- 1Department of Endocrine and Metabolic Diseases, Shanghai Institute of Endocrine and Metabolic Diseases, Ruijin Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, China
- 2Shanghai National Clinical Research Center for metabolic Diseases, Key Laboratory for Endocrine and Metabolic Diseases of the National Health Commission of the People's Republic of China, Shanghai Key Laboratory for Endocrine Tumor, State Key Laboratory of Medical Genomics, Ruijin Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, China
- 3Department of Endocrinology, Fujian Provincial Hospital, Fujian Medical University, Fuzhou, China
- 4Department of Endocrinology, The First Affiliated Hospital of Guangxi Medical University, Nanning, China
- 5Department of Endocrinology, Karamay Municipal People’s Hospital, Xinjiang, China
- 6Department of Endocrinology, Qilu Hospital of Shandong University, Jinan, China
- 7Department of Endocrinology, Union Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China
- 8Department of Endocrinology, The First Affiliated Hospital of Chongqing Medical University, Chongqing, China
- 9Department of Endocrinology, Dalian Municipal Central Hospital, Dalian, China
- 10Department of Endocrinology, Jiangxi Provincial People’s Hospital Affiliated to Nanchang University, Nanchang, China
- 11Department of Endocrinology, The Second Affiliated Hospital of Harbin Medical University, Harbin, China
- 12Department of Endocrinology, Jiangsu Province Hospital on Integration of Chinese and Western Medicine, Nanjing, China
- 13Department of Endocrinology, Chinese People’s Liberation Army General Hospital, Beijing, China
- 14Department of Endocrinology, The First Affiliated Hospital of Zhengzhou University, Zhengzhou, China
- 15Department of Endocrinology, The First Affiliated Hospital of Wenzhou Medical University, Wenzhou, China
- 16Department of Endocrinology, Affiliated Hospital of Guiyang Medical College, Guiyang, China
- 17Department of Endocrinology, Xinhua Hospital Affiliated to Shanghai Jiao Tong University, School of Medicine, Shanghai, China
- 18Department of Endocrinology, The Affiliated Hospital of Southwest Medical University, Luzhou, China
- 19Department of Endocrinology, The First Hospital of Jilin University, Changchun, China
- 20Department of Endocrinology, The First Affiliated Hospital of Anhui Medical University, Hefei, China
- 21Institute of Chronic Diseases, Zhejiang Provincial Center for Disease Control and Prevention, Hangzhou, China
- 22Clinical Trials Center, Ruijin Hospital Affiliated to Shanghai Jiao Tong University School of Medicine, Shanghai, China
- 23Department of Endocrinology, Sun Yat-sen Memorial Hospital, Sun Yat-sen University, Guangzhou, China
- 24Department of Endocrinology, The First Affiliated Hospital of Nanjing Medical University, Nanjing, China
- 25Department of Endocrinology, Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China
- 26Department of Endocrinology, Central Hospital of Shanghai Jiading District, Shanghai, China
- 27Department of Endocrinology, The First Hospital of Lanzhou University, Lanzhou, China
- 28Department of Endocrinology, Shandong Provincial Hospital, Jinan, China
Aim: To determine the effect of decade-based body weight gain from 20 to 50 years of age on later life diabetes risk.
Methods: 35,611 non-diabetic participants aged ≥ 50 years from a well-defined nationwide cohort were followed up for average of 3.6 years, with cardiovascular diseases and cancers at baseline were excluded. Body weight at 20, 30, 40, and 50 years was reported. The overall 30 years and each 10-year weight gain were calculated from the early and middle life. Cox regression models were used to estimate risks of incident diabetes.
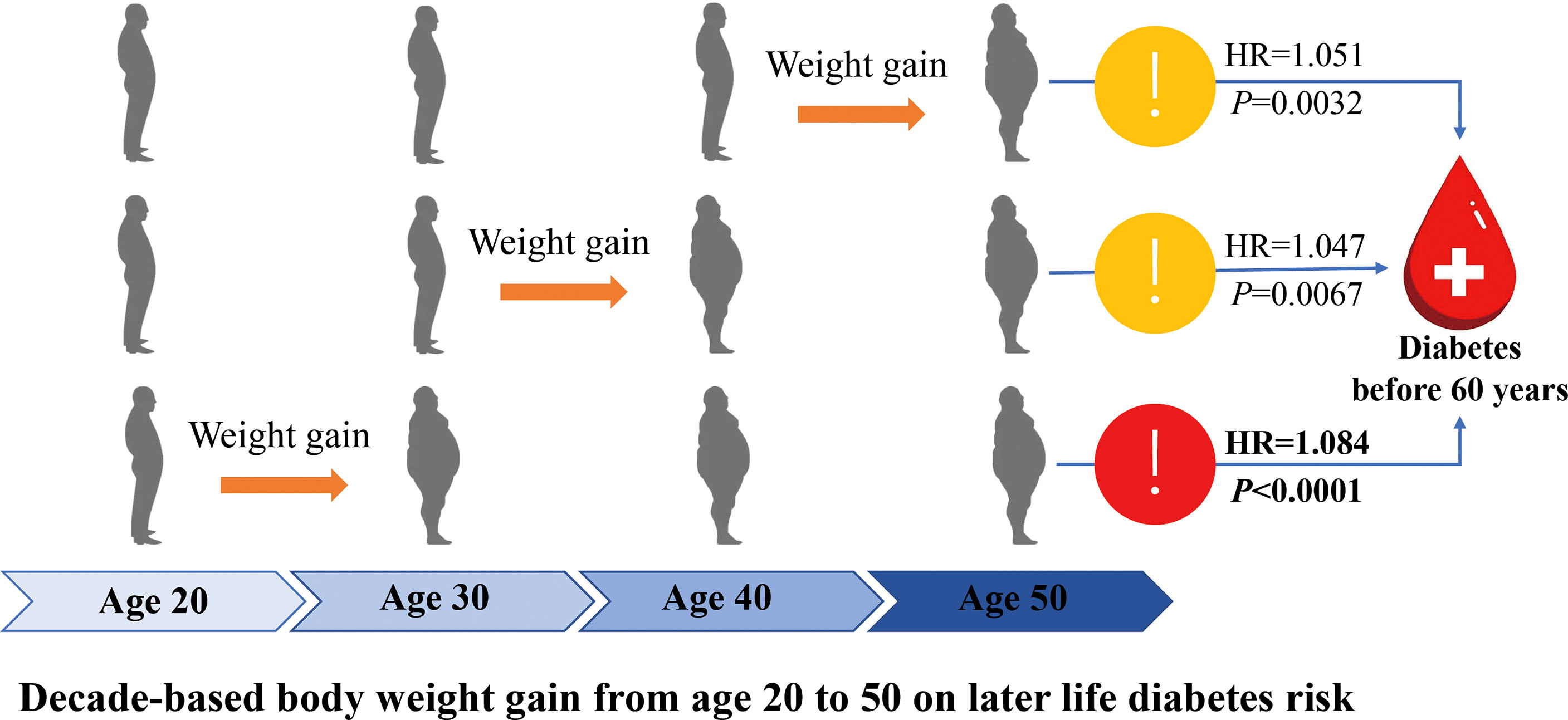
Results: After 127,745.26 person-years of follow-up, 2,789 incident diabetes were identified (incidence rate, 2.18%) in 25,289 women (mean weight gain 20-50 years, 7.60 kg) and 10,322 men (7.93 kg). Each 10-kg weight gain over the 30 years was significantly associated with a 39.7% increased risk of incident diabetes (95% confidence interval [CI], 1.33-1.47); weight gain from 20-30 years showed a more prominent effect on the risk of developing diabetes before 60 years than that of after 60 years (Hazard ratio, HR = 1.084, 95% CI [1.049-1.121], P <0.0001 vs. 1.015 [0.975-1.056], P = 0.4643; P Interaction=0.0293). It showed a stable effect of the three 10-year intervals weight gain on risk of diabetes after 60 years (HR=1.055, 1.038, 1.043, respectively, all P < 0.0036).
