
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Endocrinol., 18 August 2022
Sec. Clinical Diabetes
Volume 13 - 2022 | https://doi.org/10.3389/fendo.2022.946642
This article is part of the Research TopicEpilepsy and Endocrine FunctionView all 5 articles
Introduction: Epileptic seizures during non-ketotic hyperglycemia (NKH) represent a rare complication of uncontrolled diabetes mellitus. The definition associates a blood sugar level > 200mg/dL (11mmol/L), hyperosmolality, absence of ketosis, dehydration and seizure control after normalization of blood sugar levels.
Material and methods: This retrospective observational study included patients hospitalized for epileptic seizures and NKH in the Cayenne Hospital Center between January 2010 and June 2020. The clinical, biological, and radiological results were collected.
Results: 18 out of 228 (7.9%) patients with both diabetes and epileptic seizures had NKH. The mean age of the 12 women and 6 men was 64.8 years. In 8 patients, brain imaging did not show acute lesions and the seizures disappeared with control of hyperglycemia by hydration and insulin. In 6 patients, the seizures revealed a stroke, hemorrhagic in 4 cases, ischemic in 2 cases. 4 patients had a seizure in a context of known vascular epilepsy. The epileptic seizures were mainly focal seizures with motor symptoms that could be repeated, focal to bilateral tonic-clonic or focal status.
Conclusion: Seizures in NKH are symptomatic of an acute brain lesion or vascular epilepsy more than 1 in 2 times. However, isolated NKH can cause seizures with a suggestive brain MRI.
Hyperglycemic seizures represent a clinical condition with high blood glucose levels, normal or increased serum osmolality, negative urine ketone bodies and dehydration. Non-ketotic hyperglycemia (NKH) is a rare complication of diabetes mellitus where seizures spontaneously resolve with glycemic control. The definition associates a blood sugar level > 200mg/dL (11mmol/L), hyperosmolality, absence of ketosis, dehydratation and seizure control after normalization of blood sugar levels.
The first review of the literature on the occasion of the report of 7 observations was published in 1965 (1). Since then, small series of patients(Wang 2017, N=13) (Tiamkao 2003, N=21) (Lammouchi 2004, N=22) (2–4) and many isolated clinical cases have been reported. The most frequently observed epileptic seizures are repeated focal motor seizures or focal motor status of different types. However, the observable epileptic symptoms vary widely: language arrest (5), aphasia (6)aphasic status (7) post-ictal blindness (8), tonic clonic generalized seizures (2, 4, 9). Specific MRI(Magnetic resonance imaging) aspects have recently been described (10–12) as subcortical hypointensity in Flair with low signal on apparent diffusion coefficient (ADC). These abnormalities could be distinguished from transient abnormalities related to epileptic seizures (12). The pathophysiology of epileptic seizures in NKH is not known, an old hypothesis involves a decrease in the levels of GABA(gamma-aminobutyric acid), an inhibitory neurotransmitter, due to metabolic disorders (13).
The control of the seizures with symptomatic treatment of hyperglycemia and the reversibility of the radiological abnormalities suggest an acute transient non-lesional symptomatic disorder. Antiepileptic drugs are ineffective or even deleterious (14).
However, hyperglycemia in the setting of known or unknown diabetes is also a risk factor for ischemic stroke (15). Hyperglycemia is also frequently observed in the acute phase of hemorrhagic stroke (16). In addition, seizures are observed in the initial phase of a stroke in 4.3-6.2% of ischemic strokes and in 10.7-15.6% of hemorrhagic strokes (17).
The aim of our retrospective study was to describe the clinical, biological and radiological aspects of patients with epileptic seizures associated with NKH at Cayenne Hospital in French Guiana. In French Guiana, the prevalence of diabetes is double that of mainland France and uncontrolled diabetes is frequent (18).
