
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Endocrinol., 11 July 2022
Sec. Thyroid Endocrinology
Volume 13 - 2022 | https://doi.org/10.3389/fendo.2022.927859
The association between glucagon-like peptide-1 (GLP-1) receptor agonists and the risk of various kinds of thyroid disorders remains uncertain. We aimed to evaluate the relationship between the use of GLP-1 receptor agonists and the occurrence of 6 kinds of thyroid disorders. We searched PubMed (MEDLINE), EMBASE, the Cochrane Central Register of Controlled Trials (CENTRAL) and Web of Science from database inception to 31 October 2021 to identify eligible randomized controlled trials (RCTs). We performed meta-analysis using a random-effects model to calculate risk ratios (RRs) and 95% confidence intervals (CIs). A total of 45 trials were included in the meta-analysis. Compared with placebo or other interventions, GLP-1 receptor agonists’ use showed an association with an increased risk of overall thyroid disorders (RR 1.28, 95% CI 1.03-1.60). However, GLP-1 receptor agonists had no significant effects on the occurrence of thyroid cancer (RR 1.30, 95% CI 0.86-1.97), hyperthyroidism (RR 1.19, 95% CI 0.61-2.35), hypothyroidism (RR 1.22, 95% CI 0.80-1.87), thyroiditis (RR 1.83, 95% CI 0.51-6.57), thyroid mass (RR 1.17, 95% CI 0.43-3.20), and goiter (RR 1.17, 95% CI 0.74-1.86). Subgroup analyses and meta-regression analyses showed that underlying diseases, type of control, and trial durations were not related to the effect of GLP-1 receptor agonists on overall thyroid disorders (all P subgroup > 0.05). In conclusion, GLP-1 receptor agonists did not increase or decrease the risk of thyroid cancer, hyperthyroidism, hypothyroidism, thyroiditis, thyroid mass and goiter. However, due to the low incidence of these diseases, these findings need to be examined further.
Systematic Review Registration: PROSPERO https://www.crd.york.ac.uk/prospero/, identifier: CRD42021289121.
Thyroid diseases are common in some metabolic disorders, such as diabetes mellitus (DM) and obesity. Thyroid dysfunction (TD) and DM are closely linked. A high prevalence of TD has been reported among both type 1 DM (T1DM) and type 2 DM (T2DM) patients (1, 2). Although the mechanism is unknown, epidemiological studies have indicated that obesity and T2DM are associated with increased risks of several cancers, including thyroid cancer (3–5). Furthermore, insulin resistance and hyperinsulinemia can lead to goiter, proliferation of thyroid tissues, and an increased incidence of nodular thyroid disease (6). In addition to the effects of the disease itself, some antidiabetic drugs can impact the hypothalamic–pituitary–thyroid (HPT) axis and thyroid function. For example, multiple studies have demonstrated that metformin can inhibit the growth of thyroid cells and different types of thyroid cancer cells, and metformin therapy has been associated with a decrease in the levels of serum thyroid-stimulating hormone (TSH) (7). Thiazolidinediones can induce thyroid-associated ophthalmopathy (8, 9). Recently, the relationship between glucagon-like peptide-1 (GLP-1) receptor agonists and thyroid cancer has attracted attention, but there is still controversy.
GLP-1 is an amino acid peptide hormone secreted by L cells of the gastrointestinal mucosa that promotes insulin secretion, suppresses glucagon secretion, and delays gastric emptying (10). Rodent studies have shown that the GLP-1 receptor agonist liraglutide can activate the GLP-1 receptor on thyroid C cells, leading to the release of calcitonin with a dose-dependent effect on the pathology of C cells (11). Some animal models have proven that exenatide or liraglutide treatment is related to the abnormal appearance of thyroid C cells, with gradual development of hyperplasia and adenomas (12, 13). Moreover, a study found that patients treated with exenatide had an increased risk of thyroid cancer by examining the US Food and Drug Administration’s database of reported adverse events (14). However, the results of A Long Term Evaluation (LEADER) trial that followed for 3.5-5 years showed no effect of GLP-1 receptor activation on human serum calcitonin levels, C-cell proliferation or C-cell malignancy (15). Nevertheless, GLP-1 receptor agonists are not recommended in patients with a personal or family history of medullary thyroid cancer or type 2 multiple endocrine neoplasia.
GLP-1 receptor agonists, a new type of antidiabetic drug for treating T2DM in recent years, with additional benefits of weight loss and blood pressure reduction (16). Although many large randomized controlled trials (RCTs) of GLP-1 receptor agonists have identified the obvious benefits of GLP-1 receptor agonists on cardiovascular and renal outcomes in patients with DM or obesity (17–20), the association between GLP-1 receptor agonists and various thyroid disorders remains controversial. In addition, considering that thyroid disorders are common in some metabolic diseases such as DM and obesity, we conducted this study. Thus, by comparing GLP-1 receptor agonists with placebo or other antidiabetic drugs, we conducted a meta-analysis of all available RCT data to evaluate the relationship between the use of GLP-1 receptor agonists and the occurrence of various kinds of thyroid disorders.
We searched PubMed (MEDLINE), EMBASE, Cochrane Central Register of Controlled Trials (CENTRAL) and Web of Science from database inception to 31 October 2021 to identify eligible RCTs without restriction of language or publication period. The search terms used were “glucagon-like peptide 1 receptor agonist”, “exenatide”, “liraglutide”, “dulaglutide”, “lixisenatide”, “semaglutide”, “albiglutide”, “taspoglutide”, “loxenatide”, “diabetes mellitus”, “obesity” and “randomized controlled trial”. In addition, we manually scanned the ClinicalTrials.gov web and reference lists from established trials and review articles.
The trials we included met the following criteria: (1) RCTs that compared GLP-1 receptor agonist with a placebo or active control (other antidiabetic drugs or insulin), (2) patients with type 2 diabetes, type 1 diabetes, prediabetes, overweight or obesity, (3) with durations of at least 24 weeks, and (4) reported the occurrence of at least one case of various thyroid disorders as adverse events. We excluded duplicate reports, conference abstracts, letters, case reports, editorials, articles without treatment-emergent adverse events, and animal experimental studies.
Two investigators (Hu and Song) independently extracted the following data by reviewing the full text of each study: first author, year of publication, Clinical Trial Registration Number (NCT ID), trial duration, patient characteristics, sample size, intervention (type of GLP-1 receptor agonist), comparators, and outcomes of interest. Any discrepancies were resolved by consensus or by the third reviewer (Chen). The primary outcome was the incidence of overall thyroid disorders, and the secondary outcomes included the incidence of goiter, hyperthyroidism, hypothyroidism, thyroiditis, thyroid mass, and thyroid cancer. When multiple reports from the same population were retrieved, the most complete or recently reported data were used. If thyroid-related events were not reported in publication, these data were extracted from the ‘Serious Adverse Events’ portion of ClinicalTrials.gov.
The quality of each included RCT was assessed by the Cochrane Risk-of-Bias Tool 1.0. The Jadad scale was also used to quantify the study quality. Two authors assessed the risk of bias for each study through five aspects: random sequence generation, allocation concealment, blinding, incomplete outcome data and selective reporting.
Dichotomous outcomes were analyzed by risk ratios (RRs) and 95% confidence intervals (CIs) using the DerSimonian and Laird random-effects model. We assessed heterogeneity between the included studies using the I² statistic, where I2 values of 25%, 50%, and 75% indicated low, medium, and high heterogeneity, respectively. Subgroup analyses were conducted according to the type of underlying diseases, type of control, and trial duration. Between-subgroup heterogeneity was assessed by χ2 tests and meta-regression. All of the above analyses were performed using Stata software 13.0 (Stata Corp). A p value < 0.05 was considered statistically significant.
