- 1Guangxi Key Laboratory of Environmental Exposomics and Entire Lifecycle Heath, Guangxi Health Commission Key Laboratory of Entire Lifecycle Health and Care, School of Public Health, Guilin Medical University, Guilin, China
- 2Scientific Experiment Center, Guilin Medical University, Guilin, China
- 3Institute of Preventive Medicine, School of Public Health, Guilin Medical University, Guilin, China
Objective: The aim of this study was to investigate the relationship between potential functional single-nucleotide polymorphisms (SNPs) of the angiotensin-converting enzyme 2 (ACE2) gene and the pathogenesis of pre-eclampsia (PE) in Guangxi, China.
Materials and methods: A case–control study was conducted involving 327 PE cases and 591 age-matched, normal, singleton pregnant women. Potential functional ACE2 gene variants (rs2106809 A>G, rs6632677 G>C, and rs2074192 C>T) were selected and genotyped using kompetitive allele-specific PCR. The strength of the associations between the studied genetic variants and the risk of PE were evaluated using odds ratios (ORs) and corresponding 95% confidence intervals (CIs).
Result: After adjusting for age and body mass index (BMI), unconditional logistic regression analysis showed that rs2106809 A>G was significantly associated with PE risk (AG vs. AA, OR = 1.43, 95% CI = 1.03–1.99, p = 0.034; AG/GG vs. AA, OR = 1.45, 95% CI = 1.06–1.99, p = 0.019), especially with severe PE (AG vs. AA, adjusted OR = 1.70, 95% CI = 1.10–2.61; AG/GG vs. AA, adjusted OR = 1.71, 95% CI = 1.14–2.57). Further stratified analysis showed that rs2106809 was even more pronounced in subjects in the pre-pregnancy BMI (pre-BMI) >23 kg/m2 (adjusted OR = 2.14, 95% CI = 1.32–3.45) and triglyceride (TG) >2.84 mmol/L subgroups (adjusted OR = 1.81, 95% CI = 1.09–3.01) under the dominant genetic model. We also found that rs2106809 interacted with pre-BMI (pinteraction = 0.040), thereby affecting an individual’s genetic susceptibility to PE. Multiple dimension reduction analysis demonstrated that rs2106809 made the best one-locus model, and the three-locus model was the best interaction model for predicting PE risk. Functional analysis suggested that rs2106809 A>G causes a change in the reliability of classifications of two putative splice sites in the ACE2 gene, potentially regulating the expression of functional genes (PIR, ACE2, and CLTRN) in multiple tissues and cell lines (p< 0.05).
Conclusion: The ACE2 gene rs2106809 A>G variant is significantly associated with the risk of PE via individual locus effects and/or complex gene–gene and gene–environment interactions. Regulating the expression of functional genes such as PIR, ACE2, and CLTRN may be the molecular mechanism by which rs2106809 increases an individual’s susceptibility to PE.
Introduction
Pre-eclampsia (PE), a common disease that occurs during pregnancy, has a global incidence of 3%–5% and approximately 2.9% in China (1). PE is characterized by hypertension (140/90 mmHg) and albuminuria (0.3 g/day) after 20 weeks of pregnancy. In recent years, the incidence of PE has increased, especially in developing countries (2). Clinical studies have shown that PE can cause systemic small vessel spasms, endothelial injury, and a decrease in blood perfusion of various systems and organs caused by local ischemia, which can injure the mother and fetus. As the disease progresses, it may even lead to terminal organ dysfunction and multiple severe adverse pregnancy outcomes, such as maternal eclampsia, oligohydramnios, placental abruption, hemolysis, elevated liver enzymes, and low platelet count (HELLP) syndrome, and cardiovascular disease or fetal intrauterine growth restriction, premature birth, and fetal perinatal death (3, 4). PE seriously endangers both the short-term and long-term health of mothers and infants. However, details regarding the etiological mechanism of PE remain unclear.
PE may be affected by conditions such as advanced pregnancy, chronic hypertension, inflammatory immune overactivation, vascular endothelial cell damage, nutritional deficiency or obesity, and insulin resistance (5). In addition, previous studies have found that those with an individual or family history of PE are significantly more likely to develop PE during pregnancy (6, 7). Thus, in addition to various environmental factors, the risk of PE is also regulated by genetic factors. Single-nucleotide polymorphisms (SNPs) as DNA sequence variations caused by the conversion or transversion of a single nucleotide are the most common genetic variations in humans (8). They play important roles in the genetic anatomy of complex traits and diseases, and are useful for identifying population-based susceptibility genes (9). Genome-wide association and case–control studies have identified a series of susceptibility genes and susceptibility loci related to the risk of PE, including the zinc finger protein 831(ZNF831) gene polymorphism rs259983, FTO alpha-ketoglutarate dependent dioxygenase (FTO) rs1421085, MDS1 and EVI1 complex locus (MECOM) rs1918975, angiotensinogen (AGT) M235T, storkhead box 1 (STOX1) Y153H, and interleukin 6 (IL-6) 176 G>C (10–13).
Studies have confirmed that the renin–angiotensin system (RAS) is involved in vascular remodeling and blood pressure regulation during pregnancy (14–17). The ACE2 gene plays a key role in the RAS system. ACE2 can induce angiotensin II (Ang II) to produce angiotensin 1–7 (Ang1–7), which dilate blood vessels and thereby lower body blood pressure (18). Tamanna et al. found that the regulation of ACE2 plays an important role in PE and fetal growth restriction, suggesting that ACE2 could be a new target for the treatment and/or prevention of pregnancy complications (19). Several ACE2 gene variants, such as rs2074192, rs2106809, rs2048683, and rs4240157, are reportedly significantly associated with the risk of hypertension (20, 21). Based on current research evidence, we hypothesized that the ACE2 gene and its variants play a key role in regulating the incidence and outcome of various pregnancy complications, including PE.
To date, no studies have been published detailing the relationship between ACE2 gene variants and susceptibility to PE. We therefore conducted a case–control study involving 327 PE patients and 591 healthy pregnant women to explore the associations between three candidate variants in the ACE2 gene (rs2106809 A>G, rs6632677 G>C, and rs2074192 C>T) and the pathogenesis of PE.
Materials and methods
Study population
A total of 918 subjects with 327 cases with PE (118 women with mild PE and 61 women with severe PE) and 591 controls were recruited from the First Affiliated Hospital of Guilin University of Medical Sciences between September 2014 and April 2016. Participants who met the diagnostic criteria for PE (first presentation after 20 weeks of gestation with systolic blood pressure ≥140 mmHg or diastolic blood pressure ≥90 mmHg and urine protein ≥0.3 g/24 h) were included as PE cases. Age-matched (± 3 years) healthy pregnant women were recruited during the same period. All subjects were singleton pregnancies and unrelated. Pregnant women with diabetes, hypertension, congenital genetic diseases, cardiovascular diseases, or other pregnancy-related diseases were excluded. Study participants signed an informed consent form before the study, and the Ethics Committee Review Board of Guilin Medical University and the Affiliated Hospital of Guilin Medical University approved the study protocol.
Clinical and biochemical data
Clinical and biological characteristics were obtained from unified questionnaires and patient medical records, including weight, height, pre-pregnancy BMI (pre-BMI), diastolic blood pressure (DBP), systolic blood pressure (SBP), fasting plasma glucose (FPG), triglyceride (TG), total cholesterol (TC), and high-density lipoprotein cholesterol (HDL-c).
Genomic DNA extraction
The genomic DNA was extracted from EDTA-treated whole blood using a DNA extraction kit (Aidlab Biotechnologies Co., Ltd., China) and stored in a refrigerator at −20°C prior to PCR.
SNP selection
Potential functional SNPs of the ACE2 gene were screened using the NCBI dbSNP database (http://www.ncbi.nlm.nih.gov/projects/SNP) and SNPinfo Web Server (http://snpinfo.niehs.nih.gov/). The minor allele frequency (MAF) of candidate SNPs reported in HapMap-based Han Chinese in Beijing (HCB) was >0.05, and the linkage disequilibrium (LD) coefficient between SNPs was<0.8 (r2< 0.8). Finally, three genetic variants (rs2106809 A>G, rs6632677 G>C, and rs2074192 C>T) located in the ACE2 gene were selected for further study.