This single-center retrospective study was conducted at Cayenne Hospital in French Guiana. Records of patients aged >18 years hospitalized between January 2010 and June 2020 with coding type 1 or type 2 diabetes and seizure or epilepsy or status epilepticus were extracted, and then records with NKH were selected. We reviewed the emergency and hospital records and selected the files of patients corresponding to the definition of hyperglycemia without ketosis, including a blood sugar level > 200mg/dL (11 mmol/L)(Even if there is not really a definite threshold, it is accepted that hyperglycemia is defined by a blood sugar level above 200 mg/dl (19, 20), absence of ketosis on a urine dipstick, i.e., less than 2 crosses, or capillary ketonemia less than 0.5 mM/L. Definition of vascular epilepsy is: According to the revised classification of the International League Against Epilepsy (ILAE) a late-onset seizure (i.e at least two weeks after the stroke) in the context of stroke defines vascular epilepsy, making it no longer necessary to wait for a second seizure. For each patient, age, medical history, blood glucose and glycated hemoglobin values, type of seizure, clinical examination, brain imaging data, and length of stay were collected. All patients had had a brain CT scan. EEG was not performed in few cases of generalized status epilepticus because the diagnosis is clinical
The General Data Protection Regulation procedures included recording the study protocol on the health data hub platform under the project title EPIDIAB line 3243. We provided a declaration to the CNIL(The National Commission for Information Technology and Civil Liberties) with the EPIDIAB project title under registration number 2215827. A written information note was sent to all patients in order to confirm their non-objection, as required by French law.
The flow chart is presented in Figure 1. Among 228 diabetic patients with epileptic seizures, 18 had NKH (7.9%). The Table 1 summarizes the data collected from the 18 patients. Two different situations can be distinguished.
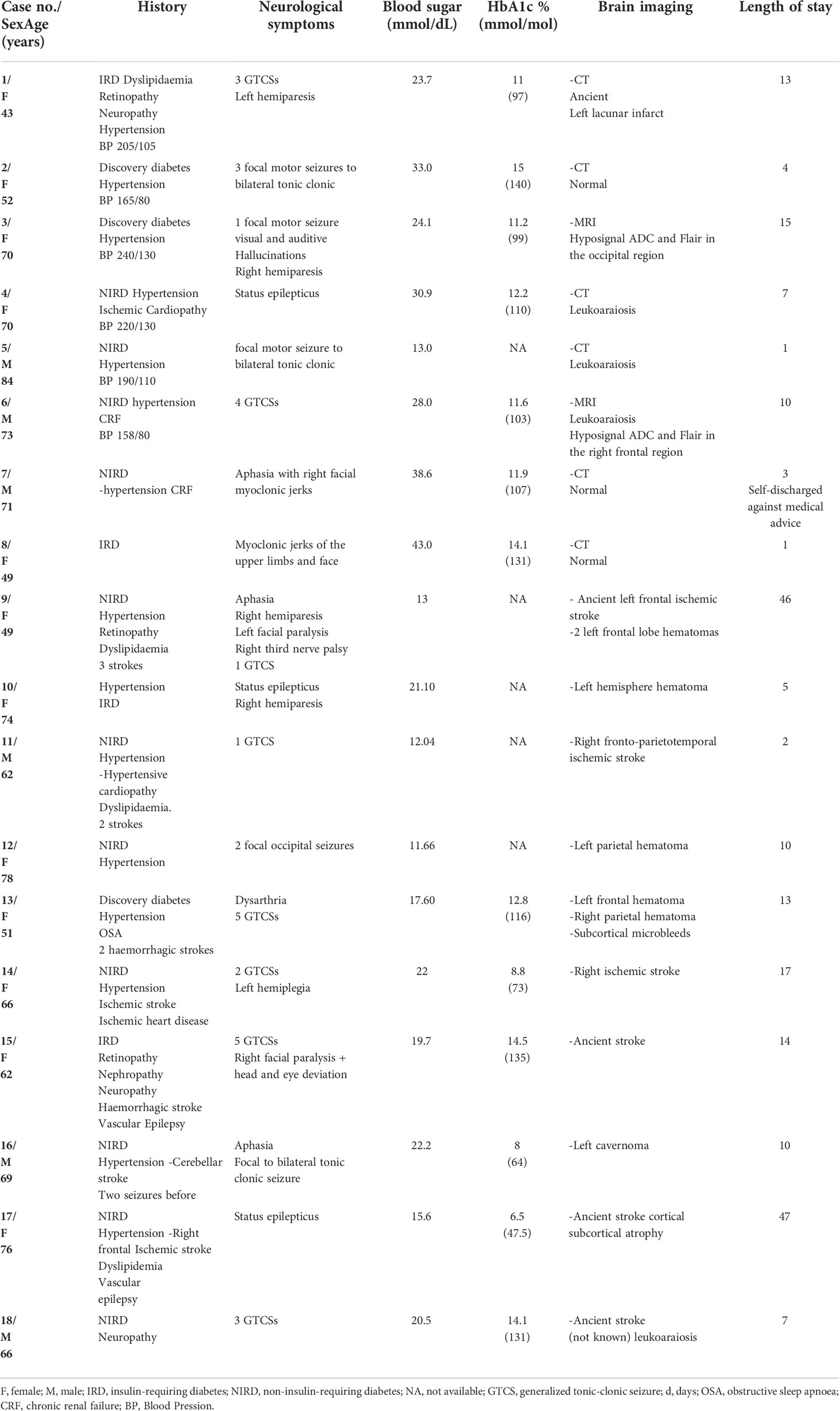
