
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Endocrinol. , 07 September 2022
Sec. Pediatric Endocrinology
Volume 13 - 2022 | https://doi.org/10.3389/fendo.2022.925102
 Hyun Wook Chae1
Hyun Wook Chae1 Il-Tae Hwang2
Il-Tae Hwang2 Ji-Eun Lee3
Ji-Eun Lee3 Cheol Hwan So4
Cheol Hwan So4 Young-Jun Rhie5
Young-Jun Rhie5 Jung Sub Lim6
Jung Sub Lim6 Eun Byul Kwon7
Eun Byul Kwon7 Kyung Hee Yi8
Kyung Hee Yi8 Eun Young Kim9
Eun Young Kim9 Chae-Ku Jo10
Chae-Ku Jo10 Kye Shik Shim11
Kye Shik Shim11 Ha-Yeong Gil12
Ha-Yeong Gil12 Min-Jeong Seong13
Min-Jeong Seong13 Chung Mo Nam14
Chung Mo Nam14 Ji-Su Moon15
Ji-Su Moon15 Jin Soon Hwang16*
Jin Soon Hwang16*Objectives: Growth hormone (GH) therapy’s capacity to increase height velocity and height at the end of the study in children with idiopathic short stature (ISS) is controversial. We aimed to investigate the height standard deviation score (SDS) and height velocity of patients with ISS in Korea who received GH treatment.
Methods: We retrospectively reviewed and performed linear mixed model and survival analyses on data from 12 tertiary hospitals in Korea, including subjects diagnosed with ISS from January 2009 to September 2019, treated with GH therapy for more than 6 months, and who were at a pre-pubertal state at the time of diagnosis.
Results: We included 578 children (330 boys and 248 girls). The mean daily dose of GH in this study was 0.051 mg/kg, which was lower than the approved dose in Korea of 0.062 - 0.067 mg/kg. Height SDS was higher in patients who started treatment before the age of 6 years. The probability of reaching the target SDS (-1 SDS) from the beginning of treatment to 2–3 years after its start was higher in children starting treatment before the age of 6 years. The hazard ratio to reach the target SDS (-1 SDS) when using automatic pen or electronic devices was 1.727 times higher than that when using the needle and syringe device.
Conclusion: ISS patients should start GH treatment at an early age, and even lower-than-recommended drug doses may be effective. The selection of automatic pen or electronic device can have a positive effect on reaching the target height SDS.
Idiopathic short stature (ISS) is generally defined as a condition in which height is not within the normal range and is below –2.0 standard deviation score (SDS) for the corresponding age and gender in a population without endocrinologic, nutritional, or chromosomal abnormalities (1–4). ISS differs from growth hormone deficiency (GHD) in that peak growth hormone levels are normal (5). Although the causes of short stature have been identified in some children, about 60%–80% of these children are considered as ISS without a definitive diagnosis (1, 2, 4, 6–11).
Recombinant human growth hormone (rhGH) was first approved in the United States in 1985 for the treatment of pediatric GHD and has been used worldwide since (4). The indications for GH treatment have gradually extended from GHD to non-GH-deficient short stature, such as Turner syndrome, chronic renal insufficiency, short children born small for gestational age, Noonan syndrome, and ISS (4, 12). The use of rhGH for ISS was approved in United States in 2003, and in Korea in 2009, while it has not been approved in Europe and Japan (13–18). In Korea, GH treatment for ISS patients is not covered by public health insurance, so the treatment is considered as the 2nd option due to its high cost (2, 4, 5, 19, 20).
Since the heterogeneous nature of nature of the ISS patient population, suggesting multiple potential pathophysiological mechanisms, it remains difficult to estimate the efficacy of GH for ISS patients worldwide, including that in Korea. Additionally, research results on the effects of the appropriate dosage of GH treatment for ISS patients and factors affecting the growth rate of children with ISS are still controversial (6, 15, 19, 21–24).
Therefore, we aimed to investigate the characteristics and treatment pattern of patients with ISS in Korea who received GH treatment, as well as identify the factors affecting their height SDS and height velocity.
Data were collected through the retrospective review of medical records from 12 nationwide tertiary hospitals in Korea from 2019 to 2021. Hospitals participating in this study represent hospitals that treat and manage patients with ISS in Korea, judging from their geographical distribution, number of patients treated, and clinical experience and academic achievements of physicians. Before patient enrollment, the study protocol was approved by the 12 institutional review boards of participating institutions. (Approval number: AJIRB-MED-OBS-19-395)
As all patients who met the inclusion and exclusion criteria were enrolled during the observation period, a separate sample size calculation was not required. Patients who were diagnosed with ISS between January 2009 and September 2019, those who were treated for more than 6 months with any GH product prescribed in Korea, and those who were at prepubertal stage during diagnosis were enrolled. ISS was defined using a practical definition, that is, height below the 3rd percentile in the absence of any endocrine, metabolic, or other disease that might explain the short stature according to the investigator. In addition, we enrolled all patients with ISS who underwent GH provocation test or combined pituitary function test and other tests during hospitalization. After the start of GH treatment, patients without additional measurement of height or with chromosomal abnormalities, malnutrition, growth disorders, or low birth weight were excluded from the study.
