
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Endocrinol. , 28 October 2022
Sec. Thyroid Endocrinology
Volume 13 - 2022 | https://doi.org/10.3389/fendo.2022.947594
This article is part of the Research Topic Treatment of Subclinical Thyroid Dysfunction in Patients with Comorbidities View all 6 articles
This systematic review and meta-analysis was conducted to evaluate the effect of COVID-19 on thyroid function and the role of thyroid hormones alterations in predicting the severity of COVID-19. Online databases, including Scopus, Medline/PubMed, EMBASE, Google Scholar, and Cochrane were searched up to August 2, 2022. After screening titles, abstracts, and full manuscripts, respectively, 30 reports were enrolled. The risk of bias (ROB) was evaluated using the QUADAS-2 tool. In addition, odds ratio (OR) and hazard ratio (HR) analysis for assessing the OR of abnormal thyroid function tests (TFT) in predicting the COVID-19 severity and poor outcomes. Among 30 enrolled studies, ROB of the current study is estimated low to moderate. The average number of patients in each study was 325 (range: 40-3,703), with an overall mean age of 57.6, and the female proportion of 40.4%. Overall, the pooled analysis showed that the prevalence of thyroid dysfunction among 9,707 COVID-19 cases was 15%. Among mild to moderate COVID-19 patients, 6.2% had abnormal TFT, and among patients who experienced severe to critical COVID-19, 20.8% had abnormal TFT. The pooled OR for abnormal TFT and the severity of COVID-19 obtained from 3,865 COVID-19 patients was 3.77 (2.03, 6.99). The pooled HR of TSH level of COVID-19 mortality was 1.57 (0.91, 2.72). Our results demonstrate a high prevalence of thyroid dysfunction in COVID-19, and that among patients severe cases had a 3.77-fold higher risk of abnormal TFT compared to mild to moderate COVID-19. Further studies are required to evaluate the longer-term prognostic role of thyroid dysfunction in severe COVID-19, and investigate potential therapeutic strategies.
Coronavirus Disease 2019 (COVID-19) is a new millennium pandemic with unprecedented public health challenges (1). The causative agent is a novel enveloped β-coronavirus called severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) (2). Since it was first detected in Wuhan, COVID-19 has spread rapidly, and outbreaks are increasing exponentially. As of December 28th, 2020, the number of patients afflicted with SARS-Cov-2 has surpassed 80M cases worldwide, and more than 1.7M people have now died of COVID-19. SARS-Cov-2 has a phylogenetic resemblance to SARS-CoV-1 and is related to SARS-CoV-1. SARS-CoV-2 infects human tissues entering cells through the angiotensin-converting enzyme 2 (ACE2) receptor (3).
COVID-19 can range from asymptomatic manifestations to severe and even fatal respiratory disorders (4). In high-risk patients (i.e., elderly or patients with a history of cardiovascular diseases, chronic hypertension, and diabetes), SARS-CoV-2 infection can induce both system and pulmonary inflammation (5). The most frequent serious complications of COVID-19 are acute respiratory distress syndrome (ARDS), respiratory failure, sepsis, acute cardiac injury, and heart failure (5).
The international scientific community has now reacted massively to the pandemic conquest of COVID-19, and a substantial number of studies have been carried out on different aspects of the disease, including prevention, diagnosis, and treatment. These vast investigations on COVID-19 has led to a growing body of evidence regarding a remarkable association between the prevalence of thyroid disorders in patients with COVID-19 (6).
A complex association has been documented between hormones and immunomodulatory signaling molecules in thyroid and viral infections (7). Viruses and their related inflammatory-immune responses are particularly noteworthy since they were noted to affect thyroid function permanently in some cases (8). Although several studies have evaluated the thyroid gland function in COVID-19, no comprehensive meta-analysis was conducted on this crucial topic so far. Thus, herein, we aimed to systematically evaluate the association between COVID-19 and thyroid function and the potential of thyroid hormones in predicting the severity of COVID-19 and present a meta-analysis of the current related data.
Electronic databases, including Scopus, Medline/PubMed, EMBASE, Google Scholar, and Cochrane database were screened until August 2, 2022. We conducted a comprehensive search of PubMed and MEDLINE articles using the combination of the search terms “thyroid” and “coronavirus” (or “SARS-CoV-2” or “COVID-19”). An English language limitation was added. This study was carried out based on Desired reporting products for the Systemic and Meta-Analysis Review (PRISMA) (9).
Two blind reviewers conducted the title-abstract screening of all selected research separately. Full-text assessments were conducted afterward. Duplicate and unrelated reports were excluded at the title-abstract screening level before reviewing the full manuscripts.
All studies evaluating or mentioning the number of patients with abnormal thyroid function in COVID-19 cases were enrolled.
Molecular reports, laboratory observations in percentages, case studies, and statements were excluded.
Two reviewers extracted the data independently, taking into account main attributes, including author, year of publication, method of analysis, sample size, lab observations, co-morbidities, and final clinical results.
The quality appraisal checklist and the critical appraisal methodological index for non-randomized studies were used for bias risk assessment. The revised “Quality Assessment of Diagnostic Accuracy Studies” (QUADAS) tool was used for the quality assessment of the included studies. This choice was based on former studies that have confirmed and suggested its utility for quality assessment of diagnostic studies in all domains of patient selection, risk of bias, reference standard, and flow and timing. Therefore, we have proceeded with this tool based on former validations (10). Moreover, the funnel plot and Egger’s regression test were used to assess publication bias (11).