Table 1 Clinical, biological, radiological data and length of stay of adult patients with epileptic seizures during non-ketotic hyperglycemia, 2010-2020, Cayenne hospital, French Guiana.
In the first group 5 patients had an oral anti-diabetic treatment and 2 had insulin glargine with oral agent, 1 was new case of diabetes. In the second group 5 had an oral anti-diabetic treatment, 3 patients had insulin glargine and oral agent and one only basal bolus, and one was a new case of diabetes.
For a first group of subjects (patients 1 to 8) the brain imaging did not show acute or ancient brain lesions. Seizures were focal to bilateral tonic-clonic in 5 out of 8 cases: 4 focal motor, one focal cognitive. One patient had generalized status epilepticus (case 4). One patient experienced four generalized tonic-clonic seizures (case 6) while another patient experienced three generalized tonic-clonic seizures (case 1). EEGs (electroencephalogram) performed in all patients were normal. Brain MRI was performed in 2 patients (patients 3 and 6) and showed subcortical hyposignals in Flair with ADC decrease (Figure 2). In 2 patients (patients 2 and 3), seizures in relation to hyperglycemia revealed previously unknown diabetes. The mean age of the five women and three men was 64 years (range, 43-84 years). On admission, the mean blood glucose concentration was 29.3 mmol/L (range, 13-43 mmol/L), while the mean osmolarity and serum sodium, urea, bicarbonate, and glycated hemoglobin levels were, respectively, 301.5 mOsmol/L (range, 293.3-316 mOsmol/L) and 133.3 mmol/L (range, 127-139 mmol/L), 6.83 mmol/L, (range, 4.6-13.8 mmol/L), 24.5 mmol/L (range, 20.2-31 mmol/L), and 12.42% (112mmol/mol) (range, 11%-15% (97mmol/mol-140mmol/mol)). All patients were rehydrated with isotonic saline associated with venous insulin therapy. Seizures were rapidly controlled with symptomatic treatment of hyperglycemia. The mean length of stay of patients in this group was 7 days (range, 1-15 days). The rehydration rates were different. Patient no. 8 received the highest rehydration volume of 3 L/24 hours with venous insulin therapy at the highest rate of 7 UI/h, and her hospital stay was the shortest (1 day). Patient No. 7 had a stay of 3 days due to discharge against medical advice.
