
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Endocrinol. , 27 September 2022
Sec. Cardiovascular Endocrinology
Volume 13 - 2022 | https://doi.org/10.3389/fendo.2022.940633
This article is part of the Research Topic Endocrine-related Cardiovascular Diseases: Recent Advances in Diagnosis and Treatment View all 6 articles
 Yijia Liu1†
Yijia Liu1† Zhu Li1†
Zhu Li1† Tong Yang1†
Tong Yang1† Lin Li1
Lin Li1 Lu Yu1
Lu Yu1 Fanfan Liu1
Fanfan Liu1 Tongyao Ni1
Tongyao Ni1 Shan Gao1
Shan Gao1 Chunjie Li2*
Chunjie Li2* Rongrong Yang1*
Rongrong Yang1* Chunquan Yu1*
Chunquan Yu1*Context: Previous studies on the association between thyroid function and carotid plaque have shown contradictory results, which may be attributable to the sensitivity to thyroid hormone indices. This study aimed to analyze the association between thyroid hormone sensitivity and risk of carotid plaque in patients with coronary heart disease (CHD) and further explore this association according to sex, age, smoking, and drinking status.
Methods: This large-scale, multi-center, retrospective, cross-sectional study included 6679 patients with CHD (age 35–75). Central sensitivity to thyroid hormone was evaluated by the thyroid feedback quantile-based index (TFQI), parametric thyroid feedback quantile-based index (PTFQI), thyroid-stimulating hormone index (TSHI), and thyrotroph thyroxine resistance index (TT4RI). Peripheral sensitivity to thyroid hormone was assessed by free triiodothyronine/free thyroxine (FT3/FT4) ratio. Taking no carotid plaque as a reference, this study used logistic regression to analyze the association between central and peripheral thyroid hormone sensitivity and carotid plaque in patients with CHD.
Results: Of the 6679 patients with CHD, 4843 (72.50%) had carotid plaque. In the multi-adjusted models, the TFQI (odds ratio [OR]: 1.50; 95% confidence interval [CI]: 1.26–1.78; P < 0.001), PTFQI (OR: 1.76; 95% CI: 1.46–2.12; P < 0.001), TSHI (OR: 1.21; 95% CI: 1.10–1.33; P < 0.001), and TT4RI (OR: 1.00; 95% CI: 1.00–1.01; P = 0.003) were positively associated with the risk of carotid plaque. Compared with that in females and people > 60 years, the OR value for carotid plaque was higher in males and people ≤ 60 years. Similarly, smokers and drinkers had higher OR values for carotid plaque than non-smokers and non-drinkers. Conversely, FT3/FT4 ratio (OR: 0.75; 95% CI: 0.70–0.81; P < 0.001) was negatively associated with carotid plaque, and the OR value for carotid plaque was lower in males, patients ≤ 60 years, smokers, and drinkers.
Conclusion: This study showed that thyroid hormone sensitivity is significantly associated with carotid plaque in patients with CHD. This association is more significant in males, patients ≤ 60 years, smokers, and drinkers.
Cardiovascular disease is one of the most common causes of death worldwide (1), seriously affecting the patient’s quality of life and longevity (2). The risk of cardiovascular and cerebrovascular events is generally increased in patients with coronary heart disease (CHD), and most cardiac deaths are caused by CHD secondary to coronary atherosclerosis (3, 4). Carotid plaque burden has been proven to be a good marker for cardiovascular or cerebrovascular disease events (5, 6), and it is also clinically relevant in the CHD population compared to in the healthy population.
CHD is closely related to thyroid hormones, and patients with CHD are often found to have abnormal levels of thyroid hormones (7, 8). Abnormal thyroid hormone levels are associated with increased systemic vascular resistance, decreased cardiac contractility, reduced cardiac output, and accelerated atherosclerosis and CHD owing to hypercholesterolemia and diastolic hypertension (9). A prospective cohort study reported that the greater the changes in thyroid hormone levels in euthyroid, middle-aged, or older participants, the higher the risk of carotid atherosclerosis (10). Sakamaki et al. (11) believed that thyroid stimulating hormone (TSH) was independently associated with carotid plaque, especially when the TSH level was ≥2.5 μIU/mL. However, another cross-sectional study (12) found that TSH and free thyroxine (FT4) were not significantly associated with carotid plaque. These conflicting results seem to be common. In addition, almost all previous analyses focused solely on TSH, free triiodothyronine (FT3), and FT4 levels to assess the risk of carotid plaque. At the same time, indices of thyroid hormone sensitivity can also be used to evaluate the complex interactions between FT3, FT4, and TSH, which can provide a new reference marker for thyroid function. However, no studies have investigated the association between thyroid hormone sensitivity and carotid plaque in patients with CHD. Although previous studies have investigated the prevalence of carotid plaque according to sex, age, and smoking and drinking status (13, 14), the results were inconsistent. Owing to these inconsistent results, the association between carotid plaque and sex, age, and smoking and drinking status remains controversial.
Therefore, this study aimed to investigate the association between central and peripheral thyroid hormone sensitivity and carotid plaque in patients with CHD, to further explore the association with sex, age, and smoking and drinking status, and to provide a basis for management of patients with clinical CHD.
This large-scale, multi-center, retrospective, cross-sectional study included 107301 CHD patients hospitalized in six Tianjin hospitals between January 1, 2014, and September 30, 2020 (15). Inclusion criteria were: i) patients with CHD meeting the diagnosis of the International Classification of Diseases 10th revision (ICD-10) codes (I20, I24-I25, and I49-I50), and ii) hospital admission during the defined period. Patients were excluded from the analyses if they: i) lacked TSH, FT3, FT4, and carotid ultrasound measurements, ii) were younger than 35 years or older than 75 years, or iii) had oncological, infectious, or severe liver or renal disease. Therefore, a total of 6679 individuals were included in the current analyses. A flowchart of the patient recruitment process is shown in Figure 1.