A total of 16,201 records were identified by retrieving the aforementioned databases. Excluding duplicates and reviewing titles and abstracts, 301 studies were read the full text. After retrieving the full text and searching on ClinicalTrials.gov, the final analysis included 45 RCTs reported in 45 publications with 94063 participants (17–61). Although the data from the two articles were presented together on ClinicalTrials.gov (62), due to the differences in population characteristics and follow-up time, we considered them separately and regarded them as two independent trials (24, 25). The search and selection process is summarized in Figure 1. The characteristics of these included studies are detailed in Table 1 and Table S1. Across the 45 trials, trial duration ranged from 26 to 360 weeks. Of all the participants, 29,348 (55.8%) were men in the experimental group, and 24121 (58.2%) were men in the control group. The mean age of study participants ranged from 41.6 to 66.2 years old in experimental groups and 41.4 to 66.2 years old in control groups. Mean patient body mass index (BMI) ranged from 24.5 to 39.3 kg/m2 in experimental groups and 24.4 to 39.0 kg/m2 in control groups.
The studies included in this analysis provide information about random sequence generation, allocation concealment, participant blindness, personnel, outcome evaluation and selective reporting. Figure S1 reports the risk details of deviation assessment. (Figure S1 in Appendix) 29 trials had a Jadad scale of 4 or 5, and others were scored ≤3.
As is shown in Figure 2, this meta-analysis included 52600 patients in the GLP-1 receptor agonist group and 41463 patients in the control group. The event rate in the GLP-1 receptor agonist group (0.39%) was higher than in the control group (0.31%). Compared with placebo or other interventions, GLP-1 receptor agonist increased the risk of overall thyroid disorders by 28% (RR 1.28, 95% CI 1.03-1.60; p = 0.027), with no statistically significant between-study heterogeneity (I2 = 0.0%). The funnel plot for this analysis indicated no significant publication bias (Figure S2).
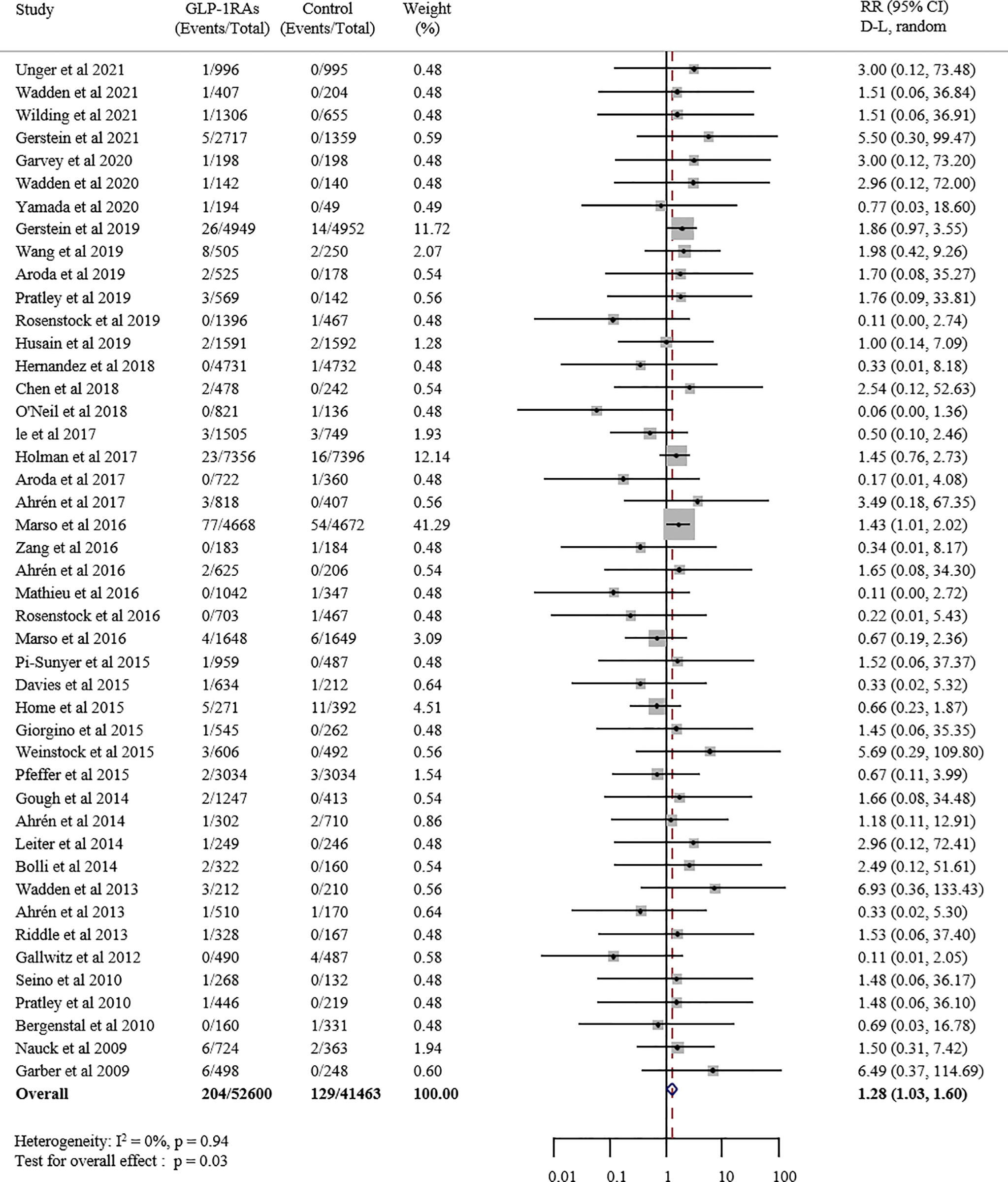
Figure 2 Forest plot of GLP-1 receptor agonists versus comparators on risk of overall thyroid disorders. GLP-1RAs, GLP-1 receptor agonists; RR, risk ratios; CI, confidence interval.
GLP-1 receptor agonists versus placebo or other interventions had no significant effects on the occurrence of thyroid cancer (RR 1.30, 95% CI 0.86-1.97, p = 0.212; I2 = 0.0%; Figure S3), hyperthyroidism (RR 1.19, 95% CI 0.61-2.35, p = 0.608; I2 = 0.0%; Figure S4), hypothyroidism (RR 1.22, 95% CI 0.80-1.87, p = 0.359; I2 = 0.0%; Figure S5), thyroiditis (RR 1.83, 95% CI 0.51-6.57, p = 0.353; I2 = 0.0%; Figure S6), thyroid mass (RR 1.17, 95% CI 0.43-3.20, p = 0.759; I2 = 0.0%; Figure S7), and goiter (RR 1.17, 95% CI 0.74-1.86, p = 0.503; I2 = 0.0%; Figure S8).
Among all 45 enrolled trials, 18 trials including 24787 patients used liraglutide as the experimental agent. Compared with placebo or other interventions, treatment with liraglutide increased the risk of overall thyroid disorders by 37% (RR 1.37, 95% CI 1.01-1.86, p = 0.044; Figure 3), and no statistically significant between-study heterogeneity was observed (I2 = 0.0%, p = 0.933).
