- 1Endocrine Bone Unit, Imperial College Healthcare NHS Trust, London, United Kingdom
- 2Section of Endocrinology and Investigative Medicine, Imperial College London, London, United Kingdom
- 3Department of Endocrinology, Imperial College Healthcare NHS Trust, London, United Kingdom
One of the most important and potentially long-lasting detrimental consequences of Functional Hypothalamic Amenorrhoea (FHA) is on skeletal homeostasis. Beyond oestrogen deficiency, FHA is associated with a cascade of additional neuro-endocrine and metabolic alterations, some adaptive, but which combine to disrupt skeletal homeostasis. Ultimately, this leads to a two-fold increased risk of fractures in women with FHA compared to healthy eumenorrhoeic women. Although the cornerstone of management of FHA-related bone loss remains recovery of menses via restoration of metabolic/psychological balance, there is rapidly developing evidence for hormonal manipulations (with a particular emphasis on route of administration) and other pharmacological treatments that can protect or improve skeletal homeostasis in FHA. In this mini-review, we provide an update on the pathophysiology, clinical management and future avenues in the field from a bone perspective.
Introduction
Functional Hypothalamic Amenorrhoea (FHA) results from the suppression of the hypothalamic control of the reproductive axis, resulting in the cessation of menses (in the absence of an organic cause). Negative energy conditions with weight loss, such as in Anorexia Nervosa (AN) or without significant weight loss (but low body fat) such as in training athletes, and psychological stress, are the main aetiologies predisposing to FHA. AN affects approximately 0.2-4% of women, with the majority experiencing amenorrhoea (1, 2). Indeed, in female athletes, the reported prevalence of secondary amenorrhoea is up to 60% (3).
Irrespective of the aetiology, FHA has detrimental effects on the skeleton through disruption of normal skeletal homeostasis, ultimately resulting in an increased risk of fractures. Therefore, it is crucial to fully appreciate the factors implicated in bone impairments in this condition. Importantly, different aetiologies of FHA and their time of onset (e.g. adolescent versus adult) are associated with characteristic neuroendocrine changes which have distinct effects on skeletal homeostasis and fracture risk (4, 5). Therefore, bone management may be tailored accordingly.
The aim of this mini-review is to give an overview of the effects of FHA on bone, specifically how they differ according to the underlying aetiology. Furthermore, we discuss current and future treatment avenues and identify gaps in the literature to inform future research, thereby providing an update for the field.
Fha and Bone, According to Aetiology
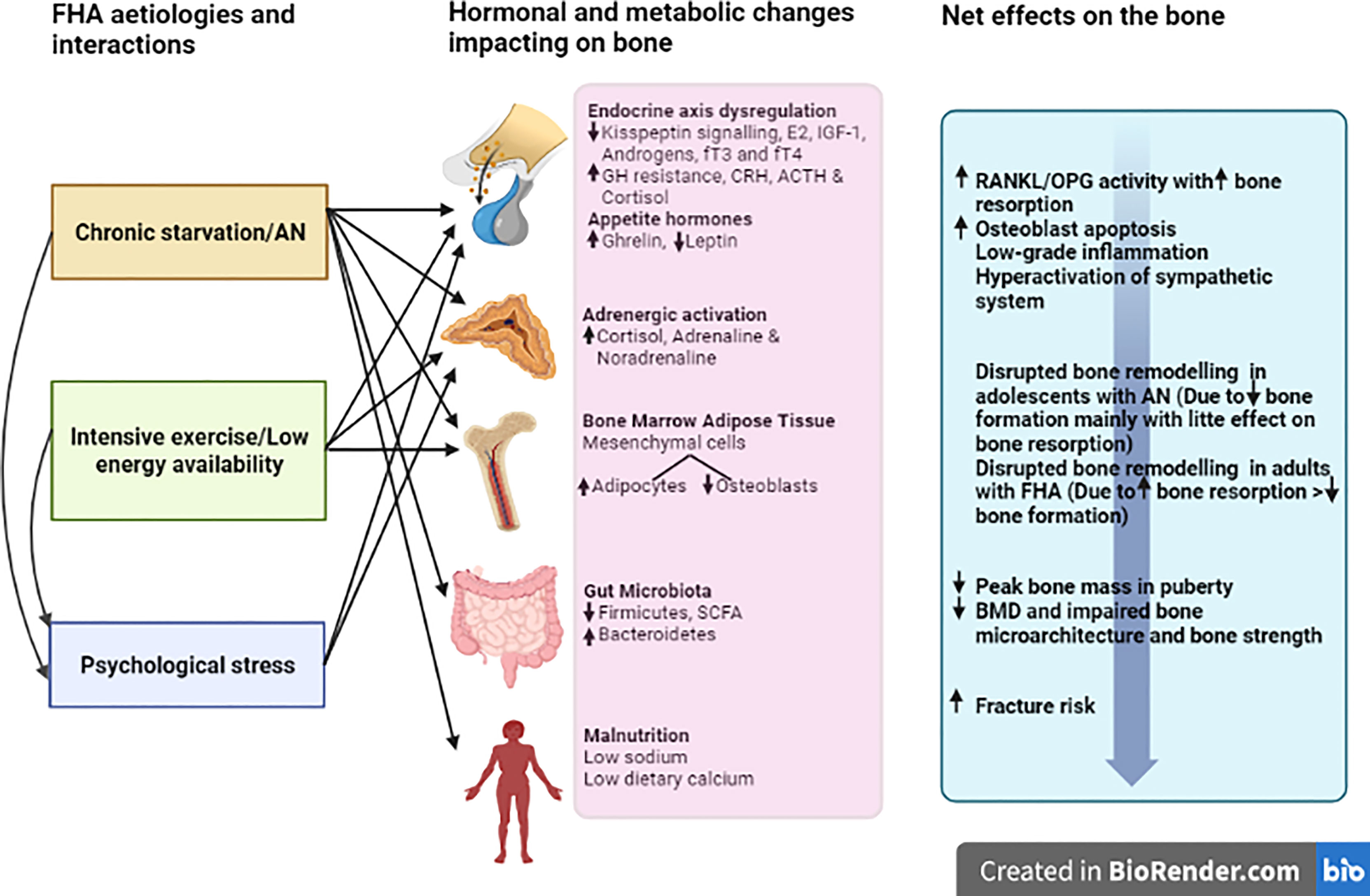
A summary of the aetiologies for FHA as discussed above, and their detrimental effects on the skeleton are displayed in Figure 1.
Anorexia Nervosa
There is an abundance of evidence for low bone mineral density (BMD) and increased risk of fractures in FHA due to AN. In a cross-sectional study of 214 women with AN aged 17-45 years, over half had osteopenia and a third were osteoporotic. Furthermore, thirty percent of the cohort reported a previous fracture (6). This was replicated in a cohort of 60 adolescent girls with AN, where 52% had a reduced BMD (based on Z score of < -1) (7). Additionally, indices of bone quality at the microarchitectural level and bone strength, as assessed by High-Resolution peripheral Quantitative CT (HR-pQCT) were also negatively affected (8). In keeping with this high fracture risk, another study observed that the incidence rate of fracture in patients with AN (mean age 21.2 ± 9.2 years, with 94% of the cohort being female), is nearly doubled, compared to controls matched for age and gender. Furthermore, this increased risk persisted beyond 10 years from diagnosis suggesting irreversible bone impairment (9).
The issue of bone fragility is compounded by the fact that AN is predominantly a condition of younger women, typically in adolescence. This corresponds to a critical time for attaining peak bone mass (PBM). Indeed, most of the PBM is acquired before the age of 19 in females (10). In a long-term retrospective follow-up study of women who acquired AN during puberty (and therefore likely failed to achieve PBM), an increased risk of fractures was observed as far as 38 years after diagnosis, with a cumulative incidence risk of 57% at 40 years (11). In another study of over 400 participants, the lifetime prevalence of fractures was 59.8% higher in adolescents with AN, compared to healthy controls. Interestingly, this was not associated with any major reduction in axial BMD (12). A possible explanation relates to the limitation of using Dual-Energy X-ray Absorptiometry (DEXA) in this young cohort, where changes in bone microarchitecture and strength, are not adequately captured. These observations have important implications with regards to treatment strategies and the importance of early interventions to minimise long-term fracture risk.
Following on from this, it is relevant from a management perspective to note a key difference between adolescents and adults with AN, with respect to underlying bone turnover. Adolescent girls with AN, have reduced bone formation with fairly normal bone resorption, whereas adult women have reduced bone formation with markedly increased bone resorption. Overall this results in low remodelling in adolescents but higher remodelling in adult women with FHA (13). This difference suggests a limited benefit for the use of anti-resorptive agents in adolescence which has been borne out in clinical studies and highlights the different responses to treatment dependent on time of onset of FHA (14).
Exercise
Exercise can be seen as a double-edged sword. In healthy populations, weight-bearing exercise has been shown to benefit BMD and a positive legacy effect is present in ex-athletes (15, 16). This was demonstrated in a study of 48 overweight adults randomised to either calorie restriction or weight-bearing exercise for a year. Despite comparable weight loss of around 8-10%, giving an approximate mean BMI of 24 kg/m2, only the calorie-restricted group experienced a reduction in lumbar BMD (mean 2.2%), suggesting a protective effect of exercise on BMD in the face of negative energy balance (17). However, it is clear that extensive exercise coupled with low energy intake can lead to FHA and bone loss, in the so-called Female Athlete Triad. This triad consists of 3 inter-linked conditions: low energy availability, menstrual disruption and low BMD (18). Studies suggest a minimum calorie intake threshold of approximately 30 kcal/kg lean body mass/day, is required to maintain reproductive axis function (19, 20). However, this concept has been disputed by others. Lieberman et al. did not identify a specific energy threshold that induced menstrual disturbances in their cohort of women (randomised into low, moderate or high energy deficit interventional groups). However, using the threshold of 30 kcal/kg lean body mass/day in their cohort, they estimated the probability of inducing menstrual disturbances to be over 50% (21). Therefore in practice, there seems to be a spectrum of energy balance set points, at which menstrual disruption occurs at an individual level, likely related to genetic and other individual factors (22).
