- 1Department of Ultrasound, Affiliated People’s Hospital of Jiangsu University, Zhenjiang, China
- 2School of Medicine, Jiangsu University, Zhenjiang, China
- 3Department of Ultrasound, Jiangsu Hospital of Integrated Traditional Chinese and Western Medicine, Nanjing, China
- 4Research and Development Center, Hangzhou D.A. Medical Laboratory, Hangzhou, China
- 5Nanjing D.A. Medical Laboratory, Nanjing, China
- 6Key Laboratory of Digital Technology in Medical Diagnostics of Zhejiang Province, Dian Diagnostics Group Co., Ltd., Hangzhou, China
- 7Department of Medicine, Zhejiang Digena Diagnosis Technology CO., LTD, Zhejiang, China
- 8Department of Ultrasound, The Second Affiliated Hospital of Nanjing Medical University, Nanjing, China
Mutations in the B-Raf proto-oncogene, serine/threonine kinase (BRAF), have been linked to a variety of solid tumors such as papillary thyroid carcinoma. The purpose of this study was to compare the DP-TOF, a DNA mass spectroscopy (MS) platform, and next-generation sequencing (NGS) methods for detecting multiple-gene mutations (including BRAFV600E) in thyroid nodule fine-needle aspiration fluid. In this study, we collected samples from 93 patients who had previously undergone NGS detection and had sufficient DNA samples remaining. The MS method was used to detect multiple-gene mutations (including BRAFV600E) in DNA remaining samples. NGS detection method was used as the standard. The MS method’s overall sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) were 95.8%, 100%, 100%, and 88%, respectively in BRAFV600E gene mutation detection. With a kappa-value of 0.92 (95%CI 0.82–0.99), the level of agreement between these methods was incredibly high. Furthermore, when compared to NGS in multiple-gene detection, the MS method demonstrated higher sensitivity and specificity, 82.9% and 100%, respectively. In addition, we collected the postoperative pathological findings of 50 patients. When the postoperative pathological findings were used as the standard, the MS method demonstrated higher sensitivity and specificity, at 80% and 80%, respectively. Our findings show that the MS method can be used as an inexpensive, accurate, and dependable initial screening method to detect genes mutations and as an adjunct to clinical diagnosis.
Introduction
Papillary thyroid carcinoma is the most common thyroid cancer of the endocrine system, with a relatively slow progression and a high survival rate (1, 2). Papillary thyroid cancer has been on the rise for three decades (3, 4). Fine-needle aspiration (FNA) cytology is the most commonly used method for diagnosing and categorizing thyroid carcinoma (1). Tumor cells in FNA biopsy samples vary in quantity, quality, and purity, making identification and diagnosis difficult (1). BRAFV600E mutation has been established as an important molecular marker for papillary thyroid cancer diagnosis over the last decade, with a frequency of 65–80% (5, 6). As a result, a sensitive and accurate detection method for the BRAFV600E mutation will aid in the early diagnosis of papillary thyroid carcinoma (7). For the detection of BRAFV600E mutations, amplification-refractory mutation system (ARMS) and next-generation sequencing (NGS) are currently used, particularly NGS, which is a sensitive method in FNA samples with few mutant cells. However, NGS is expensive and inappropriate for the initial screening of all clinical patients. As a result, a more sensitive, low-cost, and accurate detection method is required.
The DNA mass spectroscopy (MS) method is a multiplexed medium-throughput ultra-sensitive mutation detection system. This method has been used successfully to detect mutations in patients with solid tumors, with a reported limit-of-detection frequency of 0.5% (8). It is far more sensitive and specific than other clinical methods currently in use. However, the detection of BRAF V600E mutations using MS has not been investigated in papillary thyroid carcinoma FNA samples. In this study, we used MS to detect the BRAFV600E mutation in thyroid nodule FNA samples and compared its performance to that of NGS. Our study demonstrates the clinical significance of MS in the early detection of thyroid carcinoma.
