
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Endocrinol., 02 August 2022
Sec. Thyroid Endocrinology
Volume 13 - 2022 | https://doi.org/10.3389/fendo.2022.929651
This article is part of the Research TopicThermal Ablation of Thyroid NodulesView all 8 articles
 Min Ding1†
Min Ding1† Gao-Song Wu2†
Gao-Song Wu2† Jian-Hua Gu3,4†
Jian-Hua Gu3,4† Dong-Jie Shen5
Dong-Jie Shen5 Rui Zhou2
Rui Zhou2 Ying Liu6
Ying Liu6 Rong-Li Xie5
Rong-Li Xie5 Shu-Rong Wang6,7*
Shu-Rong Wang6,7* Hong-Cheng Wang8*
Hong-Cheng Wang8* Jian Fei9,10,11,12*
Jian Fei9,10,11,12*Background: The incidence of papillary thyroid carcinoma (PTC) has rapidly increased in recent years. Microwave ablation (MWA) was proposed as an alternative treatment for PTC. This study aimed to investigate the efficacy and safety of MWA by exploring the postoperative pathology results of post-ablation lesions in patients with PTC.
Methods: This study retrospectively analyzed data from 12 patients who underwent thyroid surgery after MWA treatment for primary PTC between January 2015 and November 2021 in six hospitals.
Results: The average age of the 12 patients (8 female) was 45.3 ± 9.7 years. There was one patient with PTC (size > 1 cm) and 11 patients with micro-PTC (size ≤ 1 cm), of which eight patients had unifocal micro-PTC and three patients had multifocal micro-PTC. A total of 17 tumor foci with mean size of 6.2 ± 2.6 mm were treated by MWA. The median interval time between MWA and surgery was 6.6 months (range: 0.4–21.9 months). Intraoperatively, adherence to the anterior cervical muscle group was observed in three cases (3/12). Upon postoperative pathologic examination, all the post-ablation lesions of the eight unifocal micro-PTC and two multifocal micro-PTC showed no residual carcinomas. Outside the ablation zone, PTCs were detected in three cases, including two of the eight patients with unifocal micro-PTC and one of the three patients with multifocal micro-PTC. Cervical lymph node metastases were detected in seven patients (7/12).
Conclusion: MWA was feasible for the treatment of primary unifocal low-risk micro-PTC (T1aN0M0) with good efficacy and safety. However, the use of MWA for treating PTC (size > 1 cm) and multifocal micro-PTC remains controversial.
Papillary thyroid carcinoma (PTC) is the most common subtype of thyroid cancer, whose incidence has increased rapidly worldwide in recent years (1). Currently, thyroid surgery is still the first line treatment option for patients with PTC (2). However, most PTCs, especially papillary thyroid microcarcinoma (micro-PTC) with size ≤ 1 cm, often had an indolent clinical course and excellent prognosis, and there were ongoing concerns on whether these cancers were overtreated.
Recently, thermal ablation (TA) was proposed for the treatment of primary PTC, especially low-risk micro-PTC, which has triggered an extensive discussion (3, 4). The low-risk micro-PTC is defined as micro-PTC which showed no lymph node or distant metastasis at diagnosis, no thyroid capsule contact, no extrathyroidal invasion, and no vascular invasion (3). TA, including microwave ablation (MWA), laser ablation (LA), and radiofrequency ablation (RFA), can significantly inactivate local cells through protein denaturation due to the extensive thermal effect. Nowadays, it has been widely used in treating benign thyroid nodules and recurrent micro-PTC with good efficacy and safety (2, 5). The main concerns regarding the treatment of primary PTC are locally incomplete elimination and potential cervical lymph node metastasis, which have been reported in previous literatures (6, 7). Several studies have also reported the efficacy and safety of TA in the treatment of low-risk micro-PTC (8, 9). However, the application of TA for primary PTC remains controversial.
To investigate the efficacy and safety of MWA on primary PTC, we collected data from 12 patients who underwent surgery after MWA treatment for primary PTC and provided insight into the pathological results of post-ablation lesions.
The inclusion criteria were as follows: (i) patients diagnosed as PTC by ultrasound (US) and fine-needle aspiration biopsy (FNAB) before MWA treatment, (ii) patients who underwent further surgical treatment after MWA, (iii) patients who tolerated surgery, (iv) provided written inform consent for surgery, and (v) those with available postoperative pathological results. We retrospectively analyzed data from 12 patients who underwent thyroid surgery after MWA treatment for primary PTC between January 2015 and November 2021 in six hospitals. The results of thyroid US were recorded according to the risk stratification of the Thyroid Imaging Reporting and Data System (TI-RADS) (10). The outcomes of FNAB were reported according to the 2017 Bethesda System for Reporting Thyroid Cytopathology (11). All the procedures were implemented based on the principle of the Declaration of Helsinki. The requirement to obtain patients’ consent was waived by the relevant institutional ethic committee.
