
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Endocrinol. , 16 November 2022
Sec. Reproduction
Volume 13 - 2022 | https://doi.org/10.3389/fendo.2022.918805
This article is part of the Research Topic Epigenetics of Polycystic Ovary Syndrome (PCOS) View all 7 articles
Polycystic ovary syndrome (PCOS) is a reproductive dysfunction associated with endocrine disorders and is most common in women of reproductive age. Clinical and/or biochemical manifestations include hyperandrogenism, persistent anovulation, polycystic ovary, insulin resistance, and obesity. Presently, the aetiology and pathogenesis of PCOS remain unclear. In recent years, the role of circadian rhythm changes in PCOS has garnered considerable attention. Changes in circadian rhythm can trigger PCOS through mechanisms such as oxidative stress and inflammation; however, the specific mechanisms are unclear. Exosomes are vesicles with sizes ranging from 30–120nm that mediate intercellular communication by transporting microRNAs (miRNAs), proteins, mRNAs, DNA, or lipids to target cells and are widely involved in the regulation of various physiological and pathological processes. Circadian rhythm can alter circulating exosomes, leading to a series of related changes and physiological dysfunctions. Therefore, we speculate that circadian rhythm-induced changes in circulating exosomes may be involved in PCOS pathogenesis. In this review, we summarize the possible roles of exosomes and their derived microRNAs in the occurrence and development of PCOS and discuss their possible mechanisms, providing insights into the potential role of exosomes for PCOS treatment.
Polycystic ovary syndrome (PCOS) is a common gynecological endocrine disorder that affects up to 18% of women (1). Patients with PCOS experience multiple severe clinical sequelae, including reproductive complication (menstrual disorders, infertility, and hyperandrogenism) (2, 3), metabolic dysfunction (insulin resistance [IR] and diabetes) (4), and psychological disorders (depression, anxiety disorder, social phobia) (5), which negatively impact quality of life of patients. Given the heterogeneity and clinical characteristics of PCOS, symptoms may manifest differently among patients (3). Researchers have shown that endocrine and metabolic abnormalities in women with PCOS may be associated with the severity of hyperandrogenism (6). Treatments, which include lifestyle changes, drug therapy, and surgery, are typically focused on symptom relief and do not yield satisfactory results. Although PCOS has been recognized for decades, its pathophysiological mechanisms remain unclear. The circadian rhythm(CR) affects reproduction by regulating various functions of the hypothalamic-pituitary-gonadal (HPG) axis and the ovaries (7). Dysfunction of the HPG axis is critical in the development of PCOS (8, 9). Additionally, CR disorders may affect reproductive outcomes by inducing IR, oxidative stress (OS), and systemic inflammation (10). Wang et al. found that night shift work (for >2 years) remarkably correlates with PCOS risk (11). A recent study suggests that circadian rhythm disorders may be one of the causes of excess androgen in PCOS (12). Therefore, alterations in circadian rhythm may be associated with PCOS.
Exosomes, small extracellular vesicles (EVs), typically 30–120 nm in diameter, widely participate in the regulation of various physiological processes and play an important role in PCOS pathogenesis. Exosomes carry unique macromolecules, including proteins, lipids, DNA, mRNA, and non-coding RNA (ncRNA), and deliver genetic information to recipient cells, acting as “bridges” in cellular communication and affecting the functions of target cells (13, 14). In this review, we (1) discuss the role and possible mechanisms of exosome-mediated regulation of CR change-induced PCOS, (2) address the relationship between CR and PCOS, and (3) explore novel insights into the application of exosomes for treating PCOS.
The mammalian circadian clock network is driven by several transcription factors that control the core feedback loop, including the transcription activator circadian locomotor output cycle kaput (CLOCK), brain and muscle Arnt-like protein 1 (BAML1), and the clock genes Period (Per1, Per2, Per3) and Cryptochrome (Cry1, Cry2). In the first circuit, the BMAL1 and CLOCK heterodimer bind to E-box and E-box-like promoter elements, activate the transcription of Per and Cry genes. As transcription increases, PER and CRY accumulate in the cytoplasm and their dimers enter the nucleus, inhibiting CLOCK: BMAL1 activity and stopping gene expression. The second feedback loop is composed of nuclear receptors Rev-erbα/βand Rorα-γ. Rev-erbs and RORs competitively bind to ROR response elements (ROREs) to regulate BMAL1 transcription and maintain a stable circadian clock cycle (Figure 1). The CLOCK : BMAL1 heterodimer also drives the expression of thousands of clock-regulated genes controlling many biological processes such as metabolism (15–17).
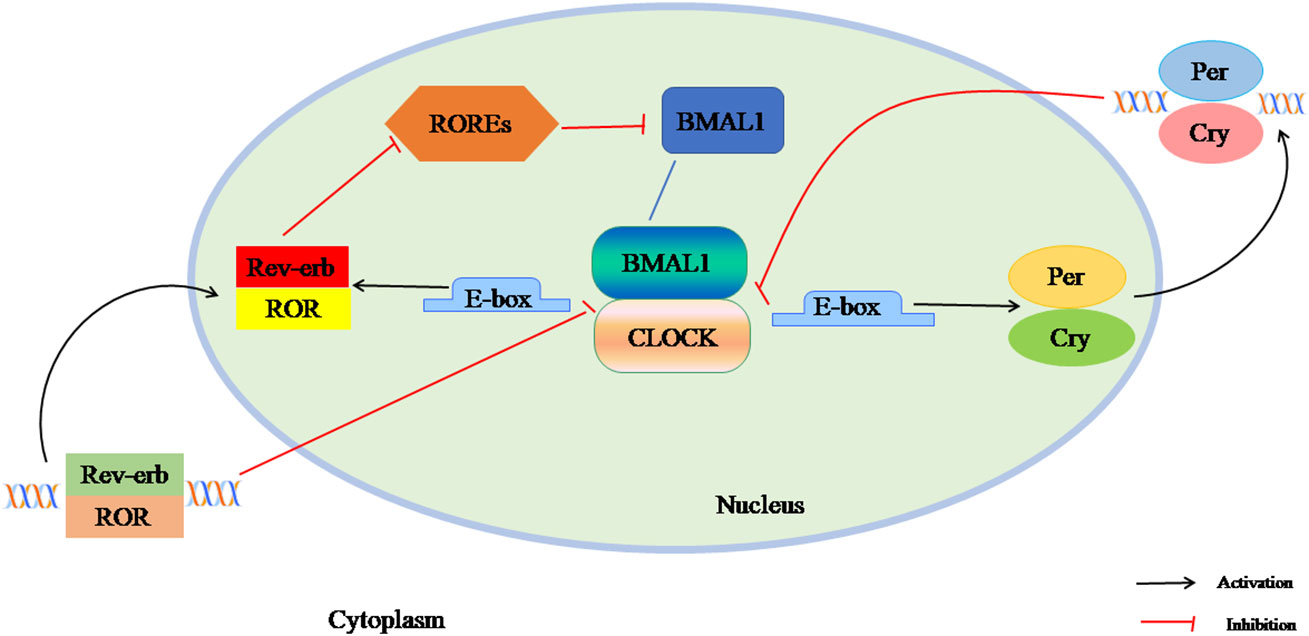
Figure 1 The composition of circadian clock. The mammalian circadian clock network is driven by several transcription factors that control the core feedback loop, including the transcription activators CLOCK, BAML1, and the clock genes Per1, Per2, Per3and Cry1, Cry2. In the first circuit, the BMAL1 and CLOCK heterodimer binds to E-box and E-box-like promoter elements, activating the transcription of Per and Cry genes. As transcription increases, PER and CRY accumulate in the cytoplasm and their dimers enter the nucleus, inhibiting CLOCK: BMAL1 activity and stopping gene expression. The second feedback loop is composed of nuclear receptor Rev-erbα / β and Rorα-γ. Rev-erbs and RORs competitively bind to ROREs to regulate BMAL1 transcription and maintain a stable circadian clock cycle.
Knockdown of BMAL1 suppresses metabolic rhythm, whereas interference with Cry1 or Cry2 typically shortens or prolongs metabolic rhythm, respectively (18). BMAL1 plays an important role in lipid and glucose metabolism. Zhang et al. found that BMAL1 promotes adipogenesis through the insulin - mTOR complex 2 (mTORC2) – protein kinase B (Akt) signaling pathway in the liver tissue (19). In addition, in specific BMAL1 knockout (KO) mice, the CR of insulin sensitivity is impaired and IR is induced (20). During fasting, glucagon induces the transcription of BMAL1 by activating cyclic adenosine response element-binding protein 1(CREB)/CREB-regulated transcription coactivator 2 (CRTC2), while insulin inhibits BMAL1 expression by suppressing the activity of CREB/CRTC2 (21). In another study, the regulatory effect of the molecular clock on white adipose tissue physiology was observed in CLOCK-KO mice. KO of Per2 or RORα/γ was beneficial for adipogenesis, whereas deletion of BMAL1 or Rev-erbα suppressed adipogenesis (22).
Both the deacetylase Sirtuin1 (SIRT1) and AMP-activated protein kinase(AMPK) are sensitive to energy metabolism and therefore have synergistic effects (23). SIRT1 is involved in the circadian transcription of core clock genes such as BMAL1, Per2 and Cry1. SIRT1 binds to CLOCK : BMAL1 through circadian rhythm (24). Nicotinamide phosphoribosyltransferase (NAMPT) and nicotinamide adenine dinucleotide(NAD)+ show circadian oscillation patterns and NAMPT participates in IR by regulating peroxisome proliferator-activated receptor γ (PPARγ) and adiponectin through SIRT1 (25). A reduction in NAMPT-mediated NAD+ biosynthesis stimulates oscillation of the clock gene Per2 by releasing SIRT1-mediated CLOCK : BMAL1. In turn, CLOCK binds and upregulates NAMPT to form a feedback loop of NNMPT/NAD+ and SIRT1/CLOCK : BMAL1 (26). Zhai et al. showed decreased expression of BMAL1 in the liver and adiposetissue of PCOS rats, resulting in suppression of the NAMPT/NAD+/SIRT1 pathway and the promoting on of IR (27). We speculate that SIRT1 may act as a bridge between BMAL1 and IR disorders in patients with PCOS. A recent study reported that activation of SIRT1 in diabetic rats can significantly up-regulate the expression of BMAL1 and increase the activation of autophagy to alleviate myocardial ischemia/reperfusion injury (28). This provides new insights into the treatment of PCOS, but more research is needed to validate these findings.
Recent studies have confirmed that biological rhythm disorders lead not only to metabolic dysfunction in PCOS, but also cause reproductive disorders (29). In the hypothalamus, the suprachiasmatic nucleus (SCN) produces timing signals to activate gonadotropin-releasing hormone (GnRH) neurons and stimulates pituitary gonadotropin cells to release luteinizing hormone(LH) (30). Bahougne et al. showed that exposure to anacutetime displacement in female mice can lead to moderate and temporary changes in LH surge, while exposure to chronic displacement can lead to severe, rapid and lasting changes in LH surge before ovulation, resulting in decreased fertility (31). In humans, disruption of circadian rhythms negatively affects fertility. A cohort study of women in North America found that shift workers had lower fertility rates than daytime workers (32). A study also confirmed an increase in LH concentrations during night/shift work (33). And another cross-sectional study reported that sleep disorders are twice as common in PCOS women compared to non-PCOS women (34).