Conclusions: The early life weight gain showed a more prominent effect on developing diabetes before 60 years than after 60 years; however, each-decade weight gain from 20 to 50 years showed a similar effect on risk developing diabetes after 60 years.
Introduction
Compelling evidence has demonstrated that overweight and obesity are major established and modifiable risk factors for cardiovascular risk, such as type 2 diabetes (T2D) (1–3). Majority of the studies focused on body weight close to the time of the disease diagnosis, usually the middle-aged and elderly life. Accumulating evidence supported that early adulthood weight could affect subsequent T2D risks regardless of weight close to the time of diagnosis, which suggested a longer history of relative overweight, starting earlier in life, poses an additional risk for developing diabetes (4–7). Besides initial body weight, weight change during early adulthood has also been demonstrated to have an independent effect on subsequent diabetes and other cardio-metabolic risk (7–12).
In China, the largest increase in the prevalence of obesity was seen in men between the ages of 20 and 40 and in women between 30 and 40 (13), which was similar with many other countries. The rising trend of obesity in China, especially in young people, will undoubtedly increase the prevalence of chronic diseases, leading to poor cardiovascular health (13, 14). Given that a large segment of the population is starting to gain weight early in adulthood, after settling into an occupation or family life, which is contributing to the obesity epidemic, it is important to determine whether elevated body weight in earl-life adulthood, or during the transition from early to middle-life adulthood, contributes independently to the disease risk. However, less is known whether body weight gain during different periods in early to middle adult life, usually 20 to 50 years old, are differently related to risk of T2D and how much such weight changes influence the diagnosis age of diabetes.
In this follow-up investigation, we used data from a large sample size well-defined community-based nation-wide cohort in China to determine the effect of decade- based body weight gain from 20 to 50 years old on developing diabetes risk of the later life.
Materials and Methods
Study Population
The study participants were from the China Cardiometabolic Disease and Cancer Cohort (4C) Study, which was an ongoing multicenter, population-based cohort study investigating associations of glucose homeostasis with clinical outcomes, including diabetes, cardiovascular diseases (CVDs), cancers, and all-cause mortality (15, 16). Briefly, a total of 20 communities from seven general geographic regions in China were selected during 2011-2012 (Supplementary Table S1). Eligible men and women aged ≥40 years were identified from local resident registration systems and invited to the study by home visiting by the trained community health workers. At baseline, a standard questionnaire was used to collect the lifestyle factors, disease and medical history, etc. Anthropometry measurements, 2-hour oral glucose tolerance tests (OGTTs), blood and urine sampling were conducted. During 2014-2015, we performed the first round of follow-up examination.
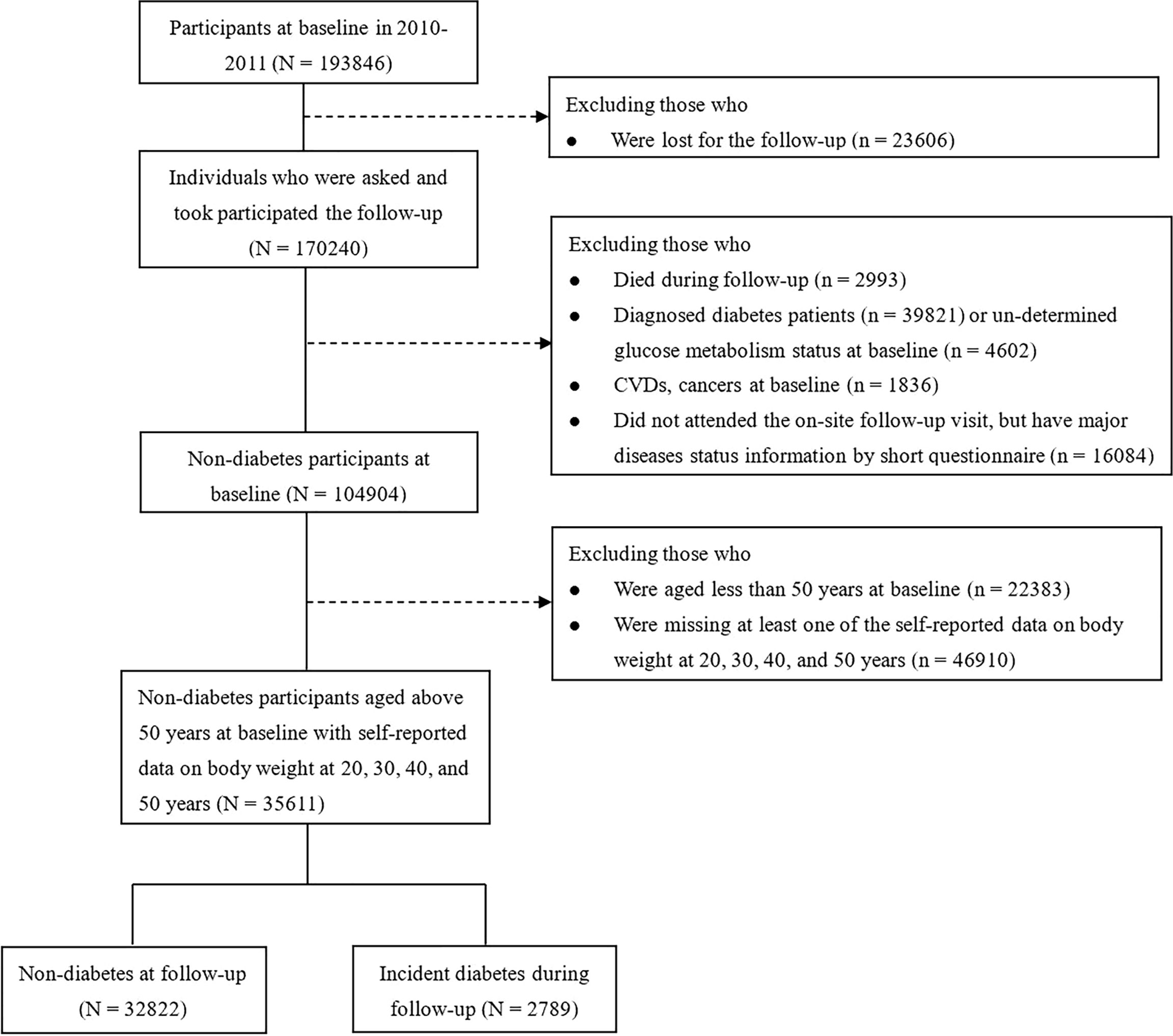
A total of 193,846 individuals were recruited for the baseline and 170,240 participants attended the follow-up examination. In the present analysis, we excluded subjects who died during the follow-up (n=2,993); the baseline diagnosed diabetic patients (n=39,821) or un-determined glucose metabolism status (n=4,602); CVDs or cancers (n=1,836); or those who did not attend the on-site follow-up visit, but have major diseases status information by short questionnaire (n=16,084). For the specific aim of studying the effect of body weight during 20 to 50 years of age on the later life diabetes risk, we further excluded those people who were aged less than 50 years at baseline (n=22,383); or missing at least one of the self-reported data on body weight at 20, 30, 40 and 50 years (n=46,910). Thus, a total of 35,611 non-diabetic participants (25,289 women, 10,322 men) aged 50 years and above were finally included in the main analyses. The flow chart of participants selecting was described in Figure 1.
The study was approved by the Medical Ethics Committee of Ruijin Hospital, Shanghai Jiao-Tong University. Each participant provided written informed consent.
Incident Diabetes Ascertainments
Incident T2D cases was defined according to the 2010 American Diabetes Association (ADA) criteria (fasting plasma glucose level ≥7.0 mmol/l or 2h OGTT glucose ≥11.1 mmol/l, or HbA1c ≥6.5%, or receiving glucose-lowering drugs or insulin injection) and/or a self-reported diagnosis by healthcare professionals.