SNP genotyping using the kompetitive allele-specific PCR method
Candidate ACE2 gene variants were genotyped using a kompetitive allele-specific PCR (KASP) method, and the corresponding specific PCR primers were designed and synthesized by Sangon Biotech Co., Ltd. (Shanghai, China) (Table 1). Reactions (10 µl each) were carried out in a 96-well plate and included 5 µl of FLu-Arms 2× PCR mix, 0.5 µl of 10 μM each of three specific primers (F1: 0.1 µl, F2: 0.1 µl, and R: 0.3 µl), 0.5 µl (25–150 ng) of DNA, and 4 µl of ddH2O. Two allele-specific forward primers were labeled with the respective fluorochromes FAM and HEX. Reactions were performed according to the following standard KASP-PCR program: pre-denaturation at 95°C for 3 min, followed by 10 touchdown cycles of 95°C for 15 s (denaturation), 61–55°C for 60 s (annealing and elongation), and 30 cycles of 95°C for 15 s and 55°C for 60 s, with a final incubation at 30°C for 30 s. Figure 1 shows a genotyping scatter plot of the candidate loci.
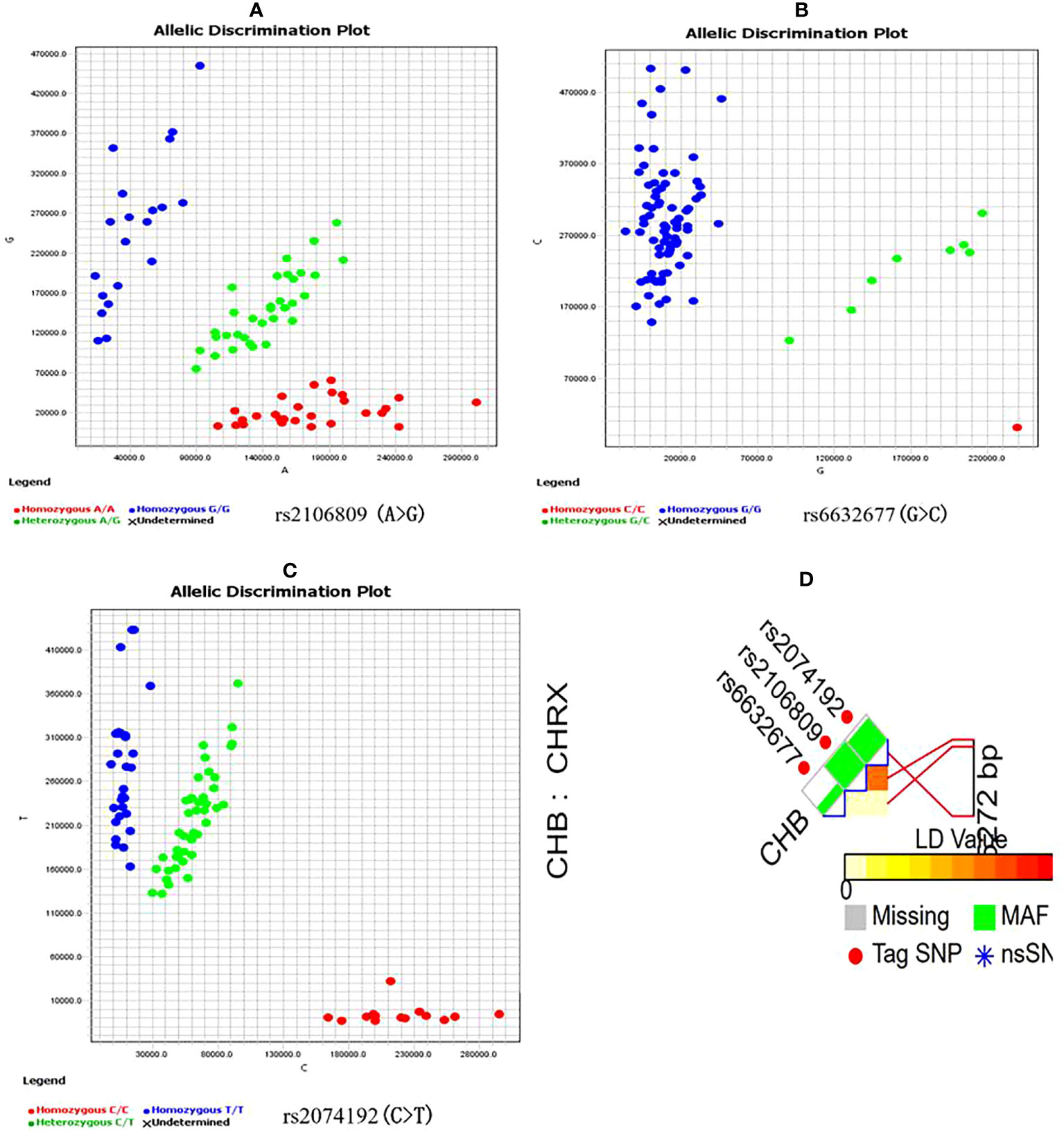
Figure 1 ACE2 gene candidate genetic variations selection and genotyping (A–C) are genotyping scatter plot of rs2106809 A>G, rs6632677G>C and rs2074192C>T respectively; (D) is ACE2 fiunctional SNPs selection by SNPinfo Web Server online tool, LD: r2<0.8.
Statistical analysis
Statistical analysis was performed with IBM SPSS Statistics 28 for Windows (IBM Corp., Armonk, NY, USA). Clinical and biochemical features were compared by calculating the mean ± standard deviation ( ± sd). Chi-square (χ2) test was used to assess the differences of genotypes between cases and controls. A χ2 goodness-of-fit test was performed to determine whether the distribution about genotypes of SNPs in controls conformed to Hardy–Weinberg equilibrium (HWE). Logistic regression model was used to evaluate the associations between genotypes of each variant with PE by computing the odds ratios (ORs) and their 95% confidence intervals (CIs). A p-value< 0.05 was considered as statistically significant and all statistical tests were two-tailed.
In addition, the false-positive reporting probability (FPRP) was estimated using the method described by Wacholder et al. (22) to assess the robustness of the statistically significant associations found. The threshold was set to 0.2 and the prior probability was set to 0.1 to detect the noteworthy with an OR value of 1.5 or 0.67, and the α level was equal to the observed p-value.
The multiple dimension reduction (MDR) software (version 3.0.2) was used to investigate the joint effect of SNPs. A 100-fold cross-validation and 1,000-fold permutation testing were adopted under the null hypothesis of no association. The cross-validation consistency (CVC) and the testing balanced accuracy helped to identify the best model among all possibilities considered (23).
If a significant association between genetic variation and PE risk is detected, the potential function of the variation regulating the putative splice sites will be analyzed by Alternative Splice Site Predictor (ASSP) tool (24). In the ASSP analysis, codon usage and stop codons for all three possible reading frames (F1—frame 1, F2—frame 2, and F3—frame 3) and scores of the preprocessing models reflecting splice site strength are calculated, and the types of putative splice sites are classified by the corresponding backpropagation network with the output activations and the confidence by ASSP analysis. In order to explore the function of significantly associated SNP and possible regulation of related gene expression, we further applied GWAS4D online analysis (http://www.mulinlab.org/gwas4d/) (25) based on GTEx database to analyze expression quantitative trait locus (eQTL) between candidate variants and gene transcription level and clarify the potential molecular regulation mechanism of variant.