A total of 578 children (330 boys and 248 girls) who started GH treatment between the ages of 4 and 14 years were included in the analysis. We collected the ISS diagnosis date, treatment start date, birth week, chronological age, bone age, height, weight, parental height, insulin like growth factor 1 (IGF-1), IGF binding protein 3 (IGFBP-3), and treatment pattern of subjects. Descriptive analysis was performed to identify the baseline characteristics of study subjects. Continuous variables were reported as mean and standard deviation and categorical variables were reported as frequency and proportion. SDS was calculated as follows: SDS = (patient’s measured value − mean value for age and sex − matched normal subjects)/(SD of the values for age and sex-matched normal subjects).
The normality of data was checked using the Shapiro–Wilk test. To compare treatment outcomes (baseline vs. treatment period), we conducted McNemar’s test and paired t-test or Wilcoxon’s signed-rank test according to the normality of data. Linear mixed model analysis was performed to identify factors affecting the height outcome of children with ISS. Survival analysis was performed to identify the variables affecting patients’ achievement of target height SDS (-1 SDS). All statistical analyses were performed using SAS 9.4 (SAS Inc., Cary, NC, USA).
At the start of the treatment, the children’s chronological age was 7.54 ± 2.43 years and bone age was 6.08 ± 2.55 years. The mean height SDS of boys was −2.48 ± 0.57 and that of girls was −2.58 ± 0.73. The mean weight SDS of boys was −1.95 ± 0.84 and that of girls was −1.92 ± 0.93 (Table 1). The mean peak GH level of children was in the normal range (18.421 ± 7.90 ng/ml) and IGF-1 SDS was −0.60, which was lower than that of peers with the same age and gender (Table 1). The proportion of patients who used each device for GH treatment was similar between needle and syringe, automatic pen, and electronic devices (Table 1).
When treatment was continued, the children’s height SDS increased, and this change was statistically significant compared to the baseline height SDS. For boys, the height SDS measured at the start of treatment was −2.48 ± 0.57, and the height SDS after 3 years of treatment was −1.02 ± 0.81. The girls’ height SDS showed a similar trend to that of boys (the girl’s height SDS measured at the start of treatment was −2.58 ± 0.73, and that measured after 3 years was −1.12 ± 0.79) (Tables 2, 3).
For both boys and girls, the group starting treatment before 6 years of age had the highest height SDS among the three groups (group 1: baseline age ≤ 6; group 2: 7 ≤ baseline age ≤ 9 for boys, 7 ≤ baseline age ≤ 8 for girls; group 3: baseline age ≥ 10 for boys, baseline age ≥ 9 for girls) (Figures 1A, B). This may mean that it is important for GH treatment to be started early and at an appropriate time.
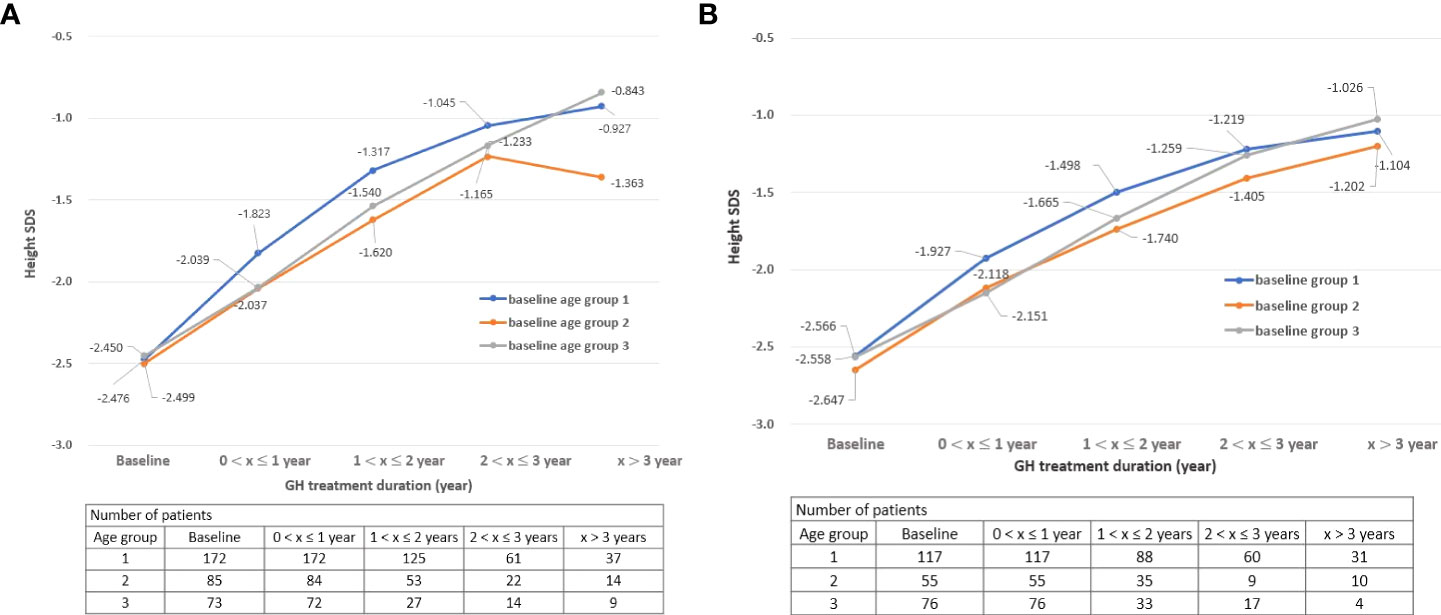
Figure 1 (A) Change in height SDS during treatment by age group: Boys. (B) Change in height SDS during treatment by age group: Girls. SDS, standard deviation score “x” denotes the duration of GH treatment (e.g., a value of 0 on the x-axis is the start time of GH treatment).
