is characterized in that the apolipoprotein B100 molecule is covalently bound to the glycoprotein apolipoprotein (a) (11). Lp(a) belongs to a subgroup of low-density lipoproteins, and the LPA gene genetically determines the concentration of Lp(a) (12). It plays important roles in the development of atherosclerosis and thrombosis as it is similar to low-density lipoprotein cholesterol (LDL-C) and plasminogen (13). A higher level of Lp(a) is linked to an increased risk of coronary, peripheral artery, cerebrovascular disease events and carotid atherosclerosis (14–19). Besides, a negative association of lipoprotein(a) with the prevalence of diabetes have been found in populations with or without the evident cardiovascular disease (20–24). Nevertheless, the intervening node of increased Lp(a) for individuals with abnormal glucose metabolism and CVD has not been determined yet in an authoritative guide.
Few studies to date have unveiled the impact of Lp(a) on the risk of carotid plaques in FLD with glucose metabolism disorders. In our cross-sectional study, we aimed to explore the relationship between lipoprotein(a) and carotid plaques in fatty liver disease individuals with different glucose metabolism.
Materials and methods
Study population
Figure 1 shows the flowchart of the research procedure. A total of 19,932 patients with fatty liver were diagnosed by abdominal ultrasonography in the physical examination center of Beijing Chao-Yang Hospital of China from April 2016 to August 2021. We excluded participants with alcohol consumption history (≥20 g a day for women and ≥30 g a day for men (6), n=1228), acute coronary syndrome in the recent 6 months (n=67), recent stroke (n=70), recent carotid artery stenosis or occlusion (<6 months, n=55), and presence of diagnosed cancer and/or under treatment (n=79). Then 1,4097 patients who did not perform carotid ultrasonography and one whose age was under 18 years old were excluded as well. At last, 4,335 patients were included in the study who concurrently measured serum Lp(a). All participants signed written informed consent. The research protocol complies with the ethical guidelines of the 1975 Declaration of Helsinki. It was approved by the Ethics Committee of Beijing Chao-Yang Hospital.
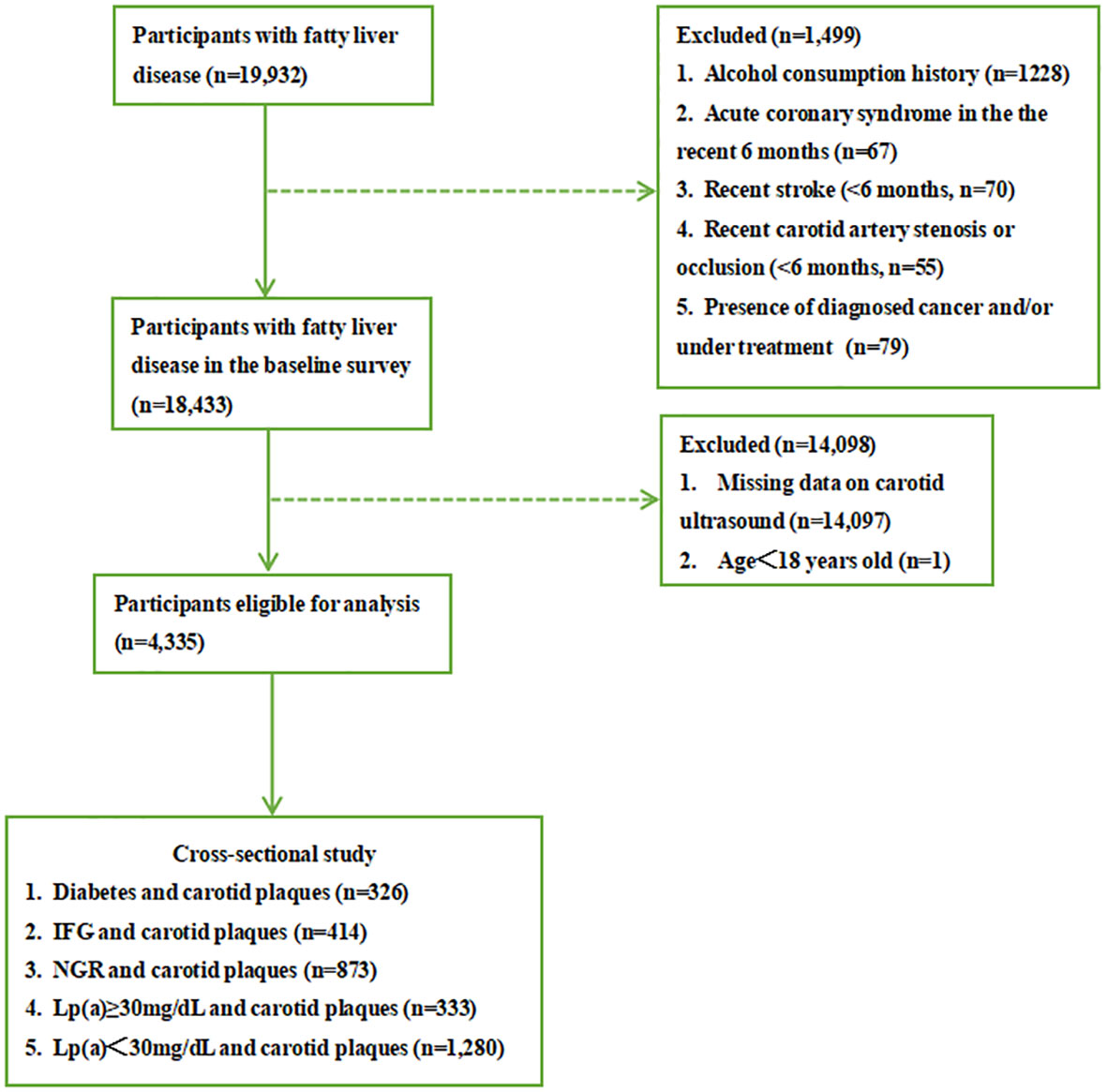
Figure 1 Flowchart of study procedure. IFG, impaired fasting glucose; NGR, normal glucose regulation.