Results
Characteristics of the study population
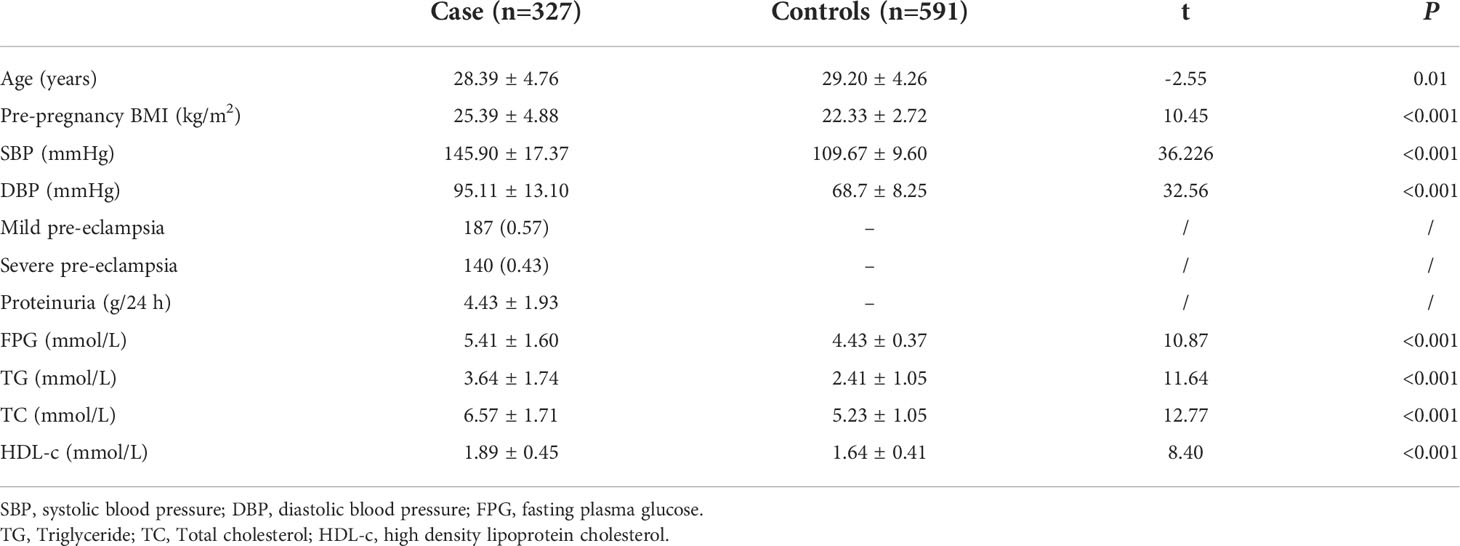
A total of 918 participants were selected, including 327 women diagnosed with PE and 591 healthy controls. Analysis of the general population data revealed that the indexes of pre-BMI, SBP, DBP, FPG, TG, and HDL-C were higher among PE cases than controls (p< 0.001), as shown in Table 2.
Association between studied variants and PE risk
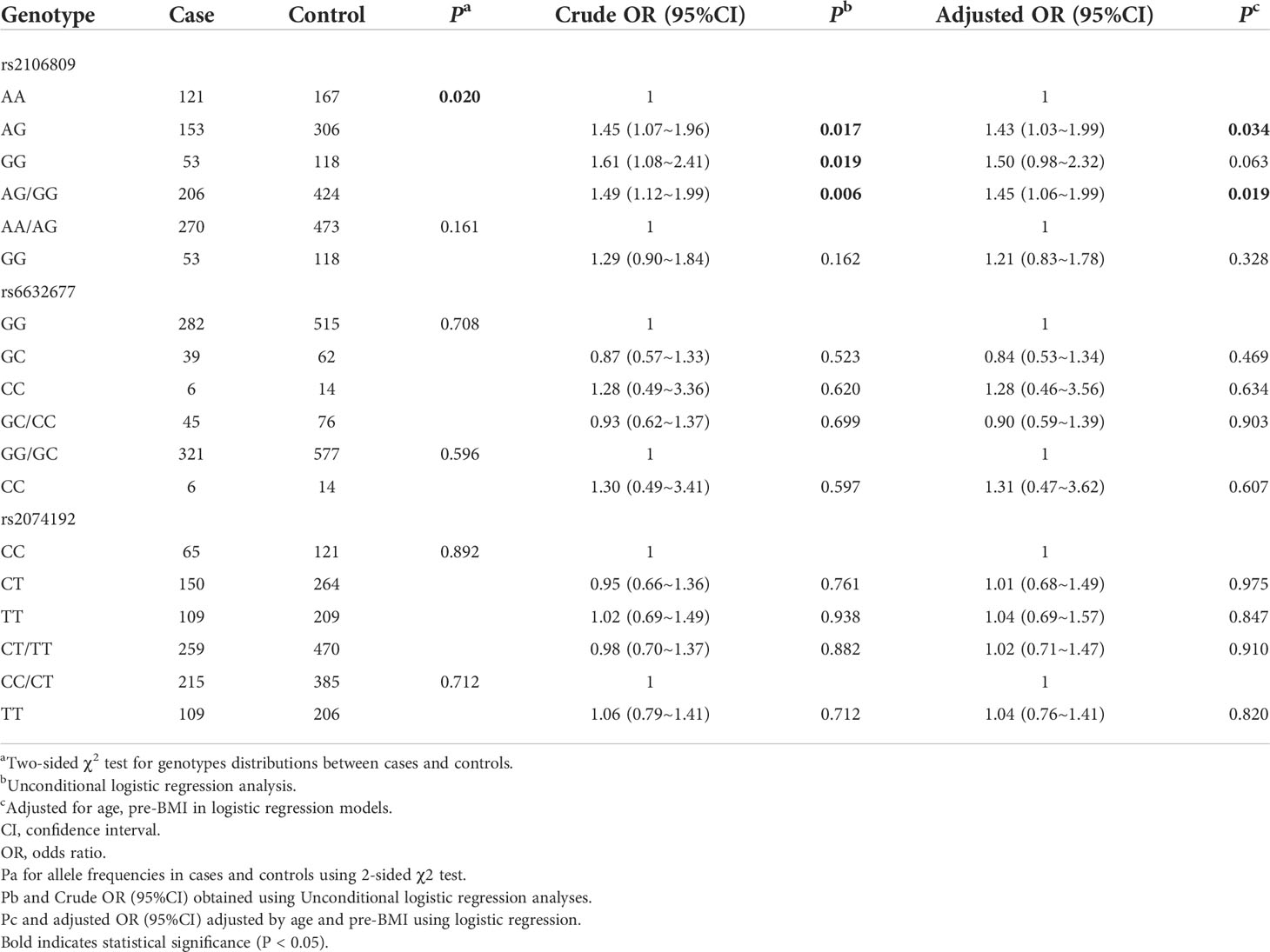
The candidate SNPs were genotyped using the KASP method. The D and r2 values were calculated to evaluate the LD of studied SNPs from the genotyping data using the SHEsis online tool, which revealed no significant degree of LD among the three SNPs (data not shown). The genotype frequencies of rs2106809, rs6632677, and rs2074192 among controls obeyed HWE (χ2 = 1.08, p = 0.299; χ2 = 0.69, p = 0.405 and χ2 = 4.55, p< 0.05, respectively). After adjustment for age and pre-BMI, logistic regression analysis showed that the associations remained significant in the heterozygote comparison (AG vs. AA, adjusted OR = 1.43, 95% CI = 1.03–1.99, p = 0.034) and in the dominant model (AG/GG vs. AA, adjusted OR = 1.45, 95% CI = 1.06–1.99, p = 0.019). However, compared with AA genotype carriers, the associations in GG genotype carriers exhibited marginal statistical significance (GG vs. AA, adjusted OR = 1.50, 95% CI = 0.98–2.32, p = 0.063). We failed to find significant associations between ACE2 rs6632677 C>G and rs2074192 C>T and susceptibility to PE in the present study (p > 0.05), as shown in Table 3.