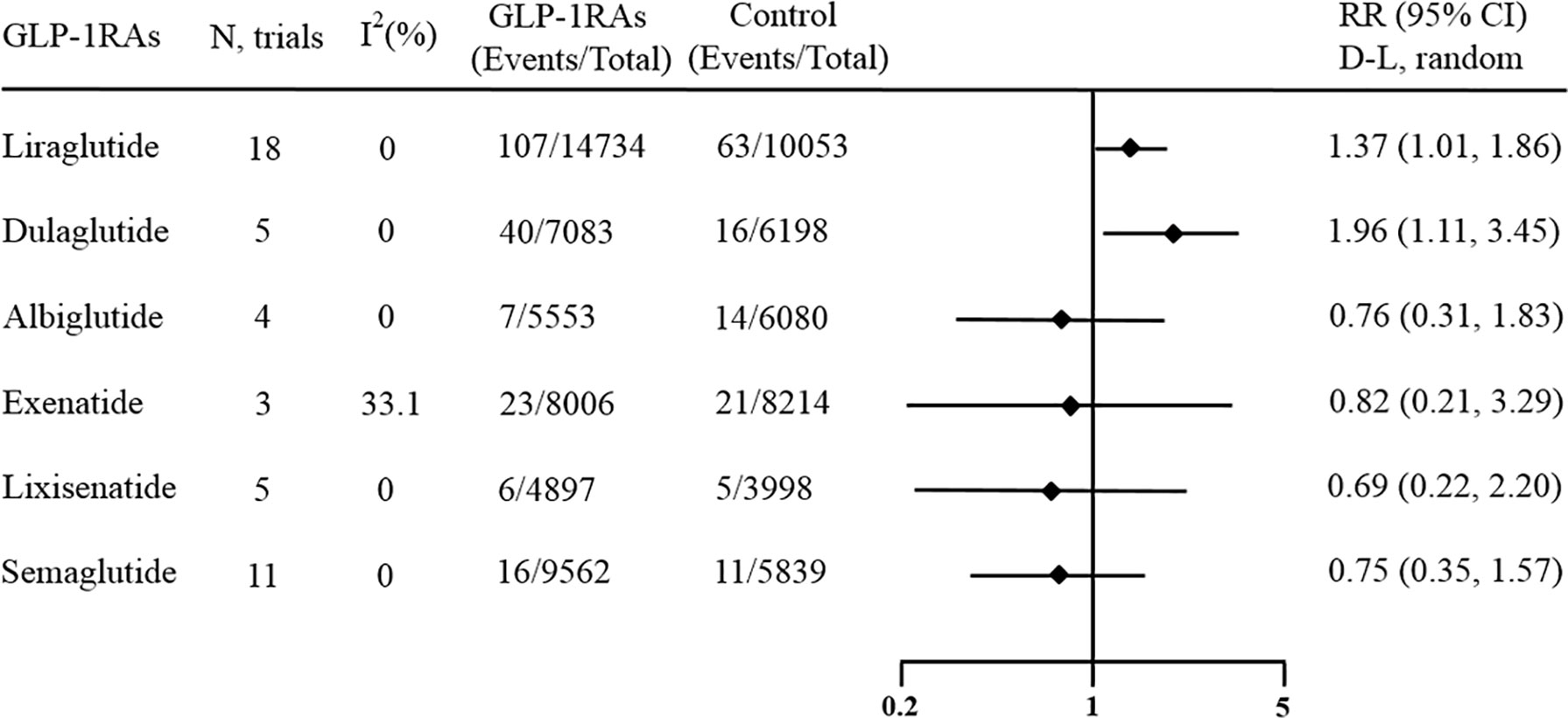
Figure 3 Forest plot of specific GLP-1 receptor agonists versus comparators on risk of overall thyroid disorders. GLP-1RAs, GLP-1 receptor agonists; RR, risk ratios; CI, confidence interval.
Moreover, another 5 trials including 13281 patients provided information about the risk of thyroid disorders in patients treated with dulaglutide. This result showed that compared with placebo or other interventions, dulaglutide significantly increased the incidence of overall thyroid disorders by 96% (RR 1.96, 95% CI 1.11-3.45, p = 0.020; Figure 3), and no statistically significant between-study heterogeneity was observed (I2 = 0.0%, p = 0.965).
However, no effect against overall thyroid disorders was found for other GLP-1 receptor agonists. There were 11 studies including 15401 patients that regarded semaglutide as the experimental agent, and the pooled RR of overall thyroid disorders in patients receiving semaglutide versus other interventions was 0.75 (95% CI 0.35‐1.57; Figure 3). Whether oral semaglutide or subcutaneous semaglutide, the results showed that they had no significant effects on the occurrence of overall thyroid disorders (Figure S9 and Figure S10). There were 5 studies including 8895 patients that regarded lixisenatide as the experimental agent, and the pooled RR of overall thyroid disorders in patients receiving lixisenatide versus other interventions was 0.69 (95% CI 0.22‐2.20; Figure 3). There were 3 studies including 16220 patients that regarded exenatide as the experimental agent, and the pooled RR of overall thyroid disorders in patients receiving exenatide versus other interventions was 0.82 (95% CI 0.21‐3.29; Figure 3). There were 3 studies including 11633 patients that regarded albiglutide as the experimental agent, and the pooled RR of overall thyroid disorders in patients receiving albiglutide versus other interventions was 0.76 (95% CI 0.31‐1.83; Figure 3). Most of the above meta-analyses had no heterogeneity (I2 = 0%), while one had medium heterogeneity (I2 = 33.1%).
Subgroup analyses based on type of underlying diseases, type of control, trial durations and pharmacokinetics. The results showed that the type of underlying diseases, type of control, trial durations and pharmacokinetics did not significantly affect the effects of GLP-1 receptor agonists on overall thyroid disorders (all P subgroup > 0.05; Figure 4). The statistical significance of the results from the meta-regression was consistent with the subgroup analyses.
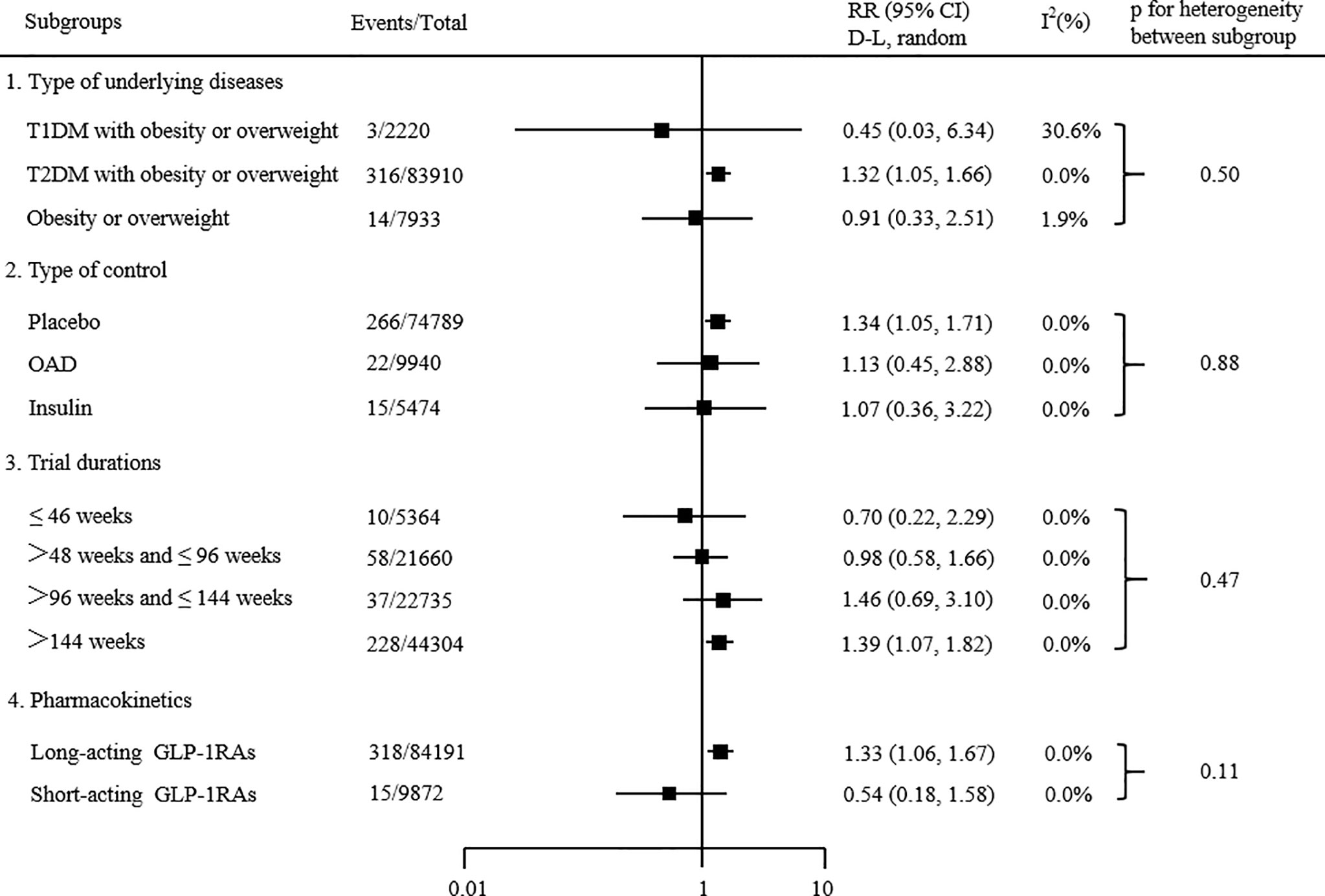
Figure 4 Subgroup analyses of the effects of GLP-1 receptor agonists on the risk of overall thyroid disorders. P value calculated by χ2 statistics is shown. Statistical significance of results from meta-regression was consistent.