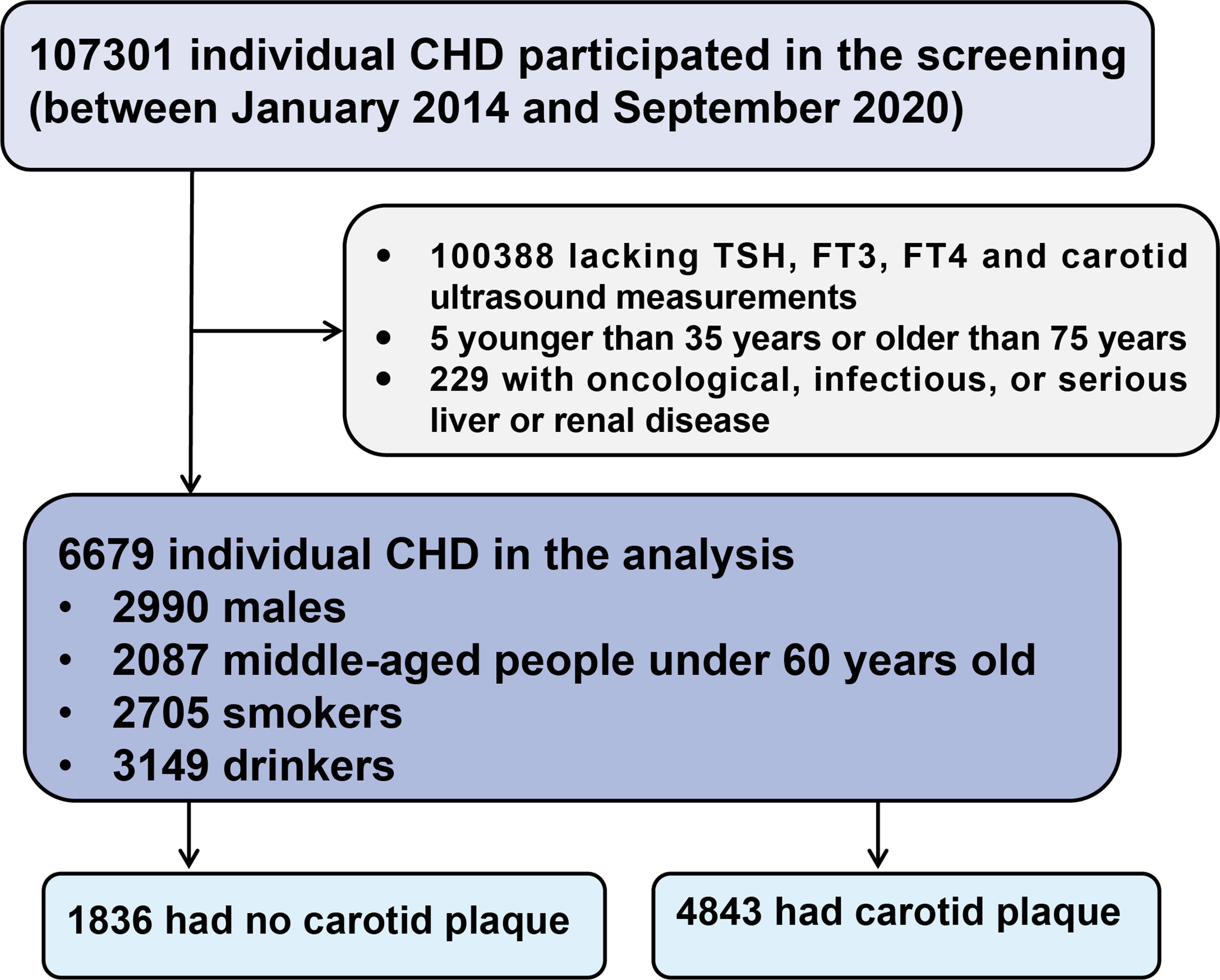
Figure 1 Flow chart of the study population. CHD, coronary heart disease; TSH, thyroid stimulating hormone; FT3, free triiodothyronine; FT4, free thyroxine.
This study was approved by the Ethics Committee of Tianjin University of Traditional Chinese Medicine (TJUTCM-EC20190008) and was registered in the Chinese Clinical Trial Registry (ChiCTR-1900024535) and Clinical Trials.gov (NCT04026724).
Trained medical personnel collected data of age, sex, and smoking and drinking status by means of a standard structured questionnaire (16). The question regarding smoking/drinking habits had two response options: 1) “no;” 2) “yes.” “Never smoked/non-smoker” and “no/mild drinking” were defined as “no,” “current smoker/former smoker” and “heavy drinking” were defined as “yes” (17, 18). The systolic and diastolic blood pressures (SBP and DBP) were measured by trained physicians using an electronic device. Hypertension was defined as SBP ≥ 130 mmHg or DBP ≥ 80 mmHg (19). Rheumatological diseases included rheumatoid arthritis (ICD-10 codes M05-06), psoriatic arthritis (ICD-10 codes L40), ankylosing spondylitis (ICD-10 codes M45), vasculitis (ICD-10 codes I77), systemic lupus erythematosus (ICD-10 codes M32), sjögren (ICD-10 codes M35), poly/dermatomyositis (ICD-10 codes M33), systemic sclerosis (ICD-10 codes M34) (20, 21). Thyroid diseases (ICD-10 codes E00-E07) included hyperthyroidism, hypothyroidism, thyroiditis, thyroid cyst, thyrophyma, or thyroid nodule (22). Autoimmune thyroid diseases (AITD) included Graves’ disease and Hashimoto thyroiditis (23, 24).
Fasting venous blood samples were obtained from all participants on the second day of hospitalization. Glycated hemoglobin (HbA1c), triglyceride (TG), total cholesterol (TC), high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C), and C-reactionprotein (CRP) levels were measured using an automatic hematology analyzer. Standard laboratory procedure for quality control were strictly followed (16). Type 2 diabetes was defined as an elevated HbA1c level ≥ 6.5% (25). Dyslipidemia was defined as TG level of ≥ 2.3 mmol/L, TC level of ≥ 6.2 mmol/L, HDL level of ≤ 1.0 mmol/L, or LDL level of ≥ 4.1 mmol/L (26).