Regarding lifetime fracture risk, this was almost double in athletes with amenorrhoea (AA) compared to athletes with eumenorrhoea (AE) and four-fold higher compared to non-athletes (NA), in a retrospective study of 175 women. Stress fractures occurred in 32% versus 5.9% versus 0% in the AA, AE and NA cohorts respectively. Furthermore, bone microarchitecture was more negatively affected in AA, especially in those who sustained multiple stress fractures highlighting the detrimental combination of excess exercise with amenorrhoea (23).
There are some salient differences worth noting in the bone sequelae of FHA depending on an AN or exercise aetiology. In a recent study, Kandemir et al. compared bone parameters in women with AN (with or without amenorrhoea) to normal-weight athletes with oligomenorrhoea (AO) and normal-weight eumenorrhoeic controls. They observed a lower BMD and greater impairment of bone microarchitecture at all sites assessed in the AN group, compared to AO and control groups. The AO group demonstrated a lower BMD at the lumbar spine only relative to controls, and bone microarchitectural parameters were less impaired, especially at the weight-bearing tibia compared to the AN group. This highlights the greater severity of bone impairments in AN, relative to a protective bone effect during weight-bearing exercise with weight preservation, despite oligomenorrhoea. However, fracture rates were similar in AN and AO, although the latter displayed a predilection for stress fractures (which athletes are inherently more at risk of). Indeed, stress fractures were 15 times higher in the AO group compared to controls, and 7.5 times higher in the AO group compared to AN (4). Limitations of this study included its cross-sectional design and self-reporting of fractures. However, it undoubtedly highlights the different severities of bone impairment depending on underlying aetiology of amenorrhoea/oligomenorrhoea.
Psychological Stress
Psychological stress is an under-appreciated but important cause of FHA. Psychological stress can independently suppress the reproductive axis but commonly co-exists and interacts synergistically with other stressors such as energy restriction and over-exercising (as above), resulting in FHA. In a recent study involving 61 exercising women by Strock et al, women with amenorrhoea showed a greater drive for thinness and a greater need for social approval than women with eumenorrhoea. Furthermore, this was positively associated with indicators of psychological stress and depression, assessed by questionnaires. This was despite both groups having comparable exercise intensity and energy intake, thus highlighting the role of stress in FHA (24). Others have also reported that women with FHA have more dysfunctional attitudes (such as drive for perfectionism, rigidity of ideas, preoccupation of being judged), more depressive symptoms and are less able to cope with stressors than eumenorrhoeic controls. These specific personality traits of women with FHA, therefore make them more susceptible to life stressors (25, 26).
These studies demonstrate an association of psychological stress with FHA but do not identify causality. However, psychological stress is a key activator of the Hypothalmic-Pituitary-Adrenal (HPA) axis, promoting cortisol secretion, which in excess has established negative effects on skeletal homeostasis (and reproductive function). However, there exist additional mechanisms linking psychological stress with bone disruption. Low grade inflammation as evidenced by increased pro-inflammatory markers (such as tumour necrosis alpha-α), has been associated with acute stress and shown to cause upregulation of Receptor activator nuclear factor kappa-B ligand (RANKL) signalling and therefore increased bone resorption in pre-clinical studies (27, 28). Stress-induced hyperactivation of the sympathetic system has also been proposed as another mechanism. Indeed, receptors for noradrenaline are present on osteoclasts and osteoblasts (29) and stress-induced bone loss is observed in the context of elevated noradrenaline levels in mice, while propranolol, a β-adrenergic antagonist, blocks this negative effect (30). Taken together, there is not only evidence that psychological stress can cause FHA as well as associate with AN/exercise, but that psychological stress itself can directly impair skeletal homeostasis.
Endocrine Mediators of Bone Loss in Fha
The key defects in FHA are attenuated hypothalamic secretion of kisspeptin and downstream gonadotropin-releasing hormone (GnRH) (31). This results in inadequate secretion of downstream follicle-stimulating hormone and luteinising hormone to sustain normal menstrual cyclicity. The negative energy balance, low body fat and/or psychological stress result in the disruption of multiple neuro-endocrine signals (Figure 1) leading to failure of the downstream reproductive axis culminating in oestrogen deficiency and detrimental effects on skeletal homeostasis (32).
Reduced Kisspeptin
Kisspeptin (secreted by kisspeptin neurons) is the master hypothalamic regulator of the reproductive axis and controls downstream GnRH secretion through kisspeptin receptors located upon GnRH neurons (33). In FHA, kisspeptin secretion has recently been shown to be reduced (34), while conversely administration of kisspeptin to patients with FHA can restore downstream pulsatile LH secretion (35). Crucially, kisspeptin neurons receive multiple neuro-endocrine and metabolic signals that can be disrupted in FHA, and so serve to orchestrate the downstream reproductive axis based on these inputs. Although kisspeptin secretion regulates downstream classical reproductive hormones crucial to skeletal homeostasis (predominantly oestrogen and testosterone), recent data has identified direct positive effects for kisspeptin in bone (36–39). However, although in FHA there is reduced kisspeptin signalling in the hypothalamus, it is currently unknown if kisspeptin signalling is also reduced in bone.
Reduced Oestrogen
Oestrogen receptors are present on the three main bone cells: osteoclasts, osteoblasts and osteocytes. Oestrogen inhibits bone resorption directly by inducing osteoclastic apoptosis and indirectly by disrupting the RANKL/Osteoprotegerin (OPG) pathway. Recent work suggests that RANKL expression on bone lining cells (derived from osteoblasts) is a key mediator of oestrogen-controlled bone resorption (40). In addition, further new data has identified oestrogen-induced secretion of semaphorin 3A, a protein known to reduce bone resorption and increase bone formation, from osteocytes (41), as well as anti-apoptotic effects by oestrogen on osteoblasts (via promotion of autophagy) (42). Taken together, the net effect of oestrogen is a reduction in bone remodelling (due to greater effect on reducing bone resorption compared to increasing bone formation). Therefore, oestrogen deficiency states are characterised by increased bone remodelling resulting in disrupted skeletal homeostasis. In the early menopause transition, BMD decreases by about 2% per year (43). Further demonstrating the impact of oestrogen deficiency, eumenorrhoeic women with AN have higher BMD than amenorrhoeic (i.e. lower oestrogen levels) women with AN, although both groups display lower than normal BMD (T score -1.2 in eumenorrhoeic versus -2.3 in amenorrhoeic women) (44). This highlights the dominating detrimental impact of oestrogen deficiency as seen in FHA beyond other nutritional and endocrine effects of anorexia nervosa.
Reduced Androgens
Low levels of testosterone and DHEA are observed in AN (45) with associated impairments in bone microarchitecture (46). However conflicting findings of high or normal levels of androgens have been observed in athletes and normal-weight women with FHA (45–47). Although androgens mediate most of their effect on bone indirectly from aromatisation into oestrogens, androgens themselves are also important in women predominantly for trabecular bone (48).
Reduced Leptin
Leptin is reduced in FHA mainly secondary to acute calorie restriction and stress, independent of weight loss (49, 50). Leptin has both central and peripheral actions on bone. Centrally, low leptin levels reduce the secretion of Insulin Growth Factor-1 (IGF-1), oestrogen and thyroid hormones, which all normally have positive bone effects (51). These hormonal reductions are part of a necessary adaptive energy-sparing response, to minimise growth, reproduction and metabolism respectively. Peripherally, leptin receptors are present on osteoblasts with possible anabolic roles in bones by enhancing osteoblast proliferation (52, 53). Furthermore, in vitro studies suggest a role for leptin-driven differentiation of human marrow stem cells into osteoblasts further supporting an anabolic role (54).
Elevated Ghrelin
In contrast to leptin, ghrelin levels are elevated in women with FHA (55). This response is presumed to be physiological to stimulate calorie intake and restore energy balance. Ghrelin is also a known growth hormone (GH) secretagogue and may contribute to excess GH secretion in AN (55). Interestingly, elevated ghrelin levels have been associated with a delayed return to menstrual cyclicity in women with persistent disordered eating in FHA, despite normalisation of weight and leptin levels. This suggests a direct effect of ghrelin on the reproductive axis in FHA (56). From a bone perspective, ghrelin directly stimulates osteoblast proliferation in vitro, and increases BMD in rodents in vivo (57). A similar anabolic effect on bone has also been observed following intracerebroventricular administration of ghrelin to rodents, independent of body weight (58). Taken together, these studies suggest central and peripheral positive effects of ghrelin on bone. However, although in FHA, ghrelin levels may be raised, this beneficial effect is far outweighed by the repercussions of other hormonal changes such as hypoestrogenism on bones.
Elevated GH and Reduced IGF-1
IGF-1 levels are reduced by up to 50% in AN despite increased GH, in keeping with a state of GH resistance (59, 60). IGF-1 has established anabolic effects on bone through increases in osteoblast activity and collagen synthesis (61). Crucially, IGF-1 has a key role in the gain of bone mass during puberty and correlates positively with BMD and bone formation markers in adolescent girls with AN and with bone microarchitecture in adult women with AN (62, 63). This further highlights the potential longer-term detrimental effects of FHA on bone when there is failure to achieve an optimal PBM in younger years.