In addition, clock genes play an important role in female fertility. Clock gene expression rhythms have been reported in the HPG axis of mice, rats, and humans (35). For example, in rats, BMAL1 mRNA expression is highest in the presence of light and Per2 mRNA expression is highest when in the absence of light (36). Long-term light exposure desynchronizes the clock genes (BMAL1, clock, Per1, Per2, Cry1 and Cry2) in the central (hypothalamus containing SCN) and peripheral (ovary and uterus) organs of hamsters, thus affecting ovarian hormone regulation, embryo implantation and pregnancy success rates (37). A BMAL1 KO can cause reproductive disorders in mice. In addition, Per1 / Per2 double mutations mice have reduced ovarian follicular reserves, leading to a decline in fertility. (38, 39) Studies have shown that BMAL1-KO mice exhibit abnormal follicle development, reduced fertilization rates, delays in early embryo/blastocyst developmental (40, 41). Chen and colleagues found that down-regulation of BMAL1 expression inhibits the synthesis of progesterone and prostaglandin E2, which are key players in the reproductive process (42). Per1 and Per2 KO mice are characterized by a significant decrease in fertility due to irregular oestrous cycles (43). Furthermore, middle-aged Cry gene-deficient female mice show early estrus abnormalities leading to reduced fertility, which can be alleviated by adjusting the light/dark cycle (44). Hence, reproductive pathophysiological treatment strategies based on the CR system is another unexplored field with a great application potential.
In short, alterations in CR may be involved in the progression of PCOS and regulate its reproductive and metabolic processes through its own genes (Figure 2), which provides a new strategy for the treatment of PCOS.
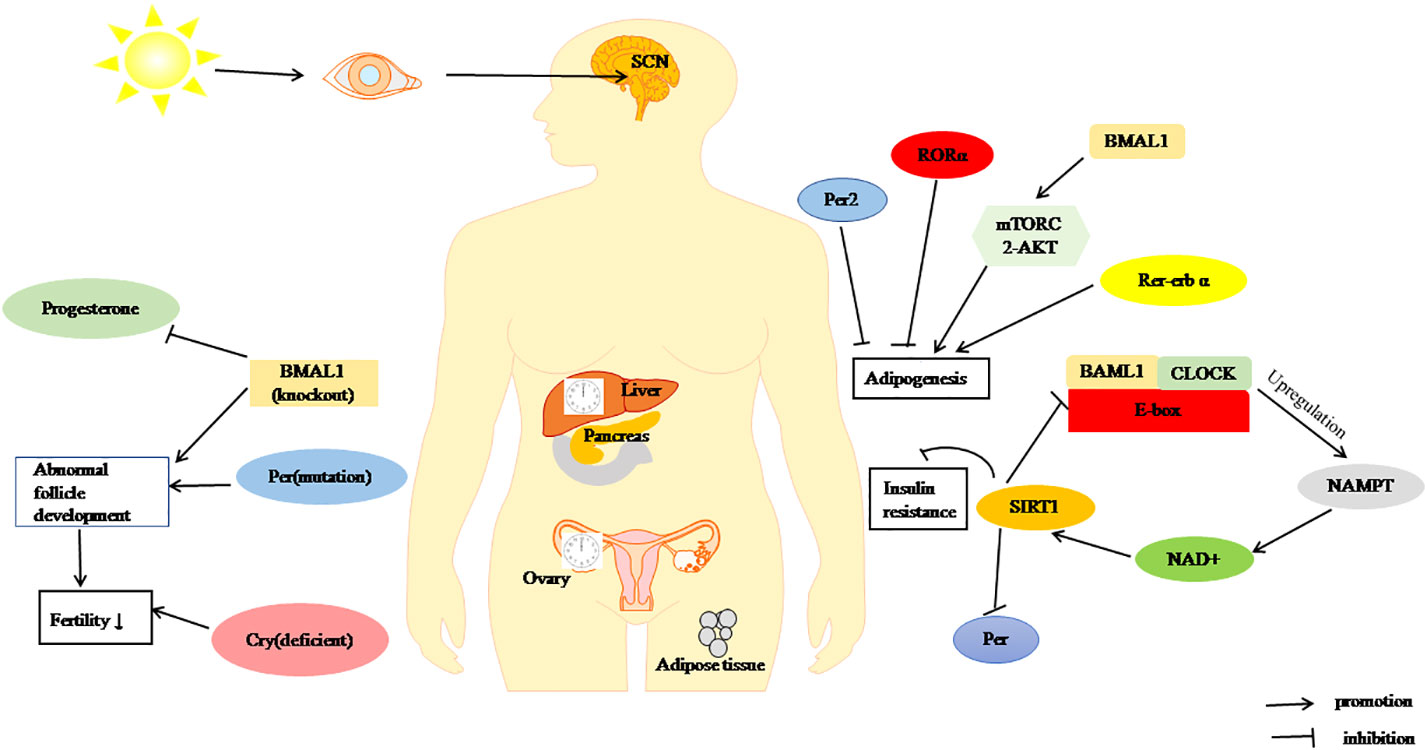
Figure 2 Regulation of metabolism and fertility by the CR. BMAL1, CLOCK and Per through SIRT1 pathway inhibit of insulin resistance. RORα, Per2, and Rer-erbα can inhibit adipogenesis, BAML1 promote adipogenesis by mTORC2/Akt signalling pathway. BMAL1-KO, Cry deficient, and Per1/2 mutation cause decreased fertility.
Chronic inflammation and OS are involved in the pathogenesis of PCOS. Abnormal expression of pro-inflammatory cytokines, characterized by increased levels of C-reactive protein (CRP), serum interleukin 1 (IL-1), interleukin 6 (IL-6), interleukin 8 (IL-8) and chemokine C-C motif ligand 2 (CCL2) has been implicated in the aetiology of PCOS (45, 46). Oxidative stress is closely associated with inflammation. Inflammation induces ROS production, and oxidative stress exacerbates inflammation (47). Clinical manifestations of PCOS may result in the development of the local and systemic OS, which in turn induces a proinflammatory state that promotes IR and hyperandrogenemia in PCOS (48). Levels of some circulating antioxidant biomarkers are decrease in patients with PCOS, including those of Paraoxonase-1(PON1) (49). However, the activity of other antioxidant biomarkers, such as catalase (CAT) and superoxide dismutase (SOD), is significantly elevated in some patients (50).
Circadian rhythm disorders may lead to oxidative stress and systemic inflammation (10).
OS is a state of imbalance between oxidation and anti-oxidation in the body. In patients with PCOS, IR is closely related to inflammation and increased OS (23, 51). The circadian clock is the major regulator of ROS homeostasis. Animals with mutated circadian protein showed elevated ROS levels and oxidative damage (52, 53). Tomas et al. demonstrated that continuous light attenuates or disrupts the CRs of the antioxidants SOD and CAT in Syrian hamsters (54).
The Kelch-like ECH-associated protein 1 (Keap1)/nuclear factor-erythroid 2-related factor 2(Nrf2) pathway proved to be a critical pathway in OS regulation (55). Studies have shown that Nrf 2 is a transcriptional regulatory target of BMAL1. BMAL1 not only activates the Nrf2-mediated antioxidant pathway, but also affects the expression of Nrf2-mediated antioxidant genes such as heme oxygenase 1(HO-1) and NAD(P)H dehydrogenase (quinone) 1(NQO-1) (56). In addition, BMAL1 regulates homeostasis by directly regulating ROS levels. For example, specific KO of BMAL1 in mouse pancreatic β cells restrained glucose-activated insulin secretion (GSIS) and elevate ROS sensitivity, resulting ROS overproduction (57). Background of BMAL1 in the peripheral tissue of PCOS, we surmise that the Nrf2 pathway can regulate BMAL1 to improve OS, which provides a new perspective for the treatment of PCOS.
The circadian clock has been suggested to play a key role in the rhythmic regulation of inflammatory responses (58). Clock genes regulate the expression of various inflammatory cytokines. Cry impacts IL-1β, IL-6 and tumor necrosis factorα (TNFα) (59), RORα affects IL-1β and IL-6 (59), Per2 regulates IFNγ and IL-1β (60); Per1 is related to monocyte chemotactic protein-1(MCP-1) (61); Rev-erbαmodulates IL-6 (62) and TH17 cells (63). Secretion of TNFα and IL-6 shows circadian oscillations. KO of Rev-erbα leads to impaired circadian regulation of inflammatory responses. Furthermore, deletion of BMAL1 in macrophages down-regulates Nrf2 induction, leading to reduced antioxidant responses and increased IL-1β production (57, 64). As reported that the shift-workers, who often suffer from circadian rhythm disorders, may resulting PCOS, and the level of inflammatory markers present increased (65).We hypothesized that PCOS could be improved by modulating clock genes associated with OS and inflammation; however, more studies are needed to confirm this hypothesis.
Studies have shown that light stimulates LH secretion (66). The possible mechanisms include inhibition of melatonin (MT) secretion, projection of SCN to the hypothalamic-pituitary-ovarian (HPO) axis, and abnormal expression of circadian genes (67). Prolonged exposure to light induces changes in the ovarian morphology and hyperandrogenemia in rodents, suggesting that CR changes may trigger PCOS (68, 69). In addition, studies have shown that continuous exposure of light in humans and animals to light also leads to increased levels of follicle-stimulating hormone (FSH) and estradiol (E2) (70, 71).However, another study showed that persistent darkness leads to increased serum LH/FSH ratio and testosterone levels in rats (29).
Melatonin levels are regulated by the photoperiod, which increases its production and secretion at night, in response to darkness, inhibits its secretion in response to light (72). Zhang and colleagues demonstrated that continuous exposure to light significantly reduces the level of MT in mice, which in turn reduces the release of LH, oestrogen, androgen, and progesterone by inhibiting the hypothalamic-pituitary-ovarian(HPO) axis, suggesting that a reduction of MT could be the underlying mechanism of hyperandrogenemia (66). MT can also increase FSH secretion by stimulating the pituitary gland (73). Tagliaferri et al. found a significant increase in FSH levels, significant recovery in menstrual cyclicity, and improvement androgen balance in women with PCOS, following a 6-month treatment with oral melatonin (74). Another research reported that administered 12 weeks of melatonin supplementation, hirsutism improved significantly and testosterone levels decreased significantly (75). Moreover, Li et al. verified that melatonin attenuates persistent darkness-induced hyperinsulinemia and hyperandrogenism in PCOS rats via BMAL1, Per1 and Per2 (29).
Take together, targeting melatonin expression by altering circadian regulation is a potential therapeutic strategy for PCOS; however, the relevant evidence is still insufficient, and more in-depth studies are needed to verify this hypothesis and explore the precise underlying mechanisms.
Extracellular vesicles are important intercellular messengers for proteins and ncRNA and can be divided into exosomes, microvesicles and apoptotic bodies according to their size, content, biogenesis and specific surface markers. Exosomes are lipid bilayer vesiclessecreted from the extracellular space in response to specific stimuli under physiological or pathological conditions. Exosomes transfer specific molecular cargoes, particularly miRNAs (76, 77). MiRNAs are small 20–23 nucleotide ncRNA molecules that negatively regulate gene expression via mRNA cleavage or translation. Abnormal expression of miRNAs is implicated in the pathogenesis of many conditions, such as obesity and diabetes, as well as in sex hormone synthesis. In recent years, the role of miRNAs in PCOS pathology has garnered considerable attention (78, 79) (Table 1).