The concentrations of TSH, FT3, and FT4 were measured with the automated immunochemiluminescent assay kits. The reference ranges of TSH, FT4, and FT3 were 0.35–4.94 mIU/L, 9.01–19.05 pmol/L, and 2.63–5.70 pmol/L, respectively. Indices reflecting central thyroid hormone sensitivity, including thyroid feedback quantile-based index (TFQI), parametric thyroid feedback quantile-based index (PTFQI), thyroid-stimulating hormone index (TSHI), and thyrotroph thyroxine resistance index (TT4RI), were calculated according to previous studies as follows:
(27)
(28)
(29)
For TSHI, TT4RI, TFQI and PTFQI, the higher the values, the lower the central sensitivity to thyroid hormones. FT3/FT4 ratio was calculated to evaluate peripheral thyroid hormone sensitivity. Higher FT3/FT4 indicates higher peripheral thyroid hormone sensitivity.
Using an ultrasound diagnostic system, trained and certified physicians performed carotid artery ultrasounds. Images were obtained at the common carotid artery, internal carotid artery, and carotid bifurcation in the supine position. The carotid arteries were carefully scanned in multiple directions using b-mode imaging. The carotid intima-media thickness (CIMT) was defined as the average IMT value of the right and left common carotid arteries (30). Carotid artery color-Doppler was analyzed by professional physicians according to Doppler ultrasound results, and the number and echo properties of carotid plaques were recorded. The number of carotid plaques was classified as single (n = 1) or multiple (n ≥ 2). The echo properties of carotid plaques included hypoechoic, isoechoic, hyperechoic, and mixed. Strict quality control procedures were implemented for image acquisition and analysis, and inter-laboratory quality evaluations were performed by certified personnel.
The Chi-squared (χ2) and Mann–Whitney U test were used to compare the characteristics of the participants in the different groups. Using logistic regression, the odds ratios (ORs) and 95% confidence intervals (CIs) of carotid plaque were estimated for the thyroid hormone indices. Age, sex, TC, TG, HDL-C, LDL-C, SBP, DBP, HbA1c, smoking, and drinking were potential confounders. Additionally, we performed the following sensitivity analysis: restricting analyses to participants with non-thyroid disease; excluding participants with AITD or rheumatological disease. Stratification was performed by sex, age categories (cutoff age: 60 years), and smoking and drinking status. Missing values for SBP (n = 17), DBP (n = 18), hypertension (n = 18), TC (n = 265), TG (n = 265), HDL-C (n = 264), LDL-C (n = 264), dyslipidemia (n = 264), HbA1c (n = 530), type 2 diabetes (n = 530), smoking status (n = 24), drinking status (n = 50), CIMT (n = 2114), carotid plaque echogenicity (n = 80), and CRP (n = 3161) were imputed using multiple imputation methods. All statistical analyses were performed using SPSS 24.0 (IBM Corp, New York, NY, USA).
The baseline clinical data of participants are presented in Table 1. A total of 6679 participants were included for data analysis, including 2990 males (44.80%) with an average age of 64.00 ± 8.00 years. Among them, 4843 patients had carotid plaque (72.51%). Compared with patients without carotid plaque, patients with carotid plaque were more likely to be older females, smokers, and drinkers and were more likely to develop hypertension, diabetes, and hyperlipidemia. In addition, participants with carotid plaque tended to have higher levels of TFQI, PTFQI, TSHI, and TT4RI, while FT3/FT4 was lower.
Three logistic regression models were constructed to assess the effect of thyroid hormone sensitivity on carotid plaque (Table 2). In the multi-adjusted models, it was found that TFQI (OR: 1.50; 95% CI: 1.26–1.78; P < 0.001), PTFQI (OR: 1.76; 95% CI: 1.46–2.12; P < 0.001), TSHI (OR: 1.21; 95% CI: 1.10–1.33; P < 0.001), and TT4RI (OR: 1.00; 95% CI: 1.00–1.01; P = 0.003) were positively associated with the risk of carotid plaque, but FT3/FT4 (OR: 0.75; 95% CI: 0.70–0.81; P < 0.001) was negatively associated with carotid plaque, which was consistent with unadjusted results. ORs for the fourth versus the first quartile of TFQI, PTFQI, TSHI, TT4RI, and FT3/FT4 were 2.08 (95% CI: 1.75–2.47) (Ptrend < 0.001), 2.36 (95% CI: 1.98–2.81) (Ptrend < 0.001), 1.83 (95% CI: 1.55–2.17) (Ptrend < 0.001), 2.31 (95% CI: 1.94–2.75) (Ptrend < 0.001), and 0.38 (95% CI: 0.32–0.46) (Ptrend < 0.001), respectively, for carotid plaque. This study excluded participants with thyroid disease, and further evaluated the association between thyroid hormone sensitivity and carotid plaque in participants with non-thyroid disease, and the results showed no significant change (Table 2). And there was no significant change in the association between thyroid hormone sensitivity and carotid plaque when participants with AITD, or rheumatological disease were excluded (Supplemental Tables S1, S2). The associations of thyroid hormone sensitivity with the number and echo properties of carotid plaques and CIMT were further evaluated. The results show that the association remained significant (Supplemental Tables S3–S5). In addition, when the present study supplemented CRP as a potential confounder for analysis, the association between thyroid hormone sensitivity and carotid plaque did not alter much in the fully adjusted model (Supplemental Table S6).
In the subgroup analysis of sex and age, the association between thyroid hormone sensitivity and carotid plaque is shown in Table 3 and Table 4. Regardless of sex, the association between thyroid hormone sensitivity and carotid plaque remained significant (P < 0.001). The OR values of thyroid hormone sensitivity and carotid plaque in males were higher than that in females. After multivariate adjustment, the association in middle-aged (≤ 60 years) patients was greater than in older (> 60 years) patients. Of all the indices representing central thyroid hormone sensitivity, the PTFQI had the highest OR value. The OR value of PTFQI in males (OR: 2.62; 95% CI: 2.04–3.35; P < 0.001) was higher than that in females (OR: 2.36; 95% CI: 1.94–2.87; P < 0.001), and the OR value of PTFQI in people ≤ 60 years old (OR: 3.23; 95% CI: 2.55–4.09; P < 0.001) was higher than that in people > 60 years old (OR: 2.16; 95% CI: 1.78–2.63; P < 0.001).