Elevated Cortisol
Increased levels of Corticotrophin-Releasing Hormone (CRH), corticotrophin (ACTH) and downstream 24-hour cortisol levels are a consistent feature of FHA (32, 64). This is due to physical or psychological stress activating the HPA axis with the increases in cortisol capable of further suppressing the reproductive axis (32).
Hypercortisolaemia itself can contribute to bone loss. In a study of normal-weight and AN-induced adult women with FHA, hypercortisolaemia was observed in both groups, and was negatively correlated with BMD (65). There are multiple mechanisms for the detrimental effects of glucocorticoids (such as cortisol) on bone beyond the scope of this mini-review but include reduced gut absorption and increased renal loss of calcium, as well as increased osteoblast apoptosis and enhanced bone resorption via the RANKL/OPG pathway (66).
Reduced Thyroid Hormones
AN is associated with reduced levels of free T3 (fT3) and free T4 (fT4) compared to controls, similar to the nonthyroidal illness syndrome observed in patients with systemic illness (67). Similarly, lower thyroidal hormonal levels have been reported in FHA due to exercise, compared to their eumenorrhoeic counterparts. In this study, reduced T3 and T4 levels were associated with a prolonged post-exercise muscle recovery rate, as assessed by phosphate recovery kinetics (68). In a more recent study involving women with FHA (but not AN), those with fT3 levels below the normal range had a lower BMD at the spine and hip as well as lower circulating osteocalcin levels (a marker of osteoblastic activity), compared to those with preserved fT3 levels (mean lumbar T score range: -0.6 to -3.4 versus 0.2 to -2.9 respectively; mean hip T score range: -0.4 to -2 versus 1.8 to -1.6 respectively). A compensatory increase in oxidative stress, driven by low fT3 levels, has been proposed as the underlying mechanism impairing skeletal homeostasis (69).
Increased Bone Marrow Adipose Tissue
Bone marrow adipose tissue (BMAT) is increased in energy deficient states (such as AN and exercise-induced FHA) due to preferential differentiation of mesenchymal stem cells to adipocytes (at the expense of osteoblasts) and this increase correlates inversely with BMD (70) (71). In vitro studies demonstrate that bone marrow adipocytes release inflammatory cytokines and RANKL, which promote osteoclastogenesis, while the secretion of saturated fatty acids can also disrupt osteoblast function and lifespan (72–74). Putative mediators of the increase in BMAT include IGF-1, leptin, oestrogens, and pre-adipocyte factor-1 (75). Interestingly, a recent exploratory study in 16 women with FHA revealed that the expected increase in BMAT in this condition can be attenuated by transdermal 17β-estradiol treatment (71). Further studies in this respect and with control groups will be of great interest.
Low Sodium
Lower circulating sodium levels are a frequent feature of AN (with or without amenorrhoea). In a large cross-sectional study of over 400 women with AN, a lower sodium level (<140mmol/L) was associated with a lower BMD at both the spine and hip compared to those with a sodium level >140mmol/L (reference range: 135-145mmol/L) (76). Overt hyponatraemia is also a recognised risk factor for bone loss, osteoporosis and fractures (77). Bone loss in hyponatraemia has been attributed to mobilisation of sodium stores from the bone via increased bone resorption (in an attempt to correct the low sodium), inappropriate vasopressin secretion and a direct effect of hyponatraemia on osteoclast activity (78).
In summary, patients with FHA have a multitude of endocrine abnormalities (beyond oestrogen deficiency) that can contribute to the disruption of skeletal homeostasis, as illustrated in Figure 1.
Treatment
Weight Gain, Restoration of Energy Balance, Reduction in Psychological Stress
Weight gain, restoration of energy balance and reduction in psychological stress leading to restoration of menstrual cycles are the most effective management strategies for FHA-related bone loss (79). In a study by Miller et al. involving 75 women with AN, weight gain especially lean body mass and resumption of menstrual function were both necessary for BMD recovery at the spine and hips (80). In contrast, improvement in BMD with weight restoration but without restoration of menses has been observed (14, 81), while others did not observe any change in BMD following weight gain alone (82, 83). These latter discrepant findings may be due to limited numbers, lack of controls, non-randomised study design and limited follow-up time, which may be insufficient to capture changes in BMD. However, it is worth noting that even if no incremental effect of weight gain was reported on BMD in some studies, a deterioration over time was nevertheless not observed, which is in itself a positive outcome (82, 83).
Unfortunately, achieving and maintaining a positive energy balance long-term is challenging for most women with FHA. Indeed, only about 60% of women with AN achieve recovery at 22 years (84). Additionally, AN is associated with a long-term increased risk of fractures in later life, irrespective of recovery (9). Even in athlete-related amenorrhoea, non-pharmacological intervention (increased dietary intake and/or decreased exercise) led to return of menses in only 17.6% of college athletes, while in the recent randomised controlled ‘REFUEL’ study, an increase in energy intake of about 330 kcal/day in exercising women with oligo/amenorrhea improved menstrual function in only 64% at 1 year (85, 86). Hence, there is a compelling need for effective long-term pharmacological replacement/treatment for women with FHA to protect their bones as the aforementioned non-pharmacological methods are challenging and not always fully effective.
Oestrogen Treatment
Oestrogen replacement/treatment studies in FHA have revealed notable bone results related to the route, formulation and dosage of oestrogen. An up-to-date summary of clinical trials and other key studies related to bone treatment are reported in Table 1.
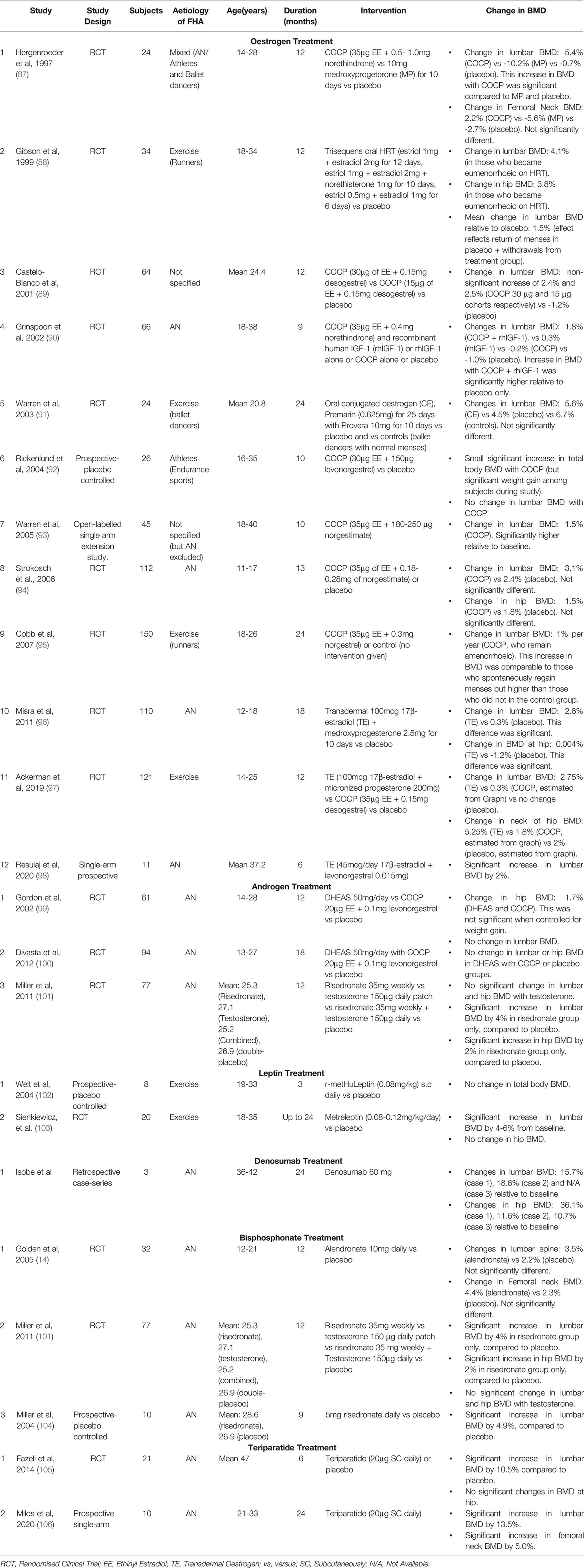
Table 1 Up-to-date summary of oestrogen treatment studies and other hormonal/pharmacological interventional studies in women with FHA.
In a recent pivotal study, 121 oligo-amenorrhoeic athletes, aged 14-25 years, were randomised to a transdermal patch providing a ‘physiological’ 100 mcg 17β-estradiol, a combined oral contraceptive pill (COCP, containing a ‘supraphysiological’ 30 µg Ethinyl-Estradiol (EE)) or no oestrogen. Only the transdermal patch group exhibited BMD improvements at 12 months (approximately 3% at the lumbar spine and 5% at the femoral neck). Surprisingly, those on the COCP had a (nonsignificant) trend to a worse BMD compared to controls mainly at the total hip (97). Crucially, there were no significant differences in weight or menstrual function change between the patch and pill groups by the end of the study, that could have confounded these results. Microarchitectural indices also improved significantly in the patch versus COCP group, especially at the tibia (107). These aforementioned findings in oligo-amenorrhoeic athletes are mirrored in females with AN. Misra et al. showed that 18 months of transdermal 17β-estradiol (100 mcg patch twice weekly) but not the COCP (35 µg of EE + 0.18-0.28 mg of norgestimate) led to an improvement of 2.6% in lumbar BMD in adolescents with AN (96). In a separate group of adolescents with AN, treatment with a triphasic COCP (35 µg of EE + 0.18–0.25 mg of norgestimate) for 13 months, did not lead to any significant change in lumbar or hip BMD (94). Similarly, in a recent 6-month pilot study, Resulaj et al. observed an increase of 2% in the lumbar BMD of women with AN (mean age 37 years), following transdermal oestradiol (45 mcg/day), although there was no control group (98). In contrast to transdermal physiological dose oestrogen, the COCP has not shown any convincing benefits (in terms of BMD) in adult women with FHA due to AN or exercise (90, 95).