The height velocity of children participating in this study up to 1 year after the start of GH treatment was 11.19 ± 5.24, and the average SDS of the height velocity was 1.22 ± 1.06. These results were similar to those of previous studies (height velocity of 6 months to 1 year treatment (cm/year): 7.57 ± 0.30 to 10.85 ± 2.34, growth velocity SDS: 1.47 ± 0.70) (4, 23, 25–27). Additionally, the height velocity was the highest in group 1 until 2 years of GH treatment, but the height velocity of group 3 was the highest after 3 years from the start of treatment. These results may have been influenced by puberty in the group 3, who started treatment at a relatively old age (Figures 2A, B).
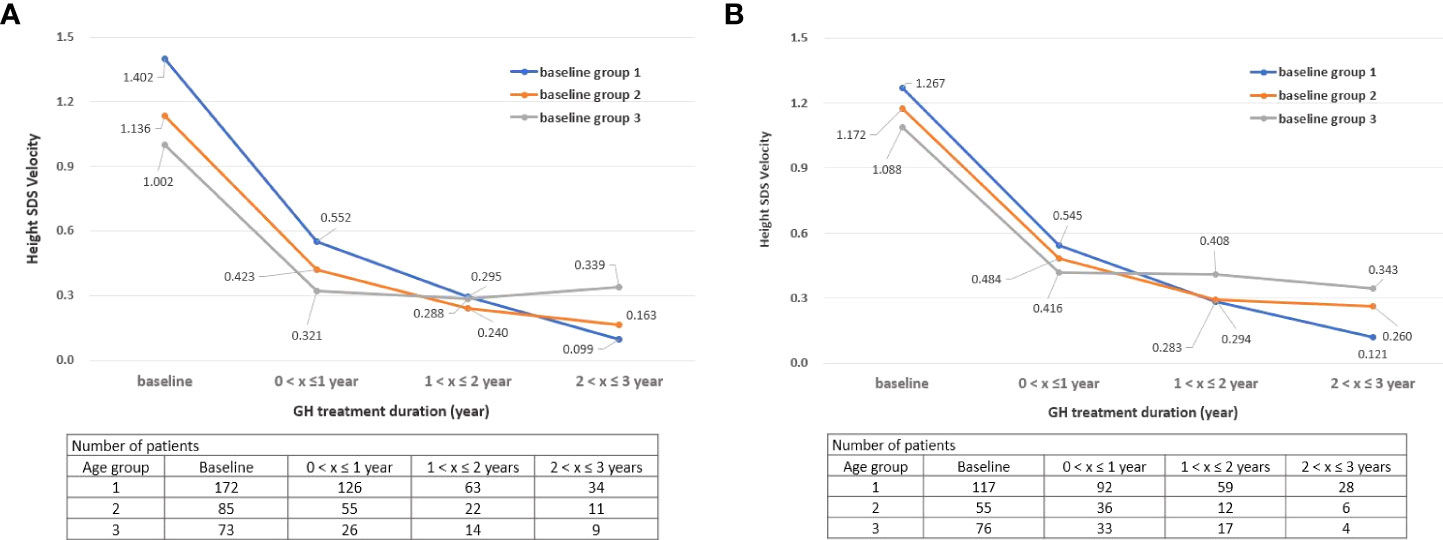
Figure 2 (A) Change in height SDS velocity during treatment by age group: Boys. (B) Change in height SDS velocity during treatment by age group: Girls. SDS, standard deviation score, “x” denotes the duration of GH treatment (e.g., a value of 0 on the x-axis is the start time of GH treatment).
As a result of multivariate analyses, the height SDS of boys was 0.224 lower than that of girls (p = 0.002), and the height SDS was as high as 0.685 when treatment was continued for 3 years or longer compared to when the treatment period was less than 1 year (p < 0.001) (Table 4).
For boys, when treatment was started after the age of 10, the height SDS was 0.429 times lower than that when treatment was started before the age of 6 years (p = 0.001). The height SDS increased the most when treatment was maintained for 2 to 3 years (p < 0.001), and for girls, the height SDS value increased the most when treatment was continued for 3 years or longer (p < 0.001) (Tables 5, 6).
The hazard ratio to reach the target SDS (-1 SDS) when using the automatic pen type or electronic device type is 1.727 times higher than that when using the needle and syringe device (Appendix Table 1). Furthermore, this result was more pronounced in boys (hazard ratio: 2.092, Appendix Tables 1-1, 1-2).
According to Kaplan–Meier plots, the probability of reaching the target SDS (-1 SDS) from the beginning of treatment to 2–3 years after the start of treatment for children who started treatment before 6 years of age was higher than that for children who started treatment after the age of 7 (Figure 3).
