- Hospital of Chengdu University of Traditional Chinese Medicine, Chengdu University of Traditional Chinese Medicine, Chengdu, China
To assess and analyse the effectiveness and safety of combined Chinese herbal formula (CHF) and metformin treatment in the modulation of the gut microbiota in the amelioration of type 2 diabetes mellitus(T2DM), all publications addressing the effect of this combination treatment on the quantitative alterations in the gut microbiota and glucose parameters were collected. Rob tool in the Cochrane handbook was performed to evaluate the methodological quality of all included studies. Relevant information and statistics were abstracted and synthesized in Review Manager 5.4 to evaluate the efficacy of combination treatment. Sensitivity analyses and subgroup analyses were used to analyse the sources of heterogeneity. Publication bias analyses were performed by Stata software to assess the robustness and quality of the outcomes. As a result, a total of 12 eligible RCTs with 1307 T2DM participants from 7 electronic databases were included. Combined CHF with metformin treatment showed better efficacies than metformin monotherapy in regulating the structure of the gut microbiota, characterized by increased Bifidobacterium, Lactobacillus and Bacteroidetes and decreased Enterobacteriaceae, Enterococcus, and Saccharomyces along with better decreases in glycated haemoglobin, fasting plasma glucose, 2-hour postprandial blood glucose, fasting insulin and homeostasis model assessment of insulin resistance. Subgroup analyses further analysed the effect of metformin doses and CHF classifications on controlling hyperglycaemia and altering the gut microbiota. In conclusion, our meta-analysis suggested that combined CHF with metformin treatment is promising for the modulation of the gut microbiota along with ameliorating hyperglycemia in T2DM patients. Importantly, more well-designed RCTs are needed to validate the outcomes and verify the treatment value for clinical purposes.
Systematic Review Registration: https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42021291524, identifier CRD42021291524.
1 Introduction
The number of people affected by type 2 diabetes mellitus (T2DM), a disease with worldwide prevalence characterized by elevated blood glucose levels (hyperglycaemia) (1, 2), is estimated to increase to more than 700 million people by 2045 (3). Pharmacotherapies, such as oral hypoglycemic agents(metformin, glimepiride, pioglitazone and Acarbose) and insulin injections, and other regimens, such as weight control and diet management, are suggested for T2DM patients (4). Despite the availability of various antidiabetic treatments, current antidiabetic medicines fail to achieve satisfactory hypoglycaemic effects in all patients, and some adverse reactions, such as gastrointestinal disturbances, can occur (5). Thus, further attempts to develop more efficient and safer therapeutic methods for T2DM to achieve satisfactory outcomes are urgently needed.
The gut microbiota is considered as a salient vehicle emerging in the pathogenesis and development of metabolic diseases. (6) The gut microbiota is formed by up to 100 trillion microorganisms, including bacteria, viruses, fungi, and protozoa (7), among which the majority are bacteria (8). An accumulating number of pivotal reports have demonstrated that the gut microbiota serves as a novel contributory factor in digestion, metabolism, pathogen defence and immunity (9). The core role of the gut microbiota in metabolic diseases originates from the realization that the gut microbiota can trigger the inflammatory reaction mediator lipopolysaccharide(LPS) to induce insulin resistance (10). The low-grade inflammation reaction, as an important pathophysiological factor resulting in hyperglycaemia and insulin resistance (11), can be activated by an increase in the permeability of gut microbiota and a reduction in the intestinal barrier function which transfers Gram-negative bacteria into the systemic circulation and elevates the LPS levels (12). The evidence that the dysbiosis has existed in the intestinal tract of T2DM patients and the oral administration of prebiotic such as Lactobacillus contributes to ameliorating hyperglycaemia and alleviating endotoxemia (6, 12) further validates that the dysbiosis of gut microbiota could disturb the integrity of the gut barrier and reshape the host metabolism, implicating in the susceptibility to the onset and progression of insulin resistance (13, 14).
Microbial-mediated therapeutic strategies are being sought to treat T2DM (8). Metformin, a widely applied oral anti-hyperglycaemic agent, exerts its hypoglycaemic action mainly by inhibiting hepatic gluconeogenesis and increasing the utilization of glucose in peripheral tissues (15). Evidence suggested that the bioavailability of metformin was higher in the intestinal tract than in the serum (16). More trials further revealed microbial changes along with the promotion of glucose metabolism in metformin treatment groups (17). In parallel, Chinese herbal formulas (CHFs), with their unique theory and accumulated considerable clinical experience (18, 19), have been concluded to have remarkable therapeutic effects on diabetes, with fewer side effects than metformin (20). Significant reductions in glycated haemoglobin(HbA1c), fasting plasma glucose(FPG), 2-hour postprandial blood glucose(2hPG), fasting insulin(FINS) and homeostasis model assessment of insulin resistance(HOMA-IR) have been found after treatment with CHFs (18, 21, 22). Early intervention with CHFs can delay diabetes-related macrovascular complications by regulating the AMPK signalling pathway (23). A recent study showed that a CHF decoction exerted beneficial effects on diabetic hindlimb ischaemia by reshaping the gut microbiota structure and stunning the VEGF/HIF-1α pathway (24). In addition, Zheng Y showed that CHFs could modulate the quantity of probiotic and pathogenic bacteria, which was accompanied by the control of blood glucose and insulin resistance at multiple levels (25), which suggested that both metformin and CHFs can be used in the treatment of diabetes mellitus by modulating gut microbiota dysbiosis.
Encouragingly, recent studies introduced the concept that metformin combined with CHFs had synergistic effects to improve the clinical efficacy of antidiabetic treatment for improving glucose and lipid metabolism through multiple pathways in line with reducing the associated adverse reactions (26, 27). Huangkui capsules combined with metformin effectively improved diabetic nephropathy via the Klotho/TGF-β1/p38 MAPK signalling pathway (28). The application of Shenqi Jiangtang granules with metformin effectively controlled blood glucose and lipid levels, enhanced antioxidant capacity, and reduced the levels of inflammatory cytokines in gestational diabetes mellitus. (29) More studies further confirmed that combined treatment with CHFs and metformin had significant additive and synergistic effects on the changes in the gut microbiota, such as increased Bifidobacterium, Bacteroides, and Lactobacillus and decreased Enterobacter and Enterococcus, compared with metformin monotherapy (30–32), supporting that the gut microbiota can be seen as a promising therapeutic target of combination therapy in the treatment of diabetes. To the best of our knowledge, few systematic and comprehensive studies have investigated the effects of combination treatment on the changes in the gut microbiota in T2DM patients. According to this background, this study aimed to conduct a meta-analysis to evaluate the effectiveness and quality of this combination treatment on the alteration of the gut microbiota in the remission of type 2 diabetes mellitus.
2 Materials and methods
This meta-analysis was registered in PROSPERO with the registration number CRD42021291524(Record ID=291524) and was performed in accordance with the instructions of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement (33).
2.1 Literature search strategy
Electronic databases, including the PubMed, Web of Science, Cochrane Library, China National Knowledge Infrastructure (CNKI), Wan Fang China, VIP Medical Information, and Chinese Biomedical Literature (CBM) databases, were searched to screen for all relevant published studies on the therapeutic value of CHFs combined with metformin in the treatment of diabetes from database inception to November 2021. The keywords or MeSH terms for the search strategy were “type 2 diabetes mellitus”, “gastrointestinal microbiome” or “gut microbiota” or “intestinal flora”, “traditional Chinese medicine”, and “hypoglycemic agents” within the English and Chinese languages.
2.2 Eligibility criteria
All randomized controlled trials meeting the following eligibility criteria were included:
i. Participants: studies that enrolled patients who were diagnosed with type 2 diabetes mellitus.
ii. Intervention: studies that used treatment with CHFs and metformin in the experimental group.
iii. Comparison: studies that used metformin in the control group.
iv. Outcomes: studies that investigated the quantitative alterations of the gut microbiota and glucose metabolism indicators. The primary outcome was the quantitative changes in the gut microbiota, especially the statistics for Bifidobacterium, Lactobacillus, Bacteroidetes Enterobacteriaceae, Enterococcus, and Saccharomyces. The secondary outcome was the quantitative changes in glucose metabolism indicators, including fasting plasma glucose (FPG), 2-hour postprandial blood glucose (2hPG), glycosylated haemoglobin (HbA1c), fasting insulin (FINS) and homeostasis model assessment of insulin resistance (HOMA-IR). All outcomes presented as continuous variables were included in the meta-analysis.
2.3 Exclusion criteria
Studies meeting any of the following exclusion criteria were excluded:
i. Studies that did not conform to this research topic, as well as studies that were case reports, animal studies, reviews and repeated publications;
ii. Studies that did not include type 2 diabetes mellitus patients in the experiment or enrolled type 1 diabetes mellitus patients in the trial;
iii. Studies that did not use treatment with CHFs and metformin in the experimental group;
iv. Studies that did not use metformin in the control group or used insulin as a control intervention.
v. Studies that did not provide explicit statistics for changes in the gut microbiota, especially those in which statistics were not presented in the form of the mean ± standard deviation.
2.4 Study selection and data extraction
The electronic databases were independently searched and screened by two reviewers (Shui Jiang and Junyu Chen) according to the inclusion and exclusion criteria. Two authors (Yunxi Xu and Shuyu Zheng) extracted data and collected the data from the included studies. The extracted information included the first author, publication year, participants, intervention measures of the experimental group and control group, information on the included formulas, data on the gut microbiota and glucose metabolism indicators in the experimental and control groups, course of treatment and adverse reactions. If any disagreement occurred, a senior reviewer (Ya Zhang) was consulted to assist in the discussion.
2.5 Methodological evaluation
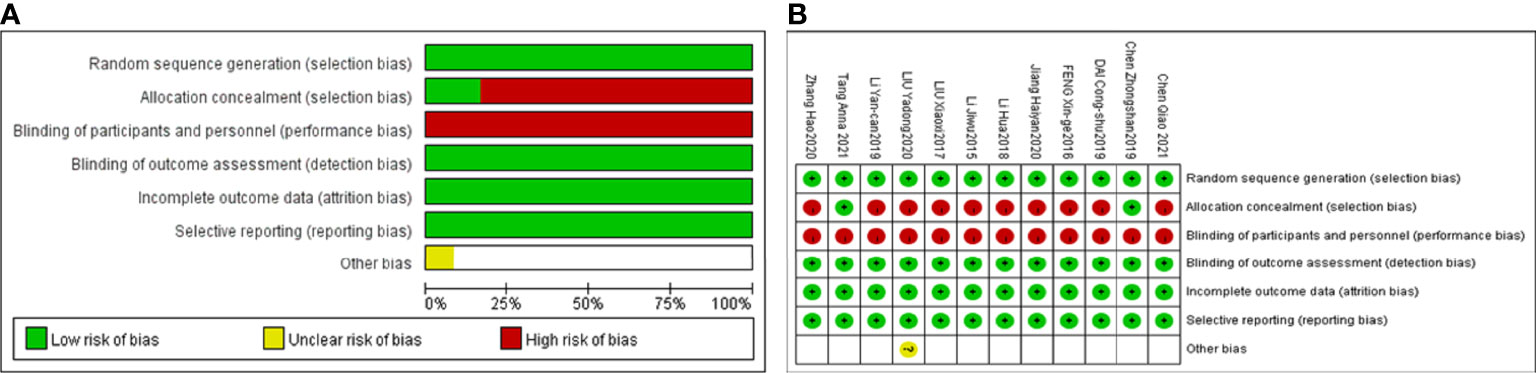
The methodological quality evaluation of each included study was performed by two authors (Shuyu Zheng and Xiaofang Zhu) through the Cochrane Collaboration risk of bias (Rob) tool (34) from six aspects: selection bias (random sequence generation, allocation concealment), performance bias, detection bias, attrition bias, reporting bias and other biases. Each item was judged as “low”, “high”, or “unclear risk of bias” to assess the risk of bias of each study. Any discrepancies were resolved and a consensus was reached by discussion between the two authors.
2.6 Data synthesis and analyses
Relevant information and statistics were synthesized by Review Manager 5.4 to evaluate the efficacy of the combination treatment. Heterogeneity tests were conducted for every outcome. If the heterogeneity was not statistically significant (I2 <50%), a fixed effect model was used for meta-analysis to analyse the outcome; if the heterogeneity was statistically significant (I2 >50%), a random effect model was used for meta-analysis. To explore the source of heterogeneity, sensitivity analysis was used to clarify the impact of individual trials on the overall outcome. If sensitivity analysis suggested that the impact on the overall outcome was stable, subgroup analysis was further planned to explore the sources of the heterogeneity.