These differing actions of oestrogen treatment have been mainly attributed to the route of its administration. Oral COCP inhibits IGF-1 production via first-pass hepatic metabolism, from which transdermal oestrogen is exempt. Indeed, a reduction in IGF-1 levels, associated with a greater fall in P1NP (a marker of osteoblastic activity) levels is observed during COCP treatment, but not with transdermal 17β-estradiol. Although the oestrogen dose is higher in studies of the COCP compared to transdermal oestrogen, even lower oral doses of oestrogen (1mg 17β-oestradiol) have suppressive effects on IGF-1 compared to transdermal oestrogen (108). Furthermore, oral oestrogens can increase hepatic sex hormone binding globulin levels, thereby reducing bioavailable oestrogen to the detriment of skeletal homeostasis (109).
In summary, the body of evidence for the positive effect of oestrogen treatment on bone in FHA defines a beneficial effect for transdermal oestrogen replacement over the COCP, with promising recent results (96, 97). This concept was confirmed in a very recent meta-analysis of the effects of oral contraceptives, conjugated oestrogens and transdermal oestrogens in FHA, with the latter showing consistent superiority in terms of BMD gains (110). However, it is worth noting inherent difficulties in these studies, with small numbers, high drop-out rates, relatively short follow-up, heterogeneity in types and doses of oestrogen (and progestin) used, and crucially the lack of fracture-related data. Therefore, further work is warranted to assess the doses (physiological (i.e. replacement) versus supraphysiological), the types (17β-estradiol versus ethinyl-oestradiol versus conjugated oestrogens) and the routes of administration of oestrogen (transdermal versus oral) to clearly define the optimal treatment strategy. Currently, the data point to transdermal oestrogen replacement as the optimal strategy.
Androgen Treatment
Transdermal testosterone replacement and DHEA do not increase BMD in women with AN (with and without amenorrhoea) at 12 months (Table 1) (99, 101). However, a combination of DHEA and COCP led to stabilisation of BMD over 18 months, relative to placebo, where a drop in BMD was observed (100). Further studies are required to clarify the independent benefits of androgen treatment.
IGF1 Treatment
Given the aforementioned suppression of IGF1 observed in FHA, it is not surprising that recombinant human IGF-1 (in combination with a COCP), led to an increase in lumbar BMD compared to placebo, by 1.8% versus -1% respectively at 9 months in women with AN and osteoporosis (aged between 18-38 years). The corresponding changes in lumbar BMD with IGF-1 or COCP monotherapy were 0.3% and -0.2% respectively (See Table 1). Longer studies of IGF-1 treatment are warranted given that the duration was only 9 months (90).
Leptin Treatment
Leptin treatment has also been the subject of study in FHA. Subcutaneous leptin administration can restore reproductive axis function with return of menses in a third of women with FHA (due to AN) with associated reductions in cortisol, and increases in IGF-1, thyroid hormones and bone formation markers (102). A 2-year study with daily subcutaneous metreleptin injection, culminated in 4-6% gain in BMD at the lumbar spine in exercising women (103). However, leptin treatment was associated with approximately 3% weight loss which has ultimately restricted its development for FHA despite these promising biochemical and bone outcomes. See Table 1 for a summary of studies investigating leptin treatment in FHA.
Bisphosphonates and Denosumab
There is a limited number of studies evaluating the benefits of bisphosphonates in FHA-related bone loss. These are small prospective studies looking at alendronate, risedronate and etidonate (14, 101, 111). Only risedronate showed a significant increase in BMD at the spine and hip by approximately 4% and 2% respectively, at 9-12 months in women with AN, most of whom were not experiencing endogenous menses (101, 104). However, no positive effect of bisphosphonates has been observed in adolescents with AN (14); presumably due to reduced underlying bone turnover as discussed previously. Key points of these studies are outlined in Table 1.
There are case reports supporting the use of denosumab in osteoporotic women with AN (aged 37-42 years, BMI 12.2-18.3 kg/m2) although menstrual status was not reported (112). However, no clinical trials have investigated denosumab in FHA to-date.
The barriers to using bisphosphonates in FHA are their prolonged half-lives with a small but potential teratogenic (observed in rodent studies but not consistently in humans) or neonatal complication risk, in a patient population often in their reproductive years (113). Similarly, denosumab is associated with complications if used in pregnancy (114).
Teriparatide
Anabolic agents such as teriparatide have been trialled with good effect (See Table 1). In a randomised controlled trial (RCT) of 21 osteoporotic women with AN and a mean BMI of 17.6 kg/m2, teriparatide resulted in a significant increase in lumbar BMD of 6% at only 6 months (105). More recently, Milos et al. provided further supporting evidence by studying a slightly younger cohort of women with AN (mean BMI 15.6 kg/m2) with or without previous fragility fractures. Teriparatide treatment for 24 months resulted in a significant increase in BMD of 13.5% at the lumbar spine and 5% at the hip. Notably, this was independent of gain in body weight and body fat (106). However, it is worth noting that this study lacked a control group and changes in menstrual function, which may have confounded the results, were not reported. Barriers to the use of teriparatide are its limited use of up to 2 years (which may lead the clinician to reserve teriparatide for when the patient with FHA is older or use it for shorter periods at different ages), cost and the inconvenience of daily injections.
Future Avenues
Romosozumab
Future pharmaceutical avenues include the humanised monoclonal antibody to sclerostin, Romosozumab, which is approved for the treatment of post-menopausal osteoporosis. Of note sclerostin levels have been reported as unaltered or raised in adolescent and young women with AN compared to healthy controls (115, 116). Data in FHA are awaited but this suggests that women with FHA (at least due to AN) may be susceptible to sclerostin pathway inhibition. Studies are therefore warranted in this regard although safety in women of reproductive age will again need to be clearly ascertained (there are no human pregnancy data as yet).
Kisspeptin
Another recent promising avenue is kisspeptin treatment. It has previously been demonstrated that kisspeptin administration can restore LH pulsatility in women with FHA acutely while twice weekly injections for 8 weeks can stimulate the secretion of reproductive hormones without significant desensitisation (117, 118). Recent data has now emerged from a bone perspective suggesting that kisspeptin administration also can have direct positive effects in human bones. In this study we showed that kisspeptin potently stimulated osteogenic differentiation of osteoblast progenitors and inhibited bone resorption in vitro (by up to 53.4%), in a dose-dependent manner. Furthermore, acute kisspeptin administration to healthy young men increased osteoblast activity in vivo. Further studies are warranted but collectively these data suggest that kisspeptin administration could benefit skeletal homeostasis in FHA by restoring reproductive hormone secretion as well as by direct effects on bone.
Gut Microbiota
Another emerging avenue is the association of the gut microbiota with abnormal body weight. Signature changes recently reported in women with AN include a relative reduction in firmicutes and short-chain fatty acids (SCFA), and an increase in bacteroidetes, Methanobrevibacter smithii and Escherichia coli (E.coli) species (119). Some of these changes may be adaptive but a positive association between E.coli and appetite suppression at the level of the MC4 receptors has been described in rodents (120). Yan et al. demonstrated that treatment with broad spectrum antibiotic for 2 months led to depletion of the microbiota in female germ-free mice with subsequent reduction in SCFA and IGF-1 levels. In contrast, SCFA supplementation in antibiotic-treated mice for 6 weeks, restored levels of IGF-1 and improved bone mass to reflect that of non-antibiotic-treated mice (121). Further clinical studies, specifically exploring the role of SCFA and pro and pre-biotics as potential treatment agents for bone health in FHA are now warranted.
Conclusion
Low BMD with an increased risk of fractures is a major complication of FHA due to a multitude of factors as updated above. Given the undoubted severity of the negative effects on bones, there remains an unmet need to clearly determine the optimal oestrogen replacement strategy as well as testing alternative and new pharmacological interventions to treat FHA-related bone loss. Current evidence favours transdermal 17β-estradiol as being the most promising intervention from an oestrogen replacement perspective, although larger and longer studies are needed to verify its long-term benefits, especially on the ultimate outcome of fractures. In addition, the potential use of romosozumab, kisspeptin and pro/prebiotics, warrant further exploration.
Author Contributions
PB drafted the manuscript. AC reviewed and amended the manuscript. All authors contributed to the article and approved the submitted version.
Funding
The Endocrine Bone Unit is funded by the National Health Service (NHS). The Section of Endocrinology and Investigative Medicine is funded by grants from the MRC, NIHR and is supported by the NIHR Biomedical Research Centre Funding Scheme and the NIHR/Imperial Clinical Research Facility. The views expressed are those of the authors and not necessarily those of the NHS, the NIHR or the Department of Health. PB and AC are supported by the NHS.
Conflict of Interest
AC has received non-promotional educational lecture honoraria and conference support from Amgen.