Cochran, Chi-Square, and I2 were used to determine the heterogeneity of tests. When I2 was more than 50%, a random-effects model was preferred to a fixed-effects model [15]. Also, we used the prevalence formula as below to evaluate the prevalence of abnormal thyroid test function (TFT) in COVID-19 cases:
In addition, we used odds ratio (OR) and hazard ratio (HR) analysis to evaluate the association of abnormal TFT on the prediction of COVID-19 severity. A P-value less than 0.05 was considered statistically meaningful (2-sided). All data have been analyzed with R (version 4.0.2; R Foundation for Statistical Computing) and R studio (Version 1.3.1073).
After removing duplicates, the initial literature search yeilded a total of 499 published records from PubMed, EMBASE, Google Scholar, Web of Science, and Scopus. The titles and abstracts were screened, and 435 records were excluded. Then we checked and reviewed the full texts of 64 remaining articles for evaluating eligibility based on the PRISMA algorithm (Figure 1). Eventually, 30 studies were deemed eligible for final inclusion. Figure 1 illustrates the selection process of enrolled studies in a flow diagram.
Most of these publications included a series of expert opinions and suggestions on new methods for treating thyroid disorders in the face of the possibility of transmission of COVID-19 and the potential of health care spikes (5, 12–25). However, all 30 articles looked at thyroid activity or identified thyroid disorders in COVID-19 patients (5, 12–40). Twenty-three studies studied the TSH level and thyroid gland hormones, including fT3 and fT4, based on COVID-19 severity (5, 14–16, 19, 20, 23–26, 28, 31, 33, 35–38, 40); and nineteen studies evaluated the exact levels of TSH and thyroid gland hormones (12, 13, 17, 18, 20, 26–35, 37–40). Finally, four studies estimated the hazard ratio (HR) in COVID-19 survivors and non-survivors based on thyroid function (22–24, 40).
The main characteristics of the 30 included studies are summarized in Table 1. Twelve studies originated in China (12, 13, 15, 19, 20, 22, 24, 32, 38–40), four from Italy (21, 27, 30, 36), two from Hong Kong (17, 35), two from Spain (22, 28), and one each from Brazil, France, Greece, India, Kuwait, Saudi Arabia, South Korea, Turkey, United Kingdom, and the United States (14, 25, 26, 29, 31, 33, 34, 37, 41, 42). All included studies stated that RT-PCR confirmed the diagnosis of COVID-19 through typical COVID-19 symptoms except one study; thus, all studies enrolled symptomatic patients, and only one enrolled asymptomatic COVID-19 patients (43). Data were recorded retrospectively in all studies except five that stated prospective data collection (28, 33–36). All patients were enrolled consecutively. The average number of patients in each study was 325 (range: 40-3,703), with an overall mean age of 57.6, and the female proportion of 40.4% (Table 1).
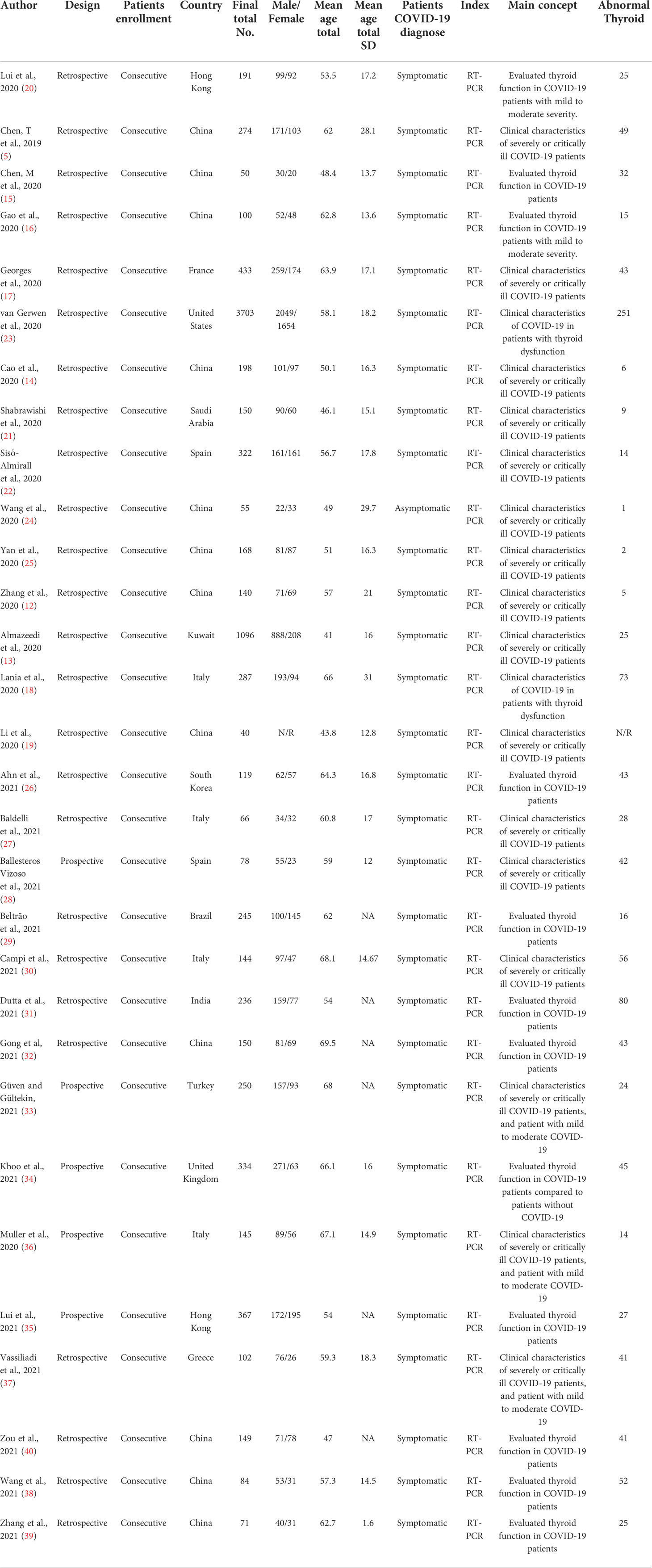
Table 1 The main characteristics of 30 studies from different countries with RT-PCR confirmed the diagnosis of COVID-19.