2.7 Publication bias
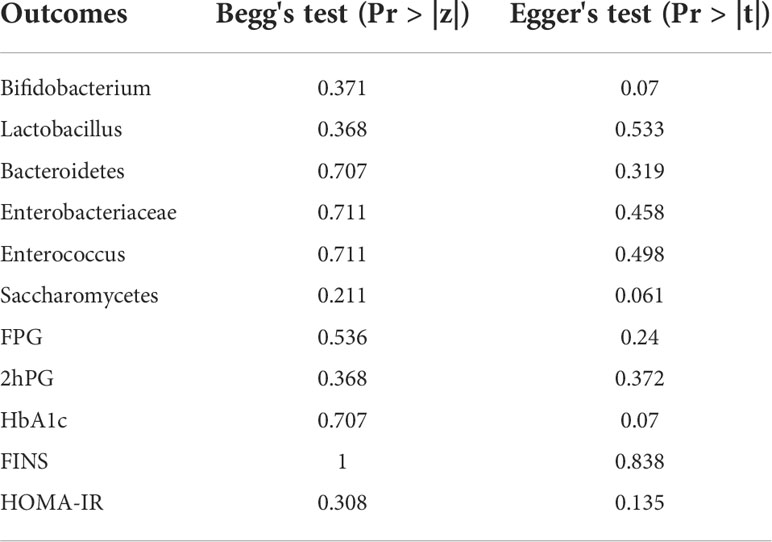
To assess possible publication biases in the analysed outcomes, Begg’s funnel plots (with pseudo 95% confidence limits) and Egger’s publication bias plots were employed by observing the symmetry of these plots formed by Stata software. Additionally, Begg’s test and Egger’s test can assess publication bias in a quantitative manner. Pr > |z| in Begg’s test and Pr > |t| in Egger’s test >0.05 indicated no obvious publication bias in the analysed outcomes (35).
3 Results
3.1 Literature search
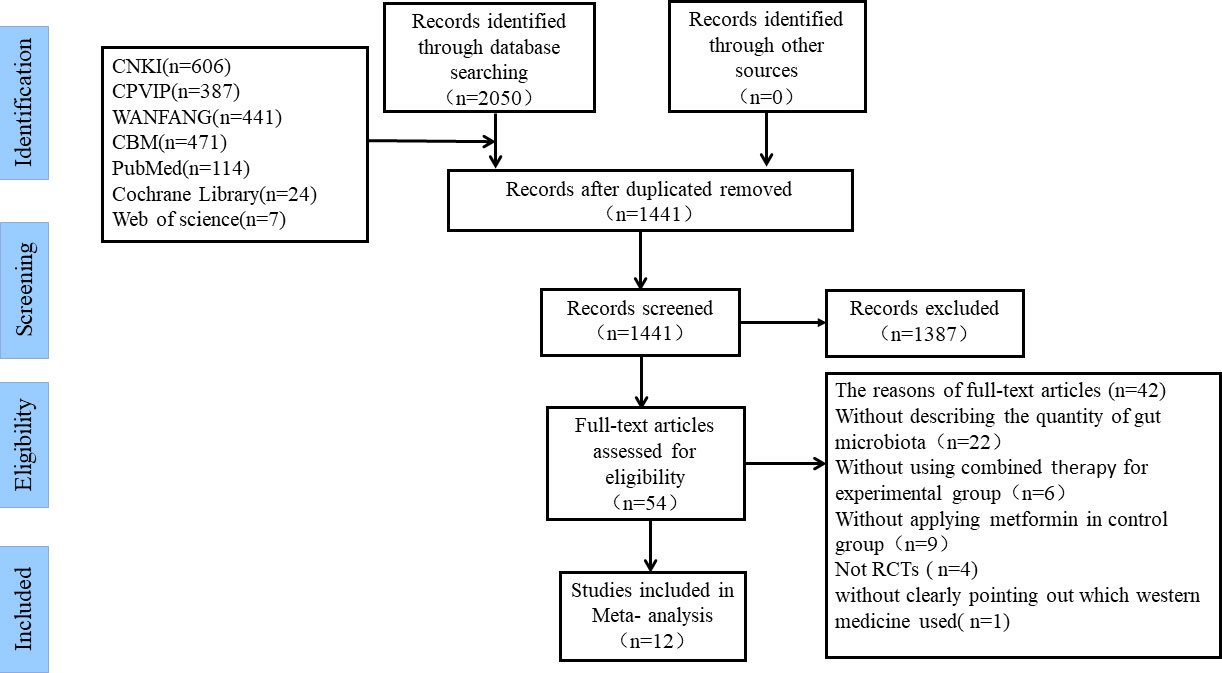
By screening 7 electronic databases, 2050 studies potentially eligible for this study were initially obtained. Then, after duplicated articles were removed, 1441 studies remained. Of the 1441 studies, 1387 irrelevant studies were excluded by reviewing the titles and abstracts. Afterwards, the remaining 54 studies were assessed according to the inclusion and exclusion criteria by carefully reading the full text. Ultimately, 12 studies were confirmed to be eligible for inclusion in this meta-analysis (36–47). The inclusion and exclusion process for each selected study is shown in Figure 1.
3.2 Main characteristics of the studies and interventions
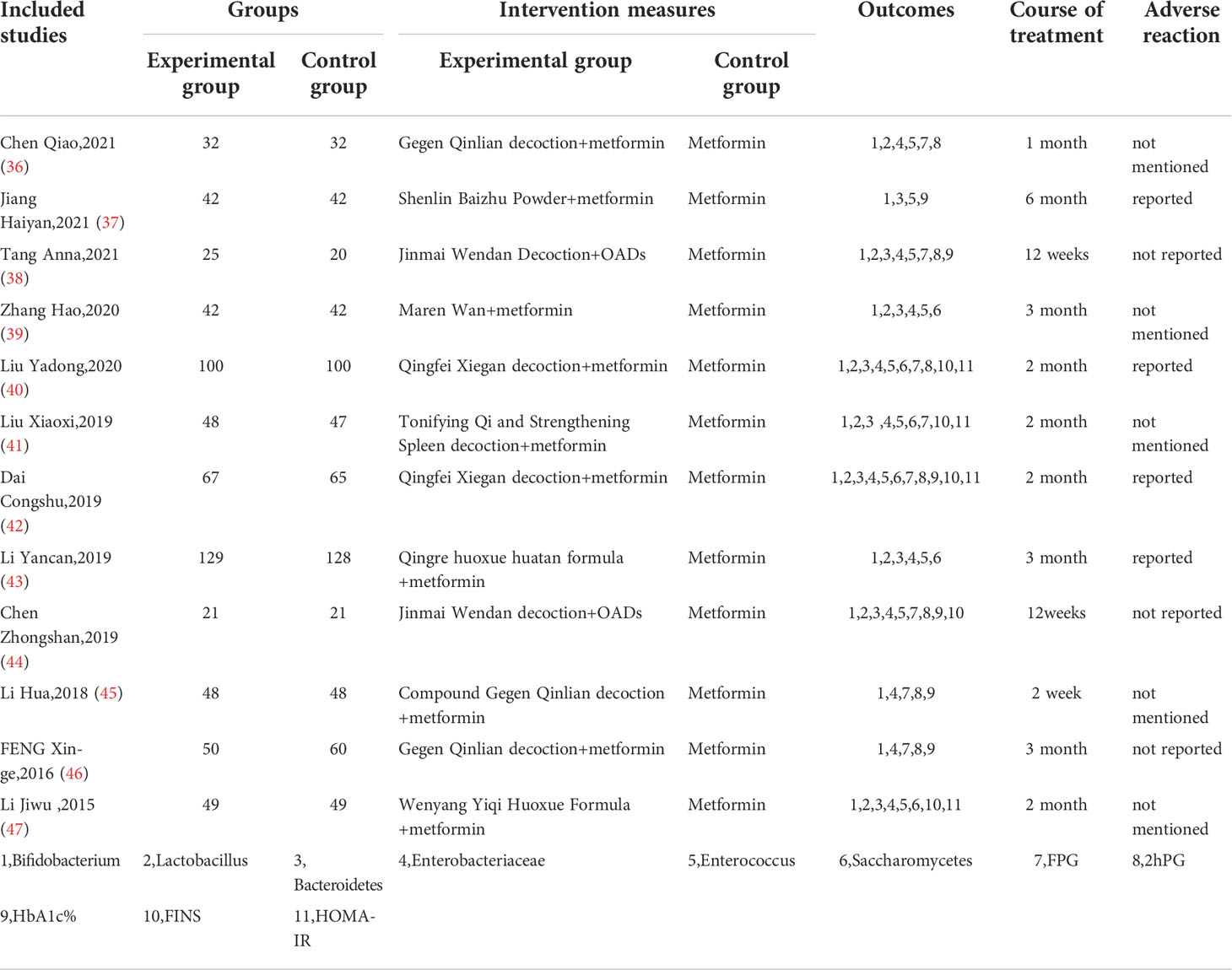
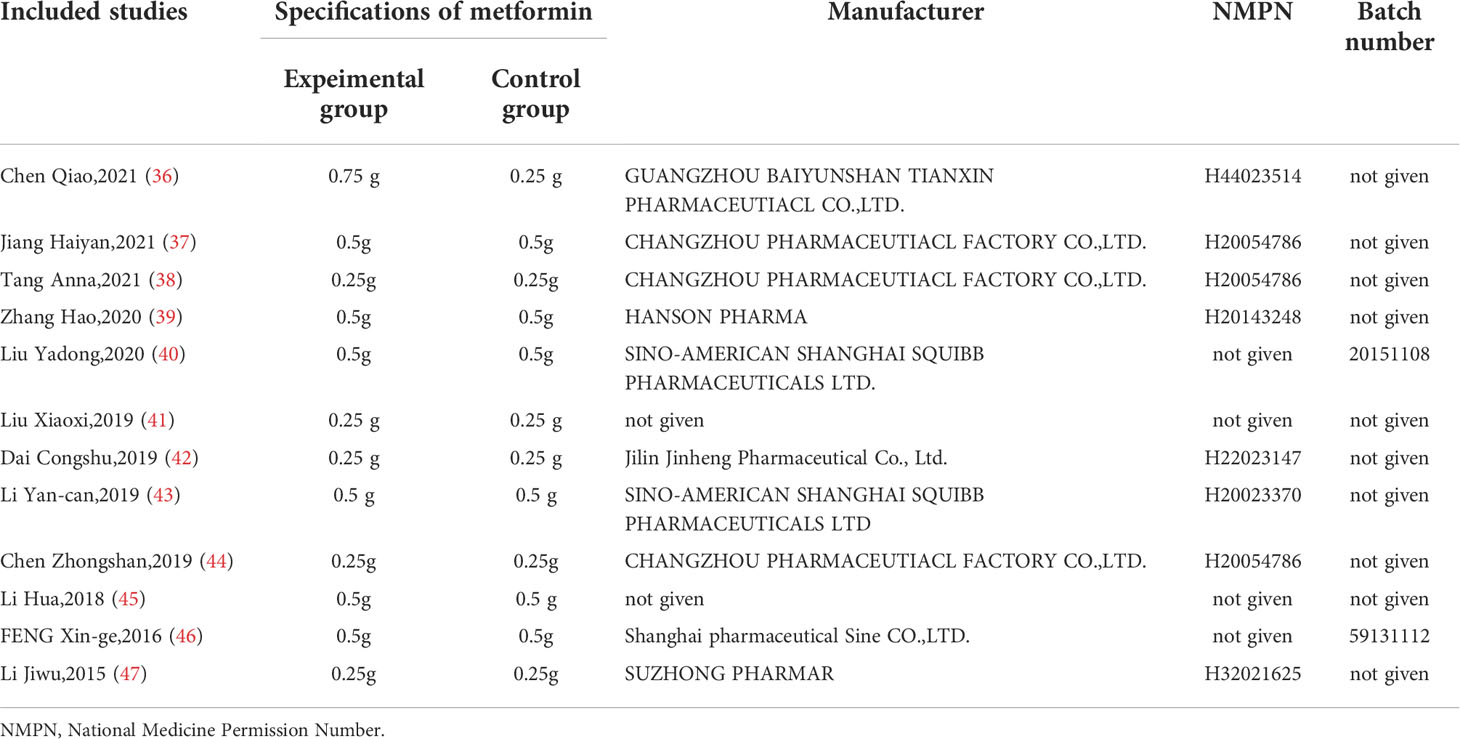
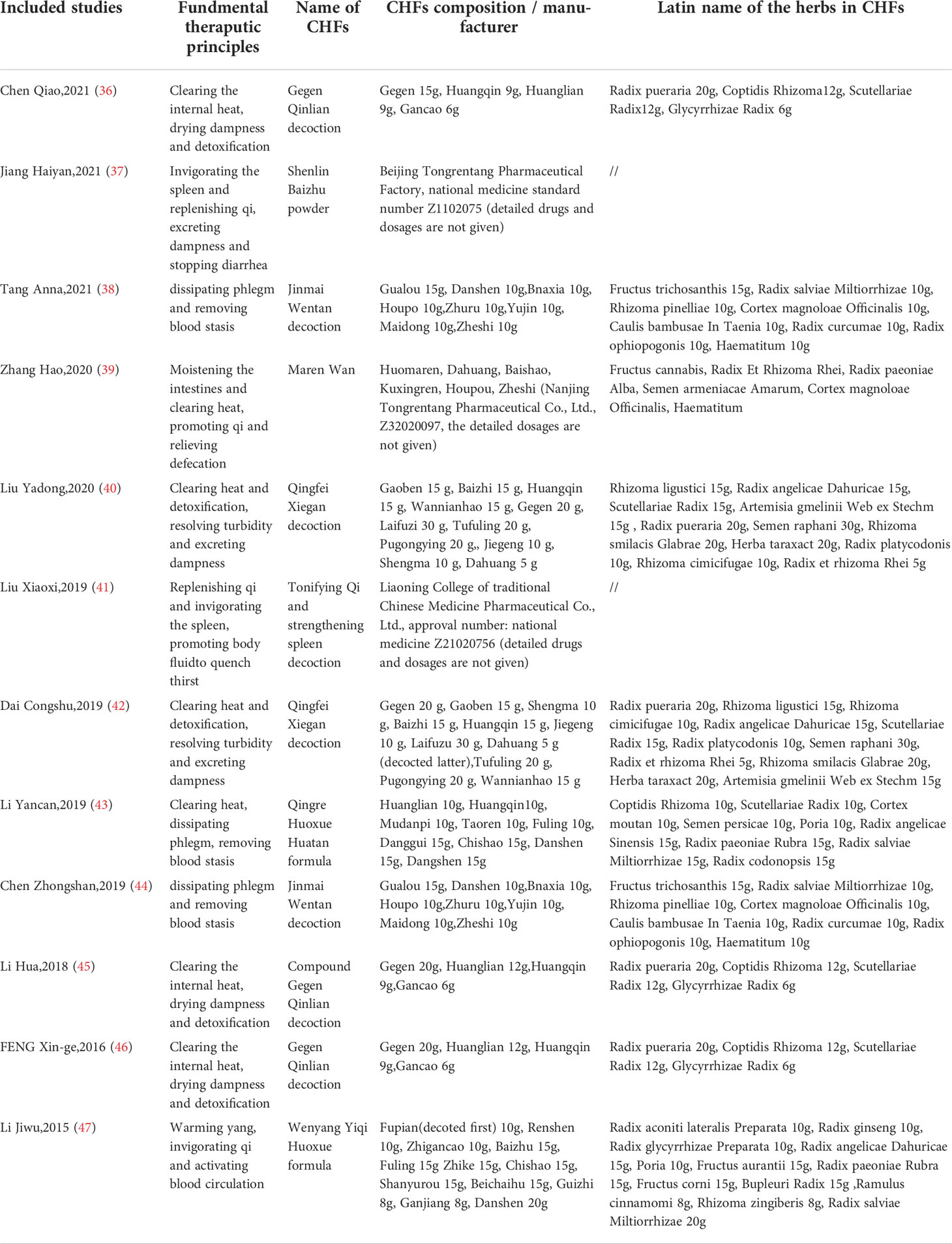
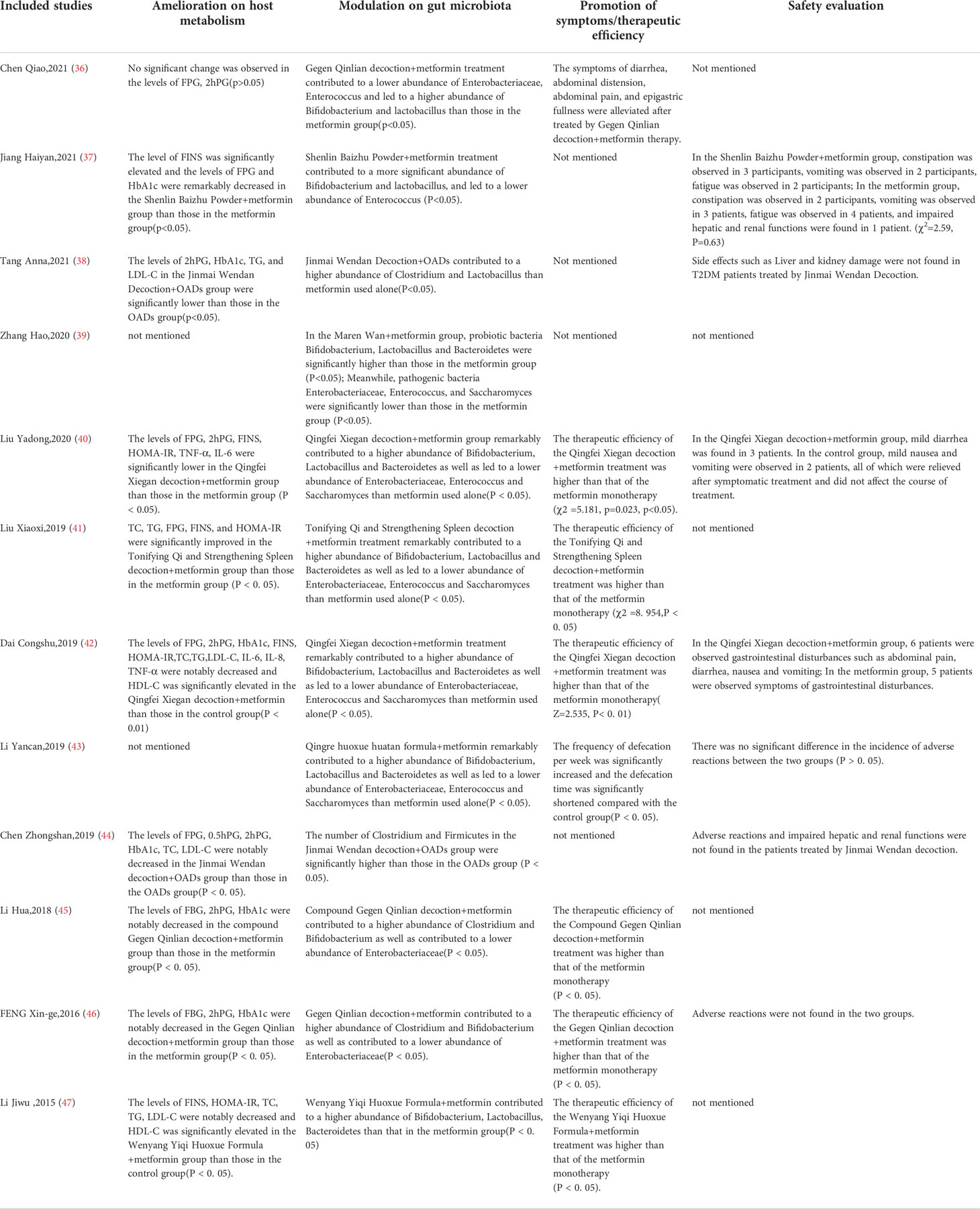
The detailed characteristics of the 12 included studies are summarized in Table 1. The 12 studies (36–47), which enrolled a total of 1307 T2DM patients (653 in experimental groups and 654 in control groups), were all RCTs and were reported in the Chinese language. Combined CHF and metformin interventions were applied in the experimental groups of the 12 studies. Metformin was used in the control groups of the 12 studies. The studies all compared the effects of combined CHF and metformin treatment and those of metformin monotherapy on the alterations of the gut microbiota in T2DM patients. Notably, the changes in glucose metabolism indicators were also analysed in 10 trials (36–38, 40–42, 44–47). The doses of metformin used varied in the 12 studies, which were mainly divided into two drug doses: 0.25 g and 0.5 g. Detailed information on metformin is summarized in Table 2. The fundamental therapeutic principles of the CHFs used in the experimental groups of 9 studies were characterized by clearing the heat and excreting dampness and blood stasis. The fundamental therapeutic principle of the CHFs used in the remaining 3 