The remaining author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Pinheiro AP, Thornton LM, Plotonicov KH, Tozzi F, Klump KL, Berrettini WH, et al. Patterns of Menstrual Disturbance in Eating Disorders. Int J Eat Disord (2007) 40(5):424–34. doi: 10.1002/eat.20388
2. Keski-Rahkonen A, Mustelin L. Epidemiology of eating disorders in Europe: prevalence, incidence, comorbidity, course, consequences, and risk factors. Curr Opin Psychiatry (2016) 29(6):340–5. doi: 10.1097/YCO.0000000000000278
3. Gibbs JC, Williams NI, De Souza MJ. Prevalence of Individual and Combined Components of the Female Athlete Triad. Med Sci Sports Exerc. (2013) 45(5):985–96. doi: 10.1249/MSS.0b013e31827e1bdc
4. Kandemir N, Slattery M, Ackerman KE, Tulsiani S, Bose A, Singhal V, et al. Bone Parameters in Anorexia Nervosa and Athletic Amenorrhea: Comparison of Two Hypothalamic Amenorrhea States. J Clin Endocrinol Metab (2018) 103(6):2392–402. doi: 10.1210/jc.2018-00338
5. Frølich J, Winkler LA, Abrahamsen B, Bilenberg N, Hermann AP, Støving RK. Fractures in Women With Eating Disorders-Incidence, Predictive Factors, and the Impact of Disease Remission: Cohort Study With Background Population Controls. Int J Eat Disord (2020) 53(7):1080–7. doi: 10.1002/eat.23223
6. Miller KK, Grinspoon SK, Ciampa J, Hier J, Herzog D, Klibanski A. Medical Findings in Outpatients With Anorexia Nervosa. Arch Intern Med (2005) 165(5):561–6. doi: 10.1001/archinte.165.5.561
7. Misra M, Aggarwal A, Miller KK, Almazan C, Worley M, Soyka LA, et al. Effects of Anorexia Nervosa on Clinical, Hematologic, Biochemical, and Bone Density Parameters in Community-Dwelling Adolescent Girls. Pediatrics. (2004) 114(6):1574–83. doi: 10.1542/peds.2004-0540
8. Faje AT, Karim L, Taylor A, Lee H, Miller KK, Mendes N, et al. Adolescent Girls With Anorexia Nervosa Have Impaired Cortical and Trabecular Microarchitecture and Lower Estimated Bone Strength at the Distal Radius. J Clin Endocrinol Metab (2013) 98(5):1923–9. doi: 10.1210/jc.2012-4153
9. Vestergaard P, Emborg C, Støving RK, Hagen C, Mosekilde L, Brixen K. Fractures in Patients With Anorexia Nervosa, Bulimia Nervosa, and Other Eating Disorders - A Nationwide Register Study. Int J Eat Disord (2002) 32(3):301–8. doi: 10.1002/eat.10101
10. Baxter-Jones ADG, Faulkner RA, Forwood MR, Mirwald RL, Bailey DA. Bone Mineral Accrual From 8 to 30 Years of Age: An Estimation of Peak Bone Mass. J Bone Miner Res (2011) 26(8):1729–39. doi: 10.1002/jbmr.412
11. Lucas AR, Melton LJ, Crowson CS, O’Fallon WM. Long-Term Fracture Risk Among Women With Anorexia Nervosa: A Population-Based Cohort Study. Mayo Clin Proc (1999) 74(10):972–7. doi: 10.1016/S0025-6196(11)63994-3
12. Faje AT, Fazeli PK, Miller KK, Katzman DK, Ebrahimi S, Lee H, et al. Fracture Risk and Areal Bone Mineral Density in Adolescent Females With Anorexia Nervosa. Int J Eat Disord (2014) 47(5):458–66. doi: 10.1002/eat.22248
13. Misra M, Klibanski A. Anorexia Nervosa and Bone. J Endocrinol (2014) 221(3):R163–76. doi: 10.1530/JOE-14-0039
14. Golden NH, Iglesias EA, Jacobson MS, Carey D, Meyer W, Schebendach J, et al. Alendronate for the Treatment of Osteopenia in Anorexia Nervosa: A Randomized, Double-Blind, Placebo-Controlled Trial. J Clin Endocrinol Metab (2005) 90(6):3179–85. doi: 10.1210/jc.2004-1659
15. Park H, Kim KJ, Komatsu T, Park SK, Mutoh Y. Effect of Combined Exercise Training on Bone, Body Balance, and Gait Ability: A Randomized Controlled Study in Community-Dwelling Elderly Women. J Bone Miner Metab (2008) 26(3):254–9. doi: 10.1007/s00774-007-0819-z
16. Etherington J, Harris PA, Nandra D, Hart DJ, Wolman RL, Doyle DV, et al. The Effect of Weight-Bearing Exercise on Bone Mineral Density: A Study of Female Ex-Elite Athletes and the General Population. J Bone Miner Res (1996) 11(9):1333–8. doi: 10.1002/jbmr.5650110918
17. Villareal DT, Fontana L, Weiss EP, Racette SB, Steger-May K, Schechtman KB, et al. Bone Mineral Density Response to Caloric Restriction-Induced Weight Loss or Exercise-Induced Weight Loss: A Randomized Controlled Trial. Arch Intern Med (2006) 166(22):2502–10. doi: 10.1001/archinte.166.22.2502
18. Nattiv A, Loucks AB, Manore MM, Sanborn CF, Sundgot-Borgen J, Warren MP. American College of Sports Medicine Position Stand. The female athlete triad. Med Sci Sports Exerc. (2007) 39(10):1867–82. doi: 10.1249/mss.0b013e318149f111
19. Koltun KJ, De Souza MJ, Scheid JL, Williams NI. Energy Availability Is Associated With Luteinizing Hormone Pulse Frequency and Induction of Luteal Phase Defects. J Clin Endocrinol Metab (2020) 105(1):185–93. doi: 10.1210/clinem/dgz030
20. Loucks AB, Thuma JR. Luteinizing Hormone Pulsatility is Disrupted at a Threshold of Energy Availability In Regularly Menstruating Women. J Clin Endocrinol Metab (2003) 88(1):297–311. doi: 10.1210/jc.2002-020369
21. Lieberman JL, De Souza MJ, Wagstaff DA, Williams NI. Menstrual Disruption With Exercise is Not Linked to an Energy Availability Threshold. Med Sci Sport Exerc. (2018) 50(3):551–61. doi: 10.1249/MSS.0000000000001451
22. Chou SH, Mantzoros C. Bone Metabolism in Anorexia Nervosa and Hypothalamic Amenorrhea. Metabolism (2018) 80:91–104. doi: 10.1016/j.metabol.2017.10.009
23. Ackerman KE, Sokoloff NC, De Nardo Maffazioli G, Clarke HM, Lee H, Misra M. Fractures in Relation to Menstrual Status and Bone Parameters in Young Athletes. Med Sci Sports Exerc. (2015) 47(8):1577–86. doi: 10.1249/MSS.0000000000000574
24. Strock NCA, De Souza MJ, Williams NI. Eating Behaviours Related to Psychological Stress are Associated With Functional Hypothalamic Amenorrhoea in Exercising Women. J Sports Sci (2020) 38(21):2396–406. doi: 10.1080/02640414.2020.1786297
25. Giles DE, Berga SL. Cognitive and Psychiatric Correlates of Functional Hypothalamic Amenorrhea: A Controlled Comparison. Fertil Steril. (1993) 60(3):486–92. doi: 10.1016/S0015-0282(16)56165-2
26. Marcus MD, Loucks TL, Berga SL. Psychological Correlates of Functional Hypothalamic Amenorrhea. Fertil Steril. (2001) 76(2):310–6. doi: 10.1016/S0015-0282(01)01921-5
27. Zhang YH, Heulsmann A, Tondravi MM, Mukherjee A, Abu-Amer Y. Tumor Necrosis Factor-Alpha (TNF) Stimulates RANKL-Induced Osteoclastogenesis via Coupling of TNF Type 1 Receptor and RANK Signaling Pathways. J Biol Chem (2001) 276(1):563–8. doi: 10.1074/jbc.M008198200
28. Marsland AL, Walsh C, Lockwood K, John-Henderson NA. The Effects of Acute Psychological Stress on Circulating and Stimulated Inflammatory Markers: A Systematic Review and Meta-Analysis. Brain Behav Immun (2017) 64:208–19. doi: 10.1016/j.bbi.2017.01.011
29. Togari A. Adrenergic Regulation of Bone Metabolism: Possible Involvement of Sympathetic Innervation of Osteoblastic and Osteoclastic Cells. Microsc Res Tech. (2002) 58(2):77–84. doi: 10.1002/jemt.10121
