
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Endocrinol. , 12 July 2022
Sec. Clinical Diabetes
Volume 13 - 2022 | https://doi.org/10.3389/fendo.2022.929864
This article is part of the Research Topic Research Advances in Gestational Diabetes Mellitus, Neonatal Diabetes Mellitus, and Metabolic Disorders Volume II View all 8 articles
 Mingyang Jiang1†‡
Mingyang Jiang1†‡ Fu Gan2‡
Fu Gan2‡ Meishe Gan3‡
Meishe Gan3‡ Huachu Deng4
Huachu Deng4 Xuxu Chen4
Xuxu Chen4 Xintao Yuan4
Xintao Yuan4 Danyi Huang4
Danyi Huang4 Siyi Liu4
Siyi Liu4 Baoyu Qin5
Baoyu Qin5 Yanhong Wei6
Yanhong Wei6 Shanggui Su4*
Shanggui Su4* Zhandong Bo1*
Zhandong Bo1*Background: Diabetic foot ulcer (DFU) in patients with type 2 diabetes mellitus (T2D) often leads to amputation. Early intervention to prevent DFU is urgently necessary. So far, there have been no studies on predictive models associated with DFU risk factors. Our study aimed to quantify the predictive risk value of DFU, promote health education, and further develop behavioral interventions to reduce the incidence of DFU.
Methods: Data from 973 consecutive patients with T2D was collected from two hospitals. Patients from the Guangxi Medical University First Affiliated Hospital formed the training cohort (n = 853), and those from the Wuming Hospital of Guangxi Medical University formed the validation cohort (n = 120). Independent variable grouping analysis and multivariate logistic regression analysis were used to determine the risk factors of DFUs. The prediction model was established according to the related risk factors. In addition, the accuracy of the model was evaluated by specificity, sensitivity, predictive value, and predictive likelihood ratio.
Results: In total, 369 of the 853 patients (43.3%) and 60 of the 120 (50.0%) were diagnosed with DFUs in the two hospitals. The factors associated with DFU were old age, male gender, lower body mass index (BMI), longer duration of diabetes, history of foot disease, cardiac insufficiency, no use of oral hypoglycemic agent (OHA), high white blood cell count, high platelet count, low hemoglobin level, low lymphocyte absolute value, and high postprandial blood glucose. After incorporating these 12 factors, the nomogram drawn achieved good concordance indexes of 0.89 [95% confidence interval (CI): 0.87 to 0.91] in the training cohort and 0.84 (95% CI: 0.77 to 0.91) in the validation cohort in predicting DFUs and had well-fitted calibration curves. Patients who had a nomogram score of ≥180 were considered to have a low risk of DFU, whereas those having ≥180 were at high risk.
Conclusions: A nomogram was constructed by combining 12 identified risk factors of DFU. These 12 risk factors are easily available in hospitalized patients, so the prediction of DFU in hospitalized patients with T2D has potential clinical significance. The model provides a reliable prediction of the risk of DFU in patients with T2D.
Diabetes mellitus (DM) is a group of metabolic diseases characterized by long-term hyperglycemia (1), and the affected patients can develop multiple complications (2). Several characteristic pathological changes in the feet of patients with DM such as infection, diabetic foot ulcer (DFU), and neuroarthropathy are called diabetic foot syndrome (3). If diabetes is not controlled, then it can cause complications through complex metabolic pathways (4, 5): Peripheral neuropathy can lead to loss of sensation; peripheral artery disease may cause ischemia; a combination of both can eventually lead to foot ulcers (6, 7). Foot ulcers are a risk factor for foot infections, which greatly increases the probability of amputation (8). Therefore, among the vascular complications of diabetes, foot ulcer is the primary cause of hospitalization (8).
In patients with diabetes, DFU is one of the main causes of morbidity and mortality and a major public health problem, exerting a heavy burden on society (9). It has been reported that more than 20%–40% of medical resources related to diabetes are allocated to foot care (8). A foot ulcer is the most common manifestation of diabetic foot disease and has a very poor prognosis (10). The global prevalence of DFU is 6.3% [higher among males than females, type 2 (T2D) is higher than type 1 diabetes] (11), and DFU is the main cause of amputation in patients with diabetes (12). It is estimated that one diabetic foot is amputated every 20 s, with an annual mortality rate of 11% for DFUs and 22% for amputees (13). Ulcers appear in numb areas of the feet and legs, are often overlooked, can easily become infected, and eventually lead to amputation. Diabetic foot lesions are responsible for more hospitalizations than any other complication of diabetes (14). Other treatments have also been tried, such as skin grafting, vacuum sealing drainage, interventional therapy, and Tibetan transverse transport, but there are many complications in the operation, such as unhealed bone end, soft tissue infection, pain, and limb shortening deformity (15). Many patients have weak tolerance and lower compliance in the later period, which affects the effect of surgery.
Patients with diabetes are in a high glucose state for a long time, and their glucose metabolism is disordered, resulting in tissue hypoxia, an increase of damaging substances such as thromboxane, and complete vascular endothelial injury and promotion of microcirculation disorder (16). In addition, a high concentration of glucose glycosylates with protein and nucleic acid molecules inside and outside the blood vessel causes vascular cell dysfunction, promoting coagulation and thrombosis, leading to microangiopathy (17). As the mobility and phagocytosis of white blood cells (WBCs) are reduced, the immunity of the body is weakened due to peripheral neuropathy and vascular lesion. Small trauma can cause invasion and infection of microorganisms, so increased local oxygen consumption aggravates ischemia and gangrene occurs (18).
Prevention of DFU is better than treatment (19). Strengthening the management of patients at high risk of diabetic foot to ensure early detection, diagnosis, and treatment and to reduce the occurrence of DFUs can achieve twice the result with half the medical resources. Preventive measures of DFUs include regulating blood glucose levels, identifying and screening high-risk groups, patient education, and footwear management (20). Tight shoes, lack of foot care knowledge, and self-examination can cause repeated foot trauma, which becomes a trigger for DFUs, especially in the presence of peripheral neuropathy (21). However, there is limited evidence to support the preventive long-term benefit of patient education. A meta-analysis indicated that among all the recommended methods to prevent DFU, only foot temperature-guided avoidance therapy was effective in randomized controlled trials (22).