studies was tonification, mainly by invigorating the viscera and replenishing qi and yang. The CHF that was most frequently utilized among the included studies was Gegen Qinlian decoction (36, 45, 46). The fundamental therapeutic principles and compositions of the formulas used in the experimental groups are shown in Table 3. The beneficial effects of CHFs combined with metformin on the amelioration on host metabolism, modulation of gut microbiota, promotion of symptoms/therapeutic efficiency and safety evaluation in type 2 diabetic patients in detail are summarized in Table 4.
3.3 Methodological quality evaluation
Twelve included studies followed the principle of randomization, among which 10 studies (36, 38, 40–47) referred to the randomization number method and 2 studies described allocation concealment methods (38, 44). Six included studies explicitly mentioned adverse reactions (37, 38, 40, 42–44). Performance bias information was not mentioned in any of the 12 articles. The detection bias, attribution bias and reporting bias were low for those 12 studies. Information on the follow-up duration was not mentioned in any of the 12 studies. The detailed quality assessments for each study are shown in Figure 2.
3.4 Meta-analysis results
3.4.1 Probiotic bacteria
To compare the effects of combined CHF and metformin treatment with those of metformin monotherapy on the quantitative changes in probiotic bacteria, 10 studies (36, 37, 39–43, 45–47) evaluating changes in Bifidobacterium, 7 studies (36, 39–43, 47) evaluating changes in Lactobacillus and 7 studies (37, 39–43, 47) evaluating changes in Bacteroidetes as the primary outcome were included in the analyses.
The heterogeneity for Bifidobacterium and Lactobacillus outcomes was found to be low (I²=19% and 39%). The heterogeneity for Bacteroidetes outcomes was found to be high (I²=94%); therefore, a sensitivity analysis was conducted by Stata software to assess the robustness of each included trial for the Bacteroidetes outcome. It was speculated that the study by LI Yan-can 2019 (43) was the overwhelming factor influencing the overall Bacteroidetes outcome, as shown in Figure 3A. After eliminating the study by LI Yan-can 2019, the heterogeneity for Bacteroidetes decreased to 0%, so a fixed effects model was utilized to perform the meta-analysis for the 3 outcomes.
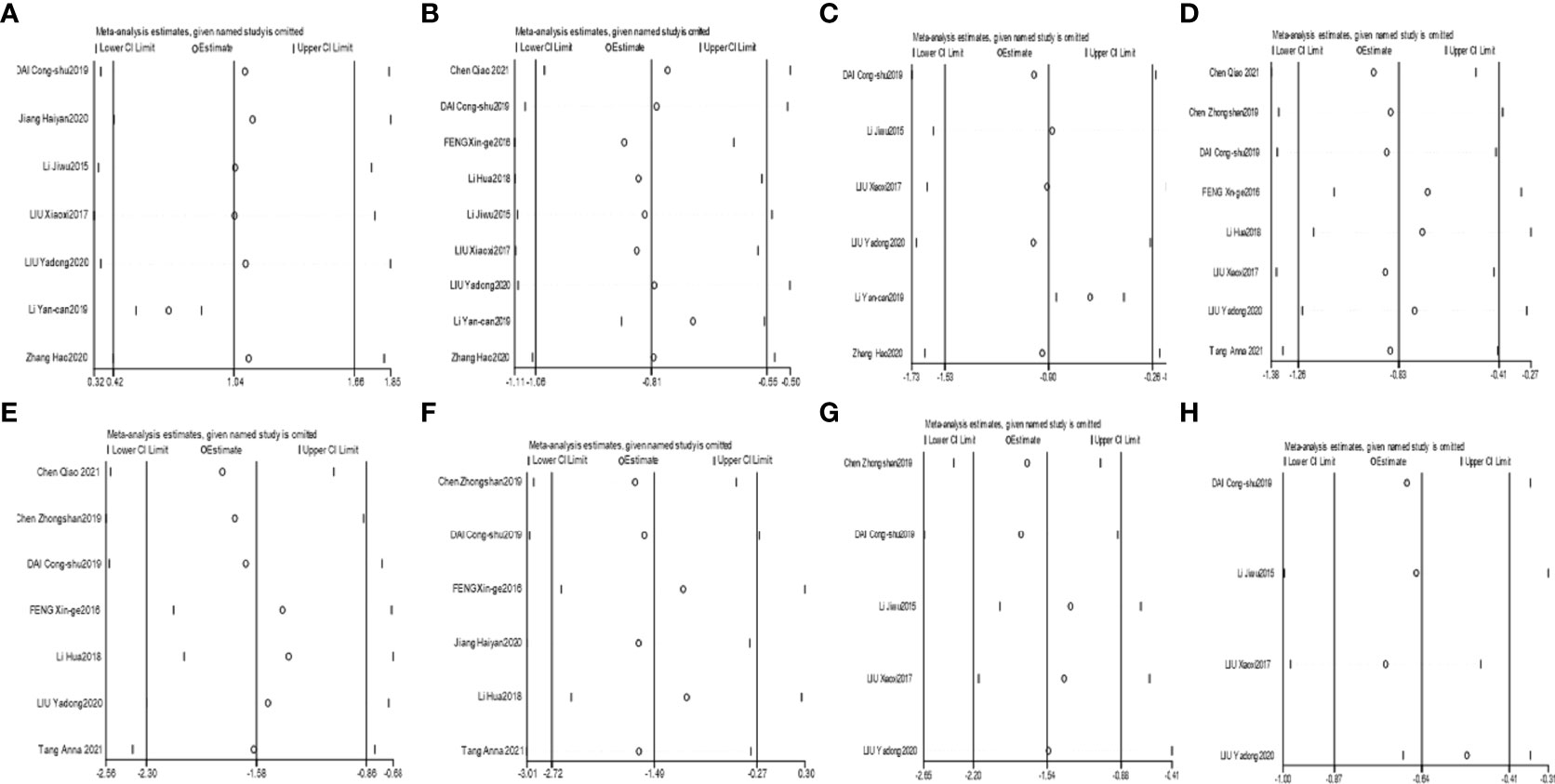
Figure 3 Sensitivity analyses of Bacteroidetes (A), Enterobacteriaceae (B), Saccharomyces (C), FPG (D), 2hPG (E), HbA1c (F), FINS (G), and HOMA-IR (H).