30. Yirmiya R, Goshen I, Bajayo A, Kreisel T, Feldman S, Tam J, et al. Depression Induces Bone Loss Through Stimulation of the Sympathetic Nervous System. Proc Natl Acad Sci U S A. (2006) 103(45):16876–81. doi: 10.1073/pnas.0604234103
31. Perkins RB, Hall JE, Martin KA. Neuroendocrine Abnormalities in Hypothalamic Amenorrhea: Spectrum, Stability, and Response to Neurotransmitter Modulation. J Clin Endocrinol Metab (1999) 84(6):1905–11. doi: 10.1210/jc.84.6.1905
32. Berga SL, Mortola JF, Girton L, Suh B, Laughlin G, Pham P, et al. Neuroendocrine Aberrations in Women With Functional Hypothalamic Amenorrhea. J Clin Endocrinol Metab (1989) 68(2):301–8. doi: 10.1210/jcem-68-2-301
33. Mills EG, Izzi-Engbeaya C, Abbara A, Comninos AN, Dhillo WS. Functions of Galanin, Spexin and Kisspeptin in Metabolism, Mood and Behaviour. Nat Rev Endocrinol (2021) 17(2):97–113. doi: 10.1038/s41574-020-00438-1
34. Podfigurna A, Maciejewska-Jeske M, Meczekalski B, Genazzani AD. Kisspeptin and LH Pulsatility in Patients With Functional Hypothalamic Amenorrhea. Gynecol Reprod Endocrinol Metab (2020) 1(1):37–42. doi: 10.1007/s12020-020-02481-4
35. Jayasena CN, Nijher GMK, Chaudhri OB, Murphy KG, Ranger A, Lim A, et al. Subcutaneous Injection of Kisspeptin-54 Acutely Stimulates Gonadotropin Secretion in Women With Hypothalamic Amenorrhea, But Chronic Administration Causes Tachyphylaxis. J Clin Endocrinol Metab (2009) 94(11):4315–23. doi: 10.1210/jc.2009-0406
36. Mills EG, Yang L, Nielsen MF, Kassem M, Dhillo WS, Comninos AN. The Relationship Between Bone and Reproductive Hormones Beyond Estrogens and Androgens. Endocr Rev (2021) 42(6):691–719. doi: 10.1210/endrev/bnab015
37. Comninos AN, Hansen MS, Courtney A, Choudhury S, Yang L, Mills EG, et al. Acute Effects of Kisspeptin Administration on Bone Metabolism in Healthy Men. J Clin Endocrinol Metab (2022) 107:1529–40. doi: 10.1210/clinem/dgac117
38. Herber CB, Krause WC, Wang L, Bayrer JR, Li A, Schmitz M, et al. Estrogen Signaling in Arcuate Kiss1 Neurons Suppresses a Sex-Dependent Female Circuit Promoting Dense Strong Bones. Nat Commun (2019) 10(1):163. doi: 10.1038/s41467-018-08046-4
39. Son HE, Kim KM, Kim EJ, Jang WG. Kisspeptin-10 (KP-10) Stimulates Osteoblast Differentiation Through GPR54-Mediated Regulation of BMP2 Expression and Activation. Sci Rep (2018) 8(1):2–10. doi: 10.1038/s41598-018-20571-2
40. Streicher C, Heyny A, Andrukhova O, Haigl B, Slavic S, Schüler C, et al. Estrogen Regulates Bone Turnover by Targeting RANKL Expression in Bone Lining Cells. Sci Rep (2017) 7(1):1–14. doi: 10.1038/s41598-017-06614-0
41. Hayashi M, Nakashima T, Yoshimura N, Okamoto K, Tanaka S, Takayanagi H. Autoregulation of Osteocyte Sema3A Orchestrates Estrogen Action and Counteracts Bone Aging. Cell Metab (2019) 29(3):627–637.e5. doi: 10.1016/j.cmet.2018.12.021
42. Gavali S, Gupta MK, Daswani B, Wani MR, Sirdeshmukh R, Khatkhatay MI. Estrogen Enhances Human Osteoblast Survival and Function via Promotion of Autophagy. Biochim Biophys Acta Mol Cell Res (2019) 1866(9):1498–507. doi: 10.1016/j.bbamcr.2019.06.014
43. Ahlborg HG, Johnell O, Turner CH, Rannevik G, Karlsson MK. Bone Loss and Bone Size After Menopause. N Engl J Med (2003) 349(4):327–34. doi: 10.1056/NEJMoa022464
44. Miller KK, Grinspoon S, Gleysteen S, Grieco KA, Ciampa J, Breu J, et al. Preservation of Neuroendocrine Control of Reproductive Function Despite Severe Undernutrition. J Clin Endocrinol Metab (2004) 89(9):4434–8. doi: 10.1210/jc.2004-0720
45. Miller KK, Lawson EA, Mathur V, Wexler TL, Meenaghan E, Misra M, et al. Androgens in Women With Anorexia Nervosa and Normal-Weight Women With Hypothalamic Amenorrhea. JCEM (2007) 92(4):1334–9. doi: 10.1210/jc.2006-2501
46. Rickenlund A, Carlström K, Ekblom B, Brismar TB, von Schoultz B, Hirschberg AL. Hyperandrogenicity is an Alternative Mechanism Underlying Oligomenorrhea or Amenorrhea in Female Athletes and may Improve Physical Performance. Fertil Steril. (2003) 79(4):947–55. doi: 10.1016/S0015-0282(02)04850-1
47. Genazzani AD, Bersi C, Luisi S, Fruzzetti F, Malavasi B. Increased Adrenal Steroid Secretion in Response to CRF in Women With Hypothalamic Amenorrhea. J Steroid Biochem Mol Biol (2001) 78:247–52. doi: 10.1016/S0960-0760(01)00094-2
48. Khosla S, Monroe DG. Regulation of Bone Metabolism by Sex Steroids. Cold Spring Harb Perspect Med (2018) 8(1):a031211. doi: 10.1101/cshperspect.a031211
49. Miller KK, Parulekar MS, Schoenfeld E, Anderson E, Hubbard J, Klibanski A, et al. Decreased Leptin Levels in Normal Weight Women With Hypothalamic Amenorrhea: The Effects of Body Composition and Nutritional Intake. J Clin Endocrinol Metab (1998) 83(7):2309–12. doi: 10.1210/jc.83.7.2309
50. Andrico S, Gambera A, Specchia C, Pellegrini C, Falsetti L, Sartori E. Leptin in Functional Hypothalamic Amenorrhoea. Hum Reprod. (2002) 17(8):2043–8. doi: 10.1093/humrep/17.8.2043
51. Triantafyllou GA, Paschou SA, Mantzoros CS. Leptin and Hormones: Energy Homeostasis. Endocrinol Metab Clin North Am (2016) 45(3):633–45. doi: 10.1016/j.ecl.2016.04.012
52. Gordeladze JO, Drevon CA, Syversen U, Reseland JE. Leptin Stimulates Human Osteoblastic Cell Proliferation, De Novo Collagen Synthesis, and Mineralization: Impact on Differentiation Markers, Apoptosis, and Osteoclastic Signaling. J Cell Biochem (2002) 85(4):825–36. doi: 10.1002/jcb.10156
53. Iwamoto I, Fujino T, Douchi T. The Leptin Receptor in Human Osteoblasts and the Direct Effect of Leptin on Bone Metabolism. Gynecol Endocrinol (2004)19(2):97–104. doi: 10.1080/09513590412331284389
54. Thomas T, Gori F, Khosla S, Jensen MD, Burguera B, Riggs BL. Leptin Acts on Human Marrow Stromal Cells to Enhance Differentiation to Osteoblasts and to Inhibit Differentiation to Adipocytes. Endocrinology (1999) 140(4):1630–8. doi: 10.1210/endo.140.4.6637
55. Tolle V, Kadem M, Bluet-Pajot M-T, Frere D, Foulon C, Bossu C, et al. Balance in Ghrelin and Leptin Plasma Levels in Anorexia Nervosa Patients and Constitutionally Thin Women. J Clin Endocrinol Metab (2003) 88(1):109–16. doi: 10.1210/jc.2002-020645
56. Schneider LF, Warren MP. Functional Hypothalamic Amenorrhea is Associated With Elevated Ghrelin and Disordered Eating. Fertil Steril. (2006) 86(6):1744–9. doi: 10.1016/j.fertnstert.2006.05.051
57. Fukushima N, Hanada R, Teranishi H, Fukue Y, Tachibana T, Ishikawa H, et al. Ghrelin Directly Regulates Bone Formation. J Bone Miner Res (2005) 20(5):790–8. doi: 10.1359/JBMR.041237
58. Choi HJ, Ki KH, Yang J-Y, Jang BY, Song JA, Baek W-Y, et al. Chronic Central Administration of Ghrelin Increases Bone Mass Through a Mechanism Independent of Appetite Regulation. PLoS One (2013) 8(7):e65505. doi: 10.1371/journal.pone.0065505
59. Counts DR, Gwirtsman H, Carlsson LM, Lesem M, Cutler GBJ. The Effect of Anorexia Nervosa and Refeeding on Growth Hormone-Binding Protein, the Insulin-Like Growth Factors (IGFs), and the IGF-Binding Proteins. J Clin Endocrinol Metab (1992) 75(3):762–7. doi: 10.1210/jcem.75.3.1381372.