All procedures were performed by doctors with more than five years of ablation experience. The MWA system used in this study was Nanjing Greatwall MTI-5AT microwave therapeutic instrument with a frequency of 2450MHz ± 30MHz and transmitting power 0-120W which could be adjusted continuously. A 16-gauge cooled-tip needle was used for treatment. The distance between the electrode and the needle tip was 3 mm. Before MWA, contrast-enhanced US (CEUS) examination was performed to evaluate the extent of the tumor and its enhancement mode. High-resolution US was performed to evaluate the relationship between the tumor and critical cervical structures to determine the best puncture site. The patients were placed in a supine position with the neck hyperextended. After skin disinfection, 1% lidocaine was applied for local anesthesia. A mixture of normal saline and lidocaine was injected outside the thyroid capsule according to the nodule’s location to form a ‘liquid isolation zone’ to protect important cervical structures from heat damage. With the guidance of US, a microwave antenna was inserted into the tumor. The output power was 30-40W. The moving-shot or fixed-applicator technique was used for the procedure. The ablated area should exceed the tumor edge until tumors were completely covered by a hyperechoic area. After MWA, CEUS was performed to evaluate the effect of MWA.
The extent of surgery was dependent on the location of the primary PTC before the MWA treatment, preoperative thyroid US and FNA results, and intraoperative freezing pathology results. Lobectomy was performed in patients with unilateral PTC, while total thyroidectomy or near-total thyroidectomy was performed in patients with bilateral PTC, isthmic PTC, and unilateral PTC accompanied with contralateral newly diagnosed carcinomas. All patients received routine ipsilateral central compartment neck dissection. Lateral neck compartment neck dissection was performed only in patients with biopsy-proven metastasis to the lateral cervical lymph nodes (levels II–V).
Statistical analysis was performed using IBM SPSS Version 23.0. Data were expressed as mean ± standard deviation for continuous variables and frequency (percentage) for categorical variables.
In this retrospective study, 12 patients (8 females) with an average age of 45.3 ± 9.7 years (range: 33–60 years) were enrolled. Among the 12 patients, one patient (case 11) had multifocal PTC, with mean size > 1 cm, and 11 patients (case 1–10, 12) had micro-PTC (size ≤ 1 cm), of which eight patients (case 2, 4–10) had unifocal micro-PTC and three patients (case 1, 3, 12) had multifocal micro-PTC. A total of 17 tumor foci with a mean size of 6.2 ± 2.6 mm (range 3–11 mm) were treated by MWA. All cases showed no evidence of cervical lymph node or distant metastasis, thyroid capsule contact, extrathyroidal invasion or vascular invasion based on US and FNAB evaluation before MWA treatment. The clinical tumor-node-metastasis stage (cTNM) of all patients was T1aN0M0 prior to MWA except for case 11 (T1bN0M0). The median interval time between MWA and surgery was 6.6 months (range: 0.4–21.9 months) (Table 1).
The reasons for further surgical treatment could be divided into five categories: (i) new thyroid carcinoma diagnosed by FNA (case 4) or suspected recurrence in the post-ablation zone detected by US (case 1 and 6); (ii) clinically evident or suspected cervical lymph node metastases during follow-up (5/12, case 4, 7-9, 11), among which three cases (case 4, 7, 8) were diagnosed as lateral cervical lymph node metastases; (iii) patients were worried about the efficacy of MWA and preferred to undergo further surgery for the definite diagnosis of post-ablation lesions (3/12, case 2, 5, 10); (iv) Case 3 developed nodular goiter, which increased in size after MWA and resulted in local compression; (v) MWA was an alternative treatment for patients who could not tolerate surgery after evaluation, and one patient (case 12) preferred to undergo further surgical treatment after her general condition improved.
None of the cases developed hoarseness after MWA treatment or showed recurrent laryngeal nerves involvement during surgery. Post-ablation lesions were found to adhere to the anterior cervical muscle group in three cases intraoperatively (3/12; case 5, 6, 9). Four patients underwent total thyroidectomy (case 1, 4, 11, 12), and three patients underwent near-total thyroidectomy (case 3, 5, 9). The remaining patients (5/12) underwent lobe thyroidectomy. All unifocal micro-PTC post-ablation lesions (8/12; case 2, 4–10), including case 6, showed no residual carcinomas according to the postoperative pathology results (Figure 1). In addition, two multifocal micro-PTCs (case 1, 3) were also disease free in the post-ablation area (Figure 2). Residual tumors were detected in two cases (2/12; case 11, 12) (Figure 3). Outside the ablation zone, new PTCs were detected in three cases, two (case 4, 7) of the eight patients with unifocal micro-PTC and one (case 12) of the three patients with multifocal micro-PTC. Seven cases (7/12; case 1, 4, 7–9, 11, 12) developed cervical lymph node metastases, of which lateral cervical lymph node metastases were found in three cases (case 4, 7, 8) (Table 2). Therefore, three cases (3/12; case 2, 5, 10) were totally disease free after MWA treatment, but still underwent subsequent surgical treatment.