Materials and methods
Subjects and study design
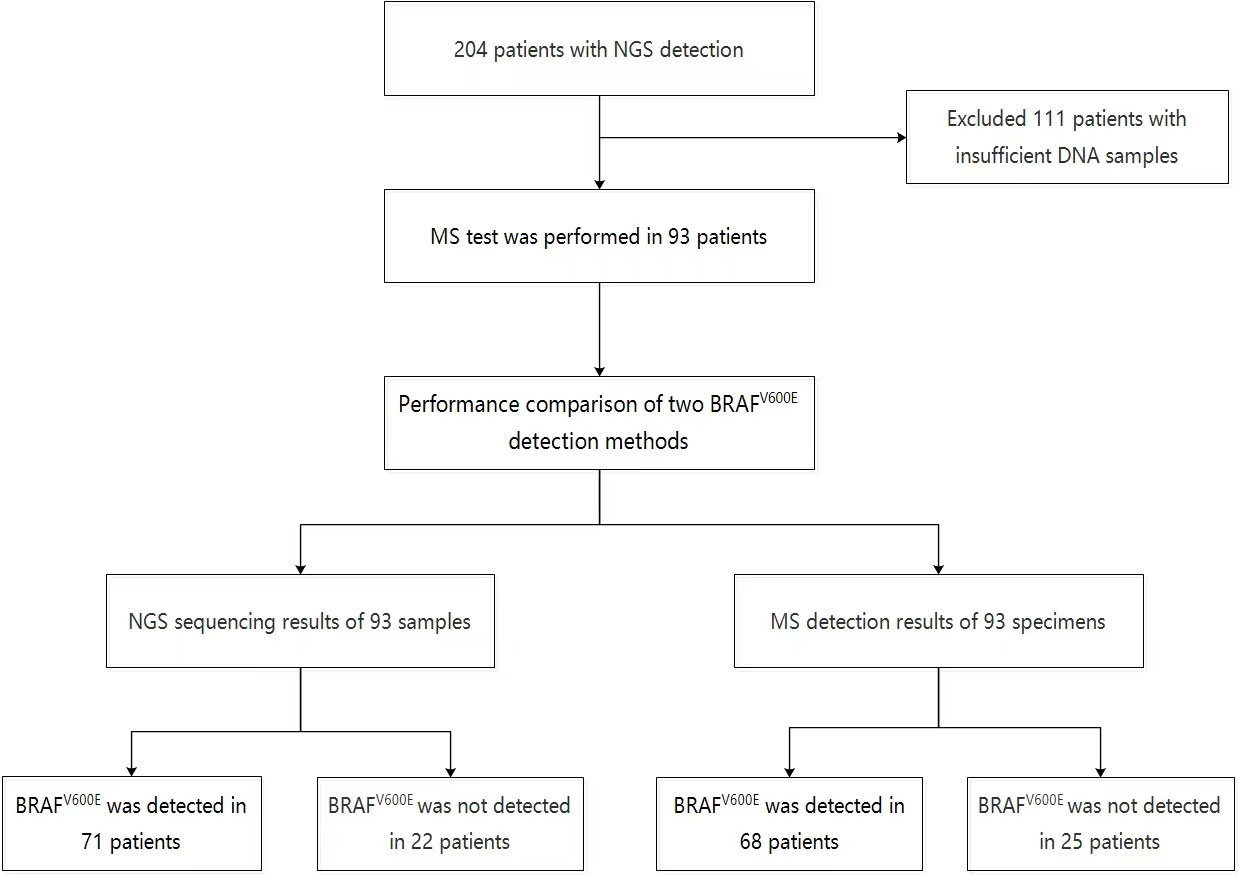
From January 2020 to January 2022, 204 patients with thyroid nodules who underwent thyroid ultrasound examination and next-generation sequencing (NGS) at Jiangsu Hospital of Integrated Traditional Chinese and Western Medicine and Jiangsu University Affiliated People’s Hospital were analyzed retrospectively. The study then enrolled 93 patients who still had enough DNA samples remaining after next-generation sequencing (NGS) (Fig 1). This study was approved by the Ethics Committee of the two hospitals, and all patients provided written informed consent.
DNA extraction
The QIAamp DNA Mini Kit (QIAGEN, Germany) was used to extract genomic DNA from thyroid FNA samples, and DNA concentrations were measured using the Qubit (Thermo Fisher Scientific, Waltham, USA).
DNA sequencing by NGS and MS
A custom-designed NGS panel containing 11 cancer-associated genes, including BRAFV600E, KRAS, NRAS, HRAS, TERT, TP53, RET, NTRK1, NTRK3, PAX8, and THADA, was used to perform comprehensive genomic profiling. TruSeq DNA Library Preparation Kit protocols were used to create genomic DNA sequencing libraries. DNA sequencing was carried out on an Illumina CN 500 sequencing system (San Diego, CA). BWA (a Burrows-Wheeler aligner) (9) was used to align the reads to the human genome build GRCh37. MuTect2 (3.4-46-gbc02625) (10) was used to identify single nucleotide variants (SNVs), while GATK was used to identify small insertions and deletions (SIDs) (Indels). The integrative genomics viewer browser was used to validate all final candidate variants.
We created an MS panel with four gene assays: BRAFV600E, TERT, TP53, and RET. All four genes are common in papillary thyroid carcinoma (PTC). BRAFV600E and RET were important molecular markers of PTC (1, 11, 12). TERT and TP53 were found to be associated with high aggressiveness (12, 13). The remaining DNA samples from NGS sequencing were used for MS detection. The DNA concentrations were determined using Qubit 3.0. (Thermo Fisher Scientific, Waltham, USA). The production was then carried out at Zhejiang Digena R&D Center, on a high-throughput DP-TOF MassARRAY platform with data analyzed using Typer 4.0 and plate manager 1.0 software.
Statistical analyses
SPSS 22.0 (SPSS Inc., USA) was used to perform all statistical analyses. The inter-rater agreement (kappa-value) test was used to assess the degree of agreement between the MS and NGS methods. A p-value <0.05 was considered statistically significant.