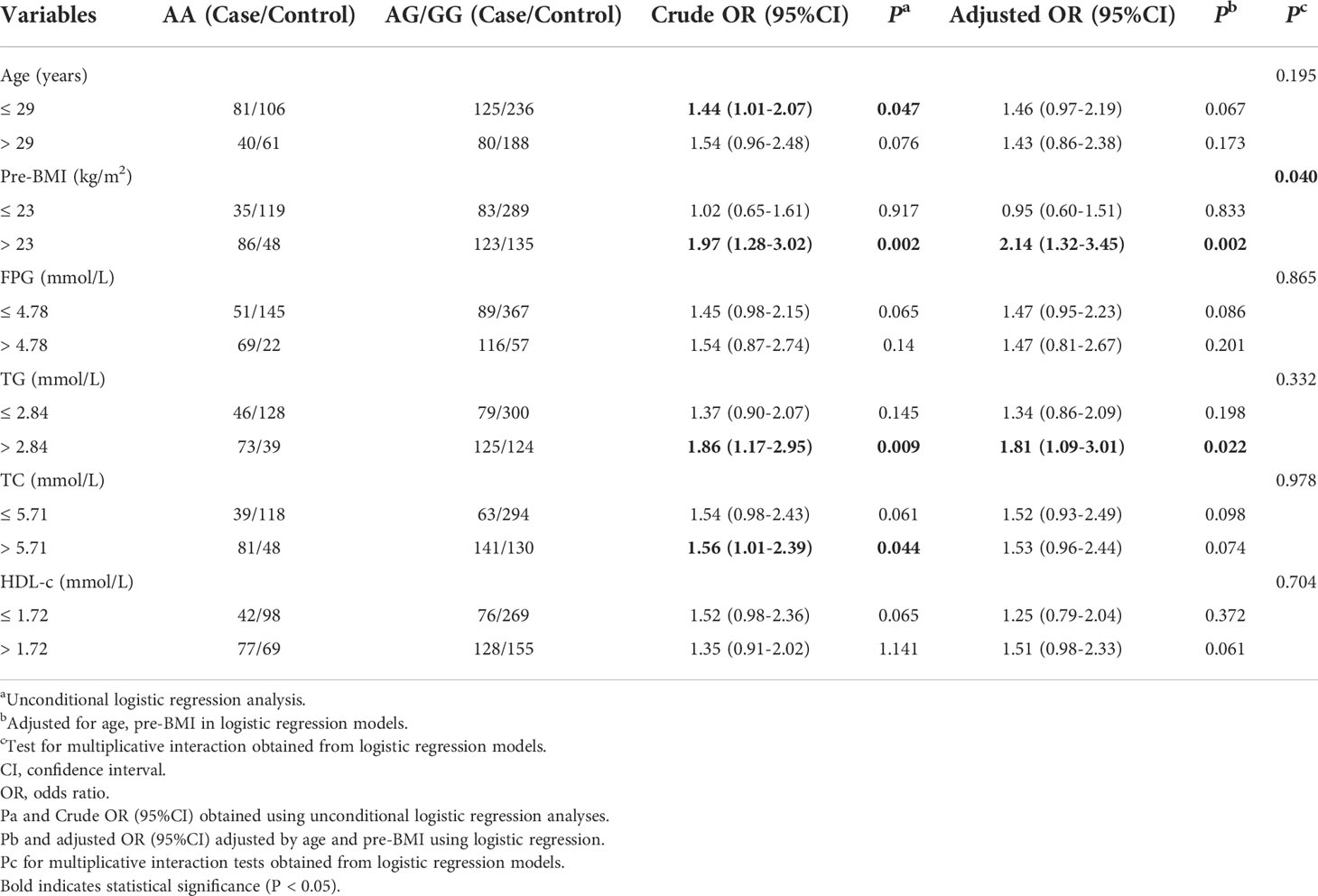
We also estimated the effect of rs2106809 A>G on PE risk using a stratified analysis under the dominant genetic model. After adjusting for age and pre-BMI, this association remained in the pre-BMI >23 kg/m2 subgroup (adjusted OR = 2.14, 95% CI = 1.32–3.45) and the TG >2.84 mmol/L subgroup (adjusted OR = 1.81, 95% CI = 1.09–3.01). However, using the same method, a stratified analysis shows that compared with the AA genotype, the AG/GG genotype exhibited a marginal statistical association with PE among subjects ≤29 years of age (adjusted OR = 1.46, 95% CI = 0.97–2.19, p = 0.067) and those with TC >5.71 mmol/L (adjusted OR = 1.53, 95% CI = 0.96–2.44, p = 0.074). In the dominant genetic model, we also found that rs2106809 interacted with pre-BMI (pinteraction = 0.040) to affect susceptibility to PE, as shown in Table 4.
Association between genotypes of selected SNPs and PE severity
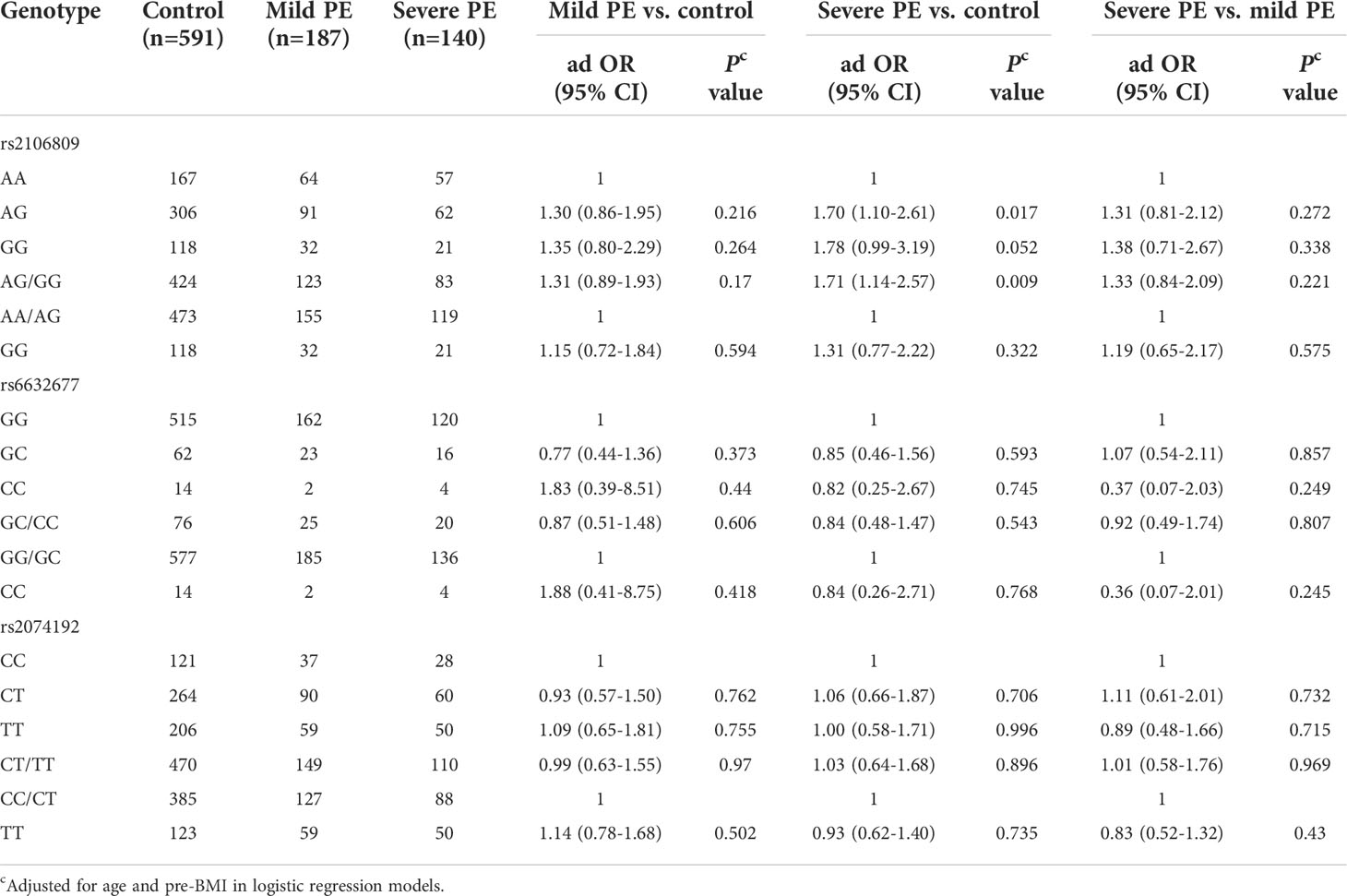
There were significant associations observed between ACE2 rs2106809 and severe PE under the heterozygote comparison (AG vs. AA, adjusted OR = 1.70, 95% CI = 1.10–2.61, p = 0.017) and the dominant model (AG/GG vs. AA, adjusted OR = 1.71, 95% CI = 1.14–2.57, p = 0.009). Furthermore, the rs2106809 GG genotype had no association with severe PE compared with the AA genotype but with marginal p-value (p = 0.052). However, no significant association was found between this variant and the onset of mild PE, p > 0.05. We also did not detect a significant association between variants of ACE2 rs6632677 and rs2074192 with PE risk of different severity (Table 5).
False-positive reporting probability analysis
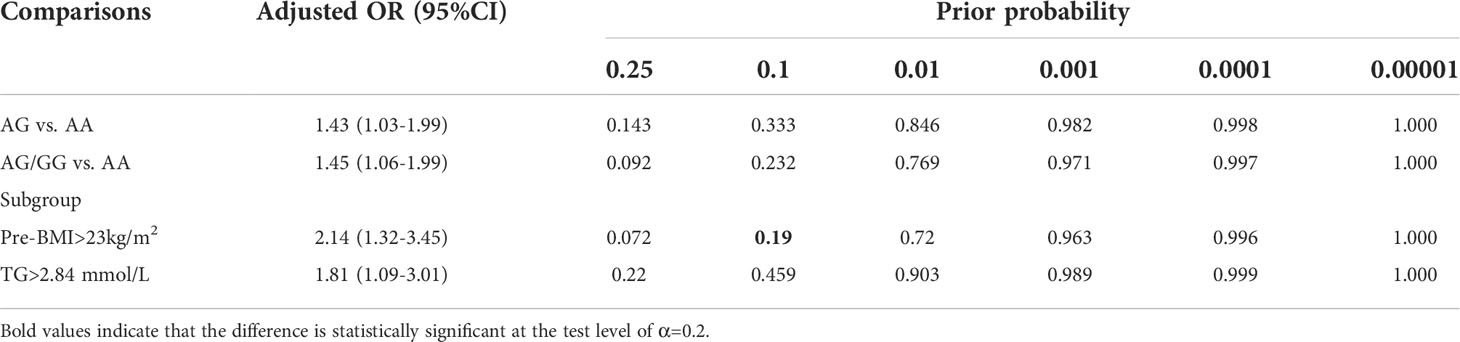
The FPRP test was adopted to assess the noteworthiness of the observed significant associations between the studied rs2106809 A>G variant and PE risk. A prior probability value of 0.1 and a relatively stringent FPRP cutoff value of 0.2 were set. The FPRP value was 0.19 for the association between rs2106809 and PE risk in the pre-BMI >23 kg/m2 subgroup, suggesting that the above associations identified in the present study are noteworthy, as shown in Table 6.