The clinical prediction model (also known as clinical prediction rules, prognosis models, or risk scores) refers to the use of multi-factor models to estimate the probability of a certain disease or its outcome in the future (23). The model includes the diagnostic and prognostic assessment. The prognostic model is concerned with the probability of recurrence, death, disability, and complications at a certain time in the future according to the current state of disease (23). Li et al. performed a predictive model to investigate diabetic retinopathy (DR) risk factors and predictive models by machine learning using a large sample dataset. They concluded that, with better comprehensive performance, the XGBoost model had high reliability to assess risk indicators of DR. An HbA1c value greater than 8%, nephropathy, a serum creatinine value greater than 100 µmol/L, insulin treatment, and diabetic lower extremity arterial disease were associated with an increased risk of DR (24). Zaidi et al. also conducted a multi−step ahead predictive model for blood glucose concentrations of patients with type 1 diabetes. They found that their model can capture the hyperglycemic and the hypoglycemic events better as compared to the ARX model but is slightly less accurate in the normoglycemic range values (25). Their studies all established predictive models associated with diabetes, but none described prognostic models associated with DFU. Our study is the first to predict DFU. In our study, by establishing a prognostic model, analyzing the risk factors of DFUs, and creating score tables and line charts, we aimed to provide more intuitive and powerful scientific tools for doctors and patients. We also aimed to quantify the risk value (probability) of DFUs in the future, promote health education, and advance behavioral interventions to reduce the incidence of DFUs.
This was a hospital retrospective case-control study spanning from 1 January 2017 to 1 September 2019. The participants were patients with T2D who were treated at Guangxi Medical University First Affiliated Hospital and Wuming Hospital of Guangxi Medical University. All data were extracted from the hospital medical record system. The study was conducted following the Declaration of Helsinki (as revised in 2013). This study was approved by the Ethics Committees of Guangxi Medical University (No. 20220144), and individual consent for this retrospective analysis was waived. The patients did not receive financial compensation.
A total of 973 patients with T2D were identified. Because of the small number of patients with DFU in the validation group, propensity score matching (PSM) was applied to balance the number of the experimental group and the control group. The inclusion criteria were as follows: patients with type 2 diabetes with DFUs (the case group), and patients with type 2 diabetes without DFUs (the control group). The exclusion criteria were as follows: patients without T2D and foot breakage caused by trauma. Data of 429 patients in the case group were compared with that of 544 patients in the control group. Sociodemographic data, foot factors, diabetes-related risk factors, complications, and biochemical indicators were collected. The confidentiality of patient information was protected.
The clinical variables of this study are shown in Table 1. Potential predictors are selected and included from published studies of diabetes (24, 25). Independent variable grouping analysis was used to determine the risk factors of DFUs: sociodemographic, foot factors, and biochemical indicators of diabetes. Sociodemographic data included gender, age, smoking history, alcohol use history, and body mass index (BMI). Age was confirmed by checking the patient’s ID number. Patients’ previous foot problems, such as non-diabetes–related foot ulcers, foot trauma, and/or foot surgery (such as surgical debridement, osteotomy, and fixation), were obtained through medical records or by self-report, without grouping. Diabetes risk factors retrieved from patients’ medical records, including course of the disease, family history, cardiac insufficiency, hypertension, hyperthyroidism, use of oral hypoglycemic agent (OHA), and use of insulin (INS). Diabetes-related complications included the following: peripheral vascular disease (PVD), peripheral neuropathy disease (PND), DR, and diabetic nephropathy; whereas biochemical indicators included the following: blood routine [red blood cell (RBC) count, WBC count, hemoglobin (Hb), platelet (PLT), neutrophil absolute value (NEUT), and lymphocyte absolute value (LY)], blood glucose {fasting (FBS), postprandial (PBS), glycosylated hemoglobin (HbA1c), urine glucose (GLU), blood lipids [total cholesterol (TCHOL), triglyceride (TG), high-density lipoprotein (HDLC), and low-density lipoprotein (LDLC)], renal function test [blood urea nitrogen (BUN) and serum creatinine (SCr)], and liver function test [total bilirubin (TBil), direct bilirubin (DBil), albumin (ALB), the ratio of glutamic oxaloacetic transaminase, glutamic pyruvic transaminase, and transaminase (ST/ALT)]. For the case group, these characteristics were determined on the basis of the data available in the patient’s medical history before the diagnosis of DFUs.
The ratio of the case to control participants was approximately 1:1. All data were encoded and analyzed using SPSS 25.0 (IBM Corp., Armonk, IL, USA) and R 3.6.1 (The R Foundation for Statistical Computing, Vienna, Austria). A chi-square test was used to check the statistical independence between the two groups. Univariate logistic regression analysis was used to evaluate the importance of each variable in the training cohort, to investigate the independent risk factors of DFUs. All significant levels of variables associated with DFUs were analyzed by multivariate logistic regression analysis. A line diagram was made using the rms software package (http://www.r-project.org/) of R 3.6.1, based on the results of multiple logistic regression analysis. The line chart was based on the proportional conversion of each regression coefficient in a multivariate logistic regression on a scale of 0 to 100 points. The influence of the variable with the highest β coefficient (absolute value) was assigned 100 points. The total points were obtained by combining these points with independent variables and were then converted into prediction probability. The predictive performance of the line chart was measured using the consistency index (C index) and calibrated with 1,000 bootstrapped samples to reduce the over-fitting deviation.
The clinical use of the model was based on the line chart to calculate the total score of each patient. The optimal critical value was calculated by analyzing the receiver operating characteristic (ROC) curve, which was determined by maximizing the Youden index. The accuracy of the optimal critical value was evaluated by specificity, sensitivity, predictive value, and predictive likelihood ratio. The acceptable threshold values of ROC, sensitivity, and specificity of the predictive model were 0.8. A two-sided P-value <0.05 was considered statistically significant (26).
During the study period, 1,288 patients with T2D were selected according to the inclusion criteria. The training cohort had 853 patients (369 in the case and 484 in the control group). The validation cohort had 435 patients, from which 120 (60 each, for the case and the control group) were selected according to PSM. Table 1 lists the clinical characteristics of the patients. The baseline clinical data of the training cohorts were similar to that of the validation cohort.
All data for the study were extracted from medical records. Univariate logistic analysis was performed to exclude significantly unrelated variables, as shown in Table 2. The results of the multivariate analysis were reported as odds ratio [95% confidence interval (CI)] as shown in Table 3.