The results demonstrated that combined CHF with metformin intervention contributed to a more significant abundance of Bifidobacterium, Lactobacillus and Bacteroidetes than metformin monotherapy, with the results being significantly different [MD=0.65, 95% CI (0.51, 0.79), P<0.00001], [MD=0.83, 95% CI (0.69, 0.97), P<0.00001], [MD=0.71, 95% CI (0.54, 0.88), p<0.00001], as shown in Figures 4A–C, respectively.
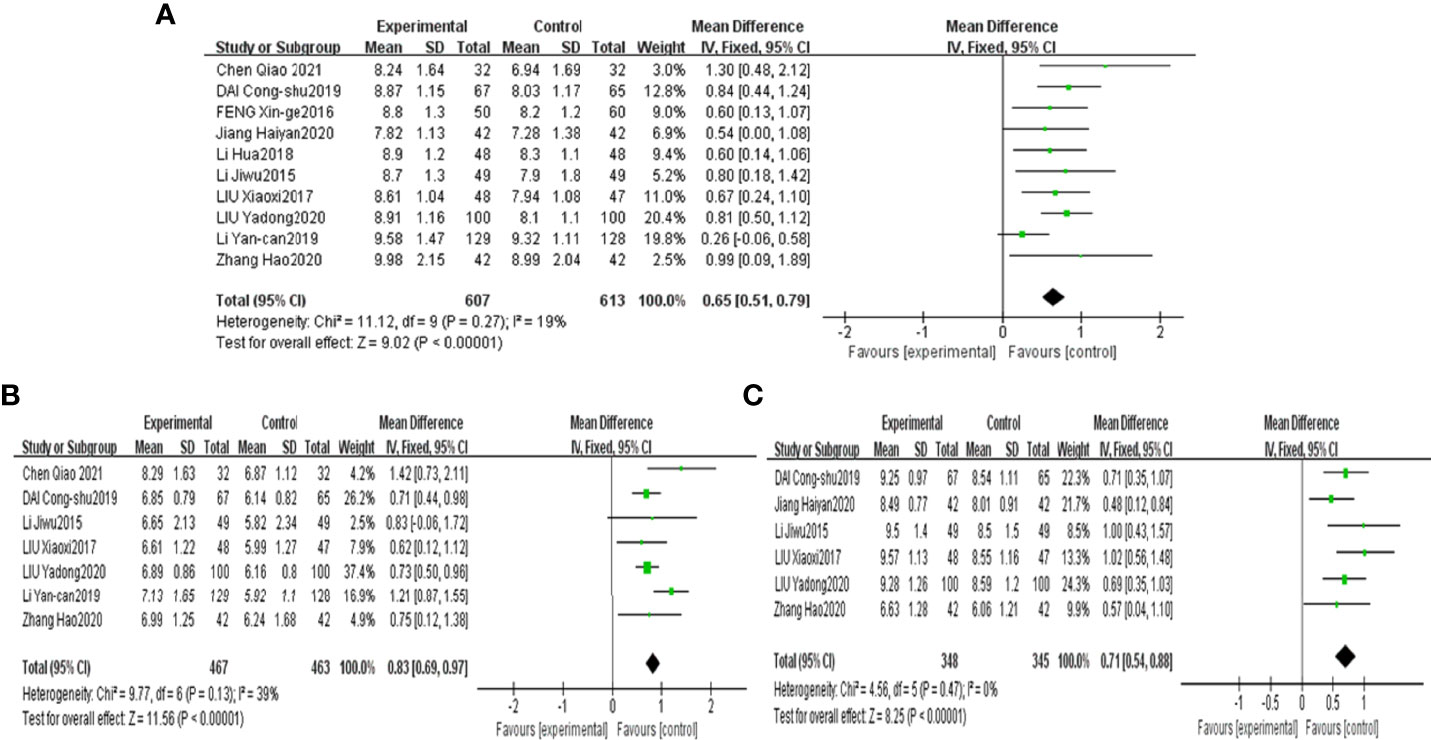
Figure 4 Forest plots of Bifidobacterium (A), Lactobacillus (B) and Bacteroidetes (C) for the comparison of CHF+metformin treatment versus metformin monotherapy.
3.4.2 Opportunistic pathogenic bacteria
To compare the effects of combined CHF and metformin treatment with those of metformin monotherapy on the quantitative changes in opportunistic pathogenic bacteria, 9 studies (36, 39–43, 45–47) describing the changes in Enterobacteriaceae, 8 studies (36, 37, 39–43, 47) describing the changes in Enterococcus and 6 studies (39–43, 47) describing the changes in Saccharomyces were included in the meta-analysis.
The heterogeneity for Enterococcus was found to be moderate (I²=59%); therefore, a random effect model was used to analyse the outcome. The heterogeneity for Enterobacteriaceae and Saccharomyces were found to be high (I²=86% and I²=98%). The sensitivity analyses suggested that the study by LI Yan-can 2019 obviously influenced the overall combined Enterobacteriaceae and Saccharomyces outcomes, as shown in Figures 3B, C. After eliminating the study by LI Yan-can 2019 (43), the I² of Enterobacteriaceae and Saccharomyces decreased to 40% and 70%, respectively.
The results of the meta-analysis revealed that combined CHF and metformin treatment led to a lower abundance of Enterobacteriaceae, Enterococcus and Saccharomyces than metformin, with the results being significantly different [MD=-0.71, 95% CI (-0.83, -0.60), P<0.00001], [MD=-0.92, 95% CI (-1.09, -0.76), p<0.00001], and [MD=-0.64, 95% CI (-0.85, -0.43), p<0.00001], as shown in Figures 5A–C, respectively.
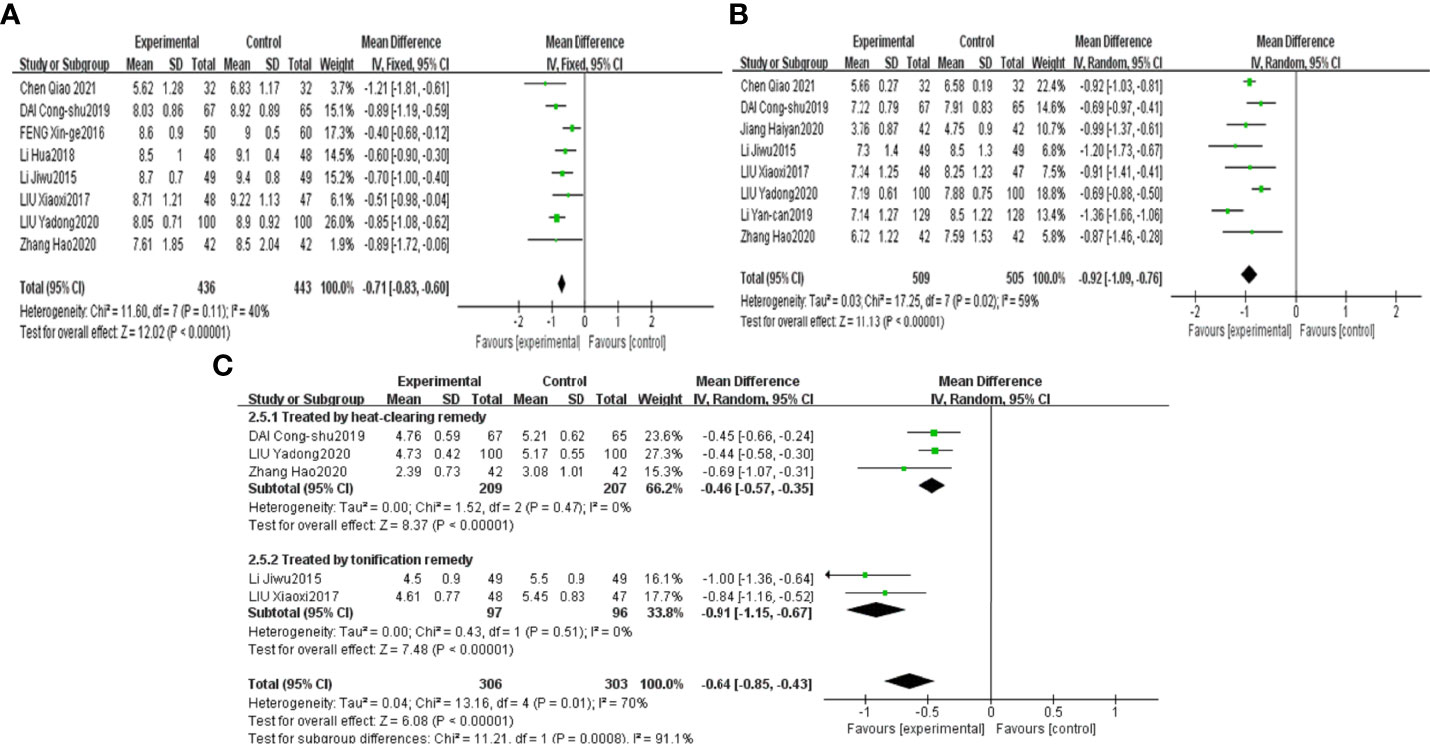
Figure 5 Forest plots of Enterobacteriaceae (A), Enterococcus (B) and Saccharomyces (C) for the comparison of CHF+metformin treatment versus metformin monotherapy.
3.4.3 Changes in glucose parameters
To compare the effects of combined CHF and metformin treatment with those of metformin monotherapy on the changes in glucose parameters, 8 studies (36, 38, 40–42, 44–46) comparing the changes in FPG, 7 studies (36, 38, 40, 42, 44–46) comparing the changes in 2hPG, 6 studies (37, 38, 42, 44–46) comparing the changes in HbA1c, 5 studies comparing the changes in FINS (40–42, 44, 47) and 4 studies comparing the changes in HOMA-IR (40–42, 47) were included in the analyses.
The heterogeneity for the six outcomes was high. According to the sensitivity analyses of the six outcomes, the influence of individual studies on the overall outcome was stable, as shown in Figures 3D–H.
The results of the meta-analyses confirmed that combined CHF and metformin treatment contributed to remarkably lower levels of FPG, 2hPG, HbA1c, FINS and HOMA-IR than metformin alone [MD=-0.83, 95% CI (-1.26, -0.41), p<0.00001], [MD=-1.58, 95% CI (-2.30, -0.86), p<0.00001], [MD=-1.49, 95% CI (-2.72, -0.27), p<0.00001], [MD=-1.54, 95% CI (-2.20, -0.88), p<0.00001], and [MD=-0.64, 95% CI (-0.87, -0.41), p<0.00001], as shown in Figures 6A–C, Figures 7A, B, respectively.
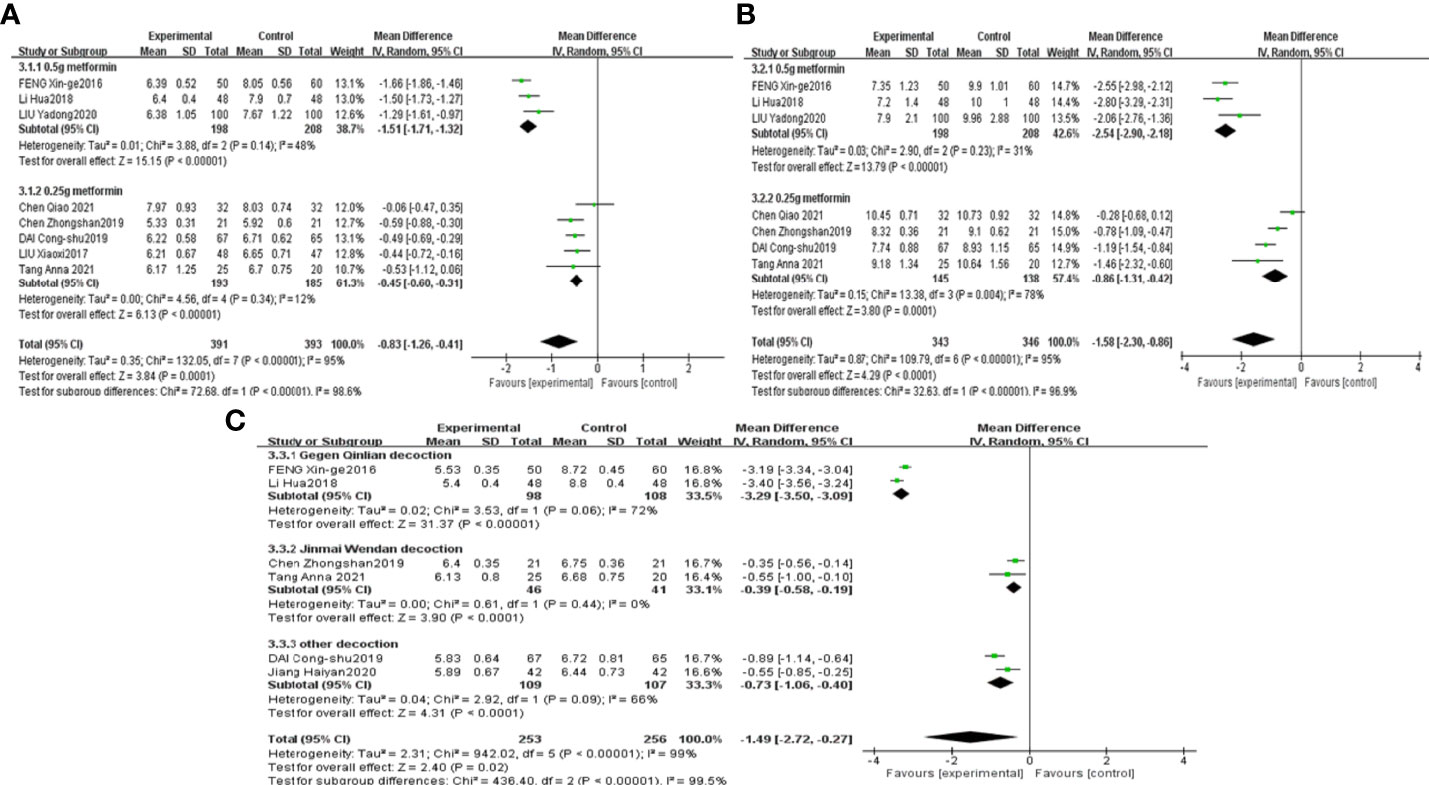
Figure 6 Forest plots of FPG (A), 2hPG (B), HbA1c (C) for the comparison of CHF+metformin treatment versus metformin monotherapy.