Measurements
The experienced technicians measured the body height, weight, systolic blood pressure (SBP), and diastolic blood pressure (DBP) of the participants according to a standard protocol. Body mass index (BMI) was the ratio of weight in kilograms to the square of height in meters (kg/m2). All blood tests were determined by standard laboratory procedures, including Lp(a), fasting blood glucose (FBG), fasting insulin (FINS), glycosylated hemoglobin (HbA1c), glycated albumin (GA), total cholesterol (TC), LDL-C, high-density lipoprotein cholesterol (HDL-C), triglycerides (TG), total protein (TP), albumin (ALB), alanine aminotransferase (ALT), and aspartate aminotransferase (AST), gamma-glutamyl transpeptidase (GGT), total bilirubin (TBIL), direct bilirubin (DBIL), indirect bilirubin (IBIL), total bile acid (TBA), creatinine (Cr), blood urea nitrogen (BUN), uric acid (UA) and free fatty acid (FFA).
Patients have regular physical exams at our health management center, where carotid and abdominal ultrasounds are performed. They were examined according to standardized protocols by trained sonographers. We used high-resolution B-mode for carotid and abdominal respectively, as described previously (25–28). Carotid plaques were defined as focal intima-media thickness ≥1.2 mm (29). Two experienced sonographers who were unacquainted with the participants’ states of illness measured the intima-media thickness. The diagnosis of FLD involved the diffuse hyperechogenicity in the liver in comparison to the kidney, vascular blurring, and deep attenuation of ultrasound signals (30).
Definitions
The definitions of hypertension, DM, dyslipidemia, low bound of IFG (IFG-L), high bound of IFG (IFG-H), and normal glucose regulation (NGR) were as followings:
● Hypertension: SBP≥140 mmHg, or DBP≥90 mmHg (31), or previous diagnosis.
● Diabetes: FBG≥7 mmol/l, or HbA1C≥6.5% (32), or previous diagnosis, or current use of anti-diabetic drugs.
● Dyslipidemia: TC≥6.22 mmol/L, or TG≥2.26 mmol/L, or LDL-C≥4.14 mmol/L, or HDL-C<1.04 mmol/L (33), or previous diagnosis.
● IFG-L: FBG 5.6–6.0 mmol/L (34, 35).
● IFG-H: FBG 6.1–6.9 mmol/L (34, 35).
● NGR: FBG<5.6 mmol/L (34, 35).
Statistics analysis
For continuous variables, the values were reported as the mean standard deviation or median (25th-75th percentile), and for categorical variables, the number (percentage). To assess the distribution of the variables, the Kolmogorov-Smirnov test was utilized. The Student t-test, Mann-Whitney U test, or chi-square test were used to analyze the differences in clinical indicators between groups where appropriate. The prevalence of carotid plaques in the comparison among groups was further estimated by the Bonferroni test. Binary logistic regression analyses were performed to calculate the odds ratio (OR). The statistical significance was defined as a value of p<0.05. The SPSS software was used to conduct the statistical analysis (version 24.0).
Results
Baseline characteristics
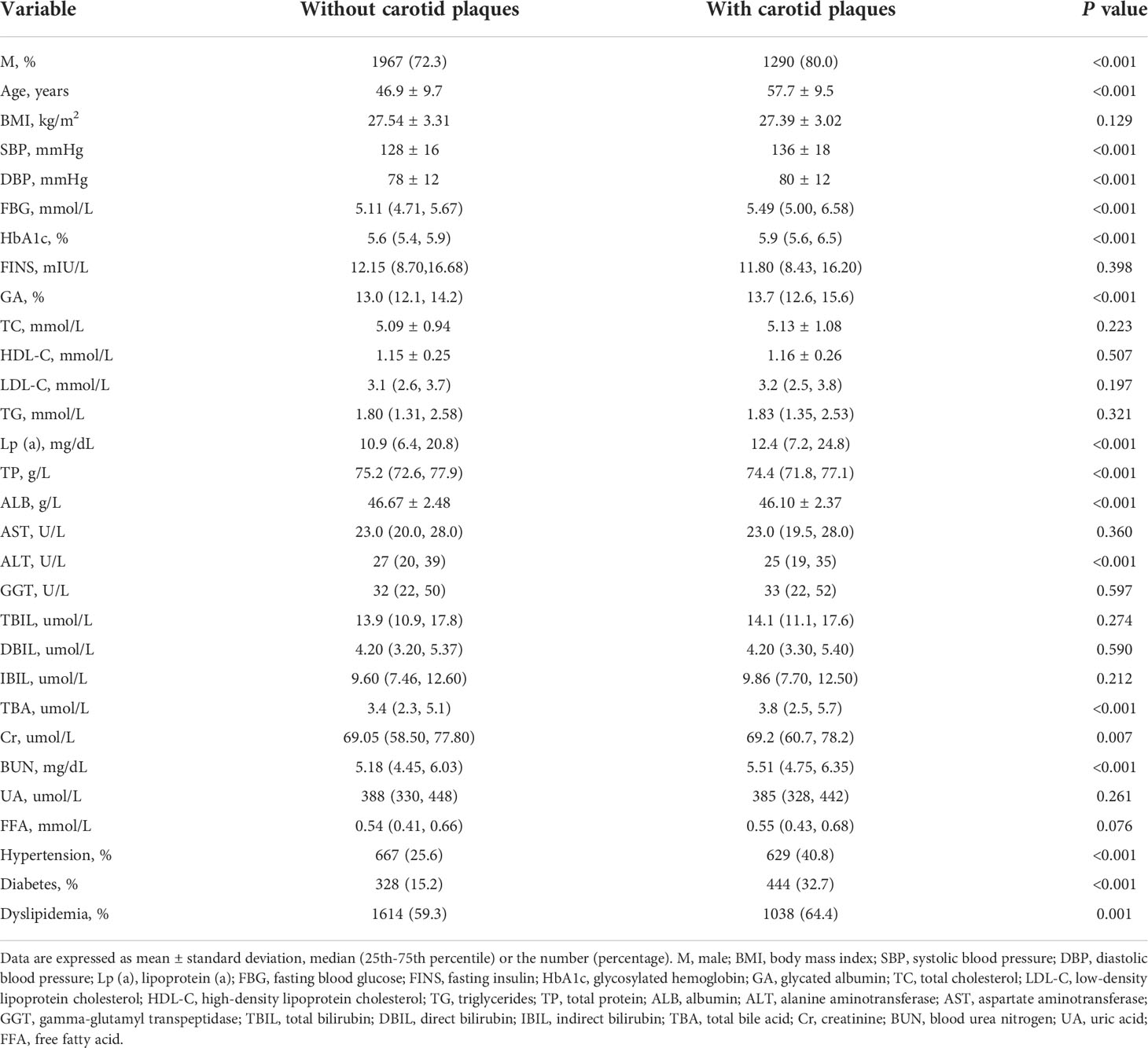
Table 1 shows the characteristics of patients with fatty liver. The age, SBP, DBP, glucose, HbA1c, GA, Lp(a), TBA, and BUN levels were linked to the presence of carotid plaques, while TP, ALB, and ALT had a negative correlation (p<0.001). The proportion of male participants and those with dyslipidemia was elevated when they suffered carotid plaques (p<0.001). But there was no discernible difference in BMI, FINS, TC, TG, HDL-C, LDL-C, FFA, AST, GGT, bilirubin, and UA between the two groups (p>0.05).