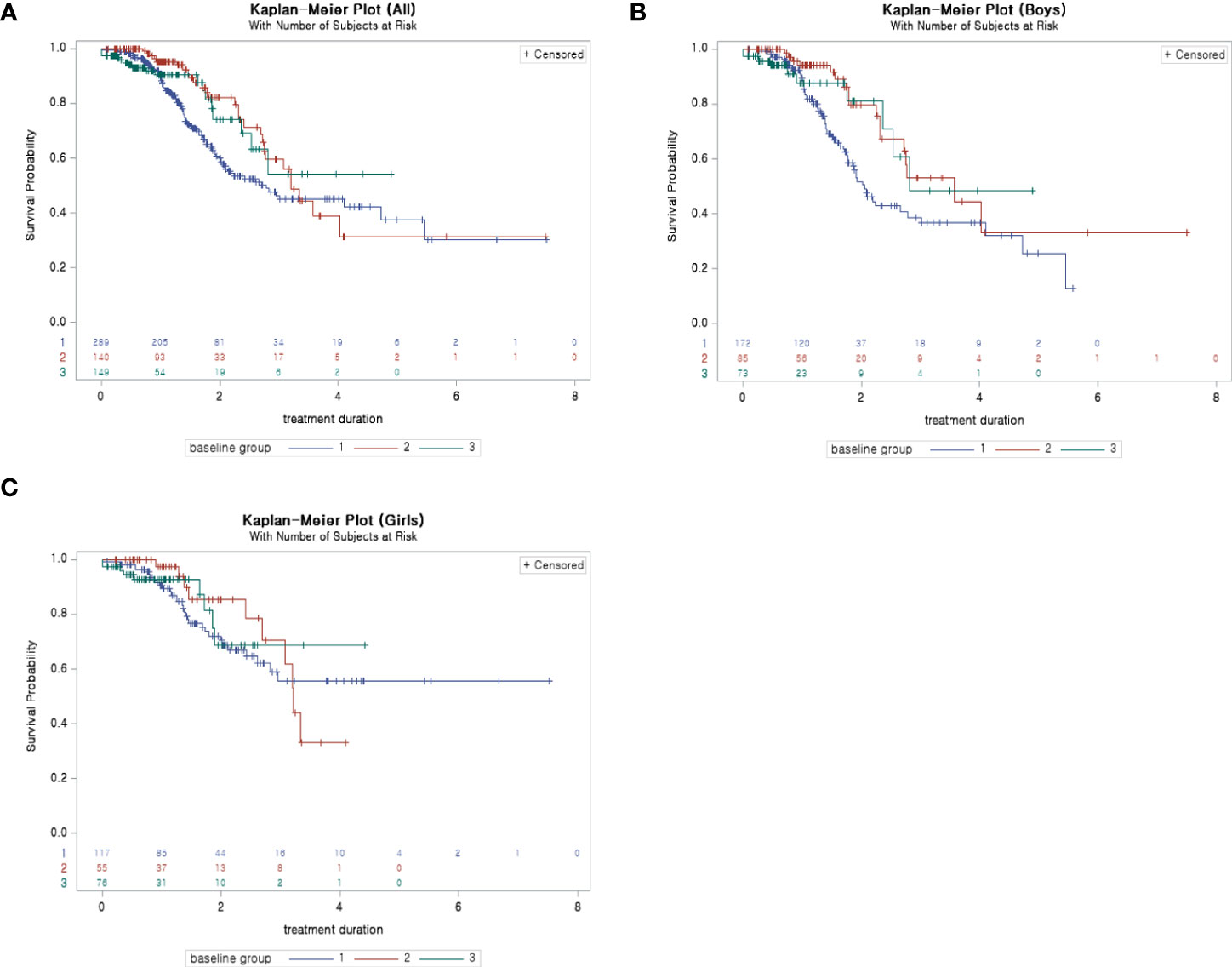
Figure 3 (A) Kaplan–Meier plot (survival probability: probability of not reaching −1 SDS): ALL. (B) Kaplan–Meier plot (survival probability: probability of not reaching −1 SDS): boys. (C) Kaplan–Meier plot (survival probability: probability of not reaching −1 SDS): girls. SDS, standard deviation score.
This was the first multi-center 10-year study on Korean pediatric patients with ISS to identify clinical characteristics and patterns of GH treatment and determine factors associated with height outcomes. We found that the height at the end of this study SDS is higher when GH treatment starts at an early age and when administered for 3 years or more, as opposed to less than 1 year. The average peak GH at baseline of children in this study was 18.42 (ng/ml), showing the characteristics of ISS pediatric patients with normal GH levels. This was consistent with the results of previous studies, in which the peak GH values of subjects at the time of ISS diagnosis were above 10 ng/ml, which were within a normal range (12.01–20.90 ng/ml) (21, 28). Regarding the administered dose, the mean daily dose in this study was 0.051 mg/kg, which was lower than the GH dose with regulatory approval of 0.062 - 0.067 mg/kg for patients with ISS in Korea.
From an efficacy point of view, the group who started before 6 years of age had the highest height SDS results after GH treatment for both boys and girls. There was a difference in height velocity according to age at the start of GH treatment, and the height velocity SDS was the highest for children who started GH treatment before the age of 6 years until 2 years of GH treatment (treatment duration 1). These results suggest that the treatment start age has a significant effect on the height velocity. Moreover, the height SDS of children showed a tendency to steadily increase while treatment was continued, as shown in Figures 1A, B. The average height SDS of children included in this study at the start of treatment was −2.52 ± 0.65, which was similar to that in other previous studies (−2.72 to −2.17) (4, 5, 21, 23, 28, 29). When GH treatment had started, boys’ height SDS increased by 0.55 and girls’ height SDS increased by 0.54 within 1 year of starting the treatment. If GH treatment had been continued for more than 3 years, the height SDS at the 3rd year increased by 1.46 compared to baseline in both boys and girls (height SDS, boys: −2.48–−1.02, girls: −2.58–−1.12). These results were consistent with those of previous studies in which the height SDS increased by 0.40 to 0.57 from 6 months to the 1st year after GH treatment and by 0.96–1.05 at the 3rd year (4, 5, 30–32).