This meta-analysis is the first large sample study that was designed to assess the relationship between the use of GLP-1 receptor agonists and the occurrence of various thyroid disorders. As a result, the following two major findings were produced. First, compared with placebo or other interventions, GLP-1 receptor agonists significantly increased the risk of overall thyroid disorders by 28%. Second, among GLP-1 receptor agonists, only liraglutide and dulaglutide showed increased trends in the risks of overall thyroid disorders compared with placebo and other antidiabetic drugs.
Despite the lack of consistent clinical and epidemiological evidence, the potential link between GLP-1 receptor agonists and thyroid cancer has received considerable attention. Rodent studies have shown that treatment with liraglutide or once-weekly exenatide is associated with thyroid C-cell proliferation and the formation of thyroid C-cell tumors (11, 63).
Therefore, the US Food and Drug Administration (FDA) prohibits these therapies for patients with an individual or family history of medullary thyroid carcinoma (MTC) or patients with multiple endocrine neoplasia syndrome type 2 (MEN2). However, these concerns are controversial in clinical trials. A retrospective analysis of the FDA’s AERS database found that the incidence of thyroid cancer treated with exenatide was 4.7 times that of the control drug (14). Similarly, analysis of data from the EudraVigilance database has found evidence from spontaneous reports that GLP-1 analogues are related to thyroid cancer in diabetic patients (64). However, a meta-analysis involving 25 studies showed that liraglutide had no significant correlation with the increased risk of thyroid cancer (65). Although our meta-analysis also showed that GLP-1 receptor agonists did not increase the risk of thyroid cancer compared to placebo or other interventions, in combination with previously available evidence, patients at risk for thyroid cancer should be prescribed GLP-1 receptor agonists with caution.
To date, the potential mechanism of the unfavorable effects of GLP-1 receptor agonists on thyroid disorders has not been completely clear. The possible mechanisms are as follows. First, it was reported that the mechanism of C-cell transformation in rodents is by activation of the GLP-1 receptor on the C cell, and a study has shown that GLP-1 receptor stimulation is a better predictor of C-cell hyperplasia than plasma drug concentrations of exenatide and liraglutide (66, 67). Second, in addition to medullary thyroid carcinoma and C-cell hyperplasia, the expression of GLP-1 receptors in papillary thyroid carcinoma (PTC) has been demonstrated. Gier et al. (68) reported positive immunoreactivity for GLP-1 receptors in PTC tissues, detected using a polyclonal anti-GLP-1 receptors antibody. Meanwhile, they reported that GLP-1 receptors were expressed differently in non-neoplastic thyroid tissues according to different inflammatory states. GLP-1 receptors were expressed in normal thyroid tissues with inflammation, but not in normal thyroid tissues without inflammation. In addition, another study also confirmed the expression of GLP-1 receptors in PTC and the expression rate of GLP-1 receptors in PTC, which was almost 30% (69). Korner et al. (70) ascertained the expression of GLP-1 receptors in various human thyroid tissues by scintigraphy and demonstrated that few normal thyroid tissue expressed GLP-1 receptors. Therefore, GLP-1 receptors may be abnormally induced in cells derived from thyroid follicles through inflammation, cell proliferation or tumorigenesis. However, some of the mentioned studies used GLP-1 receptor antibodies lacking specificity (71, 72). Using another detection method, Waser et al. found that neither normal nor hyperplastic human thyroids containing parafollicular C cells express GLP-1 receptors (73). At present, the presence and importance of GLP-1 receptors in normal human thyroid remains controversial. Third, GLP-1 might work through the phosphoinositol-3 kinase/AKT serine/threonine kinase (PI3K/Akt) pathway and/or mitogen-activated protein kinase/extracellular signal-regulated kinase (MAPK/Erk) pathway. These two signaling pathways are also critical in regulating cell growth and proliferation; accordingly, they are closely related to cancer, including PTC. These two signaling pathways are significant pathways for regulating cell growth and proliferation, and thus they are closely related to cancer formation (74). Finally, the GlP-1 receptor may be associated with triiodothyronine (T3) levels. GLP-1 stimulates type 3 iodothyronine deiodinase (D3) expression through the GLP-1 receptor, and the regulation of intracellular (T3) concentration by D3 may be involved in the stimulation of insulin secretion by GLP-1 (75). In addition, a clinical study showed that exenatide treatment for 6 months significantly reduced the serum TSH concentration in diabetic patients without thyroid disease (76). In conclusion, some animal studies have provided evidence that the use of GLP-1 receptor agonists increases the risk of thyroid disease, but this evidence has not been confirmed in humans. Therefore, we performed this meta-analysis to clarify the association of GLP-1 receptor agonists with thyroid disease in clinical studies and preparation for future studies in humans. Further prospective studies should be carried out to determine the potential effects of GLP-1 receptor agonists on thyroid disease.
In the analysis of different types of GLP-1 receptor agonists, we found that liraglutide and dulaglutide were significantly associated with an increased risk of overall thyroid disorders. However, individual tolerability and safety to GLP-1RA may vary due to differences in molecular structures (77). Furthermore, these different findings could explain with an imbalanced sample size. It is worth noting that the significantly increased risk of liraglutide is largely driven by the LEADER trial (20) and that of dulaglutide is largely driven by the REWIND test (42), both of which contributed more than 75% of the weight to the overall results. Due to the lack of sufficient research, we cannot draw a decisive conclusion until further research provides more information. Among the included studies, only one was related to short-acting exenatide (39), and two were long-acting exenatide (18, 40). Due to the small number of studies, we did not separately analyze according to pharmacokinetics.
This review has two main strengths. First, this is the first meta-analysis to comprehensively assess the risks of various thyroid diseases associated with the use of GLP-1 receptor agonists. Moreover, all included studies were RCTs. Second, no or only mild heterogeneity was found in any of the meta-analyses conducted in the present study.