In this step, we evaluated the quality assessment and ROB; in this regard, Figure 2 shows the overall quality assessment using the QUADAS-2 tool. Based on the quality assessment results achieved from each study, we considered that the overall ROB is low to moderate. Most of the studies had a low ROB (20 studies), six had medium ROB, and four had high ROB; the primary source of bias was unclear patient selection process (i.e., missing or unclear inclusion/exclusion criteria). In addition, the flow and timing domain was judged to be high risk in five studies, and the index test domain was estimated to be high risk in nine studies.
We also evaluated the potential risk of publication bias for any abnormal thyroid function test using Egger’s test method (Figure 3). Egger’s test revealed no risk for publication bias (P= 0.317).
Herein, we evaluated the prevalence of abnormal thyroid function in COVID-19 patients across all studies. Our results showed that the highest prevalence was observed in a Chinese report with a high of 64% (15), and the lowest prevalence of thyroid dysfunction was observed in a Chinese report with a prevalence of 1.2% (25) (Table 2). Our results showed significant high heterogeneity between Chinese studies (P<0.05) in comparison to reports originating from other countries (P>0.05). These results showed that Chinese reports could be the main cause of ROB in this section. Overall, the pooled analysis showed that the prevalence of thyroid dysfunction among 9,707 COVID-19 patients was 15% (Table 2).
The exact TSH and fT3 levels were merely included in nineteen studies, among which one study was selectively conducted on low T3 syndrome (43). One study has categorized the TFT disturbances into two groups: 10 patients manifesting isolated low T3 syndrome and ten patients who presented with isolated low TSH syndrome (1). Among the remaining three studies, fT3 was lower in cases with COVID-19 compared to controls. One study noted that when categorizing covid-19 patients into mild and severe, fT3 was lower in the severe group (44). The other eleven included studies that have mentioned thyroid disorders without presenting exact TSH or fT3 values. No differentiation was indicated regarding hypo/hyperthyroidism or low T3 syndrome (36, 44–50).
Among 30 studies, 23 have evaluated the role of TFT in the severity of COVID-19 directly/indirectly. Of these studies, six studies explored the mean level of TSH, fT3, and fT4 in severe and non-severe COVID-19 patients. Data obtained from fifteen studies showed that 2,841 patients experienced mild to moderate COVID-19, and 1,024 patients experienced severe critical COVID-19. Among mild to moderate COVID-19 patients, 176 patients had abnormal TFT (6.2%), and among patients who experienced severe to critical COVID-19, 213 patients had abnormal TFT (20.8%). Our results showed that the rate of abnormal TFT was significantly higher in severe to critical COVID-19 patients compared to mild to moderate COVID-19 patients.
On the other hand, to complete the risk of severity, we evaluated the odds ratio (OR) of abnormal TFT in presenting severe COVID-19 (Figure 4). Our random effect odds ratio analysis showed that the highest OR for abnormal TFT was 107.0 (2.9, 3870), and the lowest was 0.44 (0.23, 0.83). Pooled analysis showed that the overall OR obtained from 3,865 COVID-19 patients was 3.77 (2.03, 6.99) (Figure 4). Thus, there is a 3.77-fold association between the abnormal TFT and the severity of COVID-19.

Figure 4 Evaluation of OR of abnormal TFT for COVID-19 severity. Experimental: patients faced severe to critical form of COVID-19, Control: patients faced mild to moderate form of COVID-19, Events: patients with abnormal TFT.
In this section, we extracted the four manuscripts which have evaluated the severity in COVID-19 patients with normal/abnormal TSH levels based on hazard ratio (HR) analysis (Figure 5). Figure 5 illustrates the forest plot of HR analysis of each study and the pooled HR. Our results showed that the minimum HR was 0.98 (0.75, 1.30), and the maximum HR was 2.96 (1.75, 5.00) with the random effect. The pooled HR of TSH level of COVID-19 mortality was 1.57 (0.91, 2.72). This result shows that patients with abnormal TSH had a non-significant 1.57-fold higher risk of disease severity than patients with normal TSH levels.
Among 30 enrolled studies, our review revealed that the ROB of the available studies is estimated low to moderate. The pooled analysis showed a high prevalence of thyroid dysfunction among patients with COVID-19 (15%). Thyroid dysfunction was associated with the severity of COVID-19, as its prevalence was 6.2% in mild to moderate cases versus 20.8% among patients with severe COVID-19. The pooled OR for the association of abnormal TFT and severe form of COVID-19 was statistically significant.
