- 1University Center for Gastrointestinal and Liver Diseases, St. Clara Hospital and University Hospital, Basel, Switzerland
- 2Division of Endocrinology, Diabetes, and Metabolism, University Hospital Basel, Basel, Switzerland
- 3Department of Clinical Research, University Hospital Basel, University Basel, Basel, Switzerland
- 4Division of Endocrinology, Diabetes, and Metabolism, University Department of Medicine, Kantonsspital Aarau, Aarau, Switzerland
- 5Division of General Internal and Emergency Medicine, University Department of Medicine, Kantonsspital Aarau, Aarau, Switzerland
- pt?> 6Pediatric Endocrinology and Diabetology, University Children’s Hospital Basel, University of Basel, Basel, Switzerland
Objective: Diabetic ketoacidosis (DKA) is a life-threatening complication of both type 1 and type 2 diabetes. We aimed to assess population-based rates, trends and outcomes of patients with DKA.
Design and methods: This is a nationwide cohort study using hospital discharge claims data from 2010 to 2018 in Switzerland. Incidence rates and in-hospital outcomes of DKA were analyzed throughout lifetime for children (0-9 years), adolescents (10-19 years), and adults (20-29, 30-59, and 60-90 years). Analyses were stratified for type of diabetes mellitus and sex.
Results: In total, 5,544 hospitalizations with DKA were identified, of whom 3,847 were seen in patients with type 1 diabetes and 1,697 in type 2 diabetes. Incidence rates of DKA among patients with type 1 diabetes were highest during adolescence with 17.67 (girls) and 13.87 (boys) events per 100,000 person-years (incidence rate difference [IRD]: -3.80 [95% CI, -5.59 to -2.02]) and decreased with age in both sexes thereafter. Incidence rates of DKA in patients with type 2 diabetes were low up to an age of 40 years and rose to 5.26 (females) and 6.82 (males) per 100,000 person-years in adults aged 60-90 years. Diabetic ketoacidosis was associated with relevant health-care burden independent of age, sex, or type of diabetes. The population-based incidence rate of DKA increased over time from 7.22 per 100,000 person-years in 2010 to 9.49 per 100,000 person-years in 2018.
Conclusions: In type 1 diabetes highest incidence rates of DKA hospitalizations were observed among adolescent females. In comparison, in patients with type 2 diabetes the risk for DKA steadily increased with age with higher rates in adult males. Over the 9 year study period, incidence rates of DKA were increasing irrespective of type of diabetes. DKA was associated with a high burden of disease reflected by high rates of intensive care unit admission, prolonged hospital stay and high mortality rates, especially in elderly.
Introduction
Diabetic ketoacidosis (DKA) and hyperglycemic hyperosmolar state are life-threatening complications occurring at states of metabolic decompensation or at primary diagnosis of diabetes mellitus (1–3). While patients with type 1 diabetes are known to be susceptible to DKA particularly under stressful conditions such as infection, trauma, or surgery, ketoacidosis may also occur in patients with poorly controlled or newly diagnosed type 2 diabetes (4, 5). Especially in recent years, the broader use of sodium–glucose cotransporter 2 (SGLT2) inhibitors has raised awareness of DKA in type 2 diabetes, since this modern antidiabetic drug class precipitates euglycemic DKA (6).
There are disparities in occurrence of DKA, in that patients from disadvantaged socioeconomic backgrounds or with poor mental health have a higher incidence rates of DKA (7, 8). Although management of diabetes and its associated complications has significantly improved over the past decades (9, 10), DKA remains a significant health burden with high morbidity (11), mortality (12–14), and relevant utilization of health-care resources (15–17).
While recent data indicate that the population incidence for DKA has been increasing dramatically over the past years (18), data on risks throughout lifetime and granular characteristics of individuals at highest risk of DKA are scarce. Most published data about the epidemiology of DKA were either derived from longitudinal cohort studies that do not reveal population-based estimates or they were focused on either pediatric or adult patient populations, lacking an entire picture.
Hence, in this nationwide cohort study, we first aimed to investigate the risk of DKA in patients with type 1 and type 2 diabetes mellitus throughout lifespan and to assess sex-specific differences, time trends and relevant clinical outcomes.
Material and methods
Study design
This was a nationwide retrospective cohort study in pediatric, adolescent, and adult patients who were hospitalized with DKA in Switzerland between 2010 and 2018.
Hospitalization data were obtained from population-based administrative claims data provided by the Swiss Federal Office for Statistics (Bundesamt für Statistik, Neuchâtel, Switzerland). The database includes all Swiss inpatient discharge records from acute care-, general-, and specialty hospitals in Switzerland for both pediatric and adult patients. Individual-level data on patient demographics, healthcare utilization, hospital typology, medical diagnoses, diagnostic tests, clinical procedures, and in-hospital patient outcomes were provided for all hospitalized patients in Switzerland. The data were unidentifiable due to a multiple-step pseudonymization procedure. Each hospitalization in this database was identified uniquely so that re-hospitalizations could be tracked. Medical diagnoses were coded using the International Classification of Disease version 10, German Modification (ICD-10 GM) codes (http://www.who.int/classifications/icd/en/). Ethics committee Northwest and Central Switzerland (EKNZ) approved this study and granted a waiver of informed consent (Req-2021-01397).
Case ascertainment and patient population
Eligible individuals included pediatric, adolescent, and adult persons up to an age of 90 years hospitalized with DKA and the diagnosis of either type 1 or type 2 diabetes mellitus, respectively. Cases with DKA were identified by applying the ICD-10-GM codes E10.11, E11.11. The diagnosis of diabetes mellitus was identified by the codes E10.xx for type 1 diabetes and E11.xx for type 2 diabetes, respectively.
This study followed the Strengthening The Reporting of OBservational studies in Epidemiology (STROBE) reporting guideline (19). Data on the population size per age and year were obtained from census data from the Swiss Federal Office for Statistics.
Outcomes
The primary outcome was the incidence rate of DKA per 100,000 person-years and accompanying 95% confidence intervals (CIs) overall and stratified by age category, and sex. Secondary outcomes comprised assessment of time trends in DKA incidence and occurrence of clinical endpoints: intensive care unit (ICU) admission rate, intubation rate, length of ICU stay, incidence of cerebral edema, total length of hospital stay (LOS) – defined as days spent in the hospital during the hospitalization, all-cause in-hospital mortality, 30-day, 1-year, and 2-year all-cause hospital readmission rates. These analyses were stratified by age categories, sex, and type of diabetes mellitus.