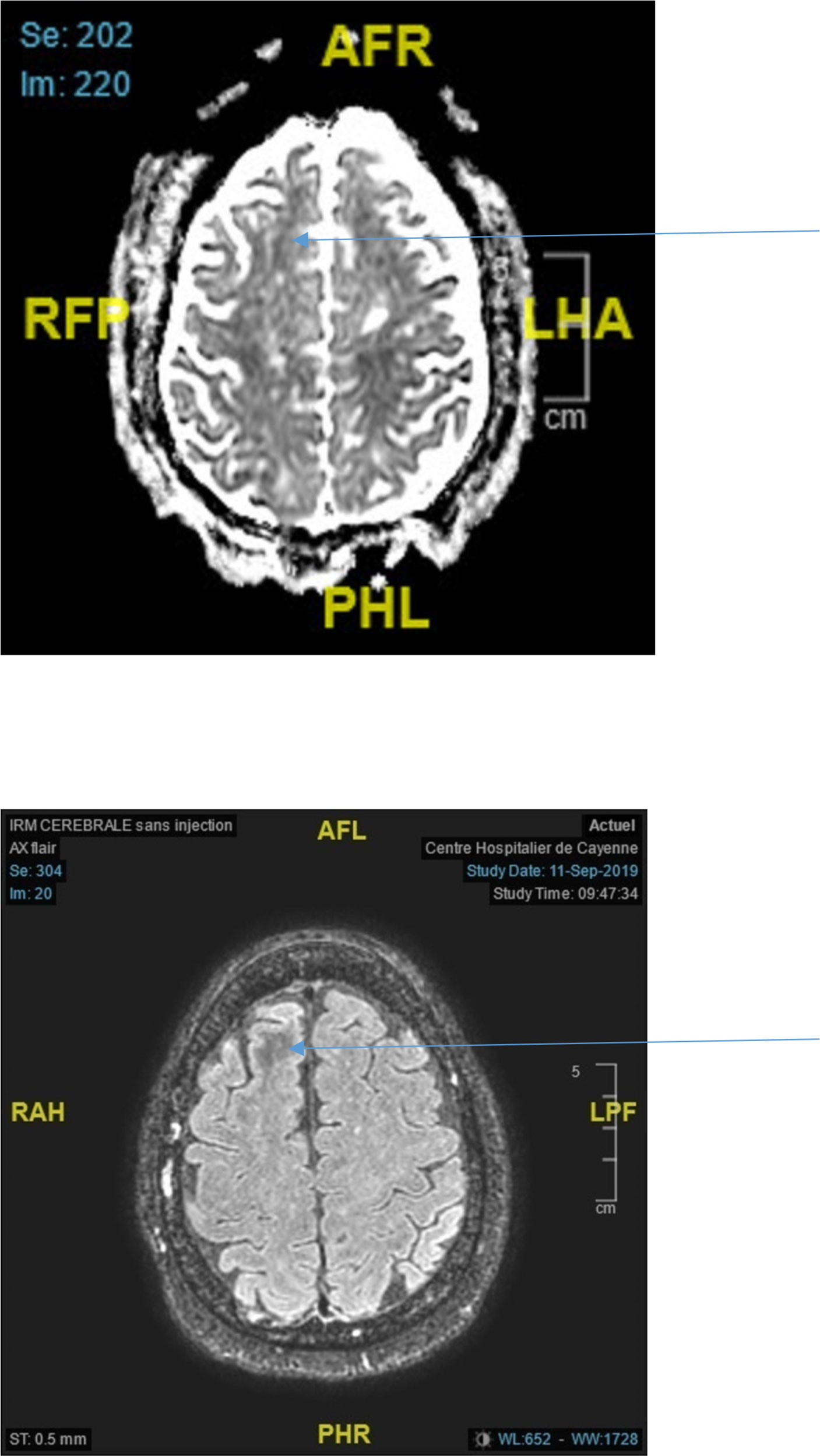
Figure 2 Top ADC decrease in the right frontal lobe (arrow) Bottom Axial Flair showing white matter hyposignal in the right frontal lobe (arrow).