Previously published documents revealed a strong bidirectional association between thyroid disorders and COVID-19. Up to now, activation of preexisting thyroid dysfunction, hypo or hyperthyroidism and, subacute thyroiditis had been noted as sequels of COVID infection. Studies suggested that thyroid dysfunction could arise by direct insult tissue or indirectly (51, 52). One of the probable mechanism is that the thyroid gland and hypothalamic-pituitary axis could be indirectly affected by the irregular systemic inflammatory immune response triggered by SRAS-CoV-2 infection (49). Moreover, several studies are published on the thyroid gland histopathological findings in patients with COVID-19. Pathological examination of thyroid tissue showed high affinity for SRAS-CoV-2 mediated by Angiotensin-converting enzyme 2 (ACE-2) receptor (53). In addition, studies have reported interstitial lymphocytic infiltration and epithelial layer disturbance (19, 20). Importantly, histopathological studies have suggested that virus infiltration in endocrine glands can be observed in severe cases of COVID-19. Such studies have detected the viral genome and proteins inside endocrine tissues and have identified signs of apoptosis as a result of this viral invasion (54, 55), which signifies the potential for direct damage to the thyroid gland in severe COVID-19 cases.
The World Health Organization (WHO) recommendation for clinical management is not to evaluate the thyroid function in COVID-19 cases (19). However, in some trials, improvements in thyroid function have been identified in the preceding coronavirus epidemic of SARS-CoV (12, 13). Wang et al., In a particular report, showed that lower serum levels of TSH, T3, and T4 were observed in COVID-19 patients compared to normal healthy individuals (24). In addition, they observed low T3 and T4 levels in different disease phases—94% and 46% in the acute phase and 90% and 38% in the convalescent phase for T3 and T4, respectively. Similarly, Leow et al. (22) reported four (6.7%) SARS patients becoming biochemically hypothyroid three months after rehabilitation, including three with core hypothyroidism and a recent persistent lymphocytic hypothyroidism. In the three cases with core hypothyroidism, the case with primary hypothyroidism spontaneously remitted after three/nine months to lifelong T4 therapy (17). Consequently, it was discovered from the SARS outbreak that a viral infection could primarily induce low TFT levels, either on its own or in combination, through primary or secondary injuries (i.e., hypothalamic or pituitary). Moreover, the low levels of TSH and T3 may be seen as part of an adaptive condition of anti-thyroidal disease syndrome caused by a significant stress situation, especially in extreme or critically ill patients (i.e., systemic virus disease) (14, 21, 25). Additionally, evidence suggests,although, preexisting thyroid disease will not increase risk of COVID infection and complication (56), COVID could activate subclinical conditions in susceptible population or cause relapse of known condition specially Grave’s disease which is mostly temporary and does not need further management, however, as it can aggregate existing condition specially inducing thyroid storm in hyperthyroidism proper management and regular monitoring of thyroid function in this population is desirable and recommended (51, 57). As for follow up in these patients, since in most patients, thyroid dysfunction remit in three month no conclusion is proposed for further follow-up by studies and experts (51).
Low T3 syndrome, also known as the euthyroid sick syndrome (ESS) could be responsible partly for high prevalence of thyroid dysfunction in COVID-19. This syndrome is known to occur in 60 to 70% of critically ill patients regardless of the cause (58) and in different studies in admitted patients due to COVID, ESS was presented in around 30% patients(up to 64%) and were related to severity, longer hospitalization and mortality (40, 59). Similarly, studies have suggested a significant inverse correlation between the severity of illness and low serum total T3. In contrast, there was no relationship between total thyroxine or TSH levels and severity of illness and TSH levels are indicated to be within the normal range or relatively low (49). Consequently, some studies suggested evaluating free triiodothyronine (fT3) as a prognostic factor in COVID (60). Moreover, it is noteworthy that like other causes of Low T3 syndrome, thyroid function tend to go back to normal after the acute phase of the disease (20). This point is particularly remarkable since our results indicated significantly higher rates of abnormal TFTs in patients with severe to critical forms of COVID-19 compared to mild to moderate forms.
However, due to a lack of past medical history and follow-up data on these patients, no diagnostic confirmation is plausible. Moreover, some included studies that have mentioned thyroid disorders without presenting exact TSH or fT3 values. No differentiation was indicated regarding hypo/hyperthyroidism or low T3 syndrome (12–14, 17, 21–25). Therefore, no subgroup analysis was conceivable. These are particularly noteworthy since, considering the lack of follow-up data on TFTs post-discharge, we are unable to discuss our results in terms of etiological aspects. The lack of data on TFTs might be due to the fact that thyroid disorders were not initially associated with COVID-19 and were only later discussed.
On the other hand, current literature on COVID- 19 patients with thyroid dysfunction presented more evidence that SARS-CoV-2 damage targets could originate from the thyroid gland and the entire hypothalamic-pituitary-thyroid (HPT) axis and can be manifested as thyrotoxic, hypothyroidism, and nonthyroidal disease. SARS-CoV-2 can directly insult thyroid tissue and like other respiratory virus causes thyroiditis. Till now several cases of subacute thyroiditis (SAH) were documented in COVID. Among 21 reported patients with SAT, about 75% were female and was mostly presented as painful SAT with the presentation of thyrotoxicosis (61). Also, in a recently published cohort study from India, around 1.6% patients were diagnosed with SAT (62). Just like in other viral thyroiditis, in most cases SAT subsided without permanent complication (61).