As shown in Tables 5 and Table 6, after multi-adjustment, the OR values of central thyroid hormone sensitivity of smokers were higher than that of non-smokers, and the OR value of peripheral thyroid hormone sensitivity was lower than that of non-smokers. Unlike non-drinkers, drinkers had higher OR values for TFQI, PTFQI, and TSHI and lower OR values for FT3/FT4. The OR value of PTFQI was the highest of central thyroid hormone sensitivity. The OR value of PTFQI in smokers (OR: 2.00; 95% CI: 1.51–2.65; P < 0.001) was higher than that in non-smokers (OR: 1.54; 95% CI: 1.17–2.03; P = 0.002), and the OR value of PTFQI in drinkers (OR: 2.02; 95% CI: 1.54–2.64; P < 0.001) was higher than that in non-drinkers (OR: 1.61; 95% CI: 1.20–2.14; P = 0.001).
To the best of our knowledge, this is the first study to evaluate the association between central and peripheral thyroid hormone sensitivity and the risk of carotid plaque in a large sample of patients with CHD in China. In this cross-sectional study, we found that the central thyroid hormone sensitivity indices TFQI, PTFQI, TSHI, and TT4RI were positively associated with the risk of carotid plaque in patients with CHD. With a gradual increase in TSHI, TT4RI, TFQI, and PTFQI, the OR value of carotid plaque also gradually increased. Peripheral thyroid hormone sensitivity index FT3/FT4 was negatively associated with the risk of carotid plaque, and the OR value of carotid plaque gradually decreased as FT3/FT4 gradually increased. Furthermore, the association remained consistent when stratified for sex, age, and smoking and drinking status.
Previous studies have shown that carotid plaque can predicte cardiovascular events and improved risk prediction for CHD (31). Due to the various changes of thyroid hormones in patients with CHD (32), the various effects of thyroid hormones in patients with CHD (33) and carotid plaque deserve attention (34). However, previous studies showed that higher TSH (11), lower TSH (35), lower FT3 (34), higher FT3, and higher FT4 (10) levels were all associated with the risk of carotid plaque. Inconsistent findings from previous studies have highlighted that TSH or thyroid hormone levels alone may not be sufficient to explain the association between the thyroid system and carotid plaque. Given these inconsistencies in previously proposed central thyroid hormone sensitivity indices (TSHI, TT4RI) and peripheral thyroid hormone sensitivity indices FT3/FT4 (27, 28), in 2019, Laclaustra et al. (29) proposed new resistance indices of central thyroid hormones, TFQI and PTFQI. TFQI is based on the empirical joint distribution of FT4 and TSH with the advantage of not yielding extreme values in cases of thyroid dysfunction. PTFQI is an index that can be calculated for any new value or adapted to other populations, with the same range and interpretation as an approximation. These new indices may have smaller deviations and can systematically reflect the regulation of thyroid hormone homeostasis compared to a single index, which can better explain the different associations between the changes in thyroid hormones and carotid plaque.
Many studies have reported an association between thyroid hormone levels and carotid plaque formation (36, 37). A cross-sectional study found that TSH was independently associated with carotid plaque, especially in participants with elevated TSH levels (11). A prospective cohort study in a Chinese population reported that higher mean levels and higher values of changes in FT3 and FT4 were associated with a higher risk of carotid atherosclerosis in euthyroid middle-aged and older participants (10). More recently, alterations in thyroid function, even within the reference ranges, were also found to be associated with atherosclerosis in the general population and patients with angina pectoris (38). Unlike this study, they did not calculate the association between central or peripheral thyroid hormone sensitivity and carotid plaque. this study showed that elevated TSHI, TT4RI, TFQI, and PTFQI calculated based on TSH and FT4 were associated with increased risk of carotid plaque, and elevated FT3/FT4 was associated with decreased risk of carotid plaque. Among central thyroid hormone sensitivity indices, PTFQI had the best sensitivity. In addition, when used as a reference in the Q1 group, central thyroid hormone sensitivity indices TSHI, TT4RI, TFQI and PTFQI were positively associated with carotid plaque in the Q4 group, and the sensitivity was the strongest in the Q4 group. Interestingly, our study also found that when used as a reference in the Q1 group, peripheral thyroid hormone sensitivity index FT3/FT4 was negatively associated with carotid plaque in the Q4 group, and the sensitivity was the strongest in the Q4 group. As many studies confirmed the positive effects of elevated FT3/FT4 on cardiovascular risk and arterial stiffness markers (39–41). However, studies have reported conflicting views. A cross-sectional analysis by Dörr et al. (35) reported an association between subclinical hyperthyroidism and carotid plaque, with an OR value of 1.67, suggesting that participants with reduced TSH levels should undergo regular screening for atherosclerotic risk factors and early treatment. A cross-sectional study of Chinese participants with type 2 diabetes and euthyroidism showed that low-normal FT3 levels were associated with a high prevalence of atherosclerosis (34). Differences in the methodologies used in various studies may also partly explain the contrasting results: differences in carotid plaque measurements, sample size, study population, differences in the normal range of the thyroid hormones, and differences in the definition of thyroid function are possible causes of the contrasting results.
To address sex- and age-specific differences noted in previous studies (42, 43), this study analyzed the association between thyroid hormone sensitivity and diabetes by sex and age, respectively. The risk of carotid plaque in patients with CHD is higher in males than in females and continues to increase with age (5, 13). The same sex-stratification was seen in this study, but contrary to most studies, our results show that, regardless of age, decreased central thyroid hormone sensitivity is associated with carotid plaques and was more strongly associated with people ≤ 60 years. This may be because this study falls within the CHD population, in which, the average age is > 60, belongs to the middle-aged and older population, and has a partial age bias.