60. Misra M, Miller KK, Bjornson J, Hackman A, Aggarwal A, Chung J, et al. Alterations in Growth Hormone Secretory Dynamics in Adolescent Girls With Anorexia Nervosa and Effects on Bone Metabolism. J Clin Endocrinol Metab (2003) 88(12):5615–23. doi: 10.1210/jc.2003-030532
61. Giustina A, Mazziotti G, Canalis E. Growth Hormone, Insulin-Like Growth Factors, and the Skeleton. Endocr Rev (2008) 29(5):535–59. doi: 10.1210/er.2007-0036
62. Gordon CM, Goodman E, Emans SJ, Grace E, Becker KA, Rosen CJ, et al. Physiologic Regulators of Bone Turnover in Young Women With Anorexia Nervosa. J Pediatr (2002) 141(1):64–70. doi: 10.1067/mpd.2002.125003
63. Lawson EA, Miller KK, Bredella MA, Phan C, Misra M, Meenaghan E, et al. Hormone Predictors of Abnormal Bone Microarchitecture in Women With Anorexia Nervosa. Bone (2010) 46(2):458–63. doi: 10.1016/j.bone.2009.09.005
64. Biller BM, Federoff HJ, Koenig JI, Klibanski A. Abnormal Cortisol Secretion and Responses to Corticotropin-Releasing Hormone in Women With Hypothalamic Amenorrhea. J Clin Endocrinol Metab (1990) 70(2):311–7. doi: 10.1210/jcem-70-2-311
65. Lawson EA, Donoho D, Miller KK, Misra M, Meenaghan E, Lydecker J, et al. Hypercortisolemia is Associated With Severity of Bone Loss and Depression in Hypothalamic Amenorrhea and Anorexia Nervosa. J Clin Endocrinol Metab (2009) 94(12):4710–6. doi: 10.1210/jc.2009-1046
66. Komori T. Glucocorticoid Signaling and Bone Biology. Horm Metab Res (2016) 48(11):755–63. doi: 10.1055/s-0042-110571
67. Estour B, Germain N, Diconne E, Frere D, Cottet-Emard J-M, Carrot G, et al. Hormonal Profile Heterogeneity and Short-Term Physical Risk in Restrictive Anorexia Nervosa. J Clin Endocrinol Metab (2010) 95(5):2203–10. doi: 10.1210/jc.2009-2608
68. Harber VJ, Petersen SR, Chilibeck PD. Thyroid Hormone Concentrations and Muscle Metabolism in Amenorrheic and Eumenorrheic Athletes. Can J Appl Physiol (1998) 23(3):293–306. doi: 10.1139/h98-017
69. Mancini A, Vergani E, Bruno C, Barini A, Silvestrini A, Meucci E, et al. Relationships Between Thyroid Hormones, Insulin-Like Growth Factor-1 and Antioxidant Levels in Hypothalamic Amenorrhea and Impact on Bone Metabolism. Horm Metab Res (2019) 51(5):302–8. doi: 10.1055/a-0859-4285
70. Bredella MA, Fazeli PK, Miller KK, Misra M, Torriani M, Thomas BJ, et al. Increased Bone Marrow Fat in Anorexia Nervosa. J Clin Endocrinol Metab (2009) 94(6):2129–36. doi: 10.1210/jc.2008-2532
71. Singhal V, Karzar NH, Bose A, Buckless C, Ackerman KE, Bredella MA, et al. Changes in Marrow Adipose Tissue in Relation to Changes in Bone Parameters Following Estradiol Replacement in Adolescent and Young Adult Females With Functional Hypothalamic Amenorrhea. Bone (2021) 145:115841. doi: 10.1016/j.bone.2021.115841
72. Wang D, Haile A, Jones LC. Dexamethasone-Induced Lipolysis Increases the Adverse Effect of Adipocytes on Osteoblasts Using Cells Derived From Human Mesenchymal Stem Cells. Bone (2013) 53(2):520–30. doi: 10.1016/j.bone.2013.01.009
73. Liu L-F, Shen W-J, Ueno M, Patel S, Kraemer FB. Characterization of Age-Related Gene Expression Profiling in Bone Marrow and Epididymal Adipocytes. BMC Genomics (2011) 12:212. doi: 10.1186/1471-2164-12-212
74. Goto H, Osaki M, Fukushima T, Sakamoto K, Hozumi A, Baba H, et al. Human Bone Marrow Adipocytes Support Dexamethasone-Induced Osteoclast Differentiation and Function Through RANKL Expression. BioMed Res (2011) 32(1):37–44. doi: 10.2220/biomedres.32.37
75. Fazeli PK, Bredella MA, Misra M, Meenaghan E, Rosen CJ, Clemmons DR, et al. Preadipocyte Factor-1 is Associated With Marrow Adiposity and Bone Mineral Density in Women With Anorexia Nervosa. J Clin Endocrinol Metab (2010) 95(1):407–13. doi: 10.1210/jc.2009-1152
76. Lawson EA, Fazeli PK, Calder G, Putnam H, Misra M, Meenaghan E, et al. Plasma Sodium Level is Associated With Bone Loss Severity in Women With Anorexia Nervosa: A Cross-Sectional Study. J Clin Psychiatry (2012) 73(11):1–9. doi: 10.4088/JCP.12m07919
77. Barsony J, Kleess L, Verbalis JG. Hyponatremia Is Linked to Bone Loss, Osteoporosis, Fragility and Bone Fractures. Front Horm Res (2019) 52:49–60. doi: 10.1159/000493237
78. Negri AL, Ayus JC. Hyponatremia and Bone Disease. Rev Endocr Metab Disord (2017) 18(1):67–78. doi: 10.1007/s11154-016-9387-7
79. Gordon CM, Ackerman KE, Berga SL, Kaplan JR, Mastorakos G, Misra M, et al. Functional Hypothalamic Amenorrhea: An Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab (2017) 102(5):1413–39. doi: 10.1210/jc.2017-00131
80. Miller KK, Lee EE, Lawson EA, Misra M, Minihan J, Grinspoon SK, et al. Determinants of Skeletal Loss and Recovery in Anorexia Nervosa. JCEM (2006) 91(8):2931–7. doi: 10.1210/jc.2005-2818
81. Bachrach LK, Katzman DK, Litt IF, Guido D, Marcus R. Recovery From Osteopenia in Adolescent Girls With Anorexia Nervosa. J Clin Endocrinol Metab (1991) 72(3):602–6. doi: 10.1210/jcem-72-3-602
82. Franzoni E, Ciccarese F, Di Pietro E, Facchini G, Moscano F, Iero L, et al. Follow-Up of Bone Mineral Density and Body Composition in Adolescents With Restrictive Anorexia Nervosa: Role of Dual-Energy X-Ray Absorptiometry. Eur J Clin Nutr (2014) 68(2):247–52. doi: 10.1038/ejcn.2013.254
83. Compston JE, McConachie C, Stott C, Hannon RA, Kaptoge S, Debiram I, et al. Changes in Bone Mineral Density, Body Composition and Biochemical Markers of Bone Turnover During Weight Gain in Adolescents With Severe Anorexia Nervosa: A 1-Year Prospective Study. Osteoporos Int (2006) 17(1):77–84. doi: 10.1007/s00198-005-1904-6
84. Eddy KT, Tabri N, Thomas JJ, Murray HB, Keshaviah A, Hastings E, et al. Recovery From Anorexia Nervosa and Bulimia Nervosa at 22-Year Follow-Up. J Clin Psychiatry (2017) 78(2):184–9. doi: 10.4088/JCP.15m10393
85. Arends JC, Cheung M-YC, Barrack MT, Nattiv A. Restoration of Menses With Nonpharmacologic Therapy in College Athletes With Menstrual Disturbances: A 5-Year Retrospective Study. Int J Sport Nutr Exerc Metab (2012) 22(2):98–108. doi: 10.1123/ijsnem.22.2.98
86. De Souza MJ, Mallinson RJ, Strock NCA, Koltun KJ, Olmsted MP, Ricker EA, et al. Randomised Controlled Trial of the Effects of Increased Energy Intake on Menstrual Recovery in Exercising Women With Menstrual Disturbances: The “REFUEL” Study. Hum Reprod (2021) 36(8):2285–97. doi: 10.1093/humrep/deab149
87. Forslund K, Hildebrand F, Nielsen T, Falony G, Le Chatelier E, Sunagawa S, et al. Disentangling Type 2 Diabetes and Metformin Treatment Signatures in the Human Gut Microbiota. Nature (2015) 528:262–6. doi: 10.1038/nature15766
88. Gibson JH, Mitchell A, Reeve J, Harries MG. Treatment of Reduced Bone Mineral Density in Athletic Amenorrhea: A Pilot Study. Osteoporosis Int (1999) 10:284–9. doi: 10.1007/s001980050228
89. Castelo-Branco C, Vicente JJ, Pons F, Martínez de Osaba MJ, Casals E, Vanrell JA. Bone Mineral Density in Young, Hypothalamic Oligoamenorrheic Women Treated With Oral Contraceptives. J Reprod Med (2001) 46(10):875–9.