Nevertheless, one point that should be noticed is that drug interactions also, might lead to TFT disturbances. For instance, Heparin-induced increase in non-essential fatty acid displaces T4 from thyroid-binding protein and causes an artefactual increase in free hormone levels. Alterations of thyroid-binding globulin can cause a corresponding change in total T4 and T3 levels (18). Corticosteroids are also known to reduce the conversion of T4 to T3, and therefore, lead to reduced T3 serum levels. This mechanism also operates regarding other common drugs used for ICU patients, including iodine and amiodarone (19). Since these drugs are commonly prescribed for severe COVID-19 cases, they can potentially be in charge of TFT alterations. Therefore, future research is required to thoroughly investigate the potential confounding role of COVID-related medications that could alter TFT levels. However, we could not address this issue in our study due to a lack of data regarding the prescribed medications in most included studies.
Our study limitations were as follows: 1- some included studies only reported the rate of patients with hypothyroidism; thus, hyperthyroidism or euthyroid diseases were missed. 2- Due to the lack of post-discharge follow-up, we were unable to analyze the progress of thyroid hormones over time. 3- We could not conclude that whether abnormal TFT could impact COVID-19 severity or COVID-19 might be severe in patients with abnormal TFT. 4- We were unable to run a meta-regression between the serum levels of TSH, T3, and T4 due to a lack of data, and a mere OR analysis was conducted. Nonetheless, our review has generated the largest available data regarding the association of COVID-19 and thyroid dysfunction.
Last but not least, several studies have suggested a potential causal association between COVID-19 infection and TFT abnormalities; however, the results of our meta-analysis do not focus on causality and do not establish a causal association between COVID-19 and thyroid dysfunction, mainly due to significant heterogeneity in populations, low sample sizes, and lack of investigation of a direct causal role for COVID-19 in most included studies. But what is apparent is that thyroid dysfunction is accompanied with more severe form of COVID thus monitoring TFT seems to be beneficial specially in some populations including patients with preexisting thyroid condition, in severe form of infection, and in patients with manifestation of viral thyroiditis (neck pain). Likewise, regarding to the new published article inventing a scoring system for thyroid dysfunction in COVID patient with five parameters including symptoms, presence of ischemic heart disease/congestive heart failure and abnormal laboratory finding (lymphocyte count, C-reactive protein, and SARS-CoV-2 cycle threshold values) should be considered for TFT evaluation (63).
Our study showed that the prevalence of thyroid dysfunction among patients with COVID-19 was as high as 15%. In addition, there is a 3.77-fold association between abnormal TFT and the severity of COVID-19. Lastly, patients with TSH lower than ~1 had a 1.57-fold higher risk of disease severity and poor outcomes than patients with normal TSH levels. Further studies are required to understand the prognostic significance of thyroid dysfunction in severe COVID-19 and investigate therapeutic approaches to reduce poor outcomes associated with this clinical condition.
MD, MRN, HS, and MN participated in the design of the review, acquired the documents and made a discussion, edited the manuscript, supervised the study and wrote the paper, collected the data and contributed to figures and tables. All authors contributed to the article and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Saberi-Movahed F, Mohammadifard M, Mehrpooya A, Rezaei-Ravari M, Berahmand K, Rostami M, et al. Decoding clinical biomarker space of COVID-19: Exploring matrix factorization-based feature selection methods Comput Biol Med. (2022) 146, 105426. doi: 10.1016/j.compbiomed.2022.105426
2. Zhu N, Zhang D, Wang W, Li X, Yang B, Song J, et al. A novel coronavirus from patients with pneumonia in China, 2019. New Engl J Med (2020) 382(8):727–33. doi: 10.1056/NEJMoa2001017
3. Ziegler CG, Allon SJ, Nyquist SK, Mbano IM, Miao VN, Tzouanas CN, et al. SARS-CoV-2 receptor ACE2 is an interferon-stimulated gene in human airway epithelial cells and is detected in specific cell subsets across tissues. Cell (2020) 181(5):1016–35.e19. doi: 10.1016/j.cell.2020.04.035
4. Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al. Clinical features of patients infected with 2019 novel coronavirus in wuhan, China. Lancet (2020) 395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5
5. Chen T, Wu D, Chen H, Yan W, Yang D, Chen G, et al. Clinical characteristics of 113 deceased patients with coronavirus disease 2019: Retrospective study. Bmj (2019) 368:m1295. doi: 10.1136/bmj.m1091
6. Hennessey JV. COVID-19 and how it is affecting me as a thyroidologist. 140 Huguenot Street, 3rd Floor New: Mary Ann Liebert, Inc. (2020).