Results
Comparison of NGS and MS for detection of BRAF V600E mutation in FNA cytology biopsy samples
To compare the efficacy of NGS and MS in detecting BRAFV600E mutation in FNA, samples from patients who had previously undergone NGS detection were collected, and 93 patients with sufficient DNA samples remaining were enrolled in the study, the 93 samples were also detected by MS in this study. In the NGS analysis of 93 patients, 71 were found to have BRAFV600E mutation (69 with only BRAFV600E, one with KRAS and BRAFV600E, and one with TERT and BRAFV600E), 11 with other mutations including KRAS, NRAS, HRAS, and NTRK3, and 11 without gene mutation. In the MS detection of these patients, 68 were found to have BRAFV600E and no gene mutation was detected in 25 cases (Figure 1).
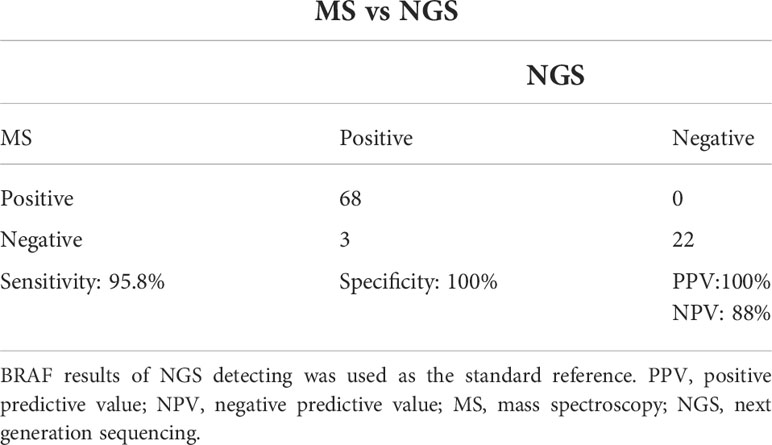
The comparative analysis of NGS and MS for the Detection of Molecular Mutations was performed using the BRAFV600E mutation status established from NGS detection as the standard. The MS method’s overall sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) were 95.8%, 100%, 100%, and 88%, respectively (Tables 1, 2). Furthermore, with a kappa-value of 0.92 (95% confidence interval (CI) 0.82–0.99, p<0.001), the level of agreement between the two methods was very high. The two methods had a 96.8% (90/93) coincidence rate, with three patients missed in MS methods (Table 2). According to NGS, the frequency of BRAFV600E in the three patients was 1.48%, 0.88%, and 0.75%, respectively. The reason for tracing was that the amount of remaining DNA was insufficient, resulting in insufficient initial abundance and a negative MS test.