For a second group of subjects (patients 9 to 18), the seizures were contemporary with an acute stroke in 6 (4 hematoma, 2 ischemia) or related to a vascular epilepsy in 4 with ancient epileptogenic vascular lesions on brain imaging. The seizures were generalized tonic in 7 patients. One patient had repeated focal occipital seizures and two patients had a generalized convulsive status. Seven out of 10 patients underwent electroencephalograms, two were normal, 3 showed slowing of background activity and 2 showed PLED’s (periodic lateralized epileptiform discharges). The brain imaging results revealed 4 hemorrhagic strokes and 2 ischemic strokes, one cavernoma, and 3 ancient strokes. The mean age of the seven women and three men was 65.2 years. The mean blood sugar concentration, glycated hemoglobin levels and corrected osmolarity of this group were, respectively, 319 mg/dL (17.54 mmol/L), 10,8% (95mmol/mol (6,5-14,5% (48-135mmol/mol)) and 295.8 mOsmol/L. The average length of stay was 13.9 days. All patients were rehydrated; half of the patients received insulin treatment.
The occurrence of seizures in a diabetic patient with NKH corresponds to 2 distinct situations: in the majority of cases, the seizures reveal an ischemic or hemorrhagic stroke or testify to a pre-existing vascular epilepsy. However, it can also be a transient metabolic phenomenon that is reversible with rehydration and insulin therapy. Brain imaging makes the diagnosis.
Seizures in NKH in the absence of brain damage represent 3.5% of our sample of diabetic patients with seizures. This is a rare situation. This percentage is much lower than those reported in in the older literature before the existence of modern brain imaging estimating NKH to represent 15-40% (1) or 25% (21) of seizures. The 8 patients with a reversible transient metabolic disorder after rehydration and insulin therapy correspond to the data in the literature (2, 4, 14): average age over 60 years, predominance of repeated focal motor seizures or in the form of focal status, rapid control of seizures with normalization of blood glucose (9). The mean values of blood glucose and osmolality were respectively 29.3 and 301 in our series versus 28.7 and 300 (4); 28.3 and 308 (2); 32.6 and 302 (3). Diabetes was in all our cases poorly controlled (HbA1 12.4% (112mmol/mol)). In 2007, Huang et al. established that diabetic patients with glycated hemoglobin values greater than 9% were at greater risk of having a seizure. Four studies investigating this neuroendocrine entity have measured this parameter. These are case reports of single patients, or series of 2 and 3 patients (19, 22). The 7 glycated hemoglobin values reported were 9.4% (79mmol/mol) and 10.5% (91mmol/mol) for the reports; 13.8% (127mmol/mol) and 14.4% (134mmol/mol) for the series of 2 patients; and 14.7% (137mmol/mol), 10.5% (91mmol/mol), and 11.9% (107mmol/mol) for the series of 3 patients.
The discovery of diabetes at the time of the seizures was observed in 2 patients/8 in our series, for 11/22 (4) and 15/21 (3). In our study the mean serum sodium level was 133mmol/L, so it did not participate in the epileptic seizure during the hyperglycemia, knowing that the cut off value responsible for epilepsy in hyponatremia is 115mmo/L (23).
In both cases where MRI was performed it showed Flair hyposignals of white matter related to the region involved in seizures (12) associated with ADC decrease. A recent review (10) collated the abnormalities observed on MRI in 30 NKH patients from 5 publications (19, 24–26): a T2/Flair hyposignal of white matter is observed in 28/30 patients. For Urbach, 2020, T2/Flair hyposignal with ADC decrease in a focal seizure context should raise the possibility of NKH (12). The ADC decrease would indicate the presence of cytotoxic edema. The transient deposition of free radicals and/or iron because of excitotoxic axonal damage during hyperglycemia-induced seizures and intracellular dehydration in glial and supporting tissues are postulated mechanisms for subcortical altered signal intensity (27). Epileptic seizures result in T2 Flair or diffusion hypersignals depending on the time course and irrespective of their cause. In case of Flair hyposignal there is no ADC decrease in an epileptic context without hyperglycemia (28).