7. De Vito P, Incerpi S, Pedersen JZ, Luly P, Davis FB, Davis PJ. Thyroid hormones as modulators of immune activities at the cellular level. Thyroid (2011) 21(8):879–90. doi: 10.1089/thy.2010.0429
8. Tomer Y, Davies TF. Infection, thyroid disease, and autoimmunity. Endocrine Rev (1993) 14(1):107–20. doi: 10.1210/edrv-14-1-107
9. Moher D, Altman DG, Liberati A, Tetzlaff J. PRISMA statement. Epidemiology (2011) 22(1):128. doi: 10.1097/EDE.0b013e3181fe7825
10. Whiting PF, Rutjes AW, Westwood ME, Mallett S, Deeks JJ, Reitsma JB, et al. QUADAS-2: A revised tool for the quality assessment of diagnostic accuracy studies. Ann Internal Med (2011) 155(8):529–36. doi: 10.7326/0003-4819-155-8-201110180-00009
11. Peters JL, Sutton AJ, Jones DR, Abrams KR, Rushton L. Comparison of two methods to detect publication bias in meta-analysis. Jama (2006) 295(6):676–80. doi: 10.1001/jama.295.6.676
12. Zhang J-J, Dong X, Cao Y-Y, Yuan Y-D, Yang Y-B, Yan Y-Q, et al. Clinical characteristics of 140 patients infected with SARS-CoV-2 in wuhan, China. Allergy (2020) 75(7):1730–41. doi: 10.1111/all.14238
13. Almazeedi S, Al-Youha S, Jamal MH, Al-Haddad M, Al-Muhaini A, Al-Ghimlas F, et al. Characteristics, risk factors and outcomes among the first consecutive 1096 patients diagnosed with COVID-19 in Kuwait. EClinicalMedicine (2020) 24:100448. doi: 10.1016/j.eclinm.2020.100448
14. Cao M, Zhang D, Wang Y, Lu Y, Zhu X, Li Y, et al. Clinical features of patients infected with the 2019 novel coronavirus (COVID-19) in shanghai, China. MedRxiv (2020). doi: 10.1101/2020.03.04.20030395
15. Chen M, Zhou W, Xu W. Thyroid function analysis in 50 patients with COVID-19: A retrospective study. Thyroid (2020) 31(1):8–11. doi: 10.1089/thy.2020.0363
16. Gao W, Guo W, Guo Y, Shi M, Dong G, Wang G, et al. Thyroid hormone concentrations in severely or critically ill patients with COVID-19. J endocrinological Invest (2020) 44(5):1031–40. doi: 10.1007/s40618-020-01460-w
17. Georges J, Cochet H, Roger G, Ben HJ, Soltani J, Azowa J, et al. Association of hypertension and antihypertensive agents and the severity of COVID-19 pneumonia. A monocentric French prospective study. Annales cardiologie d'angeiologie (2020) 69(5):247–54. doi: 10.1016/j.ancard.2020.09.030
18. Lania A, Sandri MT, Cellini M, Mirani M, Lavezzi E, Mazziotti G. Thyrotoxicosis in patients with COVID-19: the THYRCOV study. Eur J Endocrinology. (2020) 183(4):381–7. doi: 10.1530/EJE-20-0335
19. Li T, Wang L, Wang H, Gao Y, Hu X, Li X, et al. Characteristics of laboratory indexes in COVID-19 patients with non-severe symptoms in hefei city, China: diagnostic value in organ injuries. Eur J Clin Microbiol Infect Diseases. (2020) 39(12):2447–55. doi: 10.1007/s10096-020-03967-9
20. Lui DTW, Lee CH, Chow WS, Lee ACH, Tam AR, Fong CHY, et al. Thyroid dysfunction in relation to immune profile, disease status, and outcome in 191 patients with COVID-19. J Clin Endocrinol Metab (2021) 106(2):e926–35. doi: 10.1210/clinem/dgaa813
21. Shabrawishi M, Al-Gethamy MM, Naser AY, Ghazawi MA, Alsharif GF, Obaid EF, et al. Clinical, radiological and therapeutic characteristics of patients with COVID-19 in Saudi Arabia. medRxiv (2020) 15(8):e0237130. doi: 10.1101/2020.05.07.20094169
22. Sisó-Almirall A, Kostov B, Mas-Heredia M, Vilanova-Rotllan S, Sequeira-Aymar E, Sans-Corrales M, et al. Prognostic factors in Spanish COVID-19 patients: A case series from Barcelona. PloS One (2020) 15(8):e0237960. doi: 10.1371/journal.pone.0237960
23. van Gerwen M, Alsen M, Little C, Barlow J, Naymagon L, Tremblay D, et al. Outcomes of patients with hypothyroidism and COVID-19: a retrospective cohort study. Front endocrinology. (2020) 11:565. doi: 10.3389/fendo.2020.00565
24. Wang Y, Liu Y, Liu L, Wang X, Luo N, Li L. Clinical outcomes in 55 patients with severe acute respiratory syndrome coronavirus 2 who were asymptomatic at hospital admission in shenzhen, china. J Infect Diseases. (2020) 221(11):1770–4. doi: 10.1093/infdis/jiaa119
25. Yan S, Song X, Lin F, Zhu H, Wang X, Li M, et al. Clinical characteristics of coronavirus disease 2019 in hainan, China. medRxiv (2020). doi: 10.1101/2020.03.19.20038539