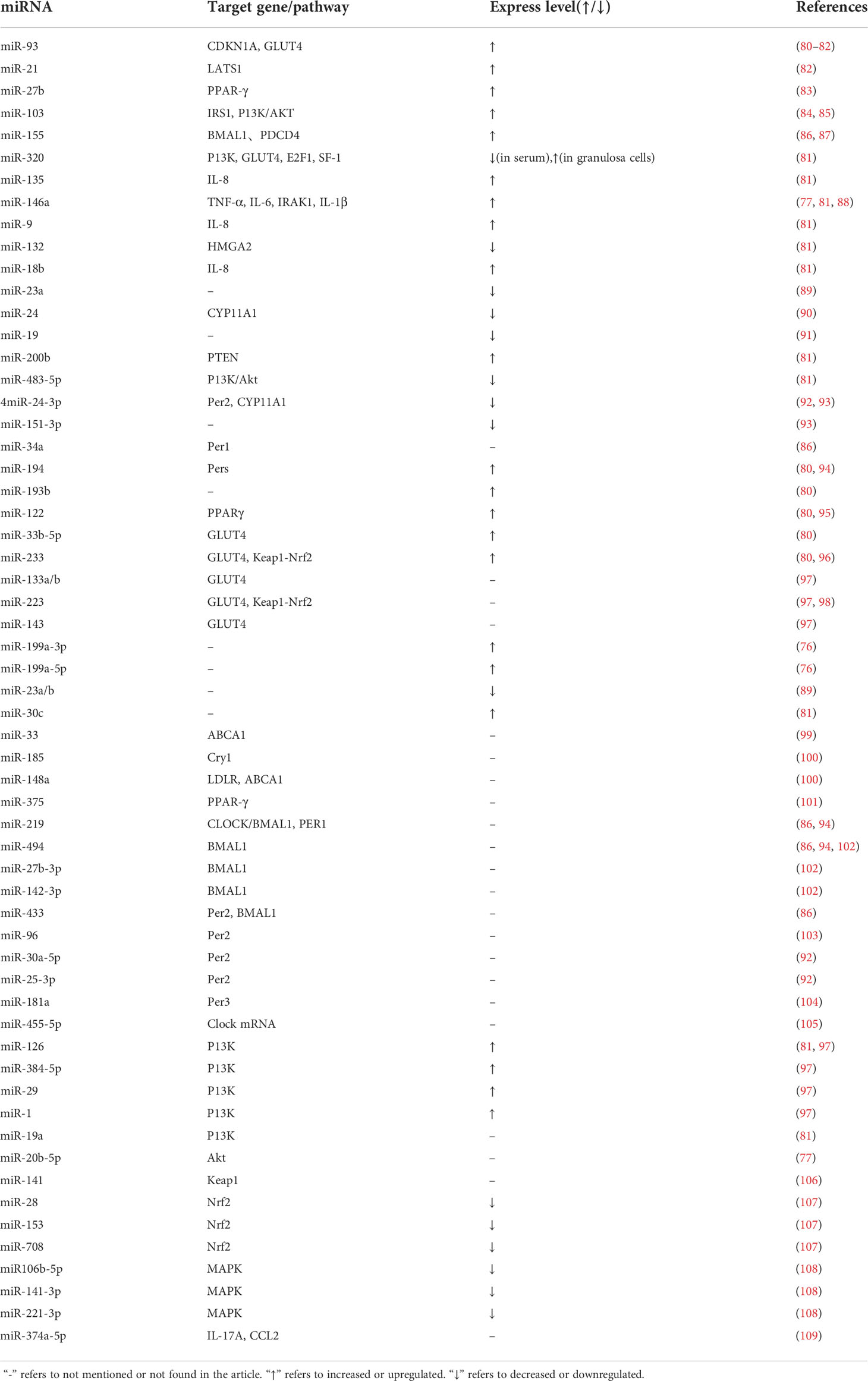
Table 1 Expression and regulation of miRNAs in the target genes/pathways related to PCOS and the circadian rhythm.
Exosomes can be produced and released by different subtypes of endosomes through different mechanisms and function as cell types and physiological states. Exosome secretion can be increased or decreased under pathological conditions. OS promotes exosome release during endoplasmic reticulum stress (110). Exosome secretion is also mediated by various physical, chemical, and biological stimuli such as ultrasound (111), ionizing radiation (112), DNA damage (113), enzymatic influence (114), and inflammatory stimulation (115). Therefore, interfering with exosome release and damaging exosome-mediated intercellular communication are potential therapeutic strategies.
In recent decades, more and more researchers have paid attention to the relationship between miRNA and abnormal androgen secretion in PCOS. Androgen affects follicular growth and health, and functions mainly through the androgen receptor (AR). AR levels are increased in patients with PCOS (79). Previous studies have confirmed that miR-21 and miR-93 are androgen response factors, which may be associated with follicular dysfunction in the pathogenesis of PCOS. Additionally, in patients with PCOS, miR-29a, miR27b, miR-103, miR-518, miR-320, and miR-155 are positively correlated with serum or free testosterone levels. In contrast, miR151 is negatively correlated with serum testosterone levels; the down-regulation of miR-23a in PCOS serum is also negatively correlated with testosterone levels. MiR-9, miR-18b, miR-132, miR-135 and miR-146a inhibit testosterone secretion. MiR-24 and miR-19 potentially reduce testosterone release in the medium of cultured human ovarian cells (79, 80, 91). The downstream target of AR is miR-200b, which is required for HPO axis-mediated ovulation. MiR-29c acts through the downstream pathways that affect androgen receptor localization. These results indicate that miR-200b and miR-29c are also closely related to hyperandrogenism in PCOS (116). Understanding the mechanisms by which miRNAs regulate androgen production can greatly contribute to improving the clinical symptoms and prognosis of PCOS. However, in-depth studies on how miRNAs cause androgen metabolism disorders in PCOS patients are currently lacking.
IR is a common feature of PCOS. According to the statistics, about 50-70% of PCOS patients have IR and are at high risk for developing metabolic syndrome, type 2 diabetes mellitus, and inflammatory and cardiovascular diseases (51, 117). Studies have revealed that the levels of miR-194, miR-193b, and miR-122 are elevated in patients with PCOS, especially in those with impaired glucose metabolism. Overexpression of miR33b-5p, miR-93, and miR-233 plays an important role in IR by inhibiting glucose transporter 4 (GLUT4) expressions in patients with PCOS. In addition, overexpression of miR-223 increased the protein expression of GLUT4; however, it should be noted that miR-223 expression was increased only in women with PCOS who had IR. MiR-133a and miR-133b participate in the expression of the GLUT4 protein through the Kruppel-like transcription factor 15 (KLF15) and reduce insulin-stimulated glucose utilization to control IR, whereas, miR-143 is involved in GLUT4 expression (80, 97), GULT4 may be a target of miRNAs for IR treatment in PCOS patients. Moreover, the level of miR-146a was negatively correlated with IR and inflammatory factors (TNF-α and IL-6). However, Jiang et al. found no correlation between the expression of miR-146a in the exosomes of patients with PCOS and their glucose metabolism indicators (77, 118). Decreased expression of miR-24 in PCOS is associated with IR and abnormal PCOS-related hormones (90). In general, miRNAs play an important role in regulating glucose metabolism and IR pathogenesis in women with PCOS, and may be a potential target for treatment of IR-related PCOS symptoms.
Obesity and dyslipidaemia are also common manifestations of PCOS. About 50 % of women with PCOS suffer from overweight or obesity (119). The expression levels of miR-21, miR-27b, miR-103, and miR-155 in women with PCOS and obesity are remarkably higher than in women with PCOS and normal weight (83). Xiong et al. found serum miR-23a/b expression was decreased in patients with PCOS, and that an increased body mass index (BMI) elevates serum miR-23b level, while miR-23a was not affected by BMI (89). MiR-30c regulates cholesterol biosynthesis and very low-density lipoprotein cholesterol (VLDL-C) secretion by reducing apolipoprotein production and becoming a target for treating hyperlipidaemia (120). Studies have shown that miR-122-5p and miR-223-3p are directly correlated with BMI, in contrast, miR-151a-3p/5p and miR-199a-3p/5p are negatively correlated with the BMI and waist-to-hip ratio (WHR) (121). MiR-122 regulates plasma low-density lipoprotein cholesterol (LDL-C) levels by inhibiting the secretion of VLDL (122). MiR-33 and lipid metabolism have been widely studied. The expression of miR-33 regulates ATP-binding cassette transporter A1 (ABCA1) and ATP-binding cassette subfamily G member 1 (ABCG1), and inhibition of miR-33 increases the expression of these proteins in the liver and elevates high-density lipoprotein levels. In animal models (mouse and rabbit), inhibition of miR-33 has been shown to modify the biosynthesis of VLDL-C and triglycerides. In addition, miR-33 affects cholesterol outflow and bile acid synthesis and excretion (99, 123–126). Furthermore, some miRNAs can regulate LDL-C metabolism. By inhibiting the expression of miR-148a, miR-128-1, and miR-185, circulating LDL-C levels were reduced (100). MiR-143 is up-regulated in the liver of obese mice, which inhibits insulin-stimulated Akt activity and glucose imbalance (127). In contrast, miR-375 expression promotes adipocyte differentiation by mediating PPARγ and extracellular signal-regulated kinases (ERK) activity (128). These findings suggest that miRNAs are closely associated with obesity and dyslipidaemia, and shed light on their potential as therapeutic targets for PCOS metabolism.
In summary, the relationship between miRNAs and PCOS progression is not fully understood, and the specific roles of miRNAs in PCOS development are unclear because one miRNA may have multiple mRNA targets and one mRNA may be controlled by multiple miRNAs. Therefore, further functional studies of miRNA-PCOS are needed.
Increasing evidence has shown that miRNAs play important roles in maintaining the homeostasis of the circadian system. Cheng et al. found that miR-132 and miR-219 are involved in regulating CRs in mammals. Brain-specific miR-219 displays rhythmic oscillations in the SCN and targets the CLOCK : BMAL1 complex involved in cycle determination, whereas the light-activated expression of miR-132 needs CREB and mitogen-activated protein kinase (MAPK)/ERK. In addition, miR-494, miR-27b-3p, miR-155 and miR-142-3p are involved in the post-transcriptional regulation of clock gene BMAL1 in the circulation. Rhythmic expression of miR-142-3p was observed in mouse SCN cells, which may be driven by a typical E-box. Changes in the expression of miR-192 and miR-194 not only affect the rhythm oscillation of BMAL1 mRNA, but also inhibit the expression of Per gene.Cry1 translation is regulated by miR-185 (86, 94, 102, 129). Another study showed that miR-959-964 exhibits significant circadian oscillation (95).
Studies have shown that miR-96, miR-24-3p and miR-30a-5p directly target the core circadian clock gene Per2. MiR-25-3p was inversely expressed with the Per2 oscillation cycle. KO of miR-183/96/182 clusters in mice led to diurnal behavior changes (92, 103) Moreover, miR-181a directly targets Per3 (104), and miR-455-5p regulates CRs by affecting the degradation of Clock mRNA (105). Yang et al. showed that circadian-regulated miRNAs in Drosophila, such as miR-263a and-263b, displayed robust, and moderate daily alteration (130). Xiao Chen et al. demonstrated that light-controlled miR-276a regulates CRs that influence Drosophila behavior by inhibiting the timeless(TIM) clock gene (131). In addition, the expression of miRNAs differs in different species. In humans, miR-107 regulates cell circadian oscillations by binding to clock genes. In mice, miR-17-5p is an important factor involved in circadian rhythm control (102) (Figure 3). Hence, the circadian rhythm can be changed by targeting miRNAs, to explore its mechanism and potential for treatment of PCOS.