Comparison of NGS and MS for detection of multiple gene mutations in FNA cytology biopsy samples
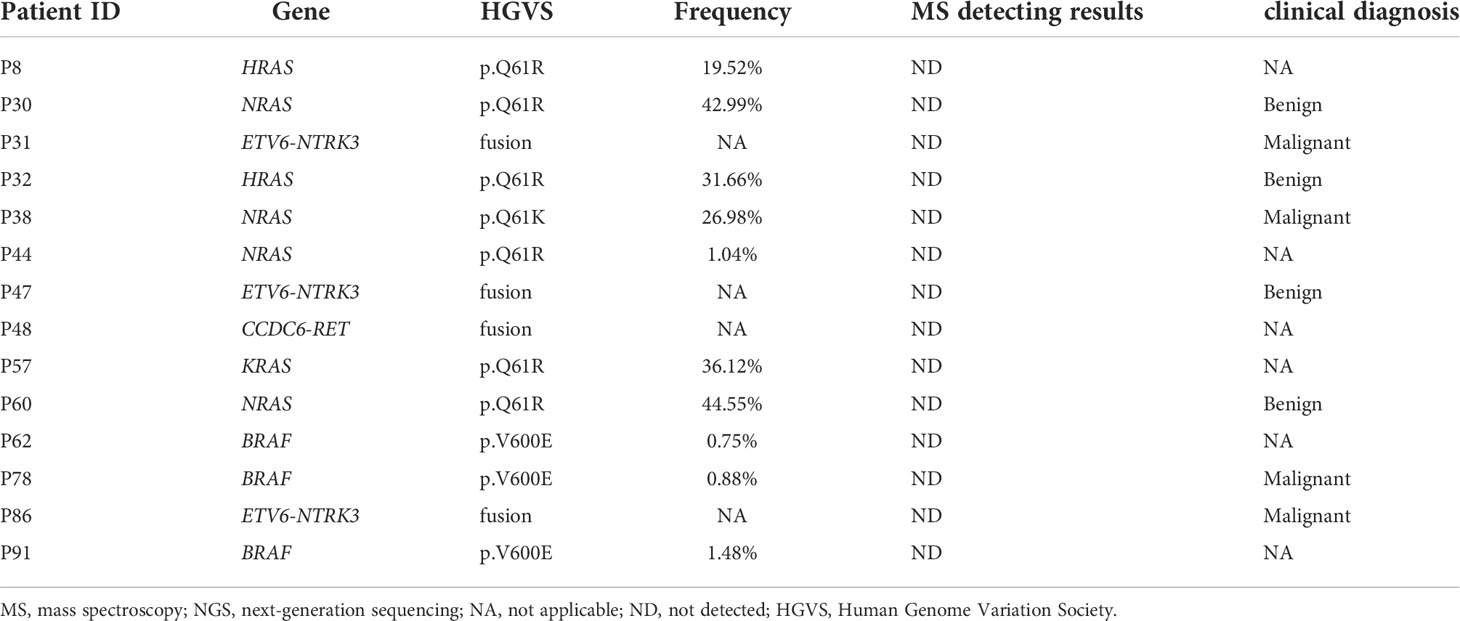
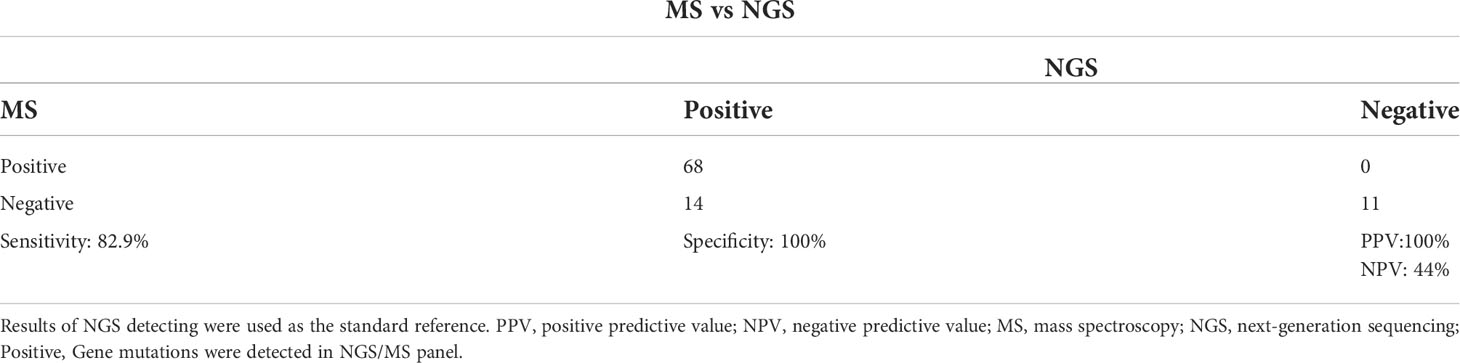
The MS panel in this study examined mutations in four genes, including TP53, TERT, and RET, in addition to BRAFV600E. The NGS panel examined 11 different genes for mutations. The results of NGS and MS were compared in order to compare their efficacy in detecting multiple gene mutations. The two methods had an 84.9% (79/93) coincidence rate. The sensitivity, specificity, PPV, and NPV of the MS method were 82.9%, 100%, 100%, and 44%, respectively, as shown in Table 3. The level of agreement between the two methods was moderate, with a kappa-value of 0.54 (95% CI 0.34–0.73, p<0.001).
In comparison to the NGS method, there were no false positives reported with the MS method. However, due to panel limitations, 11 patients with positive genes outside of the MS panel were found to be negative (Table 3). In these 11 patients, two HRAS (all p.Q61R) mutations were found, one KRAS (p.Q61R) mutation, four NRAS (3 p.Q61R and 1 p.Q61K) mutations, three ETV6-NTRK3 fusions, and one CCDC6-RET fusion (Table 3). Furthermore, two of the 93 patients had more than one gene mutation (one with KRAS and BRAF, the other with TERT and BRAFV600E). MS methods were also used to detect patients who had BRAFV600E and TERT mutations. This result demonstrated that the MS method was capable of detecting multiple genes.