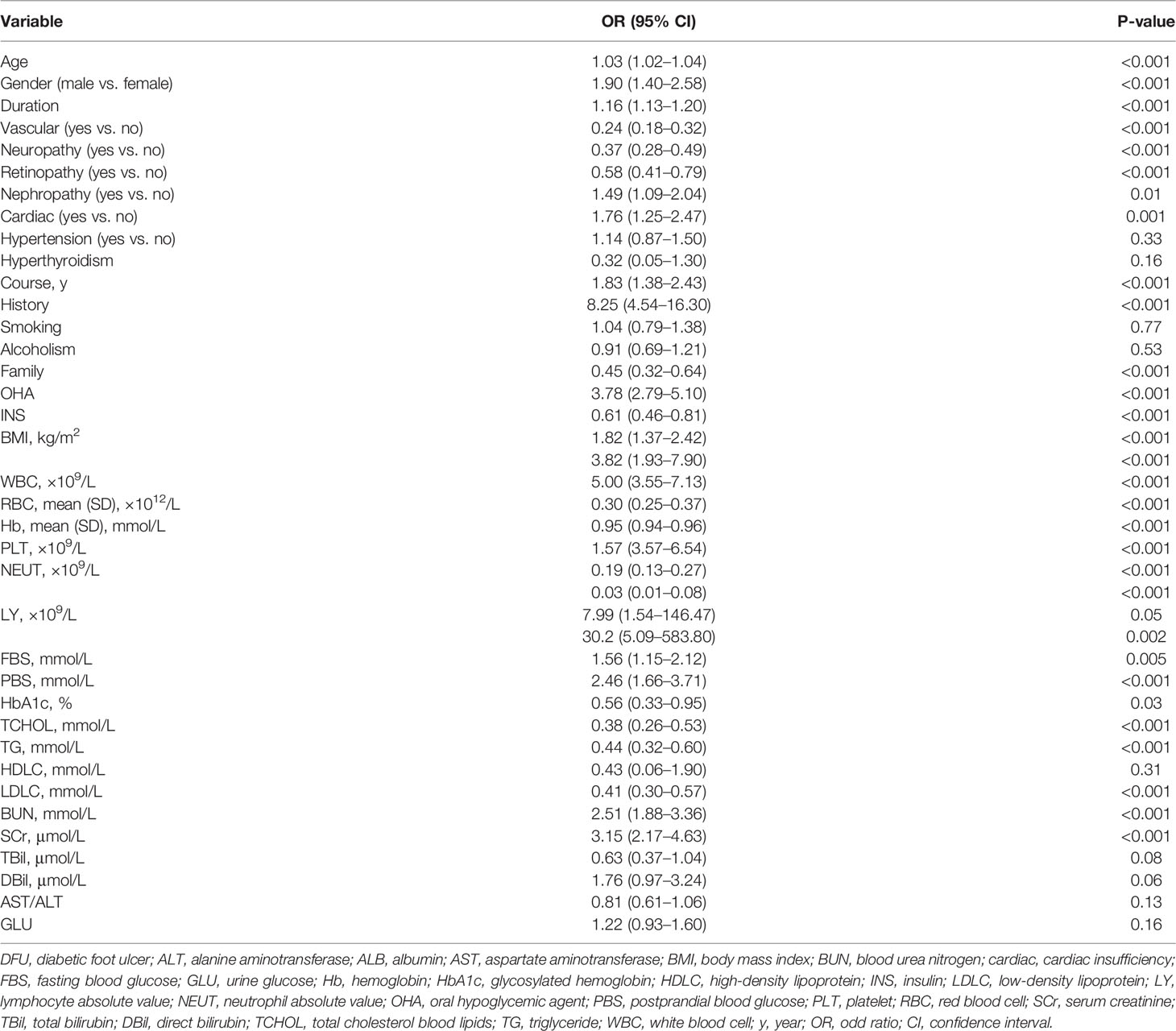
Table 2 Univariate logistic regression analysis of DFU based on preoperative data in the training cohort.
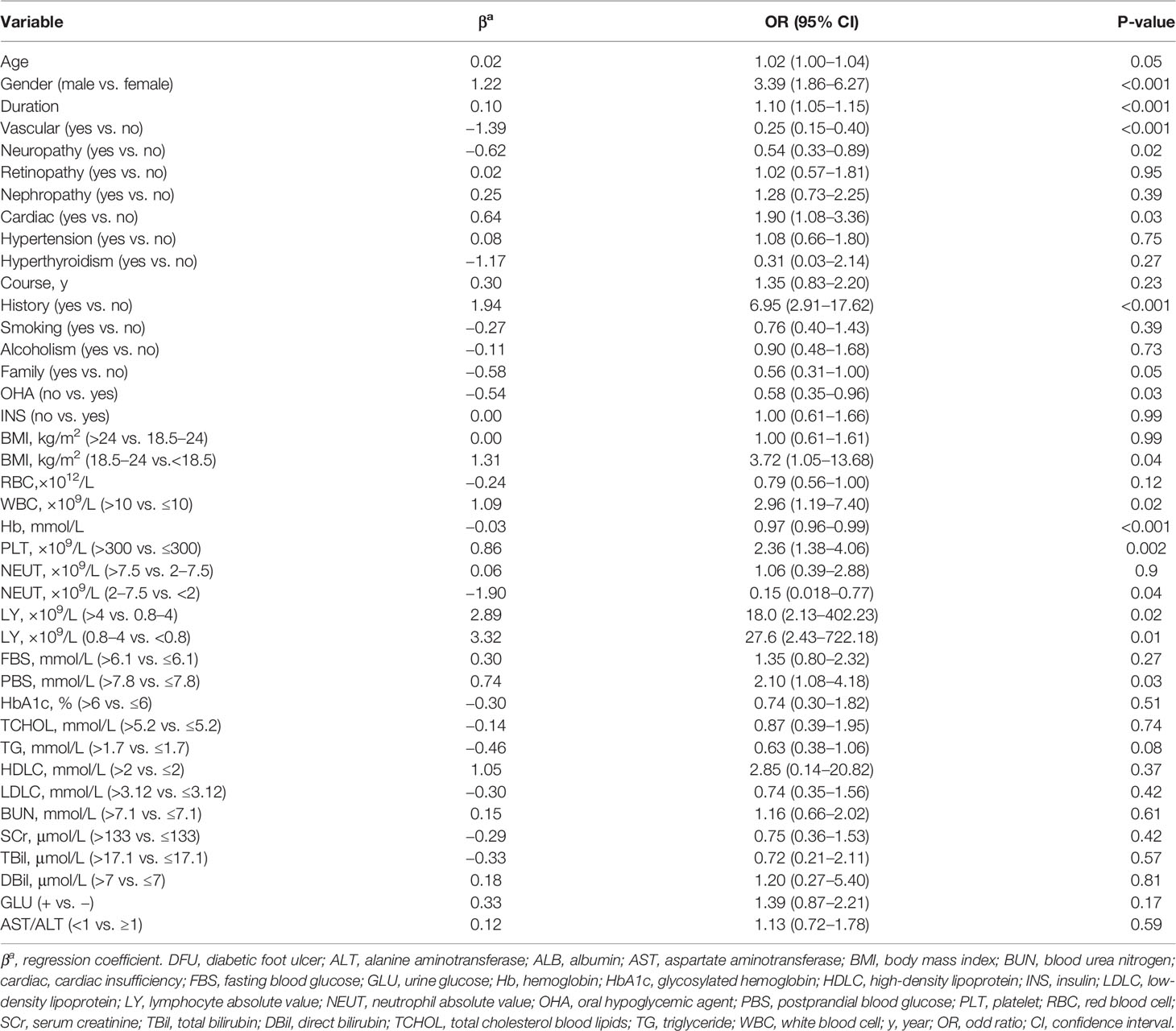
Table 3 Multivariate logistic regression analysis of DFU based on preoperative data in the training cohort.