Glucose metabolism, Lp(a) levels, and carotid plaques in fatty liver patients
The prevalence of carotid plaques in the four groups divided by different fasting blood glucose levels (NGR, IFG-L, IFG-H, and DM) was 30.7%, 43.1%, 48.1%, and 56.9%, respectively (Figure 2A). According to the Pearson chi-square test, the presence of carotid plaques in fatty liver patients rose with FBG levels (p<0.05). Individuals with Lp(a)≥30 mg/dL were at the greatest risk of carotid plaques among three groups divided by Lp(a) level (Lp(a)<10 mg/dL, 10≤Lp(a)<30 mg/dL, and Lp(a)≥30 mg/dL) (Figure 2B). However, when the patients were assessed in line with glycometabolism and Lp(a) level, the individuals with fatty liver and Lp(a)≥30 mg/dL, regardless of different glucose metabolism statuses, had a strikingly increased risk of carotid plaques compared to the reference subjects (NGR and Lp(a)<10 mg/dL). Meanwhile, the prevalence risk of carotid plaques in the groups of DM plus 10≤Lp(a)<30 mg/dL, DM plus Lp(a)≥30 mg/dL, and IFG-H plus Lp(a)≥30 mg/dL became higher than the reference subjects (Figure 2C) (p<0.05).
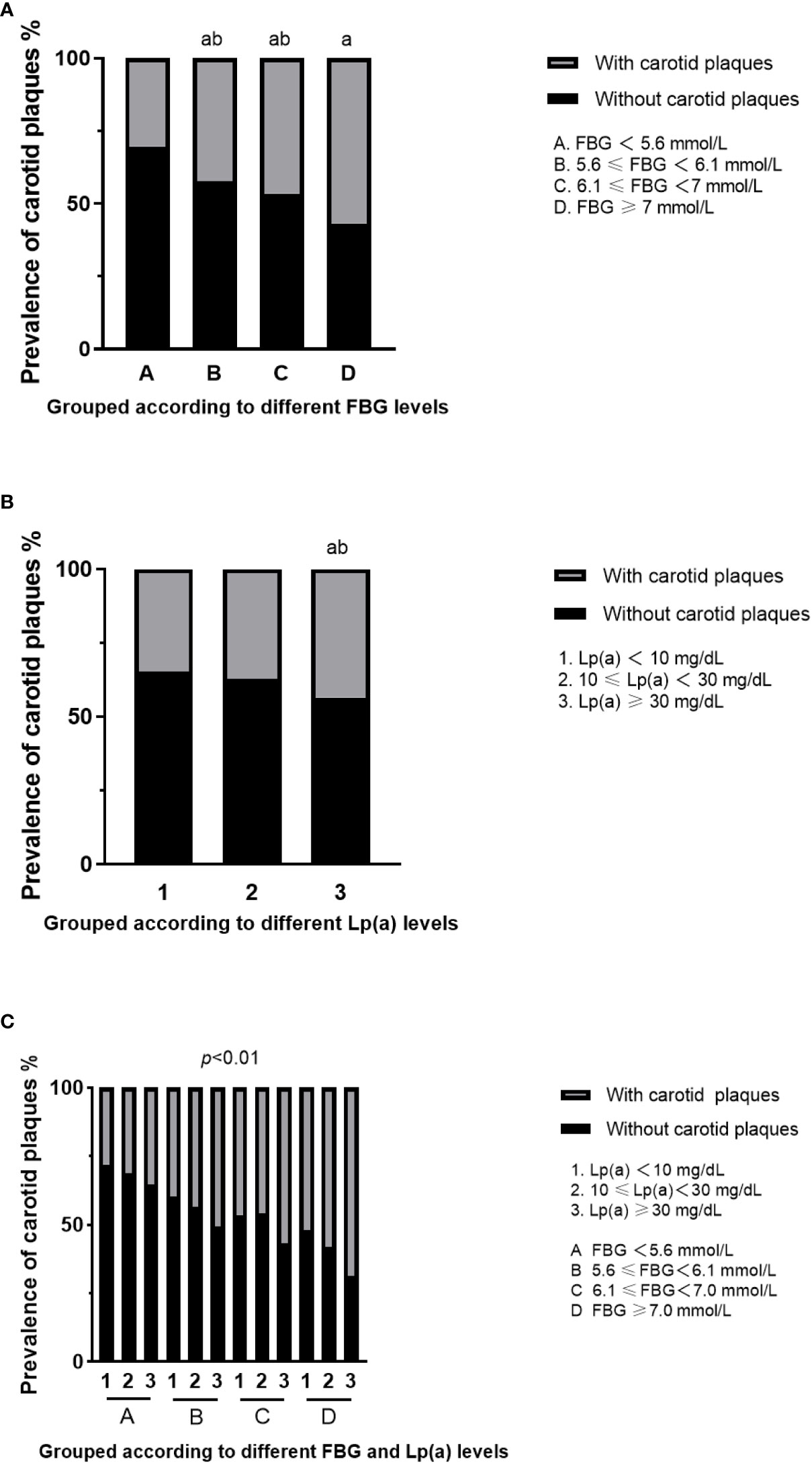
Figure 2 Prevalence of carotid plaques in fatty liver patients with different FBG and Lp(a) levels. (A) Prevalence of carotid plaques in fatty liver patients grouped according to different FBG levels. aFor p<0.05 vs A group (FBG<5.6 mmol/L), bfor p<0.05 vs D group (FBG>7.0 mmol/L). (B) Prevalence of carotid plaques in fatty liver patients grouped according to different Lp(a) levels. aFor p<0.05 vs 1 group (Lp(a)<10 mg/dL), bfor p<0.05 vs 2 group (10≤Lp(a)<30 mg/dL). (C) Prevalence of carotid plaques in fatty liver patients with both different FBG and Lp(a) levels. FBG, fasting blood glucose; Lp(a), lipoprotein(a).