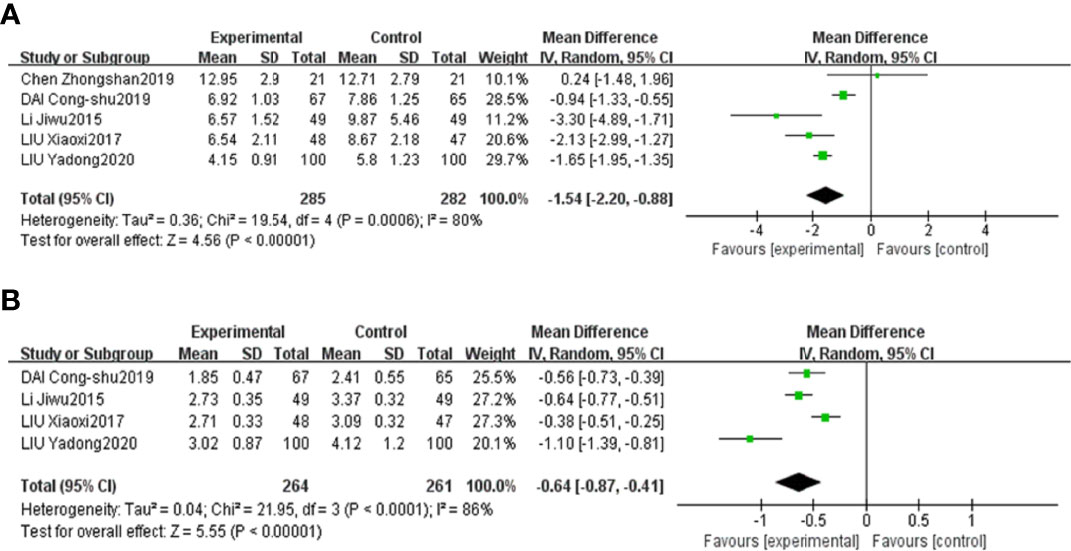
Figure 7 Forest plots of FINS (A) and HOMA-IR (B) for the comparison of CHF+metformin treatment versus metformin monotherapy.
3.5 Subgroup analyses
Sensitivity analyses showed that the overall results for Saccharomyces, FPG, 2hPG, HbA1c, FINS and HOMA-IR were stable. Only 5 studies and 4 studies individually analysed the changes in FINS and HOMA-IR, respectively; therefore, an inadequate sample size might be the source of the heterogeneity for FINS and HOMA-IR. Thus, subgroup analyses were conducted for Saccharomyces, FPG, 2hPG, and HbA1c to further verify the source of heterogeneity.
Saccharomyces and HbA1c results were grouped by the therapeutic principal classification of the CHFs. The results of 3 trials involving CHFs with the therapeutic principle of heat clearing (39, 40, 42) illustrated that Saccharomyces in the combined treatment group was notably lower than that in the metformin treatment group [MD=-0.46, 95% CI (-0.57, -0.35), P<0.00001]. The results of 2 studies involving CHFs with the therapeutic principal of “tonification” (41, 47) suggested that combination treatment contributed to a noteworthy lower level of Saccharomyces than metformin treatment [MD=-0.91, 95% CI (-1.15, -0.67), P<0.00001], as shown in Figure 5C. The results of studies (45, 46) using Gegen Qinlian decoction revealed that the level of HbA1c in the combined treatment group was notably lower than that in the metformin treatment group [MD=-3.29, 95% CI (-3.50, -3.09), p<0.00001]. The results of 2 studies (38, 44) using Jinmai Wendan decoction illustrated that the combined treatment led to a lower level of HbA1c than metformin treatment [MD=-0.39, 95% CI (-0.58, -0.19), p<0.0001]. The results of 2 other studies (37, 42) that used other CHFs revealed that combined treatment contributed to a notably lower level of HbA1c than metformin treatment [MD=-0.73, 95% CI (-1.06, -0.40), p<0.0001], as shown in Figure 6C.
FPG and 2hPG studies were grouped according to the doses of metformin administered to investigate the effect of different doses of metformin on glucose metabolism. The results of 3 studies (40, 45, 46) using 0.5 g metformin revealed that combined treatment contributed to a lower level of FPG and 2hPG than metformin treatment, [MD=-1.51, 95% CI (-1.71, -1.32), p<0.00001] and [MD=-2.54, 95% CI (-2.90, -2.18), p<0.00001], respectively. The results of 5 studies (36, 38, 41, 42, 44) using 0.25 g metformin confirmed that FPG in the combined treatment group was notably lower than that in the metformin treatment group [MD=-0.45, 95% CI (-0.60, -0.31), p<0.00001]. The results of 4 studies (36, 38, 42, 44) using 0.25 g metformin also confirmed that the 2hPG in the combined treatment group was notably lower than that in the metformin treatment group [MD=-0.86, 95% CI (-1.31, -0.42), p=0.0001], as shown in Figures 6A, B.
3.6 Publication bias analysis
Taking Bifidobacterium and Enterobacteriaceae as examples, it was observed that the included trials showed balanced distributions in Begg’s funnel and Egger’s publication bias plots, as shown in Figures 8A–D. Combined with analysing the data of Pr > |z| in Begg’s tests and Pr > |t| in Egger’s tests, which both tested > 0.05, it was found that there was no significant publication bias in the analysed outcomes in these studies. The data of Pr > |z| and Pr > |t| are summarized in Table 5.
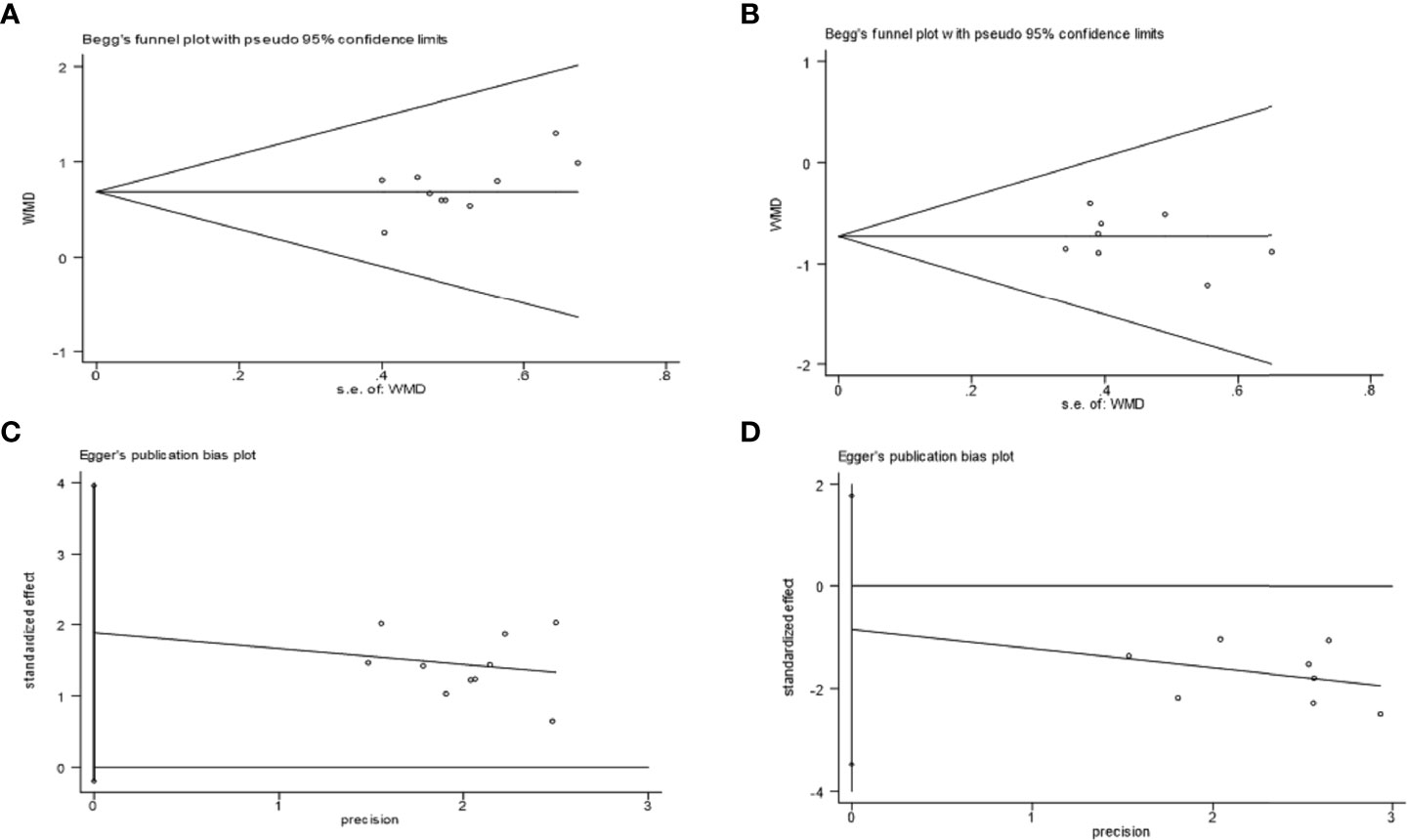
Figure 8 Examples of Begg’s funnel plots: Bifidobacterium (A) and Enterobacteriaceae (B); examples of Egger’s publication bias plots: Bifidobacterium (C) and Enterobacteriaceae (D).
4 Discussion
In recent years, new lines of evidence have shown the vital role of the gut microbiota in the pathophysiology of diabetes. The mutual relationship between the gut microbiota and glucose metabolism is mainly associated with changes in intestinal permeability, bile acid metabolism, serum lipopolysaccharide (LPS) levels and short-chain fatty acid (SCFA) production (48). The specific mechanisms are as follows: (i) The alterations in the gut microbiota in T2DM, which reduce key functional proteins in intestinal epithelial cells and increase intestinal mucosal permeability to promote the release of LPS, followed by the generation of inflammatory cytokines, can decrease the insulin sensitivity of the body as a consequence (49). (ii) The increased synthesis of intestinal branched-chain amino acids in line with the elevated concentration of branched-chain amino acids in the circulation is a powerful biomarker of insulin resistance and has the potential to increase the onset risk of T2DM (50), suggesting that changes in the synthesis and concentration of branched-chain amino acids may be a bridge between the gut microbiota and the onset of T2DM. (iii) Single-chain fatty acids (SCFAs) might be one of the bridges between the gut microbiota and T2DM. The gut microbiota can decompose carbohydrates transformed from intestinal food into SCFAs, which can regulate the inflammatory response and insulin sensitivity by stimulating the secretion of glucagon-like peptide-1 (GLP-1), accompanied by promoting liver lipid metabolism to maintain glucose homeostasis (51). (iv) The concentration and composition of bile acids were found to be modified in T2DM patients. The mutual interaction between the gut microbiota and bile acids can influence the secretion of GLP-1, which contributes to regulating the dynamic balance of glucose metabolism (52). (v) It is believed that the gut microbiota also has a potential impact on the immune system to varying degrees. Reports have revealed that when intestinal bacteria are introduced into aseptic mice, the function of the immune system is restored by triggering an immune response, which can further promote the metabolic function of the body (53). These results highlight that the gut microbiota can serve as a novel intervention target in the treatment of diabetes.