Non-smoking and non-drinking are both related to a reduced risk of atherosclerosis. The Wisconsin Smokers’ Health Study, which examined the relationship between smoking burden and carotid plaque, showed that smoking cessation was related to slower progress of carotid plaque (44). Smoking is related to an increased cardiovascular risk, and smokers should be targeted for atherosclerosis prevention (45). Drinking is related to carotid plaque formation, especially excessive drinking (46, 47). In this study, decreased central thyroid hormone sensitivity is related to an increased risk of carotid plaque in CHD patients, especially among smokers and drinkers.
Carotid plaque and CIMT are markers of carotid atherosclerosis (48, 49). More and more studies have analyzed the possible pathways of the thyroid hormone’s effect on atherosclerosis. A previous study reported that type 2 iodothyronine deiodinase (D2), a thyroid hormone-activating enzyme that converts T4 to T3, was expressed in arterial smooth muscle cells (hCASMCs) (50). Activation of intracellular thyroid hormone by D2 inhibits the DNA synthesis and migration activity of hCASMCs (51), suggesting that thyroid hormone has a direct inhibitory effect on atherosclerosis. An excessively low thyroid hormone concentration can reduce the production of reactive oxygen species and the degree of vasodilation, indicating effects on endothelial cells (52, 53). Furthermore, thyroid hormone induces rapid activation of phosphoinositide 3-kinase (PI3K)/Akt/endothelial nitric oxide synthase in endothelial cells. We recently reported that the transformation of T4 to T3 by D2 is involved in thyroid receptor α1/PI3K-mediated non-genomic effects of T4 in human umbilical vein endothelial cells, including stimulation of Akt phosphorylation and Rac activation (54). These findings suggest that thyroid hormone induces endothelial cell migration, which is important for vascular repair and angiogenesis against the progression of atherosclerosis. In addition, TSH itself stimulates the proliferation of vascular smooth muscle cells, thereby promoting atherosclerosis (55). In this study, thyroid hormone sensitivity was significantly associated with carotid plaque, especially at high hormone levels, suggesting that the direct effects of thyroid hormones on the arterial wall may be involved in the progression of atherosclerosis.
From a clinical point of view, there seems to be a gradient of increasing thyroid hormones levels when carotid plaque formation in patients with CHD. As resistance to thyroid hormone is one of the differential diagnoses when both FT4 and TSH are elevated (56), this results offer an explanation for thyroid profiles commonly found in patients with CHD who have carotid plaque. That is, at the population level, measurements of resistance to thyroid hormone are cross-sectionally associated with carotid plaque, and periodic screenings on thyroid hormones in patients with CHD were recommended to aid in early prevention of carotid plaque.
The strengths of this study include a large number of participants, detailed information on available covariates for adjustment analysis, and analysis of the new thyroid hormone sensitivity indices. This study also performed secondary analyses among euthyroid participants. However, this study has several limitations. First, ultrasound may not be as accurate as high-resolution magnetic resonance imaging or computed tomography for plaque assessment; however, ultrasound has certain advantages regarding safety and non-invasiveness. Second, as the data came from the Hospital Information System and self-reported information, sufficient body mass index data could not be obtained, and the specific amount of smoking and drinking could not be identified, which would result in underestimation for the effect. Third, The study was conducted in a non-randomized population of inpatients with CHD, which may have selection bias leading to under- or over-estimation of the observed associations. However, this study was multi-center, with strict inclusion and exclusion criteria, and the diagnosis of the disease was uniform and strict, and the results were reliable to some extent. Finally, the measurement methods may differ across laboratories in a multi-center study. However, practitioners assess the quality of external clinical laboratories at each center, and physicians keep records, greatly increasing their reliability.
The increase in central thyroid hormone indices represents an decrease in central thyroid hormone sensitivity. This study showed that thyroid hormone sensitivity is significantly associated with carotid plaque in patients with CHD. This association is more significant in males, patients aged ≤ 60 years, smokers and drinkers. Evaluation of resistance to thyroid hormone may have important clinical significance for risk stratification and individualized treatment of patients with atherosclerosis.
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
This study was approved by the ethics committee of the Tianjin University of Traditional Chinese Medicine (approval number TJUTCM-EC20190008) and registered with the Chinese Clinical Trial Registry on July 14, 2019 (registration number ChiCTR-1900024535) and in ClinicalTrials.gov on July 18, 2019 (registration number NCT04026724). Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
CY, RY, CL, and LL: review and editing. YL, ZL, and TY: writing original draft and analyzing the data. LY, FL, TN, and SG: participated in data collection. All the authors have read and approved the final manuscript.
This study was supported by the National Basic Research Program of China (973 project, grant number 2014CB542902).
We thank all the participants in the study, the members of the survey teams, and the groups for providing financial support.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2022.940633/full#supplementary-material
AITD, autoimmune thyroid disease; CHD, coronary heart disease; CI, confidence interval; CIMT, carotid intima-media thickness; CVD, cardiovascular disease; D2, type 2 iodothyronine deiodinase; DBP, diastolic blood pressure; FT3, free triiodothyronine; FT4, free thyroxine; FT3/FT4, free triiodothyronine/free thyroxine; HbA1c, glycated hemoglobin; hCASMC, human coronary arterial smooth muscle cell; HDL-C, high-density lipoprotein cholesterol; ICD-10, International Classification of Diseases 10th revision; LDL-C, low-density lipoprotein cholesterol; ORs, odd ratios; PI3K, phosphoinositide 3-kinase; PTFQI, parametric thyroid feedback quantile-based index; SBP, systolic blood pressure; TC, total cholesterol; TFQI, thyroid feedback quantile-based index; TG, triglyceride; TSH, thyroid-stimulating hormone; TSHI, TSH index; TT4RI, thyrotroph thyroxine resistance index.