Relationship between the NGS or MS detection results and postoperative pathological findings
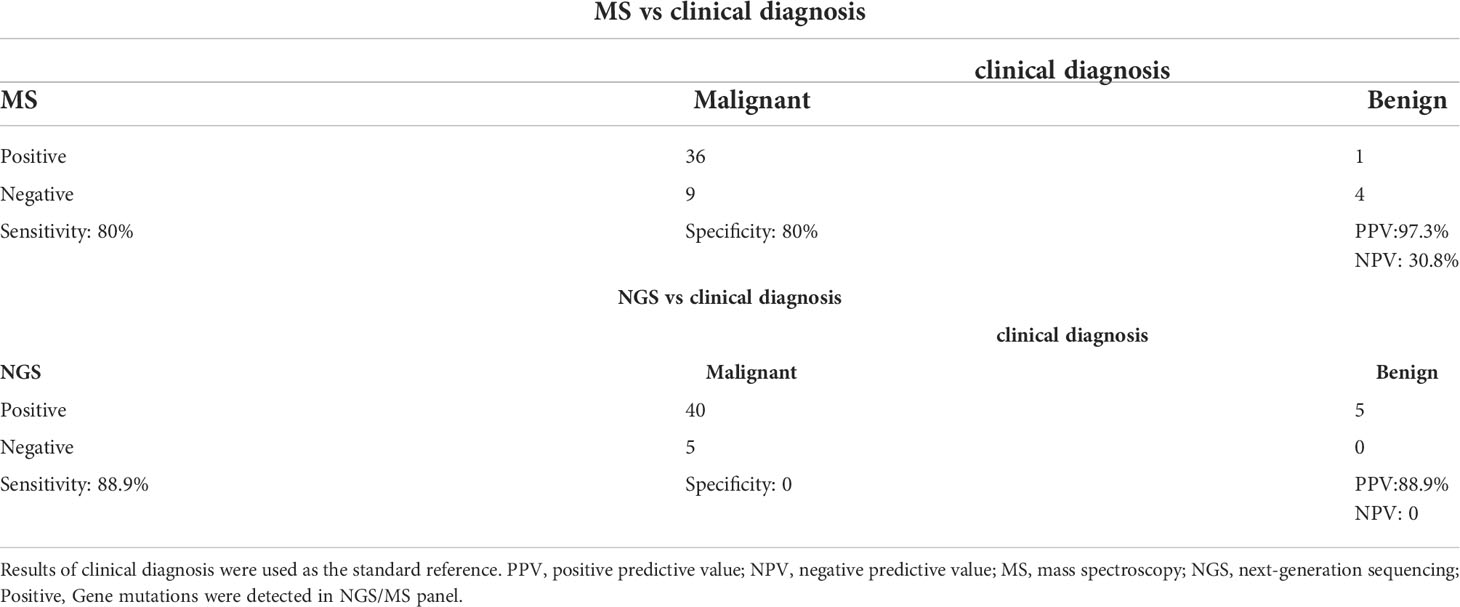
Furthermore, we collected the postoperative pathological findings of 50 patients, 45 of whom were papillary thyroid carcinoma (PTC). We also investigated the relationship between postoperative pathological findings and NGS or MS detection results, using pathological findings as the gold standard. Considering that the genes contained in NGS/MS panel are common genes for thyroid cancer diagnosis and prognosis, positive was defined as the detection of mutations in the NGS or MS panel. The sensitivity, specificity, PPV, and NPV of the MS method were 80%, 80%, 97.3%, and 30.8%, respectively, while the sensitivity, specificity, PPV, and NPV of the NGS method were 88.9%, 0%, 88.9%, and 0%. The level of agreement between clinical diagnosis and MS or NGS was lower, with kappa-values of 0.351 (95% CI 0.06–0.643, p=0.004) and 0.111 (95% CI 0.04–0.179, p=0.4), respectively.
Both MS and NGS showed higher sensitivity, as evidenced by the above results. However, some patients were missed in both methods. In the MS analysis, 9 patients were found to have thyroid cancer despite having a negative MS test result. NGS detected gene mutations in four patients out of nine, including one with BRAFV600E, one with NRAS, and two with NTRK3 fusion. Gene mutations, however, were found in all five patients with clinically benign nodules, with one having the BRAFV600E mutation (Table 4). These findings suggest that multigene testing could be used as an adjunct to clinical diagnosis, but it must be used in conjunction with other clinical methods.
Discussion
We demonstrated in this study that both NGS and MS are effective methods for detecting BRAF V600E mutations in FNA biopsy samples of patients with thyroid nodules, with a strong inter-rater agreement, high specificity, and high PPV. Furthermore, the MS method demonstrated significant potential as a screening method for gene mutation detection and as an adjunct to postoperative pathological findings.
Several next-generation sequencing studies, including whole-genome sequencing (14), whole-exome sequencing (15, 16), and targeted sequencing (17, 18) have recently been conducted to investigate the genetic changes in papillary thyroid carcinoma. B-Raf proto-oncogene, serine/threonine kinase (BRAFV600E), and telomerase reverse transcriptase (TERT) promoter mutations were the most frequently identified in papillary thyroid carcinoma. According to Cancer Genome Atlas (TCGA) cohort and several studies, the most common alterations are BRAFV600E mutation in 62% (19, 20), TERT mutation in 22% (21), and RAS (including HRAS, NRAS, and KRAS) mutation in 13% (19). A high prevalence of BRAFV600E mutations was also found in papillary thyroid carcinoma patients in a Chinese cohort study (22). In patients with papillary thyroid carcinoma, Khan and colleagues discovered that 94 percent of BRAF mutations were BRAF V600E mutations (21). Both the National Comprehensive Cancer Network (NCCN) and the Chinese Society of Clinical Oncology (CSCO) recommend testing for BRAFV600E mutations as a supplement to clinical diagnosis. As a result, preliminary BRAF screening is required for all patients with thyroid nodules to aid clinical diagnosis. The BRAFV600E mutation was found in 76.3% of the patients in this study, which was consistent with a Chinese cohort study (23).