After modification, 12 independently related risk factors (age, gender, duration, cardiac insufficiency, history of foot diseases, family history, BMI, WBC, PLT, LY, PBS, and use of OHA) were used to form a DFU risk estimation nomogram (Figure 1), which demonstrated good accuracy with a C index of 0.89 (95% CI: 0.87 to 0.91) (Figure 2A) in the training cohort. In addition, the nomogram displayed a C index of 0.84 (95% CI: 0.77 to 0.91) (Figure 2B) in the validation cohort. In addition, the calibration chart was shown on the graph, and the risk estimation of the line chart fit well (Figure 3A) in the training cohort, whereas in the validation cohort, the risk estimation also showed a good calibration curve (Figure 3B).
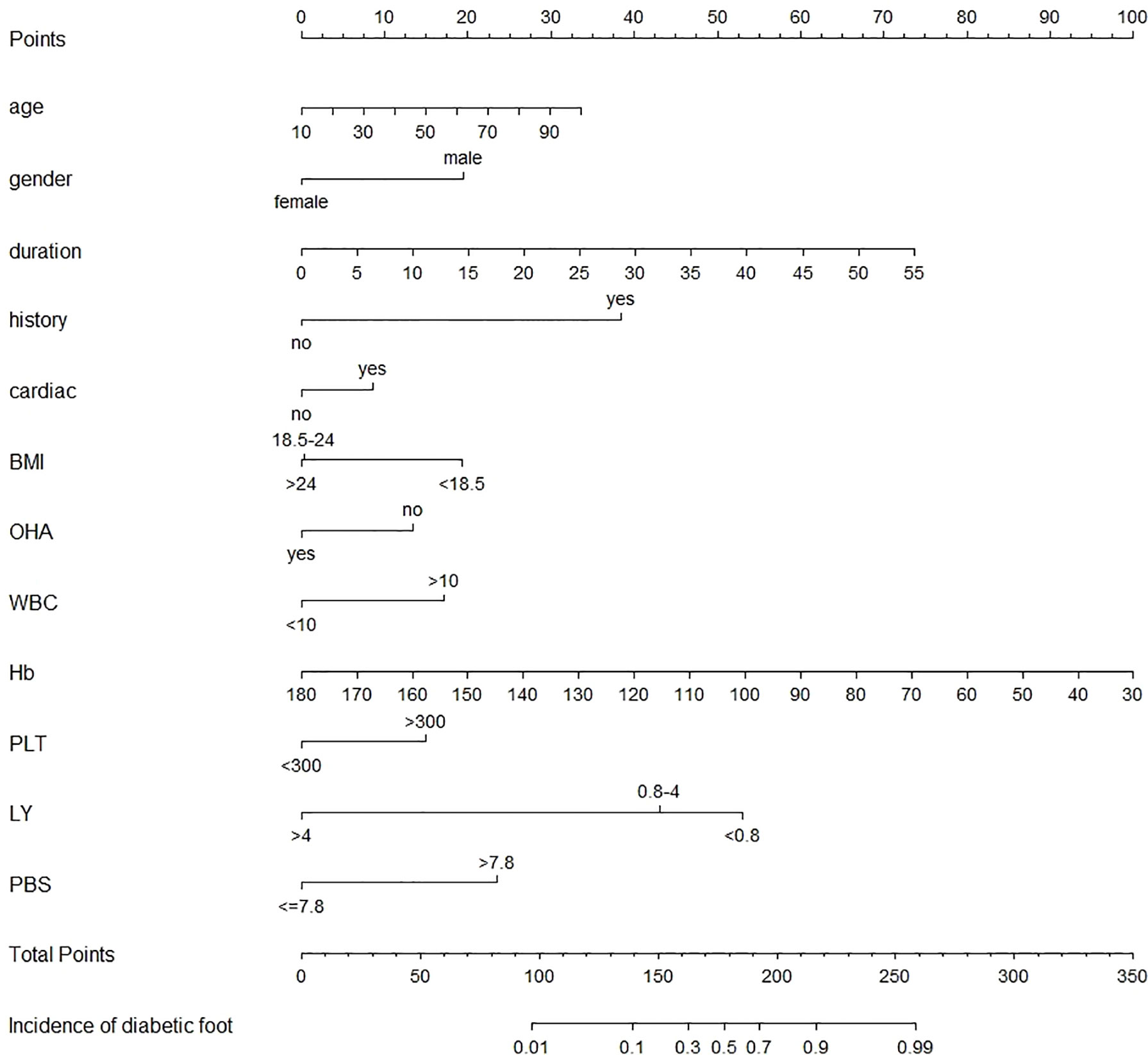
Figure 1 Nomogram to estimate the risk of DFU in patients with diabetes. To use the nomogram, find the position of each variable on the corresponding axis, draw a line to the points axis for the number of points, add the points from all variables, and draw a line from the total point axis to determine the DFU probabilities at the lower line of the nomogram. cardiac, cardiac insufficiency; DFU, diabetic foot ulcer; BMI, body mass index; Hb, hemoglobin; LY, lymphocyte absolute value; OHA, oral hypoglycemic agent; PBS, postprandial blood glucose; PLT, platelet; WBC, white blood cell.