High-order interaction with PE risk by MDR analysis
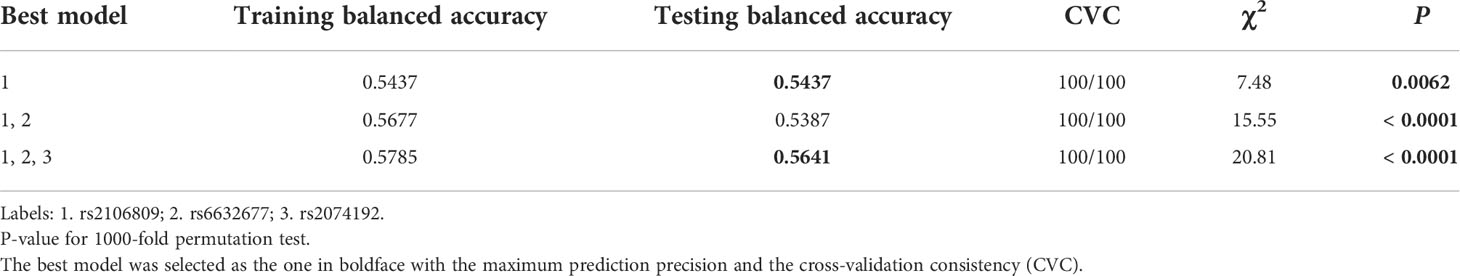
The MDR results list the three best possible interaction models. For the one-locus model, it had a training sample accuracy of 0.5437, a testing balanced accuracy of 0.5437, a maximum CVC of 100/100, and a p-value of 0.0062. For the three-locus model, which included rs2106809, rs6632677, and rs2074192, it had a training sample accuracy of 0.5785, a testing balanced accuracy of 0.5641, a maximum CVC of 100/100, and a p-value<0.001, and suggests that there are interactions among the three loci of the selected ACE2 gene that may synergistically enhance the susceptibility to PE in Chinese Han women, thereby increasing the risk of PE (Table 7).
Analysis of the potential regulatory function of rs2106809 A>G
ASSP online analysis suggested that the rs2106809 A>G variant causes a change in the reliability of classifications of two putative splice sites (positions 82 bp and 85 bp), with alt. isoform/cryptic activations of 0.811 and 0.818 and constitutive values of 0.183 and 0.174 for the rs2106809 A allele and alt. isoform/cryptic activations of 0.806 and 0.813 and constitutive values of 0.188 and 0.180 for the rs2106809 G allele, respectively. Thus, the regulation of putative splice sites may be changed, as shown in Table 8 and Figure 2.
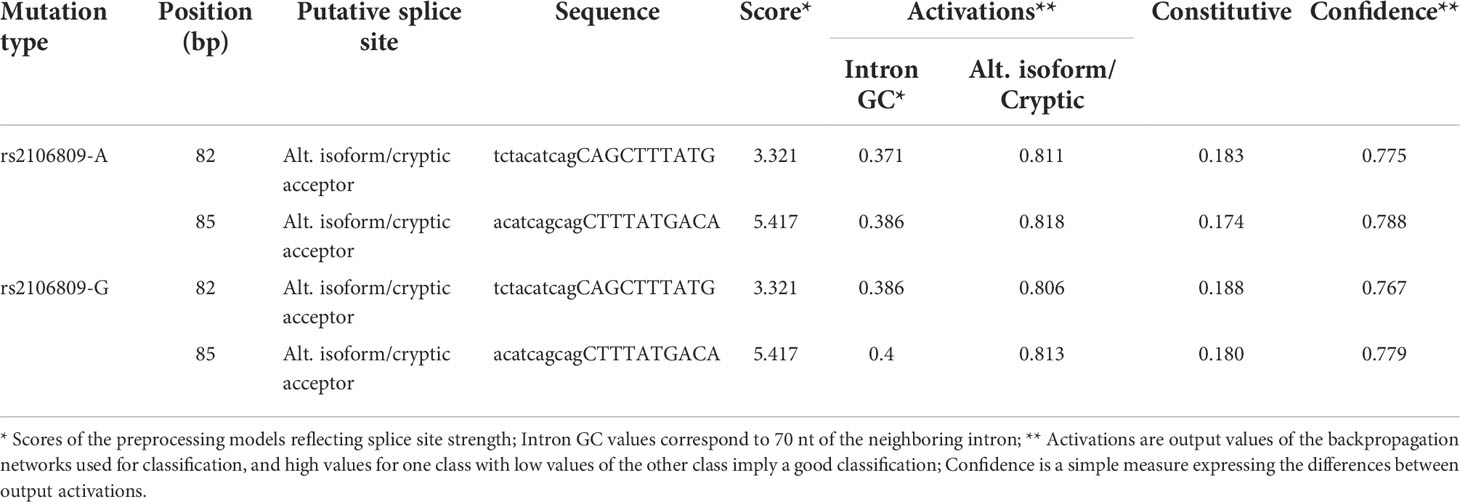
Table 8 The effect of rs2106809 A>G on alternative splicing sites of ACE2 gene evaluated by ASSP tool.
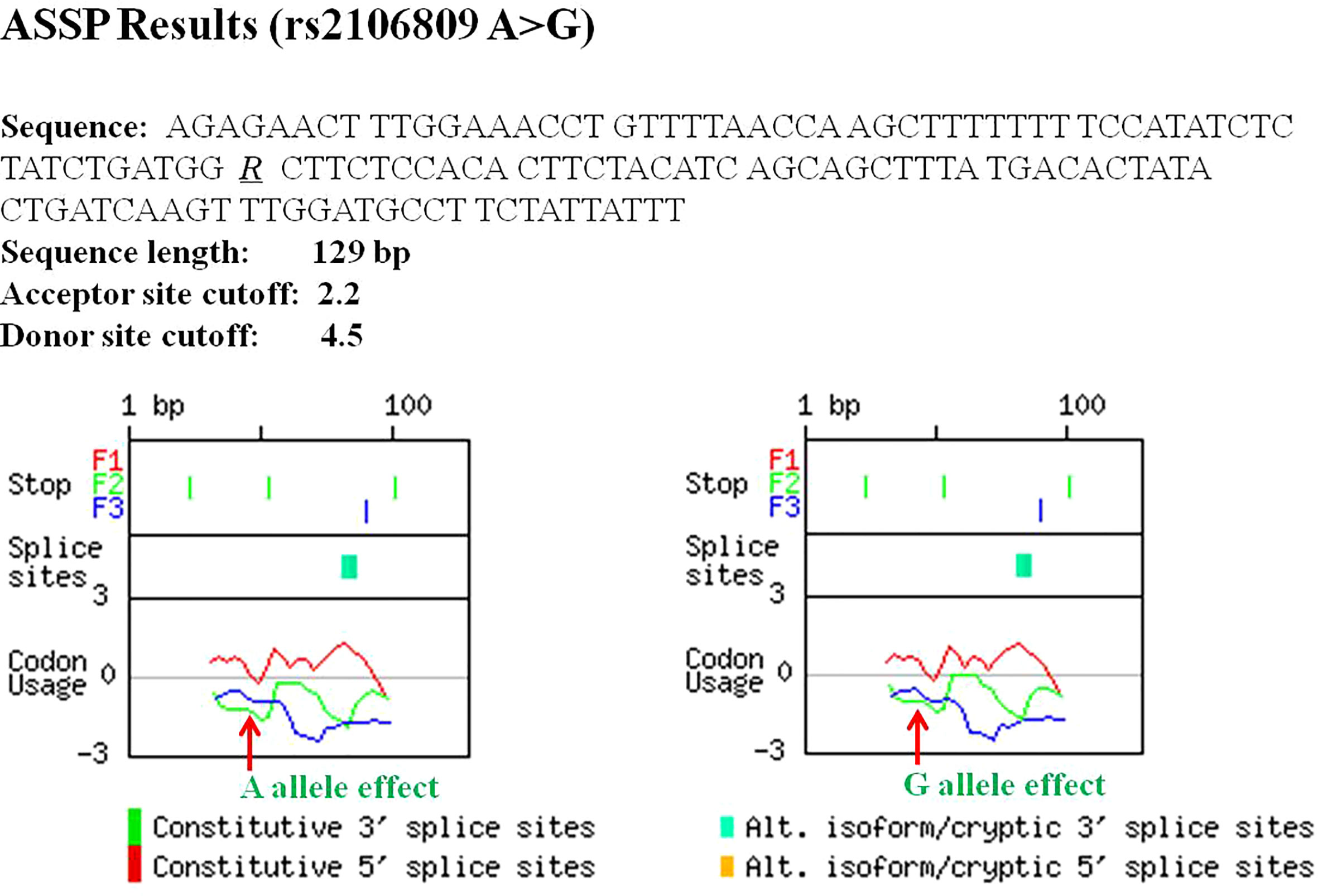
Figure 2 The effect of rs2106809 on alternative splicing sites of ACE2 gene evaluated by the Alternative Splice Site Predictor (ASSP) tool.