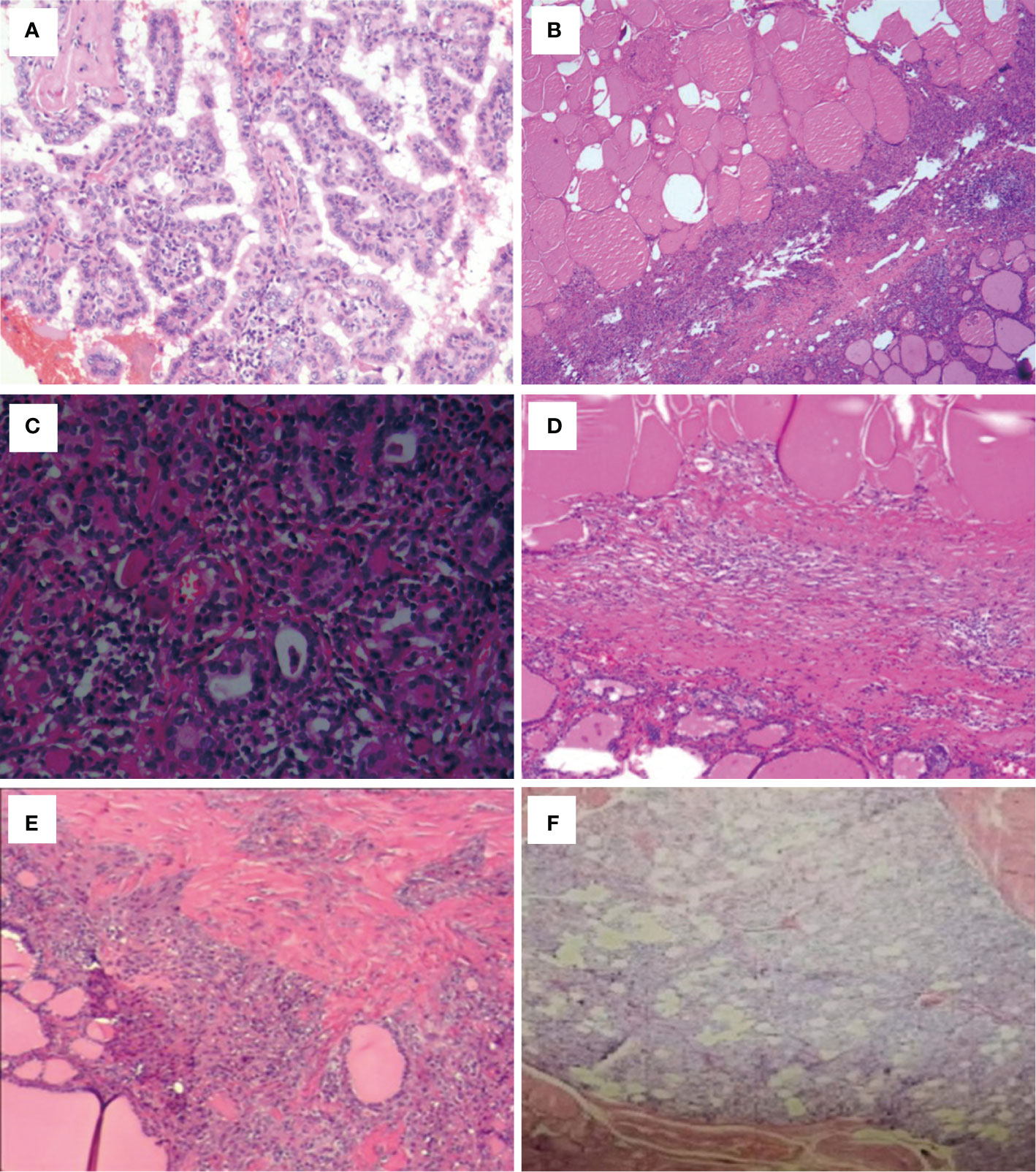
Figure 1 Post-ablation lesions of unifocal micro-PTC showed no residual carcinomas according to the postoperative pathology results (case 4–9). (A) Chronic inflammation in case 4. (B) Chronic inflammation and fibrosis in case 5. (C) Hashimoto’s thyroiditis and fibrosis in case 6. (D) Coagulative necrosis and fibrosis in case 7. (E) Fibrosis and nodular goiter in case 8. (F) Fibrosis and granuloma in case 9.