Statistical analysis
Unless stated otherwise, categorical variables are expressed as number (percentage) and continuous variables as mean (standard deviation, SD). DKA incidence rates are shown per 100,000 person-years and were calculated as the number of individuals with an incident DKA event divided by the sum of “person-time” population at risk in Switzerland, represented by the population size multiplied by the duration of follow-up per age and sex. The denominator for all incidence rates was the standard population per year. Exposure time began on January 1st 2010 and ended on December 31st of the cohort year 2018. Incidence rates were reported in five age categories: children (0-9 years), adolescents (10-19 years), and adults (20-29, 30-59, and 60-90 years), respectively. Differences between incidence rates were compared by calculating the 95% CI for the rate differences among sex and type of diabetes mellitus. Graphical depiction of incidence rates over age was performed using locally estimated scatterplot smoothing (LOESS).
To assess whether incidence rates of DKA changed with time, we used a mixed method linear regression model for three time periods 2010-2012, 2013-2015 and 2016-2018. For analyses on in-hospital burden, estimates of the effect sizes and corresponding 95% confidence intervals (CI) were determined using linear, logistic, or Cox proportional-hazards regressions as appropriate. All tests were 2-sided, p <0.05 was considered significant, and 95% confidence intervals (CIs) were reported for all incidence estimates and differences in these estimates. All statistical analyses were performed using STATA, version 15.1 (StataCorp LLC).
Results
Patient characteristics
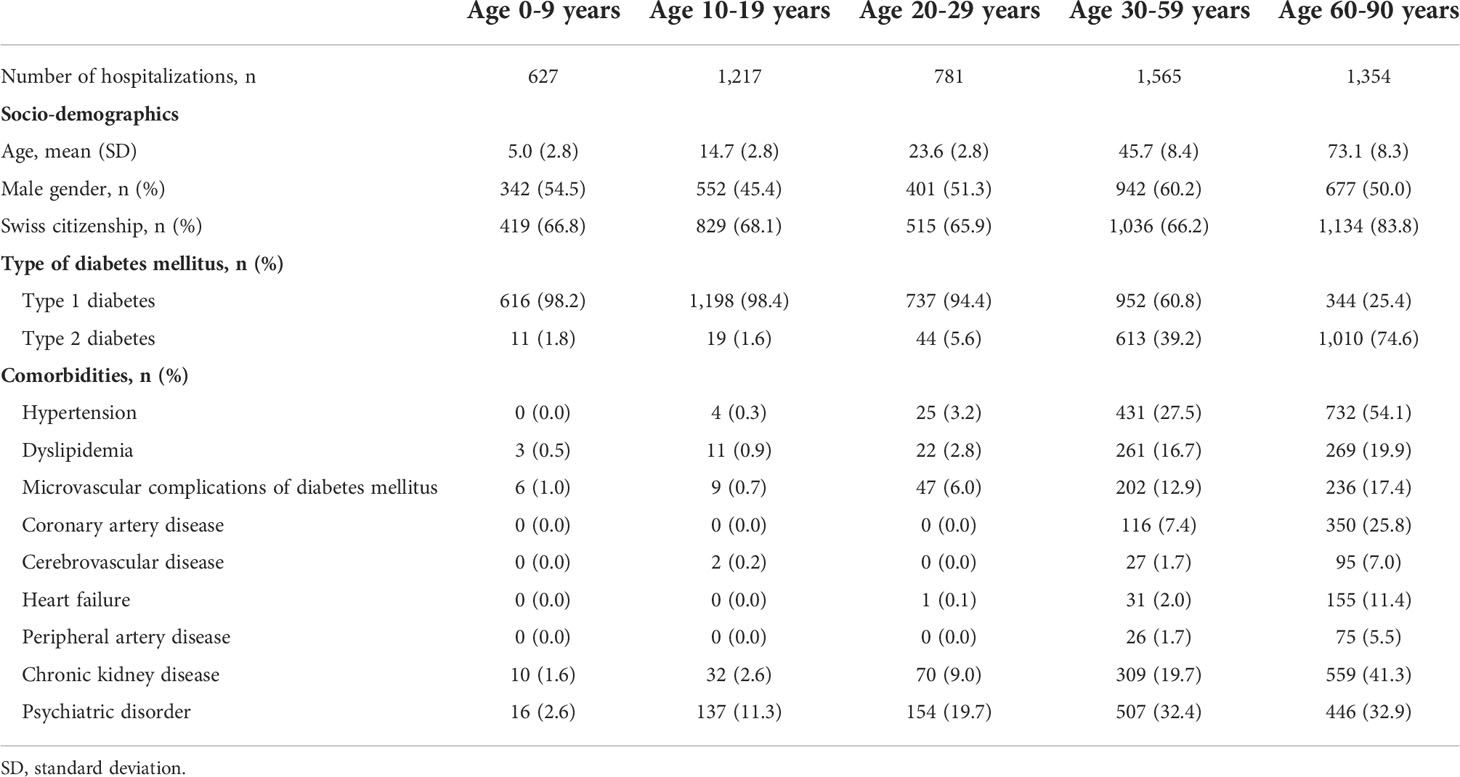
From January 1st 2010 to December 31st 2018, a total of 5,544 hospital admissions for DKA were identified in Switzerland, yielding in an average total incidence rate of 7.46 per 100,000 person-years. Of those, 3,847 DKA events occurred in patients with type 1 diabetes and 1,697 events in patients with type 2 diabetes. The clinical characteristics of the study population are outlined in Table 1. Among children, adolescents, and younger adults up to an age of 29 years, nearly all cases (97.2%) with DKA were seen in patients with metabolically decompensated type 1 diabetes. While in middle-aged adults (30-59 years), 60.8% of hospitalized cases with DKA were observed in patients with type 1 diabetes, only 25.4% of cases were linked to type 1 diabetes in older adults (≥60 years). Microvascular complications of diabetes were rare among children and adolescents (0.8%), but were more prevalent in middle-aged (12.9%) and older adults (17.4%) (definition in Supplementary Table S5). Psychiatric disorders were seen in 11.3% of adolescents who presented with a DKA event and were diagnosed among one-third of adult patients who had an event. While in adolescents anxiety, stress-related and somatoform disorders were most prevalent, in adults the main cause of psychiatric disorder was dementia.