1. Virani SS, Alonso A, Aparicio HJ, Benjamin EJ, Bittencourt MS, Callaway CW, et al. American Heart association council on epidemiology and prevention statistics committee and stroke statistics subcommittee. heart disease and stroke statistics-2021 update: A report from the American heart association. Circulation (2021) 143(8):e254–743. doi: 10.1161/CIR.0000000000000950
2. Xing DM, Zhu MJ, Liu CX, Wang H. Outcome measures in clinical trials of traditional Chinese medicine for stable angina pectoris. Acupunct Herb Med (2021) 1(2):99–106. doi: 10.1097/HM9.0000000000000014
3. Dalen JE, Alpert JS, Goldberg RJ, Weinstein RS. The epidemic of the 20(th) century: coronary heart disease. Am J Med (2014) 127(9):807–12. doi: 10.1016/j.amjmed.2014.04.015
4. Botvin Moshe C, Haratz S, Ravona-Springer R, Heymann A, Hung-Mo L, Schnaider Beeri M, et al. Long-term trajectories of BMI predict carotid stiffness and plaque volume in type 2 diabetes older adults: a cohort study. Cardiovasc Diabetol (2020) 19(1):138. doi: 10.1186/s12933-020-01104-6
5. Bos D, Arshi B, van den Bouwhuijsen QJA, Ikram MK, Selwaness M, Vernooij MW, et al. Atherosclerotic carotid plaque composition and incident stroke and coronary events. J Am Coll Cardiol (2021) 77(11):1426–35. doi: 10.1016/j.jacc.2021.01.038
6. Gepner AD, Young R, Delaney JA, Tattersall MC, Blaha MJ, Post WS, et al. Comparison of coronary artery calcium presence, carotid plaque presence, and carotid intima-media thickness for cardiovascular disease prediction in the multi-ethnic study of atherosclerosis. Circ Cardiovasc Imaging (2015) 8(1):e002262. doi: 10.1161/CIRCIMAGING.114.002262
7. Floriani C, Gencer B, Collet TH, Rodondi N. Subclinical thyroid dysfunction and cardiovascular diseases: 2016 update. Eur Heart J (2018) 39(7):503–7. doi: 10.1093/eurheartj/ehx050
8. Rodondi N, den Elzen WP, Bauer DC, Cappola AR, Razvi S, Walsh JP, et al. Subclinical hypothyroidism and the risk of coronary heart disease and mortality. JAMA (2010) 304(12):1365–74. doi: 10.1001/jama.2010.1361
9. Cappola AR, Ladenson PW. Hypothyroidism and atherosclerosis. J Clin Endocrinol Metab (2003) 88(6):2438–44. doi: 10.1210/jc.2003-030398
10. Gu Y, Meng G, Zhang Q, Liu L, Wu H, Zhang S, et al. Association of longitudinal trends in thyroid function with incident carotid atherosclerosis in middle-aged and older euthyroid subjects: the tianjin chronic low-grade systemic inflammation and health (TCLSIH) cohort study. Age Ageing (2022) 51(1):afab276. doi: 10.1093/ageing/afab276
11. Sakamaki K, Tsunekawa K, Ishiyama N, Kudo M, Ando K, Akuzawa M, et al. Association between high normal-range thyrotropin concentration and carotid intima-media thickness in euthyroid premenopausal, perimenopausal and postmenopausal women. Maturitas (2021) 144:29–36. doi: 10.1016/j.maturitas.2020.10.022
12. Delitala AP, Filigheddu F, Orrù M, AlGhatrif M, Steri M, Pilia MG, et al. No evidence of association between subclinical thyroid disorders and common carotid intima medial thickness or atherosclerotic plaque. Nutr Metab Cardiovasc Dis (2015) 25(12):1104–10. doi: 10.1016/j.numecd.2015.09.001
13. Song P, Fang Z, Wang H, Cai Y, Rahimi K, Zhu Y, et al. Global and regional prevalence, burden, and risk factors for carotid atherosclerosis: a systematic review, meta-analysis, and modelling study. Lancet Glob Health (2020) 8(5):e721–9. doi: 10.1016/S2214-109X(20)30117-0
14. Li Z, He Y, Wang S, Li L, Yang R, Liu Y, et al. Association between triglyceride glucose index and carotid artery plaque in different glucose metabolic states in patients with coronary heart disease: a RCSCD-TCM study in China. Cardiovasc Diabetol (2022) 21(1):38. doi: 10.1186/s12933-022-01470-3
15. Liu Y, Li Z, Wang X, Ni T, Ma M, He Y, et al. Effects of adjuvant Chinese patent medicine therapy on major adverse cardiovascular events in patients with coronary heart disease angina pectoris: a population-based retrospective cohort study. Acupunct Herb Med (2022) 2(2):110–8. doi: 10.1097/HM9.0000000000000028
16. Li Z, Cheng Q, Liu Y, Cheng X, Wang S, He Y, et al. Low-/high-density lipoprotein cholesterol ratio and carotid plaques in patients with coronary heart disease: a Chinese cohort study. Lipids Health Dis (2021) 20(1):144. doi: 10.1186/s12944-021-01575-w
17. Barua RS, Rigotti NA, Benowitz NL, Cummings KM, Jazayeri MA, Morris PB, et al. ACC expert consensus decision pathway on tobacco cessation treatment: A report of the American college of cardiology task force on clinical expert consensus documents. J Am Coll Cardiol (2018) 72(25):3332–65. doi: 10.1016/j.jacc.2018.10.027
18. Fan AZ, Ruan WJ, Chou SP. Re-examining the relationship between alcohol consumption and coronary heart disease with a new lens. Prev Med (2019) 118:336–43. doi: 10.1016/j.ypmed.2018.11.022
19. Flack JM, Adekola B. Blood pressure and the new ACC/AHA hypertension guidelines. Trends Cardiovasc Med (2020) 30(3):160–4. doi: 10.1016/j.tcm.2019.05.003