When considering both GH dose and the efficacy results comprehensively, GH treatment efficacy in this study was enough compared with that of previous studies, although the average GH dose in this study was lower than the recommended GH treatment dose. This revealed that the GH dose administered in a Korean clinical setting is lower than the recommended dose (29, 30, 33, 34) and that effective treatment is possible even without prescribing a high dose of GH to children with ISS. Although the dose initially approved in Korea (0.067 mg/kg/day, 0.37 mg/kg/week, 0.40 mg/kg/week) is lower that the dose recommended in international guidelines (0.47 mg/kg/week), the phenomenon of administering less than the approved dose for patients with ISS was also seen in previous Korean studies by Nam HK et al. in 2019 and Jeong HR et al. in 2014, which showed significantly improved growth outcomes even with low doses (35–40). In this study, the age at which GH treatment was started, duration of treatment, midparental height, baseline height SDS, IGF-1 SDS, and IGFBP-3 SDS were factors that affected the height outcomes of children with ISS. Remarkably, these factors have been identified in previous studies. Although the midparental height and the pediatric patients’ height and hormone status at the time of treatment cannot be altered, there were definite factors that could affect growth outcomes. It is emphasized that obtaining an accurate and early diagnosis and the early initiation of treatment are very important factors for better growth outcomes in ISS patients (4, 5, 23, 41, 42). Moreover, an important factor influencing the achievement of the target SDS in ISS patients is medication adherence. Parents of ISS children may feel sadness, guilt, and frustration for administering drugs to their children with a needle syringe, so the appropriate device type may help increase dosing compliance (27, 32, 43, 44). Treatment devices with improved dosing convenience and reduced pain, such as automatic pen and electronic device, can be helpful toward reaching the target SDS (12, 18).
The results presented above have a number of limitations. First, due to the nature of the noninterventional, multicenter, retrospective study, we could not control the time of visit of patients with ISS equally and we could not divide the patients into prepubertal and postpubertal groups. Second, because this study did not collect information on whether the GH was administered correctly daily or weekly according to the approved regimen, it is difficult to confirm patient compliance. Third, this study was conducted from 2009 to 2019 using Korea’s basic definition of ISS, so using the ISS diagnosis method from a more diverse and updated perspective was difficult. In addition, patients diagnosed with ISS were recruited on the discretion of the physicians at each hospital at the time, so patients could not be recruited according to strict and identical diagnostic criteria. Fourth, owing to the retrospective nature of this study, patients with ISS could not be continuously followed up until adulthood. Finally, considering the lack of research on patients with ISS in Korea, the study results were compared with those of previous studies in other countries where the average adult height may differ substantially from those of ethnic Koreans.
ISS is still used as a diagnostic label today, however, it is not a definitive diagnosis. While clinical and hormonal diagnostic techniques remain important, it is the emergence of genetic investigations that have led to numerous molecular discoveries in ISS subjects. The issue now is when to do some of the genetic screening, more target single gene, gene panel and array or whole exome or whole genome testing. Clinical evaluation, hormonal investigation and genetic sequencing are required to assess ISS subjects and should complement each other to identify the pathogenesis in poorly growing patients including ISS (45–53).
Although there were some limitations, this study contributes to understanding the characteristics and determining the timing and method of GH treatment in Korean pediatric patients with ISS.
Additionally, as shown in Figure 1A, the height SDS of group 2 decreased at the 4th year than at the 3rd year after treatment, which may be influenced by various biases. First, since the number of patients who received GH for more than 3 years in this study was very small, the trend of the graph is easily changed by a small number of patient results. Second, the possibility that the patients’ compliance with GH decreased with long-term administration cannot be ignored. Third, some patients in group 2 entered puberty after 3 years of treatment and the influence of puberty may have affected the graph (31).
In conclusion, ISS remains heterogeneous. Evaluating short stature in children using history, physical and laboratory examinations, and imaging and genetic studies is critical. Identifying biomarkers of rhGH response is also crucial. This study suggests that treatment for ISS should be started at an early age and that even if GH is administered at a dose lower than the approved one, its therapeutic effect can be sufficient. We highlight that using the automatic pen or electronic device instead of needle and syringe might have a positive influence on treatment effects.
The datasets presented in this article are not readily available because No access to raw dataset is allowed other than the Pfizer employee in charge of this study and the statistical analysis team. Requests to access the datasets should be directed to https://www.pfizer.com/contact.
The studies involving human participants were reviewed and approved by IRB approval numbers of 12 hospitals: CHOSUN 2019-10-007 WKUH2019-10-018 KHNMC 2019-10-027 HDT 2019-10-005-001 DAUHIRB-19-223 WMCSB201910-82 2019AS0220 2019-10-020 KIRAMS 2019-10-007 3-2019-0276 KANGDONG 2019-10-004 AJIRB-MED-OBS-19-395. Written informed consent for participation was not provided by the participants’ legal guardians/next of kin because: Because this study is retrospective medical chart review. So, the study subjects’ informed consent forms are not required in Korea.