Age distribution of DKA
The total incidence of DKA was highest among patients with type 1 diabetes, for both sexes incidence rates of DKA showed a steep rise from childhood to adolescence with a peak at an age of 16 years in females with a maximum of 20.84 events per 100,000 person-years and at 15 years in males with a maximum of 18.57 events per 100,000 person-years (Figures 1, 2). There was a significant incidence rate difference between female and male adolescents aged 10-19 years of -3.80 events (95% CI; -5.59 to -2.02) per 100,000 person-years (Supplementary Table S2). Thereafter, we observed a continuous decline in rates of DKA for both sexes, reaching a low plateau at incidence rates <5 events per 100,000 person-years from the age of 30 years until advanced age.
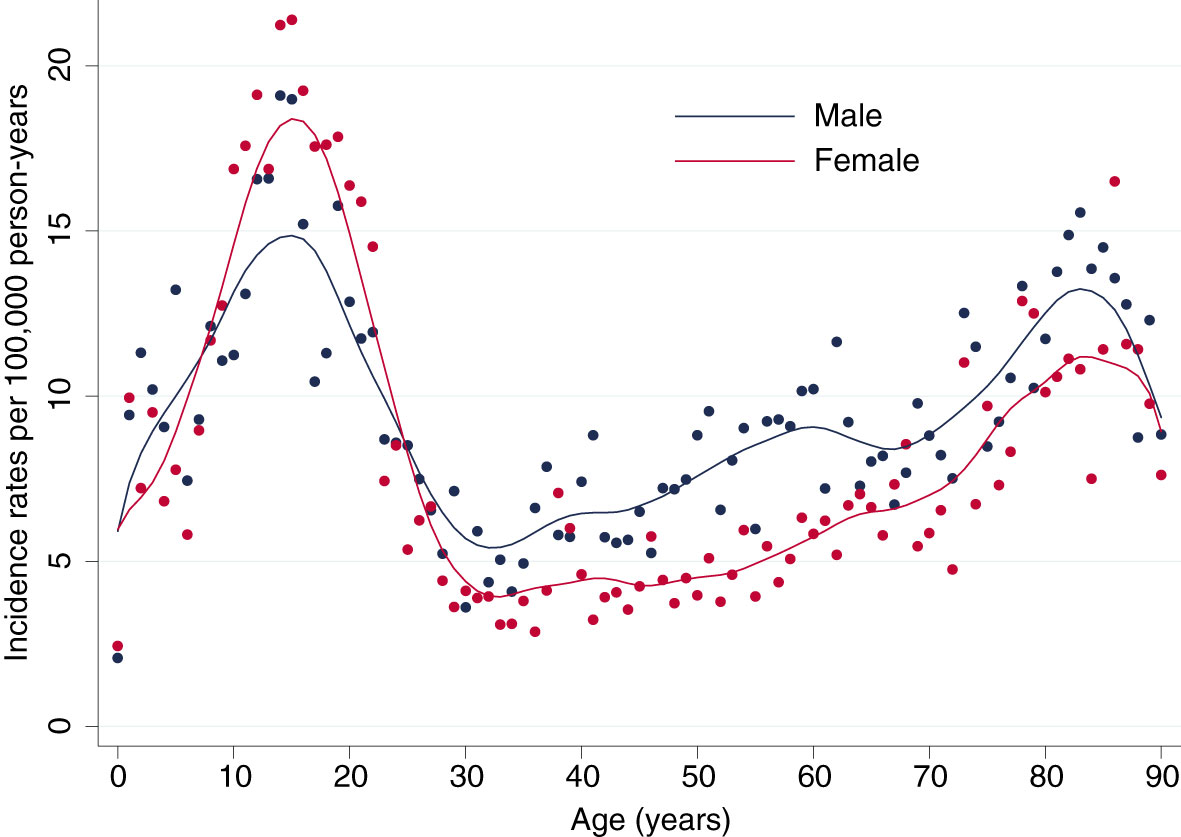
Figure 1 Lifetime Incidence Rates of Diabetic Ketoacidosis by Sex. Incidence rates per 100,000 person-years for male (blue) and female (red) patients.
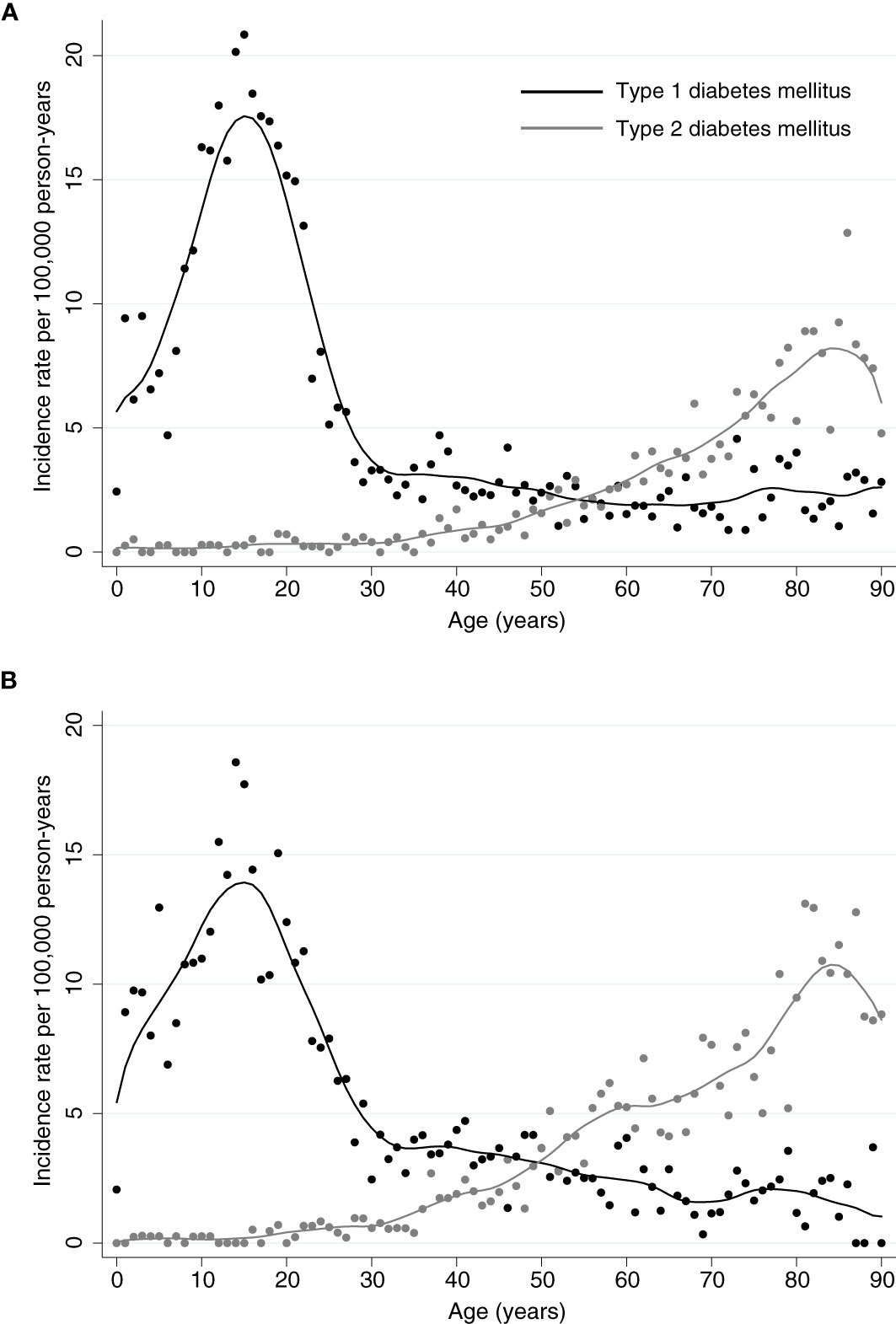
Figure 2 Incidence Rates of Diabetic Ketoacidosis. Incidence rates for diabetic ketoacidosis per 100,000 person-years for (A) female or (B) male patients with type 1 or type 2 diabetes, respectively. For both sexes, highest incidences for patients with type 1 diabetes occur within the adolescence and remain low from the age of 30 years. In patients with type 2 diabetes, incidences for ketoacidosis are lower and slowly increase with age from 30 with a peak around the age of 85 years.