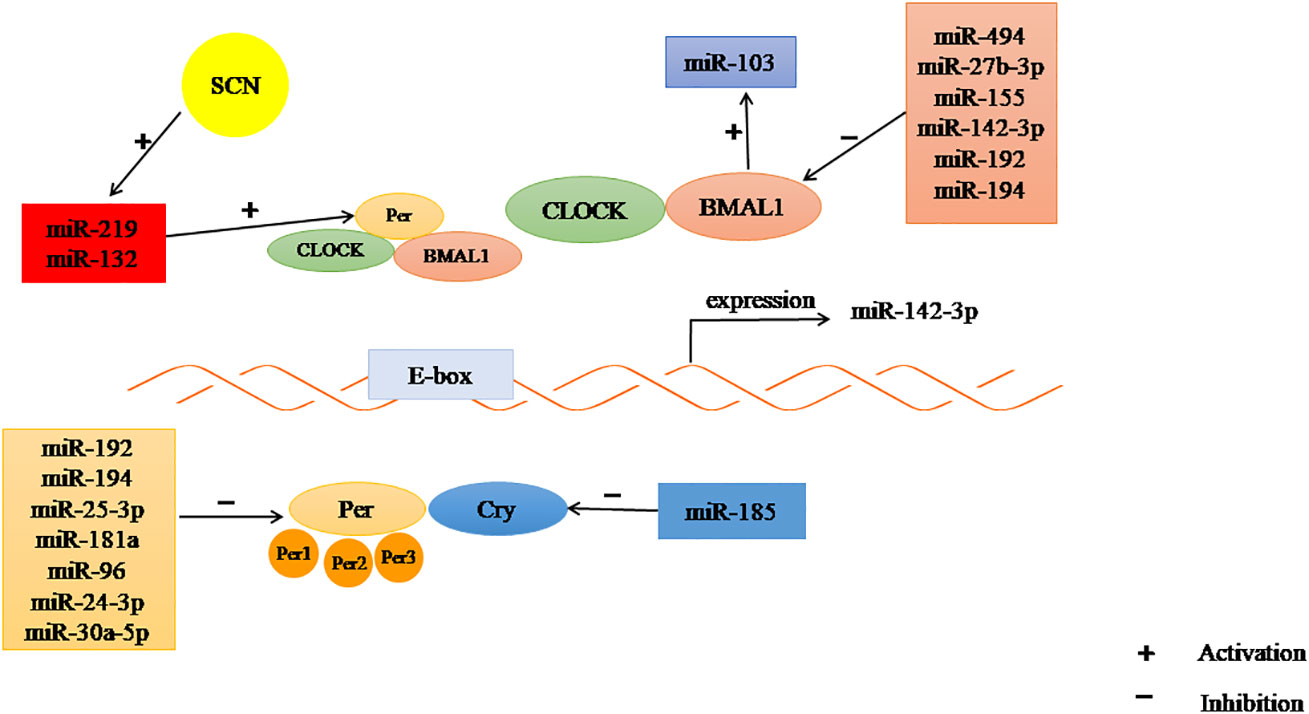
Figure 3 The regulation of CR by miRNAs. Some miRNAs may inhibit the expression of clock gene, some may promote.
Although miRNA-mediated post-transcriptional regulation regulates circadian oscillations, the circadian system in turn drives many miRNA expression-related CRs. Circadian regulation of RNA processing involves many steps, including mRNA capping, alternative splicing and tail length-controlled RNA stability changes, thereby promoting circadian gene expression. RNA degradation may also follow circadian patterns through miRNA rhythmic participation (102). Chen et al. found that BMAL1 can stimulate miR-103 expression and regulate the CR expression of the rat cerebral artery CaV1.2 channel (large or long-term voltage-dependent Ca2+ channel) (84).
Wang et al. verified that transcripts including pri-mir-122 and pri-mir-24 exhibit strong circadian expression and are regulated by circadian rhythms (132). In addition, researchers showed that after two days of dark adaptation, the expression of pre-miR-219-1 and pre-miR-132 did not change significantly in the SCN tissues of crypotochrome (mCry)1/mCry2 double mutant mice, indicating that the rhythmic expression of these two miRNAs are depended on the molecular clock (133).
Overall, miRNAs and CRs can interact with each other, but its influence on the regulation of the biological clock is complex. This complexity is largely due to various post-transcriptional, and post-translational mechanisms, and poses a challenge in exploring the mechanism of action and therapeutic potential of miRNAs in PCOS induced by circadian changes and, should be further evaluated in in-depth studies.
Phosphatidylinositol 3-kinase (PI3K) plays an important role in PCOS, mainly by affecting granulosa cells (GC) proliferation and apoptosis (134). Xie et al. found that MT regulates autophagy and apoptosis through a PI3K-Akt pathway in PCOS rats, thus improving ovarian dysfunction (135). Another study showed that the P85 subunit is a potential target of miR-320. MiR-320a may be an important factor regulating IR by reducing insulin sensitivity through the PI3K signaling pathway (136, 137). Many miRNAs, such as miR-126, miR-384-5p, miR-29, miR1, and miR-19a, are involved in regulating PI3K. In addition, miR-483-5p regulates the proliferation of PCOS granulosa cells by activating the PI3K/Akt pathway (81, 97). MiR-20b-5p regulates insulin-stimulated glucose metabolism through the Akt signaling pathway. In addition, miR-20b-5p may alter the Akt signal routing in skeletal muscle cells (138). miR-103 and miR-497 regulate the PI3K/Akt pathway by targeting insulin receptor substrate (IRS) 1 (85).
The Nrf2 signaling pathway acts as a center for regulating the antioxidant defense system in response to OS. As reported earlier, the activation of the Nrf2 signaling pathway attenuates OS and apoptosis in PCOS rats. MiR-223 may be a target of the Keap1-Nrf2 system in OS regulation (96). MiR-223 stimulates the Nrf2 signaling pathway by inhibiting Keap1 and inducing antioxidant defense system (98). MiR-141 may down-regulate the expression of its target gene Keap1, stimulate an increase of Nrf2, reduce ROS production and improve OS (106). Omar and colleagues confirmed that H2O2 treatment of bovine GCs increased the expression of Nrf2 and decreased the expression of miR-28, miR-153 and miR-708, while regulating the expression of miR-153, miR-28 and miR-708 alone led to a decrease in the expression of Nrf2 and its downstream antioxidant genes. These results indicate that miRNAs are involved in regulating Nrf2-mediated OS response (107). Additionally, ROS can induce the activation of the MAPK pathway, which is blocked by antioxidants. H2O2-induced increases in ROS may inhibit the expression of specific miRNAs (miR106b-5p, miR-141-3p, miR-221-3p), which are critical for the activation of the MAPK pathway (108).
NF-κB is a transcription factor involved in regulating the expression of pro-inflammatory mediators. Yu et al. found that miR-21 activates toll-like receptor 8 (TLR) resulting the level of TNFα and IL-12 increased in granulosa cells of PCOS (139). Furthermore, increased pro-inflammatory gene expression and induced NF-κB activation was observed in miR-146a-/- mice (140). Another study demonstrated that CCL2 as the main target of miR-374a-5p, and also demonstrated that the transcription factor NF-κB can activate CCL2, suggesting that miR-374a-5p may play a role in regulating inflammatory responses (109). Li et al. described that miR-1224-5p exerts anti-inflammatory effects and alleviates PCOS by inhibiting the activation of the NF-κB signaling pathway (141).
Taken together, exosomes, especially those carrying miRNAs, are widely involved in the regulation of PCOS-related pathways such as PI3K/Akt, NF-κb, and AMPK/Nrf2 signaling pathways (Figure 4). This provides a new target for the treatment of PCOS. Unfortunately, there is not much research in this area, and the specific mechanism is still unclear, so further research is needed.
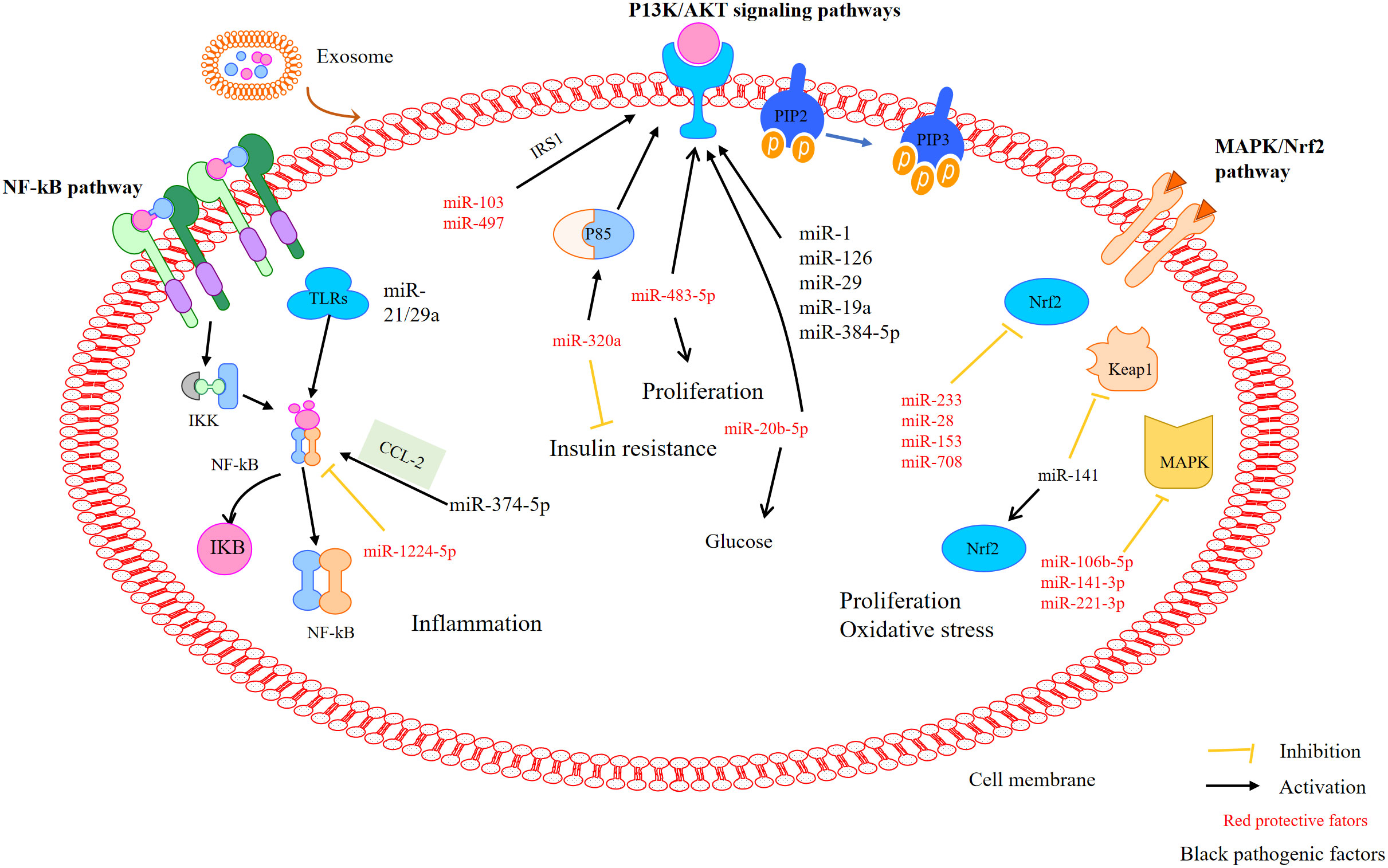
Figure 4 MiRNAs mediate pathways that may be involved in PCOS. MiRNAs mediate the activation of the P13K/Akt, NF-κB, and MAPK/Nrf2 signalling pathways in polycystic ovary syndrome.