Exposure Assessment
Participants were asked to recall their body weight (kg) at their twenty, thirty, forty and fifty years old and record on the questionnaire. Weight gain over the 30 years (from 20 to 50 year of age) and decade-based weight gain were calculated by subtracting the self-reported body weight at the former time point from the one at the later time point. We assumed that body height is relatively stable during adulthood, and weight gain can represent one’s changes in adiposity during the early to middle life.
Covariates Assessment
In the baseline questionnaires, we inquired information on risk factors for chronic diseases, such as cigarette smoking (current, former, or never smoker), and alcoholic intake, physical activity and sedentary time, education level (high school and above, or below high school) and family history of diabetes. Smoking status was defined as current, former, and never smokers. Current smokers were defined as smoking cigarettes every day or almost every day, with at least 7 cigarettes per week for at least 6 months. Former smokers were defined as cessation of smoking for more than 6 months. To assess the intensity of the smoking, the current/former smokers were used as two dummy variables, which was defined as the number of cigarettes each day, that were 0 cigarettes/day, 1–9 cigarettes/day, 10-19 cigarettes/day and ≥20 cigarettes/day. Never smokers were defined as participants who reported never smoked or had not smoked cigarettes regularly (<100 cigarettes in a lifetime). Alcohol intake was defined as drinker or non-drinker. Information regarding alcohol intake was collected by inquiring the information on the amount and type of alcoholic beverages consumed in the past 6 months, including beer, wine (e.g. red wine, or yellow wine, a typical wine made from rice and widely consumed in south China), and hard liquor. Beers are sold in 750-mL bottles, and wine and hard liquor in units of 0.5 kg. One unit of beer, wine, and liquor on average contains 30, 90, and 200 g ethanol, respectively. Average alcohol consumption was calculated by multiplying amount of alcohol consumed per drinking day by frequency (g/day). We used the Global Physical Activity Questionnaire to collect intensity, duration, and frequency of physical activity, the Metabolic Equivalent of Task (MET) minutes per week was used to measure physical activity level (17). Baseline body weight and height were measured according to a standard protocol by trained study staff. Baseline body weight and height were measured while they wore minimal clothing and no shoes, with values rounded to the nearest 0.1 kg and 0.1 cm, respectively. Body mass index (BMI) was calculated as body weight in kilograms divided by the square of body height in meters.
Biochemical Measurements
Fasting and 2h post-loading blood sampling was performed during the OGTT. Participants were required to fast for ≥10 hours prior to filed examination. Plasma glucose concentrations were analyzed locally using a glucose oxidase or hexokinase method within two hours after blood sample collection under a stringent quality control program. Hemoglobin Capillary Collection System (Bio-Rad Laboratories, CA, USA) was used to collect finger capillary whole blood and shipped at 2-8°C to a certificated central laboratory at Ruijin Hospital. HbA1c was determined by high-performance liquid chromatography (VARIANT II System, Bio-Rad Laboratories, CA, USA). Fasting serum insulin was measured at the central laboratory using an auto-analyzer (ARCHITECT ci16200 analyzer, Abbott Laboratories, Illinois, USA). Homeostasis model assessment of insulin resistance (HOMA_IR) was calculated as fasting insulin × fasting plasma glucose/22.5, and beta cell function index (HOMA_β) was 20 × fasting insulin / (fasting plasma glucose − 3.5), where fasting insulin was in mIU/L and fasting plasma glucose in mmol/L.
Statistical Analysis
Comparison of the baseline characteristics of the non-diabetic participants according to diabetes status at follow up examination by sex were conducted by ANOVA for continuous variables, and χ2 tests for categorical variables. The skewed distribution data were logarithmically transformed before statistical analysis.
We used Cox proportional hazards models to calculate multivariable-adjusted hazard ratios (HR) and 95% confidence intervals (CI) for the risk of T2D. For incident T2D, person-years were calculated from self-reported date of T2D diagnosis or the respective follow-up examination date for each individual or death or to end of follow-up (June 1, 2015), whichever came first. The following covariates were included in multivariable-adjusted models: age (continuous), sex, BMI at 20 years (continuous), baseline BMI (continuous), physical activity and sedentary time (in quintiles), smoking status (never, past, current), alcohol intake (g/day), high school and above (yes or no) and family history of diabetes (yes or no). The missing data were coded as a missing indicator category for categorical variables. We also tested the potential collinearity of BMI at 20 years age and BMI at baseline, and it showed that there was no collinearity of BMI at 20 years age and at baseline (the variance inflation factor, VIF was 1).
We tested interactions of age and other predictors of T2D by putting body weight gain in each 10 year from 20-50 years or the overall weight gain over the 30 years, each strata variable and the interaction terms (weight gain x the strata variable) in the same multivariate model. We also conducted stratified analysis according to baseline age (< or ≥60 years), sex, BMI at 20 years (< or ≥the median value, 21 kg/m2), baseline BMI (< or ≥25 kg/m2), physical activity (in quintiles), current smoking (yes or no).
All the analyses were carried out with SAS software, version 9.3 (SAS Institute), at a two-tailed alpha level of 0.05.
Results
We determined 2,789 incident diabetes after a follow up of 127,745.26 person-years from the 35,611 non-diabetic participants aged ≥ 50 years. The incidence rate of T2D was 2.18%, 95% CI (2.10% - 2.17%), with 2.43% in men and 2.08% in women.
From early to middle adulthood, the mean weight gain from 20 to 50 years [SD] was: 7.60 [7.95] kg in women and 7.93 [8.54] kg in men; each 10-year weight gain from 20-30, 30-40, and 40-50 years were: 2.78 [4.88], 2.39 [4.26], and 2.42 [4.40] kg in women; and 2.67 [4.12], 2.76 [4.66], and 2.50 [4.92] kg in men, respectively.
Baseline characteristics of participants were summarized in Table 1. Consistently among total participants, men and women, those who developed diabetes had a higher body weight at 20 years, more weight gain from 20 to 50 years, as well as each-decade based weight gain (Table 1). Body weight at 20 years and each-decade weight gain from 20 to 50 years were independently and significantly associated with an increased risk of incident diabetes (Supplementary Table S2).
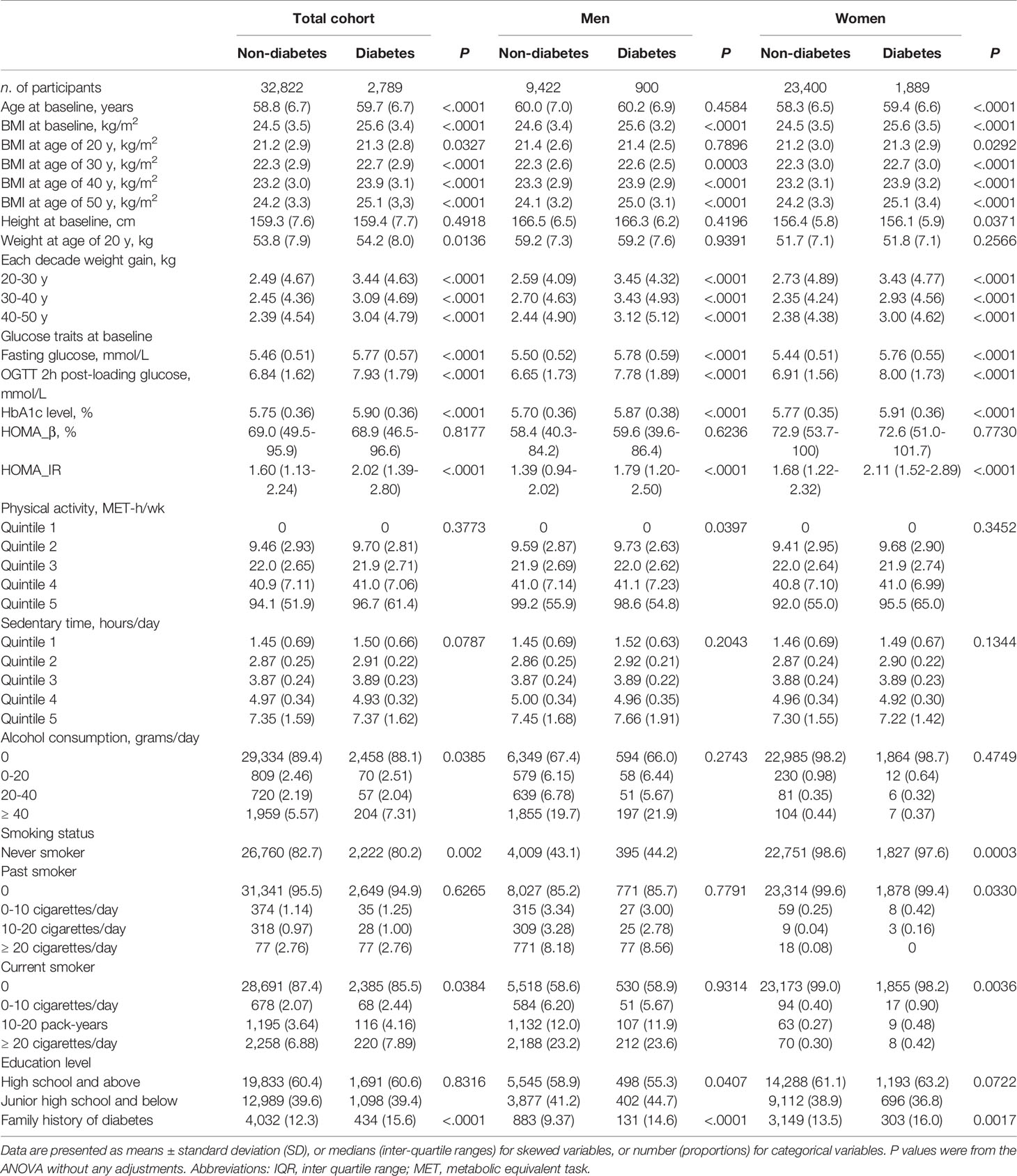
Table 1 Baseline characteristics of the non-diabetic participants according to sex and diabetes status at follow up examination.