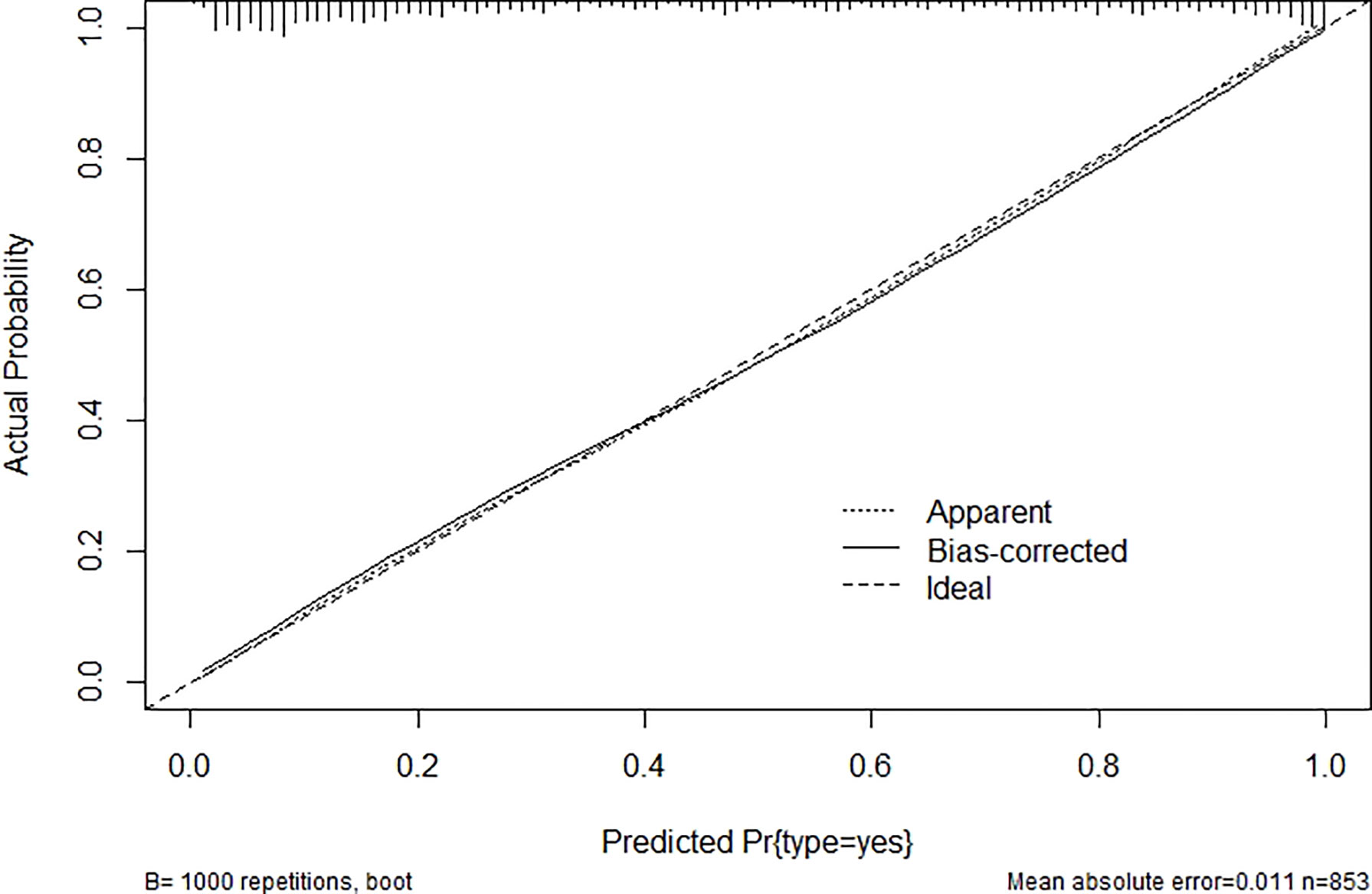
Figure 2 Area under ROC curve (concordance index) (A) in the training cohort and (B) in the validation cohort. ROC, receiver operating characteristic.
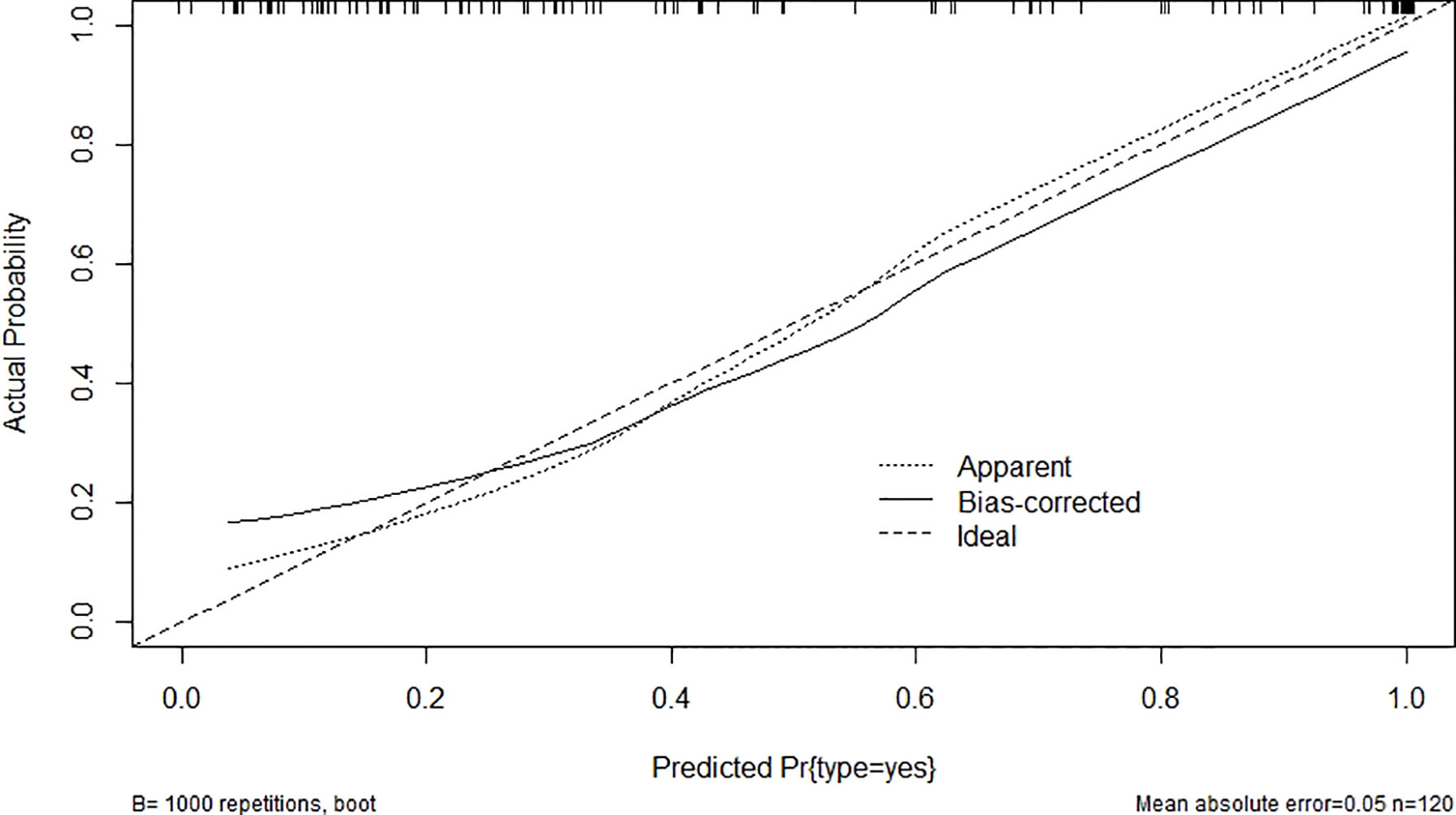
Figure 3 Validity of the predictive performance of the nomogram in estimating the risk of DFU: (A) in the training cohort (n = 853) and (B) in the validation cohort (n = 120). DFU, diabetic foot ulcer.