In contrast, among patients with type 2 diabetes incidence rates of DKA were low up to an age of 37 years (<1 event per 100,000 person-years) and from then continuously rose to a maximum of 12.86 events per 100,000 person-years for females at the age of 87 years and 13.11 per 100,000 person-years for males at the age of 82 years (Figures 1, 2).
In-hospital burden
There were no clinically relevant differences in in-hospital outcomes between sexes or type of diabetes. Hospitalization with DKA - irrespective of type of diabetes - was associated with a high burden of disease and utilization of health-care, reflected by a high rate of ICU admission of 38.1% in children and of 55.5% in adolescents up to an age of 19 years. DKA was associated with cerebral edema in 0.4% of cases and with an in-hospital mortality rate of 0.1%. During the study period, among children aged below 9 years only one boy with DKA died. In this case, cerebral edema was observed and could be the underlying cause of death. These patients were also at high risk for hospital readmission within 1 year at a rate of 22.0% (Supplementary Table S3).
In adults aged 20 years or older, DKA was associated with a mean in-hospital mortality rate of 3.9% for both sexes and types of diabetes. Length of hospital stay was long in patients with DKA, with longest hospitalizations among the oldest age group (60-90 years) of 13.1 days (standard difference [SD] 13.4) in patients with type 1 diabetes and 11.0 days (SD 9.2) in type 2 diabetes, respectively (Supplementary Table S3).
Time trends in incidence rates
Incidence rates of DKA increased from 7.22 per 100,000 person-years in 2010 to 9.49 per 100,000 person-years in 2018 (Supplementary Figure S1). Analysis of time trends in incidence of DKA among patients with type 1 diabetes revealed that over the 9-year time frame, there was a significant increase in incidence rates among male patients aged 20-29 years (p<0.001) and in elderly female patients aged 60-90 years (p<0.001). In patients with type 2 diabetes there was an increase in DKA cases among elderly male patients aged 60-90 years (p=0.02), (Figure 3 and Supplementary Table S1).
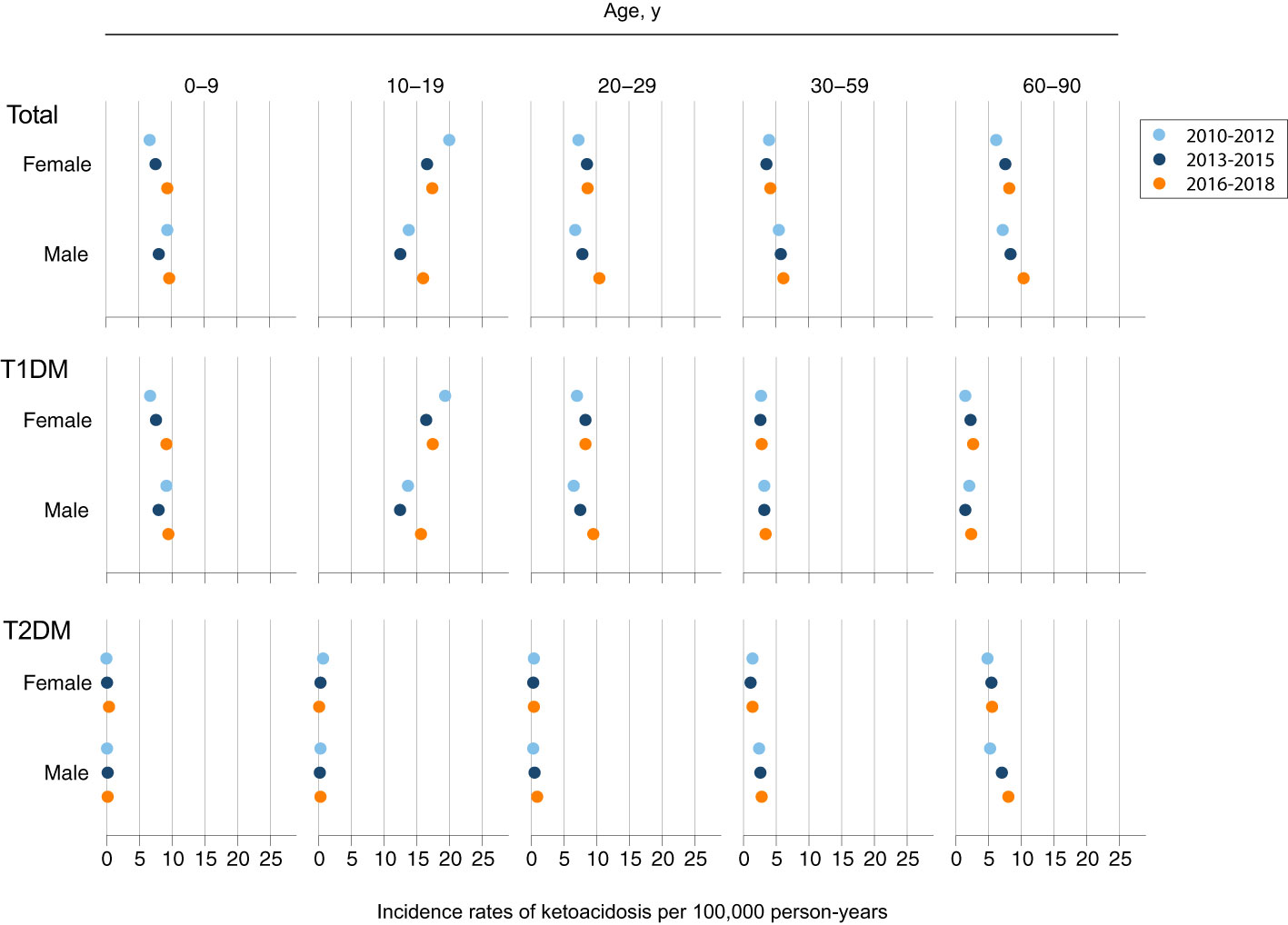
Figure 3 Trends of Ketoacidosis Incidence by Age, Sex, and Type of Diabetes. Shown are incidence rates per 100,000 person-years for an event of diabetic ketoacidosis for three time periods: 2010-2012 (light blue); 2013-2015 (dark blue); 2016-2018 (orange).