90. Grinspoon S, Thomas L, Miller K, Herzog D, Klibanski A. Effects of Recombinant Human IGF-I and Oral Contraceptive Administration on Bone Density in Anorexia Nervosa. J Clin Endocrinol Metab (2002) 87(6):2883–91. doi: 10.1210/jcem.87.6.8574
91. Warren MP, Brooks-Gunn J, Fox RP, Holderness CC, Hyle EP, Hamilton WG, et al. Persistent Osteopenia in Ballet Dancers With Amenorrhea and Delayed Menarche Despite Hormone Therapy: A Longitudinal Study. Fertil Steril. (2003) 80(2):398–404. doi: 10.1016/S0015-0282(03)00660-5
92. Rickenlund A, Carlström K, Ekblom B, Brismar TB, Von Schoultz B, Hirschberg AL. Effects of Oral Contraceptives on Body Composition and Physical Performance in Female Athletes. J Clin Endocrinol Metab (2004) 89(9):4364–70. doi: 10.1210/jc.2003-031334
93. Warren MP, Miller KK, Olson WH, Grinspoon SK, Friedman AJ. Effects of an Oral Contraceptive (Norgestimate/Ethinyl Estradiol) on Bone Mineral Density in Women With Hypothalamic Amenorrhea and Osteopenia: An Open-Label Extension of a Double-Blind, Placebo-Controlled Study. Contraception (2005) 72(3):206–11. doi: 10.1016/j.contraception.2005.03.007
94. Strokosch GR, Friedman AJ, Wu S-CC, Kamin M. Effects of an Oral Contraceptive (Norgestimate/Ethinyl Estradiol) on Bone Mineral Density in Adolescent Females With Anorexia Nervosa: A Double-Blind, Placebo-Controlled Study. J Adolesc Heal (2006) 39(6):819–27. doi: 10.1016/j.jadohealth.2006.09.010
95. Cobb KL, Bachrach LK, Sowers M, Nieves J, Greendale GA, Kent KK, et al. The Effect of Oral Contraceptives on Bone Mass and Stress Fractures in Female Runners. Med Sci Sport Exerc. (2007) 39(9):1464–73. doi: 10.1249/mss.0b013e318074e532
96. Misra M, Katzman D, Miller KK, Mendes N, Snelgrove D, Russell M, et al. Physiologic Estrogen Replacement Increases Bone Density in Adolescent Girls With Anorexia Nervosa. J Bone Miner Res (2011) 26(10):2430–8. doi: 10.1002/jbmr.447
97. Ackerman KE, Singhal V, Baskaran C, Slattery M, Campoverde Reyes KJ, Toth A, et al. Oestrogen Replacement Improves Bone Mineral Density in Oligo-Amenorrhoeic Athletes: A Randomised Clinical Trial. Br J Sports Med (2019) 53(4):229–36. doi: 10.1136/bjsports-2018-099723
98. Resulaj M, Polineni S, Meenaghan E, Eddy K, Lee H, Fazeli PK. Transdermal Estrogen in Women With Anorexia Nervosa: An Exploratory Pilot Study. JBMR Plus. (2020) 4(1):1–10. doi: 10.1002/jbm4.10251
99. Gordon CM, Grace E, Emans SJ, Feldman HA, Goodman E, Becker KA, et al. Effects of Oral Dehydroepiandrosterone on Bone Density in Young Women With Anorexia Nervosa: A Randomized Trial. J Clin Endocrinol Metab (2002) 87(11):4935–41. doi: 10.1210/jc.2002-020545
100. Divasta AD, Feldman HA, Giancaterino C, Rosen CJ, Leboff MS, Gordon CM. The Effect of Gonadal and Adrenal Steroid Therapy on Skeletal Health in Adolescents and Young Women With Anorexia Nervosa. Metab 2012/01/16. (2012) 61(7):1010–20. doi: 10.1016/j.metabol.2011.11.016
101. Miller KK, Meenaghan E, Lawson EA, Misra M, Gleysteen S, Schoenfeld D, et al. Effects of Risedronate and Low-Dose Transdermal Testosterone on Bone Mineral Density In Women With Anorexia Nervosa: A Randomized, Placebo-Controlled Study. J Clin Endocrinol Metab (2011) 96(7):2081–8. doi: 10.1210/jc.2011-0380
102. Welt CK, Chan JL, Bullen J, Murphy R, Smith P, DePaoli AM, et al. Recombinant Human Leptin in Women With Hypothalamic Amenorrhea. Obstet Gynecol Surv. (2005) 60(2):104–5. doi: 10.1097/01.ogx.0000151645.22134.0b
103. Sienkiewicz E, Magkos F, Aronis KN, Brinkoetter M, Chamberland JP, Chou S, et al. Long-Term Metreleptin Treatment Increases Bone Mineral Density and Content at the Lumbar Spine of Lean Hypoleptinemic Women. Metabolism (2011) 60(9):1211–21. doi: 10.1016/j.metabol.2011.05.016
104. Miller KK, Grieco KA, Mulder J, Grinspoon S, Mickley D, Yehezkel R, et al. Effects of Risedronate on Bone Density in Anorexia Nervosa. J Clin Endocrinol Metab (2004) 89(8):3903–6. doi: 10.1210/jc.2003-031885
105. Fazeli PK, Wang IS, Miller KK, Herzog DB, Misra M, Lee H, et al. Teriparatide Increases Bone Formation and Bone Mineral Density in Adult Women With Anorexia Nervosa. J Clin Endocrinol Metab (2014) 99(4):1322–9. doi: 10.1210/jc.2013-4105
106. Milos G, Moergeli H, Sob C, Wisler D, Wasila M, Uebelhart D, et al. Positive Effect of Teriparatide on Areal Bone Mineral Density in Young Women With Anorexia Nervosa: A Pilot Study. Calcif Tissue Int (2021) 108(5):595–604. doi: 10.1007/s00223-020-00791-3
107. Ackerman KE, Singhal V, Slattery M, Eddy KT, Bouxsein ML, Lee H, et al. Effects of Estrogen Replacement on Bone Geometry and Microarchitecture in Adolescent and Young Adult Oligoamenorrheic Athletes: A Randomized Trial. J Bone Miner Res (2020) 35(2):248–60. doi: 10.1002/jbmr.3887
108. Sonnet E, Lacut K, Roudaut N, Mottier D, Kerlan V, Oger E. Effects of the Route of Oestrogen Administration on IGF-1 and IGFBP-3 in Healthy Postmenopausal Women: Results From a Randomized Placebo-Controlled Study. Clin Endocrinol (Oxf). (2007) 66(5):626–31. doi: 10.1111/j.1365-2265.2007.02783.x
109. Singhal V, Ackerman KE, Bose A, Flores LPT, Lee H, Misra M. Impact of Route of Estrogen Administration on Bone Turnover Markers in Oligoamenorrheic Athletes and its Mediators. J Clin Endocrinol Metab (2019) 104(5):1449–58. doi: 10.1210/jc.2018-02143
110. Aalberg K, Stavem K, Norheim F, Russell MB, Chaibi A. Effect of Oral and Transdermal Oestrogen Therapy on Bone Mineral Density in Functional Hypothalamic Amenorrhoea: A Systematic Review and Meta-Analysis. BMJ Open Sport Exerc Med (2021) 7(3):1–7. doi: 10.1136/bmjsem-2021-001112
111. Nakahara T, Nagai N, Tanaka M, Muranaga T, Kojima S, Nozoe S, et al. The Effects of Bone Therapy on Tibial Bone Loss in Young Women With Anorexia Nervosa. Int J Eat Disord (2006) 39(1):20–6. doi: 10.1002/eat.20197
112. Isobe F, Nakamura Y, Suzuki T, Kato H. Effects of Denosumab on Osteoporosis in Three Cases With Anorexia Nervosa and a Review of the Literature. Mod Rheumatol Case Rep (2018) 2(1):104–6. doi: 10.1080/24725625.2017.1370784
113. Sokal A, Elefant E, Leturcq T, Beghin D, Mariette X, Seror R. Pregnancy and Newborn Outcomes After Exposure to Bisphosphonates: A Case-Control Study. Osteoporos Int (2019) 30(1):221–9. doi: 10.1007/s00198-018-4672-9
114. . Available at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2014/125320s150lbl.pdf.
115. Maïmoun L, Guillaume S, Lefebvre P, Philibert P, Bertet H, Picot M-C, et al. Role of Sclerostin and Dickkopf-1 in the Dramatic Alteration in Bone Mass Acquisition in Adolescents and Young Women With Recent Anorexia Nervosa. J Clin Endocrinol Metab (2014) 99(4):E582–90. doi: 10.1210/jc.2013-2565
116. Faje AT, Fazeli PK, Katzman DK, Miller KK, Breggia A, Rosen CJ, et al. Sclerostin Levels and Bone Turnover Markers in Adolescents With Anorexia Nervosa and Healthy Adolescent Girls. Bone (2012) 51(3):474–9. doi: 10.1016/j.bone.2012.06.006
117. Jayasena CN, Nijher GMK, Abbara A, Murphy KG, Lim A, Patel D, et al. Twice-Weekly Administration of Kisspeptin-54 for 8 Weeks Stimulates Release of Reproductive Hormones in Women With Hypothalamic Amenorrhea. Clin Pharmacol Ther (2010) 88(6):840–7. doi: 10.1038/clpt.2010.204
118. Jayasena CN, Abbara A, Veldhuis JD, Comninos AN, Ratnasabapathy R, De Silva A, et al. Increasing LH Pulsatility in Women With Hypothalamic Amenorrhoea Using Intravenous Infusion of Kisspeptin-54. J Clin Endocrinol Metab (2014) 99(6):E953–61. doi: 10.1210/jc.2013-1569
119. Aurigemma NC, Koltun KJ, VanEvery H, Rogers CJ, De Souza MJ. Linking the Gut Microbiota to Bone Health in Anorexia Nervosa. Curr Osteoporos Rep (2018) 16(1):65–75. doi: 10.1007/s11914-018-0420-5
120. Tennoune N, Chan P, Breton J, Legrand R, Chabane YN, Akkermann K, et al. Bacterial ClpB Heat-Shock Protein, an Antigen-Mimetic of the Anorexigenic Peptide α-MSH, at the Origin of Eating Disorders. Transl Psychiatry (2014) 4:e458. doi: 10.1038/tp.2014.98
Keywords: functional hypothalamic amenorrhoea, bone mineral density, osteoporosis, fractures, HRT, IGF1, kisspeptin
Citation: Behary P and Comninos AN (2022) Bone Perspectives in Functional Hypothalamic Amenorrhoea: An Update and Future Avenues. Front. Endocrinol. 13:923791. doi: 10.3389/fendo.2022.923791
Received: 19 April 2022; Accepted: 11 May 2022;
Published: 20 June 2022.
Edited by:
Anna Maria Marconi, University of Milan, ItalyReviewed by:
Anna Piotrowska, University School of Physical Education in Krakow, PolandCopyright © 2022 Behary and Comninos. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Alexander N. Comninos, YS5jb21uaW5vc0BpbXBlcmlhbC5hYy51aw==; orcid.org/0000-0002-7104-2297
 Preeshila Behary
Preeshila Behary Alexander N. Comninos
Alexander N. Comninos