Among total cohort, each 10-kg weight gain over the 30 years period (20-50 years) was significantly associated with 39.7% increased risk of incident diabetes (95% CI, 1.33-1.47) (HR 1.37, 95% CI [1.26-1.49] in men; and 1.40 [1.32-1.49] in women) after adjustments for a full list of confounders. The corresponding HRs and 95%CI for each 1-kg/m2 weight gain from 20-50 years were 1.036, 95% CI 1.001-1.071, P = 0.0427 in men and 1.047, 95% CI 1.025-1.069, P <.0001 in women (Table 2). However, the associations were not significantly different between men and women (P for interaction = 0.286). The HR and 95% CI of T2D in weight gain (each 1-kg/m2 BMI) from 20-30 years was 1.055 (1.029-1.081), 1.038 (1.012-1.064) for weight gain form 30-40 years and 1.043 (1.017-1.069) for 40-50 years, respectively. In both sexes, the weight gain from 20-30 years showed a nominally higher risk of developing diabetes than the other two-decades interval; however, no significant interaction between sex and the different life stage weight gain was detected (all P for interaction > 0.344 in men, and 0.329 in women) (Table 2).
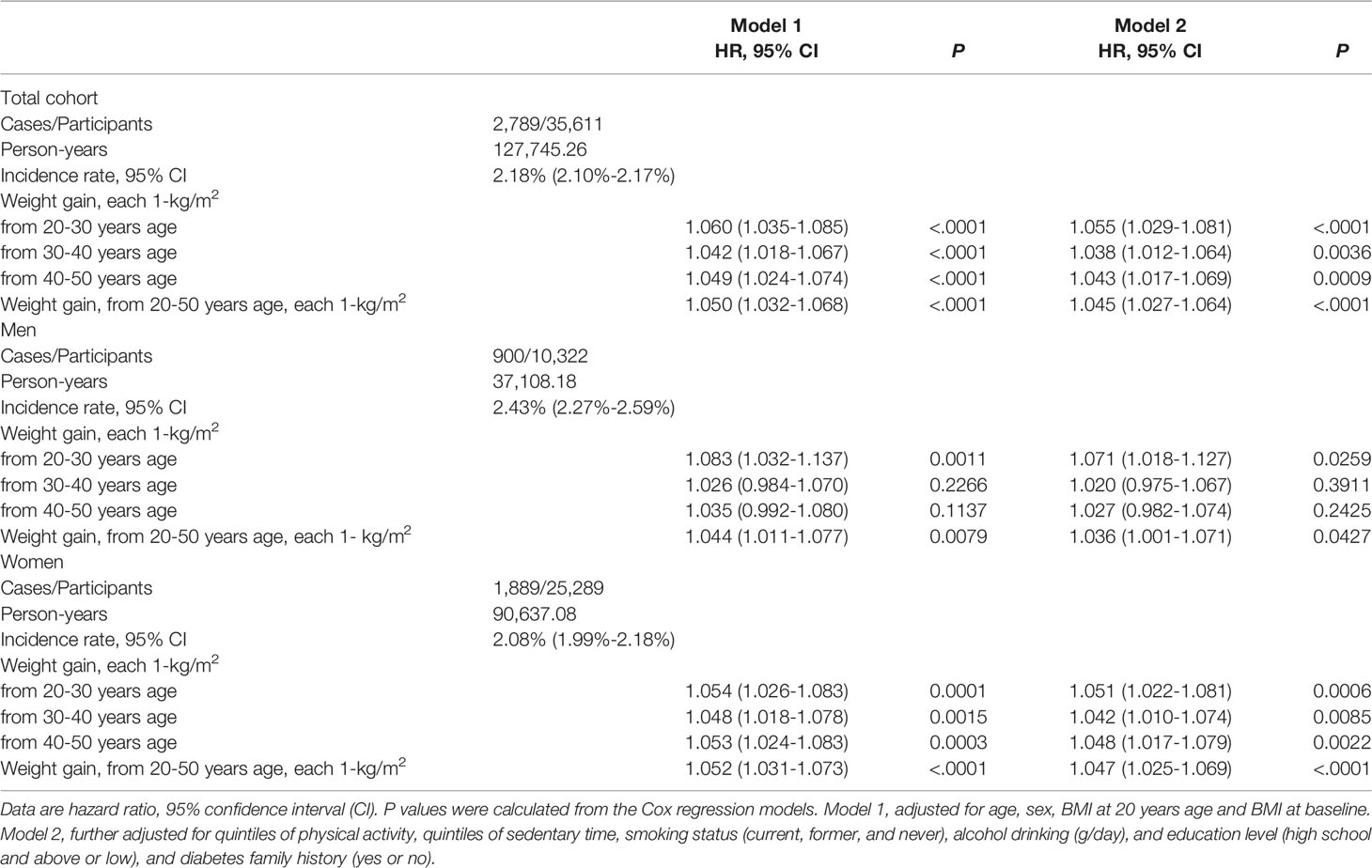
Table 2 Hazard risk of weight gain in different life stage with risk of incident diabetes in total and sex-specific samples.
We further performed stratified analysis according to age, BMI at 20 years and lifestyle factors at baseline. In the participants younger than 60 years, weight gain from 20-30 years showed more prominent effect on risk of developing diabetes than that in those older than 60 years (HR 1.084, 95% CI [1.049-1.121] vs. 1.015, 95% CI [0.975-1.056], P interaction=0.0293) (Table 3, Figure 2A). Same trend was found with regard to total weight gain over the 30 years (Table 3, Figure 2B). It showed a stable effect of the three 10-year intervals weight gain from 20 to 50 years on risk of developing diabetes after 60 years; however, the results did not remain significant after adjusted for the baseline BMI.