The binary logistic regression crude models in Table 2 indicated that fatty liver individuals with DM plus Lp(a)≥30 mg/dL were at a 5.810-fold increased risk of carotid plaques compared to reference subjects (OR 5.810 [95% CI 3.726-9.059], p<0.05). After confounding variables were adjusted, like age, sex, ALB, ALT, TBA, creatinine, hypertension, and dyslipidemia, the association between high-level Lp(a) and carotid plaques in participants with DM remained significant. The presence risk of carotid plaques in those individuals with FBG<7mmol/L plus Lp(a)<30 mg/dL did not increase in comparison to the reference individuals (p>0.05). Besides, in the group of NGR, the fatty liver people with Lp(a)≥30 mg/dL were not inclined to suffer carotid plaques. In addition, for each standard deviation increase in Lp(a) level in the DM group (25.7 mg/dL), the risk for carotid plaques went up by 35.4%. Patients with DM plus Lp(a)<10 mg/dL and DM plus 10≤Lp(a)<30 mg/dL were at 1.563-fold (95% CI 1.090-2.241) and 1.930-fold (95% CI 1.279-2.914) high risk of carotid plaques (p<0.05). Individuals with high-level Lp(a) had a relationship with 1.934, 2.667, and 4.000-fold risk of carotid plaques in the three groups with different glucose metabolism (IFG-L, IFG-H, and DM), respectively.
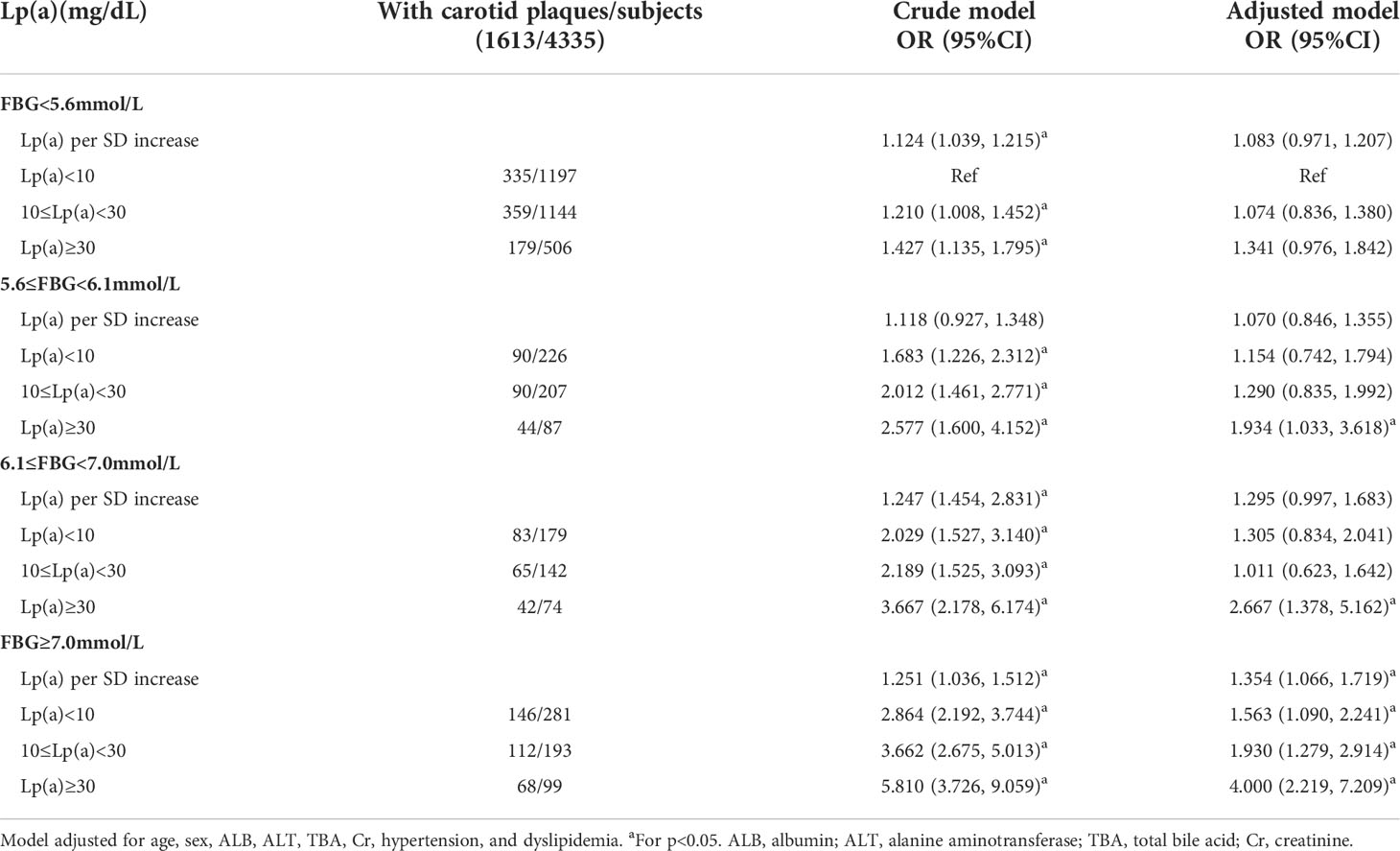
Table 2 Lp(a) levels in relation to carotid plaques in fatty liver patients with different glucose metabolism.