Furthermore, using the GTEx database, which incorporates 127 tissue/cell type–specific epigenomes datasets, the GWAS4D online tool was used to analyze eQTL between rs2106809 and the regulation of gene transcription. This analysis indicated that rs2106809 could potentially regulate the expression of functional genes associated with PE risk in a variety of specific tissues and cells, including PIR (total of 48 studies involved), ACE2 (44 studies involved), and CLTRN (collectrin) (46 studies involved), which were identified as eQTL, as shown in Table 9.
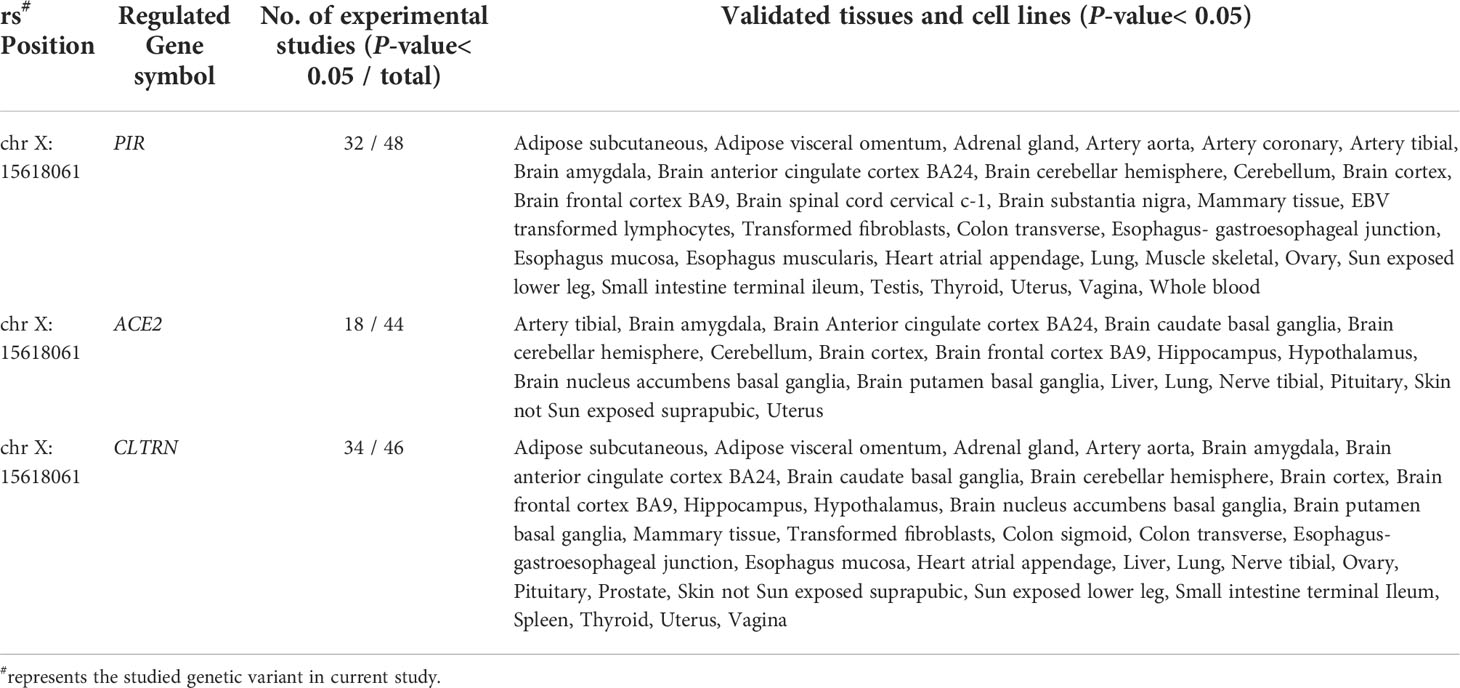
Table 9 The eQTL analysis on rs2106809 T>C and functional gene transcription levels by GWAS4D online tool.
Discussion
PE is a pregnancy-related syndrome linked to the death of more than 70,000 women and 500,000 fetuses worldwide each year (26, 27). Several studies have confirmed that PE is a complex disease caused by both environmental and genetic factors and carries a serious risk to the life and health of pregnant women and their fetuses (28, 29). Studies have shown that during normal pregnancy, ACE2 is secreted in abundance in the placenta and uterus, which represent important sources of the enzyme (30, 31). ACE2 exhibits high catalytic efficiency in the production of Ang-1–7, which is a vasodilator that inactivates the vasoconstrictor Ang II. This process contributes to systemic vasodilation and decreased blood pressure, as well as other physiological adaptations that occur during normal pregnancy. However, Ang-1–7 plasma levels are lower in pregnancies complicated by PE than in physiologically normal pregnancies (32). In addition, Song et al. (33) found a significant correlation between the ACE2 rs879922 C>T variant and late-onset PE susceptibility in a Chinese population. These findings suggest that ACE2 plays an important regulatory role in the occurrence and development of PE.
In the current study, we explored the association between ACE2 functional genetic variants (rs2106809 A>G, rs6632677 G>C, and rs2074192 C>T) and PE risk in a southern Chinese population. Demographic data showed that BMI, SBP, DBP, FPG, TG, TC, and HDL-C were much higher in PE cases than healthy controls, indicating that obesity, hypertension, hyperglycemia, and hyperlipidemia are involved in the pathogenesis of PE as risk factors. Analyses of associations between candidate SNPs and PE risk showed that the ACE2 rs2106809 variant was significantly associated with the risk of PE, and this association was even more pronounced in subjects with higher pre-BMI (>23 kg/m2), TG (>2.84 mmol/L), and TC (>2.71 mmol/L) levels. Moreover, an interaction between rs2106809 and pre-BMI was also detected. This result was consistent with the results of comparisons of general data. It is likely that overweight or obesity before pregnancy or excessive weight gain during pregnancy can lead to abnormal metabolism, accompanied by fat accumulation in the body, thereby aggravating inflammation in placental blood vessels and trophoblasts resulting from the effects of genetic variants in initiating PE (34, 35). It can be seen from these data that rs2106809 might alter an individual’s genetic background or regulate the key clinical indicators of PE. Subgroup analyses often suffer from reduced statistical power or simply errors due to chance. Therefore, this finding needs to be confirmed in larger future studies.
Genetic variation often plays a minor role in PE, but the joint amplification effects of multiple variations can be captured. Recent studies have shown that the joint effects of multiple ACE2 variants play a role in regulating blood pressure and dyslipidemia (9, 36), and the rs2074192 and rs2106809 haplotypes are associated with increased susceptibility to hypertension in women via a reduction in circulating Ang1–7 levels (37). Interestingly, although no significant association between rs6632677 G>C and rs2074192 C>T and PE risk was observed in the single-locus analysis, a complex gene–gene combination was detected in the MDR analysis. This result suggests that environmental factors, genetic factors, and combined environmental and genetic effects play a role in the pathogenesis of PE. These findings helped clarify the genetic component of ACE2 in the risk of PE.