In treating type 2 diabetes, Chinese herbal formulas, as one of the important constituents in traditional Chinese medicine (TCM), are now used as a potential adjuvant therapy for treating various diseases by modulating gut microbiota and promoting host metabolism (54–57). Drugs with oral administration can promote the host metabolism and modulate the structure of gut microbiota (58). In term, the gut microbiota could mediate the metabolism of bioactive constituents in oral drugs via various targets (59). Previous studies further provided the reliable and direct evidence for the additive and synergistic effects of CHFs and metformin on modulating gut microbiota and ameliorating metabolic disorders in type 2 diabetic patients (60–62). A multicenter, randomized, open label clinical trial has showed that metformin and CHFs changed the gut microbiota composition of T2DM patients. Experiments in vivo and in vitro have validated that Huangkui capsules combined with metformin treatment effectively improved the weight, reduced blood glucose and ameliorated renal fibrosis via the Klotho/TGF-β1/p38 MAPK signalling pathway (28). Shin NR (63) mentioned that the Flos Lonicera, a bioactive component in CHFs, could modulate the gut microbial composition by reverting LPS-related pathways when combined with metformin. Wang K (64) also suggested that Houttuynia cordata, also a key component in CHFs, could suppress MCP-1, IL-6, TLR4 accompanied by modulating the abundances of Gram-negative bacteria when combined with metformin. A randomized placebo-controlled double-blind study indicated that metformin combined with Jianyutangkang therapy had a significant antidiabetic effect and improved lipid profiles with less adverse effects (hepatotoxicity or nephrotoxicity) (60). Chen WP (65) demonstrated that Danhong Huayu Koufuye combined with metformin had a preventive and therapeutic effect on diabetic retinopathy by alleviating hyperglycaemia, reducing oxidative stress and improving lipid metabolism. Liu X (66) concluded that Huoxue Jiangtang decoction combined with metformin could significantly lower blood glucose and serum cholesterol levels and exerted protective effects on kidney tissue damage. Lian F (67) showed that Jinlida combined with metformin significantly improved β-cell function compared to metformin monotherapy. Cao Y (5) showed that Fuzhu jiangtang granules combined with metformin had beneficial effects on lowering FBG, alleviating insulin resistance and restoring glucose tolerance in diabetic rats, possibly by regulating the PI3K/Akt signalling pathway in skeletal muscle. Han K (68) reported that bile acids promoting gut microbiota, such as Lactobacillus and Bacteroides, were higher in the combined treatment group than that in the metformin treatment group. Positive changes in the gut microbiota and host metabolism observed in the CHFs combined with metformin treatment further confirmed the valuable application of the combination treatment. Thus, the application of CHFs combined with metformin therapy has a considerable development prospect for the treatment of type 2 diabetes through the modulation of gut microbiota.
In this study, sensitivity analyses and subgroup analyses identified the sources of the heterogeneity. Sensitivity analyses showed that the overall results of the three outcomes, Bacteroidetes, Enterobacteriaceae and Saccharomyces, were interfered by the study LI Yan-can 2019 (43). The elimination of this study decreased the heterogeneity of the three outcomes to varying degrees (low or modest). The heterogeneity of this study is probably associated with inconsistency in sample size, drug dosage, treatment course, massage time and operation method (69). Then, subgroup analyses found that using 0.5 g or 0.25 g metformin and using CHFs with different therapeutic classifications exerted different effects on T2DM patients, which further suggested the sources of the heterogeneity.
First, different does of metformin influence the changes in FPG and 2hPG differently. Previous studies suggested that the efficacy of the hypoglycaemic activity of metformin was dose dependent (70). Metformin, a widely prescribed oral hypoglycaemic agent that has been used as the first-line treatment option for years (71), can facilitate better glucose homeostasis for diabetes patients through pleiotropic mechanisms (72). Metformin acts predominantly on the liver to inhibit neoglucogenesis and accelerate the muscle intake of glucose (73). Metformin also activates adenosine 5’-monophosphate-activated protein kinase (AMPK), inhibits the mitochondrial respiratory chain and improves insulin sensitivity (74). The recommended dose of metformin ranges from 250 mg/day to over 2000 mg/day in European and Asian countries in different tables of metformin dosing (75, 76). Studies have found that higher doses of metformin induced modestly greater levels of glycaemic control and greater weight and BMI reduction in metabolism-syndrome-related diseases (77). The findings from another randomized controlled trial revealed that metformin may suppress the formation of human colorectal ACF for colorectal cancer patients with IGT in a dose-dependent manner (250, 500, or 1500 mg/day) (78). Chen W (79) concluded that metformin exerted a positive effect by reducing inflammatory responses in a dose-dependent manner in patients with type 2 diabetes.
Second, the therapeutic classifications of CHFs might influence their effects on glucose metabolism and the gut microbiota. In this study, CHFs belonging to the classification of “heat-clearing” remedy included Gegen Qinlian decoction, Jinmai Wentan decoction, Qingfei Xiegan decoction, and Qingre Huoxue Huatan formula. The most frequently reported “heat-clearing” CHF in treating T2DM is Gegen Qinlian decoction (GQD), which was originally used to treat bacterial diarrhoea (80), and GQD was reported to alleviate the symptoms of T2DM in current studies (56). R. Li (81) showed that GQD inhibits the inflammatory signalling pathway and enhances antioxidant effects. C. H (82) proved that GQD plays a positive role in improving glucose metabolism by increasing the insulin sensitivity index for T2DM patients. Huang ZQ (83) found that GQD has a dose-effect relationship with controlling FBG levels in 2-DM model rats, providing new insight into the evaluation of the dose-effect relationship of TCMs. In addition, CHFs belonging to the therapeutic classification of “tonification” remedy included Shenling Baizhu powder, Tonifying Qi and strengthening spleen decoction, and Wenyang Yiqi Huoxue formula. Shenling Baizhu powder (SBP), as a representative Pi-tonifying prescription, is used to treat the syndrome of Pi-deficient diarrhoea. Xiao Y’s research (84) evaluated the underlying mechanism of SBP against Pi-deficient diarrhoea and found that high-dose SBP can modulate the absorption function of the intestine and the immunity function of intestinal mesenteric lymph nodes. Wang X (85) indicated that SBP could significantly inhibit virus replication and proliferation by regulating the TLR4/MyD88/NF-κB signalling pathway. SBP was also administered as an adjuvant therapy to reduce the expression level of serum TH1 cytokines, reduce the inflammatory response, and improve the clinical efficacy in ulcerative colitis patients (86). Tang K (57) found that SBP exerts a positive effect by ameliorating insulin resistance and correcting lipid metabolism disorders.
Moreover, identifying the key components in CHFs for the improvement of T2DM may tremendously promote our understanding of the mechanisms by CHFs acting against diabetes. Cui L (87) validated that oxidative and inflammation stress can be alleviated by some active components in Gegen Qinlian decoction(GQD), such as baicalin, glabridin and berberine, which were identified as the potential bioactive compounds of GQD in treating T2DM. Berberine, which was hypothesized as the key pharmaceutical ingredient in GQD, was reported to significantly alleviate hyperglycaemia and alter gut microbiota structure as GQD did (56, 88) by preventing and alleviating obesity and insulin resistance (69). An integrated system pharmacology analysis then revealed that various targets such as PPARG, RELA, EGFR and numerous pathways such as TNF signalling, PI3K-Akt signalling, MAPK signalling and NF-κB signalling were involved in the underlying mechanisms of GQD in improving diabetic insulin resistance (89). In GQD, berberine can enhance the preferable antidiabetic activity of metformin by modulating the distribution of metformin in different organs (90). Zhang X (91) illustrated the mechanisms by which berberine prevented obesity and insulin resistance by reducing opportunistic pathogens and enriching short-chain-fatty-acid-synthesizing bacteria. H. Zhou (92) showed that berberine can activate AMPK-dependent autophagy to reduce inflammation and insulin resistance. L. Ye (93) indicated that berberine improved insulin resistance possibly through inhibiting the activation of macrophages in adipose tissue. Another typical CHF used in treating diabetes is Shenling Baizhu powder(SBP). In Shenling Baizhu powder, Baizhu which contained the inulin-type oligosaccharides, was thought as a key component in SBP to improve the abundance of SCFAs-generating gut microbiota (94). Thus, alleviating inflammation stress and shifting the gut microbiota by targeting certain pathways and signals may be the mechanisms of bioactive constituents, such as berberine and Baizhu in GQD and SBP, to alleviate insulin resistance and reshape host metabolism.
However, inadequate studies have compared and analysed the mechanism of how metformin and CHFs in different forms influence hyperglycaemia and gut microbiota differently. Thus, we aimed to explore the mechanism of metformin doses and CHF classifications on controlling hyperglycaemia and altering gut microbiota. Moreover, although the publication bias analyses did not show significant bias in these analysed studies, some limitations of this study should be pointed out. First, the methodological designs of many of the included studies still have flaws; therefore, future studies of higher methodological quality are needed. Second, the direct pharmacological mechanisms studies of the CHFs are meeting difficulties because of the diverse components of CHFs and complex interactions between constituents through physical and chemical reaction (95). Therefore, more well-designed RCTs with objective methods are needed to elucidate and investigate the effects of each component on enhancing the structure of the gut microbiota in the treatment of diabetes.
5 Conclusion
In conclusion, the results of this meta-analysis are inspiring and provide evidence supporting the potential effectiveness and safety of combined CHF and metformin therapy in controlling hyperglycemia and regulating the gut microbiota. Despite some limitations, the results contribute to supporting the value of combined CHF and metformin treatment in diabetes patients. Moreover, more well-designed RCTs are needed to explore the underlying mechanism of this combination treatment and to verify the treatment value in the amelioration of type 2 diabetes mellitus.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Author contributions
YX and YZ designed the study and were in charge of the study concept. SJ and JC searched online databases and screened the eligible studies. YX and SZ extracted data and collected the data. SZ and XZ evaluated the methodological quality of each included study. YX interpreted the results and wrote the manuscript. YZ resolved disagreements, revised the manuscript and provided funding. All authors reviewed the manuscript.
Funding
This research was supported by the Sichuan Provincial Administration of Traditional Chinese Medicine (Grant Number: 2021MS457).
Acknowledgments
I would like to acknowledge all of my team members who provided a great deal of support and assistance to fulfil this research.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
T2DM, type 2 diabetes mellitus; TCM, traditional Chinese medicine; FPG, fasting plasma glucose; 2hPG, 2-hour postprandial blood glucose; HbA1c, glycated haemoglobin; FINS, fasting insulin; HOMA-IR, homeostasis model assessment of insulin resistance; NMPN, national medicine permission number.