In recent years, research on exosomes has rapidly developed, covering almost all fields of physiology and pathophysiology. Exosomes are potential biomarkers and therapeutic agents for diseases. PCOS is a complex syndrome, involving diverse systems, primarily the reproductive and, endocrine systems. The paucity of a reliable diagnostic criteria and unclear pathogenesis pose as challenges in the development of therapeutics for PCOS. Abnormal miRNAs expression may be involved in the pathophysiology of PCOS, including reproductive functions, glucose metabolism and insulin sensitivity. However, existing research are primarily small-scale studies and the heterogeneity of their findings warrants further studies to elucidate their precise role in PCOS. Therefore, regulation of the circadian rhythm using miRNAs may be a novel therapeutic strategy for PCOS. In addition, an in-depth understanding of the interaction between genetics and the environment which lead to differential miRNAs expression may help elucidate the pathogenesis of polycystic ovarian diseases (79), which will provide new ideas for the prevention, diagnosis and treatment of PCOS.
The envisaged role of all authors in the writing of the work is as follows: SL and QS: funding acquisition, project administration, supervision, validation, writing-review, and editing. WC: writing-original draft, writing-review, and editing. QH: supervision, validation, writing-review, and editing. ZW and XZ: writing-review and editing. All authors contributed to the article and approved the submitted version.
This work was supported by the Science and Technology Bureau of Quanzhou (Grant number 2020CT003) and the Science and Technology project of the Fujian Provincial health commission (Grant number 2020CXB027).
We are thankful to The Second Affiliated Hospital of Fujian Medical University for providing infrastructure facilities.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
ABCA1, ATP-binding cascade transporter A1; AKT, Protein kinase B; AMPK, AMP-activated protein kinase; AR, Androgen receptor ; BAML1, Brain and muscle arnt-like protein 1; BMI, Body mass index ; CAT, Catalase; CCL2, C-C motif chemokine ligand 2 ; CLOCK, Circadian locomotor output cycle kaput ; CR, Circadian rhythm; CREB, CAMP response element binding protein 1 ; CRP, C-reactive protein; CRTC2, CREB-regulated transcription coactivator 2; Cry, Cryptochrome; E2, Estradiol; ERK, Extracellular signal- regulated kinase; EVs, Extracellular vesicles; FSH, Follicle-stimulating hormone ; GABA, Gamma-aminobutyric acid; GCs, Granulosa cells ; GLUT4, Glucose transporter 4; GnRH, Gonadotropin-releasing hormone; GSIS, Glucose-activated insulin secretion; HA, Hyperandrogenism; HPG, Hypothalamic-pituitary-gonadal ; HPO, Hypothalamic-pituitary-ovarian ; HO-1, Heme oxygenase-1; IL1, Interleukin 1; IL6, Interleukin 6; IR, Insulin resistance; IRAK1, IL-1 receptor-related kinase 1; IRS, Insulin receptor substrate; Keap1, Kelch-like ECH-associated protein 1 ; KO, Knockout; LDL-C, Low-density lipoprotein cholesterol-C; LH, Luteinising hormone; Lhcgr, Luteinizing hormone receptor; MAPK, Mitogen- activated protein kinase; MCP-1, Monocyte chemotactic protein-1; MiRNA, MicroRNA; MRNA, Messenger RNA; MT, Melatonin; MV, Microbubbles; NAD, Nicotinamide adenine dinucleotide; NAMPT, Nicotinamide phosphoribosyl transferase; NcRNA, Non-coding RNA; NQO-1, phosphoamide adenine dinucleotide quinone oxidoreductase-1; Nrf2, Nuclear factor-erythrocyte 2-related factor 2; OS, Oxidative stress ; PCOS, Polycystic ovary syndrome; Per, Period; PI3K, Phosphatidylinositol 3 - kinase ; PPARγ, Peroxisome proliferator-activated receptor γ; ROR, Retinoic acid-related orphan receptor; RORE, ROR reaction element; ROS, Reactive oxygen species; SCN, suprachiasmatic nucleus; SIRT1, Protein deacetylase sirtuin 1; SOD, Antioxidant enzyme; TNFα, Tumor necrosis factorα; T2DM, Type 2 diabetes mellitus; VLDL-C, Very low-density lipoprotein cholesterol-C; WAT, White adipose tissue ; WHR, Waist-hip ratio

1. Karjula S, Arffman RK, Morin-Papunen L, Franks S, Järvelin MR, Tapanainen JS, et al. A population-based follow-up study shows high psychosis risk in women with PCOS. Arch Womens Ment Health (2021) 25:301311. doi: 10.1007/s00737-021-01195-4
2. Velez LM, Seldin M, Motta AB. Inflammation and reproductive function in women with polycystic ovary syndrome†. Biol Reprod (2021) 104(6):1205–17. doi: 10.1093/biolre/ioab050
3. Teede H, Deeks A, Moran L. Polycystic ovary syndrome: a complex condition with psychological, reproductive and metabolic manifestations that impacts on health across the lifespan. BMC Med (2010) 8:41. doi: 10.1186/1741-7015-8-41
4. Sadeghi HM, Adeli I, Calina D, Docea AO, Mousavi T, Daniali M, et al. Polycystic ovary syndrome: A comprehensive review of pathogenesis, management, and drug repurposing. Int J Mol Sci (2022) 23(2):583. doi: 10.3390/ijms23020583
5. Rodriguez-Paris D, Remlinger-Molenda A, Kurzawa R, Głowińska A, Spaczyński R, Rybakowski F, et al. Psychiatric disorders in women with polycystic ovary syndrome. Psychiatr Pol (2019) 53(4):955–66. doi: 10.12740/PP/OnlineFirst/93105
6. Ozegowska K, Korman M, Szmyt A, Pawelczyk L. Heterogeneity of endocrinologic and metabolic parameters in reproductive age polycystic ovary syndrome (PCOS) women concerning the severity of hyperandrogenemia-a new insight on syndrome pathogenesis. Int J Environ Res Public Health (2020) 17(24):9291. doi: 10.3390/ijerph17249291
7. Richards JS, Ren YA, Candelaria N, Adams JE, Rajkovic A. Ovarian follicular theca cell recruitment, differentiation, and impact on fertility: 2017 update. Endocr Rev (2018) 39(1):1–20. doi: 10.1210/er.2017-00164
8. Wang HY, Jin XN, Jiang YX, Zhu ZH, Xu Y, Zhu QL, et al. Clinical values of combined diffused optical tomography and PET-CT in the diagnosis of breast cancer. Zhongguo Yi Xue Ke Xue Yuan Xue Bao (2017) 39(5):682–7. doi: 10.3881/j.issn.1000-503X.2017.05.014
9. Dinsdale NL, Crespi BJ. Endometriosis and polycystic ovary syndrome are diametric disorders. Evol Appl (2021) 14(7):1693–715. doi: 10.1111/eva.13244
10. Willis SK, Hatch EE, Wise LA. Sleep and female reproduction. Curr Opin Obstet Gynecol (2019) 31(4):222–7. doi: 10.1097/GCO.0000000000000554
11. Wang F, Xie N, Wu Y, Zhang Q, Zhu Y, Dai M, et al. Association between circadian rhythm disruption and polycystic ovary syndrome. Fertil Steril (2021) 115(3):771–81. doi: 10.1016/j.fertnstert.2020.08.1425
12. Johnson BS, Krishna MB, Padmanabhan RA, Pillai SM, Jayakrishnan K, Laloraya M. Derailed peripheral circadian genes in polycystic ovary syndrome patients alters peripheral conversion of androgens synthesis. Hum Reprod (2022) 37(8):1835–55. doi: 10.1093/humrep/deac139
13. Sun X, Ma X, Yang X, Zhang X. Exosomes and female infertility. Curr Drug Metab (2019) 20(10):773–80. doi: 10.2174/1389200220666191015155910
14. Khalyfa A, Gaddameedhi S, Crooks E, Zhang C, Li Y, Qiao Z, et al. Circulating exosomal miRNAs signal circadian misalignment to peripheral metabolic tissues. Int J Mol Sci (2020) 21(17):6396. doi: 10.3390/ijms21176396
15. Maury E, Navez B, Brichard SM. Circadian clock dysfunction in human omental fat links obesity to metabolic inflammation. Nat Commun (2021) 12(1):2388. doi: 10.1038/s41467-021-22571-9
16. Greco CM, Sassone-Corsi P. Circadian blueprint of metabolic pathways in the brain. Nat Rev Neurosci (2019) 20(2):71–82. doi: 10.1038/s41583-018-0096-y
17. Duszka K, Wahli W. Peroxisome proliferator-activated receptors as molecular links between caloric restriction and circadian rhythm. Nutrients (2020) 12(11):3476. doi: 10.3390/nu12113476
18. Krishnaiah SY, Wu G, Altman BJ, Growe J, Rhoades SD, Coldren F, et al. Clock regulation of metabolites reveals coupling between transcription and metabolism. Cell Metab (2017) 25(4):961–74.e4. doi: 10.1016/j.cmet.2017.03.019
19. Zhang D, Tong X, Arthurs B, Guha A, Rui L, Kamath A, et al. Liver clock protein BMAL1 promotes de novo lipogenesis through insulin-mTORC2-AKT signaling. J Biol Chem (2014) 289(37):25925–35. doi: 10.1074/jbc.M114.567628
20. Zhou B, Zhang Y, Zhang F, Xia Y, Liu J, Huang R, et al. CLOCK/BMAL1 regulates circadian change of mouse hepatic insulin sensitivity by SIRT1. Hepatology (2014) 59(6):2196–206. doi: 10.1002/hep.26992
21. Sun X, Dang F, Zhang D, Yuan Y, Zhang C, Wu Y, et al. Glucagon-CREB/CRTC2 signaling cascade regulates hepatic BMAL1 protein. J Biol Chem (2015) 290(4):2189–97. doi: 10.1074/jbc.M114.612358
22. de Assis LVM, Oster H. The circadian clock and metabolic homeostasis: entangled networks. Cell Mol Life Sci (2021) 78(10):4563–87. doi: 10.1007/s00018-021-03800-2
23. Tao X, Chen L, Cai L, Ge S, Deng X. Regulatory effects of the AMPKα-SIRT1 molecular pathway on insulin resistance in PCOS mice: An in vitro and in vivo study. Biochem Biophys Res Commun (2017) 494(3-4):615–20. doi: 10.1016/j.bbrc.2017.09.154
24. Asher G, Gatfield D, Stratmann M, Reinke H, Dibner C, Kreppel F, et al. SIRT1 regulates circadian clock gene expression through PER2 deacetylation. Cell (2008) 134(2):317–28. doi: 10.1016/j.cell.2008.06.050