The optimal cutoff value for the nomogram was determined to be 180. The sensitivity, specificity, positive, and negative predictive values used in differentiating the presence of DFUs were 81.1%, 81.1%, 73.4%, and 87.0%, respectively, in the training cohort, and 80.0%, 75.4%, 73.3%, and 81.7%, respectively, in the validation cohort (Table 4).
As a serious diabetic complication, DFUs seriously affect the quality of life of patients with T2D (27). The lifetime incidence of foot ulcers is estimated to be 15%–25% among patients with diabetes (28); incorporating additional data, 19%–34% of patients are likely to be affected. Peripheral artery disease is an important risk factor for the development of DFU (29). However, the diagnosis of DFU in diabetes is challenging due to neuropathy and arterial calcification (30). The commonly used bedside tests are either insensitive or have little supporting evidence for their use. There is a good correlation between duplex ultrasound and angiography, but a full scan is difficult to fathom and time-consuming to perform (31). None of the existing methods can predict early-stage DFUs, and patients are often at risk of amputation when symptoms develop. A nomogram has good discrimination characteristics, has high accuracy in the prediction of results among the available prediction tools, and is easy to use. Our proposed nomogram incorporated comprehensive and easily available preoperative variables performed well (C index values of 0.89 in the training and 0.84 in the validation cohorts), and the optimal calibration curves demonstrated agreements between actual observation and prediction (26).
A meta-analysis published in 2019 showed that risk factors for DFUs include male gender, smoking, duration of past DFUs, plantar ulcers, peripheral artery disease, and diabetic peripheral neuropathy. There were no significant differences in age, BMI, total cholesterol, DR, diabetic nephropathy, or hypertension (32). Nanwani et al. (33) suggested that crucial risk factors include male gender, smoking, hyperlipidemia, hypertension, cardiac history, and the co-occurrence of diabetic nephropathy and DR. However, literature has shown that, due to too many risk factors, their interaction, and different baseline patient characteristics, the conclusions drawn from studies around the world are controversial (24, 25). Our study suggested that older age, male gender, lower BMI, longer duration of diabetes, history of foot disease, cardiac insufficiency, no use of OHA, higher WBC count, higher PLT count, lower Hb concentration, lower LY absolute value, and higher PBS were significantly associated with high incidence rates of DFUs. Our results were somewhat consistent with previous studies (34), but there were also great differences. For example, the research of Zubair et al. (34) stated that there is a positive association with gender, diabetes duration, ulcer size, grade of ulcer, amputation rate, hospital stay, Hb, SGOT/AST, and triglyceride. However, our study showed that lower BMI (especially BMI <18.5) is related to a higher risk of developing DFUs, which may be due to thinner patients having had a longer course of the disease. Factors such as smoking, diabetic nephropathy, and DR, which have been linked to the occurrence of DFU, were excluded from the final model due to the insignificant statistical test (P > 0.05) in the multivariate logistic regression analysis. PVD and PNDs were recognized as significant signs for DFUs (35); but in our study, multivariate logistic regression analysis showed that the DFUs group had a lower risk of the two symptoms, which was a challenge to the previous findings. We postulate that this was because the conditions were common complications in T2D, with or without DFU. Because this was a retrospective study, it was not possible to determine whether the occurrence of PVD and PND was the cause of DFUs, but there was a causal relationship, so more far-reaching prospective studies are needed. The goal of the clinical prediction model was to warn patients with diabetes, with risk factors but without foot symptoms, about early prevention. If they already had PVD or PND, then imaging methods such as angiography were used for visualization, and the patient was promptly treated.
For patients with diabetes mellitus with high scores, we have the following suggestions to avoid DFU (36): (I) Pay attention to improving circulation: to prevent the affected part from being compressed, and pay attention to frequently turning over during lying to reduce the time of local compression, and use stents when necessary. (II) Foot movement: patients must pay attention to it when taking each step. It is best that all toes and forepaw consciously and actively exert force, especially the big toe so that the foot arch can participate in exerting force and effectively exercise the muscles of the foot; the heel almost does not touch the ground or just touches the ground lightly, which allows the foot arch to bear most of the weight and increases the weight-bearing efficiency of the foot. (III) Effective control of blood glucose: Good control of blood glucose is the most beneficial measure to reduce complications of diabetes, and control of glycated hemoglobin within the normal range can reduce the occurrence of complications. (IV) Actively prevent foot trauma: form the habit of checking the foot every day; quit smoking; soak your feet in warm water for a limited period each day. Trim toenails correctly and select footwear appropriately; do not walk barefoot, barefoot shoes; keep skin clean and moist, and prevent dry itching and scratches.
This is the first early clinical predictive model of DFU. Previous articles, in recent decades, have been limited to identifying foot risk factors in patients with diabetes, which are interrelated; separate, single, or multiple factors cannot facilitate accurate diagnosis. In this study, by collecting data from nearly a thousand patients and integrating significant risk factors, we made a predictive scoring model. Those with high scores can take early DFU preventive measures, such as foot care, greatly reducing the incidence of amputation and related financial burden.