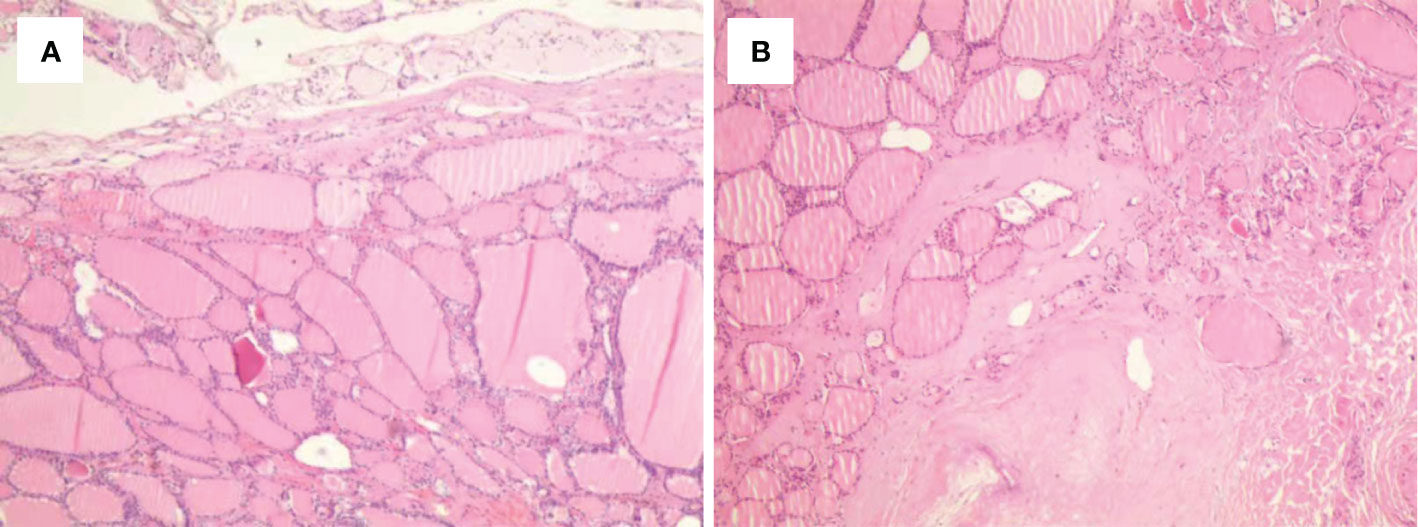
Figure 2 Histological outcomes of the post-ablation lesions in multifocal micro-PTC (case 3). (A, B) Nodular goiter was found in the post-ablation zone of the left (A) and right (B) thyroid lobes.
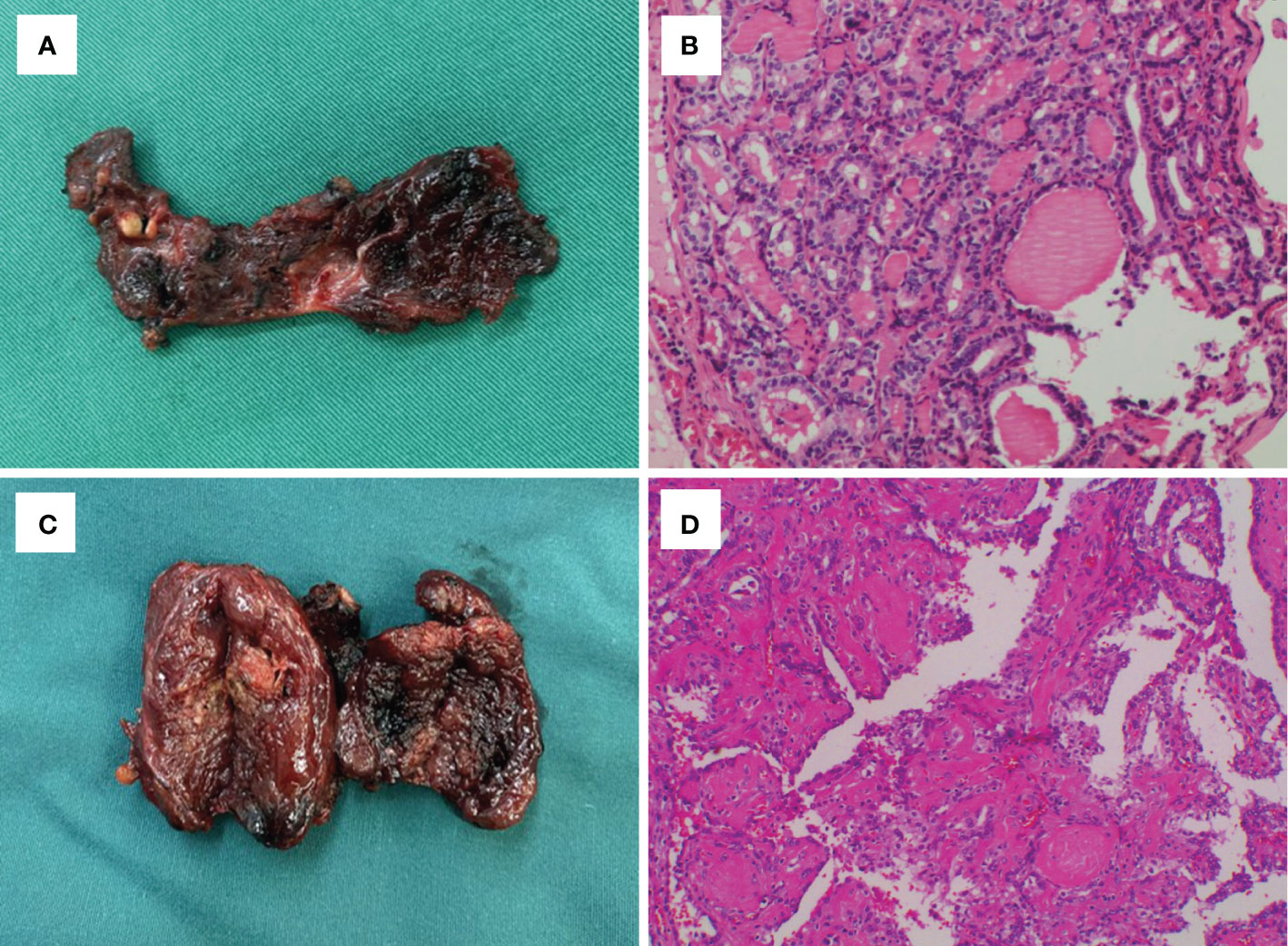
Figure 3 Gross findings and histological outcomes in case 11 (A, B) and case 12 (C, D), which showed tumor recurrence in the post-ablation lesions.
This multicenter retrospective study pathologically confirmed that MWA could achieve complete local tumor elimination for primary unifocal low-risk micro-PTC (T1aN0M0). However, MWA treatment for PTC (size > 1 cm) and multifocal micro-PTC was still limited. To our knowledge, this study is the first to systematically analyze the postoperative pathology results of patients with PTC who underwent MWA treatment. TA was not recommended for the treatment of primary PTC in the 2015 American Thyroid Association (ATA) management guidelines because there was still insufficient medical evidence (2). Nevertheless, the efficacy and safety of TA on primary low-risk micro-PTC have been intensively studied in recent years, which showed excellent outcomes (5, 12). The 2019 Chinese expert consensus also claimed that a prospective clinical study should be conducted in patients with micro-PTC who meet specific criteria (13). Our study demonstrated the efficacy and safety of MWA on unifocal low-risk micro-PTC pathologically, providing evidence for new guidelines on the management of unifocal micro-PTC.
In our study, complete local tumor elimination was found in all unifocal micro-PTC (8/12). Yue et al. (14) conducted a long-term prospective study involving patients with unifocal low-risk micro-PTC treated with MWA. In this study, three patients underwent surgery, and two patients underwent core needle aspiration (CNB) in the follow-up visit. It was confirmed by pathology that malignant cells were absent in all five lesions. Lu et al. (15) also reported that no characteristics of PTC were found by FNA and CNB at the 3 months and 6 months follow-up in 44 patients with unifocal micro-PTC who underwent MWA. In other studies, the local recurrence rate after MWA treatment was also very low (16–18). Therefore, we concluded that MWA treatment was feasible for the complete destruction of unifocal low-risk micro-PTC.