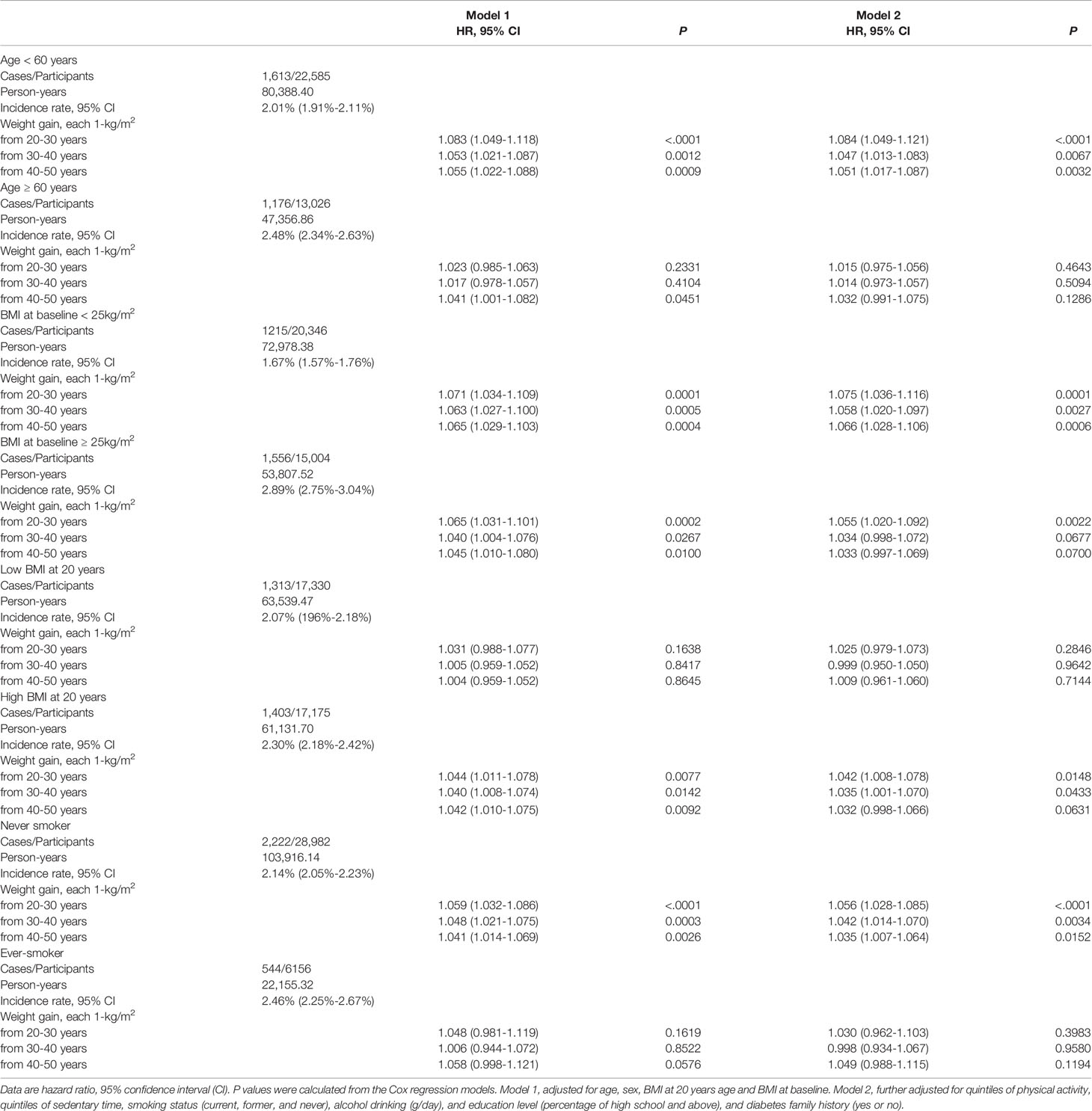
Table 3 Stratified analysis of the hazard risk of weight gain in different life with risk of incident diabetes.
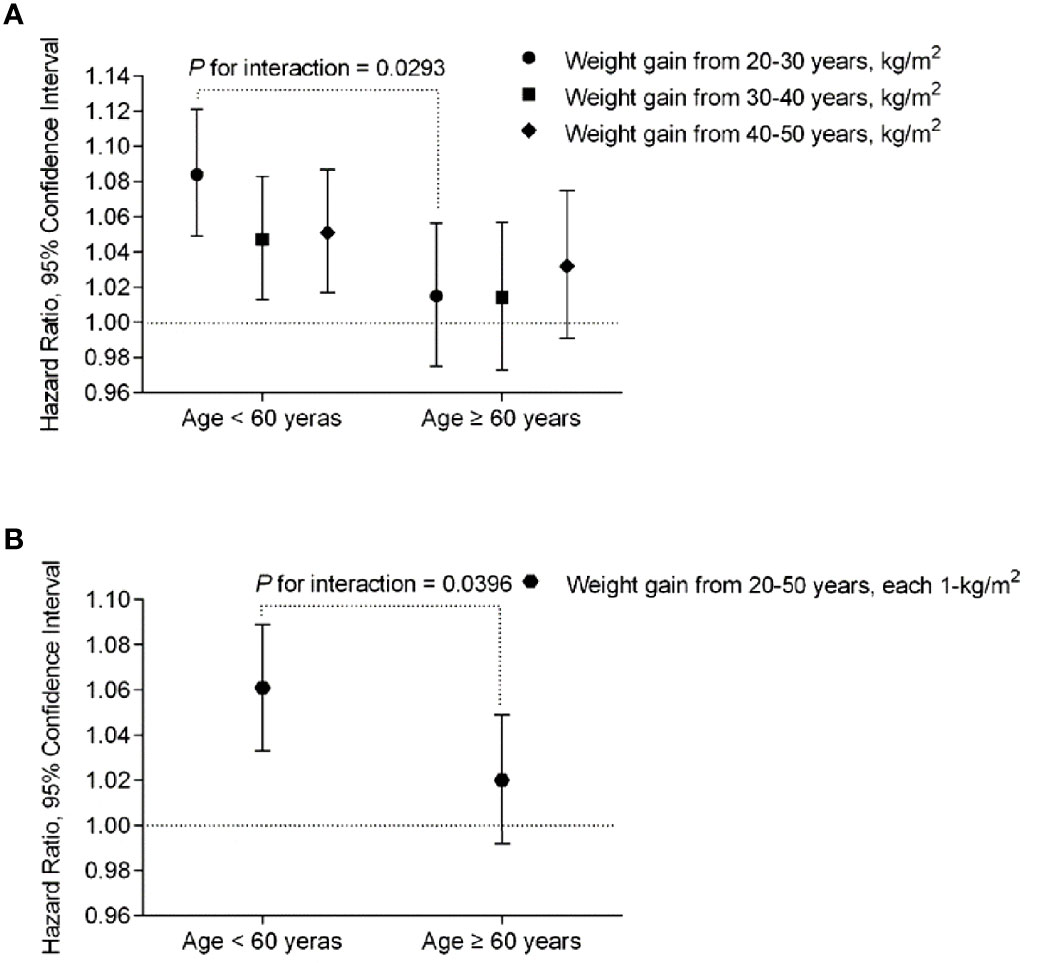
Figure 2 The risk of each 1-kg/m2 body weight gain during the 3-decade intervals from early to middle life with diabetes risk stratified by age < 60 or ≥ 60 years at baseline, respectively. The multivariable-adjusted hazard ratios (HR) and 95% confidence intervals (CI) for the risk of T2D were calculated by Cox proportional hazards models, after adjustments for age (continuous), sex, body height, BMI at 20 years (continuous), baseline BMI (continuous), physical activity and sedentary time (in quintiles), smoking status (never, past, current), alcohol intake (g/day), high school and above (yes or no) and family history of diabetes (yes or no). P interactions were from the Cox proportional hazards models, by putting age and body weight gain in each 10 years from 20-50 years or the overall weight gain over the 30 years, and the interaction terms (weight gain x age) in the same multivariable-adjusted model, simultaneously. (A) Association of each-decade increase of body weight with risk of incident diabetes stratified by age < 60 or ≥ 60 years at baseline. (B) Association of total body weight gain from age 20-50 (each 1 kg/m2) with risk of incident diabetes stratified by age < 60 or ≥ 60 years at baseline.