We acknowledge that our study has several limitations. First, almost every included study did not consider thyroid events as the main result, only regarded them as safety results and did not monitor the changes in thyroid function at the same time. In addition, only trials reporting thyroid events were included in this analysis, leading to an unclear risk of reporting bias. Second, although this analysis included 45 studies with a fairly large sample size, the low incidence of thyroid events resulted in a wide confidence interval that reduced the certainty of our findings. Moreover, the study groups considerably differ in size (52600 vs. 41463). Considering the slight difference in the rate of thyroid disorders (0.39 vs. 0.31%), a significant influence on the primary endpoint cannot be ruled out. The third limitation is that there may be the potential for numerous indirect effects or confounding. For example, reduction in BMI in obesity patients, caloric restriction, and illness are all associated with different thyroid function test (TFT) changes. Patients may be more stringently screened, particularly for thyroid nodules/cancer in patients receiving GLP-1 receptor agonists. Another limitation is that for thyroid cancer, reporting specifically the cases of MTC vs. PTC would further the goal of elucidating mechanisms of thyroid disease. However, we found that some studies did not specify the type of thyroid cancer, which would affect the accuracy of the results. Due to the lack of standardization of adverse event reports and original data, we cannot make comparisons according to different types. Finally, although our meta-analysis showed that GLP-1 receptor agonists increased the risk of overall thyroid disorder, due to the decrease in sample size, it did not show statistically significant results for specific thyroid disorder. Future large long-term RCTs with primary or secondary outcomes, including thyroid disorders and real-world data, are needed to elucidate the association between GLP-1 receptor agonists and the risk of various thyroid disorders, particularly thyroid cancer.
In conclusion, compared with placebo or other interventions, GLP-1 receptor agonists did not increase or decrease the risk of thyroid cancer, hyperthyroidism, hypothyroidism, thyroiditis, thyroid mass and goiter. Due to the low incidence of various thyroid disorders, these findings still need to be verified by further studies.
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/Supplementary Material.
JL and XL designed and outlined the work; WH, RS, RC, CL, RG, WT, JZ and QZ drafted and revised the manuscript. Both authors approved the final version of the article and agree to be accountable for all aspects of the work. All authors contributed to the article and approved the submitted version.
This work was supported in part by National Natural Science Foundation of China (No. 82000799), Research Project Supported by Shanxi Scholarship Council of China (No. 2020-187), Scientific Research Project of Shanxi Provincial Health Committee (No.2021068), The Doctoral Foundation of the Second Hospital of Shanxi Medical University (No. 20200112) and Natural Science Foundation of Shanxi Province (No. 202103021224243).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2022.927859/full#supplementary-material
1. Gu Y, Li H, Bao X, Zhang Q, Liu L, Meng G, et al. The Relationship Between Thyroid Function and the Prevalence of Type 2 Diabetes Mellitus in Euthyroid Subjects. J Clin Endocrinol Metab (2017) 102(2):434–42. doi: 10.1210/jc.2016-2965
2. Nederstigt C, Corssmit EP, de Koning EJ, Dekkers OM. Incidence and Prevalence of Thyroid Dysfunction in Type 1 Diabetes. J Diabetes Complications (2016) 30(3):420–5. doi: 10.1016/j.jdiacomp.2015.12.027
3. Vigneri P, Frasca F, Sciacca L, Pandini G, Vigneri R. Diabetes and Cancer. Endocr Relat Cancer (2009) 16(4):1103–23. doi: 10.1677/ERC-09-0087
4. Aschebrook-Kilfoy B, Sabra MM, Brenner A, Moore SC, Ron E, Schatzkin A, et al. Diabetes and Thyroid Cancer Risk in the National Institutes of Health-AARP Diet and Health Study. Thyroid (2011) 21(9):957–63. doi: 10.1089/thy.2010.0396
5. Avgerinos KI, Spyrou N, Mantzoros CS, Dalamaga M. Obesity and Cancer Risk: Emerging Biological Mechanisms and Perspectives. Metabolism (2019) 92:121–35. doi: 10.1016/j.metabol.2018.11.001
6. Kalra S, Aggarwal S, Khandelwal D. Thyroid Dysfunction and Type 2 Diabetes Mellitus: Screening Strategies and Implications for Management. Diabetes Ther (2019) 10(6):2035–44. doi: 10.1007/s13300-019-00700-4
7. Meng X, Xu S, Chen G, Derwahl M, Liu C. Metformin and Thyroid Disease. J Endocrinol (2017) 233(1):R43–51. doi: 10.1530/JOE-16-0450
8. Lee S, Tsirbas A, Goldberg RA, McCann JD. Thiazolidinedione Induced Thyroid Associated Orbitopathy. BMC Ophthalmol (2007) 4:7:8. doi: 10.1186/1471-2415-7-8
9. Mimura LY, Villares SM, Monteiro ML, Guazzelli IC, Bloise W. Peroxisome Proliferator-Activated Receptor-Gamma Gene Expression in Orbital Adipose/Connective Tissues is Increased During the Active Stage of Graves' Ophthalmopathy. Thyroid (2003) 13(9):845–50. doi: 10.1089/105072503322401032
10. Cho YM, Fujita Y, Kieffer TJ. Glucagon-Like Peptide-1: Glucose Homeostasis and Beyond. Annu Rev Physiol (2014) 76:535–59. doi: 10.1146/annurev-physiol-021113-170315
11. Bjerre Knudsen L, Madsen LW, Andersen S, Almholt K, de Boer AS, Drucker DJ, et al. Glucagon-Like Peptide-1 Receptor Agonists Activate Rodent Thyroid C-Cells Causing Calcitonin Release and C-Cell Proliferation. Endocrinology (2010) 151(4):1473–86. doi: 10.1210/en.2009-1272
12. Martín-Lacave I, Bernab R, Sampedro C, Conde E, Fernández-Santos JM, San Martín MV, et al. Correlation Between Gender and Spontaneous C-Cell Tumors in the Thyroid Gland of the Wistar Rat. Cell Tissue Res (1999) 297(3):451–7. doi: 10.1007/s004410051371
13. Capen CC, Martin SL. The Effects of Xenobiotics on the Structure and Function of Thyroid Follicular and C-Cells. Toxicol Pathol (1989) 17(2):266–93. doi: 10.1177/019262338901700205