However, local tumor residue after TA treatment for primary PTC was also reported in several previous studies. Ma et al. (6) retrospectively analyzed postoperative pathology data of 12 patients with primary PTC treated with TA and confirmed residual PTCs in all cases pathologically. Similar results were also reported by Sun et al. (7) and Kim et al. (19). The two distinct conclusions may mainly be due to the selection of patients and ablation methods. In the study of Ma et al., most of the cases (9/12) were PTC (size > 1 cm), whereas only two cases (2/12) were multifocal micro-PTC. In addition, some cases (2/12) had clinically evident cervical lymph node metastasis before MWA. Therefore, these patients had more “high-risk factors” than our patients (20). In our study, one case (1/12) was also diagnosed as PTC (size > 1 cm) and showed residual tumor in postoperative pathology. Although a few studies also reported that TA was feasible in treating patients with solitary T1bN0M0 PTC (21, 22), the need to protect surrounding thyroid tissue made it more difficult to completely eliminate local lesions for PTC (size>1 cm) than for micro-PTC (7). The application of MWA in the treatment of PTC (size>1 cm) is still limited and controversial. There were three cases of multifocal micro-PTC in our study, and in two of these cases (2/12), complete tumor elimination was achieved. Regarding multifocal micro-PTC, the main challenges were the potential occult micro-PTC and the tendency of recurrence. Multifocality is also a risk factor of cervical lymph node metastasis (20). The two most recent guidelines did not recommend using TA for treating multifocal micro-PTC (13, 23). Interestingly, the two cases with multifocal micro-PTC that were successfully treated both had one lesion on each lobe. Another case had more than one tumor lesion on one lobe. Sugitani et al. (24) concluded that multiplicity is not associated with tumor enlargement. Whether MWA is feasible for multifocal micro-PTC having one lesion on each lobe is still controversial and needs further research. Regarding the ablation methods, it remains controversial whether MWA is superior to RFA. In hepatic tumors, the potential to completely destroy the tumor tissues may be higher for MWA than for RFA (25). MWA is thought to be less affected by the heat-sink effect, which may be related to local recurrence after RFA (26). However, some studies also reported that RFA and MWA showed comparable results in terms of volume reduction in the treatment of thyroid nodules (27).
In our study, cervical lymph node metastasis was one of the main reasons for further surgical treatment. Moreover, seven cases (7/12) were confirmed to have cervical lymph node metastases by postoperative pathology. The high lymph node metastasis rate may mainly be due to our patients selection criteria. In previous studies, the rate of lymph node metastasis was very low in low-risk micro-PTC treated by TA, ranging from 0.08%–0.4% (8, 14, 16). Li et al. (18) also showed that there was no statistically significant difference in the rate of lymph node metastasis between MWA and surgery. Therefore, MWA is still feasible for the treatment of low-risk micro-PTC, although long-term US monitoring is recommended to detect potential lymph node metastasis. In addition, three cases (3/12) sought for further surgical treatment because of their uncertainty about the efficacy of MWA, and they were all found to be completely disease free after the surgery. Therefore, these patients need more humanistic care to relieve their anxiety. On the other hand, sonologists need to distinguish the ultrasonographic features of thyroid nodules after MWA treatment because they may present suspicious features such as marked hypoechogenicity, heterogeneity, irregular margins, and hardness as measured by elastography, which are misleading and result in anxiety among patients (28).
Regarding the safety of MWA, no complications were noted after MWA treatment in our study. Ablation lesions were found to adhere to the anterior cervical muscle group in three cases, but it did not significantly increase the difficulty of the surgery. The rates of hoarseness were 2.7% and 4.2%, respectively, in the studies by Teng et al. (29) and Li et al. (18). The complications decreased significantly after MWA treatment compared with surgery (18). Therefore, we conclude that MWA is a safe tool for the treatment of micro-PTC.
This study has some limitations. This was merely a cases series study, and more high-quality medical evidence from studies such as prospective randomized controlled trials is needed to prove the efficacy and safety of MWA. In addition, only 12 patients were enrolled in this study; thus, a larger number of cases is also needed in future studies.
This study demonstrated that MWA could achieve complete local tumor elimination for primary unifocal low-risk micro-PTC (T1aN0M0). After MWA, long-term US monitoring was necessary. It was recommended to undergo further surgery if there was suspected recurrence or lymph node metastasis in the follow-up visit. More humanistic care and professional post-ablation ultrasonography interpretation are needed for patients undergoing MWA. The safety of MWA was also confirmed by postoperative pathology. However, the application of MWA on PTC (size > 1 cm) and multifocal micro-PTC remains controversial.
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding authors.
The studies involving human participants were reviewed and approved by Ruijin Hospital, Shanghai Jiao Tong University School of Medicine. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
MD reviewed the literature and contributed to data analysis and manuscript drafting; G-SW and J-HG were responsible for data collection and the design of the study; D-JS and RZ contributed to conception of the study. R-LX contributed to funding acquisition. JF, S-RW, and H-CW were responsible for the revision of the manuscript, considering and providing important intellectual content; All authors contributed to the article and approved the submitted version.
This work was supported by the Project of Shanghai Municipal Health Commission (Grant No. 20214Y0223) and Medical-Engineering Cross Foundation of Shanghai Jiao Tong University (Grant No. YG2019ZDA17).