JH reviewed the study design and data collection instruments, collected data, and reviewed the manuscript. H-WC collected data, reviewed, and revised the manuscript. I-TH collected data, critically reviewed the analysis results for important intellectual content and reviewed the manuscript. J-EL, CS, Y-JR, JL, EK, KY, EK, C-KJ, and KS collected data and reviewed the study results. CN reviewed statistical analysis plan, reviewed the analysis results, and reviewed the manuscript. J-SM designed statistical analysis plan, carried out the analysis, and reviewed the manuscript. H-YG conceptualized and designed the study, coordinated and supervised data collection, developed, reviewed, and revised the manuscript. M-JS coordinated with investigators, reviewed the study results, and revised the manuscript. All authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work.
This study received funding from Pfizer Pharmaceuticals Korea Ltd. The funder had the following involvement with the study: study design. All the data were available to the authors and the authors analyzed the data with academic statisticians independently.
H-YG and M-JS are employees of Pfizer Pharmaceuticals Korea Ltd.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The authors declare that this study received funding from Pfizer Pharmaceuticals Korea Ltd. The funder had the following involvement with the study: study design.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2022.925102/full#supplementary-material
Appendix Figure 1 | (A) Change in height SDS during treatment – boys. (B) Change in height SDS during treatment– girls “x” denotes the duration of GH treatment (e.g., a value of 0 on the x-axis is the start time of GH treatment).
Appendix Figure 2 | (A) Change in height SDS velocity during treatment – boys. (B) Change in height SDS velocity during treatment – girls “x” denotes the duration of GH treatment (e.g., a value of 0 on the x-axis is the start time of GH treatment).
1. Ranke MB. Towards a consensus on the definition of idiopathic short stature. Horm Res (1996) 45(Suppl 2):64–6. doi: 10.1159/000184851
2. Bryant J, Baxter L, Cave CB, Milne R. Recombinant growth hormone for idiopathic short stature in children and adolescents. Cochrane Database Syst Rev (2007) 3:CD004440. doi: 10.1002/14651858.CD004440.pub2
3. Wit JM. ESPE classification of paediatric endocrine diagnoses: Supplement issue: Hormone research, Vol. 68. (2007). doi: 10.3349/ymj.2014.55.1.53.
4. Kim HS, Yang SW, Yoo HW, Suh BK, Ko CW, Chung WY, et al. Efficacy of short-term growth hormone treatment in prepubertal children with idiopathic short stature. Yonsei Med J (2014) 55:53–60. doi: 10.3349/ymj.2014.55.1.53
5. Kim SA, Choe YR, Yang EM, Kim CJ. Comparison of growth hormone treatment in patients with idiopathic short stature and idiopathic growth hormone deficiency. Chonnam Med J (2014) 50:63–6. doi: 10.4068/cmj.2014.50.2.63
6. Cohen P, Rogol AD, Deal CL, Saenger P, Reiter EO, Ross JL, et al. Consensus statement on the diagnosis and treatment of children with idiopathic short stature: a summary of the growth hormone research society, the Lawson Wilkins pediatric endocrine society, and the European society for paediatric endocrinology workshop. J Clin Endocrinol Metab ISS Consens Workshop Participants (2008) 93:4210–7. doi: 10.1210/jc.2008-0509
7. Hwang JS, Lee HS, Lee KH, Yoo HW, Lee DY, Suh BK, et al. Once-weekly administration of sustained-release growth hormone in Korean prepubertal children with idiopathic short stature: a randomized, controlled phase II study. Horm Res Paediatr (2018) 90:54–63. doi: 10.1159/000489262
8. Cohen LE. Idiopathic short stature: a clinical review. JAMA (2014) 311:1787–96. doi: 10.1001/jama.2014.3970
9. Sotos JF, Tokar NJ. Growth hormone significantly increases the adult height of children with idiopathic short stature: comparison of subgroups and benefit. Int J Pediatr Endocrinol (2014) 2014:15. doi: 10.1186/1687-9856-2014-15
10. Rosenfeld RG, Belgorosky A, Camacho-Hubner C, Savage MO, Wit JM, Hwa V. Defects in growth hormone receptor signaling. Trends Endocrinol Metab (2007) 18:134–41. doi: 10.1016/j.tem.2007.03.004
11. Rosenbloom AL. Pediatric endo-cosmetology and the evolution of growth diagnosis and treatment. J Pediatr (2011) 158:187–93. doi: 10.1016/j.jpeds.2010.10.004
12. Main KM, Jørgensen JT, Hertel NT, Jensen S, Jakobsen L. Automatic needle insertion diminishes pain during growth hormone injection. Acta Paediatr (1995) 84:331–4. doi: 10.1111/j.1651-2227.1995.tb13638.x
13. Al Shaikh A, Daftardar H, Alghamdi AA, Jamjoom M, Awidah S, Ahmed ME, et al. Effect of growth hormone treatment on children with idiopathic short stature (ISS), idiopathic growth hormone deficiency (IGHD), small for gestational age (SGA) and turner syndrome (TS) in a tertiary care center. Acta BioMed (2020) 91:29–40. doi: 10.23750/abm.v91i1.9182
14. Food and Drug Administration. FDA Approves humatrope for non-growth hormone deficient short stature. (2003). Available at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2014/019640s092lbl.pdf.