Discussion
This population-based cohort study has three key findings: First, while among patients with type 1 diabetes the risk for DKA was highest among adolescents at around 15 years of age, in patients with type 2 diabetes the lifetime risk for DKA steadily increased with age. Second, in adolescence, females were predominantly prone to develop DKA, however in adults there was a switch towards higher rates in males. Third, incidence rates for DKA were increasing over time, especially among the elderly with relevant health-care burden.
So far, few data exist about the lifetime risks for DKA among patients with diabetes. While patients with diabetes are usually managed in an outpatient care setting, DKA is a life-threatening condition and requires emergency admission and most often relies on inpatient acute care. Therefore, incidence rates and in-hospital mortality rates reflect the real-world data with high certainty using nationwide hospital claims data. A previous meta-analysis investigating the incidences of DKA among patients with type 1 diabetes mellitus reported a very wide range in incidence rates of DKA ranging from 0 to 26,300 events per 100,000 person-years, however, almost all included studies were susceptible to potential selection bias or were of limited generalizability (20).
We found that in type 1 diabetes, the risks for the development of DKA were most pronounced during adolescence and in particular among girls. This finding is in line with previous data on HbA1c trajectories among youth with type 1 diabetes. Several longitudinal studies have shown that worsening of glycemic control during puberty is common and that girls were more likely to have significant deterioration of their glycemic control (21–24). Underlying factors for this gender disparity have been discussed extensively. Potential contributors to poor glucose control during puberty being more predominant in females include poorer diabetes acceptance, psychiatric disorders (e.g. depression, personality disorders), eating disorders (e.g. patients omitting inulin to induce weight loss), cognitive problems (e.g. attention deficit), binge alcohol consumption, and hazardous and risk-taking behavior inherent to adolescence (1, 25–27). In addition, increased autonomy in the management of diabetes with refusal of further parental support and the strong need to be accepted outside the family, especially by the peer group, result in less stringent diabetes treatment. Finally, somatic factors such as increased insulin requirements due to changing insulin sensitivity during puberty have an impact on glycemic control and differ between females and males (28–30). In addition, previous DKA episodes are well known risk factors for repeated DKA events in adolescents (1). Transition from pediatric to adult care during this sensitive developmental phase might further increase the risk for DKA by disengagement for clinical care of diabetes, while structured transition programs and young adult diabetes clinics show in some studies the potential to improve metabolic control and psychosocial well-being during the intervention (31–33). In summary, the DKA peak between 10-20 years reflects the multifactorial difficulties in chronic disease acceptance and management during this vulnerable developmental time window and underlines the importance of awareness for age specific multidisciplinary patient care (25).
In comparison, higher rates of DKA among males in adult age may be reflected by higher prevalence of type 2 diabetes with worse metabolic control and higher rates of obesity in men when compared with women (34). DKA no longer can be considered pathognomonic of type 1 diabetes, since a substantial number of DKA episodes especially in adult patients occur with a history of type 2 diabetes. In type 2 diabetes, DKA is known to occur commonly among patients with low social status from urban populations with high rates of obesity (35). Previous studies identified risk factors for DKA such as lack of adherence to therapy, low socioeconomic status, substance abuse, and low education (12). It has been shown that in patients with type 2 diabetes DKA is more severe with worse outcomes and higher mortality (36, 37).
It can only be speculated whether in older patients hospitalized with DKA the high rate of organic mental disorders, i.e. various forms of dementia, may be related to the fact that patients with dementia more often forget their insulin application or if patients with recurrent DKA episodes and possibly worse glycemic control are at higher risk to develop dementia. It is well established that cognitive impairment is a long-term comorbidity of diabetes mellitus (38). In addition, it has been demonstrated that in adults with type 1 diabetes, recurrent DKA events were associated with lower global cognitive function (39). Hence, approaches to prevent, early diagnose and to manage diabetes-associated cognitive impairments become increasingly important with longevity and ageing of populations.
During the 9-year study period, the population-based incidence rate of DKA increased over time. This is an intriguing finding. It may be explained by the epidemic rise in cases with type 2 diabetes or it could as well be associated with the increasing prescription rate of SGLT2 inhibitors that are widely recommended in current guidelines but are known to increase the risk of euglycemic ketoacidosis (40, 41). On the other hand, incidence rates of type 1 diabetes in children have been also increasing over time (42). There is a significant global variation in rates of DKA, being highest in developing countries (24, 43–49), which may be explained by lower disease awareness, and as a consequence delayed diagnosis (50).
Our data confirm the high in-hospital burden of DKA with a high utilization of health-care resources. While length of hospital stay might be influenced by other co-morbidities among adults, rates of ICU admissions among children may even have been underestimated since many Swiss hospitals do not provide pediatric intensive care, but rather intermediate care that was not captured in the hospital claims dataset.