26. Ahn J, Lee MK, Lee JH, Sohn SY. Thyroid hormone profile and its prognostic impact on the coronavirus disease 2019 in Korean patients. Endocrinol Metab (Seoul). (2021) 36(4):769–77. doi: 10.3803/EnM.2021.1109
27. Baldelli R, Nicastri E, Petrosillo N, Marchioni L, Gubbiotti A, Sperduti I, et al. Thyroid dysfunction in COVID-19 patients. J Endocrinol Invest (2021) 44(12):2735–9. doi: 10.1007/s40618-021-01599-0
28. Ballesteros Vizoso MA, Castilla AF, Barceló A, Raurich JM, Argente del Castillo P, Morell-García D, et al. Thyroid disfunction in critically ill COVID-19 patients. relationship with in-hospital mortality. J Clin Med (2021) 10(21):5057. doi: 10.3390/jcm10215057
29. Beltrão FEL, Beltrão DCA, Carvalhal G, Beltrão FEL, Brito ADS, Capistrano K, et al. Thyroid hormone levels during hospital admission inform disease severity and mortality in COVID-19 patients. Thyroid (2021) 31(11):1639–49. doi: 10.1089/thy.2021.0225
30. Campi I, Bulgarelli I, Dubini A, Perego GB, Tortorici E, Torlasco C, et al. The spectrum of thyroid function tests during hospitalization for SARS COV-2 infection. Eur J Endocrinol (2021) 184(5):699–709. doi: 10.1530/EJE-20-1391
31. Dutta A, Jevalikar G, Sharma R, Farooqui KJ, Mahendru S, Dewan A, et al. Low FT3 is an independent marker of disease severity in patients hospitalized for COVID-19. Endocrine Connections (2021) 10(11):1455–62. doi: 10.1530/EC-21-0362
32. Gong J, Wang DK, Dong H, Xia QS, Huang ZY, Zhao Y, et al. Prognostic significance of low TSH concentration in patients with COVID-19 presenting with non-thyroidal illness syndrome. BMC Endocr Disord (2021) 21(1):111. doi: 10.1186/s12902-021-00766-x
33. Güven M, Gültekin H. The prognostic impact of thyroid disorders on the clinical severity of COVID-19: Results of single-centre pandemic hospital. Int J Clin Pract (2021) 75(6):e14129. doi: 10.1111/ijcp.14129
34. Khoo B, Tan T, Clarke SA, Mills EG, Patel B, Modi M, et al. Thyroid function before, during, and after COVID-19. J Clin Endocrinol Metab (2021) 106(2):e803–e11. doi: 10.1210/clinem/dgaa830
35. Lui DTW, Lee CH, Chow WS, Lee ACH, Tam AR, Fong CHY, et al. Role of non-thyroidal illness syndrome in predicting adverse outcomes in COVID-19 patients predominantly of mild-to-moderate severity. Clin Endocrinol (Oxf). (2021) 95(3):469–77. doi: 10.1111/cen.14476
36. Muller I, Cannavaro D, Dazzi D, Covelli D, Mantovani G, Muscatello A, et al. SARS-CoV-2-related atypical thyroiditis. Lancet Publishing Group (2020) p:739–41. doi: 10.1016/S2213-8587(20)30266-7
37. Vassiliadi DA, Ilias I, Pratikaki M, Jahaj E, Vassiliou AG, Detsika M, et al. Thyroid hormone alterations in critically and non-critically ill patients with SARS-CoV-2 infection. Endocrine Connections (2021) 10(6):646–55. doi: 10.1530/EC-21-0029
38. Wang W, Su X, Ding Y, Fan W, Zhou W, Su J, et al. Thyroid function abnormalities in COVID-19 patients. Front Endocrinol (Lausanne) (2020) 11:623792. doi: 10.3389/fendo.2020.623792
39. Zhang Y, Lin F, Tu W, Zhang J, Choudhry AA, Ahmed O, et al. Thyroid dysfunction may be associated with poor outcomes in patients with COVID-19. Mol Cell Endocrinol (2021) 521:111097. doi: 10.1016/j.mce.2020.111097
40. Zou R, Wu C, Zhang S, Wang G, Zhang Q, Yu B, et al. Euthyroid sick syndrome in patients with COVID-19. Front Endocrinol (Lausanne). (2020) 11:566439. doi: 10.3389/fendo.2020.566439
41. Wei L, Sun S, Zhang J, Zhu H, Xu Y, Ma Q, et al. Endocrine cells of the adenohypophysis in severe acute respiratory syndrome (SARS). Biochem Cell Biol (2010) 88(4):723–30. doi: 10.1139/O10-022
42. Yao X TYL. Histopathological study of new coronavirus pneumonia (COVID-19) in three patients. Chin J Pathol (2020) 49:34–8.
43. Hanley B, Naresh KN, Roufosse C, Nicholson AG, Weir J, Cooke GS, et al. Histopathological findings and viral tropism in UK patients with severe fatal COVID-19: a post-mortem study. Lancet Microbe (2020) 1(6):e245–e53. doi: 10.1016/S2666-5247(20)30115-4
44. Wang W, Ye Y, Yao H, Sun L, Wang A, Wang Z. Evaluation and observation of serum thyroid hormone and parathyroid hormone in patients with severe acute respiratory syndrome. J Chin Antituberculous Assoc (2003) 25:232–4.