14. Elashoff M, Matveyenko AV, Gier B, Elashoff R, Butler PC. Pancreatitis, Pancreatic, and Thyroid Cancer With Glucagon-Like Peptide-1-Based Therapies. Gastroenterology (2011) 141(1):150–6. doi: 10.1053/j.gastro.2011.02.018
15. Hegedüs L, Sherman SI, Tuttle RM, von Scholten BJ, Rasmussen S, Karsbøl JD, et al. No Evidence of Increase in Calcitonin Concentrations or Development of C-Cell Malignancy in Response to Liraglutide for Up to 5 Years in the LEADER Trial. Diabetes Care (2018) 41(3):620–2. doi: 10.2337/dc17-1956
16. Brown E, Heerspink HJL, Cuthbertson DJ, Wilding JPH. SGLT2 Inhibitors and GLP-1 Receptor Agonists: Established and Emerging Indications. Lancet (2021) 398(10296):262–76. doi: 10.1016/S0140-6736(21)00536-5
17. Marso SP, Bain SC, Consoli A, Eliaschewitz FG, Jódar E, Leiter LA, et al. Semaglutide and Cardiovascular Outcomes in Patients With Type 2 Diabetes. N Engl J Med (2016) 375(19):1834–44. doi: 10.1056/NEJMoa1607141
18. Holman RR, Bethel MA, Mentz RJ, Thompson VP, Lokhnygina Y, Buse JB, et al. Effects of Once-Weekly Exenatide on Cardiovascular Outcomes in Type 2 Diabetes. N Engl J Med (2017) 377(13):1228–39. doi: 10.1056/NEJMoa1612917
19. Leiter LA, Carr MC, Stewart M, Jones-Leone A, Scott R, Yang F, et al. Efficacy and Safety of the Once-Weekly GLP-1 Receptor Agonist Albiglutide Versus Sitagliptin in Patients With Type 2 Diabetes and Renal Impairment: A Randomized Phase III Study. Diabetes Care (2014) 37(10):2723–30. doi: 10.2337/dc13-2855
20. Marso SP, Daniels GH, Brown-Frandsen K, Kristensen P, Mann JF, Nauck MA, et al. Liraglutide and Cardiovascular Outcomes in Type 2 Diabetes. N Engl J Med (2016) 375(4):311–22. doi: 10.1056/NEJMoa1603827
21. Unger J, Allison DC, Kaltoft M, Lakkole K, Panda JK, Ramesh C, et al. Maintenance of Glycaemic Control With Liraglutide Versus Oral Antidiabetic Drugs as Add-on Therapies in Patients With Type 2 Diabetes Uncontrolled With Metformin Alone: A Randomized Clinical Trial in Primary Care (LIRA-PRIME). Diabetes Obes Metab (2022) 24(2):204–11. doi: 10.1111/dom.14566
22. Garvey WT, Birkenfeld AL, Dicker D, Mingrone G, Pedersen SD, Satylganova A, et al. Efficacy and Safety of Liraglutide 3.0 Mg in Individuals With Overweight or Obesity and Type 2 Diabetes Treated With Basal Insulin: The SCALE Insulin Randomized Controlled Trial. Diabetes Care (2020) 43(5):1085–93. doi: 10.2337/dc19-1745
23. Wadden TA, Tronieri JS, Sugimoto D, Lund MT, Auerbach P, Jensen C, et al. Liraglutide 3.0 Mg and Intensive Behavioral Therapy (IBT) for Obesity in Primary Care: The SCALE IBT Randomized Controlled Trial. Obes (Silver Spring) (2020) 28(3):529–36. doi: 10.1002/oby.22726
24. le Roux CW, Astrup A, Fujioka K, Greenway F, Lau DCW, Van Gaal L, et al. 3 Years of Liraglutide Versus Placebo for Type 2 Diabetes Risk Reduction and Weight Management in Individuals With Prediabetes: A Randomised, Double-Blind Trial. Lancet (2017) 389(10077):1399–409. doi: 10.1016/S0140-6736(17)30069-7
25. Pi-Sunyer X, Astrup A, Fujioka K, Greenway F, Halpern A, Krempf M, et al. A Randomized, Controlled Trial of 3.0 Mg of Liraglutide in Weight Management. N Engl J Med (2015) 373(1):11–22. doi: 10.1056/NEJMoa1411892
26. Zang L, Liu Y, Geng J, Luo Y, Bian F, Lv X, et al. Efficacy and Safety of Liraglutide Versus Sitagliptin, Both in Combination With Metformin, in Chinese Patients With Type 2 Diabetes: A 26-Week, Open-Label, Randomized, Active Comparator Clinical Trial. Diabetes Obes Metab (2016) 18(8):803–11. doi: 10.1111/dom.12674
27. Ahrén B, Hirsch IB, Pieber TR, Mathieu C, Gómez-Peralta F, Hansen TK, et al. Efficacy and Safety of Liraglutide Added to Capped Insulin Treatment in Subjects With Type 1 Diabetes: The ADJUNCT TWO Randomized Trial. Diabetes Care (2016) 39(10):1693–701. doi: 10.2337/dc16-0690
28. Mathieu C, Zinman B, Hemmingsson JU, Woo V, Colman P, Christiansen E, et al. Efficacy and Safety of Liraglutide Added to Insulin Treatment in Type 1 Diabetes: The ADJUNCT ONE Treat-To-Target Randomized Trial. Diabetes Care (2016) 39(10):1702–10. doi: 10.2337/dc16-0691
29. Davies MJ, Bergenstal R, Bode B, Kushner RF, Lewin A, Skjøth TV, et al. Efficacy of Liraglutide for Weight Loss Among Patients With Type 2 Diabetes: The SCALE Diabetes Randomized Clinical Trial. JAMA (2015) 314(7):687–99. doi: 10.1001/jama.2015.9676
30. Gough SC, Bode B, Woo V, Rodbard HW, Linjawi S, Poulsen P, et al. Efficacy and Safety of a Fixed-Ratio Combination of Insulin Degludec and Liraglutide (IDegLira) Compared With its Components Given Alone: Results of a Phase 3, Open-Label, Randomised, 26-Week, Treat-to-Target Trial in Insulin-Naive Patients With Type 2 Diabetes. Lancet Diabetes Endocrinol (2014) 2(11):885–93. doi: 10.1016/S2213-8587(14)70174-3
31. Wadden TA, Hollander P, Klein S, Niswender K, Woo V, Hale PM, et al. Weight Maintenance and Additional Weight Loss With Liraglutide After Low-Calorie-Diet-Induced Weight Loss: The SCALE Maintenance Randomized Study. Int J Obes (Lond) (2015) 39(1):187. doi: 10.1038/ijo.2013.120
32. Seino Y, Rasmussen MF, Nishida T, Kaku K. Efficacy and Safety of the Once-Daily Human GLP-1 Analogue, Liraglutide, vs Glibenclamide Monotherapy in Japanese Patients With Type 2 Diabetes. Curr Med Res Opin (2010) 26(5):1013–22. doi: 10.1185/03007991003672551
33. Pratley RE, Nauck M, Bailey T, Montanya E, Cuddihy R, Filetti S, et al. Liraglutide Versus Sitagliptin for Patients With Type 2 Diabetes Who did Not Have Adequate Glycaemic Control With Metformin: A 26-Week, Randomised, Parallel-Group, Open-Label Trial. Lancet (2010) 375(9724):1447–56. doi: 10.1016/S0140-6736(10)60307-8
34. Nauck M, Frid A, Hermansen K, Shah NS, Tankova T, Mitha IH, et al. Efficacy and Safety Comparison of Liraglutide, Glimepiride, and Placebo, All in Combination With Metformin, in Type 2 Diabetes: The LEAD (Liraglutide Effect and Action in Diabetes)-2 Study. Diabetes Care (2009) 32(1):84–90. doi: 10.2337/dc08-1355
35. Garber A, Henry R, Ratner R, Garcia-Hernandez PA, Rodriguez-Pattzi H, Olvera-Alvarez I, et al. Liraglutide Versus Glimepiride Monotherapy for Type 2 Diabetes (LEAD-3 Mono): A Randomised, 52-Week, Phase III, Double-Blind, Parallel-Treatment Trial. Lancet (2009) 373(9662):473–81. doi: 10.1016/S0140-6736(08)61246-5