Limitations
Our data must be interpreted in the context of the study design. First and foremost, in Switzerland, having a decentralized health-care system steered by their 26 cantons (federal states), there is a strong need for improved completeness, accessibility, and linkability of health-care data. For instance, there is minimal information on the incidence of type 1 and of type 2 diabetes within the Swiss population, hence incidence rates in our study were calculated in the general population and not in patients with diabetes only. Second, our study is based on administrative hospital claims data that do neither contain information on the duration of diabetes - thus DKA events may have occurred in newly diagnosed as well as in established disease – nor do they contain medication data (e.g. insulin pump usage). Unfortunately, in Switzerland there is no national database on utilization of medications. Third, data on causes of ICU admission, mechanical ventilation, and death were not available. Fourth, data on laboratory parameters such as glycemic control were likewise not available, therefore associations of DKA with poor glycemic control cannot be assessed in our study. Finally, we cannot exclude a certain risk of misclassification and underreporting since administrative data were used in our analyses and we were not able to validate diagnoses, as a consequence it is possible that patients with latent autoimmune diabetes in adults (LADA) may have been misclassified. Nevertheless, a previous validation study verified that the diagnosis of DKA based on hospital claims data had a positive predictive value of around 90% (88.9%, 95% CI; 71.9 to 96.1%) (51).
There are several strengths of note: Our analysis was based on nationwide hospital care data with high external validity, a strong power, and it encompasses a long study period. Furthermore, due to the fact that DKA almost always requires hospitalization, our data allow for highly accurate estimates at the national level. The results highlight the need for further studies on national incidences of DKA especially from countries, where national estimates of diabetes prevalence exist and where linkage to other registries, such as medication usage are available, in order to decipher causes and evolve possible strategies to combat the trend of rising DKA incidences.
In conclusion, DKA is a life-threatening complication of diabetes that can occur at all ages. This study characterizes subpopulations at highest risk for DKA, which showed an overall increase over time with a high burden of disease and significant health-care utilization. Our results highlight the need for intensified efforts to optimize ambulatory care with incorporation of educational, clinical, and social support and to individualize transition from pediatric to adult care clinics. Most likely such an approach has to be both interdisciplinary and interprofessional.
Data availability statement
The data analyzed in this study is subject to the following licenses/restrictions: The data that support the findings of this study are available from the Swiss Federal Office for Statistics. Restrictions apply to the availability of these data, which were used under license for this study. Requests to access these datasets should be directed to Swiss Federal Office for Statistics.
Author contributions
FE, AK, and GS designed the study and wrote the manuscript. FE and AK analyzed the data and were responsible for the decision to submit the manuscript. All authors provided substantial comments on drafts and approved the final report.
Funding
This study was supported by the Kantonsspital Aarau AG. The funding sources had no role in study design, data collection, data analysis, data interpretation, or writing of the report.
Acknowledgments
We thank the Swiss Federal Office for Statistics (Bundesamt für Statistik, Neuchâtel, Switzerland) for the acquisition and provision of data.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2022.940990/full#supplementary-material
References
1. Wolfsdorf JI, Glaser N, Agus M, Fritsch M, Hanas R, Rewers A, et al. ISPAD clinical practice consensus guidelines 2018: Diabetic ketoacidosis and the hyperglycemic hyperosmolar state. Pediatr Diabetes (2018) 19:155–77. doi: 10.1111/pedi.12701
2. Dhatariya KK, Glaser NS, Codner E, Umpierrez GE. Diabetic ketoacidosis. Nat Rev Dis Prim (2020) 6(1):40. doi: 10.1038/s41572-020-0165-1
3. Holman N, Wild SH, Gregg EW, Valabhji J, Sattar N, Khunti K, et al. Comparison of mortality in people with type 1 and type 2 diabetes by age of diagnosis: An incident population-based study in England and Wales. Lancet Diabetes Endocrinol (2021) 10:95–97. doi: 10.1016/S2213-8587(21)00293-X
4. Tittel SR, Sondern KM, Weyer M, Poeplau T, Sauer BM, Schebek M, et al. Multicentre analysis of hyperglycaemic hyperosmolar state and diabetic ketoacidosis in type 1 and type 2 diabetes. Acta Diabetol (2020) 57(10):1245–53. doi: 10.1007/s00592-020-01538-0
5. Kitabchi AE, Umpierrez GE, Miles JM, Fisher JN. Hyperglycemic crises in adult patients with diabetes. Diabetes Care (2009) 32(7):1335–43. doi: 10.2337/dc09-9032
6. Modi A, Agrawal A, Morgan F. Euglycemic diabetic ketoacidosis: A review. Curr Diabetes Rev (2016) 13(3):315–21. doi: 10.2174/1573399812666160421121307
7. Allcock B, Stewart R, Jackson M. Psychosocial factors associated with repeat diabetic ketoacidosis in people living with type 1 diabetes: A systematic review. Diabetes Med (2021) 39:e14663. doi: 10.1111/dme.14663
8. McCoy RG, Galindo RJ, Swarna KS, Van Houten HK, O’Connor PJ, Umpierrez GE, et al. Sociodemographic, clinical, and treatment-related factors associated with hyperglycemic crises among adults with type 1 or type 2 diabetes in the US from 2014 to 2020. JAMA Netw Open (2021) 4(9):e2123471. doi: 10.1001/jamanetworkopen.2021.23471