45. Leow MKS, Kwek DSK, Ng AWK, Ong KC, Kaw GJL, Lee LSU. Hypocortisolism in survivors of severe acute respiratory syndrome (SARS). Clin Endocrinol (2005) 63(2):197–202. doi: 10.1111/j.1365-2265.2005.02325.x
46. Gu J, Gong E, Zhang B, Zheng J, Gao Z, Zhong Y, et al. Multiple organ infection and the pathogenesis of SARS. J Exp Med (2005) 202(3):415–24. doi: 10.1084/jem.20050828
47. Wei L, Sun S, Xu C-h, Zhang J, Xu Y, Zhu H, et al. Pathology of the thyroid in severe acute respiratory syndrome. Hum Pathol (2007) 38(1):95–102. doi: 10.1016/j.humpath.2006.06.011
48. Tang C, Wang Y, Lv H, Guan Z, Gu J. Caution against corticosteroid-based COVID-19 treatment. Lancet (2020) 395(10239):1759–60. doi: 10.1016/S0140-6736(20)30749-2
49. Economidou F, Douka E, Tzanela M, Nanas S, Kotanidou A. Thyroid function during critical illness. Hormones (Athens) (2011) 10(2):117–24. doi: 10.14310/horm.2002.1301
50. Suresh M, Srivastava N, Jain A, Nandy P. Thyroid dysfunction in critically ill patients in a tertiary care hospital in sikkim, India. Thyroid Res Pract (2017) 14:58. doi: 10.4103/trp.trp_16_16
51. Duntas LH, Jonklaas J. COVID-19 and thyroid diseases: A bidirectional impact. J Endocrine Soc (2021) 5(8):bvab076.
52. Trimboli P, Camponovo C, Scappaticcio L, Bellastella G, Piccardo A, Rotondi M. Thyroid sequelae of COVID-19: A systematic review of reviews. Rev Endocrine Metab Disord (2021) 22(2):485–91. doi: 10.1007/s11154-021-09653-1
53. Rotondi M, Coperchini F, Ricci G, Denegri M, Croce L, Ngnitejeu ST, et al. Detection of SARS-COV-2 receptor ACE-2 mRNA in thyroid cells: a clue for COVID-19-related subacute thyroiditis. J Endocrinological Invest (2021) 44(5):1085–90. doi: 10.1007/s40618-020-01436-w.
54. Poma AM, Bonuccelli D, Giannini R, Macerola E, Vignali P, Ugolini C, et al. COVID-19 autopsy cases: detection of virus in endocrine tissues. J Endocrinological Invest (2022) 45(1):209–14. doi: 10.1007/s40618-021-01628-y
55. Jakovac H, Ferenčić A, Stemberger C, Vitezić BM, Cuculić D. Detection of sars-Cov-2 antigens in thyroid gland showing histopathological features of subacute thyroiditis. Eur Thyroid J (2022) 11(2):e220005. doi: 10.1530/ETJ-22-0005
56. Bogojevic M, Bansal V, Pattan V, Singh R, Tekin A, Sharma M, et al. Association of hypothyroidism with outcomes in hospitalized adults with COVID-19: Results from the international SCCM discovery viral infection and respiratory illness universal study (VIRUS): COVID-19 registry. Clin Endocrinol (2022). doi: 10.1111/cen.14699
57. Naguib R. Potential relationships between COVID-19 and the thyroid gland: an update. J Int Med Res (2022) 50(2):030006052210828. doi: 10.1177/03000605221082898
58. Krishnamurthy A, Bhattacharya S, Lathia T, Deka N. Bnormal thyroid test results in euthyroid state: An appraisal of the role of drugs. (2020). doi: 10.22541/au.159414506.65558424
59. Schwarz Y, Percik R, Oberman B, Yaffe D, Zimlichman E, Tirosh A. Sick euthyroid syndrome on presentation of patients with COVID-19: A potential marker for disease severity. Endocrine Pract (2021) 27(2):101–9. doi: 10.1016/j.eprac.2021.01.001
60. Świstek M, Broncel M, Gorzelak-Pabiś P, Morawski P, Fabiś M, Woźniak E. Euthyroid sick syndrome as a prognostic indicator of COVID-19 pulmonary involvement, associated with poorer disease prognosis and increased mortality. Endocrine Pract (2022) 28(5):494–501. doi: 10.1016/j.eprac.2022.02.006
61. Aemaz Ur Rehman M, Farooq H, Ali MM, Ebaad Ur Rehman M, Dar QA, Hussain A. The association of subacute thyroiditis with COVID-19: A systematic review. SN Compr Clin Med (2021) 3(7):1515–27. doi: 10.1007/s42399-021-00912-5
62. Mondal S, DasGupta R, Lodh M, Ganguly A. Subacute thyroiditis following recovery from COVID-19 infection: novel clinical findings from an Eastern Indian cohort. Postgraduate Med J (2022), postgradmedj–2021-141429. doi: 10.1136/postgradmedj-2021-141429
Keywords: COVID-19, thyroid, TSH, T3, T4, severity
Citation: Darvishi M, Nazer MR, Shahali H and Nouri M (2022) Association of thyroid dysfunction and COVID-19: A systematic review and meta-analysis. Front. Endocrinol. 13:947594. doi: 10.3389/fendo.2022.947594
Received: 18 May 2022; Accepted: 16 September 2022;
Published: 28 October 2022.
Edited by:
Leonidas H. Duntas, National University Of Athens, GreeceReviewed by:
Mahsa Mozaffari, Iran University of Medical Sciences, IranCopyright © 2022 Darvishi, Nazer, Shahali and Nouri. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Majid Nouri, ZHIubWFqaWQubm91cmlAZ21haWwuY29t
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.