References
1. Campbell MD, Sathish T, Zimmet PZ, Thankappan KR, Oldenburg B, Owens DR, et al. Benefit of lifestyle-based T2DM prevention is influenced by prediabetes phenotype. Nat Rev Endocrinol (2020) 16(7):395–400. doi: 10.1038/s41574-019-0316-1
2. Forslund K, Hildebrand F, Nielsen T, Falony G, Le Chatelier E, Sunagawa S, et al. Disentangling type 2 diabetes and metformin treatment signatures in the human gut microbiota. Nature (2015) 528(7581):262–6. doi: 10.1038/nature15766
3. Magkos F, Hjorth MF, Astrup A. Diet and exercise in the prevention and treatment of type 2 diabetes mellitus. Nat Rev Endocrinol (2020) 16(10):545–55. doi: 10.1038/s41574-020-0381-5
4. Permutt MA, Wasson J, Cox N. Genetic epidemiology of diabetes. J Clin Invest (2005) 115(6):1431–9. doi: 10.1172/JCI24758
5. Cao Y, Sun W, Xu G. Fuzhu jiangtang granules combined with metformin reduces insulin resistance in skeletal muscle of diabetic rats via PI3K/Akt signaling. Pharm Biol (2019) 57(1):660–8. doi: 10.1080/13880209.2019.1659831
6. Sharma N, Navik U, Tikoo K. Unveiling the presence of epigenetic mark by lactobacillus supplementation in high-fat diet-induced metabolic disorder in sprague-dawley rats. J Nutr Biochem (2020) 84:108442. doi: 10.1016/j.jnutbio.2020.108442
7. Liu X, Chen Y, Zhang S, Dong L. Gut microbiota-mediated immunomodulation in tumor. J Exp Clin Cancer Res (2021) 40(1):221. doi: 10.1186/s13046-021-01983-x
8. Ianiro G, Tilg H, Gasbarrini A. Antibiotics as deep modulators of gut microbiota: between good and evil. Gut (2016) 65(11):1906–15. doi: 10.1136/gutjnl-2016-312297
9. Xu J, Lian F, Zhao L, Zhao Y, Chen X, Zhang X, et al. Structural modulation of gut microbiota during alleviation of type 2 diabetes with a Chinese herbal formula. ISME J (2015) 9(3):552–62. doi: 10.1038/ismej.2014.177
10. Adeshirlarijaney A, Gewirtz AT. Considering gut microbiota in treatment of type 2 diabetes mellitus. Gut Microbes (2020) 11(3):253–64. doi: 10.1080/19490976.2020.1717719
11. Cani PD, Osto M, Geurts L, Everard A. Involvement of gut microbiota in the development of low-grade inflammation and type 2 diabetes associated with obesity. Gut Microbes (2012) 3(4):279–88. doi: 10.4161/gmic.19625
12. Sharma S, Tripathi P. Gut microbiome and type 2 diabetes: where we are and where to go? J Nutr Biochem (2019) 63:101–8. doi: 10.1016/j.jnutbio.2018.10.003
13. Canfora EE, Meex RCR, Venema K, Blaak EE. Gut microbial metabolites in obesity, NAFLD and T2DM. Nat Rev Endocrinol (2019) 15(5):261–73. doi: 10.1038/s41574-019-0156-z.10
14. Cani PD, Possemiers S, Van de Wiele T, Guiot Y, Everard A, Rottier O, et al. Changes in gut microbiota control inflammation in obese mice through a mechanism involving GLP-2-driven improvement of gut permeability. Gut (2009) 58(8):1091–103. doi: 10.1136/gut.2008.165886
15. Foretz M, Hébrard S, Leclerc J, Zarrinpashneh E, Soty M, Mithieux G, et al. Metformin inhibits hepatic gluconeogenesis in mice independently of the LKB1/AMPK pathway via a decrease in hepatic energy state. J Clin Invest (2010) 120(7):2355–69. doi: 10.1172/JCI40671
16. Sun L, Xie C, Wang G, Wu Y, Wu Q, Wang X, et al. Gut microbiota and intestinal FXR mediate the clinical benefits of metformin. Nat Med (2018) 24(12):1919–29. doi: 10.1038/s41591-018-0222-4
17. Wu H, Esteve E, Tremaroli V, Khan MT, Caesar R, Mannerås-Holm L, et al. Metformin alters the gut microbiome of individuals with treatment-naive type 2 diabetes, contributing to the therapeutic effects of the drug. Nat Med (2017) 23(7):850–8. doi: 10.1038/nm.4345
18. Pan L, Li Z, Wang Y, Zhang B, Liu G, Liu J. Network pharmacology and metabolomics study on the intervention of traditional Chinese medicine huanglian decoction in rats with type 2 diabetes mellitus. J Ethnopharmacol (2020) 258:112842. doi: 10.1016/j.jep.2020.112842
19. Hu RF, Sun XB. Design of new traditional Chinese medicine herbal formulae for treatment of type 2 diabetes mellitus based on network pharmacology. Chin J Nat Med (2017) 15(6):436–41. doi: 10.1016/S1875-5364(17)30065-1
20. Jin D, Tian J, Bao Q, Zhang H, Ding Q, Lian F, et al. Does adjuvant treatment with Chinese herbal medicine to antidiabetic agents have additional benefits in patients with type 2 diabetes? a system review and meta-analysis of randomized controlled trials. Evid Based Complement Alternat Med (2019) 2019:1825750. doi: 10.1155/2019/1825750
21. Tong XL, Wu ST, Lian FM, Zhao M, Zhou SP, Chen XY, et al. The safety and effectiveness of TM81, a Chinese herbal medicine, in the treatment of type 2 diabetes: a randomized double-blind placebo-controlled trial. Diabetes Obes Metab (2013) 15(5):448–54. doi: 10.1111/dom.12051
22. Lian F, Li G, Chen X, Wang X, Piao C, Wang J, et al. Chinese Herbal medicine tianqi reduces progression from impaired glucose tolerance to diabetes: a double-blind, randomized, placebo-controlled, multicenter trial. J Clin Endocrinol Metab (2014) 99(2):648–55. doi: 10.1210/jc.2013-3276
23. Ren DD, Li J, Chang B, Li CS, Yang JH. Early intervention with didang decoction delays macrovascular lesions in diabetic rats through regulating AMP-activated protein kinase signaling pathway. Chin J Nat Med (2017) 15(11):847–54. doi: 10.1016/S1875-5364(18)30018-9
24. Zheng J, Chen M, Ye C, Sun X, Jiang N, Zou , et al. BuZangTongLuo decoction improved hindlimb ischemia by activating angiogenesis and regulating gut microbiota in diabetic mice. J Ethnopharmacol (2020) 248:112330. doi: 10.1016/j.jep.2019.112330
25. Zheng Y, Ding Q, Wei Y, Gou X, Tian J, Li M, et al. Effect of traditional Chinese medicine on gut microbiota in adults with type 2 diabetes: A systematic review and meta-analysis. Phytomedicine (2021) 88:153455. doi: 10.1016/j.phymed.2020.153455
26. Zhang X, Cao D, Yan M, Liu M. The feasibility of Chinese massage as an auxiliary way of replacing or reducing drugs in the clinical treatment of adult type 2 diabetes: A systematic review and meta-analysis. Med (Baltimore) (2020) 99(34):e21894. doi: 10.1097/MD.0000000000021894
27. Zhang Y, An H, Pan SY, Zhao DD, Zuo JC, Li XK, et al. Jiang tang xiao ke granule, a classic Chinese herbal formula, improves the effect of metformin on lipid and glucose metabolism in diabetic mice. Evid Based Complement Alternat Med (2016) 2016:1592731. doi: 10.1155/2016/1592731
28. Gu LY, Yun-Sun, Tang HT, Xu ZX. Huangkui capsule in combination with metformin ameliorates diabetic nephropathy via the Klotho/TGF-β1/p38MAPK signaling pathway. J Ethnopharmacol (2021) 281:113548. doi: 10.1016/j.jep.2020.113548
29. Tang J, Fan L, Lv C, Wan R, Liu J, Liu X. Efficacy of shenqi jiangtang granules-assisted Western medicine in the treatment of gestational diabetes mellitus (GDM). Iran J Public Health (2021) 50(11):2191–201. doi: 10.18502/ijph.v50i11.7573
30. Yue SJ, Wang WX, Yu JG, Chen YY, Shi XQ, Yan D, et al. Gut microbiota modulation with traditional Chinese medicine: A system biology-driven approach. Pharmacol Res (2019) 148:104453. doi: 10.1016/j.phrs.2019.104453
31. Tong X, Xu J, Lian F, Yu X, Zhao Y, Xu L, et al. Structural alteration of gut microbiota during the amelioration of human type 2 diabetes with hyperlipidemia by metformin and a traditional Chinese herbal formula: a multicenter, randomized, open label clinical trial. mBio (2018) 9(3):e02392–17. doi: 10.1128/mBio.02392-17
32. Shin NR, Gu N, Choi HS, Kim H. Combined effects of Scutellaria baicalensis with metformin on glucose tolerance of patients with type 2 diabetes via gut microbiota modulation. Am J Physiol Endocrinol Metab (2020) 318(1):E52–61. doi: 10.1152/ajpendo.00221.2019
33. Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PloS Med (2009) 6(7):e1000097. doi: 10.1371/journal.pmed.1000097
34. Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, et al. The cochrane collaboration's tool for assessing risk of bias in randomised trials. BMJ (2011) 343:d5928. doi: 10.1136/bmj.d5928
35. Wang D, Mou Z-Y, Zhai J-X, Zong H, Zhao X. Study on stata software in investigating publication bias in meta-analysis. Modern Prev Med (2008) 35(15):2819–22. doi: 10.3969/j.issn.1003-8507.2008.15.002.
36. Chen Q, Zhang S, Fan S, Zeng J. Clinical observation of gegen qinlian decoction in the treatment of diarrhea caused by metformin in patients with T2DM. Chin Manipulation Rehabil Med (2021) 12(15):23–5. doi: 10.19787/j.issn.1008-1879.2021.15.008.
37. Jiang H, Ge S, Yu X, Sun L, Peng J. A study of the gut microbiota changings and adverse effects from type 2 diabetes mellitus obesity patients treated with shenling baizhu powder and metformin. J Gansu Sci (2020) 32(6):9–13. doi: 10.16468/j.cnki.issn1004-0366.2020.06.002.
38. Tang A. Effect of jinmai wentan decoction on intestinal micro flora, gastrin and glucagon in patients with type 2 diabetes mellitus with phlegm and stasis interaction. Fujian Univ Traditional Med (2021); 01:01–54. doi: 10.27021/d.cnki.gfjzc.2021.000331.
39. Zhang H, Wei J, Ni M, Jin H. Study on the changes of intestinal flora after maren pill combined with metformin in the treatment of type 2 diabetes mellitus. J China Prescription Drug (2017) 15(12):91–2. doi: CNKI:SUN:ZGCF.0.2017-12-063.
40. Yadong L, Fang L, Xiao R, Xiegan Q. Decoction in treatment of type 2 diabetes with non-alcoholic fatty liver. Acta Chin Med (2020); 35(06):1312–6. doi: 10.16368/j.issn.1674-8999.2020.06.294.
41. Liu X, Li X, Shi Y, Wang L. Intervention study of tonifying qi and strengthening spleen method on intestinal flora in patients with type 2 diabetes. Liaoning J Trad Chin Med (2017) 44(11):2311–3. doi: 10.13192/j.issn.1000-1719.2017.11.023.
42. Cong-shu D, Zhuo Y, Guang-yao L, Chang-qing L. Regulatory effect of QingfeiXiegan tang on inflammatory response and intestinal flora of type 2 diabetesmellitus. Chin J Exp Traditional Med Formulae (2019) 25(18):83–8. doi: 10.13422/j.cnki.syfjx.20191327.
43. Li Y-c, Ye R, Ye H. Effect of qingre huoxue huatan recipe combined with metformin hydrochloride on intestinal flora in type 2 diabetic mellitus patients with constipation. Strait Pharm J (2019) 31(10):165–6. doi: CNKI:SUN:HAIX.0.2019-10-061.
44. Chen Z. Effect of jinmai wendan decoction on metabolic index, early insulin and intestinal flora in patients with type 2 diabetes mellitus with phlegm and blood stasis. Fujian Univ Traditional Med (2019); 08:01–46.
45. Li H. Clinical observation on 48 cases of type 2 diabetes treated with compound gegen qinlian decoction. Hunan J Traditional Chin Med (2018) 34(08):65–6. doi: 10.16808/j.cnki.issn1003-7705.2018.08.029.
46. Feng X-G, Yan Y-Z, Zeng Y-P, Gou Y-F. The effect of gegen qinlian decoction on intestinal flora in damp-heat syndrome of type 2 diabetes. World J Integrated Traditional Western Med (2016) 11(08):1110–2. doi: 10.13935/j.cnki.sjzx.160820.
47. Li J, Tang A, Zhao W, Zheng C. Effect of wenyang yiqi huoxue formula on the intestinal flora and lipid metabolism of obese type 2 diabetic patients. J Traditional Chin Med (2015) 56(04):409–13. doi: 10.13288/j.11-2166/r.2015.05.014.