9. Holt RIG, DeVries JH, Hess-Fischl A, Hirsch IB, Kirkman MS, Klupa T, et al. The management of type 1 diabetes in adults. A consensus report by the American diabetes association (ADA) and the European association for the study of diabetes (EASD). Diabetologia (2021) 64:2609–2652. doi: 10.1007/s00125-021-05568-3
10. American Diabetes Association. Comprehensive medical evaluation and assessment of comorbidities: Standards of medical care in diabetes-2020. Diabetes Care (2020) 43:S37–47. doi: 10.2337/dc20-S004
11. Chang LH, Lin LY, Tsai MT, How CK, Chiang JH, Hsieh VCR, et al. Association between hyperglycaemic crisis and long-term major adverse cardiovascular events: A nationwide population-based, propensity score-matched, cohort study. BMJ Open (2016) 6(8):e012233. doi: 10.1136/bmjopen-2016-012233
12. Mays JA, Jackson KL, Derby TA, Behrens JJ, Goel S, Molitch ME, et al. An evaluation of recurrent diabetic ketoacidosis, fragmentation of care, and mortality across Chicago, Illinois. Diabetes Care (2016) 39(10):1671–6. doi: 10.2337/dc16-0668
13. Kao Y, Hsu CC, Weng SF, Lin HJ, Wang JJ, Bin S, et al. Subsequent mortality after hyperglycemic crisis episode in the non-elderly: A national population-based cohort study. Endocrine (2016) 51(1):72–82. doi: 10.1007/s12020-015-0669-8
14. Wang J, Williams DE, Narayan KMV, Geiss LS. Declining death rates from hyperglycemic crisis among adults with diabetes, U.S., 1985-2002. Diabetes Care (2006) 29(9):2018–22. doi: 10.2337/dc06-0311
15. Lyerla R, Johnson-Rabbett B, Shakally A, Magar R, Alameddine H, Fish L. Recurrent DKA results in high societal costs – a retrospective study identifying social predictors of recurrence for potential future intervention. Clin Diabetes Endocrinol (2021) 7(1):13. doi: 10.1186/s40842-021-00127-6
16. Desai D, Mehta D, Mathias P, Menon G, Schubart UK. Health care utilization and burden of diabetic ketoacidosis in the U.S. over the past decade: A nationwide analysis. Diabetes Care (2018) 41(8):1631–8. doi: 10.2337/dc17-1379
17. Dhatariya KK, Skedgel C, Fordham R. The cost of treating diabetic ketoacidosis in the UK: A national survey of hospital resource use. Diabetes Med (2017) 34(10):1361–6. doi: 10.1111/dme.13427
18. Benoit SR, Hora I, Pasquel FJ, Gregg EW, Albright AL, Imperatore G. Trends in emergency department visits and inpatient admissions for hyperglycemic crises in adults with diabetes in the U.S., 2006–2015. Diabetes Care (2020) 43(5):1057–64. doi: 10.2337/dc19-2449
19. von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: Guidelines for reporting observational studies. Int J Surg (2014) 12(12):1495–9. doi: 10.1016/j.ijsu.2014.07.013
20. Fazeli Farsani S, Brodovicz K, Soleymanlou N. Correction: Incidence and prevalence of diabetic ketoacidosis (DKA) among adults with type 1 diabetes mellitus (T1D): A systematic literature review. BMJ Open (2017) 7(8):e016587. doi: 10.1136/bmjopen-2017-016587corr1
21. Moore JM, Snell-Bergeon JK. Trajectories of hemoglobin A1c and body mass index z-score over four decades among 2 to 18 year olds with type 1 diabetes. Pediatr Diabetes (2019) 20(5):594–603. doi: 10.1111/pedi.12862
22. Schwandt A, Hermann JM, Rosenbauer J, Boettcher C, Dunstheimer D, Grulich-Henn J, et al. Longitudinal trajectories of metabolic control from childhood to young adulthood in type 1 diabetes from a Large German/Austrian registry: A group-based modeling approach. Diabetes Care (2017) 40(3):309–16. doi: 10.2337/dc16-1625
23. Clements MA, Schwandt A, Donaghue KC, Miller K, Lück U, Couper JJ, et al. Five heterogeneous HbA1c trajectories from childhood to adulthood in youth with type 1 diabetes from three different continents: A group-based modeling approach. Pediatr Diabetes. (2019) 20(7):920–31. doi: 10.1111/pedi.12907
24. Al-Adsani A, Famuyiwa O. Hospitalization of diabetics 12-30 years of age in Kuwait: Patients’ characteristics, and frequency and reasons for admission. Acta Diabetol (2000) 37(4):213–7. doi: 10.1007/s005920070008
25. Cameron FJ, Garvey K, Hood KK, Acerini CL, Codner E. ISPAD clinical practice consensus guidelines 2018: Diabetes in adolescence. Pediatr Diabetes. (2018) 19:250–61. doi: 10.1111/pedi.12702
26. Rewers A, Peter Chase H, Mackenzie T, Walravens P, Roback M, Rewers M, et al. Predictors of acute complications in children with type 1 diabetes. J Am Med Assoc (2002) 287(19):2511–8. doi: 10.1001/jama.287.19.2511
27. Pasquini S, Rinaldi E, Da Prato G, Csermely A, Indelicato L, Zaffani S, et al. Growing up with type 1 diabetes mellitus: Data from the Verona diabetes transition project. Diabetes Med (2021) 39:e14719. doi: 10.1111/dme.14719
28. Chiang JL, Maahs DM, Garvey KC, Hood KK, Laffel LM, Weinzimer SA, et al. Type 1 diabetes in children and adolescents: A position statement by the American diabetes association. Diabetes Care (2018) 41(9):2026–44. doi: 10.2337/dci18-0023
29. Chiang JL, Kirkman MS, Laffel LMB, Peters AL. Type 1 diabetes through the life span: A position statement of the American diabetes association. Diabetes Care (2014) 37(7):2034–54. doi: 10.2337/dc14-1140
30. Hoffman RP, Vicini P, Sivitz WI, Cobelli C. Pubertal adolescent male-female differences in insulin sensitivity and glucose effectiveness determined by the one compartment minimal model. Pediatr Res (2000) 48(3):384–8. doi: 10.1203/00006450-200009000-00022
31. Sequeira PA, Pyatak EA, Weigensberg MJ, Vigen CP, Wood JR, Ruelas V, et al. Let’s empower and prepare (LEAP): Evaluation of a structured transition program for young adults with type 1 diabetes. Diabetes Care (2015) 38(8):1412–9. doi: 10.2337/dc14-2577
32. White M, O’Connell MA, Cameron FJ. Clinic attendance and disengagement of young adults with type 1 diabetes after transition of care from paediatric to adult services (TrACeD): A randomised, open-label, controlled trial. Lancet Child Adolesc Heal (2017) 1(4):274–83. doi: 10.1016/S2352-4642(17)30089-5