25. Stromsdorfer KL, Yamaguchi S, Yoon MJ, Moseley AC, Franczyk MP, Kelly SC, et al. NAMPT-mediated NAD(+) biosynthesis in adipocytes regulates adipose tissue function and multi-organ insulin sensitivity in mice. Cell Rep (2016) 16(7):1851–60. doi: 10.1016/j.celrep.2016.07.027
26. Ramsey KM, Yoshino J, Brace CS, Abrassart D, Kobayashi Y, Marcheva B, et al. Circadian clock feedback cycle through NAMPT-mediated NAD+ biosynthesis. Science (2009) 324(5927):651–4. doi: 10.1126/science.1171641
27. Zhai J, Li S, Hu M, Di F, Liu J, Du Y. Decreased brain and muscle ARNT-like protein 1 expression mediated the contribution of hyperandrogenism to insulin resistance in polycystic ovary syndrome. Reprod Biol Endocrinol (2020) 18(1):32. doi: 10.1186/s12958-020-00592-1
28. Qiu Z, Ming H, Zhang Y, Yu Y, Lei S, Xia ZY. The protective role of Bmal1-regulated autophagy mediated by HDAC3/SIRT1 pathway in myocardial Ischemia/Reperfusion injury of diabetic rats. Cardiovasc Drugs Ther (2022) 36(2):229–43. doi: 10.1007/s10557-021-07159-1
29. Li S, Zhai J, Chu W, Geng X, Chen ZJ, Du Y. Altered circadian clock as a novel therapeutic target for constant darkness-induced insulin resistance and hyperandrogenism of polycystic ovary syndrome. Transl Res (2020) 219:13–29. doi: 10.1016/j.trsl.2020.02.003
30. Shao S, Zhao H, Lu Z, Lei X, Zhang Y. Circadian rhythms within the female HPG axis: From physiology to etiology. Endocrinology (2021) 162(8):bqab117. doi: 10.1210/endocr/bqab117
31. Bahougne T, Kretz M, Angelopoulou E, Jeandidier N, Simonneaux V. Impact of circadian disruption on female mice reproductive function. Endocrinology (2020) 161(4):bqaa028. doi: 10.1210/endocr/bqaa028
32. Yaw AM, McLane-Svoboda AK, Hoffmann HM. Shiftwork and light at night negatively impact molecular and endocrine timekeeping in the female reproductive axis in humans and rodents. Int J Mol Sci (2020) 22(1):324. doi: 10.3390/ijms22010324
33. Michels KA, Mendola P, Schliep KC, Yeung EH, Ye A, Dunietz GL, et al. The influences of sleep duration, chronotype, and nightwork on the ovarian cycle. Chronobiol Int (2020) 37(2):260–71. doi: 10.1080/07420528.2019.1694938
34. Moran LJ, March WA, Whitrow MJ, Giles LC, Davies MJ, Moore VM. Sleep disturbances in a community-based sample of women with polycystic ovary syndrome. Hum Reprod (2015) 30(2):466–72. doi: 10.1093/humrep/deu318
35. Sen A, Sellix MT. The circadian timing system and environmental circadian disruption: From follicles to fertility. Endocrinology (2016) 157(9):3366–73. doi: 10.1210/en.2016-1450
36. Karman BN, Tischkau SA. Circadian clock gene expression in the ovary: Effects of luteinizing hormone. Biol Reprod (2006) 75(4):624–32. doi: 10.1095/biolreprod.106.050732
37. Das M, Mohanty SR, Minocha T, Mishra NK, Yadav SK, Haldar C. Circadian desynchronization in pregnancy of golden hamster following long time light exposure: Involvement of Akt/FoxO1 pathway. J Photochem Photobiol B (2022) 234:112508. doi: 10.1016/j.jphotobiol.2022.112508
38. Jiang Z, Zou K, Liu X, Gu H, Meng Y, Lin J, et al. Aging attenuates the ovarian circadian rhythm. J Assist Reprod Genet (2021) 38(1):33–40. doi: 10.1007/s10815-020-01943-y
39. Zheng Y, Liu C, Li Y, Jiang H, Yang P, Tang J, et al. Loss-of-function mutations with circadian rhythm regulator Per1/Per2 lead to premature ovarian insufficiency†. Biol Reprod (2019) 100(4):1066–72. doi: 10.1093/biolre/ioy245
40. Xu J, Li Y, Wang Y, Xu Y, Zhou C. Loss of Bmal1 decreases oocyte fertilization, early embryo development and implantation potential in female mice. Zygote (2016) 24(5):760–7. doi: 10.1017/S0967199416000083
41. Boden MJ, Varcoe TJ, Voultsios A, Kennaway DJ. Reproductive biology of female Bmal1 null mice. Reproduction (2010) 139(6):1077–90. doi: 10.1530/REP-09-0523
42. Chen H, Zhao L, Kumazawa M, Yamauchi N, Shigeyoshi Y, Hashimoto S, et al. Downregulation of core clock gene Bmal1 attenuates expression of progesterone and prostaglandin biosynthesis-related genes in rat luteinizing granulosa cells. Am J Physiol Cell Physiol (2013) 304(12):C1131–40. doi: 10.1152/ajpcell.00008.2013
43. Sciarra F, Franceschini E, Campolo F, Gianfrilli D, Pallotti F, Paoli D, et al. Disruption of circadian rhythms: A crucial factor in the etiology of infertility. Int J Mol Sci (2020) 21(11):3493. doi: 10.3390/ijms21113943
44. Takasu NN, Nakamura TJ, Tokuda IT, Todo T, Block GD, Nakamura W. Recovery from age-related infertility under environmental light-dark cycles adjusted to the intrinsic circadian period. Cell Rep (2015) 12(9):1407–13. doi: 10.1016/j.celrep.2015.07.049
45. Yan S, Ding J, Zhang Y, Wang J, Zhang S, Yin T, et al. C1QTNF6 participates in the pathogenesis of PCOS by affecting the inflammatory response of granulosa cells‡. Biol Reprod (2021) 105(2):427–38. doi: 10.1093/biolre/ioab094
46. Abraham Gnanadass S, Divakar Prabhu Y, Valsala Gopalakrishnan A. Association of metabolic and inflammatory markers with polycystic ovarian syndrome (PCOS): an update. Arch Gynecol Obstet (2021) 303(3):631–43. doi: 10.1007/s00404-020-05951-2
47. Zhao Y, Zhang C, Huang Y, Yu Y, Li R, Li M, et al. Up-regulated expression of WNT5a increases inflammation and oxidative stress via PI3K/AKT/NF-κB signaling in the granulosa cells of PCOS patients. J Clin Endocrinol Metab (2015) 100(1):201–11. doi: 10.1210/jc.2014-2419
48. González F, Considine RV, Abdelhadi OA, Acton AJ. Oxidative stress in response to saturated fat ingestion is linked to insulin resistance and hyperandrogenism in polycystic ovary syndrome. J Clin Endocrinol Metab (2019) 104(11):5360–71. doi: 10.1210/jc.2019-00987
49. Murri M, Luque-Ramírez M, Insenser M, Ojeda-Ojeda M, Escobar-Morreale HF. Circulating markers of oxidative stress and polycystic ovary syndrome (PCOS): a systematic review and meta-analysis. Hum Reprod Update (2013) 19(3):268–88. doi: 10.1093/humupd/dms059
50. Herman R, Jensterle M, Janež A, Goričar K, Dolžan V. Genetic variability in antioxidative and inflammatory pathways modifies the risk for PCOS and influences metabolic profile of the syndrome. Metabolites (2020) 10(11):439. doi: 10.3390/metabo10110439
51. Bannigida DM, Nayak BS, Vijayaraghavan R. Insulin resistance and oxidative marker in women with PCOS. Arch Physiol Biochem (2020) 126(2):183–6. doi: 10.1080/13813455.2018.1499120
52. Patel SA, Velingkaar NS, Kondratov RV. Transcriptional control of antioxidant defense by the circadian clock. Antioxid Redox Signal (2014) 20(18):2997–3006. doi: 10.1089/ars.2013.5671
53. Hardeland R, Coto-Montes A, Poeggeler B. Circadian rhythms, oxidative stress, and antioxidative defense mechanisms. Chronobiol Int (2003) 20(6):921–62. doi: 10.1081/CBI-120025245
54. Tomás-Zapico C, Coto-Montes A, Martínez-Fraga J, Rodríguez-Colunga MJ, Tolivia D. Effects of continuous light exposure on antioxidant enzymes, porphyric enzymes and cellular damage in the harderian gland of the Syrian hamster. J Pineal Res (2003) 34(1):60–8. doi: 10.1034/j.1600-079X.2003.02951.x
55. Wang Y, Li N, Zeng Z, Tang L, Zhao S, Zhou F, et al. Humanin regulates oxidative stress in the ovaries of polycystic ovary syndrome patients via the Keap1/Nrf2 pathway. Mol Hum Reprod (2021) 27(2):gaaa081. doi: 10.1093/molehr/gaaa081
56. Chhunchha B, Kubo E, Singh DP. Clock protein Bmal1 and Nrf2 cooperatively control aging or oxidative response and redox homeostasis by regulating rhythmic expression of Prdx6. Cells (2020) 9(8):1861. doi: 10.3390/cells9081861
57. Liu F, Zhang X, Zhao B, Tan X, Wang L, Liu X. Role of food phytochemicals in the modulation of circadian clocks. J Agric Food Chem (2019) 67(32):8735–9. doi: 10.1021/acs.jafc.9b02263
58. Fagiani F, Di Marino D, Romagnoli A, Travelli C, Voltan D, Di Cesare Mannelli L, et al. Molecular regulations of circadian rhythm and implications for physiology and diseases. Signal Transduct Target Ther (2022) 7(1):41. doi: 10.1038/s41392-022-00899-y
59. Stapleton CM, Jaradat M, Dixon D, Kang HS, Kim SC, Liao G, et al. Enhanced susceptibility of staggerer (RORalphasg/sg) mice to lipopolysaccharide-induced lung inflammation. Am J Physiol Lung Cell Mol Physiol (2005) 289(1):L144–52. doi: 10.1152/ajplung.00348.2004
60. Liu J, Malkani G, Shi X, Meyer M, Cunningham-Runddles S, Ma X, et al. The circadian clock period 2 gene regulates gamma interferon production of NK cells in host response to lipopolysaccharide-induced endotoxic shock. Infect Immun (2006) 74(8):4750–6. doi: 10.1128/IAI.00287-06
61. Yamaoka M, Maeda N, Takayama Y, Sekimoto R, Tsushima Y, Matsuda K, et al. Adipose hypothermia in obesity and its association with period homolog 1, insulin sensitivity, and inflammation in fat. PloS One (2014) 9(11):e112813. doi: 10.1371/journal.pone.0112813
62. Gibbs JE, Blaikley J, Beesley S, Matthews L, Simpson KD, Boyce SH, et al. The nuclear receptor REV-ERBα mediates circadian regulation of innate immunity through selective regulation of inflammatory cytokines. Proc Natl Acad Sci USA (2012) 109(2):582–7. doi: 10.1073/pnas.1106750109
63. Sato S, Sakurai T, Ogasawara J, Takahashi M, Izawa T, Imaizumi K, et al. A circadian clock gene, rev-erbα, modulates the inflammatory function of macrophages through the negative regulation of Ccl2 expression. J Immunol (2014) 192(1):407–17. doi: 10.4049/jimmunol.1301982
64. Baxter M, Ray DW. Circadian rhythms in innate immunity and stress responses. Immunology (2020) 161(4):261–7. doi: 10.1111/imm.13166