20. Santos CS, Férnandez XC, Moriano Morales C, Álvarez ED, Álvarez Castro C, López Robles A, et al. Biological agents for rheumatic diseases in the outbreak of COVID-19: friend or foe? RMD Open (2021) 7(1):e001439. doi: 10.1136/rmdopen-2020-001439
21. Sen G, Gordon P, Sado DM. Cardiac manifestations of rheumatological disease: a synopsis for the cardiologist. Heart (2021) 107(14):1173–81. doi: 10.1136/heartjnl-2019-316460
22. Chambers T, Anney R, Taylor PN, Teumer A, Peeters RP, Medici M, et al. Effects of thyroid status on regional brain volumes: A diagnostic and genetic imaging study in UK biobank. J Clin Endocrinol Metab (2021) 106(3):688–96. doi: 10.1210/clinem/dgaa903
23. Yun JS, Bae JM, Kim KJ, Jung YS, Kim GM, Kim HR, et al. Increased risk of thyroid diseases in patients with systemic lupus erythematosus: A nationwide population-based study in Korea. PloS One (2017) 12(6):e0179088. doi: 10.1371/journal.pone.0179088
24. Stassi G, De Maria R. Autoimmune thyroid disease: new models of cell death in autoimmunity. Nat Rev Immunol (2002) 2(3):195–204. doi: 10.1038/nri750
25. American Diabetes Association 2. Classification and diagnosis of diabetes: Standards of medical care in diabetes-2019. Diabetes Care (2019) 42(Suppl 1):S13–28. doi: 10.2337/dc19-S002
26. Yang L, Li Z, Song Y, Liu Y, Zhao H, Liu Y, et al. Study on urine metabolic profiling and pathogenesis of hyperlipidemia. Clin Chim Acta (2019) 495:365–73. doi: 10.1016/j.cca.2019.05.001
27. Cappelli C, Rotondi M, Pirola I, Agosti B, Gandossi E, Valentini U, et al. TSH-lowering effect of metformin in type 2 diabetic patients: differences between euthyroid, untreated hypothyroid, and euthyroid on l-T4 therapy patients. Diabetes Care (2009) 32(9):1589–90. doi: 10.2337/dc09-0273
28. Yagi H, Pohlenz J, Hayashi Y, Sakurai A, Refetoff S. Resistance to thyroid hormone caused by two mutant thyroid hormone receptors beta, R243Q and R243W, with marked impairment of function that cannot be explained by altered in vitro 3,5,3’-triiodothyroinine binding affinity. J Clin Endocrinol Metab (1997) 82:1608–14. doi: 10.1210/jcem.82.5.3945
29. Laclaustra M, Moreno-Franco B, Lou-Bonafonte JM, Mateo-Gallego R, Casasnovas JA, Guallar-Castillon P, et al. Impaired sensitivity to thyroid hormones is associated with diabetes and metabolic syndrome. Diabetes Care (2019) 42(2):303–10. doi: 10.2337/dc18-1410
30. Liu Y, Zhu Y, Jia W, Sun D, Zhao L, Zhang C, et al. Association between lipid profiles and presence of carotid plaque. Sci Rep (2019) 9(1):18011. doi: 10.1038/s41598-019-54285-w
31. Polak JF, Szklo M, Kronmal RA, Burke GL, Shea S, Zavodni AE, et al. The value of carotid artery plaque and intima-media thickness for incident cardiovascular disease: the multi-ethnic study of atherosclerosis. J Am Heart Assoc (2013) 2(2):e000087. doi: 10.1161/JAHA.113.000087
32. Ertugrul O, Ahmet U, Asim E, Gulcin HE, Burak A, Murat A, et al. Prevalence of subclinical hypothyroidism among patients with acute myocardial infarction. ISRN Endocrinol (2011) 2011:810251. doi: 10.1186/1475-2840-11-93
33. Cappola AR, Desai AS, Medici M, Cooper LS, Egan D, Sopko G, et al. Thyroid and cardiovascular disease: Research agenda for enhancing knowledge, prevention, and treatment. Thyroid (2019) 29(6):760–77. doi: 10.1089/thy.2018.0416
34. Wang L, Chen T, Yu J, Yuan H, Deng X, Zhao Z. Clinical associations of thyroid hormone levels with the risk of atherosclerosis in euthyroid type 2 diabetic patients in central China. Int J Endocrinol (2020) 2020:2172781. doi: 10.1155/2020/2172781
35. Dörr M, Empen K, Robinson DM, Wallaschofski H, Felix SB, Völzke H. The association of thyroid function with carotid artery plaque burden and strokes in a population-based sample from a previously iodine-deficient area. Eur J Endocrinol (2008) 159(2):145–52. doi: 10.1530/EJE-08-0140
36. Shimizu Y, Kawashiri SY, Noguchi Y, Nagata Y, Maeda T, Hayashida N. Association between thyroid cysts and hypertension by atherosclerosis status: a cross-sectional study. Sci Rep (2021) 11(1):13922. doi: 10.1038/s41598-021-92970-x
37. Manolis AA, Manolis TA, Melita H, Manolis AS. Subclinical thyroid dysfunction and cardiovascular consequences: An alarming wake-up call? Trends Cardiovasc Med (2020) 30(2):57–69. doi: 10.1016/j.tcm.2019.02.011
38. Cappola AR, Arnold AM, Wulczyn K, Carlson M, Robbins J, Psaty BM. Thyroid function in the euthyroid range and adverse outcomes in older adults. J Clin Endocrinol Metab (2015) 100(3):1088–96. doi: 10.1210/jc.2014-3586
39. Yuan D, Zhang C, Jia S, Liu Y, Jiang L, Xu L, et al. Predictive value of free triiodothyronine (FT3) to free thyroxine (FT4) ratio in long-term outcomes of euthyroid patients with three-vessel coronary artery disease. Nutr Metab Cardiovasc Dis (2021) 31(2):579–86. doi: 10.1016/j.numecd.2020.10.011