Discussion
In our research, we analyzed the impact of Lp(a) on the prevalence risk of carotid plaques in fatty liver patients with four groups of various glucose metabolism. Our main findings showed that the fatty liver population with diabetes plus Lp(a)≥30 mg/dL were at a quadruple higher risk of carotid plaques in comparison to the reference subjects. Besides, the carotid plaques risk in those with IFG plus Lp(a)≥30 mg/dL was higher than in the fatty liver participants with diabetes plus Lp(a)<30 mg/dL, indicating that measurement of Lp(a) was significant in the patients including not only diabetes but also the pre-diabetes.
Although the apparent vascular disease had not appeared in the patients with carotid atherosclerosis, they had more risk of CVD than the ones without carotid atherosclerosis (26, 36). Among individuals with non-alcoholic fatty liver disease (NAFLD), DM was connected with prevalent subclinical atherosclerosis evaluated by the detection of carotid plaques (37). That was in keeping with our present findings that fatty liver patients with rising FBG were more likely to suffer carotid plaques. There were similar risk factors in diabetes and NAFLD, and both diseases were closely related consequently. The simultaneous presence of both NAFLD and type 2 diabetes exacerbated lipid disorders as well as hepatic insulin resistance, in turn worsening atherosclerosis and raising the incidence of cardiovascular events among type 2 diabetes individuals (38–40).
Substantial evidence suggested that the determining factor of residual CVD risk might be Lp(a) when LDL-C level was optimum (41). Even though target LDL-C levels were reached, the increasing concentration of Lp(a) remained relevant to the existence of carotid atherosclerosis (19). The recent studies indicated that higher Lp(a) level was associated with subclinical coronary atherosclerosis in asymptomatic subjects (42), and elevated baseline Lp(a) was associated with subclinical vascular and valvular calcification in the White and Black participants (43). It is known that Lp(a) and its associated oxidized phospholipids could induce a proinflammatory response, causing cellular apoptosis and necrosis which accelerate necrotic core formation (44, 45). Furthermore, Lp(a) contains proatherogenic components of LDL, and its prothrombotic effects through the plasminogen-like apolipoprotein(a) also contribute to the atherosclerosis (46, 47). Our results also manifested that high Lp(a) levels lead to an increased risk of subclinical atherosclerosis which was consistent with previous studies. As fatty liver disease, diabetes, and Lp(a) levels had a link with the presence of carotid plaques dissimilarly based on earlier reports, it is worthy of exploring the forecast value of Lp(a) in various subpopulations.
We studied the link between serum Lp(a) and carotid plaques in fatty liver patients with different glucose metabolism. We found that the risks for carotid plaques whose FBG≥7 mmol/L reached the highest among the whole study population when grouped by FBG levels (NGR, IFG-L, IFG-H, and DM) through binary logistic regression analysis. When grouped by different concentrations of FBG and Lp(a), patients with IFG-L plus Lp(a)≥30 mg/dL and IFG-H plus Lp(a)≥30 mg/dL were at 1.934 and 2.667-fold higher risk of carotid plaques than the reference groups respectively. In contrast, the risk prediction was unaffected with the addition of Lp(a) when FBG<5.6 mmol/L. It was the first time to indicate that fatty liver individuals suffering from IFG and high Lp(a) levels simultaneously were at an increased risk of carotid plaques. Pre-diabetes population with elevated Lp(a) levels were more vulnerable to cardiovascular events, and the diabetic population with high-level Lp(a) had the worst prognosis in different countries and races (48–50), which was similar to our results. However, several studies reported that plasma Lp(a) decreased in T2DM patients compared to controls, which is called Lp(a) paradox in T2DM (51). It is necessary to measure Lp(a) concentrations in diabetes patients and evaluate the risk considering the paradox effect. A study had found that lowering the Lp(a) levels from above 50 nmol/l to 14 nmol/l could reduce risk of CVD and would not increase risk of T2DM (20), but guidelines for measurements or treatments of high-Lp(a) levels in diabetes or prediabetes patients have not been published yet at present.
There were several limitations in this study including that only Chinese patients were chosen as candidates and whether the other races could show the same tendency needs further investigation. While ultrasound is simple, harmless and reproducible, we did not perform liver biopsy which is the gold standard for the diagnosis of fatty liver disease. We could not identify the patients with or without non-alcoholic steatohepatitis as well. In addition, the data on smoking and family history were not gathered, and more research is required with the data taken into account. The history of hepatitis had little influence on our study as the infection rate of Hepatitis B Virus and Hepatitis C Virus in China was only 6.89% from 2013 to 2017 (52). Beyond that, due to the cross-sectional design of our study, we did the research only at baseline and found the relationship between Lp(a), glucose metabolism and carotid plaques at one point of time. Further research is necessary to explore more robust evidence from prospective studies.