65. Tavakoli A, Mirzababaei A, Sajadi F, Mirzaei K. Circulating inflammatory markers may mediate the relationship between low carbohydrate diet and circadian rhythm in overweight and obese women. BMC Womens Health (2021) 21(1):87. doi: 10.1186/s12905-021-01240-5
66. Zhang L, Liu Y, Li M, Zhu X, Shi Y. Effect of a high-calorie diet and constant light exposure on female reproduction, metabolism and immune inflammation: A comparative study of different mouse models. Am J Reprod Immunol (2021) 86(5):e13479. doi: 10.1111/aji.13479
67. Evans MC, Anderson GM. Integration of circadian and metabolic control of reproductive function. Endocrinology (2018) 159(11):3661–73. doi: 10.1210/en.2018-00691
68. Paixão L, Ramos RB, Lavarda A, Morsh DM, Spritzer PM. Animal models of hyperandrogenism and ovarian morphology changes as features of polycystic ovary syndrome: a systematic review. Reprod Biol Endocrinol (2017) 15(1):12. doi: 10.1186/s12958-017-0231-z
69. Chu W, Zhai J, Xu J, Li S, Li W, Chen ZJ, et al. Continuous light-induced PCOS-like changes in reproduction, metabolism, and gut microbiota in sprague-dawley rats. Front Microbiol (2019) 10:3145. doi: 10.3389/fmicb.2019.03145
70. Zhang S, Chen X, Zhang J, Li H. Differences in the reproductive hormone rhythm of tree sparrows (Passer montanus) from urban and rural sites in Beijing: the effect of anthropogenic light sources. Gen Comp Endocrinol (2014) 206:24–9. doi: 10.1016/j.ygcen.2014.05.020
71. Li Y, Cheng S, Li L, Zhao Y, Shen W, Sun X. Light-exposure at night impairs mouse ovary development via cell apoptosis and DNA damage. Biosci Rep (2019) 39(5):ESR20181464. doi: 10.1042/BSR20181464
72. Mojaverrostami S, Asghari N, Khamisabadi M, Heidari Khoei H. The role of melatonin in polycystic ovary syndrome: A review. Int J Reprod BioMed (2019) 17(12):865–82. doi: 10.18502/ijrm.v17i12.5789
73. Guo YM, Sun TC, Wang HP, Chen X. Research progress of melatonin (MT) in improving ovarian function: a review of the current status. Aging (Albany NY) (2021) 13(13):17930–47. doi: 10.18632/aging.203231
74. Tagliaferri V, Romualdi D, Scarinci E, Cicco S, Florio CD, Immediata V, et al. Melatonin treatment may be able to restore menstrual cyclicity in women with PCOS: A pilot study. Reprod Sci (2018) 25(2):269–75. doi: 10.1177/1933719117711262
75. Jamilian M, Foroozanfard F, Mirhosseini N, Kavossian E, Aghadavod E, Bahmani F, et al. Effects of melatonin supplementation on hormonal, inflammatory, genetic, and oxidative stress parameters in women with polycystic ovary syndrome. Front Endocrinol (Lausanne) (2019) 10:273. doi: 10.3389/fendo.2019.00273
76. Esfandyari S, Elkafas H, Chugh RM, Park HS, Navarro A, Al-Hendy A. Exosomes as biomarkers for female reproductive diseases diagnosis and therapy. Int J Mol Sci (2021) 22(4):2165. doi: 10.3390/ijms22042165
77. Jiang X, Li J, Zhang B, Hu J, Ma J, Cui L, et al. Differential expression profile of plasma exosomal microRNAs in women with polycystic ovary syndrome. Fertil Steril (2021) 115(3):782–92. doi: 10.1016/j.fertnstert.2020.08.019
78. Jiang L, Huang J, Chen Y, Yang Y, Li R, Li Y, et al. Identification of several circulating microRNAs from a genome-wide circulating microRNA expression profile as potential biomarkers for impaired glucose metabolism in polycystic ovarian syndrome. Endocrine (2016) 53(1):280–90. doi: 10.1007/s12020-016-0878-9
79. Chen Z, Ou H, Wu H, Wu P, Mo Z. Role of microRNA in the pathogenesis of polycystic ovary syndrome. DNA Cell Biol (2019) 38(8):754–62. doi: 10.1089/dna.2019.4622
80. Chen B, Xu P, Wang J, Zhang C. The role of MiRNA in polycystic ovary syndrome (PCOS). Gene (2019) 706:91–6. doi: 10.1016/j.gene.2019.04.082
81. Gebremedhn S, Ali A, Hossain M, Hoelker M, Salilew-Wondim D, Anthony RV, et al. MicroRNA-mediated gene regulatory mechanisms in mammalian female reproductive health. Int J Mol Sci (2021) 22(2):938. doi: 10.3390/ijms22020938
82. Tu J, Cheung AH, Chan CL, Chan WY. The role of microRNAs in ovarian granulosa cells in health and disease. Front Endocrinol (Lausanne) (2019) 10:174. doi: 10.3389/fendo.2019.00174
83. Murri M, Insenser M, Fernández-Durán E, San-Millán JL, Escobar-Morreale HF. Effects of polycystic ovary syndrome (PCOS), sex hormones, and obesity on circulating miRNA-21, miRNA-27b, miRNA-103, and miRNA-155 expression. J Clin Endocrinol Metab (2013) 98(11):E1835–44. doi: 10.1210/jc.2013-2218
84. Chen L, Zhang B, Yang L, Bai YG, Song JB, Ge YL, et al. BMAL1 disrupted intrinsic diurnal oscillation in rat cerebrovascular contractility of simulated microgravity rats by altering circadian regulation of miR-103/Ca(V)1.2 signal pathway. Int J Mol Sci (2019) 20(16):3947. doi: 10.3390/ijms20163947
85. Mu J, Yu P, Li Q. microRNA-103 contributes to progression of polycystic ovary syndrome through modulating the IRS1/PI3K/AKT signal axis. Arch Med Res (2021) 52(5):494–504. doi: 10.1016/j.arcmed.2021.01.008
86. Tao SC, Guo SC. Extracellular vesicles: Potential participants in circadian rhythm synchronization. Int J Biol Sci (2018) 14(12):1610–20. doi: 10.7150/ijbs.26518
87. Xia H, Zhao Y. miR-155 is high-expressed in polycystic ovarian syndrome and promotes cell proliferation and migration through targeting PDCD4 in KGN cells. Artif Cells Nanomed Biotechnol (2020) 48(1):197–205. doi: 10.1080/21691401.2019.1699826
88. Carloni S, Favrais G, Saliba E, Albertini MC, Chalon S, Longini M, et al. Melatonin modulates neonatal brain inflammation through endoplasmic reticulum stress, autophagy, and miR-34a/silent information regulator 1 pathway. J Pineal Res (2016) 61(3):370–80. doi: 10.1111/jpi.12354
89. Xiong W, Lin Y, Xu L, Tamadon A, Zou S, Tian F, et al. Circulatory microRNA 23a and microRNA 23b and polycystic ovary syndrome (PCOS): the effects of body mass index and sex hormones in an Eastern han Chinese population. J Ovarian Res (2017) 10(1):10. doi: 10.1186/s13048-016-0298-8
90. Nanda D, Chandrasekaran SP, Ramachandran V, Kalaivanan K, Carani Venkatraman A. Evaluation of serum miRNA-24, miRNA-29a and miRNA-502-3p expression in PCOS subjects: Correlation with biochemical parameters related to PCOS and insulin resistance. Indian J Clin Biochem (2020) 35(2):169–78. doi: 10.1007/s12291-018-0808-0
91. Sørensen AE, Udesen PB, Wissing ML, Englund ALM, Dalgaard LT. MicroRNAs related to androgen metabolism and polycystic ovary syndrome. Chem Biol Interact (2016) 259:8–16. doi: 10.1016/j.cbi.2016.06.008
92. Park I, Kim D, Kim J, Jang S, Choi M, Choe HK, et al. microRNA-25 as a novel modulator of circadian Period2 gene oscillation. Exp Mol Med (2020) 52(9):1614–26. doi: 10.1038/s12276-020-00496-5
93. Sørensen AE, Wissing ML, Englund AL, Dalgaard LT. MicroRNA species in follicular fluid associating with polycystic ovary syndrome and related intermediary phenotypes. J Clin Endocrinol Metab (2016) 101(4):1579–89. doi: 10.1210/jc.2015-3588
94. Kinoshita C, Aoyama K, Nakaki T. Neuroprotection afforded by circadian regulation of intracellular glutathione levels: A key role for miRNAs. Free Radic Biol Med (2018) 119:17–33. doi: 10.1016/j.freeradbiomed.2017.11.023
95. Anna G, Kannan NN. Post-transcriptional modulators and mediators of the circadian clock. Chronobiol Int (2021) 38(9):1244–61. doi: 10.1080/07420528.2021.1928159
96. Ding X, Jian T, Wu Y, Zuo Y, Li J, Lv H, et al. Ellagic acid ameliorates oxidative stress and insulin resistance in high glucose-treated HepG2 cells via miR-223/keap1-Nrf2 pathway. BioMed Pharmacother (2019) 110:85–94. doi: 10.1016/j.biopha.2018.11.018
97. Chakraborty C, Doss CG, Bandyopadhyay S, Agoramoorthy G. Influence of miRNA in insulin signaling pathway and insulin resistance: micro-molecules with a major role in type-2 diabetes. Wiley Interdiscip Rev RNA (2014) 5(5):697–712. doi: 10.1002/wrna.1240
98. Ashrafizadeh M, Ahmadi Z, Samarghandian S, Mohammadinejad R, Yaribeygi H, Sathyapalan T, et al. MicroRNA-mediated regulation of Nrf2 signaling pathway: Implications in disease therapy and protection against oxidative stress. Life Sci (2020) 244:117329. doi: 10.1016/j.lfs.2020.117329
99. Abdalla M, Deshmukh H, Atkin SL, Sathyapalan T. miRNAs as a novel clinical biomarker and therapeutic targets in polycystic ovary syndrome (PCOS): A review. Life Sci (2020) 259:118174. doi: 10.1016/j.lfs.2020.118174
100. Iacomino G, Siani A. Role of microRNAs in obesity and obesity-related diseases. Genes Nutr (2017) 12:23. doi: 10.1186/s12263-017-0577-z
101. Lionett S, Kiel IA, Camera DM, Vanky E, Parr EB, Lydersen S, et al. Circulating and adipose tissue miRNAs in women with polycystic ovary syndrome and responses to high-intensity interval training. Front Physiol (2020) 11:904. doi: 10.3389/fphys.2020.00904
102. Torres M, Becquet D, Franc JL, François-Bellan AM. Circadian processes in the RNA life cycle. Wiley Interdiscip Rev RNA (2018) 9(3):e1467. doi: 10.1002/wrna.1467
103. Zhou L, Miller C, Miraglia LJ, Romero A, Mure LS, Panda S, et al. A genome-wide microRNA screen identifies the microRNA-183/96/182 cluster as a modulator of circadian rhythms. Proc Natl Acad Sci U.S.A. (2021) 118(1):1–9. doi: 10.1073/pnas.2020454118