40. Yuan D, Jia S, Zhu P, Zhang C, Liu Y, Liu R, et al. Usefulness of FT3 to FT4 ratio to predict mortality in euthyroid patients with prior cardiovascular events undergoing PCI: Five-year findings from a Large single-center cohort study. Front Endocrinol (Lausanne) (2021) 12:700349. doi: 10.3389/fendo.2021.700349
41. Yu N, Wang L, Zeng Y, Zhao Y, Chen S, Pan H, et al. The association of thyroid hormones with coronary atherosclerotic severity in euthyroid patients. Horm Metab Res (2022) 54(1):12–9. doi: 10.1055/a-1718-6283
42. Man JJ, Beckman JA, Jaffe IZ. Sex as a biological variable in atherosclerosis. Circ Res (2020) 126(9):1297–319. doi: 10.1161/CIRCRESAHA.120.315930
43. Lu M, Peng P, Qiao H, Cui Y, Ma L, Cui B, et al. Association between age and progression of carotid artery atherosclerosis: a serial high resolution magnetic resonance imaging study. Int J Cardiovasc Imaging (2019) 35(7):1287–95. doi: 10.1007/s10554-019-01538-4
44. Stein JH, Smith SS, Hansen KM, Korcarz CE, Piper ME, Fiore MC, et al. Longitudinal effects of smoking cessation on carotid artery atherosclerosis in contemporary smokers: The Wisconsin smokers health study. Atherosclerosis (2020) 315:62–7. doi: 10.1016/j.atherosclerosis.2020.11.010
45. Jiang Y, Pang T, Shi R, Qian WL, Yan WF, Li Y, et al. Effect of smoking on coronary artery plaques in type 2 diabetes mellitus: Evaluation with coronary computed tomography angiography. Front Endocrinol (Lausanne) (2021) 12:750773. doi: 10.3389/fendo.2021.750773
46. Bardach AE, Caporale JE, Rubinstein AL, Danaei G. Impact of level and patterns of alcohol drinking on coronary heart disease and stroke burden in Argentina. PloS One (2017) 12(3):e0173704. doi: 10.1371/journal.pone.0173704
47. Sohn SM, Choi BG, Choi SY, Byun JK, Mashaly A, Park Y, et al. Impact of alcohol drinking on acetylcholine-induced coronary artery spasm in Korean populations. Atherosclerosis (2018) 268:163–9. doi: 10.1016/j.atherosclerosis.2017.11.032
48. Amato M, Veglia F, de Faire U, Giral P, Rauramaa R, Smit AJ, et al. Carotid plaque-thickness and common carotid IMT show additive value in cardiovascular risk prediction and reclassification. Atherosclerosis (2017) 263:412–9. doi: 10.1016/j.atherosclerosis.2017.05.023
49. Lind L, Gigante B, Borné Y, Feldreich T, Leppert J, Hedberg P, et al. Plasma protein profile of carotid artery atherosclerosis and atherosclerotic outcomes: Meta-analyses and mendelian randomization analyses. Arterioscler Thromb Vasc Biol (2021) 41(5):1777–88. doi: 10.1161/ATVBAHA.120.315597
50. Mizuma H, Murakami M, Mori M. Thyroid hormone activation in human vascular smooth muscle cells: expression of type II iodothyronine deiodinase. Circ Res (2001) 88(3):313–8. doi: 10.1161/01.res.88.3.313
51. Kasahara T, Tsunekawa K, Seki K, Mori M, Murakami M. Regulation of iodothyronine deiodinase and roles of thyroid hormones in human coronary artery smooth muscle cells. Atherosclerosis (2006) 186(1):207–14. doi: 10.1016/j.atherosclerosis.2005.07.018
52. Lekakis J, Papamichael C, Alevizaki M, Piperingos G, Marafelia P, Mantzos J, et al. Flow-mediated, endothelium-dependent vasodilation is impaired in subjects with hypothyroidism, borderline hypothyroidism, and high-normal serum thyrotropin (TSH) values. Thyroid (1997) 7(3):411–4. doi: 10.1089/thy.1997.7.411
53. De Sibio MT, Luvizotto RA, Olimpio RM, Correa CR, Marino J, de Oliveira M, et al. A comparative genotoxicity study of a supraphysiological dose of triiodothyronine (t(3)) in obese rats subjected to either calorie-restricted diet or hyperthyroidism. PloS One (2013) 8:e56913. doi: 10.1371/journal.pone.0056913
54. Aoki T, Tsunekawa K, Araki O, Ogiwara T, Nara M, Sumino H, et al. Type 2 iodothyronine deiodinase activity is required for rapid stimulation of PI3K by thyroxine in human umbilical vein endothelial cells. Endocrinology (2015) 156(11):4312–24. doi: 10.1210/en.2014-1988
55. Tian L, Ni J, Guo T, Liu J, Dang Y, Guo Q, et al. TSH stimulates the proliferation of vascular smooth muscle cells. Endocrine (2014) 46(3):651–8. doi: 10.1007/s12020-013-0135-4
Keywords: coronary heart disease, carotid plaque, thyroid hormone sensitivity, association, TFQI, PTFQI, resistance to thyroid hormone
Citation: Liu Y, Li Z, Yang T, Li L, Yu L, Liu F, Ni T, Gao S, Li C, Yang R and Yu C (2022) Impaired sensitivity to thyroid hormones and carotid plaque in patients with coronary heart disease: A RCSCD-TCM study in China. Front. Endocrinol. 13:940633. doi: 10.3389/fendo.2022.940633
Received: 10 May 2022; Accepted: 06 September 2022;
Published: 27 September 2022.
Edited by:
Arti Dhar, Birla Institute of Technology and Science, IndiaReviewed by:
Fumihiko Furuya, Fukushima Medical University, JapanCopyright © 2022 Liu, Li, Yang, Li, Yu, Liu, Ni, Gao, Li, Yang and Yu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chunjie Li, eWt3bkBzaW5hLmNvbQ==; Rongrong Yang, cm9uZ3JvbmcwNDIzQGhvdG1haWwuY29t; Chunquan Yu, eWNxdGp1dGNtQGZveG1haWwuY29t
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.