15. Pedicelli S, Peschiaroli E, Violi E, Cianfarani S. Controversies in the definition and treatment of idiopathic short stature (ISS). J Clin Res Pediatr Endocrinol (2009) 1:105–15. doi: 10.4008/jcrpe.v1i3.53
16. Inzaghi E, Reiter E, Cianfarani S. The challenge of defining and investigating the causes of idiopathic short stature and finding an effective therapy. Horm Res Paediatr (2019) 92:71–83. doi: 10.1159/000502901
17. Korean Intellectual Property Office. Registered Patent Publication, 10-1641415 (2016). Available at: https://patentimages.storage.googleapis.com/16/44/a1/f9b8a537e08d4b/KR101641415B1.pdf
18. Dumas H, Panayiotopoulos P, Parker D, Pongpairochana V. Understanding and meeting the needs of those using growth hormone injection devices. BMC Endocr Disord (2006) 6:5. doi: 10.1186/1472-6823-6-5
19. Kemp SF, Kuntze J, Attie KM, Maneatis T, Butler S, Frane J, et al. Efficacy and safety results of long-term growth hormone treatment of idiopathic short stature. J Clin Endocrinol Metab (2005) 90:5247–53. doi: 10.1210/jc.2004-2513
20. Cutfield WS, Derraik JG, Gunn AJ, Reid K, Delany T, Robinson E, et al. Non-compliance with growth hormone treatment in children is common and impairs linear growth. PloS One (2011) 6:e16223. doi: 10.1371/journal.pone.0016223
21. Kim J, Suh BK, Ko CW, Lee KH, Shin CH, Hwang JS, et al. Recombinant growth hormone therapy for prepubertal children with idiopathic short stature in Korea: a phase III randomized trial. J Endocrinol Invest (2018) 41:475–83. doi: 10.1007/s40618-017-0786-8
22. Lee MM. Clinical practice. idiopathic short stature. N Engl J Med (2006) 354:2576–82. doi: 10.1056/NEJMcp060828
23. Finkelstein BS, Imperiale TF, Speroff T, Marrero U, Radcliffe DJ, Cuttler L. Effect of growth hormone therapy on height in children with idiopathic short stature: a meta-analysis. Arch Pediatr Adolesc Med (2002) 156:230–40. doi: 10.1001/archpedi.156.3.230
24. Grimberg A, DiVall SA, Polychronakos C, Allen DB, Cohen LE, Quintos JB, et al. Guidelines for growth hormone and insulin-like growth factor-I treatment in children and adolescents: growth hormone deficiency, idiopathic short stature, and primary insulin-like growth factor-I deficiency. Horm Res Paediatr (2016) 86:361–97. doi: 10.1159/000452150
25. Deal C, Kirsch S, Chanoine JP, Lawrence S, Cummings E, Rosolowsky ET, et al. Growth hormone treatment of Canadian children: results from the GeNeSIS phase IV prospective observational study. CMAJ Open (2018) 6:E372–83. doi: 10.9778/cmajo.20180020
26. Barstow C, Rerucha CM. Evaluation of short and tall stature in children. Am Fam Phys (2015) 92:43–50.
27. Acerini CL, Wac K, Bang P, Lehwalder D. Optimizing patient management and adherence for children receiving growth hormone. Front Endocrinol (2017) 8:313. doi: 10.3389/fendo.2017.00313
28. Rhie YJ, Yoo JH, Choi JH, Chae HW, Kim JH, Chung S, et al. Long-term safety and effectiveness of growth hormone therapy in Korean children with growth disorders: 5-year results of LG growth study. PloS One (2019) 14:e0216927. doi: 10.1371/journal.pone.0216927
29. Ranke MB, Lindberg A, Tanaka T, Camacho-Hübner C, Dunger DB, Geffner ME. Baseline characteristics and gender differences in prepubertal children treated with growth hormone in Europe, USA, and Japan: 25 years’ KIGS® experience (1987-2012) and review. Horm Res Paediatr (2017) 87:30–41. doi: 10.1159/000452887
30. Ben-Ari T, Chodick G, Shalev V, Goldstein D, Gomez R, Landau Z. Real-world treatment patterns and outcomes of growth hormone treatment among children in Israel over the past decade (2004-2015). Front Pediatr (2021) 9:711979. doi: 10.3389/fped.2021.711979
31. Im M, Kim YD, Han HS. Effect of growth hormone treatment on children with idiopathic short stature and idiopathic growth hormone deficiency. Ann Pediatr Endocrinol Metab (2017) 22:119–24. doi: 10.6065/apem.2017.22.2.119
32. Deodati A, Cianfarani S. Impact of growth hormone therapy on adult height of children with idiopathic short stature: systematic review. BMJ (2011) 342:c7157. doi: 10.1136/bmj.c7157
33. Wit JM. Growth hormone therapy. Best Pract Res Clin Endocrinol Metab (2002) 16:483–503. doi: 10.1053/beem.2002.0206
34. Park YH. Short stature and growth hormone therapy. Yeungnam Univ J Med (2005) 22:1–12. doi: 10.12701/yujm.2005.22.1.1
35. Grimberg A, Kanter GP. US Growth hormone use in the idiopathic short stature era: trends in insurer payments and patient financial burden. J Endocr Soc (2019) 3:2023–31. doi: 10.1210/js.2019-00246
36. Nam HK, Kim HR, Lee KH, Rhie YJ. Idiopathic short stature phenotypes among Korean children: cluster analysis. Tohoku J Exp Med (2019) 248:193–200. doi: 10.1620/tjem.248.193
37. Genotropin injection (somatropin). In: Prescr inf pfizer Korea. Revised 2021/12/24. (2021). Available at: https://labeling.pfizer.com/showlabeling.aspx?id=577
38. Eutropin pen injection (somatropin). In: Prescr inf LG chem. Revised 2020/4/8. (2020). Available at: https://innovation.lgchem.com/resource/assets/web/images/pdf/LG%20Chem%20Life%20Sciences%20Factsheet.pdf
39. Saizen liquid cartridge injection (somatropin). In: Prescribing information. Merck Korea: Saizen Prescribing Information (emdserono.com). Revised2020/5/22. Available at: https://www.emdserono.com/us-en/pi/saizen-ce-pi.pdf.