For patients with NKH, the main explanation is an increased metabolism of GABA which is the major inhibitory neurotransmitter in the central nervous system. Cellular dehydration caused by increased intra-extraneuronal osmolality and hypoxia depresses the Krebs cycle. To compensate for the consequent deficit in brain glucose, GABA is metabolized to succinic acid, fulfilling 40% of the nervous system’s energy requirements (11). This former explanation does not adequately account for the abnormalities seen on MRI and the small number of NKH patients who have seizures.
In the most common situation (10 patients/18) seizures are associated with an acute vascular brain injury or are related to vascular epilepsy. In the series of 21 patients by Singh and Strobos, 1980, at least 13 patients had a documented brain lesion and the metabolic disorder was considered as the triggering factor of the seizures but not as its sole cause (29).
The existence of diabetes and hyperglycemia are two independent factors for poor prognosis of intracerebral hematomas (30). In addition, the incidence of late-onset post-stroke seizures has been estimated at 8.2% for all types of strokes (31) while the rate at 5 years has been 9.5% for ischemic strokes. In hemorrhagic strokes, the rate is slightly higher (11.8%) Haapaniemi 2014 (32). Hyperglycemia in this context is a factor favoring epileptic seizures. The glycemic control of the previous months is also an important factor to take into account, with an increased risk in patients with diabetes of having epileptic seizures in NKH. It should be noted that we do not find epileptic seizures in ketotic hyperglycemia that must be extremely rare (29).
This retrospective study shows that epileptic seizures in NKH may correspond to a rare and specific neuro-endocrine entity, reversible within 24 hours with rehydration and insulin therapy and not requiring antiepileptic treatment. Brain MRI shows abnormalities suggestive of the diagnosis. However, in the most common situation, epileptic seizures in NKH are related to acute or ancient brain damage observed on the brain MRI and in a context of glycemic imbalance.
The original contributions presented in the study are included in the article/Supplementary Materials. Further inquiries can be directed to the corresponding author.
The studies involving human participants were reviewed and approved by 2215827. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Conceptualization: DB, BD, MN, and NS. Data curation: DB, MN, and NS. Formal analysis: DB, BD, MN, and NS. Investigation: DB, BD, MN, and NS. Resources: DB, BD, MN, and NS. Supervision: MN and NS. Validation: DB, BD, MN, and NS. Visualization: DB, BD, MN, and NS. Manuscript writing: DB, BD, MN, and NS.
This work was realized in close collaboration with the Centre d’Investigation Clinique Antilles Guyane, Inserm CIC 1424.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
NKH, Non ketotic hyperglycemia; MRI, Magnetic resonance imaging; GABA, gamma-aminobutyric acid; ADC, Apparent Diffusion Coefficient; EEG, electroencephalogram CNIL, The National Commission for Information Technology and Civil Liberties; PLED’s, periodic lateralized epileptiform discharges.
1. Maccario M, Messis CP, Vastola EF. Focal seizures as a manifestation of hyperglycemia without ketoacidosis. A report of seven cases with review of the literature. Neurology (1965) 15:195-206. doi: 10.1212/wnl.15.3.195