Conclusions
Our cross-sectional study indicated that fatty liver patients with diabetes and high-level Lp(a) were the most vulnerable to carotid plaques. But more than that, we first suggested that the the presence of carotid plaques was closely related to the high-level Lp(a) and IFG in the fatty liver patients, implying that testing of Lp(a) and treatment towards high-level Lp(a) might make sense for fatty liver patients with not merely DM but mildly impaired glucose metabolism.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving human participants were reviewed and approved by Medical Ethics Committee, Beijing Chaoyang Hospital, Capital Medical University. The patients/participants provided their written informed consent to participate in this study.
Author contributions
YW gathered the data of the participants. YA further organized the data. JXW and HLS conducted the design of the research, data analyses, and statistical analyses. JXW wrote the article, and prepared the presentation parts, all supervised by JL and GW. All authors contributed to the article and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
CVD, cardiovascular disease; DM ,diabetes; Lp(a), Lipoprotein(a); IFG, impaired fasting glucose; FLD, fatty liver disease; NAFLD, non-alcoholic fatty liver disease; LDL-C, low-density lipoprotein cholesterol; SBP, systolic blood pressure; DBP, diastolic blood pressure; BMI, body mass index; FBG, fasting blood glucose; FINS, fasting insulin; HbA1c, glycosylated hemoglobin; GA, glycated albumin; TC, total cholesterol; HDL-C, high-density lipoprotein cholesterol; TG, triglycerides; TP, total protein; ALB, albumin; ALT, alanine aminotransferase; AST, aspartate aminotransferase; GGT, gamma-glutamyl transpeptidase; TBIL, total bilirubin; DBIL, direct bilirubin; IBIL, indirect bilirubin; TBA, total bile acid; Cr, creatinine; BUN, blood urea nitrogen; UA, uric acid; FFA, free fatty acid; IFG-L, lower bound of impaired fasting glucose; IFG-H, higher bound of impaired fasting glucose; NGR, normal glucose regulation; OR, odds ratio.
References
1. Townsend N, Wilson L, Bhatnagar P, Wickramasinghe K, Rayner M, Nichols M, et al. Cardiovascular disease in Europe: epidemiological update 2016. Eur Heart J (2016) 37(42):3232–45. doi: 10.1093/eurheartj/ehw334
2. Ingelsson E, Sullivan LM, Fox CS, Murabito JM, Benjamin EJ, Polak JF, et al. Burden and prognostic importance of subclinical cardiovascular disease in overweight and obese individuals. Circulation (2007) 116(4):375–84. doi: 10.1161/CIRCULATIONAHA.107.688788
3. Stein JH, Tattersall MC. Carotid intima-media thickness and cardiovascular disease risk prediction. J Am Coll Cardiol (2014) 63(21):2301–2. doi: 10.1016/j.jacc.2014.02.528
4. Younossi Z, Anstee QM, Marietti M, Hardy T, Henry L, Eslam M, et al. Global burden of NAFLD and NASH: trends, predictions, risk factors and prevention. Nat Rev Gastroenterol Hepatol (2018) 15(1):11–20. doi: 10.1038/nrgastro.2017.109
5. Blachier M, Leleu H, Peck-Radosavljevic M, Valla DC, Roudot-Thoraval F. The burden of liver disease in Europe: a review of available epidemiological data. J Hepatol (2013) 58(3):593–608. doi: 10.1016/j.jhep.2012.12.005
6. Anstee QM, McPherson S, Day CP. How big a problem is non-alcoholic fatty liver disease? BMJ (2011) 18:343. doi: 10.1136/bmj.d3897
7. Adams LA, Anstee QM, Tilg H, Targher G. Non-alcoholic fatty liver disease and its relationship with cardiovascular disease and other extrahepatic diseases. Gut (2017) 66(6):1138–53. doi: 10.1136/gutjnl-2017-313884
8. Lee SB, Park GM, Lee JY, Lee BU, Park JH, Kim BG, et al. Association between non-alcoholic fatty liver disease and subclinical coronary atherosclerosis: An observational cohort study. J Hepatol (2018) 68(5):1018–24. doi: 10.1016/j.jhep.2017.12.012
9. International Diabetes Federation. IDF diabetes atlas (2019). Brussels, Belgium. Available at: http://www.diabetesatlas.org (Accessed 20 March 2020).