TERT in 1.1%, and RAS (including HRAS, NRAS, and KRAS) mutation in 8.6% of the patients in this study.
Because of its high throughput, multi-gene coverage, and high precision, next-generation sequencing (NGS) is a widely known method for detecting solid tumor mutations. However, due to the high throughput and high cost of the equipment, NGS is not suitable for all thyroid tumors or nodules patients for initial screening, particularly for the detection of a BRAF gene (24). The MS method is a multiplexed ultrasensitive mutation detection system with a medium throughput. This method had previously been used successfully to detect mutations in patients with solid tumors, with a reported limit-of-detection frequency of 0.1% (8). In this study, we compared the efficacy of MS and NGS methods for detecting BRAF mutations. Our findings show that the MS method is a reliable and sensitive method for detecting BRAFV600E mutations in thyroid tumors or nodules in patients’ FNA biopsy samples. Compared with NGS detection, the MS demonstrated greater sensitivity and specificity. However, the MS method did not detect three patients with BRAFV600E. False-negative results were complicated because the study used retrospective samples. According to the NGS results, the BRAF mutation frequency in these three patients was relatively low. We hypothesized that the false-negative detection was a lack of sufficient tumor DNA in the remaining samples. The absence of false-positive cases demonstrates the MS method’s potential in clinical applications.
Furthermore, we compared the efficiency of MS and NGS methods in detecting multiple-gene mutations. The MS method maintained high specificity and sensitivity, as expected. However, 11.8% of patients were reported negative because they had mutations that were not found in the MS panel. Intriguingly, MS results from NGS were consistent in a patient with BRAFV600E and TERT mutations. Previous research has shown that patients with both BRAFV600E and TERT mutations have a poor prognosis, so simultaneous multigene screening is necessary (13).
The MS and NGS results were also evaluated using the postoperative pathological findings as the standard. The MS method had higher sensitivity and specificity with pathological findings, and the majority of false-negative patients had out-of-panel mutations. Although the 11-gene panel of NGS demonstrated higher sensitivity due to the greater number of non-BRAF mutations covered, NGS demonstrated lower specificity due to non-BRAF mutations detected in benign lesions (12, 25). Three of the five patients with benign lesions had RAS mutations, and one had an NTRK3 fusion. Previous research has shown that these mutations can be found in both malignant and benign lesions (11, 12, 25). In addition, all malignant in our study were PTC. Previous studies have confirmed that non-BRAF genes were more common in other subtype thyroid carcinoma patients, which may have higher diagnostic value. For example, RAS mutation in follicular thyroid carcinoma was more common than BRAF (12). Moreover, 7 patients had the results of clinical diagnosis in 11 patients with non-BRAF mutations. Of the 7 patients, 4 (57.14%) patients were benign lesions, and 3 of 4 patients harbored RAS mutations. Although several studies have demonstrated that multigene testing can improve the specificity of clinically assisted diagnosis (26–28). Previous research and CSCO guidelines have shown that RAS mutations have an unsatisfactory clinical impact on the management of thyroid nodules (29). As a result, the NCCN and CSCO guidelines recommend BRAF as the primary screening gene for adjunctive diagnosis, and these non-BRAF genes are not required for initial screening. These findings imply that the MS method can be used as a primary screening method for molecular detection in patients.
There were a few limitations to this study as well. Due to the small number of patients in our cohort who had TERT and TP53 mutations, the feasibility of the MS method in this population needs to be investigated further. Second, the importance of multiple-gene detection should be discussed further. Because our study was retrospective, residual DNA from NGS detection was used as samples in MS detection, limiting the sample size of this study. The possibility of full-process detection of clinical FNA samples should be investigated further.
Conclusion
Finally, our findings showed that the MS method was a precise and dependable alternative for detecting BRAF mutations in patients with thyroid nodules. Furthermore, the MS method as primary screening for molecular detection was promising.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving human participants were reviewed and approved by Ethics Committee of Jiangsu Hospital of Integrated Traditional Chinese and Western Medicine and Jiangsu University Affiliated People’s Hospital. All procedures were in accordance with the 1964 Declaration of Helsinki and its later amendments. The patients/participants provided their written informed consent to participate in this study.
Author contributions
X-QQ and EA contributed to the conception and design of the study. Y-GW and F-JX contributed to the acquisition of data. L-LZ, R-LY, and X-YL assisted in sample management and experiment operation. F-JX, NY, MX, and D-JG organized the database. NY, F-JX, YY and MX contributed to the confirmation of the authenticity of the data. Y-GW, X-QQ, EA, WX-P and D-JG performed data analysis and interpretation. EA performed review and editing. All authors contributed to the manuscript and read and approved the final manuscript. All authors contributed to the article and approved the submitted version.
Funding
This study was financially supported by National Natural Science Foundation of China (Project No. 81971629) and Zhenjiang Commission of Science and Technology (Project No. SH2020046).