2. Wang X, Yu H, Cai Z, Wang Z, Ma B, Zhang Y. Nonketotic hyperglycemia-related epileptic seizures. Epilepsy Behav Case Rep (2013) 1:77-8. doi: 10.1016/j.ebcr.2013.03.001
3. Tiamkao S, Pratipanawatr T, Tiamkao S, Nitinavakarn B, Chotmongkol V, Jitpimolmard S. Seizures in nonketotic hyperglycaemia. Seizure (2003) 12(6):409-10. doi: 10.1016/S1059-1311(02)00353-9
4. Lammouchi T, Zoghlami F, Ben Slamia L, Grira M, Harzallah MS, Benammou S. [Epileptic seizures in non-ketotic hyperglycemia]. Neurophysiol Clin (2004) 34(3−4):183-7. doi: 10.1016/j.neucli.2004.04.002
5. Kang KW, Kim SH, Kim JM, Nam TS, Choi KH, Kim MK. Ictal SPECT in diagnosis of non-ketotic hyperglycemia-related seizure manifesting as speech arrest. J Clin Neurol (2019) 15(2):253-5. doi: 10.3988/jcn.2019.15.2.253
6. Oh C, Lee SY, Jang JW. Hyperglycemia-induced aphasia presenting with seizure-like brain perfusion findings on single photon emission computed tomography. Dement Neurocogn Disord (2019) 18(2):69-72. doi: 10.12779/dnd.2019.18.2.69
7. Huang CW, Tsai JJ, Ou HY, Wang ST, Cheng JT, Wu SN, et al. Diabetic hyperglycemia is associated with the severity of epileptic seizures in adults. Epilepsy Res (2008) 79(1):71-7. doi: 10.1016/j.eplepsyres.2007.12.012
8. Jain A, Sankhe S. Hyperglycemia-induced seizures and blindness. Indian J Radiol Imaging (2020) 30(2):245-7. doi: 10.4103/ijri.IJRI_41_20
9. Tiamkao S, Janon C, Sawanyawisuth K, Pratipanawatr T, Jitpimolmard S. Prediction of seizure control in non-ketotic hyperglycemic induced seizures. BMC Neurol (2009) 9:61. doi: 10.1186/1471-2377-9-61
10. Hiremath SB, Gautam AA, George PJ, Thomas A, Thomas R, Benjamin G. Hyperglycemia-induced seizures - understanding the clinico- radiological association. Indian J Radiol Imaging (2019) 29(4):343-9. doi: 10.4103/ijri.IJRI_344_19
11. Martínez-Fernández R, Gelabert A, Pablo MJ, Carmona O, Molins A. Status epilepticus with visual seizures in ketotic hyperglycemia. Epilepsy Behav (2009) 16(4):660-2. doi: 10.1016/j.yebeh.2009.10.005
12. Urbach H, Berger B, Solymosi L, Zimmermann J. « negative T2 shine through » in patients with hyperglycemia and seizures: a frequently overlooked MRI pattern. Neuroradiology (2020) 62(7):895-9. doi: 10.1007/s00234-020-02408-w
13. Guisado R, Arieff AI. Neurologic manifestations of diabetic comas: Correlation with biochemical alterations in the brain. Metabolism (1975) 24(5):665-79. doi: 10.1016/0026-0495(75)90146-8
14. Scherer C. [Seizures and non-ketotic hyperglycemia]. Presse Med (2005) 34(15):1084-6. doi: 10.1016/S0755-4982(05)84123-5
15. Maida CD, Daidone M, Pacinella G, Norrito RL, Pinto A, Tuttolomondo A. Diabetes and ischemic stroke: An old and new relationship an overview of the close interaction between these diseases. Int J Mol Sci (2022) 23(4):2397. doi: 10.3390/ijms23042397
16. Zarean E, Lattanzi S, Looha MA, Napoli MD, Chou SHY, Jafarli A, et al. Glycemic gap predicts in-hospital mortality in diabetic patients with intracerebral hemorrhage. J Stroke Cerebrovasc Dis (2021) 30(5):105669. doi: 10.1016/j.jstrokecerebrovasdis.2021.105669
17. Quirins M, Dussaule C, Denier C, Masnou P. Epilepsy after stroke: Definitions, problems and a practical approach for clinicians. Rev Neurol (Paris) (2019) 175(3):126-32. doi: 10.1016/j.neurol.2018.02.088
18. Sudre C, Duplan H, Bukasakakamba J, Nacher M, Peyre-Costa P, Sabbah N. Diabetes care in French Guiana: The gap between national guidelines and reality. Front Endocrinol (Lausanne) (2021) 12:789391. doi: 10.3389/fendo.2021.789391