10. American Diabetes Association. 9: Cardiovascular disease and risk management. Diabetes Care (2017) 40:S75–87. doi: 10.2337/dc17-S012
11. Kronenberg F. Human genetics and the causal role of lipoprotein(a) for various diseases. Cardiovasc Drugs Ther (2016) 30(1):87–100. doi: 10.1007/s10557-016-6648-3
12. Boerwinkle E, Leffert CC, Lin J, Lackner C, Chiesa G, Hobbs HH, et al. Apolipoprotein(a) gene accounts for greater than 90% of the variation in plasma lipoprotein(a) concentrations. J Clin Invest (1992) 90(1):52–60. doi: 10.1172/JCI115855
13. Boffa MB, Koschinsky ML. Lipoprotein (a): truly a direct prothrombotic factor in cardiovascular disease? J Lipid Res (2016) 57(5):745–57. doi: 10.1194/jlr.R060582
14. Gurdasani D, Sjouke B, Tsimikas S, Hovingh GK, Luben RN, Weinwright NWJ, et al. Lipoprotein(a) and risk of coronary, cerebrovascular, and peripheral artery disease: the EPIC-Norfolk prospective population study. Arterioscler Thromb Vasc Biol (2012) 32(12):3058–65. doi: 10.11619/ATVBAHA.112.255521
15. Jun JE, Kang H, Hwang YC, Ahn KJ, Chung HY, Jeong IK, et al. The association between lipoprotein (a) and carotid atherosclerosis in patients with type 2 diabetes without pre-existing cardiovascular disease: A cross-sectional study. Diabetes Res Clin Pract (2021) 171:108622. doi: 10.1016/j.diabres.2020.108622
16. Gudbjartsson DF, Thorgeirsson G, Sulem P, Helgadottir A, Gylfason A, Saemundsdottir J, et al. Lipoprotein(a) concentration and risks of cardiovascular disease and diabetes. J Am Coll Cardiol (2019) 74(24):2982–94. doi: 10.1016/j.jacc.2019.10.019
17. Kaya A, Onat A, Yüksel H, Can G, Yüksel M, Ademoğlu E. Lipoprotein(a)-activated immunity, insulin resistance and new-onset diabetes. Postgrad Med (2017) 129(6):611–8. doi: 10.1080/00325481.2017.1342508
18. Mora S, Kamstrup PR, Rifai N, Nordestgaard BG, Buring JE, Ridker PM. Lipoprotein(a) and risk of type 2 diabetes. Clin Chem (2010) 56(8):1252–60. doi: 10.1373/clinchem.2010.146779
19. Paige E, Masconi KL, Tsimikas S, Kronenberg F, Santer P, Weger S, et al. Lipoprotein(a) and incident type-2 diabetes: results from the prospective bruneck study and a meta-analysis of published literature. Cardiovasc Diabetol (2017) 16(1):38. doi: 10.1186/s12933-017-0520-z
20. Schwartz GG, Szarek M, Bittner VA, Bhatt DL, Diaz R, Goodman SG, et al. ODYSSEY OUTCOMES committees and investigators. relation of lipoprotein(a) levels to incident type 2 diabetes and modification by alirocumab treatment. Diabetes Care (2021) 44(5):1219–27. doi: 10.2337/dc20-2842
21. Lakka HM, Laaksonen DE, Lakka TA, Niskanen LK, Kumpusalo E, Tuomilehto J, et al. The metabolic syndrome and total and cardiovascular disease mortality in middle-aged men. JAMA (2002) 288(21):2709–16. doi: 10.1001/jama.288.21.2709
22. Folsom AR, Eckfeldt JH, Weitzman S, Ma J, Chambless LE, Barnes RW, et al. Relation of carotid artery wall thickness to diabetes mellitus, fasting glucose and insulin, body size, and physical activity. Atheroscl Risk Communities (ARIC) Study Investigators. Stroke (1994) 25(1):66–73. doi: 10.1161/01.str.25.1.66
23. Fracanzani AL, Burdick L, Raselli S, Pedotti P, Fargion S. Carotid artery intima-media thickness in nonalcoholic fatty liver disease. Am J Med (2008) 121(1):72–8. doi: 10.1016/j.amjmed.2007.08.041
24. Lee SS, Park SH. Radiologic evaluation of nonalcoholic fatty liver disease. World J Gastroenterol (2014) 20(23):7392–402. doi: 10.3748/wjg.v20.i23.7392
25. Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, et al. National heart, lung, and blood institute joint national committee on prevention, detection, evaluation, and treatment of high blood pressure; national high blood pressure education program coordinating committee. the seventh report of the joint national committee on prevention, detection, evaluation, and treatment of high blood pressure: the JNC 7 report. JAMA (2003) 289(19):2560–72. doi: 10.1001/jama.289.19.2560
26. American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care (2010) 33:S62–9. doi: 10.2337/dc10-S062
27. Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Executive summary of the third report of the national cholesterol education program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (Adult treatment panel III). JAMA (2001) 285(19):2486–97. doi: 10.1001/jama.285.19.2486
28. Genuth S, Alberti KG, Bennett P, Buse J, Defronzo R, Kahn R, et al. Expert committee on the diagnosis and classification of diabetes mellitus. follow-up report on the diagnosis of diabetes mellitus. Diabetes Care (2003) 26(11):3160–7. doi: 10.2337/diacare.26.11.3160
29. Davidson MB. Correction to the 2010 report on the diagnosis and classification of diabetes. Diabetes Care (2010) 33(4):e57. doi: 10.2337/dc09-2368
30. Bonora E, Kiechl S, Willeit J, Oberhollenzer F, Egger G, Bonadonna RC, et al. Bruneck study. carotid atherosclerosis and coronary heart disease in the metabolic syndrome: prospective data from the bruneck study. Diabetes Care (2003) 26(4):1251–7. doi: 10.2337/diacare.26.4.1251
31. Sirimarco G, Amarenco P, Labreuche J, Touboul PJ, Alberts M, Goto S, et al. REACH registry investigators. carotid atherosclerosis and risk of subsequent coronary event in outpatients with atherothrombosis. Stroke (2013) 44(2):373–9. doi: 10.1161/STROKEAHA.112.673129
32. Wang B, Zhao Z, Liu S, Wang S, Chen Y, Xu Y, et al. Impact of diabetes on subclinical atherosclerosis and major cardiovascular events in individuals with and without non-alcoholic fatty liver disease. Diabetes Res Clin Pract (2021) 177:108873. doi: 10.1016/j.diabres.2021.108873