48. Warmbrunn MV, Herrema H, Aron-Wisnewsky J, Soeters MR, Van Raalte DH, Nieuwdorp M, et al. Gut microbiota: a promising target against cardiometabolic diseases. Expert Rev Endocrinol Metab (2020) 15(1):13–27. doi: 10.1080/17446651.2020.1720511
49. Zhang Q, Hu N. Effects of metformin on the gut microbiota in obesity and type 2 diabetes mellitus. Diabetes Metab Syndr Obes (2020) 13:5003–14. doi: 10.2147/DMSO.S286430
50. Fan Y, Pedersen O. Gut microbiota in human metabolic health and disease. Nat Rev Microbiol (2021) 19(1):55–71. doi: 10.1038/s41579-020-0433-9
51. Koh A, De Vadder F, Kovatcheva-Datchary P, Bäckhed F. From dietary fiber to host physiology: Short-chain fatty acids as key bacterial metabolites. Cell (2016) 165(6):1332–45. doi: 10.1016/j.cell.2016.05.041
52. Fava S. Glucagon-like peptide 1 and the cardiovascular system. Curr Diabetes Rev (2014) 10(5):302–10. doi: 10.2174/1573399810666141030125830
53. Scheithauer TPM, Rampanelli E, Nieuwdorp M, Vallance BA, Verchere CB, van Raalte DH, et al. Gut microbiota as a trigger for metabolic inflammation in obesity and type 2 diabetes. Front Immunol (2020) 11:571731. doi: 10.3389/fimmu.2020.571731
54. Zhang Y, Tang K, Deng Y, Chen R, Liang S, Xie H, et al. Effects of shenling baizhu powder herbal formula on intestinal microbiota in high-fat diet-induced NAFLD rats. BioMed Pharmacother (2018) 102:1025–36. doi: 10.1016/j.biopha.2018.03.158
55. Meng M, Bai C, Wan B, Zhao L, Li Z, Li D, et al. A network pharmacology-based study on irritable bowel syndrome prevention and treatment utilizing shenling baizhu powder. BioMed Res Int (2021) 2021:4579850. doi: 10.1155/2021/4579850
56. Xu X, Gao Z, Yang F, Yang Y, Chen L, Han L, et al. Antidiabetic effects of gegen qinlian decoction via the gut microbiota are attributable to its key ingredient berberine. Genomics Proteomics Bioinf (2020) 18(6):721–36. doi: 10.1016/j.gpb.2019.09.007
57. Tang K, Deng Y, Zheng C, Nie H, Pan M, Chen R, et al. Prevention of nonalcoholic hepatic steatosis by shenling baizhu powder: Involvement of adiponectin-induced inhibition of hepatic SREBP-1c. Oxid Med Cell Longev (2020) 2020:9701285. doi: 10.1155/2020/9701285
58. Feng W, Ao H, Peng C, Yan D. Gut microbiota, a new frontier to understand traditional Chinese medicines. Pharmacol Res (2019) 142:176–91. doi: 10.1016/j.phrs.2019.02.024
59. Liu CS, Liang X, Wei XH, Chen FL, Tang QF, Tan XM. Comparative metabolism of the eight main bioactive ingredients of gegen qinlian decoction by the intestinal flora of diarrhoeal and healthy piglets. BioMed Chromatogr (2019) 33(3):e4421. doi: 10.1002/bmc.4421
60. Hu Y, Zhou X, Liu P, Wang B, Duan DM, Guo DH. A comparison study of metformin only therapy and metformin combined with Chinese medicine jianyutangkang therapy in patients with type 2 diabetes: A randomized placebo-controlled double-blind study. Complement Ther Med (2016) 24:13–8. doi: 10.1016/j.ctim.2015.11.005
61. Xu X, Niu L, Liu Y, Pang M, Lu W, Xia C, et al. Study on the mechanism of gegen qinlian decoction for treating type II diabetes mellitus by integrating network pharmacology and pharmacological evaluation. J Ethnopharmacol (2020) 262:113129. doi: 10.1016/j.jep.2020.113129
62. Xu Y, Huang J, Wang N, Tan HY, Zhang C, Li S, et al. Network pharmacology-based analysis and experimental exploration of antidiabetic mechanisms of gegen qinlian decoction. Front Pharmacol (2021) 12:649606. doi: 10.3389/fphar.2021.649606
63. Shin NR, Bose S, Wang JH, Ansari A, Lim SK, Chin YW, et al. Flos lonicera combined with metformin ameliorates hepatosteatosis and glucose intolerance in association with gut microbiota modulation. Front Microbiol (2017) 8:2271. doi: 10.3389/fmicb.2017.02271
64. Wang K, Yang Q, Ma Q, Wang B, Wan Z, Chen M, et al. Protective effects of salvianolic acid a against dextran sodium sulfate-induced acute colitis in rats. Nutrients (2018) 10(6):791. doi: 10.3390/nu10060791
65. Chen WP, Wang YD, Ma Y, Zhang ZY, Hu LY, Lin JL, et al. Danhong huayu koufuye combined with metformin attenuated diabetic retinopathy in zucker diabetic fatty rats. Int J Ophthalmol (2015) 8(6):1094–100. doi: 10.3980/j.issn.2222-3959.2015.06.03
66. Liu X, Liu D, Shuai Y, Li H, Zhao H, Qu X, et al. Effects of HuoxueJiangtang decoction alone or in combination with metformin on renal function and renal cortical mRNA expression in diabetic nephropathy rats. Pharm Biol (2020) 58(1):1123–30. doi: 10.1080/13880209.2020.1844242
67. Lian F, Tian J, Chen X, Li Z, Piao C, Guo J, et al. The efficacy and safety of Chinese herbal medicine jinlida as add-on medication in type 2 diabetes patients ineffectively managed by metformin monotherapy: A double-blind, randomized, placebo-controlled, multicenter trial. PloS One (2015) 10(6):e0130550. doi: 10.1371/journal.pone.0130550
68. Han K, Bose S, Wang JH, Lim SK, Chin YW, Kim YM, et al. In vivo therapeutic effect of combination treatment with metformin and scutellaria baicalensis on maintaining bile acid homeostasis. PloS One (2017) 12(9):e0182467. doi: 10.1371/journal.pone.0182467
69. Zhang X, Zhao Y, Zhang M, Pang X, Xu J, Kang C, et al. Structural changes of gut microbiota during berberine-mediated prevention of obesity and insulin resistance in high-fat diet-fed rats. PloS One (2012) 7(8):e42529. doi: 10.1371/journal.pone.0042529
70. Graham GG, Punt J, Arora M, Day RO, Doogue MP, Duong JK, et al. Clinical pharmacokinetics of metformin. Clin Pharmacokinet (2011) 50(2):81–98. doi: 10.2165/11534750-000000000-00000
71. Chaudhari K, Wang J, Xu Y, Winters A, Wang L, Dong X, et al. Determination of metformin bio-distribution by LC-MS/MS in mice treated with a clinically relevant paradigm. PloS One (2020) 15(6):e0234571. doi: 10.1371/journal.pone.0234571
72. Garza-Ocañas L, González-Canudas J, Tamez-de la O E, Badillo-Castañeda C, Gómez-Meza MV, Romero-Antonio Y, et al. Comparative bioavailability of metformin hydrochloride oral solution versus metformin hydrochloride tablets in fasting Mexican healthy volunteers. Adv Ther (2019) 36(2):407–15. doi: 10.1007/s12325-018-0853-3
73. Rena G, Hardie DG, Pearson ER. The mechanisms of action of metformin. Diabetologia (2017) 60(9):1577–85. doi: 10.1007/s00125-017-4342z
74. Campagnoli C, Pasanisi P, Abbà C, Ambroggio S, Biglia N, Brucato T, et al. Effect of different doses of metformin on serum testosterone and insulin in non-diabetic women with breast cancer: a randomized study. Clin Breast Cancer (2012) 12(3):175–82. doi: 10.1016/j.clbc.2012.03.004
75. Kanto K, Ito H, Noso S, Babaya N, Hiromine Y, Taketomo Y, et al. Effects of dosage and dosing frequency on the efficacy and safety of high-dose metformin in Japanese patients with type 2 diabetes mellitus. J Diabetes Investig (2017) 9(3):587–93. doi: 10.1111/jdi.12755
76. Nishimura R, Taniguchi M, Takeshima T, Iwasaki K. Efficacy and safety of metformin versus the other oral antidiabetic drugs in Japanese type 2 diabetes patients: A network meta-analysis. Adv Ther (2021), 39(1):632–654. doi: 10.1007/s12325-021-01979-1
77. Bruno RV, de Avila MA, Neves FB, Nardi AE, Crespo CM, Sobrinho AT. Comparison of two doses of metformin (2.5 and 1.5 g/day) for the treatment of polycystic ovary syndrome and their effect on body mass index and waist circumference. Fertil Steril (2007) 88(2):510–2. doi: 10.1016/j.fertnstert.2006.11.133
78. Zhao X, Li Y, Chen M, Chen Y, Dai Y, Wang Y, et al. Effects of different doses of metformin treatment for 6 months on aberrant crypt foci in Chinese patients with impaired glucose tolerance. Eur J Cancer Prev (2015) 24(1):27–36. doi: 10.1097/CEJ.0000000000000078
79. Chen W, Liu X, Ye S. Effects of metformin on blood and urine pro-inflammatory mediators in patients with type 2 diabetes. J Inflammation (Lond) (2016) 13:34. doi: 10.1186/s12950-016-0142-3
80. Zhao Y, Luan H, Jiang H, Xu Y, Wu X, Zhang Y, et al. Gegen qinlian decoction relieved DSS-induced ulcerative colitis in mice by modulating Th17/Treg cell homeostasis via suppressing IL-6/JAK2/STAT3 signaling. Phytomedicine (2021) 84:153519. doi: 10.1016/j.phymed.2021.153519
81. Li R, Chen Y, Shi M, Xu X, Zhao Y, Wu X, et al. Gegen qinlian decoction alleviates experimental colitis via suppressing TLR4/NF-κB signaling and enhancing antioxidant effect. Phytomedicine (2016) 23(10):1012–20. doi: 10.1016/j.phymed.2016.06.010
82. Zhang CH, Xu GL, Liu YH, Rao Y, Yu RY, Zhang ZW, et al. Anti-diabetic activities of gegen qinlian decoction in high-fat diet combined with streptozotocin-induced diabetic rats and in 3T3-L1 adipocytes. Phytomedicine (2013) 20(3-4):221–9. doi: 10.1016/j.phymed.2012.11.002
83. Huang ZQ, Fan XM, Wang YM, Liang QL, Tong XL, Bai Y, et al. A new method to evaluate the dose-effect relationship of a TCM formula gegen qinlian decoction: "Focus" mode of integrated biomarkers. Acta Pharmacol Sin (2017) 38(8):1141–9. doi: 10.1038/aps.2016.165
84. Xiao Y, Zhang K, Zhu SY, Deng XL, Chen XY, Fu NL, et al. Shenling baizhu powder ameliorates pi (Spleen)-Deficiency-Induced functional diarrhea in rats. Chin J Integr Med (2021) 27(3):206–11. doi: 10.1007/s11655-020-3259-4
85. Wang X, Yang Q, Zhou X, Chen T, Dou L, Wang F, et al. Shenling baizhu powder inhibits RV-SA11-Induced inflammation and rotavirus enteritis via TLR4/MyD88/NF-κB signaling pathway. Front Pharmacol (2021) 12:642685. doi: 10.3389/fphar.2021.642685
86. Li S, Hao X, Gong Y, Liu S, Niu W, Jia J, et al. Effect of shenling baizhu powder on the serum TH1 cytokines of elderly patients with ulcerative colitis complicated by bloody purulent stool. Am J Transl Res (2021) 13(8):9701–7. PMID: 34540098; PMCID: PMC8430119
87. Cui L, Feng L, Zhang ZH, Jia XB. The anti-inflammation effect of baicalin on experimental colitis through inhibiting TLR4/NF-kappaB pathway activation. Int Immunopharmacol (2014) 23:294–303. doi: 10.1016/j.intimp.2014.09.005
88. Zhang Y, Li X, Zou D, Liu W, Yang J, Zhu N, et al. Treatment of type 2 diabetes and dyslipidemia with the natural plant alkaloid berberine. J Clin Endocrinol Metab (2008) 93(7):2559–65. doi: 10.1210/jc.2007-2404
89. Cao Z, Zeng Z, Wang B, Liu C, Liu C, Wang Z, et al. Identification of potential bioactive compounds and mechanisms of GegenQinlian decoction on improving insulin resistance in adipose, liver, and muscle tissue by integrating system pharmacology and bioinformatics analysis. J Ethnopharmacol (2021) 264:113289. doi: 10.1016/j.jep.2020.113289
90. Shi R, Xu Z, Xu X, Jin J, Zhao Y, Wang T, et al. Organic cation transporter and multidrug and toxin extrusion 1 co-mediated interaction between metformin and berberine. Eur J Pharm Sci (2019) 127:282–90. doi: 10.1016/j.ejps.2018.11.010
91. Zhang X, Zhao Y, Xu J, Xue Z, Zhang M, Pang X, et al. Modulation of gut microbiota by berberine and metformin during the treatment of high-fat diet-induced obesity in rats. Sci Rep (2015) 5:14405. doi: 10.1038/srep14405
92. Zhou H, Feng L, Xu F, Sun Y, Ma Y, Zhang X, et al. Berberine inhibits palmitate-induced NLRP3 inflammasome activation by triggering autophagy in macrophages: A new mechanism linking berberine to insulin resistance improvement. BioMed Pharmacother (2017) 89:864–74. doi: 10.1016/j.biopha.2017.03.003
93. Ye L, Liang S, Guo C, Yu X, Zhao J, Zhang H, et al. Inhibition of M1 macrophage activation in adipose tissue by berberine improves insulin resistance. Life Sci (2016) 166:82–91. doi: 10.1016/j.lfs.2016.09.025
94. Lin Z, Liu YF, Qu Y, Shi LY, Dou DQ, Kuang HX. Characterisation of oligosaccharides from baizhu by HILIC-MS. Nat Prod Res (2015) 29(13):1194–200. doi: 10.1080/14786419.2014.995652
Keywords: type 2 diabetes mellitus, gut microbiota, metformin, Chinese herbal formula, combination treatment, meta-analysis
Citation: Xu Y, Zheng S, Jiang S, Chen J, Zhu X and Zhang Y (2022) The effect of Chinese herbal formulas combined with metformin on modulating the gut microbiota in the amelioration of type 2 diabetes mellitus: A systematic review and meta-analysis. Front. Endocrinol. 13:927959. doi: 10.3389/fendo.2022.927959
Received: 25 April 2022; Accepted: 15 August 2022;
Published: 15 September 2022.
Edited by:
Anouar Abidi, Facuty of Medicine of Tunis, TunisiaReviewed by:
Hsien-Hui Chung, Kaohsiung Veterans General Hospital, Taiwan;Uma Shanker Navik, Central University of Punjab, IndiaCopyright © 2022 Xu, Zheng, Jiang, Chen, Zhu and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ya Zhang, emhhbmd5YTE5NzgwNzExQDE2My5jb20=
 Yunxi Xu
Yunxi Xu Ya Zhang
Ya Zhang