19. Seo DW, Na DG, Na DL, Moon SY, Hong SB. Subcortical hypointensity in partial status epilepticus associated with nonketotic hyperglycemia. J Neuroimaging (2003) 13(3):259-63. doi: 10.1111/j.1552-6569.2003.tb00188.x
20. Harden CL, Rosenbaum DH, Daras M. Hyperglycemia presenting with occipital seizures. Epilepsia (1991) 32(2):215-20. doi: 10.1111/j.1528-1157.1991.tb05247.x
21. Singh BM, Gupta DR, Strobos RJ. Nonketotic hyperglycemia and epilepsia partialis continua. Arch Neurol (1973) 29(3):187-90. doi: 10.1001/archneur.1973.00490270069011
22. Hung WL, Hsieh PF, Lee YC, Chang MH. Occipital lobe seizures related to marked elevation of hemoglobin A1C: report of two cases. Seizure (2010) 19(6):359-62. doi: 10.1016/j.seizure.2010.05.006
23. Beghi E, Carpio A, Forsgren L, Hesdorffer DC, Malmgren K, Sander JW, et al. Recommendation for a definition of acute symptomatic seizure. Epilepsia (2010) 51(4):671-5. doi: 10.1111/j.1528-1167.2009.02285.x
24. Raghavendra S, Ashalatha R, Thomas SV, Kesavadas C. Focal neuronal loss, reversible subcortical focal T2 hypointensity in seizures with a nonketotic hyperglycemic hyperosmolar state. Neuroradiology (2007) 49(4):299-305. doi: 10.1007/s00234-006-0189-6
25. Chen CCC, Chai JW, Wu CH, Chen WS, Hung HC, Lee SK. Neuroimaging in seizure patients associated with nonketotic hyperglycemia. Neuroradiol J (2011) 24(2):215-20. doi: 10.1177/197140091102400208
26. Lee EJ, Kim KK, Lee EK, Lee JE. Characteristic MRI findings in hyperglycaemia-induced seizures: Diagnostic value of contrast-enhanced fluid-attenuated inversion recovery imaging. Clin Radiol (2016) 71(12):1240-7. doi: 10.1016/j.crad.2016.05.006
27. Lavin PJM. Hyperglycemic hemianopia: a reversible complication of non-ketotic hyperglycemia. Neurology (2005) 65(4):616-9. doi: 10.1212/01.wnl.0000173064.80826.b8
28. Nicholson P, Abdulla S, Alshafai L, Mandell DM, Krings T. Decreased subcortical T2 FLAIR signal associated with seizures. AJNR Am J Neuroradiol (2020) 41(1):111-4. doi: 10.3174/ajnr.A6328
29. Singh BM, Strobos RJ. Epilepsia partialis continua associated with nonketotic hyperglycemia: clinical and biochemical profile of 21 patients. Ann Neurol (1980) 8(2):155-60. doi: 10.1002/ana.410080205
30. Saxena A, Anderson CS, Wang X, Sato S, Arima H, Chan E, et al. Prognostic significance of hyperglycemia in acute intracerebral hemorrhage: The INTERACT2 study. Stroke (2016) 47(3):682−8. doi: 10.1161/STROKEAHA.115.011627
31. Jungehulsing GJ, Heuschmann PU, Holtkamp M, Schwab S, Kolominsky-Rabas PL. Incidence and predictors of post-stroke epilepsy. Acta Neurol Scand (2013) 127(6):427-30. doi: 10.1111/ane.12070
Keywords: diabetes, non-ketotic hyperglycemia, epileptic seizures, neuroendocrine entity, French Guiana
Citation: Baltyde D, De Toffol B, Nacher M and Sabbah N (2022) Epileptic seizures during Non-Ketotic Hyperglycemia (NKH) in French Guiana: A retrospective study. Front. Endocrinol. 13:946642. doi: 10.3389/fendo.2022.946642
Received: 17 May 2022; Accepted: 01 August 2022;
Published: 18 August 2022.
Edited by:
Liang-Jun Yan, University of North Texas Health Science Center, United StatesReviewed by:
Lulup K. Sahoo, Institute of Medical Sciences and Sum Hospital, IndiaCopyright © 2022 Baltyde, De Toffol, Nacher and Sabbah. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Nadia Sabbah, bmFkaWEuc2FiYmFoQGNoLWNheWVubmUuZnI=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.