Studies have confirmed that genetic variants in the intron region of a gene can regulate gene expression and affect mRNA transcription levels (38, 39). It is speculated that the genetic variant rs2106809 A>G in the intron of ACE2 affects the function of the ACE2 protein by modifying the transcription process of the gene, thereby ultimately affecting an individual’s susceptibility to PE. Another study showed that rs2106809 in ACE2 may be a determinant of circulating Ang1–7 levels in female patients with essential hypertension (40). This could explain the mechanism by which rs2106809 alters susceptibility to PE by affecting the expression of ACE2 and subsequent executive function of the ACE2 protein. Therefore, it is essential to explore the biological function of rs2106809 in future studies to verify its role in the pathogenesis of PE.
FPRP analysis is an effective method for determining the biological importance of associations (41). In this study, a relatively strict FPRP threshold of 0.2 was set. Among patients with pre-BMI >23 kg/m2, a significant correlation was observed between rs2106809 A>G and PE risk. This shows that these positive findings are possible, true, and reliable. In addition, the FPRP values obtained in some comparisons were much greater than 0.2, indicating that these important findings may have been observed by chance. Therefore, the conclusions drawn here are preliminary and need to be verified.
In view of the above findings, we explored the potential molecular mechanism of the significant association between rs2106809 A>G and PE risk and revealed the biological rationality of the observed association. As an intron variant, rs2106809 is thought to affect the post-transcriptional splicing of genes or regulate gene expression and affect mRNA transcription levels (38). The ASSP can be used to identify putative splice sites of genes and subsequently classify them as either constitutive or alternative isoform/cryptic splice sites using backpropagation networks (24). Subsequent analyses suggested some possible regulatory changes in putative splice sites due to the rs2106809 variant. Furthermore, eQTL analyses using the GTEx database, which incorporates 127 tissue/cell type-specific epigenome datasets, suggested that rs2106809 could regulate the expression of various functional genes (PIR, ACE2, and CLTRN) in certain tissues and cell lines. Studies of collectrin levels and PE onset showed that serum collectrin levels play a significant role in controlling blood pressure in pregnant women, with a significant inverse correlation between serum collectrin concentrations and blood pressure (42). The PIR gene is a transcriptional co-regulator of nuclear factor kappa-B (NF-κB), which is a tightly regulated molecule also related to inflammation and the control of innate and adaptive immune responses during onset of labor. Early activation of NF-κB may have an adverse effect by inducing premature termination of pregnancy, with bad outcomes for the mother and the fetus, including pregnancy loss (43). The above evidence reveals that as an eQTL, rs2106809 can affect physiologic processes such as immunity, glycolipid metabolism, and blood pressure by regulating the expression of functional genes and is therefore significantly associated with the pathogenesis of PE.
This study explored the relationship between functional variants of ACE2 and the risk of PE, and etiological clues were provided from the perspective of genetics. An advantage of this study was its good design and application of multiple statistical analyses, which fully demonstrated the correlation between the studied ACE2 gene variants and PE risk. However, this study still has some limitations. First, this was a hospital-based, case–control study and therefore might have selection bias. Second, although a relatively large sample was recruited for this study, the lower frequencies of some genotypes tested may still limit the efficiency of detecting significant associations in some subgroups. Third, this study did not experimentally explore the biological function of the significant association locus, and only functional prediction analysis was carried out using bioinformatics methods.
Conclusion
The ACE2 gene rs2106809 A>G variant is significantly associated with the risk of PE via individual locus effects and/or complex gene–gene and gene–environment interactions. Regulating the expression of functional genes such as PIR, ACE2, and CLTRN may be the molecular mechanism by which rs2106809 affects individual susceptibility to PE. A multi-center study with a larger sample size and functional experiments are needed to confirm these findings.
Data availability statement
The original contributions presented in the study are publicly available. This data can be found here: https://doi.org/10.5061/dryad.crjdfn377.
Ethics statement
This study was reviewed and approved by the Ethics Committee Review Board of Guilin Medical University and the Affiliated Hospital of Guilin Medical University. The patients/participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
XY: protocol/project development and manuscript editing. GH and YW: data collection and analysis, and manuscript writing. GH and LQ: data analysis and manuscript writing. BH: data collection and management. All authors contributed to the article and approved the submitted version.
Funding
This study was supported by the Guangxi Young and middle-aged teachers’ basic ability improvement project (2020KY12028), Guangxi Natural Science Foundation of China (2020GXNSFAA238025), National Natural Science Foundation of China (81760614), the Key Research and Development Program (2018AB62004), and College Students’ Innovation Project (201810601031) of Guangxi, China.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Ye C, Ruan Y, Zou L, Li G, Li C, Chen Y, et al. The 2011 survey on hypertensive disorders of pregnancy (HDP) in China: Prevalence, risk factors, complications, pregnancy and perinatal outcomes. PloS One (2014) 9(6):e100180. doi: 10.1371/journal.pone.0100180
2. Sánchez-Aranguren L, Prada C, Riaño-Medina C, Lopez M. Endothelial dysfunction and preeclampsia: role of oxidative stress. Front Physiol (2014) 5:372. doi: 10.3389/fphys.2014.00372
3. Sibai B, Dekker G, Kupferminc M. Pre-eclampsia. Lancet (London England) (2005) 365(9461):785–99. doi: 10.1016/S0140-6736(05)17987-2
4. Vázquez Rodríguez J, Flores Granados C. [Maternal complications and HELLP syndrome]. Ginecol Y Obstetricia Mexico (2011) 79(4):183–9.
5. Hutcheon J, Lisonkova S, Joseph K. Epidemiology of pre-eclampsia and the other hypertensive disorders of pregnancy. Best Pract Res Clin Obstetr Gynaecol (2011) 25(4):391–403. doi: 10.1016/j.bpobgyn.2011.01.006
6. Bartsch E, Medcalf K, Park A, Ray J. Clinical risk factors for pre-eclampsia determined in early pregnancy: systematic review and meta-analysis of large cohort studies. BMJ (Clin Res Ed) (2016) 353:i1753. doi: 10.1136/bmj.i1753
7. Rana S, Lemoine E, Granger J, Karumanchi S. Preeclampsia: Pathophysiology, challenges, and perspectives. Circ Res (2019) 124(7):1094–112. doi: 10.1161/CIRCRESAHA.118.313276
8. Schork N, Fallin D, Lanchbury J. Single nucleotide polymorphisms and the future of genetic epidemiology. Clin Genet (2000) 58(4):250–64. doi: 10.1034/j.1399-0004.2000.580402.x
9. Shastry B. SNPs: impact on gene function and phenotype. Methods Mol Biol (Clifton NJ) (2009) 578:3–22. doi: 10.1007/978-1-60327-411-1_1
10. Jansaka N, Pornwattanakrilert W, Tongsong T, Piyamongkol S, Piyamongkol W. AGTA study of the association between angiotensinogen () gene polymorphism (M235T) and preeclampsia in Thai pregnant women. J Obstetr Gynaecol: J Institute Obstetrics Gynaecol (2021) 41(7):1062–6. doi: 10.1080/01443615.2020.1837757