36. Hernandez AF, Green JB, Janmohamed S, D'Agostino RB, Granger CB, Jones NP, et al. Albiglutide and Cardiovascular Outcomes in Patients With Type 2 Diabetes and Cardiovascular Disease (Harmony Outcomes): A Double-Blind, Randomised Placebo-Controlled Trial. Lancet (2018) 392(10157):1519–29. doi: 10.1016/S0140-6736(18)32261-X
37. Home PD, Shamanna P, Stewart M, Yang F, Miller M, Perry C, et al. Efficacy and Tolerability of Albiglutide Versus Placebo or Pioglitazone Over 1 Year in People With Type 2 Diabetes Currently Taking Metformin and Glimepiride: HARMONY 5. Diabetes Obes Metab (2015) 17(2):179–87. doi: 10.1111/dom.12414
38. Ahrén B, Johnson SL, Stewart M, Cirkel DT, Yang F, Perry C, et al. HARMONY 3: 104-Week Randomized, Double-Blind, Placebo- and Active-Controlled Trial Assessing the Efficacy and Safety of Albiglutide Compared With Placebo, Sitagliptin, and Glimepiride in Patients With Type 2 Diabetes Taking Metformin. Diabetes Care (2014) 37(8):2141–8. doi: 10.2337/dc14-0024
39. Gallwitz B, Guzman J, Dotta F, Guerci B, Simó R, Basson BR, et al. Exenatide Twice Daily Versus Glimepiride for Prevention of Glycaemic Deterioration in Patients With Type 2 Diabetes With Metformin Failure (EUREXA): An Open-Label, Randomised Controlled Trial. Lancet (2012) 379(9833):2270–8. doi: 10.1016/S0140-6736(12)60479-6
40. Bergenstal RM, Wysham C, Macconell L, Malloy J, Walsh B, Yan P, et al. Efficacy and Safety of Exenatide Once Weekly Versus Sitagliptin or Pioglitazone as an Adjunct to Metformin for Treatment of Type 2 Diabetes (DURATION-2): A Randomised Trial. Lancet (2010) 376(9739):431–9. doi: 10.1016/S0140-6736(10)60590-9
41. Wang W, Nevárez L, Filippova E, Song KH, Tao B, Gu L, et al. Efficacy and Safety of Once-Weekly Dulaglutide Versus Insulin Glargine in Mainly Asian Patients With Type 2 Diabetes Mellitus on Metformin and/or a Sulphonylurea: A 52-Week Open-Label, Randomized Phase III Trial. Diabetes Obes Metab (2019) 21(2):234–43. doi: 10.1111/dom.13506
42. Gerstein HC, Colhoun HM, Dagenais GR, Diaz R, Lakshmanan M, Pais P, et al. Dulaglutide and Cardiovascular Outcomes in Type 2 Diabetes (REWIND): A Double-Blind, Randomised Placebo-Controlled Trial. Lancet (2019) 394(10193):121–30. doi: 10.1016/S0140-6736(19)31149-3
43. Chen YH, Huang CN, Cho YM, Li P, Gu L, Wang F, et al. Efficacy and Safety of Dulaglutide Monotherapy Compared With Glimepiride in East-Asian Patients With Type 2 Diabetes in a Multicentre, Double-Blind, Randomized, Parallel-Arm, Active Comparator, Phase III Trial. Diabetes Obes Metab (2018) 20(9):2121–30. doi: 10.1111/dom.13340
44. Weinstock RS, Guerci B, Umpierrez G, Nauck MA, Skrivanek Z, Milicevic Z. Safety and Efficacy of Once-Weekly Dulaglutide Versus Sitagliptin After 2 Years in Metformin-Treated Patients With Type 2 Diabetes (AWARD-5): A Randomized, Phase III Study. Diabetes Obes Metab (2015) 17(9):849–58. doi: 10.1111/dom.12479
45. Giorgino F, Benroubi M, Sun JH, Zimmermann AG, Pechtner V. Efficacy and Safety of Once-Weekly Dulaglutide Versus Insulin Glargine in Patients With Type 2 Diabetes on Metformin and Glimepiride (AWARD-2). Diabetes Care (2015) 38(12):2241–9. doi: 10.2337/dc14-1625
46. Rosenstock J, Aronson R, Grunberger G, Hanefeld M, Piatti P, Serusclat P, et al. Benefits of LixiLan, a Titratable Fixed-Ratio Combination of Insulin Glargine Plus Lixisenatide, Versus Insulin Glargine and Lixisenatide Monocomponents in Type 2 Diabetes Inadequately Controlled on Oral Agents: The LixiLan-O Randomized Trial. Diabetes Care (2016) 39(11):2026–35. doi: 10.2337/dc16-0917
47. Pfeffer MA, Claggett B, Diaz R, Dickstein K, Gerstein HC, Køber LV, et al. Lixisenatide in Patients With Type 2 Diabetes and Acute Coronary Syndrome. N Engl J Med (2015) 373(23):2247–57. doi: 10.1056/NEJMoa1509225
48. Bolli GB, Munteanu M, Dotsenko S, Niemoeller E, Boka G, Wu Y, et al. Efficacy and Safety of Lixisenatide Once Daily vs. Placebo in People With Type 2 Diabetes Insufficiently Controlled on Metformin (GetGoal-F1). Diabetes Med (2014) 31(2):176–84. doi: 10.1111/dme.12328
49. Ahrén B, Leguizamo Dimas A, Miossec P, Saubadu S, Aronson R. Efficacy and Safety of Lixisenatide Once-Daily Morning or Evening Injections in Type 2 Diabetes Inadequately Controlled on Metformin (GetGoal-M). Diabetes Care (2013) 36(9):2543–50. doi: 10.2337/dc12-2006
50. Riddle MC, Aronson R, Home P, Marre M, Niemoeller E, Miossec P, et al. Adding Once-Daily Lixisenatide for Type 2 Diabetes Inadequately Controlled by Established Basal Insulin: A 24-Week, Randomized, Placebo-Controlled Comparison (GetGoal-L). Diabetes Care (2013) 36(9):2489–96. doi: 10.2337/dc12-2454
51. Wilding JPH, Batterham RL, Calanna S, Davies M, Van Gaal LF, Lingvay I, et al. Once-Weekly Semaglutide in Adults With Overweight or Obesity. N Engl J Med (2021) 384(11):989. doi: 10.1056/NEJMoa2032183
52. Wadden TA, Bailey TS, Billings LK, Davies M, Frias JP, Koroleva A, et al. Effect of Subcutaneous Semaglutide vs Placebo as an Adjunct to Intensive Behavioral Therapy on Body Weight in Adults With Overweight or Obesity: The STEP 3 Randomized Clinical Trial. JAMA (2021) 325(14):1403–13. doi: 10.1001/jama.2021.1831
53. Yamada Y, Katagiri H, Hamamoto Y, Deenadayalan S, Navarria A, Nishijima K, et al. Dose-Response, Efficacy, and Safety of Oral Semaglutide Monotherapy in Japanese Patients With Type 2 Diabetes (PIONEER 9): A 52-Week, Phase 2/3a, Randomised, Controlled Trial. Lancet Diabetes Endocrinol (2020) 8(5):377–91. doi: 10.1016/S2213-8587(20)30075-9
54. Husain M, Birkenfeld AL, Donsmark M, Dungan K, Eliaschewitz FG, Franco DR, et al. Oral Semaglutide and Cardiovascular Outcomes in Patients With Type 2 Diabetes. N Engl J Med (2019) 381(9):841–51. doi: 10.1056/NEJMoa1901118
55. Rosenstock J, Allison D, Birkenfeld AL, Blicher TM, Deenadayalan S, Jacobsen JB, et al. Effect of Additional Oral Semaglutide vs Sitagliptin on Glycated Hemoglobin in Adults With Type 2 Diabetes Uncontrolled With Metformin Alone or With Sulfonylurea: The PIONEER 3 Randomized Clinical Trial. JAMA (2019) 321(15):1466–80. doi: 10.1001/jama.2019.2942