It also showed that weight gain in each-decade was significantly associated with risk of later life diabetes in participants with higher BMI at their 20 years (≥21 kg/m2); while no significant association was found in those with lower level (< 21 kg/m2) (Table 3). No significant interactions were found between body weight at 20 years, baseline BMI or the smoking status at baseline with the weight gains of each 10-year intervals on the risk of incident diabetes (all P interaction ≥0.05). Characteristics of the participants by their BMI at 20 years and baseline BMI categories was shown in Supplementary Table S3.
On considering a high proportion of the participants failed to report their body weight at least one time per year (44.7%, 46,910/104,904), we validated the robustness of the present analysis by conducting the multiple imputation analysis. We imputed missing data not only in BMI at 20, 30, 40 and 50 years of age, and but also the above mentioned covariates (n=82,521), using the Markov Chain Monte Carlo (MCMC) method, to estimate the risk ratio of weight gain on diabetes (SAS software, version 9.2, SAS Institute, Cary, North Carolina) (18). Figure 3 shows the main and stratified analysis results using the procedure of MIANALYZE, which were consistent with that calculating from the previous procedure in which the missing data for weight at each time point were excluded.
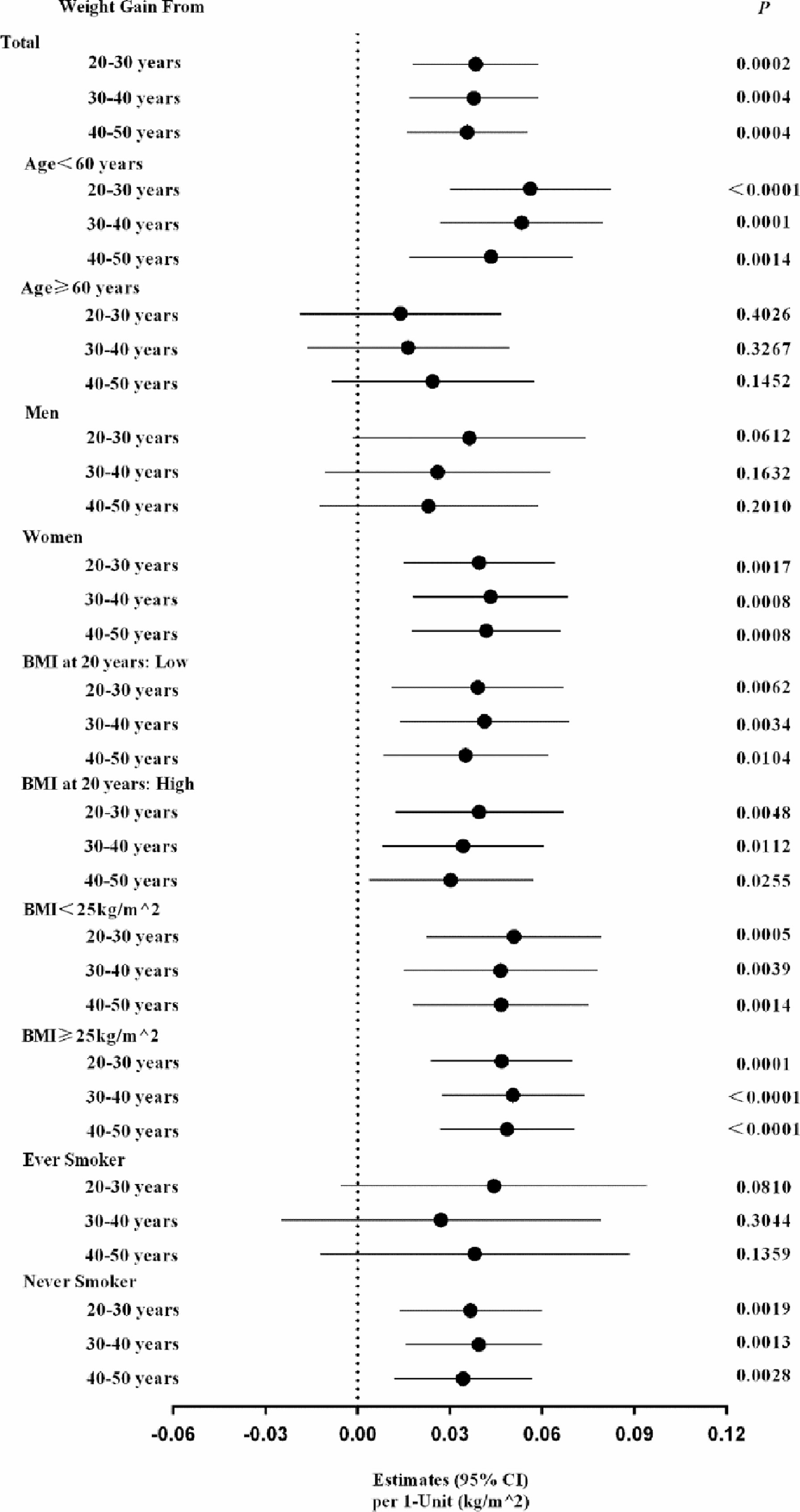
Figure 3 The estimated risk of each 1-kg/m2 of each-decade body weight gain from early to middle life with diabetes risk: data from the multiple imputation methods. The multiple imputation methods were conducted in dealing with the missing data in BMI at 20, 30, 40 and 50 years of age, and the above-mentioned covariates, using the Markov chain Monte Carlo (MCMC) method, to estimate the risk of weight gains on diabetes in total participants and each risk factors strata.
Discussion
In the present large population-based nation-wide follow up study, we found that weight gain from early to middle life was significantly associated with increased later life diabetes risk. Weight gain in early life of the adulthood, usually 20 to 30 years showed more prominent effect on developing diabetes before 60 years than that for developing diabetes after 60 years; however, each-decade weight gain over the adulthood from 20 to 50 years showed similar effect on the risk of developing diabetes after 60 years. The main findings were summarized graphically in Figure 4.
The prevalence of obesity gradually increases from 20 to 60 years of old. A large percentage of T2D develops after 40 to 70 years (13, 19–21). Previous studies have demonstrated that besides initial body weight, weight change during early adulthood poses an independent role on subsequent diabetes risk (7, 12), including recent two large, long-term cohorts, which showed that weight change from early to middle adulthood (age of 18 or 21 to 55 years) was associated with increased risk of morbidity and mortality, independent of weight at early adulthood (11). In addition, we have summarized the scientific literature of similar research works as Supplementary Table 4. In the present study, we confirmed that body weight gain over 30 years in the early and middle adulthood were significantly associated with later life diabetes risk, independent of the baseline and the early life (in 20s) BMI.
Previous study showed an inverse linear relation was found between BMI and age at diabetes onset (22). Adults with early diagnosed diabetes were more obese and more likely to be female than those with a later onset of T2D. Weight gain in early rather than middle-to-late adulthood played an important role in developing diabetes (23). The relative risk of T2D for a 5-kg m−2 increment in BMI was 3.07 for early weight gain (in 18–24s), and 2.12 for late weight-gain (in their 50s) (23). Our results were consistent with the previous studies that investigated the associations of weight change at different period with the risk of T2D (24). In the EPIC-Potsdam study, it was suggested that severe weight gain between ages 25 and 40 y was more strongly associated with risk of diabetes in men (1.5 times) and in women (4.3 times) than weight gain in later life. In addition, greater weight gain, particularly in early adulthood, may be more likely to lead to a lower age at onset of diabetes in men (5 y) and in women (3 y) (24). Though the definition of early adulthood phase was different in our study and other similar studies, it provides us very impressive evidence that the early life, from the 20 to 30 s years, weight gain exerted more prominent effect on risk of earlier developing diabetes.
One possible explanation is that early adulthood marks the end of a critical period for the development of metabolism (25), such as androgen-induced intra-abdominal fat deposition during adolescence may contribute to subsequent hepatic insulin resistance that, in turn, lead to glucose intolerance (26). Individuals who showed weight gain in early life may differ from those who gained weight in later life in behavior and genetic predisposition that may affect metabolism and disease risk (27). Moreover, weight gain in early life tends to be metabolic disturbances reflected by HbA1c, high density lipoprotein cholesterol, high sensitive C-reactive protein, and adiponectin (28).