There were limitations to this study: (I) The reliability of the nomogram was not confirmed and requires confirmation via prospective studies. (II) This analysis was based on the data of two single institutions in Guangxi, China. It is necessary to verify the results through comparison with those of other centers. (III) Although the nomogram had good predictive accuracy (cutoff point of 180), there were still false positives and false negatives in the training and validation cohorts. (IV) Using the nomogram to estimate the risk of secondary DFUs in patients with diabetes to guide clinical treatment is a new concept. Diabetes has other complications and other factors that were not included in the model, such as the general performance of patients and the functional reserve of the liver, which should also be considered in the future. If major clinical decisions are to be made, then larger sample sizes are needed for more in-depth research. (V) The model was based on clinical data, and the use of angiography and other imaging techniques may further improve accuracy, but this needs the cooperation of patients and financial support.
A nomogram was constructed by combining 12 risk factors of DFU. The model provides a reliable prediction of the risk of DFUs in patients with T2D.
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
This study was approved by the Ethics Committees of Guangxi medical university (No. 20220144), and individual consent for this retrospective analysis was waived.
Conception and design: MJ, FG, and MG; Administrative support: ZB and SS; Provision of study materials or patients: BQ and YW; Collection and assembly of data: HD, XY, DH, XC, and SL; Data analysis and interpretation: MJ, FG, and MG; Manuscript writing: All authors; Final approval of manuscript: All authors.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
1. Tai N, Wong FS, Wen L. The Role of Gut Microbiota in the Development of Type 1, Type 2 Diabetes Mellitus and Obesity. Rev Endocr Metab Disord (2015) 16:55–65. doi: 10.1007/s11154-015-9309-0
2. Deshpande AD, Harris-Hayes M, Schootman M. Epidemiology of Diabetes and Diabetes-Related Complications. Phys Ther (2008) 88:1254–64. doi: 10.2522/ptj.20080020
3. Hobizal KB, Wukich DK. Diabetic Foot Infections: Current Concept Review. Diabetes Foot Ankle (2012) 3:18409. doi: 10.3402/dfa.v3i0.18409
4. Hittel N, Donnelly R. Treating Peripheral Arterial Disease in Patients With Diabetes. Diabetes Obes Metab (2002) 4 Suppl 2:S26–31. doi: 10.1046/j.1463-1326.2002.0040s2s26.x
5. Poulsen MK, Nybo M, Dahl J, Hosbond S, Henriksen JE. Plasma Osteoprotegerin is Related to Carotid and Peripheral Arterial Disease, But Not to Myocardial Ischemia in Type 2 Diabetes Mellitus. Cardiovasc Diabetol (2011) 10:76. doi: 10.1186/1475-2840-10-76
6. Armstrong DG, Boulton AJM, Bus SA. Diabetic Foot Ulcers and Their Recurrence. N Engl J Med (2017) 376:2367–75. doi: 10.1056/NEJMra1615439
7. Singh R, Kishore L, Kaur N. Diabetic Peripheral Neuropathy: Current Perspective and Future Directions. Pharmacol Res (2014) 80:21–35. doi: 10.1016/j.phrs.2013.12.005
8. Tuttolomondo A, Maida C, Pinto A. Diabetic Foot Syndrome: Immune-Inflammatory Features as Possible Cardiovascular Markers in Diabetes. World J Orthop (2015) 6:62–76. doi: 10.5312/wjo.v6.i1.62
9. Narayan KM, Gregg EW, Fagot-Campagna A, Engelgau MM, Vinicor F. Diabetes–a Common, Growing, Serious, Costly, and Potentially Preventable Public Health Problem. Diabetes Res Clin Pract (2000) 50 Suppl 2:S77–84. doi: 10.1016/S0168-8227(00)00183-2
10. Grodsinsky C, Nibler JG, Brush BE. Complications of Peptic Ulcer Disease in Geriatric Patients. J Am Geriatr Soc (1973) 21:398–404. doi: 10.1111/j.1532-5415.1973.tb01698.x
11. Zhang P, Lu J, Jing Y, Tang S, Zhu D, Bi Y. Global Epidemiology of Diabetic Foot Ulceration: A Systematic Review and Meta-Analysis †. Ann Med (2017) 49:106–16. doi: 10.1080/07853890.2016.1231932
12. Madanchi N, Tabatabaei-Malazy O, Pajouhi M, Heshmat R, Larijani B, Mohajeri-Tehrani MR. Who are Diabetic Foot Patients? A Descriptive Study on 873 Patients. J Diabetes Metab Disord (2013) 12:36. doi: 10.1186/2251-6581-12-36
13. Abraham J, Golubnitschaja O, Akhmetov I, Akhmetov I, Andrews RJ, Quintana L, Baban B, et al. EPMA-World Congress 2015. Epma J (2016) 7:9. doi: 10.1186/s13167-016-0054-6
14. Rice JB, Desai U, Cummings AK, Birnbaum HG, Skornicki M, Parsons NB. Burden of Diabetic Foot Ulcers for Medicare and Private Insurers. Diabetes Care (2014) 37:651–8. doi: 10.2337/dc13-2176
15. Sohrabi K, Belczyk R. Surgical Treatment of Diabetic Foot and Ankle Osteomyelitis. Clin Podiatr Med Surg (2022) 39:307–19. doi: 10.1016/j.cpm.2021.11.003
16. He A, Ding X, Huang J, Luo X, Meng J, Cao Y, et al. [Clinical Characteristics and Risk Factors of Lower Extremity Arterial Disease in Patients With Diabetic Foot Ulcer].[J]. .Nan Fang Yi Ke Da Xue Xue Bao (2022) 42:604–9. doi: 10.12122/j.issn.1673-4254.2022.04.18
17. Mariadoss AVA, Sivakumar AS, Lee C, Kim SJ. Diabetes Mellitus and Diabetic Foot Ulcer: Etiology, Biochemical and Molecular Based Treatment Strategies via Gene and Nanotherapy. BioMed Pharmacother (2022) 151:113–34. doi: 10.1016/j.biopha.2022.113134
18. Macdonald KE, Boeckh S, Stacey HJ, Jones JD. The Microbiology of Diabetic Foot Infections: A Meta-Analysis. BMC Infect Dis (2021) 21:770. doi: 10.1186/s12879-021-06516-7
19. Meaney B. Diabetic Foot Care: Prevention is Better Than Cure. J Ren Care (2012) 38 Suppl 1:90–8. doi: 10.1111/j.1755-6686.2012.00276.x
20. Wu SC, Driver VR, Wrobel JS, Armstrong DG. Foot Ulcers in the Diabetic Patient, Prevention and Treatment. Vasc Health Risk Manag (2007) 3:65–76.