Conflict of interests
NY was employed by Dian Diagnostics Group Co., Ltd and MX was employed by Zhejiang Digena Diagnosis Technology Co. Ltd.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Jarząb B, Dedecjus M, Handkiewicz-Junak D, Lange D, Lewiński A, Nasierowska-Guttmejer A, et al. Diagnostics and treatment of thyroid carcinoma. Endokrynol Polska (2016) 67(1):74–107. doi: 10.5603/EP.2016.0011
2. Jarząb B, Dedecjus M, Słowińska-Klencka D, Lewiński A, Adamczewski Z, Anielski R, et al. Guidelines of polish national societies diagnostics and treatment of thyroid carcinoma. 2018 Update Endokrynol Polska (2018) 69(1):34–74. doi: 10.5603/EP.2018.0014
3. Wiltshire JJ, Drake TM, Uttley L, Balasubramanian SP. Systematic review of trends in the incidence rates of thyroid cancer. Thyroid: Off J Am Thyroid Assoc (2016) 26:1541–52. doi: 10.1089/thy.2016.0100
4. Liu Y, Su L, Xiao H. Review of factors related to the thyroid cancer epidemic. Int J Endocrinol (2017) 2017:5308635. doi: 10.1155/2017/5308635
5. Jinih M, Foley N, Osho O, Houlihan L, Toor AA, Khan JZ, et al. BRAF(V600E) mutation as a predictor of thyroid malignancy in indeterminate nodules: A systematic review and meta-analysis. Eur J Surg Oncol: J Eur Soc Surg Oncol Br Assoc Surg Oncol (2017) 43:1219–27. doi: 10.1016/j.ejso.2016.11.003
6. Mungan S, Ersoz S, Saygin I, Sagnak Z, Cobanoglu U. Nuclear morphometric findings in undetermined cytology: A possible clue for prediction of BRAF mutation in papillary thyroid carcinomas. Endocrine Res (2017) 42:138–44. doi: 10.1080/07435800.2016.1255895
7. Bentz BG, Miller BT, Holden JA, Rowe LR, Bentz JS. B-RAF V600E mutational analysis of fine needle aspirates correlates with diagnosis of thyroid nodules. Otolaryngology–head Neck Surg: Off J Am Acad Otolaryngology-Head Neck Surg (2009) 140:709–14. doi: 10.1016/j.otohns.2009.01.007
8. Mehrotra M, Singh RR, Loghavi S, Duose DY, Barkoh BA, Behrens C, et al. Detection of somatic mutations in cell-free DNA in plasma and correlation with overall survival in patients with solid tumors. Oncotarget (2018) 9:10259–71. doi: 10.18632/oncotarget.21982
9. Li H, Durbin R. Fast and accurate short read alignment with burrows-wheeler transform. Bioinf (Oxford England) (2009) 25:1754–60. doi: 10.1093/bioinformatics/btp324
10. Cibulskis K, Lawrence MS, Carter SL, Sivachenko A, Jaffe D, Sougnez C, et al. Sensitive detection of somatic point mutations in impure and heterogeneous cancer samples. Nat Biotechnol (2013) 31:213–9. doi: 10.1038/nbt.2514
11. Marotta V, Guerra A, Sapio MR, Vitale M. RET/PTC rearrangement in benign and malignant thyroid diseases: a clinical standpoint. Eur J Endocrinol (2011) 165:499–507. doi: 10.1530/EJE-11-0499
12. Decaussin-Petrucci M, Descotes F, Depaepe L, Lapras V, Denier ML, Borson-Chazot F, et al. Molecular testing of BRAF, RAS and TERT on thyroid FNAs with indeterminate cytology improves diagnostic accuracy. Cytopathol: Off J Br Soc Clin Cytol (2017) 28:482–7. doi: 10.1111/cyt.12493
13. Xing M. Genetic-guided risk assessment and management of thyroid cancer. Endocrinol Metab Clinics North America (2019) 48:109–24. doi: 10.1016/j.ecl.2018.11.007
14. Yoo SK, Song YS, Lee EK, Hwang J, Kim HH, Jung G, et al. Integrative analysis of genomic and transcriptomic characteristics associated with progression of aggressive thyroid cancer. Nat Commun (2019) 10:2764. doi: 10.1038/s41467-019-10680-5
15. Kunstman JW, Juhlin CC, Goh G, Brown TC, Stenman A, Healy JM, et al. Characterization of the mutational landscape of anaplastic thyroid cancer via whole-exome sequencing. Hum Mol Genet (2015) 24:2318–29. doi: 10.1093/hmg/ddu749
16. Ravi N, Yang M, Gretarsson S, Jansson C, Mylona N, Sydow SR, et al. Identification of targetable lesions in anaplastic thyroid cancer by genome profiling. Cancers (2019) 11(3):402. doi: 10.3390/cancers11030402