40. Norditropin NordiFlex (somatropin). In: Prescribing information. Novo Nordisk South Korea. Norditropin Nordiflex Full Prescribing Information, Dosage & Side Effects | MIMS Singapore. Available at: https://www.mims.com/singapore/drug/info/norditropin%20nordiflex?type=full.
41. Polak M, Blair J, Kotnik P, Pournara E, Pedersen BT, Rohrer TR. Early growth hormone treatment start in childhood growth hormone deficiency improves near adult height: analysis from NordiNet® international outcome study. Eur J Endocrinol (2017) 177:421–9. doi: 10.1530/EJE-16-1024
42. Zhao Q, Zhang M, Chu Y, Sun H, Pan H, Ban B. A retrospective analysis of patients with short stature in Eastern China between 2013 and 2019. BioMed Res Int (2021) 2021:6640026. doi: 10.1155/2021/6640026
43. Quitmann J, Bloemeke J, Silva N, Bullinger M, Witt S, Akkurt I, et al. Quality of life of short-statured children born small for gestational age or idiopathic growth hormone deficiency within 1 year of growth hormone treatment. Front Pediatr (2019) 7:164. doi: 10.3389/fped.2019.00164
44. Rohrer TR, Ceplis-Kastner S, Jorch N, Müller HL, Pfäffle R, Reinehr T, et al. Needle-free and needle-based growth hormone therapy in children: a pooled analysis of three long-term observational studies. Horm Res Paediatr (2018) 90:393–406. doi: 10.1159/000496614
45. Chung WY, Yoo HW, Hwang JS, Ko CW, Kim HS, Jin DK, et al. Effect of growth hormone therapy on height velocity in Korean children with idiopathic short stature: a phase III randomised controlled trial. Horm Res Paediatr (2018) 90:44–53. doi: 10.1159/000491016
46. Lee KH. Growth assessment and diagnosis of growth disorders in childhood. Korean J Pediatr (2003) 46:1171–7.
47. Chandar MCSR, Kaplowitz PB, Vaidyanathan P. Challenges of securing growth hormone coverage for idiopathic short stature: review of the 7-year experience at one institution. Endocr Pract (2019) 25:156–60. doi: 10.4158/EP-2018-0271
48. Pricci F, Villa M, Maccari F, Agazio E, Rotondi D, Panei P, et al. The Italian registry of GH treatment: electronic clinical report form (e-CRF) and web-based platform for the national database of GH prescriptions. J Endocrinol Invest (2019) 42:769–77. doi: 10.1007/s40618-018-0980-3
49. Al-Abdulrazzaq D, Al-Taiar A, Hassan K, Al-Basari I. Recombinant growth hormone therapy in children with short stature in Kuwait: a cross-sectional study of use and treatment outcomes. BMC Endocr Disord (2015) 15:76. doi: 10.1186/s12902-015-0073-7
50. Wu D, Chen RM, Chen SK, Liu GL, Chen LQ, Yang Y, et al. Final adult height of children with idiopathic short stature: a multicenter study on GH therapy alone started during peri-puberty. BMC Pediatr (2020) 20:138. doi: 10.1186/s12887-020-02034-8
51. Lindsay R, Feldkamp M, Harris D, Robertson J, Rallison M. Utah Growth study: growth standards and the prevalence of growth hormone deficiency. J Pediatr (1994) 125:29–35. doi: 10.1016/s0022-3476(94)70117-2
52. Jeong HR, Shim YS, Lee HS, Hwang JS. The effect of growth hormone treatment on height in children with idiopathic short stature. J Pediatr Endocrinol Metab (2014) 27:629–33. doi: 10.1515/jpem-2013-0461
Keywords: ISS (idiopathic short stature), growth hormone, height velocity, Korean ISS (idiopathic short stature) patients, dose of growth hormone, GH device type
Citation: Chae HW, Hwang I-T, Lee J-E, So CH, Rhie Y-J, Lim JS, Kwon EB, Yi KH, Kim EY, Jo C-K, Shim KS, Gil H-Y, Seong M-J, Nam CM, Moon J-S and Hwang JS (2022) Height outcomes in Korean children with idiopathic short stature receiving growth hormone treatment. Front. Endocrinol. 13:925102. doi: 10.3389/fendo.2022.925102
Received: 21 April 2022; Accepted: 11 August 2022;
Published: 07 September 2022.
Edited by:
Regis Coutant, Centre Hospitalier Universitaire d’Angers, FranceReviewed by:
Zhu Huijuan, Peking Union Medical College Hospital (CAMS), ChinaCopyright © 2022 Chae, Hwang, Lee, So, Rhie, Lim, Kwon, Yi, Kim, Jo, Shim, Gil, Seong, Nam, Moon and Hwang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jin Soon Hwang, cGVkaHdhbmdAYWpvdS5hYy5rcg==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.