21. Chiwanga FS, Njelekela MA. Diabetic Foot: Prevalence, Knowledge, and Foot Self-Care Practices Among Diabetic Patients in Dar Es Salaam, Tanzania - a Cross-Sectional Study. J Foot Ankle Res (2015) 8:20. doi: 10.1186/s13047-015-0080-y
22. Arad Y, Fonseca V, Peters A, Vinik A. Beyond the Monofilament for the Insensate Diabetic Foot: A Systematic Review of Randomized Trials to Prevent the Occurrence of Plantar Foot Ulcers in Patients With Diabetes. Diabetes Care (2011) 34:1041–6. doi: 10.2337/dc10-1666
23. Zhou ZR, Wang WW, Li Y, Jin KR, Wang XY, Wang ZW, et al. In-Depth Mining of Clinical Data: The Construction of Clinical Prediction Model With R. Ann Transl Med (2019) 7:796. doi: 10.21037/atm.2019.08.63
24. Li WY, Song YN, Chen K, Ying J, Zheng Z, Qiao S, et al. Predictive Model and Risk Analysis for Diabetic Retinopathy Using Machine Learning: A Retrospective Cohort Study in China. BMJ Open (2021) 11:e050989. doi: 10.1136/bmjopen-2021-050989
25. Zaidi SMA, Chandola V, Ibrahim M, Romanski B, Mastrandrea LD, Singh T. Multi-Step Ahead Predictive Model for Blood Glucose Concentrations of Type-1 Diabetic Patients. Sci Rep (2021) 11:24332. doi: 10.1038/s41598-021-03341-5
26. Lei Z, Li J, Wu D, Xia Y, Wang Q, Si A, et al. Nomogram for Preoperative Estimation of Microvascular Invasion Risk in Hepatitis B Virus-Related Hepatocellular Carcinoma Within the Milan Criteria. JAMA Surg (2016) 151:356–63. doi: 10.1001/jamasurg.2015.4257
27. Price P. The Diabetic Foot: Quality of Life. Clin Infect Dis (2004) 39 Suppl 2:S129–31. doi: 10.1086/383274
28. Yazdanpanah L, Nasiri M, Adarvishi S, et al. Literature Review on the Management of Diabetic Foot Ulcer. World J Diabetes (2015) 6:37–53. doi: 10.4239/wjd.v6.i1.37
29. Bus SA, van Netten JJ, Lavery LA, Monteiro-Soares M, Rasmussen A, Jubiz Y, et al. IWGDF Guidance on the Prevention of Foot Ulcers in at-Risk Patients With Diabetes. Diabetes Metab Res Rev (2016) 32 Suppl 1:16–24. doi: 10.1002/dmrr.2696
30. Sari Y, Upoyo AS, Isworo A, Taufik A, Sumeru A, Anandari D, et al. Foot Self-Care Behavior and its Predictors in Diabetic Patients in Indonesia. BMC Res Notes (2020) 13:38. doi: 10.1186/s13104-020-4903-y
31. Normahani P, Poushpas S, Alaa M, Bravis V, Aslam M, Jaffer U. Study Protocol for a Comparative Diagnostic Accuracy Study of Bedside Tests Used to Detect Arterial Disease in Diabetes: TEsting for Arterial Disease in Diabetes (TrEAD) Study. BMJ Open (2020) 10:e033753. doi: 10.1136/bmjopen-2019-033753
32. Huang ZH, Li SQ, Kou Y, Huang L, Yu T, Hu A. Risk Factors for the Recurrence of Diabetic Foot Ulcers Among Diabetic Patients: A Meta-Analysis. Int Wound J (2019) 16:1373–82. doi: 10.1111/iwj.13200
33. Nanwani B, Shankar P, Kumar R, Shaukat F. Risk Factors of Diabetic Foot Amputation in Pakistani Type II Diabetes Individuals. Cureus (2019) 11:e4795. doi: 10.7759/cureus.4795
34. Zubair M, Malik A, Ahmad J. Correlation of HbA1c and S. Creatinine Along With Microbiological Profiling of Infected Ulcers; Cases of Diabetic Patients. Diabetes Metab Syndr (2019) 13:30–4. doi: 10.1016/j.dsx.2018.08.011
35. Ylitalo KR, Sowers M, Heeringa S. Peripheral Vascular Disease and Peripheral Neuropathy in Individuals With Cardiometabolic Clustering and Obesity: National Health and Nutrition Examination Survey 2001-2004. Diabetes Care (2011) 34:1642–7. doi: 10.2337/dc10-2150
36. Monteiro-Soares M, Vale-Lima J, Martiniano J, Pinheiro-Torres S, Dias V, Boyko EJ. A Systematic Review With Meta-Analysis of the Impact of Access and Quality of Diabetic Foot Care Delivery in Preventing Lower Extremity Amputation. J Diabetes Complications (2021) 35:107837. doi: 10.1016/j.jdiacomp.2020.107837
Keywords: diabetic foot ulcer (DFU), nomogram, risk factor, prediction model, dysmetabolism
Citation: Jiang M, Gan F, Gan M, Deng H, Chen X, Yuan X, Huang D, Liu S, Qin B, Wei Y, Su S and Bo Z (2022) Predicting the Risk of Diabetic Foot Ulcers From Diabetics With Dysmetabolism: A Retrospective Clinical Trial. Front. Endocrinol. 13:929864. doi: 10.3389/fendo.2022.929864
Received: 27 April 2022; Accepted: 09 June 2022;
Published: 12 July 2022.
Edited by:
Ihtisham Bukhari, Henan Provincial People’s Hospital, ChinaReviewed by:
Bing Li, Liuzhou Workers Hospital, ChinaCopyright © 2022 Jiang, Gan, Gan, Deng, Chen, Yuan, Huang, Liu, Qin, Wei, Su and Bo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhandong Bo, ZHJyYm96aGFuZG9uZ0AxMjYuY29t; Shanggui Su, ZHJyc3VzaGFuZ2d1aUAxMjYuY29t
†ORCID: Mingyang Jiang, orcid.org/0000-0002-9207-8782
‡These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.