11. Pinarbasi E, Cekin N, Bildirici A, Akin S, Yanik A. STOX1 gene Y153H polymorphism is associated with early-onset preeclampsia in Turkish population. Gene (2020) 754:144894. doi: 10.1016/j.gene.2020.144894
12. Steinthorsdottir V, McGinnis R, Williams N, Stefansdottir L, Thorleifsson G, Shooter S, et al. Genetic predisposition to hypertension is associated with preeclampsia in European and central Asian women. Nat Commun (2020) 11(1):5976. doi: 10.1038/s41467-020-19733-6
13. Veisian M, Javaheri A, Amjadi N, Tabatabaei R, Zanbagh L, Hadadan A, et al. Association of IL-6 -176G > C polymorphism with susceptibility to preeclampsia: A systematic review and meta-analysis. Fetal Pediatr Pathol (2020) 39(6):491–502. doi: 10.1080/15513815.2019.1675110
14. Leal C, Costa L, Ferreira G, Ferreira A, Reis F, Simões E Silva A. Renin-angiotensin system in normal pregnancy and in preeclampsia: A comprehensive review. Pregnancy Hypertension (2022) 28:15–20. doi: 10.1016/j.preghy.2022.01.011
15. Martell Claros N, Asenjo de la Fuente J, Abad Cardiel M, García Donaire J, Herráiz M. Role of the renin-angiotensin system in pregnancy and preeclampsia. Hipertension Y Riesgo Vascular (2020) 37(2):72–7. doi: 10.1016/j.hipert.2020.02.003
16. Tagle R, Tagle VR, Acevedo M, Valdés G. Hypertension in women. Rev Med Chile (2013) 141(2):237–47. doi: 10.4067/S0034-98872013000200014
17. van Thiel B, van der Pluijm I, te Riet L, Essers J, Danser A. The renin-angiotensin system and its involvement in vascular disease. Eur J Pharmacol (2015) 763:3–14. doi: 10.1016/j.ejphar.2015.03.090
18. Vickers C, Hales P, Kaushik V, Dick L, Gavin J, Tang J, et al. Hydrolysis of biological peptides by human angiotensin-converting enzyme-related carboxypeptidase. J Biol Chem (2002) 277(17):14838–43. doi: 10.1074/jbc.M200581200
19. Tamanna S, Lumbers E, Morosin S, Delforce S, Pringle K. ACE2: a key modulator of the renin-angiotensin system and pregnancy. Am J Physiol Regulatory Integr Comp Physiol (2021) 321(6):R833–R43. doi: 10.1152/ajpregu.00211.2021
20. Fan Z, Wu G, Yue M, Ye J, Chen Y, Xu B, et al. Hypertension and hypertensive left ventricular hypertrophy are associated with ACE2 genetic polymorphism. Life Sci (2019) 225:39–45. doi: 10.1016/j.lfs.2019.03.059
21. Lozano-Gonzalez K, Padilla-Rodríguez E, Texis T, Gutiérrez M, Rodríguez-Dorantes M, Cuevas-Córdoba B, et al. ACE2Allele frequency of intron variants and its association with blood pressure. DNA Cell Biol (2020) 39(11):2095–101. doi: 10.1089/dna.2020.5804
22. Wacholder S, Chanock S, Garcia-Closas M, El Ghormli L, Rothman N. Assessing the probability that a positive report is false: an approach for molecular epidemiology studies. J Natl Cancer Institute (2004) 96(6):434–42. doi: 10.1093/jnci/djh075
23. Hahn L, Ritchie M, Moore J. Multifactor dimensionality reduction software for detecting gene-gene and gene-environment interactions. Bioinf (Oxford England) (2003) 19(3):376–82. doi: 10.1093/bioinformatics/btf869
24. Wang M, Marin A. Characterization and prediction of alternative splice sites. Gene (2006) 366(2):219–27. doi: 10.1016/j.gene.2005.07.015
25. Huang D, Yi X, Zhang S, Zheng Z, Wang P, Xuan C, et al. GWAS4D: multidimensional analysis of context-specific regulatory variant for human complex diseases and traits. Nucleic Acids Res (2018) 46(W1):W114–W20. doi: 10.1093/nar/gky407
26. Steegers E, von Dadelszen P, Duvekot J, Pijnenborg R. Pre-eclampsia. Lancet (London England) (2010) 376(9741):631–44. doi: 10.1016/S0140-6736(10)60279-6
27. Wanderer J, Leffert L, Mhyre J, Kuklina E, Callaghan W, Bateman B. Epidemiology of obstetric-related ICU admissions in Maryland: 1999-2008*. Crit Care Med (2013) 41(8):1844–52. doi: 10.1097/CCM.0b013e31828a3e24
28. McClure J, Cooper G, Clutton-Brock T. Saving mothers' lives: reviewing maternal deaths to make motherhood safer: 2006-8: A review. Br J Anaesthesia (2011) 107(2):127–32. doi: 10.1093/bja/aer192
29. Zhang J, He J. Risk factors of recurrent preeclampsia and its relation to maternal and offspring outcome. Zhejiang Da Xue Xue Bao Yi Xue Ban J Zhejiang Univ Med Sci (2015) 44(3):258–63. doi: 10.3785/j.issn.1008-9292.2015.05.04
30. Jing Y, Run-Qian L, Hao-Ran W, Hao-Ran C, Ya-Bin L, Yang G, et al. Potential influence of COVID-19/ACE2 on the female reproductive system. Mol Hum Reprod (2020) 26(6):367–73. doi: 10.1093/molehr/gaaa030
31. Valdés G, Corthorn J, Bharadwaj M, Joyner J, Schneider D, Brosnihan K. Utero-placental expression of angiotensin-(1-7) and ACE2 in the pregnant guinea-pig. Reprod Biol Endocrinol: RB&E (2013) 11:5. doi: 10.1186/1477-7827-11-5
32. Todros T, Masturzo B, De Francia S. COVID-19 infection: ACE2, pregnancy anpd preeclampsia. Eur J Obstetr Gynecol Reprod Biol (2020) 253:330. doi: 10.1016/j.ejogrb.2020.08.007
33. Song W, Wang H, Ma L, Chen Y. Associations between the TNMD rs4828038 and ACE2 rs879922 polymorphisms and preeclampsia susceptibility: A case-control study. J Obstetr Gynaecol: J Institute Obstetrics Gynaecol (2022), 42(5):1–5. doi: 10.1080/01443615.2021.2012438
34. Altmäe S, Segura M, Esteban F, Bartel S, Brandi P, Irmler M, et al. Maternal pre-pregnancy obesity is associated with altered placental transcriptome. PloS One (2017) 12(1):e0169223. doi: 10.1371/journal.pone.0169223
35. Mitsuya K, Parker A, Liu L, Ruan J, Vissers M, Myatt L. Alterations in the placental methylome with maternal obesity and evidence for metabolic regulation. PloS One (2017) 12(10):e0186115. doi: 10.1371/journal.pone.0186115
36. Pan Y, Wang T, Li Y, Guan T, Lai Y, Shen Y, et al. Association of ACE2 polymorphisms with susceptibility to essential hypertension and dyslipidemia in xinjiang, China. Lipids Health Dis (2018) 17(1):241. doi: 10.1186/s12944-018-0890-6
37. Chen Y, Zhang P, Zhou X, Liu D, Zhong J, Zhang C, et al. Relationship between genetic variants of ACE2 gene and circulating levels of ACE2 and its metabolites. J Clin Pharm Ther (2018) 43(2):189–95. doi: 10.1111/jcpt.12625
38. Jacob AG, Smith CWJ. Intron retention as a component of regulated gene expression programs. Hum Genet (2017) 136(9):1043–57. doi: 10.1007/s00439-017-1791-x
39. Shaul O. How introns enhance gene expression. Int J Biochem Cell Biol (2017) 91(Pt B):145–55. doi: 10.1016/j.biocel.2017.06.016
40. Liu D, Chen Y, Zhang P, Zhong J, Jin L, Zhang C, et al. Association between circulating levels of ACE2-Ang-(1-7)-MAS axis and ACE2 gene polymorphisms in hypertensive patients. Medicine (2016) 95(24):e3876. doi: 10.1097/MD.0000000000003876
41. Tong X, Ma Y, Deng H, Wang X, Liu S, Yan Z, et al. The SDF-1 rs1801157 polymorphism is associated with cancer risk: An update pooled analysis and FPRP test of 17,876 participants. Sci Rep (2016) 6:27466. doi: 10.1038/srep27466
42. Al-Bayati MMJ, Al-Ani ART, Ahmed HN. Correlation of serum collectrin level and preeclampsia onset: A case control study. J Gynecol Obstetr Hum Reprod (2021) 50(3):101770. doi: 10.1016/j.jogoh.2020.101770
Keywords: angiotensin-converting enzyme 2, variation, pre-eclampsia (PE), risk, functional analysis
Citation: Huang G, Wang Y, Qin L, Huang B and Yu X (2022) Association and functional analysis of angiotensin-converting enzyme 2 genetic variants with the pathogenesis of pre-eclampsia. Front. Endocrinol. 13:926512. doi: 10.3389/fendo.2022.926512
Received: 22 April 2022; Accepted: 07 October 2022;
Published: 07 November 2022.
Edited by:
Jyoti Rajan Sharma, South African Medical Research Council, South AfricaReviewed by:
Singh Rajender, Central Drug Research Institute (CSIR), IndiaMing Li, Hunan University of Medicine, China
Zhaolei Cui, Fujian Medical University, China
Copyright © 2022 Huang, Wang, Qin, Huang and Yu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiangyuan Yu, MTEyMDEzMDMzQGdsbWMuZWR1LmNu; Z3VpbGlueGlhbmd5dWFuMTIzQDE2My5jb20=
†These authors have contributed equally to this work and share first authorship
 Gongchen Huang1†
Gongchen Huang1† Linyuan Qin
Linyuan Qin Bo Huang
Bo Huang Xiangyuan Yu
Xiangyuan Yu