Several limitations should be acknowledged. Firstly, the early and middle life body weight data was self-reported, which might yield recall bias. The recall bias should be cautiously concerned in this study. However, several previous studies have characterized the validity of retrospective-recalled self-reported weight and measured weight in longitudinal cohorts (29–32), and provided evidence that self-reported weight is reasonably valid surrogate in epidemiology studies (32). In a subgroup of younger women aged 25 - 42 years in the Nurses’ Health Study II, the self-reported and measured weights were highly correlated: the correlation between measured and recalled weight at 18 years was 0.87 (30), and as well in middle aged and elderly study participants: 0.97 for men aged 40-75 years and for women aged 41-65 years (29). In the longitudinal Charleston Heart Study, in elderly study participants aged 60 to 100 years, correlations between reported and measured weights were 0.979 for current, 0.935 for 4-year, and 0.822 for 28-year recall (31). In the present analysis, the average age at baseline were 58.8 years (interquartile range 53.9-62.6). We compared the self-reported body weight at 60 years and the weight measured at baseline examination, the correlation was 0.84 for BMI (kg/m2) and 0.85 for body weight (kg). We could not provide the exact valid data on the early life stage. In one of our ongoing community- based cohort studies at Shanghai local area, we assessed the correlation of two repeated recalled body weight during early life to middle-aged and elderly life with a 5-year interval. We found that the two recalled body weight at 50 years were highly correlated (r = 0.72, n = 2,746), and moderately correlated for body weight at 20 years (r = 0.57, n = 2,783).
Secondly, we carefully excluded the missing values on the early to middle life stage in at least one time points. This procedure could yield solid results, along with some missing value bias. However, the relative homogeneity of the cohort in educational attainment and so on actually may serve to enhance the internal validity of this study. Moreover, we conducted the sensitivity analysis by using multiple imputation methods. Because of a large proportion of missing data in recalled weights, we increased the number of imputations (n of impute = 50) to achieve reasonable statistical efficiency. Additionally, when we selected the predictors of diabetes, we imputed not only the main exposure but also all the covariates that were input in the analysis models. The results yielded from the multiple imputation method consistently supported the main analysis.
In addition, the values of age, body weight and height in the present study were shown as means and standard deviation with one decimal place. As the values of weight and height were usually rounded during the empirical work, it should be cautious in the data interpretation.
Conclusions
Weight gain from early to middle life was linearly and significantly associated with increased risk of incident diabetes. The earlier life weight gain the more strong effect was found on earlier developing diabetes; however, for the risk of developing diabetes after 60 years, each-decade weight gain over the adulthood showed similar effect. It provided evidence that people should pay equal attention to the body weight during the life span, not only after the middle life but also the early stage. More well designed and longitudinal cohort studies with validated early life body weight records or measurements and well documented diseases outcomes are warranted to replicate the present study.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
Ethics Statement
The studies involving human participants were reviewed and approved by Medical Ethics Committee of Ruijin Hospital, Shanghai Jiao-Tong University School of Medicine. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
WW, JL, GN, and MX conceived and designed the study. MX, YQ, ML, YX, JL, TW, and ZZ analyzed data. QW, FS, ZG, GC, JZ, LC, LS, RH, ZY, XT, QS, GQ, GW, ZL, YQ, YH, QL, YZ, YC, CL, YM, YW, SWu, TY, LC, XY, LY, and HD collected data. All authors were involving in writing and revising the manuscript and had final approval of the submitted and published versions. WW, JL and GN are the guarantors of this work and, as such, had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. All authors contributed to the article and approved the submitted version.
Funding
This work was funded by the National Natural Science Foundation of China (81941017, 81930021, 81970706, 81970728, 91857205, and 82088102), and the Clinical Research Plan of SHDC (SHDC2020CR1001A and SHDC2020CR3064B), and the Shanghai Municipal Education Commission–Gaofeng Clinical Medicine Grant Support (20152508 Round 2). MX, JL, ML, TW, YX, ZZ, YB, WW, and GN are members of innovative research team of high-level local universities in Shanghai.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
The authors thank all study participants.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2022.927067/full#supplementary-material
References
1. Naser KA, Gruber A, Thomson GA. The Emerging Pandemic of Obesity and Diabetes: Are We Doing Enough to Prevent a Disaster? Int J Clin Pract (2006) 60(9):1093–7. doi: 10.1111/j.1742-1241.2006.01003.x
2. Schulze MB, Hu FB. Primary Prevention of Diabetes: What can be Done and How Much can be Prevented? Annu Rev Public Health (2005) 26:445–67. doi: 10.1146/annurev.publhealth.26.021304.144532
3. Weinstein AR, Sesso HD, Lee IM, Cook NR, Manson JE, Buring JE, et al. Relationship of Physical Activity vs Body Mass Index With T2D in Women. JAMA (2004) 292(10):1188–94. doi: 10.1001/jama.292.10.1188
4. Tirosh A, Shai I, Afek A, Dubnov-Raz G, Ayalon N, Gordon B, et al. Adolescent BMI Trajectory and Risk of Diabetes Versus Coronary Disease. N Engl J Med (2011) 364(14):1315–25. doi: 10.1056/NEJMoa1006992
5. de Lauzon-Guillain B, Balkau B, Charles MA, Romieu I, Boutron-Ruault MC, Clavel-Chapelon F. Birth Weight, Body Silhouette Over the Life Course, and Incident Diabetes in 91,453 Middle-Aged Women From the French Etude Epidemiologique De Femmes De La Mutuelle Generale De L’education Nationale (E3N) Cohort. Diabetes Care (2010) 33(2):298–303. doi: 10.2337/dc09-1304
6. He S, Shu Y, He J, Chen X, Cui K, Feng J, et al. The Effects of Initial and Subsequent Adiposity Status on Diabetes Mellitus. Int J Cardiol (2013) 168(1):511–4. doi: 10.1016/j.ijcard.2012.09.196
7. de Mutsert R, Sun Q, Willett WC, Hu FB, van Dam RM. Overweight in Early Adulthood, Adult Weight Change, and Risk of T2D, Cardiovascular Diseases, and Certain Cancers in Men: A Cohort Study. Am J Epidemiol (2014) 179(11):1353–65. doi: 10.1093/aje/kwu052
8. Black E, Holst C, Astrup A, Toubro S, Echwald S, Pedersen O, et al. Long-Term Influences of Body-Weight Changes, Independent of the Attained Weight, on Risk of Impaired Glucose Tolerance and T2D. Diabetes Med (2005) 22(9):1199–205. doi: 10.1111/j.1464-5491.2005.01615.x
9. Zhou L, Li Y, Guo M, Wu Y, Zhao L. Relations of Body Weight Status in Early Adulthood and Weight Changes Until Middle Age With Hypertension in the Chinese Population. Hypertension Res (2016) 39(12):913–18. doi: 10.1038/hr.2016.80
10. Chen C, Ye Y, Zhang Y, Pan XF, Pan A. Weight Change Across Adulthood in Relation to All Cause and Cause Specific Mortality: Prospective Cohort Study. BMJ (2019) 367:l5584. doi: 10.1136/bmj.l5584
11. Zheng Y, Manson JE, Yuan C, Liang MH, Grodstein F, Stampfer MJ, et al. Associations of Weight Gain From Early to Middle Adulthood With Major Health Outcomes Later in Life. JAMA (2017) 318(3):255–69. doi: 10.1001/jama.2017.7092
12. Sun W, Shi L, Ye Z, Mu Y, Liu C, Zhao J, et al. Association Between the Change in Body Mass Index From Early Adulthood to Midlife and Subsequent T2D Mellitus. Obesity (2016) 24(3):703–9. doi: 10.1002/oby.21336
13. Tian Y, Jiang C, Wang M, Cai R, Zhang Y, He Z, et al. BMI, Leisure-Time Physical Activity, and Physical Fitness in Adults in China: Results From a Series of National Surveys, 2000-14. Lancet Diabetes Endocrinol (2016) 4(6):487–97. doi: 10.1016/S2213-8587(16)00081-4
14. Bi Y, Jiang Y, He J, Xu Y, Wang L, Xu M, et al. Status of Cardiovascular Health in Chinese Adults. J Am Coll Cardiol (2015) 65(10):1013–25. doi: 10.1016/j.jacc.2014.12.044
15. Lu J, He J, Li M, Tang X, Hu R, Shi L, et al. Predictive Value of Fasting Glucose, Postload Glucose, and Hemoglobin A 1c on Risk of Diabetes and Complications in Chinese Adults. Diabetes Care (2019) 42(8):1539–48. doi: 10.2337/dc18-1390
16. Wang T, Lu J, Shi L, Chen G, Xu M, Xu Y, et al. Association of Insulin Resistance and β-Cell Dysfunction With Incident Diabetes Among Adults in China: A Nationwide, Population-Based, Prospective Cohort Study. Lancet Diabetes Endocrinol (2020) 8(2):115–24. doi: 10.1016/S2213-8587(19)30425-5
17. World Health Organization. Global Physical Activity Questionnaire and Analysis Guide (2019). Available at: https://www.who.int/ncds/surveillance/steps/GPAQ/en/ (Accessed 15 Feb 2019).