We thank Yahui Kong for helping with data collection.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Pellegriti G, Frasca F, Regalbuto C, Squatrito S, Vigneri R. Worldwide increasing incidence of thyroid cancer: update on epidemiology and risk factors. J Cancer Epidemiol (2013) 2013:965212. doi: 10.1155/2013/965212
2. Haugen BR, Alexander EK, Bible KC, Doherty GM, Mandel SJ, Nikiforov YE, et al. 2015 American Thyroid association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: The American thyroid association guidelines task force on thyroid nodules and differentiated thyroid cancer. Thyroid (2016) 26:1–133. doi: 10.1089/thy.2015.0020
3. Ramundo V, Sponziello M, Falcone R, Verrienti A, Filetti S, Durante C, et al. Low-risk papillary thyroid microcarcinoma: Optimal management toward a more conservative approach. J Surg Oncol (2020) 121:958–63. doi: 10.1002/jso.25848
4. Brito JP, Hay ID. Management of papillary thyroid microcarcinoma. Endocrinol Metab Clin North Am (2019) 48:199–213. doi: 10.1016/j.ecl.2018.10.006
5. Bo XW, Lu F, Xu HX, Sun LP, Zhang K. Thermal ablation of benign thyroid nodules and papillary thyroid microcarcinoma. Front Oncol (2020) 10:580431. doi: 10.3389/fonc.2020.580431
6. Ma B, Wei W, Xu W, Wang Y, Guan H, Fan J, et al. Surgical confirmation of incomplete treatment for primary papillary thyroid carcinoma by percutaneous thermal ablation: A retrospective case review and literature review. Thyroid (2018) 28:1134–42. doi: 10.1089/thy.2017.0558
7. Sun W, Zhang H, He L, Zhang T, Wang Z, Dong W, et al. Surgery after ultrasound-guided radiofrequency ablation for papillary thyroid carcinoma in 21 patients: A retrospective study from a single center in China. Med Sci Monit (2020) 26:e928391. doi: 10.12659/msm.928391
8. Cho SJ, Baek JH, Chung SR, Choi YJ, Lee JH. Thermal ablation for small papillary thyroid cancer: A systematic review. Thyroid (2019) 29:1774–83. doi: 10.1089/thy.2019.0377
9. Tong M, Li S, Li Y, Li Y, Feng Y, Che Y. Efficacy and safety of radiofrequency, microwave and laser ablation for treating papillary thyroid microcarcinoma: a systematic review and meta-analysis. Int J Hyperthermia (2019) 36:1278–86. doi: 10.1080/02656736.2019.1700559
10. Tessler FN, Middleton WD, Grant EG, Hoang JK, Berland LL, Teefey SA, et al. ACR thyroid imaging, reporting and data system (TI-RADS): White paper of the ACR TI-RADS committee. J Am Coll Radiol (2017) 14:587–95. doi: 10.1016/j.jacr.2017.01.046
11. Cibas ES, Ali SZ. The 2017 Bethesda system for reporting thyroid cytopathology. Thyroid (2017) 27:1341–6. doi: 10.1089/thy.2017.0500
12. Min Y, Wang X, Chen H, Chen J, Xiang K, Yin G. Thermal ablation for papillary thyroid microcarcinoma: How far we have come? Cancer Manag Res (2020) 12:13369–79. doi: 10.2147/cmar.S287473
13. Xu D, Ge M, Yang A, Cheng R, Sun H, Wang H, et al. Expert consensus workshop report: Guidelines for thermal ablation of thyroid tumors (2019 edition). J Cancer Res Ther (2020) 16:960–6. doi: 10.4103/jcrt.JCRT_558_19
14. Yue WW, Qi L, Wang DD, Yu SJ, Wang XJ, Xu HX, et al. US-Guided microwave ablation of low-risk papillary thyroid microcarcinoma: Longer-term results of a prospective study. J Clin Endocrinol Metab (2020) 105:dgaa128. doi: 10.1210/clinem/dgaa128
15. Lu C, Li X, Chu X, Li R, Li J, Wang J, et al. Clinical effects of microwave ablation in the treatment of low-risk papillary thyroid microcarcinomas and related histopathological changes. Front Endocrinol (2021) 12:751213. doi: 10.3389/fendo.2021.751213
16. Cho SJ, Baek SM, Lim HK, Lee KD, Son JM, Baek JH. Long-term follow-up results of ultrasound-guided radiofrequency ablation for low-risk papillary thyroid microcarcinoma: More than 5-year follow-up for 84 tumors. Thyroid (2020) 30:1745–51. doi: 10.1089/thy.2020.0106