56. Pratley R, Amod A, Hoff ST, Kadowaki T, Lingvay I, Nauck M, et al. Oral Semaglutide Versus Subcutaneous Liraglutide and Placebo in Type 2 Diabetes (PIONEER 4): A Randomised, Double-Blind, Phase 3a Trial. Lancet (2019) 394(10192):39–50. doi: 10.1016/S0140-6736(19)31271-1
57. Aroda VR, Rosenstock J, Terauchi Y, Altuntas Y, Lalic NM, Morales Villegas EC, et al. PIONEER 1: Randomized Clinical Trial Comparing the Efficacy and Safety of Oral Semaglutide Monotherapy With Placebo in Patients With Type 2 Diabetes. Diabetes Care (2019) 42(9):1724–32. doi: 10.2337/dc19-0749
58. O'Neil PM, Birkenfeld AL, McGowan B, Mosenzon O, Pedersen SD, Wharton S, et al. Efficacy and Safety of Semaglutide Compared With Liraglutide and Placebo for Weight Loss in Patients With Obesity: A Randomised, Double-Blind, Placebo and Active Controlled, Dose-Ranging, Phase 2 Trial. Lancet (2018) 392(10148):637–49. doi: 10.1016/S0140-6736(18)31773-2
59. Ahrén B, Masmiquel L, Kumar H, Sargin M, Karsbøl JD, Jacobsen SH, et al. Efficacy and Safety of Once-Weekly Semaglutide Versus Once-Daily Sitagliptin as an Add-on to Metformin, Thiazolidinediones, or Both, in Patients With Type 2 Diabetes (SUSTAIN 2): A 56-Week, Double-Blind, Phase 3a, Randomised Trial. Lancet Diabetes Endocrinol (2017) 5(5):341–54. doi: 10.1016/S2213-8587(17)30092-X
60. Aroda VR, Bain SC, Cariou B, Piletič M, Rose L, Axelsen M, et al. Efficacy and Safety of Once-Weekly Semaglutide Versus Once-Daily Insulin Glargine as Add-on to Metformin (With or Without Sulfonylureas) in Insulin-Naive Patients With Type 2 Diabetes (SUSTAIN 4): A Randomised, Open-Label, Parallel-Group, Multicentre, Multinational, Phase 3a Trial. Lancet Diabetes Endocrinol (2017) 5(5):355–66. doi: 10.1016/S2213-8587(17)30085-2
61. Gerstein HC, Sattar N, Rosenstock J, Ramasundarahettige C, Pratley R, Lopes RD, et al. Cardiovascular and Renal Outcomes With Efpeglenatide in Type 2 Diabetes. N Engl J Med (2021) 385(10):896–907. doi: 10.1056/NEJMoa2108269
62. Effect of Liraglutide on Body Weight in Non-Diabetic Obese Subjects or Overweight Subjects With Co-Morbidities: SCALE™-Obesity and Pre-Diabetes (2011). Available at: https://ClinicalTrials.gov/show/NCT01272219.
63. Bulchandani D, Nachnani JS, Herndon B, Molteni A, Pathan MH, Quinn T, et al. Effect of Exendin (Exenatide)–GLP 1 Receptor Agonist on the Thyroid and Parathyroid Gland in a Rat Model. Eur J Pharmacol (2012) 691(1-3):292–6. doi: 10.1016/j.ejphar.2012.07.024
64. Mali G, Ahuja V, Dubey K. Glucagon-Like Peptide-1 Analogues and Thyroid Cancer: An Analysis of Cases Reported in the European Pharmacovigilance Database. J Clin Pharm Ther (2021) 46(1):99–105. doi: 10.1111/jcpt.13259
65. Alves C, Batel-Marques F, Macedo AF. A Meta-Analysis of Serious Adverse Events Reported With Exenatide and Liraglutide: Acute Pancreatitis and Cancer. Diabetes Res Clin Pract (2012) 98(2):271–84. doi: 10.1016/j.diabres.2012.09.008
66. Madsen LW, Knauf JA, Gotfredsen C, Pilling A, Sjögren I, Andersen S, et al. GLP-1 Receptor Agonists and the Thyroid: C-Cell Effects in Mice are Mediated via the GLP-1 Receptor and Not Associated With RET Activation. Endocrinology (2012) 153(3):1538–47. doi: 10.1210/en.2011-1864
67. van den Brink W, Emerenciana A, Bellanti F, Della Pasqua O, van der Laan JW. Prediction of Thyroid C-Cell Carcinogenicity After Chronic Administration of GLP1-R Agonists in Rodents. Toxicol Appl Pharmacol (2017) 320:51–9. doi: 10.1016/j.taap.2017.02.010
68. Gier B, Butler PC, Lai CK, Kirakossian D, DeNicola MM, Yeh MW. Glucagon Like Peptide-1 Receptor Expression in the Human Thyroid Gland. J Clin Endocrinol Metab (2012) 97(1):121–31. doi: 10.1210/jc.2011-2407
69. Jung MJ, Kwon SK. Expression of Glucagon-Like Peptide-1 Receptor in Papillary Thyroid Carcinoma and its Clinicopathologic Significance. Endocrinol Metab (Seoul) (2014) 29(4):536–44. doi: 10.3803/EnM.2014.29.4.536
70. Körner M, Stöckli M, Waser B, Reubi JC. GLP-1 Receptor Expression in Human Tumors and Human Normal Tissues: Potential for In Vivo Targeting. J Nucl Med (2007) 48(5):736–43. doi: 10.2967/jnumed.106.038679
71. Pyke C, Knudsen LB. The Glucagon-Like Peptide-1 Receptor–or Not? Endocrinology (2013) 154(1):4–8. doi: 10.1210/en.2012-2124
72. McLean BA, Wong CK, Campbell JE, Hodson DJ, Trapp S, Drucker DJ. Revisiting the Complexity of GLP-1 Action From Sites of Synthesis to Receptor Activation. Endocr Rev (2021) 42(2):101–32. doi: 10.1210/endrev/bnaa032
73. Waser B, Blank A, Karamitopoulou E, Perren A, Reubi JC. Glucagon-Like-Peptide-1 Receptor Expression in Normal and Diseased Human Thyroid and Pancreas. Mod Pathol (2015) 28(3):391–402. doi: 10.1038/modpathol.2014.113
74. He L, Zhang S, Zhang X, Liu R, Guan H, Zhang H. Effects of Insulin Analogs and Glucagon-Like Peptide-1 Receptor Agonists on Proliferation and Cellular Energy Metabolism in Papillary Thyroid Cancer. Onco Targets Ther (2017) 10:5621–31. doi: 10.2147/OTT.S150701
75. Akiyama S, Ogiwara T, Aoki T, Tsunekawa K, Araki O, Murakami M. Glucagon-Like Peptide-1 Stimulates Type 3 Iodothyronine Deiodinase Expression in a Mouse Insulinoma Cell Line. Life Sci (2014) 115(1-2):22–8. doi: 10.1016/j.lfs.2014.09.004
76. Sencar ME, Sakiz D, Calapkulu M, Hepsen S, Kizilgul M, Ozturk IU, et al. The Effect of Exenatide on Thyroid-Stimulating Hormone and Thyroid Volume. Eur Thyroid J (2019) 8(6):307–11. doi: 10.1159/000501895
Keywords: GLP-1 receptor agonists, thyroid disorders, thyroid cancer, meta-analysis, randomized controlled trials
Citation: Hu W, Song R, Cheng R, Liu C, Guo R, Tang W, Zhang J, Zhao Q, Li X and Liu J (2022) Use of GLP-1 Receptor Agonists and Occurrence of Thyroid Disorders: a Meta-Analysis of Randomized Controlled Trials. Front. Endocrinol. 13:927859. doi: 10.3389/fendo.2022.927859
Received: 25 April 2022; Accepted: 13 June 2022;
Published: 11 July 2022.
Edited by:
Johannes Wolfgang Dietrich, Ruhr University Bochum, GermanyReviewed by:
Daniel Quast, St. Josef-Hospital Bochum, GermanyCopyright © 2022 Hu, Song, Cheng, Liu, Guo, Tang, Zhang, Zhao, Li and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jing Liu, MTM5MzQ2NjM5MDVAMTYzLmNvbQ==; Xing Li, MTM1MDM1MDQxODBAMTYzLmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.