33. Targher G, Lonardo A, Byrne CD. Nonalcoholic fatty liver disease and chronic vascular complications of diabetes mellitus. Nat Rev Endocrinol (2018) 14(2):99–114. doi: 10.1038/nrendo.2017.173
34. Targher G, Byrne CD. Non-alcoholic fatty liver disease: an emerging driving force in chronic kidney disease. Nat Rev Nephrol (2017) 13(5):297–310. doi: 10.1038/nrneph.2017.16
35. Vanjiappan S, Hamide A, Ananthakrishnan R, Periyasamy SG, Mehalingam V. Nonalcoholic fatty liver disease in patients with type 2 diabetes mellitus and its association with cardiovascular disease. Diabetes Metab Syndr (2018) 12(4):479–82. doi: 10.1016/j.dsx.2018.01.001
36. Ruotolo G, Lincoff MA, Menon V, McErlean E, Wolski K, Haas JV, et al. Lipoprotein (a) is a determinant of residual cardiovascular risk in the setting of optimal LDL-c in statin-treated patients with atherosclerotic cardiovascular disease. Circulation (2017) 136:A17400–A. doi: 10.1161/circ.136.suppl_1.17400
37. Lee H, Park KS, Jeon YJ, Park EJ, Park S, Ann SH, et al. Lipoprotein(a) and subclinical coronary atherosclerosis in asymptomatic individuals. Atherosclerosis (2022) 349:190–5. doi: 10.1016/j.atherosclerosis.2021.09.027
38. Obisesan OH, Kou M, Wang FM, Boakye E, Honda Y, Uddin SMI, et al. Lipoprotein(a) and subclinical vascular and valvular calcification on cardiac computed tomography: The atherosclerosis risk in communities study. J Am Heart Assoc (2022) 11(11):e024870. doi: 10.1161/JAHA.121.024870
39. van Dijk RA, Kolodgie F, Ravandi A, Leibundgut G, Hu PP, Prasad A, et al. Differential expression of oxidation-specific epitopes and apolipoprotein(a) in progressing and ruptured human coronary and carotid atherosclerotic lesions. J Lipid Res (2012) 53(12):2773–90. doi: 10.1194/jlr.P030890
40. Que X, Hung MY, Yeang C, Gonen A, Prohaska TA, Sun X, et al. Oxidized phospholipids are proinflammatory and proatherogenic in hypercholesterolaemic mice. Nature (2018) 558(7709):301–6. doi: 10.1038/s41586-018-0198-8
41. Tsimikas S. A test in context: Lipoprotein(a): Diagnosis, prognosis, controversies, and emerging therapies. J Am Coll Cardiol (2017) 69(6):692–711. doi: 10.1016/j.jacc.2016.11.042
42. Willeit P, Ridker PM, Nestel PJ, Simes J, Tonkin AM, Pedersen TR, et al. Baseline and on-statin treatment lipoprotein(a) levels for prediction of cardiovascular events: individual patient-data meta-analysis of statin outcome trials. Lancet (2018) 392(10155):1311–20. doi: 10.1016/S0140-6736(18)31652-0
43. Jin JL, Cao YX, Zhang HW, Sun D, Hua Q, Li YF, et al. Lipoprotein(a) and cardiovascular outcomes in patients with coronary artery disease and prediabetes or diabetes. Diabetes Care (2019) 42(7):1312–8. doi: 10.2337/dc19-0274
44. Saeed A, Sun W, Agarwala A, Virani SS, Nambi V, Coresh J, et al. Lipoprotein(a) levels and risk of cardiovascular disease events in individuals with diabetes mellitus or prediabetes: The atherosclerosis risk in communities study. Atherosclerosis (2019) 282:52–6. doi: 10.1016/j.atherosclerosis.2018.12.022
45. Kostner KM, Kostner GM. Lp(a) and the risk for cardiovascular disease: Focus on the lp(a) paradox in diabetes mellitus. Int J Mol Sci (2022) 23(7):3584. doi: 10.3390/ijms23073584
46. Wang H, Men P, Xiao Y, Gao P, Lv M, Yuan Q, et al. Hepatitis b infection in the general population of China: a systematic review and meta-analysis. BMC Infect Dis (2019) 19(1):811. doi: 10.1186/s12879-019-4428-y
47. Langsted A, Nordestgaard BG, Kamstrup PR. Elevated lipoprotein(a) and risk of ischemic stroke. J Am Coll Cardiol (2019) 74(1):54–66. doi: 10.1016/j.jacc.2019.03.524
48. Laschkolnig A, Kollerits B, Lamina C, Meisinger C, Rantner B, Stadler M, et al. Lipoprotein (a) concentrations, apolipoprotein (a) phenotypes, and peripheral arterial disease in three independent cohorts. Cardiovasc Res (2014) 103(1):28–36. doi: 10.1093/cvr/cvu107
49. Tsimikas S, Kiechl S, Willeit J, Mayr M, Miller ER, Kronenberg F, et al. Oxidized phospholipids predict the presence and progression of carotid and femoral atherosclerosis and symptomatic cardiovascular disease: five-year prospective results from the bruneck study. J Am Coll Cardiol (2006) 47(11):2219–28. doi: 10.1016/j.jacc.2006.03.001
50. Yamamoto M, Egusa G, Yamakido M. Carotid atherosclerosis and serum lipoprotein(a) concentrations in patients with NIDDM. Diabetes Care (1997) 20(5):829–31. doi: 10.2337/diacare.20.5.829
51. Scuteri A, Najjar SS, Muller DC, Andres R, Hougaku H, Metter EJ, et al. Metabolic syndrome amplifies the age-associated increases in vascular thickness and stiffness. J Am Coll Cardiol (2004) 43(8):1388–95. doi: 10.1016/j.jacc.2003.10.061
Keywords: lipoprotein(a), impaired fasting glucose, diabetes, carotid plaques, fatty liver disease
Citation: Wang J, Sun H, Wang Y, An Y, Liu J and Wang G (2022) Glucose metabolism status modifies the relationship between lipoprotein(a) and carotid plaques in individuals with fatty liver disease. Front. Endocrinol. 13:947914. doi: 10.3389/fendo.2022.947914
Received: 19 May 2022; Accepted: 31 October 2022;
Published: 16 November 2022.
Edited by:
Christiano Argano, ARNAS Ospedali Civico Di Cristina Benfratelli, ItalyReviewed by:
Shuangling Xiu, Capital Medical University, ChinaAntonino Tuttolomondo, University of Palermo, Italy
Copyright © 2022 Wang, Sun, Wang, An, Liu and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Guang Wang, d2FuZ2d1YW5nQGJqY3loLmNvbQ==
 Jiaxuan Wang
Jiaxuan Wang Honglin Sun
Honglin Sun Ying Wang2
Ying Wang2 Yu An
Yu An Jia Liu
Jia Liu Guang Wang
Guang Wang