33. Spaic T, Robinson T, Goldbloom E, Gallego P, Hramiak I, Lawson ML, et al. Closing the gap: Results of the multicenter Canadian randomized controlled trial of structured transition in young adults with type 1 diabetes. Diabetes Care (2019) 42(6):1018–26. doi: 10.2337/dc18-2187
34. Chatterjee S, Khunti K, Davies MJ. Type 2 diabetes. Lancet. (2017) 389(10085):2239–51. doi: 10.1016/S0140-6736(17)30058-2
35. Balasubramanyam A, Zern JW, Hyman DJ, Pavlik V. New profiles of diabetic ketoacidosis: Type 1 vs type 2 diabetes and the effect of ethnicity. Arch Intern Med (1999) 159(19):2317–22. doi: 10.1001/archinte.159.19.2317
36. Barski L, Nevzorov R, Harman-Boehm I, Jotkowitz A, Rabaev E, Zektser M, et al. Comparison of diabetic ketoacidosis in patients with type-1 and type-2 diabetes mellitus. Am J Med Sci (2013) 345(4):326–30. doi: 10.1097/MAJ.0b013e31827424ab
37. Schwarzfuchs D, Rabaev E, Sagy I, Zimhony-Nissim N, Lipnitzki I, Musa H, et al. Clinical and epidemiological characteristics of diabetic ketoacidosis in older adults. J Am Geriatr Soc (2020) 68(6):1256–61. doi: 10.1111/jgs.16376
38. Biessels GJ, Despa F. Cognitive decline and dementia in diabetes mellitus: mechanisms and clinical implications. Nat Rev Endocrinol (2018) 14(10):591–604. doi: 10.1038/s41574-018-0048-7
39. Lacy ME, Gilsanz P, Eng CW, Beeri MS, Karter AJ, Whitmer RA. Recurrent diabetic ketoacidosis and cognitive function among older adults with type 1 diabetes: findings from the study of longevity in diabetes. BMJ Open Diabetes Res Care (2020) 8(1):e001173. doi: 10.1136/bmjdrc-2020-001173
40. Musso G, Saba F, Cassader M, Gambino R. Diabetic ketoacidosis with SGLT2 inhibitors. BMJ (2020) 371:m4147. doi: 10.1136/bmj.m4147
41. Marx N, Davies MJ, Grant PJ, Mathieu C, Petrie JR, Cosentino F, et al. Guideline recommendations and the positioning of newer drugs in type 2 diabetes care. Lancet Diabetes Endocrinol (2021) 9(1):46–52. doi: 10.1016/S2213-8587(20)30343-0
42. Wojcik M, Sudacka M, Wasyl B, Ciechanowska M, Nazim J, Stelmach M, et al. Incidence of type 1 diabetes mellitus during 26 years of observation and prevalence of diabetic ketoacidosis in the later years. Eur J Pediatr (2015) 174(10):1319–24. doi: 10.1007/s00431-015-2537-1
43. Große J, Hornstein H, Manuwald U, Kugler J, Glauche I, Rothe U. Incidence of diabetic ketoacidosis of new-onset type 1 diabetes in children and adolescents in different countries correlates with human development index (HDI): An updated systematic review, meta-analysis, and meta-regression. Horm Metab Res (2018) 50(3):209–22. doi: 10.1055/s-0044-102090
44. Aminzadeh M, Navidi N, Valavi E, Aletayeb SMH. Childhood onset type 1 diabetes at a tertiary hospital in south-western Iran during 2000–2015: Rapid increase in admissions and high prevalence of DKA at diagnosis. Prim Care Diabetes (2019) 13(1):43–8. doi: 10.1016/j.pcd.2018.07.013
45. Usher-Smith JA, Thompson M, Ercole A, Walter FM. Variation between countries in the frequency of diabetic ketoacidosis at first presentation of type 1 diabetes in children: A systematic review. Diabetologia (2012) 55(11):2878–94. doi: 10.1007/s00125-012-2690-2
46. Zayed H. Epidemiology of diabetic ketoacidosis in Arab patients with type 1 diabetes: A systematic review. Int J Clin Pract (2016) 70(3):186–95. doi: 10.1111/ijcp.12777
47. Yamamoto T. Diabetic ketoacidosis in a community of suburban osaka. analysis of 39 episodes of ketoacidosis and related conditions. Endocrinol Jpn (1983) 30(1):43–54. doi: 10.1507/endocrj1954.30.43
48. Al Shaikh A, Farahat F, Saeedi M, Bakar A, Al Gahtani A, Al-Zahrani N, et al. Incidence of diabetic ketoacidosis in newly diagnosed type 1 diabetes children in western Saudi Arabia: 11-year experience. J Pediatr Endocrinol Metab (2019) 32(8):857–62. doi: 10.1515/jpem-2018-0548
49. Praveen PA, Hockett CW, Ong TC, Amutha A, Isom SP, Jensen ET, et al. Diabetic ketoacidosis at diagnosis among youth with type 1 and type 2 diabetes: Results from SEARCH (United states) and YDR (India) registries. Pediatr Diabetes (2021) 22(1):40–6. doi: 10.1111/pedi.12979
50. Cherubini V, Grimsmann JM, Åkesson K, Birkebæk NH, Cinek O, Dovč K, et al. Temporal trends in diabetic ketoacidosis at diagnosis of paediatric type 1 diabetes between 2006 and 2016: results from 13 countries in three continents. Diabetologia (2020) 63(8):1530–41. doi: 10.1007/s00125-020-05152-1
51. Bobo WV, Cooper WO, Epstein RA, Arbogast PG, Mounsey J, Ray WA. Positive predictive value of automated database records for diabetic ketoacidosis (DKA) in children and youth exposed to antipsychotic drugs or control medications: A tennessee medicaid study. BMC Med Res Methodol (2011) 11:157. doi: 10.1186/1471-2288-11-157
Keywords: hyperglycemic crisis, ketoacidosis (DKA), type 1 diabetes mellitus (T1D), type 2 diabetes mellitus, coma (diabetic)
Citation: Ebrahimi F, Kutz A, Christ ER and Szinnai G (2022) Lifetime risk and health-care burden of diabetic ketoacidosis: A population-based study. Front. Endocrinol. 13:940990. doi: 10.3389/fendo.2022.940990
Received: 10 May 2022; Accepted: 03 August 2022;
Published: 24 August 2022.
Edited by:
Sathish Thirunavukkarasu, Emory University, United StatesReviewed by:
Marion Elizabeth Walsh, Wake Forest University, United StatesMarc Rendell, The Rose Salter Medical Research Foundation, United States
Copyright © 2022 Ebrahimi, Kutz, Christ and Szinnai. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Alexander Kutz, a3V0ei5hbGV4YW5kZXJAZ21haWwuY29t; YWxleGFuZGVyLmt1dHpAa3NhLmNo
†These authors have contributed equally to this work
‡ORCID: Fahim Ebrahimi, orcid.org/0000-0001-5862-966X
Alexander Kutz, orcid.org/0000-0002-7549-5648
Emanuel Remigius Christ, orcid.org/0000-0002-5604-4606
Gabor Szinnai, orcid.org/0000-0003-0559-2597
 Fahim Ebrahimi
Fahim Ebrahimi Alexander Kutz
Alexander Kutz Emanuel Remigius Christ
Emanuel Remigius Christ Gabor Szinnai3,6‡
Gabor Szinnai3,6‡