104. Knarr M, Nagaraj AB, Kwiatkowski LJ, DiFeo A. miR-181a modulates circadian rhythm in immortalized bone marrow and adipose derived stromal cells and promotes differentiation through the regulation of PER3. Sci Rep (2019) 9(1):307. doi: 10.1038/s41598-018-36425-w
105. Cheng Q, Fan X, Liu Y, Xu L, Dong P, Song L, et al. miR-455-5p regulates circadian rhythms by accelerating the degradation of clock mRNA. IUBMB Life (2021) 74:245–58. doi: 10.1002/iub.2587
106. Zhou B, Liu HY, Zhu BL, Yue AX. MicroRNA-141 protects PC12 cells against hypoxia/reoxygenation-induced injury via regulating Keap1-Nrf2 signaling pathway. J Bioenerg Biomembr (2019) 51(4):291–300. doi: 10.1007/s10863-019-09804-9
107. Khadrawy O, Gebremedhn S, Salilew-Wondim D, Taqi MO, Neuhoff C, Tholen E, et al. Endogenous and exogenous modulation of Nrf2 mediated oxidative stress response in bovine granulosa cells: Potential implication for ovarian function. Int J Mol Sci (2019) 20(7):1635. doi: 10.3390/ijms20071635
108. Sohel MMH, Akyuz B, Konca Y, Arslan K, Sariozkan S, Cinar MU. Oxidative stress modulates the expression of apoptosis-associated microRNAs in bovine granulosa cells in vitro. Cell Tissue Res (2019) 376(2):295–308. doi: 10.1007/s00441-019-02990-3
109. Doumatey AP, He WJ, Gaye A, Lei L, Zhou J, Gibbons GH, et al. Circulating MiR-374a-5p is a potential modulator of the inflammatory process in obesity. Sci Rep (2018) 8(1):7680. doi: 10.1038/s41598-018-26065-5
110. Kanemoto S, Nitani R, Murakami T, Kaneko M, Asada R, Matsuhisa K, et al. Multivesicular body formation enhancement and exosome release during endoplasmic reticulum stress. Biochem Biophys Res Commun (2016) 480(2):166–72. doi: 10.1016/j.bbrc.2016.10.019
111. Zeng Q, Hong S, Wang X, Cheng Y, Sun J, Xia W. Regulation of exosomes secretion by low-intensity pulsed ultrasound in lung cancer cells. Exp Cell Res (2019) 383(1):111448. doi: 10.1016/j.yexcr.2019.05.029
112. Beer L, Zimmermann M, Mitterbauer A, Ellinger A, Gruber F, Narzt MS, et al. Analysis of the secretome of apoptotic peripheral blood mononuclear cells: Impact of released proteins and exosomes for tissue regeneration. Sci Rep (2015) 5:16662. doi: 10.1038/srep16662
113. Lehmann BD, Paine MS, Brooks AM, McCubrey JA, Renegar RH, Wang R, et al. Senescence-associated exosome release from human prostate cancer cells. Cancer Res (2008) 68(19):7864–71. doi: 10.1158/0008-5472.CAN-07-6538
114. Zhang C, Xiao X, Chen M, Aldharee H, Chen Y, Long W. Liver kinase B1 restoration promotes exosome secretion and motility of lung cancer cells. Oncol Rep (2018) 39(1):376–82. doi: 10.3892/or.2017.6085
115. Almohammai A, Rahbarghazi R, Keyhanmanesh R, Rezaie J, Ahmadi M. Asthmatic condition induced the activity of exosome secretory pathway in rat pulmonary tissues. J Inflammation (Lond) (2021) 18(1):14. doi: 10.1186/s12950-021-00275-7
116. Xue Y, Lv J, Xu P, Gu L, Cao J, Xu L, et al. Identification of microRNAs and genes associated with hyperandrogenism in the follicular fluid of women with polycystic ovary syndrome. J Cell Biochem (2018) 119(5):3913–21. doi: 10.1002/jcb.26531
117. Diamanti-Kandarakis E, Dunaif A. Insulin resistance and the polycystic ovary syndrome revisited: an update on mechanisms and implications. Endocr Rev (2012) 33(6):981–1030. doi: 10.1210/er.2011-1034
118. Balasubramanyam M, Aravind S, Gokulakrishnan K, Prabu P, Sathishkumar C, Ranjani H, et al. Impaired miR-146a expression links subclinical inflammation and insulin resistance in type 2 diabetes. Mol Cell Biochem (2011) 351(1-2):197–205. doi: 10.1007/s11010-011-0727-3
119. Glueck CJ, Goldenberg N. Characteristics of obesity in polycystic ovary syndrome: Etiology, treatment, and genetics. Metabolism (2019) 92:108–20. doi: 10.1016/j.metabol.2018.11.002
120. Soh J, Iqbal J, Queiroz J, Fernandez-Hernando C, Hussain MM. MicroRNA-30c reduces hyperlipidemia and atherosclerosis in mice by decreasing lipid synthesis and lipoprotein secretion. Nat Med (2013) 19(7):892–900. doi: 10.1038/nm.3200
121. Murri M, Insenser M, Fernández-Durán E, San-Millán JL, Luque-Ramírez M, Escobar-Morreale HF. Non-targeted profiling of circulating microRNAs in women with polycystic ovary syndrome (PCOS): effects of obesity and sex hormones. Metabolism (2018) 86:49–60. doi: 10.1016/j.metabol.2018.01.011
122. Zhang X, Price NL, Fernández-Hernando C. Non-coding RNAs in lipid metabolism. Vascul Pharmacol (2019) 114:93–102. doi: 10.1016/j.vph.2018.06.011
123. Rayner KJ, Suárez Y, Dávalos A, Parathath S, Fitzgerald ML, Tamehiro N, et al. MiR-33 contributes to the regulation of cholesterol homeostasis. Science (2010) 328(5985):1570–3. doi: 10.1126/science.1189862
124. Horie T, Ono K, Horiguchi M, Nishi H, Nakamura T, Nagao K, et al. MicroRNA-33 encoded by an intron of sterol regulatory element-binding protein 2 (Srebp2) regulates HDL in vivo. Proc Natl Acad Sci U.S.A. (2010) 107(40):17321–6. doi: 10.1073/pnas.1008499107
125. Gerin I, Clerbaux LA, Haumont O, Lanthier N, Das AK, Burant CF, et al. Expression of miR-33 from an SREBP2 intron inhibits cholesterol export and fatty acid oxidation. J Biol Chem (2010) 285(44):33652–61. doi: 10.1074/jbc.M110.152090
126. Aryal B, Singh AK, Rotllan N, Price N, Fernández-Hernando C. MicroRNAs and lipid metabolism. Curr Opin Lipidol (2017) 28(3):273–80. doi: 10.1097/MOL.0000000000000420
127. Jordan SD, Krüger M, Willmes DM, Redemann N, Wunderlich FT, Brönneke HS, et al. Obesity-induced overexpression of miRNA-143 inhibits insulin-stimulated AKT activation and impairs glucose metabolism. Nat Cell Biol (2011) 13(4):434–46. doi: 10.1038/ncb2211
128. Ling HY, Wen GB, Feng SD, Tuo QH, Ou HS, Yao CH, et al. MicroRNA-375 promotes 3T3-L1 adipocyte differentiation through modulation of extracellular signal-regulated kinase signalling. Clin Exp Pharmacol Physiol (2011) 38(4):239–46. doi: 10.1111/j.1440-1681.2011.05493.x
129. Yoo SH, Kojima S, Shimomura K, Koike N, Buhr ED, Furukawa T, et al. Period2 3'-UTR and microRNA-24 regulate circadian rhythms by repressing PERIOD2 protein accumulation. Proc Natl Acad Sci U.S.A. (2017) 114(42):55–64. doi: 10.1073/pnas.1706611114
130. Gao Q, Zhou L, Yang SY, Cao JM. A novel role of microRNA 17-5p in the modulation of circadian rhythm. Sci Rep (2016) 6:30070. doi: 10.1038/srep30070
131. Chen X, Rosbash M. Mir-276a strengthens drosophila circadian rhythms by regulating timeless expression. Proc Natl Acad Sci U.S.A. (2016) 113(21):E2965–72. doi: 10.1073/pnas.1605837113
132. Wang H, Fan Z, Zhao M, Li J, Lu M, Liu W, et al. Oscillating primary transcripts harbor miRNAs with circadian functions. Sci Rep (2016) 6:21598. doi: 10.1038/srep21598
133. Cheng HY, Papp JW, Varlamova O, Dziema H, Russell B, Curfman JP, et al. microRNA modulation of circadian-clock period and entrainment. Neuron (2007) 54(5):813–29. doi: 10.1016/j.neuron.2007.05.017
134. Gao Y, Chen J, Ji R, Ding J, Zhang Y, Yang J. USP25 regulates the proliferation and apoptosis of ovarian granulosa cells in polycystic ovary syndrome by modulating the PI3K/AKT pathway via deubiquitinating PTEN. Front Cell Dev Biol (2021) 9:779718. doi: 10.3389/fcell.2021.779718
135. Xie F, Zhang J, Zhai M, Liu Y, Hu H, Yu Z, et al. Melatonin ameliorates ovarian dysfunction by regulating autophagy in PCOS via the PI3K-akt pathway. Reproduction (2021) 162(1):73–82. doi: 10.1530/REP-20-0643
136. Ling HY, Ou HS, Feng SD, Zhang XY, Tuo QH, Chen LX, et al. CHANGES IN microRNA (miR) profile and effects of miR-320 in insulin-resistant 3T3-L1 adipocytes. Clin Exp Pharmacol Physiol (2009) 36(9):e32–9. doi: 10.1111/j.1440-1681.2009.05207.x
137. Luo Y, Cui C, Han X, Wang Q, Zhang C. The role of miRNAs in polycystic ovary syndrome with insulin resistance. J Assist Reprod Genet (2021) 38(2):289–304. doi: 10.1007/s10815-020-02019-7
138. Katayama M, Wiklander OPB, Fritz T, Caidahl K, El-Andaloussi S, Zierath JR, et al. Circulating exosomal miR-20b-5p is elevated in type 2 diabetes and could impair insulin action in human skeletal muscle. Diabetes (2019) 68(3):515–26. doi: 10.2337/db18-0470
139. Yu Y, Li G, He X, Lin Y, Chen Z, Lin X, et al. MicroRNA-21 regulate the cell apoptosis and cell proliferation of polycystic ovary syndrome (PCOS) granulosa cells through target toll like receptor TLR8. Bioengineered (2021) 12(1):5789–96. doi: 10.1080/21655979.2021.1969193
140. Runtsch MC, Nelson MC, Lee SH, Voth W, Alexander M, Hu R, et al. Anti-inflammatory microRNA-146a protects mice from diet-induced metabolic disease. PloS Genet (2019) 15(2):e1007970. doi: 10.1371/journal.pgen.1007970
Keywords: Polycystic ovary syndrome, miRNAs, circadian rhythm, exosomes, inflammation, oxidative stress
Citation: Chen W-h, Huang Q-y, Wang Z-y, Zhuang X-x, Lin S and Shi Q-y (2022) Therapeutic potential of exosomes/miRNAs in polycystic ovary syndrome induced by the alteration of circadian rhythms. Front. Endocrinol. 13:918805. doi: 10.3389/fendo.2022.918805
Received: 12 April 2022; Accepted: 19 October 2022;
Published: 16 November 2022.
Edited by:
Srabani Mukherjee, National Institute for Research in Reproductive Health (ICMR), IndiaReviewed by:
Francisco A Martin, Cajal Institute (CSIC), SpainCopyright © 2022 Chen, Huang, Wang, Zhuang, Lin and Shi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Qi-yang Shi, d3NxeTIxNEAxNjMuY29t; Shu Lin, c2h1bGluMTk1NkAxMjYuY29t
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.