18. Yuan YC. Multiple Imputation for Missing Data: Concepts and New Development (Version 9.0). Rockville, MD: SAS Institute (2000).
19. Centers for Disease Control and Prevention. 2011 National Diabetes Statistic Report (2017). Available at: https://www.cdc.gov/features/diabetes-statistic-report/index.html (Accessed 15 Feb 2019).
20. Wang L, Gao P, Zhang M, Huang Z, Zhang D, Deng Q, et al. Prevalence and Ethnic Pattern of Diabetes and Prediabetes in China in 2013. JAMA (2017) 317(24):2515–23. doi: 10.1001/jama.2017.7596
21. Xu Y, Wang L, He J, Bi Y, Li M, Wang T, et al. 2010 China Noncommunicable Disease Surveillance Group. Prevalence and Control of Diabetes in Chinese Adults. JAMA (2013) 310(9):948–59. doi: 10.1001/jama.2013.168118
22. Hillier TA, Pedula KL. Characteristics of an Adult Population With Newly Diagnosed T2D: The Relation of Obesity and Age of Onset. Diabetes Care (2001) 24(9):1522–7. doi: 10.2337/diacare.24.9.1522
23. Kodama S, Horikawa C, Fujihara K, Yoshizawa S, Yachi Y, Tanaka S, et al. Quantitative Relationship Between Body Weight Gain in Adulthood and Incident T2D: A Meta-Analysis. Obes Rev (2014) 15(3):202–14. doi: 10.1111/obr.12129
24. Schienkiewitz A, Schulze MB, Hoffmann K, Kroke A, Boeing H. Body Mass Index History and Risk of T2D: Results From the European Prospective Investigation Into Cancer and Nutrition (EPIC)-Potsdam Study. Am J Clin Nutr (2006) 84(2):427–33. doi: 10.1093/ajcn/84.1.427
25. Brancati FL, Wang NY, Mead LA, Liang KY, Klag MJ. Body Weight Patters From 20 to 49 Years of Age and Subsequent Risk for Diabetes Mellitus. Arch Inter Med (1999) 159(9):957–63. doi: 10.1001/archinte.159.9.957
26. Dietz WH. Critical Periods in Childhood for the Development of Obesity. Am J Clin Nutr (1994) 59(5):955–9. doi: 10.1093/ajcn/59.5.955
27. Stunkard AJ, Sørensen TI, Hanis C, Teasdale TW, Chakraborty R, Schull WJ, et al. An Adoption Study of Human Obesity. N Engl J Med (1986) 314(4):193–8. doi: 10.1056/NEJM198601233140401
28. Montonen J, Boeing H, Schleicher E, Fritsche A, Pischon T. Association of Changes in Body Mass Index During Earlier Adulthood and Later Adulthood With Circulating Obesity Biomarker Concentrations in Middle-Aged Men and Women. Diabetologia (2011) 54(7):1676–83. doi: 10.1007/s00125-011-2124-6
29. Rimm EB, Stampfer MJ, Colditz GA, Chute CG, Litin LB, Willett WC. Validity of Self-Reported Waist and Hip Circumferences in Men and Women. Epidemiology (1990) 1(6):466–73. doi: 10.1097/00001648-199011000-00009
30. Troy LM, Hunter DJ, Manson JE, Colditz GA, Stampfer MJ, Willett WC. The Validity of Recalled Weight Among Younger Women. Int J Obes Relat Metab Disord (1995) 19(8):570–2.
31. Stevens J, Keil JE, Waid LR, Gazes PC. Accuracy of Current, 4-Year, and 28-Year Self-Reported Body Weight in an Elderly Population. Am J Epidemiol (1990) 132(6):1156–63. doi: 10.1093/oxfordjournals.aje.a115758
Keywords: Body weight gain, early life adulthood, prospective cohort, type 2 diabetes, later life risk, obesity
Citation: Xu M, Qi Y, Chen G, Qin Y, Wu S, Wang T, Zhao Z, Xu Y, Li M, Chen L, Chen L, Chen Y, Deng H, Gao Z, Huo Y, Li Q, Liu C, Luo Z, Mu Y, Qin G, Shen F, Shi L, Su Q, Wan Q, Wang G, Wang S, Wang Y, Hu R, Xu Y, Yan L, Yang T, Yu X, Zhang Y, Zeng T, Tang X, Ye Z, Zhao J, Bi Y, Ning G, Lu J and Wang W (2022) The Relative Body Weight Gain From Early to Middle Life Adulthood Associated With Later Life Risk of Diabetes: A Nationwide Cohort Study. Front. Endocrinol. 13:927067. doi: 10.3389/fendo.2022.927067
Received: 23 April 2022; Accepted: 15 June 2022;
Published: 19 July 2022.
Edited by:
Alexandre Gabarra Oliveira, São Paulo State University, BrazilReviewed by:
Heng Wan, Southern Medical University, ChinaJatinderkumar R. Saini, Symbiosis Institute of Computer Studies and Research (SICSR), India
Copyright © 2022 Xu, Qi, Chen, Qin, Wu, Wang, Zhao, Xu, Li, Chen, Chen, Chen, Deng, Gao, Huo, Li, Liu, Luo, Mu, Qin, Shen, Shi, Su, Wan, Wang, Wang, Wang, Hu, Xu, Yan, Yang, Yu, Zhang, Zeng, Tang, Ye, Zhao, Bi, Ning, Lu and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Weiqing Wang, d3Fpbmd3NjFAMTYzLmNvbQ==; Jieli Lu, amllbGlsdUBob3RtYWlsLmNvbQ==; Guang Ning, Z25pbmdAc2licy5hYy5jbg==
†These authors have contributed equally to this work
 Min Xu
Min Xu Yan Qi1,2†
Yan Qi1,2† Gang Chen
Gang Chen Yingfen Qin
Yingfen Qin Tiange Wang
Tiange Wang Zhiyun Zhao
Zhiyun Zhao Mian Li
Mian Li Li Chen
Li Chen Yuhong Chen
Yuhong Chen Zhengnan Gao
Zhengnan Gao Yanan Huo
Yanan Huo Zuojie Luo
Zuojie Luo Yiming Mu
Yiming Mu Guijun Qin
Guijun Qin Qin Wan
Qin Wan Guixia Wang
Guixia Wang Shuangyuan Wang
Shuangyuan Wang Li Yan
Li Yan Tao Yang
Tao Yang Xuefeng Yu
Xuefeng Yu Tianshu Zeng
Tianshu Zeng Xulei Tang
Xulei Tang Jiajun Zhao
Jiajun Zhao Yufang Bi
Yufang Bi Jieli Lu
Jieli Lu Weiqing Wang
Weiqing Wang