17. Pozdeyev N, Gay LM, Sokol ES, Hartmaier R, Deaver KE, Davis S, et al. Genetic analysis of 779 advanced differentiated and anaplastic thyroid cancers. Clin Cancer Res: Off J Am Assoc Cancer Res (2018) 24:3059–68. doi: 10.1158/1078-0432.CCR-18-0373
18. Landa I, Ibrahimpasic T, Boucai L, Sinha R, Knauf JA, Shah RH, et al. Genomic and transcriptomic hallmarks of poorly differentiated and anaplastic thyroid cancers. J Clin Invest (2016) 126:1052–66. doi: 10.1172/JCI85271
19. Integrated genomic characterization of papillary thyroid carcinoma. Cell (2014) 159:676–90. doi: 10.1016/j.cell.2014.09.050
20. Deeken-Draisey A, Yang GY, Gao J, Alexiev BA. Anaplastic thyroid carcinoma: an epidemiologic, histologic, immunohistochemical, and molecular single-institution study. Hum Pathol (2018) 82:140–8. doi: 10.1016/j.humpath.2018.07.027
21. Khan SA, Ci B, Xie Y, Gerber DE, Beg MS, Sherman SI, et al. Unique mutation patterns in anaplastic thyroid cancer identified by comprehensive genomic profiling. Head Neck (2019) 41:1928–34. doi: 10.1002/hed.25634
22. Duan H, Li Y, Hu P, Gao J, Ying J, Xu W, et al. Mutational profiling of poorly differentiated and anaplastic thyroid carcinoma by the use of targeted next-generation sequencing. Histopathology (2019) 75:890–9. doi: 10.1111/his.13942
23. Xu X, Ma X, Zhang X, Cao G, Tang Y, Deng X, et al. Detection of BRAF V600E mutation in fine-needle aspiration fluid of papillary thyroid carcinoma by droplet digital PCR. Clin Chim Acta Int J Clin Chem (2019) 491:91–6. doi: 10.1016/j.cca.2019.01.017
24. Ng JY, Lu CT, Lam AK. BRAF mutation: Current and future clinical pathological applications in colorectal carcinoma. Histol Histopathol (2019) 34(5):469–77. doi: 10.14670/HH-18-079
25. Guan H, Toraldo G, Cerda S, Godley FA, Rao SR, McAneny D, et al. Utilities of RAS mutations in preoperative fine needle biopsies for decision making for thyroid nodule management: Results from a single-center prospective cohort. Thyroid: Off J Am Thyroid Assoc (2020) 30:536–47. doi: 10.1089/thy.2019.0116
26. Nikiforov YE, Ohori NP, Hodak SP, Carty SE, LeBeau SO, Ferris RL, et al. Impact of mutational testing on the diagnosis and management of patients with cytologically indeterminate thyroid nodules: a prospective analysis of 1056 FNA samples. J Clin Endocrinol Metab (2011) 96:3390–7. doi: 10.1210/jc.2011-1469
27. Alexander EK, Kennedy GC, Baloch ZW, Cibas ES, Chudova D, Diggans J, et al. Preoperative diagnosis of benign thyroid nodules with indeterminate cytology. New Engl J Med (2012) 367:705–15. doi: 10.1056/NEJMoa1203208
28. Beaudenon-Huibregtse S, Alexander EK, Guttler RB, Hershman JM, Babu V, Blevins TC, et al. Centralized molecular testing for oncogenic gene mutations complements the local cytopathologic diagnosis of thyroid nodules. Thyroid: Off J Am Thyroid Assoc (2014) 24:1479–87. doi: 10.1089/thy.2013.0640
Keywords: thyroid nodules, BRAF gene, next generation sequencing, mass spectroscopy, fine needle aspiration
Citation: Qian X-q, Agyekum EA, Zhao L-l, Yu R-l, Li X-y, Gu D-j, Yan N, Xu M, Yuan Y, Wang Y-g, Xin-ping W and Xu F-j (2022) A comparison of DP-TOF Mass Spectroscopy (MS) and Next Generation Sequencing (NGS) methods for detecting molecular mutations in thyroid nodules fine needle aspiration biopsies. Front. Endocrinol. 13:928788. doi: 10.3389/fendo.2022.928788
Received: 26 April 2022; Accepted: 05 July 2022;
Published: 04 August 2022.
Edited by:
Akira Sugawara, Tohoku University, JapanReviewed by:
Erivelto Martinho Volpi, Centro de referencia no ensino do diagnóstico por imagem (CETRUS), BrazilFumihiko Furuya, Fukushima Medical University, Japan
Copyright © 2022 Qian, Agyekum, Zhao, Yu, Li, Gu, Yan, Xu, Yuan, Wang, Xin-ping and Xu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Fei-ju Xu, eGZqMDQyM0AxNjMuY29t; Wu Xin-ping, eGl5dWx1b3lvdW1AMTI2LmNvbQ==; Yu-guo Wang, d3lzb3NAMTYzLmNvbQ==
†These authors have contributed equally to this work and share first authorship
 Xiao-qin Qian
Xiao-qin Qian Enock Adjei Agyekum
Enock Adjei Agyekum Ling-ling Zhao4
Ling-ling Zhao4 Na Yan
Na Yan