17. Teng DK, Li WH, Du JR, Wang H, Yang DY, Wu XL. Effects of microwave ablation on papillary thyroid microcarcinoma: A five-year follow-up report. Thyroid (2020) 30:1752–8. doi: 10.1089/thy.2020.0049
18. Li J, Liu Y, Liu J, Yang P, Hu X, Qian L. A comparative study of short-term efficacy and safety for thyroid micropapillary carcinoma patients after microwave ablation or surgery. Int J Hyperthermia (2019) 36:640–6. doi: 10.1080/02656736.2019.1626492
19. Kim HY, Ryu WS, Woo SU, Son GS, Lee ES, Lee JB, et al. Primary papillary thyroid carcinoma previously treated incompletely with radiofrequency ablation. J Cancer Res Ther (2010) 6:310–2. doi: 10.4103/0973-1482.73328
20. Wu X, Li B, Zheng C, He X. Predicting factors of lateral neck lymph node metastases in patients with papillary thyroid microcarcinoma. Med (Baltimore) (2019) 98:e16386. doi: 10.1097/md.0000000000016386
21. Cao XJ, Liu J, Zhu YL, Qi L, Liu G, Wang HL, et al. Efficacy and safety of thermal ablation for solitary T1bN0M0 papillary thyroid carcinoma: A multicenter study. J Clin Endocrinol Metab (2021) 106:e573–e81. doi: 10.1210/clinem/dgaa776
22. Xiao J, Zhang M, Zhang Y, Yan L, Lan Y, Zhu Y, et al. Efficacy and safety of ultrasonography-guided radiofrequency ablation for the treatment of T1bN0M0 papillary thyroid carcinoma: a retrospective study. Int J Hyperthermia (2020) 37:392–8. doi: 10.1080/02656736.2020.1752945
23. Mauri G, Hegedüs L, Bandula S, Cazzato RL, Czarniecka A, Dudeck O, et al. European Thyroid association and cardiovascular and interventional radiological society of Europe 2021 clinical practice guideline for the use of minimally invasive treatments in malignant thyroid lesions. Eur Thyroid J (2021) 10:185–97. doi: 10.1159/000516469
24. Sugitani I, Ito Y, Takeuchi D, Nakayama H, Masaki C, Shindo H, et al. Indications and strategy for active surveillance of adult low-risk papillary thyroid microcarcinoma: Consensus statements from the Japan association of endocrine surgery task force on management for papillary thyroid microcarcinoma. Thyroid (2021) 31:183–92. doi: 10.1089/thy.2020.0330
25. Yu J, Liang P, Yu X, Liu F, Chen L, Wang Y. A comparison of microwave ablation and bipolar radiofrequency ablation both with an internally cooled probe: results in ex vivo and in vivo porcine livers. Eur J Radiol (2011) 79:124–30. doi: 10.1016/j.ejrad.2009.12.009
26. Wright AS, Sampson LA, Warner TF, Mahvi DM, Lee FT Jr. Radiofrequency versus microwave ablation in a hepatic porcine model. Radiology (2005) 236:132–9. doi: 10.1148/radiol.2361031249
27. Korkusuz Y, Gröner D, Raczynski N, Relin O, Kingeter Y, Grünwald F, et al. Thermal ablation of thyroid nodules: are radiofrequency ablation, microwave ablation and high intensity focused ultrasound equally safe and effective methods? Eur Radiol (2018) 28:929–35. doi: 10.1007/s00330-017-5039-x
28. Andrioli M, Valcavi R. The peculiar ultrasonographic and elastographic features of thyroid nodules after treatment with laser or radiofrequency: similarities and differences. Endocrine (2014) 47:967–8. doi: 10.1007/s12020-014-0241-y
Keywords: thyroid, papillary carcinoma, microwave ablation, surgery, pathology
Citation: Ding M, Wu G-S, Gu J-H, Shen D-J, Zhou R, Liu Y, Xie R-L, Wang S-R, Wang H-C and Fei J (2022) Pathology confirmation of the efficacy and safety of microwave ablation in papillary thyroid carcinoma. Front. Endocrinol. 13:929651. doi: 10.3389/fendo.2022.929651
Received: 27 April 2022; Accepted: 01 July 2022;
Published: 02 August 2022.
Edited by:
Hui Wang, Jilin University, ChinaReviewed by:
Wen Hui Li, Jilin University, ChinaCopyright © 2022 Ding, Wu, Gu, Shen, Zhou, Liu, Xie, Wang, Wang and Fei. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jian Fei, RmVpamlhbkBob3RtYWlsLmNvbQ==; Shu-Rong Wang, d3NyNzc2MjgwOEBob3RtYWlsLmNvbQ==; Hong-Cheng Wang, d2FuZ2hjMTk2NkAxNjMuY29t
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.